Corresponding Author: Shahab Shahgaldi
Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, P.O. Box 14115-331 (Iran)
Tel. +9821-82883846, Fax +9821-82884555, E-Mail shahab.shahgaldi@gmail.com; shahab.shahgaldi@modares.ac.ir
Sirtuins: Subtle Regulators Involved in Convoluted Mechanisms of Pregnancy
Fatemeh Rezaei Kahminia Hadis Darvishi Ghalehb Shahab Shahgaldic
aAutoimmune Diseases Research Center, Shiraz University of Medical Sciences, Shiraz, Iran, bSchool of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran, cDepartment of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
Introduction
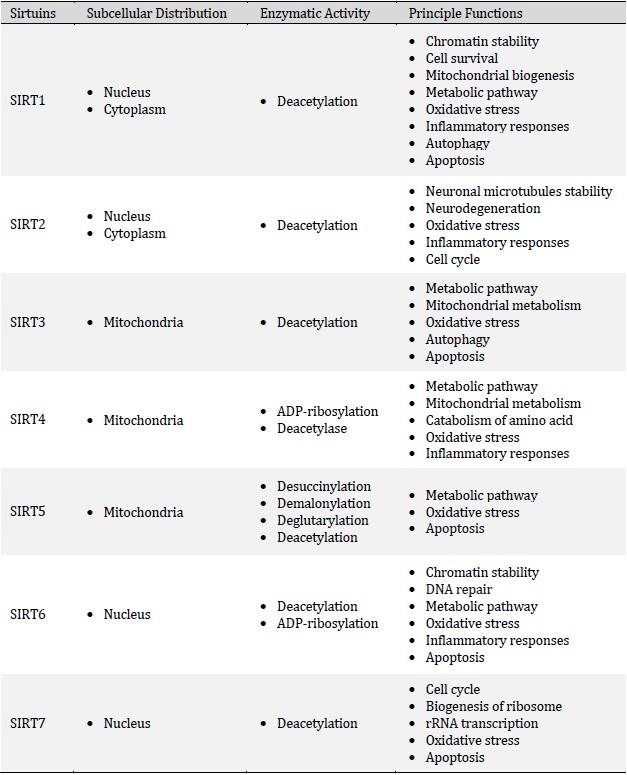
Sirtuins are a conserved family of proteins that are involved in the regulation of several physiological conditions such as metabolism, cell cycle, mitochondrial biogenesis, apoptosis, inflammatory response, stress and aging [1, 2]. Historically, the first member of the Sirtuin family was detected in saccharomyces cerevisiae and later other isoforms have been reported in other species including yeast, bacteria, plants and mammalians, which indicates their conservation throughout evolution [3, 4]. Indeed, it is well recognized that mammals have seven distinct members of the Sirtuin family, namely SIRT1-SIRT7 (Table 1) that possess different C- and N-terminal domains which leads to their different distribution in organelles [5]. In general, Sirtuins have been found in the nucleus (SIRT1, SIRT6, and SIRT7), cytoplasm (SIRT2) and mitochondria (SIRT3, SIRT4, and SIRT5) [6, 7]. However, depending on the physiological conditions, they might translocate to other organelles to exert their functions. For example, SIRT3 is mainly expressed in mitochondria, but under cellular stress, it could localize to the nucleus or SIRT2 might shuttle from the cytoplasm to the nucleus during cell cycle transition [8]. Sirtuins are primarily classified as nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylases/monoADP ribosyltransferases. In fact, Sirtuins sense cellular stress following micro-environmental changes (oxidative stress, inflammation, lack of nutrients, hypoxia, and etc.), leading to the regulation of cellular responses [9]. Of note, several mechanisms are involved in the Sirtuins activation including a higher ratio of NAD+/NADH and phosphorylation kinases proteins such as AMP-activated protein kinase or c-Jun N-terminal kinase 1 [10-12]. In general, the enzymatic activity of Sirtuins ensures cellular homeostasis via modifying a plethora of epigenetic factors and transcription factors implicated in several aspects of gene expression. Sirtuins are prominently involved in the regulation of myriad fundamental biological processes including inflammation, proliferation, cell survival, DNA repair and metabolism that we extensively reviewed earlier [13]. Recent studies also indicate the biological role of Sirtuins in female reproduction. Currently, a rapidly expanding body of evidence supports that Sirtuins can improve the pregnancy outcome by reducing placental inflammation and oxidative stress and enhancing cell survival (anti-apoptotic effects), placental angiogenesis and trophoblast functions (differentiation and invasion) (Fig. 1). In general, male and female SIRT1-null (SIRT1−/−) mice are sterile (or have very low fertility rates) and exhibit neonatal or early postnatal lethality due to multiple severe abnormalities. Indeed, SIRT1-deficient and knockout mice display severe developmental defects and irregularities including defective germ cell differentiation, small placenta, labyrinthine layer and junctional zone abnormalities, exencephaly, abnormal shaped eye and defective cardiac septation. Furthermore, intrauterine growth retardation appears to be a common phenotype among all SIRT1-null offspring [14-19]. Regarding the localization, it has been elucidated that SIRT1 and SIRT2 are greatly expressed in decidual cells, cytotrophoblasts, syncytiotrophoblasts, amniotic epithelial cells and the chorionic trophoblast cell layer. In addition, SIRT2 has also been localized in the placental endothelium [20]. Similarly, positive SIRT6 staining has been detected in the decidua, amnion epithelium and chorionic trophoblasts [21].
Similar to SIRT1 knockout mice, SIRT6-deficient and knockout mice are reported to mainly suffer post-weaning lethality (die at about 4 weeks of age) and develop several profound abnormalities such as hypoglycemia (increased glucose uptake and AKT activation), massive inflammation (enhanced c-JUN and NF-κB transcriptional activity), severe lymphopenia (elevated lymphocyte apoptosis), osteopenia (reduced bone density), colitis (erosion of the superficial colonic epithelium), lordokyphosis (hunchbacked spine), eye abnormalities (retinal transmission defects), developmental retardation (reduced body size and weight), progeroid degenerative syndrome (an aged appearance at birth), premature aging, hepatic fibrosis and visceral and subcutaneous fat loss [22-26]. Furthermore, SIRT6-null cynomolgus monkeys display increased acetylation levels of H3K56 in various tissues including the brain and muscle and die hours after birth due to a pan-tissue developmental delay in utero. Mechanistically, this article illustrates that H3K56 hyperacetylation delays neuronal maturation and differentiation through transcriptionally activating a potent developmental suppressor, the long non-coding RNA H19 [27]. In humans, a homozygous inactivating mutation in the SIRT6 gene leads to perinatal lethality and multiple critical congenital aberrations including intrauterine growth restriction (IUGR), sex reversal in male fetuses, cardiac insufficiencies (valve defects, cardiac enlargement and septal defects) and craniofacial and cephalic anomalies (cerebellar hypoplasia, microcephaly and frontal bossing). In vitro experiments conducted in this research further clarify that SIRT6 deacetylase activity towards H3K9Ac and H3K56Ac is required for direct silencing of the core pluripotent genes (Sox2, Oct4 and Nanog) and consequently SIRT6-deficient stem cells fail to differentiate into embryoid bodies, functional cardiomyocyte foci and neural progenitor cells [28]. With regard to SIRT7, heterozygous mutant SIRT7 mice exhibit no obvious phenotype whereas SIRT7 knockout mice show an accelerated aging phenotype and die prematurely (reduced lifespan) during the postnatal period. The most pivotal abnormalities observed in SIRT7 knockout mice include partial embryonic lethality (perinatal lethality), kyphosis (excessive convex curvature of the spine), stem cell dysfunction (decreased tissue regeneration), fatty liver development (increased hepatic lipogenesis and ER stress and decreased VLDL secretion), increased heart and liver inflammation, progeroid-like phenotype, reduced plasma IGF‐1 levels, subcutaneous fat loss, cardiac hypertrophy, reduced body weight, leukopenia in lymphoid organs, increased mutant frequency and decreased stress-resistance mechanisms [29-31]. In particular, SIRT7 is demonstrated to boost stress resistance of neonatal primary cardiomyocytes by deacetylating p53 (inhibition of apoptosis) and lessen replication stress through promoting NHEJ repair in a PARP1-dependent manner [29, 30]. With respect to the development of hepatosteatosis, SIRT7 is shown to be recruited to the promoters of ribosomal protein genes upon physical interaction with Myc to suppress the expression of these proteins and alleviate ER stress [31].
Contrary to the severe irregularities caused by SIRT1, SIRT6 and SIRT7 deficiency, knockout mice deficient in SIRT2, SIRT3, SIRT4 and SIRT5 are viable and grossly healthy and display no obvious phenotypic defects and abnormalities. Nevertheless, SIRT2 knockout mice display H4K16 hyperacetylation in various tissues and are prone to tumorigenesis, chromosomal aberrations and genomic instability. In fact, it is demonstrated that SIRT2 participates in a mitotic checkpoint mechanism by directly deacetylating H4K16 and the histone methyltransferase PR-Set7 and thus increasing the monomethylation of H4K20 [32]. SIRT3 deficiency is reported not to affect the fertility, adaptive thermogenesis and overall metabolism of SIRT3−/− mice. However, these mice display not only mitochondrial protein hyperacetylation but also diminished ATP levels (inhibition of complex I subunit NDUFA9) [33, 34]. Likewise, SIRT4 knockout mice are shown to be fertile and phenotypically normal but display slight fasting hypoglycemia because of an increase in circulating insulin levels. Indeed, SIRT4 can downregulate amino acid-stimulated insulin secretion in mouse β cells through ADP-ribosylation and inhibition of GDH [35]. Regardless of normal fertility and health, SIRT5 knockout mice are unable to upregulate the enzymatic activity of CPS1 and thus experience increased blood ammonia levels under fasting conditions [33, 36]. Interestingly, even dual deletion of SIRT3 and SIRT5 is reported not to largely alter development, fertility, mitochondrial mass, immune cell development and hematopoiesis [37]. Overall, it seems that identifying the underlying mechanisms of Sirtuins functions on pregnancy modulation is required to develop more efficient diagnostic and therapeutic strategies. In this review, we shall first address Sirtuin regulation and functions including their interactions with the most important signaling pathways involved in pregnancy. Then, we will focus on the pivotal roles of Sirtuins in female reproductive functions, normal pregnancy, parturition and pregnancy complications.
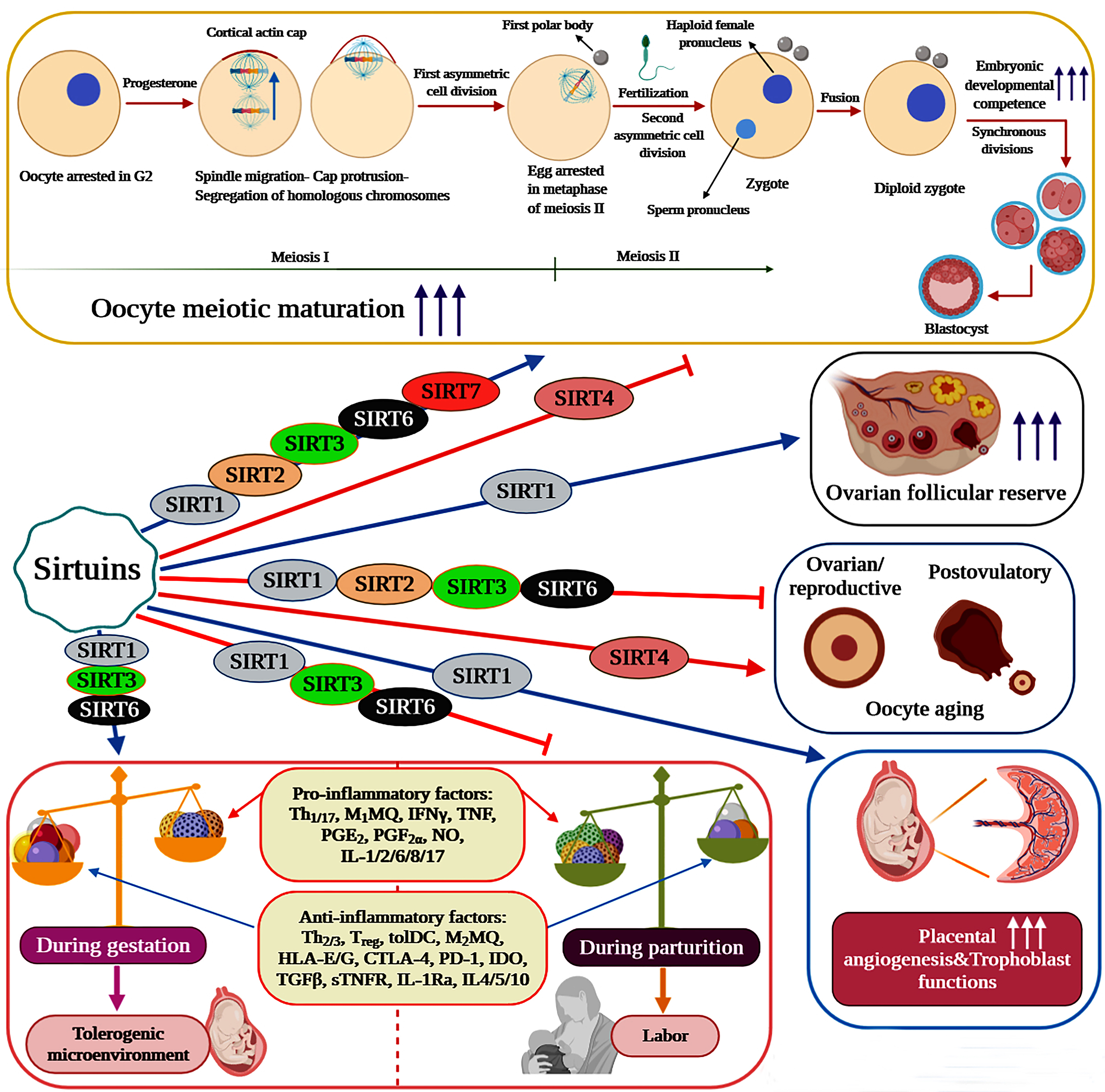
Pivotal roles of Sirtuins in female reproductive functions
Sirtuins have emerged as vital regulators of folliculogenesis, oocyte meiotic maturation and oocyte aging (ovarian/reproductive and postovulatory aging). Indeed, the expression of Sirtuins has been detected in endometrium (SIRT1-7), ovaries (SIRT1, 3, 6), oocytes (SIRT1-7), cumulus cells (SIRT1, 2, 3, 5, 6), embryos (SIRT1-3) and granulosa cells (SIRT1-3) [38, 39]. It is well illustrated that caloric restriction (CR) or specific activation of SIRT1 can suppress the activation of primordial follicles (transition from primordial to developing follicles) and thus preserve the ovarian follicular reserve through increasing the expression levels of SIRT1, SIRT6, FOXO3a and NRF-1 and suppressing mTOR signaling [40, 41]. In oocytes, evidence indicates that Sirtuins are decisively involved in the regulation of meiotic progression and aging. Consequently, each member of this family plays distinct yet fundamental roles in key biological processes controlling oocyte quality, quantity and developmental competence. Employment of the pan-Sirtuin inhibitor nicotinamide is shown to severely disrupt oocyte meiotic maturation and progression in mice and pigs. During porcine oocyte maturation, this effect is reported to be associated with several abnormalities including disrupted oocyte cortical polarity (inhibition of actin cap and cortical granule-free domain (CGFD) formation), decreased polar body extrusion, impaired cumulus cell expansion, abnormal spindle organization and chromosomal misalignment [42]. On the contrary, NAM impairs the establishment of metaphase II arrest and entry into meiosis I in murine oocytes by disrupting Cdk1 regulation (lowering Cdk1 activation) [43].
In oocytes undergoing meiotic maturation, SIRT1 activation is demonstrated to decrease cellular reactive oxygen species (ROS) levels and enhance polar body extrusion, spindle and chromosome organization, mitochondrial quantity, mitochondrial function (mitochondrial membrane potential (ΔΨm) and ATP content), intracellular glutathione (GSH) content and energy homeostasis. Therefore, SIRT1 can positively affect the meiotic maturation of oocytes by improving their quantity, quality and developmental competence. As evident by increased blastocyst rate and embryonic developmental competence, SIRT1 can also improve the oocyte’s ability to develop into a viable embryo and subsequently into a blastocyst [44-48]. With regard to oocyte aging, SIRT1 plays a protective role against both reproductive and postovulatory aging. In murine ovaries, resveratrol treatment counteracts ovarian aging and age-associated fertility decline via increasing SIRT1 expression levels, follicular reserve, telomere length and telomerase activity [46]. As a sensor of oocyte redox state, SIRT1 is reported to activate a FOXO3a-MnSod axis to orchestrate an antioxidant response in mouse oocytes. Nevertheless, oocytes isolated from reproductively old mice display a massive reduction in SIRT1 protein levels under normal conditions and are unable to efficiently upregulate SIRT1 mRNA levels in response to oxidative stress. Consequently, aged oocytes fail to upregulate the FOXO3a-MnSod axis under oxidative stress, which ultimately could result in impaired ROS detoxification [48]. During postovulatory aging, mRNA expression levels of SIRT1, 2 and 3 are all dramatically reduced in murine oocytes. This reduction has been associated with an increase in aging-induced morphological changes, abnormal mitochondrial distribution pattern, spindle defects, ROS accumulation, apoptotic rate and autophagy induction and a decrease in developmental competence, mitochondrial function (ΔΨm), H3K9me3 levels and maturation-promoting factor activity [49-51]. Importantly, culture medium supplementation with melatonin is illustrated to avert the alterations observed in postovulatory aged mouse oocytes by elevating SIRT1 transcription and subsequently activating a SIRT1-MnSOD-dependent pathway [51]. In a similar fashion, resveratrol treatment can significantly improve the defects detected in spindle and chromosome organization and cortical granule and mitochondrial distribution during pig oocyte in vitro aging [52].
During bovine and mouse oocyte maturation, SIRT2 is uniformly located in both the nucleus and cytoplasm of the oocyte. Nevertheless, SIRT2 becomes concentrated on the meiotic spindle of the mouse oocyte upon entrance into metaphase and its expression decreases in the bovine oocyte after the first cleavage. Consistently, SIRT2 knockdown or selective inhibition by SirReal2 is reported to induce spindle disorganization and chromosome misalignment and compromise the microtubule-kinetochore interaction, a crucial mechanism in charge of chromosome segregation, in oocytes undergoing meiotic maturation. Importantly, these defects are accompanied by the hyperacetylation of α-tubulin, involved in spindle morphology, and H4K16, involved in chromosome alignment and kinetochore function [39, 53]. Other abnormalities caused by SIRT2 inhibition in bovine oocytes include disturbed cytoplasmic maturation, mitochondrial dysfunction, and meiotic arrest, decreased oocyte cleavage and elevated ROS accumulation. Mechanistically, SIRT2 inhibition intensifies cellular ROS levels through blocking the FOXO3a–Sod2/Cat axis and regulates mitochondrial biogenesis and function by upregulating DRP1 and simultaneously downregulating TFAM and Mfn2 [39]. With regard to oocyte aging, SIRT2 also plays a protective role against both reproductive and postovulatory aging. In oocytes obtained from reproductively-aged mice, downregulation of SIRT2 protein is found to be responsible for increased aneuploidy rate, spindle/chromosome disorganization and compromised kinetochore-microtubule attachments. In detail, SIRT2 is suggested to mediate the deacetylation of BubR1, α-tubulin and H4K16 to ameliorate the occurrence of maternal age-associated meiotic defects in aged oocytes [53, 54]. In in vitro-matured bovine MII oocytes, post-maturation aging is negatively associated with the mRNA expression levels of SIRT1-6, especially SIRT1, SIRT2 and SIRT5 mRNA levels. Accordingly, exposure of bovine MII oocytes to SirReal2 can significantly increase autophagy-dependent cellular apoptosis, mitochondrial dysfunction, abnormal mitochondrial distribution pattern, ROS accumulation, oocyte activation, cytoplasmic fragmentation and spindle defects during the early stage of in vitro aging [55].
Diabetes and obesity have been linked to reduced SIRT3 expression and elevated ROS levels in murine oocytes. The same studies further elucidate that, during meiotic maturation, SIRT3-dependent deacetylation of SOD2 produces an antioxidant effect in oocytes from obese and diabetic mice, thus improving spindle assembly and chromosome alignment [56-58]. Notably, SIRT3 is proposed to be indispensable for the development of mammalian MII oocytes and preimplantation embryos under stress conditions like in vitro culture. Consistently, in human in vitro-matured MII oocytes, a decrease in SIRT3 mRNA levels has been identified as the major underlying cause of diminished mitochondrial biogenesis and reduced developmental competence [59]. Correspondingly, SIRT3 knockdown is illustrated to provoke developmental arrest in mouse embryos under in vitro culture conditions by promoting ROS-induced p53 activation whereas SIRT3 overexpression, under the same conditions, improves the developmental efficiency of mouse MII oocytes, at least in part, through upregulating mitochondrial biogenesis [59, 60]. SIRT6 has also been proven to positively affect the meiotic progression of murine and porcine oocytes and is further shown to play a protective role against reproductive aging. Indeed, specific depletion of SIRT6 in mouse oocytes is demonstrated to markedly increase spindle/chromosome disorganization, aneuploidy incidence and impaired kinetochore-microtubule attachments. Consistent with altered kinetochore function, SIRT6 is suggested to participate in the deacetylation of histone H4K16 in mouse oocytes undergoing meiotic maturation [61]. During pig oocyte meiotic maturation, SIRT6 is expressed at mRNA and protein levels in both cumulus cells and oocytes. In cumulus-free porcine oocytes, SIRT6 inhibition by OSS_128167 is discovered to dramatically reduce spindle/chromosome organization and the first polar body extrusion rate while, in cumulus-enclosed oocytes, its inhibition results not only in the mentioned defects but also in dwindled developmental competence, cumulus expansion and germinal vesicle breakdown (GVBD) [62]. With respect to its protective role against oocyte aging, SIRT6, whose expression is reduced in aged oocytes, is believed to take part in the quality control of aged murine oocytes and embryos by enhancing the telomere elongation and decreasing the incidence of apoptotic blastomeres [63].
SIRT7-knockdown oocytes are prone to producing aneuploid eggs and display impaired meiotic progression and developmental competence. Specifically, SIRT7 knockdown prominently elevates γH2AX signals, H3K18 acetylation levels (at asynaptic regions), mitochondrial dysfunction and ROS production in mouse oocytes, severely compromising DNA integrity, chromosome synapsis, cortical actin cap formation, first polar body extrusion and spindle/chromosome organization during meiotic maturation [64, 65]. In addition, SIRT7 protein level is reported to be markedly lower in oocytes from obese mice while its ectopic expression can ameliorate maternal obesity–associated meiotic defects and oxidative stress [64]. Intriguingly, at odds with the protective roles of SIRT1, 2, 3, 6, and 7, increased SIRT4 expression is linked to oocyte aging and meiotic defects in mice whereas its depletion not only ameliorates the deficient phenotypes of reproductively-aged oocytes but is also expendable in meiotic maturation. Molecularly, it is illustrated that mouse oocytes overexpressing SIRT4 exhibit spindle/chromosome disorganization, increased ROS accumulation and lowered ATP content due to an upregulation in the phosphorylation of Ser293‐PDHE1α [66].
Anti-inflammatory and angiogenic properties of some Sirtuin isoforms can improve the pregnancy outcome
The anti-inflammatory properties of Sirtuins mostly rise from the ability of SIRT1, 2, and 6 to suppress the activation of the inflammatory transcription factors NF-κB and AP-1. Consistently, the SIRT1 activators resveratrol and SRT1720 are reported to strongly reduce LPS-induced expression and release of proinflammatory cytokines (IL-6, IL-8 and TNF-α) and prostaglandins (PGE2 and PGF2α) in human fetal tissues [20]. Likewise, in a study on pregnant non-human primates, resveratrol treatment is demonstrated to protect the mother and fetus from the adverse effects of maternal high-fat diet (HFD) by lessening placental inflammation and promoting maternal weight reduction, uterine blood flow and glucose tolerance [67]. In primary cytotrophoblasts, resveratrol is found to significantly decrease the secreted levels of IL-6 and TNF-α and the mRNA expression of IL-6, IL-1β and NF-κB [68]. In addition to SIRT1, evidence supports the active involvement of both SIRT3 and SIRT6 in the regulation of feto-maternal inflammation. SIRT6 silencing is determined to enhance the production and release of IL-1β-induced proinflammatory cytokines and mediators including IL-6, IL-8, TNF-α, MMP9, PGE2 and PGF2α in primary amnion cells. Molecularly, this article shows that SIRT6 overexpression can inhibit the transcriptional activity of NF-κB in primary amnion cells [21]. In human primary myometrial cells treated with IL-1β or TNF, SIRT3 knockdown has been associated with augmented NF-κB transcriptional activity and proinflammatory mediator expression and release (IL-6, CXCL8, CCL2, PGF2α, MMP9 and ICAM-1) [69]. Importantly, NF-κB is not considered to be a direct target of SIRT3, yet SIRT3 is reported to suppress inflammation and NF-κB signaling probably via downregulation of ROS levels [70-73]. Thus, the mechanism by which SIRT3 contributes to the resolution of feto-maternal inflammation remains to be investigated.
The angiogenic properties of SIRT1 can also provide the living fetus with a proper environment to develop. Indeed, SIRT1, which is highly expressed in endothelial cells during angiogenesis, promotes sprouting angiogenesis, blood vessel development and vascular remodeling by deacetylating FOXO1, a potent anti-angiogenic transcription factor [74]. Regarding the angiogenic properties of SIRT1 in pregnancy, it is demonstrated that resveratrol can reduce sFlt-1 levels both in vivo, in pregnant mice, and in vitro, in human primary term trophoblasts and placental explants treated with either inflammatory cytokines or hypoxia. Additionally, SIRT1 activation significantly attenuates sFlt-1 release from primary cytotrophoblasts probably by a mechanism involving the activation of PGC-1α [68, 75, 76]. Equally important, AMPK (AMP-activated kinase) activation, which induces SIRT1 activation and expression, has also been associated with the downregulation of sFlt-1 secretion from cytotrophoblast cells and is shown to significantly decrease the expression of IL-6, IL-8 and MMP9 in fetal membranes and primary amnion cells [75, 77].
Sirtuins enhance trophoblast survival and functions during pregnancy
Sirtuins play key roles in the enhancement of trophoblast survival, differentiation and invasion during pregnancy. In primary human trophoblasts exposed to hypoxia, increased SIRT1 expression is reported to be involved in the upregulation of NDRG1 (N-Myc down-regulated gene 1), which in turn promotes trophoblast differentiation and apoptotic resistance. Importantly, this study further substantiates that the observed increase in trophoblast apoptotic resistance could be attributed to NDRG1 ability to diminish p53 expression possibly via SIRT1/p53 signaling [78, 79]. Perfluorooctanesulfonate (PFOS), an organic pollutant used in industrial products, is known to adversely affect the pregnancy outcome by increasing inflammation and oxidative stress. Interestingly, knockdown of miR-29b, a microRNA increased in preeclampsia (PE) patients, can significantly decrease PFOS-induced ROS generation in first trimester human trophoblasts and this effect has been associated with a decrease in global protein hyperacetylation and an increase in global DNA methylation via upregulation of SIRT1/SIRT3 and DNA methyltransferases, respectively [80]. On the contrary, it is illustrated that inhibition of LSD1 in trophoblast stem cells (TSCs) provokes senescence and markedly curtails glutamine anaplerosis required for the maintenance of functional mitochondria and redox balance through direct upregulation of SIRT4 expression and subsequent reduction of GDH1 enzymatic activity [81].
While human villous cytotrophoblast and syncytiotrophoblast cells, analogous to labyrinthine trophoblasts in mice, surround fetal villous vessels to facilitate gas and nutrient exchange, human endovascular and invasive extravillous cytotrophoblast cells, analogous to invasive and junctional zone trophoblasts in mice, provide sufficient blood flow to the feto-placental unit by invading and remodeling the spiral arteries. Therefore, abnormal differentiation and deficient invasion of placental trophoblast cells can give rise to multiple obstetric complications distinguished by inadequate placentation and blood flow [82]. Notably, a recent study has shed new light on the importance of SIRT1 in the regulation of trophoblast functions. In brief, SIRT1-null mouse TSCs display impaired invasive properties and are not able to properly differentiate into labyrinthine and junctional zone trophoblasts. In fact, SIRT1-null TSCs persistently express high levels of cMet and Epcam during differentiation and thus appear to be trapped in an Epcamhigh (labyrinthine) trophoblast progenitor state [19, 83]. Furthermore, according to this recent study, these cells show clear reductions in STAT3, Smad2/3 and PPARγ expression levels, which, to some extent, can explain their blunted differentiation and the significant reduction observed in their invasive ability [19]. Generally, IL-6 and phosphorylated STAT3 are known for their ability to enhance the invasive abilities of trophoblast cells during pregnancy by regulating the release and activity of trophoblast proteases such as MMP-9 and MMP-2 [84]. Provided that Sirtuins are known to negatively impact IL-6 levels, further investigation can divulge the association between Sirtuin levels and trophoblast invasive capabilities with regard to the regulation of IL-6. Equally important, as the differentiation of trophoblast cells into the invasive lineage also firmly depends on the hypoxia inducible factor (HIF) complex, further investigation could illuminate the effect of Sirtuins on this complex during trophoblast differentiation [85, 86].
Correlation between proinflammatory cytokines and Sirtuin levels in the context of pregnancy and abortion
In general, proinflammatory cytokines and factors negatively affect the levels of SIRT1 and SIRT2. However, in the context of pregnancy, these interactions seem to be more complicated. While LPS and the inflammatory cytokines TNF-α and IL-1β negatively regulate the mRNA expression of SIRT1, SIRT2 expression level may be regulated by a mechanism independent of inflammation in the human placenta [20]. Women suffering from recurrent implantation failure (RIF) are reported to have higher levels of SIRT1 in their serum as compared to non-pregnant women and healthy pregnant women; however, it should be mentioned that this report may show poor reproducibility due to the low number of subjects [87]. Importantly, the expression patterns of local and systemic cytokines are markedly changed in patients with RIF. It is described that the plasma levels of IFN-γ, IL-1β, TNF-α, IL-6 and IL-4 are higher whereas TGF-β plasma levels are lower in women suffering from RIF [88, 89]. Conversely, local expressions of IL-6, IL-8 and TGF-β are decreased in the endometrium of patients with RIF compared to normal fertile women [90]. Therefore, further investigation is required to determine the systemic and local correlation between these cytokines and SIRT1 levels in the context of pregnancy and abortion. Similar to the effect on SIRT1 expression, LPS significantly diminishes SIRT6 expression both at the mRNA and protein levels in human fetal membranes [21]. Likewise, the proinflammatory cytokines IL-1β and TNF are reported to reduce SIRT3 mRNA and protein expression levels in human primary myometrial cells [69].
Parturition and maternal obesity are accountable for major alterations in Sirtuin levels
Sirtuins also seem to play major roles in the regulation of parturition and pregnancy complications caused by obesity. Provided that normal pregnancy comprises a sequence of distinct events, namely implantation (inflammatory), gestation (anti-inflammatory) and parturition (inflammatory), any alterations in the nature of these events and responses could provoke dire consequences during pregnancy [91]. Importantly, parturition is an event represented by both systemic and localized intrauterine inflammation, which juxtaposes the anti-inflammatory properties of SIRT1, 3, and 6 during pregnancy [92]. In fact, it can be hypothesized that, once inflammation is induced by the onset of labor, not only the expression levels of SIRT1, 3, and 6 should be reduced in response, but also the constraints imposed by these Sirtuins on feto-maternal inflammation should be diminished. Consistently, it is demonstrated that both SIRT1 and SIRT6, but not SIRT2, levels are significantly downregulated in human gestational tissues and cells by the physiologic onset of labor [20, 21]. Similarly, in human myometrium, the spontaneous onset of labor has been associated with a significant reduction in SIRT3 expression levels while no change has been observed in the expression levels of SIRT4, 5, and 7 [69]. Visfatin/NAMPT (VSF) is a systemic adipocytokine, which is involved in SIRT1 activation via catalyzing the rate-limiting step in the nicotinamide adenine dinucleotide (NAD)+ salvage pathway. Interestingly, it is also shown that, although SIRT1 levels are decreased in placental samples collected from term preeclamptic women compared to term controls, VSF and SIRT1 levels are not significantly affected by labor [93]. The observed discrepancies in this study could partially be due to the small sample size and use of semi-quantitative methods. Contrary to uterine SIRT3, hepatic SIRT3 is found to reach its peak level on the day of parturition and gradually decline to the basal level during the first two weeks after parturition in dairy goats, indicating that parturition may regulate SIRT3 expression by a different mechanism in the liver [94]. Notably, NF-κB can directly bind to a cis-acting element in the SIRT3 promoter to activate its expression in tumor cells and perhaps SIRT3 is regulated by NF-κB itself in the liver [95]. Inevitably, altered levels of Sirtuins may either prolong, as observed in postterm delivery associated with obesity, or expedite, as observed in preterm delivery, the onset of parturition. Prior to term labor, placental syncytiotrophoblast VSF levels are reported to be positively correlated with both BMI and syncytiotrophoblast SIRT1 levels and consequently obese women tend to have higher levels of VSF in their placenta, which are suggested to be culpable for postterm delivery in obese pregnancy. However, and unexpectedly, this report has also divulged that the placental SIRT1 levels are not affected by maternal BMI [92]. In conclusion, further investigation is essential to unravel more details on the relationship between Sirtuin levels and the initiation of labor.
Maternal obesity is considered as a contributing factor to several complications in pregnancy including increased placental oxidative stress, inflammation, lipotoxicity and vasculopathy. Besides, maternal HFD increases the levels of proinflammatory cytokines and factors such as IL-6, IL-8, IL-18, TNF-α and NF-κB in the placenta. In this context, diminished expression of SIRT1 and SIRT3 is suggested to participate in the exacerbation of oxidative stress, inflammation and p53-mediated cell cycle arrest at the feto-maternal unit [96]. Furthermore, SIRT3 is hyperacetylated in aged and obese mice due to reduced SIRT1 activity and this hyperacetylation is reported to influence both the stability and enzymatic activity of SIRT3 including its ability to promote fatty acid β-oxidation via deacetylating LCAD. At the molecular level, SIRT1 is demonstrated to directly interact with and deacetylate SIRT3 in the mitochondria, improving its stability and deacetylase activity [97]. Therefore, maternal obesity may abrogate the beneficial effects of SIRT1 and SIRT3 on pregnancy by decreasing not only their expression but also their activity. Consistently, Maternal HFD feeding is found to significantly decrease SIRT1 protein and mRNA levels in the murine placenta, with concomitant increased levels of placental LPL and proliferator activated receptor (PPAR) γ levels. Using selective activators and inhibitors, this study further explains that SIRT1 negatively regulates LPL expression in JEG-3 trophoblasts through suppressing PPARγ expression [98]. In addition, obese pregnant women, regardless of gestational diabetes mellitus (GDM) status, display diminished skeletal muscle SIRT3 activity and expression, which is proposed to be a contributing factor to the increased oxidative stress observed in obese pregnancy [99]. Importantly, hierarchical cluster analysis of the hepatic and placental gene expression has identified that maternal obesity in mice prominently upregulates lysine acetyltransferases and Bromodomain-containing protein 2 in the fetal liver and placental labyrinth, while downregulating most histone deacetylases in the same tissues. Among the histone deacetylases analyzed in this study, SIRT4 expression is shown to be downregulated in dams fed a HFD, yet the significance of this finding remains to be elucidated [100]. Moreover, maternal obesity, similar to SIRT1-deficiency, is also related to poor placental angiogenesis and fetal developmental problems. According to a recent study, regardless of SIRT1 genotypes, dams fed a HFD develop pronounced placental angiogenic problems, which are reversible by eicosapentaenoic acid (EPA) treatment. Compared to HFD, EPA diet significantly decreases placental HIF-1α expression and increases placental angiogenesis via boosting NF-κB-mediated inflammatory responses [101]. Therefore, it is possible that obesity-induced placental vasculopathy is independent of SIRT1 angiogenic properties, yet the lack of a normal diet group has limited this study. In addition to placental complications, maternal obesity can induce alterations in fetal metabolic programming by exposing the fetus to maternal overnutrition. With this regard, expression and enzymatic activity of both SIRT1 and SIRT3 are found to be decreased in the fetal liver in response to maternal obesity [102, 103].
Sirtuins can hold mTOR functions in check during pregnancy
SIRT1 and mammalian target of rapamycin (mTOR) are known to negatively regulate each other. mTOR is the catalytic subunit of two distinct enzymatic complexes, a rapamycin-sensitive complex mammalian target of rapamycin complex 1 (mTORC1) and a rapamycin-insensitive complex mTORC2, and is implicated in the regulation of a wide variety of functions including gene transcription, protein formation, proliferation, inflammation and cellular metabolism. Furthermore, rapamycin, a potent SIRT1 activator and an immunosuppressant widely used in transplantation medicine, downregulates mTOR activity by inhibiting mTORC1 [104-107]. It is reported that high glucose levels significantly enhance the expression and activity of mTOR while they have a negative effect on the expression and activity of SIRT1, which collectively leads to the senescence of mesangial cells [108]. In embryonic cells, chitosan nanoparticles (CSNPs) reduce the expression of SIRT1 and SIRT3, which provokes oxidative stress and apoptosis. In contrast, rapamycin is demonstrated to neutralize the apoptotic effects of CSNPs and reduce reactive oxygen species levels in embryonic cells by considerably upregulating the expression levels of Bcl-2, SIRT1 and SIRT3 and downregulating Caspase-3 mRNA expression [109]. Similar to SIRT1, both SIRT3 and SIRT4 have also been implicated in restraining mTOR signaling and functions [110, 111]. Nevertheless, the importance of these interactions should be investigated in detail in the context of pregnancy.
Sirtuins and pregnancy complications
Alterations in Sirtuin levels may be a pivotal intermediary step in the pathogenesis of several pregnancy disorders such as recurrent spontaneous abortion (RSA), PE, RIF and fetal growth restriction (FGR). PE is a disorder of vascular endothelial malfunction and is associated with an imbalance between inflammatory and regulatory cells and several other events including hyper-lipidemia, anti-angiogenic factor production and inadequate trophoblast invasion and differentiation. PE eventually results in chronic hyper-inflammation, hypoxia, oxidative stress and abnormal placental development and function [112-114]. Indeed, Abnormal differentiation of syncytiotrophoblasts (labyrinthine trophoblasts) and shallow invasion of extravillous cytotrophoblasts (junctional zone trophoblasts) are regarded as hallmarks of this disease [115]. Compared to normal pregnancies, placental mRNA expression levels of SIRT1 and PGC-1α, together with placental mitochondrial DNA and protein, are found to be markedly reduced in pregnancies complicated by both PE and IUGR [116]. In fact, results from other studies have further substantiated that preeclamptic women, regardless of being at term or preterm delivery, exhibit reduced SIRT1 and VSF levels in placental syncytiotrophoblasts compared to normal controls whereas SIRT3 decreases in preeclamptic placentas most significantly at preterm [93, 117]. Similar to the reductions observed in placental SIRT1 and SIRT3 levels, SIRT2 protein expression is reported to be significantly lower in preterm preeclamptic and fetal growth restricted placentas relative to gestation matched preterm controls. In preeclamptic placentas, this reduction is illustrated to be concomitant with elevated mRNA levels of receptor-interacting serine/threonine-protein kinase 1 (RIPK1), an enzyme involved in the induction of necroptosis [118]. Contrary to these reductions, expression of the placental SIRT4 gene is reported to be significantly higher in PE superimposed on chronic hypertension than normal pregnancy, but the mechanism and importance of this observation remain to be explored in greater detail [119].
Furthermore, the expression and activity of xanthine oxidase, an enzyme known to induce oxidative stress via ROS generation, are elevated in cytotrophoblasts during PE [120]. Treatment of human placental explants with hypoxanthine and xanthine oxidase is explained to reduce placental GLUT1 expression and resultant glucose uptake by inhibiting SIRT1 expression at both the transcriptional and translational levels [121]. Even so, it is still unclear whether SIRT1 can regulate the expression of xanthine oxidase in feto-maternal tissues during pregnancy. Similar to xanthine oxidase, HMGB1 and HSP70 also play an important role in the pathogenesis of PE. Under cellular stress, heat-shock protein 70 (HSP70) inhibits caspase-dependent and FasL-induced apoptosis by preventing several apoptotic events [122-126]. Conversely, HSP70 can also promote TNF-triggered apoptosis by suppressing IKK and NF-κB anti-apoptotic activities [127]. Equally important, high mobility group box-1 (HMGB1) is considered both as an actively secreted cytokine produced by inflammatory cells and a chromatin-associated protein involved in the transcriptional regulation of several important genes [128, 129]. Upon being released into the extracellular space, HMGB1 binds to multiple surface receptors including TLR2, TLR4, and RAGE (Receptor for Advanced Glycation End-products) and induces either apoptosis by regulating the expression of mTOR and discoidin domain receptor 1 (DDR1) or inflammation via activating inflammatory pathways like NF-κB [130-132]. Importantly, plasma levels of both HMGB1 and HSP70 are strongly and positively correlated with the severity of PE and HMGB1 expression levels are markedly increased in the placental syncytiotrophoblasts of preeclamptic patients, which is described to contribute to RAGE-mediated endothelial cell activation. Moreover, the expression of inflammatory mediators such as IL-1β, IL-18, TNF-α and HMGB1 is found to be significantly elevated in monocytes isolated from pregnant women with PE [133-135]. Interestingly, using a murine model of PE, a recent study has revealed that a decrease in placental SIRT1 protein levels can be the underlying cause of increased serum HMGB1 and HSP70 concentrations in preeclamptic patients. Mechanistically, SIRT1 has been found to hinder both IL-6- and preeclamptic serum-induced release of HMGB1 and HSP70 from in vitro-cultured human umbilical vein endothelial cells (HUVECs) [136]. Indeed, HMGB1 hyper-acetylation, which can be regulated by Sirtuins, is elucidated to be responsible for the translocation of HMGB1 into the cytosol and the lysosomal release of HMGB1 [132]. Thus, Sirtuins may avert the adverse effects (inflammation and apoptosis) of these proteins by direct regulation of their acetylation status, so further research is needed to clarify the exact underlying mechanisms involved in the regulation of HMGB1 and HSP70 by Sirtuins during pregnancy.
In contrast to preeclamptic pregnancies, very little is known about Sirtuin alterations in the hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. Importantly, short-term hypoxia and impaired fatty acid oxidation are considered to be involved in the pathogenesis of this syndrome. With this regard, after a short-term hypoxia treatment, HUVECs from HELLP pregnancies are shown to have higher SIRT4 protein levels than HUVECs isolated from normal pregnancies. As a result, elevated SIRT4 levels can be a possible contributor to the mitochondrial defects observed in pregnancies complicated by the HELLP syndrome, but this possibility should be further explored [137]. Likewise, SIRT4 also appears to play a role in the fetal complications caused by maternal diabetes in conjunction with SIRT1, 2, and 3. Specifically, it is demonstrated that GDM reduces the transcription levels of SIRT1, 3, and 4 and the activity of SIRT1, and 3 in fetal endothelial cells, which may contribute to long-term cardiovascular complications in the offspring [138]. In addition, SIRT2 is proposed to play a protective role against maternal diabetes-induced apoptosis, cellular organelle stress and neural tube defects by deacetylating myristoylated alanine-rich C-kinase substrate (MARCKS) [139]. Interestingly, exposure of pregnant mice to chronic mild stress enhances the expression of SIRT7 in the placenta, which can be attributed to SIRT7’s ability to promote cell survival and DNA repair [140]. As a concluding remark, each member of the Sirtuin family plays distinct yet integral roles in key biological processes related to the pregnancy and thus their dysregulated expression or activity can give rise to adverse pregnancy outcomes such as PE, IUGR, diabetic embryopathy and the HELLP syndrome.
Conclusion
Recent evidence supports that Sirtuins can improve the pregnancy outcome by regulating fundamental biological processes in charge of folliculogenesis, oocyte meiotic maturation, oocyte aging, trophoblast functions, feto-maternal inflammation and placental angiogenesis and oxidative stress. Sirtuins also play major roles in the regulation of labor and obstetric complications caused by maternal obesity and diabetes. Consequently, alterations in Sirtuin levels and activity can be a pivotal intermediary step in the pathogenesis of several pregnancy complications including recurrent spontaneous abortion, fetal growth retardation, preeclampsia, recurrent implantation failure and the HELLP syndrome.
Author Contributions
F. R. K.: Manuscript writing, performed the literature search, final approval of the manuscript; H. D. G.: Manuscript writing, final approval of the manuscript; S. S.: Conceptualized the study, performed the literature search, manuscript writing, final approval of manuscript.
Funding
No funding was received in preparing this manuscript.
The authors declare that no conflict of interests exists.
| 1 Teixeira CS, Cerqueira NM, Gomes P, Sousa SF: A Molecular Perspective on Sirtuin Activity. Int J Mol Sci 2020;21:8609. https://doi.org/10.3390/ijms21228609 |
||||
| 2 Alqarni MH, Foudah AI, Muharram MM, Labrou NE: The Pleiotropic Function of Human Sirtuins as Modulators of Metabolic Pathways and Viral Infections. Cells 2021;10:460. https://doi.org/10.3390/cells10020460 |
||||
| 3 Ivy JM, Klar A, Hicks JB: Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol 1986;6:688-702. https://doi.org/10.1128/mcb.6.2.688-702.1986 |
||||
| 4 Greiss S, Gartner A: Sirtuin/Sir2 phylogeny, evolutionary considerations and structural conservation. Mol Cells 2009;28:407-415. https://doi.org/10.1007/s10059-009-0169-x |
||||
| 5 Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A, Altucci L: Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics 2016;8:1-21. https://doi.org/10.1186/s13148-016-0224-3 |
||||
| 6 Vassilopoulos A, Fritz KS, Petersen DR, Gius D: The human sirtuin family: evolutionary divergences and functions. Hum Genomics 2011;5:485-496. https://doi.org/10.1186/1479-7364-5-5-485 |
||||
| 7 Lin J, Xiong Z, Gu J, Sun Z, Shuai J, Fan D, Li W: Sirtuins: Potential Therapeutic Targets for Defense against Oxidative Stress in Spinal Cord Injury. Oxid Med Cell Longev 2021;2021:7207692. https://doi.org/10.1155/2021/7207692 |
||||
| 8 Vazquez BN, Vaquero A, Schindler K: Sirtuins in female meiosis and in reproductive longevity. Mol Reprod Dev 2020;87:1175-1187. https://doi.org/10.1002/mrd.23437 |
||||
| 9 Kupis W, Pałyga J, Tomal E, Niewiadomska E: The role of sirtuins in cellular homeostasis. J Physiol Biochem 2016;72:371-380. https://doi.org/10.1007/s13105-016-0492-6 |
||||
| 10 Vaquero A, Reinberg D: Calorie restriction and the exercise of chromatin. Genes Dev 2009;23:1849-1869. https://doi.org/10.1101/gad.1807009 |
||||
| 11 Kitada M, Ogura Y, Monno I, Koya D: Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne) 2019;10:187. https://doi.org/10.3389/fendo.2019.00187 |
||||
| 12 Ogura Y, Kitada M, Koya D: Sirtuins and Renal Oxidative Stress. Antioxidants 2021;10:1198. https://doi.org/10.3390/antiox10081198 |
||||
| 13 Shahgaldi S, Kahmini FR: A comprehensive review of Sirtuins: With a major focus on redox homeostasis and metabolism. Life Sci 2021;282:119803. https://doi.org/10.1016/j.lfs.2021.119803 |
||||
| 14 Cheng H-L, Mostoslavsky R, Saito Si, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF: Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A 2003;100:10794-10799. https://doi.org/10.1073/pnas.1934713100 |
||||
| 15 McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M: The mammalian SIR2α protein has a role in embryogenesis and gametogenesis. Mol Cell Biol 2003;23:38-54. https://doi.org/10.1128/MCB.23.1.38-54.2003 |
||||
| 16 Tang S, Fang Y, Huang G, Xu X, Padilla‐Banks E, Fan W, Xu Q, Sanderson SM, Foley JF, Dowdy S: Methionine metabolism is essential for SIRT1‐regulated mouse embryonic stem cell maintenance and embryonic development. EMBO J 2017;36:3175-3193. https://doi.org/10.15252/embj.201796708 |
||||
| 17 Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y: Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A 2008;105:15599-15604. https://doi.org/10.1073/pnas.0800612105 |
||||
| 18 Tatone C, Di Emidio G, Vitti M, Di Carlo M, Santini S, D'Alessandro AM, Falone S, Amicarelli F: Sirtuin functions in female fertility: possible role in oxidative stress and aging. Oxid Med Cell Longev 2015;2015:659687. https://doi.org/10.1155/2015/659687 |
||||
| 19 Rajan KAN, Khater M, Soncin F, Pizzo D, Moretto-Zita M, Pham J, Stus O, Iyer P, Tache V, Laurent LC: Sirtuin1 is required for proper trophoblast differentiation and placental development in mice. Placenta 2018;62:1-8. https://doi.org/10.1016/j.placenta.2017.12.002 |
||||
| 20 Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M: SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod 2011;84:167-178. https://doi.org/10.1095/biolreprod.110.086983 |
||||
| 21 Lim R, Barker G, Lappas M: SIRT6 is decreased with preterm labor and regulates key terminal effector pathways of human labor in fetal membranes. Biol Reprod 2013;88:17. https://doi.org/10.1095/biolreprod.112.105163 |
||||
| 22 Xiao C, Wang R-H, Lahusen TJ, Park O, Bertola A, Maruyama T, Reynolds D, Chen Q, Xu X, Young HA: Progression of chronic liver inflammation and fibrosis driven by activation of c-JUN signaling in Sirt6 mutant mice. J Biol Chem 2012;287:41903-41913. https://doi.org/10.1074/jbc.M112.415182 |
||||
| 23 Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY: SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 2009;136:62-74. https://doi.org/10.1016/j.cell.2008.10.052 |
||||
| 24 Xiao C, Kim HS, Lahusen T, Wang RH, Xu X, Gavrilova O, Jou W, Gius D, Deng CX: SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem 2010;285:36776-36784. https://doi.org/10.1074/jbc.M110.168039 |
||||
| 25 Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM: Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006;124:315-329. https://doi.org/10.1016/j.cell.2005.11.044 |
||||
| 26 Peshti V, Obolensky A, Nahum L, Kanfi Y, Rathaus M, Avraham M, Tinman S, Alt FW, Banin E, Cohen HY: Characterization of physiological defects in adult SIRT6-/-mice. PLoS One 2017;12:e0176371. https://doi.org/10.1371/journal.pone.0176371 |
||||
| 27 Zhang W, Wan H, Feng G, Qu J, Wang J, Jing Y, Ren R, Liu Z, Zhang L, Chen Z: SIRT6 deficiency results in developmental retardation in cynomolgus monkeys. Nature 2018;560:661. https://doi.org/10.1038/s41586-018-0437-z |
||||
| 28 Ferrer CM, Alders M, Postma AV, Park S, Klein MA, Cetinbas M, Pajkrt E, Glas A, van Koningsbruggen S, Christoffels VM: An inactivating mutation in the histone deacetylase SIRT6 causes human perinatal lethality. Genes Dev 2018;32:373-388. https://doi.org/10.1101/gad.307330.117 |
||||
| 29 Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E: Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 2008;102:703-710. https://doi.org/10.1161/CIRCRESAHA.107.164558 |
||||
| 30 Vazquez BN, Thackray JK, Simonet NG, Kane‐Goldsmith N, Martinez‐Redondo P, Nguyen T, Bunting S, Vaquero A, Tischfield JA, Serrano L: SIRT7 promotes genome integrity and modulates non‐homologous end joining DNA repair. EMBO J 2016;35:1488-1503. https://doi.org/10.15252/embj.201593499 |
||||
| 31 Shin J, He M, Liu Y, Paredes S, Villanova L, Brown K, Qiu X, Nabavi N, Mohrin M, Wojnoonski K: SIRT7 represses Myc activity to suppress ER stress and prevent fatty liver disease. Cell Rep 2013;5:654-665. https://doi.org/10.1016/j.celrep.2013.10.007 |
||||
| 32 Serrano L, Martínez-Redondo P, Marazuela-Duque A, Vazquez BN, Dooley SJ, Voigt P, Beck DB, Kane-Goldsmith N, Tong Q, Rabanal RM: The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev 2013;27:639-653. https://doi.org/10.1101/gad.211342.112 |
||||
| 33 Lombard DB, Alt FW, Cheng H-L, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A: Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 2007;27:8807-8814. https://doi.org/10.1128/MCB.01636-07 |
||||
| 34 Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng C-X, Finkel T: A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proceedings of the National Academy of Sciences 2008;105:14447-14452. https://doi.org/10.1073/pnas.0803790105 |
||||
| 35 Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G: SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell 2006;126:941-954. https://doi.org/10.1016/j.cell.2006.06.057 |
||||
| 36 Nakagawa T, Lomb DJ, Haigis MC, Guarente L: SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 2009;137:560-570. https://doi.org/10.1016/j.cell.2009.02.026 |
||||
| 37 Heinonen T, Ciarlo E, Le Roy D, Roger T: Impact of the dual deletion of the mitochondrial sirtuins SIRT3 and SIRT5 on anti-microbial host defenses. Front Immunol 2019;10:2341. https://doi.org/10.3389/fimmu.2019.02341 |
||||
| 38 Tatone C, Di Emidio G, Barbonetti A, Carta G, Luciano AM, Falone S, Amicarelli F: Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update 2018;24:267-289. https://doi.org/10.1093/humupd/dmy003 |
||||
| 39 Xu D, Wu L, Jiang X, Yang L, Cheng J, Chen H, Hua R, Geng G, Yang L, Li Q: SIRT2 Inhibition Results in Meiotic Arrest, Mitochondrial Dysfunction, and Disturbance of Redox Homeostasis during Bovine Oocyte Maturation. Int J Mol Sci 2019;20:1365. https://doi.org/10.3390/ijms20061365 |
||||
| 40 Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX, Zhang XM, Liu WJ, Luo LL, Fu YC: SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. J Ovarian Res 2014;7:97. https://doi.org/10.1186/s13048-014-0097-z |
||||
| 41 Liu WJ, Zhang XM, Wang N, Zhou XL, Fu YC, Luo LL: Calorie restriction inhibits ovarian follicle development and follicle loss through activating SIRT1 signaling in mice. Eur J Med Res 2015;20:22. https://doi.org/10.1186/s40001-015-0114-8 |
||||
| 42 Zhang L, Ma R, Hu J, Ding X, Xu Y: Sirtuin inhibition adversely affects porcine oocyte meiosis. PLoS One 2015;10:e0132941. https://doi.org/10.1371/journal.pone.0132941 |
||||
| 43 Riepsamen A, Wu L, Lau L, Listijono D, Ledger W, Sinclair D, Homer H: Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes. PLoS One 2015;10:e0126194. https://doi.org/10.1371/journal.pone.0126194 |
||||
| 44 Itami N, Shirasuna K, Kuwayama T, Iwata H: Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology 2015;83:1360-1367. https://doi.org/10.1016/j.theriogenology.2015.01.029 |
||||
| 45 Li Y, Wang J, Zhang Z, Yi J, He C, Wang F, Tian X, Yang M, Song Y, He P: Resveratrol compares with melatonin in improving in vitro porcine oocyte maturation under heat stress. J Anim Sci Biotechnol 2016;7:33. https://doi.org/10.1186/s40104-016-0093-9 |
||||
| 46 Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L: Resveratrol protects against age-associated infertility in mice. Hum Reprod 2013;28:707-717. https://doi.org/10.1093/humrep/des437 |
||||
| 47 Sato D, Itami N, Tasaki H, Takeo S, Kuwayama T, Iwata H: Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS One 2014;9:e94488. https://doi.org/10.1371/journal.pone.0094488 |
||||
| 48 Di Emidio G, Falone S, Vitti M, D'Alessandro AM, Vento M, Di Pietro C, Amicarelli F, Tatone C: SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod 2014;29:2006-2017. https://doi.org/10.1093/humrep/deu160 |
||||
| 49 Zhang T, Zhou Y, Li L, Wang H-H, Ma XS, Qian WP, Shen W, Schatten H, Sun QY: SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging (Albany NY) 2016;8:685. https://doi.org/10.18632/aging.100911 |
||||
| 50 Wang H, Jo YJ, Oh JS, Kim NH: Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity. Oncotarget 2017;8:38631. https://doi.org/10.18632/oncotarget.16219 |
||||
| 51 Yang Q, Dai S, Luo X, Zhu J, Li F, Liu J, Yao G, Sun Y: Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction (Cambridge, England) 2018;156:81-92. https://doi.org/10.1530/REP-18-0211 |
||||
| 52 Ma R, Zhang Y, Zhang L, Han J, Rui R: Sirt1 protects pig oocyte against in vitro aging. Anim Sci J 2015;86:826-832. https://doi.org/10.1111/asj.12360 |
||||
| 53 Zhang L, Hou X, Ma R, Moley K, Schedl T, Wang Q: Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J 2014;28:1435-1445. https://doi.org/10.1096/fj.13-244111 |
||||
| 54 Qiu D, Hou X, Han L, Li X, Ge J, Wang Q: Sirt2‐BubR1 acetylation pathway mediates the effects of advanced maternal age on oocyte quality. Aging Cell 2018;17:e12698. https://doi.org/10.1111/acel.12698 |
||||
| 55 Xu D, Jiang X, He H, Liu D, Yang L, Chen H, Wu L, Geng G, Li Q: SIRT2 functions in aging, autophagy, and apoptosis in post-maturation bovine oocytes. Life Sci 2019;232:116639. https://doi.org/10.1016/j.lfs.2019.116639 |
||||
| 56 Liu X, Zhang L, Wang P, Li X, Qiu D, Li L, Zhang J, Hou X, Han L, Ge J: Sirt3-dependent deacetylation of SOD2 plays a protective role against oxidative stress in oocytes from diabetic mice. Cell Cycle 2017;16:1302-1308. https://doi.org/10.1080/15384101.2017.1320004 |
||||
| 57 Zhang L, Han L, Ma R, Hou X, Yu Y, Sun S, Xu Y, Schedl T, Moley KH, Wang Q: Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle 2015;14:2959-2968. https://doi.org/10.1080/15384101.2015.1026517 |
||||
| 58 Han L, Wang H, Li L, Li X, Ge J, Reiter RJ, Wang Q: Melatonin protects against maternal obesity‐associated oxidative stress and meiotic defects in oocytes via the SIRT 3‐SOD 2‐dependent pathway. J Pineal Res 2017;63:e12431. https://doi.org/10.1111/jpi.12431 |
||||
| 59 Zhao H-C, Ding T, Ren Y, Li TJ, Li R, Fan Y, Yan J, Zhao Y, Li M, Yu Y: Role of Sirt3 in mitochondrial biogenesis and developmental competence of human in vitro matured oocytes. Hum Reprod 2016;31:607-622. https://doi.org/10.1093/humrep/dev345 |
||||
| 60 Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H: Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest 2010;120:2817-2828. https://doi.org/10.1172/JCI42020 |
||||
| 61 Han L, Ge J, Zhang L, Ma R, Hou X, Li B, Moley K, Wang Q: Sirt6 depletion causes spindle defects and chromosome misalignment during meiosis of mouse oocyte. Sci Rep 2015;5:15366. https://doi.org/10.1038/srep15366 |
||||
| 62 Cao Z, Zhang D, Tong X, Wang Y, Qi X, Ning W, Xu T, Gao D, Zhang L, Ma Y: Cumulus cell-derived and maternal SIRT6 differentially regulates porcine oocyte meiotic maturation. Theriogenology 2020;142:158-168. https://doi.org/10.1016/j.theriogenology.2019.09.048 |
||||
| 63 Ge J, Li C, Li C, Huang Z, Zeng J, Han L, Wang Q: SIRT6 participates in the quality control of aged oocytes via modulating telomere function. Aging (Albany NY) 2019;11:1965. https://doi.org/10.18632/aging.101885 |
||||
| 64 Gao M, Li X, He Y, Han L, Qiu D, Ling L, Liu H, Liu J, Gu L: SIRT7 functions in redox homeostasis and cytoskeletal organization during oocyte maturation. FASEB J 2018;32:6228-6238. https://doi.org/10.1096/fj.201800078RR |
||||
| 65 Vazquez BN, Blengini CS, Hernandez Y, Serrano L, Schindler K: SIRT7 promotes chromosome synapsis during prophase I of female meiosis. Chromosoma 2019;128:369-383. https://doi.org/10.1007/s00412-019-00713-9 |
||||
| 66 Zeng J, Jiang M, Wu X, Diao F, Qiu D, Hou X, Wang H, Li L, Li C, Ge J: SIRT 4 is essential for metabolic control and meiotic structure during mouse oocyte maturation. Aging Cell 2018;17:e12789. https://doi.org/10.1111/acel.12789 |
||||
| 67 Roberts VH, Pound LD, Thorn SR, Gillingham MB, Thornburg KL, Friedman JE, Frias AE, Grove KL: Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J2014;28:2466-2477. https://doi.org/10.1096/fj.13-245472 |
||||
| 68 Hannan NJ, Brownfoot FC, Cannon P, Deo M, Beard S, Nguyen TV, Palmer KR, Tong S, Tu'uhevaha J: Resveratrol inhibits release of soluble fms-like tyrosine kinase (sFlt-1) and soluble endoglin and improves vascular dysfunction-implications as a preeclampsia treatment. Sci Rep 2017;7:1819. https://doi.org/10.1038/s41598-017-01993-w |
||||
| 69 Lim R, Barker G, Menon R, Lappas M: A novel role for SIRT3 in regulating mediators involved in the terminal pathways of human labor and delivery. Biol Reprod 2016;95:95, 91-11. https://doi.org/10.1095/biolreprod.116.142372 |
||||
| 70 Chen CJ, Fu YC, Yu W, Wang W: SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-κB. Biochem Biophys Res Commun 2013;430:798-803. https://doi.org/10.1016/j.bbrc.2012.11.066 |
||||
| 71 Fan D, Yang Z, Liu F-y, Jin Y-G, Zhang N, Ni J, Yuan Y, Liao HH, Wu QQ, Xu M: Sesamin protects against cardiac remodeling via Sirt3/ROS pathway. Cell Physiol Biochem 2017;44:2212-2227. https://doi.org/10.1159/000486026 |
||||
| 72 Yuping S, Jingli S, Ying W, Chong H, Junjie Z, Yongquan S, Zhimin L: Metformin ameliorates insulin resistance in L6 rat skeletal muscle cells through upregulation of SIRT3. Chin Med J 2014;127:1523-1529. | ||||
| 73 Ma B, Zhu Z, Zhang J, Ren C, Zhang Q: Aucubin alleviates diabetic nephropathy by inhibiting NF-κB activation and inducing SIRT1/SIRT3-FOXO3a signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. J Funct Foods 2020;64:103702. https://doi.org/10.1016/j.jff.2019.103702 |
||||
| 74 Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW: SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 2007;21:2644-2658. https://doi.org/10.1101/gad.435107 |
||||
| 75 Hastie R, Brownfoot FC, Pritchard N, Hannan NJ, Cannon P, Nguyen V, Palmer K, Beard S, Tong S, Kaitu'u-Lino TJ: EGFR (Epidermal Growth Factor Receptor) Signaling and the Mitochondria Regulate sFlt-1 (Soluble FMS-Like Tyrosine Kinase-1) Secretion. Hypertension 2019;73:659-670. https://doi.org/10.1161/HYPERTENSIONAHA.118.12300 |
||||
| 76 Cudmore MJ, Ramma W, Cai M, Fujisawa T, Ahmad S, Al-Ani B, Ahmed A: Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol 2012;206:253.e10-15. https://doi.org/10.1016/j.ajog.2011.11.010 |
||||
| 77 Menon R, Richardson LS, Lappas M: Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta 2019;79:40-45. https://doi.org/10.1016/j.placenta.2018.11.003 |
||||
| 78 Chen B, Nelson DM, Sadovsky Y: N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem 2006;281:2764-2772. https://doi.org/10.1074/jbc.M507330200 |
||||
| 79 Shi XH, Larkin JC, Chen B, Sadovsky Y: The expression and localization of N-myc downstream-regulated gene 1 in human trophoblasts. PLoS One 2013;8:e75473. https://doi.org/10.1371/journal.pone.0075473 |
||||
| 80 Sonkar R, Kay MK, Choudhury M: PFOS modulates interactive epigenetic regulation in first-trimester human trophoblast cell line HTR-8/SVneo. Chem Res Toxicol 2019;32:2016-2027. https://doi.org/10.1021/acs.chemrestox.9b00198 |
||||
| 81 Castex J, Willmann D, Kanouni T, Arrigoni L, Li Y, Friedrich M, Schleicher M, Wöhrle S, Pearson M, Kraut N: Inactivation of Lsd1 triggers senescence in trophoblast stem cells by induction of Sirt4. Cell Death Dis 2017;8:e2631. https://doi.org/10.1038/cddis.2017.48 |
||||
| 82 Pham J, Rajan KAN, Li P, Parast MM: The role of Sirtuin1-PPARγ axis in placental development and function. J Mol Endocrinol 2018;60:R201-R212. https://doi.org/10.1530/JME-17-0315 |
||||
| 83 Liu Z, Wang C, Pei J, Li M, Gu W: SIRT1: A Novel Protective Molecule in Pre-eclampsia. Int J Med Sci 2022;19:993-1002. https://doi.org/10.7150/ijms.73012 |
||||
| 84 Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR: Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum Reprod Update 2008;14:335-344. https://doi.org/10.1093/humupd/dmn010 |
||||
| 85 Maltepe E, Krampitz GW, Okazaki KM, Red-Horse K, Mak W, Simon MC, Fisher SJ: Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development 2005;132:3393-3403. https://doi.org/10.1242/dev.01923 |
||||
| 86 Wakeland AK, Soncin F, Moretto-Zita M, Chang C-W, Horii M, Pizzo D, Nelson KK, Laurent LC, Parast MM: Hypoxia directs human extravillous trophoblast differentiation in a hypoxia-inducible factor-dependent manner. Am J Pathol 2017;187:767-780. https://doi.org/10.1016/j.ajpath.2016.11.018 |
||||
| 87 Engin-Ustun Y, Ozgu-Erdinc AS, Caglayan EK, Gulerman C, Sarikaya E, Aktulay A, Demirtas C, Erkaya S, Yilmaz N: Sirtuin 1 Levels in Recurrent Implantation Failure. Rev Bras Ginecol Obstet 2017;39:541-544. https://doi.org/10.1055/s-0037-1606349 |
||||
| 88 Liang PY, Diao LH, Huang CY, Lian RC, Chen X, Li GG, Zhao J, Li YY, He XB, Zeng Y: The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod Biomed Online 2015;31:823-826. https://doi.org/10.1016/j.rbmo.2015.08.009 |
||||
| 89 Bashiri A, Halper KI, Orvieto R: Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol 2018;16:121. https://doi.org/10.1186/s12958-018-0414-2 |
||||
| 90 Rajaei S, Zamani AH, Jeddi-Tehrani M, Tavakoli M, Mohammadzadeh A, Dabbagh A, Mirahmadian M: Cytokine profile in the endometrium of normal fertile and women with repeated implantation failure. Iran J Immunol 2011;8:201-208. | ||||
| 91 Cheng SB, Sharma S: Preeclampsia and health risks later in life: an immunological link: Semin Immunopathol, Springer, 2016, 38, pp 699-708. https://doi.org/10.1007/s00281-016-0579-8 |
||||
| 92 Tsai PJS, Davis J, Thompson K, Bryant-Greenwood G: Visfatin/Nampt and SIRT1: roles in postterm delivery in pregnancies associated with obesity. Reprod Sci 2015;22:1028-1036. https://doi.org/10.1177/1933719115570908 |
||||
| 93 Broady A, Loichinger M, Bryant-Greenwood G: 774: Placental visfatin/NAMPT and sirtuin-1 expression in pre-eclampsia. Am J Obstet Gynecol 2015;212:S375. https://doi.org/10.1016/j.ajog.2014.10.980 |
||||
| 94 Liu L, Yao L, Peng T, Wen L, Cai W, Jia X, He J: Hepatic Sirt3 expression declines postpartum in dairy goats. J Dairy Res 2018;85:163-166. https://doi.org/10.1017/S0022029918000171 |
||||
| 95 Liu R, Fan M, Candas D, Qin L, Zhang X, Eldridge A, Zou JX, Zhang T, Juma S, Jin C: CDK1-mediated SIRT3 activation enhances mitochondrial function and tumor radioresistance. Mol Cancer Ther 2015;14:2090-2102. https://doi.org/10.1158/1535-7163.MCT-15-0017 |
||||
| 96 Nguyen LT, Chen H, Pollock CA, Saad S: Sirtuins-mediators of maternal obesity-induced complications in offspring? FASEB J 2016;30:1383-1390. https://doi.org/10.1096/fj.15-280743 |
||||
| 97 Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK: Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem 2017;292:17312-17323. https://doi.org/10.1074/jbc.M117.778720 |
||||
| 98 Qiao L, Guo Z, Bosco C, Guidotti S, Wang Y, Wang M, Parast M, Schaack J, Hay WW, Moore TR: Maternal high-fat feeding increases placental lipoprotein lipase activity by reducing SIRT1 expression in mice. Diabetes 2015;64:3111-3120. https://doi.org/10.2337/db14-1627 |
||||
| 99 Boyle KE, Newsom SA, Janssen RC, Lappas M, Friedman JE: Skeletal muscle MnSOD, mitochondrial complex II, and SIRT3 enzyme activities are decreased in maternal obesity during human pregnancy and gestational diabetes mellitus. The J Clin Endocrinol Metab 2013;98:E1601-E1609. https://doi.org/10.1210/jc.2013-1943 |
||||
| 100 Panchenko PE, Voisin S, Jouin M, Jouneau L, Prézelin A, Lecoutre S, Breton C, Jammes H, Junien C, Gabory A: Expression of epigenetic machinery genes is sensitive to maternal obesity and weight loss in relation to fetal growth in mice. Clin Epigenetics 2016;8:22. https://doi.org/10.1186/s13148-016-0188-3 |
||||
| 101 Peng J, Zhou Y, Hong Z, Wu Y, Cai A, Xia M, Deng Z, Yang Y, Song T, Xiong J: Maternal eicosapentaenoic acid feeding promotes placental angiogenesis through a Sirtuin-1 independent inflammatory pathway. Biochim Biophys Acta Mol Cell Biol Lipids 2019;1864:147-157. https://doi.org/10.1016/j.bbalip.2018.11.003 |
||||
| 102 Suter MA, Chen A, Burdine MS, Choudhury M, Harris RA, Lane RH, Friedman JE, Grove KL, Tackett AJ, Aagaard KM: A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J 2012;26:5106-5114. https://doi.org/10.1096/fj.12-212878 |
||||
| 103 Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJ, Badger TM, Shankar K: Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One 2011;6:e24068. https://doi.org/10.1371/journal.pone.0024068 |
||||
| 104 Maiese K: The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): oversight for neurodegenerative disorders. Biochem Soc Trans 2018;46:351-360. https://doi.org/10.1042/BST20170121 |
||||
| 105 Santos RX, Correia SC, Cardoso S, Carvalho C, Santos MS, Moreira PI: Effects of rapamycin and TOR on aging and memory: implications for Alzheimer's disease. J Neurochem 2011;117:927-936. https://doi.org/10.1111/j.1471-4159.2011.07262.x |
||||
| 106 Ghosh HS, McBurney M, Robbins PD: SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 2010;5:e9199. https://doi.org/10.1371/journal.pone.0009199 |
||||
| 107 Zheng H, Fu Y, Huang Y, Zheng X, Yu W, Wang W: mTOR signaling promotes foam cell formation and inhibits foam cell egress through suppressing the SIRT1 signaling pathway. Mol Med Report 2017;16:3315-3323. https://doi.org/10.3892/mmr.2017.7032 |
||||
| 108 Zhang S, Cai G, Fu B, Feng Z, Ding R, Bai X, Liu W, Zhuo L, Sun L, Liu F: SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev 2012;133:387-400. https://doi.org/10.1016/j.mad.2012.04.005 |
||||
| 109 Choi YJ, Gurunathan S, Kim D, Jang HS, Park WJ, Cho SG, Park C, Song H, Seo HG, Kim J-H: Rapamycin ameliorates chitosan nanoparticle-induced developmental defects of preimplantation embryos in mice. Oncotarget 2016;7:74658. https://doi.org/10.18632/oncotarget.10813 |
||||
| 110 Herrera KNG, Zaganjor E, Ishikawa Y, Spinelli JB, Yoon H, Lin J-R, Satterstrom FK, Ringel A, Mulei S, Souza A: Small-molecule screen identifies de novo nucleotide synthesis as a vulnerability of cells lacking SIRT3. Cell Rep 2018;22:1945-1955. https://doi.org/10.1016/j.celrep.2018.01.076 |
||||
| 111 Wang YS, Du L, Liang X, Meng P, Bi L, Wang Yl, Wang C, Tang B: Sirtuin 4 Depletion Promotes Hepatocellular Carcinoma Tumorigenesis Through Regulating Adenosine‐Monophosphate-Activated Protein Kinase Alpha/Mammalian Target of Rapamycin Axis in Mice. Hepatology 2019;69:1614-1631. https://doi.org/10.1002/hep.30421 |
||||
| 112 Wieser F, Waite L, Depoix C, Taylor RN: PPAR action in human placental development and pregnancy and its complications. PPAR Res 2008;2008:527048. https://doi.org/10.1155/2008/527048 |
||||
| 113 Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW, Wallace K, LaMarca B: The role of inflammation in the pathology of preeclampsia. Clin Sci 2016;130:409-419. https://doi.org/10.1042/CS20150702 |
||||
| 114 Silveira SIC: Redox changes in aged placental bed: consequences for cell signalling (dissertation). Universidade do Minho, 2013. | ||||
| 115 Fisher SJ: Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol 2015;213:S115-S122. https://doi.org/10.1016/j.ajog.2015.08.042 |
||||
| 116 Poidatz D, Dos Santos E, Duval F, Moindjie H, Serazin V, Vialard F, De Mazancourt P, Dieudonné MN: Involvement of estrogen-related receptor-γ and mitochondrial content in intrauterine growth restriction and preeclampsia. Fertil Steril 2015;104:483-490. https://doi.org/10.1016/j.fertnstert.2015.05.005 |
||||
| 117 Broady AJ, Loichinger MH, Ahn HJ, Davy PM, Allsopp RC, Bryant-Greenwood GD: Protective proteins and telomere length in placentas from patients with pre-eclampsia in the last trimester of gestation. Placenta 2017;50:44-52. https://doi.org/10.1016/j.placenta.2016.12.018 |
||||
| 118 Hannan NJ, Beard S, Binder NK, Onda K, Tu'uhevaha J, Chen Q, Tuohey L, De Silva M, Tong S: Key players of the necroptosis pathway RIPK1 and SIRT2 are altered in placenta from preeclampsia and fetal growth restriction. Placenta 2017;51:1-9. https://doi.org/10.1016/j.placenta.2017.01.002 |
||||
| 119 Chang SD, Chao AS, Peng HH, Chang YL, Wang CN, Cheng PJ, Lee YS, Chao A, Wang TH: Analyses of placental gene expression in pregnancy-related hypertensive disorders. Taiwan J Obstet Gynecol 2011;50:283-291. https://doi.org/10.1016/j.tjog.2011.07.005 |
||||
| 120 Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y: Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol 2000;156:321-331. https://doi.org/10.1016/S0002-9440(10)64733-5 |
||||
| 121 Lappas M, Andrikopoulos S, Permezel M: Hypoxanthine-xanthine oxidase down-regulates GLUT1 transcription via SIRT1 resulting in decreased glucose uptake in human placenta. J Endocrinol 2012;213:49-57. https://doi.org/10.1530/JOE-11-0355 |
||||
| 122 Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD: Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem 2005;280:38729-38739. https://doi.org/10.1074/jbc.M509497200 |
||||
| 123 Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B: The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol 2000;20:7146-7159. https://doi.org/10.1128/MCB.20.19.7146-7159.2000 |
||||
| 124 Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, Vickers SM, Saluja AK: Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology 2009;136:1772-1782. https://doi.org/10.1053/j.gastro.2009.01.070 |
||||
| 125 Vasaikar S, Ghosh S, Narain P, Basu A, Gomes J: HSP70 mediates survival in apoptotic cells-Boolean network prediction and experimental validation. Front Cell Neurosci 2015;9:319. https://doi.org/10.3389/fncel.2015.00319 |
||||
| 126 Li CY, Lee JS, Ko YG, Kim JI, Seo JS: Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem 2000;275:25665-25671. https://doi.org/10.1074/jbc.M906383199 |
||||
| 127 Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC: Hsp70 promotes TNF-mediated apoptosis by binding IKKγ and impairing NF-κB survival signaling. Genes Dev 2004;18:1466-1481. https://doi.org/10.1101/gad.1188204 |
||||
| 128 Bell CW, Jiang W, Reich CF, Pisetsky DS: The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 2006;291:C1318-1325. https://doi.org/10.1152/ajpcell.00616.2005 |
||||
| 129 Schiller M, Heyder P, Ziegler S, Niessen A, Claßen L, Lauffer A, Lorenz H-M: During apoptosis HMGB1 is translocated into apoptotic cell-derived membraneous vesicles. Autoimmunity 2013;46:342-346. https://doi.org/10.3109/08916934.2012.750302 |
||||
| 130 Ouyang F, Huang H, Zhang M, Chen M, Huang H, Huang F, Zhou S: HMGB1 induces apoptosis and EMT in association with increased autophagy following H/R injury in cardiomyocytes. Int J Mol Med 2016;37:679-689. https://doi.org/10.3892/ijmm.2016.2474 |
||||
| 131 Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 2009;28:367-388. https://doi.org/10.1146/annurev.immunol.021908.132603 |
||||
| 132 Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A: HMGB1: endogenous danger signaling. Mol Med 2008;14:476-484. https://doi.org/10.2119/2008-00034.Klune |
||||
| 133 Romão-Veiga M, Matias ML, Ribeiro VR, Nunes PR, Borges VTM, Peraçoli JC, Peraçoli MTS: Induction of systemic inflammation by hyaluronan and hsp70 in women with pre-eclampsia. Cytokine 2018;105:23-31. https://doi.org/10.1016/j.cyto.2018.02.007 |
||||
| 134 Chen Q, Yin Y, Wei J, Tong M, Shen F, Zhao M, Chamley L: Increased expression of high mobility group box 1 (HMGB1) in the cytoplasm of placental syncytiotrophoblast from preeclamptic placentae. Cytokine 2016;85:30-36. https://doi.org/10.1016/j.cyto.2016.06.001 |
||||
| 135 Shao J, Zhao M, Tong M, Wei J, Wise MR, Stone P, Chamley L, Chen Q: Increased levels of HMGB1 in trophoblastic debris may contribute to preeclampsia. Reproduction (Cambridge, England) 2016;152:775-784. https://doi.org/10.1530/REP-16-0083 |
||||
| 136 Yin Y, Feng Y, Zhao H, Zhao Z, Yua H, Xu J, Che H: SIRT1 inhibits releases of HMGB1 and HSP70 from human umbilical vein endothelial cells caused by IL-6 and the serum from a preeclampsia patient and protects the cells from death. Biomed Pharmacother 2017;88:449-458. https://doi.org/10.1016/j.biopha.2017.01.087 |
||||
| 137 Sandvoß M, Potthast AB, von Versen-Höynck F, Das AM: HELLP Syndrome: Altered Hypoxic Response of the Fatty Acid Oxidation Regulator SIRT 4. Reprod Sci 2017;24:568-574. https://doi.org/10.1177/1933719116667216 |
||||
| 138 Gui J, Potthast A, Rohrbach A, Borns K, Das AM, von Versen-Höynck F: Gestational diabetes induces alterations of sirtuins in fetal endothelial cells. Pediatr Res 2016;79:788. https://doi.org/10.1038/pr.2015.269 |
||||
| 139 Yang P, Xu C, Reece EA, Chen X, Zhong J, Zhan M, Stumpo DJ, Blackshear PJ, Yang P: Tip60-and sirtuin 2-regulated MARCKS acetylation and phosphorylation are required for diabetic embryopathy. Nat Commun 2019;10:282. https://doi.org/10.1038/s41467-018-08268-6 |
||||
| 140 Schroeder M, Jakovcevski M, Polacheck T, Drori Y, Luoni A, Röh S, Zaugg J, Ben-Dor S, Albrecht C, Chen A: Placental miR-340 mediates vulnerability to activity based anorexia in mice. Nat Commun 2018;9:1596. https://doi.org/10.1038/s41467-018-03836-2 |
||||