Regulation of αKlotho
Keywords
Abstract
Since its discovery in 1997, αKlotho has gained a lot of attention due to its powerful anti-aging and health-promoting properties. It exists as a membrane-bound protein or as a soluble factor. Membrane-bound αKlotho is an essential cofactor for fibroblast growth factor 23 (FGF23), thereby being involved in the regulation of renal phosphate and vitamin D metabolism. Soluble αKlotho (sKL) is present in different body fluids and exerts hormone-like effects. Through the αKlotho-FGF23 signaling axis, FGF23 regulates phosphate excretion by downregulating Na+-dependent phosphate transporter (NaPi-2a). In addition, this axis suppresses expression of 1α-hydroxylase, thereby reducing active vitamin D (calcitriol) serum concentration. Disruptions of this axis lead to deranged mineral metabolism. Low levels of αKlotho and elevated FGF23 are early biomarkers for different diseases, including chronic kidney disease (CKD) and cardiovascular diseases (CVD). In CKD, decreased renal αKlotho expression and enhanced FGF23 production contribute to worsening kidney function. Activated transforming growth factor b1 (TGF-b1) signaling, promoting renal fibrosis, contributes to the pathophysiology. Moreover, FGF23 directly induces left ventricular hypertrophy (LVH) through FGF receptor-induced calcineurin/nuclear factor of activated T cells (NFAT) signaling in CKD. Our review aims to comprehensively summarize the regulation and function of αKlotho, highlighting its central role in maintaining mineral metabolism and its therapeutic potential in age-related and chronic diseases.Introduction
The anti-aging protein αKlotho owes its name to Greek mythology, in which the goddess Klotho decided over life and death and held the threads of life [1, 2]. Its discovery goes back to experiments with a kl/kl mouse strain in 1997 [1]. This mouse strain is characterized by changes in behavior and appearance at a few weeks of age only [1]. Particularly striking is a drastic loss of bone mineral density and further signs of premature aging, leading to early death [1]. Conversely, overexpression of αKlotho delays aging and induces longevity, making αKlotho an interesting target in longevity research [3].
Klotho family
Three different Klotho proteins exist, termed α-, β- and γ-Klotho, all being expressed in different organs and fulfilling various functions [4], but this review focuses only on αKlotho.
The latter is strongly expressed in the brain and kidney, and to a much lesser extent in the pituitary gland, aorta or pancreas [1]. αKlotho belongs to the group of type I membrane proteins with several structural domains: two extracellular domains KL1 and KL2, a transmembrane domain (TM) and a short cytoplasmic site (CYT) [5–7]. Depending on the cleavage site, membrane-bound αKlotho protein can be split into full-length soluble αKlotho (sKL) or into the respective single fragments KL1 and KL2 by a disintegrin and metalloproteinase (ADAM)10 or 17 [8–10]. In addition, a product of alternative RNA splicing exists, namely secreted αKlotho and identical to KL1 [5, 10]. Both human and mouse transcripts of membrane αKlotho comprise five exons, whereas the human secreted form of αKlotho consists of five and mouse secreted αKlotho only consists of three exons [5, 11]. Secreted αKlotho transcripts can only be detected in mice and humans, but not in rats [12] and the expression of secreted αKlotho in humans is even higher than that of membrane αKlotho [5].
In contrast to soluble and secreted αKlotho (summarized as circulating KL) [13] being humoral factors [14], the function of the membrane-bound form is much better understood: It acts as an essential cofactor for the binding of fibroblast growth factor 23 (FGF23) to its receptor since only αKlotho generates a specific FGF23 receptor FGFR) complex FGFR1c, FGFR3c or FGFR4 [15, 16].
Membrane-bound αKlotho and FGF23
FGF23 was first described in 2000 [17] and is predominantly expressed by bone cells, i.e. osteoblasts and osteocytes [18]. The discovery of missense mutations in the FGF23 gene accounting for derangements of phosphate metabolism, rickets and further disorders of bone, led to the assumption that FGF23 is a major factor for phosphate and vitamin D metabolism [17, 19].
Altogether, 22 FGF genes exist that can be divided into intracellular and secreted FGFs, the latter having paracrine and endocrine functions [20, 21] and comprising FGF15/FGF19, FGF21, and FGF23 [22]. In contrast to the other FGF subfamilies, endocrine FGFs only have low affinity for heparin, resulting in a weak FGF receptor interaction [21–23]. It is the primary task of αKlotho to facilitate efficient and specific FGF23 signaling in the kidney by forming a FGFR1(IIIc)-αKlotho complex (Fig. 1) [16, 24]. It controls calcitriol synthesis by regulating the expression of its key enzyme, 1α-hydroxylase, in the proximal tubule [25, 26]. By downregulating the major renal Na+-dependent phosphate transporter NaPi-2a, FGF23 suppresses phosphate reabsorption [26]. Both, αKlotho or FGF23 deficiency result in similar disorders in mice that are mainly due to deranged vitamin D and phosphate homeostasis and further characterized by growth retardation and a severely reduced life span [4, 24]. Moreover, αKlotho and FGF23 may both serve as biomarkers for the early detection of various diseases. Particularly in chronic kidney disease (CKD), an early rise in FGF23 serum levels as well as a decrease αKlotho serum levels are predictors of CKD progression [27, 28]. In an αKlotho-independent manner, elevated FGF23 binds to FGFR4 on cardiomyocytes and thereby activates phospholipase Cγ (PLCγ)/calcineurin/nuclear factor of activated T cells (NFAT) signaling, inducing left ventricular hypertrophy [29, 30].
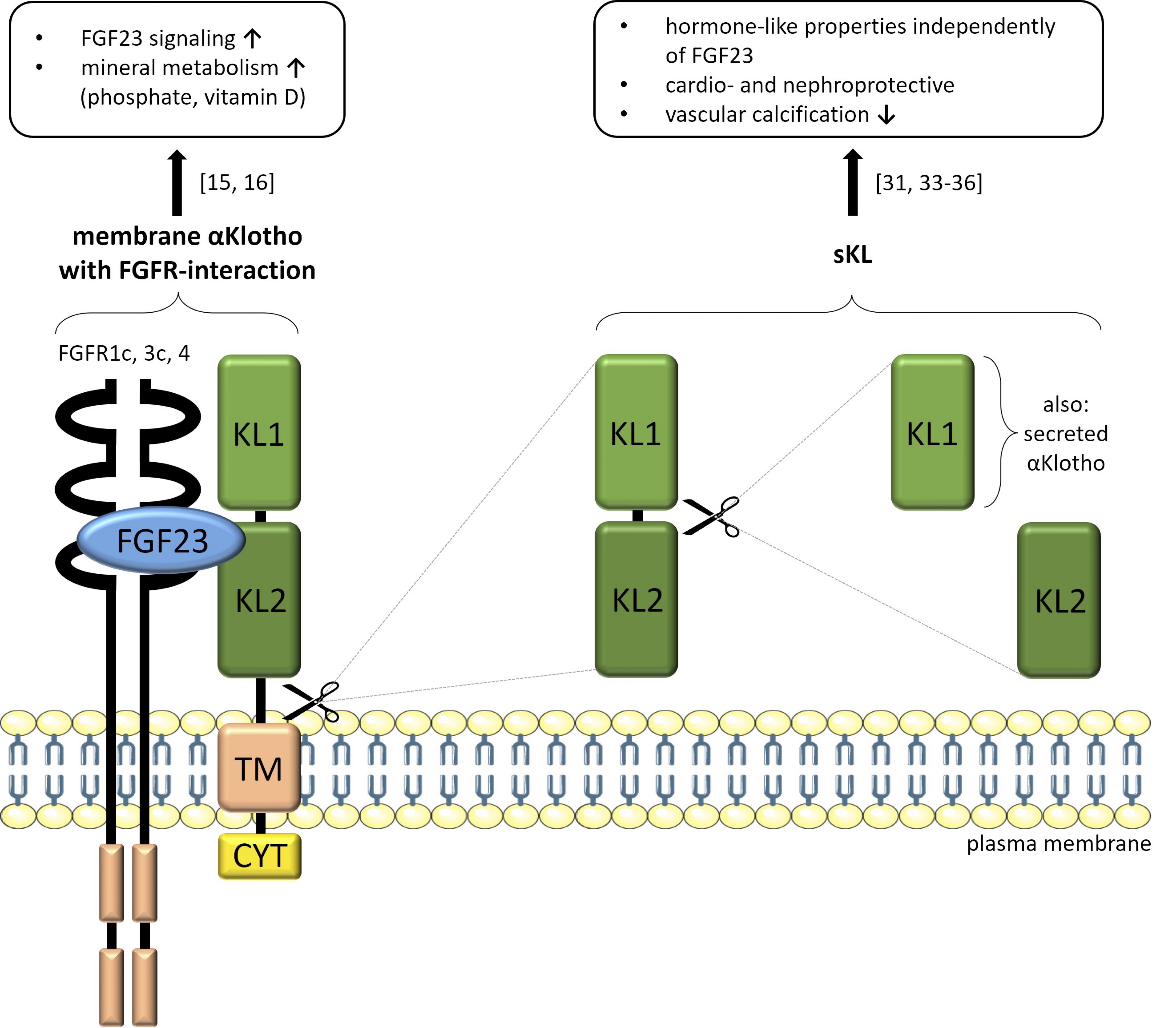
Fig. 1: Structures of membrane-bound αKlotho forming a complex with FGFR and FGF23 (left) and the cleaved forms of soluble αKlotho (KL1/KL2, right) with their respective functions in the organism. Fibroblast growth factor 23 (FGF23), FGF23 receptor (FGFR), transmembrane domain (TM), short cytoplasmic site (CYT), soluble αKlotho (sKL). Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/), figure adapted from [6].
Soluble αKlotho (sKL)
As the product of cleaved renal membrane-bound αKlotho, sKL serves as a hormone-like factor independently of FGF23 [31]. It can be detected in blood, cerebrospinal fluid, or urine [31] and is effective in different organs, including heart and blood vessels [32]. SKL has organoprotective properties in the heart by reducing susceptibility to stress signals and lowering intracellular calcium levels by inhibition of transient receptor potential channel TRPC6 [33]. It has beneficial effects in blood vessels by reducing vascular calcification [34, 35] and is nephroprotective [36]. SKL controls important intracellular signaling pathways including transforming growth factor-β (TGF-β) or Wnt signaling [31]. Antitumor [37] or antifibrosis effects of sKL may also be due to TGF-β receptor or Wnt signaling inhibition [38, 39]
Hitherto, no receptor for sKL has been characterized, but sKL binds to so-called lipid rafts and thereby negatively affects phosphoinositide 3-kinase (PI3K) signaling [40]. Lipid rafts are considered a promising target for many sKL-induced pathways [40].
Regulation of αKlotho in the kidney
Regulators of renal αKlotho expression are reviewed below and listed in an alphabetical order (summarized in Table 1).

Table 1: Regulators of transmembrane αKlotho
1, 25-dihydroxyvitamin D3
In cell lines of proximal or distal tubular origin or of the collecting duct, 1,
25-dihydroxyvitamin D3 (1, 25D)
enhances renal αKlotho gene expression, an effect dependent on vitamin D receptor (VDR) [41,
42].
Also, the administration of 1, 25D is paralleled by an increase in αKlotho gene expression
in mice [43].
Albumin
Albumin reduces αKlotho mRNA and protein abundance in vitro and in vivo [44, 45], an effect
attributed to
albumin-induced endoplasmic reticulum (ER) stress. Conversely, inhibition of ER stress or
silencing of
activating transcription factor 3 (ATF3) enhance αKlotho protein [44].
AMP-dependent kinase
AMP-dependent kinase (AMPK) is activated in cellular states of energy deficiency
characterized by high levels of
AMP [46]. It stimulates renal αKlotho gene and protein expression in vitro [47], but αKlotho
itself can activate
AMPK signaling, too [48].
Cytotoxic agents
In certain renal cell lines, αKlotho expression is enhanced by cisplatin, paclitaxel, or
doxorubicin [49], an
effect at least in part involving peroxisome proliferator-activated receptor γ (PPARγ) [49].
The induction of
apoptosis with PAC-1 shows a similar effect on αKlotho expression in vitro [49]. In
contrast, these cytotoxic
drugs suppress renal αKlotho gene expression and reduce sKL in human kidney 2 (HK2) cells
[49].
D-galactose
D-galactose stimulates renal fibrosis by inducing silent mating type information regulation
2 homolog-2 (SIRT2)
and TGF-β1, an effect paralleled by reduced renal αKlotho protein abundance in vivo [50].
Conversely, SIRT2
inhibitor acylglycerol kinase (AGK)-2 upregulates αKlotho protein [50].
Epidermal growth factor
Epidermal growth factor (EGF) elevates renal αKlotho mRNA levels in vitro [51].
Erythropoietin
Recombinant human erythropoietin (EPO) induces renal αKlotho protein expression in rats with
acute nephropathy
[52].
Histone deacetylase 3
Histone deacetylase (HDAC) inhibition up-regulates αKlotho mRNA and protein in a kidney cell
line or in vivo and
delays CKD progression [53, 54]. HDAC3 is a regulator of ROS production and is involved in
renal fibrosis [55].
TGF-β activates HDAC3 that subsequently decreases αKlotho protein [56]. In contrast,
inhibition of HDAC3
stimulates both αKlotho gene and protein expression in vitro, while increased αKlotho
protein expression is
reported in vivo [56].
Inflammation
Lipopolysaccharides (LPS) downregulate renal αKlotho gene expression [12] and protein [57]
in vivo and in vitro.
Also, tumor necrosis factor α (TNFα) and TNF-like weak inducer of apoptosis (TWEAK) suppress
αKlotho mRNA and
protein expression through NFκB signaling in vitro and in vivo [58], as does interferon
(IFN)-γ in vitro [59].
Metabolic factors
High levels of glucose, especially in type 2 diabetes, are negatively associated with
αKlotho mRNA and protein
abundance in a proximal tubular cell line [60].
PPARγ agonists including troglitazone upregulate renal αKlotho gene and protein expression
in vitro and in vivo
[61, 62]
Klotho-derived peptide 1
Klotho-derived peptide 1 (KP1), an inhibitor of TGF-β1 signaling pathway as a ligand of
TGF-β receptor 2, is
positively associated with αKlotho protein expression in vitro and in vivo [63, 64].
Lithium
Lithium reduces renal αKlotho protein abundance in vivo [65].
Nutrition and lifestyle
Berberine, a natural plant compound has anti-inflammatory, anti-oxidative and anti-apoptotic
properties [66]. In
acute kidney injury, it upregulates renal αKlotho gene expression [66].
Resveratrol, a polyphenol available in many plant-based foods, stimulates renal αKlotho gene
and protein
expression in vitro and in vivo [67, 68].
A high phosphate diet suppresses renal αKlotho protein abundance in wild type mice [69]. Its
impact is stronger
in adolescent mice compared to adult animals [70].
Nicotinamide attenuates the decrease of αKlotho protein expression in mice with
glycerol-induced AKI by altering
NFκB and histone deacetylase 1 activity [71].
Aerobic exercise elevates renal αKlotho gene and protein expression and reduces ROS
production [72] as well as
TGF-β1 signaling [73].
PKC
Protein kinase C (PKC), especially isoform PKCγ, activation downregulates αKlotho gene
expression in vitro [74].
Rapamycin
Rapamycin is an mTOR (molecular target of rapamycin) inhibitor [75]. One study found
upregulation of renal
αKlotho protein in mice by rapamycin [75] whereas another one reported rapamycin-induced
downregulation of
αKlotho transcripts and protein abundance in rats [76].
Renin-angiotensin system
Water homeostasis controls renal αKlotho expression. Dehydration induces angiotensin II, an
effect paralleled by
suppression of αKlotho mRNA and protein levels [77]. Angiotensin II is a direct negative
regulator of αKlotho
gene and protein expression in vitro [78].
In vitro or in vivo, vasopressin [77] and aldosterone [77, 79] reduce the expression of
renal αKlotho gene and
protein, while aldosterone antagonist spironolactone induces it [80]. Both losartan
(angiotensin II receptor
antagonist [81]) and fosinopril (inhibitor of angiotensin-converting enzyme (ACE) [82]),
enhance renal αKlotho
gene and protein expression in a mouse model of primary hypertension [83].
Reactive oxygen species
Reactive oxygen species (ROS) are negative regulators of renal αKlotho gene and protein
expression in vitro [84,
85] with nuclear factor erythroid 2-related factor 2 (Nrf2) being involved [85].
Sodium-glucose co-transporter-2 inhibitors
Sodium-glucose co-transporter-2 inhibitors (SGLT2i) canagliflozin, dapagliflozin,
empagliflozin or sotagliflozin
are reported to exert contrasting effects on αKlotho gene and protein expression in
different renal cell lines
and attenuate the decrease of αKlotho triggered by albuminuria or inflammation [60, 86].
Statins
Statins upregulate renal αKlotho mRNA and protein expression in vitro and in vivo [87–89].
The upregulation is
dependent on inhibition of RhoA pathway [88].
Toxins
αKlotho gene and protein expression is downregulated in the presence of uremic toxin indoxyl
sulfate in vitro
and in vivo [90, 91]. AST-120, an adsorbent of indole, reverses the suppressive effect on
αKlotho protein [91,
92].
Shiga toxin 2 downregulates renal αKlotho mRNA and protein abundance in mice [93].
Transcription factor Sp1
Overexpression of the ubiquitously expressed transcription factor Sp1 upregulates αKlotho
transcripts and
protein in vitro [94].
Regulation of αKlotho in organs and tissues other than kidney
Regulators of αKlotho expression in extrarenal organs or tissues are reviewed below and listed in alphabetical order (summarized in Table 2).
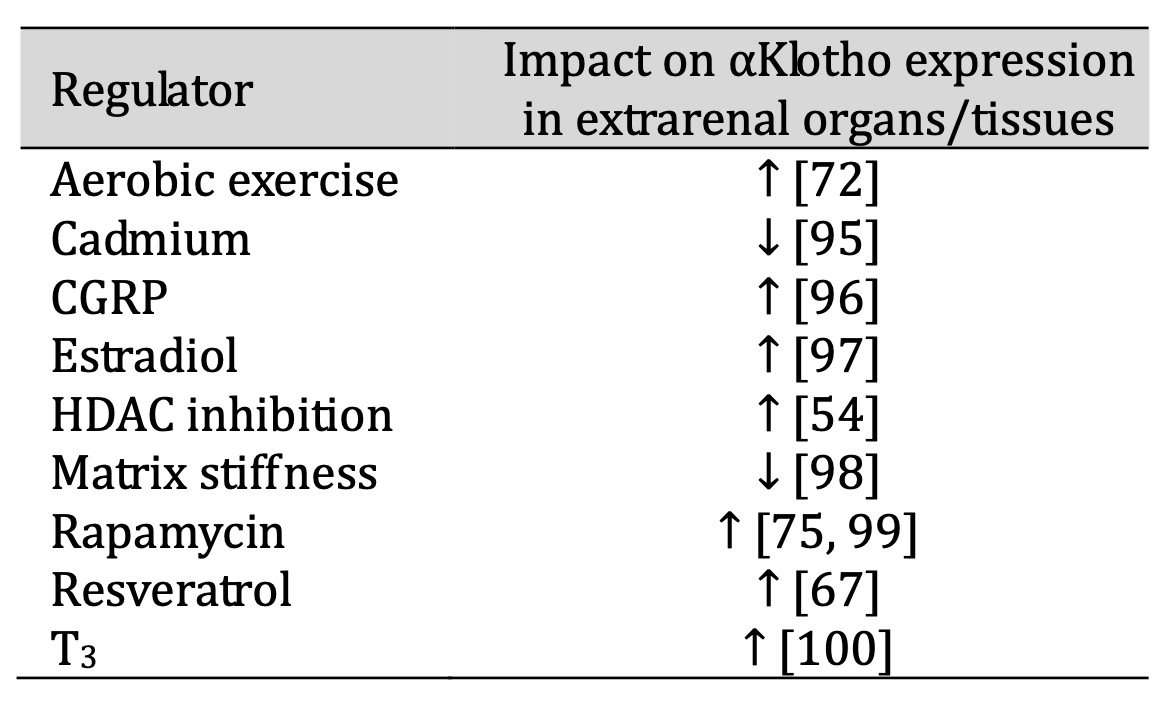
Table 2: Regulators of αKlotho in extrarenal organs/tissues
Aerobic exercise
Aerobic exercise upregulates αKlotho gene and protein expression in rat brain [72].
Cadmium
Cadmium exposure is negatively associated with αKlotho protein expression in rat hippocampus
and in a cell line
derived from the adrenal gland [95].
Calcitonin gene-related peptide
In endothelial progenitor cells, calcitonin gene-related peptide (CGRP) upregulates αKlotho
gene and protein
expression and reverses angiotensin II-induced senescence [96].
Estradiol
αKlotho protein is enhanced by estradiol E2 in rat hippocampus, an effect related to
cognitive function and
synapse formation [97].
Histone deacetylase inhibition
Inhibition of HDAC elevates αKlotho mRNA levels in femurs of mice [54].
Matrix stiffness
Matrix stiffness, a typical feature of aging, is implicated in decreased αKlotho expression
in chondrocytes, and
abolishment of stiffness enhances αKlotho expression in vivo [98].
Rapamycin
In addition to the kidney, αKlotho protein is also upregulated by rapamycin in adipose
tissue, lung, muscle,
brain and heart [75].
Rapamycin also increases αKlotho mRNA and protein levels in some cell lines derived from the
aorta or in the
aorta of mice or rats and thus counteracts vascular calcification [99].
Resveratrol
Treatment with resveratrol elevates αKlotho gene and protein abundance in mouse brain
dose-dependently [67].
Triiodothyronine
Triiodothyronine (T3) increases mRNA levels of the membrane form of αKlotho in preadipocytes
during
differentiation [100].
Regulation of sKL and secreted αKlotho
Regulators of sKL or secreted αKlotho expression are reviewed below and listed in alphabetical order (summarized in Table 3).
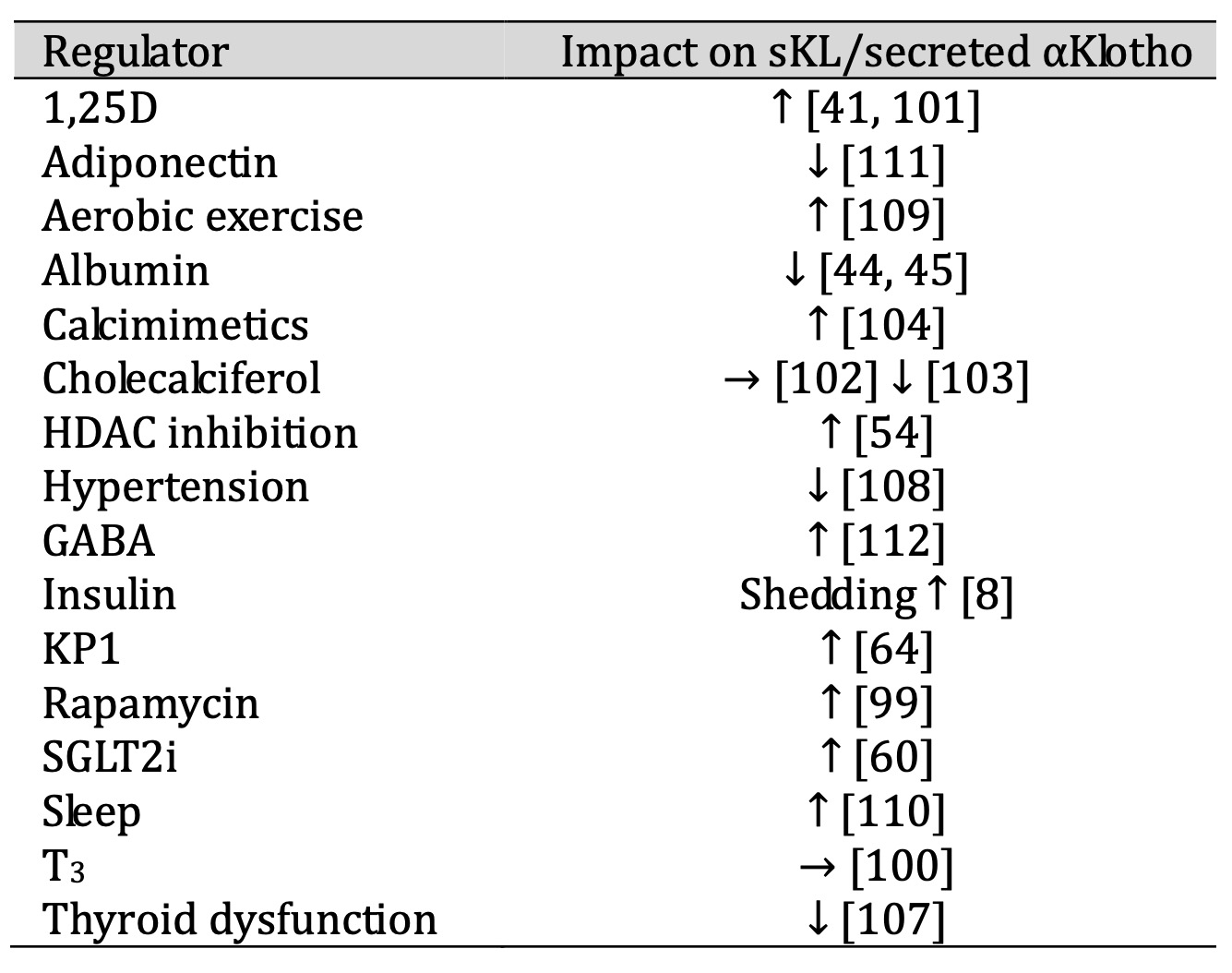
Table 3: Regulators of sKL and secreted αKlotho
1, 25-dihydroxyvitamin D3
In a cell line of distal tubular origin or of the collecting duct, 1,25-dihydroxyvitamin D3
(1, 25D) enhances
mRNA levels of secreted αKlotho identified with a primer pair that specifically amplifies
the secreted αKlotho
splice form [41].
An increase in serum and urinary αKlotho also occurs in mice with CKD on a high phosphate
diet treated with
vitamin D receptor agonists [101]. In contrast, cholecalciferol does not significantly
change sKL [102] or even
reduces it [103] in patients on dialysis.
Albumin
Albumin reduces secreted αKlotho mRNA expression in vivo [44]. Furthermore, αKlotho protein
levels are reduced
in the urine of patients with renal dysfunction as a consequence of severe albuminuria [45].
Calcimimetics
The calcium-sensing receptor CaSR activates ADAM10 in the kidney, thereby being involved in
Klotho shedding
[104]. SKL is elevated upon treatment with calcimimetics or alkali in vitro and in vivo
[104], an effect
dependent on CaSR, ADAM10, and tetraspanin 5 [104, 105].
Histone deacetylase inhibition
Inhibition of HDAC elevates αKlotho protein levels in mouse serum [54].
Hormones
According to a human study, the sKL serum concentration is positively correlated with total
and free
triiodothyronine (T3) [106]. T3 increases αKlotho but not sKL gene expression in a
preadipocyte cell line during
differentiation [100]. In patients with hyper- or hypothyroidism sKL protein is reduced
[107].
Hypertension
Elevated blood pressure lowers serum sKL levels [108].
KP1
KP1 increases sKL protein levels in mice with fibrotic kidney [64].
Lifestyle
Aerobic exercise is associated with elevated sKL plasma levels in a human study [109] as is
adequate sleep
[110].
Metabolic factors
Insulin elevates sKL by enhancing ADAM10- and ADAM17-mediated shedding of transmembrane
Klotho [8]. SKL and
secreted αKlotho are downregulated by adiponectin in vivo and in vitro [111].
In mice treated with streptozotocin that induces type 1 diabetes, gamma-aminobutyric acid
(GABA) enhances
sKL[112].
Rapamycin
In rats with CKD, rapamycin elevates serum sKL levels [99].
SGLT2 inhibitors
In patients treated with SGLT2 inhibitors for type 2 diabetes, sKL in serum and urine is
upregulated [60].
Administration of exogenous Klotho as therapeutic agent
The administration of exogenous Klotho protein may be a promising approach in the treatment of different diseases. Exogenous Klotho may be comparable to sKL and may thus provide resistance of cells to oxidative stress via inhibition of the insulin/PI3K/Akt signaling pathway and FoxO-mediated upregulation of anti-oxidative enzymes [113, 114]. Moreover, sKL not only ameliorates renal fibrosis and CKD [115, 116], but also acts as a tumor suppressor in various types of cancer [117–119]. Further health-promoting effects of exogenous Klotho administration are part of current research and already reviewed elsewhere [120].
Conclusion
αKlotho in both of its form (membrane-bound or soluble) is an important regulator of health and disease. Due to its anti-aging effects, αKlotho has gained attention as a putative therapeutic target. It not only preserves kidney function, but also positively affects the heart, blood vessels or cognitive functions and improves outcomes in cancer or diabetes. As summarized in this article, regulation of αKlotho is complex and dependent on several factors. For sure, more research is needed to better understand the physiological and pathophysiological roles of membrane-bound αKlotho and sKL.
Acknowledgements
Fig. 1 was partly generated using Servier Medical Art (https://smart.servier.com/), licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). No AI was applied.
Author Contributions
Julia Vogt and Michael Föller wrote the paper.
Funding Sources
The author’s research into regulation of αKlotho was supported by Deutsche
Forschungsgemeinschaft.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
Michael Föller received speaker fees from Kyowa Kirin without relationship to this article.
References
| 1 | Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y,
Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S,
Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature 1997;390:45-51.
https://doi.org/10.1038/36285 |
| 2 | Lichtenauer M, Altwein A-K, Kopp K, Salmhofer H: Uncoupling fate: Klotho-Goddess
of fate and regulator of life and ageing. Australasian journal on ageing
2020;39:161-3.
https://doi.org/10.1111/ajag.12772 |
| 3 | Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP,
Chikuda H, Yamaguchi M, Ka-waguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR,
Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho.
Science (New York, N.Y.) 2005;309:1829-33.
https://doi.org/10.1126/science.1112766 |
| 4 | Hu MC, Shiizaki K, Kuro-o M, Moe OW: Fibroblast growth factor 23 and Klotho:
physiology and pathophysiology of an endocrine network of mineral metabolism.
Annual review of physiology 2013;75:503-33.
https://doi.org/10.1146/annurev-physiol-030212-183727 |
| 5 | Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y:
Identification of the human klotho gene and its two transcripts encoding
membrane and secreted klotho protein. Biochemical and biophysical research
communications 1998;242:626-30.
https://doi.org/10.1006/bbrc.1997.8019 |
| 6 | Prud'homme GJ, Kurt M, Wang Q: Pathobiology of the Klotho Antiaging Protein and
Therapeutic Considerations. Frontiers in aging 2022;3:931331.
https://doi.org/10.3389/fragi.2022.931331 |
| 7 | Tohyama O, Imura A, Iwano A, Freund J-N, Henrissat B, Fujimori T, Nabeshima Y:
Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid
beta-glucuronides. The Journal of biological chemistry 2004;279:9777-84.
https://doi.org/10.1074/jbc.M312392200 |
| 8 | Chen C-D, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the
cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17.
Proceedings of the National Academy of Sciences of the United States of America
2007;104:19796-801.
https://doi.org/10.1073/pnas.0709805104 |
| 9 | van Loon EPM, Pulskens WP, van der Hagen EAE, Lavrijsen M, Vervloet MG, van Goor
H, Bindels RJM, Hoenderop JGJ: Shedding of klotho by ADAMs in the kidney.
American journal of physiology. Renal physiology 2015;309:F359-68.
https://doi.org/10.1152/ajprenal.00240.2014 |
| 10 | Wang Y, Sun Z: Current understanding of klotho. Ageing research reviews
2009;8:43-51.
https://doi.org/10.1016/j.arr.2008.10.002 |
| 11 | Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R,
Kuro-o M, Nabeshima Y: Structure of the mouse klotho gene and its two
transcripts encoding membrane and secreted protein. FEBS letters 1998;424:6-10.
https://doi.org/10.1016/S0014-5793(98)00127-6 |
| 12 | Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai
M, Aizawa H, Matsumura Y, Kuro-o M, Nabeshima YI, Nagail R: Molecular cloning of
rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory
stress. Biochemical and biophysical research communications 1998;251:920-5.
https://doi.org/10.1006/bbrc.1998.9576 |
| 13 | Xu Y, Sun Z: Molecular basis of Klotho: from gene to function in aging.
Endocrine reviews 2015;36:174-93.
https://doi.org/10.1210/er.2013-1079 |
| 14 | Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I,
Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer
ameliorates angiotensin II-induced renal damage. Hypertension (Dallas, Tex.
1979) 2002;39:838-43.
https://doi.org/10.1161/01.HYP.0000013734.33441.EA |
| 15 | Kuro-o M: Klotho as a regulator of fibroblast growth factor signaling and
phosphate/calcium metabolism. Cur-rent opinion in nephrology and hypertension
2006;15:437-41.
https://doi.org/10.1097/01.mnh.0000232885.81142.83 |
| 16 | Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T,
Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific
receptor for FGF23. Nature 2006;444:770-4.
https://doi.org/10.1038/nature05315 |
| 17 | Autosomal dominant hypophosphataemic rickets is associated with mutations in
FGF23. Nature genetics 2000;26:345-8.
https://doi.org/10.1038/81664 |
| 18 | Liu S, Guo R, Simpson LG, Xiao Z-S, Burnham CE, Quarles LD: Regulation of
fibroblastic growth factor 23 ex-pression but not degradation by PHEX. The
Journal of biological chemistry 2003;278:37419-26.
https://doi.org/10.1074/jbc.M304544200 |
| 19 | White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ:
Autosomal-dominant hypophospha-temic rickets (ADHR) mutations stabilize FGF-23.
Kidney international 2001;60:2079-86.
https://doi.org/10.1046/j.1523-1755.2001.00064.x |
| 20 | Ornitz DM, Itoh N: Fibroblast growth factors. Genome biology 2001;2:REVIEWS3005.
https://doi.org/10.1186/gb-2001-2-3-reviews3005 |
| 21 | Ornitz DM, Itoh N: The Fibroblast Growth Factor signaling pathway. Wiley
interdisciplinary reviews. Develop-mental biology 2015;4:215-66.
https://doi.org/10.1002/wdev.176 |
| 22 | Martin A, David V, Quarles LD: Regulation and function of the FGF23/klotho
endocrine pathways. Physiological reviews 2012;92:131-55.
https://doi.org/10.1152/physrev.00002.2011 |
| 23 | Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM: Receptor
specificity of the fibroblast growth factor family. The complete mammalian FGF
family. The Journal of biological chemistry 2006;281:15694-700.
https://doi.org/10.1074/jbc.M601252200 |
| 24 | Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG,
Schiavi S, Hu M-C, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23
signaling by klotho. The Journal of biological chemistry 2006;281:6120-3.
https://doi.org/10.1074/jbc.C500457200 |
| 25 | Erben RG, Andrukhova O: FGF23-Klotho signaling axis in the kidney. Bone
2017;100:62-8.
https://doi.org/10.1016/j.bone.2016.09.010 |
| 26 | Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T,
Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D
metabolism and phosphate homeostasis. Journal of bone and mineral research the
official journal of the American Society for Bone and Mineral Research
2004;19:429-35.
https://doi.org/10.1359/JBMR.0301264 |
| 27 | Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM,
Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu C, Lash J,
Leonard M, Kusek JW, Feldman HI, Wolf M: Fibroblast growth factor 23 and risks
of mortality and end-stage renal disease in patients with chronic kidney
disease. JAMA 2011;305:2432-9.
https://doi.org/10.1001/jama.2011.826 |
| 28 | Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto
S, Ikebe M, Yuasa K, Yamana-ka S, Sugiura T, Terada Y: Serum levels of soluble
secreted α-Klotho are decreased in the early stages of chronic kidney disease,
making it a probable novel biomarker for early diagnosis. Clinical and
experimental nephrology 2012;16:722-9.
https://doi.org/10.1007/s10157-012-0621-7 |
| 29 | Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, Gutiérrez OM,
Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M,
Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John
Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand
M, Hill JA, Moe OW, Kuro-o M, Kusek JW, Keane MG, Wolf M: FGF23 induces left
ventricular hypertrophy. The Journal of clinical investigation
2011;121:4393-408.
https://doi.org/10.1172/JCI46122 |
| 30 | Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA,
Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer
AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leif-heit-Nestler M,
Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M,
Faul C: Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left
Ventricular Hypertrophy. Cell metabolism 2015;22:1020-32.
https://doi.org/10.1016/j.cmet.2015.09.002 |
| 31 | Dalton GD, Xie J, An S-W, Huang C-L: New Insights into the Mechanism of Action
of Soluble Klotho. Frontiers in endocrinology 2017;8:323.
https://doi.org/10.3389/fendo.2017.00323 |
| 32 | Erben RG: Update on FGF23 and Klotho signaling. Molecular and cellular
endocrinology 2016;432:56-65.
https://doi.org/10.1016/j.mce.2016.05.008 |
| 33 | Xie J, Cha S-K, An S-W, Kuro-o M, Birnbaumer L, Huang C-L: Cardioprotection by
Klotho through downregulation of TRPC6 channels in the mouse heart. Nature
communications 2012;3:1238.
https://doi.org/10.1038/ncomms2240 |
| 34 | Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho
deficiency causes vascular calcification in chronic kidney disease. Journal of
the American Society of Nephrology JASN 2011;22:124-36.
https://doi.org/10.1681/ASN.2009121311 |
| 35 | Liu Q, Yu L, Yin X, Ye J, Li S: Correlation Between Soluble Klotho and Vascular
Calcification in Chronic Kidney Disease: A Meta-Analysis and Systematic Review.
Frontiers in physiology 2021;12:711904.
https://doi.org/10.3389/fphys.2021.711904 |
| 36 | Hu M-C, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW: Klotho deficiency is an
early biomarker of renal ische-mia-reperfusion injury and its replacement is
protective. Kidney international 2010;78:1240-51.
https://doi.org/10.1038/ki.2010.328 |
| 37 | Tang X, Wang Y, Fan Z, Ji G, Wang M, Lin J, Huang S, Meltzer SJ: Klotho: a tumor
suppressor and modulator of the Wnt/β-catenin pathway in human hepatocellular
carcinoma. Laboratory investigation; a journal of technical me-thods and
pathology 2016;96:197-205.
https://doi.org/10.1038/labinvest.2015.86 |
| 38 | Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R,
Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M: Klotho inhibits
transforming growth factor-beta1 (TGF-beta1) signaling and suppres-ses renal
fibrosis and cancer metastasis in mice. The Journal of biological chemistry
2011;286:8655-65.
https://doi.org/10.1074/jbc.M110.174037 |
| 39 | Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury
by derepression of Wnt/β-catenin signaling. Journal of the American Society of
Nephrology JASN 2013;24:771-85.
https://doi.org/10.1681/ASN.2012080865 |
| 40 | Dalton G, An S-W, Al-Juboori SI, Nischan N, Yoon J, Dobrinskikh E, Hilgemann DW,
Xie J, Luby-Phelps K, Kohler JJ, Birnbaumer L, Huang C-L: Soluble klotho binds
monosialoganglioside to regulate membrane microdomains and growth factor
signaling. Proceedings of the National Academy of Sciences of the United States
of America 2017;114:752-7.
https://doi.org/10.1073/pnas.1620301114 |
| 41 | Forster RE, Jurutka PW, Hsieh J-C, Haussler CA, Lowmiller CL, Kaneko I, Haussler
MR, Kerr Whitfield G: Vitamin D receptor controls expression of the anti-aging
klotho gene in mouse and human renal cells. Biochemical and biophysical research
communications 2011;414:557-62.
https://doi.org/10.1016/j.bbrc.2011.09.117 |
| 42 | Haussler MR, Haussler CA, Whitfield GK, Hsieh J-C, Thompson PD, Barthel TK,
Bartik L, Egan JB, Wu Y, Kubicek JL, Lowmiller CL, Moffet EW, Forster RE,
Jurutka PW: The nuclear vitamin D receptor controls the expression of genes
encoding factors which feed the "Fountain of Youth" to mediate healthful aging.
The Journal of steroid bio-chemistry and molecular biology 2010;121:88-97.
https://doi.org/10.1016/j.jsbmb.2010.03.019 |
| 43 | Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y: Klotho, a gene
related to a syndrome resembling human premature aging, functions in a negative
regulatory circuit of vitamin D endocrine system. Molecular en-docrinology
(Baltimore, Md.) 2003;17:2393-403.
https://doi.org/10.1210/me.2003-0048 |
| 44 | Delitsikou V, Jarad G, Rajaram RD, Ino F, Rutkowski JM, Chen C-D, Santos CXC,
Scherer PE, Abraham CR, Shah AM, Feraille E, Miner JH, Seigneux S de: Klotho
regulation by albuminuria is dependent on ATF3 and endoplasmic reticulum stress.
FASEB journal official publication of the Federation of American Societies for
Experimental Biology 2020;34:2087-104.
https://doi.org/10.1096/fj.201900893R |
| 45 | Fernandez-Fernandez B, Izquierdo MC, Valiño-Rivas L, Nastou D, Sanz AB, Ortiz A,
Sanchez-Niño MD: Albumin downregulates Klotho in tubular cells. Nephrology,
dialysis, transplantation official publication of the European Dialysis and
Transplant Association - European Renal Association 2018;33:1712-22.
https://doi.org/10.1093/ndt/gfx376 |
| 46 | Hardie DG, Hawley SA, Scott JW: AMP-activated protein kinase--development of the
energy sensor concept. The Journal of physiology 2006;574:7-15.
https://doi.org/10.1113/jphysiol.2006.108944 |
| 47 | Vogt J, Wolf L, Hoelzle LE, Feger M, Föller M: AMP-dependent kinase stimulates
the expression of αKlotho. FEBS open bio 2024;14:1691-700.
https://doi.org/10.1002/2211-5463.13872 |
| 48 | Lee J, Tsogbadrakh B, Yang S, Ryu H, Kang E, Kang M, Kang HG, Ahn C, Oh K-H:
Klotho ameliorates diabetic ne-phropathy via LKB1-AMPK-PGC1α-mediated renal
mitochondrial protection. Biochemical and biophysical rese-arch communications
2021;534:1040-6.
https://doi.org/10.1016/j.bbrc.2020.10.040 |
| 49 | Münz S, Wolf L, Hoelzle LE, Chernyakov D, Edemir B, Föller M: Impact of
cytotoxic agents or apoptosis stimulants on αklotho in MDCK, NRK-52E and HK2
kidney cells. Aging 2022;14:7282-99.
https://doi.org/10.18632/aging.204238 |
| 50 | Protective Effect of Pharmacological SIRT2 Inhibition on Renal Dysfunction,
Fibrosis, TGF-β1/β-Catenin, and Klotho Signaling in D-Galactose-Induced Aging
Model. J Biol Regul Homeost Agents 2023;37.
https://doi.org/10.23812/j.biol.regul.homeost.agents.20233711.580 |
| 51 | Choi BH, Kim CG, Lim Y, Lee YH, Shin SY: Transcriptional activation of the human
Klotho gene by epidermal growth factor in HEK293 cells; role of Egr-1. Gene
2010;450:121-7.
https://doi.org/10.1016/j.gene.2009.11.004 |
| 52 | Sugiura H, Yoshida T, Mitobe M, Shiohira S, Nitta K, Tsuchiya K: Recombinant
human erythropoietin mitigates reductions in renal klotho expression. American
journal of nephrology 2010;32:137-44.
https://doi.org/10.1159/000315864 |
| 53 | Kale A, Sankrityayan H, Anders H-J, Gaikwad AB: Epigenetic and non-epigenetic
regulation of Klotho in kidney disease. Life sciences 2021;264:118644.
https://doi.org/10.1016/j.lfs.2020.118644 |
| 54 | Lin W, Li Y, Chen F, Yin S, Liu Z, Cao W: Klotho preservation via histone
deacetylase inhibition attenuates chronic kidney disease-associated bone injury
in mice. Scientific reports 2017;7:46195.
https://doi.org/10.1038/srep46195 |
| 55 | He R, Liu B, Geng B, Li N, Geng Q: The role of HDAC3 and its inhibitors in
regulation of oxidative stress and chronic diseases. Cell death discovery
2023;9:131.
https://doi.org/10.1038/s41420-023-01399-w |
| 56 | Chen F, Gao Q, Wei A, Chen X, Shi Y, Wang H, Cao W: Histone deacetylase 3
aberration inhibits Klotho transcripti-on and promotes renal fibrosis. Cell
death and differentiation 2021;28:1001-12.
https://doi.org/10.1038/s41418-020-00631-9 |
| 57 | Tsai K-D, Lee W-X, Chen W, Chen B-Y, Chen K-L, Hsiao T-C, Wang S-H, Lee Y-J,
Liang S-Y, Shieh J-C, Lin T-H: Upregu-lation of PRMT6 by LPS suppresses Klotho
expression through interaction with NF-κB in glomerular mesangial cells. Journal
of cellular biochemistry 2018;119:3404-16.
https://doi.org/10.1002/jcb.26511 |
| 58 | Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C,
Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A,
Sanz AB: The inflammatory cytokines TWEAK and TNFα reduce renal klotho
expression through NFκB. Journal of the American Society of Nephrology JASN
2011;22:1315-25.
https://doi.org/10.1681/ASN.2010101073 |
| 59 | Thurston RD, Larmonier CB, Majewski PM, Ramalingam R, Midura-Kiela M, Laubitz D,
Vandewalle A, Besselsen DG, Mühlbauer M, Jobin C, Kiela PR, Ghishan FK: Tumor
necrosis factor and interferon-gamma down-regulate Klotho in mice with colitis.
Gastroenterology 2010;138:1384-94, 1394.e1-2.
https://doi.org/10.1053/j.gastro.2009.12.002 |
| 60 | Mora-Fernández C, Sánchez-Niño MD, Donate-Correa J, Martín-Núñez E,
Pérez-Delgado N, Valiño-Rivas L, Fernández-Fernández B, Ortiz A,
Navarro-González JF: Sodium-glucose co-transporter-2 inhibitors increase Klotho
in patients with diabetic kidney disease: A clinical and experimental study.
Biomedicine & pharma-cotherapy = Biomedecine & pharmacotherapie 2022;154:113677.
https://doi.org/10.1016/j.biopha.2022.113677 |
| 61 | Yamagishi T, Saito Y, Nakamura T, Takeda S, Kanai H, Sumino H, Kuro-o M,
Nabeshima Y, Kurabayashi M, Nagai R: Troglitazone improves endothelial function
and augments renal klotho mRNA expression in Otsuka Long-Evans Tokushima Fatty
(OLETF) rats with multiple atherogenic risk factors. Hypertension research
official journal of the Japanese Society of Hypertension 2001;24:705-9.
https://doi.org/10.1291/hypres.24.705 |
| 62 | Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N: Klotho is a target
gene of PPAR-gamma. Kidney inter-national 2008;74:732-9.
https://doi.org/10.1038/ki.2008.244 |
| 63 | Yuan Q, Ren Q, Li L, Tan H, Lu M, Tian Y, Huang L, Zhao B, Fu H, Hou FF, Zhou L,
Liu Y: A Klotho-derived peptide protects against kidney fibrosis by targeting
TGF-β signaling. Nature communications 2022;13:438.
https://doi.org/10.1038/s41467-022-28096-z |
| 64 | Zhang X, Li L, Tan H, Hong X, Yuan Q, Hou FF, Zhou L, Liu Y: Klotho-derived
peptide 1 inhibits cellular senescence in the fibrotic kidney by restoring
Klotho expression via posttranscriptional regulation. Theranostics
2024;14:420-35.
https://doi.org/10.7150/thno.89105 |
| 65 | Fakhri H, Pathare G, Fajol A, Zhang B, Bock T, Kandolf R, Schleicher E, Biber J,
Föller M, Lang UE, Lang F: Regulati-on of mineral metabolism by lithium.
Pflugers Archiv European journal of physiology 2014;466:467-75.
https://doi.org/10.1007/s00424-013-1340-y |
| 66 | Salah TM, Rabie MA, El Sayed NS: Renoprotective effect of berberine in
cisplatin-induced acute kidney injury: Role of Klotho and the
AMPK/mtor/ULK1/Beclin-1 pathway. Food and chemical toxicology an international
journal published for the British Industrial Biological Research Association
2025;196:115179.
https://doi.org/10.1016/j.fct.2024.115179 |
| 67 | Chu S-H, Yang D, Wang Y-P, Yang R, Qu L, Zeng H-J: Effect of resveratrol on the
repair of kidney and brain injuries and its regulation on klotho gene in
d-galactose-induced aging mice. Bioorganic & medicinal chemistry letters
2021;40:127913.
https://doi.org/10.1016/j.bmcl.2021.127913 |
| 68 | Hsu S-C, Huang S-M, Chen A, Sun C-Y, Lin S-H, Chen J-S, Liu S-T, Hsu Y-J:
Resveratrol increases anti-aging Klotho gene expression via the activating
transcription factor 3/c-Jun complex-mediated signaling pathway. The
interna-tional journal of biochemistry & cell biology 2014;53:361-71.
https://doi.org/10.1016/j.biocel.2014.06.002 |
| 69 | Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, Takeshige K, Kamiya T:
The progression of aging in klotho mutant mice can be modified by dietary
phosphorus and zinc. The Journal of nutrition 2001;131:3182-8.
https://doi.org/10.1093/jn/131.12.3182 |
| 70 | Fukuda-Tatano S, Yamamoto H, Nakahashi O, Yoshikawa R, Hayashi M, Kishimoto M,
Imi Y, Yamanaka-Okumura H, Ohnishi K, Masuda M, Taketani Y: Regulation of
α-Klotho Expression by Dietary Phosphate During Growth Pe-riods. Calcified
tissue international 2019;104:667-78.
https://doi.org/10.1007/s00223-019-00525-0 |
| 71 | Lin W, Wu X, Wen J, Fei Y, Wu J, Li X, Zhang Q, Dong Y, Xu T, Fan Y, Wang N:
Nicotinamide retains Klotho expressi-on and ameliorates rhabdomyolysis-induced
acute kidney injury. Nutrition (Burbank, Los Angeles County, Calif.)
2021;91-92:111376.
https://doi.org/10.1016/j.nut.2021.111376 |
| 72 | Ji N, Luan J, Hu F, Zhao Y, Lv B, Wang W, Xia M, Zhao X, Lao K: Aerobic
exercise-stimulated Klotho upregulation extends life span by attenuating the
excess production of reactive oxygen species in the brain and kidney.
Expe-rimental and therapeutic medicine 2018;16:3511-7.
https://doi.org/10.3892/etm.2018.6597 |
| 73 | Zhao J, Guan Y, Jia Y, Chen Y, Cai Y: Aerobic exercise up-regulates Klotho to
improve renal fibrosis associated with aging and its mechanism. PloS one
2024;19:e0311055.
https://doi.org/10.1371/journal.pone.0311055 |
| 74 | Wolf L, Vogt J, Alber J, Franjic D, Feger M, Föller M: PKC regulates αKlotho
gene expression in MDCK and NRK-52E cells. Pflugers Archiv European journal of
physiology 2024;476:75-86.
https://doi.org/10.1007/s00424-023-02863-3 |
| 75 | Szőke K, Bódi B, Hendrik Z, Czompa A, Gyöngyösi A, Haines DD, Papp Z, Tósaki Á,
Lekli I: Rapamycin treatment increases survival, autophagy biomarkers and
expression of the anti-aging klotho protein in elderly mice. Phar-macology
research & perspectives 2023;11:e01091.
https://doi.org/10.1002/prp2.1091 |
| 76 | Espartero A, Vidal A, Lopez I, Raya AI, Rodriguez M, Aguilera-Tejero E, Pineda
C: Rapamycin downregulates α-klotho in the kidneys of female rats with normal
and reduced renal function. PloS one 2023;18:e0294791.
https://doi.org/10.1371/journal.pone.0294791 |
| 77 | Tang C, Pathare G, Michael D, Fajol A, Eichenmüller M, Lang F: Downregulation of
Klotho expression by dehydra-tion. American journal of physiology. Renal
physiology 2011;301:F745-50.
https://doi.org/10.1152/ajprenal.00037.2011 |
| 78 | Zhou Q, Lin S, Tang R, Veeraragoo P, Peng W, Wu R: Role of Fosinopril and
Valsartan on Klotho Gene Expression Induced by Angiotensin II in Rat Renal
Tubular Epithelial Cells. Kidney & blood pressure research 2010;33:186-92.
https://doi.org/10.1159/000316703 |
| 79 | Lai L, Cheng P, Yan M, Gu Y, Xue J: Aldosterone induces renal fibrosis by
promoting HDAC1 expression, deacetyla-ting H3K9 and inhibiting klotho
transcription. Molecular medicine reports 2019;19:1803-8.
https://doi.org/10.3892/mmr.2018.9781 |
| 80 | Alesutan I, Feger M, Pakladok T, Mia S, Ahmed MSE, Voelkl J, Lang F:
25-Hydroxyvitamin D3 1-α-hydroxylase-dependent stimulation of renal klotho
expression by spironolactone. Kidney & blood pressure research 2013;37:475-87.
https://doi.org/10.1159/000355728 |
| 81 | Yoon HE, Ghee JY, Piao S, Song J-H, Han DH, Kim S, Ohashi N, Kobori H, Kuro-o M,
Yang CW: Angiotensin II blo-ckade upregulates the expression of Klotho, the
anti-ageing gene, in an experimental model of chronic cyclospo-rine nephropathy.
Nephrology, dialysis, transplantation official publication of the European
Dialysis and Trans-plant Association - European Renal Association
2011;26:800-13.
https://doi.org/10.1093/ndt/gfq537 |
| 82 | Weber MA: Fosinopril: a new generation of angiotensin-converting enzyme
inhibitors. Journal of cardiovascular pharmacology 1992;20 Suppl 10:S7-12.
https://doi.org/10.1097/00005344-199200101-00003 |
| 83 | Tang R, Zhou Q-L, Ao X, Peng W-S, Veeraragoo P, Tang T-F: Fosinopril and
losartan regulate klotho gene and nico-tinamide adenine dinucleotide phosphate
oxidase expression in kidneys of spontaneously hypertensive rats. Kidney & blood
pressure research 2011;34:350-7.
https://doi.org/10.1159/000326806 |
| 84 | Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H: Oxidative stress
decreases klotho expression in a mouse kidney cell line. Nephron. Experimental
nephrology 2005;101:e67-74.
https://doi.org/10.1159/000086500 |
| 85 | Zhang D, Li Z, Gao Y, Sun H: MiR-556-3p mediated repression of klotho under
oxidative stress promotes fibrosis of renal tubular epithelial cells. Scientific
reports 2025;15:12182.
https://doi.org/10.1038/s41598-025-85479-0 |
| 86 | Wolf L, Föller M, Feger M: The impact of SGLT2 inhibitors on αKlotho in renal
MDCK and HK-2 cells. Frontiers in endocrinology 2023;14:1069715.
https://doi.org/10.3389/fendo.2023.1069715 |
| 87 | Kuwahara N, Sasaki S, Kobara M, Nakata T, Tatsumi T, Irie H, Narumiya H, Hatta
T, Takeda K, Matsubara H, Hushiki S: HMG-CoA reductase inhibition improves
anti-aging klotho protein expression and arteriosclerosis in rats with chronic
inhibition of nitric oxide synthesis. International journal of cardiology
2008;123:84-90.
https://doi.org/10.1016/j.ijcard.2007.02.029 |
| 88 | Narumiya H, Sasaki S, Kuwahara N, Irie H, Kusaba T, Kameyama H, Tamagaki K,
Hatta T, Takeda K, Matsubara H: HMG-CoA reductase inhibitors up-regulate
anti-aging klotho mRNA via RhoA inactivation in IMCD3 cells. Car-diovascular
research 2004;64:331-6.
https://doi.org/10.1016/j.cardiores.2004.07.011 |
| 89 | Yoon HE, Lim SW, Piao SG, Song J-H, Kim J, Yang CW: Statin upregulates the
expression of klotho, an anti-aging gene, in experimental cyclosporine
nephropathy. Nephron. Experimental nephrology 2012;120:e123-33.
https://doi.org/10.1159/000342117 |
| 90 | Adijiang A, Shimizu H, Higuchi Y, Nishijima F, Niwa T: Indoxyl sulfate reduces
klotho expression and promotes senescence in the kidneys of hypertensive rats.
Journal of renal nutrition the official journal of the Council on Renal
Nutrition of the National Kidney Foundation 2011;21:105-9.
https://doi.org/10.1053/j.jrn.2010.10.020 |
| 91 | Shimizu H, Bolati D, Adijiang A, Adelibieke Y, Muteliefu G, Enomoto A,
Higashiyama Y, Higuchi Y, Nishijima F, Niwa T: Indoxyl sulfate downregulates
renal expression of Klotho through production of ROS and activation of nuclear
factor-ĸB. American journal of nephrology 2011;33:319-24.
https://doi.org/10.1159/000324885 |
| 92 | Adijiang A, Niwa T: An oral sorbent, AST-120, increases Klotho expression and
inhibits cell senescence in the kidney of uremic rats. American journal of
nephrology 2010;31:160-4.
https://doi.org/10.1159/000264634 |
| 93 | Feger M, Mia S, Pakladok T, Nicolay JP, Alesutan I, Schneider SW, Voelkl J, Lang
F: Down-regulation of renal klotho expression by Shiga toxin 2. Kidney & blood
pressure research 2014;39:441-9.
https://doi.org/10.1159/000368457 |
| 94 | Li Y, Liu Y, Wang K, Huang Y, Han W, Xiong J, Yang K, Liu M, Xiao T, Liu C, He
T, Bi X, Zhang J, Zhang B, Zhao J: Klotho is regulated by transcription factor
Sp1 in renal tubular epithelial cells. BMC molecular and cell biology
2020;21:45.
https://doi.org/10.1186/s12860-020-00292-z |
| 95 | Liu S, Yu D, Wei P, Cai J, Xu M, He H, Tang X, Nong C, Wei Y, Xu X, Mo X, Zhang
Z, Qin J: JAK2/STAT3 Signaling Pa-thway and Klotho Gene in Cadmium-induced
Neurotoxicity In vitro and In vivo. Biological trace element rese-arch
2023;201:2854-63.
https://doi.org/10.1007/s12011-022-03370-9 |
| 96 | Zhou Z, Hu C-P, Wang C-J, Li T-T, Peng J, Li Y-J: Calcitonin gene-related
peptide inhibits angiotensin II-induced endothelial progenitor cells senescence
through up-regulation of klotho expression. Atherosclerosis 2010;213:92-101.
https://doi.org/10.1016/j.atherosclerosis.2010.08.050 |
| 97 | Tan Z, Li Y, Guan Y, Iqbal J, Wang C, Yan R, Ma X-M: Klotho Regulated by
Estrogen Plays a Key Role in Sex Diffe-rences in Stress Resilience in Rats.
International journal of molecular sciences 2023;24.
https://doi.org/10.3390/ijms24021206 |
| 98 | Iijima H, Gilmer G, Wang K, Bean AC, He Y, Lin H, Tang W-Y, Lamont D, Tai C, Ito
A, Jones JJ, Evans C, Ambrosio F: Age-related matrix stiffening epigenetically
regulates α-Klotho expression and compromises chondrocyte integ-rity. Nature
communications 2023;14:18.
https://doi.org/10.1038/s41467-022-35359-2 |
| 99 | Zhao Y, Zhao M-M, Cai Y, Zheng M-F, Sun W-L, Zhang S-Y, Kong W, Gu J, Wang X, Xu
M-J: Mammalian target of ra-pamycin signaling inhibition ameliorates vascular
calcification via Klotho upregulation. Kidney international 2015;88:711-21.
https://doi.org/10.1038/ki.2015.160 |
| 100 | Mizuno I, Takahashi Y, Okimura Y, Kaji H, Chihara K: Upregulation of the klotho
gene expression by thyroid hor-mone and during adipose differentiation in 3T3-L1
adipocytes. Life sciences 2001;68:2917-23.
https://doi.org/10.1016/S0024-3205(01)01092-X |
| 101 | Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM: Vitamin D
receptor agonists increase klotho and osteopontin while decreasing aortic
calcification in mice with chronic kidney disease fed a high phosphate diet.
Kidney international 2012;82:1261-70.
https://doi.org/10.1038/ki.2012.322 |
| 102 | Seibert E, Heine GH, Ulrich C, Seiler S, Köhler H, Girndt M: Influence of
cholecalciferol supplementation in hemo-dialysis patients on monocyte subsets: a
randomized, double-blind, placebo-controlled clinical trial. Nephron. Clinical
practice 2013;123:209-19.
https://doi.org/10.1159/000354717 |
| 103 | Hryszko T, Rydzewska-Rosołowska A, Goździkiewicz J, Brzósko S, Koc-Żórawska E,
Zelazowska-Rutkowska B, Myśliwiec M: Cholecalciferol supplementation reduces
soluble Klotho concentration in hemodialysis patients. Polskie Archiwum Medycyny
Wewnetrznej 2013;123:277-81.
https://doi.org/10.20452/pamw.1768 |
| 104 | Yoon J, Liu Z, Lee E, Liu L, Ferre S, Pastor J, Zhang J, Moe OW, Chang AN,
Miller RT: Physiologic Regulation of Systemic Klotho Levels by Renal CaSR
Signaling in Response to CaSR Ligands and pH o. Journal of the American Society
of Nephrology JASN 2021;32:3051-65.
https://doi.org/10.1681/ASN.2021020276 |
| 105 | Liu Z, Yoon J, Lee E, Chang AN, Miller RT: Calcium-sensing receptor- and
ADAM10-mediated klotho shedding is regulated by tetraspanin 5. FEBS letters
2025;599:866-75.
https://doi.org/10.1002/1873-3468.15078 |
| 106 | Dong J, Liu M, Xiang G, Yue L, Xu X, Xiang L: The association between serum
soluble α-Klotho and thyroid profile among adults from NHANES 2007-2012. BMC
endocrine disorders 2024;24:161.
https://doi.org/10.1186/s12902-024-01687-1 |
| 107 | Abdullah NAA-H, Hassan EA: Serum Klotho protein level in patients with thyroid
dysfunction. Irish journal of medical science 2025.
https://doi.org/10.1007/s11845-025-03937-0 |
| 108 | Awasthi R, Manger PT, Khare RK, Alam R: Klotho protein: a new insight into the
pathogenesis of essential hyper-tension. Clinical hypertension 2024;30:36.
https://doi.org/10.1186/s40885-024-00294-5 |
| 109 | Amaro-Gahete FJ, De-la-O A, Jurado-Fasoli L, Espuch-Oliver A, Haro T de,
Gutierrez A, Ruiz JR, Castillo MJ: Exer-cise training increases the S-Klotho
plasma levels in sedentary middle-aged adults: A randomised controlled trial.
The FIT-AGEING study. Journal of sports sciences 2019;37:2175-83.
https://doi.org/10.1080/02640414.2019.1626048 |
| 110 | Mochón-Benguigui S, Carneiro-Barrera A, Castillo MJ, Amaro-Gahete FJ: Is Sleep
Associated with the S-Klotho Anti-Aging Protein in Sedentary Middle-Aged Adults?
The FIT-AGEING Study. Antioxidants (Basel, Switzerland) 2020;9.
https://doi.org/10.3390/antiox9080738 |
| 111 | Rutkowski JM, Pastor J, Sun K, Park SK, Bobulescu IA, Chen CT, Moe OW, Scherer
PE: Adiponectin alters renal calcium and phosphate excretion through regulation
of klotho expression. Kidney international 2017;91:324-37.
https://doi.org/10.1016/j.kint.2016.09.016 |
| 112 | Prud'homme GJ, Glinka Y, Kurt M, Liu W, Wang Q: The anti-aging protein Klotho is
induced by GABA therapy and exerts protective and stimulatory effects on
pancreatic beta cells. Biochemical and biophysical research commu-nications
2017;493:1542-7.
https://doi.org/10.1016/j.bbrc.2017.10.029 |
| 113 | Olejnik A, Radajewska A, Krzywonos-Zawadzka A, Bil-Lula I: Klotho inhibits
IGF1R/PI3K/AKT signalling pa-thway and protects the heart from oxidative stress
during ischemia/reperfusion injury. Scientific reports 2023;13:20312.
https://doi.org/10.1038/s41598-023-47686-5 |
| 114 | Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa
Y, Castrillon DH, Rosenblatt KP, Kuro-o M: Regulation of oxidative stress by the
anti-aging hormone klotho. The Journal of biological che-mistry
2005;280:38029-34.
https://doi.org/10.1074/jbc.M509039200 |
| 115 | Neyra JA, Hu MC: Potential application of klotho in human chronic kidney
disease. Bone 2017;100:41-9.
https://doi.org/10.1016/j.bone.2017.01.017 |
| 116 | Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu
MC: αKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy.
Journal of the American Society of Nephrology JASN 2016;27:2331-45.
https://doi.org/10.1681/ASN.2015060613 |
| 117 | Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, Kaufman B,
Wolf I: KL1 internal repeat medi-ates klotho tumor suppressor activities and
inhibits bFGF and IGF-I signaling in pancreatic cancer. Clinical cancer research
an official journal of the American Association for Cancer Research
2011;17:4254-66.
https://doi.org/10.1158/1078-0432.CCR-10-2749 |
| 118 | Shu G, Xie B, Ren F, Liu D, Zhou J, Li Q, Chen J, Yuan L, Zhou J: Restoration of
klotho expression induces apopto-sis and autophagy in hepatocellular carcinoma
cells. Cellular oncology (Dordrecht, Netherlands) 2013;36:121-9.
https://doi.org/10.1007/s13402-012-0118-0 |
| 119 | Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M,
Karlan B, Kaufman B, Koeffler HP, Rubinek T: Klotho: a tumor suppressor and a
modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene
2008;27:7094-105.
https://doi.org/10.1038/onc.2008.292 |
| 120 | Hajare AD, Dagar N, Gaikwad AB: Klotho antiaging protein: molecular mechanisms
and therapeutic potential in diseases. Molecular biomedicine 2025;6:19.
https://doi.org/10.1186/s43556-025-00253-y |