Dual Modification of Tau by Pseudophosphorylation and Glycation Does Not Enhance Amorphous Aggregation
bThe School of Bioengineering Sciences and Research, Maharashtra Institute of Technology, Loni Kalbhor, Pune, India,
cAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, India,
dDepartment of Neurochemistry, National Institute of Mental Health and Neuro Sciences (NIMHANS), Institute of National Importance, Hosur Road, Bangalore, Karnataka, India
Keywords
Abstract
Background/Aims:
The neurofibrillary tangles consisting of Tau protein are an important pathology in Alzheimer’s disease. The paired helical filaments of Tau form most of the NFTs. These PHFs of Tau are found to carry numerous post-translational modifications, which stabilize them and aid in aggregation. The mechanistic function of Tau is to bind and stabilize the axonal microtubules. Hyperphosphorylation of Tau causes it to compromise its physiological function and accumulate in the neurons in the form of aggregates. Such residue-specific phosphorylation has been studied by employing Tau pseudophosphorylation mutants. But in addition to phosphorylation, several other modifications also aid in stabilizing the Tau PHF. Glycation is one such non-enzymatic PTM caused by sugars and their reactive intermediates. In this study, we employed the pseudophosphorylated Tau double mutants (262/404D, 262/396D, and 231/262) for studying their modification by methyl glyoxal, a reactive intermediate of glucose metabolism.Methods:
We studied various biophysical properties like aggregation propensity, Advanced glycation end-product formation, and global conformation of the Tau with dual modifications. Our study includes the use of in vitro techniques e.g., ThS fluorescence assay, electron microscopy, CD spectroscopy, SDS-PAGE.Results:
The overall result of the study suggest that the MG-induced Tau aggregation is influenced by the residue-specific Tau phosphorylation.Conclusion:
In conclusion, the combinatorial effect of discreet PTMs on Tau function could lead to a better understanding of Tauopathy.Introduction
The neurofibrillary tangles comprising of Tau protein are escalated in the brains of Alzheimer’s disease patients [1, 2]. The Tau protein in the NFTs is highly modified by the post-translational modifications leading to its self-aggregation and accumulation [3-7]. Tau regulates microtubule assembly and dynamics in the neuronal axons [8-10]. The regulation of microtubule stability and dynamics is essential for neuronal functioning. In abnormal conditions, Tau undergoes severe post-translational modifications, which alters its functions and increases its propensity for self-aggregation [11-13]. The screening of small molecules against Tau aggregation is an important therapeutic intervention and has yielded molecules belonging to various sources like naturally occurring molecules [14-17], synthetic molecules [18, 19], peptidomimetics etc . The function of Tau is regulated by its phosphorylation state and the site-specific phosphorylation affects its microtubule-binding as well as its aggregation propensity. The sites responsible for Tau phosphorylation are located in its proline-rich domain as well as the C-terminal region. Differential phosphorylation at the sites in these regions affects the microtubule-binding capacity of Tau as well as its aggregating propensity [20, 21]. The hyperphosphorylation of Tau is a major modification that not only affects its mechanistic functions but also accelerates its self-assembly [22]. Thus, it becomes extremely important to study the impact of residue-specific Tau phosphorylation on its physiological and pathological functions. One of the challenges in studying the effect of phosphorylation of specific amino acids on Tau functions is controlled phosphorylation, which cannot be attained by in vitro kinase assays. Thus, an alternative approach is followed, which includes the substitution of the phospho-epitopes to negatively charged amino acids like aspartic acid and glutamic acid such that the negative charges mimic the phosphorylation state of Tau [23, 24]. These mutants are referred to as pseudophosphorylation mutants and have been utilized in various Tau studies. Phospho-mimicking mutants have been found to alter the aggregation propensity of Tau in a domain-specific manner. Tau pseudophosphorylation at N-terminal showed inhibition of self-assembly whereas at C-terminal it enhanced the self-aggregation [20]. Along with its aggregation propensity, phospho-mimicking mutants have also been studied for their effect on Tau-microtubule interaction and assembly. The conformational studies revealed that pseudophosphorylation at sites 262, 293, 324, and 356 modulates the structural domains of repeat 1 and 2, and pseudophosphorylation at serine 262 imparts the structural changes to repeat Tau such that it hampers the microtubule interaction [25]. Thus, the effect of phosphorylation on Tau function depends on the residues modified in the particular domain. Along with phosphorylation, other modifications also affect Tau function and modulate its aggregation propensity. One such modification is glycation wherein; Tau is modified by glucose and its metabolic reactive intermediates. Tau glycation is observed in PHFs of AD brains, which hampers microtubule-binding and enhances its aggregation [26]. Moreover, glycation is more pronounced in the functional domain of Tau, which is involved in microtubule-binding, and regulation of its dynamics [27, 28]. Thus, it becomes important to study the effect of dual modification of Tau on its structure and aggregation kinetics. In vitro studies have suggested that glycation of pseudophosphorylated Tau aids in Tau fibrillization rather than seeding or nucleating the fibrillization [29]. The Tau tangle formation by glycating agents such as methyl glyoxal and acrolein is found to be enhanced by hyperphosphorylation. Moreover, the self-aggregation capacity is enhanced for triple pseudophosphorylated Tau mutants as compared to single or double mutants [30]. On the other hand, the single pseudophosphorylated mutants displayed a loss of Tau function whereas the double mutants exhibited enhanced microtubule assembly and more efficiency in governing dynamic microtubule instability. Thus, not just the extent of phosphorylation but the combination of phosphorylated residues also determines the fate of its microtubule binding and self-assembly [31] and plays a key role in regulating the fate of Tau. Additionally, the extent of glycation-mediated aggregation of Tau is subject to the isoform composition [32]. In our study, we attempted to understand the effect of methyl glyoxal-induced glycation of pseudophosphorylated Tau double mutants. We studied the fluorophore-based aggregation kinetics of this modified Tau as well as biophysical properties such as conformational modulations and morphological analysis of Tau aggregates. We have also studied the relative MG-induced AGEs formation of Tau and its pseudophosphorylation mutants by employing the autofluorescence property of AGEs.
Materials and Methods
Chemicals and reagents
Luria-Bertani broth was purchased from HiMedia. DTT and IPTG were obtained from Calbiochem; MES, BES, methyl glyoxal, SDS, glycine, ThT were purchased from Sigma. Heparin, NaCl, Ampicillin, sodium azide, ethanol, PMSF, MgCl2, and PMSF were obtained from MP Biomedicals.
Tau purification
The full-length (hTau40) wild-type Tau and its pseudophosphorylated double mutants were purified as described [52]. The proteins were expressed in E.coli by induction with IPTG. The cell pellet containing the expressed proteins was lysed under high pressure in a microfluidics device and the lysate was collected. The lysate was subjected to heating at 90 °C after the addition of 0.5 M NaCl and 5 mM DTT. After 20 minutes of heating, the lysate was cooled and centrifuged at 45000 rpm for 50 minutes to clear the lysate of precipitated proteins. The cleared lysate was dialyzed against the sepharose buffer A overnight and further clarified by centrifugation at 45000 rpm for 50 minutes. Further, the lysate was subjected to cation exchange chromatography and the eluted fractions were pooled, concentrated, and preceded for size-exclusion chromatography. The purified proteins were concentrated and the concentration was measured by BCA assay.
Aggregation of Tau and pseudophosphorylated mutants by methyl glyoxal
Tau and its phospho-mimicking mutants were made to a final concentration of 20 μM in BES buffer pH 7.4 supplemented with 25 mM NaCl, 1 mM DTT, 0.01% sodium azide, and protease inhibitor cocktail. Methyl glyoxal was added to a final concentration of 2.5 mM. Thioflavin T was added to the reaction mixture at a concentration of 2.5 μM. The reactions were protected from light and incubated at 37 °C. The fluorescent measurements were carried out by exciting the fluorophore at 435 nm and collecting the emission data at 485 nm [53] in TECAN Infinite series Pro plate reader.
AGEs-specific fluorescence measurements
The AGEs-specific fluorescence measurements were recorded as previously described [48]. Separate reaction mixtures were set up for monitoring AGEs-specific fluorescence without the addition of ThT. The fluorescence was recorded by exciting the reaction mixture at 370 nm and collecting emission at 430 nm. The readings were recorded in triplicates and the buffer background was subtracted.
SDS-PAGE analysis of MG-induced aggregation
The wild-type Tau and the phospho-mimicking mutants subjected to MG-induced aggregation were analyzed by SDS-PAGE. 10 μL reaction mixtures were separated on 10% SDS-PAGE and stained with Coomassie Brilliant Blue.
Circular dichroism spectroscopy
The conformational changes of MG-modified and unmodified Tau and its phospho-mimicking mutants were mapped in Jasco J-815 CD spectrometer under a nitrogen atmosphere. 3 μM of reaction mixtures were made in sodium phosphate buffer pH 6.8. The spectra were recorded in cuvette of 1 mm path length at 100 nm/min scan speed and 1 nm bandwidth. The average of 5 acquisitions was obtained over a scan range of 190-250 nm.
Transmission electron microscopy
The morphology of MG-modified proteins was visualized by Tecnai G2 20 S-Twin transmission electron microscope. 2 μM reaction mixtures were spotted onto the 400 mesh carbon-coated copper grids and stained with 2 % uranyl acetate. The grids were dried well before scanning.
Results
Methyl glyoxal does not enhance the aggregation of phospho-mimicking Tau mutants
Methyl glyoxal is a known protein glycating agent involved in protein modification diabetic conditions [33]. It modifies proteins and renders them non-functional due to various structural changes in the proteins [34]. It is also known to induce protein aggregation [35] and methyl glyoxal is found to be present in AD brains [36]. To study the effect of dual modification on Tau aggregation, we modified the 3 phospho-mimicking double mutants with the glycating agent methyl glyoxal and studied the aggregation propensity of mutants versus the wild-type Tau. The aggregation was monitored by thioflavin T fluorescence assay for 168 hours. The kinetics revealed a gradual increase in ThT fluorescence indicating an increase in the aggregation of proteins. Though no significant difference was observed in aggregation propensity at the end of 168 hours, the proteins demonstrated a different pattern of fluorescence at the initial time points (Fig. 1C). The phospho-mutants 262/396D and 231/262 had initial kinetics similar to the wild-type Tau till 36 hours. After 36 hours the phospho-mutant 262/396 D showed enhanced aggregation propensity as compared to wild-type Tau whereas the mutant 262/404D showed decreased MG-induced aggregation propensity concerning wild-type Tau. At the end time point, no difference was observed in the aggregation propensities of Tau mutants 262/396D and 231/262D concerning the wild type. The mutant 262/404D showed a minimal increase in fluorescence throughout the kinetics suggesting decreased MG-induced aggregation rate. The end-time fluorescence intensity analysis suggested no significant difference among the wild type and phospho-mutants except 262/404D (Fig 1D). These results confirm the previous reports to some extent wherein; double pseudophosphorylated Tau does not show changes in methyl glyoxal-induced Tau aggregation as compared to wild-type Tau. This might be due to the effect of residue-specific pseudophosphorylation on overall Tau aggregation [30]. It has been reported that the sites phosphorylated at C-terminal for example S396 and S404 enhance Tau fibrillization more efficiently as compared to sites in the proline-rich region (T231) and repeat domain of Tau (S262) [20, 37]. Additionally, pS262 is found to have increased inhibition of heparin-induced Tau aggregation with an increase in the number of phosphorylated sites [25]. All the mutants used in our study have pseudophosphorylated S262 that might be withholding the MG-induced Tau aggregation as compared to wild-type Tau. Thus, although glycation by MG is a strong modification that drastically alters protein structure by driving them to aggregation, the pseudophosphorylation at S262 prevents MG-induced Tau aggregation which is in concordance with its effect on heparin-induced Tau aggregation. Thus, MG-induced Tau aggregation is influenced by residue-specific Tau phosphorylation.
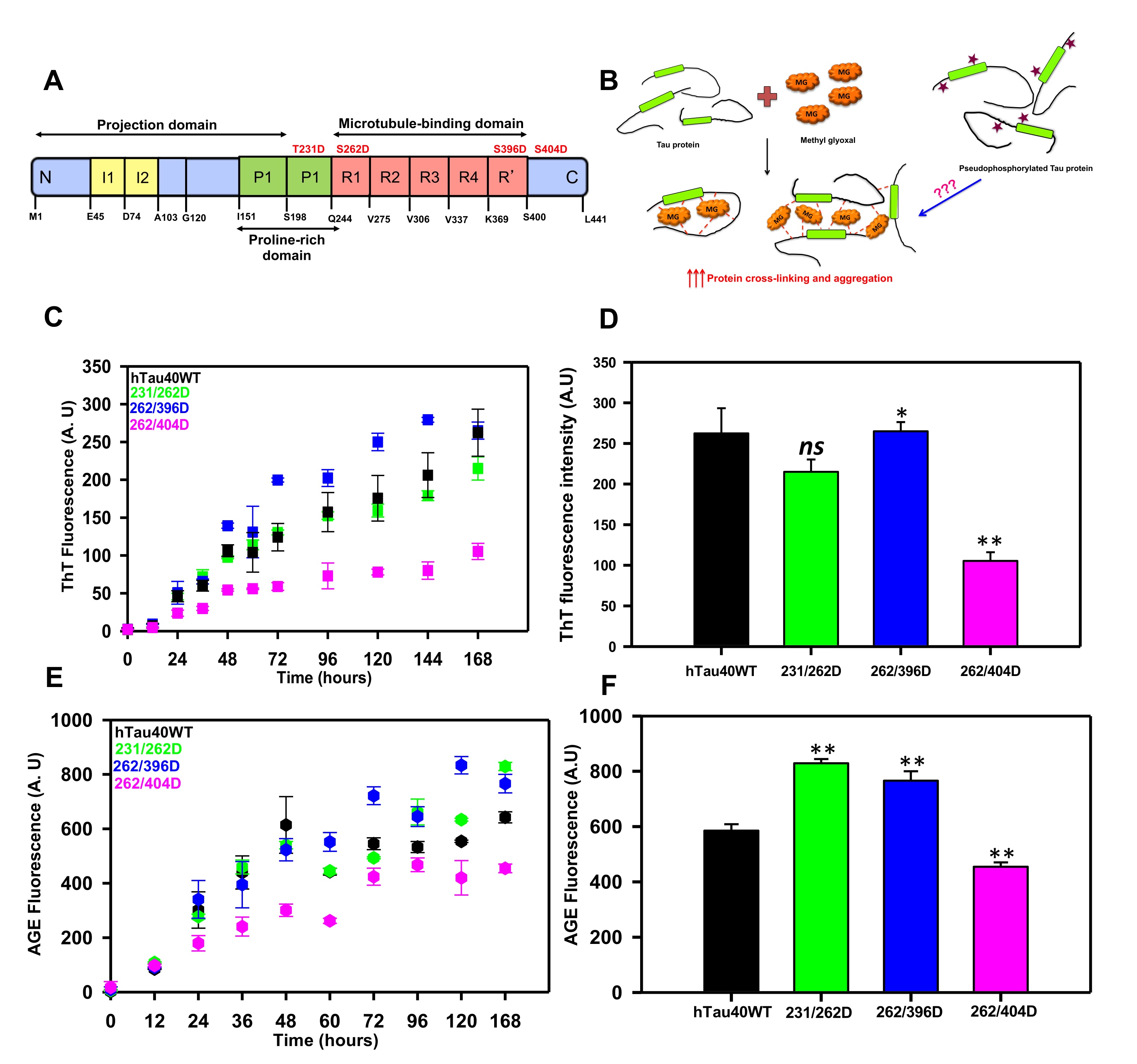
Fig. 1: Glycation of Pseudophosphorylated Tau. A) Tau protein is composed of two insert regions toward the N-terminal followed by a poly-proline stretch. The C-terminal regions harbor a repeat region consisting of 4 imperfect repeats (R1 to R4) and a truncated 4th repeat also known as the pseudorepeat (R’). These repeats are involved in microtubule-binding and hence this region is also known as the microtubule-binding region. The pseudophosphorylation sites studied are mentioned in red. B) The hypothesis model depicting the glycation of pseudophosphorylated mutants by methyl glyoxal. Methyl glyoxal leads to intra-molecular as well as intermolecular protein cross-linking by modifying the amine side chains. The phospho-mutants might interfere with this cross-linking to either enhance or reduce the MG-induced aggregation. C) The ThT fluorescence assay for MG-induced Tau aggregation showed enhanced aggregation of mutant 262/396D and decreased aggregation in 396/404D as compared to wild-type Tau. D) The end time point fluorescence intensity of MG-induced aggregation does show a lower aggregation propensity of the phospho-Tau mutant 262/396D. E) The AGEs fluorescence kinetics did not differ for the proteins until at later time points. F) The 168 hours analysis of AGEs fluorescence revealed increased AGEs fluorescence in 231/262D and 262/396D. The statistical analysis was carried out by Student's unpaired T- test concerning untreated control. *** p≤0.001, **p≤0.01, *p≤0.05. ns: non-significant p-value.
Glycation enhances AGEs formation in Tau phospho-mimicking mutants
The glycation of proteins comprises several steps of reversible and irreversible chemical reactions, which form the final product of advanced glycation end products. These AGEs tend to accumulate in the body due to their protease-resistant nature and hamper cellular functioning [38]. The AGEs formation can be determined by antibody-based methods [39] or by the AGEs autofluorescence [40, 41]. We employed the AGEs autofluorescence method to determine the Tau AGEs formed in presence of methyl glyoxal. The AGEs fluorescence did not differ among the proteins till 36 hours of incubation after which the fluorescence pattern varied for Tau and its phospho-mimicking mutants (Fig. 1E). The AGEs fluorescence reached saturation at 60 hours for wild-type Tau whereas it was found to stably increase for the phospho-Tau 231/262D and 262/396D. The AGEs formation did not increase considerably for 262/404D mutant Tau. The analysis for the end time-point suggested elevated AGEs formation in mutants 231/262D and 262/396D as compared to wild-type (Fig. 1F). Glycation is well known to induce protein cross-linking and form protein aggregates. The AGEs formation in the phospho-mimicking mutants was also analyzed by SDS-PAGE at various time intervals. It was observed that glycation had led to the formation of SDS-resistant Tau species for all the proteins. The mutant 262/404D had less intense AGEs formation as compared to the wild type and the other two phospho-Tau mutants (Fig. 2A, B). The study of glycation of pseudophosphorylated Tau by exposure to MG also reports the formation of higher-order aggregates on SDS-PAGE [30]. Thus, MG-induced glycation is more pronounced in the double mutants 231/262D and 262/396D as compared to the wild-type Tau. Since, these PTMs are postulated to play a role in Tau propagation [42] their study would aid in screening molecules effective in overcoming these PTMs and ameliorating Tau pathology in vitro and in vivo [43, 44]. Moreover, these PTMs are postulated to play a role in Tau propagation.
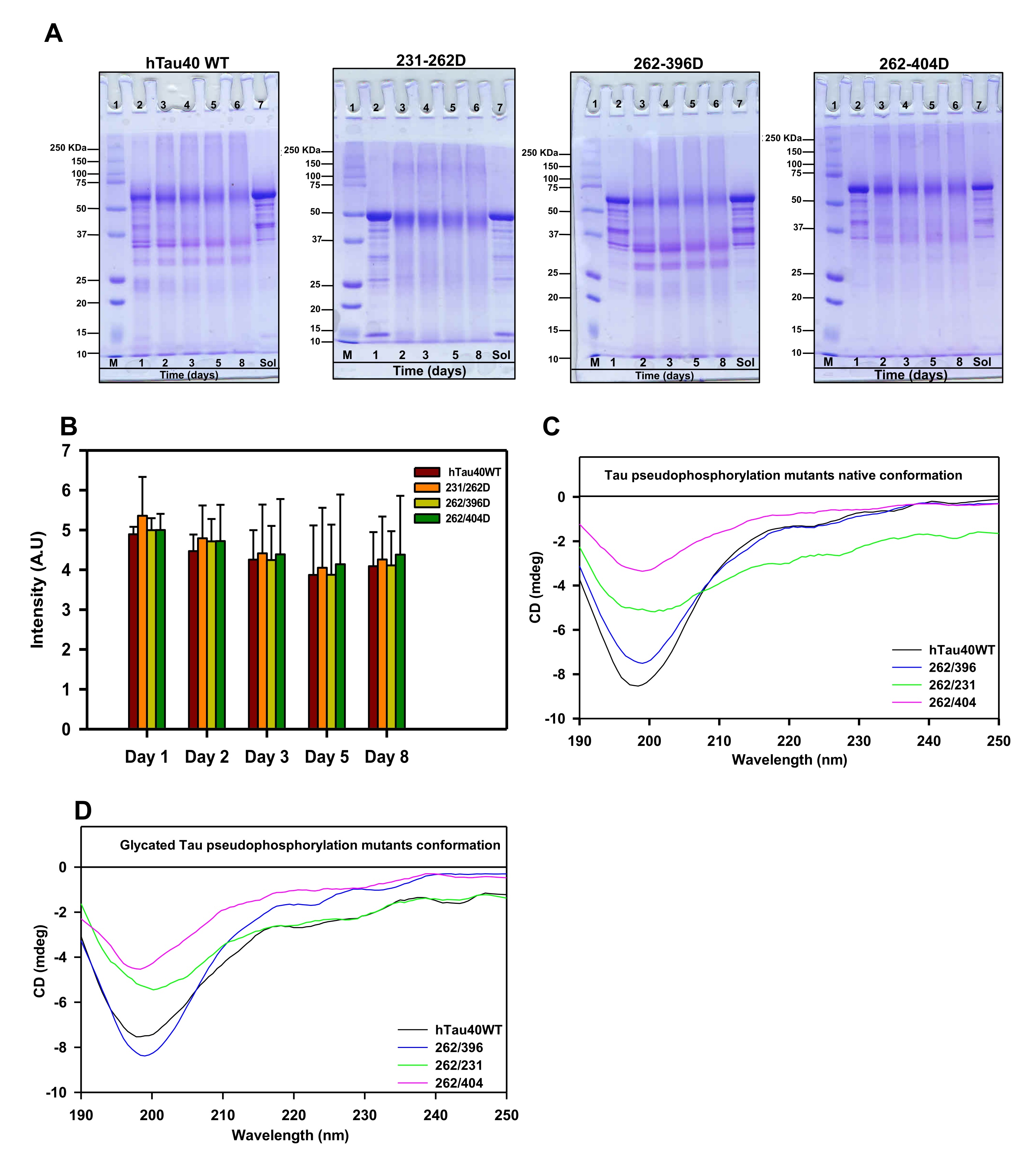
Fig. 2: Glycation does not alter the native conformation of Tau and its phospho-mutants. A) The SDS-PAGE analysis of MG-treated Tau showed the presence of higher-order cross-linked proteins at various time points. The mutant 262/396D and 231/396D show the presence of cross-linked products even at lower molecular weights. The lane Sol represents the soluble Tau protein in absence of inducer methyl glyoxal. B) The quantification of SDS-PAGE analysis for the dual modification. C) The CD spectra of Tau and its phospho-mutants showed a native conformation of a random coil. The mutations did not alter the global conformation of Tau. D) The conformation of Tau and the phospho-mutants was not altered by MG-induced glycation.
The dual modification of Tau does not alter its conformation
The AGE-modification of proteins leads to loss of structure due to excessive protein cross-linking and bond rearrangements resulting in the loss of function [48]. Tau is an intrinsically disordered protein with native random coil conformation [49]. Aggregation of Tau leads it to adopt partial secondary conformation. The full-length Tau adopts a partial β-sheet structure on aggregation. We studied whether glycation could induce any conformational change in Tau by CD spectroscopy. The CD analyses of unmodified Tau and its phospho-mimicking mutants demonstrated a complete random coil conformation (Fig. 2C). This has been previously reported for various sites of Tau pseudophosphorylation. Phosphorylation at T231 does not have any significant effect on overall Tau conformation. Phosphorylation at this residue leads to some local interactions of salt-bridge formation with surrounding residues but does not change the Tau conformation [50]. Similarly, phosphorylation at S262 induces turn formation and induces local rigidity in conformation but does not affect the global Tau conformation. Moreover, phosphorylation at the C-terminal has been reported to induce flexibility in Tau [25]. Thus, pseudophosphorylation did not alter the native conformation of Tau. The modification of Tau and its mutants by MG did not have any effect on the global conformation of Tau (Fig. 2D). Thus, even the dual modification of pseudophosphorylation and glycation did not induce conformational changes in Tau. This might be attributed to the stable random coil conformation of these pseudophosphorylated residues. Further, the MG-modified Tau was visualized by electron microscopy. It revealed the presence of amorphous aggregates in all the modified proteins (Fig. 3). Similar amorphous aggregates were also observed in Tau pseudophosphorylated mutants glycated by various reactive carbonyls including methyl glyoxal [30]. Glycation of Tau dementia mutants by methyl glyoxal also is being reported to form amorphous aggregates as opposed to heparin-induced fibrillar aggregates [35]. Tau aggregation landscape exhibits two channels of assemblies amorphous oligomers and prefibrillar oligomers. The amorphous Tau species in AD are more thermodynamically favored as compared to the prefibrillar oligomers [51]. This agrees with the fact that the early Tau deposits in AD are characterized by amorphous non-fibrillar structures [52] highlighting the role of amorphous aggregates as the initial Tau species formed in the Tau-mediated neurodegeneration (Fig. 4).
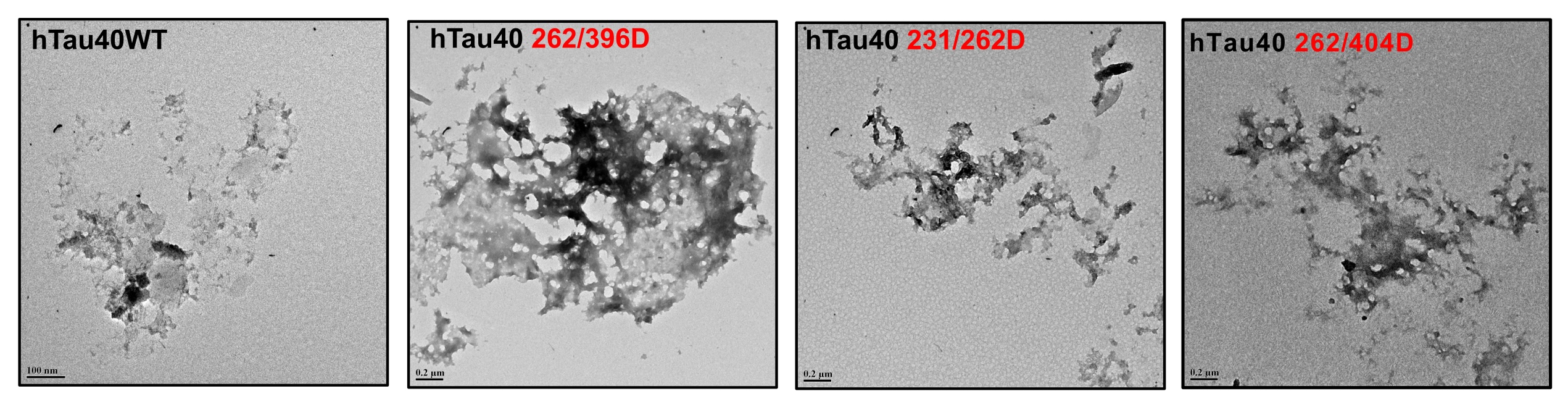
Fig. 3:Electron micrographs of modified Tau. The glycated Tau showed the presence of amorphous aggregates under a transmission electron microscope.
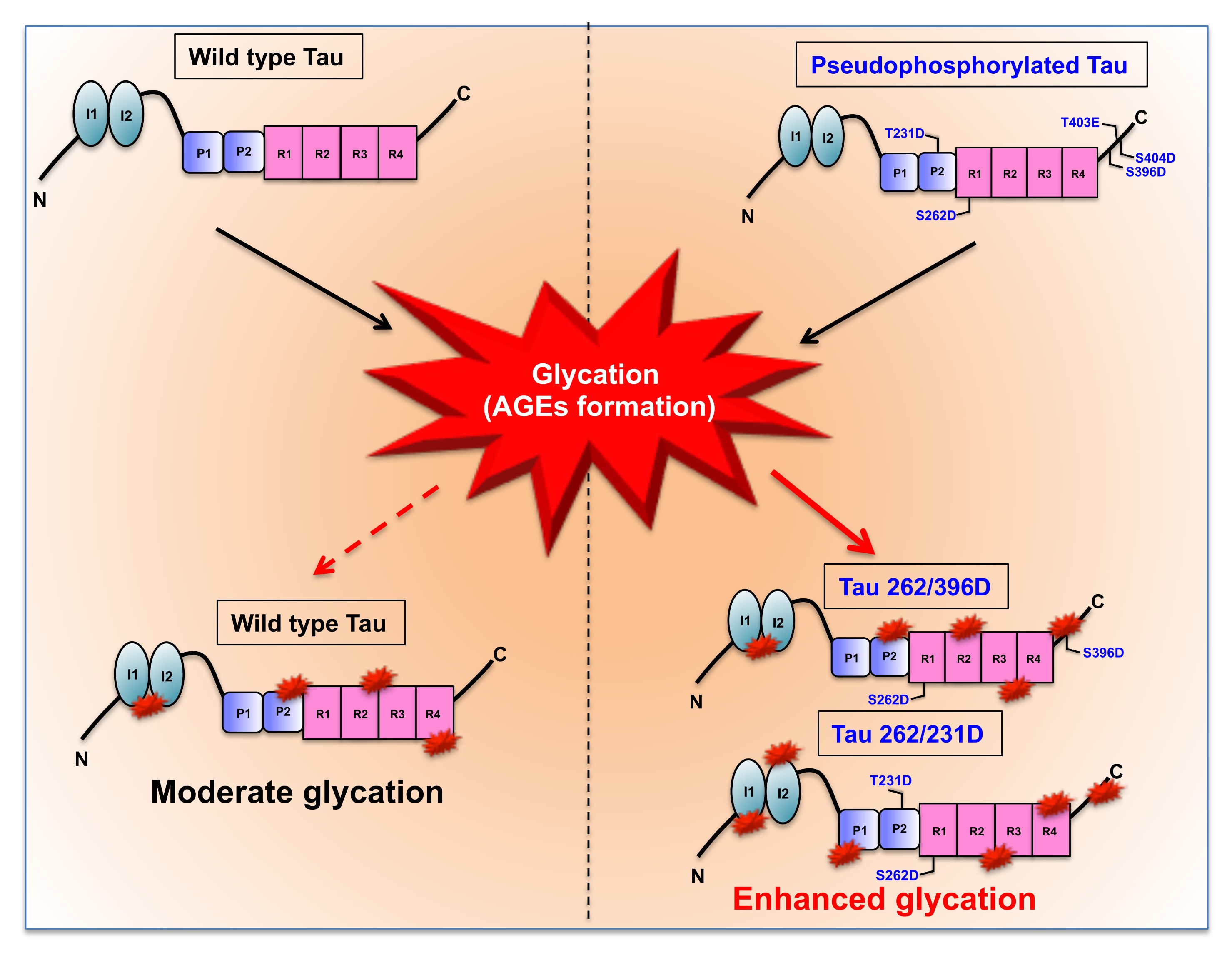
Fig. 4: MG-induced glycation of Pseudophosphorylated mutants. The wild-type Tau consisting of 441 amino acids is composed of two major functional domains namely the N-terminal projection domain and the C-terminal microtubule-binding domain. The projection domain consists of 2 inserts and the microtubule region comprises of the proline-rich region and 4 imperfect repeats. These repeats play a major role in microtubule binding and stabilization. The reaction of wild-type Tau with methylglyoxal leads to moderate glycation whereas the glycation of pseudophosphorylated double (262/396D, 262/231D) and triple mutants (S396D/T403E/S404D) leads to enhanced glycation as compared to wild type Tau.
Discussion
Tau is a phosphoprotein requiring a balance of phosphorylation and dephosphorylation for its physiological functioning. In AD, an imbalance of this process results in abnormal phosphorylation of Tau, which increases the overall negative charge of the protein. Low net charge of the protein is reported to be one of the important parameters for protein aggregation186. The effect of hyperphosphorylation on Tau function and self-assembly is studied by utilizing the charge mimicking pseudophosphorylation mutants. The negative charge of phosphate group is added to the protein by replacing the specific sites of phosphorylation, serine and threonine by negatively charged amino acids aspartic acid or glutamic acid. This approach has helped to study the effect of site-specific phosphorylation on Tau aggregation and microtubule assembly rather than in vitro Tau phosphorylation by kinases which uncontrollably phosphorylate several sites. We employed similar approach to study the effect of dual modification, glycation and phosphorylation on Tau. The phospho-mimicking Tau sites T231D, S262D, S396D and S404D were studied as double mutants. The combinations were, (262/404D, 262/396D and 231/262). Methyl glyoxal was used to glycate the phospho-mimicking mutants [51]. The glycation reaction causes intra-molecular and inter-molecular cross-linking which favors the self-assembly of protein forming aggregates [52]. Pseudophosphorylation is also found to enhance the Tau aggregation. The question we posed whether pseudophosphorylation can enhance MG-induced cross-linking of Tau.
Thus, the combinatorial effect of discreet PTMs on Tau function could pave a way for a better understanding of the pathology. Such approaches could help in designing effective and potent therapeutics to overcome AD manifestation [53].
Acknowledgements
Funding Sources
This project is supported in part by grants from the Department of Biotechnology from Neuroscience Task Force (Medical Biotechnology-Human Development & Disease Biology (DBT-HDDB))-BT/PR/19562/MED/122/13/2016 and in-house CSIR-National Chemical Laboratory grant MLP029526. SS acknowledges DBT for the fellowship.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Author contributions
SBC and SS conducted the experiments, analyzed the data and wrote the manuscript. MC contributed in the data analysis and revision of the work. SC conceived, designed, supervised, initial draft, review editing and wrote the paper. All authors read and approved the final paper.
Disclosure Statement
The authors declare no competing interests.
References
| 1 | Wood JG, Mirra SS, Pollock NJ, Binder LI: Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). PNAS 1986;83:4040-4043.
https://doi.org/10.1073/pnas.83.11.4040 |
| 2 | Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW: Tau, tangles, and Alzheimer's disease. Biochim Biophys Acta 2005;1739:216-223.
https://doi.org/10.1016/j.bbadis.2004.08.014 |
| 3 | Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski HM, Binder LI: Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. PNAS 1986;83:4913-4917.
https://doi.org/10.1073/pnas.83.13.4913 |
| 4 | Morishima M, Ihara Y: Posttranslational modifications of tau in paired helical filaments. Dement Geriatr Cogn Dis Extra 1994;5:282-288.
https://doi.org/10.1159/000106736 |
| 5 | Ercan-Herbst E, Ehrig J, Schöndorf DC, Behrendt A, Klaus B, Ramos BG, Oriol NP, Weber C, Ehrnhoefer DE: A post-translational modification signature defines changes in soluble tau correlating with oligomerization in early stage Alzheimer's disease brain. Acta Neuropathol Commun 2019;7:1-19.
https://doi.org/10.1186/s40478-019-0823-2 |
| 6 | Kontaxi C, Piccardo P, Gill AC: Lysine-directed post-translational modifications of tau protein in Alzheimer's disease and related Tauopathies. Front Mol Biosci 2017;4:56.
https://doi.org/10.3389/fmolb.2017.00056 |
| 7 | Ramesh M, Gopinath P, Govindaraju T: Role of Post‐translational Modifications in Alzheimer's Disease. ChemBioChem 2020;21:1052-1079.
https://doi.org/10.1002/cbic.201900573 |
| 8 | Barbier P, Zejneli O, Martinho M, Lasorsa A, Belle V, Smet-Nocca C, Tsvetkov PO, Devred F, Landrieu I: Role of Tau as a microtubule associated protein: structural and functional aspects. Front Aging Neurosci 2019;11:204.
https://doi.org/10.3389/fnagi.2019.00204 |
| 9 | Prezel E, Elie A, Delaroche J, Stoppin-Mellet V, Bosc C, Serre L, Fourest-Lieuvin A, Andrieux A, Vantard M, Arnal I: Tau can switch microtubule network organizations: from random networks to dynamic and stable bundles. Mol Biol Cell 2018;29:154-165.
https://doi.org/10.1091/mbc.E17-06-0429 |
| 10 | Bunker JM, Wilson L, Jordan MA, Feinstein SC: Modulation of microtubule dynamics by tau in living cells: implications for development and neurodegeneration. Mol Biol Cell 2004;15:2720-2728.
https://doi.org/10.1091/mbc.e04-01-0062 |
| 11 | Gong C-X, Liu F, Grundke-Iqbal I, Iqbal K: Post-translational modifications of tau protein in Alzheimer's disease. J Neural Transm 2005;112:813-838.
https://doi.org/10.1007/s00702-004-0221-0 |
| 12 | Wang J-Z, Xia Y-Y, Grundke-Iqbal I, Iqbal K: Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis 2013;33:123-139.
https://doi.org/10.3233/JAD-2012-129031 |
| 13 | Martin L, Latypova X, Terro F: Post-translational modifications of tau protein: implications for Alzheimer's disease. Neurochem Int 2011;58:458-471.
https://doi.org/10.1016/j.neuint.2010.12.023 |
| 14 | Balmik AA, Das R, Dangi A, Gorantla NV, Marelli UK, Chinnathambi S: Melatonin interacts with repeat domain of Tau to mediate disaggregation of paired helical filaments. Biochim Biophys Acta 2020;1864:129467.
https://doi.org/10.1016/j.bbagen.2019.129467 |
| 15 | Das R, Balmik AA, Chinnathambi S: Effect of Melatonin on Tau aggregation and Tau-mediated cell surface morphology. International J Biol Macromol 2020;152:30-39.
https://doi.org/10.1016/j.ijbiomac.2020.01.296 |
| 16 | Sonawane SK, Chidambaram H, Boral D, Gorantla NV, Balmik AA, Dangi A, Ramasamy S, Marelli UK, Chinnathambi S: EGCG impedes human Tau aggregation and interacts with Tau. Sci Rep 2020;10:1-17.
https://doi.org/10.1038/s41598-020-69429-6 |
| 17 | Sonawane SK, Balmik AA, Boral D, Ramasamy S, Chinnathambi S: Baicalein suppresses Repeat Tau fibrillization by sequestering oligomers. Arch Biochem Biophys 2019;675:108119.
https://doi.org/10.1016/j.abb.2019.108119 |
| 18 | Gorantla NV, Landge VG, Nagaraju PG, Priyadarshini CG P, Balaraman E, Chinnathambi S: Molecular cobalt (II) complexes for tau polymerization in Alzheimer's disease. ACS omega 2019;4:16702-16714.
https://doi.org/10.1021/acsomega.9b00692 |
| 19 | Sonawane SK, Ahmad A, Chinnathambi S: Protein-capped metal nanoparticles inhibit tau aggregation in Alzheimer's disease. ACS omega 2019;4:12833-12840.
https://doi.org/10.1021/acsomega.9b01411 |
| 20 | Liu F, Li B, Tung EJ, Grundke‐Iqbal I, Iqbal K, Gong CX: Site‐specific effects of tau phosphorylation on its microtubule assembly activity and self‐aggregation. Eur J Neurosci 2007;26:3429-3436.
https://doi.org/10.1111/j.1460-9568.2007.05955.x |
| 21 | Chang E, Kim S, Schafer KN, Kuret J: Pseudophosphorylation of tau protein directly modulates its aggregation kinetics. Biochim Biophys Acta 2011;1814:388-395.
https://doi.org/10.1016/j.bbapap.2010.10.005 |
| 22 | Haase C, Stieler J, Arendt T, Holzer M: Pseudophosphorylation of tau protein alters its ability for self‐aggregation. J Neurochem 2004;88:1509-1520.
https://doi.org/10.1046/j.1471-4159.2003.02287.x |
| 23 | Eidenmüller J, Fath T, Maas T, Pool M, Sontag E, Brandt R: Phosphorylation-mimicking glutamate clusters in the proline-rich region are sufficient to simulate the functional deficiencies of hyperphosphorylated tau protein. Biochem J 2001;357:759-767.
https://doi.org/10.1042/bj3570759 |
| 24 | Léger J, Kempf M, Lee G, Brandt R: Conversion of serine to aspartate imitates phosphorylation-induced changes in the structure and function of microtubule-associated protein tau. J Biol Chem 1997;272:8441-8446.
https://doi.org/10.1074/jbc.272.13.8441 |
| 25 | Fischer D, Mukrasch MD, Biernat J, Bibow S, Blackledge M, Griesinger C, Mandelkow E, Zweckstetter M: Conformational changes specific for pseudophosphorylation at serine 262 selectively impair binding of tau to microtubules. Biochem 2009;48:10047-10055.
https://doi.org/10.1021/bi901090m |
| 26 | Ledesma MD, Bonay P, Colaco C, Avila J: Analysis of microtubule-associated protein tau glycation in paired helical filaments. J Biol Chem 1994;269:21614-21619.
https://doi.org/10.1016/S0021-9258(17)31849-5 |
| 27 | Ledesma MD, Bonay P, Avila J: τ Protein from Alzheimer's disease patients is glycated at its tubulin‐binding domain. J Neurochem 1995;65:1658-1664.
https://doi.org/10.1046/j.1471-4159.1995.65041658.x |
| 28 | Nacharaju P, Ko Lw, Yen SHC: Characterization of in vitro glycation sites of tau. J Neurochem 1997;69:1709-1719.
https://doi.org/10.1046/j.1471-4159.1997.69041709.x |
| 29 | Necula M, Kuret J: Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. J Biol Chem 2004;279:49694-49703.
https://doi.org/10.1074/jbc.M405527200 |
| 30 | Kuhla B, Haase C, Flach K, Lüth H-J, Arendt T, Münch G: Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J Biol Chem 2007;282:6984-6991.
https://doi.org/10.1074/jbc.M609521200 |
| 31 | Kiris E, Ventimiglia D, Sargin ME, Gaylord MR, Altinok A, Rose K, Manjunath B, Jordan MA, Wilson L, Feinstein SC: Combinatorial Tau Pseudophosphorylation Markedly Different Regulatory Effects on Microtubule Assembly and Dynamic Instability Than The Sum Of The Individual Parts. J Biol Chem 2011;286:14257-14570.
https://doi.org/10.1074/jbc.M111.219311 |
| 32 | Liu K, Liu Y, Li L, Qin P, Iqbal J, Deng Y, Qing H: Glycation alter the process of Tau phosphorylation to change Tau isoforms aggregation property. Biochim Biophys Acta 2016;1862:192-201.
https://doi.org/10.1016/j.bbadis.2015.12.002 |
| 33 | Vander Jagt DL, Hunsaker LA: Methylglyoxal metabolism and diabetic complications: roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem Biol Interact 2003;143:341-351.
https://doi.org/10.1016/S0009-2797(02)00212-0 |
| 34 | Lo T, Westwood ME, McLellan AC, Selwood T, Thornalley PJ: Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem 1994;269:32299-32305.
https://doi.org/10.1016/S0021-9258(18)31635-1 |
| 35 | Oliveira LM, Gomes RA, Yang D, Dennison SR, Família C, Lages A, Coelho AV, Murphy RM, Phoenix DA, Quintas A: Insights into the molecular mechanism of protein native-like aggregation upon glycation. Biochim Biophys Acta 2013;1834:1010-1022.
https://doi.org/10.1016/j.bbapap.2012.12.001 |
| 36 | Angeloni C, Zambonin L, Hrelia S: Role of methylglyoxal in Alzheimer's disease. Biomed Res Int 2014:2014:238485
https://doi.org/10.1155/2014/238485 |
| 37 | Kumar S, Tepper K, Kaniyappan S, Biernat J, Wegmann S, Mandelkow E-M, Müller DJ, Mandelkow E: Stages and conformations of the Tau repeat domain during aggregation and its effect on neuronal toxicity. J Biol Chem 2014;289:20318-20332.
https://doi.org/10.1074/jbc.M114.554725 |
| 38 | Fournet M, Bonté F, Desmoulière A: Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis 2018;9:880.
https://doi.org/10.14336/AD.2017.1121 |
| 39 | Nagai R, Shirakawa J-i, Ohno R-i, Hatano K, Sugawa H, Arakawa S, Ichimaru K, Kinoshita S, Sakata N, Nagai M: Antibody-based detection of advanced glycation end-products: promises vs. limitations. Glycoconj J 2016;33:545-552.
https://doi.org/10.1007/s10719-016-9708-9 |
| 40 | Yanagisawa K, Makita Z, Shiroshita K, Ueda T, Fusegawa T, Kuwajima S, Takeuchi M, Koike T: Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabol 1998;47:1348-1353.
https://doi.org/10.1016/S0026-0495(98)90303-1 |
| 41 | Fokkens BT, Smit AJ: Skin fluorescence as a clinical tool for non-invasive assessment of advanced glycation and long-term complications of diabetes. Glycoconj J 2016;33:527-535.
https://doi.org/10.1007/s10719-016-9683-1 |
| 42 | Sonawane SK, Chinnathambi S: Prion-like propagation of post-translationally modified tau in Alzheimer's disease: a hypothesis. Journal of Molecular Neurosci 2018;65:480-490.
https://doi.org/10.1007/s12031-018-1111-5 |
| 43 | Sonawane SK, Chinnathambi S: A green tea polyphenol Epigallocatechin-3-gallate modulates Tau Post-translational modifications and cytoskeletal network. Oncotarget 2021:1083-1099.
https://doi.org/10.18632/oncotarget.27963 |
| 44 | Sonawane SK, Raina A, Majumdar A, Chinnathambi S: EGCG modulates nuclear formaldehyde-induced Tau phosphorylation in Neuronal cells. bioRxiv 2020:2020.10.04.325134. DOI: 10.1101/2020.10.04.325134.
https://doi.org/10.1101/2020.10.04.325134 |
| 45 | Awasthi S, Saraswathi N: Non-enzymatic glycation mediated structure-function changes in proteins: case of serum albumin. Rsc Adv 2016;6:90739-90753.
https://doi.org/10.1039/C6RA08283A |
| 46 | Gorantla NV, Shkumatov AV, Chinnathambi S. Conformational dynamics of intracellular tau protein revealed by CD and SAXS. Methods Mol Biol 2017;1523:3-20.
https://doi.org/10.1007/978-1-4939-6598-4_1 |
| 47 | Schwalbe M, Kadavath H, Biernat J, Ozenne V, Blackledge M, Mandelkow E, Zweckstetter M: Structural impact of tau phosphorylation at threonine 231. Structure 2015;23:1448-1458.
https://doi.org/10.1016/j.str.2015.06.002 |
| 48 | Sonawane SK, Chinnathambi S: P301 L, an FTDP-17 Mutant, Exhibits Enhanced Glycation in vitro. J Alzheimers Dis 2020;75:61-71.
https://doi.org/10.3233/JAD-191348 |
| 49 | Chen X, Chen M, Schafer NP, Wolynes PG: Exploring the interplay between fibrillization and amorphous aggregation channels on the energy landscapes of tau repeat isoforms. PNAS 2020;117:4125-4130.
https://doi.org/10.1073/pnas.1921702117 |
| 50 | Mena R, Edwards PC, Harrington CR, Mukaetova-Ladinska EB, Wischik CM: Staging the pathological assembly of truncated tau protein into paired helical filaments in Alzheimer's disease. Acta Neuropathol 1996;91:633-641.
https://doi.org/10.1007/s004010050477 |
| 51 | Rajasekhar K, Govindaraju T: Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer's disease. RSC Adv 2018;8:23780-23804.
https://doi.org/10.1039/C8RA03620A |
| 52 | Gorantla NV, Das R, Chidambaram H, Dubey T, Mulani FA, Thulasiram HV, Chinnathambi S: Basic Limonoid modulates Chaperone-mediated Proteostasis and dissolve Tau fibrils. Sci Rep 2020;10:1-19.
https://doi.org/10.1038/s41598-020-60773-1 |
| 53 | Sabate R, Rodriguez-Santiago L, Sodupe M, Saupe SJ, Ventura S: Thioflavin-T excimer formation upon interaction with amyloid fibers. Chem Commun 2013;49:5745-5747.
https://doi.org/10.1039/c3cc42040j |