Original Article - DOI:10.33594/000000680
Accepted 18 December 2023 - Published online 16 January 2024
Insights on Protective Effect of Platelet Rich Plasma and Tadalafil on Testicular Ischemia/Reperfusion Injury in Rats Exposed to Testicular Torsion/Detorsion
bDepartment of Physiology, Faculty of Medicine, Mansoura University, Mansoura, Egypt,
cDepartment of Basic Medical Sciences, Ibn Sina University for Medical Sciences, Amman 11104, Jordan,
dDepartment of Dermatology, Andrology and STDs, Faculty of Medicine, Mansoura University, Mansoura, Egypt,
eDepartment of Biochemistry, Faculty of Pharmacy, Delta University for Science and Technology, Gamasa, Egypt,
fDepartment of Anatomy and embryology, Faculty of Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt,
gDepartment of Clinical Pharmacology, Faculty of Medicine, Mansoura University, Mansoura, Egypt,
hDepartment of Pharmacy Practice, Faculty of Pharmacy, Delta University for Science and Technology, Gamasa, Egypt,
iDepartment of Physiology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
Keywords
Abstract
Background/Aims:
Ischemic reperfusion (I-R) injury is greatly influenced by the testicular torsion/detorsion process (TDP). In this instance, the anti-inflammatory properties of platelet-rich plasma (PRP) combined with tadalafil (Td) significantly promote tissue healing in the I-R injury model.Methods:
Five groups of rats were created: the control group, the I-R group not receiving any therapy, the I-R group receiving a single dosage of Td (0.25 mg/kg, I.P.), the I-R group receiving a single dose of PRP (80 l, intratesticular), and the I-R group receiving both Td and PRP. Sperm morphology, motility, and histology were assessed. The levels of TNF-, BAX, antioxidant status, and testosterone were measured. Additionally, E-selectin expression was done.Results:
PRP reduced oxidative stress, inflammation, and apoptosis while also boosting testosterone levels, which alleviated I-R injury. Otherwise, PRP reduces E-selectin expression, which modifies the pathways that control endothelial function. Td also partially demonstrated its testicular-protective activity at the same time.Conclusion:
PRP's proven anti-inflammatory, antioxidant, and antiapoptotic potentials make it a natural treatment for testicular harm caused by tadalafil. For the first time, it was demonstrated that PRP therapy restored the functionality of the vascular endothelium, specifically the control of E-selectin expression. Combining Td and PRP therapy may be a promising strategy for improving response to PDE5 inhibitors.Introduction
Testicular torsion/detorsion is a typical urological reserve; that affects both male children and adolescents. Testicular torsion annual incidence is 4 in 100.000 by 18 years [1]. It results from spermatic cord rotation and interruption of testicular blood flow. So, early diagnosis and surgical detorsion are essential for restoring the testis's blood flow and avoiding testicular necrosis to preserve future fertility [2]. However, this is not entirely through surgery regarding manual detorsion in the setting of limited operating room availability or at presentation in the emergency department to turn an emergent case into an elective surgery [3]. The primary pathophysiological exploration of testicular torsion and detorsion is testicular ischemic reperfusion (I-R) injury. Proinflammatory cytokines, reactive oxygen species (ROS) formation, lipid peroxidation, neutrophil activation, anoxia, and cell apoptosis are considered important risk factors for erectile dysfunction (E.D) and may lead to infertility [4].
However, the ROS formed throughout I-R is a substantial component of this procedure. It recognized that I-R damage activates the production of multiple toxic substances in the circulation of numerous tissues. Also, vascular endothelial cell damage and microcirculatory disorders can arise through reperfusion and induce organ atrophy [5].
Otherwise, the involvement of proinflammatory cytokines in I-R. injury is receiving much attention. Tumor necrosis factor-alpha (TNFα), Proinflammatory cytokines and Interleukin-1β (IL-1β) have been related to I-R. in several models [6-8]. These cytokines activate neutrophils at positions of inflammation through stimulation of endothelium cell adhesion molecules expression as endothelial (E)-selectin and platelet (P)-selectin [9]. E-selectin is a participant of transmembrane glycoproteins of the selectin family, and it is expressed constitutively on endothelial cells, whereas it helps monocyte rolling and adhesion to the endothelium [10]. The activation of the c-Jun N-terminal kinases (JNK) pathway through such cytokines is correlated with the expression of E-selectin and neutrophil recruitment [8]. Thoughtful the intracellular signaling pathways triggered after I-R. of the testis may lead to the proposal of definite therapies that will help save testicular function and spermatogenesis afterwards I-R.
Multiple investigations have been done to explore new effective strategies and medications to decrease and prevent testicular I-R injury, even after manual and surgical detorsion of testicular torsion to maintain normal testicular functions [11]. Previous studies explained testicular I-R damage and evaluated treatment with various therapeutic medications in torsion-detorsion, such as anti-inflammatory drugs, antioxidant drugs, and nitric oxide donors. Still, no one has been entirely successful in clinical practice. It may be due to the unclear detailed pathophysiological explanations underlying testicular I-R injury [12-14].
In the current urology practice, a phosphodiesterase-5 inhibitor (PDE5i); Tadalafil (Td) is used for the management of E.D. The cGMP levels in the tissue increases by inhibition of phosphodiesterase (PDE) enzymes, and leads to relaxation of smooth muscles and increasing the vessels' local perfusion and dilation [15, 16]. Otherwise, some studies investigate the I-R injury in testes torsion models by means of different PDE5 inhibitors as Vardenafil and Sildenafil [16, 17].
On the other hand, in clinical cases, platelet-rich plasma (PRP) has received significant attention to stimulate human tissue healing. PRP is a unique derivative of whole blood rich in a high convergence of vital growth factors and cytokines cell adhesion molecules. PRP has proven its role in regenerative medicine and clinical applications in cell therapy for its powerful healing properties [18]. PRP has a prominent potential role in tissue repair through the proliferation and differentiation of tissue progenitor cells. It is a potent antioxidant that protects against ischemia-reperfusion injury in different organs [19-21].
The effect of PRP and Td on testicular ischemic reperfusion damage has not been evaluated before. So, this study’s objectives are to discuss whether combining the regenerative impact of PRP with increasing testicular blood flow by vasodilator effect of Td can give a synergistic effect and higher protection for the testis from I-R injury in rats than given by each of them when used alone.
Materials and Methods
Animals and Experimental Design
Experimental protocols monitored the ARRIVE guidelines of the National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs). All experiments were achieved following appropriate policies and regulations of the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020) [22]. Fifty adult male albino rats 12-16 weeks old, with a weight of 220–300 g, were obtained from the facility at the faculty of pharmacy, Delta University (FPDU), and were housed in polypropylene cages with ad-lib access to food and tap water. Animals were acclimatized for one week. Moreover, all trials were permitted by the Institutional Animal Care and Use Committee at the FPDU number (FPDU 10/2021). After acclimatization, the sample size was calculated by G*power, and rats were randomized into five experimental groups; each had six rats, except group I, which had 16 rats separated into four subgroups. Animals were divided as follows:
Group I (Control group): was subdivided into four equal subgroups as follows:
- Subgroup 1-A: Sham-operated, untreated group.
- Subgroup 1-B: Subjected to intraperitoneal (IP) injection of tadalafil (Td) 0.25 mg/kg single dose [23]. 0.25 mg/kg Td in the form of tablets were grinded and converted into a solution for injection.
- Subgroup 1-C: Subjected to intra-testicular platelet-rich plasma (PRP) injection into the left testis parenchyma 80μl single dose [24].
- Subgroup 1-D: Subjected to both I.P. injection of Td 0.25 mg/kg and intra-testicular PRP injection into the left testis parenchyma 80μl each as a single dose.
Group III (I-R group with Td injection): testicular TDP as in group II with I.P. injection of Td 0.25 mg/kg single dose 30 minutes after torsion then after 90 minutes of its injection, detorsion occurred.
Group IV (I-R group with PRP injection): testicular TDP as in group II with intra-testicular PRP injection into the left testis parenchyma 80μl single dose, 5 min before detorsion.
Group V (I-R group with Td and PRP injection): testicular TDP as in group II with I.P. injection of Td 0.25 mg/kg single dose and intra-testicular PRP injection into the left testis parenchyma 80μl single dose.
Induction of ischemic reperfusion (I-R) injury
Before the surgical intervention, general anesthesia helping physical inactivity in rats was induced by I.P. Injection of ketamine 50 mg/kg and xylazine hydrochloride 10 mg/kg. After the inducement of anesthesia and ensuring stabilization, the scrotal area was shaved, and antiseptic was used with a 10% povidone-iodine solution. Group, IA was assigned to the sham operation group. A midline-scrotal incision was performed, and the left testis and spermatic cord were released. Without creating testicular torsion, the testis was placed into the scrotum and returned to its anatomical position [25], and the scrotum was primarily closed with 4/0 polypropylene sutures. After 2 hours, both testes were removed and fixed in Bouin's solution for histopathologic examination. I-R injury was created in all other groups. The left testis and spermatic cord were released after the mid-scrotal incision. The testicular torsion experimental model was done by rotating the left testicle with its cord 720 degrees clockwise. The testicle was fixed to the scrotum's internal side with a 4 / 0 polypropylene suture. After torsion the testis was placed into the scrotum and returned in the anatomic position then sutured the scrotum during the whole period of torsion120 minutes. PRP was injected 5 min before detorsion blindly intratesticular without opening the scrotum to avoid dryness of testicular tissue. Tadalafil was injected intraperitoneally 30 mi after torsion then after ending of 120 minutes of torsion, the opening of the scrotum was done then detorsion occurred. Reperfusion was done by detorsion to its natural position with the closure of the scrotum then it remained for another 2 hours before scarification in rats groups except groups receiving PRP remained for another 2 weeks till the regenerative effect began [25, 26]. No unexpected adverse events occurred. The euthanasia procedure was based on AVMA guidelines [22] using a 5:1 (mg/mg) solution of a ketamine-xylazine mix. We obtained left testicles from each rat in all groups then immediately after euthanasia excised a part of each testicle to be used in testicular tissue homogenate assay for oxidative stress markers evaluation. The remaining part of each testicular tissue was fixed in previously prepared Bouin's solution for pathology evaluation. All authors have read the ARRIVE guidelines specification and fulfilled its instructions.
Platelet Rich Plasma Preparation
Ten age-matched healthy male rats were used as PRP donors according to several previous studies that carried out the usage of albino rats as blood donors for PRP without occurrence of any unpredictable complications [24, 27-31]. The whole blood samples of rats were withdrawn using specific techniques to prevent hemolysis. Venous blood was taken via cardiac puncture and mixed with acid citrate dextrose at a citrate/blood ratio of 1/9. Samples were transferred for laboratory analysis before centrifugation for platelet concentration. The blood was centrifuged at 1480 rpm by a soft spin for 6 minutes to separate the plasma holding the platelets from erythrocytes. The plasma was drawn off the top and relocated to another tube lacking any anticoagulant, centrifuged once more using a spin at a more incredible speed of 3400 rpm for 15 min to have a platelet concentrate. The upper platelet-poor plasma in the 2/3 rd of the tube was removed. Otherwise, the lower platelet-rich plasma (PRP) in the 1/3 rd was taken and used as fresh PRP at the time of detorsion [25, 32].
At the end of the reperfusion time, 2 hrs after detorsion, animals were anesthetized with ketamine-xylazine. Otherwise, groups receiving PRP (G-1C, G-1D, IV, V) were evaluated and sacrified two weeks after detortion. Blood was sampled from their hearts to measure the serum levels of testosterone. Blood samples were centrifuged at 1252g for 5 min, and then, the serum was separated from the blood cells and kept at −70°C until testing was done. Semen was also collected at the time of scarification from the epididymis.
Assessment methods
1) Histological and immunohistochemical analysis
A standard histological technique was used. Testicular tissue samples were fixed in Bouin's solution and processed to obtain serial paraffin sections of 7 μm. Each blocked testicular sample was stained with hematoxylin and eosin (H&E) stain to observe histological changes. Histological examination was done under light microscopy. The quantitative estimation of sperm count was estimated within animals of each group [33]. Tissues from paraffin blocks were deparaffinized, hydrated, and then wrapped up in an antigen retrieval solution. Testes sections were incubated overnight by primary rabbit anti-bax (BCL2 Associated X gene) (Sigma-Aldrich, USA) and anti-tumor necrosis factor-α (TNF-α) as inflammatory mediators (Thermo Fisher Scientific, Rockford, IL, USA). Primary binding was distinguished using a horseradish peroxidase-conjugated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) and imagined by development with 3, 3-diaminobenzidine (DAB, Sigma). The immunoreactivity was evaluated by calculating the immunopositive cells/ 1000 cell count. Testicular tissues were assessed histopathologically with the Johnsen scoring system to estimate the mean Johnsen score. The Johnsen scoring covers 1-10 histological measures starting with no cells within seminiferous tubules; no germ cells and only Sertoli cells are present; only spermatogonia as germ cells; no spermatozoa and spermatids with a few spermatocytes; no spermatozoa and spermatids with many spermatocytes, no spermatozoa with a few (<10) spermatids; no spermatozoa with no late spermatids but many early spermatids; late spermatids without any mature spermatozoa, slightly impaired spermatogenesis with many late spermatids have disorganized epithelium finally score 10 be fully spermatogenesis [34].
2) Evaluation of sperm morphology and motility using a computer-assisted semen analysis (CASA) [35]
Semen samples were analyzed using VideoTest® ZooSperm® (rat module) software (Russia). VideoTest® ZooSperm® CASA is designed to equip the workplace of specialists dealing with sperm analysis. The analyzer allows experts to count the number of spermatozoa, evaluate their motility parameters in a native specimen, and analyze stained specimens' morphology. Motility and Morphology are considered two standard methods in VideoTest® ZooSperm® CASA for automatic report generation and an in-built database. The analysis of the parameters is performed automatically. The parameters that can be determined with the help of the VideoTest® ZooSperm® CASA are the basic ones for estimating sperm fertility[36].
Motility standard method. The process evaluates the spermatozoa motility and concentration in a natural specimen. A counting chamber with the sample is positioned under the microscope to accomplish the analysis, and a video is recorded afterward. The registered items are thresholded, and their sign paths are reassembled automatically. The particular spermatozoon color of the path matches its motility class. To get statistical results, numerous clips can be verified. The analysis results of each clip are concise and shifted to the database.
Morphology standard method. Rigorous criteria for analyzing heads of spermatozoa are utilized in this standard method. The spermatozoa's tails, middle parts, and heads are automatically measured. The pathology is revealed based on the head parameters. The obtained data is transferred to the database automatically.
Computer-assisted digital image analysis. Slides were photographed using an Olympus® digital camera installed on an Olympus® microscope with 0.5 X photo adaptor, using 40 X objective and saved as TIFF. The result images were analyzed on Intel® Core I3® based computer using VideoTest Morphology® software (Russia) with a specific built-in routine for area measurement and Stain quantification.
3) Oxidative stress parameters in testis
Superoxide Dismutase (SOD) activity and glutathione (GSH) level were determined in testicular tissue homogenate assay. SOD is detected spectrophotometrically using SOD Kit (Bio-diagnostic Giza, Egypt) following the instructions from the manufacturer expressed as units/ml. Tissue GSH level in testicular tissue homogenate was determined by spectrophotometer using GSH reduced kit (Bio-diagnostic Giza, Egypt) according to the manufacturer's instructions.
4) Enzyme-linked immunoassay (ELISA) for the quantitative determination of serum testosterone
The serum testosterone level was determined using an ELISA kit (MyBioSource, Canada) with a microplate reader (Tecan, Infinite 200 PRO, Switzerland) at 450 nm. The limit of detection for testosterone was 1.56 pg/ml - 50 pg/ml.
5) Analysis of the expression of E-selectin by qRT-PCR
Total RNA was extracted from isolated primary rat testis tissue with a nucleic acid extraction kit (NucleoSpin® REF. 740901.250) purchased from (Macherey- Nagel GmbH & Co. K.G.- Germany) in an RNase-free environment according to the manufacturer's protocol. The purity (A260/A280 ratio) and RNA concentration were measured by spectrophotometry (dual wavelength Beckman, Spectrophotometer, USA). The purified DNA samples were stored at –80 °C for use. Reverse transcription of 2μg total RNA into complementary DNA (cDNA) was performed with a SensiFAST™ SYBR® Hi-ROX One-Step Kit, catalog no.PI-50217 V) (Bioline, a median life science company, U.K.) in a reaction volume of 20μl. Expression levels were normalized concerning the housekeeping gene (GAPDH). Table 1 shows the primer sequences used in qRT-PCR. The relative gene expression was evaluated using the comparative cycle threshold (Ct) (2−ΔΔCT) method.
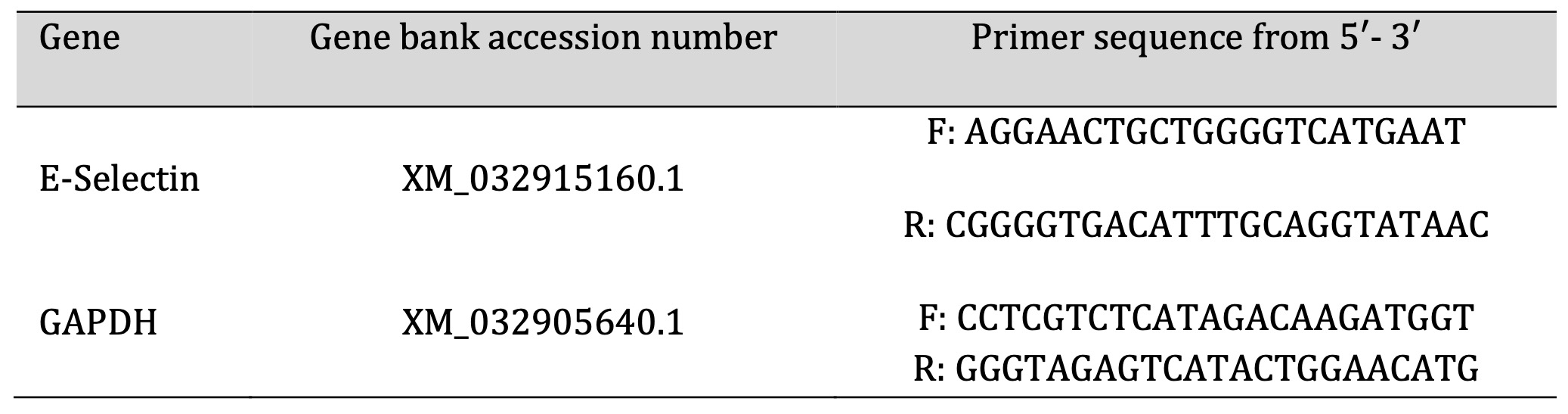
Table 1: PCR primer sequence
Statistical analysis
Statistical data analysis is carried out in SPSS Software (version16; SAS Institute Inc., Cary, North Carolina, USA). All data are presented as mean± standard deviation (SD) and are compared using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. A value of P < 0.05 was considered to indicate significant statistical analysis.
Results
Histological and immunohistochemical analysis
Histopathological examination. As shown in Fig. 1, testicular sections from the control groups displayed normal regular crossly sectioned seminiferous tubules with normal lining germinal epithelium and normal little interstitial space containing Leydig cells. Regarding TDP groups (Fig. 2), sections from the un-received treatment group (G2) demonstrated diffusely irregular shrunken crossly sectioned seminiferous tubules, mostly with severe tubular degeneration and necrosis, giving rise to an increase in interstitial space containing pale eosinophilic material. Testicular sections from G3 received Td demonstrated reduced testicular relapse, mostly as irregular shrunken crossly sectioned seminiferous tubules and decreased interstitial space with pale eosinophilic material. However, sections from G4 received PRP revealed marked degenerated irregular shrunken crossly sectioned seminiferous tubules and still widened interstitial space containing pale eosinophilic material. However, these degeneration changes were scarcely observed in testicular sections from G5 receiving both PRP+Td. G5 showed regular crossly sectioned seminiferous tubules lined with normal germinal epithelium and minimal interstitial space containing pale eosinophilic material.
As shown in Table 2 and consistent with the histological analyses, Td and PRP significantly attenuated the TDP- increase in the Johnosn score with a decrease in the diameter of seminiferous tubules. Moreover, drug treatment with Td or PRP individually also attenuated the TDP-induced pathological score and elevated diameter to a lesser degree than using them collectively.
Immunohistochemical labeling of TNF-α and BAX. Fig. 3, Fig. 4, Fig. 5 and Fig. 6 show the immunolabeling of TNF-α and BAX within the testicular sections of different groups of rats, respectively. TNF-α and BAX were expressed mainly in Leydig's tubular epithelium and interstitial cells. Marked expressions of TNF-α and BAX were noticed in G2 TDP received group, and the positive reaction mildly decreased in G3, and G4 received drugs individually. While scanty expression of TNF-α and BAX were detected in the normal and drug control groups, G5 received TDP+PRP+Td.
As shown in Table 3, Td and PRP significantly attenuated the TDP-encouraged increase in the % area of BAX and TNF alpha. Moreover, drug treatment with Td or PRP individually also attenuated the TDP-induced levels of BAX and TNF alpha to a lesser degree than using them collectively. These results support the collaborative activity of Td and PRP that was intermediated mostly by the deactivation of BAX and TNF alpha.
As shown in Table 4, Td and PRP organized non-significant changes in the TDP and encouraged sperm count and motility reduction with disturbance in their morphology. Moreover, drug treatment with Td or PRP slightly elevated the TDP-reduced levels of sperm count, either used individually or collectively.
The mean tissue antioxidant parameters (GSH, SOD) were significantly reduced in the TDP group in contrast to the control groups (P<0.05). Furthermore, the mean tissue antioxidant parameters were elevated in the TDP/Td and TDP/PRP groups compared to the TDP group (P<0.05). Else, the TDP/Td and TDP/PRP groups were significantly decreased compared to the control groups (P<0.05). On the other hand, the tissue antioxidant parameters were found to be substantially elevated in TDP/Td +PRP group compared to TDP, TDP/Td, and TDP/PRP groups (P<0.05) with non-significant change when compared to control normal groups (Table 5).
The mean serum testosterone hormone level was significantly decreased in the TDP group compared to the control groups (P<0.001). Moreover, the mean serum testosterone hormone level was increased in the TDP/Td and TDP/PRP groups compared to the TDP group (P<0.001). Otherwise, the TDP/Td and TDP/PRP groups were significantly decreased compared to the control groups (P<0.001). On the other hand, the serum testosterone level was found to be significantly elevated in TDP/Td +PRP group compared to TDP, TDP/Td, and TDP/PRP groups (P<0.001) with non-significant change when compared to control normal groups (Table 6).
As shown in Table 7, Td and PRP significantly attenuated the TDP-induced increase in the gene expression of E-selectin. Drug treatment with Td or PRP individually also attenuated the TDP-induced gene expression levels of E-selectin but to a lesser extent than using them collectively. These results confirm the synergistic activity of Td and PRP that might be mediated mainly by the inactivation of the E-selectin synthesis pathway.
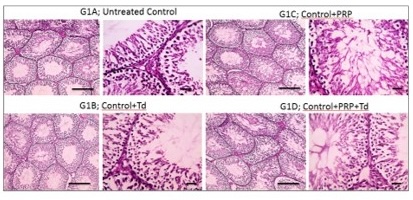
Fig. 1: Photomicrographs of testicular sections from control group. The sections had normal regular crossly sectioned seminiferous tubules with normal lining germinal epithelium and normal little interstitial space containing Leydig cells (bold line) in control group (G1), and groups received platelet-rich plasma (PRP), Tadalafil (Td) and PRP+Td. Hematoxylin and eosin stain; low magnification X: 100 bar 100 μm and high magnification X: 400 bar 50 μm.
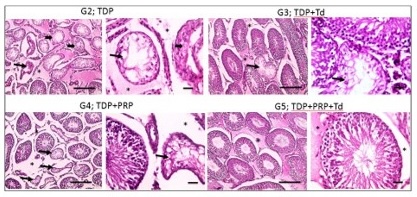
Fig. 2: Photomicrographs of testicular sections from the torsion/detorsion process (TDP) groups. G2 is TDP without treatment had diffusely irregular shrunken crossly sectioned seminiferous tubules with severe tubular degeneration (thin arrow) and necrosis (thick arrows) and increased interstitial space containing pale eosinophilic material (*). Testicular sections from G3 received TDP+Tadalafil (Td) had very few degenerated irregulars, shrunken crossly sectioned seminiferous tubules (thin arrow) and decreased interstitial space containing pale eosinophilic material (*). Testicular sections from G4 received TDP+platelet-rich plasma (PRP) showing some degenerated irregular shrunken crossly sectioned seminiferous tubules (thin arrow) and still widened interstitial space containing pale eosinophilic material (*). Testicular sections from G5 received TDP+PRP+Td had regular crossly sectioned seminiferous tubules lined with normal lining germinal epithelium (thin arrow), and much decreased interstitial space containing pale eosinophilic material (*). Hematoxylin and eosin stain; low magnification X: 100 bar 100 μm and high magnification X: 400 bar 50 μm.
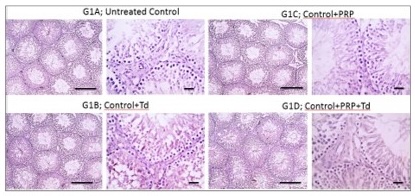
Fig. 3: Photomicrographs of immunostained testicular sections against TNF-α from the control groups. The untreated control group (G1A), groups G1B, G1C, G1D received Tadalafil (Td), platelet-rich plasma (PRP) and PRP+Td, respectively, had negative immunostaining of TNF-α. IHC counterstained with Mayer's hematoxylin. Low magnification X: 100 bar 100 and high magnification X: 400 bar 50.
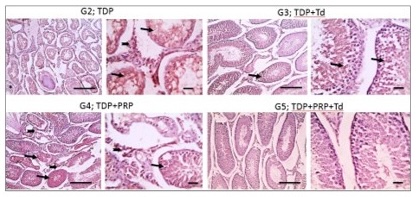
Fig. 4: Photomicrographs of immunostained testicular sections against TNF-α from the different groups. Torsion/detorsion process (TDP) group G2 had strong positive brown immunolabelling in the tubular epithelium (thin arrow) and interstitial cells of Leydig (thick arrow). The G3 group received TDP, and Tadalafil (Td) showed markedly decreased positive brown immunolabelling and appeared mainly in the tubular epithelium (thin arrow). The positive reaction mildly decreased in G4 received TDP+ platelet-rich plasma (PRP) and appeared in both tubular epithelium (thin arrow) and interstitial cells of Leydig (thick arrow). The positive reaction returns negative in G5 received TDP+PRP+Td. IHC counterstained with Mayer's hematoxylin. Low magnification X: 100 bar 100 and high magnification X: 400 bar 50.
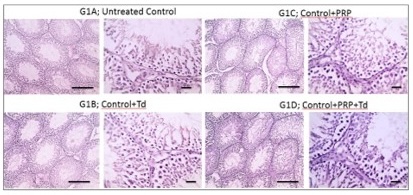
Fig. 5: Photomicrographs of immunostained testicular sections against BAX from the different control groups. The pictures showed negative staining in the untreated control group (G1A), and groups G1B, G1C, G1D received Tadalafil (Td), platelet-rich plasma (PRP) and PRP+Td, respectively. IHC counterstained with Mayer's hematoxylin. Low magnification X: 100 bar 100 and high magnification X: 400 bar 50.
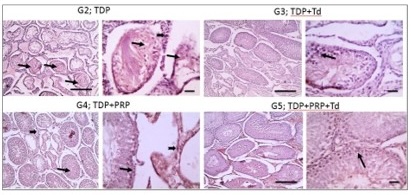
Fig. 6: Photomicrographs of immunostained testicular sections against BAX from the different groups. The G2 torsion/detorsion process (TDP) group showed prominent positive brown immunolabelling in the tubular epithelium (thin arrow) and interstitial cells of Leydig (thick arrow). The positive brown immunolabelling markedly decreased in G3 received TDP+tadalafil (Td) and appeared mainly in the tubular epithelium (thin arrow). The positive reaction mildly decreased in G4 received TDP+Platelet rich plasma (PRP) and appeared in both tubular epithelium (thin arrow) and interstitial cells of Leydig (thick arrow). Bax weakly stained in the tubular epithelium (thin arrow) in G5 received TDP+PRP+Td. IHC counterstained with Mayer's hematoxylin. Low magnification X: 100 bar 100 and high magnification X: 400 bar 50.
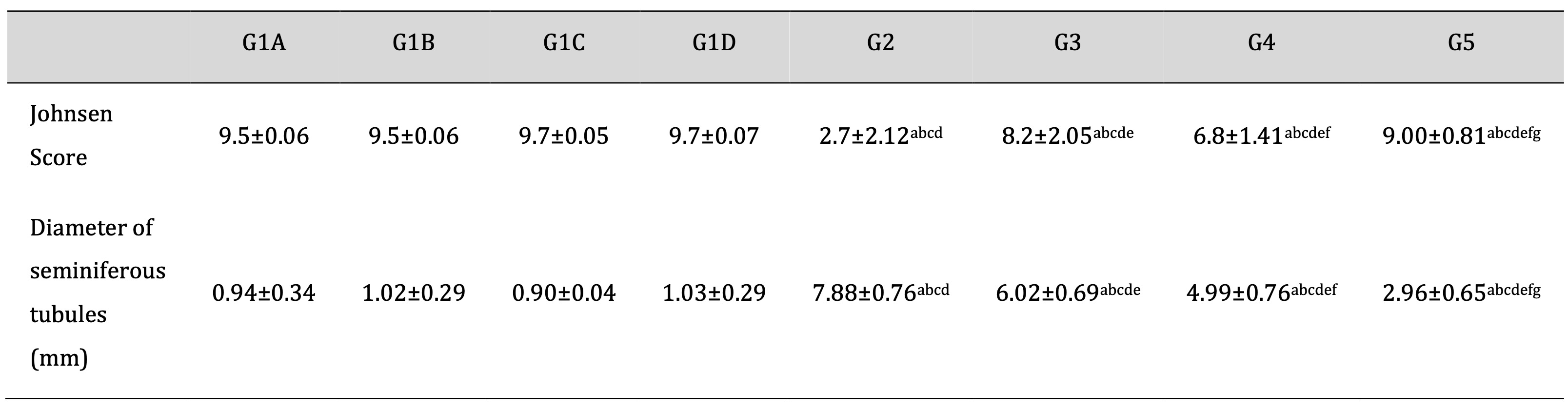
Table 2: Effect of different treatments on Johnsen Scoring system and diameter of seminiferous tubules in the TDP and normal control groups. G1A: Untreated control group, G1B: Tadalafil (Td) control group, G1C: platelet-rich plasma (PRP) control group, G1D: PRP+Td control group, G2: torsion/detorsion process (TDP) group, G3: TDP+Td, G4: TDP+PRP, G5: TDP+PRP+Td. Data expressed as mean ± S.D., *: significance <0.001. A test used: One-way ANOVA followed by post-hoc tukey. a: significance vs G1A, b: significance vs G1B, c: significance vs G1C, d: significance vs G1D, e: significance vs G2, f: significance vs G3, g: significance vs G4
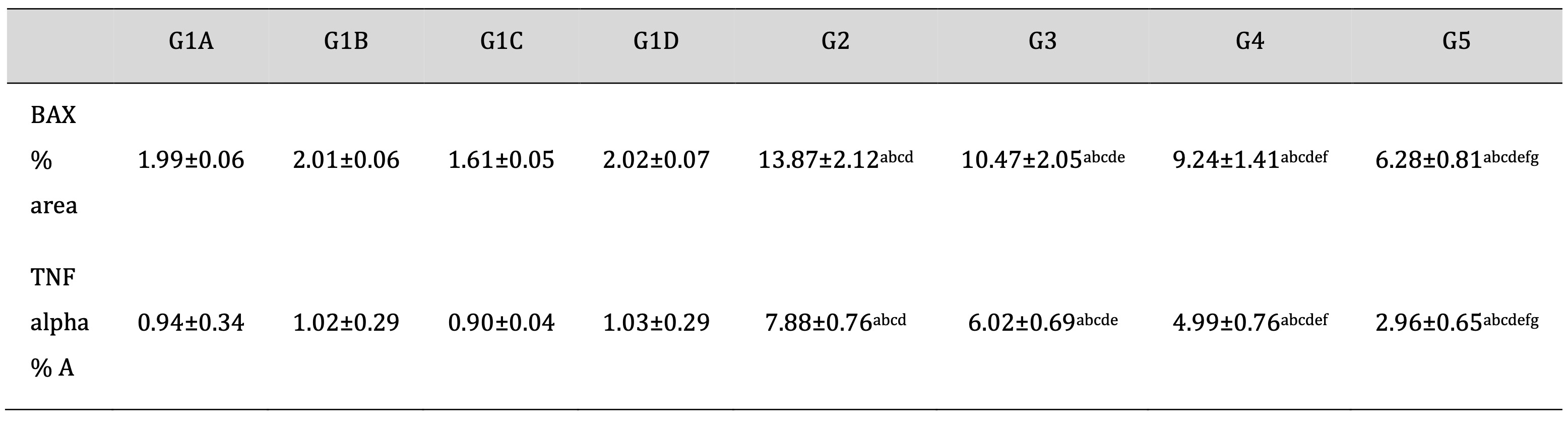
Table 3: Effect of different treatments on an antiapoptotic marker (BAX) and tumor necrosis factor area (%) score in the TDP and normal control groups. G1A: Untreated control group, G1B: Tadalafil (Td) control group, G1C: platelet-rich plasma (PRP) control group, G1D: PRP+Td control group, G2: torsion/detorsion process (TDP) group, G3: TDP+Td, G4: TDP+PRP, G5: TDP+PRP+Td. Data expressed as mean ± S.D., *: significance <0.001. A test used: One-way ANOVA followed by post-hoc tukey. a: significance vs G1A, b: significance vs G1B, c: significance vs G1C, d: significance vs G1D, e: significance vs G2, f: significance vs G3, g: significance vs G4
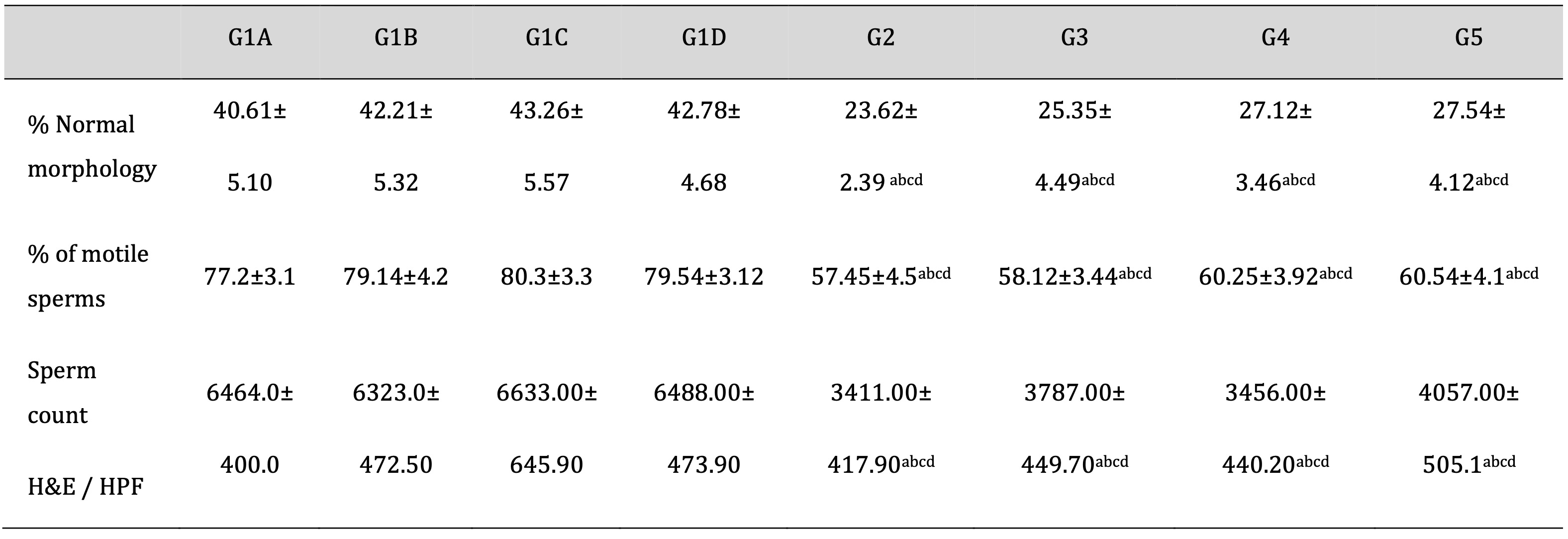
Table 4: Evaluation of sperm morphology and motility using a computer-assisted semen analysis (CASA) in epididymal samples and sperm count in testicular tissue (H&E). CASA: computer-assisted semen analysis; H&E: Hematoxylin and eosin stain; G1A: Untreated control group, G1B: Tadalafil (Td) control group, G1C: platelet-rich plasma (PRP) control group, G1D: PRP+Td control group, G2: torsion/detorsion process (TDP) group, G3: TDP+Td, G4: TDP+PRP, G5: TDP+PRP+Td. Data expressed as mean ± S.D., *: significance <0.001. A test used: One-way ANOVA followed by post-hoc Tukey. a: significance vs G1A, b: significance vs G1B, c: significance vs G1C, d: significance vs G1D, e: significance vs G2, f: significance vs G3, g: significance vs G4
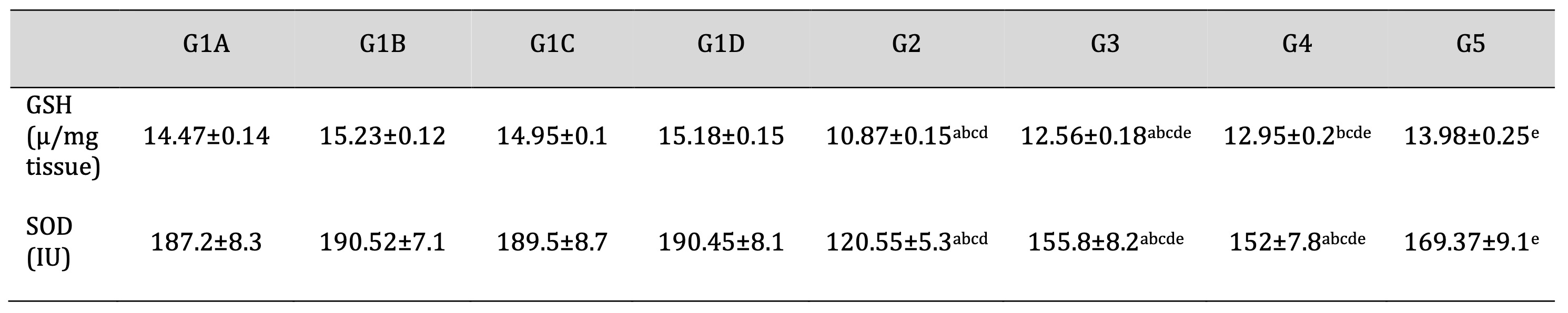
Table 5: Oxidative stress parameters (GSH, SOD) in the different studied groups. G1A: Untreated control group, G1B: Tadalafil (Td) control group, G1C: platelet-rich plasma (PRP) control group, G1D: PRP+Td control group, G2: torsion/detorsion process (TDP) group, G3: TDP+Td, G4: TDP+PRP, G5: TDP+PRP+Td. Data expressed as mean ± S.D., *: significance <0.05. A test used: One-way ANOVA followed by post-hoc Tukey. a: significance vs G1A, b: significance vs G1B, c: significance vs G1C, d: significance vs G1D, e: significance vs G2, f: significance vs G3, g: significance vs G4
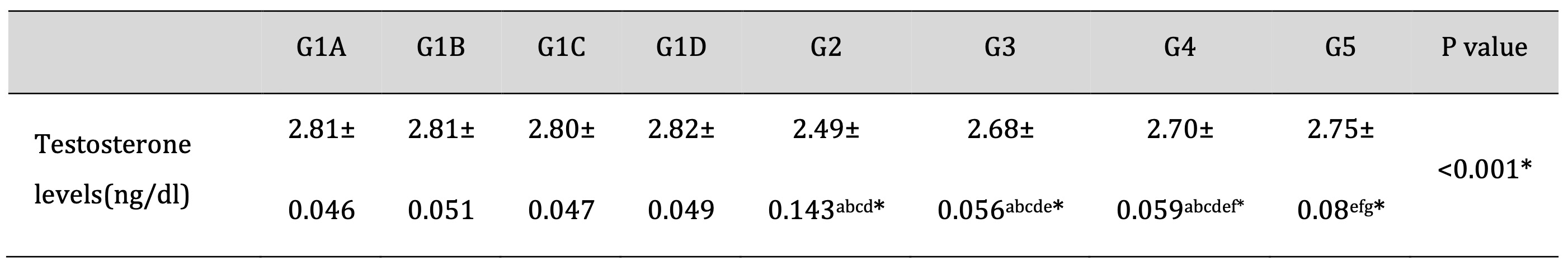
Table 6: Testosterone hormone level in the different studied groups. G1A: Untreated control group, G1B: Tadalafil (Td) control group, G1C: platelet-rich plasma (PRP) control group, G1D: PRP+Td control group, G2: torsion/detorsion process (TDP) group, G3: TDP+Td, G4: TDP+PRP, G5: TDP+PRP+Td. Data expressed as mean ± S.D., *: significance <0.001. A test used: One-way ANOVA followed by post-hoc Tukey. a: significance vs G1A, b: significance vs G1B, c: significance vs G1C, d: significance vs G1D, e: significance vs G2, f: significance vs G3, g: significance vs G4
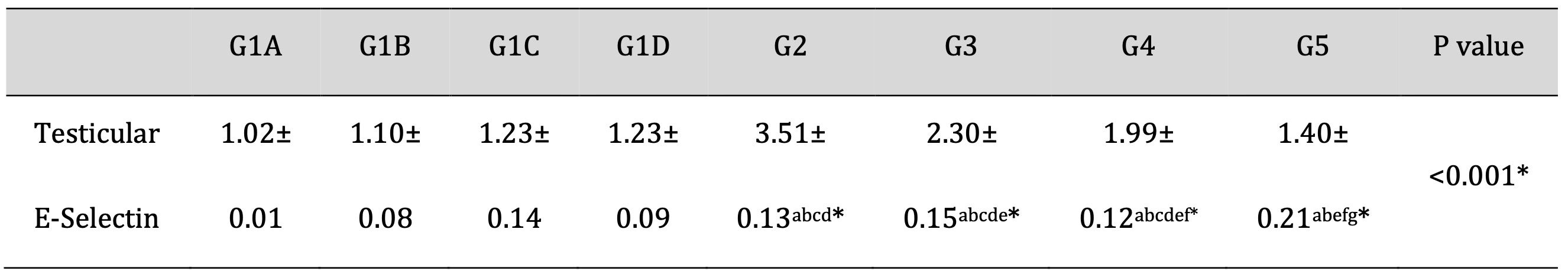
Table 7: Effects of Td and PRP on the mRNA expression levels of E-selectin. G1A: Untreated control group, G1B: Tadalafil (Td) control group, G1C: platelet-rich plasma (PRP) control group, G1D: PRP+Td control group, G2: torsion/detorsion process (TDP) group, G3: TDP+Td, G4: TDP+PRP, G5: TDP+PRP+Td. Data expressed as mean ± S.D., *: significance <0.001. A test used: One-way ANOVA followed by post-hoc Tukey. a: significance vs G1A, b: significance vs G1B, c: significance vs G1C, d: significance vs G1D, e: significance vs G2, f: significance vs G3, g: significance vs G4
Discussion
This study aims to evaluate the protective efficacy of platelet-rich plasma (PRP) and Tadalafil (Td), alone or combined on testis in experimentally induced Ischemia/reperfusion (I-R) injury following experimental torsion/detorsion process (TDP) of testis in the experimental rat model.
Despite the return of blood flow after testicular detorsion, testicular atrophy is an expected outcome. Multiple studies proved that the duration of symptoms before presentation to the hospital was the most important and influential factor in prediction of patients at risk of acute testicular torsion. Delayed management puts patients at risk of orchidectomy [37, 38]. Also, several studies proved that tissue damage caused by torsion cannot be entirely prevented. There is no recognized therapeutic agent that clinically can be used. Moreover, although the testis is the most easily torsional organ in the body, there is no satisfactory clinical research in the literature as regards this subject [23, 39]. For this reason, studies using experimental models continue regarding the management of testicular torsion.
Vascular endothelial function plays an essential role in testicular tissue viability, and TDP is closely linked to the abnormality of vascular endothelial function. Additionally, TDP can damage the vascular endothelium of the testis, make the vascular wall rough, and decrease the vascular endothelium's ability to inhibit platelet aggregation, quickly leading to thrombus [5].
Firstly, the results of our present study show that using Td, intratesticular injection of PRP and the combination can protect the testicular tissue against I-R injury. Otherwise, other studied drug as calcium channel blocker verapamil was less likely to reduce testicular torsion injury [40]. So the degeneration of the seminiferous tubules was scarcely observed in testicular sections from G5 received both PRP+Td than TDP group. In agreement with our results, Ameli et al. [25, 41] show that Tadalafil can increase the layers of "seminiferous epithelial," the histological score of the testis, and the diameter of the seminiferous tubule. As well, another study represents a response to intracavernosal injection of vasoactive drugs [42]. Also, other studies show that PRP therapy has been recorded to be efficient in I-R models of some organs. This is achieved by growth factors like transforming growth factor beta 1 (TGF-β1), insulin-like growth factor (IGF), and vascular/endothelial growth factor (VEGF) generally released during I-R injury for various protective roles. With its high convergence of growth factor, PRP can benefit regenerative therapy, particularly I-R damage following experimental torsion/detorsion of testis in animal models [43].
Then, reperfusion of the ischemic tissues releases different free radicals having hydroxyl radical ions, superoxide (O2−) and H2O2, which activate the inflammatory mediators. For this purpose, studies primarily focus on antioxidant therapy in I-R injury. In this manner, different antioxidants were effectively used to struggle with I-R injury[15]. The current study revealed that testicular torsion could lead to biochemical, histological, and physiological changes in rat testes. Correspondingly, the TDP group showed noticeable testicular impairment and go-over or unsuccessful spermatogenesis. This effect was compatible with the outcome of other researchers who indicated that the pathophysiologic machinery of testicular injury is an ischemia-reperfusion hurt [18, 44-46]. On the other hand, other studies represented the beneficial effect of PRP on spermatogenesis while used for longer time (one month) [47] than our study (2 hours). From a biochemical point of view, testicular ischemia-reperfusion corresponds to excess generation of ROS. Overproduction of ROS can impair DNA, lipids and proteins, from cellular dysfunction to even death [48].
Secondly, this work assessed oxidative tissue damage passing through SOD and GSH levels and parameters of antioxidant activity and found that the action of SOD and GSH levels was significantly low in the TDP group compared with the control group. This intervention with antioxidants may be able to cause cell damage induced by free radicals. These effects are in agreement with the results of earlier studies [49]. The oxidative tissue damage of TDP makes a need to use compounds to reduce free radical-induced cell damage. Clinical and experimental data showed that PRP, a potent antioxidant, has been reported to protect against I-R injury in the brain, liver, heart, and kidney [18, 44-46]. Also, PRP has no adverse effect on fertility [48], and PRP potentially impacts tissue healing through the differentiation and proliferation of tissue progenitor cells [49]. Therefore, the present study supports PRP treatment, as it successfully elevates the SOD activity, achieves GSH levels, and significantly enhances spermatogenesis in the testes, compared with the TDP group.
Otherwise, the effects of PRP and Td on biochemical parameters showed that the serum level of testosterone in therapeutic groups was significantly higher than in the TDP group. It may be due to these drugs' protective effects on preventing Leydig cell damage induced by torsion–detorsion in testis tissue. Evaluation of the sperm parameters in different groups shows that Td and PRP produced no significant improvement in sperm count, motility or morphology. In rats, one cycle of the spermatogenesis process takes about 56-58 days to form new motile normal sperms [50]. In agreement with our belief, Pomara et al. [51] show in a study that the Td has increased the quality and motility of spermatozoa with continued follow-up over several days. On the other hand, the administration of Td in our study reputable the projected blood level in Group 3, so the biochemical indicators were adjusted. Furthermore, it was significant that Td administration reduced necrosis in Group 3.
It is demonstrated that Td decreased the adverse effects of I-R injury through recognized actions on platelets, vascular smooth muscles and leukocytes. The antioxidant markers were significantly increased in group 3, and necrosis was raised in the ischemic group. These consequences propose that Td reduced testicular damage by diminishing ROS level also. The antioxidant defense systems, such as SOD and GSH, were found to be significantly low in tissue in the TDP group in comparison with the control group and were higher in the Td-treated group than the TDP group. Also, Wu et al. [52] show in a study that Tadalafil can increase the activity of SOD. In another study, a mouse model of induced nephropathy, Sildenafil and tadalafil pretreatment were capable of diminishing nephropathy possibility and overturned oxidative stress by the oxidant/antioxidant balance modulation [53].
E-selectin is an endothelial cell adhesion molecule responsible for the tying and slow progression of neutrophils to endothelial cells. Earlier studies have shown E-selectin to be expressed on endothelial cells next to I-R. injury in different organs [10, 54] or after treatment with TNFα [55]. Notably, mice lacking E-selectin show a reduction in neutrophil recruitment after I-R and a significant decrease in germ cell-specific apoptosis [56]. These findings are agreed with our results in which E selectin expression is significantly elevated in the TDP group with a significant elevation in the BCL2 apoptotic marker. These consequences indicate that E-selectin is the vital cell adhesion molecule for neutrophil enrolment to the endothelium in the testis after I-R. Neutrophils are chief mediators of germ cell apoptosis.
On the other hand, proinflammatory cytokines, particularly TNFα, are increased after I-R, leading to an increase in neutrophils and, subsequently, an increase in apoptosis in the smitten organ [8]. Earlier studies show that the rise in TNFα may occur via stimulation of the JNK/p38 stress-related kinase pathway [57, 58]. Otherwise, the NFκB pathway may be triggered by proinflammatory cytokines and end with a similar effect [59]. Additionally, TNFα activates the intracellular signaling pathway responsible for the increase in E-selectin expression, which matched with our results that E-selectin expression is stimulated in rat testis endothelial cells as a consequence of the elevation of TNFα.
Finally, the Td treatment was linked with a decrease in the E-selectin levels, which were higher in the TDP group than in the healthy group. Tadalafil treatment in the injury group had a modest result on the laboratory procedures of E-selectin compared with the normal healthy groups. Earlier studies demonstrate PDE5 inhibition down-regulates the endothelium system [60, 61], which clarifies E-selectin reduced levels in the rats with I-R injury treated with PDE5i. Whereas available studies, including our research, documented the reduction of biochemical measures of endothelial activation/damage system in the testes injury treated with PDE5i, which are sPlatelet (P)-selectin, sE-selectin and s-Intercellular adhesion molecule-1 (ICAM-1) [62-64]. This finding is confirmed in our data and established that the E-selectin levels were returned below those observed in the TDP group, even though its quantifiable significance has yet to be distinct compared to the healthy groups. Otherwise, guidelines indicate that men with a severe testicular injury due to older age, diabetes mellitus, and/or after radical prostatectomy often have a less continuing response to PDES5 inhibitors [65] and may require different managing approaches.
We recognized a potential role for endothelial E-selectin in PRP. PRP is autologous blood plasma that comprises additional four times than usual human physiological serum platelet concentration and is rich in numerous growth factors. The biological molecules active within PRP upregulate cell revival downstream [66]. In our study, it has been demonstrated that normal control was associated with excellent response to PRP therapy and better results when compared with the TDP group, primarily due to decreased endothelial dysfunction. Recently, Zaghloul (2022) pointed out that the PRP could be an effective treatment for erectile dysfunction patients non-responding to PDE5 inhibitors [42].
An earlier study assesses that the expression of selectin on the surface of platelets is often used as a marker of platelet 'activation'. So far, its clinical application is indefinite [67]. In the present study, PRP was found to improve the testicular injury of I-R rats by affecting endothelial function and platelet activation. Under normal conditions, E-selectin expression in endothelial cells is low. However, E-selectin expression can be activated through the activation of proinflammatory factors and oxidative stress and led to vascular endothelial function damage, which is significant in the TDP group. As an adhesion molecule in endothelial cells, E-selectin is considered of endothelial activation. Wang et al. [68] established that the elevation of E-selectin in serum plays a vital role in the differentiation and development of embryos, the maintenance of typical tissue structure, the pathological process of inflammation and the immune response, coagulation and thrombosis.
Additionally, Fathollahi et al. [69] demonstrated that sE-selectin and sICAM-1 were closely related to diabetes mellitus vascular lesions [69]. In our study, we found that the E-selectin level in the TDP group was significantly increased. HE staining showed that the number of blood sinuses in the corpus cavernosum of the TDP group showed a significant reduction in the number of small blood vessels, the distribution of blood sinuses was disrupted, and the densities of endothelial cells and smooth muscle cells were significantly decreased. The above verified that the T.D. process damaged the endothelial function of blood vessels, as reflected in the serum of model rats. In the PRP group, the E-selectin level was significantly reduced. Although a significant difference was present between the groups, the effect of the PRP group was better, and this indicates that PRP had a protective effect on the vascular endothelium of TDP rats.
In the pathophysiology of testicular reperfusion injury, endothelial dysfunction secondary to inflammation occurs significantly; so the regenerative PRP treatment in our study is to improve endothelial functions by reducing the E-selectin level. Experimental studies and reviews [70-72] demonstrate that the cellular mechanisms of PRP activated in tissue regeneration and healing with improving testicular blood flow by Td coincide with our results.
Conclusion
Our results, for the first time, according to our knowledge, showed that PRP therapy reformed the endothelial purpose and enhanced the testicular degeneration respective to E-selectin expression management. However, the beneficial effect obtained in the current study cannot be attributed alone to PRP treatment only, but it can be more due to Td and PRP combination. Combination treatment of Td with PRP is reliable. It could be a promising modality where it could be concluded that such a therapeutic regimen could be effective for the improvement of response to PDE5 inhibitors. However, further detailed studies and explanations are needed to reach the best regimen and preparation method for subjects with testicular I-R injury. PRP can be an accepted therapy for testicular damage with Tadalafil via its antioxidant, anti-inflammatory and antiapoptotic abilities. Our findings also suggest that PRR can fast-track testicular and endothelial regeneration and avoid corporal smooth muscle atrophy by spot on the primary molecular signaling pathway in the progression of vascular repair through the endothelial function of E-selectin expression.
Positiveness and limitations
To our knowledge, this study is the first one that evaluates the combination of traditional treatment of PDE5i Tadalafil and PRP in an animal model of ischemia-reperfusion injury. Although other studies have evaluated the combination therapy of PRP with other medicines, thus lack control groups. Otherwise, this study has some limitations, such as the need for more scale to find the prognostic factors that could direct the best application of this treatment approach. A continuing follow-up also appears critical to distinguish the probable further and/or adversative effects. Likewise, additional studies should be helpful for PRP usage as a vital need for the participation of the female companion in assessing the results. Otherwise, a long period of treatment can evaluate the spermatogenesis more definitely. Despite these limitations, the study has shown a tendency for PRP to improve testicular I-R injury and outcomes in rats with I-R injury, and the results have been hopeful. Future studies in our laboratory will advance trials for in vivo infusion of PRP into the human testis to precisely block endothelial injury after I-R of the testis.
Abbreviations
AVMA (American Veterinary Medical Association,); CASA (Computer-assisted semen analysis); E.D (Erectile dysfunction); ELISA (Enzyme-linked immunoassay); GSH (Glutathione); H&E (Hematoxylin and eosin); ICAM-1 (Intercellular adhesion molecule-1); IGF (Insulin-like growth factor); IL-1β (Interleukin-1β); I-R (Ischemic reperfusion); JNK (c-Jun N-terminal kinases); NC3R (National Centre for the Replacement, Refinement and Reduction of Animals in Research); PDE (Phosphodiesterase); PDE5i (Phosphodiesterase-5 inhibitor); PRP (Platelet-rich plasma); ROS (Reactive oxygen species); SOD (Superoxide Dismutase); Td (Tadalafil); TDP (Torsion/detorsion process); TGF-β1 (Transforming growth factor beta 1); TNF (Tumor necrosis factor-alpha); VEGF (Vascular/endothelial growth factor);
Acknowledgements
Statement of Ethics
Experimental protocols monitored the ARRIVE guidelines of the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs). All experiments were achieved following appropriate policies and regulations of the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals. This study was approved by the Institutional Animal Care and Use Committee at the FPDU (Ref No.: FPDU 10/2021).
Author contributions
Dalia M. Abdel Ghaffar: conceptualization of this research idea, methodology development, experiments, data collection, investigation, validation, resources, project administration, statistical analysis, interpretation of results, writing review & editing. Zienab Helmy Eldken: Conceptualizing the research idea, experiments, methodology development, data collection, interpretation of results and writing review & editing. Mohammed S. Sultan: methodology development, histopathological and immunohistochemical examinations, data collection, writing-review & editing. Rania M. Khalil: methodology development, analysis, data collection, literature review, interpretation of results, writing-review & editing. Noha Hammad Sakr: Conceptualization of the research idea, experiments, methodology development, data collection, sperm analysis, and interpretation of results. Hanan Eissa: methodology development, data collection, writing-review & editing. Sally M. Safwat: Conceptualization of the research idea, experiments, methodology development, data collection, and interpretation of results.
Funding Sources
This research did not have any grant from funding agencies in the commercial, public or not for profit sectors.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Zhao LC, Lautz TB, Meeks JJ, Maizels M: Pediatric testicular torsion epidemiology using a national database: incidence, risk of orchiectomy and possible measures toward improving the quality of care. The Journal of urology 2011;186:2009-2013.
https://doi.org/10.1016/j.juro.2011.07.024 |
| 2 | Riaz-Ul-Haq M, Mahdi DE, Elhassan EU: Neonatal testicular torsion; a review article. Iranian journal of pediatrics 2012;22:281-289.
|
| 3 | Vasconcelos-Castro S, Flor-de-Lima B, Campos JM, Soares-Oliveira M: Manual detorsion in testicular torsion: 5 years of experience at a single center. Journal of pediatric surgery 2020;55:2728-2731.
https://doi.org/10.1016/j.jpedsurg.2020.02.026 |
| 4 | Chi KK, Zhang WH, Wang GC, Chen Z, He W, Wang SG, Cui Y, Lu P, Wang XJ, Chen H: Comparison of Intraperitoneal and Intraepididymal Quercetin for the Prevention of Testicular Torsion/Detorsion-induced Injury. Urology 2017;99:106-111.
https://doi.org/10.1016/j.urology.2016.09.017 |
| 5 | Tamamura M, Saito M, Kinoshita Y, Shimizu S, Satoh I, Shomori K, Dimitriadis F, Satoh K: Protective effect of edaravone, a free-radical scavenger, on ischaemia-reperfusion injury in the rat testis. BJU international 2010;105:870-876.
https://doi.org/10.1111/j.1464-410X.2009.08798.x |
| 6 | Wang Y, Kawamura N, Schmelzer JD, Schmeichel AM, Low PA: Decreased peripheral nerve damage after ischemia-reperfusion injury in mice lacking TNF-alpha. Journal of the neurological sciences 2008;267:107-111.
https://doi.org/10.1016/j.jns.2007.10.004 |
| 7 | Oswari L, Hidayat R, Fatmawati F, Hayati L, Alisa BS: Gambir Extract (Uncaria Gambir) Decreases Inflammatory Response and Increases Gastric Mucosal Integrity in Wistar Rats - Model Gastritis. Open access Macedonian journal of medical sciences 2019;7:3149-3152.
https://doi.org/10.3889/oamjms.2019.758 |
| 8 | Dasdelen D, Solmaz M, Menevse E, Mogulkoc R, Baltaci AK, Erdogan E: Increased apoptosis, tumor necrosis factor-α, and DNA damage attenuated by 3',4'-dihydroxyflavonol in rats with brain İschemia-reperfusion. Indian J Pharmacol 2021;53:39-49.
https://doi.org/10.4103/ijp.IJP_727_20 |
| 9 | Dohlman TH, Di Zazzo A, Omoto M, Hua J, Ding J, Hamrah P, Chauhan SK, Dana R: E-Selectin Mediates Immune Cell Trafficking in Corneal Transplantation. Transplantation 2016;100:772-780.
https://doi.org/10.1097/TP.0000000000001107 |
| 10 | Lysiak JJ, Nguyen QA, Kirby JL, Turner TT: Ischemia-reperfusion of the murine testis stimulates the expression of proinflammatory cytokines and activation of c-jun N-terminal kinase in a pathway to E-selectin expression. Biology of reproduction 2003;69:202-210.
https://doi.org/10.1095/biolreprod.102.013318 |
| 11 | Bakacak M, Bostanci MS, İnanc F, Yaylali A, Serin S, Attar R, Yildirim G, Yildirim OK: Protective Effect of Platelet Rich Plasma on Experimental Ischemia/Reperfusion Injury in Rat Ovary. Gynecologic and obstetric investigation 2016;81:225-231.
https://doi.org/10.1159/000440617 |
| 12 | Shimizu S, Tsounapi P, Dimitriadis F, Higashi Y, Shimizu T, Saito M: Testicular torsion-detorsion and potential therapeutic treatments: A possible role for ischemic postconditioning. International journal of urology : official journal of the Japanese Urological Association 2016;23:454-463.
https://doi.org/10.1111/iju.13110 |
| 13 | Ozdemirkan A, Kurtipek AC, Kucuk A, Ozdemir C, Yesil S, Sezen SC, Kavutcu M, Arslan M: Effect of Cerium Oxide on Kidney and Lung Tissue in Rats with Testicular Torsion/Detorsion. BioMed research international 2022;2022:3176455.
https://doi.org/10.1155/2022/3176455 |
| 14 | Arabaci Tamer S, Yildirim A, Köroğlu MK, Çevik Ö, Ercan F, Yeğen B: Nesfatin-1 ameliorates testicular injury and supports gonadal function in rats induced with testis torsion. Peptides 2018;107:1-9.
https://doi.org/10.1016/j.peptides.2018.07.005 |
| 15 | Kayiran O, Cuzdan SS, Uysal A, Kocer U: Tadalafil significantly reduces ischemia reperfusion injury in skin island flaps. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India 2013;46:75-81.
https://doi.org/10.4103/0970-0358.113714 |
| 16 | Tetsi L, Charles AL, Georg I, Goupilleau F, Lejay A, Talha S, Maumy-Bertrand M, Lugnier C, Geny B: Effect of the Phosphodiesterase 5 Inhibitor Sildenafil on Ischemia-Reperfusion-Induced Muscle Mitochondrial Dysfunction and Oxidative Stress. Antioxidants (Basel, Switzerland) 2019;8
https://doi.org/10.3390/antiox8040093 |
| 17 | Erol B, Tokgoz H, Hanci V, Bektas S, Akduman B, Yencilek F, Mungan G, Mungan A: Vardenafil reduces testicular damage following ischemia/reperfusion injury in rats. The Kaohsiung journal of medical sciences 2009;25:374-380.
https://doi.org/10.1016/S1607-551X(09)70530-3 |
| 18 | Hargrave B, Varghese F, Barabutis N, Catravas J, Zemlin C: Nanosecond pulsed platelet-rich plasma (nsPRP) improves mechanical and electrical cardiac function following myocardial reperfusion injury. Physiological reports 2016;4
https://doi.org/10.14814/phy2.12710 |
| 19 | Salem N, Helmi N, Assaf N: Renoprotective Effect of Platelet-Rich Plasma on Cisplatin-Induced Nephrotoxicity in Rats. Oxidative medicine and cellular longevity 2018;2018:9658230.
https://doi.org/10.1155/2018/9658230 |
| 20 | Bayat M, Zabihi S, Karbalaei N, Haghani M: Time-dependent effects of platelet-rich plasma on the memory and hippocampal synaptic plasticity impairment in vascular dementia induced by chronic cerebral hypoperfusion. Brain research bulletin 2020;164:299-306.
https://doi.org/10.1016/j.brainresbull.2020.08.033 |
| 21 | Li XH, Zhou X, Zeng S, Ye F, Yun JL, Huang TG, Li H, Li YM: Effects of intramyocardial injection of platelet-rich plasma on the healing process after myocardial infarction. Coronary artery disease 2008;19:363-370.
https://doi.org/10.1097/MCA.0b013e3282fc6165 |
| 22 | Underwood W, Anthony RJRoM: AVMA guidelines for the euthanasia of animals: 2020 edition. 2020;2013:2020-2021.
|
| 23 | Yildirim C, Yuksel OH, Urkmez A, Sahin A, Somay A, Verit A: Protective effects of Tadalafil and darbepoetin against ischemia - reperfusion injury in a rat testicular torsion model. International braz j urol : official journal of the Brazilian Society of Urology 2018;44:1005-1013.
https://doi.org/10.1590/s1677-5538.ibju.2018.0200 |
| 24 | Dehghani F, Sotoude N, Bordbar H, Panjeshahin MR, Karbalay-Doust S: The use of platelet-rich plasma (PRP) to improve structural impairment of rat testis induced by busulfan. Platelets 2019;30:513-520.
https://doi.org/10.1080/09537104.2018.1478400 |
| 25 | Gazia MA: Histological Study on the Possible Ameliorating Effect of Platelet Rich Plasma on Ischemia/Reperfusion Injury in Testicular Torsion Model in Adult Albino Rat %J Egyptian Journal of Histology. 2020;43:614-629.
https://doi.org/10.21608/ejh.2019.9860.1091 |
| 26 | Beheshtian A, Salmasi AH, Payabvash S, Kiumehr S, Ghazinezami B, Rahimpour S, Tavangar SM, Dehpour AR: Protective effects of sildenafil administration on testicular torsion/detorsion damage in rats. World journal of urology 2008;26:197-202.
https://doi.org/10.1007/s00345-008-0243-6 |
| 27 | Meadows MC, Levy DM, Ferry CM, Gardner TR, Teratani T, Ahmad CS: Effects of Platelet-Rich Plasma and Indomethacin on Biomechanics of Rotator Cuff Repair. American journal of orthopedics (Belle Mead, NJ) 2017;46:E336-e343.
|
| 28 | Barrionuevo DV, Laposy CB, Abegão KG, Nogueira RM, Nai GA, Bracale BN, Delfim IG: Comparison of experimentally-induced wounds in rabbits treated with different sources of platelet-rich plasma. Laboratory animals 2015;49:209-214.
https://doi.org/10.1177/0023677214567747 |
| 29 | Gamal N, Abourabia NM, Elebiary FH, Khalaf G, Raafat MH: The Possible Therapeutic Role of Platelet Rich Plasma on a Model of Osteoarthritis in Male Albino Rat. Histological and Immunohistochemical Study. QJM: An International Journal of Medicine 2020;113
https://doi.org/10.1093/qjmed/hcaa051 |
| 30 | RANIA N. ABD-ELLATIF MD, ISLAM I. HEGAB, M.D., ATEF MD, MARWA M.: Effect of Platelet Rich Plasma on an Experimental Rat Model of Adriamycin Induced Chronic Kidney Disease %J The Medical Journal of Cairo University. 2019;87:2207-2217.
https://doi.org/10.21608/mjcu.2019.54382 |
| 31 | Chen NF, Sung CS, Wen ZH, Chen CH, Feng CW, Hung HC, Yang SN, Tsui KH, Chen WF: Therapeutic Effect of Platelet-Rich Plasma in Rat Spinal Cord Injuries. Frontiers in neuroscience 2018;12:252.
https://doi.org/10.3389/fnins.2018.00252 |
| 32 | Oz M, Cetinkaya N, Bas S, Korkmaz E, Ozgu E, Terzioglu GS, Buyukkagnici U, Akbay S, Caydere M, Gungor T: A randomized controlled experimental study of the efficacy of platelet-rich plasma and hyaluronic acid for the prevention of adhesion formation in a rat uterine horn model. Archives of gynecology and obstetrics 2016;294:533-540.
https://doi.org/10.1007/s00404-016-4079-9 |
| 33 | Bancroft JD, Floyd AD: 3 - Light Microscopy; in Bancroft JD, Gamble M (eds): Theory and Practice of Histological Techniques (Sixth Edition). Edinburgh, Churchill Livingstone, 2008, pp 33-52.
https://doi.org/10.1016/B978-0-443-10279-0.50010-5 |
| 34 | Johnsen SG: Testicular Biopsy Score Count - A Method for Registration of Spermatogenesis in Human Testes: Normal Values and Results in 335 Hypogonadal Males. Hormone Research in Paediatrics 1970;1:2-25.
https://doi.org/10.1159/000178170 |
| 35 | Krause W: [Value of computer-assisted sperm analysis (CASA). Reproducibility--online documentation--prognostic value]. Fortschritte der Medizin 1996;114:470-473.
|
| 36 | Menkveld R, Franken DR, Kruger TF, Oehninger S, Hodgen GD: Sperm selection capacity of the human zona pellucida. Molecular reproduction and development 1991;30:346-352.
https://doi.org/10.1002/mrd.1080300409 |
| 37 | Grimsby GM, Schlomer BJ, Menon VS, Ostrov L, Keays M, Sheth KR, Villanueva C, Granberg C, Dajusta D, Hill M, Sanchez E, Harrison CB, Jacobs MA, Burgu B, Hennes H, Baker LA: Prospective Evaluation of Predictors of Testis Atrophy After Surgery for Testis Torsion in Children. Urology 2018;116:150-155.
https://doi.org/10.1016/j.urology.2018.03.009 |
| 38 | Goetz J, Roewe R, Doolittle J, Roth E, Groth T, Mesrobian HG, Rein LE, Szabo A, Kryger J: A comparison of clinical outcomes of acute testicular torsion between prepubertal and postpubertal males. Journal of pediatric urology 2019;15:610-616.
https://doi.org/10.1016/j.jpurol.2019.07.020 |
| 39 | Aggarwal D, Parmar K, Sharma AP, Tyagi S, Kumar S, Singh SK, Gupta S: Long-term impact of testicular torsion and its salvage on semen parameters and gonadal function. Indian journal of urology : IJU : journal of the Urological Society of India 2022;38:135-139.
https://doi.org/10.4103/iju.iju_328_21 |
| 40 | Davoodi F, Raisi A, Rajabzadeh A, Hablolvarid MH, Zakian A: The effects of verapamil and heparin co-administration on sperm parameters and oxidative stress in prevention of testicular torsion/detorsion damage in rats. Andrologia 2020;52:e13479.
https://doi.org/10.1111/and.13479 |
| 41 | Lian BS, Ong CC, Chiang LW, Rai R, Nah SA: Factors Predicting Testicular Atrophy after Testicular Salvage following Torsion. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie 2016;26:17-21.
https://doi.org/10.1055/s-0035-1566096 |
| 42 | Zaghloul AS, El-Nashaar AM, Said SZ, Osman IA, Mostafa T: Assessment of the intracavernosal injection platelet-rich plasma in addition to daily oral tadalafil intake in diabetic patients with erectile dysfunction non-responding to on-demand oral PDE5 inhibitors. Andrologia 2022;54:e14421.
https://doi.org/10.1111/and.14421 |
| 43 | Khedr NF, Khalil RM: Effect of hesperidin on mice bearing Ehrlich solid carcinoma maintained on doxorubicin. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2015;36:9267-9275.
https://doi.org/10.1007/s13277-015-3655-0 |
| 44 | Soliman AF, Saif-Elnasr M, Abdel Fattah SM: Platelet-rich plasma ameliorates gamma radiation-induced nephrotoxicity via modulating oxidative stress and apoptosis. Life sciences 2019;219:238-247.
https://doi.org/10.1016/j.lfs.2019.01.024 |
| 45 | Yip HK, Chen KH, Dubey NK, Sun CK, Deng YH, Su CW, Lo WC, Cheng HC, Deng WP: Cerebro- and renoprotective activities through platelet-derived biomaterials against cerebrorenal syndrome in rat model. Biomaterials 2019;214:119227.
https://doi.org/10.1016/j.biomaterials.2019.119227 |
| 46 | Schulte am Esch J, 2nd, Akyildiz A, Tustas RY, Ganschow R, Schmelzle M, Krieg A, Robson SC, Topp SA, Rogiers X, Knoefel WT, Fischer L: ADP-dependent platelet function prior to and in the early course of pediatric liver transplantation and persisting thrombocytopenia are positively correlated with ischemia/reperfusion injury. Transplant international : official journal of the European Society for Organ Transplantation 2010;23:745-752.
https://doi.org/10.1111/j.1432-2277.2010.01054.x |
| 47 | Kutluhan MA, Özsoy E, Şahin A, Ürkmez A, Topaktaş R, Toprak T, Gümrükçü G, Verit A: Effects of platelet-rich plasma on spermatogenesis and hormone production in an experimental testicular torsion model. Andrology 2021;9:407-413.
https://doi.org/10.1111/andr.12895 |
| 48 | Kamath MS, Mascarenhas M, Franik S, Liu E, Sunkara SK: Clinical adjuncts in in vitro fertilization: a growing list. Fertility and sterility 2019;112:978-986.
https://doi.org/10.1016/j.fertnstert.2019.09.019 |
| 49 | Sharun K, Jambagi K, Dhama K, Kumar R, Pawde AM, Amarpal: Therapeutic Potential of Platelet-Rich Plasma in Canine Medicine. Archives of Razi Institute 2021;76:721-730.
|
| 50 | Ameli M, Hashemi MS, Moghimian M, Shokoohi M: Protective effect of tadalafil and verapamil on testicular function and oxidative stress after torsion/detorsion in adult male rat. Andrologia 2018;50:e13068.
https://doi.org/10.1111/and.13068 |
| 51 | Pomara G, Morelli G, Canale D, Turchi P, Caglieresi C, Moschini C, Liguori G, Selli C, Macchia E, Martino E, Francesca F: Alterations in sperm motility after acute oral administration of sildenafil or tadalafil in young, infertile men. Fertility and sterility 2007;88:860-865.
https://doi.org/10.1016/j.fertnstert.2006.12.019 |
| 52 | Wu ZG, Wang GB, Xiao YB, Chen TK, Cai J, Li CD: [Protective effect of tadalafil against ischemia-reperfusion injury in rats]. Zhonghua nan ke xue = National journal of andrology 2015;21:214-218.
|
| 53 | Iordache AM, Docea AO, Buga AM, Zlatian O, Ciurea ME, Rogoveanu OC, Burada F, Sosoi S, Mitrut R, Mamoulakis C, Albulescu D, Vasile RC, Tsatsakis A, Calina D: Sildenafil and tadalafil reduce the risk of contrast-induced nephropathy by modulating the oxidant/antioxidant balance in a murine model. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2020;135:111038.
https://doi.org/10.1016/j.fct.2019.111038 |
| 54 | Herter JM, Rossaint J, Spieker T, Zarbock A: Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. Journal of innate immunity 2014;6:597-606.
https://doi.org/10.1159/000358238 |
| 55 | Celebi M, Paul AG: Blocking E-selectin inhibits ischaemia-reperfusion-induced neutrophil recruitment to the murine testis. Andrologia 2008;40:235-239.
https://doi.org/10.1111/j.1439-0272.2008.00849.x |
| 56 | Sukhotnik I, Greenblatt R, Voskoboinik K, Lurie M, Coran AG, Mogilner JG: Relationship between time of reperfusion and E-selectin expression, neutrophil recruitment, and germ cell apoptosis after testicular ischemia in a rat model. Fertility and sterility 2008;90:1517-1522.
https://doi.org/10.1016/j.fertnstert.2007.07.1334 |
| 57 | Zhong L, Huot J, Simard MJ: p38 activation induces production of miR-146a and miR-31 to repress E-selectin expression and inhibit transendothelial migration of colon cancer cells. Scientific reports 2018;8:2334.
https://doi.org/10.1038/s41598-018-20837-9 |
| 58 | Zhong L, Simoneau B, Huot J, Simard MJ: p38 and JNK pathways control E-selectin-dependent extravasation of colon cancer cells by modulating miR-31 transcription. Oncotarget 2017;8:1678-1687.
https://doi.org/10.18632/oncotarget.13779 |
| 59 | Saber S, Khalil RM, Abdo WS, Nassif D, El-Ahwany E: Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicology and applied pharmacology 2019;364:120-132.
https://doi.org/10.1016/j.taap.2018.12.020 |
| 60 | Liang F, Yang S, Yao L, Belardinelli L, Shryock J: Ambrisentan and tadalafil synergistically relax endothelin-induced contraction of rat pulmonary arteries. Hypertension (Dallas, Tex : 1979) 2012;59:705-711.
https://doi.org/10.1161/HYPERTENSIONAHA.111.182261 |
| 61 | Duan XG: [Tadalafil for erectile dysfunction: an overview]. Zhonghua nan ke xue = National journal of andrology 2013;19:380-383.
|
| 62 | Pelliccione F, D'Angeli A, D'Andrea S, Barbonetti A, Pezzella A, Necozione S, Falone S, Amicarelli F, Francavilla F, Francavilla S: Tadalafil treatment had a modest effect on endothelial cell damage and repair ability markers in men with erectile dysfunction and vascular risk. Asian journal of andrology 2014;16:290-294.
https://doi.org/10.4103/1008-682X.122347 |
| 63 | Choi H, Kim HJ, Bae JH, Kim JH, Moon du G, Cheon J, Yeo JK: A Meta-Analysis of Long- Versus Short-Acting Phosphodiesterase 5 Inhibitors: Comparing Combination Use With α-Blockers and α-Blocker Monotherapy for Lower Urinary Tract Symptoms and Erectile Dysfunction. International neurourology journal 2015;19:237-245.
https://doi.org/10.5213/inj.2015.19.4.237 |
| 64 | De Bon E, Bonanni G, Saggiorato G, Bassi P, Cella G: Effects of tadalafil on platelets and endothelium in patients with erectile dysfunction and cardiovascular risk factors: a pilot study. Angiology 2010;61:602-606.
https://doi.org/10.1177/0003319710362977 |
| 65 | Burnett AL, Nehra A, Breau RH, Culkin DJ, Faraday MM, Hakim LS, Heidelbaugh J, Khera M, McVary KT, Miner MM, Nelson CJ, Sadeghi-Nejad H, Seftel AD, Shindel AW: Erectile Dysfunction: AUA Guideline. The Journal of urology 2018;200:633-641.
https://doi.org/10.1016/j.juro.2018.05.004 |
| 66 | Campbell JD, Milenkovic U, Usta MF, Albersen M, Bivalacqua TJ: The good, bad, and the ugly of regenerative therapies for erectile dysfunction. Translational andrology and urology 2020;9:S252-s261.
https://doi.org/10.21037/tau.2019.10.06 |
| 67 | Cardigan R, Turner C, Harrison P: Current methods of assessing platelet function: relevance to transfusion medicine. Vox sanguinis 2005;88:153-163.
https://doi.org/10.1111/j.1423-0410.2005.00618.x |
| 68 | Wang JS, Li X, Chen ZL, Feng JL, Bao BH, Deng S, Dai HH, Meng FC, Wang B, Li HS: Effect of leech-centipede medicine on improving erectile function in DIED rats via PKC signalling pathway-related molecules. Journal of ethnopharmacology 2021;267:113463.
https://doi.org/10.1016/j.jep.2020.113463 |
| 69 | Fathollahi A, Massoud A, Amirzargar AA, Aghili B, Nasli Esfahani E, Rezaei N: sICAM-1, sVCAM-1 and sE-Selectin Levels in Type 1 Diabetes. Fetal and pediatric pathology 2018;37:69-73.
https://doi.org/10.1080/15513815.2017.1405467 |
| 70 | Namazi H: Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation: a novel molecular mechanism. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2011;27:1456; author reply 1456-1457.
https://doi.org/10.1016/j.arthro.2011.09.002 |
| 71 | Geyik S: Comparison of the efficacy of low-intensity shock wave therapy and its combination with platelet-rich plasma in patients with erectile dysfunction. Andrologia 2021;53:e14197.
https://doi.org/10.1111/and.14197 |
| 72 | Anastasiadis E, Ahmed R, Khoja AK, Yap T: Erectile dysfunction: Is platelet-rich plasma the new frontier for treatment in patients with erectile dysfunction? A review of the existing evidence. 2022;4.
https://doi.org/10.3389/frph.2022.944765 |