Inactivation of the Reactive Oxygen Species-Dependent PI3K/Akt/Mtor Signaling Pathway by Phloroglucinol Contributes to Cytotoxicity in Hep3B Human Hepatocellular Carcinoma Cells
bBasic Research Laboratory for the Regulation of Microplastic-Mediated Diseases and Anti‑Aging Research Center, Dong-eui University, Busan 47340, Republic of Korea,
cDepartment of Biochemistry, Dong-eui University College of Korean Medicine, Busan 47227, Republic of Korea,
dDepartment of Marine Life Sciences, School of Marine Biomedical Sciences, Jeju National University, Jeju 63243, Republic of Korea
Keywords
Abstract
Background/Aims:
Phloroglucinol is a phenolic derivative isolated from brown algae and reportedly has the potential to induce apoptosis in cancer cells, but its mechanism is unclear. This study aimed to elucidate the complete anticancer mechanism of phloroglucinol in Hep3B human hepatocellular carcinoma (HCC) cells.Methods:
We investigated whether phloroglucinol inhibits the proliferation of Hep3B cells by inducing DNA damage and apoptosis, and conducted a study on the mechanism involved. We also explored whether phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway is involved in phloroglucinol-induced apoptosis. In addition, we evaluated whether reactive oxygen species (ROS) was involved in the anticancer activity of phloroglucinol.Results:
Our results revealed that phloroglucinol disrupted mitochondrial integrity and induced caspase-dependent apoptosis by altering the expression of Bcl-2 family proteins and increasing the cytosolic release of cytochrome c . Phloroglucinol also inactivated the PI3K/Akt/mTOR signaling pathway, and pretreatment with a PI3K inhibitor remarkably augmented the phloroglucinol-induced cytotoxic effect in Hep3B cells. In addition, phloroglucinol significantly stimulated generation of ROS and reduced glutathione ratios. However, a ROS scavenger attenuated phloroglucinol-induced oxidative stress, DNA damage, and apoptosis, thus restoring the reduced cellular viability by blockading phloroglucinol-mediated inactivation of PI3K/Akt/mTOR signaling.Conclusion:
Our findings support a mechanism in which phloroglucinol enhances Hep3B cell apoptosis by inactivating the ROS-dependent PI3K/Akt/mTOR pathway, which implies that ROS generation acts as an inducer of phloroglucinol-mediated anticancer activity. Taken together, our findings support further research on the potential of phloroglucinol as a candidate for treating HCC.Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer worldwide, including in Korea, and is often fatal [1, 2]. In addition to genetic factors that increase the risk of developing HCC, environmental factors, such as excessive drinking, hepatitis virus infection, and aflatoxin intake, also are frequently observed influences in patients with non-alcoholic fatty liver disease. Notably, HCC is a complex, multifactorial carcinoma with a heterogeneous prognosis, and although treatment options for HCC include liver transplantation, radiotherapy, surgical resection, chemotherapy, and immunotherapy, there are very few treatments for patients with advanced HCC [3, 4]. Recently, standard treatments have used drugs, such as multi-kinase inhibitors, but serious side effects and drug resistance issues have been raised [5, 6]. Consequently, there is a need to develop effective alternative or adjuvant therapies with low-toxicity that offer improvements over the currently available treatments.
The recent discovery of seaweed-based natural bioactive substances has contributed to the control of many diseases, including cancer [7, 8]. This is because they contain various bioactive substances, including antiviral, antioxidant, anti-inflammatory, hepatoprotective, immunostimulatory, and anticancer activities [9, 10]. Among them, brown algae are rich in active substances, such as polyphenols, fucoxanthin, terpenes, proteins, and polysaccharides, and have been widely used as pharmaceutical and food materials in East Asia for a long time due to their health benefits [11, 12]. In particular, polyphenolic compounds, which are important components of brown algae cell walls, comprise phlorotannins and polymers whose basic unit is phloroglucinol, all of which have high medicinal potential due to their rich biological activities [13-15]. As demonstrated by numerous studies, extensive evidence has shown that these phenolic compounds have potential in cancer prevention and treatment. These beneficial effects of phlorotannins have been found to be closely related to reduced inflammatory responses caused by their antioxidant activity, which inhibits development of cancer to some degree [16, 17]. The mechanism involves generation of free radicals that can help stimulate cancer cell development and increase inflammatory responses [18, 19]. However, the anticancer activity of phlorotannins may be caused by oxidative stress, which is characterized by the accumulation of reactive oxygen species (ROS) mediated by mitochondrial damage and involving various cellular signaling mechanisms [14, 20, 21]. Despite these facts, there has been insufficient investigation of the underlying mechanism, so there is very little data on the anticancer activity of phloroglucinol, a monomer of phlorotannins. However, there is increasing evidence that the potent anti-inflammatory action of phloroglucinol is linked to its antioxidant properties [22-25]. In particular, the inhibition of cancer cell growth by some phloroglucinol derivatives has been suggested to depend on ROS, which are associated with changes in the activity of specific cellular signaling pathways [14; 26-29].
Therefore, this study aimed to explore the cytotoxic-inducing mechanism of phloroglucinol in Hep3B HCC cells and the role of ROS as an upstream regulator of the anticancer activity of this phenolic compound. Additionally, among the cellular signaling pathways, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling, which is hyperactivated in HCC as in other tumors and contributes to tumor proliferation, has been previously investigated for its possible mechanistic involvement in HCC [14, 28, 29]. Given that ROS are widely implicated in the activation of this pathway and are a known contributor to chemotherapeutic resistance in HCC [30, 31], the role of ROS was further examined in this study.
Materials and Methods
Cell culture and phloroglucinol treatment
Hep3B cells (American Type Culture Collection (Manassas, VA, USA) were maintained in RPMI 1640 medium containing fetal bovine serum and antibiotic mixtures (WelGENE Inc., Gyeongsan, Republic of Korea) at 37°C in an atmosphere of 5% CO2 in air. Phloroglucinol (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (Sigma-Aldrich) to prepare a 100 mg/ml stock solution, which was then diluted to various concentrations in the medium before treating the cells.
Cell viability assay
Hep3B cells were treated with different concentrations of phloroglucinol for 48 h or pretreated with or without carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (z-VAD-fmk) (50 µM, Sigma-Aldrich), necrostatin-1 (30 µM, Thermo Fisher Scientific, Waltham, MA, USA), N-acetyl-L-cysteine (NAC) (10 mM, Sigma-Aldrich) or LY294002 (10 µM, Cell Signaling Technology, Inc., Danvers, MA, USA) for 1 h, and then exposed to phloroglucinol (30 µg/ml). After treatment, the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was performed [32]. Inverse phase-contrast microscopy (Carl Zeiss, Oberkochen, Germany) was performed to obtain images of cell morphological changes.
Quantitative assessment of apoptosis
To quantitatively investigate the degree of apoptosis induction, an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD Bioscience, Franklin Lakes, NJ, USA) was used. Following the procedure suggested by the manufacturer, the harvested cells were suspended in a buffer, and Annexin V-FITC and propidium iodide (PI) buffer were added and reacted for 20 min. A Muse™ Cell Analyzer (Millipore Corporation, Hayward, CA, USA) was used to analyze the cell suspension to determine the frequency of apoptosis induction.
Observation of nuclear morphological changes
To examine the effects of phloroglucinol on the nuclear morphology of Hep3B cells, 4′,6′-diamidino-2-phenylindole (DAPI) staining was performed. Briefly, the cells were collected, fixed with 4% paraformaldehyde (Sigma-Aldrich) solution, and then stained with 1 μg/ml DAPI (Thermo Fisher Scientific) solution [33]. The morphology of the DAPI-stained nuclei was observed by fluorescence microscopy (Carl Zeiss).
Protein extraction and immunoblotting
Whole cell lysates were isolated for immunoblotting as previously described [34], and mitochondrial and cytosolic fractions were separated by use of a Mitochondrial Fractionation Kit (Active Motif, Inc., Carlsbad, CA, USA). According to a previous method [34], the separated proteins were fractionated by electrophoresis, transferred to PVDF membranes (Thermo Fisher Scientific), and then reacted with primary and secondary antibodies against the target proteins. An enhanced chemiluminescence kit (Sigma-Aldrich) was used to detect immunoreactive proteins. Antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), Cell Signaling Technology, Inc., and Abcam, Inc. (Cambridge, MA, UK). -actin and cytochrome c oxidase subunit IV (COX IV) were probed as loading controls for cytosolic and mitochondrial proteins.
Caspase activity assay
A Colorimetric Caspase Activity Assay Kit (Abcam, Inc.) was used to measure the changes in caspase activity after phloroglucinol treatment versus the control on the basis of the hydrolysis of fluorescent substrate peptides by activated caspases. Briefly, after suspending the cells in cell lysis buffer, the supernatants containing an equal amount of protein were reacted with each caspase substrate according to the kit instructions. A microplate reader was used to detect the level of cleaved p -nitroaniline from the substrates representing each caspase activity [35].
Mitochondrial membrane potential (MMP) assay
To investigate the changes in mitochondrial activity following phloroglucinol treatment in Hep3B cells, 5, 5,6, 6’-tetrachloro-1, 1’,3, 3’-tetraethylbenzimidazoylcarbocyanine iodine (JC-1) fluorescent dye (Abcam, Inc.) was used to analyze MMP. According to the protocol, the cells were stained with JC-1 solution (10 μM) for 30 min. The MMP values were then calculated from flow cytometric results. As previously described [36], images of JC-1-stained cells were also monitored by fluorescence microscopy.
Measurement of the ROS production
To measure ROS levels generated in cells cultured under various conditions, we used 2›,7›-dichlorofluorescein diacetate (DCF-DA), which can be oxidized by ROS to the fluorescent DCF. Following the manufacturer’s suggested procedure, the cells were reacted with 10 μM DCF-DA (Cayman Chemical Co., Ann Arbor, MI, USA) solution, and we used a flow cytometer to measure the ROS levels. In addition, the nuclei of cells stained with DCF-DA were counterstained with DAPI, and the DCF fluorescence intensity was observed under a fluorescence microscope following the same procedure as in the previous method [37].
Assessment of the glutathione (GSH)/oxidized glutathione (GSSH) ratio
A commercially available assay kit (Glutathione Fluorescence Detection Kit, Thermo Fisher Scientific) was used to assay the antioxidant capacity of phloroglucinol. In brief, after reacting the cells under the conditions suggested by the manufacturer, the standard curve of the GSH and GSSG amounts was used to calculate the GSH/GSSG ratio.
Comet assay
To assess DNA damage, the Comet assay, also called the single-gel electrophoresis assay, was performed. Briefly, phloroglucinol-exposed cells in the presence or absence of NAC were collected, and a Comet Assay Kit (Trevigen, Inc., Gaithersburg, MD, USA) was used to perform the Comet assay according to the manufacturer’s instructions.
Statistical analysis
GraphPad Prism 5.03 software (GraphPad Software Inc., La Jolla, CA, USA) and the unpaired two-tailed Student’s t-test and one-way ANOVA were used to statistically analyze all results. All results are presented as the mean ± standard deviation (SD) of at least three independent experiments. Values of p ≤ 0.05 were accepted as indicating statistical significance.
Results
Phloroglucinol decreased cell survival and induced apoptosis in Hep3B cells
The MTT analysis to determine if phloroglucinol would inhibit proliferation of Hep3B cells showed that cell viability was gradually inhibited as the treatment concentration of phloroglucinol increased (Fig. 1A). In addition, we found that the percentage of annexin-positive cells, i.e., the percentage indicating that apoptosis was induced, significantly increased as the treatment concentration of phloroglucinol increased compared with the control cells (Fig. 1B). In addition, compared with the control cells, the phloroglucinol-treated cells showed severe cell morphology distortion, branching, and loss of contact with adjacent cells (Fig. 1C). Moreover, the results of DAPI staining indicated that the phloroglucinol-treated cells exhibited chromosomal fragmentation and condensation nuclear blebs, characteristic of apoptosis-induced cells in the nucleus (Fig. 1D). These results indicate that the decrease in Hep3B cell viability induced by phloroglucinol was linked to the induction of apoptosis.
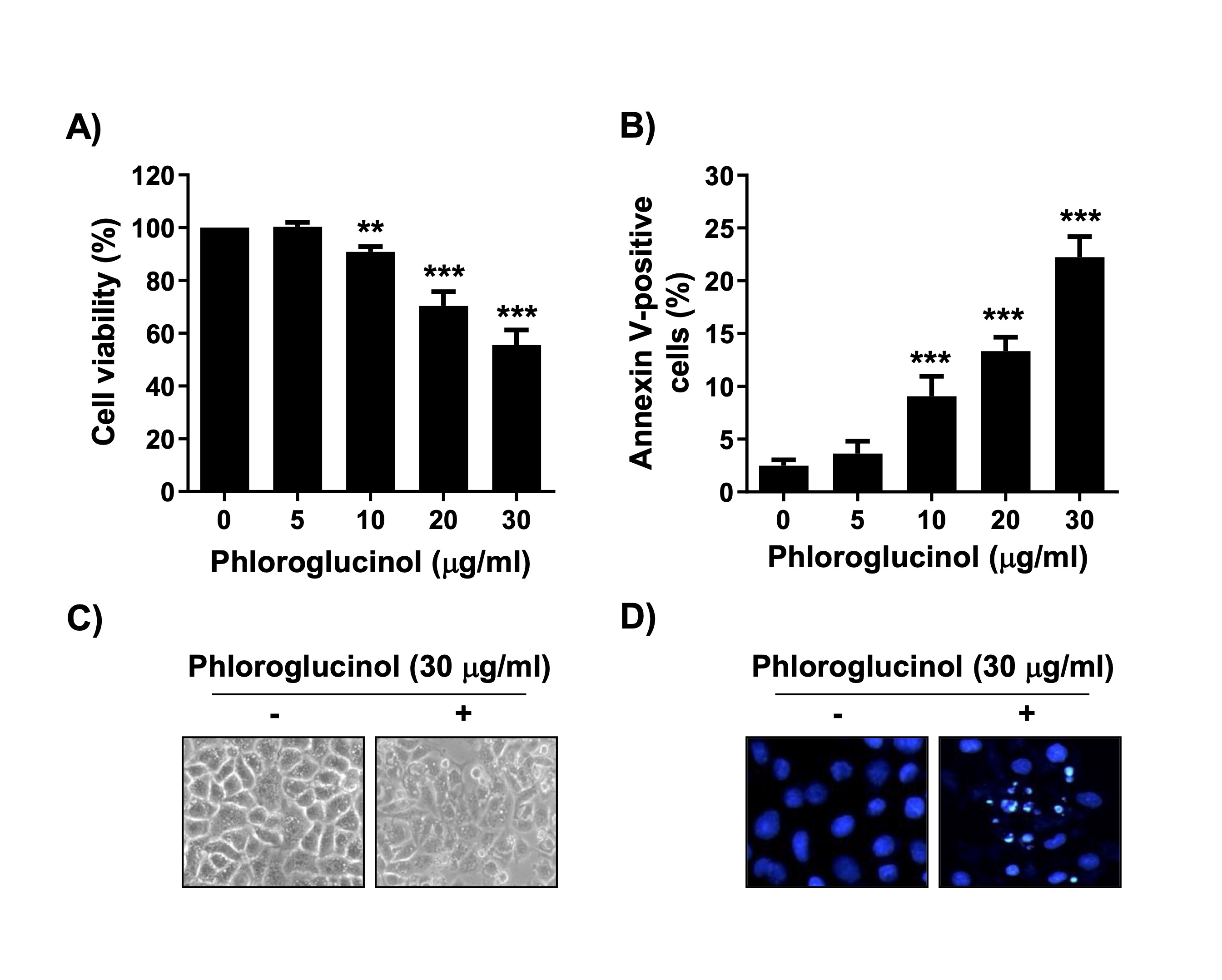
Fig. 1: Suppression of cell viability and induction of apoptosis by phloroglucinol in Hep3B cells. Cells were cultured in medium containing phloroglucinol at the indicated concentrations for 48 h. (A) Cell viability was analyzed by the MTT assay (**p < 0.01 and ***p < 0.001 vs. control cells). (B) After staining with annexin V-FITC/PI, the frequencies of annexin-positive cells are presented as the ratio of annexin V-positive cells to the total number of cells expressed as a percentage (***p < 0.001 vs. control cells). (C) Changes in cell morphology after phloroglucinol treatment were observed under an inverted phase-contrast microscope. (D) Morphological changes in the nuclei after DAPI staining were imaged by performing fluorescence microscopy.
Caspase activity was increased in Hep3B cells treated with phloroglucinol
Next, we determined if increased caspase activity was involved in phloroglucinol-induced apoptosis in Hep3B cells. Immunoblotting results showed that the levels of the inactive forms of caspase-8, -9, and -3 were markedly suppressed in phloroglucinol-treated cells, although their active forms were not detected (Fig. 2A). In addition, the cleavage of poly(ADP-ribose) polymerase (PARP) and the activity of caspases were significantly increased after phloroglucinol treatment (Fig. 2A and B). Therefore, we used the pan-caspase inhibitor z-VAD-fmk to determine if the cell death induced by phloroglucinol treatment was caspase dependent. As shown in the MTT assay, z-VAD-fmk potently rescued cells from phloroglucinol-induced cytotoxicity (Fig. 2C). However, the necrosis inhibitor necrostatin-1 did not prevent the phloroglucinol-induced inhibition of cell viability (Fig. 2D), demonstrating that phloroglucinol-induced caspase-dependent apoptosis but not necrotic cell death in Hep3B cells.
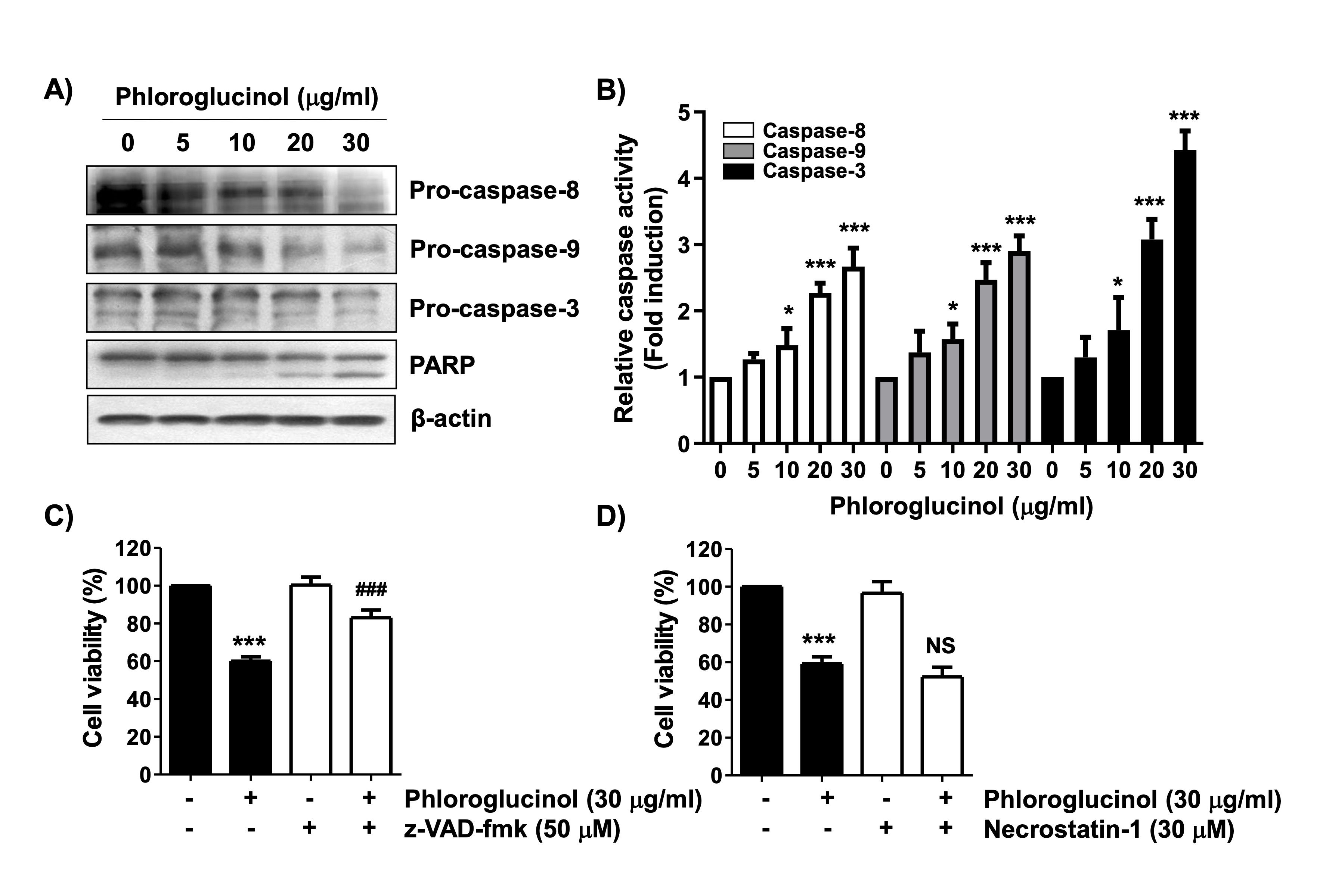
Fig. 2: Activation of caspase and induction of caspase-dependent apoptosis in phloroglucinol-treated Hep3B cells. (A and B) After 48 h of treatment with phloroglucinol at the indicated concentration, the change in expression of the indicated proteins was measured according to the total protein and corresponding antibody levels. Equal loading was confirmed with -actin. (B) The activity of each caspase was presented as a value relative to that of the untreated control group (*p < 0.05, **p < 0.01 and ***p < 0.001 vs. control cells). (C and D) Cells were pretreated with 50 M z-VAD-fmk or 30 M necrostatin-1 for 1 h and subsequently treated with 30 μg/ml phloroglucinol for 48 h. Cell viability was analyzed by performing the MTT assay (*p < 0.05, **p < 0.01 and ***p < 0.001 vs. control cells; NS, not significant).
Mitochondrial impairment was induced in Hep3B cells treated with phloroglucinol
We investigated whether phloroglucinol-induced apoptosis in Hep3B cells was accompanied by mitochondrial dysfunction. Our flow cytometry results showed that MMP, an indicator of mitochondrial stability, was significantly disrupted by phloroglucinol treatment (Figs. 3A and B). We also observed the fluorescence intensity of JC-1 under a fluorescence microscope and found that J-aggregates (red) shifted to monomers (green) in the phloroglucinol-treated cells, indicating MMP loss (Fig. 3C). In addition, among the Bcl-2 family proteins, the level of the Bax protein, a representative proapoptotic protein, increased dose-dependently with phloroglucinol treatment, whereas the expression of the Bcl-2 protein, an anti-apoptotic protein, was suppressed (Fig. 3D). Moreover, phloroglucinol-induced MMP loss was associated with upregulation of cytochrome c expression in the cytosolic fraction and concomitant downregulation at the mitochondrial level (Fig. 3E), indicating that phloroglucinol can destroy mitochondrial integrity by modulating Bcl-2 family proteins in Hep3B cells.
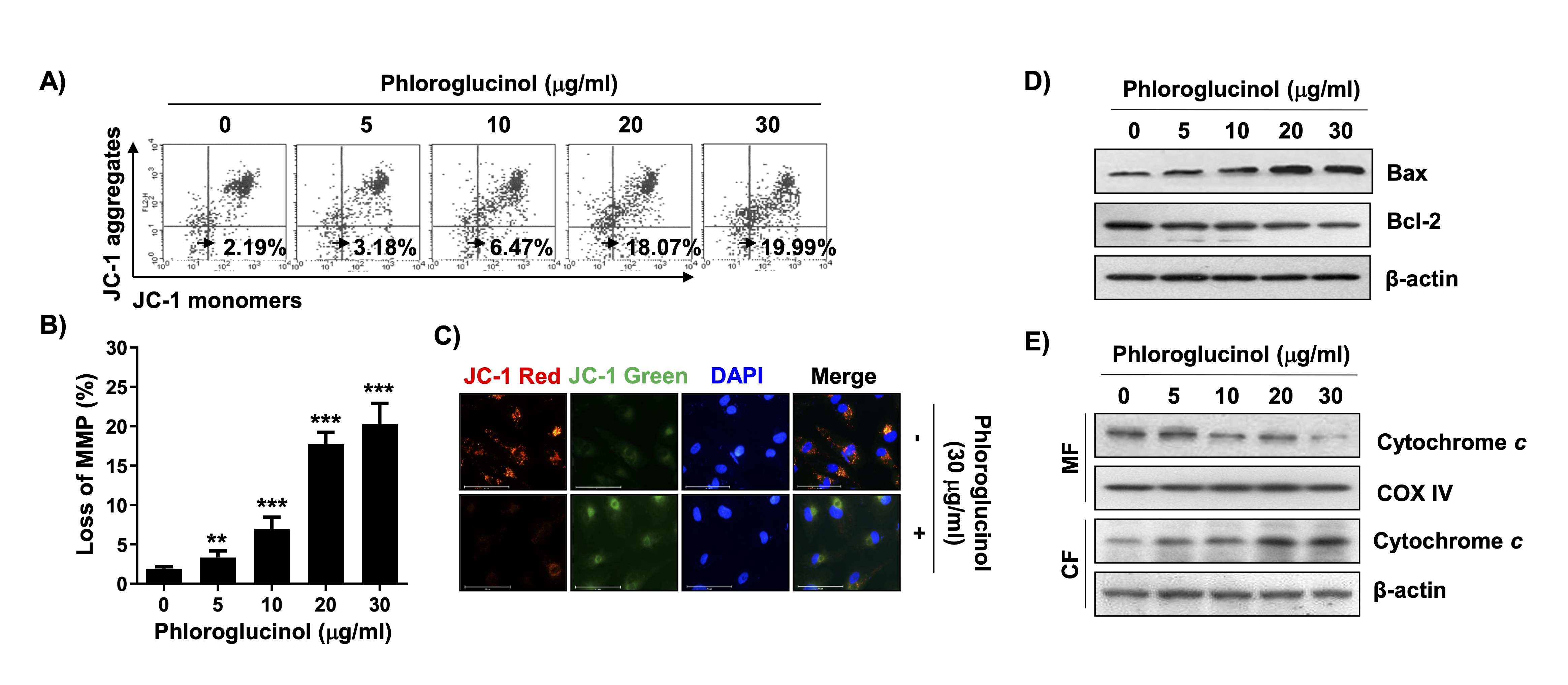
Fig. 3: Induction of mitochondrial dysfunction by phloroglucinol in Hep3B cells. Cells were cultured in medium containing phloroglucinol at the indicated concentrations for 48 h. (A and B) To measure MMP changes, cells stained with JC-1 were subjected to flow cytometry. (A) Representative histogram profiles are presented (values at the bottom of the box indicate the percentage of cells with depolarized mitochondrial membranes). (B) Statistical analysis results of cells with loss of MMP after treatment with phloroglucinol are presented (**p < 0.01 and ***p < 0.001 vs. control cells). (C) JC-1-stained cells as observed under a fluorescence microscope (cells with high MMP show red fluorescence, and cells with low MMP show green fluorescence). The nuclei were counterstained with DAPI (blue). (D) Changes in the expression levels of the indicated proteins as measured according to the total protein and corresponding antibody levels. (E) After separation of the mitochondrial (MF) and cytoplasmic fractions (CF), the expression of cytochrome c was detected by immunoblotting. COX IV and -actin were probed as loading controls for each fraction.
Phloroglucinol increased ROS accumulation in Hep3B cells
We then determined if ROS generation was involved in the phloroglucinol-induced apoptosis of Hep3B cells. Flow cytometric analysis showed that ROS production began to increase within 30 min after phloroglucinol treatment, reached a peak after 1 h, and then gradually decreased (data not shown). However, no significant ROS production was observed when phloroglucinol alone was used, and under conditions pretreated with NAC, a ROS scavenger, ROS production induced by phloroglucinol was significantly suppressed to the control level (Figs. 4A and B). Additionally, after 1 h of phloroglucinol treatment, the green fluorescence intensity indicating ROS accumulation was stronger than that in the untreated control cells, whereas the phloroglucinol-induced green fluorescence intensity was very low in the cells pretreated with NAC (Fig. 4C). Furthermore, the GSH/GSSG ratio, an indicator of oxidative stress, was reduced in the cells treated with phloroglucinol, whereas the decrease in this ratio was significantly attenuated in the NAC pretreatment group (Fig. 4D), suggesting that phloroglucinol-induced apoptosis may be associated with increased oxidative stress.
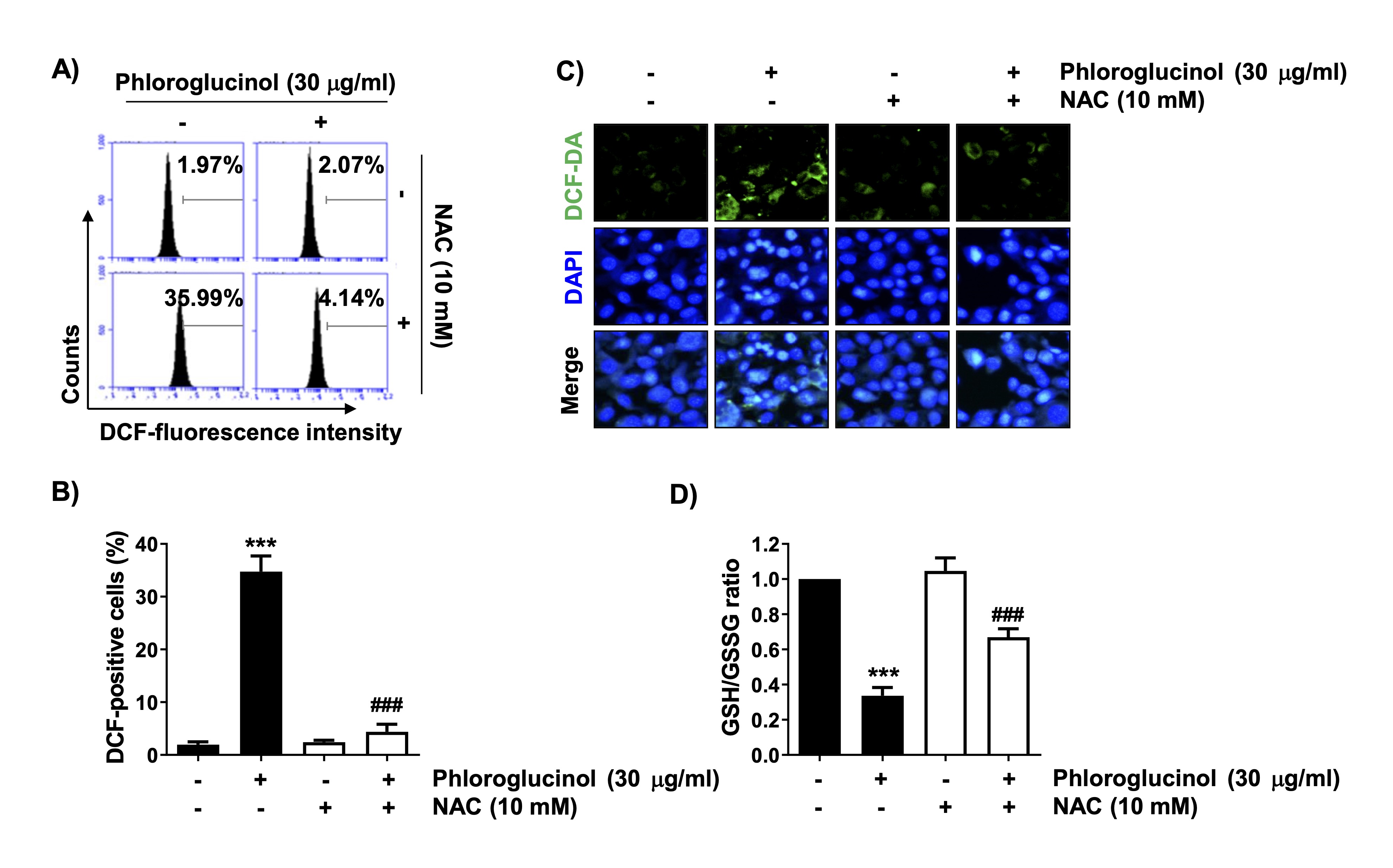
Fig. 4: Increased ROS production and decreased GSH content by phloroglucinol in Hep3B cells. The cells were pretreated with 10 mM NAC for 1 h and then stimulated with 30 μg/ml phloroglucinol for 1 h (A–C) or 48 h (D). (A and B) After treatment, the extent of ROS production as detected by flow cytometry. (A) Representative DNA histograms are shown. (B) The frequencies of DCF-positive cells in each experimental group are presented (***p<0.001 vs. control cells, ###p<0.001 vs. phloroglucinol-treated cells). (C) The ROS generation level (green) as confirmed by fluorescence microscopy. Nuclei are identified by DAPI staining (blue). (D) The GSH/GSSG ratio was calculated by use of a commercially available kit (***p<0.001 vs. control cells, ###p<0.001 vs. phloroglucinol-treated cells)
Phloroglucinol-induced ROS-dependent DNA damage in Hep3B cells
We further determined if the induction of cytotoxicity by phloroglucinol in Hep3B cells was associated with DNA damage. Results from immunoblotting and fluorescence microscopy showed that the expression of phosphor-H2AX (γH2AX), which indicates DNA damage, was significantly increased by phloroglucinol treatment (Figs. 5A and B). This evidence showing that phloroglucinol-induced DNA damage was consistent with the results of the Comet assay, which detects DNA strand breaks that form tail-like structures (Fig. 5C). However, these changes induced by phloroglucinol were clearly abolished in the presence of NAC. Therefore, these results suggest that ROS generation by phloroglucinol is essential for inducing DNA damage in Hep3B cells.
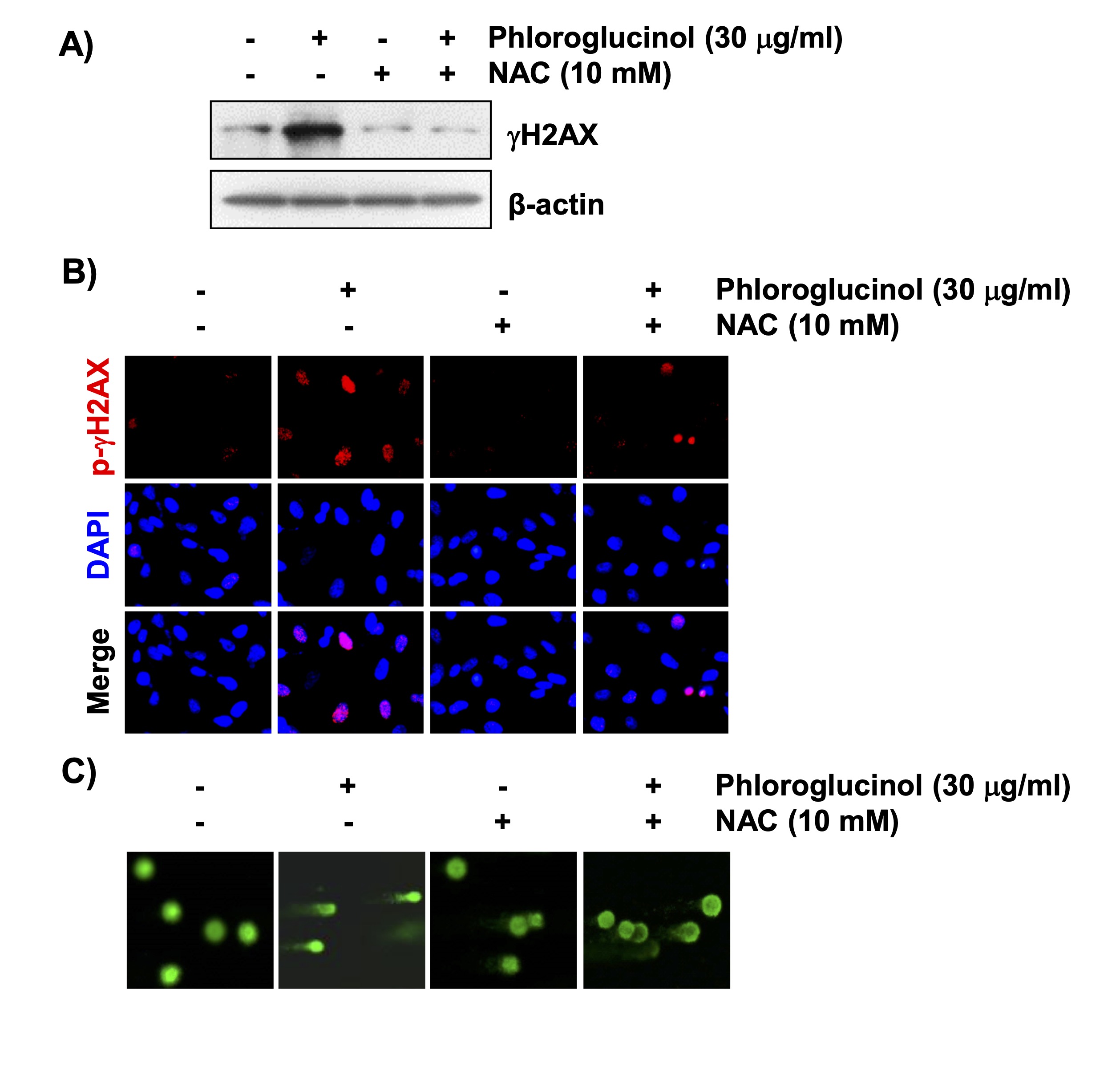
Fig. 5: Induction of ROS-dependent DNA damage by phloroglucinol in Hep3B cells. Cells were pretreated with 10 mM NAC for 1 h and then treated with 30 μg/ml phloroglucinol for 48 h. (A) The expression level of γH2AX is shown by immunoblotting. (B) Changes in the expression of γH2AX (green) as shown by fluorescence microscopy. The nuclei were counterstained with DAPI. (C) The degree of DNA damage was also evaluated by performing the Comet assay, and representative fluorescence images of the Comet assay are shown.
Inactivation of ROS-dependent PI3K/Akt/mTOR signaling contributed to phloroglucinol-induced cytotoxicity in Hep3B cells
Furthermore, we investigated the effects of phloroglucinol on PI3K/Akt/mTOR signaling to further explore potential targets of its anticancer activity in Hep3B cells. Immunoblotting results showed that the expression of phosphorylated PI3K (p-PI3K) was dose-dependently decreased in phloroglucinol-treated cells relative to that in the control cells (Fig. 6A). Similarly, the phosphorylation levels of Akt and mTOR, which are well-known downstream factors of PI3K, were also dramatically reduced by phloroglucinol. However, the overall expression levels of their proteins did not change significantly, and pretreatment with NAC completely rescued the dephosphorylation of these proteins cells (Fig. 6B). Further, PARP protein expression was also preserved without degradation. In addition, LY294002, a PI3K inhibitor, significantly increased phloroglucinol-induced apoptosis and enhanced phloroglucinol-induced cell viability reduction. However, NAC was found to significantly attenuate these aggravating effects (Fig. 6C and D). These findings demonstrated that phloroglucinol decreased cell proliferation and promoted apoptosis of Hep3B cells by inactivating the PI3K/Akt/mTOR signaling pathway in a ROS-dependent manner.
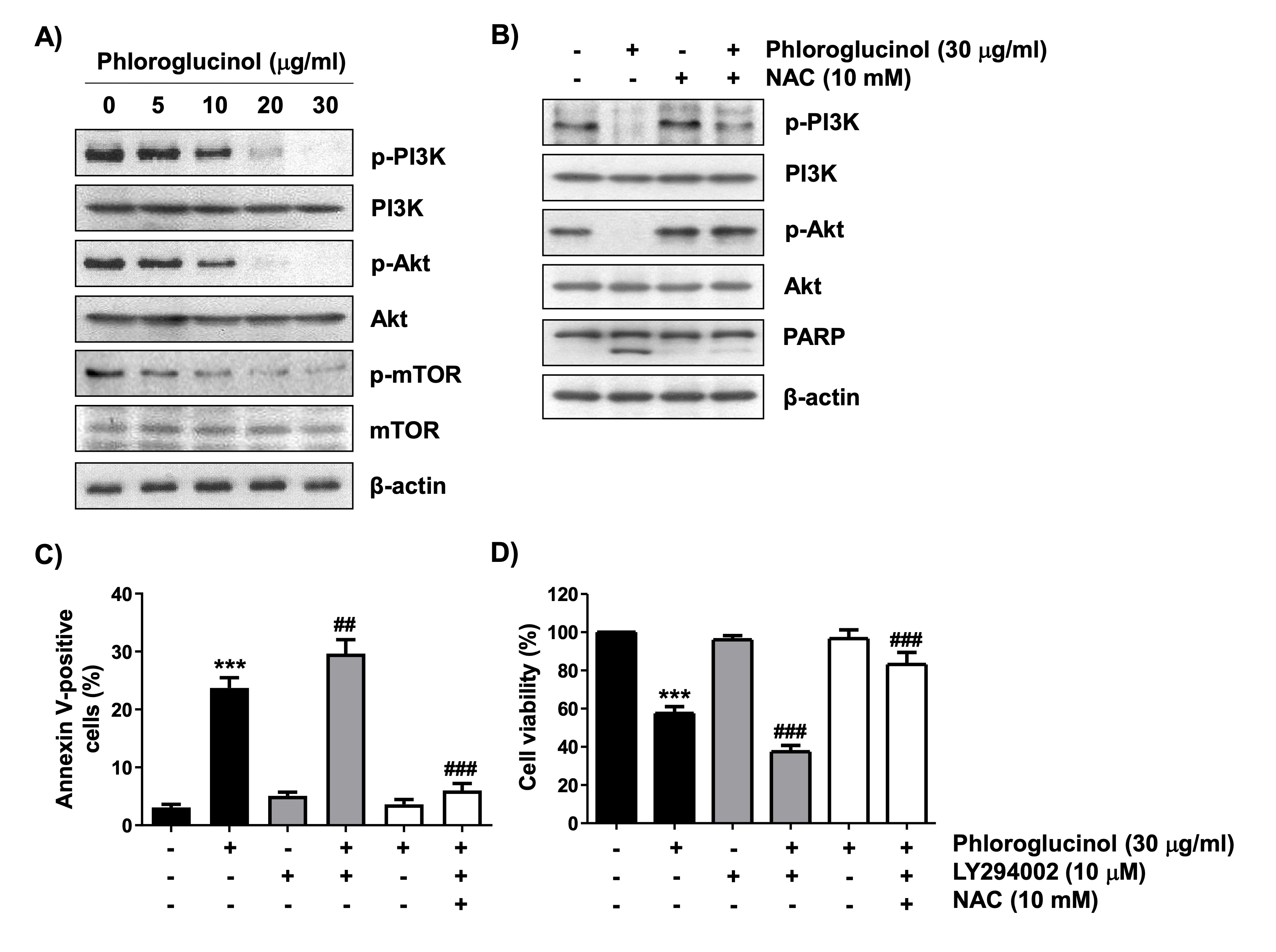
Fig. 6: Role of PI3K/Akt signaling and ROS in phloroglucinol-induced apoptosis and cell viability inhibition in Hep3B cells. The cells were cultured in medium containing the indicated concentration of phloroglucinol for 48 h (A) or treated with 10 mM NAC or 10 mM LY294002 for 1 h and further treated with 30 μg/ml phloroglucinol for 48 h (B–D). (A and B) The corresponding antibodies were used to investigate the changes in the expressions of the indicated proteins. Equal loading was confirmed with β-actin. (C) After staining with Annexin V-FITC/PI, the frequencies of annexin-positive cells are presented as the ratio of annexin V-positive cells to the total number of cells expressed as a percentage. (D) Cell viability was analyzed by performing the MTT assay (***p<0.001 vs. control cells, ##p<0.01 and ###p<0.001 vs. phloroglucinol-treated cells).
Discussion
In this study, our data demonstrated that the suppression of the viability of Hep3B cells by phloroglucinol, a phenolic derivative isolated from brown algae, was due to caspase-dependent apoptosis, not to necrosis. In Hep3B cells exposed to phloroglucinol, both caspase-8 and caspase-9 were activated, indicating that the death receptor-mediated extrinsic and mitochondrial-mediated intrinsic pathways were simultaneously activated during phloroglucinol-induced apoptosis. Both enzymes were activated because they are the initiating caspases for each respective pathway [38, 39]. Effector caspases, including caspase-3 and -7, are activated by caspase-8 and caspase-9 to sequentially cleave intracellular matrix proteins, such as PARP, thereby completing apoptosis; thus, degradation of PARP is recognized as a representative biomarker of apoptosis induction [39, 40]. Phloroglucinol also increased PARP cleavage in response to caspase-3 activation, which may provide additional evidence that phloroglucinol-induced typical caspase-dependent apoptosis in Hep3B cells.
Among the intracellular organelles, mitochondria are both energy generators and key mediators of cellular fate [41, 42]. The main event in the regulation of apoptosis by mitochondria is the cytosolic release of apoptosis-inducing proteins, such as cytochrome c , which reside in the mitochondrial intermembrane space, resulting from the permeabilization of the mitochondrial outer membrane [38, 41]. A widely used method to detect the disruption of mitochondrial stability is to determine if there is a loss of MMP, and our results clearly indicated that the loss of MMP was induced in the Hep3B cells treated with phloroglucinol. The Bcl-2 family of proteins primarily controls mitochondrial apoptotic events, including cytochrome c release [39, 43]. Among them, proapoptotic proteins, including Bax, induce apoptosis by attacking the mitochondria and increasing their outer membrane permeability, whereas pro-survival proteins, such as Bcl-2, support cell survival by inhibiting apoptotic activity. In the extrinsic pathway, Bid cleaved by caspase-8 also bridges the proapoptotic activation of mitochondria by inducing cytochrome c release [38, 43]. Therefore, the increase in the Bax/Bcl-2 expression ratio caused by phloroglucinol may have contributed to the dissipation in MMP caused by the mitochondrial portion. This may have resulted in cytochrome c leakage into the cytosol, supporting the idea that phloroglucinol-induced cell death was achieved through the simultaneous activation of the extrinsic and intrinsic pathways.
Accumulated studies have shown that phloroglucinol and its derivatives act as antioxidants that reduce ROS production in various experimental models, thereby blocking cell damage by oxidative stress [23, 24, 44-46]. Alternatively, other studies have shown that the anticancer potential of several phloroglucinol derivatives in many different types of cancer was closely related to mitochondrial dysfunction induced by oxidative stress accompanied by increased ROS production [26, 47]. Although mitochondria are critical producers of ROS production, the loss of mitochondrial function caused by natural products with anticancer potential is closely correlated with the accumulation of ROS because mitochondria are organelles that are also vulnerable to ROS [48, 49]. Our results showed that phloroglucinol significantly increased ROS production and conversely decreased the GSH/GSSG ratio, which occurs when cells are exposed to oxidative stress. However, in the presence of NAC, these changes were restored. Similar events have been noted in previous studies [26, 47], suggesting that increased oxidative stress by phloroglucinol in Hep3B cells possibly contributes to its anticancer activity. Nevertheless, evaluation is needed to confirm if the increase in ROS production following phloroglucinol treatment is related to mitochondrial damage or to other factors.
Abnormalities in cellular function due to oxidative stress are also highly correlated with DNA damage [50]. Although phloroglucinol derivatives reportedly might increase DNA damage in cancer cells [27, 51], there is no clear supporting evidence. The nuclear morphological changes presented in this study are evidence that phloroglucinol-induced apoptosis in Hep3B cells were due to DNA strand breaks. In addition, because the PARP enzyme is involved in the repair of damaged DNA [52], its cleavage might contribute to DNA damage induction. Therefore, we investigated the changes in the expression of histone H2AX, which is phosphorylated to γ-H2AX when double-stranded DNA breaks are induced [17], to provide direct evidence that phloroglucinol causes DNA damage. Our results clearly demonstrated that the expression of this protein was largely upregulated in phloroglucinol-exposed Hep3B cells. In addition, the Comet assay, which is widely used to detect DNA strand breaks [53], showed that phloroglucinol increased DNA damage. However, in the presence of NAC, these DNA damage markers were significantly rescued, indicating that oxidative damage has an important functional role in phloroglucinol-induced Hep3B cytotoxicity.
Changes in the activity of various intracellular signaling pathways have been associated with induction of apoptosis. Among them, PI3K/Akt/mTOR signaling may be implicated in the anticancer activity induced by phloroglucinol, which has been shown to be related to induction of oxidative damage [28, 29]. This signaling pathway, which is upregulated in various tumor tissues and clinical samples, promotes glucose uptake and favors glycolysis in cancer development, thereby increasing tumor cell survival and evading apoptotic cell death, as shown in other studies [54, 55]. In addition, PI3K/Akt/mTOR signaling stimulates tumor-cell metastasis and angiogenesis, and activation of this signaling reportedly decreases the radiosensitivity of HCC cells and becomes a causal factor in drug resistance to anticancer drugs [30, 31, 54]. Therefore, inactivation of this signaling pathway is known to limit cancer-cell growth and induce apoptosis; thus, it could be a therapeutic target. In the current study, activation of this pathway was reduced in phloroglucinol-treated Hep3B cells, as evidenced by the diminished phosphorylation of not only PI3K but also its downstream substrates, Akt and mTOR. Furthermore, we proved that this phenomenon was ROS-dependent by artificially blocking ROS production, which restored the inactivated pathway. In parallel with these results, cleavage of PARP by phloroglucinol was attenuated by NAC, which suggests that phloroglucinol-induced apoptosis was ROS-dependent. Furthermore, phloroglucinol-induced apoptosis was blocked by NAC, whereas the diminished cell viability was restored. In contrast, LY294002, a PI3K inhibitor, further enhanced phloroglucinol-induced cytotoxicity, including apoptosis and decreased cell viability, in Hep3B cells. However, the increase in phloroglucinol-mediated cytotoxicity by LY294002 was abolished by NAC.
Taken together, our results indicate that ROS levels elevated by phloroglucinol in Hep3B cells are critical events in phloroglucinol-induced apoptosis and DNA damage and are upstream regulators of PI3K/Akt/mTOR signaling pathway inactivation (Fig. 7). This finding contrasts with the known role of phloroglucinol in many normal cell types, where it counteracts oxidative stress-induced cytotoxicity by inhibiting ROS generation [23, 44, 45]. It is well established that excessive production of ROS is a mediator of mitochondrial dysfunction because ROS are highly reactive toward various macromolecules involved in the mitochondrial electron transport chain [48, 49]. However, because ROS generation is involved in various redox homeostasis and cellular signaling pathways in addition to mitochondrial damage associated with aerobic respiration [48, 56], further detailed investigations into the origin of ROS generation would be interesting. Notably, this study’s use of a single HCC cell line limits the generalizability of the findings across all HCCs. Furthermore, the lack of evidence regarding the origin and specificity of ROS production is a limitation of this study. Future studies should include insightful investigations into the role of the PI3K/Akt/mTOR and other intracellular signaling pathways in phloroglucinol’s anticancer activity, as well as comprehensive analyses using animal tumor models and various cancer cell types to better understand the therapeutic potential of phloroglucinol.
Conclusion
Our study results showed that phloroglucinol-induced DNA damage by increasing ROS production in HCC Hep3B cells, which was associated with induction of apoptosis and decreased cell survival. However, such effects of phloroglucinol were markedly abrogated by the ROS scavenger, demonstrating that phloroglucinol stimulated ROS-dependent anticancer activity in Hep3B cells. Furthermore, our data showed that phloroglucinol was able to inactivate the ROS-mediated PI3K/Akt/mTOR signaling pathway that inhibited cell survival and promoted apoptosis. Collectively, these findings demonstrated that ROS generation acted as an upstream event of phloroglucinol-induced apoptosis and inactivation of the PI3K/Akt/mTOR signaling pathway in Hep3B cells. Therefore, these findings support the potential efficacy of phloroglucinol as a treatment for HCC.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00213236). The authors would like to thank Core-Facility Center for Tissue Regeneration, Dong-eui University (Busan, Republic of Korea), for letting us use their flow cytometer and fluorescence microscope.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Author Contributions
So Young Kim and Yung Hyun Choi designed the study; So Young Kim, Hyun Hwangbo and Gi-Young Kim performed the experiments; So Young Kim and Hyun Hwangbo wrote the original manuscript; Yung Hyun Choi reviewed and edited the manuscript.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Chan YT, Zhang C, Wu J, Lu P, Xu L, Yuan H, Feng Y, Chen ZS, Wang N: Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol Cancer 2024;23:189.
https://doi.org/10.1186/s12943-024-02101-z |
| 2 | Koshy A: Evolving global etiology of hepatocellular carcinoma (HCC): Insights and trends for 2024. J Clin Exp Hepatol 2025;15:102406.
https://doi.org/10.1016/j.jceh.2024.102406 |
| 3 | Fenton SE, Burns MC, Kalyan A: Epidemiology, mutational landscape and staging of hepatocellular carcinoma. Chin Clin Oncol 2021;10:2.
https://doi.org/10.21037/cco-20-162 |
| 4 | Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun O, Tan Y, Ma GX, Nguyen MT: Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol 2019;25:2279-2293.
https://doi.org/10.3748/wjg.v25.i19.2279 |
| 5 | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6.
https://doi.org/10.1038/s41572-020-00240-3 |
| 6 | Dong Y, Liu TH, Yau T, Hsu C: Novel systemic therapy for hepatocellular carcinoma. Hepatol Int 2020;14:638-651.
https://doi.org/10.1007/s12072-020-10073-7 |
| 7 | Cotas J, Lomartire S, Gonçalves AMM, Pereira L: From ocean to medicine: Harnessing seaweed's potential for drug development. Int J Mol Sci 2024;25:797.
https://doi.org/10.3390/ijms25020797 |
| 8 | Silva M, Avni D, Varela J, Barreira L: The ocean's pharmacy: Health discoveries in marine algae. Molecules 2024;29:1900.
https://doi.org/10.3390/molecules29081900 |
| 9 | Baghel RS, Choudhary B, Pandey S, Pathak PK, Patel MK, Mishra A: Rehashing our insight of seaweeds as a potential source of foods, nutraceuticals, and pharmaceuticals. Foods 2023;12:3642.
https://doi.org/10.3390/foods12193642 |
| 10 | Sadeghi A, Rajabiyan A, Nabizade N, Meygoli Nezhad N, Zarei-Ahmady A: Seaweed-derived phenolic compounds as diverse bioactive molecules: A review on identification, application, extraction and purification strategies. Int J Biol Macromol 2024;266:131147.
https://doi.org/10.1016/j.ijbiomac.2024.131147 |
| 11 | Jaison JP, Balasubramanian B, Gangwar J, Pappuswamy M, Meyyazhagan A, Kamyab H, Paari KA, Liu WC, Taheri MM, Joseph KS. Bioactive nanoparticles derived from marine brown seaweeds and their biological applications: A review. Bioprocess Biosyst Eng 2024;47:1605-1618.
https://doi.org/10.1007/s00449-024-03036-x |
| 12 | Lomartire S, Gonçalves AMM: Marine macroalgae polyphenols as potential neuroprotective antioxidants in neurodegenerative diseases. Mar Drugs 2023;21:261.
https://doi.org/10.3390/md21050261 |
| 13 | Negara BFSP, Sohn JH, Kim JS, Choi JS: Effects of phlorotannins on organisms: Focus on the safety, toxicity, and availability of phlorotannins. Foods 2021;10:452.
https://doi.org/10.3390/foods10020452 |
| 14 | Simón L, Arazo-Rusindo M, Quest AFG, Mariotti-Celis MS: Phlorotannins: Novel orally administrated bioactive compounds that induce mitochondrial dysfunction and oxidative stress in cancer. Antioxidants (Basel) 2023;12:1734.
https://doi.org/10.3390/antiox12091734 |
| 15 | Zheng H, Zhao Y, Guo L: A bioactive substance derived from brown seaweeds: Phlorotannins. Mar Drugs 2022;20:742.
https://doi.org/10.3390/md20120742 |
| 16 | Kumar LRG, Paul PT, Anas KK, Tejpal CS, Chatterjee NS, Anupama TK, Mathew S, Ravishankar CN: Phlorotannins-bioactivity and extraction perspectives. J Appl Phycol 2022;34:2173-2185.
https://doi.org/10.1007/s10811-022-02749-4 |
| 17 | Prabhu KS, Kuttikrishnan S, Ahmad N, Habeeba U, Mariyam Z, Suleman M, Bhat AA, Uddin S: H2AX: A key player in DNA damage response and a promising target for cancer therapy. Biomed Pharmacother 2024;175:116663.
https://doi.org/10.1016/j.biopha.2024.116663 |
| 18 | Tuli HS, Kaur J, Vashishth K, Sak K, Sharma U, Choudhary R, Behl T, Singh T, Sharma S, Saini AK, Dhama K, Varol M, Sethi G: Molecular mechanisms behind ROS regulation in cancer: A balancing act between augmented tumorigenesis and cell apoptosis. Arch Toxicol 2023;97:103-120.
https://doi.org/10.1007/s00204-022-03421-z |
| 19 | Yu W, Tu Y, Long Z, Liu J, Kong D, Peng J, Wu H, Zheng G, Zhao J, Chen Y, Liu R, Li W, Hai C: Reactive oxygen species bridge the gap between chronic inflammation and tumor development. Oxid Med Cell Longev 2022;2022:2606928.
https://doi.org/10.1155/2022/2606928 |
| 20 | Catarino MD, Amarante SJ, Mateus N, Silva AMS, Cardoso SM: Brown algae phlorotannins: A marine alternative to break the oxidative stress, inflammation and cancer network. Foods 2021;10:1478.
https://doi.org/10.3390/foods10071478 |
| 21 | Pradhan B, Ki JS: Antioxidant and chemotherapeutic efficacies of seaweed-derived phlorotannins in cancer treatment: A review regarding novel anticancer drugs. Phytother Res 2023;37:2067-2091.
https://doi.org/10.1002/ptr.7809 |
| 22 | Marasinghe CK, Jung WK, Je JY: Phloroglucinol possesses anti-inflammatory activities by regulating AMPK/Nrf2/HO-1 signaling pathway in LPS-stimulated RAW264.7 murine macrophages. Immunopharmacol Immunotoxicol 2023;45:571-580.
https://doi.org/10.1080/08923973.2023.2196602 |
| 23 | Park C, Cha HJ, Hwangbo H, Ji SY, Kim DH, Kim MY, Bang E, Hong SH, Kim SO, Jeong SJ, Lee H, Moon SK, Shim JH, Kim GY, Cho S, Choi YH: Phloroglucinol inhibits oxidative-stress-induced cytotoxicity in C2C12 murine myoblasts through Nrf-2-mediated activation of HO-1. Int J Mol Sci 2023;24:4637.
https://doi.org/10.3390/ijms24054637 |
| 24 | Lee HA, Lee JH, Han JS: 2, 7"-Phloroglucinol-6, 6'-bieckol protects INS-1 cells against high glucose-induced apoptosis. Biomed Pharmacother 2018;103:1473-1481.
https://doi.org/10.1016/j.biopha.2018.04.129 |
| 25 | Park MH, Han JS: Phloroglucinol protects INS-1 pancreatic b-cells against glucotoxicity-induced apoptosis. Phytother Res 2015;29:1700-1706.
https://doi.org/10.1002/ptr.5407 |
| 26 | El Gaafary M, Saber FR, Mahrous EA, Ashour RM, Okba MM, Jin L, Lang SJ, Schmiech M, Simmet T, Syrovets T: The phloroglucinol calcitrinone A, a novel mitochondria-targeting agent, induces cell death in breast cancer cells. Food Chem Toxicol 2022;162:112896.
https://doi.org/10.1016/j.fct.2022.112896 |
| 27 | Lopes-Costa E, Abreu M, Gargiulo D, Rocha E, Ramos AA: Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J Toxicol Environ Health A 2017;80:776-787.
https://doi.org/10.1080/15287394.2017.1357297 |
| 28 | Kim RK, Suh Y, Yoo KC, Cui YH, Hwang E, Kim HJ, Kang JS, Kim MJ, Lee YY, Lee SJ: Phloroglucinol suppresses metastatic ability of breast cancer cells by inhibition of epithelial-mesenchymal cell transition. Cancer Sci 2015;106:94-101.
https://doi.org/10.1111/cas.12562 |
| 29 | Kang MH, Kim IH, Nam TJ: Phloroglucinol induces apoptosis through the regulation of insulin-like growth factor 1 receptor signaling pathways in human colon cancer HT-29 cells. Int J Oncol 2014;45:1036-1042.
https://doi.org/10.3892/ijo.2014.2521 |
| 30 | Paskeh MDA, Ghadyani F, Hashemi M, Abbaspour A, Zabolian A, Javanshir S, Razzazan M, Mirzaei S, Entezari M, Goharrizi MASB, Salimimoghadam S, Aref AR, Kalbasi A, Rajabi R, Rashidi M, Taheriazam A, Sethi G: Biological impact and therapeutic perspective of targeting PI3K/Akt signaling in hepatocellular carcinoma: Promises and challenges. Pharmacol Res 2023;187:106553.
https://doi.org/10.1016/j.phrs.2022.106553 |
| 31 | Narayanankutty A: Natural products as PI3K/Akt inhibitors: Implications in preventing hepatocellular carcinoma. Curr Mol Pharmacol 2021;14:760-769.
https://doi.org/10.2174/1874467214666210120152657 |
| 32 | Kang JB, Son HK, Park DJ, Jin YB, Koh PO: Chlorogenic acid regulates the expression of protein phosphatase 2A subunit B in the cerebral cortex of a rat stroke model and glutamate-exposed neurons. Lab Anim Res 2024;40:8.
https://doi.org/10.1186/s42826-024-00196-5 |
| 33 | Jeon SJ, Jung GH, Choi EY, Han EJ, Lee JH, Han SH, Woo JS, Jung SH, Jung JY: Kaempferol induces apoptosis through the MAPK pathway and regulates JNK-mediated autophagy in MC-3 cells. Toxicol Res 2024;40:45-55.
https://doi.org/10.1007/s43188-023-00206-z |
| 34 | Manigandan S, Yun JW: Sodium-potassium adenosine triphosphatase α2 subunit (ATP1A2) negatively regulates UCP1-dependent and UCP1-independent thermogenesis in 3T3-L1 adipocytes. Biotechnol Bioprocess Eng 2023;28:644-657.
https://doi.org/10.1007/s12257-023-0095-3 |
| 35 | Cao M, Fan B, Zhen T, Das A, Wang J: Ruthenium biochanin-A complex ameliorates lung carcinoma through the downregulation of the TGF-β/PPARγ/PI3K/TNF-α pathway in association with caspase-3-mediated apoptosis. Toxicol Res 2023;39:455-475.
https://doi.org/10.1007/s43188-023-00177-1 |
| 36 | Park C, Kim DH, Kim TH, Jeong SU, Yoon JH, Moon SK, Kwon CY, Park SH, Hong SH, Shim JH, Kim GY, Choi YH: Improvement of oxidative stress-induced cytotoxicity of Angelica keiskei (Miq.) Koidz. leaves extract through activation of heme oxygenase-1 in C2C12 murine myoblasts. Biotechnol Bioprocess Eng 2023;28:51-62.
https://doi.org/10.1007/s12257-022-0310-7 |
| 37 | Park C, Cha HJ, Hwangbo H, Bang E, Kim HS, Yun SJ, Moon SK, Kim WJ, Kim GY, Lee SO, Shim JH, Choi YH: Activation of heme oxygenase-1 by mangiferin in human retinal pigment epithelial cells contributes to blocking oxidative damage. Biomol Ther (Seoul) 2024;32:329-340.
https://doi.org/10.4062/biomolther.2023.175 |
| 38 | Glover HL, Schreiner A, Dewson G, Tait SWG: Mitochondria and cell death. Nat Cell Biol. 2024;26:1434-1446.
https://doi.org/10.1038/s41556-024-01429-4 |
| 39 | Sahoo G, Samal D, Khandayataray P, Murthy MK: A review on caspases: Key regulators of biological activities and apoptosis. Mol Neurobiol 2023;60:5805-5837.
https://doi.org/10.1007/s12035-023-03433-5 |
| 40 | Ai Y, Meng Y, Yan B, Zhou Q, Wang X: The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol Cell 2024;84:170-179.
https://doi.org/10.1016/j.molcel.2023.11.040 |
| 41 | Basei FL, E Silva IR, Dias PRF, Ferezin CC, Peres de Oliveira A, Issayama LK, Moura LAR, da Silva FR, Kobarg J: The mitochondrial connection: The nek kinases' new functional axis in mitochondrial homeostasis. Cells 2024;13:473.
https://doi.org/10.3390/cells13060473 |
| 42 | Fontana F, Limonta P: The multifaceted roles of mitochondria at the crossroads of cell life and death in cancer. Free Radic Biol Med 2021;176:203-221.
https://doi.org/10.1016/j.freeradbiomed.2021.09.024 |
| 43 | Voss AK, Strasser A: The essentials of developmental apoptosis. F1000Res 2020;9:F1000 Faculty Rev-148.
https://doi.org/10.12688/f1000research.21571.1 |
| 44 | Piao MJ, Ahn MJ, Kang KA, Kim KC, Zheng J, Yao CW, Cha JW, Hyun CL, Kang HK, Lee NH, Hyun JW: Phloroglucinol inhibits ultraviolet B radiation-induced oxidative stress in the mouse skin. Int J Radiat Biol 2014;90:928-935.
https://doi.org/10.3109/09553002.2014.911990 |
| 45 | Piao MJ, Kim KC, Kang KA, Fernando PDSM, Herath HMUL, Hyun JW: Phloroglucinol attenuates ultraviolet B-induced 8-oxoguanine formation in human HaCaT keratinocytes through Akt and Erk-mediated Nrf2/Ogg1 signaling pathways. Biomol Ther (Seoul) 2021;29:90-97.
https://doi.org/10.4062/biomolther.2020.059 |
| 46 | Wang H, Shao B, Yu H, Xu F, Wang P, Yu K, Han Y, Song M, Li Y, Cao Z: Neuroprotective role of hyperforin on aluminum maltolate-induced oxidative damage and apoptosis in PC12 cells and SH-SY5Y cells. Chem Biol Interact 2019;299:15-26.
https://doi.org/10.1016/j.cbi.2018.11.016 |
| 47 | Zhang Y, Luo M, Zu Y, Fu Y, Gu C, Wang W, Yao L, Efferth T: Dryofragin, a phloroglucinol derivative, induces apoptosis in human breast cancer MCF-7 cells through ROS-mediated mitochondrial pathway. Chem Biol Interact 2012;199:129-136.
https://doi.org/10.1016/j.cbi.2012.06.007 |
| 48 | Li Y, Zhang H, Yu C, Dong X, Yang F, Wang M, Wen Z, Su M, Li B, Yang L: New insights into mitochondria in health and diseases. Int J Mol Sci 2024;25:9975.
https://doi.org/10.3390/ijms25189975 |
| 49 | Guan S, Zhao L, Peng R: Mitochondrial respiratory chain supercomplexes: From structure to function. Int J Mol Sci 2022;23:13880.
https://doi.org/10.3390/ijms232213880 |
| 50 | Yang J, Luo J, Tian X, Zhao Y, Li Y, Wu X: Progress in understanding oxidative stress, aging, and aging-related diseases. Antioxidants (Basel) 2024;13:394.
https://doi.org/10.3390/antiox13040394 |
| 51 | Ferreira J, Ramos AA, Almeida T, Azqueta A, Rocha E: Drug resistance in glioblastoma and cytotoxicity of seaweed compounds, alone and in combination with anticancer drugs: A mini review. Phytomedicine 2018;48:84-93.
https://doi.org/10.1016/j.phymed.2018.04.062 |
| 52 | Arya P, Malhotra H, Chaudhary B, Sarwara A, Goyal R, Wan C, Mishra DK, Gautam RK: Mechanism-based suppression of cancer by targeting DNA-replicating enzymes. Curr Protein Pept Sci 2024;25:4-11.
https://doi.org/10.2174/1389203724666230512144011 |
| 53 | Mišík M, Staudinger M, Kundi M, Worel N, Nersesyan A, Ferk F, Dusinska M, Azqueta A, Møller P, Knasmueller S: Use of the single cell gel electrophoresis assay for the detection of DNA-protective dietary factors: Results of human intervention studies. Mutat Res Rev Mutat Res 2023;791:108458.
https://doi.org/10.1016/j.mrrev.2023.108458 |
| 54 | Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S, Hao H, Xiong J: Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B 2022;12:558-580.
https://doi.org/10.1016/j.apsb.2021.09.019 |
| 55 | Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai W, Guo C: Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res 2020;39:126.
https://doi.org/10.1186/s13046-020-01629-4 |
| 56 | García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG: The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxid Med Cell Longev 2020;2020: 2082145.
https://doi.org/10.1155/2020/2082145 |