The Role of Exercise-Induced Reactive Oxygen Species (ROS) Hormesis in Aging: Friend or Foe
bCenter of Excellence in Highter Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia,
cBiological Activity Division, Central Laboratory, Universitas Padjadjaran, Bandung, Indonesia,
dUndergraduate Medical Program, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia,
ePhysiology Department, Faculty of Medicine, Maranatha Christian University, Bandung, Indonesia
Keywords
Abstract
Reactive oxygen species (ROS) are oxygen derivatives that arise intrinsically from the oxidative phosphorylation process and extrinsically as a response to xenobiotics and pollution. ROS is involved in various conditions such as exercise, aging, inflammation, and neurodegenerative diseases. In the aging process, increased cellular senescence and decreased endogenous antioxidants also occur. Meanwhile, physical activity, specifically exercise, can modulate ROS. The impact of exercise on ROS varies from harmful to beneficial and depends on the type of exercise as they induce different types of ROS. Long-term exercise regulates signaling pathways that enhance antioxidant defense systems and control ROS production. This review will discuss studies on how exercise can regulate ROS and which type of exercise has a role in delaying the aging process. This review also exposes the impact of nutraceutical antioxidant agents that likely enhance the benefit of exercise. The nutraceutical antioxidants agents that likely enhance the benefit of exercise are creatine, whey, and ascorbic acid. Exercise is rewarding for the aging population concerning increasing their quality of life. Special consideration to exercise needs to be given to the type of exercise, and the exercise must be done continuously.Introduction
The aging process generates a progressive loss of many biological functions, starting from the cellular level. Several cardinal biomarkers of aging are telomere length shortening, DNA damage, and oxidative stress [1–3]. Telomere length shortening, also known as telomere attrition, is hypothesized to trigger cellular aging as its length correlates with many vertebrates’ survival [4]. DNA damage elicits activation of DNA damage response (DDR) which includes increasing levels of reactive oxygen species (ROS). Moreover, high levels of ROS could also damage DNA, resulting in a vicious cycle of ROS production [5]. These processes may lead to cellular cycle arrest or cellular senescence. Cellular senescence causes immunosenescence and mitochondrial dysfunction. Immunosenescence is a term used to describe a chronic deterioration of immune function, such as neutrophil dysfunction associated with the pathogenesis of age-related diseases [6]. Cellular senescence attenuates GRSF1, which functions to maintain mitochondrial phosphorylation in complex I, causing impairment of the electron transport chain that leads to mitochondrial dysfunction marked by increased ROS production [7, 8].
ROS are oxygen derivatives that arise intrinsically from the oxidative phosphorylation process as an excess product and extrinsically as a response to xenobiotics and pollution. As a reactive molecule, the generation of ROS is observed as a cascade of transitions from one species to another. The first generation of ROS, such as superoxide radical (O2•-), nitric oxide radical (NO•), and hydrogen peroxide (H2O2), is mainly crucial for redox signaling [9]. Mitochondria is the primary contributor to this type of ROS, mainly O2•-, via incomplete oxygen reduction at the electron transport chains (ETC). Meanwhile, in the cytoplasm, O2•- and H2O2 are produced by NOX (nicotinamide adenine dinucleotide phosphate [NADPH] oxidases) family. These enzymes contain seven isoforms, NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2, distributed differently throughout the body [10–14]. Moreover, NOXs are triggered by specific stimuli from the environment. A study by Rathore et al. [15] showed hypoxia induces ROS production, which further causes AMPK (AMP-activated protein kinase) activity. Several fundamental roles that AMPK carried out include modulating cell growth and adjusting metabolism [16]. On the other hand, reactive nitrogen species (RNS) is well known as NO• Derivatives that arise from L-arginine (L-Arg) and catalyzed by nitric oxide synthase (NOS).
The second generation of ROS, including peroxynitrite (ONOO−), peroxynitrous acid (ONOOH), hydroxyl radical (OH•), and hypochlorous acid (HOCl), are mostly had high reactivity as well as low selectivity to their molecule target which contributes to oxidative stress. The third generation of ROS, such as nitrogen dioxide radical (NO2•), carbonate radical (CO3•), alkoxyl radical (RO•), and peroxyl radical (ROO•), is a potent oxidative stress inducer that causes cellular damage [9]. Therefore, ROS is an advantage and disadvantage due to its contribution to physiological and pathological conditions. Based on the analysis and synthesis of several references listed in Supplementary Table 1, we discuss the beneficial and harmful effects of ROS and how exercise can regulate ROS (for all supplementary material see www.cellphysiolbiochem.com).
The living organism must balance ROS production and its elimination rate and magnitude. Endogenous antioxidant enzymes and dietary antioxidants are needed to scavenge the excess ROS, thereby maintaining redox homeostasis. The human body has its antioxidant defense system, which includes superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT). SOD is an essential antioxidant that acts as a first-line defense mechanism against oxidative stress and ROS-mediated disease by catalyzing the conversion of highly reactive O2•- to H2O2, which is relatively stable. These enzymes have three isoforms, SOD1-3, located differently throughout the human body. The primary clearance of O2•- in the cytosol is SOD1, while SOD2 is mainly within the mitochondrial matrix. On the other hand, SOD3 is the primary protection against extracellular O2•- as it is secreted in the extracellular [17]. CAT is an enzyme that decomposes H2O2 into H2O. Meanwhile, GPX is a selenium-containing peroxide responsible for reducing H2O2 that comes in eight isoforms GPX1-8 [18]. Exogenous antioxidants, including vitamin C and E supplementation, and other minerals, like selenium (Se) and zinc (Zn), are shown to lower the concentration of ROS, thus preventing inflammation and muscles damages [19, 20]. Besides that, fruit and vegetables are rich sources of antioxidants that keep ROS at a flat rate [21, 22].
Oxidative stress is one of both promising and controversial theories of aging pathomechanism. Briefly, the theory says that the imbalance between pro-oxidants and antioxidants leads to oxidative damage, which results in cellular processes and the development of aging. Pro-oxidants include mitochondrial ROS and ROS-producing enzymes, such as xanthine oxidase (XO) and NOX [1]. To counter these pro-oxidants, there is an involvement of an endogenous antioxidant defense system composed of low molecular weight antioxidants (reduced glutathione [GSH], ascorbic acid tocopherols, etc.) and antioxidant enzymes. Endogenous antioxidants reduce ROS species to their less harmful form [9]. Kozakiewicz, et al. [23] showed a significant decrease in several antioxidant enzymes, such as Zn, Cu-superoxide dismutase (SOD-1), CAT, and glutathione peroxidase (GSH-Px), in older adults. The decreased activity of endogenous antioxidants is shown to cause higher levels of oxidative stress [24, 25]. In addition, some authors said that aging is closely related to increased ROS that scavenges NO. These findings indicate lower activities of primary antioxidant enzymes in older adults, indicating antioxidant defense impairment contributing to aging processes. Moreover, chronic oxidative stress leads to various consequences such as oxidant damage in several tissues, accelerated muscle damage, sarcopenia, and age-related degenerative diseases. The pathomechanism of oxidative stress in the aging process is depicted in Fig. 1.
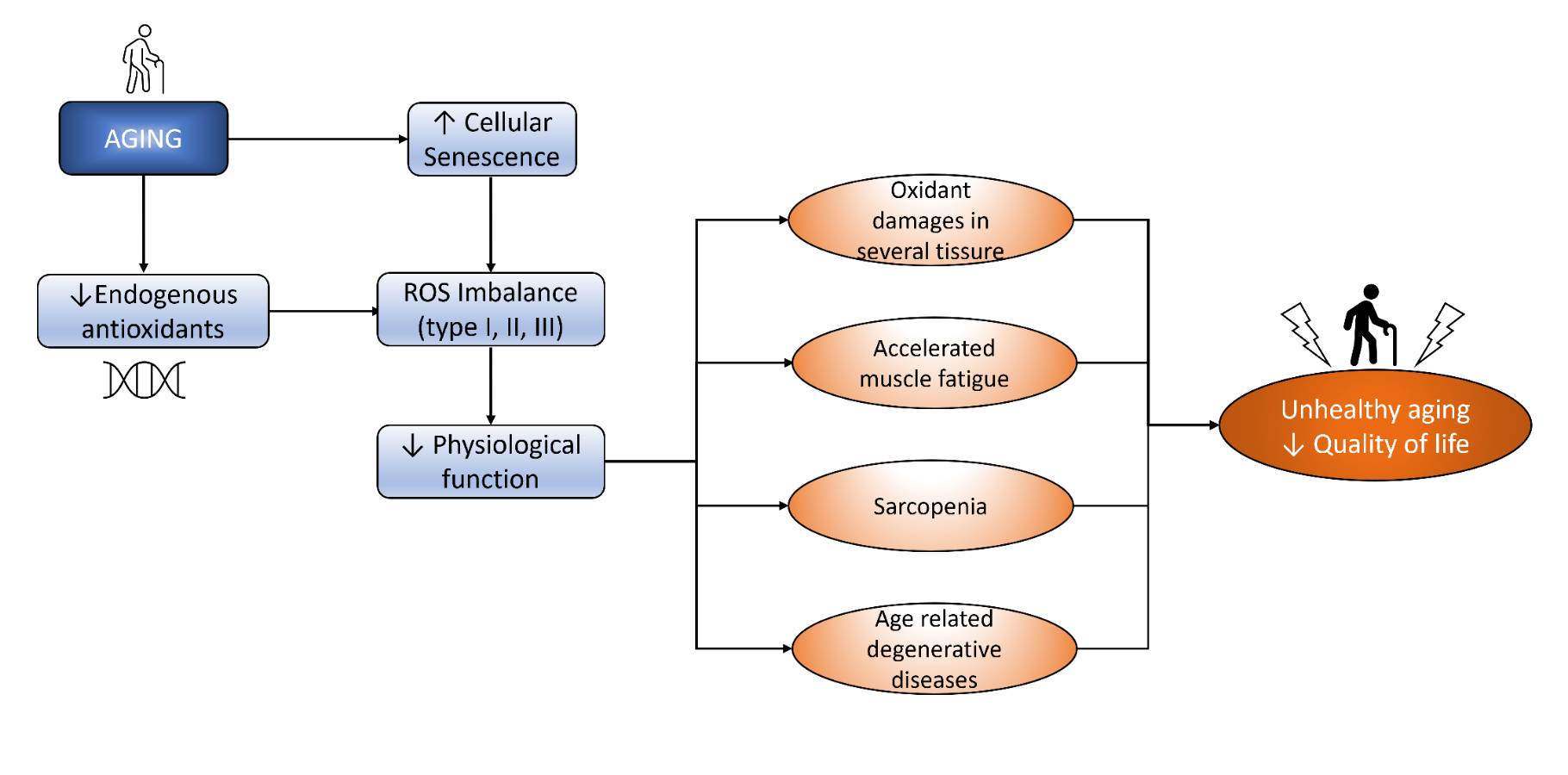
Fig. 1: Aging induces ROS imbalance that decreases physiological function and finally decreases QoL.
Oxidant Damages in Several Tissue
Oxidative damage occurs when the capacity of oxidative stress is exceeded due to the generation of ROS and RNS caused by increased oxygen flux. This condition leads to cell damage and cell death. An untrained individual is likelier to have significantly raised oxidative stress resulting in muscle damage, neutrophil activation, inflammation, and muscle soreness. One period of complete exercise may lead to peripheral fatigue, the temporary redox imbalance accumulation, and oxidative DNA damage as reflected in the elevated biomarkers of redox state and oxidative DNA damage based on the C242T polymorphism in the gene encoding NOXs subunit p22phox (CYBA) [26]. Furthermore, Moflehi et al. [27] stated that a single session of aerobic exercise causes significant increases in malondialdehyde (MDA), a marker of oxidative stress, and creatine kinase (CK), a marker of muscle damage.
On the other side, exercise can modulate oxidative stress and may protect against DNA damage. Besides pro-oxidants, exercise also causes an increase in antioxidants in the body, including SOD. Frequency, intensity, time, and type of exercise are the factors influencing whether the exercise cause more pro-oxidants or antioxidants. A study conducted by Cho et al. [26] revealed that a single bout of exhaustive exercise via inducing the increase of both pro-oxidant and antioxidant status, but the increase in antioxidant status is not enough to scavenge oxidant increase, thus leading to oxidative damage. On the other hand, Shin et al. [28] had shown that long-term aerobic exercise training with moderate intensity causes a significant increase in SOD activity and reduces oxidative damage from acute exercise with moderate and high intensity. This finding is in line with the theory from Metin et al. [29], which suggested that the increase of antioxidant status may be caused by the elevation of oxidative stress caused by regular exercise.
In addition, two significant factors affect oxidant and antioxidant balance in the body, including individual fitness level and BMI. A study conducted by Watson et al. [30] showed that antioxidant levels in an athlete are significantly higher compared to a sedentary individual with sex matches. These elevated antioxidant levels are parallel with lower lipid peroxidation, which is proven by lower MDA levels in athletes. Furthermore, BMI is closely related to oxidant production in the body. Obese individuals, compared to non-obese showed significantly higher ROS levels immediately after exercise [31]. Mechanisms regarding this finding remain unclear, but it is suggested that higher ROS levels in an obese individual are related to neurotrophic factors that influence Blood-Brain Barrier (BBB) disruption.
Accelerated Muscle Fatigue
Fatigue is shown to be correlated with oxidative stress. A study held on the non-aging population showed that higher oxidative stress index (OSI) and reactive oxygen metabolites-derived compounds (d-ROMs) with lower biological antioxidant potential (BAP) are found in chronic fatigue syndrome patients (patients) at rest than in healthy individuals. High OSI and d-ROMs are also found in healthy individuals following sub-acute and acute fatigue [32]. Together, ROS accumulation potentially accelerates muscle fatigue in older adults since they showed a significant decrease in antioxidant defense mechanisms.
There are many studies concerning exercise’s effect on age-related accelerated muscle fatigue. Several types of resistance training are given to the aging population, and there are positive outcomes regarding muscle activity. In mobility-limited older adults, progressive resistance training (PRT) increases their muscle strength and torque capacity. However, it does not influence the ability to recover from- and the magnitude of fatigue [33]. Drop-set resistance training increases muscle mass, strength, endurance, and functionality tasks. In addition to resistance exercise, different nutraceuticals are given to see if they would improve the gain of training alone. Combining creatine supplementation with training augments muscle mass gains and enables individuals to train to greater capacity over time. Note that this intervention is more effective in aging males than females [34]. The addition of whey to resistance exercise in frail elderly significantly improves muscle function, measured by handgrip strength, chair-stand ability, and walking speed [35]. However, the study conducted by Kirk et al. [36] revealed that a combination of leucine-enriched whey protein did not augment the improvement result of muscle fatigue and health-related quality of life (HR-QOL) after exercise alone.Nutraceuticals and pharmaceuticals can also be used to counteract muscle deterioration during aging. A healthy aging population who given an essential amino acid (EAA)-based multi-ingredient nutritional supplementation containing EAA, creatine, vitamin D, and muscle restores complex gain muscle strength, muscle power, and muscle mass that balance out more than a year of age-related loss of muscle mass and strength [37]. In the average aging population, oxidative stress is lowered from polyphenol-rich nutraceuticals, making them a potential agent. Green tea and sour tea (Hibiscus sabdariffa L.), both rich in flavonoid and polyphenols, significantly lessened malondialdehyde (MDA) and significantly increased total antioxidant capacity (TAC) level with sour tea consumption only [38]. Grape juice consumption also shares the same result as acidic tea consumption and lowers DNA damage, isoprostane, and lower muscle fatigue with higher upper limb strength results [22].
In the middle-aged population, beta-alanine (β-alanine) and phosphodiesterase-5 inhibitors sildenafil supplementation increases exercise endurance and muscle proteome [39, 40]. In summary, exercises, nutraceuticals, and pharmaceuticals can improve muscle performance in the aging population. A combination of nutraceuticals and exercises may add the improvement by exercise only.
Sarcopenia
Sarcopenia is an age-related loss of muscle mass and function caused by factors inherent to skeletal [41]. The pathogenesis of sarcopenia is multifactorial; however, several studies suggest that oxidative stress plays a major role in the development of sarcopenia [42].
ROS-induced oxidative in skeletal muscles leads to decreased muscle mass and reduced muscle fiber diameter. A proposed mechanism of oxidative-stress-enhanced proteolytic systems is initiated by modulating cysteine proteases, calpain, and caspase-3 that cause protein degradation and the breakdown of the sarcomere [43]. In addition, a study by Bak et al. [44] reported that oxidative stress in muscles stimulated myostatin expression. Interestingly, myostatin functions as a negative muscle regulator; if its concentration increases, sarcomeric protein synthesis will be inhibited [45]. These conclude that excessive ROS results in decreased muscle protein turnover by simultaneously augmenting proteolysis and attenuating protein synthesis.
Accumulation of ROS also induces oxidative damage to proteins involved in the excitation-coupling response, resulting in decreased muscle contractile function [46]. Furthermore, it also causes impairment in the neuromuscular junction and SERCA pump, further impairing muscle function [47]. Thus, a disused muscle would eventually become atrophy and bear mitochondrial dysfunction, leading to further ROS production.
Mitochondria, as a ROS generator, could accumulate ROS that damages the mtDNA and cause defective production of the electron transport chains, resulting in a vicious cycle of continual ROS production [48]. Excessive ROS is also correlated to an altered mitochondria morphology marked by a thickened outer membrane, decreased membrane potential, and increased mitochondrial fission proteins, such as Drp1, that cause mitochondria fragmentation [44].
Age-related Degenerative Disease
It is well known that aging and age-related diseases are the consequences of oxidative stress [5]. Neurodegenerative diseases have been linked with the disturbances in redox homeostasis in the brain with increasing age. ROS production in specific brain regions and lowered antioxidant function cause oxidative damage leading to neuronal death and neurodegeneration associated with Parkinson’s disease and Alzheimer’s [49–51]. The study showed a progressive age-related rise in protein nitration and oxidation with a gradual decrease in SOD, CAT, and GSH in the postmortem human brain of individuals between 0.01 to 80 years [50]. Moreover, the accumulation of unrepaired damage due to oxidative damage in the aging brain is responsible for neurodegeneration [52]. In addition, interleukin (IL)-1, IL-6, TNF-α, and chemokines are released as ROS production increases, leading to neuroinflammatory processes. A large amount of ROS causes chronic stress, thus leading to cell death and dementia [53].
Excess ROS also contributes to cardiovascular diseases by deteriorating endothelial function. As aging increases ROS, the NO bioavailability is compromised in the systemic circulation and the musculature in older humans, thus further inducing endothelial dysfunction [54]. Studies have also shown that vascular oxidative stress is mainly caused by O 2•- as the inhibition of its main generator (NADPH oxidase) has been shown to reduce arterial oxidative stress and normalize endothelial dysfunction in mice [55]. Respiratory diseases are caused by increased ROS production triggered by environmental exposure, primarily cigarette smoking, various toxicants, and infectious agents resulting in oxidative stress. Oxidative stress causes cellular damage by lipid peroxidation, protein oxidation, and DNA histone modification [56, 57]. Moreover, elevated ROS levels trigger a cascade of events that produce pro-inflammatory mediators that promote inflammation. Inflammation further enhances ROS production and contributes to detrimental pathological characteristics in pulmonary disease. A study demonstrated erdosteine could reduce oxidative stress in patients with severe COPD, thus alleviating the symptoms and highlighting that oxidative stress is one major contributor to the pathogenesis of respiratory disease [58].
Exercise Produces both ROS and Antioxidant
Although it was believed that ROS is pathological, recent studies have also shown that a relative amount of ROS elicits a physiological role. Exercise has increased ROS production [59], producing a ROS signaling response [60]. These findings are further confirmed by exploring exercise markers upon NOX knockout. Even after exercise, the examination of mice skeletal tissue with NOX knockout showed no significant increases in specific antioxidant enzymes (SOD2, catalase) and mitochondrial protein levels (mitochondrial complex I, pyruvate dehydrogenase, OPA1, and mitofusin 2) [61], and no difference in metabolic responses [62, 63]. Investigation of the white adipose tissue also showed impaired exercise adaptation markers (Nrf2, HMOX, SOD1, CAT) upon NOX knockout [64]. These findings suggest that exercise-induced ROS induces beneficial adaptation.
Considering that ROS could be either pathological or physiological, it is suggested that ROS has a biphasic effect, beneficial up to a certain point and delirious with increasing levels. A study on ischemia/reperfusion injury showed that dosing mitoPQ results in an increase in ROS production. Low doses protect myocytes, but higher doses cause myocyte dysfunction and death [65]. Another study showed the biphasic effect of stimulating ROS production by gelatin treatment to injured skeletal muscle. A low dose of gelatin results in an increased antioxidant response, tissue adaptation to mild stress, myogenesis, and muscle regeneration. A high amount of gelatin causes ROS overproduction, leading to a worse tissue injury [66]. Therefore, ROS has a biphasic effect.
Although ROS has been shown to have a biphasic effect, the cutoff point between physiological and pathological ROS has not been defined. Most studies showed that the level of ROS is a determining factor between physiological and pathological ROS. However, there might also be other parameters for the biphasic cutoff point. A recent review classified ROS into several types based on its structure, compartmentalization, and reactivity [67]. The kind of ROS is potentially crucial in determining the cutoff point. However, current studies have not investigated the importance of ROS type in elucidating this biphasic effect. Therefore, further studies could explore the impact of ROS types in determining the cutoff point of the ROS biphasic effect.
ROS can be modulated by physical activity. There are different impacts of acute and chronic physical activity on oxidative stress. Acute exercise induces reactive oxygen, nitrogen species, and oxidative stress. Still, regular exercise training causes the endogenous antioxidative system and protects the body against adverse effects of oxidative damage [68].
This statement is supported by Nyberg et al.’s study [54], which stated that lifelong physical activity opposes compromised NO in systemic circulation and skeletal muscle of sedentary old individuals. He et al. [69] found that exercise training can alter antioxidant capacity in skeletal muscle. This finding is supported by Powers et al. [70]. They stated that high-intensity exercise training with all kinds of duration and low and moderate-intensity exercise training with an extended period elevate SOD activity in the myocardium. Lots of pathways suggest mechanisms that lie behind the protective effect of ROS. Research by Yavari et al. [71] showed that mitochondrial ROS produced during regular exercise is needed to activate primary signaling pathways associated with muscle adaptation. Acute exercise stress (AES) will activate signaling of Nrf2/ARE (antioxidant response element) and subsequent enhancement of antioxidant defense pathways in wild-type (WT) mouse hearts [72].
In contrast, oxidative stress and blunted defense mechanisms were observed in Nrf2-/- mice [72]. Furthermore, Nrf2 is found to control neuronal survival in aging. On the other hand, Nrf2 also scavenges and controls ROS production via NADPH oxidase [73].
Besides that, ROS can also be regulated by exogenous antioxidants. Supplementation of antioxidants is reported to attenuate injury from strenuous resistance exercise [74]. Before exercise, acutely elevated antioxidant levels post-exercise, ascorbic acid supplementation–antioxidant protecting cellular components from radical damage. The beneficial effect of ascorbic acid supplementation is that they interfere with glucose utilization or transportation, thus leading to lower production of ROS.
Moderate elevation of mitochondrial oxidants has been shown to enhance systemic defense through adaptive response, termed mitohormesis [75, 76]. However, this does not apply to hydrogen peroxide. Based on a recent review, supraphysiological H 2O2 level, measured at more than 100 nM, leads to cellular growth arrest and cell death. This state, that is to say, oxidative distress, correlates to pathologies [77]. Bladier et al. [78] showed a senescence-like state response in fibroblast that is given 50-100 μM H2O2 and apoptosis on 300-400 μM H 2O2.
The redox signaling generates a response from transcription factors p53 and Nrf-2 that regulate antioxidant gene expressions. The activation of tumor suppressor 53 (p53) is modulated by H 2O2 [79]. The Nrf2-KEAP1 system is the primary sensor of oxidative stress. Oxidation leads to the conformational change of KEAP1 (Nrf2 inhibitor), which prevents Nrf2 ubiquitylation and, therefore, will increase Nrf2 stability [80]. Within the cellular level, the membrane-associated phospholipid hydroperoxide glutathione peroxidase (GPX-4) prevents lipid hydroperoxides accumulation in the plasma membrane [81]. In the nucleus, DNA replication and telomere length maintenance regulation by peroxiredoxin 2 and peroxiredoxin 1, respectively, is sensitive to oxidants. In response to low oxidant levels, peroxiredoxin 2 promotes replication fork progression. Elevated H 2O2 oxidizes and dissociates peroxiredoxin 2 and thus slowing down the replication fork. Higher oxidants level suppresses the synthesis of deoxynucleotide triphosphate (dNTPs) [82]. Peroxiredoxin 1 counters oxidative damage in telomeric DNA and promotes telomere elongation with 7,8-dihydro-8-oxoguanine triphosphate (MTH1) [83]. In the mitochondria, different levels of oxidant elicit different responses. Low oxidant level triggers mitophagy and selective removal of mitochondria. Higher oxidant level ends up in non-selective autophagy termed macroautophagy [84]. The endoplasmic reticulum has glutathione peroxidase and peroxiredoxins that scavenge H 2O2 to water [85].
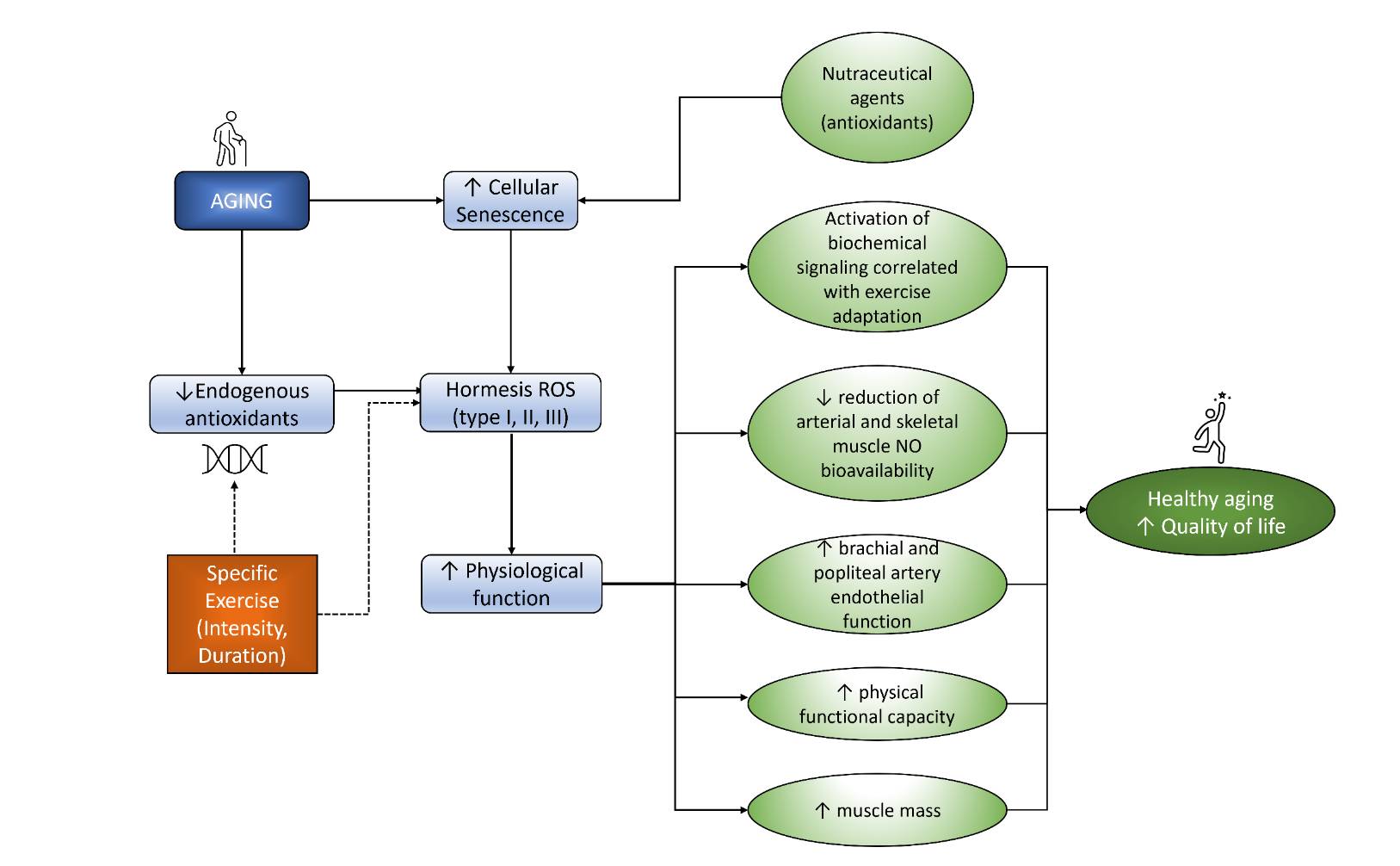
Fig. 2: Exercise and nutraceutical agents modulate hormesis ROS that increased physiological function leads to increased QoL.
Conclusion
All this evidence showed that giving specific exercise to older people can delay aging by inhibiting the decrease of endogenous antioxidants and ROS formation. This inhibition eventually will lead to physiological function improvement. In addition, the supplementation of antioxidant nutraceutical agents will support the inhibition of cellular aging. As a result, as seen in Fig. 2, exercise and antioxidant supplementation will lead to a healthy aging process and enhance the quality of life of the elderly.Method
Identification of the articles used in this review starts from searching the electronic database using the keywords aging, antioxidant, exercise, oxidative stress, and ROS. After screening and assessing the articles, thirty-four articles were used for discussion in this review. The detailed flow of article selection is represented in Fig. 3.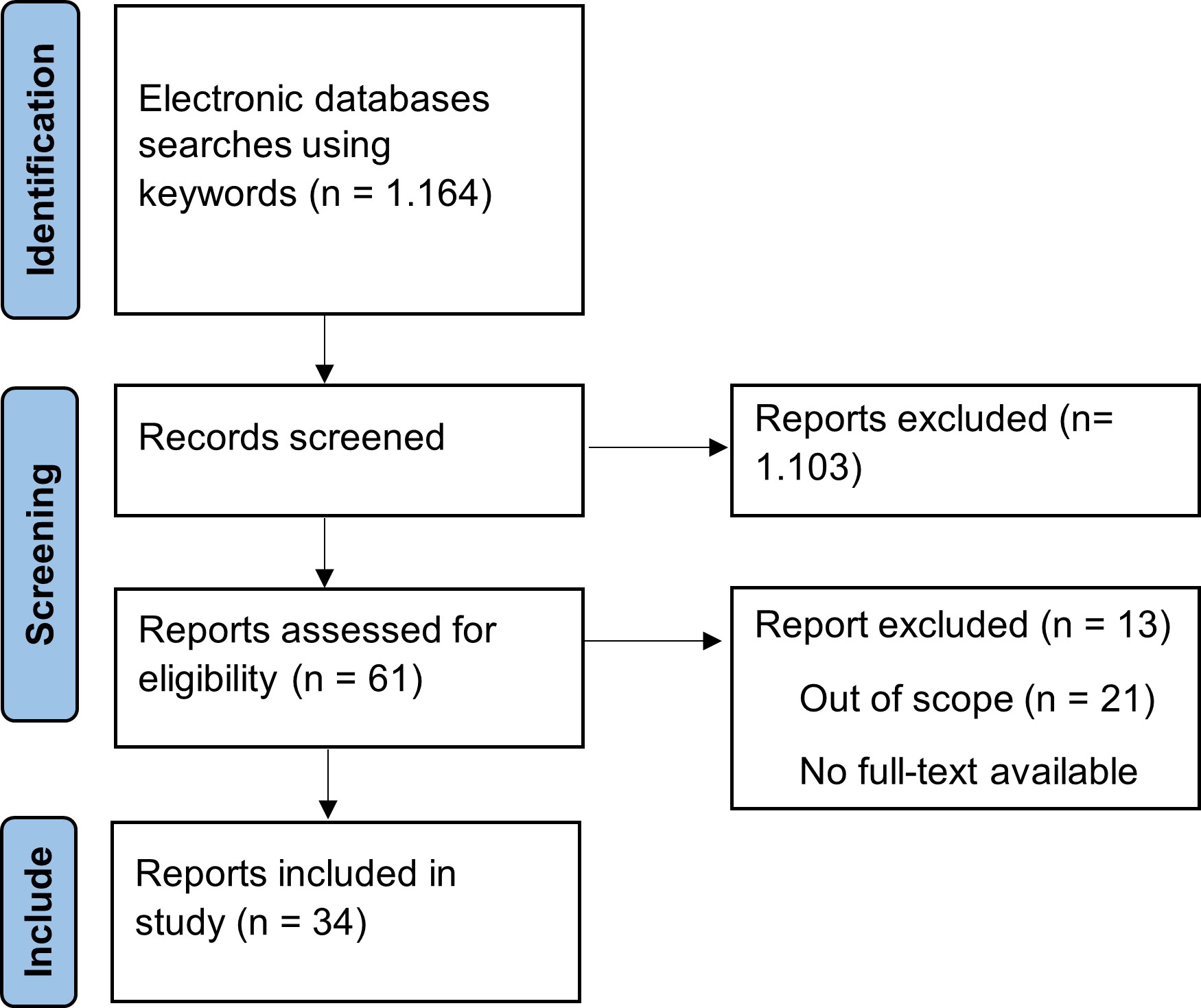
Fig. 3: Article collection flow.
Acknowledgements
We thank our colleagues from the Physiology Division Department of Biomedical Science Faculty of Medicine Universitas Padjadjaran, who provided insight for this paper.
Author Contributions
All authors contributed significantly to the conception and design of this review. RL, CP, GFM, JFW, and NJP gathered and analyzed the information. All authors contributed to the original draft, and RL, PTR, and JWG critically revised it. All authors approved the final version submitted for publication and take responsibility for statements made in the published article.
Funding Sources
This work was supported by funding from Grant Research from Ministry Education, Culture and Technology (Number: 1207/UN6.3.1/PT.00/2021) to RL.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare that no conflict of interests exists.
References
| 1 | Robinson AR, Yousefzadeh MJ, Rozgaja TA, Wang J, Li X, Tilstra JS, Feldman CH, Gregg SQ, Johnson CH, Skoda EM, Frantz MC, Bell-Temin H, Pope-Varsalona H, Gurkar AU, Nasto LA, Robinson RAS, Fuhrmann-Stroissnigg H, Czerwinska J, McGowan SJ, Cantu-Medellin N, et al.: Spontaneous DNA damage to the nuclear genome promotes senescence, redox imbalance and aging. Redox Biol 2018;17:259-273. https://doi.org/10.1016/j.redox.2018.04.007 |
| 2 | Chen L, Yang R, Qiao W, Zhang W, Chen J, Mao L, Goltzman D, Miao D: 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 2019;18:12951. https://doi.org/10.1111/acel.12951 |
| 3 | Salunkhe S, Mishra S v, Nair J, Shah S, Gardi N, Thorat R, Sarkar D, Rajendra J, Kaur E, Dutt S: Nuclear localization of p65 reverses therapy-induced senescence. J Cell Sci 2021;134:jcs253203. https://doi.org/10.1242/jcs.253203 |
| 4 | Chatelain M, Drobniak SM, Szulkin M: The association between stressors and telomeres in non-human vertebrates: a meta-analysis. Ecol Lett 2020;23:381-398. https://doi.org/10.1111/ele.13426 |
| 5 | Nair RR, Bagheri M, Saini DK: Temporally distinct roles of ATM and ROS in genotoxic-stress-dependent induction and maintenance of cellular senescence. J Cell Sci 2015;128:342-353. https://doi.org/10.1242/jcs.159517 |
| 6 | Bartlett DB, Slentz CA, Willis LH, Hoselton A, Huebner JL, Kraus VB, Moss J, Muehlbauer MJ, Spielmann G, Muoio DM, Koves TR, Wu H, Huffman KM, Lord JM, Kraus WE: Rejuvenation of Neutrophil Functions in Association With Reduced Diabetes Risk Following Ten Weeks of Low-Volume High Intensity Interval Walking in Older Adults With Prediabetes - A Pilot Study. Front Immunol 2020;11:729. https://doi.org/10.3389/fimmu.2020.00729 |
| 7 | Noh JH, Kim KM, Idda ML, Martindale JL, Yang X, Kotb A, Gorospe M: GRSF1 suppresses cell senescence. Aging 2018;10:1856-1866. https://doi.org/10.18632/aging.101516 |
| 8 | Flack KD, Davy BM, Deberardinis M, Boutagy NE, Mcmillan RP, Hulver MW, Frisard MI, Anderson AS, Savla J, Davy KP: Resistance exercise training and in vitro skeletal muscle oxidative capacity in older adults. Physiol Rep 2016;4:12849. https://doi.org/10.14814/phy2.12849 |
| 9 | Zhang L, Wang X, Cueto R, Effi C, Zhang Y, Tan H, Qin X, Ji Y, Yang X, Wang H: Biochemical basis and metabolic interplay of redox regulation. Redox Biol 2019;26:101284. https://doi.org/10.1016/j.redox.2019.101284 |
| 10 | de Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F: Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 2000;275:23227-23233. https://doi.org/10.1074/jbc.M000916200 |
| 11 | Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naitoi S, Hattori M, Sakaki Y, Sumimoto H: A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 2001;276:1417-1423. https://doi.org/10.1074/jbc.M007597200 |
| 12 | Kikuchi H, Hikage M, Miyashita H, Fukumoto M: NADPH oxidase subunit, gp91phox homologue, preferentially expressed in human colon epithelial cells. Gene 2000;254:237-243. https://doi.org/10.1016/S0378-1119(00)00258-4 |
| 13 | Bánfi B, Molnár G, Maturana A, Steger K, Hegedûs B, Demaurex N, Krause KH: A Ca2+-activated NADPH Oxidase in Testis, Spleen, and Lymph Nodes. J Biol Chem 2001;276:37594-37601. https://doi.org/10.1074/jbc.M103034200 |
| 14 | Bánfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH: NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 2004;279:46065-46072. https://doi.org/10.1074/jbc.M403046200 |
| 15 | Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX: Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCe{open} signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223-1231. https://doi.org/10.1016/j.freeradbiomed.2008.06.012 |
| 16 | Mihaylova MM, Shaw RJ: The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011;13:1016-1023. https://doi.org/10.1038/ncb2329 |
| 17 | Fukai T, Ushio-Fukai M: Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 2011;15:1583-1606. https://doi.org/10.1089/ars.2011.3999 |
| 18 | Toppo S, Vanin S, Bosello V, Tosatto SCE: Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid Redox Signal 2008;10:1501-1513. https://doi.org/10.1089/ars.2008.2057 |
| 19 | Yimcharoen M, Kittikunnathum S, Suknikorn C, Nak-On W, Yeethong P, Anthony TG, Bunpo P: Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J Int Soc Sports Nutr 2019;16:2. https://doi.org/10.1186/s12970-019-0269-8 |
| 20 | White SH, Warren LK: Submaximal exercise training, more than dietary selenium supplementation, improves antioxidant status and ameliorates exercise-induced oxidative damage to skeletal muscle in young equine athletes. J Anim Sci 2017;95:657-670. https://doi.org/10.2527/jas.2016.1130 |
| 21 | Harms-Ringdahl M, Jenssen D, Haghdoost S: Tomato juice intake suppressed serum concentration of 8-oxodG after extensive physical activity. Nutr J 2012;11:29. https://doi.org/10.1186/1475-2891-11-29 |
| 22 | Goulart MJVC, Pisamiglio DS, Moller GB, Dani C, Alves FD, Bock PM, Schneider CD: Effects of grape juice consumption on muscle fatigue and oxidative stress in judo athletes: a randomized clinical trial. An Acad Bras Cienc 2020;92:e20191551. https://doi.org/10.1590/0001-3765202020191551 |
| 23 | Kozakiewicz M, Kornatowski M, Krzywińska O, Kędziora-Kornatowska K: Changes in the blood antioxidant defense of advanced age people. Clin Interv Aging 2019;14:763-771. https://doi.org/10.2147/CIA.S201250 |
| 24 | Xiong W, Garfinkel AEMC, Li Y, Benowitz LI, Cepko CL: NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest 2015;125:1433-1445. https://doi.org/10.1172/JCI79735 |
| 25 | Matsuzaki S, Eyster C, Newhardt MF, Giorgione JR, Kinter C, Young ZT, Kinter M, Humphries KM: Insulin signaling alters antioxidant capacity in the diabetic heart. Redox Biol 2021;47:102140. https://doi.org/10.1016/j.redox.2021.102140 |
| 26 | Cho SY, So WY, Roh HT: Effect of C242T Polymorphism in the Gene Encoding the NAD(P)H Oxidase p22 phox Subunit and Aerobic Fitness Levels on Redox State Biomarkers and DNA Damage Responses to Exhaustive Exercise: A Randomized Trial. Int J Environ Res Public Health 2020;17:4215. https://doi.org/10.3390/ijerph17124215 |
| 27 | Moflehi D, Kok LY, Tengku-Kamalden TF, Amri S: Effect of single-session aerobic exercise with varying intensities on lipid peroxidation and muscle-damage markers in sedentary males. Glob J Health Sci 2012;4:48-54. https://doi.org/10.5539/gjhs.v4n4p48 |
| 28 | Shin YA, Lee JH, Song W, Jun TW: Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech Ageing Dev 2008;129:254-260. https://doi.org/10.1016/j.mad.2008.01.001 |
| 29 | Metin G, Atukeren P, Alturfan AA, Gülyaşar T, Kaya M, Gümüştaş MK: Lipid Peroxidation, Erythrocyte Superoxide-Dismutase Activity and Trace Metals in Young Male Footballers. Yonsei Med J 2003;44:979. https://doi.org/10.3349/ymj.2003.44.6.979 |
| 30 | Watson TA, MacDonald-Wicks LK, Garg ML: Oxidative Stress and Antioxidants in Athletes Undertaking Regular Exercise Training. Int J Sport Nutr Exerc Metab 2005;15:131-146. https://doi.org/10.1123/ijsnem.15.2.131 |
| 31 | Roh HT, Cho SY, So WY: Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise. J Sport Health Sci 2017;6:225-230. https://doi.org/10.1016/j.jshs.2016.06.005 |
| 32 | Fukuda S, Nojima J, Motoki Y, Yamaguti K, Nakatomi Y, Okawa N, Fujiwara K, Watanabe Y, Kuratsune H: A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol Psychol 2016;118:88-93. https://doi.org/10.1016/j.biopsycho.2016.05.005 |
| 33 | Englund DA, Price LL, Grosicki GJ, Iwai M, Kashiwa M, Liu C, Reid KF, Fielding RA: Progressive Resistance Training Improves Torque Capacity and Strength in Mobility-Limited Older Adults. J Gerontol A Biol Sci Med Sci 2019;74:1316-1321. https://doi.org/10.1093/gerona/gly199 |
| 34 | Johannsmeyer S, Candow DG, Brahms CM, Michel D, Zello GA: Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Exp Gerontol 2016;83:112-119. https://doi.org/10.1016/j.exger.2016.08.005 |
| 35 | Kang L, Gao Y, Liu X, Liang Y, Chen Y, Liang Y, Zhang L, Chen W, Pang H, Peng LN: Effects of whey protein nutritional supplement on muscle function among community-dwelling frail older people: A multicenter study in China. Arch Gerontol Geriatr 2019;83:7-12. https://doi.org/10.1016/j.archger.2019.03.012 |
| 36 | Kirk B, Mooney K, Cousins R, Angell P, Jackson M, Pugh JN, Coyles G, Amirabdollahian F, Khaiyat O: Effects of exercise and whey protein on muscle mass, fat mass, myoelectrical muscle fatigue and health-related quality of life in older adults: a secondary analysis of the Liverpool Hope University-Sarcopenia Ageing Trial (LHU-SAT). Eur J Appl Physiol 2020;120:493-503. https://doi.org/10.1007/s00421-019-04293-5 |
| 37 | Negro M, Perna S, Spadaccini D, Castelli L, Calanni L, Barbero M, Cescon C, Rondanelli M, D'Antona G: Effects of 12 Weeks of Essential Amino Acids (EAA)-Based Multi-Ingredient Nutritional Supplementation on Muscle Mass, Muscle Strength, Muscle Power and Fatigue in Healthy Elderly Subjects: A Randomized Controlled Double-Blind Study. J Nutr Health Agin 2019;23:414-424. https://doi.org/10.1007/s12603-019-1163-4 |
| 38 | Hadi A, Pourmasoumi M, Kafeshani M, Karimian J, Maracy MR, Entezari MH: The Effect of Green Tea and Sour Tea (Hibiscus sabdariffa L.) Supplementation on Oxidative Stress and Muscle Damage in Athletes. J Diet Suppl 2016;14:346-357. https://doi.org/10.1080/19390211.2016.1237400 |
| 39 | Furst T, Massaro A, Miller C, Williams BT, LaMacchia ZM, Horvath PJ: β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J Int Soc Sports Nutr 2018;15:32. https://doi.org/10.1186/s12970-018-0238-7 |
| 40 | Sheffield-Moore M, Wiktorowicz JE, Soman KV, Danesi CP, Kinsky MP, Dillon EL, Randolph KM, Casperson SL, Gore DC, Horstman AM, Lynch JP, Doucet BM, Mettler JA, Ryder JW, Ploutz-Snyder LL, Hsu JW, Jahoor F, Jennings K, White GR, Mccammon SD, et al.: Sildenafil Increases Muscle Protein Synthesis and Reduces Muscle Fatigue. Clin Transl Sci 2013;6:463-468. https://doi.org/10.1111/cts.12121 |
| 41 | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP 2: Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. https://doi.org/10.1093/ageing/afy169 |
| 42 | Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, Rosenthal N, Scicchitano BM, Musarò A: Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019;18:e12954. https://doi.org/10.1111/acel.12954 |
| 43 | Jang YC, Rodriguez K, Lustgarten MS, Muller FL, Bhattacharya A, Pierce A, Choi JJ, Lee NH, Chaudhuri A, Richardson AG, Van Remmen H: Superoxide-mediated oxidative stress accelerates skeletal muscle atrophy by synchronous activation of proteolytic systems. Geroscience 2020;42:1579-1591. https://doi.org/10.1007/s11357-020-00200-5 |
| 44 | Bak DH, Na J, Im SI, Oh CT, Kim JY, Park SK, Han HJ, Seok J, Choi SY, Ko EJ, Mun SK, Ahn SW, Kim BJ: Antioxidant effect of human placenta hydrolysate against oxidative stress on muscle atrophy. J Cell Physiol 2019;234:1643-1658. https://doi.org/10.1002/jcp.27034 |
| 45 | Rodriguez J, Vernus B, Chelh I, Cassar-Malek I, Gabillard JC, Hadj Sassi A, Seiliez I, Picard B, Bonnieu A: Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci 2014;71:4361-4371. https://doi.org/10.1007/s00018-014-1689-x |
| 46 | Qaisar R, Bhaskaran S, Premkumar P, Ranjit R, Natarajan KS, Ahn B, Riddle K, Claflin DR, Richardson A, Brooks SV, Van Remmen H: Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J Cachexia Sarcopenia Muscle 2018;9:1003-1017. https://doi.org/10.1002/jcsm.12339 |
| 47 | Xu H, Ranjit R, Richardson A, Van Remmen H: Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia. J Cachexia Sarcopenia Muscle 2021;12:1582-1596. https://doi.org/10.1002/jcsm.12768 |
| 48 | Szczesny B, Olah G, Walker DK, Volpi E, Rasmussen BB, Szabo C, Mitra S: Deficiency in repair of the mitochondrial genome sensitizes proliferating myoblasts to oxidative damage. PLoS One 2013;8:e75201. https://doi.org/10.1371/journal.pone.0075201 |
| 49 | Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, Srinivas Bharath MM, Shankar SK: Evaluation of Markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson's disease brains. Neurochem Res 2011;36:1452-1463. https://doi.org/10.1007/s11064-011-0471-9 |
| 50 | Venkateshappa C, Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK: Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: Implications for neurodegeneration in Alzheimer's disease. Neurochem Res 2012;37:1601-1614. https://doi.org/10.1007/s11064-012-0755-8 |
| 51 | Angelova PR, Horrocks MH, Klenerman D, Gandhi S, Abramov AY, Shchepinov MS: Lipid peroxidation is essential for α-synuclein-induced cell death. J Neurochem 2015;133:582-589. https://doi.org/10.1111/jnc.13024 |
| 52 | Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Flint Beal M, Wallace DC: Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet 1992;2:324-329. https://doi.org/10.1038/ng1292-324 |
| 53 | Sochocka M, Koutsouraki E, Gasiorowski K, Leszek J: Vascular Oxidative Stress and Mitochondrial Failure in the Pathobiology of Alzheimer's Disease: A New Approach to Therapy. CNS Neurol Disord Drug Targets 2013;12:870-881. https://doi.org/10.2174/18715273113129990072 |
| 54 | Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP: Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 2012;590:5361-5370. https://doi.org/10.1113/jphysiol.2012.239053 |
| 55 | Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA: Voluntary wheel running restores endothelial function in conduit arteries of old mice: Direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 2009;587:3271-3285. https://doi.org/10.1113/jphysiol.2009.169771 |
| 56 | Siu GM, Draper HH: Metabolism of malonaldehyde in vivo and in vitro. Lipids 1982;17:349. https://doi.org/10.1007/BF02535193 |
| 57 | Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER: Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186:464-478. https://doi.org/10.1016/0076-6879(90)86141-H |
| 58 | Dal Negro RW, Visconti M: Erdosteine reduces the exercise-induced oxidative stress in patients with severe COPD: Results of a placebo-controlled trial. Pulm Pharmacol Ther 2016;41:48-51. https://doi.org/10.1016/j.pupt.2016.09.007 |
| 59 | Wang P, Li CG, Qi Z, Cui D, Ding S: Acute exercise induced mitochondrial H2O2 production in mouse skeletal muscle: Association with p66Shc and FOXO3a signaling and antioxidant enzymes. Oxid Med Cell Longev 2015;2015:536456. https://doi.org/10.1155/2015/536456 |
| 60 | Wang P, Li CG, Qi Z, Cui D, Ding S: Acute exercise stress promotes Ref1/Nrf2 signalling and increases mitochondrial antioxidant activity in skeletal muscle. Exp Physiol 2016;101:410-420. https://doi.org/10.1113/EP085493 |
| 61 | Henríquez-Olguín C, Renani LB, Arab-Ceschia L, Raun SH, Bhatia A, Li Z, Knudsen JR, Holmdahl R, Jensen TE: Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol 2019;24:101188. https://doi.org/10.1016/j.redox.2019.101188 |
| 62 | Henríquez-Olguin C, Knudsen JR, Raun SH, Li Z, Dalbram E, Treebak JT, Sylow L, Holmdahl R, Richter EA, Jaimovich E, Jensen TE: Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat Commun 2019;10:1-11. https://doi.org/10.1038/s41467-019-12523-9 |
| 63 | Specht KS, Kant S, Addington AK, McMillan RP, Hulver MW, Learnard H, Campbell M, Donnelly SR, Caliz AD, Pei Y, Reif MM, Bond JM, DeMarco A, Craige B, Keaney JF, Craige SM: Nox4 mediates skeletal muscle metabolic responses to exercise. Mol Metab 2021;45:101160. https://doi.org/10.1016/j.molmet.2020.101160 |
| 64 | Matta L, Fonseca TS, Faria CC, Lima-Junior NC, De Oliveira DF, Maciel L, Boa LF, Pierucci APTR, Ferreira ACF, Nascimento JHM, Carvalho DP, Fortunato RS: The Effect of Acute Aerobic Exercise on Redox Homeostasis and Mitochondrial Function of Rat White Adipose Tissue. Oxid Med Cell Longev 2021;2021:4593496. https://doi.org/10.1155/2021/4593496 |
| 65 | Antonucci S, Mulvey JF, Burger N, Di Sante M, Hall AR, Hinchy EC, Caldwell ST, Gruszczyk A V., Deshwal S, Hartley RC, Kaludercic N, Murphy MP, Di Lisa F, Krieg T: Selective mitochondrial superoxide generation in vivo is cardioprotective through hormesis. Free Radic Biol Med 2019;134:678-687. https://doi.org/10.1016/j.freeradbiomed.2019.01.034 |
| 66 | Liu X, Zu E, Chang X, Ma X, Wang Z, Song X, Li X, Yu Q, Kamei KI, Hayashi T, Mizuno K, Hattori S, Fujisaki H, Ikejima T, Wang DO: Bi-phasic effect of gelatin in myogenesis and skeletal muscle regeneration. Dis Model Mech 2021;14:dmm049290. https://doi.org/10.1242/dmm.049290 |
| 67 | Zhang L, Wang X, Cueto R, Effi C, Zhang Y, Tan H, Qin X, Ji Y, Yang X, Wang H: Biochemical basis and metabolic interplay of redox regulation. Redox Biol 2019;26:101284. https://doi.org/10.1016/j.redox.2019.101284 |
| 68 | Gökbel H: ACUTE EXERCISE INDUCED OXIDATIVE STRESS AND ANTIOXIDANT CHANGES. Eur J Gen Med 2006;3:126-131. https://doi.org/10.29333/ejgm/82392 |
| 69 | He F, Li J, Liu Z, Chuang CC, Yang W, Zuo L: Redox Mechanism of Reactive Oxygen Species in Exercise. Front Physiol 2016;7:486. https://doi.org/10.3389/fphys.2016.00486 |
| 70 | Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, Herb RA: Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol 1993;265:H2094-H2098. https://doi.org/10.1152/ajpheart.1993.265.6.H2094 |
| 71 | Yavari A, Javadi M, Mirmiran P, Bahadoran Z: Exercise-Induced Oxidative Stress and Dietary Antoxidative. Asian J Sports Med 2015;6:e24898. https://doi.org/10.5812/asjsm.24898 |
| 72 | Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L, Rajasekaran NS: Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med 2012;52:366-376. https://doi.org/10.1016/j.freeradbiomed.2011.10.440 |
| 73 | Kovac S, Angelova PR, Holmström KM, Zhang Y, Dinkova-Kostova AT, Abramov AY: Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta 2015;1850:794. https://doi.org/10.1016/j.bbagen.2014.11.021 |
| 74 | Ives SJ, Bloom S, Matias A, Morrow N, Martins N, Roh Y, Ebenstein D, O'Brien G, Escudero D, Brito K, Glickman L, Connelly S, Arciero PJ: Effects of a combined protein and antioxidant supplement on recovery of muscle function and soreness following eccentric exercise. J Int Soc Sports Nutr 2017;14:21. https://doi.org/10.1186/s12970-017-0179-6 |
| 75 | Tapia PC: Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary p. Med Hypotheses 2006;66:832-843. https://doi.org/10.1016/j.mehy.2005.09.009 |
| 76 | Ristow M, Schmeisser K: Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose-Response 2014;12:288-341. https://doi.org/10.2203/dose-response.13-035.Ristow |
| 77 | Sies H, Jones DP: Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 2020;21:363-383. https://doi.org/10.1038/s41580-020-0230-3 |
| 78 | Bladier C, Wolvetang EJ, Hutchinson P, De Haan JB, Kola I: Response of a primary human fibroblast cell line to H2O2: senescence-like growth arrest or apoptosis? Cell Growth Differ 1997;8:589-598. |
| 79 | Sablina AA, Budanov A V, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM: The antioxidant function of the p53 tumor suppressor. Nat Med 2005;11:1306-1313. https://doi.org/10.1038/nm1320 |
| 80 | Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M: The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 2009;29:493-502. https://doi.org/10.1128/MCB.01080-08 |
| 81 | Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, Mehr L, Aichler M, Walch A, Lamp D, Jastroch M, Miyamoto S, Wurst W, Ursini F, Arnér ESJ, Fradejas-Villar N, et al.: Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018;172:409-422.e21. https://doi.org/10.1016/j.cell.2017.11.048 |
| 82 | Somyajit K, Gupta R, Sedlackova H, Neelsen KJ, Ochs F, Rask MB, Choudhary C, Lukas J: Redox-sensitive alteration of replisome architecture safeguards genome integrity. Science 2017;358:797-802. https://doi.org/10.1126/science.aao3172 |
| 83 | Ahmed W, Lingner J: PRDX1 and MTH1 cooperate to prevent ROS-mediated inhibition of telomerase. Genes Dev 2018;32:658-669. https://doi.org/10.1101/gad.313460.118 |
| 84 | Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS: Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta 2012;1823:2297-2310. https://doi.org/10.1016/j.bbamcr.2012.08.007 |
| 85 | Siegenthaler KD, Sevier CS: Working Together: Redox Signaling between the Endoplasmic Reticulum and Mitochondria. Chem Res Toxicol 2019;32:342-344. https://doi.org/10.1021/acs.chemrestox.8b00379 |
| 86 | Bartlett DB, Willis LH, Slentz CA, Hoselton A, Kelly L, Huebner JL, Kraus VB, Moss J, Muehlbauer MJ, Spielmann G, Kraus WE, Lord JM, Huffman KM: Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: A pilot study. Arthritis Res Ther 2018;20:127. https://doi.org/10.1186/s13075-018-1624-x |
| 87 | Orlando P, Silvestri S, Galeazzi R, Antonicelli R, Marcheggiani F, Cirilli I, Bacchetti T, Tiano L: Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intense exercise in young athletes. Redox Rep 2018;23:136-145. https://doi.org/10.1080/13510002.2018.1472924 |
| 88 | Park SY, Pekas EJ, Headid 3rd RJ, Son WM, Wooden TK, Song J, Layec G, Santosh X, Yadav K, Mishra PK, Pipinos II: Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am J Physiol Heart Circ Physiol. 2020;319:456-467. https://doi.org/10.1152/ajpheart.00235.2020 |
| 89 | Morrison D, Hughes J, della Gatta PA, Mason S, Lamon S, Russell AP, Wadley GD: Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med 2015;89:852-862. https://doi.org/10.1016/j.freeradbiomed.2015.10.412 |
| 90 | Vezzoli A, Dellanoce C, Mrakic-Sposta S, Montorsi M, Moretti S, Tonini A, Pratali L, Accinni R, Mcanulty S: Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production according to Duration of a Competitive Race. Oxid Med Cell Longev 2016;2016:6439037 https://doi.org/10.1155/2016/6439037 |
| 91 | Koenig RT, Dickman JR, Kang C-H, Zhang T, Chu Y-, Ji LL: Avenanthramide supplementation attenuates eccentric exercise-inflicted blood inflammatory markers in women. Eur J Appl Physiol 2016;116:67-76. https://doi.org/10.1007/s00421-015-3244-3 |
| 92 | Trewin AJ, Lundell LS, Perry BD, Vikhe K, Chibalin AV, Levinger I, Mcquade LR, Stepto NK: Effect of N-acetylcysteine infusion on exercise-induced modulation of insulin sensitivity and signaling pathways in human skeletal muscle. Am J Physiol Endocrinol Metab 2015;309:388-397. https://doi.org/10.1152/ajpendo.00605.2014 |
| 93 | Martin N, Smith AC, Dungey MR, Young HML, Burton JO, Bishop NC: Exercise during hemodialysis does not affect the phenotype or prothrombotic nature of microparticles but alters their proinflammatory function. Physiol Rep 2018;6:e13825. https://doi.org/10.14814/phy2.13825 |
| 94 | Jenkins NT, Landers RQ, Thakkar SR, Fan X, Brown MD, Prior SJ, Spangenburg EE, Hagberg JM: Prior endurance exercise prevents postprandial lipaemia-induced increases in reactive oxygen species in circulating CD31 + cells. J Physiol 2011;589:5539-5553. https://doi.org/10.1113/jphysiol.2011.215277 |
| 95 | Petersen AC, McKenna MJ, Medved I, Murphy KT, Brown MJ, della Gatta P, Cameron-Smith D: Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol 2012;204:382-392. https://doi.org/10.1111/j.1748-1716.2011.02344.x |
| 96 | Mrakic-Sposta S, Gussoni M, Montorsi M, Porcelli S, Vezzoli A: Assessment of a Standardized ROS Production Profile in Humans by Electron Paramagnetic Resonance. Oxid Med Cell Longev 2012;201973927. https://doi.org/10.1155/2012/973927 |
| 97 | Place N, Ivarsson N, Venckunas T, Neyroud D, Brazaitis M, Cheng AJ, Ochala J, Kamandulis S, Girard S, Volungevičius G, Pau Zas FH, Mekideche A, Kayser B, Martinez-Redondo V, Ruas JL, Bruton J, Truffert A, Lanner JT, Skurvydas A, Westerblad H: Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca 2+ leak after one session of high-intensity interval exercise. Proc Natl Acad Sci U S A 2015;112:15492-15498. https://doi.org/10.1073/pnas.1507176112 |
| 98 | Hajizadeh Maleki B, Tartibian B, Chehrazi M: The effects of three different exercise modalities on markers of male reproduction in healthy subjects: a randomized controlled trial. Reproduction 2017;153:157-174. https://doi.org/10.1530/REP-16-0318 |
| 99 | Pileggi CA, Hedges CP, D'Souza RF, Durainayagam BR, Markworth JF, Hickey AJR, Mitchell CJ, Cameron-Smith D: Exercise recovery increases skeletal muscle H2O2 emission and mitochondrial respiratory capacity following two-weeks of limb immobilization. Free Radic Biol Med 2018;124:241-248. https://doi.org/10.1016/j.freeradbiomed.2018.06.012 |
| 100 | Clifford T, Bowman A, Capper T, Allerton DM, Foster E, Birch-Machin M, Lietz G, Howatson G, Stevenson EJ: A pilot study investigating reactive oxygen species production in capillary blood after a marathon and the influence of an antioxidant-rich beetroot juice. Appl Physiol Nutr Metab 2018;43:303-306. https://doi.org/10.1139/apnm-2017-0587 |
| 101 | Caruana H, Marshall JM: Effects of modest hyperoxia and oral vitamin C on exercise hyperaemia and reactive hyperaemia in healthy young men. Eur J Appl Physiol 2015;115:1995-2006. https://doi.org/10.1007/s00421-015-3182-0 |
| 102 | Garten R, Goldfarb A, Crabb B, Waller J: The Impact of Partial Vascular Occlusion on Oxidative Stress Markers during Resistance Exercise. Int J Sports Med 2015;36:542-549. https://doi.org/10.1055/s-0034-1396827 |
| 103 | Ranadive SM, Joyner MJ, Walker BG, Taylor JL, Casey DP: Effect of vitamin C on hyperoxia-induced vasoconstriction in exercising skeletal muscle. J Appl Physiol 2014;117:1207-1211. https://doi.org/10.1152/japplphysiol.00073.2014 |
| 104 | Hajizadeh Maleki B, Tartibian B: High-intensity interval training modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: A randomized controlled trial. Cytokine 2020;125:154861. https://doi.org/10.1016/j.cyto.2019.154861 |
| 105 | Cobley JN, McGlory C, Morton JP, Close GL: N-Acetylcysteine's Attenuation of Fatigue After Repeated Bouts of Intermittent Exercise: Practical Implications for Tournament Situations. Int J Sport Nutr Exerc Metab 2011;21:451-461. https://doi.org/10.1123/ijsnem.21.6.451 |
| 106 | Dopheide JF, Scheer M, Doppler C, Obst V, Stein P, Vosseler M, Abegunewardene N, Gori T, Münzel T, Daiber A, Radsak MP, Espinola-Klein C: Change of walking distance in intermittent claudication: impact on inflammation, oxidative stress and mononuclear cells: a pilot study. Clin Res Cardiol 2015;104:751-763. https://doi.org/10.1007/s00392-015-0840-5 |
| 107 | Ahmad NS, Abdul Aziz A, Kong KW, Hamid MSA, Cheong JPG, Hamzah SH: Dose-Response Effect of Tualang Honey on Postprandial Antioxidant Activity and Oxidative Stress in Female Athletes: A Pilot Study. J Altern Complement 2017;23:989-995. https://doi.org/10.1089/acm.2017.0129 |
| 108 | Sanguigni V, Manco M, Sorge R, Gnessi L, Francomano D: Natural antioxidant ice cream acutely reduces oxidative stress and improves vascular function and physical performance in healthy individuals. Nutrition 2017;33:225-233. https://doi.org/10.1016/j.nut.2016.07.008 |
| 109 | Broome SC, Braakhuis AJ, Mitchell CJ, Merry TL: Mitochondria-targeted antioxidant supplementation improves 8 km time trial performance in middle-aged trained male cyclists. J Int Soc Sports Nutr 2021;18:58. https://doi.org/10.1186/s12970-021-00454-0 |
| 110 | Vasconcelos Gouveia SS, Pertinni de Morais Gouveia G, Souza LM, Cunha da Costa B, Iles B, Pinho VA, Vasconcelos SS, Rolim Medeiros JV, Lago da Silva R, Porto Pinheiro LG: The effect of pilates on metabolic control and oxidative stress of diabetics type 2 - A randomized controlled clinical trial. J Body Mov Ther 2021;27:60-66. https://doi.org/10.1016/j.jbmt.2021.01.004 |
| 111 | Tolahunase M, Sagar R, Dada R: Impact of Yoga and Meditation on Cellular Aging in Apparently Healthy Individuals: A Prospective, Open-Label Single-Arm Exploratory Study. Oxid Med Cell Longev 2017;2017:1-9. https://doi.org/10.1155/2017/7928981 |