Role of O-GlcNAcylation in Breast Cancer Biology
Keywords
Abstract
Introduction
O-GlcNAcylation is one of the most abundant post-translational modifications observed in animals, plants, and even viruses. This modification affects proteins in nearly every functional class and can be found in every cellular compartment [1]. Although technological limitations have made O-GlcNAcylation imperceptible for a long time, it has become extensively researched over the past decades. O-GlcNAcylation is involved in many physiological processes like learning and memory formation, muscle contraction, metabolic homeostasis, and stem and immune cell maintenance. Moreover, the role of this modification in pathophysiology has emerged, especially in cancers [2].
O-GlcNAcylation is a dynamic, reversible modification in which acetylated hexosamine sugar N-acetylglucosamine (GlcNAc) is added through a glycosyl linkage to the serine or threonine residues in amino-acid chains. O-GlcNAcylation differs significantly from other types of glycosylation. Most of the O-GlcNAcylation affects cytosolic or nuclear proteins rather than extracellular ones, the glycosylation is not further elongated or modified, and the enzymes regulating it are reduced to a few [1]. O-GlcNAcylation regulates protein localization, activity, and stability [3]. Similarly, to phosphorylation, O-GlcNAcylation occurs in response to a signal event [4]. Due to the strict dependency on glucose concentration, O-GlcNAcylation is frequently referred to as a metabolic indicator or a metabolic sensor [3]. The dependence is due to the fact that O-GlcNAcylation utilizes uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) which is the final product of the hexosamine biosynthetic pathway (HBP). HBP, a derivative path of glycolysis, is sensitive to nutrient availability. Around 2% of the entering glucose — up to 6% in pathological conditions — is redirected to the hexosamine biosynthetic pathway [5].
O-GlcNAc cycling enzymes
The enzyme catalyzing O-GlcNAcylation is the highly conservative O-GlcNAc transferase (OGT). In humans, it is encoded by a single OGT gene, expressed in three variants resulting from alternative splicing. The difference in these three isoforms is the number of N-terminal tetratricopeptide repeat (TRP) sequences responsible for protein interaction and substrate recognition [6]. The longest isoform, nucleocytoplasmic OGT (ncOGT), is the best-known variant accountable for most O-GlcNAc-related interactions and effects, and most of the research refers to that isoform [2]. The following text will mainly address ncOGT, which will be referred to as “OGT.” The second longest variant is the mitochondrial isoform (mOGT). It contains a unique mitochondrial targeting sequence (MTS) and interacts with approximately 35% of mitochondrial proteins. However, even in a glucose-rich environment, the mRNA level of mOGT is 5-10 times lower than ncOGT [7]. The shortest isoform is called short OGT (sOGT). The enzymes' affinity to protein substrates depends on the concentration of UDP-GlcNAc, the presence of binding partners, modifications of the protein substrates, or modifications of OGT itself, considering it has been observed to be phosphorylated and O-GlcNAcylated [2, 6, 8].
The enzyme with an opposing function to OGT is O-GlcNAcase (OGA) which reverses O-GlcNAcylation. OGA is encoded by the single gene MGEA5 (meningioma-expressed antigen 5), first found in human meningiomas and identified as non-insulin-dependent diabetes and obesity susceptibility locus [9]. The alternative splicing produces the long (OGA-L) and short (OGA-S) isoforms. Those variants vary in the dominant location; OGA-L can be found in the cytosol and nucleus, OGA-S in lipid droplets, and domain content in the C-terminal region. The long isoform C-terminal region bears a sequence matching the histone acetyltransferases (HATs), so OGA was accredited with potential HAT-like activity [2]. Such activity was observed in an in vitro study [10] but was not further confirmed [11]. Although contemporary studies cannot support the internal acetyltransferase activity of OGA, it still seems to be associated with acetylation and is even essential for this modification in a specific context [12]. OGT and OGA present a strong relationship. OGT knockdown results in decreased OGA levels [13] but glucose concentration, which affects both OGT and O-GlcNAcylation levels, doesn’t seem to alter the OGA level [7].
One of the most highlighted features of the O-GlcNAcylation in humans is that only two antagonist enzymes orchestrate it, especially compared to the complex machinery regulating phosphorylation. However, a recent study by Shin et al. described another protein endowed with O-GlcNAc transferase activity. A Gene regulated in breast cancer 1 protein (GREB1) was found to mediate and directly transfer O-GlcNAc to the estrogen receptor alpha (ERα) in the breast cancer cell line MCF7. The O-GlcNAc transferase activity was confirmed by observing O-GlcNAcylation taking place despite OGT knockdown and by identifying specific sites that are glycosylated in ERα by GREB1 [14].
Relation of O-GlcNAcylation and phosphorylation
O-GlcNAcylation is often compared to an equally abundant modification: phosphorylation. Cross-talk between those two modifications integrates metabolic and cell-signaling pathways [6]; however, this relationship presents a significant level of complexity. Both OGT and OGA are phosphorylated, and the high number of O-GlcNAcylated kinases reflects mutual dependence [2]. O-GlcNAcylation and phosphorylation share a vast pool of target proteins and, at times, they are described as antagonistic. For example, phosphorylation on the transactivation domain of the transcriptional factors results in increased activity while glycosylation tends to result in lower activity [15]. O-GlcNAcylation of Snail in breast cancer inhibits its O-phosphorylation, leading to Snail stabilization and accumulation [16, 17]. There are a few types of interplay that can be observed between those two modifications and competitive is just one of them, but it can also be alternating or even cooperative. For instance, in breast cancer, TAB3 [18] or MEK2 O-GlcNAcylation [19] regulates their phosphorylation which is demonstrated by phosphorylation reduction following mutation in the O-GlcNAcylation sites. Sometimes, although interplay would be expected, O-GlcNAcylation and phosphorylation occur independently, like in the case of cofilin regulation in breast cancer [20]. Nevertheless, the presence of both modification sites in proteins exponentially increases molecular diversity [1] so the interplay between them should not be overlooked.
Dysregulations of the O-GlcNAcylation in breast cancer
O-GlcNAcylation and OGT/OGA dysregulations have been described in many types of cancers; increased O-GlcNAcylation levels and changes in the enzymes that regulate it were observed in breast, lung, colon, liver, bladder, endometrial, and prostate cancers [3]. Breast cancer presents higher O-GlcNAcylation levels than other types of cancer, comparing cancer cell lines (breast vs. colon and liver) [21] and clinical samples (breast vs. lung and colon cancers) [12]. The O-GlcNAcylation level is increased even 1.5 times in breast cancer cell lines (SKBR3, MDA-MB-453, MCF-7, MDA-MB-231) compared to non-cancerous ones (MCF-10A, HMEC) [22, 23]; however, another study on HMT-3522 sublines established from one patient found no differences in malignant (T4-2) versus non-malignant (S1) breast cells sublines [24]. Differences can also depend on cell culture type. Akella et al. demonstrated that 3D cultures of BC cells have higher O-GlcNAcylation and OGT levels compared to 2D cultures which cannot be appointed to the lack of adhesion [25]. The O-GlcNAcylation patterns seem to differ between clinical samples and cell culture samples, which should be taken into account when concluding [26]. The complexity of the O-GlcNAcylation is indeed reflected in tendencies observed in clinical samples of breast cancer. The pattern of O-GlcNAcylated proteins differs between tumor and non-tumor samples. Although benign tumors present a similar level of O-GlcNAcylation to adjacent normal tissue, with higher grades of tumor modification level elevates [26]. The immunohistochemistry of clinical samples revealed that tumors had increased O-GlcNAcylation levels compared to non-tumor samples and the level is even higher in lymph node metastases compared to the primary tumors [27]. Despite observing the link between O-GlcNAcylation and breast cancer progression, samples of tumors with positive lymph node status had decreased O-GlcNAcylation, OGT, and OGA levels compared to negative lymph node status samples. However, both groups showed significant differences in modification patterns [8]. These indicate that the O-GlcNAcylation statuses of individual proteins were independent of the overall O-GlcNAcylation levels and aberrant O-GlcNAc modification of these proteins might be associated with lymph node metastasis progression [8].
Regarding enzymes orchestrating O-GlcNAcylation, breast cancer cell lines show much higher levels of OGT compared to non-cancerous cells and the difference is even more significant with highly invasive BC lines like MDA-MD-231 or MDA-MB-435 [13, 22, 28]. In our earlier study, we demonstrated that OGT expression was increased in tumor samples and the highest was in III-grade tumors. However, lymph node status didn’t show any association with OGT expression, and no correlation was found with hormone receptors status [29]. Interestingly, the OGA mRNA level seems negatively correlated with the OGT mRNA level. OGA was expressed in all normal tissues and only in 81% of tumor samples, and its level decreased with increasing tumor grade. Lymph node status was associated with a drop in the OGA level [29]. The other study showed that OGT levels were similar in benign tumors and adjacent normal tissue but revealed an intensifying tendency with increasing tumor grade [26]. Increased OGT levels in tumors were also observed in an animal model [30]. Despite the lack of correlation between the O-GlcNAcylation level and the progesterone receptor (PR) status, the OGT level correlated with PR-positive clinical samples [31]. No significant differences between breast cancer subtypes could be observed, with a modest increase of OGT in the basal subtype whereas the highest content of OGA can be observed in luminal A/B subtypes and the lowest in the basal subtype [32]. In conclusion, breast cancer tends to have elevated O-GlcNAcylation and different modification patterns than normal breast tissue, and those differences intensify in more advanced and malignant tumors. OGT is upregulated, while OGA levels manifest a negative correlation. Moreover, dysregulation of the O-GlcNAcylation and its cycling enzymes present prognostic significance in breast cancer. Increased O-GlcNAcylation correlates with increased relapse rate, distant metastases, and poor outcomes [33]; decreased O-GlcNAcylation correlates with increasing lymph node status [8]. Low OGA level correlates with decreased metastasis-free and overall survival [34]. O-GlcNAcylation seems to have the potential to serve as a prognostic marker in luminal breast cancer [35].
Origin of the O-GlcNAcylation dysregulations in breast cancer
Although defining the origin of the O-GlcNAcylation dysregulations in breast cancer is challenging, research investigating this phenomenon gathered a few possible mechanisms of their occurrences, summarized in Fig. 1. Even though mutations in critical genes are a common source of characteristic features of cancers, no mutations were found in the OGT or OGA genes [6]. The most frequently indicated reason for increased O-GlcNAcylation is increased glucose consumption observed in cancers. Altered metabolism, and aerobic glycolysis, referred to as the Warburg Effect, enable adaptation to cell growth and proliferation and enhance other hallmarks of cancers but require extensive glucose uptake [36]. The elevated rate of glycolysis is associated with an increase in its branches, including the HBP pathway which produces the O-GlcNAcylation substrate. Onodera et al. point out that an increased glucose uptake can be recognized as an oncogenic event on its own [24]. A high glucose level is necessary for the occurrence of a tumor-like O-GlcNAcylation pattern. Manipulation of the glucosamine level didn’t affect the O-GlcNAcylation level suggesting that glucose is, in fact, the master regulator of O-GlcNAcylation dysregulation in breast cancer [24]. A high glucose environment results in increased O-GlcNAcylation [37, 38], confirmed in a more systemic study in which a high-glucose diet in mice led to the O-GlcNAcylation elevation [39]. Interestingly, while an extensive glucose uptake results in increased O-GlcNAcylation and OGT protein levels, it doesn’t increase the mRNA level of OGT but even tends to lower it, suggesting the occurrence of a post-translation mechanism of regulation, perhaps through the OGT protein stabilization [7, 2]. Sodi et al. suggest that the activation of the Akt and mTOR pathways, common in many types of cancers, is sufficient enough to elevate O-GlcNAcylation, showing another possible mechanism of dysregulation occurrence [30]. However, hyperglycemia also increases these signaling pathways which retract glucose as a regulator of O-GlcNAcylation [37]. In MYC-driven cancers, elevated heat shock protein 70 (HSP70) levels also increase both OGT and O-GlcNAcylation levels [30]. O-GlcNAcylation was observed to be increased in response to stress occurrences, such as hypoxia [32] and the presence of cytotoxic [28, 40] or genotoxic compounds [41]. Worth mentioning is the study in which treating breast cancer cells with nicotine significantly elevated O-GlcNAcylation without altering the levels of OGT or OGA [17]. Although 10% to 30% of breast cancer cases can be smoking-related [42], concluding the nicotine effect on the O-GlcNAcylation level in the systemic approach would require more investigation.
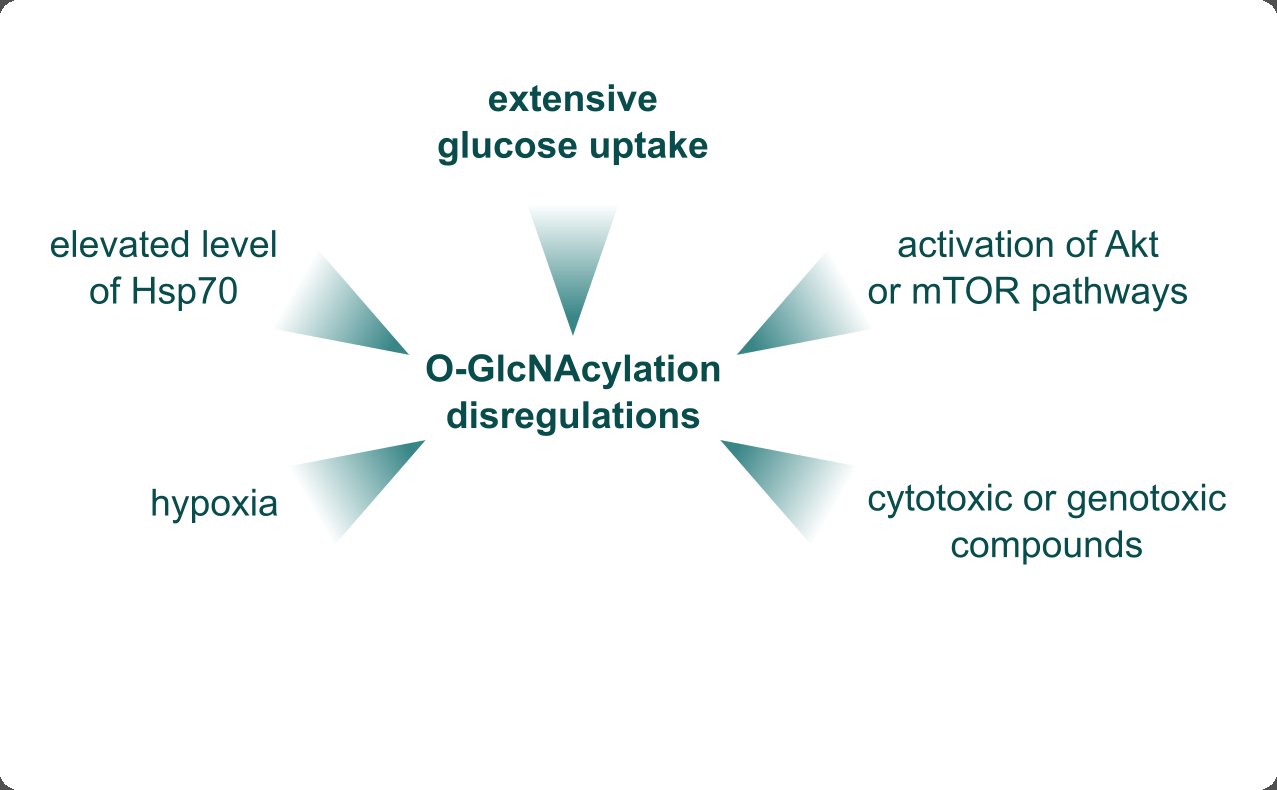
Fig. 1: Possible sources of the O-GlcNAcylation dysregulations in breast cancer. The primary origin of the elevated O-GlcNAcylation level is increased glucose uptake, which enables extensive production of the acetylated hexosamine sugar N-acetylglucosamine (GlcNAc) substrate. The Akt or mTOR pathways' activation is sufficient to increase the O-GlcNAcylation level. O-GlcNAcylation gets elevated in the presence of stressors, such as cytotoxic or genotoxic compounds, nicotine, or hypoxia. The elevated heat shock protein 70 (HSP70) also contributes to the O-GlcNAcylation upregulation.
Outcomes of the O-GlcNAcylation dysregulation in breast cancer
The dysregulations of O-GlcNAcylation alter breast cancer biology in different aspects and through distinct mechanisms. The significance of this type of glycosylation is illustrated by strong selective pressure toward expressing OGT. Tumors formed from the OGT-knockdown cells [22] and OGT-knockdown cells treated with OGA inhibitors can still restore OGT expression [27]. Cells treated with an OGT inhibitor (OSMI1) overexpress OGT in a robust compensating mechanism [43]. The OGT knockdown affects global protein expression [21] and breast cancer cells overexpressing OGT require fewer cells to initiate tumors [25]. O-GlcNAcylation dysregulations affect several aspects of breast cancer biology. The effects are summarized in Table 1. and discussed in more detail in the following paragraphs.
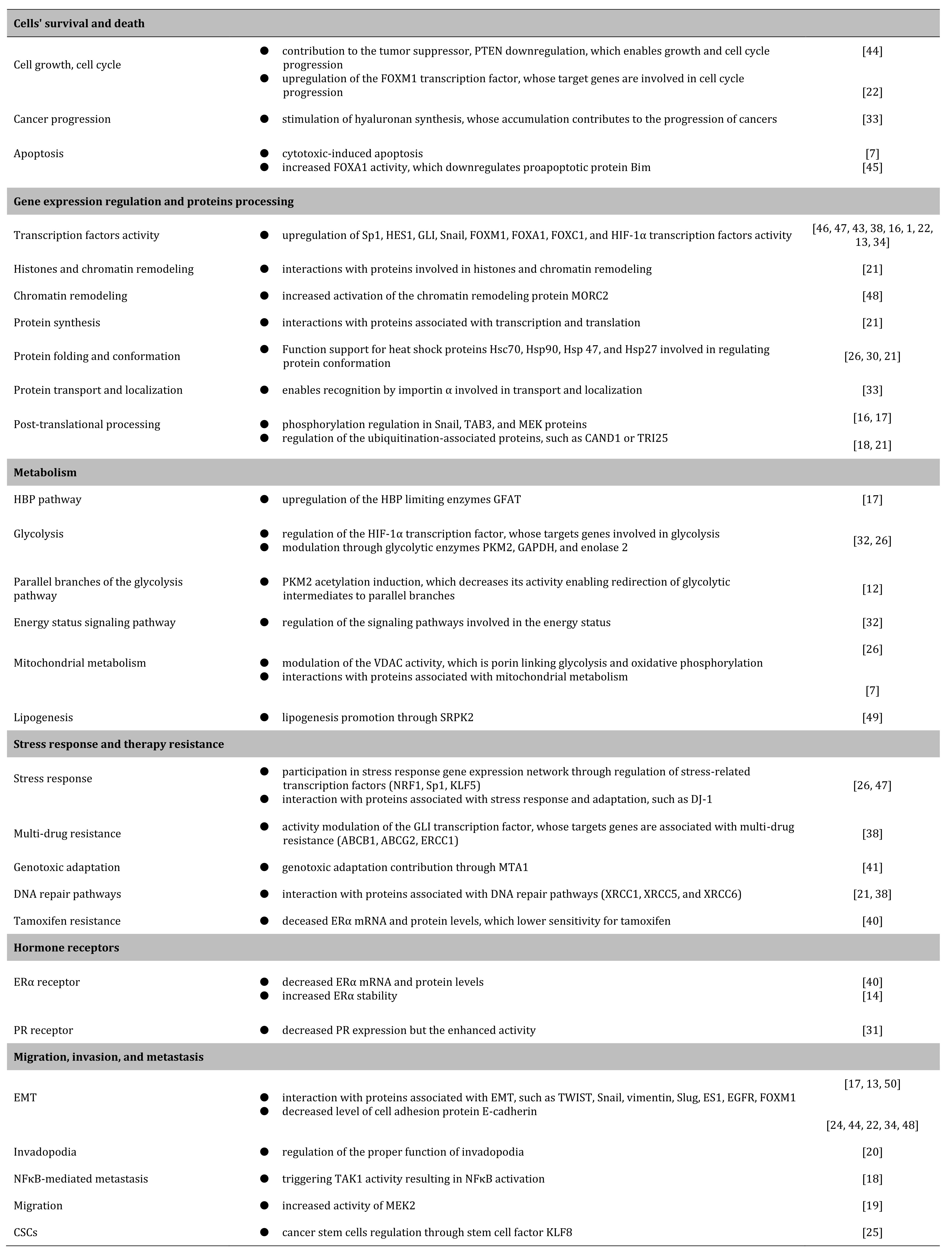
Table 1: Aspects of breast cancer biology affected by O-GlcNAcylation dysregulations.
Cells' survival and death
Among the hallmarks of cancers, abnormal growth, proliferation, and apoptosis avoidance seem the most prominent. The influence of O-GlcNAcylation on breast cancer survival was widely investigated using OGT inhibition, through the knockdown or specific inhibitors, in breast cancer cell lines. However, the gathered results do not allow to make clear conclusions. In the case of some cells, OGT silencing didn’t affect viability, proliferation, apoptosis, colony formation, or morphology [21, 23, 26, 27]. In opposition, many studies showed that OGT inhibition induced growth arrest [22, 24, 44], suppressed oncogenic pathways [24], altered proliferation [28], decreased colony formation [26], and induced apoptosis [30]. Some of these effects depend on the cellular context; for example, OGT inhibition reduced growth in less malignant MFC-7 line but didn’t affect MDA-MB-231 cells [23], or decreased viability applied to spheroid and reseeded, but not to the primary monolayer culture [21]. Vast proteomic analyses revealed that O-GlcNAcylation modifies proteins associated with cell cycle and checkpoints (e.g., phosphatase 2A, peroxiredoxin 3, MEK2), cell survival signaling (e.g., NFκB, p53, p38, MAPK, c-Myc), cytoskeleton (e.g., β-tubulin, keratin proteins), proliferation (e.g., FUSE binding protein 1, thymidine kinase), senescence, and apoptosis. Part of the O-GlcNAc-associated proteins also presents elements involved in the cell cycle and apoptosis [8, 19, 22, 26, 43, 45-51]. Enhanced O-GlcNAcylation contributes to cancer cells’ proliferation [38] and can modulate cell death through chromatin remodeling and transcriptional regulations [41]. A few mechanisms of O-GlcNAcylation or its cycling enzymes’ dysregulation effects on breast cell survival are better understood. OGT-mediated tumor growth regulation partially depends on glucose transporter GLUT1’s activity. GLUT transporters facilitate cellular glucose uptake whose upregulation is crucial for sustaining cancer cells’ growth. GLUT1 overexpression enables overcoming the effect of OGT depletion and restores tumor growth [32]. OGT knockdown results in increased expression of frequently downregulated breast cancer tumor suppressor PTEN [44]. The downregulation of PTEN, in which OGT seems to be involved, increases breast cancer cell growth and modulates the cell cycle [52]. FOXM1 is a transcriptional factor regulating the cell cycle through target genes involved in cycle progressions, such as Skp2, Nek2, Survivin, or PLK1. Although FOXM1 is not directly O-GlcNAcylated, OGT upregulates it indirectly [22]. O-GlcNAcylation also activates hyaluronan synthesis. Hyaluronan is a glycosaminoglycan that is a part of the extracellular matrix. Hyaluronan is involved in the proliferation and survival of cells and its accumulation contributes to the progression of cancers [33]. OGT upregulation contributes to breast cancer cells’ survival; however, overexpression of the mitochondrial isoform, mOGT, leads to cytotoxic-induced apoptosis [7]. Studies on O-GlcNAcylation dysregulation focus on OGT alteration but it was shown that mutation-induced defects in OGA activity resulted in decreased proliferation of breast cancer cells [12].
Gene expression regulation and protein processing
The expression of genes is orchestrated by proteins called transcription factors (TFs). In cancers, various transcription factors are frequently upregulated, enabling molecular advantages and adaptation. O-GlcNAcylation globally enhances the chromatin interaction of many transcription factors, even with a twofold increase under stress conditions [47]. Several TFs were observed to be O-GlcNAcylated in breast cancer, for instance, Sp1 [46, 47] which contributes to the hallmarks of cancer such as proliferation and survival, infinite replicative potential, evasion of growth suppression, avoidance of immune response and death, invasion, and metastasis, genomic instability [53], HES1 whose glycosylation inhibits its proteasomal degradation [44], or GLI proteins whose increased activity resulting from O-GlcNAcylation contributes to the upregulation of the Hedgehog signaling pathway [38]. Other factors, although not directly O-GlcNAcylated, are regulated by the activity of the OGT. This applies to the FOX family of transcription factors, such as the already mentioned FOXM1 factor [22], FOXA1, and FOXC1 [13]. O-GlcNAcylation directly modifies the Snail transcription factor [16, 17], and OGT depletion results in a decreased level of Slug transcription factor [13]. Reduction of either OGT or O-GlcNAcylation leads to the inhibition of the hypoxia-inducible factor 1α (HIF-1α) expression, even under hypoxic conditions [32]. The activity of the hypoxia-inducible factors in cancers is associated with altered metabolism, invasion, metastasis, and other cancer hallmarks [54]. Despite the lack of direct O-GlcNAcylation, OGT regulates HIF-1α by hindering pVHL-mediated proteasomal degradation of the transcriptional factor [32]. O-GlcNAcylation also regulates NRF1 and KLF5 transcription factors which are involved in the stress response [47].
O-GlcNAcylation also affects gene expression through OGT interactions with chromatin remodeling proteins and histones. The OGT-knockdown cells presented altered interactions of histones (H2A, H2B, H3), histone methylation protein (DPY30), histones chaperone (NP1L1), proteins associated with the nuclear import of histones (importin), and chromatin organization (BAF) [21]. The chromatin remodeling protein MORC2 is directly O-GlcNAcylated, which results in its stabilization, increased activity, and downstream events [48].
Rapid growth, proliferation, and adaptation to hostile conditions require extensive production of biological building blocks such as proteins and nucleic acids. Response to the demanding condition is mediated through the regulation of gene expression, which often ends with protein synthesis. Proteasomal analysis of the alterations in protein-protein interactions resulting from OGT knockdown revealed its association with transcription and translations, which are essential steps of protein biosynthesis. OGT silencing was followed by dysregulation of proteins crucial for transcription regulation (WDR61, DDX1, Pirin), helicase activity (DHX9), pre-mRNA splicing (SNRPD1, SMD1, PTB1), translation initiation (IF5A1, IF4G1, EIF3E, and others), translation processing (SYAC), elongation (elongation factor 2), and mRNA localization (CAP1). In addition, some of the affected proteins can be classified as associated with ribosomes (RL5, RRSA, NOP58, NOLC1) and RNA metabolism (LPPRC) [21].
The aspect crucial for the activity of the synthesized protein is proper folding and conformation. Regulating protein conformation is the primary function of heat shock proteins (HSPs) [55]. The dysregulations of the O-GlcNAcylation were observed in the case of Hsc70 protein [26], Hsp90 [30], and its co-chaperone unc-45 homolog A, Hsp47 (also known as Serpin H1) and Hsp27 [21]. Properly synthesized protein still requires precise localization for its activity. Tiainen et al. suggest that O-GlcNAcylation may be involved in the general transport mechanism mediated by importin α which recognizes glycosylated proteins and regulates their localization [33].
An essential element involved in protein functionality is post-translational processing. The O-GlcNAcylation is one of the modifications regulating the activity of ensuing glycoproteins and, as it was already described, it also has an extensive cross-talk with phosphorylation. Efimova et al. showed that O-GlcNAcylation cyclin enzymes might also be associated with other post-translational modifications: SUMOylation and ubiquitination [51]. The relationship with ubiquitination can be indicated by the effects of the OGT-silencing on the activity of the ubiquitination-associated proteins, such as CAND1 or TRI25 [21].
Metabolism
Maintaining cancer cells' pathophysiology requires upregulation or alteration of most metabolic pathways. One of the most extensively researched aspects of cancer metabolism is the Warburg Effect — the phenomenon of metabolizing glucose to lactate even in the presence of sufficient oxygenation. The so-called reprogrammed metabolism contributes to other hallmarks of cancer, assisting molecular adaptation to the fast growth and proliferation of cancer cells and enabling malignant features. Since O-GlcNAcylation utilizes the end product of the branch of glycolysis, the HBP pathway, the modification remains closely related to glucose metabolism. Extensive glucose uptake is possibly the primary source of O-GlcNAcylation dysregulations in breast cancer [37, 38, 39]. Moreover, O-GlcNAcylation regulates transcription of the glutamine fructose-6-phosphate amidotransferase (GFAT), the limiting enzyme of the HBP pathway, creating a positive feedback loop in which O-GlcNAcylation increases the amount of available UDP-GlcNAc [17]. The link between O-GlcNAcylation and metabolism is not limited to dependency on the modification’s substrate. The inhibition of either GFAT or OGT resulted in reversing the malignant phenotype of breast cancer cells which also manifested in reduced glycolytic activity [24]. OSMI1-induced OGT inhibition significantly affected glucose homeostasis [44] and OGT-knockdown resulted in a Warburg Effects reversion which was indicated by a decrease in glycolytic metabolites and an increase in the tricarboxylic acid cycle metabolites associated with metabolic flux toward oxidative phosphorylation. Interestingly, reducing OGT levels in normal cells didn’t affect glucose metabolism [32]. Proteomic analysis of the OGT inhibition effects showed altered regulation of the proteins associated with pyruvate metabolism (acetyl-CoA acetyltransferase), glycolysis (aldolase, lactate dehydrogenase), sugar catabolism (glucosidase II), cellular energy homeostasis (adenylate kinase), and ATP production (ATP synthase) [21, 43]. O-GlcNAcylation regulates signaling pathways involved in the energy status through the LKB1-AMPK pathway and affects glycolytic proteins by interacting with the HIF-1α transcription factor [32]. A few proteins involved in glycolysis undergo direct O-GlcNAcylation, such as PKM2, GAPDH, and enolase 2 [26]. The de-O-GlcNAcylation cycling enzyme, OGA, induces PKM2 acetylation which hinders its activity, enabling the redirection of glycolytic intermediates to parallel branches of the main glycolysis pathway, like the pentose phosphate pathway [12].
O-GlcNAcylation and its cycling enzymes are also involved in mitochondrial metabolism. O-GlcNAcylation modifies the Voltage Dependent Anion Channel 1 (VDAC1) [26], an essential gatekeeper between mitochondrion and cytosol, which contributes to the reprogrammed metabolism of cancers [56]. The mitochondrial isoform of OGT, the mOGT, regulates mitochondrial and bioenergetic function through interaction with proteins associated with mitochondrial transport, ATP synthesis, amino acids metabolism, and fatty acids metabolism [7]. The nucleoplasmatic isoform is also involved in amino acid and lipid metabolism. OGT inhibition resulted in the dysregulation of proteins associated with amino acid metabolism (asparagine synthetase, aminobutyrate aminotransferase) and transport (SLC3A2), fatty acids synthesis (fatty acid synthetase, ATP-citrate synthetase, HCD2), and lipid transport (PCTP-like protein) [20, 22, 42]. O-GlcNAcylation of the SR protein-specific kinase 2 (SRPK2) promotes de novo lipogenesis, and silencing OGT expression impairs this process [49].
Stress response and therapy resistance
O-GlcNAcylation contributes to stress response and therapy resistance through various mechanisms. However, one of the most critical might be regulating the transcription factors. NRF1, Sp1, and KLF5 transcription factors might play a central role in the O-GlcNAc-regulated stress response gene expression network. It was shown that O-GlcNAcylation increases NRF1 stabilization which protects cells from the genotoxic effect of adriamycin [47]. Modification contributes to multi-drug resistance through GLI transcription factor targets such as ABCB1, ABCG2, and ERCC1 [38]. Most prominent differences in expression patterns observed after altering OGT or OGA levels occurred among proteins associated with chromatin regulation and DNA damage sensing and response [51]. Indeed, an increase in O-GlcNAc chromatin-associated protein interactions with chromatin under stress was observed [47]. O-GlcNAcylation of the metastasis-associated protein 1 (MTA1) enhances its interaction with histone-deacetylase multiprotein complex (NuRD) which contributes to genotoxic adaptation [41]. OGT regulates radiosensitivity and O-GlcNAcylation protects from persistent DNA damage [51]. Such an activity can be confirmed by the fact that OGT reduction results in significant expression dysregulation of proteins associated with DNA repair pathways: XRCC1, XRCC5, and XRCC6 [21, 38]. The OGT inhibition affected peroxiredoxin 2 associated with cellular protection and detoxification and Epiplakin involved in filament reorganization in response to stress [21, 23]. O-GlcNAcylation directly modifies proteins such as DJ-1 [26] which protects cells from oxidative stress and prevents histone misregulations [57], and protein disulfide isomerase A6 [26] which controls responsiveness to the unfolded protein stress [58]. Liu et al. 2019 observed that treating breast cancer cells with a proteasome inhibitor, bortezomib (BTZ), resulted in elevated O-GlcNAcylation. Although it didn’t affect OGT or OGA levels, OGT silencing restored BTZ sensitivity, while OGT enrichment decreased BTZ-induced apoptosis. Further investigation of the mechanism by which OGT regulates BTZ sensitivity revealed that under inhibitor treatment, the FOXA1 transcription factor is hyper-O-GlcNAcylated, resulting in the downregulation of the proapoptotic protein Bim [45].
One of the most used compounds in breast cancer therapy is the estrogen receptor modulator tamoxifen. It was demonstrated that OGA inhibition protected breast cancer cells from tamoxifen-induced death, and OGT inhibition increased sensitivity [40]. Interestingly, tamoxifen-resistance cells showed increased sensitivity to the OGT inhibition, compared to the tamoxifen-sensitive ones, despite similar O-GlcNAcylation levels between both cancer cell lines [43]. It is suspected that the mechanism behind O-GlcNAcylation-induced tamoxifen resistance results from an O-GlcNAcylation-mediated decrease in ERα mRNA and protein levels, which reduces the number of receptor molecules available for tamoxifen [40].
Migration, invasion, and metastasis
Since metastases are responsible for the majority of breast cancer-related deaths, it is vital to investigate and better understand the mechanisms behind cancer cell spread from primal tumors to adjacent or distant tissues. O-GlcNAcylation seems significant for the invasion and metastasis of breast cancer. OGT inhibition results in enhanced adhesion, decreased migration, invasion, and metastases [22, 27, 28, 48]; however, in some studies, OGT inhibition reduced invasiveness only in 3D and reseeded culture, and this effect was not observed in monolayer culture [21]. Interestingly, the migration reduction effect following OGT inhibition is limited to invasive migration but it won't hinder other types of migrations, such as chemotaxis [20]. OGT overexpression induced migration and invasion, an increase in the invasion markers like TWIST1 and ZEB1 [13], and the overexpression affecting invasion and migration was observed even in the non-invasive MCF-7 breast cancer cell line. OGA inhibition resulted in increased invasion and migration and reduced adhesion of breast cancer cells [16].
Metastasis consists of many steps, and one of the initial ones is the epithelial-mesenchymal transition (EMT). Treating cells with an OGT inhibitor decreased EMT-associated proteins like TWIST, Snail, and vimentin [17]. OGT depletion elevated the expression of E-cadherin, a protein crucial for cell adhesion, and whose downregulation is an essential part of the EMT [13]. Comparing proteomic profiles of the breast cancer samples and adjacent tissue revealed that one of the uniquely O-GlcNAcylated proteins in cancers is vimentin [25], a well-known regulator of the EMT [59]. Although, in another study, silencing OGT did not affect classic EMT markers (E-cadherin, N-cadherin, β-catenin), and an effect on other metastasis-associated proteins was observed, namely Hsp47 (invasion and metastasis promotion), vinculin (cell adhesion), and actin (cell motility) [21]. O-GlcNAcylation influences EMT through various molecular pathways. O-GlcNAcylation of transcription factor Snail inhibits its phosphorylation-mediated proteasomal degradation which results in its accumulation. The elevated level of Snail suppresses the activity of the E-cadherin gene’s promoter, followed by a decrease in the E-cadherin level [16]. Although E-cadherin is not directly O-GlcNAcylated, the modification applies to the β-catenin and p120 proteins which may modulate E-cadherin binding [27]. O-GlcNAcylation of another protein, MORC2, may also contribute to the E-cadherin downregulation by activating the Snail transcription factor [48]. OGT targets the HES1 transcription factor and the epidermal growth factor receptor (EGFR), both involved in the EMT [24, 50, 44]. OGT inhibition decreased the expression of the EMT activator Slug transcription factor [13]. The loss of adhesion that enables cancer cells' invasion of other tissues is partially facilitated by metalloproteinases. It was shown that OGT regulates invasion through FOXM1-mediated regulation of the matrix metalloproteinase 2 (MMP-2) [22] but this process requires suppression of SIRT1 which is indirectly regulated by O-GlcNAcylation [34]. O-GlcNAcylation affects other proteins involved in the proper function of invadopodia (cofilin) [20], NFκB-mediated metastasis (TAB3) [18], and breast cancer cells migration (MEK2) [19]. Recently the role of cancer stem cells (CSCs) in metastasis emerged. OGT regulates CSCs through stem cell factor KLF8. Knockdown of OGT reduces the population of cancer stem cells, inhibits mammosphere formation, and decreases levels of fibronectin, vimentin, and CSC markers [25].
Relationship between O-GlcNAcylation, hormonal regulation, and diabetes: mechanistic insights and possibilities of breast cancer therapeutic interventions
Out of many O-GlcNAcylation dysregulation effects on cancer biology, two aspects are especially interesting in the context of breast cancer therapy. The first one is the relationship between O-GlcNAcylation and hormonal dependence of breast cancer and the second - is the impact of O-GlcNAcylation on insulin resistance. Breast cancer represents a heterogeneous group of human cancer at both histological and molecular levels. Estrogen receptor (ER), progesterone receptor (PR), and proto-oncogene HER2 are the basic molecular markers that are used as prognostic factors and predictors of response for therapy [60]. It has been shown that both ER and PR are O-GlcNAcylated and this modification may impact their turnover or transcriptional activity [61, 31]. ERα is a key regulatory transcription factor in hormonal response and breast cancer proliferation and thus is subjected to many levels of regulation. ERα expression is necessary to predict the responsiveness to anti-estrogen treatment and its low expression level is generally associated with a poor prognosis. Kanwal et al. have found that O-GlcNAcylation-inducing treatment of breast cancer cells results in the inhibition of ERα expression and tamoxifen resistance [40]. Thus targeting the O-GlcNAc pathway might be a promising therapeutic approach for the sensitization of anti-estrogen-resistant breast tumors [40]. Interestingly, triple-negative breast cancers (TNBC) contain higher OGT and O-GlcNAcylation levels than luminal types [32]. Barkovskaya et al. compared the significance of O-GlcNAcylation in a panel of breast cancer cells of different phenotypes [44]. They found a greater dependency on OGT among triple-negative breast cancer (TNBC) cell lines, which respond to OGT inhibition by undergoing cell cycle arrest and apoptosis. They suggested that transcriptional repressor - hairy and enhancer of split-1 (HES1) - is a mediator of the OGT inhibition response in the TNBC cells. Thus, OGT inhibition has a differential effect on breast cancer cells of different phenotypes, with TNBC cells having the highest sensitivity [44]. It is of particular interest taking into account that this type of breast cancer usually appears in the form of high-grade invasive ductal carcinoma and is associated with a poorer prognosis compared to other breast cancer subtypes [62]
Numerous clinical studies demonstrate that obesity and diabetes may worsen the incidence, prognosis, and mortality rates of breast cancer, particularly in relation to menopausal status and disease subtypes [63]. The effects of obesity on the risk of breast cancer in premenopausal and postmenopausal women differ based on ER status. In premenopausal women, obesity is associated with a higher risk of TNBC, and in postmenopausal women, obesity is associated with a markedly higher risk of ER‐positive breast cancer [63]. Obesity has been correlated to increased O-GlcNAcylation in multiple tissues [64]. Obesity is also associated with insulin resistance which is the hallmark of diabetes type II [65]. The involvement of O-GlcNAcylation in insulin resistance has been broadly studied. O-GlcNAcylation is regulated by nutrient availability in a tissue-dependent manner, being active in every major tissue involved in the regulation of systemic glucose homeostasis. Nutritional stress and hyperglycemia induce changes in O-GlcNAcylation of some transcription factors which in turn may further increases glucose levels and increase glucose toxicity and aggravates the progression of diabetes or diabetic-related cancers [66]. Interestingly, clinical and experimental observations have revealed that endogenous estrogens can protect against insulin resistance primarily through ER-α activation in multiple tissues, including in the brain, liver, skeletal muscle, and adipose tissue, in addition to pancreatic β cells [65]. Thus O-GlcNAcylation may be possibly involved in insulin resistance through reducing expression of ER. The link between type II diabetes and breast cancer is of particular significance since diabetic women have a 40% increased risk of developing breast cancer compared to non-diabetic women and a 74% increase in overall mortality [67]. The high mortality rate is mostly associated with aggressive subtypes of breast cancer, such as estrogen receptor-negative (ER-) and triple-negative breast cancer (TNBC) [67]. The cellular and molecular mechanisms of comorbidity of diabetes and breast cancer are still poorly understood. Knowledge of the O-GlcNAcylation role in the interdependence of hormone receptors, diabetes, and breast cancer may be crucial for developing future new anticancer strategies.
Conclusion
Dysregulations of the O-GlcNAcylation and its cycling enzymes are common features of breast cancers. Most of the differences in cancer cells or clinical samples are associated with the upregulation of OGT and elevated O-GlcNAcylation levels. Those tendencies seem to be stronger with the increase of tumor grade which points to the possible role of the modification in breast cancer progression.
The importance of O-GlcNAcylation in breast cancer biology is reflected in the selective pressure toward expressing OGT in cells in which it has been inhibited. O-GlcNAcylation significantly affects several different aspects of breast cancer biology, all of which are summarized in Table 1.
O-GlcNAcylation and OGT/OGA dysregulations have the potential to serve as prognostic markers and be used in practical applications [35]. Treating breast cancer cells with OGT inhibitors resulted in growth reduction, reversion of the Warburg Effect, and cell sensitization to a proteasome inhibitor, bortezomib [22, 24, 32, 44, 45]. Moreover, reducing OGT levels enhanced breast cancer cells’ adhesion and decreased their migratory and invasive potential [22, 27, 28, 48]. Those findings suggest that OGT could be a potential anticancer target in breast cancer. However, evaluating the applicability of such a therapy should be preceded by considering several aspects. O-GlcNAcylation is not only a common modification whose depletion affects global protein expression [21], but it also displays extensive cross-talk with another prevailing modification — phosphorylation [1]. Targeting OGT could be associated with unpredictable outcomes in terms of systemic effects. Extensive glucose uptake is probably the main cause of O-GlcNAcylation dysregulation in cancers [24, 37, 38] and the association of O-GlcNAc with insulin resistance makes this modification especially important in the context of breast cancer and diabetes comorbidity. On the other hand, the effect of reducing viability resulting from OGT inhibition was the strongest in the triple-negative BC lines [44]. That may point out the possibility of targeting OGT in basal-like breast cancers which are especially challenging due to the lack of standard therapy targets like ER and HER2 receptors. In conclusion, even if targeting OGT does not present an acceptable monotherapy strategy in breast cancer, it might be considered support in other strategies based on the molecular context.
Acknowledgements
Author Contributions
Conceptualization, A.K. and K.K., writing—original draft preparation, K.K.; writing—review and editing, A.K. All authors have read and agreed to the published version of the manuscript.
Funding Sources
This research received no external funding.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Hart GW, Housley MP, Slawson C: Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007;446:1017-1022.
https://doi.org/10.1038/nature05815 |
| 2 | Bond MR, Hanover JA: A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol 2015;208:869-880.
https://doi.org/10.1083/jcb.201501101 |
| 3 | Fardini Y, Dehennaut V, Lefebvre T, Issad T: O-GlcNAcylation: A New Cancer Hallmark? Front Endocrinol 2013;4:99.
https://doi.org/10.3389/fendo.2013.00099 |
| 4 | Slawson C, Pidala J, Potter R: Increased N-acetyl-beta-glucosaminidase activity in primary breast carcinomas corresponds to a decrease in N-acetylglucosamine containing proteins. Biochim Biophys Acta 2001;1537:147-157.
https://doi.org/10.1016/S0925-4439(01)00067-9 |
| 5 | McClain DA, Crook ED: Hexosamines and insulin resistance. Diabetes 1996;45:1003-1009.
https://doi.org/10.2337/diabetes.45.8.1003 |
| 6 | Chaiyawat P, Netsirisawan P, Svasti J, Champattanachai V: Aberrant O-GlcNAcylated Proteins: New Perspectives in Breast and Colorectal Cancer. Front Endocrinol 2014;5:193.
https://doi.org/10.3389/fendo.2014.00193 |
| 7 | Jóźwiak P, Ciesielski P, Zakrzewski PK, Kozal K, Oracz J, Budryn G, Żyżelewicz D, Flament S, Vercoutter-Edouart AS, Bray F, Lefebvre T, Krześlak A: Mitochondrial O-GlcNAc Transferase Interacts with and Modifies Many Proteins and Its Up-Regulation Affects Mitochondrial Function and Cellular Energy Homeostasis. Cancers 2021;13:2956.
https://doi.org/10.3390/cancers13122956 |
| 8 | Jiang K, Gao Y, Hou W, Tian F, Ying W, Li L, Bai B, Hou G, Wang PG, Zhang L: Proteomic analysis of O-GlcNAcylated proteins in invasive ductal breast carcinomas with and without lymph node metastasis. Amino Acids 2016;48:365-374.
https://doi.org/10.1007/s00726-015-2089-8 |
| 9 | Heckel D, Comtesse N, Brass N, Blin N, Zang KD, Meese E: Novel immunogenic antigen homologous to hyaluronidase in meningioma. Hum Mol Genet 1998;7:1859-1872.
https://doi.org/10.1093/hmg/7.12.1859 |
| 10 | Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE: Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem 2004;279:53665-53673.
https://doi.org/10.1074/jbc.M410406200 |
| 11 | Butkinaree C, Cheung WD, Park S, Park K, Barber M, Hart GW: Characterization of beta-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J Biol Chem 2008;283:23557-23566.
https://doi.org/10.1074/jbc.M804116200 |
| 12 | Singh JP, Qian K, Lee JS, Zhou J, Han X, Zhang B, Ong Q, Ni W, Jiang M, Ruan HB, Li MD, Zhang K, Ding Z, Lee P, Singh K, Wu J, Herzog RI, Kaech S, Wendel HG, Yates JR 3rd, Han W, Sherwin RS, Nie Y, Yang X: O-GlcNAcase targets pyruvate kinase M2 to regulate tumor growth. Oncogene 2020;39:560-573.
https://doi.org/10.1038/s41388-019-0975-3 |
| 13 | Forma E, Jóźwiak P, Ciesielski P, Zaczek A, Starska K, Bryś M, Krześlak A: Impact of OGT deregulation on EZH2 target genes FOXA1 and FOXC1 expression in breast cancer cells. PLoS One 2018;13:e0198351.
https://doi.org/10.1371/journal.pone.0198351 |
| 14 | Shin EM, Huynh VT, Neja SA, Niu CY, Raju A, Tan K, Tan NS, Gunaratne J, Bi X, Iyer LM, Aravind L, Tergaonkar V: GREB1: An evolutionarily conserved protein with a glycosyltransferase domain links ERα glycosylation and stability to cancer. Sci Adv 2021;7:eabe2470.
https://doi.org/10.1126/sciadv.abe2470 |
| 15 | Ozcan S, Andrali SS, Cantrell JE: Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta 2010;1799:353-364.
https://doi.org/10.1016/j.bbagrm.2010.02.005 |
| 16 | Park SY, Kim HS, Kim NH, Ji S, Cha SY, Kang JG, Ota I, Shimada K, Konishi N, Nam HW, Hong SW, Yang WH, Roth J, Yook JI, Cho JW: Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. EMBO J 2010;29:3787-3796.
https://doi.org/10.1038/emboj.2010.254 |
| 17 | Zhang N, Zhu T, Yu K, Shi M, Wang X, Wang L, Huang T, Li W, Liu Y, Zhang J: Elevation of O-GlcNAc and GFAT expression by nicotine exposure promotes epithelial-mesenchymal transition and invasion in breast cancer cells. Cell Death Dis 2019;10:343.
https://doi.org/10.1038/s41419-019-1577-2 |
| 18 | Tao T, He Z, Shao Z, Lu H: TAB3 O-GlcNAcylation promotes metastasis of triple negative breast cancer. Oncotarget 2016;7:22807-22818.
https://doi.org/10.18632/oncotarget.8182 |
| 19 | Xu Y, Sheng X, Zhao T, Zhang L, Ruan Y, Lu H: O-GlcNAcylation of MEK2 promotes the proliferation and migration of breast cancer cells. Glycobiology 2021;31:571-581.
https://doi.org/10.1093/glycob/cwaa103 |
| 20 | Huang X, Pan Q, Sun D, Chen W, Shen A, Huang M, Ding J, Geng M: O-GlcNAcylation of cofilin promotes breast cancer cell invasion. J Biol Chem 2013; 288:36418-36425.
https://doi.org/10.1074/jbc.M113.495713 |
| 21 | Netsirisawan P, Chokchaichamnankit D, Saharat K, Srisomsap C, Svasti J, Champattanachai V: Quantitative proteomic analysis of the association between decreasing O‑GlcNAcylation and metastasis in MCF‑7 breast cancer cells. Int J Oncol 2020;56:1387-1404.
https://doi.org/10.3892/ijo.2020.5022 |
| 22 | Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ: Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 2010;29:2831-2842.
https://doi.org/10.1038/onc.2010.41 |
| 23 | Netsirisawan P, Chaiyawat P, Chokchaichamnankit D, Lirdprapamongkol K, Srisomsap C, Svasti J, Champattanachai V: Decreasing O-GlcNAcylation affects the malignant transformation of MCF-7 cells via Hsp27 expression and its O-GlcNAc modification. Oncol Rep 2018;40:2193-2205.
https://doi.org/10.3892/or.2018.6617 |
| 24 | Onodera Y, Nam JM, Bissell MJ: Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest 2014;124:367-384.
https://doi.org/10.1172/JCI63146 |
| 25 | Akella NM, Le Minh G, Ciraku L, Mukherjee A, Bacigalupa ZA, Mukhopadhyay D, Sodi VL, Reginato MJ: O-GlcNAc Transferase Regulates Cancer Stem-like Potential of Breast Cancer Cells. Mol Cancer Res 2020;18:585-598.
https://doi.org/10.1158/1541-7786.MCR-19-0732 |
| 26 | Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J: Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics 2013;13:2088-2099.
https://doi.org/10.1002/pmic.201200126 |
| 27 | Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W: GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res 2010;70:6344-6351.
https://doi.org/10.1158/0008-5472.CAN-09-1887 |
| 28 | Liu Y, Huang H, Cao Y, Wu Q, Li W, Zhang J: Suppression of OGT by microRNA24 reduces FOXA1 stability and prevents breast cancer cells invasion. Biochem Biophys Res Commun 2017;487:755-762.
https://doi.org/10.1016/j.bbrc.2017.04.135 |
| 29 | Krześlak A, Forma E, Bernaciak M, Romanowicz H, Bryś M: Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin Exp Med 2012;12:61-65.
https://doi.org/10.1007/s10238-011-0138-5 |
| 30 | Sodi VL, Khaku S, Krutilina R, Schwab LP, Vocadlo DJ, Seagroves TN, Reginato MJ: mTOR/MYC Axis Regulates O-GlcNAc Transferase Expression and O-GlcNAcylation in Breast Cancer. Mol Cancer Res 2015;13:923-933.
https://doi.org/10.1158/1541-7786.MCR-14-0536 |
| 31 | Trinca GM, Goodman ML, Papachristou EK, D'Santos CS, Chalise P, Madan R, Slawson C, Hagan CR: O-GlcNAc-Dependent Regulation of Progesterone Receptor Function in Breast Cancer. Horm Cancer 2018;9:12-21.
https://doi.org/10.1007/s12672-017-0310-9 |
| 32 | Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ: O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell 2014;54:820-831.
https://doi.org/10.1016/j.molcel.2014.04.026 |
| 33 | Tiainen S, Oikari S, Tammi M, Rilla K, Hämäläinen K, Tammi R, Kosma VM, Auvinen P: High extent of O-GlcNAcylation in breast cancer cells correlates with the levels of HAS enzymes, accumulation of hyaluronan, and poor outcome. Breast Cancer Res Treat 2016;160:237-247.
https://doi.org/10.1007/s10549-016-3996-4 |
| 34 | Ferrer CM, Lu TY, Bacigalupa ZA, Katsetos CD, Sinclair DA, Reginato MJ: O-GlcNAcylation regulates breast cancer metastasis via SIRT1 modulation of FOXM1 pathway. Oncogene 2017;36:559-569.
https://doi.org/10.1038/onc.2016.228 |
| 35 | Kuo WL, Tseng LL, Chang CC, Chen CJ, Cheng ML, Cheng HH, Wu MJ, Chen YL, Chang RT, Tang HY, Hsu YC, Lin WJ, Kao CY, Hsieh WP, Kung HJ, Wang WC: Prognostic Significance of O-GlcNAc and PKM2 in Hormone Receptor-Positive and HER2-Nonenriched Breast Cancer. Diagnostics 2021;11:1460.
https://doi.org/10.3390/diagnostics11081460 |
| 36 | Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell 2011;144:646-674.
https://doi.org/10.1016/j.cell.2011.02.013 |
| 37 | Rehman S, Obaid A, Naz A, Ali A, Kanwal S, Ahmad J: Model-based in silico analysis of the PI3K/Akt pathway: the elucidation of cross-talk between diabetes and breast cancer. PeerJ 2018;6:e5917.
https://doi.org/10.7717/peerj.5917 |
| 38 | Das S, Bailey SK, Metge BJ, Hanna A, Hinshaw DC, Mota M, Forero-Torres A, Chatham JC, Samant RS, Shevde LA: O-GlcNAcylation of GLI transcription factors in hyperglycemic conditions augments Hedgehog activity. Lab Invest 2019;99:260-270.
https://doi.org/10.1038/s41374-018-0122-8 |
| 39 | Alsheikh HAM, Metge BJ, Ha CM, Hinshaw DC, Mota MSV, Kammerud SC, Lama-Sherpa T, Sharafeldin N, Wende AR, Samant RS, Shevde LA: Normalizing glucose levels reconfigures the mammary tumor immune and metabolic microenvironment and decreases metastatic seeding. Cancer Lett 2021;517:24-34.
https://doi.org/10.1016/j.canlet.2021.05.022 |
| 40 | Kanwal S, Fardini Y, Pagesy P, N'tumba-Byn T, Pierre-Eugène C, Masson E, Hampe C, Issad T: O-GlcNAcylation-inducing treatments inhibit estrogen receptor α expression and confer resistance to 4-OH-tamoxifen in human breast cancer-derived MCF-7 cells. PLoS One 2013;8:e69150.
https://doi.org/10.1371/journal.pone.0069150 |
| 41 | Xie X, Wu Q, Zhang K, Liu Y, Zhang N, Chen Q, Wang L, Li W, Zhang J, Liu Y: O-GlcNAc modification regulates MTA1 transcriptional activity during breast cancer cell genotoxic adaptation. Biochim Biophys Acta Gen Subj 2021;1865:129930.
https://doi.org/10.1016/j.bbagen.2021.129930 |
| 42 | Passarelli MN, Newcomb PA, Hampton JM, Trentham-Dietz A, Titus LJ, Egan KM, Baron JA, Willett WC: Cigarette Smoking Before and After Breast Cancer Diagnosis: Mortality From Breast Cancer and Smoking-Related Diseases. J Clin Oncol 2016;34:1315-1322.
https://doi.org/10.1200/JCO.2015.63.9328 |
| 43 | Barkovskaya A, Seip K, Prasmickaite L, Mills IG, Moestue SA, Itkonen HM: Inhibition of O-GlcNAc transferase activates tumor-suppressor gene expression in tamoxifen-resistant breast cancer cells. Sci Rep 2020;10:16992.
https://doi.org/10.1038/s41598-020-74083-z |
| 44 | Barkovskaya A, Seip K, Hilmarsdottir B, Maelandsmo GM, Moestue SA, Itkonen HM: O-GlcNAc Transferase Inhibition Differentially Affects Breast Cancer Subtypes. Sci Rep 2019; 9:5670.
https://doi.org/10.1038/s41598-019-42153-6 |
| 45 | Liu Y, Wang X, Zhu T, Zhang N, Wang L, Huang T, Cao Y, Li W, Zhang J: Resistance to bortezomib in breast cancer cells that downregulate Bim through FOXA1 O-GlcNAcylation. J Cell Physiol 2019;234:17527-17537.
https://doi.org/10.1002/jcp.28376 |
| 46 | Jhu JW, Yan JB, Lin ZH, Lin SC, Peng IC: SREBP1-Induced Glutamine Synthetase Triggers a Feedforward Loop to Upregulate SREBP1 through Sp1 O-GlcNAcylation and Augments Lipid Droplet Formation in Cancer Cells. Int J Mol Sci 2021;22:9814.
https://doi.org/10.3390/ijms22189814 |
| 47 | Liu Y, Chen Q, Zhang N, Zhang K, Dou T, Cao Y, Liu Y, Li K, Hao X, Xie X, Li W, Ren Y, Zhang J: Proteomic profiling and genome-wide mapping of O-GlcNAc chromatin-associated proteins reveal an O-GlcNAc-regulated genotoxic stress. Nat Commun 2020;11:5898.
https://doi.org/10.1038/s41467-020-19579-y |
| 48 | Liu YY, Liu HY, Yu TJ, Lu Q, Zhang FL, Liu GY, Shao ZM, Li DQ: O-GlcNAcylation of MORC2 at threonine 556 by OGT couples TGF-β signaling to breast cancer progression. Cell Death Differ 2022;29:861-873.
https://doi.org/10.1038/s41418-021-00901-0 |
| 49 | Tan W, Jiang P, Zhang W, Hu Z, Lin S, Chen L, Li Y, Peng C, Li Z, Sun A, Chen Y, Zhu W, Xue Y, Yao Y, Li X, Song Q, He F, Qin W, Pei H: Posttranscriptional regulation of de novo lipogenesis by glucose-induced O-GlcNAcylation. Mol Cell 2021;81:1890-1904.
https://doi.org/10.1016/j.molcel.2021.02.009 |
| 50 | Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT: Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat 2012;136:331-345.
https://doi.org/10.1007/s10549-012-2289-9 |
| 51 | Efimova EV, Appelbe OK, Ricco N, Lee SS, Liu Y, Wolfgeher DJ, Collins TN, Flor AC, Ramamurthy A, Warrington S, Bindokas VP, Kron SJ: O-GlcNAcylation Enhances Double-Strand Break Repair, Promotes Cancer Cell Proliferation, and Prevents Therapy-Induced Senescence in Irradiated Tumors. Mol Cancer Res 2019;17:1338-1350.
https://doi.org/10.1158/1541-7786.MCR-18-1025 |
| 52 | Chiang KC, Chen HY, Hsu SY, Zhang K, Dou T, Cao Y, Liu Y, Li K, Hao X, Xie X, Li W, Ren Y, Zhang J: PTEN insufficiency modulates ER+ breast cancer cell cycle progression and increases cell growth in vitro and in vivo. Drug Des Devel Ther 2015;9:4631-4638.
https://doi.org/10.2147/DDDT.S86184 |
| 53 | Beishline K, Azizkhan-Clifford J: Sp1 and the 'hallmarks of cancer'. FEBS J 2015;282:224-258.
https://doi.org/10.1111/febs.13148 |
| 54 | Semenza GL: Hypoxia-inducible factors in physiology and medicine. Cell 2012;148:399-408.
https://doi.org/10.1016/j.cell.2012.01.021 |
| 55 | Dubrez L, Causse S, Borges Bonan N, Dumétier B, Garrido C: Heat-shock proteins: chaperoning DNA repair. Oncogene 2020;39:516-529.
https://doi.org/10.1038/s41388-019-1016-y |
| 56 | Arif T, Paul A, Krelin Y, Shteinfer-Kuzmine A, Shoshan-Barmatz V: Mitochondrial VDAC1 Silencing Leads to Metabolic Rewiring and the Reprogramming of Tumour Cells into Advanced Differentiated States. Cancers 2018;10:499.
https://doi.org/10.3390/cancers10120499 |
| 57 | Scumaci D, Olivo E, Fiumara CV, La Chimia M, De Angelis MT, Mauro S, Costa G, Ambrosio FA, Alcaro S, Agosti V, Costanzo FS, Cuda G: DJ-1 Proteoforms in Breast Cancer Cells: The Escape of Metabolic Epigenetic Misregulation. Cells 2020;9:1968.
https://doi.org/10.3390/cells9091968 |
| 58 | Eletto D, Eletto D, Dersh D, Gidalevitz T, Argon Y: Protein disulfide isomerase A6 controls the decay of IRE1α signaling via disulfide-dependent association. Mol Cell 2014;53:562-576.
https://doi.org/10.1016/j.molcel.2014.01.004 |
| 59 | Chen Z, Fang Z, Ma J: Regulatory mechanisms and clinical significance of vimentin in breast cancer. Biomed Pharmacother 2021;133:111068.
https://doi.org/10.1016/j.biopha.2020.111068 |
| 60 | Mohanty SS, Sahoo CR, Padhy RN: Role of hormone receptors and HER2 as prospective molecular markers for breast cancer: An update. Genes Dis 2020; 9:648-658.
https://doi.org/10.1016/j.gendis.2020.12.005 |
| 61 | Cheng X, Hart GW: Glycosylation of the murine estrogen receptor-alpha. J Steroid Biochem Mol Biol 2000;75:147-158.
https://doi.org/10.1016/S0960-0760(00)00167-9 |
| 62 | Baranova A, Krasnoselskyi M, Starikov V, Kartashov S, Zhulkevych I, Vlasenko V, Oleshko K, Bilodid O, Sadchikova M, Vinnyk Y: Triple-negative breast cancer: current treatment strategies and factors of negative prognosis. J Med Life 2022;15:153-161.
|
| 63 | Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM: Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin 2017; 67:378-397.
https://doi.org/10.3322/caac.21405 |
| 64 | Lockridge A, Hanover JA: A nexus of lipid and O-Glcnac metabolism in physiology and disease. Front Endocrinol 2022;13:943576.
https://doi.org/10.3389/fendo.2022.943576 |
| 65 | Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H: Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther 2022; 7:216.
https://doi.org/10.1038/s41392-022-01073-0 |
| 66 | Gonzalez-Rellan MJ, Fondevila MF, Dieguez C, Nogueiras R. O-GlcNAcylation: A Sweet Hub in the Regulation of Glucose Metabolism in Health and Disease. Front Endocrinol 2022; 13:873513.
https://doi.org/10.3389/fendo.2022.873513 |
| 67 | Ennis CS, Llevenes P, Qiu Y, Dries R, Denis GV: The crosstalk within the breast tumor microenvironment in type II diabetes: Implications for cancer disparities. Front Endocrinol 2022; 13:1044670.
https://doi.org/10.3389/fendo.2022.1044670 |