Original Article - DOI:10.33594/000000838
Accepted 19 December 2025 - Published online
31 December 2025
The Effect of 6-Gingerol on Human AML Cell Lines
Keywords
Abstract
Background/Aims:
Acute myeloid leukemia (AML) is a devastating hematological malignancy without a definitive cure. 6-Gingerol, a bioactive compound, has shown promise in treating various cancers, yet its impact on AML remains elusive.Methods:
To elucidate the potential of 6-gingerol in AML, we conducted comprehensive experiments. Cell growth and clonogenic capacity were assessed using CCK-8 testing and colony formation assays. Flow cytometry was employed to analyze cell cycle progression and apoptosis. The invasive capability of AML cells was evaluated through the Transwell migration assay. Fluorescent probe staining was used to determine intracellular reactive oxygen species (ROS) concentration, while Western Blot was utilized to assess the expression levels of key proteins including Bcl-2, caspase3, MAPK, and p-MAPK in AML cells. Potential targets of 6-gingerol in AML were identified through various bioinformatics databases such as STP, SEA, STICH, OMIM GeneMap, and GeneCards. Enrichment analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was performed using the cluster Profiler package (v4.16.0).Results:
Our findings indicated that 6-gingerol effectively inhibits proliferation, colony formation, and invasive capacity of AML cells, arresting them at the G1 phase of the cell cycle. Furthermore, 6-gingerol enhanced the expression levels of ROS, caspase 3, MAPK, and p-MAPK in AML cells. 67 overlapping targets between 6-gingerol and AML were identified, which are enriched within the MAPK signaling pathway and ROS-related pathways. Notably, NFKB1 emerged as a pivotal hub gene through which 6-gingerol exerts its influence on AML.Conclusion:
6-Gingerol could act as a promising agent sourced from Traditional Chinese Medicine (TCM) for AML treatment.Introduction
Acute myeloid leukemia (AML), a malignancy arising from hematopoietic stem or progenitor cells within the bone marrow [1], presents a challenging clinical scenario with a median survival of approximately one year post-diagnosis [2]. Globally, AML incidence stands at 1.5 per 100, 000 individuals, exhibiting a higher rate of 2.4 per 100, 000 in Western nations [3]. The worldwide mortality rate for AML is 1.3 per 100, 000, escalating to approximately 2.2 per 100, 000 in Western Europe and North America [3]. In accordance with the 2022 classification by the European Leukemia Net, complete remission rates vary among risk groups: 73% for favorable-risk, 66% for intermediate-risk, and 45% for poor-risk AML patients. Correspondingly, five-year progression-free survival (PFS) rates are approximately 52%, 32%, and 16%, while overall survival (OS) outcomes reach 55%, 34%, and 15%, respectively [4]. The standard induction therapy for AML employs the "3+7" regimen, a combination of cytarabine and anthracyclines. The US Food and Drug Administration has approved several targeted therapies for AML in recent years, including FMS-like tyrosine kinase 3 inhibitors [5], isocitrate dehydrogenase inhibitors [6, 7], and B-cell lymphoma 2 (BCL2) inhibitors [8]. Furthermore, drugs such as TP53 are under clinical investigation, demonstrating potential to enhance complete remission rates, recurrence-free survival, and OS in select AML patients [9]. Nevertheless, the prognosis remains bleak for older patients (≥60 years) and those with relapsed/refractory AML, with five-year OS rates of merely 4-18% and 10%, respectively [10]. Therefore, there is an urgent need to develop novel treatment strategies to address this unmet medical need in AML management.
6-Gingerol, an active constituent derived from ginger (a plant renowned for its dual roles in both medicine and cuisine), exhibits a broad spectrum of biological activities, including anticancer, anti-inflammatory, anti-apoptotic, antioxidant, and anti-obesity effects [11-14]. Specifically, 6-gingerol has been shown to inhibit the progression of cervical cancer by generating reactive oxygen species (ROS), which not only damages DNA but also suppresses cell proliferation and promotes cell death [15]. Nevertheless, the efficacy of 6-gingerol in treating AML cells remains largely uncharted territory.
In this study, we delved into the impact of 6-gingerol on AML cell lines, examining its effects on proliferation, colony formation, cell cycle progression, apoptosis induction, invasive capacity, as well as the modulation of key biomarkers including ROS, caspase 3, MAPK, and p-MAPK. This investigation aims to provide novel insights into the potential therapeutic benefits of 6-gingerol in AML management.
Materials and Methods
Cells and cell culture
We obtained human AML cells (HL-60 and SKM-1) from Procell. We cultured HL-60 cells using IMDM (Procell)
supplemented with 20% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S). SKM-1 cells were
grown in
RPMI 1640 (Procell) containing 10% FBS and 1% P/S.
Cell proliferation assay
HL-60 and SKM-1 cells were exposed to various concentrations of 6-gingerol for 0, 24, 48, and 72 hours,
respectively. Cell proliferation was assessed using the CCK-8 (BKMAM) assay, following the manufacturer's
instructions. A microplate reader (Shanpu) measured the absorbance at 450 nm, enabling the calculation of
cell
viability.
Colony formation assay
To prepare the lower gel layer, 1.2% agar was combined with 2× medium. This mixture was added to 6-well
plates
and allowed to solidify at 37°C for 30 minutes. Once the lower gel layer had solidified, cell suspensions
were
mixed with a 0.7% agar-medium mixture to create the upper gel layer. The plates were then incubated until
visible colonies formed (2-4 weeks). Subsequently, the colonies were stained with nitroblue tetrazolium
(NBT)
staining solution at 37°C for 1-4 hours, photographed, and counted.
Cell cycle analysis
Cells were either treated with 200 μM 6-gingerol (Med Chem Express) or left untreated for 72 hours.
Following
this treatment, the cells were collected and preserved in 75% ethanol. DNA staining was performed using
the Cell
Cycle Detection Kit (Solarbio). The proportions of each phase in the cell cycle were determined through
flow
cytometric analysis and processed using NovoExpress software.
Cell apoptosis assay
Cells were harvested and stained with Annexin V-phycoerythrin/7-aminoactinomycin D from the Annexin
V-phycoerythrin/Apoptosis Detection Kit (Solarbio) according to the manufacturer’s instructions. Apoptosis
cells
were analyzed using flow cytometry (Agilent NovoCyte) and processed with NovoExpress software (Agilent
NovoCyte).
Transwell migration assay
After gel formation and a 72-hour incubation period, cell suspension was introduced into transwell
inserts. A
24-well plate was then filled with 600 µL of 4% paraformaldehyde, and the inserts were submerged within
this
solution for 20-30 minutes to secure cell integrity. Subsequently, cells were stained with 0.1% crystal
violet,
air-dried, and observed to enumerate the number of migrated cells.
ROS detection
Cells were collected post-treatment with 6-gingerol and suspended in serum-free medium. The ROS Detection
Kit
(Solarbio) was employed for ROS assessment. A DCFH-DA solution was added to the cell suspension, and cells
were
subsequently washed. The intracellular fluorescence, an indicator of ROS levels, was visualized using a
fluorescence microscope.
Western blot analysis
Cells were lysed and combined with protein phosphatase inhibitors. The protein concentration of the sample
was
determined using the bicinchoninic acid Protein Quantification Kit (CWBIO). Following lysis, proteins were
subjected to SDS-PAGE and transferred onto PVDF membranes via electroblotting. The membranes were then
blocked
with 5% non-fat milk at room temperature for one hour. Subsequently, they were incubated overnight at 4°C
with
gentle agitation using primary antibodies targeting Bcl-2, caspase-3, MAPK, and p-MAPK (Servicebio). After
thorough washing, the membranes were exposed to a secondary antibody for 1.5 hours. Following another wash
with
1×Tris-buffered saline containing Tween 20 (TBST), an ECL reagent from Sangon Biotech was added for
chemiluminescent detection. ACTIN (TransGen Biotech) served as an internal control in this process.
Identification of 6-gingerol and AML-related potential target
To identify potential therapeutic targets for 6-gingerol in AML, we utilized several bioinformatic
databases and
tools. The potential targets of 6-gingerol were predicted using the SwissTargetPrediction, SEA, and STITCH
databases. AML-related genes were retrieved from the OMIM GeneMap and GeneCards databases using the
keyword
"Acute Myeloid Leukemia."The overlapping targets between 6-gingerol and AML were considered as
candidate therapeutic targets. To evaluate potential target interactions, we utilized the STRING (Search
Tool
for the Retrieval of Interacting Genes/Proteins) database to construct a protein-protein interaction
network.
The analysis results were then imported into Cytoscape software (version 3.10.3) to construct a
"component-target-pathway" network.To identify key genes within this network, we employed the
CytoHubba extension in Cytoscape, utilizing four scoring models: Degree, Edge Percolated Component (EPC),
Maximum Clique Centrality (MCC), and Maximum Neighborhood Component (MNC). Genes that ranked concurrently
by all
four criteria were designated as core hubs.The intersections among these results were illustrated through
an
UpSet diagram. Additionally, GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes)
enrichment
analyses were performed using the clusterProfiler (version 4.16.0) package in R, retaining only pathways
with p
< 0.01 for further interpretation.
Statistical analysis
The data were expressed as mean ± standard deviation (x̄ ± SD). Statistical analysis was conducted using
SPSS
19.0 with one-way ANOVA. P < 0.05 was considered statistically significant. All figures were created
using
GraphPad Prism 9.0.
Results
6-Gingerol inhibited the proliferation and colony formation of AML cells
To evaluate the impact of 6-gingerol on AML cell proliferation, we employed the CCK-8 assay. As shown in
Fig.
1A, treating HL-60 cells with 25 μM 6-gingerol for 0 or 24 hours resulted in a slight increase in cell
viability, followed by a gradual decrease as the 6-gingerol concentration increased. After 48 and 72 hours
of
treatment, HL-60 cell viability decreased gradually. Notably, at a concentration of 200 μM, cell viability
was
reduced to 67.3% and 43.9%, respectively. For SKM-1 cells (Fig. 1B), cell viability initially increased
slowly
and then decreased rapidly within the 6-gingerol concentration range of 0-100 μM. When the concentration
was
increased from 100 μM to 200 μM for 72 hours, SKM-1 cell viability decreased from 83.1% to 52.6%.
Compared to untreated controls, exposure to 6-gingerol significantly reduced the number of colonies.
Specifically, the number of HL-60 colonies decreased from 1110±102.20 to 839±78.58, and SKM-1 colonies
decreased
from 215±45.50 to 90±48.09 (Fig.1C-F). These findings demonstrate that 6-gingerol effectively inhibits the
proliferation and colony-forming capacity of AML cells.
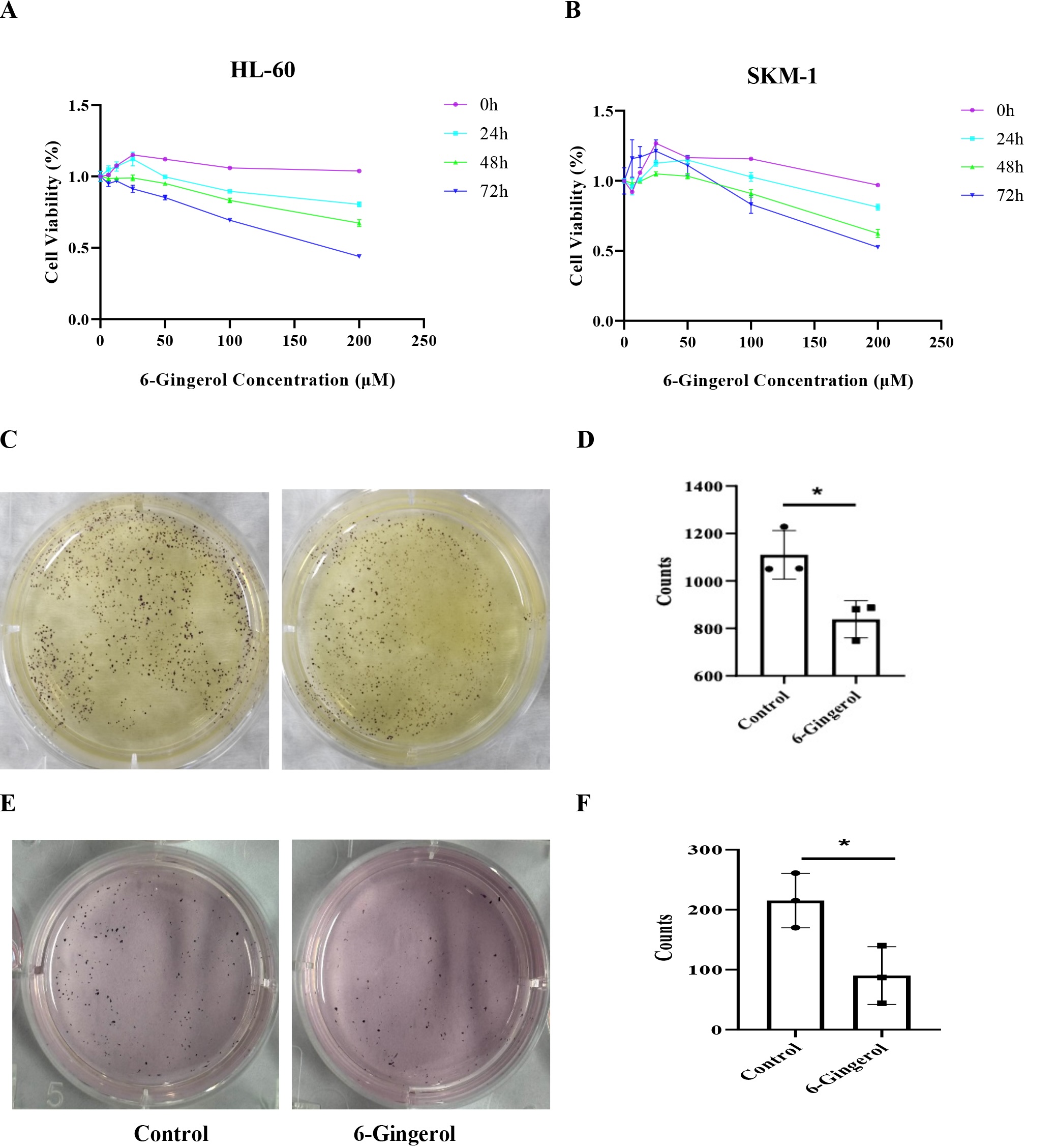
Fig. 1: 6-Gingerol inhibited the proliferation and colony formation of AML cells. 6-gingerol inhibited the proliferation of HL-60(A) and SKM-1 (B); 6-gingerol inhibited the colony formation of HL-60 (C-D) and SKM-1(E-F).∗𝑃 < 0.05.
6-Gingerol arrested G1 phase of HL-60 and SKM-1
To further investigate the impact of 6-gingerol on cell cycle progression in HL-60 and SKM-1 cells, we
employed
flow cytometry to study the cell cycle progression of these two cell lines (Fig. 2). In HL-60 cells,
treatment
with 6-gingerol resulted in a significant increase in the G1 phase population from 33.6% to 65.1%,
accompanied
by a decrease in the S and G2 phases from 30.5% to 17.0% and from 18.5% to 10.6%, respectively.
Similarly,
in
SKM-1 cells, treatment with 6-gingerol caused an accumulation of cells in the G1 phase, with concomitant
reductions in both the S and G2 fractions. These findings suggest that 6-gingerol may induce DNA damage
in
AML
cells, either triggering the apoptotic pathway or blocking cell cycle checkpoints, ultimately leading to
G1
phase arrest.
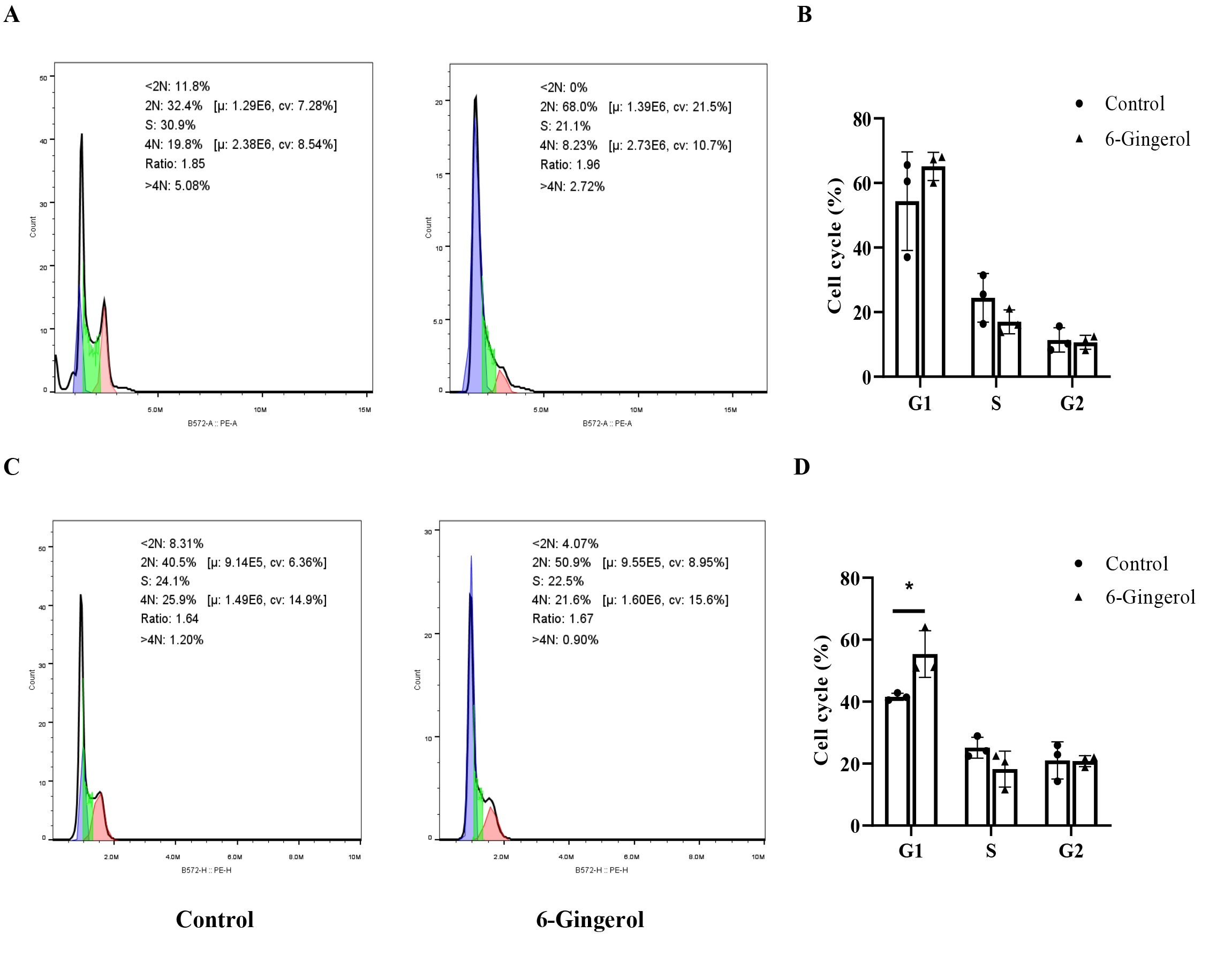
Fig. 2: 6-Gingerol arrested G1 phase of HL-60 and SKM-1. (A-B) HL-60;(C-D) SKM-1.∗P<0.05.
6-Gingerol induced apoptosis of AML cell lines
Flow cytometry analysis revealed a substantial increase in the apoptosis rate of AML cells treated
with
6-gingerol compared to the control group (Fig. 3). Specifically, the apoptosis rate of HL-60 cells
increased
from 1.767±0.18% to 25.563±7.05%, and that of SKM-1 cells increased from 3.75±0.485% to 33.287±10.061%
(P
<0.01). These data unequivocally confirm that 6-gingerol effectively promotes apoptosis in AML cell
lines.
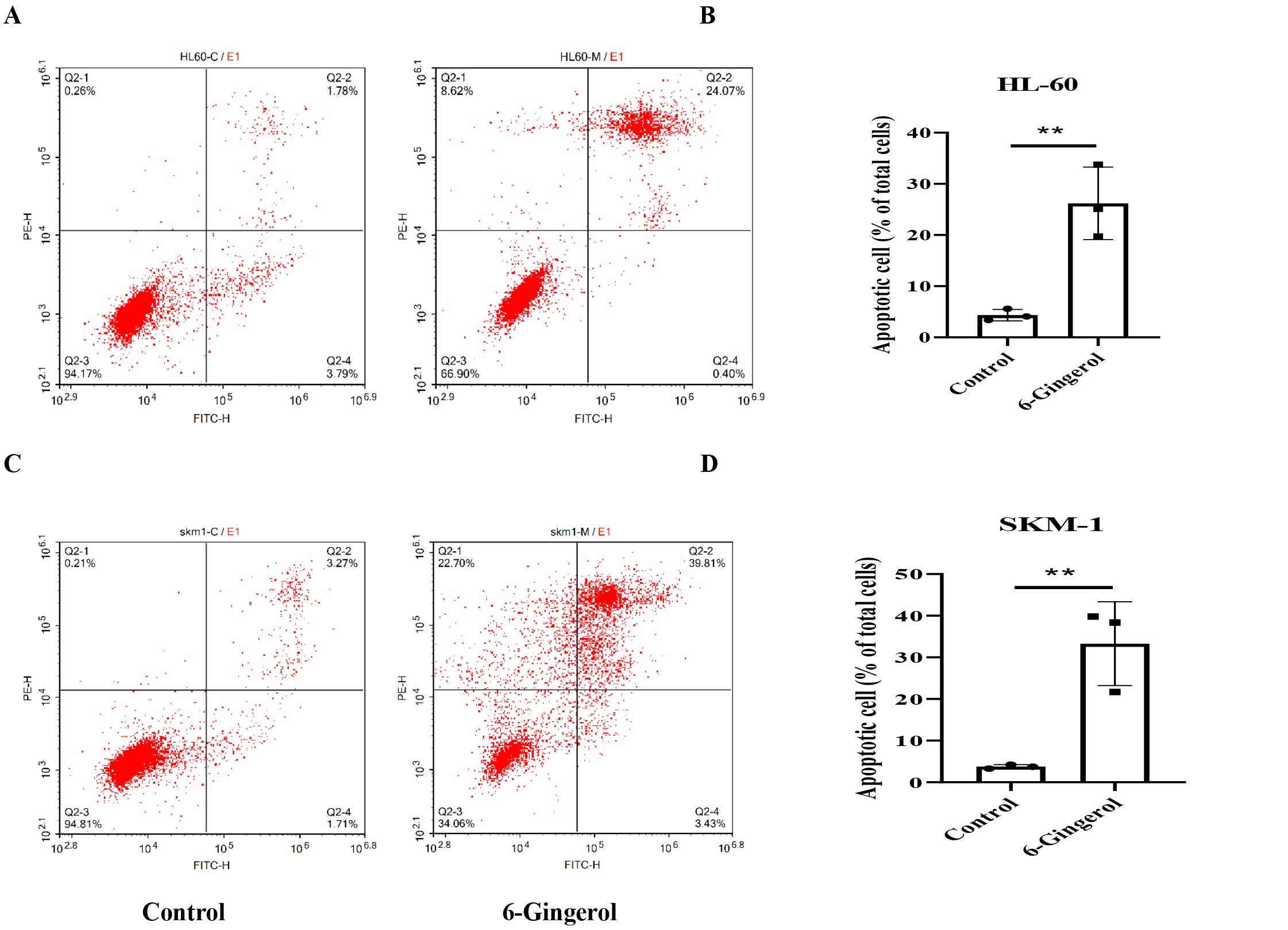
Fig. 3: 6-gingerol induced apoptosis of AML cell lines. (A-B) HL-60;(C-D) SKM-1. ∗∗P<0.01.
6-Gingerol suppressed invasive capacity of AML cells
The invasive potential of HL-60 and SKM-1 cells, subjected to treatment with 6-gingerol, was
evaluated
using a
transwell assay. In comparison to the control group, 6-gingerol significantly reduced the migration
of
HL-60
cells from 385±50.587 to 130±11.533 (P<0.01) and SKM-1 cells from 173.667±48.645 to 62.333±4.041
(P<0.05),
respectively (Fig. 4). These results unequivocally demonstrate that 6-gingerol exerts a profound
inhibitory
effect on the invasive capabilities of both HL-60 and SKM-1 cell lines.

Fig. 4: 6-Gingerol suppressed invasive capacity of AML cells. (A-B) HL-60;(C-D) SKM-1.∗∗P <0.01.
6-Gingerol induced the ROS levels of AML cell lines
The administration of 6-gingerol was observed to elicit a significant elevation in the
intracellular ROS
levels
within AML cell lines, as assessed through immunofluorescence assays. As illustrated in Fig. 5,
the mean
fluorescent intensity (MFI) of HL-60 and SKM-1 cells subjected to 6-gingerol treatment notably
rose from
8.57 ±
3.388 to 21.516 ± 6.197 (P < 0.05) and from 3.588 ± 2.605 to 20.87 ± 5.723, respectively (P
< 0.01).
Notably, the MFI of both cell lines decreased when the ROS inhibitor (NAC, Solarbio) was
introduced,
suggesting
that 6-gingerol is a stimulant of ROS levels in AML cell lines.
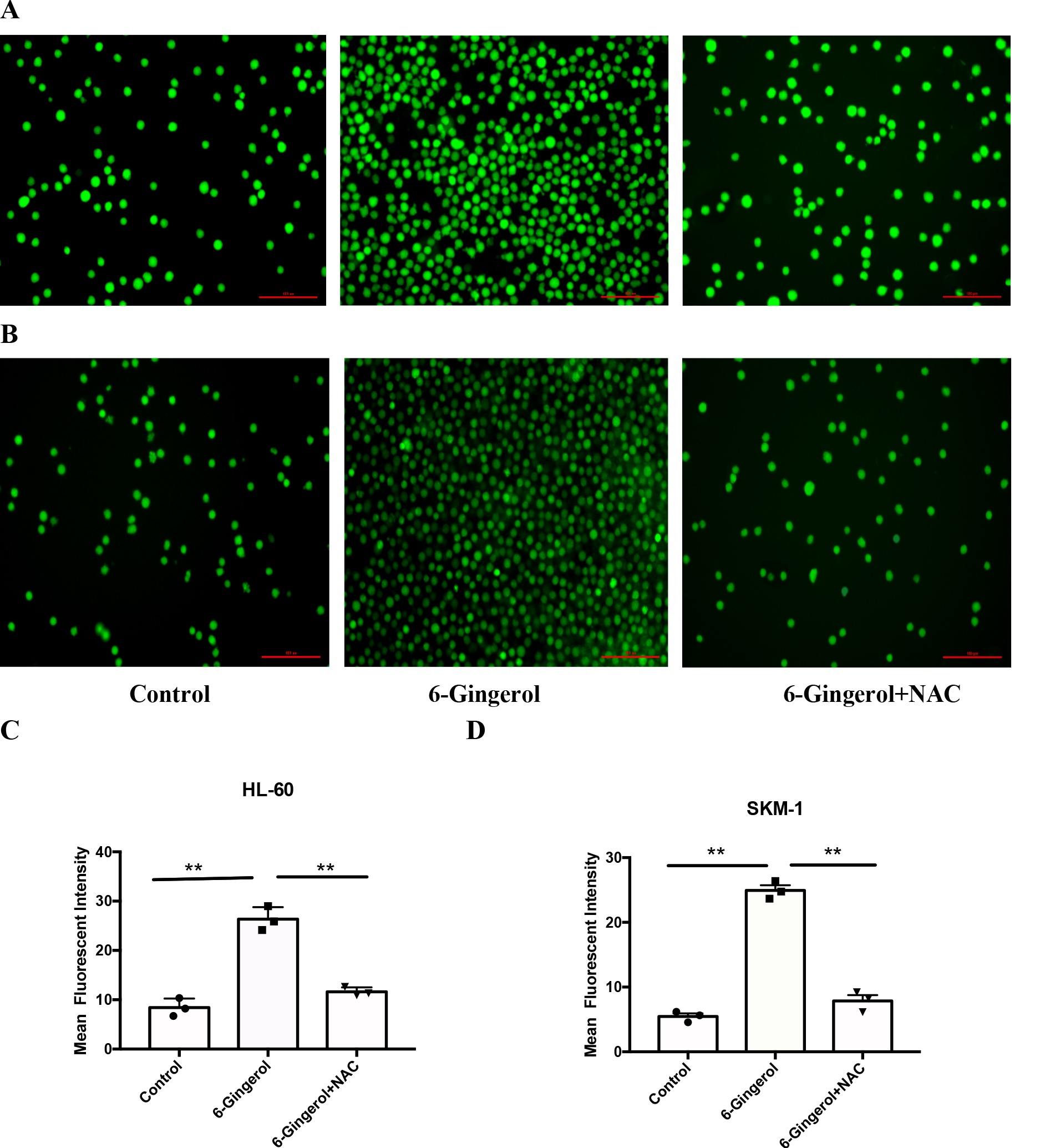
Fig. 5: 6-Gingerol induced the ROS levels of AML cell lines. (A, C) HL-60;(B, D) SKM-1.∗∗𝑃 < 0.01.
6-Gingerol improved the protein expression levels of caspase3, mitogen-activated protein
kinase
(MAPK), p-MAPK of AML cells
To further elucidate the influence of 6-gingerol on AML cells, particularly in relation to
Bcl-2,
caspase3,
MAPK, and p-MAPK, Western blotting was conducted (Fig. 6). In comparison to the control group,
6-gingerol
decreased the level of Bcl-2 in HL-60 cells (from 0.548 ± 0.013 to 0.24 ± 0.048) (P < 0.001)
and SKM-1
cells
(from 0.401 ± 0.058 to 0.122 ± 0.008) (P < 0.01) (Fig. 6). Simultaneously, it augmented the
expression
of
caspase3 (from 0.123 ± 0.054 to 0.615 ± 0.013 in HL-60 cells and from 0.21 ± 0.03 to 0.52 ± 0.17
in SKM-1
cells), MAPK (from 0.138 ± 0.015 to 0.399 ± 0.023 in HL-60 cells and from 0.441 ± 0.157 to 0.921
± 0.295
in
SKM-1 cells), and p-MAPK (from 0.193 ± 0.045 to 0.356 ± 0.068 in HL-60 cells and from 0.259 ±
0.084 to
0.971 ±
0.227 in SKM-1 cells) in both cell lines. These findings collectively indicate that 6-gingerol
promotes
the
protein expression levels of caspase3, MAPK, and p-MAPK in AML cells.
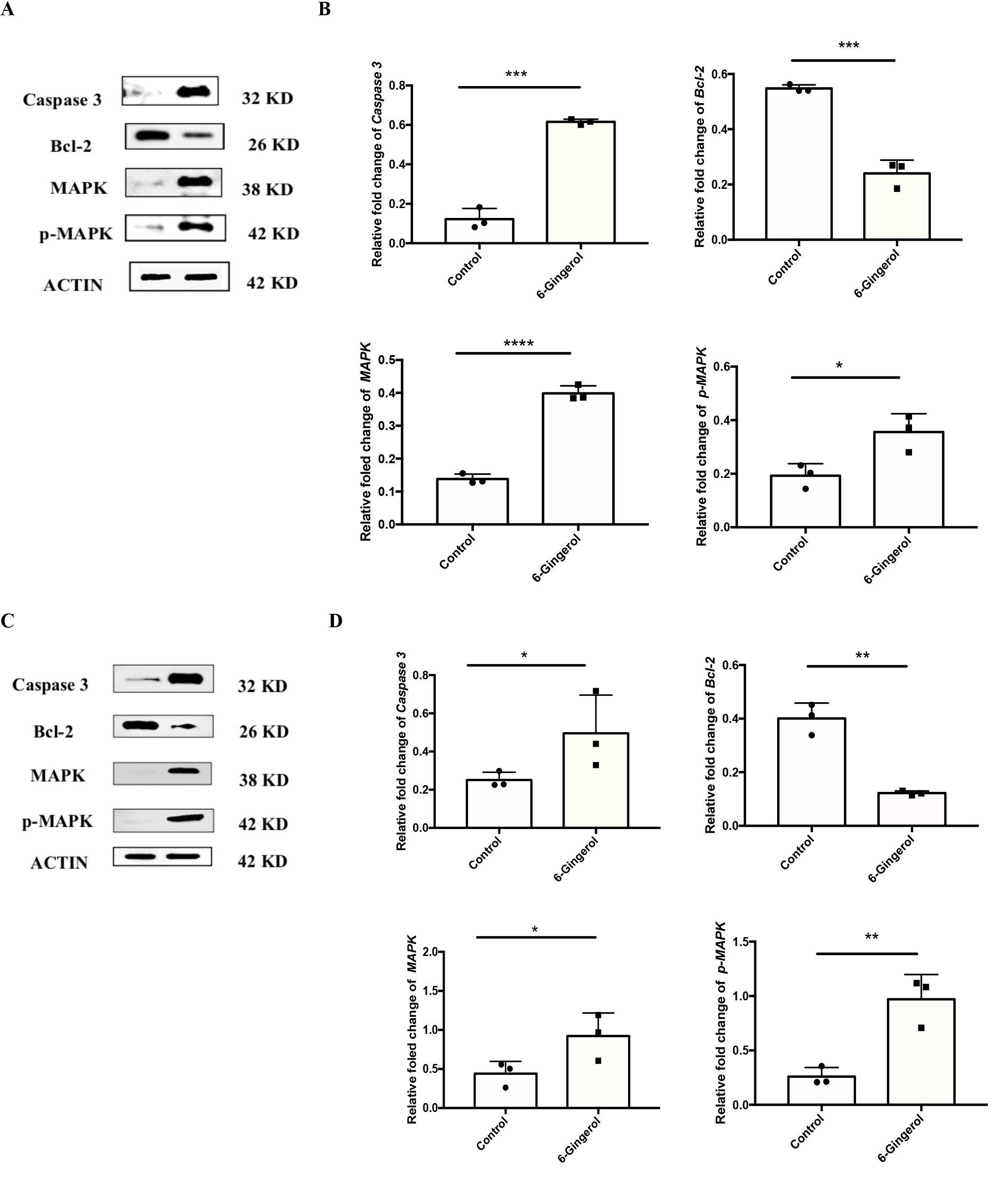
Fig. 6: 6-Gingerol improved the protein expression levels of Caspase3, MAPK, p-MAPK of AML cell lines. (A-B) HL-60;(C-D) SKM-1. ∗𝑃 < 0.05, ∗∗𝑃 < 0.01, ∗∗∗𝑃 < 0.001, ∗∗∗∗𝑃 < 0.0001.
Analysis of 6-gingerol and AML-related potential targets
194 6-gingerol-related potential targets were predicted through the SwissTarget Prediction,
SEA, and STICH
databases while 2930 AML-related targets were retrieved from the GeneCards and OMIM databases.
Notably,
there
was an overlap of 67 targets pertinent to both 6-gingerol and AML (illustrated in Fig. 7A).
These shared
targets
underwent PPI analysis and were visualized using Cytoscape software (as shown in Fig. 7B).
In GO enrichment analysis, the overlapping targets were primarily enriched in the biological
process (BP)
"response to peptide hormone," the cellular component (CC) "extrinsic component
of
membrane," and the molecular function (MF) "histone kinase activity" (depicted
in Fig. 7C).
Furthermore, KEGG pathway enrichment analysis revealed their association with tumor-related
pathways,
including
the PI3K-Akt signaling pathway, the MAPK signaling pathway, and ROS-related pathways
(presented in Fig.
7D).
To identify key genes, the CytoHubba plugin (which employs four algorithms: Degree, EPC, MCC,
and MNC) was
employed to rank the top 10 genes based on their scores (as shown in Fig. 7E). Importantly,
six hub genes
consistently identified by all four algorithms were selected (displayed in Fig. 7F). The
constructed
"component - target - pathway" network (illustrated in Fig. 7G) underscores that
these targets
are
predominantly enriched within the MAPK signaling pathway and ROS-related pathways. Notably,
NFKB1 emerges
as a
pivotal hub gene through which 6-gingerol exerts its influence on AML.
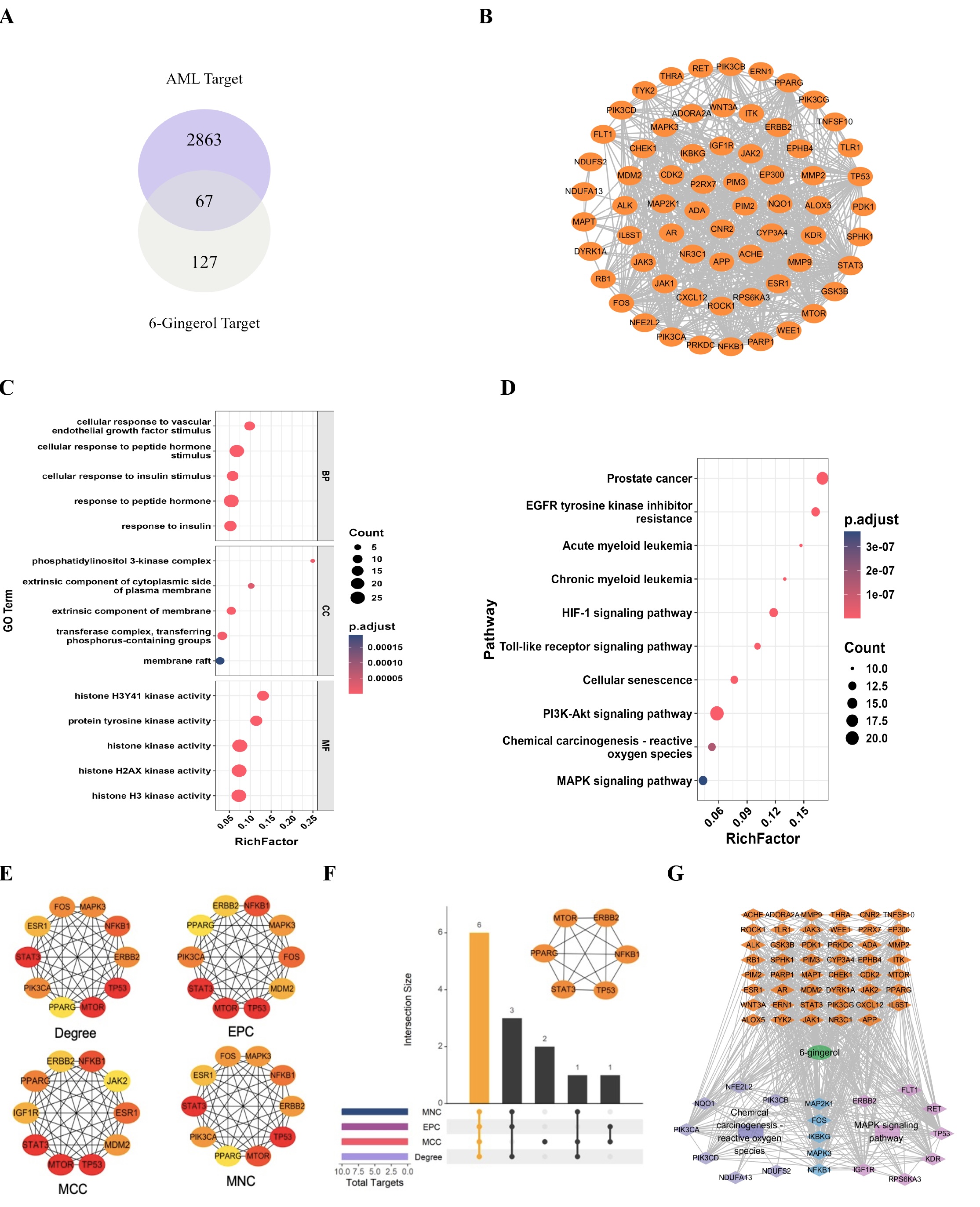
Fig. 7: Analysis of 6-gingerol and AML-related potential targets. (A) overlapping targets of 6-gingerol and AML;(B) Potential target network diagram of 6-gingerol for AML treatment;(C) GO enrichment bubble plot;(D) KEGG enrichment bubble plot;(E) the top 10 genes;(F) 6 hub genes;(G) "Component-target-pathway" network analysis plot.
Discussion
This study was the first to demonstrate that 6-gingerol effectively inhibits the proliferation, colony formation, and invasive capacity of AML cells, culminating in their arrest at the G1 phase of the cell cycle. Furthermore, 6-gingerol enhanced the expression levels of ROS, caspase 3, MAPK, and p-MAPK in AML cells.
AML presents with a dismal prognosis, and disease recurrence following short-term clinical remission remains a significant challenge in its treatment. The persistence of leukemia stem cells (LSCs) is primarily responsible for AML recurrence. 6-gingerol, a commonly used herb in TCM [16], exhibits multiple pharmacological activities including anti-oxidation [17], anti-inflammation [18], anti-obesity, anti-cancer [12, 13], anti-hyperglycemia and immune regulation [14, 19]. Previous evidence indicated that 6-gingerol suppresses tumor cell proliferation in oral cancer by inducing apoptosis and halting progression at the G2/M checkpoint [20]. In contrast, our data revealed that in AML cells, 6-gingerol inhibits proliferation and colony formation, induces G1 phase arrest, and substantially diminishes their invasive capacity.
AML cells primarily rely on oxidative phosphorylation (OXPHOS) as their energy source, with ROS as a by-product. ROS plays a pivotal role in AML pathogenesis and therapeutic targeting [21]. Elevated ROS levels can damage cells and intracellular components, leading to DNA damage, protein denaturation, and tissue injury, ultimately resulting in G2/M phase arrest, apoptosis, senescence, or cell death. ROS are also implicated in mitochondria-, death receptor-, and endoplasmic reticulum-mediated apoptosis [22]. Consistently with these reports, our study demonstrated that 6-gingerol significantly increases ROS levels in AML cell lines. Furthermore, ROS levels decreased when an inhibitor of ROS was added.
It is known that MAPK are crucial for cancer cell survival [23] and involved in proliferation, differentiation, apoptosis, angiogenesis, inflammation, stress responses, and immune defense [24, 25]. Caspase 3 is a cysteinyl aspartate-specific proteinase that serves as an executioner caspase in apoptosis and mediates the anti-cancer effect of cytotoxic drugs [26]. Earlier research study reported that 6-gingerol downregulated anti-apoptotic protein BCL‑2 and survivin, while enhancing Bax expression and activating caspase 3 and caspase 9, thereby inducing apoptosis in bladder cancer via MAPK- and ROS-dependent signaling cascades [27]. Our study indicated that 6-gingerol treatment also results in a reduction in Bcl-2 expression and an increase in the levels of caspase 3, MAPK, and p-MAPK in AML cell lines. When inhibitors of ROS, caspase 3, and MAPK were added, ROS levels, caspase 3 activity, and apoptosis rates decreased, improving cell viability in both cell lines (data not shown). Our findings suggested that the impact of 6-gingerol on AML cell lines may be mediated through caspase 3, ROS, and MAPK. However, the observed changes in protein expression represent molecular observations in the absence of pathway inhibition or knockdown, leaving mechanistic validation as a future direction for study. There are 67 overlapping targets between 6-gingerol and AML, which are enriched within the MAPK signaling pathway and ROS-related pathways. Furthermore, NFKB1 emerges as a central node through which 6-gingerol exerted its influence on AML cells. This represented a hypothesis-generating exercise rather than an experimentally validated target or pathway.
In conclusion, we have successfully demonstrated a previously uncharacterized fact that the effect of 6-gingerol on AML cell lines is apparent. This implies that 6-gingerol could potentially serve as a promising therapeutic agent derived from TCM for the treatment of AML.
Acknowledgements
The authors have no acknowledgements to report.
Author contributions
M.Wu analyzed data, prepared figures and write the manuscript.
T.T. Zhang cultured cells and write manuscript and prepare figures.
C.F. Kong conducted cell proliferation and colony formation assay.
A.N. Li contributed to cell cycle and apoptosis assay.
H.B. Cheng participated in the design and data analysis.
W.R. Ding contributed to ROS assay.
B. Ke conducted Western Blot experiments.
C.Chen Analyzed of 6-gingerol and AML-related potential targets.
Funding Sources
This work was supported by the Science and Technology Research Project of Jiangxi Provincial
Department of
Education (Grant No. GJJ2403641).
Statement of Ethics
Cell lines in this study were purchased from Procell. It does not require ethics committee
approval.
Disclosure Statement
The authors declare no conflicts of interest.
References
| 1 | Negotei C, Colita A, Mitu I et al. A Review of FLT3 Kinase Inhibitors in AML. J Clin
Med 2023; 12.
https://doi.org/10.3390/jcm12206429 |
| 2 | Adamska M, Kowal-Wisniewska E, Przybylowicz-Chalecka A et al. Clinical outcomes of
therapy-related
acute myeloid leukemia: an over 20-year single-center retrospective analysis. Pol Arch
Intern Med
2023; 133.
https://doi.org/10.20452/pamw.16344 |
| 3 | Korbecki J, Kupnicka P, Barczak K et al. The Role of CXCR1, CXCR2, CXCR3, CXCR5, and
CXCR6 Ligands
in Molecular Cancer Processes and Clinical Aspects of Acute Myeloid Leukemia (AML).
Cancers (Basel)
2023; 15.
https://doi.org/10.3390/cancers15184555 |
| 4 | Lo MY, Tsai XC, Lin CC et al. Validation of the prognostic significance of the 2022
European
LeukemiaNet risk stratification system in intensive chemotherapy treated aged 18 to 65
years
patients with de novo acute myeloid leukemia. Am J Hematol 2023; 98: 760-769.
https://doi.org/10.1002/ajh.26892 |
| 5 | Click ZR, Seddon AN, Bae YR et al. New Food and Drug Administration-Approved and
Emerging Novel
Treatment Options for Acute Myeloid Leukemia. Pharmacotherapy 2018; 38: 1143-1154.
https://doi.org/10.1002/phar.2180 |
| 6 | DiNardo CD, Stein EM, de Botton S et al. Durable Remissions with Ivosidenib in
IDH1-Mutated
Relapsed or Refractory AML. N Engl J Med 2018; 378: 2386-2398.
https://doi.org/10.1056/NEJMoa1716984 |
| 7 | Stein EM, DiNardo CD, Pollyea DA et al. Enasidenib in mutant IDH2 relapsed or
refractory acute
myeloid leukemia. Blood 2017; 130: 722-731.
https://doi.org/10.1182/blood-2017-04-779405 |
| 8 | Kantarjian HM, DiNardo CD, Kadia TM et al. Acute myeloid leukemia management and
research in 2025
CA Cancer J Clin 2025; 75: 46-67.
https://doi.org/10.3322/caac.21873 |
| 9 | Kantarjian H, Kadia T, DiNardo C et al. Acute myeloid leukemia: current progress and
future
directions. Blood Cancer J 2021; 11: 41.
https://doi.org/10.1038/s41408-021-00425-3 |
| 10 | Ganzel C, Sun Z, Cripe LD et al. Very poor long-term survival in past and more recent
studies for
relapsed AML patients: The ECOG-ACRIN experience. Am J Hematol 2018; 93: 1074-1081.
https://doi.org/10.1002/ajh.25162 |
| 11 | Wu S, Zhu J, Wu G et al. 6-Gingerol Alleviates Ferroptosis and Inflammation of
Diabetic
Cardiomyopathy via the Nrf2/HO-1 Pathway. Oxid Med Cell Longev 2022; 2022: 3027514.
https://doi.org/10.1155/2022/3027514 |
| 12 | Tsai Y, Xia C, Sun Z. The Inhibitory Effect of 6-Gingerol on Ubiquitin-Specific
Peptidase 14
Enhances Autophagy-Dependent Ferroptosis and Anti-Tumor in vivo and in vitro. Front
Pharmacol 2020;
11: 598555.
https://doi.org/10.3389/fphar.2020.598555 |
| 13 | Bhaskar A, Kumari A, Singh M et al 6.-Gingerol exhibits potent anti-mycobacterial and
immunomodulatory activity against tuberculosis. Int Immunopharmacol 2020; 87: 106809.
https://doi.org/10.1016/j.intimp.2020.106809 |
| 14 | Han JJ, Li X, Ye ZQ et al. Treatment with 6-Gingerol Regulates Dendritic Cell Activity
and
Ameliorates the Severity of Experimental Autoimmune Encephalomyelitis. Mol Nutr Food Res
2019; 63:
e1801356.
https://doi.org/10.1002/mnfr.201801356 |
| 15 | Zivarpour P, Nikkhah E, Maleki Dana P et al. Molecular and biological functions of
gingerol as a
natural effective therapeutic drug for cervical cancer. J Ovarian Res 2021; 14: 43.
https://doi.org/10.1186/s13048-021-00789-x |
| 16 | Li Q, Wang M, Huang X et al. 6-Gingerol, an active compound of ginger, attenuates
NASH-HCC
progression by reprogramming tumor-associated macrophage via the NOX2/Src/MAPK signaling
pathway.
BMC Complement Med Ther 2025; 25: 154.
https://doi.org/10.1186/s12906-025-04890-2 |
| 17 | Alsahli MA, Almatroodi SA, Almatroudi A et al. 6-Gingerol, a Major Ingredient of
Ginger Attenuates
Diethylnitrosamine-Induced Liver Injury in Rats through the Modulation of Oxidative
Stress and
Anti-Inflammatory Activity. Mediators Inflamm 2021; 2021: 6661937.
https://doi.org/10.1155/2021/6661937 |
| 18 | Zahoor A, Yang C, Yang Y et al. 6-Gingerol exerts anti-inflammatory effects and
protective
properties on LTA-induced mastitis. Phytomedicine 2020; 76: 153248.
https://doi.org/10.1016/j.phymed.2020.153248 |
| 19 | Farombi EO, Ajayi BO, Adedara IA. 6-Gingerol delays tumorigenesis in benzo [a]pyrene
and dextran
sulphate sodium-induced colorectal cancer in mice. Food Chem Toxicol 2020; 142: 111483.
https://doi.org/10.1016/j.fct.2020.111483 |
| 20 | Zhang H, Kim E, Yi J et al. 6-Gingerol Suppresses Oral Cancer Cell Growth by Inducing
the
Activation of AMPK and Suppressing the AKT/mTOR Signaling Pathway. In vivo 2021; 35:
3193-3201.
https://doi.org/10.21873/invivo.12614 |
| 21 | Khorashad JS, Rizzo S, Tonks A. Reactive oxygen species and its role in pathogenesis
and
resistance to therapy in acute myeloid leukemia. Cancer Drug Resist 2024; 7: 5.
https://doi.org/10.20517/cdr.2023.125 |
| 22 | Moon DO, Kim MO, Choi YH et al. Butein induces G(2)/M phase arrest and apoptosis in
human hepatoma
cancer cells through ROS generation. Cancer Lett 2010; 288: 204-213.
https://doi.org/10.1016/j.canlet.2009.07.002 |
| 23 | Mitchell I, Bihari D, Chang R et al. Earlier identification of patients at risk from
acetaminophen-induced acute liver failure. Crit Care Med 1998; 26: 279-284.
https://doi.org/10.1097/00003246-199802000-00026 |
| 24 | Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase:
conservation of a
three-kinase module from yeast to human. Physiol Rev 1999; 79: 143-180.
https://doi.org/10.1152/physrev.1999.79.1.143 |
| 25 | Moustardas P, Aberdam D, Lagali N. MAPK Pathways in Ocular Pathophysiology: Potential
Therapeutic
Drugs and Challenges. Cells 2023; 12.
https://doi.org/10.3390/cells12040617 |
| 26 | Dou H, Yu PY, Liu YQ et al. Recent advances in caspase-3, breast cancer, and
traditional Chinese
medicine: a review. J Chemother 2024; 36: 370-388.
https://doi.org/10.1080/1120009X.2023.2278014 |
| 27 | Choi NR, Choi WG, Kwon MJ et al. 6-Gingerol induces Caspase-Dependent Apoptosis in
Bladder Cancer
cells via MAPK and ROS Signaling. Int J Med Sci 2022; 19: 1093-1102.
https://doi.org/10.7150/ijms.73077 |