[6]-Shogaol Induces Apoptosis of Murine Bladder Cancer Cells
bLaboratory of Applied Immunology, Department of Genetics and Evolution, Federal University of São Carlos (UFSCar), CEP 13565-905 São Carlos-SP, Brazil,
cLaboratory of Bioactive Natural Products, Department of Pharmacy, Federal University of Sergipe (UFS), CEP 49400-000, Av. Gov. Marcelo Deda, 330-São José, Lagarto-SE, Brazil.
Keywords
Abstract
Background/Aims:
Bladder cancer is considered one of the most aggressive neoplasms due to its recurrence and progression profile, and even with the improvement in diagnosis and treatment methods, the mortality rate has not shown a declining trend in recent decades. From this perspective, the search and development of more effective and safer therapeutic alternatives are necessary. Phytochemicals are excellent sources of active principles with therapeutic potential. [6]-Shogaol is a phenolic compound extracted from the ginger rhizomes that has shown antitumor effects in a wide variety of cancer models. However, there is no record in the literature of studies reporting these effects in models of bladder cancer. Thus, this study aimed to investigate the in vitro cytotoxic and pro-apoptotic potential of [6]-Shogaol against murine bladder cancer urothelial cells (MB49).Methods:
The cytotoxic effects of [6]-Shogaol on cell viability (MTT method), cell morphology (light microscopy), alteration of proliferative processes (clonogenic assay), oxidative stress pathway (levels of reactive oxygen species) and the induction of apoptotic events (flow cytometry and high-resolution epifluorescence imaging) were evaluated in murine urothelial bladder cancer cell lines (MB49), relative to non-tumor murine fibroblasts (L929).Results:
The results showed that [6]-Shogaol was able to induce concentration-dependent cytotoxic effects, which compromised cell viability, exhibiting an inhibitory concentration of 50% of cells (IC50) of 146.8 µM for MB49 tumor cells and 236.0 µM for L929 non-tumor fibroblasts. In addition to inhibiting and altering the proliferative processes if colony formation, it presented pro-apoptotic activity identified through a quantitative analysis and the observation of apoptotic phenotypes, events apparently mediated by the induction of nuclear fragmentation.Conclusion:
The data presented suggest that [6]-Shogaol has a higher concentration-dependent cytotoxic and apoptosis-inducing potential in MB49 cells than in L929 fibroblasts. These results may contribute to the development of therapeutic alternatives for bladder cancer.Introduction
Cancer is one of the main causes of morbidity and mortality worldwide [1, 2]. According to the latest data presented by the World Health Organization (WHO) on its interactive platform Global Cancer Observatory (GLOBOCAN) [1, 3], it was estimated for the year 2020 the occurrence of 573, 278 new cases of bladder cancer and approximately 212, 536 new deaths, cataloging it as the tenth most frequently diagnosed type of cancer in the world and as the thirteenth deadliest [1, 2, 4]. Bladder cancer is characterized by affecting mostly adults over the age of 55 years, however, this age group has gradually decreased over the years, due to the wide variety of factors involved that help the development of this neoplasm, which include the factors environmental and genetic [5]. Another peculiarity of this disease is that it preferentially affects men over women (4:1) [5]. However, the prognosis for women is more unfavorable, due to late detection, in more advanced stages due to a masking of signs and symptoms [5-7].
Bladder cancer significantly affects the life quality of patients and most therapeutic options that make up the current treatment regimens significantly compromise health, since there is a high rate of treatment failure due to low specificity and the side effects caused [8].
Despite progress in both diagnosis and treatment regimens, their availability, cost, and effectiveness are a limitation. Therefore, the search for new therapeutic alternatives is extremely important. Natural products are an excellent source of therapeutic bioactive compounds and have been used to treat a wide variety of diseases. The Ginger (Zingiber officinale Roscoe), belonging to the Zingiberaceae family, is a spice used worldwide for the preparation of food and beverages. However, it is also known for different studies that report its various therapeutic properties, which include antioxidant [9-11], anti-inflammatory [9, 11], antimicrobial [10], neuroprotective [12], antidiabetic [13], anti-nausea [14], antiemetic [14], antiangiogenic and anticancer [15]. Ginger is abundant in bioactive compounds, such as gingerols, paradols, shogaols, zingerone, quercetin and others [15]. One of its most promising compounds found in processed ginger is [6]-Shogaol (6S), which has already demonstrated its competence as an phytoterapic for several models of cancer in vitro and in vivo, not only acting directly in the elimination of neoplastic tissues, but also in its sensitization and/or modulation, making it a good hit for study within phytoterapics [16-19].
Currently, the existence of studies reporting the use of [6]-Shogaol as a treatment for bladder cancer, and the effects it exerts on cell cultures, cell models or living systems that simulate bladder cancer is not known in the literature. Thus, the present study aimed to evaluate the cytotoxic potential in murine bladder cancer cells (MB49).
Materials and Methods
Reagents
[6]-Shogaol was extracted with absolute ethanol, using Soxhlet apparatus, and isolated by classical chromatographic techniques and HPLC as described in Supplementary Material [20, 21]. It has a purity greater than 97% which was determined by HPLC-DAD. The compound was isolated and purified as described previously by Silva et al [21].. The purified compound was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 100 mM and stored in -20 °C until further use. The final concentrations of DMSO (Sigma-Aldrich , USA) in the culture medium did not exceed 1%. We used the following reagents to perform the different proposed tests: Dulbecco´s Modified Eagle Medium (DMEM, Gibco, Life Technologies , EUA), fetal bovine serum (FBS, Cultilab), penicillin and streptomycin (Vitrocell Embriolife , Campinas, Brazil), 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-dipheyltetrazolium bromide (MTT) (Thermo Fisher Scientific ), acridine orange (Sigma-Aldrich, USA), propidium iodide (Sigma-Aldrich, USA), paraformaldehyde (Sigma-Aldrich, USA), camptothecin (Sigma-Aldrich, USA), 4´,6´-Diamidine -2´-phenylindole dihydrochloride (DAPI) (Thermo Fisher Scientific ), 2´,7´-Dichlorodihydrofluorescein diacetate (H2DCFDA) (Sigma-Aldrich, USA), hydrogen peroxide (H2O2).
Cell culture
Murine transitional carcinoma cell line (MB49 – NCI Thesaurus Code: C25823), donated by Dr. Yi Lou (University of Iowa), and mouse fibroblast cell line (L929, ATCC # CCL-1 CCL-1) were cultured in Dulbecco´s Modified Eagle Medium supplemented with 10% fetal bovine serum, and 1% of penicillin and streptomycin (complete DMEM) in an incubator, at 37°C with a humid atmosphere and 5% CO2.
Cytotoxicity and Cell morphology
The effects of [6]-Shogaol on the cytotoxicity of MB49 and L929 cells were determined by colorimetric assay using MTT [22]. The cells were seeded in complete medium (1 x 104 cells/well) in 96 well-plate culture (Corning Incorporated , NY, USA) and incubated for the period of adherence (24 hours). Afterwards the cells were exposed to serial dilution of [6]-Shogaol (1000 – 3.9 μM) for 24 hours. Four hours of metabolic activity were measured using the reagent MTT (0.5 mg/mL). The absorbance was measured by spectrophotometry at a wavelength of 570 nm using the Multiskan FC Microplate Photometer (Thermo Fisher Scientific). Images of [6]-Shogaol treated cells were taken during the assay using a microscope (Carl Zeiss Primovert) with a magnitude of 40x and captured with a camera (CANON Powershot A650 IS 12, 1 Megapixels Live Resolution ).
Selectivity Index
Selectivity index (SI) was calculated as the radio between the IC50 of the [6]-Shogaol obtained from non-tumor cells (L929) and the IC50 of the [6]-shogaol obtained for tumor cells (MB49) [23].
Colony formation
MB49 Cells were seeded in 6-well plates culture (Corning Incorporated, NY, USA) at 300 cells/ well and incubated for the period of adherence at 37°C. The cells were then treated with [6]-Shogaol (0; 15.625; 31.25 and 62.5 μM). After 24 hours of incubation, the culture media was replaced by new fresh media and cells were incubated for 5 days [24, 25]. Adherent cells were fixed with absolute methanol for 5 minutes and stained with a solution of crystal violet (0.1% m/v) (Sigma-Aldrich, USA) for 1 minute. The colonies (˃50 cells) were counted and measured using ImageJ Software.
Reactive oxygen species (ROS) production
MB49 and L929 cells (1x105cells/well) were plated in a black 96 well-plate culture (Corning Incorporated, NY, USA), and incubated for the period of adherence. The cells were treated with 62.5 and 125 μM of [6]-Shogaol for 2 and 4 hours. After treatment, was added H2DCFDA reagent (100 μM) and incubated for 30 minutes. The fluorescence was measure using SpectraMax i3x (Multi-Mode Microplate Fluorimeter, Molecular Devices, CA, EUA) at a wavelength of excitation 485 nm and emission 530 nm. The emitted fluorescence was expressed at relative fluorescence units and compared to control cells (untreated). Hydrogen peroxide (100 μM) was used as a positive control of intracellular ROS production [26].
Detection of nuclear condensation and nuclear fragmentation events
MB49 and L929 cells (5x103 cells/well) were seeded in a black 96 well-plate culture (Corning Incorporated, NY, USA), and maintained at 37°C and 5% CO2 for the period of adherence. After incubation, cells were treated with [6]-Shogaol (15.625; 32.25; 62.5 and 125 μM) for 12 hours. Following the cells were washed with PBS and fixed with 4% (v/v) paraformaldehyde for 10 minutes and stained with a solution of DAPI (0.15 μg/mL) for 10 minutes in the dark. Images were captured with automated high-resolution epifluorescence microscopy ImageXpress Micro (Molecular Devices, CA, EUA) equipment with a magnification of 400x. Camptothecin (100 μM) was used as a positive control for induction of nuclear condensation and nuclear fragmentation events [27].
Detection of apoptosis by fluorescent labeling
MB49 and L929 cells (5x103 cells/well) were seeded in a black 96 well-plate culture (Corning Incorporated, NY, USA), and maintained at 37°C and 5% CO2 for the period of adherence. After incubation, cells were treated with [6]-Shogaol (62.5 and 125 μM) for 12 hours. Following the cells were washed with PBS and stained with a solution of acridine orange (1 mg/ mL), and propidium iodide (1 mg/mL) for 15 minutes in the dark and washed thoroughly. Images were captured with automated high-resolution epifluorescence microscopy ImageXpress Micro (Molecular Devices, CA, EUA) equipment with a magnification of 400x. Camptothecin (100 μM) was used as a positive control of apoptosis [28].
Detection of apoptosis by flow cytometry
The pro-apoptotic activity of [6]-Shogaol was analyzed by flow cytometry with the PE-Annexin-V Apoptosis Detection Kit (BD Bioscience ). MB49 and L929 cells (1x105 cells/ well) were seeded in 24 well plates culture (Corning Incorporated, NY, USA) and incubated for the period of adherence. The medium was removed, and the cells incubated with [6]-Shogaol (62.5 and 125 μM) for 12 hours. Cells were harvested with scraper, washed with cold PBS, and resuspended in binding buffer (200 μL). Cells were incubated with PE-Annexin-V (1 μL) and 7-aminoactinomycin D (7AAD) (1μL) for 15 minutes at room temperature, in the dark. Three hundred microliters of binding buffer were added to microtubes and the cells were analyzed on a BD Accuri C6 flow cytometer (Becton, Dickinson and Company, Franklin Lakes , NJ, USA), selecting a gate with 10, 000 events [29, 30]. Specific fluorescence was quantified using CSampler software and the analyses of Dot Plots were performed with FCS Express software.
Statistical analyses
Statistical analysis was performed using GraphPad Prism version 7.0 software. To assess the parametric distribution of the data, statistical values and a frequency distribution in histograms were evaluated and analyzed for the Shapiro Wilk normality test. According to its classification, the parametric data were represented as the mean ± SD, however, non-parametric data were provided as the median and 95% CI. Subsequently, for the parametric data, One-way ANOVA analysis of variance and Dunnett´s post-test were performed to determine if the results were statistically different compared to controls, in the case of comparisons between two groups, the 2-way ANOVA and Sidak post-test were used. For the non-parametric data, the Kruskal Wallis test and Dunn´s post-test (Dunn´s Multiple Comparison Test) were used. The significance level adopted for all tests was 5%, being.
Results
Effect of compound [6]-Shogaol on the viability of MB49 and L929 cells
To investigate the effects of [6]-Shogaol on tumor (MB49) and non-tumor (L929) cells, different concentrations of the compound were used and exposed to cells for 24 hours. From the MTT assay it was possible to demonstrated that [6]-Shogaol induced cell damage that compromises the metabolism and cell viability of both cell lines, and that this effect was not significantly selective against tumor cells (Fig. 1A). This can be verified from the superposition of the IC50 curves for both cell lines, and the IC50 concentration values (146.8 μM for MB49 cells and 236.0 μM for L929 cells) used to calculate the selectivity index (1.61) that there is no marked and significant selectivity (Fig. 1B). These interpretations could also be deduced from the morphological differences observed in the micrographs of both cell lines (Fig. 1C), where it is possible to observe the morphological changes in both cell lines, being a little more prominent in MB49 cells compared to the L929 cells.
Subsequently, the effects of different concentration of [6]-Shogaol were evaluated on the continuous proliferation capacity of tumor cells, through the colony formation assay, which allowed us to directly assess the cytotoxic and cytostatic effects of the compound [31, 32]. The results showed that for concentrations 15.625; 31.25 and 62.5 μM, [6]-Shogaol presented mechanisms as a cytotoxic and cytostatic agent, as it significantly inhibited the number and size of colonies of MB49 cells compared to the untreated group (Fig. 2A-C).
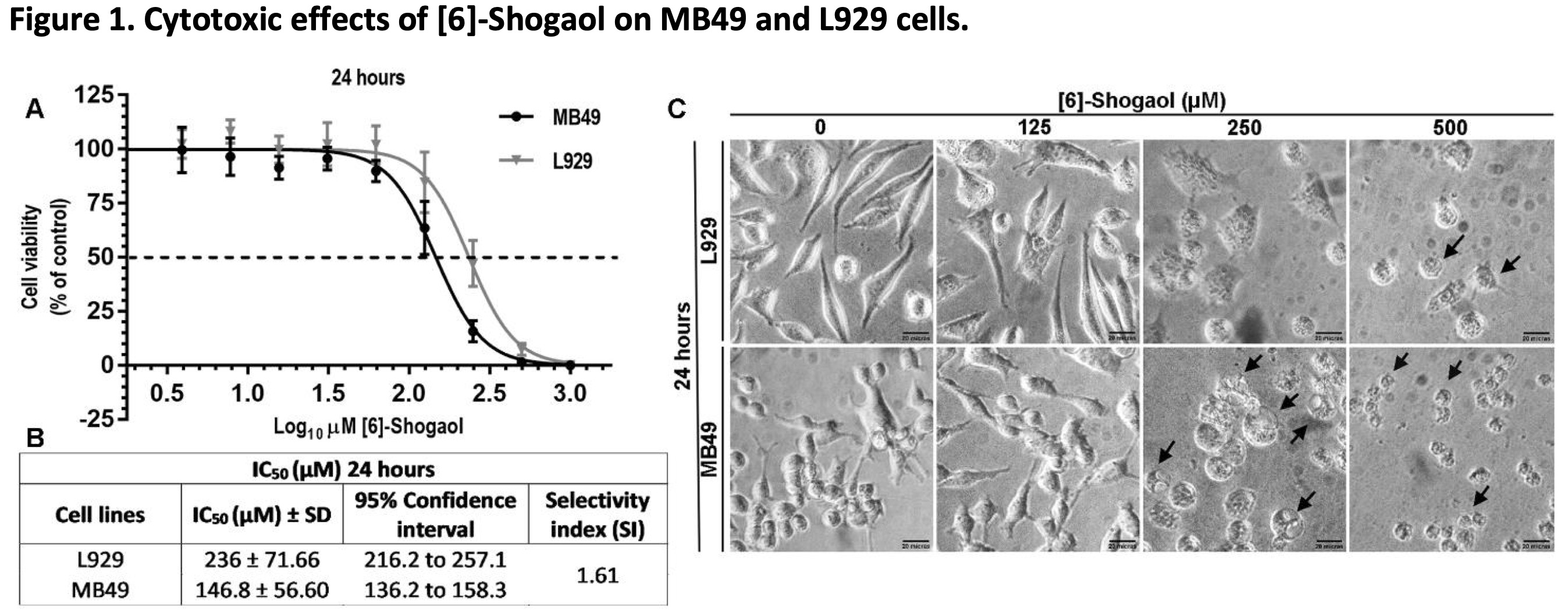
Fig. 1: Cytotoxic effects of [6]-Shogaol on MB49 and L929 cells. (A) Concentration-response curves and the effects of different concentrations of [6]-Shogaol on the viability of cell lines, treated for 24 hours and measured by the MTT colorimetric assay. The curve points were expressed as the median ± 95% CI of four independent experiments (n = 4). (B) IC50 values from 24 hours of [6]-Shogaol treatment in L929 and MB49 cell lines. (C) Morphological changes and cell detachment (black arrows) caused by exposure to [6]-Shogaol. Cells were examined at 400x amplification. Scale bar = 20 µm.
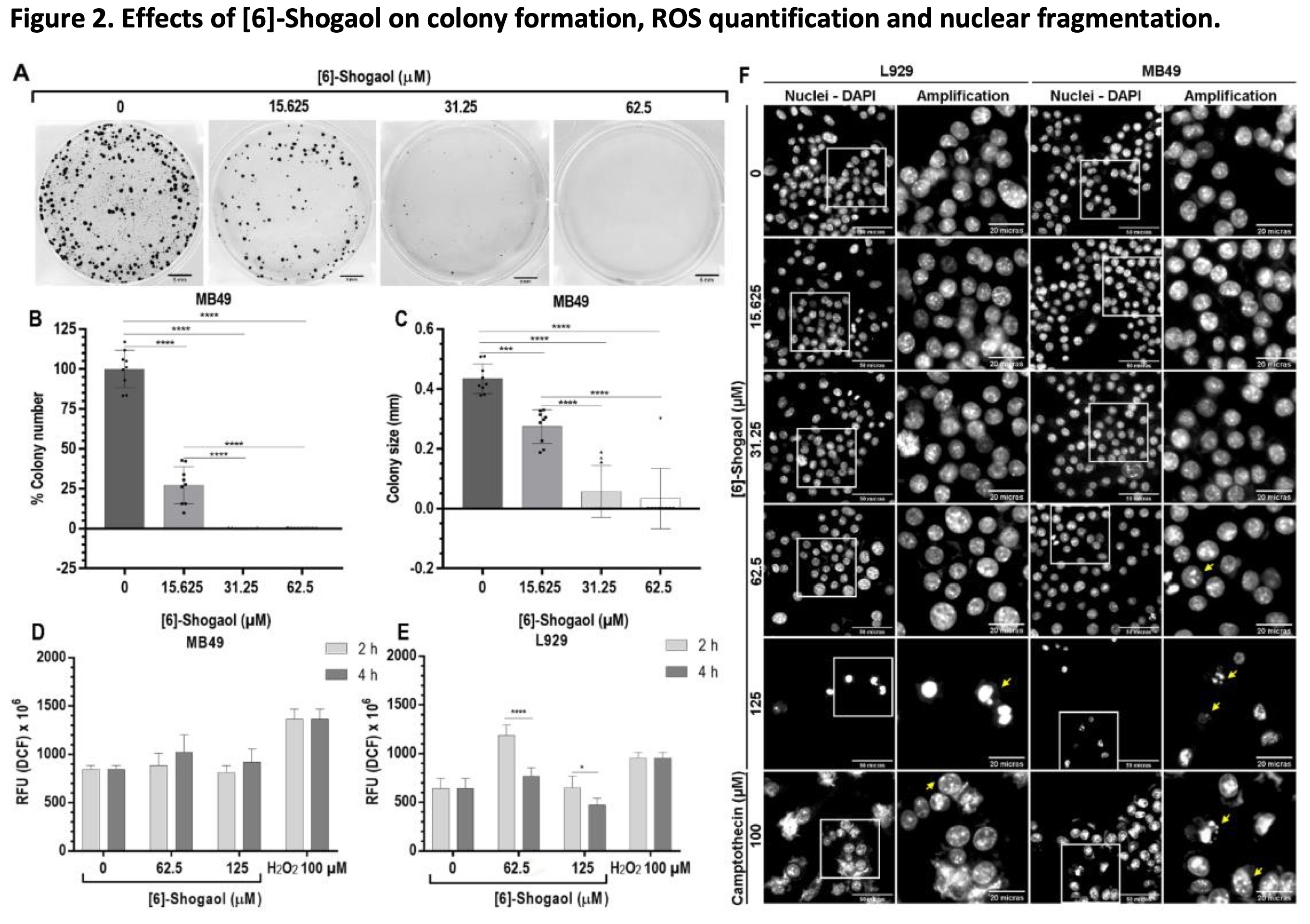
Fig. 2: Effects of [6]-Shogaol on colony formation, ROS quantification and nuclear fragmentation. (A) Representative image of three independent experiments (n = 3) in the formation of MB49 colonies, formed after 5 days of exposure to [6]-Shogaol. Bar scale = 5 mm. (B) Graph of the number of colonies normalized in relation to the control. (C) Colony size graph normalized to control. Data are presented as mean ± SD (n = 3). Statistical analysis was performed using the One-way ANOVA parametric test and Tukey's multiple comparison post-test. *** p ˂ 0.001; **** p ˂ 0.0001. (D, E) Effects of [6]-Shogaol on ROS generation in MB49 and L929 cells, respectively, treated with the indicated concentrations of compound for 2 and 4 hours, followed by incubation with H2DCFDA and analyzed by fluorescence. Data were considered parametric and presented as mean ± SD (n = 4). Comparative statistical analysis was performed using the 2-way ANOVA test and the Sidak post-test. * p ˂ 0.05; **** p ˂ 0.0001. (F) Photomicrographs of the effects of [6]-Shogaol on the induction of nuclear condensation and nuclear fragmentation events. L929 and MB49 cells were treated with the indicated concentration of compound for 12 hours and images captured at 400x magnification. Yellow arrows represent nuclear fragmentation and condensation events. Scale bar = 50 and 20 µm.
The cytotoxic effects of [6]-Shogaol induce apoptosis
To elucidate the main mechanisms triggering the cytotoxic effects of [6]-Shogaol, ROS level in the cell lines were measured after exposure periods of 2 and 4 hours. As shown in Fig. 2D, no concentrations tested were able to cause a significant increase in intracellular ROS content in MB49 tumor cells compared to controls groups. However, for L929 cells, there was increase in the fluorescence emitted by the ROS present inside the cells was observed when exposed to concentration of 62.5 μM, after 2 hours of treatment (Fig. 2E). From the comparative analysis of the time 2 and 4 hours of exposure of [6]-Shogaol in MB49 and L929 cells (Figures 2D-E), it can be stated that the compound apparently does not stimulate an imbalance in the REDOX system, mainly in MB49 cells, although an increase was observed for the L929 cells with the treatment of 62.5 μM. From these results we can indicate that, for the bladder cancer cells used in this study, oxidative stress is not a causative mechanism of the cytotoxic effects of [6]-Shogaol.
One of the main cell death processes related to the cytotoxicity of organic compounds is apoptosis. Among the alterations associated with the apoptotic processes are the partial condensation of chromatin around the nuclear membrane (type I apoptotic nuclear morphology) and the fragmentation of the nucleus into condensed chromatin masses (type II apoptotic nuclear morphology), the latter being one the most representative of this processes of cell death [27, 33]. The evaluation of induction of changes in nuclear morphology in MB49 and L929 cells by [6]-Shogaol, with the aid of the fluorescent dye DAPI, showed us that at concentrations above 62.5 μM, it was possible to notice the presence of fragmented nuclei and decrease in the number of cells compared to the control groups for both cell lines (Fig. 2F). These results suggest that damage to the nuclear structure could trigger cell death processes, preferably by apoptosis.
However, to conform these claims, the structural integrity of both cells was visualized with the aid of fluorophores and based on their ability to penetrate into the cells, according to degree of integrity of the membranes [28]. After 12 hours of exposure with [6]-Shogaol, at concentrations above 62.5 μM, phenotypes associated with cell death events were observed in both cell lines (Fig. 3A). Cells exposed to concentration of 62.5 μM have nuclear regions linked to the orange fluorophore of acridine accompanied by faint cytoplasmic regions that are diffusely stained with the afore mentioned dye, characterizing an early apoptosis event (Fig. 3A – b and f). Likewise, nuclear fragmentation events at concentration of 125 μM were visualized by marking nuclear regions with both fluorophores, characterizing the process of late apoptosis (Fig. 3A – c and g). However, cells with prominent structural damage were also found, from the marking of the propidium iodide fluorophore in the nuclear region, associated with necrosis events (Fig. 3A c and g).
To quantify the pro-apoptotic capacity of [6]-Shogaol, the flow cytometry assay was performed with the aid of PE-annexin-V and 7AAD markers, considered the high specificity of the Annexin-V protein to recognize apoptosis events [30, 34-36]. In Fig. 3B, it is possible to observe from the distribution of cells in the quadrants that the predominant cytotoxic effects of [6]-Shogaol is apoptotic cell death for both cell lines. As can be seen, concentrations of 62.5 μM and 125 μM of [6]-Shogaol are able of cause 35.48% and 60.94% of apoptotic cell death events in MB49 cells, respectively, and only a 1, 53% and 6% of necrotic cell death events (Fig. 3C). In the same concentrations, for L929 cells, [6]-Shogaol only incited 19.41% and 39.76% of apoptotic cell death events, respectively, while it induced only 3.75% and 19.95% of necrotic cell death events, (Fig. 3D). From the results, our suggest that [6]-Shogaol predominantly induces concentration-dependent apoptotic cell death events.
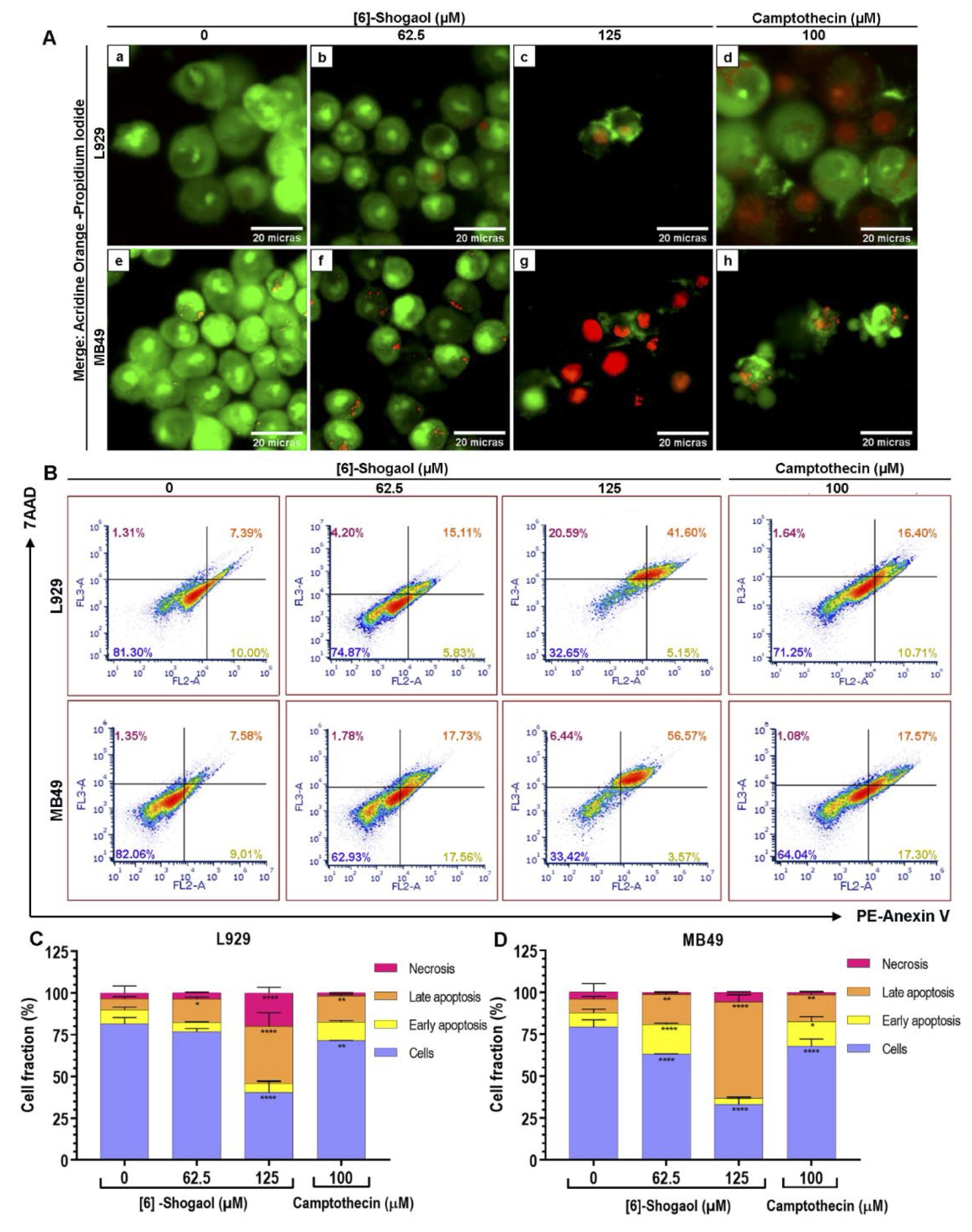
Fig. 3: Analysis of the type of cell death caused by [6]-Shogaol exposure in MB49 and L929 cells. (A) Photomicrographs of apoptosis and necrosis events detected by acridine orange/propidium iodide staining assay in cells treated with different concentrations of [6]-Shogaol for 12 hours. Images correspond to a representative replica (n = 5), captured at 400x magnification. (a) control group L929 cell without treatment; (b) L929 treated with 62.5 µM [6]-Shogaol, showing weak and diffuse fluorescence in the cytoplasm representing early events of apoptosis; (c) L929 treated with 125 µM [6]-Shogaol, presenting orange color in the nuclei, phenotypes associated with late apoptosis events; (d) L929 treated with 100 µM camptothecin, late apoptosis and necrosis events are observed; (e) untreated control of M4B9 cells; (f) MB49 treated with 62.5 µM [6]-Shogaol, showing almost inexistent weak fluorescence in the cytoplasm, phenotype associated with apoptosis and nuclear fragmentation; (g) MB49 treated with 125 µM [6]-Shogaol, showing reddish coloration in the nuclei, associated with necrosis events; (h) MB49 treated with 100 µM of camptothecin, showing apoptotic bodies. Scale bar = 20 µm. (B) Dot blot density diagrams showing the percentages of apoptosis and necrosis induced by [6]-Shogaol in L929 and MB49 cells. (C, D) Bar diagram representing the percentages of L929 and MB49 cells at different stages of apoptotic cell death and necrosis. Data represent mean ± SD (n = 3). Data were considered parametric and statistical analysis was performed using the 2-way ANOVA test and Dunnett's post-test. *p ≤ 0.05; **p ˂ 0.01; ****p ˂ 0.0001.
Discussion
Currently, many of the available treatment regimens for the bladder cancer are not considered effective due to the lack of response to existing anticancer drugs, the generation of resistance mechanisms by tumor cells and the induction of side effects in non-target tissues, which makes it difficult to reverse the disease and on the contrary, in many cases considerably affects their quality of life. Thus, the search for new drugs with longer shelf life, safety, specificity, and selectivity has become a necessity. Several studies report the potential of phytochemicals for the prevention and treatment of a wide variety of cancers, among the beneficial characteristics associated with these products are their low toxicity, their ability to simultaneously target several cell signaling pathways, which may compromise the viability of cancer cells, and their alternative ways of promoting cell death, converting them into fascinating therapeutic options for different types of cancers [37-39], there are also promising references to the use of combined therapies, employing medicinal herbs, natural extracts or compounds of natural origin for the purpose of to mitigate the adverse effects of chemotherapy [38]. According to information presented by the Food and Drug Administration (FDA) indicate that almost 40% of molecules with approved therapeutic use are isolated from natural compounds or are inspired by them [38], and according to Newman et al., the total number of approved anticancer drugs in the period of 1940 to 2019, about 50% are derived from natural products [38, 40, 41]. The study of phytochemicals as therapeutic alternatives for bladder cancer is a promising area of investigation, since many of these compounds are specific and selective for tumor cells, and some of them have reduced toxicity, facts reported in different studies using cell cultures and living organisms to simulate the conditions of the neoplasm [42-44]. According to the Committee for Herbal Medicines (HMPC) of the European Medicine Agency (EMA), dried ginger rhizome powder can be used for the prevention of nausea and vomiting in motion sickness, side effects associated with chemotherapy [11]. However, it is usefulness is not only limited to these properties, due to which there are references of in vitro , in vivo , and pre-clinical studies in which they report that this spice has anti-cancer, antioxidant, and anti-inflammatory activities, with the compound [6]-Shogaol being one of the main bioactive principles of dried ginger rhizome [11, 45]. Further investigation of this compound is extensive since that the ginger is considered by the FDA as Generally Recognized as Safe (GRAS) in it is Spices and other natural seasonings section and in the Essential oils, oleoresins, and natural extracts section according to it is regulation [45]. Previously published studies reported that [6]-Shogaol, a lipophilic compound isolated from rhizomes of dry ginger (Zingiber officinale) , showed cytotoxic potential and apoptosis-inducing activity for lung [46], prostate [18], colorectal [47], larynx [48] cervicouterin [49], breast [50] and hematologic cancer cells [51].
However, no study was found in the literature reporting the cytotoxic effect of [6]-Shogaol on cell lines or in vivo models of bladder cancer. This study is the first to evaluate these effects in bladder cell cultures, and the results presented are important as they allow establishing a baseline for a study with this approach, allowing the future evaluation of the effect of modifying the different variables presented to improve the results obtained. In this work we demonstrated in an unprecedented way, that [6]-Shogaol can reduce cell viability of concentration and time-dependent manner, on murine bladder tumor cells MB49 (IC50 of 146.8 μM), with a selectivity index of 1.61 in relation to non-tumor murine fibroblasts L929 (IC50 of 236.0 μM) (Fig. 1). Considering that there are no records of previous studies with which to compare our results, an analysis of the effects identified in our study was performed in comparison with other studies that reported the effect of the compound in other cancer models, to assess whether the induced cytotoxic effect, and the mechanisms are similar or different, being important to emphasize that the variation in IC50 values of the other cited studies and those estimated in our study are different, since that the cells that represent the different types of cancer mentioned have different functions, characteristics and locations, therefore their gene expression profile and tumor microenvironment are completely different, in addition to the fact that targets of [6]-Shogaol and their mechanisms of action are different in many cases, facts that may explain the variations in these values. Our IC50 estimation findings are consistent with previous studies, which reported that [6]-Shogaol induces lethal cytotoxic effects to tumor cells in different types of cancers, in a concentration and exposure-time-dependent manner, as described by Annamalai et al. (2016), which demonstrated that this compound exhibits IC50 of 20 μM for human laryngeal carcinoma cells (Hep-2) and IC50 of 80 μM for non-tumor gingival fibroblasts (HGF-1) [48]. As reported by Bawadood et al. (2020), [6]-Shogaol showed considerable cytotoxic effects on triple positive ductal carcinoma cells (T47D) and human breast adenocarcinoma cells (MCF-7), exhibiting IC50 of 0.5 and 23.3 μM, respectively, considerably low values that highlight the compound´s potency against these breast cancer cell lines [50]. In addition, it was observed cytotoxic effects of [6]-Shogaol on gastric cancer cells (HCG-27) of concentration-dependent manner, presenting IC50 of 32 μM [52].
In the present study, [6]-Shogaol showed cytostatic and lethal cytotoxic potential at concentrations below 62.5 µM, since the absence of colonies in the clonogenic assay may be due to lethal cytotoxic effects, or even to interference in the process of the cell division (aberrant mitosis is capable of causing cell death) [50]. These findings agree with the literature, as different studies have reported that [6]-Shogaol induces alterations in cell cycle flow through different mechanisms [49, 50, 52].
The elucidation of the mechanisms involved in the cytotoxic effects induced by [6]-Shogaol is the paramount importance to discover possible targets and metabolic pathways altered by the compound which compromise the viability of the cells under study. Tumor cells are for the most part, characterized by having greater amount of intracellular ROS when compared to normal cells [37, 53, 54], however, they also express increased levels of antioxidant proteins to detoxify the ROS present in the intracellular medium, an event that suggests the existence of a fragile balance of levels of ROS, a necessary event that guarantees the survival and functioning of cancer cells [37]. An event that does not happen in non-tumor cells because are regulated by different enzymatic and non-enzymatic antioxidant pathways, maintaining their antioxidant homeostasis and viability [53, 55]. Thus, it is common to associate that an increase in ROS levels in tumor cells may trigger a REDOX imbalance, which compromises their viability, since the pre-existing higher ROS content in tumor cells makes them more sensitive to damage caused by the ROS [53, 54]. Overall, it is reasonable to assume that oxidative processes may mediate the release of pro-apoptotic factors from mitochondria and thus take advantage of this oxidative cellular state of cancer cells to direct new therapeutic strategies to alter the REDOX balance for the apoptotic pathway [37]. However, in this study, it was not identified that [6]-Shogaol induces an increase in the level of intracellular ROS and from this information we can suggest that the cytotoxic effects evidenced are not mediated by this process. These results are in agreement with the dichotomous effect of this compound described by other authors [37], with the study by Chen et al. (2014), in which they report the antioxidant properties of [6]-Shogaol, by positively regulating the expression of nuclear factor erythroid factor 2 (Nrf2) and its translocation to the nucleus, which in turn regulates the transcription of different genes that participate in the synthesis of Glutathione (GSH), such as the gene that encodes the Glutamate-Cysteine Ligase Catalytic Subunit (GCLC) and the gene that encodes the Glutamate-Cysteine Ligase Modifying Subunit (GCLM), events identified in human colon carcinoma cells HCT-116 [16].
Condensation and nuclear fragmentation are considered typical events of the cell death, especially in late apoptosis [56], together with morphological changes, cell shrinkage and the formation of apoptotic bodies in plasmatic membrane [56]. From the events of nuclear fragmentation evidenced in the results, it is possible suggest that this effect is one of the mechanisms involved in the cytotoxic activity that [6]-Shogaol presents, typical events of the apoptotic cell death process, and reported in the literature as apoptotic nuclear morphology stage I and II to chromatin condensation and nuclear fragmentation events, respectively [27, 33]. DNA breakdown by divalent cation-dependent endonucleases (Ca+2 and Mg+2) is one of the distinctive mechanisms of apoptosis [57]. According to the study by Iglesias-Guimaraes (2013), stage II apoptotic nuclear morphology is associated with activation of DNA fragmentation factor (DFF40 / CAD), a caspase-3 activated endonuclease that hydrolyzes DNA into oligonucleosomal length fragments (≈ 180 pb), being the catalytic activity of this endonuclease necessary for the occurrence of nuclear fragmentation [27]. Our results agree with other studies, in which they report that [6]-Shogaol is able to induce damage to the nuclear structure as part of the cytotoxic effects that trigger in the process of apoptotic cell death in laryngeal cancer cells Hep-2 and human colorectal cancer cells HT29 [47, 48].
The ability of a compound to modulate the viability and mortality of tumor cells is widely recognized for its therapeutic potential [57], and the induction of apoptotic processes is considered more beneficial in relation to necrosis, since, being a programmed process does not incite the surging of inflammatory episodes, which may subsequently favor carcinogenesis and dissemination of neoplastic tissues [57]. According to results previously described in other studies [6]-Shogaol has pro-apoptotic potential in several cancer cell lines [46, 47, 49, 50], statements that agree with our study, in which it was possible to identify that [6]-shogaol induces more prominent pro-apoptotic effects in a concentration-dependent manner in bladder tumor cells (MB49) relative to non-tumor cells (L929). Although there is no registers in the literature of any study that reveals the effects of [6]-Shogaol in bladder cancer cell cultures or in vivo assays, these results are in agreement with different studies [58, 59] that assessed the pro-apoptotic potential of [6]-Shogaol in other cancer models, such as the study of Liu et al. (2013) in which they described a concentration-dependent relationship in the induction of apoptosis in Jurkat cells U937 and human leukemia cells HL-60 [51]. The pro-apoptotic effect of [6]-Shogaol has also been reported in human liposarcoma cells (SW872), by reducing the expression level and phosphorylation of the STAT3 gene, a gene that plays a crucial role in proliferation and which is activated constitutively in different neoplasms, however, when this gene is inhibited, it induces growth suppression and leads to apoptosis [60]. Our results also agree with the pro-apoptotic potential reported by the study of Najafi et al., and are considered more promising, since in this study [6]-Shogaol at higher concentrations (200 µM), after 96 hours of exposure, the compound only incites 10.56% of apoptotic events in Nalm6 cells of Acute Lymphoblastic Leukemia B, in relation to our study that in a shorter exposure time (12 hours) it was shown that the compound induces 60.94% of apoptosis events at a concentration of 125 µM in the bladder cancer cells (MB49) [45].
Conclusion
In summary, this study reported that [6]-Shogaol, a lipophilic compound present in the rhizomes of dry ginger, showed cytotoxic potential in bladder tumor cells MB49, triggered damage to the nuclear structure of cells that possibly trigger a subsequent apoptotic process, and it was evidenced that these cytotoxic effects are not mediated by an exacerbated increase in the content of intracellular ROS (Fig. 4) as reported by several studies [51, 60] [6].-Shogaol can be used as a promising strategy for bladder cancer therapy, as it induces a controlled death process in tumor cells, which is characterized by the non-release of cellular content into the intercellular space, which would trigger lesions to the surrounding tissues that favor the dissemination of neoplastic tissue. This study offers important information to further study the metabolic pathways involved in these cytotoxic effects, providing a new study target, considering that this compound has different molecular mechanisms of cytotoxicity in tumor and non-tumor cells.
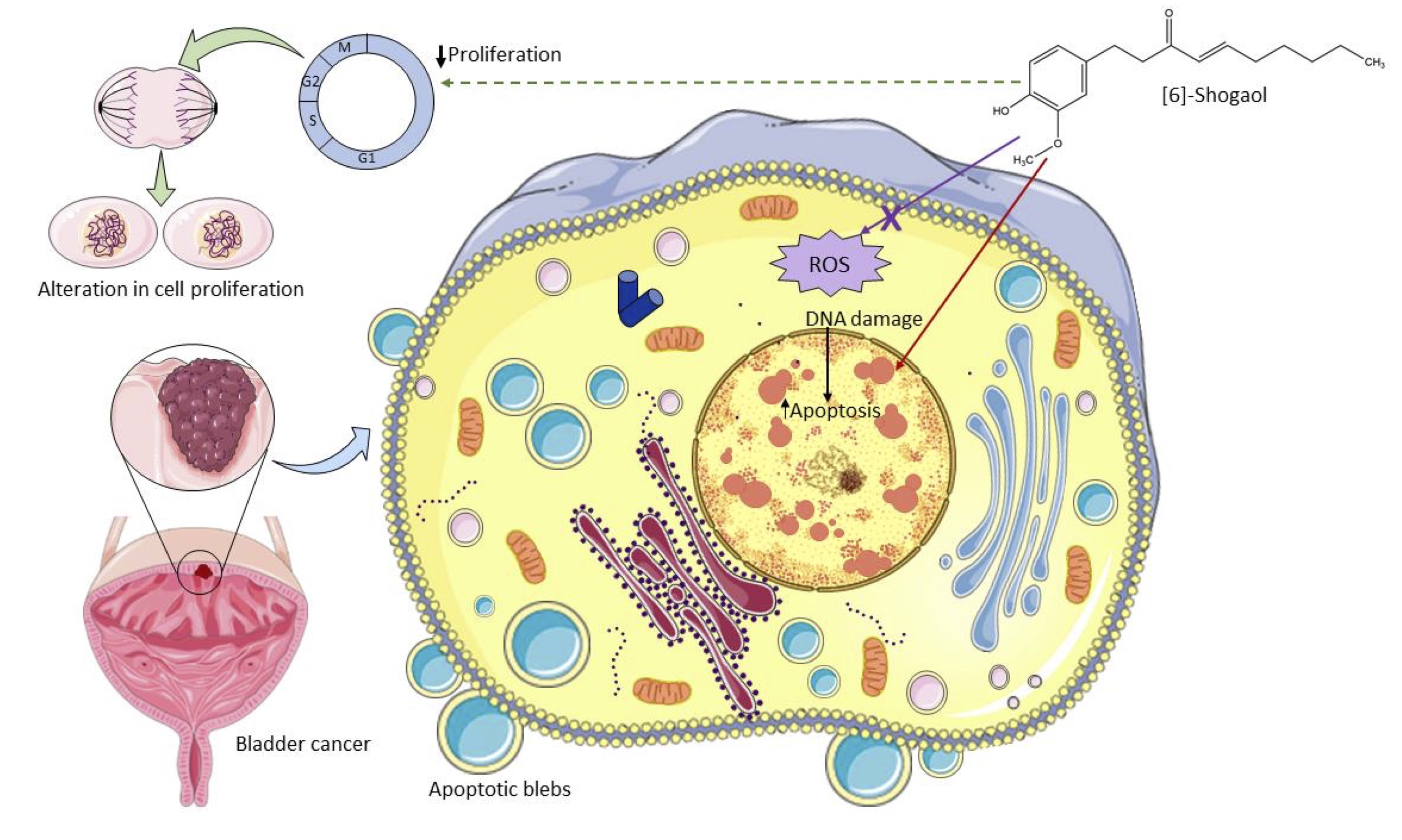
Fig. 4: Schematic diagram of the action of [6]-Shogaol on murine bladder cancer cells MB49 in vitro. [6]-Shogaol induced concentration-dependent cytotoxic effects mediated by damage to the nuclear structure that triggered apoptotic cell death, but these effects were not mediated by an exacerbated increase in ROS. It also showed a cytostatic potential mediated by an alteration in the cell division process on in vitro assays. Source: Author (2022), performed with the help of Servier Medical Art image bank.
Acknowledgements
The authors would like to thank Dr. Marcia Regina Cominetti, Dr. Liany Luna Dulcey, and Dr. Ana Carolina B.M. Martin from Laboratory of Biology of Aging, Department of Gerontology, Federal University of São Carlos, São Carlos, SP, Brazil, for the availability of equipment and Dr. Heloisa S. Selistre de Araujo and Dr. Wanessa F. Altei from Laboratory of Biochemistry and Molecular Biology, Department of Physiological Sciences, Federal University of São Carlos, São Carlos, SP, Brazil, which provided yours equipment’s and for the technical support. The authors acknowledge the use of the Servier Medical Art image bank used to create schematic figure 4. Finally, we thank CAPES and PETROBRAS for the financial support.
Author Contributions
Nina D. is responsible for the designed the research, laboratory work, data analysis and preparation of the manuscript first draft. Robeldo T. is responsible for the designed the research and manuscript writing. Anibal F. is responsible for the designed the research, made substantial contributions and critically revised the manuscript for important intellectual content. Borra R. also contributed to the design of experiments, data analysis, data curation, and critically revised the manuscript for important intellectual content. da Silva J. critically revised the manuscript for important intellectual content and extracted the compound used in this study. Gonçalves V. extracted the compound used in this study. All authors approved the final content for journal submission and publication.
Funding Sources
This research was supported by Coordenação de Aperfeiçoamento do Pessoal de Nível Superior (CAPES, Finance Code 001).
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-249.
https://doi.org/10.3322/caac.21660 |
| 2 | Fidler MM, Bray F, Soerjomataram I: The global cancer burden and human development: A review. Scand J Public Health 2018;46:27-36.
https://doi.org/10.1177/1403494817715400 |
| 3 | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021;71:209-249.
https://doi.org/10.3322/caac.21660 |
| 4 | Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F: Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108.
https://doi.org/10.1016/j.eururo.2016.06.010 |
| 5 | Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A: Epidemiology of Bladder Cancer. Medical sciences (Basel, Switzerland) 2020;8:15.
https://doi.org/10.3390/medsci8010015 |
| 6 | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424.
https://doi.org/10.3322/caac.21492 |
| 7 | Wong MCS, Fung FDH, Leung C, Cheung WWL, Goggins WB, Ng CF: The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Scientific reports. DOI: 10.1038/s41598-018-19199-z.
https://doi.org/10.1038/s41598-018-19199-z |
| 8 | Apolo AB, Vogelzang NJ, Theodorescu D: New and promising strategies in the management of bladder cancer. Am Soc Clin Oncol Educ Book 2015:105-112.
https://doi.org/10.14694/EdBook_AM.2015.35.105 |
| 9 | Nile SH, Park SW: Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Industrial Crops and Products 2015;70:238-244.
https://doi.org/10.1016/j.indcrop.2015.03.033 |
| 10 | Ghasemzadeh A, Jaafar HZE, Baghdadi A, Tayebi-Meigooni A: Formation of 6-, 8- and 10-Shogaol in Ginger through Application of Different Drying Methods: Altered Antioxidant and Antimicrobial Activity. Molecules (Basel, Switzerland) 2018;23:1646.
https://doi.org/10.3390/molecules23071646 |
| 11 | Bischoff-Kont I, Fürst R: Benefits of Ginger and Its Constituent 6-Shogaol in Inhibiting Inflammatory Processes. Pharmaceuticals (Basel, Switzerland) 2021;14:571.
https://doi.org/10.3390/ph14060571 |
| 12 | Ho S-C, Chang K-S, Lin C-C: Anti-neuroinflammatory capacity of fresh ginger is attributed mainly to 10-gingerol. Food Chemistry 2013;141:3183-3191.
https://doi.org/10.1016/j.foodchem.2013.06.010 |
| 13 | Carvalho GCN, Lira-Neto JCG, Araújo MFMd, Freitas RWJFd, Zanetti ML, Damasceno MMC: Effectiveness of ginger in reducing metabolic levels in people with diabetes: a randomized clinical trial. Revista latino-americana de enfermagem 2020;28:e3369-e3369.
https://doi.org/10.1590/1518-8345.3870.3369 |
| 14 | Walstab J, Krüger D, Stark T, Hofmann T, Demir IE, Ceyhan GO, Feistel B, Schemann M, Niesler B: Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterology & Motility 2013;25:439-e302.
https://doi.org/10.1111/nmo.12107 |
| 15 | Mao Q-Q, Xu X-Y, Cao S-Y, Gan R-Y, Corke H, Beta T, Li H-B: Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods (Basel, Switzerland) 2019;8:185.
https://doi.org/10.3390/foods8060185 |
| 16 | Chen H, Fu J, Chen H, Hu Y, Soroka DN, Prigge JR, Schmidt EE, Yan F, Major MB, Chen X, Sang S: Ginger compound [6]-shogaol and its cysteine-conjugated metabolite (M2) activate Nrf2 in colon epithelial cells in vitro and in vivo. Chemical research in toxicology 2014;27:1575-1585.
https://doi.org/10.1021/tx500211x |
| 17 | Ray A, Vasudevan S, Sengupta S: 6-Shogaol Inhibits Breast Cancer Cells and Stem Cell-Like Spheroids by Modulation of Notch Signaling Pathway and Induction of Autophagic Cell Death. PLoS One 2015;10:e0137614.
https://doi.org/10.1371/journal.pone.0137614 |
| 18 | Saha A, Blando J, Silver E, Beltran L, Sessler J, DiGiovanni J: 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-κB signaling. Cancer Prev Res (Phila) 2014;7:627-638.
https://doi.org/10.1158/1940-6207.CAPR-13-0420 |
| 19 | Zhou L, Qi L, Jiang L, Zhou P, Ma J, Xu X, Li P: Antitumor activity of gemcitabine can be potentiated in pancreatic cancer through modulation of TLR4/NF-κB signaling by 6-shogaol. The AAPS journal 2014;16:246-257.
https://doi.org/10.1208/s12248-013-9558-3 |
| 20 | Ok S, Jeong W-S: Optimization of Extraction Conditions for the 6-Shogaol-rich Extract from Ginger (Zingiber officinale Roscoe). Preventive nutrition and food science 2012;17:166-171.
https://doi.org/10.3746/pnf.2012.17.2.166 |
| 21 | da Silva JA, dos Santos CY, Mohammadi M, Fernandes JB, das Graças Fernandes da Silva MF, Vieira PC: Cathepsin K Inhibitors Isolated from Ginger Rhizome. Revista Brasileira de Farmacognosia 2021;31:859-864.
https://doi.org/10.1007/s43450-021-00223-9 |
| 22 | Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
https://doi.org/10.1016/0022-1759(83)90303-4 |
| 23 | Bézivin C, Tomasi S, Lohézic-Le Dévéhat F, Boustie J: Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003;10:499-503.
https://doi.org/10.1078/094471103322331458 |
| 24 | Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C: Clonogenic assay of cells in vitro. Nat Protoc 2006;1:2315-2319.
https://doi.org/10.1038/nprot.2006.339 |
| 25 | Rafehi H, Orlowski C, Georgiadis GT, Ververis K, El-Osta A, Karagiannis TC: Clonogenic assay: adherent cells. JoVE (Journal of Visualized Experiments) 2011:e2573.
https://doi.org/10.3791/2573 |
| 26 | Eruslanov E, Kusmartsev S: Identification of ROS using oxidized DCFDA and flow-cytometry; Advanced protocols in oxidative stress II, Springer, 2010, pp 57-72.
https://doi.org/10.1007/978-1-60761-411-1_4 |
| 27 | Iglesias-Guimarais V, Gil-Guiñon E, Sánchez-Osuna M, Casanelles E, García-Belinchón M, Comella JX, Yuste VJ: Chromatin Collapse during Caspase-dependent Apoptotic Cell Death Requires DNA Fragmentation Factor, 40-kDa Subunit-/Caspase-activated Deoxyribonuclease-mediated 3′-OH Single-strand DNA Breaks * . Journal of Biological Chemistry 2013;288:9200-9215.
https://doi.org/10.1074/jbc.M112.411371 |
| 28 | Liu K, Liu PC, Liu R, Wu X: Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res 2015;21:15-20.
https://doi.org/10.12659/MSMBR.893327 |
| 29 | van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP: A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996;24:131-139.
https://doi.org/10.1002/(SICI)1097-0320(19960601)24:2<131::AID-CYTO5>3.0.CO;2-M |
| 30 | Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH: Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994;84:1415-1420.
https://doi.org/10.1182/blood.V84.5.1415.1415 |
| 31 | Puck TT, Marcus PI: Action of x-rays on mammalian cells. J Exp Med 1956;103:653-666.
https://doi.org/10.1084/jem.103.5.653 |
| 32 | Puck TT, Morkovin D, Marcus PI, Cieciura SJ: Action of x-rays on mammalian cells. II. Survival curves of cells from normal human tissues. The Journal of experimental medicine 1957;106:485-500.
https://doi.org/10.1084/jem.106.4.485 |
| 33 | Garcia-Belinchón M, Sánchez-Osuna M, Martínez-Escardó L, Granados-Colomina C, Pascual-Guiral S, Iglesias-Guimarais V, Casanelles E, Ribas J, Yuste VJ: An Early and Robust Activation of Caspases Heads Cells for a Regulated Form of Necrotic-like Cell Death. The Journal of biological chemistry 2015;290:20841-20855.
https://doi.org/10.1074/jbc.M115.644179 |
| 34 | Chaurio RA, Janko C, Muñoz LE, Frey B, Herrmann M, Gaipl US: Phospholipids: key players in apoptosis and immune regulation. Molecules (Basel, Switzerland) 2009;14:4892-4914.
https://doi.org/10.3390/molecules14124892 |
| 35 | Schlegel RA, Williamson P: Phosphatidylserine, a death knell. Cell Death Differ 2001;8:551-563.
https://doi.org/10.1038/sj.cdd.4400817 |
| 36 | Cossarizza A, Chang HD, Radbruch A, Acs A, Adam D, Adam-Klages S, Agace WW, Aghaeepour N, Akdis M, Allez M, Almeida LN, Alvisi G, Anderson G, Andrä I, Annunziato F, Anselmo A, Bacher P, Baldari CT, Bari S, Barnaba V, Barros-Martins J, Battistini L, Bauer W, Baumgart S, Baumgarth N, Baumjohann D, Baying B, Bebawy M, Becher B, Beisker W, et al.: Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). European Journal of Immunology 2019;49:1457-1973.
https://doi.org/10.1002/eji.201970107 |
| 37 | Zadorozhna M, Mangieri D: Mechanisms of Chemopreventive and Therapeutic Proprieties of Ginger Extracts in Cancer. International journal of molecular sciences 2021;22:6599.
https://doi.org/10.3390/ijms22126599 |
| 38 | Seca AML, Pinto DCGA: Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. International journal of molecular sciences 2018;19:263.
https://doi.org/10.3390/ijms19010263 |
| 39 | Huang H, Kim M-O, Kim K-R: Anticancer effects of 6-shogaol via the AKT signaling pathway in oral squamous cell carcinoma. Journal of applied oral science : revista FOB 2021;29:e20210209-e20210209.
https://doi.org/10.1590/1678-7757-2021-0209 |
| 40 | Newman DJ, Cragg GM: Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. Journal of Natural Products 2020;83:770-803.
https://doi.org/10.1021/acs.jnatprod.9b01285 |
| 41 | Al-Bari MAA, Ito Y, Ahmed S, Radwan N, Ahmed HS, Eid N: Targeting Autophagy with Natural Products as a Potential Therapeutic Approach for Cancer. International journal of molecular sciences 2021;22:9807.
https://doi.org/10.3390/ijms22189807 |
| 42 | Abbaoui B, Lucas CR, Riedl KM, Clinton SK, Mortazavi A: Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Molecular nutrition & food research 2018;62:e1800079-e1800079.
https://doi.org/10.1002/mnfr.201800079 |
| 43 | Wu Q, Wong JPC, Kwok HF: Putting the Brakes on Tumorigenesis with Natural Products of Plant Origin: Insights into the Molecular Mechanisms of Actions and Immune Targets for Bladder Cancer Treatment. Cells 2020;9:1213.
https://doi.org/10.3390/cells9051213 |
| 44 | Xia Y, Chen R, Lu G, Li C, Lian S, Kang T-W, Jung YD: Natural Phytochemicals in Bladder Cancer Prevention and Therapy. Frontiers in oncology 2021;11:652033-652033.
https://doi.org/10.3389/fonc.2021.652033 |
| 45 | Najafi Dorcheh S, Rahgozar S, Talei D: 6-Shogaol induces apoptosis in acute lymphoblastic leukaemia cells by targeting p53 signalling pathway and generation of reactive oxygen species. Journal of cellular and molecular medicine 2021;25:6148-6160.
https://doi.org/10.1111/jcmm.16528 |
| 46 | Kim MO, Lee M-H, Oi N, Kim S-H, Bae KB, Huang Z, Kim DJ, Reddy K, Lee S-Y, Park SJ: [6]-Shogaol inhibits growth and induces apoptosis of non-small cell lung cancer cells by directly regulating Akt1/2. Carcinogenesis 2014;35:683-691.
https://doi.org/10.1093/carcin/bgt365 |
| 47 | Li TY, Chiang BH: 6-shogaol induces autophagic cell death then triggered apoptosis in colorectal adenocarcinoma HT-29 cells. Biomed Pharmacother 2017;93:208-217.
https://doi.org/10.1016/j.biopha.2017.06.038 |
| 48 | Annamalai G, Kathiresan S, Kannappan N: [6]-Shogaol, a dietary phenolic compound, induces oxidative stress mediated mitochondrial dependant apoptosis through activation of proapoptotic factors in Hep-2 cells. Biomed Pharmacother 2016;82:226-236.
https://doi.org/10.1016/j.biopha.2016.04.044 |
| 49 | Liu Q, Peng Y-B, Qi L-W, Cheng X-L, Xu X-J, Liu L-L, Liu EH, Li P: The Cytotoxicity Mechanism of 6-Shogaol-Treated HeLa Human Cervical Cancer Cells Revealed by Label-Free Shotgun Proteomics and Bioinformatics Analysis. Evidence-Based Complementary and Alternative Medicine 2012;2012:278652.
https://doi.org/10.1155/2012/278652 |
| 50 | Bawadood AS, Al-Abbasi FA, Anwar F, El-Halawany AM, Al-Abd AM: 6-Shogaol suppresses the growth of breast cancer cells by inducing apoptosis and suppressing autophagy via targeting notch signaling pathway. Biomedicine & Pharmacotherapy 2020;128:110302.
https://doi.org/10.1016/j.biopha.2020.110302 |
| 51 | Liu Q, Peng Y-B, Zhou P, Qi L-W, Zhang M, Gao N, Liu EH, Li P: 6-Shogaol induces apoptosis in human leukemia cells through a process involving caspase-mediated cleavage of eIF2α. Molecular Cancer 2013;12:135.
https://doi.org/10.1186/1476-4598-12-135 |
| 52 | Ishiguro K, Ando T, Watanabe O, Goto H: Specific reaction of alpha,beta-unsaturated carbonyl compounds such as 6-shogaol with sulfhydryl groups in tubulin leading to microtubule damage. FEBS letters 2008;582:3531-3536.
https://doi.org/10.1016/j.febslet.2008.09.027 |
| 53 | Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A: ROS in cancer therapy: the bright side of the moon. Exp Mol Med 2020;52:192-203.
https://doi.org/10.1038/s12276-020-0384-2 |
| 54 | Wang G, McKenney JK: Urinary Bladder Pathology: World Health Organization Classification and American Joint Committee on Cancer Staging Update. Arch Pathol Lab Med 2019;143:571-577.
https://doi.org/10.5858/arpa.2017-0539-RA |
| 55 | Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan M, Sethi G: Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules 2019;9:735.
https://doi.org/10.3390/biom9110735 |
| 56 | Pavlina M, Jan C, Filip P, Jiri H, Tomas R: Quantitative spectrofluorometric assay detecting nuclear condensation and fragmentation in intact cells. Scientific reports 2021;11:11921-11921.
https://doi.org/10.1038/s41598-021-91380-3 |
| 57 | Elmore S: Apoptosis: a review of programmed cell death. Toxicologic pathology 2007;35:495-516.
https://doi.org/10.1080/01926230701320337 |
| 58 | Kim MO, Lee M-H, Oi N, Kim S-H, Bae KB, Huang Z, Kim DJ, Reddy K, Lee S-Y, Park SJ, Kim JY, Xie H, Kundu JK, Ryoo ZY, Bode AM, Surh Y-J, Dong Z: [6]-shogaol inhibits growth and induces apoptosis of non-small cell lung cancer cells by directly regulating Akt1/2. Carcinogenesis 2014;35:683-691.
https://doi.org/10.1093/carcin/bgt365 |
| 59 | Hu R, Zhou P, Peng Y-B, Xu X, Ma J, Liu Q, Zhang L, Wen X-D, Qi L-W, Gao N, Li P: 6-Shogaol induces apoptosis in human hepatocellular carcinoma cells and exhibits anti-tumor activity in vivo through endoplasmic reticulum stress. PloS one 2012;7:e39664-e39664.
https://doi.org/10.1371/journal.pone.0039664 |
| 60 | Yadav AK, Jang B-C: Anti-Survival and Pro-Apoptotic Effects of 6-Shogaol on SW872 Human Liposarcoma Cells via Control of the Intrinsic Caspase Pathway, STAT-3, AMPK, and ER Stress. Biomolecules 2020;10:1380.
https://doi.org/10.3390/biom10101380 |