Magnesium (Mg2+) Deficiency, Not Well-Recognized Non-Infectious Pandemic: Origin and Consequence of Chronic Inflammatory and Oxidative Stress-Associated Diseases
bPostgraduate in Biological Sciences, National Autonomous University of Mexico, Ciudad Universitaria, Mexico City, 04510, Mexico
Keywords
Abstract
Introduction
Crucial micronutrients such as magnesium (Mg2+) are essential for correct body function. Its deficiency is associated with the development of comorbidities such as diabetes, obesity, and cardiovascular diseases (CVD, i.e., heart failure, arrhythmias, atherosclerosis, stroke, and hypertension) [1–6]. These comorbidities are frequently associated with an increase in inflammatory markers and oxidative stress (OS), in which Mg2+ deficiency may play an important role [2, 7,8]. Subclinical Mg2+ deficiency is widespread worldwide, mainly due to insufficient dietary intake [6, 9–16]. Unfortunately, this deficiency is difficult to detect but stimulates the production of cytokines in cells, causing chronic inflammation and, consequently, OS [17, 18].
This narrative review focuses on Mg2+ deficiency, its complications, and its relationship with OS and chronic inflammatory diseases. We highlight the potential importance of increasing Mg2+ intake worldwide to attenuate manifestations and symptoms derivate from Mg2+ deficiency. Our exhaustive review of the scientific literature was conducted in the “PubMed databases”. Search keyword terms included all possible combinations, abbreviations, and synonyms between “magnesium”, “magnesium deficiency”, “magnesium supplementation”, “cardiovascular diseases”, “Diabetes”, “oxidative stress”, and “inflammation.” We also considered the publication date from 1957 to 2022.
Mg2+ body functions
Mg2+ is the fourth most abundant intracellular ion in the human body [18, 19]. Mg2+ is essential to cellular processes, including energetic metabolism, protein and amino acid synthesis, and maintenance of the electrical potential of nerve tissues and cell membranes [18, 20]. Many enzymes that are vital for life require Mg2+. It is estimated that Mg2+ acts as a cofactor for over 600 enzymes and an activator in other 200 enzymes [21]. Fundamentally, Mg2+ participates as a cofactor in several complex electron transport chain subunits, including methylenetetrahydrofolate dehydrogenase 2 and pyruvate dehydrogenase phosphatase [22]. In this respect, Mg2+ is needed to feed the electron transport chain with nicotinamide adenine dinucleotide reduced (NADH) and flavine-adenine dinucleotide reduced (FADH2) due to acetyl coenzyme A (acetyl-CoA) requires Mg2+ to enter the tricarboxylic acid cycle [23, 24]. Also, Mg2+ is fundamental to signal transduction processes requiring kinases because almost all transphosphorylation reactions require Mg2+ [25]. Mg2+ is needed for all the reactions in which ATP participates; binding sites of the substrate in kinases, ATPases, guanylyl cyclases, and adenylyl cyclases are specific to the Mg-ATP complex [21]. In this sense, 538 kinases have been identified that comprise the human kinome, and an example of them are glycolytic enzymes, i.e., hexokinase, phosphofructokinase, aldolase, phosphoglycerate kinase, and pyruvate kinase [21, 26]. Mg2+ is also necessary for the structure and activity of DNA and RNA polymerases. Mg2+ is required for the enzyme to make conformational changes during catalytic reactions [27]. Mg2+ also participates in muscle relaxation, neurotransmission, and stabilizing of the cellular membrane (reducing its fluidity and permeability indirectly by disturbances in lipid metabolism) [28–31]. Mg2+ is a key component in mediating protein synthesis through stabilizing the structure of ribosomes, stabilizing the secondary structure of ribosomal RNA (rRNA), and ribosomal binding proteins to rRNA [32]. Mg2+ binds to rRNA and ribosomal proteins alleviating electrostatic phosphates repulsion; they translate the genetic information encoded by mRNA [32, 33]. When Mg2+ concentration is low (e.g., 10 mM in 70S ribosomes from Escherichia coli), the ribosome dissociates with the release of ribosomal components, stopping polypeptide synthesis [33, 34].
Moreover, Mg2+ is also necessary to transport vitamin D and activate it [35, 36]. Vitamin D binding protein (VDBP) and vitamin D receptor (VDR) are Mg2+ dependent for binding vitamin D [37]. Also, the enzymes responsible for vitamin D metabolism require Mg2+ as a cofactor for 25 hydroxylations of vitamin D in the liver and 1 hydroxylation in the kidneys [37]. Besides, Mg2+ may act as a second messenger in different cell signal pathways [38, 39]. For example, the Mg2+ cation has been described as a second signaling messenger in T cells [4, 21, 39]. Thus, Mg2+ has a closer relationship with adaptative immunity, mainly related to signaling and immunomodulatory pathways [20, 40, 41]. To summarize, Mg2+ has multiple functions, primarily associated with energy metabolism; its deficiency causes mitochondrial dysfunction and damage, increasing reactive oxygen species (ROS) production, which, in addition to the inflammatory response observed in Mg2+ deficiency, leads to chronic metabolic diseases [3, 17, 42, 43].
Mg2+ homeostasis
Mg2+ homeostasis is maintained by the intestine, bone, and kidneys [40]. In the small intestine, Mg2+ reabsorption is mediated by the passive paracellular pathway dependent on an electrochemical gradient. However, a small portion is absorbed by the large intestine mediated by transient receptor potential melastatin 6 and 7 channel (TRPM6 and TRPM7), which also involve calcium absorption [21, 40]. Proteins that transport Mg2+ are required to recognize the large, hydrated cation, remove its hydration layer, and deliver the dehydrated ion to the Mg2+ transporters for transcellular transport across the membrane [44]. It has been reported that in normal consumption of 370 mg, the intestine only absorbs between 30-50% of Mg2+, and the not absorbed Mg2+ is eliminated in the feces [21].
Bone is the most important Mg2+ reservoir, containing around 65%, residing in the bone at hydroxyapatite crystals surface; 34% is intracellular, less than 1% is extracellular, and only 0.3% is found in serum. Bone surface Mg2+ or exchangeable Mg2+ pool is continuously exchanged with blood Mg2+. During Mg2+ depletion, the Mg2+ concentration in bone exchangeable Mg2+ pool decreases to maintain blood Mg2+, reducing bone formation [45]. Additionally, during Mg2+ deficiency, increased proinflammatory cytokines such as substance P, tumor necrosis factor-alpha (TNF-α), and interleukin (IL)1 promote osteoclastic bone resorption [46].
The kidney maintains the serum concentration of Mg2+. Approximately 70% of the total serum Mg2+ is not protein bound, making it available for glomerular filtration. However, Mg2+ can be reabsorbed in the ascending limb of the loop of Henle (65-75%) and the proximal convoluted tubule (5-15%) using paracellular pathways. Also, the distal convoluted tubule reabsorbs 5-10% of Mg2+ through TRPM6/7 channels [47]. Under normal conditions, 96% of the filtered Mg2+ is reabsorbed, and the body's Mg2+ balance is delicately adjusted by urinary excretion [47].
To summarize, the intestine, bones, and kidneys maintain the serum Mg2+ concentration; kidneys play a central role because gastrointestinal absorption is balanced by renal excretion (Fig. 1).
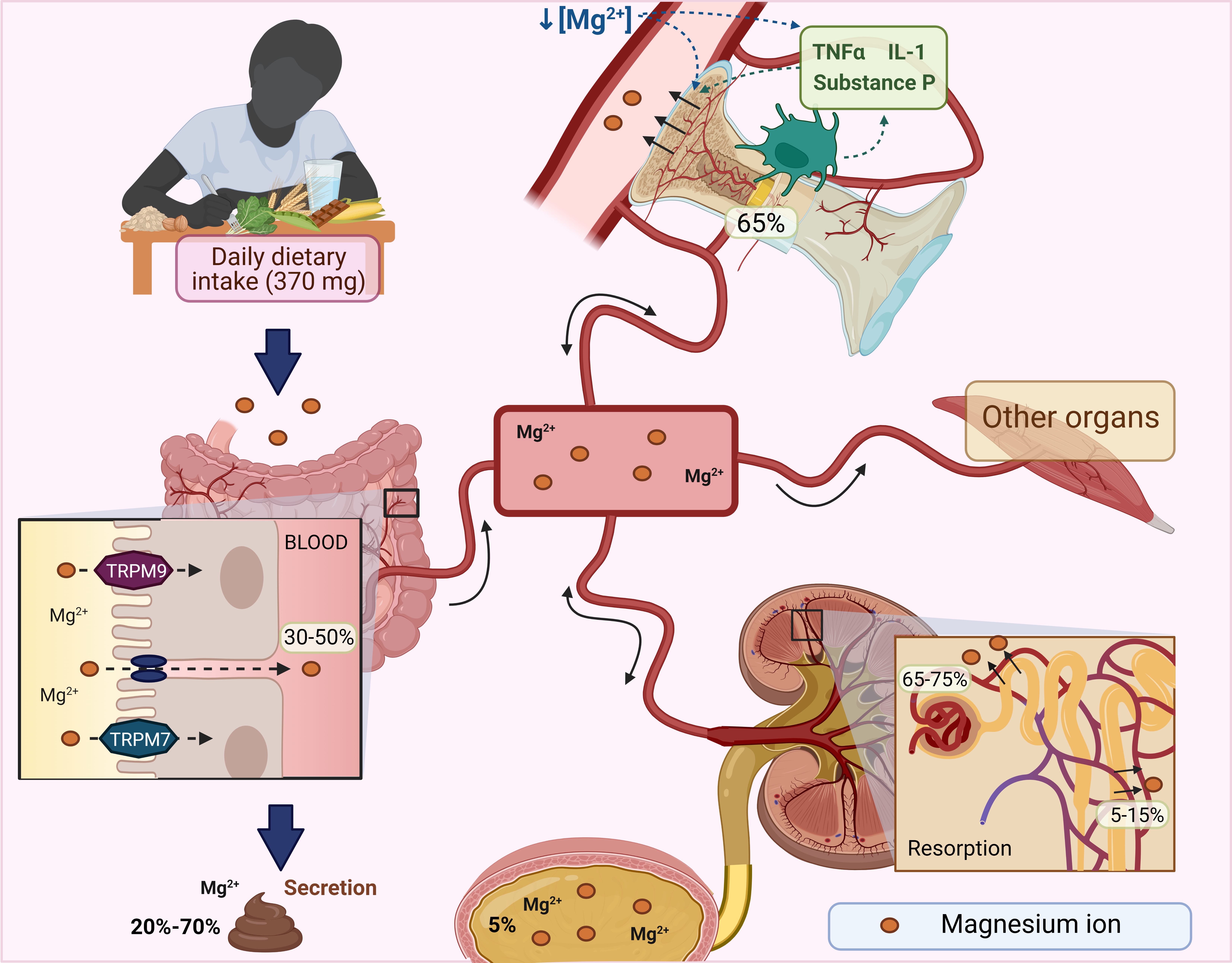
Fig. 1: Magnesium homeostasis (Mg2+). The Mg2+ consumed through the diet is absorbed throughout the entire gastrointestinal tract and into the blood, while that not absorbed is excreted in the feces. Once in the blood, the Mg2+ passes quickly to the different tissues. The kidney is essential to Mg2+ homeostasis since the most significant amount is filtered here, and only about 5% is excreted in the urine. Under conditions of Mg2+ deficiency, the concentration of exchangeable Mg2+ in bone decreases to maintain Mg2+ in the blood, reducing bone formation. In addition, they increase proinflammatory cytokines that promote osteoclastic bone resorption. IL: interleukins, TNFα: tumor necrosis factor-α, TRPM: transient receptor potential melastatin. Created with biorender.com (published with permission from biorender.com).
Mg2+ intake
The primary source of Mg2+ is the diet [48]. Mg2+ intake recommendations are provided in the Dietary Reference Intakes (DRI), which are developed by the Food and Nutrition Board (FNB) at the National Academies Institute of Medicine (formerly the National Academy of Sciences) [49]. DRI is the set of reference values used to plan and assess the nutrient intake of healthy people. These values vary by age and gender and include a) the recommended dietary allowance (RDA), which refers to the average daily level of intake sufficient to meet the nutrient requirements of nearly all healthy people (97–98%); b) adequate intake (AI), which is the intake that guarantees nutritional adequacy; c) the estimated average requirement (EAR) which is equivalent to the average daily level of consumption estimated to meet the requirements of 50% of healthy individuals; and finally d) the tolerable upper intake level (UL), which refers to supplemented Mg2+, that is, that which is not consumed in food because it is more for pharmacological use [49, 50]. Table 1 lists the different reference values for Mg2+ [49].
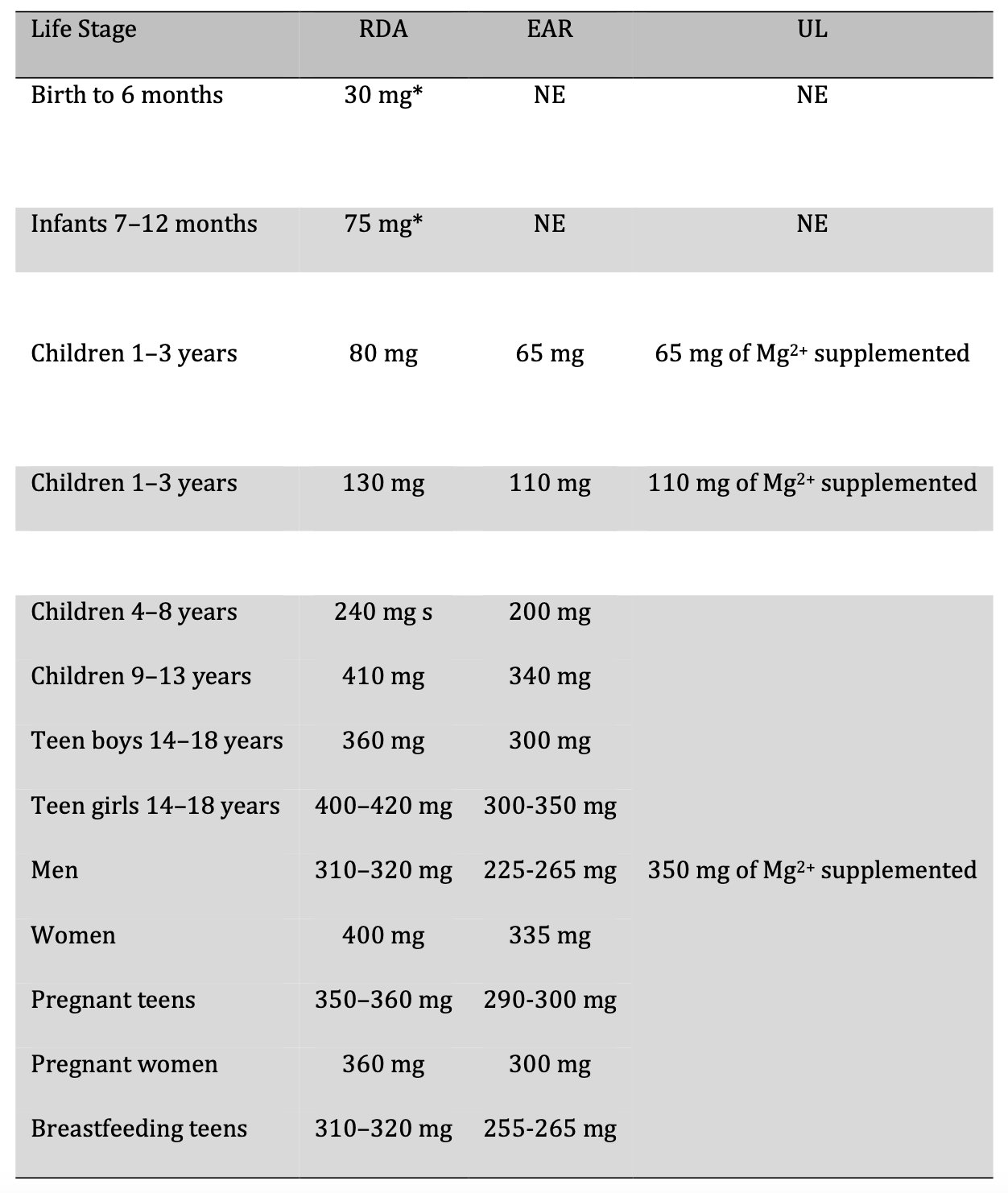
Table. 1: Dietary Reference Intakes (DRI) for Magnesium Intake (Mg2+). RDA: recommended dietary intake, EAR: estimated average requirement, UL: tolerable upper intake level, NE: not established. * Adequate Intake (AI)
Whole grains are considered the best dietary source of Mg2+. In fact, Mg2+ has been linked to most of the benefits of whole grain intake, including reduced risk of diabetes, coronary heart disease, stroke, and various types of cancer [51]. Also, leafy-green foods (e.g., chard, spinach, purslane), nuts, peas, and green lentils are good sources of Mg2+. Other foods with high levels of Mg2+ are dark chocolate, black beans, avocados, and some other fruits, also seeds such as pumpkin and chia seeds [52–55].
Mineral water is another important source of Mg2+ in the diet [56, 57]. Due to the relatively frequent consumption of water for drinking and food preparation, mineral water as a source of Mg2+ may be an essential part of the daily Mg2+ intake. However, the quality of the water is essential since the available Mg2+ content depends on it. Using hard water (calcium and Mg2+ concentration of 100-200 mg/L) to boil food rich in Mg2+ may prevent its loss, while boiling this food in soft water (calcium and Mg2+ concentration less than 100 mg/L) may leach out it [58]. In this respect, many studies have found a relationship between drinking water mineral content and CVD risk [59–68]. Catling et al. [69] conclude with an extensive review of epidemiological studies that there was significant evidence of an inverse association between Mg2+ content in drinking water and cardiovascular mortality. Sabatier et al. [70] showed in a study with ten healthy white women (aged 25-45) that Mg2+ from Mg2+ rich mineral water (110 mg/L) is highly bioavailable, with a ≈50% Mg2+ absorption from mineral water consumed, being even better when water was consumed with a light meal (may due the transit time of Mg2+ in the intestine). Thus, mineral Mg2+-rich water is a calorie-free good source of Mg2+. Mg2+ bioavailability is comparable for mineral waters with different mineralization levels or other food such as bread and dietary supplements [56].
However, most of the population does not consume these rich Mg2+ foods and water daily; therefore, it is insufficient to cover the dietary reference intake (DRI), leading to Mg2+ deficiency. Blache et al. [8] have shown in a preclinic study that a long-term moderate Mg2+ deficiency diet is closely related to increased mortality, blood pressure, inflammation, and lipid oxidation. Also, they demonstrated that these effects were mainly due to chronic impairment of redox status associated with inflammation, and these effects can be normalized or improved with Mg2+ supplementation. In addition, it has been seen that a high intake of processed foods provides low amounts of Mg2+. Food processing, which can range from cooking to refining, causes a substantial loss of Mg2+ [71, 72]. Since a large part of the population has opted for refined cereals consumption, the intake of trace elements such as Mg2+, which are found in the pericarp of cereal grains, has decreased notably [72]. For this reason, subclinical Mg2+ deficiency has been observed more frequently, mainly in populations that consume processed foods, such as the U.S. and countries with a Western diet [6, 10–15, 73, 74].
Mg2+ deficiency
Mg2+ deficiency means body deficiency, including hypomagnesemia (specifically serum deficiency). Low levels of Mg2+ characterize Mg2+ deficiency and depends on its chronicity and status. For instance, Nielsen et al. [75] demonstrated a significant deprivation of red blood cell membrane Mg2+ in healthy postmenopausal women. They were on a restrictive diet of approximately 33% of the DRI of Mg2+ for 78 days. Thus, these authors concluded that Mg2+ deficiency is mainly associated with chronic inadequate Mg2+ intake [75].
Due to its facility and cost, total serum Mg2+ is the most used measure to diagnose Mg2+ deficiency clinically. The normal serum Mg2+ concentration is between 0.850 and 0.955 mmol/L [76]; if the serum Mg2+ concentration is below 0.7 mM, it is hypomagnesemia. According to Liu and Dudley Jr [3]., mild to moderate hypomagnesemia is when serum Mg2+ is between 0.5–0.69 mM, and severe hypomagnesemia is when serum Mg2+ is lower than 0.5 mM. Hypermagnesemia is characterized by high levels than normal serum concentrations of Mg2+ [3].
Unfortunately, even with a total serum Mg2+ level in the acceptable range, there may exist deficiency since approximately 55% of serum Mg2+ is in its bioactive form. At the same time, the rest is bound to proteins such as albumin or an anionic complex [77, 78]. Although Mg2+ serum concentrations are the main form to describe abnormalities in the Mg2+ status, these are very unspecific, providing inaccurate body Mg2+ status data. For instance, body Mg2+ homeostasis in other tissues, including bone, the main reservoir, provides Mg2+ through bone resorption during Mg2+ deficiency or insufficient Mg2+ intake, but this is related to a lower bone mineral density [79–81]. Mg2+ deficiency has detrimental effects on skeletal health, contributing to osteoporosis [81]. Thus, normal serum Mg2+ concentrations could mask Mg2+ deficiency in other tissues like bone.
Also, some conditions affect circulating Mg2+ concentrations; an example of this is an abnormal state in the acid-base balance in the blood as slight acidosis. Defects can cause acidosis in renal tubules that facilities the reabsorption of bicarbonate or secretion of protons [82], also during a failure of respiratory ventilation due to carbon dioxide accumulation [83]. Acidosis generally occurs due to increased acid production, decreased acid excretion, acid ingestion, and bicarbonate losses [84]. That serum acid increase can release Mg2+ from the bone surface, artificially increasing the Mg2+ detected in serum that can mask hypomagnesemia [9]. In addition, the acidosis significantly increasing urine Mg2+ excretion [28, 85]. Thus, acidosis masks hypomagnesemia and induces Mg2+ excretion, harming Mg2+ homeostasis.
The positive correlation between hypomagnesemia, higher morbidity, and mortality in hospitalized patients in an intensive care unit (ICU) [86, 87] makes it fundamental to know the general Mg2+ status. Thus preventing increased risk parameters associated with mortality (i.e., high C-reactive protein (CRP) serum levels and electrolytic abnormalities) [86, 87]. Various methods of assessing Mg2+ status, from surveys to clinical concentration data, have been extensively reviewed [88–91]. Not all the methods are of clinical utility to diagnose hypomagnesemia, but these indicate clinical or subclinical Mg2+ deficiency. These are considered measures for the evaluation of the status of the nutrient [88, 91, 92]. To obtain a valid assessment of Mg2+ status, Costello and Nielsen [88] proposed the combined determination of the concentration of serum Mg2+, the 24-hour urine Mg2+ excretion, and the intake diet. Due to difficulties in hypomagnesemia detection, it has proposed a sensible measurement of the bioactive form concentrations of whole blood from acute oral Mg2+ intake compared to serum and urine total Mg2+ [88].
Mg2+ deficiency can represent a potential risk to health [1, 4,93, 94]. An association between Mg2+ deficiency and sudden death has even been suggested [95]. In a preclinical study by Fiset et al. [96], rats assigned to an Mg2+-free diet with consequent hypomagnesemia commonly died from episodes of sudden death after inadvertent startles. Because seizures preceded sudden death, the authors concluded that sudden cardiac death was probably due to a neurological trigger's interaction and ventricular repolarization dispersion [96]. Depending on the degree of Mg2+ deficiency and its chronicity, it can present from a mild clinical presentation, such as weakness or fatigue, and escalate to severe and life-threatening complications such as arrhythmias, heart failure, or electrolyte disorders (Table 2) [3, 9,17, 18, 21, 36, 40, 93, 94, 97].
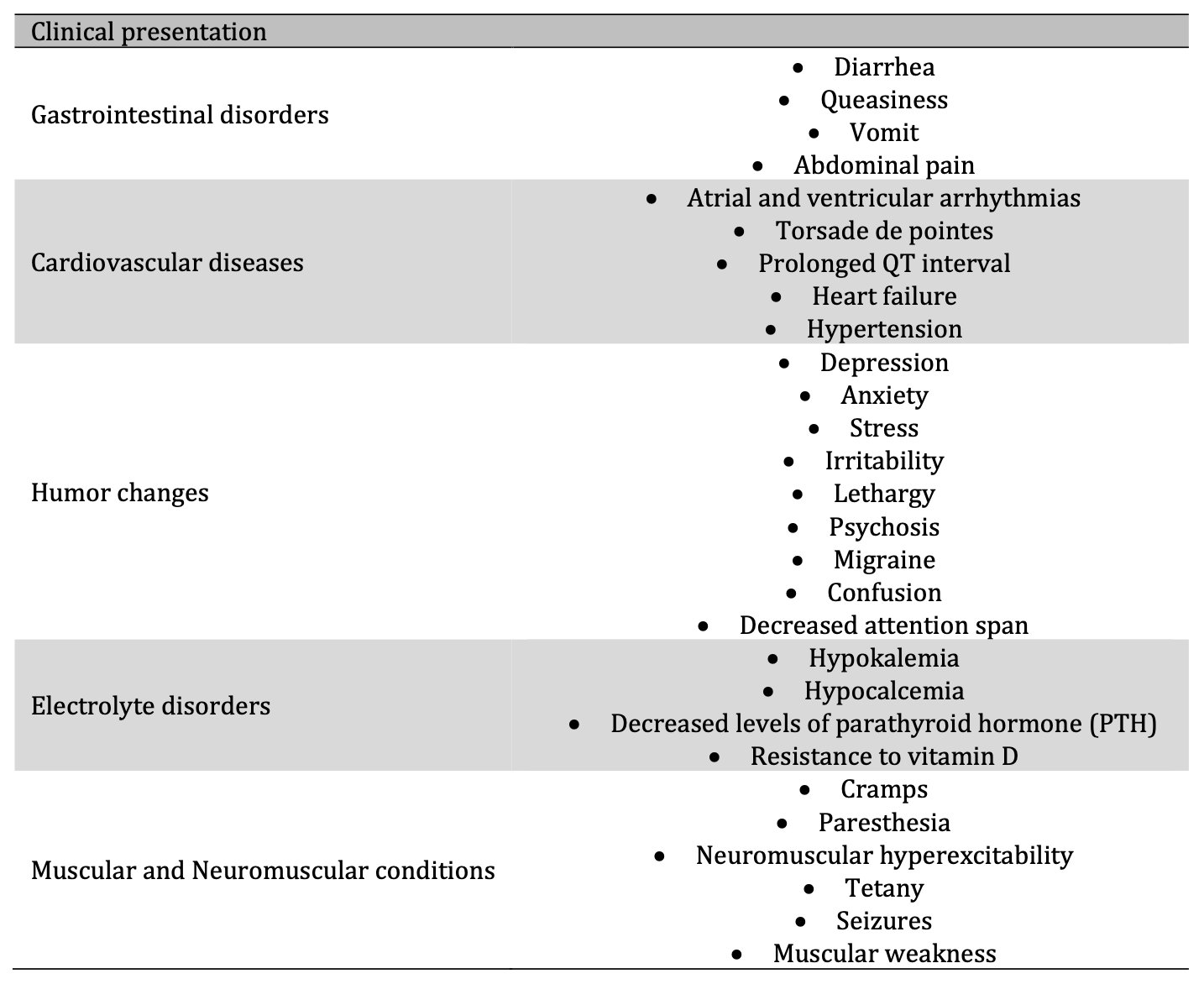
Table. 2: Mg2+ deficiency clinical presentation
Mg2+ deficiency can decrease the synthesis of proteins, carbohydrates, lipids, and genetic material [40]. It could also affect the functioning of the other micronutrients, such as reducing the number of VDRs available in vitamin D target cells [98, 99]. When Mg2+ deficiency is acute, muscle cramps help to its diagnosis [18]. However, in a chronic clinical deficiency, the symptoms are less severe, infrequent, and nonspecific, making its diagnosis difficult [18].
The causes of Mg2+ deficiency are many and very frequent
Abnormal Mg2+ levels during Mg2+ deficiency can be attributed to various factors. Intrinsic factors are insufficient intake or gastrointestinal insufficiency, decreased absorption due to injury to the intestinal epithelium (e.g., damage from alcoholism), kidney damage, and replacement therapies [17, 20, 100, 101]. At the same time, extrinsic factors may be diuretics that alter the renal tubule's reabsorption due to alterations in the electrochemical gradient. Loop diuretics decrease Mg2+ reabsorption, and thiazide diuretics reduce Mg2+ reabsorption and enhance its excretion [102, 103]. Also, some others are related to lower levels of Mg2+ in soil due to Mg2+ leaching, consequently affecting food levels [104]. An example is the decreased mineral concentration reported in wheat for the past several decades [105–107]. Fan et al. [106] showed a significant decrease of 27% in the concentration of Mg2+ in wheat from 1968. The authors conclude that significant changes were made that year in cultivars due to the "Green Revolution," with higher grain yields but a dilution effect on mineral concentration.
As in wheat, other comparative studies of ancient and modern times observed a historical depletion in the concentration of minerals in food [108–110]. Unfortunately, this decrease in the concentration of Mg2+ is observed in fruits, vegetables, and cereals, affecting other food groups such as their derivatives and animal origin [108]. The latter means that people need to eat more servings of food to obtain the same Mg2+ content as in the past, which is especially problematic due to metabolic syndrome problems in the current population [107].
In industrialized countries, clinical and subclinical Mg2+ deficiency is increasing, which can be associated with pathological states [1, 4,73, 74, 76, 93]. Multiple factors contribute to Mg2+ deficiency. For example, in people with diets high in phosphate (PO43-), Mg2+ absorption may be decreased due to the ability of PO43- to bind to Mg2+, reducing its availability [9, 28, 93, 111]. In general, the main source of phosphorus comes from soda (phosphoric acid) and inorganic PO43- contained in many ingredients used in processed foods (i.e., meat products). Dairy (especially cheese) also contributes to increasing Mg2+ requirements due to their phosphorus-magnesium-calcium ratio [93, 111]. Diets high in dietary fiber decrease the absorbed fraction of Mg2+. Fiber phytate decreases Mg2+ absorption because Mg2+ binds to the PO43- groups of phytic acid [28, 112]. In addition to the abovementioned cases, other factors contribute to Mg2+ deficiency, such as chronic diseases, gastrointestinal disorders, elderly age, and emotional stress (Table 3) [6, 9,17, 20, 93, 97, 100, 111]. The following list shows factors that contribute to Mg2+ deficiency:
- Diets with refined and processed foods
- Chronic diseases (kidney disease, cancer, insulin resistance, diabetes)
- Gastrointestinal disorders (intestinal lesions, Chhorn’s disease, irritable bowel syndrome, celiac syndrome, celiac disease, gastroenteritis ulcerative colitis)
- Drugs (diuretics, insulin, proton pump inhibitors)
- Chronic stress
- Strenuous physical exercise
- Deficiency or excess of vitamin D (lack causes less absorption of Mg2+, the excess causes excessive absorption of Ca 2+)
- Excessive supplementation or high levels content of other micronutrients in the diet such as Ca 2+ and phosphorus
- Elderly age
- Alcoholism
- Intake of coffee and tea (caffeine)
- High saturated fat in the diet
- Excessive menstruation
- Emotional stress (overactivation of the sympathetic nervous system)
- Laxative abuse
- High intake of dietary fiber and phytic acid
- Metabolic acidosis
Subclinical Mg2+ deficiency is the most common in the population, especially in countries that consume refined or ultra-processed products [9, 73, 74, 93]. The 2013-2016 National Health and Nutrition Examination Survey (NHANES) conducted on the US population showed that approximately 48% of the general population over one year does not reach the adequate intake of Mg2+. Moreover, in people older than 19 years (adult population), just over 50% of the population does not have consumption habits that cover the DRI [113].
According to an analysis of the 2006 national health and nutrition survey conducted on the Mexican population, 35% of adult men and women older than 20 have low serum concentrations of Mg2+ [10]. In addition, 64.2% of women and 25.2% of men presented a low ingestion of Mg2+ compared with the DRI [10]. Based on the same survey, Cruz-Góngora et al. [114] reported that in the 12 to 19-year-old population, the overall prevalence of low serum Mg2+ was 37.68%, and at least 50% of the analyzed population did not comply with the DRI [114]. In the case of the child population, Morales-Ruán et al. [11] reported that the nutritional status of Mg2+ in Mexican children from 1 to 11 years old is deficient, and the prevalence of low serum Mg2+ concentrations is 22.6% for this population. The lowest prevalence (9.1%) of low serum Mg2+ concentrations is in the population 1 to 2 years old [11]. The latter evidence shows the trend toward increasing Mg2+ deficiency prevalence with age.
At a global level, the consumption of Mg2+ in the diet is deficient and generalized in the populations (Table 4) [6, 9–16, 115]. Subclinical Mg2+ deficiency has been observed more frequently, mainly in populations consuming processed food, such as the US and countries with a Western diet [1, 4,9, 73, 74, 76, 93].
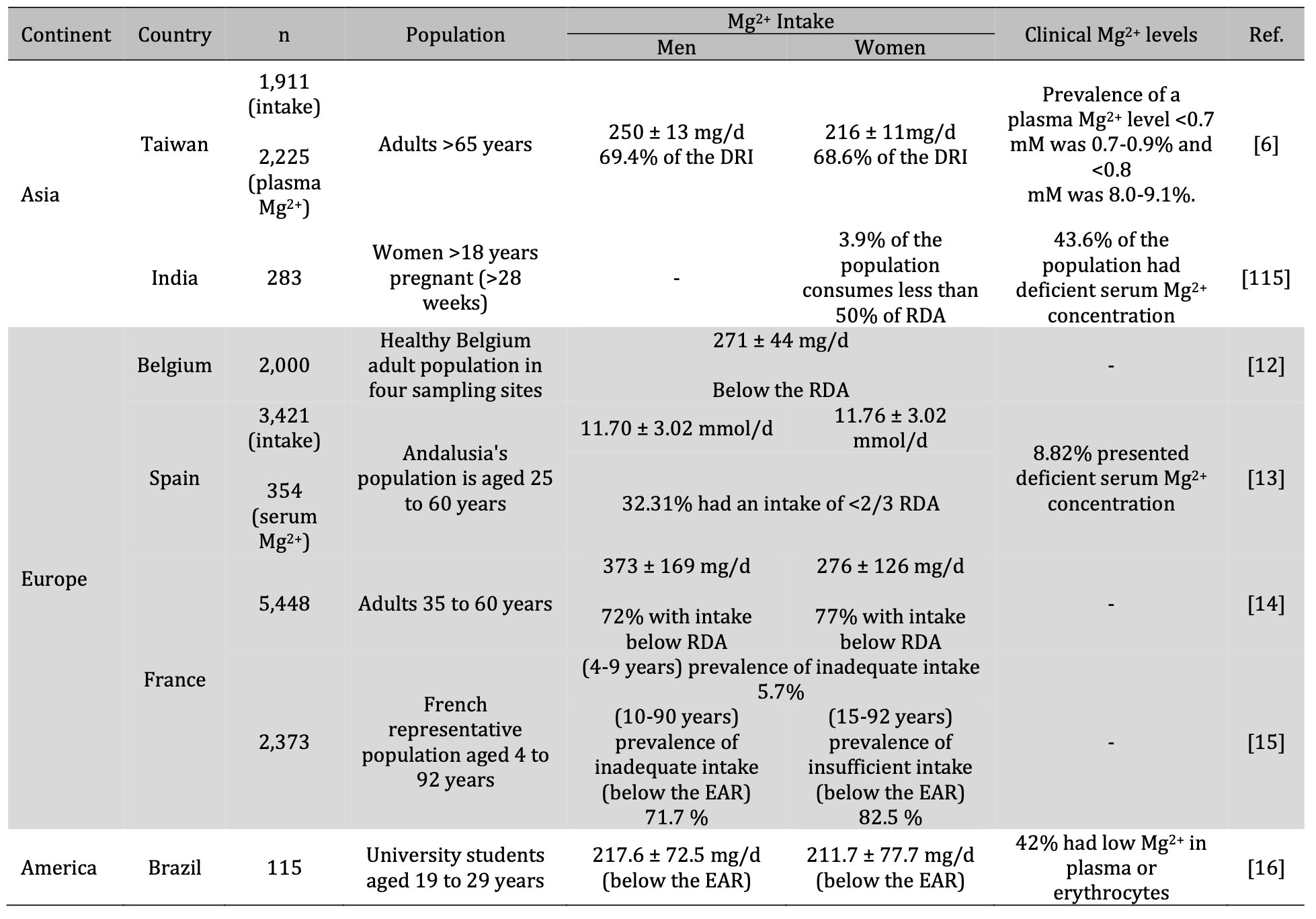
Table. 3: Mg2+ deficiency is global and general. Mg2+: magnesium; mg/d: milligrams per day; mmol/d: millimole per day; DRI: Dietary Reference Intakes; RDA: Recommended Dietary Allowances; EAR: Estimated Average Requirement
In addition to the countries mentioned above, DiNicolantonio et al. [93] included Japan and Ukraine as countries consuming insufficient amounts of Mg2+. The latter derives from the results obtained in the National Nutrition Survey in Japan in 2002, where it was found that for people aged 15 to 49 years, the intake of Mg2+ was below the Japanese recommended daily dose. Moreover, in Kiev (Ukraine), men between the ages of 20 and 59 years (n= 780) consumed 10% less than the recommended Mg2+ intake.
Mg2+ deficiency is difficult to detect at an early stage since bone compensation of Mg2+ maintains normal serum Mg2+ levels; and the absence of signs or symptoms [45, 116]. Knowing the general body Mg2+ status is essential to avoid other related Mg2+ deficiency complications, such as chronic inflammation and excessive production of ROS. To properly diagnose and treat Mg2+ deficiency, it is necessary to carry out more than one measurement of the Mg2+ levels method. It is suggested that due to the compensation of the homeostasis of Mg2+, the detection of low levels of Mg2+with a single method cannot be a good indicator of deficiency
In summary, many factors could contribute to developing a chronic deficiency. It is clear that Mg2+ intake is inadequate worldwide, and Mg2+ deficiency is a potential public health problem; nevertheless, the consequences of this deficiency are more frequently reflected in older adults.
Relationship between Mg2+ deficiency with OS and inflammation
Mg2+ deficiency has been widely correlated to the development of OS [3, 117]. OS is defined as “an imbalance between the generation of oxidants (ROS and reactive nitrogen species) and their removal systems (antioxidants) in favor of oxidants, leading to disruption of redox signaling and control and/or molecular damage” [118]. Mitochondria are the primary source of ROS production, and mainly, when mitochondria suffer structural or functional damage, excessive ROS production is generated [119]. Studies have shown that Mg2+ deficiency causes mitochondrial dysfunction [43, 120]. Mitochondria are the main reservoirs of Mg2+ in most cells (with mitochondrial Mg2+ concentrations between 0.2 and 1.5 mM) [121]. However, intracellular Mg2+ deficiency inhibits Mg2+ transport to the mitochondria through mitochondrial RNA splicing protein 2 (MRS2) and promotes mitochondrial Mg2+ efflux via solute carrier family 41, member 3 (SLC41A3), leading to decreased mitochondrial Mg2+ [3]. Mitochondrial Mg2+ deficiency decreases the activity of the electron transport chain, which alters coupled respiration [122–124] and increases the production of mitochondrial ROS [125, 126]. In addition, the antioxidant defense system (such as superoxide dismutase (SOD), catalase, and glutathione) is suppressed, and ATP synthase (F0F1) is downregulated, causing a decrease in ATP concentration [127–129]. In turn, the decrease in ATP causes an increase in the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) [130].
Mg2+ deficiency also causes depolarization of the mitochondrial membrane potential (ΔΨm) [131] by promoting the opening of the mitochondrial ATP-sensitive potassium (K) channel [132], the anion channel of the inner membrane (IMAC) [133] and the mitochondrial permeability transition pore (PTP) [134]. These effects exacerbate ROS production and lead to apoptosis, where Bcl-2-associated X (Bax) and the voltage-gated anion channel (VDAC) increase cytochrome C release, leading to apoptosome formation [135]. In addition, antiapoptotic proteins such as the Bcl-2 family are decreased, and proapoptotic proteins such as HIF-1α and p38/JNK are increased [136].
On the other hand, Mg2+ deficiency also increases the concentration of calcium (Ca) in the mitochondria through the mitochondrial Ca uniporter (MCU) [131, 137], which could alter ΔΨm. In contrast, Ca leakage from mitochondria via VDAC increases with apoptosis induced by Mg2+ deficiency. Other mechanisms that explain the increase in intracellular calcium in situations of Mg2+ deficiency include the activation of N-methyl-D-aspartate (NMDA) receptors in neural cells and L-type calcium channels in adipose tissue [2, 138].
The excess of intracellular Ca results in the activation of Ca-dependent processes, such as the release of inflammatory cytokines and the activation of NOX by phosphorylation of protein kinase C (PKC), the activation of nitric oxide synthase (NOS) and the calcium-dependent calmodulin complex, which exacerbates ROS production [1]. Likewise, the increase in Ca stimulates the release of catecholamines, and it has been proven that catecholamines increase the production of ROS [139]. Furthermore, elevated levels of catecholamines, such as epinephrine, cause Mg2+ deficiency to intensify, creating a vicious circle [140].
Likewise, Zheltova et al. [117] suggest that Mg2+ deficiency and Ca increase cause an increase in the number of available substrates for radical oxidation. A greater amount of Ca stimulates the activity of phospholipase A2 [141], an enzyme responsible for mobilizing unsaturated fatty acids (UFA) from phospholipids. UFAs, either free or bound to triglycerides and phospholipids, can be readily oxidized by ROS to form lipid hydroperoxides. In turn, hydroperoxides can decompose to form new radicals, thus initiating branching chain reactions that lead to self-sustaining peroxidation [142, 143].
OS can also be generated because the renin-angiotensin-aldosterone system (RAAS) is activated by Mg2+ deficiency [138, 144]. It is well established that angiotensin II activates NOX, monocytes, macrophages, and endothelial cells to produce ROS [145, 146]. In addition, RAAS has been shown to decrease the expression of TRPM6 and TRPM7, Mg2+ transporters, which further increases intracellular Mg2+ deficiency [147]. Fig. 2 shows the possible mechanisms by which Mg2+ deficiency increases ROS production.
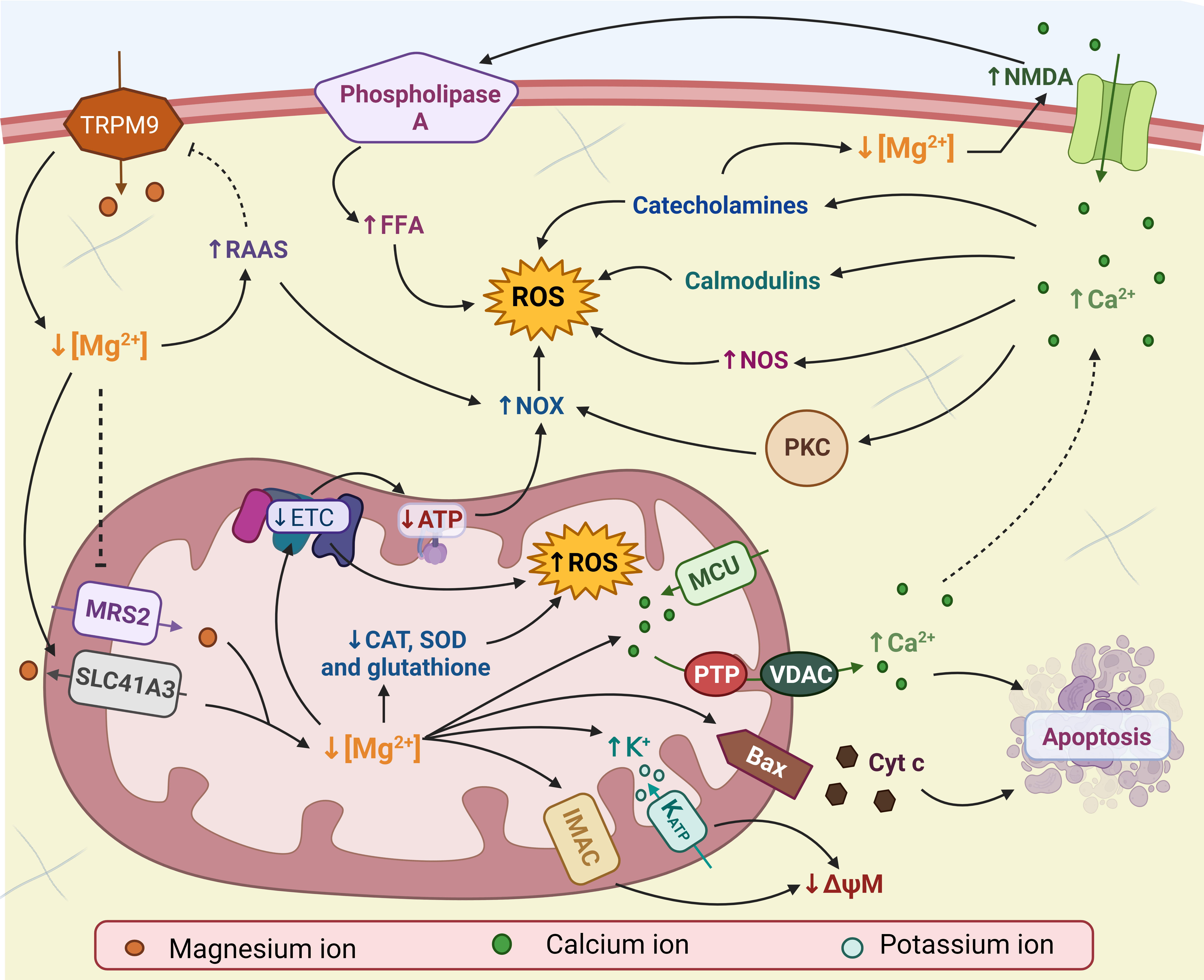
Fig. 2: Magnesium deficiency (Mg2+) and oxidative stress (OS). Mg2+ deficiency in mitochondria leads to the inhibition of the electron transport chain (ETC) and the opening of different channels, decreasing the mitochondrial membrane potential (ΔψM), Bax recruitment and calcium efflux (Ca2+). These factors increase the production of reactive oxygen species (ROS) in mitochondria and induce apoptosis. Intracellular Mg2+ deficiency activates N-methyl-D-aspartate (NMDA) receptors, contributing to the increase in Ca2+. High concentrations of Ca2+ they increase ROS through calmodulins, catecholamines, nitric oxide synthase (NOS), and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX). NOX is also activated by decreased production of adenosine triphosphate (ATP) and the renin-angiotensin-aldosterone system (RAAS). NMDA also activates phospholipase A, increasing the concentration of free fatty acids (FFA) and ROS. Low concentrations of Mg2+ are enhanced by inhibition of mitochondrial RNA splicing protein 2 (MRS2), activation of solute transporter family 41 member 3 (SLC41A3), RAAS, and catecholamines. Bax: Bcl-2 associated X, CAT: catalase, Cyc c: cytochrome c, IMAC: inner membrane anion channel, K: potassium, MCU: mitochondrial Ca2+ uniporter, PKC: protein kinase C phosphorylation, PTP: pore permeability transition, SOD: superoxide dismutase, TRPM9: melastatin transient receptor potential, VDAC: voltage-gated anion channel. Created with biorender.com (published with permission from biorender.com).
On the other hand, inflammation is also a highly reported consequence in situations where the concentration of Mg2+ is insufficient [7, 148]. In addition, the OS generated by low concentrations of Mg2+ could have a strong relationship with inflammation [3, 149]. As mentioned above, Mg2+ deficiency causes excessive ROS production mainly due to mitochondrial dysfunction, abnormal calcium homeostasis, and RAAS activation. The increase in ROS activates transcription factors such as NF-κB [150]. For example, Mg2+ deficiency has been shown to induce lipid peroxidation and NF-κB activation in cultured canine cerebral vascular tissue [151]. NF-κB is inactive in the cytoplasm, and its activation generates the transcription of proinflammatory cytokines such as TNF-α and interleukins (IL-1 and 6) [150, 152]. Bussière et al. [153] showed that an early consequence of Mg2+ deficiency is the activation of polymorphonuclear leukocyte activity and elevated circulating levels of IL-6. Likewise, Malpuech-Brugère et al. [154] observed macrophage activation and an elevation of IL-6 in rats after a few days of Mg2+ deficiency. Therefore, Mg2+ deficiency induces an acute phase inflammatory response that turns into chronic inflammation [7, 153].
In the brain, NF-κB can also be activated by substance P (SP), vascular cell adhesion molecule-1, and inhibitor of plasminogen activator-1, which is induced by NMDA activation and the increased intracellular calcium by decreasing the concentration of Mg2+ [155]. Indeed, in a mouse model of Mg2+ deficiency, immunochemistry revealed that substance P is increased by 230 and 200% in megakaryocytes and lymphocytes, respectively, after 1 day of Mg2+ depletion [46]. Furthermore, SP has a direct role in promoting the activation of neutrophils and endothelium and inducing nitric oxide (NO) production; these processes could participate in the OS that contributes to the depletion of blood glutathione [156].
Mg2+ deficiency also increases endothelin levels, an endothelial cell-derived cytokine [157]. Likewise, it has been reported that animals with Mg2+ deficiency present greater recruitment and activity of phagocytic cells [1, 158]. The origin of this phenomenon is not well understood, but it is probably also related to OS [1]. Finally, inflammation related to Mg2+ deficiency is also generated by reducing anti-inflammatory mediators such as NO, lipoxins, resolvins, and protectins [159, 160].
In summary, Mg2+ deficiency is strongly related to OS due to impaired calcium homeostasis, mitochondrial dysfunction, and RAAS activation. OS can cause inflammation, and inflammation, in turn, improves OS (Fig. 3). However, some aspects of this relationship are not yet fully elucidated. Therefore, more preclinical and clinical studies are needed to clarify the mechanisms involved in the relationship between Mg2+ deficiency with OS and inflammation.
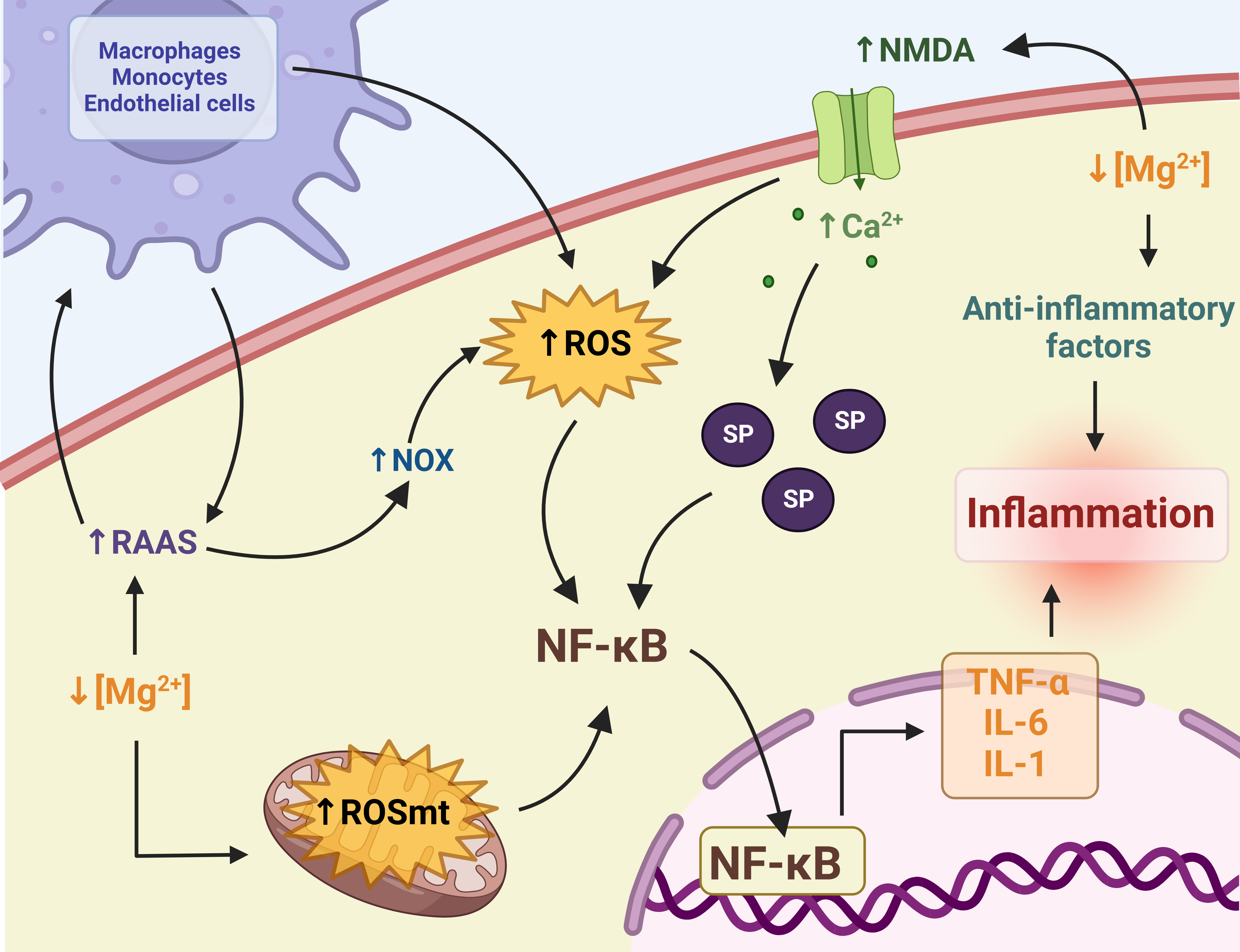
Fig. 3: Relationship between magnesium (Mg2+) deficiency with oxidative stress (OS) and inflammation. Mg2+ deficiency causes an increase in reactive oxygen species (ROS) due to mitochondrial damage, an increase in N-methyl-D-aspartate (NMDA), and the activation of the renin-angiotensin-aldosterone system (RAAS). The latter also increases the recruitment of phagocytic cells, which exacerbates ROS. ROS activates the transcription factor nuclear transcription factor kappa B (NF-κB), which increases the transcription of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukins (1 and 6). This leads to inflammation. NF-κB is also activated by substance P (SP). Finally, Mg2+ deficiency causes a decrease in anti-inflammatory factors, exacerbating inflammation. Ca: calcium, mt: mitochondria, NOX: adenine dinucleotide phosphate (NADPH) oxidase. Created with biorender.com (published with permission from biorender.com).
Mg2+ deficiency, chronic inflammatory, and OS-associated diseases
Mg2+ deficient diets lead to low Mg2+ body concentrations, decreased antioxidants, and OS that progresses to oxidative damage, such as lipid peroxidation [2, 4,75, 161–163]. Also, there is evidence that low Mg2+ body concentrations are associated with increased OS and cytokine storm due to the alteration of antioxidant and immune defenses [111, 162, 164, 165]. Thus, Mg2+ deficiency is strongly associated with increased OS and metabolic syndrome mainly associated with low-grade systemic inflammation, such as obesity, diabetes, and CVD [2–4, 166]. These CVD includes heart failure, arrhythmias, atrial fibrillation, atherosclerosis, hypertension, and preeclampsia [3–5].
Mg2+ deficiency and cardiovascular diseases
Low serum Mg2+ levels have been associated with increased cardiovascular mortality by causing cardiovascular problems and exacerbating pre-existing ones [3, 5,8, 43, 75, 93, 120, 167–169]. In contrast, restoration of adequate Mg2+ levels or supplementation has been associated with improvements in CVD [3, 5,43, 75, 120, 169–171]. In a preclinical study with mice, Liu et al. [43] observed that a low Mg2+ diet for six weeks significantly decreased serum Mg2+ concentration. In addition, as a consequence, cardiac functions were affected with prolonged QTc intervals; mitochondrial dysfunction was observed in mouse cardiomyocytes with low cellular ATP production, overproduction of mitochondrial ROS, and mitochondrial membrane depolarization. Finally, normalizing these affectations with the replacement of Mg2+ [43]. In another study by Watanabe et al. [120], similar results were observed since an Mg2+ deficient diet for eight weeks significantly decreased plasma Mg2+ levels. In addition to increased systolic and diastolic blood pressure, left ventricular hypertrophy, macrocytic anemia, and impaired basal cardiac contractile activities. Similarly, observing that with the replacement of Mg2+, the conditions described above were normalized [120].
One of the causes of these CVDs is that intracellular Mg2+ deficiency leads to inflammation and cardiovascular fibrosis. The latter was identified thanks to the anti-inflammatory and anti-fibrotic role of coenzyme TRPM7 mediated partly through Mg2+ dependent mechanisms since mice deficient in TRPM7 presented significant cardiac hypertrophy, fibrosis, and inflammation; Mg2+ treatment at a cellular level ameliorated effects [172]. Also, the electrophysiologic changes resulting from Mg2+ deficiency can increase the risk of malignant ventricular arrhythmias and sudden cardiac death [173, 174].
A higher incidence of sudden death in some geographic regions attracts attention, and researchers begin to relate them to geological environments such as drinking water due to their mineral content [62]. Residents in soft water areas presented higher sudden death rates due to an increased susceptibility to lethal arrhythmias [62, 63, 95]. Electrolyte disturbances are a frequent complication of chronic heart failure [175]. Patients with isolated hypomagnesemia (without other electrolyte disturbance) frequently present electrocardiogram disturbances with a P wave, corrected QT, and corrected T peak-to-end-interval duration prolonged, suggesting atrial depolarization and ventricular repolarization dispersion increased [176]. Even though the electrophysiologic action on cellular function is unclear, it suggests that these disturbances may have importance in the relationship between hypomagnesemia and sudden death [176]. Mg2+ deficiency has been implicated in sudden death, and it is suspected that the electrophysiological changes induced by calcium are involved [177, 178].
Mg2+ deficiency and diabetes
Mg2+ deficiency is widely associated with diabetes, mainly in type 2 diabetes [179–186]. Hypomagnesemia is frequently identified in diabetic patients and contributes to the progression of diabetes complications [187, 188]. Also, numerous studies have described a high prevalence of Mg2+ deficiency in diabetic patients [6, 180, 185, 189–192]. There has been evidence that Mg2+ deficiency alters calcium homeostasis by competitively inhibiting the voltage-dependent calcium channel, leading to lower insulin secretion [42, 193]. Mg2+ deficiency also may influence the insulin signaling pathway, modifying sensitivity to insulin, such as increasing the association between insulin receptor substrate-1 and p58 subunit of phosphatidyl-inositol 3 kinase or reducing the phosphorylation of protein kinase B (Akt), leading to a diminished response to insulin [194, 195]. As if that were not enough, it has been observed that Mg2+ excretion is more significant in diabetic patients than in healthy subjects due to type 2 diabetes frequently causing damage to the glomerular filtration barrier, altering Mg2+ reabsorption [196–198]. The latter indicates that Mg2+ deficiency is promoted by diabetes, and at the same time, Mg2+ lack exacerbates IR and impaired insulin secretion diabetes.
Also, as mentioned previously, inflammation and OS are related to the incidence of diabetes, a consequence of cellular signaling pathways interference [179, 199, 200]. The secretion of IL-1, IL-6, IL-8, IL-18, TNF-α, beta-adrenergic, and ROS in IR is enhanced in Mg2+ deficiency [42]. King et al. [201] observed that diabetic patients with elevated glycated hemoglobin levels present elevated CRP concentrations, indicating systemic inflammation. Han et al. [202] even suggest that inflammation is essential in diabetic pathogenesis and a high CRP level increases the risk of developing diabetes. Although the linking mechanisms of inflammation and IR are unclear, inflammation plays an important role via cytokines and molecular pathways [203].
Mg2+ supplementation to prevent diseases progression
Fortunately, Mg2+ replenishment in inflammatory pathologies associated with Mg2+ deficiency through supplementation is favorable. Clinic and pre-clinic studies showed decreased inflammatory biomarkers and disease improvement (Table 5) [8, 170, 171, 204–211]. These optimistic and encouraging results suggest using Mg2+ as an immunomodulatory agent, a regulator of inflammation and associated conditions, thus preventing the development of severe or chronic inflammation [3, 163, 205]. Mg2+ therapy decreases nuclear transcription factor kappa B (NF-κB), IL-6, TNF-α, and CRP and enhances vitamin D functionality [36, 99, 111, 212].
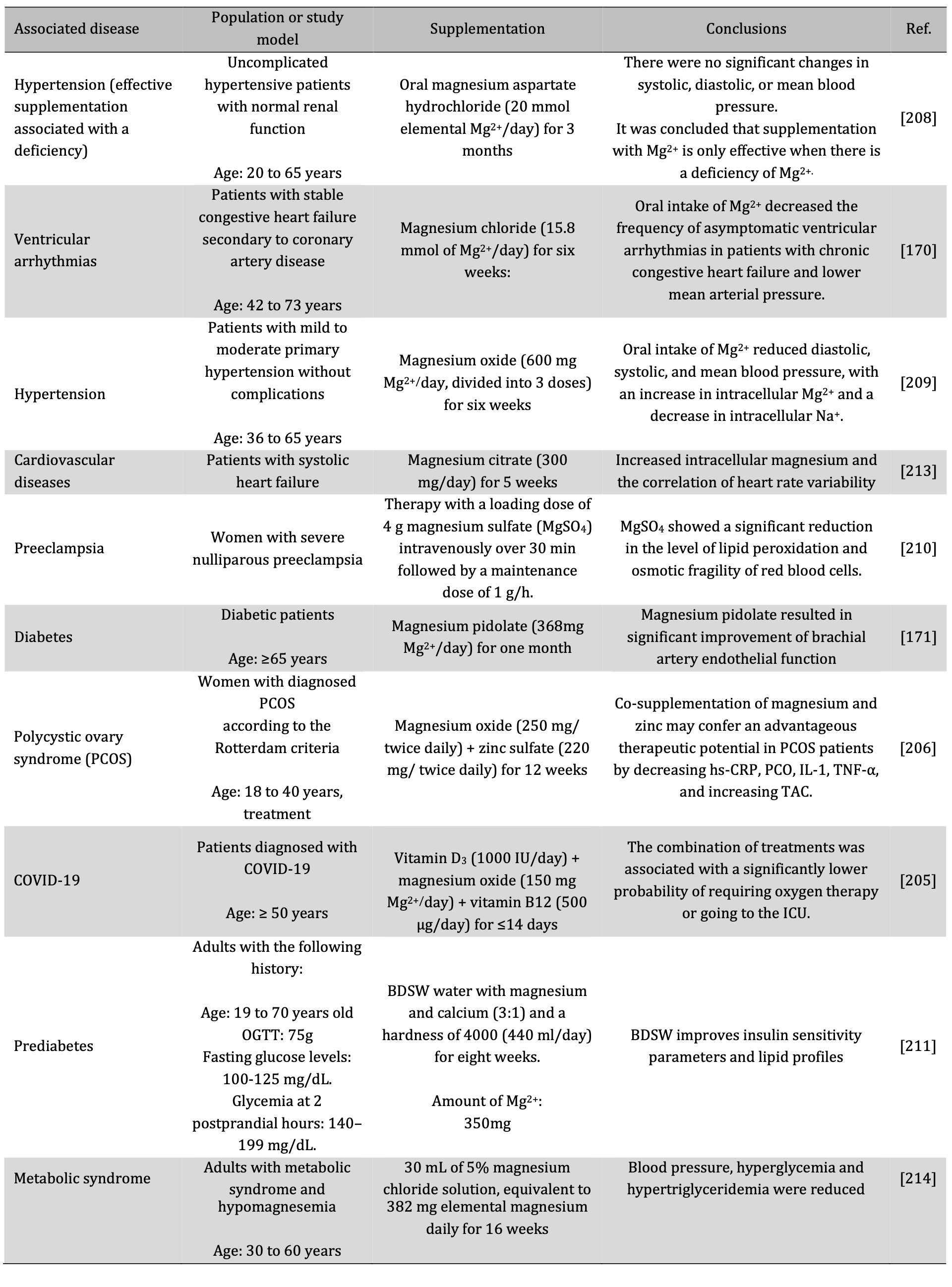
Table. 4: Diseases associated with Mg2+ deficiency and the effect of supplementation. BDSW: Balanced Deep Water, hs-CRP: High Sensitivity Serum C-Reactive Protein, IL-1: Interleukin 1, Mg2+: Magnesium, OGTT: Oral Glucose Tolerance Test, PCO: Protein Carbonyl, TAC: plasma total antioxidant capacity, TNF-α: tumor necrosis factor-alpha, ICU: intensive care unit
Also, Mg2+ supplementation has been observed to be effective as a treatment in diabetic rats due to increased insulin receptors and glucose transporter-4 improving glucose tolerance and lowering blood glucose levels almost to the normal range [215]. Even it has observed reduced oxidative damage and increased glutathione concentrations [215]. Liu et al. [216] also observed that Mg2+ supplementation positively affects insulin sensitivity by increasing insulin receptor expression. Additionally, Kamran et al. [217] observed that Mg2+ supplementation improved blood glucose levels and intraperitoneal glucose tolerance test of diabetic rats and improved Akt-2 and insulin receptor substrate-1 gene and protein expression, increasing glucose transportation in skeletal muscle. In summary, Mg2+ supplementation promotes the correct insulin signaling pathway increasing the expression of proteins involved in enhancing its activity.
Concluding remarks and future directions
Although it is still uncertain whether Mg2+ deficiency is the origin or consequence of diseases associated with OS and inflammation, there is clear evidence that it represents a greater risk for their development, in addition to the high prevalence of Mg2+ deficiency in these patients and that this leads to exacerbating clinical symptoms. So, maintaining optimal Mg2+ body concentration may be favorable in preventing of OS, inflammation, and, thus, chronic comorbidities. Furthermore, Mg2+ deficiency is directly associated with physiological mechanisms such as electrophysiology, insulin excretion, and sensitivity. Therefore, it is associated with an increased risk of developing or exacerbating diabetes and CVD. Although favorable results have been observed with Mg2+ supplementation in inflammatory markers, more specific studies are required to evaluate and understand the Mg2+ supplementation effect as a joint therapy in comorbidities and to prevent disease development. Also, assessing the impact of Mg2+ supplementation in healthy subjects as a preventive treatment is necessary.
Acknowledgements
This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT) México, Grants Numbers A1-S-7495, by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), Grant Numbers IN202219 and IN200922 of the Universidad Nacional Autónoma de México (UNAM), and by Programa de Apoyo a la Investigación y el Posgrado (PAIP), Grant Number 5000-9105. Estefani Yaquelin Hernández-Cruz is a doctoral student from Programa de Doctorado en Ciencias Biológicas from the National Autonomous University of Mexico (UNAM), and she received a fellowship from CONACYT (779741).
Disclosure Statement
The authors declare no conflicts of interest.
References
| 1 | Mazur A, Maier JAM, Rock E, Gueux E, Nowacki W, Rayssiguier Y: Magnesium and the inflammatory response: Potential physiopathological implications. Arch Biochem Biophys 2007;458:48-56. https://doi.org/10.1016/j.abb.2006.03.031 |
| 2 | Nielsen FH: Magnesium, inflammation, and obesity in chronic disease. Nutr Rev 2010;68:333-340. https://doi.org/10.1111/j.1753-4887.2010.00293.x |
| 3 | Liu M, Dudley SC: Magnesium, Oxidative Stress, Inflammation, and Cardiovascular Disease. Antioxidants 2020;9:907-938. https://doi.org/10.3390/antiox9100907 |
| 4 | Shahi A, Aslani S, Ataollahi M, Mahmoudi M: The role of magnesium in different inflammatory diseases. Inflammopharmacology 2019;27:649-661. https://doi.org/10.1007/s10787-019-00603-7 |
| 5 | Efstratiadis G, Sarigianni M, Gougourelas I: Hypomagnesemia and cardiovascular system. Hippokratia 2006;10:147-152. |
| 6 | Wang J-L, Shaw N-S, Yeh H-Y, Kao M-D: Magnesium status and association with diabetes in the Taiwanese elderly. Asia Pac J Clin Nutr 2005;14:263-269. |
| 7 | Maier JA, Castiglioni S, Locatelli L, Zocchi M, Mazur A: Magnesium and inflammation: Advances and perspectives. Semin Cell Dev Biol 2021;115:37-44. https://doi.org/10.1016/j.semcdb.2020.11.002 |
| 8 | Blache D, Devaux S, Joubert O, Loreau N, Schneider M, Durand P, Prost M, Gaume V, Adrian M, Laurant P, Berthelot A: Long-term moderate magnesium-deficient diet shows relationships between blood pressure, inflammation and oxidant stress defense in aging rats. Free Radic Biol Med 2006;41(2):277-284. https://doi.org/10.1016/j.freeradbiomed.2006.04.008 |
| 9 | Vormann J: Magnesium: nutrition and metabolism. Mol Aspects Med 2003;24:27-37. https://doi.org/10.1016/S0098-2997(02)00089-4 |
| 10 | Mejía-Rodríguez F, Shamah-Levy T, Villalpando S, García-Guerra A, Méndez-Gómez Humarán I: Iron, zinc, copper and magnesium deficiencies in Mexican adults from the National Health and Nutrition Survey 2006. Salud Publica Mex 2013;55:275-284. https://doi.org/10.21149/spm.v55i3.7210 |
| 11 | Morales-Ruán M del C, Villalpando S, García-Guerra A, Shamah-Levy T, Robledo-Pérez R, Ávila-Arcos MA, Rivera JA: Iron, zinc, copper and magnesium nutritional status in Mexican children aged 1 to 11 years. Salud Publica Mex 2012;54:125-134. https://doi.org/10.1590/S0036-36342012000200008 |
| 12 | Hendrix P, Cauwenbergh R, Robberecht HJ, Deelstra HA: Measurement of the daily dietary calcium and magnesium intake in Belgium, using duplicate portion sampling. Zeitschrift für Leb und-forsch 1995;201:213-217. https://doi.org/10.1007/BF01192990 |
| 13 | Mataix J, Aranda P, López-Jurado M, Sánchez C, Planells E, Llopis J: Factors influencing the intake and plasma levels of calcium, phosphorus and magnesium in southern Spain. Eur J Nutr 2006;45:349-354. https://doi.org/10.1007/s00394-006-0605-z |
| 14 | Galan P, Preziosi P, Durlach V, Ribas L, Bouzid D, Favier A, Hercberg S: Dietary magnesium intake in french adult population. Magnes Res 1997;10: 321-328. https://doi.org/10.1007/978-94-009-0057-8_36 |
| 15 | Touvier M, Lioret S, Vanrullen I, Boclé JC, Boutron-Ruault MC, Berta JL, Volatier JL. Vitamin and Mineral Inadequacy in the French Population: Estimation and Application for the Optimization of Food Fortification. Int J Vitam Nutr Res 2006;76:343-351. https://doi.org/10.1024/0300-9831.76.6.343 |
| 16 | Hermes Sales C, Azevedo Nascimento D, Queiroz Medeiros AC, Costa Lima K, Campos Pedrosa LF, Colli C: There is chronic latent magnesium deficiency in apparently healthy university students. Nutr Hosp 2014;30:200-204. |
| 17 | Nielsen FH: Dietary Magnesium and Chronic Disease. Adv Chronic Kidney Dis 2018;25:230-235. https://doi.org/10.1053/j.ackd.2017.11.005 |
| 18 | Crosby V, Elin RJ, Twycross R, Mihalyo M, Wilcock A: Magnesium. J Pain Symptom Manage 2013;45:137-144. https://doi.org/10.1016/j.jpainsymman.2012.10.005 |
| 19 | Martin KJ, González EA, Slatopolsky E: Clinical Consequences and Management of Hypomagnesemia. J Am Soc Nephrol 2009;20:2291-2295. https://doi.org/10.1681/ASN.2007111194 |
| 20 | Jahnen-Dechent W, Ketteler M: Magnesium basics. Clin Kidney J 2012;5:i3-14. https://doi.org/10.1093/ndtplus/sfr163 |
| 21 | de Baaij JHF, Hoenderop JGJ, Bindels RJM: Magnesium in Man: Implications for Health and Disease. Physiol Rev 2015;95:1-46. https://doi.org/10.1152/physrev.00012.2014 |
| 22 | Shin M, Momb J, Appling DR: Human mitochondrial MTHFD2 is a dual redox cofactor-specific methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase. Cancer Metab 2017;5:11-17. https://doi.org/10.1186/s40170-017-0173-0 |
| 23 | Ingraham LL, Green DE: Role of Magnesium in Acetyl Coenzyme A Formation by Acetothiokinase. Science 1959;129:896-896. https://doi.org/10.1126/science.129.3353.896 |
| 24 | Pilchova I, Klacanova K, Tatarkova Z, Kaplan P, Racay P: The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxid Med Cell Longev 2017;2017:1-8. https://doi.org/10.1155/2017/6797460 |
| 25 | Wolf F: Cell physiology of magnesium. Mol Aspects Med 2003;24:11-26. https://doi.org/10.1016/S0098-2997(02)00088-2 |
| 26 | Zhang H, Cao X, Tang M, Zhong G, Si Y, Li H, Zhu F, Liao Q, Li L, Zhao J, Feng J, Li S, Wang C, Kaulich M, Wang F, Chen L, Li L, Xia Z, Liang T, Lu H, Feng XH, Zhao B: A subcellular map of the human kinome. eLlife 2021;10: e64943-64974. https://doi.org/10.7554/eLife.64943 |
| 27 | Yang L, Arora K, Beard WA, Wilson SH, Schlick T: Critical Role of Magnesium Ions in DNA Polymerase β's Closing and Active Site Assembly. J Am Chem Soc 2004;126:8441-8453. https://doi.org/10.1021/ja049412o |
| 28 | Vormann J: Magnesium: Nutrition and Homoeostasis. AIMS Public Heal 2016;3:329-340 https://doi.org/10.3934/publichealth.2016.2.329 |
| 29 | Bara M, Guiet-Bara A, Durlach J: Regulation of sodium and potassium pathways by magnesium in cell membranes. Magnes Res 1993;6:167-177. |
| 30 | Mahfouz MM, Smith TL, Kummerow FA: Changes in phospholipid composition and calcium flux in LLC-PK cells cultured at low magnesium concentrations. Biochim Biophys Acta - Lipids Lipid Metab 1989;1006:75-83. https://doi.org/10.1016/0005-2760(89)90325-1 |
| 31 | Tongyai S, Rayssiguier Y, Motta C, Gueux E, Maurois P, Heaton FW: Mechanism of increased erythrocyte membrane fluidity during magnesium deficiency in weanling rats. Am J Physiol Physiol 1989;257:C270-276. https://doi.org/10.1152/ajpcell.1989.257.2.C270 |
| 32 | Akanuma G: Diverse relationships between metal ions and the ribosome. Biosci Biotechnol Biochem 202185:1582-1593. https://doi.org/10.1093/bbb/zbab070 |
| 33 | Cowan JA: The biological chemistry of magnesium. New York, N.Y; 1995. |
| 34 | Rheinberger H-J, Nierhaus KH: The Ribosomal E Site at Low Mg2+: Coordinate Inactivation of Ribosomal Functions at Mg2+ Concentrations Below 10 mM and its Prevention by Polyamines. J Biomol Struct Dyn 1987;5:435-446. https://doi.org/10.1080/07391102.1987.10506403 |
| 35 | Zittermann A: Magnesium deficit - overlooked cause of low vitamin D status?. BMC Med 2013;11:229-232. https://doi.org/10.1186/1741-7015-11-229 |
| 36 | Reddy P, Edwards LR: Magnesium Supplementation in Vitamin D Deficiency. Am J Ther 2019;26:e124-132. https://doi.org/10.1097/MJT.0000000000000538 |
| 37 | Uwitonze AM, Razzaque MS: Role of Magnesium in Vitamin D Activation and Function. J Am Osteopath Assoc 2018;118:181-189. https://doi.org/10.7556/jaoa.2018.037 |
| 38 | Stangherlin A, O'Neill JS: Signal Transduction: Magnesium Manifests as a Second Messenger. Curr Biol 2018;28:R1403-1405. https://doi.org/10.1016/j.cub.2018.11.003 |
| 39 | Li F-Y, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ: Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011;475:471-476. https://doi.org/10.1038/nature10246 |
| 40 | Gröber U, Schmidt J, Kisters K: Magnesium in Prevention and Therapy. Nutrients 2015;7:8199-8226. https://doi.org/10.3390/nu7095388 |
| 41 | de Baaij JHF, Hoenderop JGJ, Bindels RJM: Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J 2012;5(Suppl 1):i15-24. https://doi.org/10.1093/ndtplus/sfr164 |
| 42 | Günther T: The biochemical function of Mg2+ in insulin secretion, insulin signal transduction and insulin resistance. Magnes Res 2010;23:5-18. https://doi.org/10.1684/mrh.2009.0195 |
| 43 | Liu M, Liu H, Feng F, Xie A, Kang G, Zhao Y, Hou CR, Zhou X, Dudley SC Jr: Magnesium Deficiency Causes a Reversible, Metabolic, Diastolic Cardiomyopathy. J Am Heart Assoc 2021;10: e020205. https://doi.org/10.1161/JAHA.120.020205 |
| 44 | Maguire ME, Cowan JA: Magnesium chemistry and biochemistry. Biometals 2002;15:203-210. https://doi.org/10.1023/A:1016058229972 |
| 45 | Alfrey AC, Miller NL, Trow R: Effect of Age and Magnesium Depletion on Bone Magnesium Pools in Rats. J Clin Invest 1974;54:1074-1081. https://doi.org/10.1172/JCI107851 |
| 46 | Rude RK, Gruber HE, Wei LY, Frausto A, Mills BG: Magnesium Deficiency: Effect on Bone and Mineral Metabolism in the Mouse. Calcif Tissue Int 2003;72:32-41. https://doi.org/10.1007/s00223-001-1091-1 |
| 47 | Blaine J, Chonchol M, Levi M: Renal Control of Calcium, Phosphate, and Magnesium Homeostasis. Clin J Am Soc Nephrol 2015;10:1257-1272. https://doi.org/10.2215/CJN.09750913 |
| 48 | Al Alawi AM, Majoni SW, Falhammar H: Magnesium and Human Health: Perspectives and Research Directions. Int J Endocrinol 2018;2018:1-17. https://doi.org/10.1155/2018/9041694 |
| 49 | Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes: Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press (US) 1997. |
| 50 | National Institutes of Health. Magnesium: Fact Sheet for Health Professionals [Internet]. Diet Suppl Fact Sheets. 2022Available at: https://ods.od.nih.gov/factsheets/Magnesium-Consumer/ |
| 51 | McCarty MF: Magnesium may mediate the favorable impact of whole grains on insulin sensitivity by acting as a mild calcium antagonist. Med Hypotheses 2005;64:619-627. https://doi.org/10.1016/j.mehy.2003.10.034 |
| 52 | Suliburska J, Krejpcio Z: Evaluation of the content and bioaccessibility of iron, zinc, calcium and magnesium from groats, rice, leguminous grains and nuts. J Food Sci Technol 2014;51:589-594. https://doi.org/10.1007/s13197-011-0535-5 |
| 53 | Glasdam S-M, Glasdam S, Peters GH: The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv Clin Chem 2016;73:169-193. https://doi.org/10.1016/bs.acc.2015.10.002 |
| 54 | Arshad MS, Khan U, Sadiq A, Khalid W, Hussain M, Yasmeen A, Asghar Z, Rehana H: Coronavirus disease (COVID‐19) and immunity booster green foods: A mini review. Food Sci Nutr 2020;8:3971-3976. https://doi.org/10.1002/fsn3.1719 |
| 55 | Glew RH, Glew RS, Chuang LT, Huang YS, Millson M, Constans D, Vanderjagt DJ: Amino Acid, Mineral and Fatty Acid Content of Pumpkin Seeds (Cucurbita spp) and Cyperus esculentus Nuts in the Republic of Niger. Plant Foods Hum Nutr 2006;61:49-54. https://doi.org/10.1007/s11130-006-0010-z |
| 56 | Schneider I, Greupner T, Hahn A: Magnesium bioavailability from mineral waters with different mineralization levels in comparison to bread and a supplement. Food Nutr Res 2017;61:1384686-1384692. https://doi.org/10.1080/16546628.2017.1384686 |
| 57 | Maraver F, Vitoria I, Ferreira-Pêgo C, Armijo F, Salas-Salvadó J: Magnesium in tap and bottled mineral water in Spain and its contribution to nutritional recommendations. Nutr Hosp 2015;3:2297-2312. |
| 58 | Haring BSA, Delft WV:Changes in the Mineral Composition of Food as a Result of Cooking in "Hard" and "Soft" Waters. Arch Environ Heal An Int J 1981;36:33-35. https://doi.org/10.1080/00039896.1981.10667603 |
| 59 | Rubenowitz E, Axelsson G, Rylander R: Magnesium and calcium in drinking water and death from acute myocardial infarction in women. Epidemiology 1999;10:31-36. https://doi.org/10.1097/00001648-199901000-00007 |
| 60 | Crawford M: mortality and hardness of local water-supplies. Lancet 1968;291:827-831. https://doi.org/10.1016/S0140-6736(68)90297-3 |
| 61 | Rubenowitz E, Axelsson G, Rylander R: Magnesium in Drinking Water and Death from Acute Myocardial Infarction. Am J Epidemiol 1996;143:456-462. https://doi.org/10.1093/oxfordjournals.aje.a008765 |
| 62 | Karppanen H, Pennanen R, Passinen L: Minerals, Coronary Heart Disease and Sudden Coronary Death1,2. In: Manninen V, Halonen PI, argitaratzaileak. Advances in Cardiology 1978;9-24. https://doi.org/10.1159/000402000 |
| 63 | Punsar S, Karvonen MJ: Drinking Water Quality and Sudden Death: Observations from West and East Finland. Cardiology 1979;64:24-34. https://doi.org/10.1159/000170575 |
| 64 | Luoma H, AromaaProfessor A, Helminen S, Murtomaa H, Kiviluoto L, Punsar S, Knekt P: Risk of Myocardial Infarction in Finnish Men in Relation to Fluoride, Magnesium and Calcium Concentration in Drinking Water. Acta Med Scand 2009;213:171-176. https://doi.org/10.1111/j.0954-6820.1983.tb03712.x |
| 65 | Yang C: Calcium and magnesium in drinking water and the risk of death from hypertension. Am J Hypertens 1999;12:894-899. https://doi.org/10.1016/S0895-7061(99)00065-5 |
| 66 | Yang CY, Chang CC, Tsai SS, Chiu HF: Calcium and magnesium in drinking water and risk of death from acute myocardial infarction in Taiwan. Environ Res 2006;101:407-411. https://doi.org/10.1016/j.envres.2005.12.019 |
| 67 | Masironi R, Shaper AG: Epidemiological studies of health effects of water from different sources. Annu Rev Nutr 1981;1:375-400. https://doi.org/10.1146/annurev.nu.01.070181.002111 |
| 68 | Schroeder HA: Relation between mortality from cardiovascular disease and treated water supplies. J Am Med Assoc 1960;172:1902-1908. https://doi.org/10.1001/jama.1960.03020170028007 |
| 69 | Catling LA, Abubakar I, Lake IR, Swift L, Hunter PR: A systematic review of analytical observational studies investigating the association between cardiovascular disease and drinking water hardness. J Water Health 2008;6:433-442. https://doi.org/10.2166/wh.2008.054 |
| 70 | Sabatier M, Arnaud MJ, Kastenmayer P, Rytz A, Barclay DV: Meal effect on magnesium bioavailability from mineral water in healthy women. Am J Clin Nutr 2002;75:65-71. https://doi.org/10.1093/ajcn/75.1.65 |
| 71 | Nestares T, Barrionuevo M, López-Frías M, Vidal C, Urbano G: Effect of Different Soaking Solutions on Nutritive Utilization of Minerals (Calcium, Phosphorus, and Magnesium) from Cooked Beans ( Phaseolus vulgaris L.) in Growing Rats. J Agric Food Chem 2003;51:515-520. https://doi.org/10.1021/jf020693y |
| 72 | Temple NJ: Refined carbohydrates - A cause of suboptimal nutrient intake. Med Hypotheses 1983;10:411-424. https://doi.org/10.1016/0306-9877(83)90007-5 |
| 73 | Syedmoradi L, Ghasemi A, Zahediasl S, Azizi F: Prevalence of hypo- and hypermagnesemia in an Iranian urban population. Ann Hum Biol 2011;38:150-155. https://doi.org/10.3109/03014460.2010.500472 |
| 74 | Rosanoff A, Weaver CM, Rude RK: Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev 2012;70:153-164. https://doi.org/10.1111/j.1753-4887.2011.00465.x |
| 75 | Nielsen FH, Milne DB, Klevay LM, Gallagher S, Johnson L: Dietary Magnesium Deficiency Induces Heart Rhythm Changes, Impairs Glucose Tolerance, and Decreases Serum Cholesterol in Post Menopausal Women. J Am Coll Nutr 2007;26:121-132. https://doi.org/10.1080/07315724.2007.10719593 |
| 76 | Costello RB, Elin RJ, Rosanoff A, Wallace TC, Guerrero-Romero F, Hruby A, Lutsey PL, Nielsen FH, Rodriguez-Moran M, Song Y, Van Horn LV: Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv Nutr An Int Rev J 2016;7:977-993. https://doi.org/10.3945/an.116.012765 |
| 77 | Cao S, Hodges JK, McCabe LD, Weaver CM: Magnesium Requirements in Children. Nutr Today 2019;54:195-206. https://doi.org/10.1097/NT.0000000000000363 |
| 78 | Schlingmann KP, Konrad M: Magnesium homeostasis. Principles of Bone Biology. Elsevier; 2020;509-525. https://doi.org/10.1016/B978-0-12-814841-9.00021-X |
| 79 | Belluci MM, de Molon RS, Rossa Jr C, Tetradis S, Giro G, Cerri PS, Marcantonio E Jr, Orrico SRP: Severe magnesium deficiency compromises systemic bone mineral density and aggravates inflammatory bone resorption. J Nutr Biochem 2020;77:108301. https://doi.org/10.1016/j.jnutbio.2019.108301 |
| 80 | Gruber HE, Rude RK, Wei L, Frausto A, Mills BG, Norton HJ: Magnesium deficiency: effect on bone mineral density in the mouse appendicular skeleton. BMC Musculoskelet Disord 2003;4:7-12. https://doi.org/10.1186/1471-2474-4-7 |
| 81 | Castiglioni S, Cazzaniga A, Albisetti W, Maier J: Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013;5:3022-3033. https://doi.org/10.3390/nu5083022 |
| 82 | Alexander RT, Bitzan M: Renal Tubular Acidosis. Pediatr Clin North Am 2019;66:135-157. https://doi.org/10.1016/j.pcl.2018.08.011 |
| 83 | Patel S, Sharma S. Respiratory Acidosis [Internet]. tatPearls, StatPearls Publ Treasure Isl. 2021 [aipatua 2022 urr 12]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK482430/ |
| 84 | Burger M, Schaller DJ. Metabolic Acidosis [Internet]. StatPearls, StatPearls Publ Treasure Isl. 2021 [aipatua 2022 urr 12]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK482146/ |
| 85 | Rylander R, Remer T, Berkemeyer S, Vormann J: Acid-Base Status Affects Renal Magnesium Losses in Healthy, Elderly Persons. J Nutr 2006;136:2374-2377. https://doi.org/10.1093/jn/136.9.2374 |
| 86 | Zafar MS, Wani J, Karim R, Mir M, Koul P: Significance of serum magnesium levels in critically ill-patients. Int J Appl Basic Med Res 2014;4:34-37. https://doi.org/10.4103/2229-516X.125690 |
| 87 | Curiel-García JA, Rodríguez-Morán M, Guerrero-Romero F: Hypomagnesemia and mortality in patients with type 2 diabetes. Magnes Res 2008;21:163-166. |
| 88 | Costello RB, Nielsen F: Interpreting magnesium status to enhance clinical care. Curr Opin Clin Nutr Metab Care 2017;20:504-511. https://doi.org/10.1097/MCO.0000000000000410 |
| 89 | Ismail Y, Ismail AA, Ismail AAA: The underestimated problem of using serum magnesium measurements to exclude magnesium deficiency in adults; a health warning is needed for "normal" results. Clin Chem Lab Med 2010;48: 323-327. https://doi.org/10.1515/CCLM.2010.077 |
| 90 | Fiorentini D, Cappadone C, Farruggia G, Prata C: Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021;13:1136-1180. https://doi.org/10.3390/nu13041136 |
| 91 | Workinger J, Doyle R, Bortz J: Challenges in the Diagnosis of Magnesium Status. Nutrients 2018;10:1202-1225. https://doi.org/10.3390/nu10091202 |
| 92 | Fairley J, Glassford NJ, Zhang L, Bellomo R. Magnesium status and magnesium therapy in critically ill patients: A systematic review. J Crit Care 2015;30:1349-1358. https://doi.org/10.1016/j.jcrc.2015.07.029 |
| 93 | DiNicolantonio JJ, O'Keefe JH, Wilson W: Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Hear 2018;5:e000668. https://doi.org/10.1136/openhrt-2017-000668 |
| 94 | Laupland KB, Tabah A, Jacobs N, Ramanan M: Determinants of serum magnesium abnormalities and outcome among admissions to the intensive care unit. Anaesth Crit Care Pain Med 2020;39:793-797. https://doi.org/10.1016/j.accpm.2020.07.020 |
| 95 | Eisenberg MJ: Magnesium deficiency and sudden death. Am Heart J 1992;124:544-549. https://doi.org/10.1016/0002-8703(92)90633-7 |
| 96 | Fiset C, Kargacin ME, Kondo CS, Lester WM, Duff HJ: Hypomagnesemia: Characterization of a model of sudden cardiac death. J Am Coll Cardiol 1996;27:1771-1776. https://doi.org/10.1016/0735-1097(96)00089-7 |
| 97 | Johnson S: The multifaceted and widespread pathology of magnesium deficiency. Med Hypotheses 2001;56:163-170. https://doi.org/10.1054/mehy.2000.1133 |
| 98 | Wallace TC: Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J Am Coll Nutr 2020;39:685-693. https://doi.org/10.1080/07315724.2020.1785971 |
| 99 | Dai Q, Zhu X, Manson JE, Song Y, Li X, Franke AA, Costello RB, Rosanoff A, Nian H, Fan L, Murff H, Ness RM, Seidner DL, Yu C, Shrubsole MJ: Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr 2018;108:1249-1258. https://doi.org/10.1093/ajcn/nqy274 |
| 100 | Alamdari NM, Afaghi S, Rahimi FS, Tarki FE, Tavana S, Zali A, Fathi M, Besharat S, Bagheri L, Pourmotahari F, Irvani SSN, Dabbagh A, Mousavi SA: Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran. Tohoku J Exp Med 2020;252:73-84. https://doi.org/10.1620/tjem.252.73 |
| 101 | Di Mario F, Regolisti G, Greco P, Maccari C, Superchi E, Morabito S, Pistolesi V, Fiaccadori E. Prevention of hypomagnesemia in critically ill patients with acute kidney injury on continuous kidney replacement therapy: the role of early supplementation and close monitoring. J Nephrol 2021;34: 1271-1279. https://doi.org/10.1007/s40620-020-00864-4 |
| 102 | Alexander RT, Dimke H: Effect of diuretics on renal tubular transport of calcium and magnesium. Am J Physiol Physiol 2017;312:F998-1015. https://doi.org/10.1152/ajprenal.00032.2017 |
| 103 | Nijenhuis T, Vallon V, van der Kemp AWCM, Loffing J, Hoenderop JGJ, Bindels RJM: Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 2005;115:1651-1658. https://doi.org/10.1172/JCI24134 |
| 104 | Guo W, Nazim H, Liang Z, Yang D: Magnesium deficiency in plants: An urgent problem. Crop J 2016;4:83-91. https://doi.org/10.1016/j.cj.2015.11.003 |
| 105 | Ficco DBM, Riefolo C, Nicastro G, De Simone V, Di Gesù AM, Beleggia R, Platani C, Cattivelli L, De Vita P: Phytate and mineral elements concentration in a collection of Italian durum wheat cultivars. F Crop Res 2009;111:235-242. https://doi.org/10.1016/j.fcr.2008.12.010 |
| 106 | Fan M-S, Zhao FJ, Fairweather-Tait SJ, Poulton PR, Dunham SJ, McGrath SP: Evidence of decreasing mineral density in wheat grain over the last 160 years. J Trace Elem Med Biol 2008;22:315-324. https://doi.org/10.1016/j.jtemb.2008.07.002 |
| 107 | Murphy KM, Reeves PG, Jones SS: Relationship between yield and mineral nutrient concentrations in historical and modern spring wheat cultivars. Euphytica 2008;163:381-390. https://doi.org/10.1007/s10681-008-9681-x |
| 108 | Thomas D: The Mineral Depletion of Foods Available to US as A Nation (1940-2002) - A Review of the 6th Edition of McCance and Widdowson. Nutr Health 2007;19:21-55. https://doi.org/10.1177/026010600701900205 |
| 109 | Mayer A: Historical changes in the mineral content of fruits and vegetables. Br Food J 1997;99:207-211. https://doi.org/10.1108/00070709710181540 |
| 110 | Davis DR: Impact of Breeding and Yield on Fruit, Vegetable, and Grain Nutrient Content. Breeding for Fruit Quality. Hoboken, NJ, USA: John Wiley & Sons, Inc 2011;127-150. https://doi.org/10.1002/9780470959350.ch6 |
| 111 | DiNicolantonio JJ, O'Keefe JH: Magnesium and Vitamin D Deficiency as a Potential Cause of Immune Dysfunction, Cytokine Storm and Disseminated Intravascular Coagulation in covid-19 patients. Mo Med 2021;118:68-73. |
| 112 | Siener R, Hesse A: Influence of a mixed and a vegetarian diet on urinary magnesium excretion and concentration. Br J Nutr 1995;73:783-790. https://doi.org/10.1079/BJN19950081 |
| 113 | WWEIA/NHANES. Overview : USDA ARS [Internet]. [aipatua 2022 ira 11]. Available at: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/ |
| 114 | Cruz-Góngora VD, Gaona B, Villalpando S, Shamah-Levy T, Robledo R: Anemia and iron, zinc, copper and magnesium deficiency in Mexican adolescents: National Health and Nutrition Survey 2006. Salud Publica Mex 2012;54:125-134. https://doi.org/10.1590/S0036-36342012000200009 |
| 115 | Pathak P, Kapil U, Kapoor SK, Saxena R, Kumar A, Gupta N, Dwivedi SN, Singh R, Singh P. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr 2004;71:1007-1014. https://doi.org/10.1007/BF02828117 |
| 116 | Vormann J, Anke M: Dietary Magnesium: Supply, Requirements and Recommendations - Results From Duplicate and Balance Studies in Man. J Clin Basic Cardiol 2002;5:49-53. |
| 117 | Zheltova AA, Kharitonova M V., Iezhitsa IN, Spasov AA: Magnesium deficiency and oxidative stress: an update. BioMedicine 2016;6:8-14. https://doi.org/10.7603/s40681-016-0020-6 |
| 118 | Sies H, Berndt C, Jones DP: Oxidative Stress. Annu Rev Biochem 2017;86:715-748. https://doi.org/10.1146/annurev-biochem-061516-045037 |
| 119 | Lambert AJ, Brand MD: Reactive Oxygen Species Production by Mitochondria. Methods Mol Biol 2009;554:165-181. https://doi.org/10.1007/978-1-59745-521-3_11 |
| 120 | Watanabe M, Nakamura K, Kato M, Okada T, Lesaki T: Chronic magnesium deficiency causes reversible mitochondrial permeability transition pore opening and impairs hypoxia tolerance in the rat heart. J Pharmacol Sci 2022;148:238-247. https://doi.org/10.1016/j.jphs.2021.12.002 |
| 121 | Kolisek M: Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J 2003;22:1235-1244. https://doi.org/10.1093/emboj/cdg122 |
| 122 | Gout E, Rébeillé F, Douce R, Bligny R: Interplay of Mg 2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg 2+ in cell respiration. Proc Natl Acad Sci 2014;111: e4560-4567. https://doi.org/10.1073/pnas.1406251111 |
| 123 | Panov A, Scarpa A: Mg 2+ Control of Respiration in Isolated Rat Liver Mitochondria. Biochemistry 1996;35:12849-12856. https://doi.org/10.1021/bi960139f |
| 124 | Rodrı́guez-Zavala JS, Moreno-Sánchez R: Modulation of Oxidative Phosphorylation by Mg2+ in Rat Heart Mitochondria. J Biol Chem 1998;273:7850-7855. https://doi.org/10.1074/jbc.273.14.7850 |
| 125 | Kramer JH, Mišík V, Weglicki WB: Magnesium-deficiency potentiates free radical production associated with postischemic injury to rat hearts: Vitamin E affords protection. Free Radic Biol Med 1994;16:713-723. https://doi.org/10.1016/0891-5849(94)90186-4 |
| 126 | Liu YX, Guo YM, Wang Z: Effect of magnesium on reactive oxygen species production in the thigh muscles of broiler chickens. Br Poult Sci 2007;48:84-89. https://doi.org/10.1080/00071660601148187 |
| 127 | Shah NC, Liu J-P, Iqbal J, Hussain M, Jiang X-C, Li Z, Li Y, Zheng T, Li W, Sica AC, Perez-Albela JL, Altura BT, Altura BM: Mg deficiency results in modulation of serum lipids, glutathione, and NO synthase isozyme activation in cardiovascular tissues: relevance to de novo synthesis of ceramide, serum Mg and atherogenesis. Int J Clin Exp Med 2011;4:103-118. |
| 128 | Kumar BP, Shivakumar K: Depressed antioxidant defense in rat heart in experimental magnesium deficiency implications for the pathogenesis of myocardial lesions. Biol Trace Elem Res 1997;60:139-144. https://doi.org/10.1007/BF02783317 |
| 129 | Björkblom B, Maple-Grødem J, Puno MR, Odell M, Larsen JP, Møller SG: Reactive Oxygen Species-Mediated DJ-1 Monomerization Modulates Intracellular Trafficking Involving Karyopherin β2. Mol Cell Biol 2014;34:3024-3040. https://doi.org/10.1128/MCB.00286-14 |
| 130 | Tamura M, Kanno M, Kai T: Destabilization of neutrophil NADPH oxidase by ATP and other trinucleotides and its prevention by Mg2+. Biochim Biophys Acta - Biomembr 2001;1510:270-277. https://doi.org/10.1016/S0005-2736(00)00358-8 |
| 131 | Racay P: Effect of magnesium on calcium-induced depolarisation of mitochondrial transmembrane potential. Cell Biol Int 2008;32:136-145. https://doi.org/10.1016/j.cellbi.2007.08.024 |
| 132 | Bednarczyk P, Dołowy K, Szewczyk A: Matrix Mg2+ regulates mitochondrial ATP-dependent potassium channel from heart. FEBS Lett 2005;579:1625-1632. https://doi.org/10.1016/j.febslet.2005.01.077 |
| 133 | Beavis AD, Powers MF: On the Regulation of the Mitochondrial Inner Membrane Anion Channel by Magnesium and Protons. J Biol Chem 1989;264:17148-17155. https://doi.org/10.1016/S0021-9258(18)71471-3 |
| 134 | Zoratti M, Szabò I: The mitochondrial permeability transition. Biochim Biophys Acta - Rev Biomembr 1995;1241:139-176. https://doi.org/10.1016/0304-4157(95)00003-A |
| 135 | Chen Y, Wei X, Yan P, Han Y, Sun S, Wu K, Fan D: Human mitochondrial Mrs2 protein promotes multidrug resistance in gastric cancer cells by regulating p27, cyclin D1 expression and cytochrome C release. Cancer Biol Ther 2009;8:607-614. https://doi.org/10.4161/cbt.8.7.7920 |
| 136 | Huang CY: Attenuation of Magnesium Sulfate on CoCl_2-Induced Cell Death by Activating ERK1/2/MAPK and Inhibiting HIF-1α via Mitochondrial Apoptotic Signaling Suppression in a Neuronal Cell Line. Chin J Physiol 2015;58:244-253. https://doi.org/10.4077/CJP.2015.BAD296 |
| 137 | Blomeyer CA, Bazil JN, Stowe DF, Dash RK, Camara AKS: Mg2+ differentially regulates two modes of mitochondrial Ca2+ uptake in isolated cardiac mitochondria: implications for mitochondrial Ca2+ sequestration. J Bioenerg Biomembr 2016;48:175-188. https://doi.org/10.1007/s10863-016-9644-1 |
| 138 | Rayssiguier Y, Libako P, Nowacki W, Rock E: Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnes Res 2010;23:73-80. |
| 139 | Blaustein MP: The role of calcium in catecholamine release from adrenergic nerve terminals. The Release of Catecholamines from Adrenergic Neurons. Elsevier; 1979;39-58. https://doi.org/10.1016/B978-0-08-021536-5.50008-3 |
| 140 | Joborn H, Åkerström G, Ljunghall S: Effects of exogenous catecholamines and exercise on plasma magnesium concentrations. Clin Endocrinol (Oxf). 1985;23(3):219-226. https://doi.org/10.1111/j.1365-2265.1985.tb00217.x |
| 141 | Chakraborti S, Michael JR, Patra SK: Protein kinase C dependent and independent activation of phospholipase A 2 under calcium ionophore (A23187) exposure in rabbit pulmonary arterial smooth muscle cells. FEBS Lett 1991;285:104-107. https://doi.org/10.1016/0014-5793(91)80735-L |
| 142 | Girotti AW: Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res 1998;39:1529-1542. https://doi.org/10.1016/S0022-2275(20)32182-9 |
| 143 | Schneider C, Porter NA, Brash AR: Routes to 4-Hydroxynonenal: Fundamental Issues in the Mechanisms of Lipid Peroxidation. J Biol Chem 2008;283:15539-15543. https://doi.org/10.1074/jbc.R800001200 |
| 144 | Ahokas RA, Sun Y, Bhattacharya SK, Gerling IC, Weber KT: Aldosteronism and a Proinflammatory Vascular Phenotype. Circulation 2005;111:51-57. https://doi.org/10.1161/01.CIR.0000151516.84238.37 |
| 145 | Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994;74:1141-1148. https://doi.org/10.1161/01.RES.74.6.1141 |
| 146 | Hazan-Halevy I, Levy T, Wolak T, Lubarsky I, Levy R, Paran E: Stimulation of NADPH oxidase by angiotensin II in human neutrophils is mediated by ERK, p38 MAP-kinase and cytosolic phospholipase A2. J Hypertens 2005;23:1183-1190. https://doi.org/10.1097/01.hjh.0000170381.53955.68 |
| 147 | Sontia B, Montezano ACI, Paravicini T, Tabet F, Touyz RM: Downregulation of Renal TRPM7 and Increased Inflammation and Fibrosis in Aldosterone-Infused Mice. Hypertension 2008;51:915-921. https://doi.org/10.1161/HYPERTENSIONAHA.107.100339 |
| 148 | Nielsen FH: Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res. 2018;11:25-34. https://doi.org/10.2147/JIR.S136742 |
| 149 | Guerrero-Romero F, Rodríguez-Morán M: Hypomagnesemia, oxidative stress, inflammation, and metabolic syndrome. Diabetes Metab Res Rev 2006;22:471-476. https://doi.org/10.1002/dmrr.644 |
| 150 | Chatterjee S: Oxidative Stress, Inflammation, and Disease. Oxidative Stress and Biomaterials. Elsevier; 2016; 35-58. https://doi.org/10.1016/B978-0-12-803269-5.00002-4 |
| 151 | Altura BM, Gebrewold A, Zhang A, Altura BT: Low extracellular magnesium ions induce lipid peroxidation and activation of nuclear factor-kappa B in canine cerebral vascular smooth muscle: possible relation to traumatic brain injury and strokes. Neurosci Lett 2003;341:189-192. https://doi.org/10.1016/S0304-3940(03)00134-4 |
| 152 | Kabe Y, Ando K, Hirao S, Yoshida M, Handa H: Redox Regulation of NF-κB Activation: Distinct Redox Regulation Between the Cytoplasm and the Nucleus. Antioxid Redox Signal 2005;7:395-403. https://doi.org/10.1089/ars.2005.7.395 |
| 153 | Bussière FI, Gueux E, Rock E, Girardeau J-P, Tridon A, Mazur A, Rayssiguier Y: Increased phagocytosis and production of reactive oxygen species by neutrophils during magnesium deficiency in rats and inhibition by high magnesium concentration. Br J Nutr 2002;87:107-113. https://doi.org/10.1079/BJN2001498 |
| 154 | Malpuech-Brugère C, Nowacki W, Daveau M, Gueux E, Linard C, Rock E, Lebreton J, Mazur A, Rayssiguier Y: Inflammatory response following acute magnesium deficiency in the rat. Biochim Biophys Acta - Mol Basis Dis 2000;1501:91-98. https://doi.org/10.1016/S0925-4439(00)00018-1 |
| 155 | Lo CCW, Moosavi SM, Bubb KJ: The Regulation of Pulmonary Vascular Tone by Neuropeptides and the Implications for Pulmonary Hypertension. Front Physiol 2018;9: 1167-1186. https://doi.org/10.3389/fphys.2018.01167 |
| 156 | Mak IT, Kramer JH, Weglicki WB: Suppression of neutrophil and endothelial activation by substance P receptor blockade in the Mg-deficient rat. Magnes Res 2003;16:91-97. |
| 157 | Weglicki WB, Phillips TM, Freedman AM, Cassidy MM, Dickens BF: Magnesium-deficiency elevates circulating levels of inflammatory cytokines and endothelin. Mol Cell Biochem 1992;110:169-173. https://doi.org/10.1007/BF02454195 |
| 158 | Libako P, Nowacki W, Rock E, Rayssiguier Y, Mazur A: Phagocyte priming by low magnesium status: input to the enhanced inflammatory and oxidative stress responses. Magnes Res 2010;23:1-4. https://doi.org/10.1684/mrh.2009.0201 |
| 159 | Das UN: Nutritional factors in the prevention and management of coronary artery disease and heart failure. Nutrition 2015;31:283-291. https://doi.org/10.1016/j.nut.2014.08.011 |
| 160 | Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 2009;158:960-971. https://doi.org/10.1111/j.1476-5381.2009.00290.x |
| 161 | Morais JBS, Severo JS, de Oliveira ARS, Cruz KJC, da Silva Dias TM, de Assis RC, Colli C, do Nascimento Marreiro D: Magnesium Status and Its Association with Oxidative Stress in Obese Women. Biol Trace Elem Res 2017;175:306-311. https://doi.org/10.1007/s12011-016-0797-x |
| 162 | Wiles ME, Wagner TL, Weglicki WB: Effect of acute magnesium deficiency (MgD) on aortic endothelial cell (EC) oxidant production. Life Sci 1996;60:221-236. https://doi.org/10.1016/S0024-3205(96)00619-4 |
| 163 | Beltrán-García J, Osca-Verdegal R, Pallardó F V., Ferreres J, Rodríguez M, Mulet S, Sanchis-Gomar F, Carbonell N, García-Giménez JL: Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants 2020;9:936-956. https://doi.org/10.3390/antiox9100936 |
| 164 | Hsu JM, Rubenstein B, Paleker AG: Role of Magnesium in Glutathione Metabolism of Rat Erythrocytes. J Nutr 1982;112:488-496. https://doi.org/10.1093/jn/112.3.488 |
| 165 | Minnich V, Smith MB, Brauner MJ, Majerus PW: Glutathione biosynthesis in human erythrocytes. J Clin Invest 1971;50:507-513. https://doi.org/10.1172/JCI106519 |
| 166 | Piuri G, Zocchi M, Della Porta M, Ficara V, Manoni M, Zuccotti GV, Pinotti L, Maier JA, Cazzola R: Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021;13:320-336. https://doi.org/10.3390/nu13020320 |
| 167 | Wilimzig C, Latz R, Vierling W, Mutschler E, Trnovec T, Nyulassy S: Increase in magnesium plasma level after orally administered trimagnesium dicitrate. Eur J Clin Pharmacol 1996;49:317-323. https://doi.org/10.1007/BF00226334 |
| 168 | Oost LJ, van der Heijden AAWA, Vermeulen EA, Bos C, Elders PJM, Slieker RC, Kurstjens S, van Berkel M, Hoenderop JGJ, Tack CJ, Beulens JWJ, de Baaij JHF: Serum Magnesium Is Inversely Associated With Heart Failure, Atrial Fibrillation, and Microvascular Complications in Type 2 Diabetes. Diabetes Care 2021;44:1757-1765. https://doi.org/10.2337/dc21-0236 |
| 169 | Houston M: The Role of Magnesium in Hypertension and Cardiovascular Disease. J Clin Hypertens 2011;13:843-847. https://doi.org/10.1111/j.1751-7176.2011.00538.x |
| 170 | Bashir Y, Sneddon JF, Staunton HA, Haywood GA, Simpson IA, McKenna WJ, Camm AJ. Effects of long-term oral magnesium chloride replacement in congestive heart failure secondary to coronary artery disease. Am J Cardiol 1993;72:1156-1162. https://doi.org/10.1016/0002-9149(93)90986-M |
| 171 | Barbagallo M, Dominguez LJ, Galioto A, Pineo A, Belvedere M: Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res 2010;23:131-137. |
| 172 | Rios FJ, Zou Z-G, Harvey AP, Harvey KY, Nosalski R, Anyfanti P, Camargo LL, Lacchini S, Ryazanov AG, Ryazanova L, McGrath S, Guzik TJ, Goodyear CS, Montezano AC, Touyz RM: Chanzyme TRPM7 protects against cardiovascular inflammation and fibrosis. Cardiovasc Res 2020;116:721-735. https://doi.org/10.1093/cvr/cvz164 |
| 173 | Sheehan JP, Seelig MS: Interactions of magnesium and potassium in the pathogenesis of cardiovascular disease. Magnesium 1984;3:301-314. |
| 174 | Iseri LT, Allen BJ, Ginkel ML, Brodsky MA: Ionic biology and ionic medicine in cardiac arrhythmias with particular reference to magnesium. Am Heart J 1992;123:1404-1409. https://doi.org/10.1016/0002-8703(92)91059-A |
| 175 | Leier C V., Dei Cas L, Metra M: Clinical relevance and management of the major electrolyte abnormalities in congestive heart failure: Hyponatremia, hypokalemia, and hypomagnesemia. Am Heart J 1994;128:564-574. https://doi.org/10.1016/0002-8703(94)90633-5 |
| 176 | Yang Y, Chen C, Duan P, Thapaliya S, Gao L, Dong Y, Yin X, Yang X, Zhang R, Tan R, Hui S, Wang Y, Sutton R, Xia Y: The ECG Characteristics of Patients With Isolated Hypomagnesemia. Front Physiol 2021;11: 617374-617383 https://doi.org/10.3389/fphys.2020.617374 |
| 177 | Schwinger RH, Erdmann E: Heart failure and electrolyte disturbances. Methods Find Exp Clin Pharmacol 1992;14:315-325. |
| 178 | Dei Cas L, Metra M, Leier CV: Electrolyte disturbances in chronic heart failure: Metabolic and clinical aspects. Clin Cardiol 1995;18:370-376. https://doi.org/10.1002/clc.4960180704 |
| 179 | Kostov K: Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int J Mol Sci 2019;20:1351-1366. https://doi.org/10.3390/ijms20061351 |
| 180 | Zhang H, Cao Y, Man Q, Li Y, Jia S, Wang R, Lu J, Yang L: Magnesium Nutritional Status, Risk Factors, and the Associations with Glucose Parameters of Childbearing Women in the China Adult Chronic Disease and Nutrition Surveillance (2015). Nutrients 2022;14:847-856. https://doi.org/10.3390/nu14040847 |
| 181 | Feng J, Wang H, Jing Z, Wang Y, Cheng Y, Wang W, Sun W: Role of Magnesium in Type 2 Diabetes Mellitus. Biol Trace Elem Res 2020;196:74-85. https://doi.org/10.1007/s12011-019-01922-0 |
| 182 | Veronese N, Watutantrige-Fernando S, Luchini C, Solmi M, Sartore G, Sergi G, Manzato E, Barbagallo M, Maggi S, Stubbs B: Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: a systematic review and meta-analysis of double-blind randomized controlled trials. Eur J Clin Nutr 2016;70:1354-1359. https://doi.org/10.1038/ejcn.2016.154 |
| 183 | Chaudhary DP, Sharma R, Bansal DD: Implications of Magnesium Deficiency in Type 2 Diabetes: A Review. Biol Trace Elem Res 2010;134:119-129. https://doi.org/10.1007/s12011-009-8465-z |
| 184 | Morais JBS, Severo JS, de Alencar GRR, de Oliveira ARS, Cruz KJC, Marreiro DDN, Freitas BJESA, de Carvalho CMR, Martins MDCCE, Frota KMG: Effect of magnesium supplementation on insulin resistance in humans: A systematic review. Nutrition 2017;38:54-60. https://doi.org/10.1016/j.nut.2017.01.009 |
| 185 | Ebrahimi Mousavi S, Ghoreishy SM, Hemmati A, Mohammadi H: Association between magnesium concentrations and prediabetes: a systematic review and meta-analysis. Sci Rep 2021;11:24388-24397. https://doi.org/10.1038/s41598-021-03915-3 |
| 186 | Tangvarasittichai S: Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 2015;6: 456-480. https://doi.org/10.4239/wjd.v6.i3.456 |
| 187 | Zahra H, Berriche O, Mizouri R, Boukhayatia F, Khiari M, Gamoudi A, Lahmar I, Ben Amor N, Mahjoub F, Zayet S, Jamoussi H. Plasmatic Magnesium Deficiency in 101 Outpatients Living with Type 2 Diabetes Mellitus. Clin Pract 2021;11:791-800. https://doi.org/10.3390/clinpract11040095 |
| 188 | Naqvi DA, Shadani DAK, Gupta DS: Study of Serum Magnesium Level in Type 2 Diabetes Mellitus in Relation to Its Microvascular Complication. Int J Sci Healthc Res 2022;7:108-117. https://doi.org/10.52403/ijshr.20220417 |
| 189 | Sales CH, Pedrosa LFC, Lima JG, Lemos TMAM, Colli C: Influence of magnesium status and magnesium intake on the blood glucose control in patients with type 2 diabetes. Clin Nutr 2011;30:359-364. https://doi.org/10.1016/j.clnu.2010.12.011 |
| 190 | Wei J, Zeng C, Li X, Gong Q, Lei G, Yang T: Association among dietary magnesium, serum magnesium, and diabetes: a cross-sectional study in middle-aged and older adults. J Heal Popul Nutr 2016;35:33-39. https://doi.org/10.1186/s41043-016-0071-z |
| 191 | Fang X, Han H, Li M, Liang C, Fan Z, Aaseth J, He J, Montgomery S, Cao Y: Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies. Nutrients 2016;8:739-757. https://doi.org/10.3390/nu8110739 |
| 192 | Fang X, Wang K, Han D, He X, Wei J, Zhao L, Imam MU, Ping Z, Li Y, Xu Y, Min J, Wang F: Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. BMC Med 2016;14:210-223. https://doi.org/10.1186/s12916-016-0742-z |
| 193 | Murakami M, Ishizuka J, Sumi S, Nickols GA, Cooper CW, Townsend CM, Thompson JC: Role of Extracellular Magnesium in Insulin Secretion from Rat Insulinoma Cells. Exp Biol Med 1992;200:490-494. https://doi.org/10.3181/00379727-200-43459 |
| 194 | Reis MAB, Reyes FGR, Saad MJA, Velloso LA: Magnesium Deficiency Modulates the Insulin Signaling Pathway in Liver but Not Muscle of Rats. J Nutr 2000;130:133-138. https://doi.org/10.1093/jn/130.2.133 |
| 195 | Sales CH, Santos AR dos, Cintra DEC, Colli C: Magnesium-deficient high-fat diet: Effects on adiposity, lipid profile and insulin sensitivity in growing rats. Clin Nutr 2014;33:879-888. https://doi.org/10.1016/j.clnu.2013.10.004 |
| 196 | Singh A, Satchell SC: Microalbuminuria: causes and implications. Pediatr Nephrol 2011;26:1957-1965. https://doi.org/10.1007/s00467-011-1777-1 |
| 197 | Oka T, Hamano T, Sakaguchi Y, Yamaguchi S, Kubota K, Senda M, Yonemoto S, Shimada K, Matsumoto A, Hashimoto N, Mori D, Monden C, Takahashi A, Obi Y, Yamamoto R, Takabatake Y, Kaimori JY, Moriyama T, Horio M, Matsui I, Isaka Y. Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: a common electrolyte abnormality in chronic kidney disease. Nephrol Dial Transplant 2019;34:1154-1162. https://doi.org/10.1093/ndt/gfy119 |
| 198 | Corsonello A, Ientile R, Buemi M, Cucinotta D, Mauro VN, Macaione S, Corica F: Serum Ionized Magnesium Levels in Type 2 Diabetic Patients with Microalbuminuria or Clinical Proteinuria. Am J Nephrol 2000;20:187-192. https://doi.org/10.1159/000013582 |
| 199 | Bloch-Damti A, Bashan N: Proposed Mechanisms for the Induction of Insulin Resistance by Oxidative Stress. Antioxid Redox Signal 2005;7:1553-1567. https://doi.org/10.1089/ars.2005.7.1553 |
| 200 | Rains JL, Jain SK: Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 2011;50:567-575. https://doi.org/10.1016/j.freeradbiomed.2010.12.006 |
| 201 | King DE, Mainous AG, Buchanan TA, Pearson WS: C-Reactive Protein and Glycemic Control in Adults With Diabetes. Diabetes Care 2003;26:1535-1539. https://doi.org/10.2337/diacare.26.5.1535 |
| 202 | Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean MEJ, Haffner SM: Prospective Study of C-Reactive Protein in Relation to the Development of Diabetes and Metabolic Syndrome in the Mexico City Diabetes Study. Diabetes Care 2002;25:2016-2021. https://doi.org/10.2337/diacare.25.11.2016 |
| 203 | Chen L, Chen R, Wang H, Liang F: Mechanisms Linking Inflammation to Insulin Resistance. Int J Endocrinol 2015;2015:1-9. https://doi.org/10.1155/2015/508409 |
| 204 | Guerrero-Romero F, Nevárez-Sida A: Cost-effectiveness analysis of using oral magnesium supplementation in the treatment of prediabetes. Prim Care Diabetes 2022;16: 435-439, https://doi.org/10.1016/j.pcd.2022.03.013 |
| 205 | Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, Wong HM, Tern PJW, Chandran M, Chay JWM, Nagarajan C, Sultana R, Low JGH, Ng HJ: Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020;79-80:111017-111022. https://doi.org/10.1016/j.nut.2020.111017 |
| 206 | Afshar Ebrahimi F, Foroozanfard F, Aghadavod E, Bahmani F, Asemi Z: The Effects of Magnesium and Zinc Co-Supplementation on Biomarkers of Inflammation and Oxidative Stress, and Gene Expression Related to Inflammation in Polycystic Ovary Syndrome: a Randomized Controlled Clinical Trial. Biol Trace Elem Res 2018;184:300-307. https://doi.org/10.1007/s12011-017-1198-5 |
| 207 | Abad C, Vargas FR, Zoltan T, Proverbio T, Piñero S, Proverbio F, Marín R: Magnesium sulfate affords protection against oxidative damage during severe preeclampsia. Placenta 2015;36:179-185. https://doi.org/10.1016/j.placenta.2014.11.008 |
| 208 | Zemel PC, Zemel MB, Urberg M, Douglas FL, Geiser R, Sowers JR: Metabolic and hemodynamic effects of magnesium supplementation in patients with essential hypertension. Am J Clin Nutr 1990;51:665-669. https://doi.org/10.1093/ajcn/51.4.665 |
| 209 | Sanjuliani AF, de Abreu Fagundes VG, Francischetti EA: Effects of magnesium on blood pressure and intracellular ion levels of Brazilian hypertensive patients. Int J Cardiol 1996;56:177-183. https://doi.org/10.1016/0167-5273(96)02716-7 |
| 210 | Abad C, Carrasco MJ, Piñero S, Delgado E, Chiarello DI, Teppa-Garrán A, Proverbio T, Proverbio F, Marín R: Effect of Magnesium Sulfate on the Osmotic Fragility and Lipid Peroxidation of Intact Red Blood Cells from Pregnant Women with Severe Preeclampsia. Hypertens Pregnancy 2010;29:38-53. https://doi.org/10.3109/10641950902777713 |
| 211 | Ham JY, Shon YH: Natural Magnesium-Enriched Deep-Sea Water Improves Insulin Resistance and the Lipid Profile of Prediabetic Adults: A Randomized, Double-Blinded Crossover Trial. Nutrients 2020;12:515-530. https://doi.org/10.3390/nu12020515 |
| 212 | Sugimoto J, Romani AM, Valentin-Torres AM, Luciano AA, Ramirez Kitchen CM, Funderburg N, Mesiano S, Bernstein HB: Magnesium Decreases Inflammatory Cytokine Production: A Novel Innate Immunomodulatory Mechanism. J Immunol 2012;188:6338-6346. https://doi.org/10.4049/jimmunol.1101765 |
| 213 | Almoznino-Sarafian D, Sarafian G, Berman S, Shteinshnaider M, Tzur I, Cohen N, Gorelik O: Magnesium administration may improve heart rate variability in patients with heart failure. Nutr Metab Cardiovasc Dis 2009;19:641-645. https://doi.org/10.1016/j.numecd.2008.12.002 |
| 214 | Rodríguez-Morán M, Simental-Mendía LE, Gamboa-Gómez CI, Guerrero-Romero F: Oral Magnesium Supplementation and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Adv Chronic Kidney Dis 2018;25:261-266. https://doi.org/10.1053/j.ackd.2018.02.011 |
| 215 | Morakinyo AO, Samuel TA, Adekunbi DA: Magnesium upregulates insulin receptor and glucose transporter-4 in streptozotocin-nicotinamide-induced type-2 diabetic rats. Endocr Regul 2018;52:6-16. https://doi.org/10.2478/enr-2018-0002 |
| 216 | Liu H, Li N, Jin M, Miao X, Zhang X, Zhong W: Magnesium supplementation enhances insulin sensitivity and decreases insulin resistance in diabetic rats. Iran J Basic Med Sci 2020;23:990-998. |
| 217 | Kamran M, Kharazmi F, Malekzadeh K, Talebi A, Khosravi F, Soltani N: Effect of Long-term Administration of Oral Magnesium Sulfate and Insulin to Reduce Streptozotocin-Induced Hyperglycemia in Rats: the Role of Akt2 and IRS1 Gene Expressions. Biol Trace Elem Res 2019;190:396-404. https://doi.org/10.1007/s12011-018-1555-z |