Adaptive Effects of Intermittent Hypoxia Training on Oxygen-Dependent Processes as a Potential Therapeutic Strategy Tool
bDepartment of Ecology, Geography and Nature Management, T.H. Shevchenko National University “Chernihiv Colehium”, 53 Hetmana Polubotka Str., Chernihiv, 14013, Ukraine,
cNicolausCopernicusUniversity in Toruń, Collegium Medicum in Bydgoszcz, Department of Medical Biology and Biochemistry, Department of Ecology and Environmental Protection, M. Skłodowska-Curie St. 9, PL 85-094 Bydgoszcz, Poland,
dUniversity of Zielona Góra, Faculty of Biological Sciences, Institute of Biological Sciences, Department of Biotechnology, Prof. Z. Szafran St. 1, PL 65-516 Zielona Góra, Poland
Keywords
Abstract
Background/Aims:
Important benefits of intermittent hypoxic training (IHT) have emerged as an effective tool for enhancing adaptive potential in different pathological states, among which acute hypoxia dominates. Therefore, the aim of our study was to evaluate the mechanisms related to the effects of the nitric oxide system (nitrites, nitrates, carbamide, and total polyamine content) on ADP-stimulated oxygen consumption and oxidative phosphorylation in heart and liver mitochondria and biomarkers of oxidative stress in the blood, heart, and liver of rats exposed to the IHT method and acute hypoxia and treated with the amino acid L-arginine (600 mg/kg, 30 min) or the NO synthase inhibitor L-NNA (35 mg/kg, 30 min) prior to each IHT session.Methods:
We analysed the modulation of the system of oxygen-dependent processes (mitochondrial respiration with the oxygraphic method, microsomal oxidation, and lipoperoxidation processes using biochemical methods) in tissues during IHT in the formation of short-term and long-term effects (30, 60, and 180 days after the last IHT session) with simultaneous administration of L-arginine. In particular, we investigated how mitochondrial functions are modulated during intermittent hypoxia with the use of oxidation substrates (succinate or α-ketoglutarate) in bioenergetic mechanisms of cellular stability and adaptation.Results:
The IHT method is associated with a significant increase in the production of endogenous nitric oxide measured by the levels of its stable metabolite, nitrite anion, in both plasma (almost 7-fold) and erythrocytes (more than 7-fold) of rats. The intensification of nitric oxide-dependent pathways of metabolic transformations in the energy supply processes in the heart and liver, accompanied by oscillatory mechanisms of adaptation in the interval mode, causes a probable decrease in the production of urea and polyamines in plasma and liver, but not in erythrocytes. The administration of L-arginine prior to the IHT sessions increased the level of the nitrite-reducing component of the nitric oxide cycle, which persisted for up to 180 days of the experiment.Conclusion:
Thus, the efficacy of IHT and its nitrite-dependent component shown in this study is associated with the formation of long-term adaptive responses by preventing the intensification of lipoperoxidation processes in tissues due to pronounced changes in the main enzymes of antioxidant defence and stabilisation of erythrocyte membranes, which has a pronounced protective effect on the system of regulation of oxygen-dependent processes as a whole.Introduction
Hypoxia refers to a condition in which there is an insufficient supply of oxygen to tissues and cells in the body (Lee et al., 2019). It can be caused by various factors, such as high altitude, lung disease, or reduced blood flow (Huang et al., 2022). When cells do not get enough oxygen, their normal function is compromised, leading to potential health problems. Researchers are studying hypoxia to understand its effects and explore therapeutic strategies for treatment of related conditions. Adaptation to hypoxia – a condition characterised by an insufficient supply of oxygen to tissues and cells – is a widespread phenomenon in living organisms. It is considered to be one of the oldest evolutionary forms of adaptation. Initially associated with pathological conditions, the concept of hypoxia has evolved to recognise its physiological significance in adaptive responses to external hypoxic factors. Gradually, researchers have explored the clinical implications of hypoxia, particularly in cardio- and cerebrovascular diseases (Della Rocca et al., 2022).
Intermittent hypoxic training (IHT) has emerged as an effective tool for improving adaptive potential, endurance, and work capacity, and its beneficial effects have been discussed in many publications (Rybnikova et al., 2022; Sales de Campos et al., 2023; Timón et al., 2024). The therapeutic effects of intermittent hypoxic training (cycles of hypoxic exposure followed by reoxygenation) have been studied by many teams of authors (Zhao et al., 2019; Urdampilleta Otegui and Roche Collado, 2024) and are reported in the literature in various research areas. Firstly, it improves oxygen transport and utilisation (Zhang et al., 2023). IHT stimulates the production of erythropoietin (EPO), which leads to increased red blood cell production (Song et al., 2017). This increases oxygen transport capacity and delivery to tissues.
Important benefits are associated with improved oxygen utilisation through adaptation of mitochondrial function and oxidative phosphorylation pathways (Sokolova, 2018). Intermittent hypoxia affects mitochondrial function, mitophagy, and NO dynamics (Gong et al., 2020; Wang et al., 2021). Mitochondrial respiration during intermittent hypoxia has been shown to enhance mitophagy processes, with IHT greatly facilitating the induction of mitophagy, leading to the removal of damaged mitochondria. This is related to the enhancement of mitochondrial depolarisation by affecting the electrochemical gradient across the inner mitochondrial membrane (Wu et al., 2021). Increased release of reactive oxygen species (ROS) by mitochondria has been found, which may contribute to oxidative stress in a dose-dependent manner (Iturriaga et al., 2015).
IHT may affect nitric oxide (NO) levels through increased NO production and increased NO synthesis (Kurhaluk et al., 2023). The vasodilatory effects of IHT are associated with increased NO production, which promotes vasodilation, improving blood flow and oxygen delivery. A number of studies have linked the potential protective effects of IHT to the protective effects of NO during IHT, which may have a protective effect against oxidative stress (Manukhina et al., 2006; Liu et al., 2022). In addition, IHT induces neuroprotective effects and improves cognitive function, which benefits brain health by promoting neurogenesis, synaptic plasticity, and antioxidant defences. Importantly, this exposure to interval hypoxia may attenuate age-related cognitive decline and reduce the risk of neurodegenerative diseases, as shown by Shobatake et al. (2022).
The metabolic benefits of IHT are associated with increased insulin sensitivity and resulting changes in glucose metabolism, making it useful for people with type 2 diabetes (Prabhakar et al., 2020). In addition, these effects of IHT may help to control weight and prevent obesity by modulating the metabolism of adipose tissue in patients. The effects of IHT also cause modulation of cardiovascular adaptation associated with improved endothelial function, vasodilation, and blood flow due to IHT-induced changes in NO bioavailability. A reduction in blood pressure and improvement in cardiac function have also been observed during IHT, which is important for patients with these diseases (Zhu et al., 2020).
The anti-inflammatory effects of IHT have been implicated in the effects of chronic low-grade inflammation, which may contribute to general health by reducing excessive immune responses (Xiao et al., 2019). At the level of cellular adaptation, IHT mechanisms activate hypoxia-inducible factor (HIF) pathways (Prabhakar et al., 2020), including enhanced antioxidant defence and increased cellular resistance (González-Candia et al., 2022). Potential therapeutic applications include the prevention and treatment of obesity, type 2 diabetes, hypertension, and cardiovascular and respiratory diseases; additionally, IHT can be part of a promising adjunctive therapy for neurological diseases such as Alzheimer’s and Parkinson’s (Zhang et al., 2023). Thus, the wide range of effects of hypoxia on physiology makes IHT an effective therapy.
In our previous work (Kurhaluk et al., 2023), we showed the important role of Krebs cycle intermediates, i.e. succinate (SC) and α-ketoglutarate (KGL), in the processes of oxidative phosphorylation in mitochondria under acute hypoxia, which is significantly modified when the processes of adaptation to hypoxia in rats are modelled in the interval mode. The present study is a continuation of this work in which we aimed to show the effects of the nitric oxide system on the processes of mitochondrial function in the heart and liver and the modulation of the system of oxygen-dependent processes (mitochondrial respiration, microsomal oxidation, and lipid peroxidation processes) during the development of oxidative stress in different tissues. The goal of our research was to clarify the metabolic justifications for using the method of intermittent hypoxia and determine the key moments of redistribution of pathways for functioning of oxygen-dependent processes both in the formation of short-term and long-term metabolic traces in rats. The aim of this article was to study the mechanisms of phosphorylation in mitochondria in conditions of intermittent hypoxia in the formation of short-term and long-term effects of interval hypoxia. In particular, we investigated how mitochondrial functions are modulated during intermittent hypoxia with the use of oxidation substrates in bioenergetic mechanisms of cellular stability and adaptation. Understanding these processes may provide insight into potential therapeutic strategies for the treatment of hypoxia-related conditions.
Materials and Methods
Animals and experimental design
Animals. he experiments were conducted in accordance with the guidelines of the Council of the European Union, the current laws of Poland and Ukraine, and the recommendations of the Ethical Committee. They were approved by the Ethical Commission of T.H. Shevchenko National University “Chernihiv Colehium” (05/02/2019). The experiment was conducted in accordance with both Directive 2010/63/EU on the protection of animals used for scientific purposes and the Polish Act of 15 January 2015 on the protection of animals used for scientific or educational purposes (Journal of Laws, 26 February 2015, item 266).
Experimental groups. Male white rats (180-220 g) were used in the present study. The animals were maintained at a constant temperature of 21 ± 2oC with free access to food and water throughout the experiments. The rats were randomly divided into ten groups as follows:
- A control group (untreated);
- An acute hypoxia (AH) group. These rats were exposed to a mixture of breathing gas containing 7% oxygen in nitrogen for 30 minutes. Prior to the exposure to hypoxia, they received an injection of 1 mL of saline. An absorbent in the chamber absorbed carbon dioxide and water vapour during the exposure;
- An intermittent hypoxic training (IHT) group. The rats in this group were placed daily for 14 days in a chamber with alternating levels between 10% oxygen in nitrogen and room air (21% O2) at 15-minute intervals. Prior to each hypoxic training session, the rats received a parenteral injection of 1 mL of normal saline;
- IHT and AH groups. The rats in this group underwent intermittent hypoxic training (IHT) for 14 days. Each day, they were placed in a chamber with alternating levels between 10% oxygen in nitrogen and room air (21% O2) at 15-minute intervals. Before each hypoxic training session, the rats received a parenteral injection of 1 mL of normal saline. The day after the last training session, all the animals were tested with acute hypoxia exposure (7% oxygen in nitrogen, 30 min). After the test, the rats were euthanised by intraperitoneal injection of a lethal dose of sodium pentobarbital;
- An L-arginine and IHT group. The rats received L-arginine (600 mg/kg body weight, b.w.) injections before each session of IHT;
- An L-arginine, IHT, and AH group. The rats received L-arginine (600 mg/kg b.w., 30 min) before each day of IHT and the day after the final training session; all the animals were tested with acute hypoxia exposure (7% oxygen in nitrogen, 30 min);
- An L-NNA (Nω-nitro-L-arginine as an inhibitor of NO-synthase), HT, and AH group. The rats were injected with L-NNA (0.35 mg/kg b.w., 30 min) before each day of IHT and the day after the final training session; all the animals were tested with acute hypoxia exposure (7% oxygen in nitrogen, 30 min);
- An L-arginine and IHT group, 30 days. The rats received L-arginine (600 mg/kg b.w., 30 min before each IHT session) before each day of IHT; 30 days after the last IHT session, the rats were decapitated;
- An L-arginine and IHT group, 60 days. The rats received L-arginine (600 mg/kg b.w., 30 min before each IHT session) before each day of IHT; 60 days after the last IHT session, the rats were decapitated;
- An L-arginine and IHT group, 180 days. The rats received L-arginine (600 mg/kg b.w., 30 min before each IHT session) before each day of IHT; 180 days after the last IHT session, the rats were decapitated;
Samples. Peripheral whole blood samples were collected from the rats by cardiac puncture (under anaesthesia) into 10% EDTA vacutainer tubes and 3.8% citrate sodium tubes and stored at 4oC until processed (within 8 hours). The samples were centrifuged at 3, 000 rpm for 10 minutes to obtain plasma. Whole blood, plasma, and erythrocytes were used immediately for analysis. Erythrocytes were used for erythrogram analysis after the action of haemolytic agents.
Livers and hearts were removed from the rats immediately after decapitation. One animal was used for each mitochondrial preparation. The isolated liver was excised, weighed, and washed in ice-cold buffer containing 2 mM K2CO3, 10 mM HEPES, and 1 mM EGTA (pH 7.2). The isolated heart was excised, weighed, and washed in ice-cold buffer containing 180 mM KCl, 0.5% bovine serum albumin, 10 mM HEPES, and 1 mM EGTA (pH 7.2). Finally, the mitochondria were resuspended in the isolation buffer. The mitochondrial suspension (4-6 mg protein/mL) was kept on ice before the experiment (Kondrashova and Doliba, 1989). A detailed description of these isolation and analysis techniques can be found in our previous paper (Kurhaluk et al., 2023).
The liver microsomal fraction was isolated according to the methods proposed by Wisniewski et al. (1987). A detailed description of the reference to these isolation and analysis techniques is given in Kurhaluk et al. (2023). The protein concentration was determined with the Bradford assay (1976) using bovine serum albumin as a standard.
Mitochondrial respiration assessment with the oxygraphic method. Oxygen uptake was assessed using a Clark-type oxygen probe immersed in a magnetically stirred sample chamber (1 mL) in a water bath. The oxygen uptake rate was quantified as nanograms of oxygen per minute per milligram of mitochondrial protein according to the methods proposed by Chance and Williams (1955a,b). The mitochondria were placed in a respiration chamber containing 120 mM KCl, 2 mM K2CO3, 2 mM KH2PO4, and 10 mM HEPES. To maintain pH 7.20 at 26°C, we used 1.0 N potassium hydroxide. The oxidative substrates included α-ketoglutarate (KGL at a final concentration of 1 mM), succinate (SC at 0.35 mM), and ADP (at a concentration of 0.2 mM) as a phosphate acceptor. In addition, inhibition analyses were performed using rotenone (10 µM), which inhibits complex I activity in the mitochondrial electron transport chain, and malonate (2 mM), i.e. a competitive inhibitor of succinate oxidation by complex II, to assess the role of NADH- and FADH-generated substrates in the mitochondrial oxidation processes. A detailed analysis of oxygen consumption parameters (state 2, state 3, and state 4, the V3/V4, ADP/O ratio, Vph) was described in our previous paper (Kurhaluk et al., 2023).
Biochemical assays
Aminopyrine N-demethylase activity assay. The activity of cytochrome P450-dependent aminopyrine N-demethylase was determined with a method described elsewhere (Karuzina and Archakov, 1977). This method is based on the formaldehyde reaction using the Nash reagent (Matsubara et al., 1977). The reaction takes place in the presence of cytochrome P450, NADPH, and oxygen. During incubation, the media contained 3 mM NADPH(H), 0.1 M Tris-hydrochloride, 0.25 M Tris, 8 mM aminopyrine, and 5 mM magnesium chloride. The hepatic microsomal activity was then expressed as nanomoles of formaldehyde produced per minute per milligram of microsomal protein.
Nitric oxide system assays.
The nitric oxide system function was assessed using spectrophotometry by measuring the concentration of its stable nitrite anion metabolite (NO2–). This assessment followed the method proposed by Green et al. (1982) and the result was expressed in picomoles per milligram of protein. In addition, the NO3– content was determined using a method described by other authors (Brown and Cooper, 1994). The NO3– content was quantified in nanomoles per milligram of protein. In addition, carbamides were determined in a reaction with diacethylmonooxime (Kolb and Kamyshnikov, 1982), and the total polyamine content was determined spectrophotometrically on the basis of the amount of putrescine in a reaction with 2, 4-dinitrofluorobenzene (Faber et al., 1980).
Analysis of biomarkers of oxidative stress.
The 2-thiobarbituric acid reactive substances (TBARS) assay was used to measure lipid peroxidation in the samples according to the method used by Kamyshnikov (2004). The TBARS levels were expressed as nmol malondialdehyde (MDA) per mL (for blood samples) or nmol MDA per mg protein (for tissue samples). The following activities in blood and tissue samples were measured: superoxide dismutase (SOD) activity as in Kostiuk et al. (1990), catalase (CAT) activity as in Koroliuk et al. (1988), glutathione reductase (GR) activity as in Glatzle et al. (1974), glutathione peroxidase (GPx) activity as in Moin (1986), and ceruloplasmin activity as in Ravin (1961). The analyses provided valuable information on antioxidant defence and oxidative stress levels.
Assay of acid-induced resistance of erythrocytes.
The acid resistance of erythrocytes was measured spectrophotometrically with 0.1M HCl (Terskov and Gitelson, 1957). The assay is based on the measurement of the dynamics of erythrocyte disintegration caused by the action of the haemolytic reagent.
Statistical analysis
The study results were shown as mean ± standard deviation (S.D.). The Kolmogorov-Smirnov and Lilliefors tests were used to assess the normal distribution of the variables (p > 0.05). In addition, the homogeneity of variance was tested using Levene’s test. ANOVA, Student’s t-test, and Mann-Whitney U -test were used to determine differences in enzyme and substrate levels between the control and study groups. Significance was considered at p < 0.05. In addition, Spearman correlation analysis was used to examine the relationships between the data of all individuals (Zar, 1999). All statistical calculations were performed using STATISTICA 13.3 software (TIBCO Inc., USA).
Results
IHT and the functioning of myocardial and liver mitochondria
By studying the state of energy metabolism under acute hypoxia in heart and liver mitochondria, we observed changes in biochemical mechanisms associated with a sharp increase in ADP-stimulated respiration when primarily succinate (SC) was used as a substrate for phosphorylation processes, compared to the control group. The results of the study are summarised in Tables 1 and 2. The oxidation of SC did not increase the value of the respiratory coefficient by Chance and significantly reduced the cost of phosphorylation of added ADP in both heart and liver tissues, i.e. the efficiency of oxygen utilisation in the mitochondria (ADP/O value).
The decrease in the oxygen concentration in the hypoxic conditions was accompanied by a significant increase in the oxidation of the NAD-dependent substrate α-ketoglutarate (KGL), compared to the value recorded for the SC oxidation, particularly in the cardiac mitochondria. The rate of oxidative phosphorylation increased by 64.3% (p < 0.05) during the SC oxidation in the heart, but this value was significantly higher and amounted to 78.3% (p < 0.05) during the KGL oxidation process. The values of the rate of oxidative phosphorylation in the liver mitochondria were also higher when using SC as the oxidation substrate, compared to the control, whereas no similar changes were observed during the KGL oxidation in the liver mitochondria. Thus, the parameters of ATP-stimulated oxidative phosphorylation in the rat myocardial mitochondria under acute hypoxia indicate the initiation of KGL oxidation processes to maintain a tissue energy supply in the conditions of acute oxygen deprivation. This defines the important role of cardiac functional load in this type of hypoxia-induced effects.
The formation of the effects of intermittent hypoxia on mitochondrial energy supply involves significant changes in the processes of functional state reorganisation within the mitochondria themselves. The use of SC as a substrate for oxidation was followed by a statistically significant increase in the rate of mitochondrial phosphorylation, especially in the myocardial mitochondria compared to the liver, where the highest values of the V3, the respiratory control rate (V3/V4), and the ADP/O ratio were demonstrated. The effects of acute hypoxic stress on mitochondrial respiratory values in the rats exposed to adaptation to hypoxia performed by interval hypoxic sessions brought benefits for the functional state of mitochondrial processes during the SC oxidation in the heart. The effects of SC oxidation were associated with a significantly pronounced efficacy in preventing a decrease in ADP/O levels, as previously shown for the acute hypoxia group. In these conditions, such changes were less pronounced in the liver mitochondria.
The oxidation of KGL in the cardiac mitochondria during the formation of the adaptation “trace” after the use of the interval hypoxic training was associated with preferential significant oxidation of this substrate, which is also clearly manifested in the rats during acute hypoxia. Thus, the generation of effective adaptation mechanisms is linked to the predominant role of NAD-dependent oxidation processes in the mitochondria.
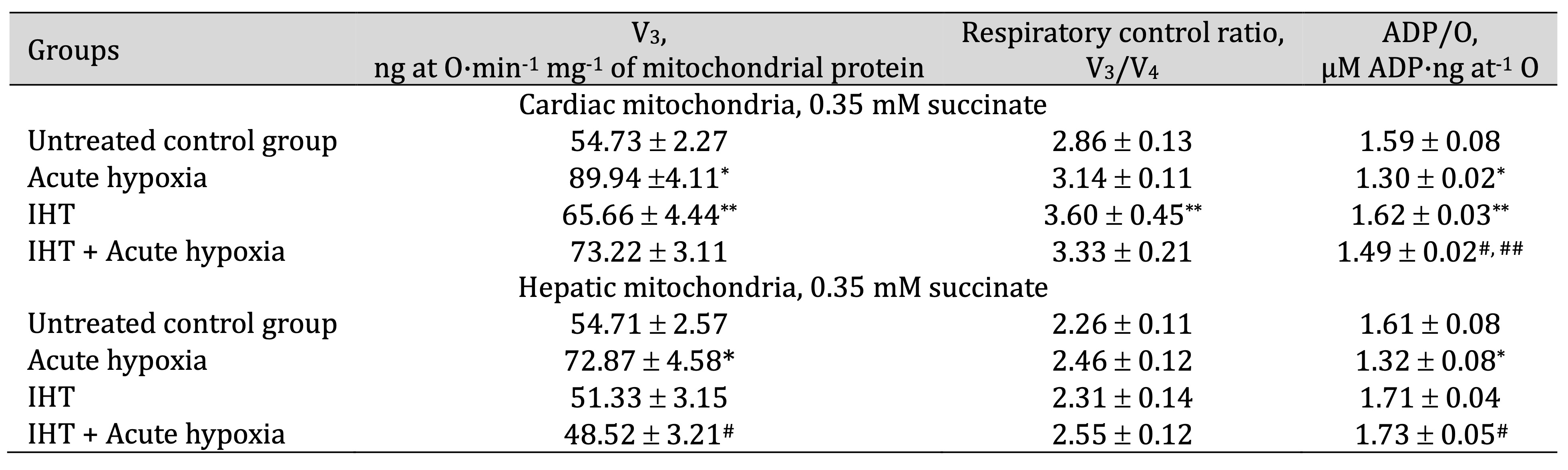
Table 1: Parameters of ATP-stimulated oxidative phosphorylation in cardiac and hepatic mitochondria from rats (M ± m; n = 6) exposed to the method of intermittent hypoxia training (IHT, 15 min 10% oxygen, 5 cycles per day), acute hypoxia (AH, 7% O2 in N2, 30 min), and acute hypoxia in IHT-exposed rats. Oxidation substrate – 0.35 mM succinate. As a phosphate acceptor, ADP was administered at a concentration of 0.2 mM. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between the untreated control group and the IHT group; # – changes are statistically significant (p < 0.05) between the IHT group exposed to acute hypoxia and acute hypoxia group; ## – changes are statistically significant (p < 0.05) between the IHT group exposed to acute hypoxia and IHT group
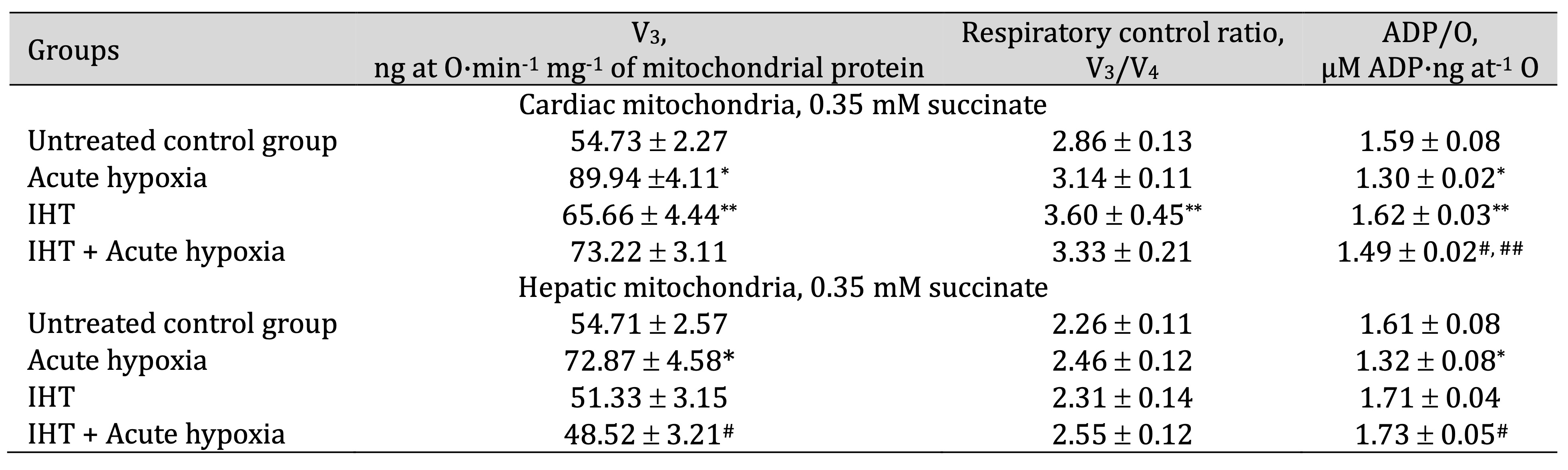
Table 2: Parameters of ATP-stimulated oxidative phosphorylation in cardiac and hepatic mitochondria from rats (M ± m; n = 6) exposed to the method of intermittent hypoxia training (IHT, 15 min 10% oxygen, 5 cycles per day), acute hypoxia (AH, 7% O2 in N2, 30 min), and acute hypoxia in IHT-exposed rats. Oxidation substrate – 1 mM α-ketoglutarate. As a phosphate acceptor, ADP was administered at a concentration of 0.2 mM. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between the untreated control group and the IHT group; # – changes are statistically significant (p < 0.05) between the IHT group exposed to acute hypoxia and acute hypoxia group; ## – changes are statistically significant (p < 0.05) between the IHT group exposed to acute hypoxia and IHT group
IHT and nitric oxide donors
The above-mentioned dependencies of the formation of effects of intermittent hypoxic exposure on oxygen-dependent processes in different tissues determined our further steps in this study related to the use of nitric oxide donors via parenteral administration of the amino acid L-arginine (600 mg/kg, 30 min) and the NO synthase inhibitor Nω-nitro-L-arginine (35 mg/kg, 30 min) to the rats. The results of this series of experiments on the oxygen consumption and oxidative phosphorylation parameters in the liver mitochondria are shown in Table 3.
The influence of the precursor of nitric oxide biosynthesis, L-arginine, in hypoxic conditions leads to different effects on the oxidation of substrates of the Krebs cycle. This may play a role in the compensatory and adaptive responses of the body under the influence of hypoxic factors. A significant decrease in the rate of ADP-stimulated respiration in the rat liver mitochondria was found when both SC and KGL oxidation substrates were used. In particular, oxygen consumption during the SC oxidation was accompanied by a decrease in the ADP-induced phosphorylation efficiency. A significant decrease in the rate of ADP-stimulated respiration (V3) was found during the oxidation of KGL (compared to the effects of the SC oxidation). Increased coupling of respiratory and oxidative phosphorylation processes with a decrease in their efficiency during the KGL oxidation was demonstrated under the influence of L-arginine and acute hypoxia.
The effects of the parenteral administration of the nitric oxide synthase inhibitor were abolished by those of L-arginine. The administration of the nitric oxide synthase inhibitor L-NNA was associated with an increase in the rate of ADP-stimulated respiration in the rat liver mitochondria using both SC and KGL substrates, compared with that in the rats given L-arginine. However, the effects of L-NNA showed that the coupling of respiration and phosphorylation during the SC oxidation remained low, and at the same time the ADP/O ratio also decreased. It should be noted that the L-NNA administration resulted in the maintenance of the value of the efficiency of ADP-induced oxidation and phosphorylation during the KGL oxidation, approaching the values determined in the untreated animal group (Table 3).
Therefore, under acute hypoxia, SC oxidation is accompanied by an increase in the efficiency of oxygen utilisation for the synthesis of macroergs, especially after the administration of L-arginine. This may be due to short-term adaptive responses. The effect of the nitric oxide synthase inhibitor L-NNA, which enhances the role of reductase reactions compared with those involving NO synthase, is more appropriate for the implementation of the effects of KGL oxidation in similar conditions.
Table 4 shows the dependencies of the formation of the IHT method effects on mitochondrial oxygen consumption and oxidative phosphorylation and the effect of acute hypoxia during daily parenteral administration of the amino acid L-arginine (600 mg/kg, 30 min) or the NO synthase inhibitor Nω-nitro-L-arginine (35 mg/kg, 30 min) before each session of IHT in the rats. IHT induced a statistically significant decrease in the rate of ADP-stimulated respiration and oxidative phosphorylation at both SC and KGL oxidation in the mitochondria, compared to the effects of acute hypoxia.
The effects of L-arginine administration on two substrates for oxidation in mitochondria are related to the increased efficiency of phosphorylation (ADP/O ratio) in the SC oxidation and the respiratory control ratio (V3/V4) in the KGL oxidation, compared to acute hypoxia conditions. The nitric oxide synthase inhibitor L-NNA induced a statistically significant increase in the rate of oxygen consumption and oxidative phosphorylation, mainly in the KGL oxidation, together with an increase in the respiratory control ratio and the ADP/O ratio, compared to both the acute hypoxia and IHT groups. L-NNA affected the oxidation of SC in the mitochondria, causing a decrease in the respiratory control ratio and phosphorylation efficiency (ADP/O ratio). Thus, IHT sessions are mediated by alternate activation of nitrate synthase, mediated by the initiating role of L-arginine, and nitrate reductase reactions under the influence of L-NNA, but the benefits of these dependencies are seen only under acute hypoxia.
The intensity of lipoperoxidation assessed by the level of TBARS products in the whole blood, erythrocytes, liver, and heart of the rats showed statistically significant dependencies for these four tissues. It was shown that the enhancement of lipoperoxidation induced by acute hypoxia was significantly reduced after the adaptation induced by IHT (Fig. 1). While L-arginine administered before each session of IHT caused a statistically significant decrease in the level of TBARS in the tissues analysed after acute hypoxia, the effects of the nitric oxide synthase inhibitor L-NNA in similar conditions were accompanied by an increase in the level of lipoperoxidation end products or had no effect on this process, compared with the effects of IHT or acute hypoxia.
In contrast to mitochondrial respiration, microsomal oxidation is a type of oxidation of biological substrates and xenobiotics by molecular oxygen that is not coupled to energy production but can account for a significant proportion of the total oxygen consumption in the cell. To clarify the role of exogenous L-arginine and the nitric oxide synthase inhibitor L-NNA in the regulation of oxygen-dependent cellular processes, we also studied changes in microsomal oxidation as a function of aminopyrine N-demethylase activity during animal adaptation to intermittent hypoxic exercise. This enzyme is considered a marker of the intensity of microsomal oxidation in liver tissue, and these changes are shown in Fig. 2.
It is shown that the changes in the ratio between mitochondrial and microsomal oxidation were shifted towards a predominance of mitochondrial processes (Fig. 2), indicating a statistically significant decrease in aminopyrine N-demethylase activity, compared to the untreated controls. Additional stimulation of nitric oxide production by administration of the amino acid L-arginine prior to each IHT session significantly reduced the microsomal oxidation rate, and these effects were “reversed” by the administration of the nitric oxide synthase inhibitor L-NNA. Thus, the adaptive effect of IHT is directly related to limiting the role of microsomal oxidation, which can be further enhanced by additional pharmacological correction with exogenous nitric oxide donors.
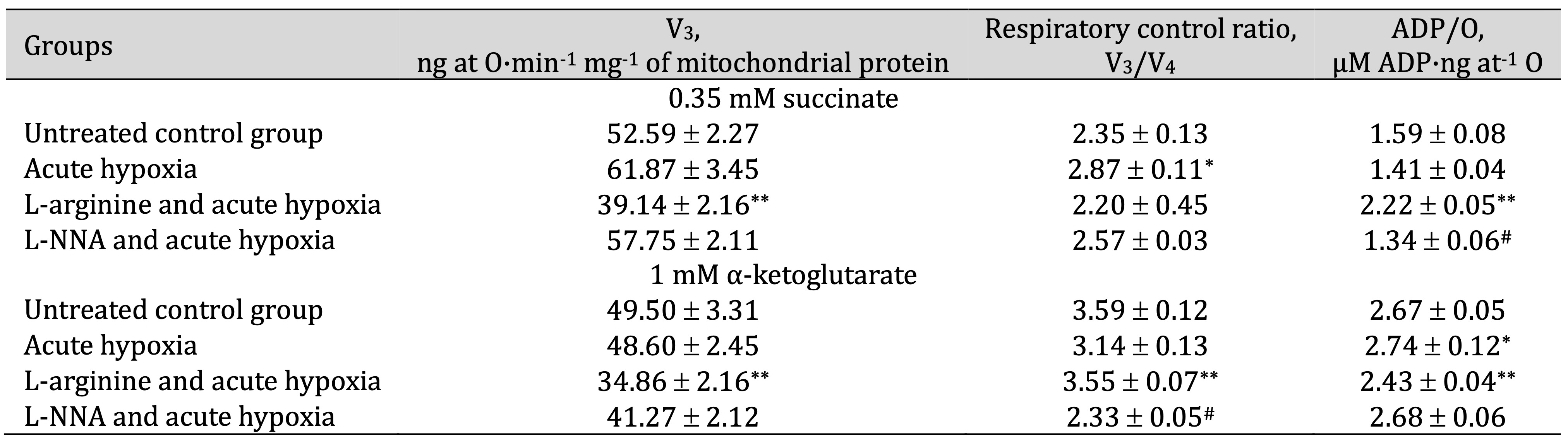
Table 3: Effects of L-arginine (600 mg/kg, 30 min) and L-NNA (35 mg/kg, 30 min) on parameters of ADP-stimulated oxidative phosphorylation processes in hepatic mitochondria during acute hypoxia (7% O2 in N2, 30 min). Oxidation substrates – 0.35 mM succinate and 1 mM α-ketoglutarate. As a phosphate acceptor, ADP was administered at a concentration of 0.2 mM. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; **– changes are statistically significant (p < 0.05) between the acute hypoxia group and the L-arginine + acute hypoxia group; #– changes are statistically significant (p < 0.05) between the acute hypoxia group and the L-NNA + acute hypoxia group
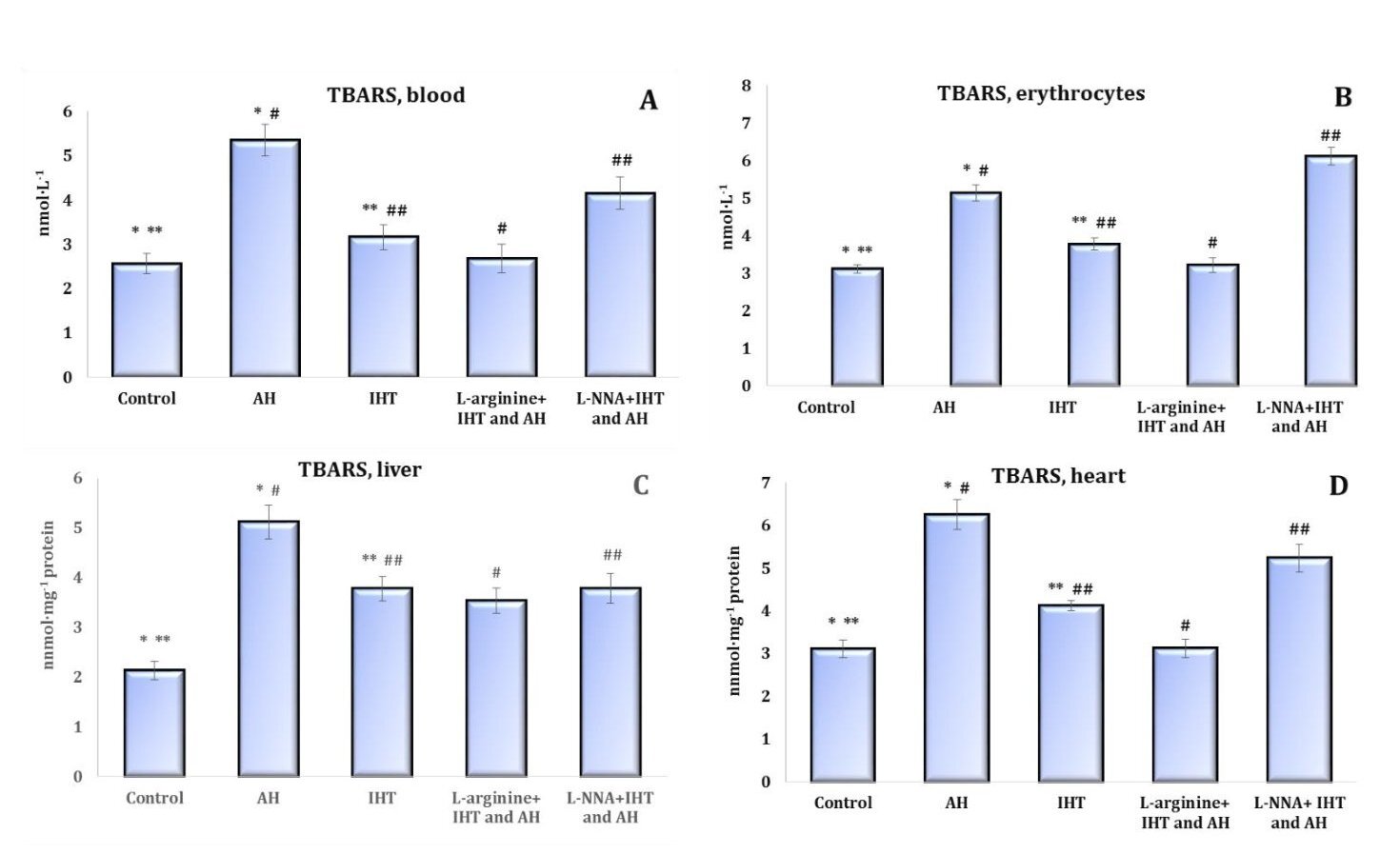
Fig. 1: Parameters of ATP-stimulated oxidative phosphorylation in cardiac and hepatic mitochondria from rats (M ± m; n = 6) exposed to the method of intermittent hypoxia training (IHT, 15 min 10% oxygen, 5 cycles per day), acute hypoxia (AH, 7% O2 in N2, 30 min), and acute hypoxia in IHT-exposed rats. Oxidation substrate – 0.35 mM succinate. As a phosphate acceptor, ADP was administered at a concentration of 0.2 mM. * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between the untreated control group and the IHT group; # – changes are statistically significant (p < 0.05) between the IHT group exposed to acute hypoxia and acute hypoxia group; ## – changes are statistically significant (p < 0.05) between the IHT group exposed to acute hypoxia and IHT group
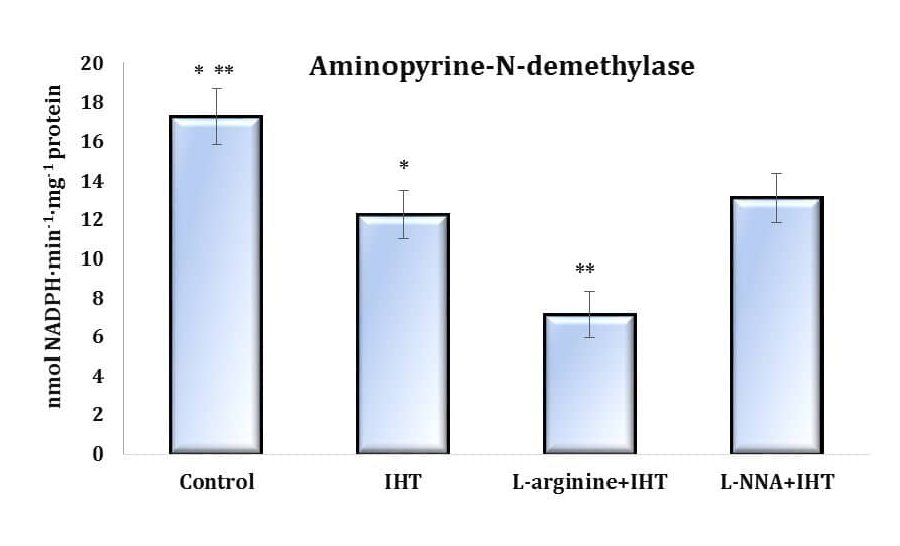
Fig. 2: Effects of L-arginine (600 mg/kg, 30 min) and L-NNA (35 mg/kg, 30 min) on aminopyrine-N-demethylase activity (nmol NADPH·min-1·mg-1 protein) in the liver of rats (M ± m; n = 6) exposed to intermittent hypoxia (IHT, 15 min, 10% oxygen, 5 cycles per day) and acute hypoxia (AH, 7% O2 in N2, 30 min). * – changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between the L-arginine +IHT group and the IHT-exposed group.
Nitric oxide metabolites in oxygen-dependent pathways during intermittent hypoxia and effects of L-arginine
The effects of adaptation to hypoxia are mediated by increasing the role of NO-dependent mechanisms and modulating the ratio of oxidative (via NO synthase) and non-oxidative (via arginase) pathways. It has been shown that body’s responses to acute hypoxia and adaptive responses to the effects of IHT are mediated by the role of NO-dependent mechanisms of nitrate, nitrite, urea, and total polyamine metabolism. These changes in the ratio of oxidative metabolism (based on the sum of nitrite and nitrate, the ratio of nitrite to the sum of nitrite and nitrate) and non-oxidative metabolism (based on the sum of urea and polyamines formed) are suitable for analysing the role of the nitric oxide-dependent biochemical component in the adaptive responses to IHT.
To elucidate the possible role of the NO-dependent pathway and its metabolites in the oxygen-dependent and oxygen-independent pathways in the organism, we studied the effects of exposure to the precursor of nitric oxide biosynthesis, L-arginine, in conditions of adaptation to IHT and subsequent exposure to acute hypoxia. To this end, we analysed the pools of nitrite, nitrate, urea, and polyamines in rats’ plasma, erythrocytes, and liver, as shown in Figures 3-5.
The adaptive mechanisms of intermittent hypoxia were accompanied by a significant increase in the production of endogenous nitric oxide, as assessed by the content of its stable metabolite, nitrite anion, in both plasma (almost 7-fold, as shown in Fig. 3) and erythrocytes (more than 7-fold, data in Fig. 4). However, the increase in NO production in the erythrocytes compared to the plasma was found to be at a higher percentage level, as it is known that the mechanisms of NO ion reduction to NO2– result from the involvement of haemoglobin. A statistical increase in the nitrate anion and urea content in the erythrocytes was also found in these conditions (Fig. 4).
The total polyamine levels increased significantly in both plasma and erythrocytes (Fig. 3D and Fig. 4D). Thus, the adaptation to intermittent hypoxia led to activation of nitric oxide synthesis and modulation of endogenous biosynthetic pathways (via the ornithine cycle and the nitric oxide cycle). In the liver (Fig. 5), a significant increase in the nitrite anion content was observed, compared to the untreated controls (Fig. 5A).
However, the results obtained in the intermittent hypoxia group with concomitant administration of L-arginine showed a reduction in the levels of NO produced, compared to those in animals subjected to IHT. However, only when the precursor of nitric oxide biosynthesis, L-arginine, was administered prior to each session of IHT under acute hypoxia was there an increase in endogenous NO, as measured by the levels of its stable metabolite, nitrite anion, compared to the control group of animals. This means that the combination of IHT, which induces the body’s own adaptive properties, with additional pharmacological effects using the precursor of nitric oxide biosynthesis, i.e. the amino acid L-arginine, can play a very important role in preventing hypoxic conditions.
It should be noted that no significant increase in the nitrate component of the nitric oxide pathway was observed during acute hypoxia (Figures 3-5). The statistically significant decrease in the nitrate levels in the plasma (Fig. 3B), erythrocytes (Fig. 4B), and liver (Fig. 5B) demonstrated in our studies may indirectly indicate a decrease in the reduction of NO to NO3– and its excretion from the body in the form of nitrate. The results also showed that the probable decrease in the urea and total polyamine levels caused by acute hypoxia did not increase to the levels seen in the untreated control group (Figures 3-5). This is also the case for the changes observed in the levels of these metabolites during the IHT sessions and the IHT and L-arginine administration.
It was shown in our studies that the formation of adaptive mechanisms to intermittent hypoxia was accompanied by a significant increase in the level of the nitrite reductase component of the nitric oxide cycle in rats’ erythrocytes (p < 0.001), against a background of no change in the blood plasma and a significant decrease in the liver. The sum of nitrite and nitrate anions in the liver was even halved (p < 0.001). In particular, the ratio of nitrite to the sum of nitrite and nitrate anions in the blood plasma and liver increased significantly, compared to the untreated control group of rats (Figures 3-5). Thus, IHT enhances the regulatory functions of nitric oxide in the mechanisms of effective adaptive defence.
The above-mentioned intensification of nitric oxide-dependent pathways of metabolic transformations in the implementation of energy supply processes in the heart and liver and the production of reactive oxygen species (ROS) accompanied by mechanisms of adaptation to hypoxia in the interval mode caused a probable decrease in the production of urea and polyamines in the plasma and liver but not in the erythrocytes. A decrease in the total levels of urea and polyamines was observed in the plasma and liver. In the erythrocytes, however, the levels of these compounds increased more than fourfold, compared with the untreated control rats (Figures 3-5).
In conclusion, the formation of bioenergetic adaptive mechanisms to intermittent hypoxia training in our studies is primarily associated with the nitrite reductase component of nitric oxide metabolism and a significant increase in the intensity of enzymes in erythrocytes against the background of changes in blood plasma and a significant decrease in the liver.
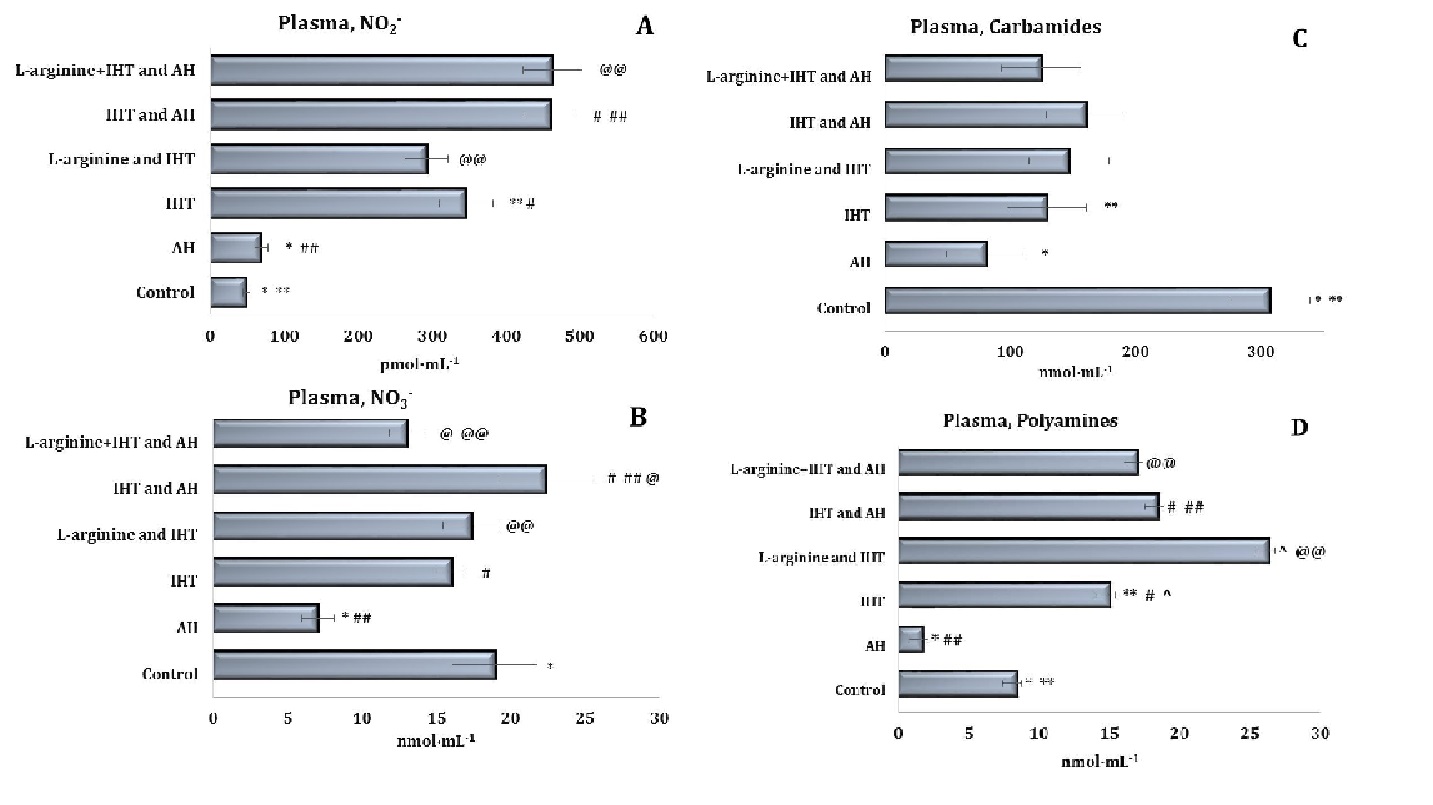
Fig. 3: Effects of L-arginine administration (600 mg/kg) on the levels of nitrites (A, pmol∙mL-1), nitrates (B, nmol∙mL-1), carbamides (C, nmol∙mL-1) and polyamines (D, nmol∙mL-1) in plasma of rats (M ± m; n = 6) exposed to intermittent hypoxia training (IHT, 15 min, 10% oxygen, 5 cycles per day) and acute hypoxia (AH, 7% O2 in N2, 30 min). *– changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between untreated control group and the IHT-exposed group; #– changes are statistically significant (p < 0.05) between IHT-exposed + AH group and the IHT-exposed group; ##– changes are statistically significant (p < 0.05) between IHT-exposed + AH group and the acute hypoxia group; ^ – changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed group and the IHT-exposed group; @– changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed + AH group and the IHT-exposed + AH group; @@– changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed + AH group and the L-arginine + IHT-exposed group.
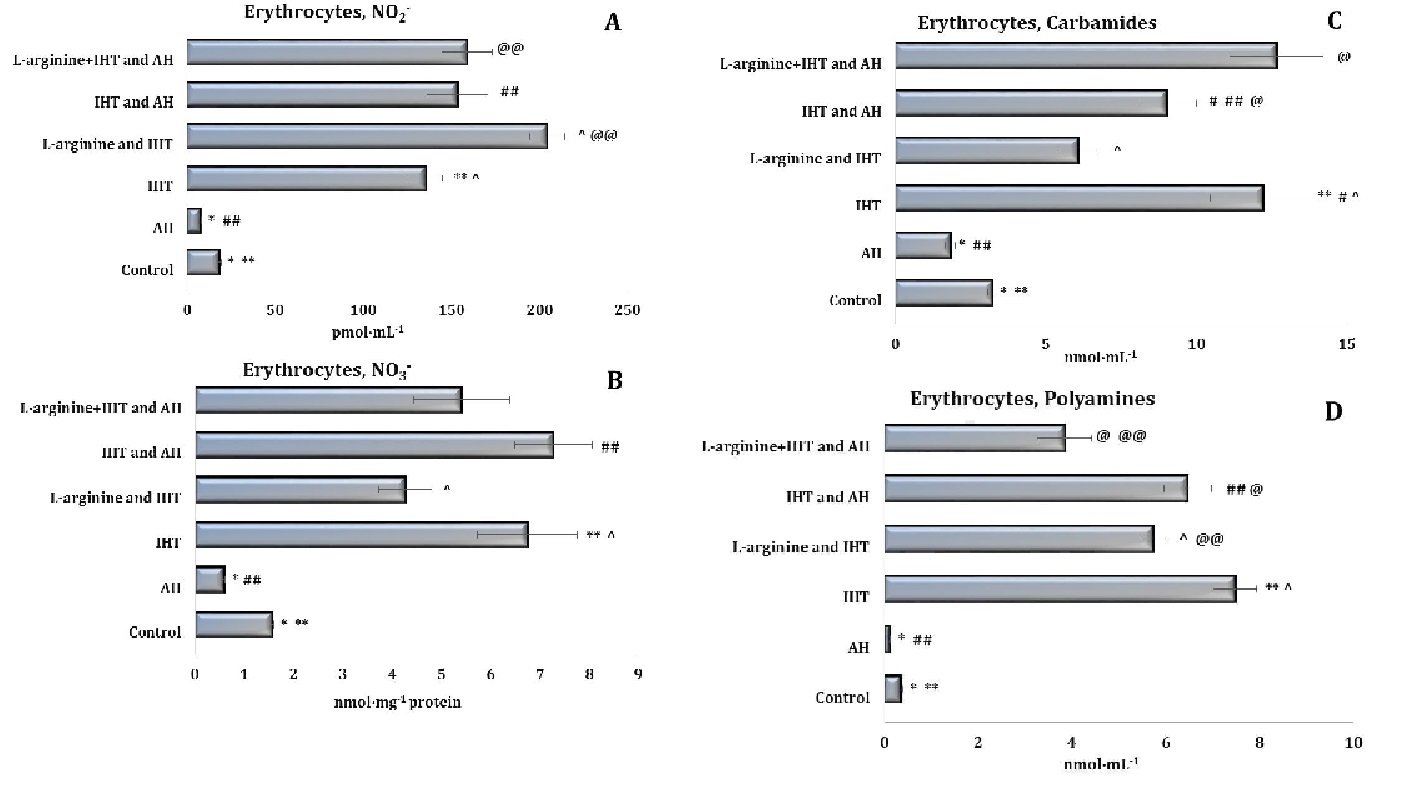
Fig. 4: Effects of L-arginine administration (600 mg/kg) on the levels of nitrites (A, pmol∙mL-1), nitrates (B, nmol∙mL-1), carbamides (C, nmol∙mL-1) and polyamines (D, nmol∙mL-1) in erythrocytes of rats (M ± m; n = 6) exposed to intermittent hypoxia training (IHT, 15 min, 10% oxygen, 5 cycles per day) and acute hypoxia (AH, 7% O2 in N2, 30 min). *– changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between untreated control group and the IHT-exposed group; #– changes are statistically significant (p < 0.05) between IHT-exposed + AH group and the IHT-exposed group; ##– changes are statistically significant (p < 0.05) between IHT-exposed + AH group and the acute hypoxia group; ^ – changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed group and the IHT-exposed group; @– changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed + AH group and the IHT-exposed + AH group; @@– changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed + AH group and the L-arginine + IHT-exposed group.
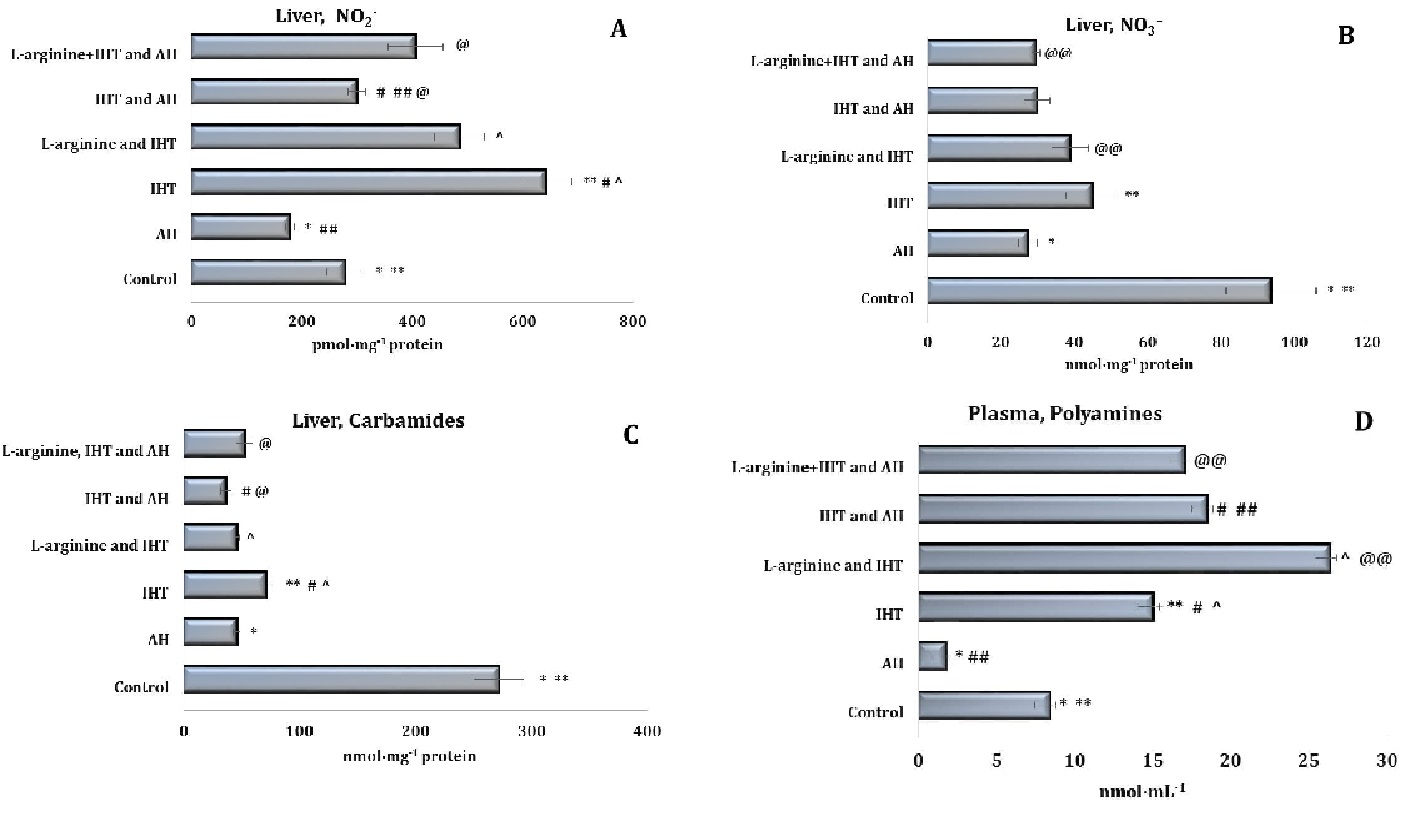
Fig. 5: Effects of L-arginine administration (600 mg/kg) on the levels of nitrites (A, pmol∙mg-1), nitrates (B, nmol∙mg-1), carbamides (C, nmol∙mg-1) and polyamines (D, nmol∙mg-1) in liver tissue of rats (M ± m; n = 6) exposed to intermittent hypoxia training (IHT, 15 min, 10% oxygen, 5 cycles per day) and acute hypoxia (AH, 7% O2 in N2, 30 min). *– changes are statistically significant (p < 0.05) between the untreated control group and the acute hypoxia group; ** – changes are statistically significant (p < 0.05) between untreated control group and the IHT-exposed group; #– changes are statistically significant (p < 0.05) between IHT-exposed + AH group and the IHT-exposed group; ##– changes are statistically significant (p < 0.05) between IHT-exposed + AH group and the acute hypoxia group; ^ – changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed group and the IHT-exposed group; @– changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed + AH group and the IHT-exposed + AH group; @@– changes are statistically significant (p < 0.05) between L-arginine + IHT-exposed + AH group and the L-arginine + IHT-exposed group.
Biomarkers of oxidative stress, NO donors, and intermittent hypoxia
The study of the enzymatic component of antioxidant defence is shown in Figures 6 and 7. The analysis of the major antioxidants, such as SOD, CAT, GR, and GPx, in the blood and liver tissue during IHT and administration of the precursor nitric oxide and the nitric oxide synthase inhibitor L-NNA showed multidirectional changes in antioxidant defence.
The next stage of our research was to examine the levels of ceruloplasmin (CP), a copper-containing protein that regulates free radical metabolism and has a number of antioxidant properties (Fig. 6E), including the ability to interact with superoxide and hydrogen peroxide. CP oxidises ferrous iron to trivalent iron with concomitant reduction of oxygen to water. This enzymatic activity has been shown to be necessary for the binding of iron to ferritin and determines the oxidative properties of blood. A decrease in the concentration of CP causes a sharp decrease in haem synthesis in the mitochondria. The effect of L-arginine in our studies led to accumulation of CP (p < 0.05) during IHT. At the same time, the exposure of the animals to the NO synthase inhibitor prior to the IHT sessions significantly reduced the concentration of CP. Thus, the oxidative properties of the blood are largely related to the functioning of NO-ergic mechanisms and can be corrected by the introduction of modulators of the nitric oxide system. This may be one of the main mechanisms for the formation of adaptive trace mechanisms under the influence of extreme environmental factors related to the functioning of iron-containing blood proteins.
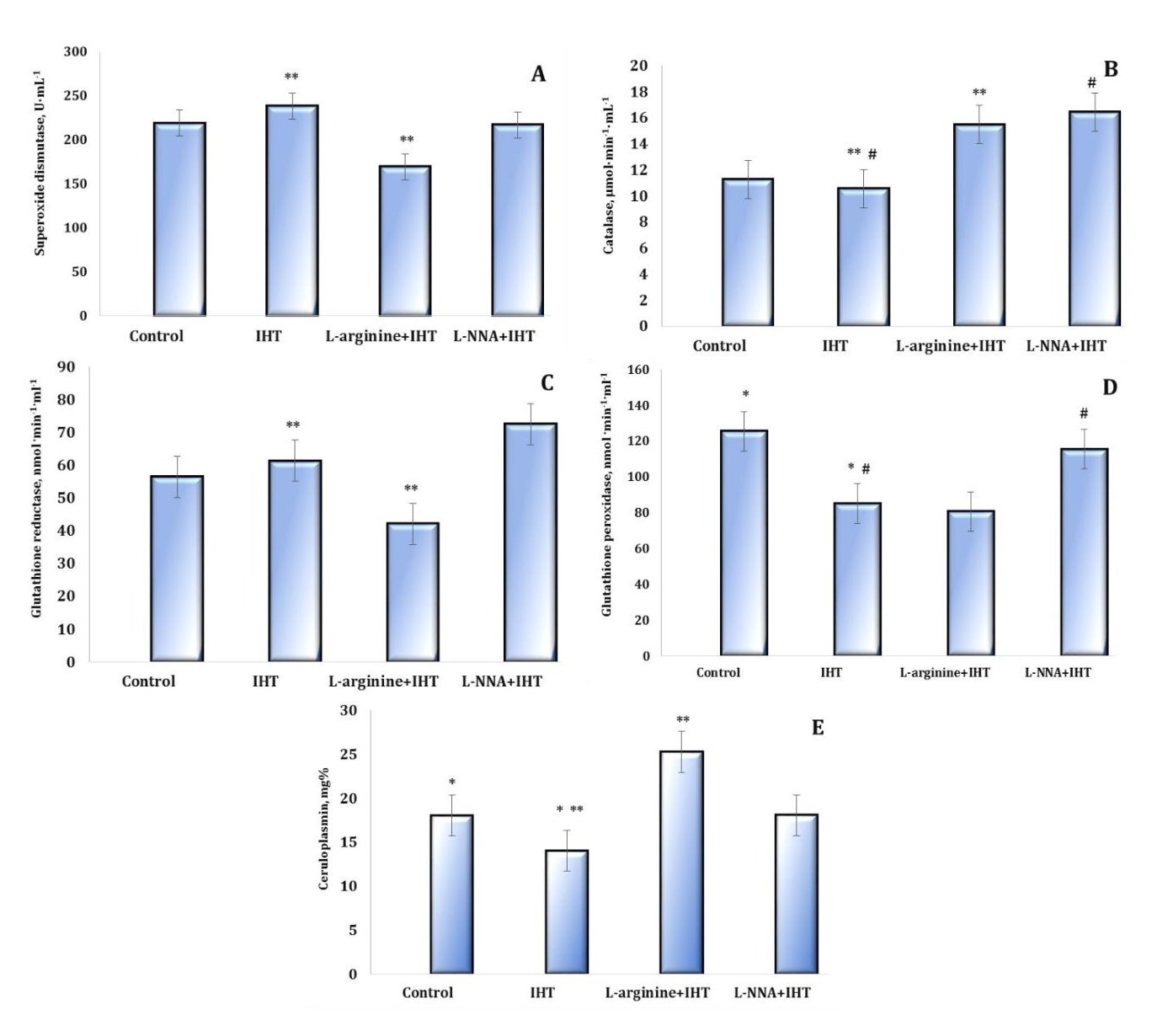
Fig. 6: Effects of L-arginine (600 mg/kg, 30 min) and L-NNA (35 mg/kg, 30 min) on the activities of antioxidant enzymes such as superoxide dismutase (SOD, U∙mL-1, A), catalase (CAT, μmol∙min-1∙mL-1, B), glutathione reductase (GR, nmol NADPH2·min-1·mL-1, C), glutathione peroxidase (GPx, nmol GSH·min-1·mL-1, D) and ceruloplasmin content (mg%, E) in the blood of rats exposed to intermittent hypoxia training (IHT) (M ± m, n = 6). * – changes are statistically significant (p < 0.05) between the untreated control group and the IHT-exposed group; **– changes are statistically significant (p < 0.05) between the L-arginine + IHT-exposed group and the IHT-exposed group; #– changes are statistically significant (p < 0.05) between the L-NNA + IHT-exposed group and the IHT-exposed group.
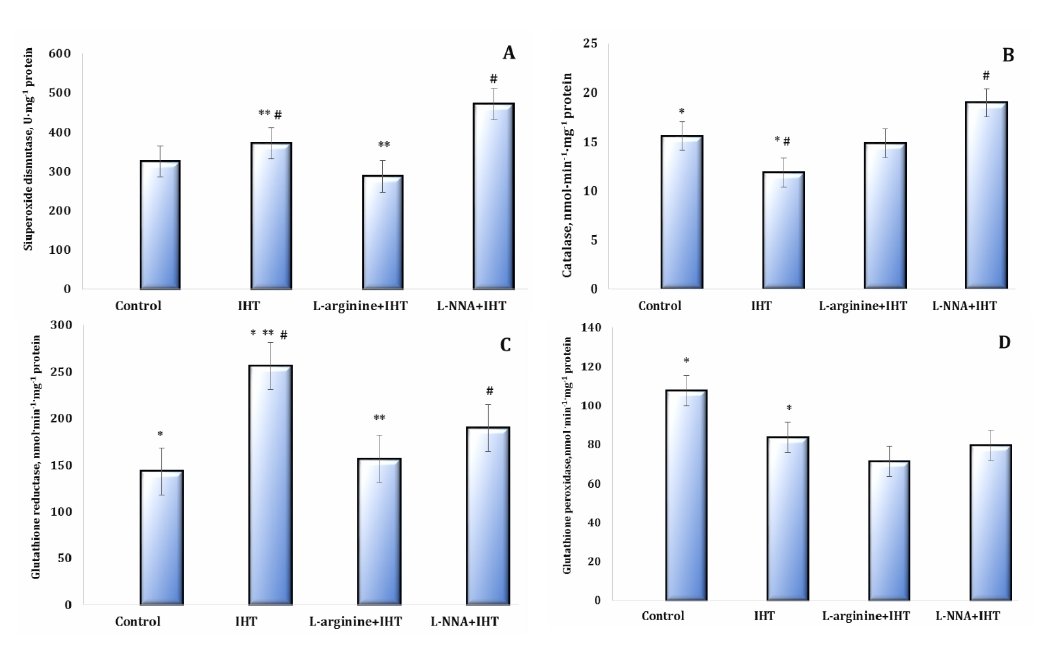
Fig. 7: Effects of L-arginine (600 mg/kg, 30 min) and L-NNA (35 mg/kg, 30 min) on activities of the antioxidant enzymes such as superoxide dismutase (SOD, U∙ mg-1 protein, A), catalase (CAT, nmol∙min-1∙mg-1 protein, B), glutathione reductase (GR, nmol NADPH2·min-1· mg-1 protein, C), glutathione peroxidase (GPx, nmol GSH·min-1· mg-1 protein, D) in the liver tissue of rats exposed to intermittent hypoxia training (IHT) (M ± m, n = 6). * – changes are statistically significant (p < 0.05) between the untreated control group and the IHT-exposed group; **– changes are statistically significant (p < 0.05) between the L-arginine + IHT-exposed group and the IHT-exposed group; #– changes are statistically significant (p < 0.05) between the L-NNA + IHT-exposed group and the IHT-exposed group.
Time-dependent properties of mitochondrial function during intermittent exposure to hypoxia
The time dependence of the maintenance of the effects of the “adaptation curve” induced by IHT sessions is important, since the study of the degree of biochemical changes induced by this therapeutic method is of constant importance for analysis. This was the next step in our work when we studied the long-term effects of intermittent hypoxia training on oxygen-dependent processes after the end of the IHT sessions in a group of animals that had been administered L-arginine before the IHT session. The results of the studies in this group of rats are presented as data at 30, 60, and 180 days after the end of the IHT exposure treatment and are shown in Figures 8 and 9. We studied the functional characteristics of the two mitochondrial complexes associated with oxidation substrates, i.e. SC and KGL. Therefore, the next step of our study was to investigate the “independent” oxidation of NAD-dependent substrates in the mitochondrial respiratory chain by determining their oxidation parameters in the presence of the succinate dehydrogenase (SDH) inhibitor malonate. The contribution of direct oxidation of NAD-dependent substrates remains stable in most cases because endogenous succinate has a high oxidation rate and can therefore modify the processes of phosphorylation respiration.
On the other hand, the contribution of NAD-dependent substrates to SC oxidation is equally important and can be studied using rotenone, an inhibitor of the NAD-dependent electron transfer pathway in the mitochondrial respiratory chain. This was confirmed by the results of our studies. Rotenone significantly limited the main indicators of energy supply in the SC oxidation conditions, whereas malonate significantly increased both the coupling and efficiency of oxygen consumption and phosphorylation processes.
It has been shown that the malonate-sensitive component of mitochondrial respiration in rats during acute hypoxic stress, as well as the formation of IHT-related mechanisms, changes significantly depending on the form of hypoxic exposure, i.e. acute or intermittent. The data from this series of studies are shown in Figures 8 and 9 for the main parameters of mitochondrial respiration as the respiratory control by Chance and the efficiency of oxygen consumption and phosphorylation using KGL and SC as substrates of oxidation in mitochondria.
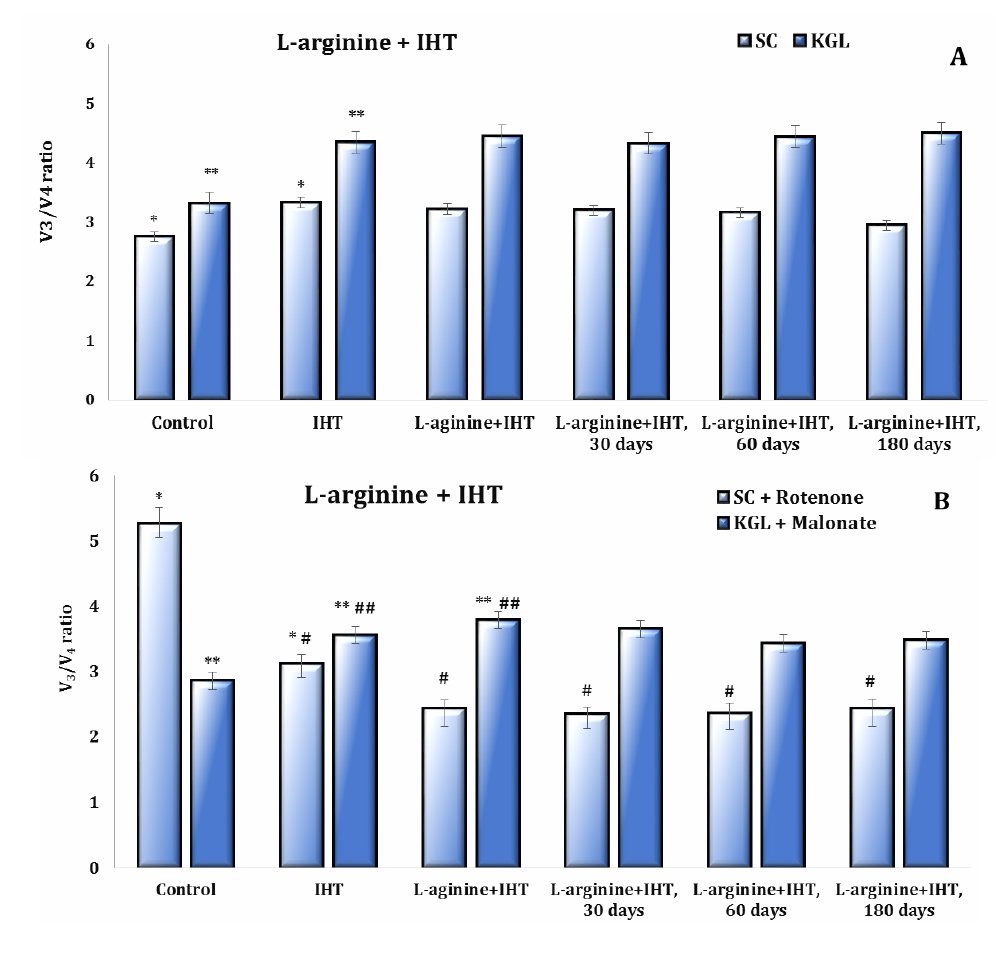
Fig. 8: The respiratory control ratio (V3/V4) of the ADP-stimulated mitochondrial respiration and oxidative phosphorylation in the cardiac tissue of rats using substrates such as 0.35 mM succinate and 1 mM α-ketoglutarate, and in the inhibition assay using 10 μM rotenone as a tissue respiratory inhibitor that blocks electron transfer from the reduced form of nicotinamide adenine dinucleotide (NADH) to cytochrome b and 2 mM malonate as a competitive inhibitor of succinate dehydrogenase enzyme in the untreated control group, IHT-exposed group and L-arginine administration (600 mg/kg, 30 min) in the IHT-exposed group at 30, 60 and 180 days after the end of the IHT sessions. * – changes are statistically significant (p < 0.05) between the untreated control group and the IHT-exposed group (at succinate oxidation); **– changes are statistically significant (p < 0.05) between the untreated control group and the IHT-exposed group (at α-ketoglutarate oxidation); #– changes are statistically significant (p < 0.05) between the IHT-exposed group and L-arginine administration in the IHT-exposed group at 30, 60 and 180 days after the end of the IHT sessions (in the inhibition assay using 10 μM rotenone); ##– changes are statistically significant (p < 0.05) between the IHT-exposed group and L-arginine administration in the IHT-exposed group (in the inhibition assay using 2 mM malonate).
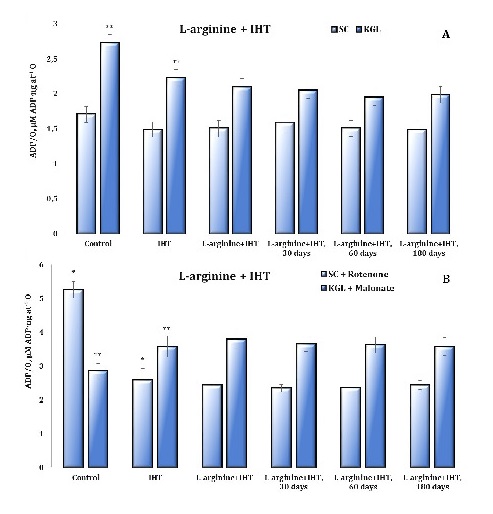
Fig. 9: The ADP/O ratio (µM ADP·ng at-1 O) of the ADP-stimulated mitochondrial respiration and oxidative phosphorylation in the cardiac tissue of rats using substrates such as 0.35 mM succinate and 1 mM α-ketoglutarate, and in the inhibition assay using 10 μM rotenone as a tissue respiratory inhibitor that blocks electron transfer from the reduced form of nicotinamide adenine dinucleotide (NADH) to cytochrome b and 2 mM malonate as a competitive inhibitor of succinate dehydrogenase enzyme in the untreated control group, IHT-exposed group and L-arginine administration (600 mg/kg, 30 min) in the IHT-exposed group at 30, 60 and 180 days after the end of the IHT sessions. * – changes are statistically significant (p < 0.05) between the untreated control group and the IHT-exposed group (at succinate oxidation); **– changes are statistically significant (p < 0.05) between the untreated control group and the IHT-exposed group (at α-ketoglutarate oxidation).
Time-dependent properties of NO donors at IHT
The effects of adaptation to hypoxia were found to be mediated by increasing the role of NO-dependent mechanisms and modulating the ratio of oxidative (NO synthase) to non-oxidative (arginase) metabolism. In order to elucidate the possible role of NO and its metabolites in the oxygen-dependent and oxygen-independent pathways of mitochondrial function, we studied the effects of the precursor of nitric oxide biosynthesis, L-arginine, after adaptation to intermittent hypoxia at 30, 60, and 180 days after the end of the IHT sessions in comparison with the values in the untreated control animals and the effects of L-arginine before each IHT session. It should be noted that the animals that underwent the IHT session and pre-treatment with the amino acid L-arginine for 30, 60, and 180 days did not receive any further medication after the end of the IHT course. We attribute the changes analysed to a significant increase in the nitrite-reducing component of the nitric oxide cycle compared to the levels of nitrates, urea, and total polyamines. These changes are shown in Table 5. The administration of L-arginine before the IHT sessions reduced this component, but the statistically increased level of the nitrite-reducing component of the nitric oxide cycle persisted until day 180 after the end of the IHT sessions.
The antioxidant system plays a crucial role in the adaptation to periodic hypoxia, and the amino acid L-arginine has an additional role in this process. The results of this series of experiments on SOD, CAT, GR, and GPx activities are summarised in the table, with data obtained 30, 60, and 180 days after the end of the IHT sessions, which involved repeated cycles of hypoxia followed by reoxygenation. As can be seen from the results of the study, the high level of stimulation of the nitric oxide system via a nitrite-dependent component supports the low levels of lipid peroxidation and the associated high potential for antioxidant enzyme activity in liver tissue. The effectiveness of hypoxic training and its nitrite-dependent component demonstrated in this study is associated with the formation of long-term adaptive responses to the action of extreme factors. IHT also prevents the intensification of lipoperoxidation processes in the liver due to pronounced changes in the main antioxidant defence enzymes (Table 6), which has a significant protective effect and is an important element of the systemic response for the regulation of oxygen-dependent processes as a whole.
Thus, the activation of SC oxidation in mitochondria caused by acute hypoxia is reduced in animals after adaptation by IHT. By preventing the intensification of lipoperoxidation processes in the liver during acute hypoxia, IHT increases the efficiency of liver and myocardial mitochondrial function by enhancing the role of NO-dependent regulatory mechanisms. The NADPH oxidase pathway is a limiting step in the early stages of hypoxia and largely determines the impairment of mitochondrial energy synthesising function. The development of mechanisms of adaptation to oxygen deprivation is associated with an increase in the efficiency of the NADPH oxidase pathway, as shown by the inhibition analysis with malonate and rotenone at 30, 60, and 180 days after cessation of adaptation. The increased role of NO-dependent effects enhances the role of NADPH oxidase oxidation, which contributes to the formation of long-term adaptive responses to hypoxia.
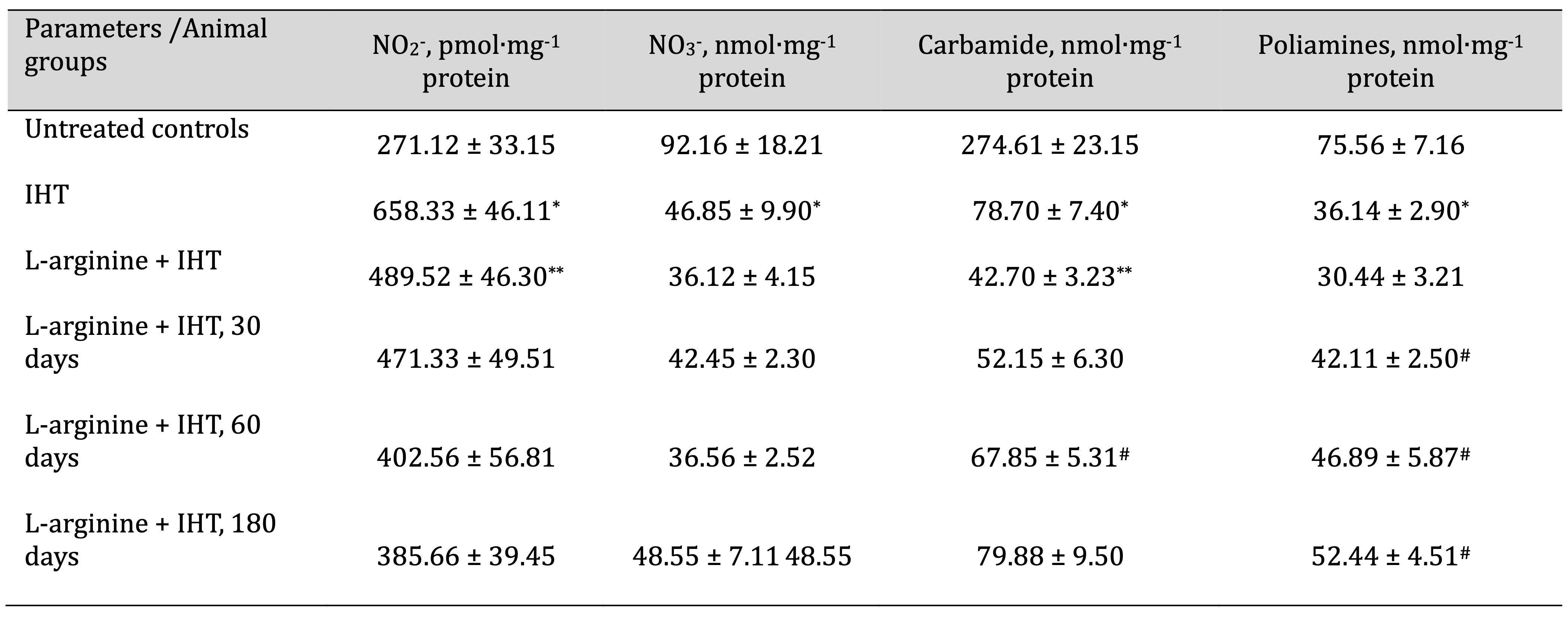
Table 5: Effects of L-arginine (600 mg/kg, 30 min) on the levels of nitrites (pmol∙mg-1 protein), nitrates (nmol∙mg-1 protein), carbamides (nmol∙mg-1 protein) and polyamines (nmol∙mg-1 protein) in the liver of rats (M ± m; n = 6) exposed to intermittent hypoxia training (IHT, 15 min, 10% oxygen, 5 cycles per day) and at 30, 60 and 180 days after the end of the IHT sessions. * – Changes are statistically significant (p < 0.05) between the control group and the IHT group; ** – Changes are statistically significant (p < 0.05) between the IHT exposed group and the L-arginine + IHT group; # – Changes are statistically significant (p < 0.05) between the L-arginine + IHT group and the IHT group at 30, 60 and 180 days after the end of the IHT sessions
Acid-induced resistance of erythrocytes
We also analysed the acid-induced resistance of erythrocytes derived from rats exposed to the IHT sessions and acute hypoxia (Fig. 10A), rats treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before each IHT session (Fig. 10B), and rats exposed to IHT at 30, 60, and 180 days after the end of the IHT sessions (Fig. 10C). The IHT method and the administration of L-arginine before each IHT session significantly increased the resistance of erythrocytes, which may indicate an increase in the stability of their membranes in the current study (Fig. 10A, B). The IHT method and the administration of L-NNA before each IHT session resulted in a decrease in haemolysed erythrocytes with a simultaneous reduction in the time of onset of maximal haemolysis (Fig. 10B). The long-term effects of the IHT method with the administration of L-arginine before each IHT session showed a decrease in haemolysed erythrocytes with a simultaneous increase in the time of onset of maximal haemolysis (Fig. 10C).
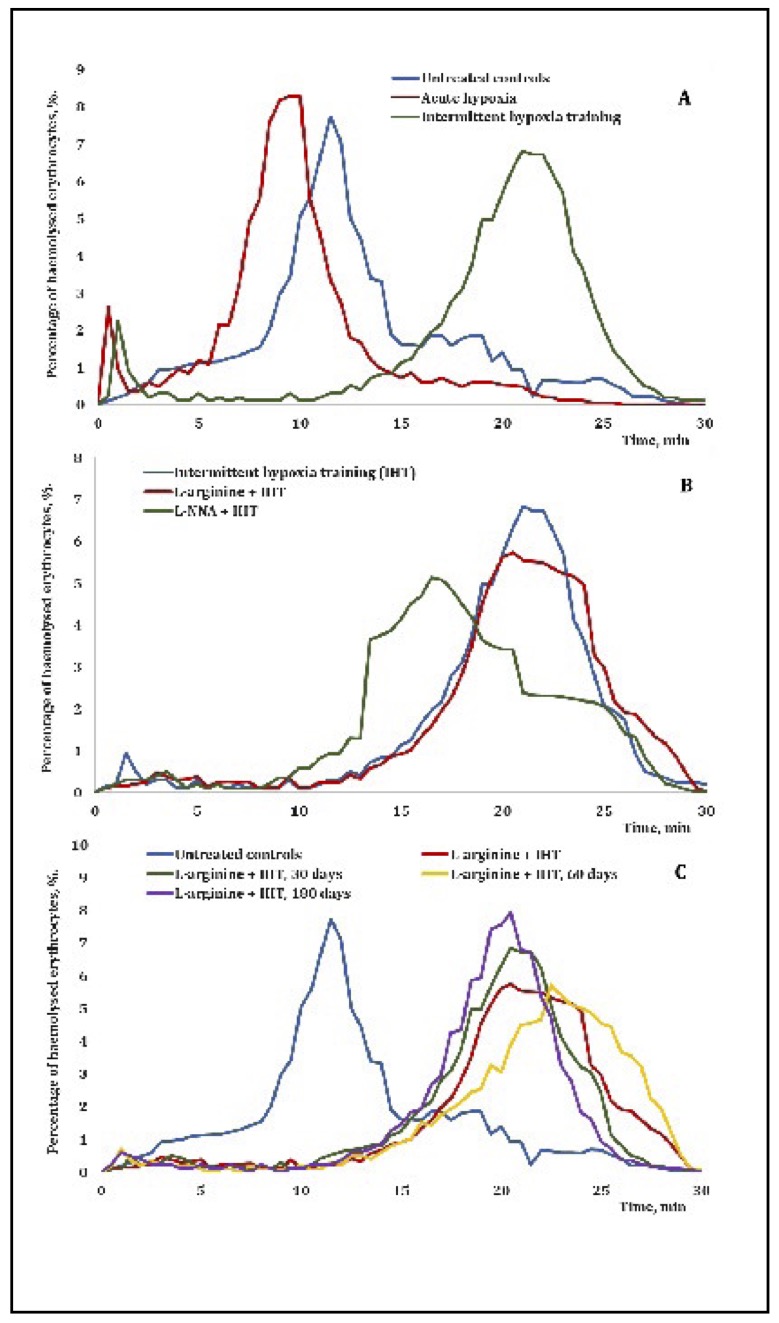
Fig. 10: Erythrograms of acid-induced hemolysis of erythrocytes sampled from rats exposed to the IHT method and acute hypoxia (A), in rats treated with L-arginine (600 mg/kg, 30 min) or L-NNA (35 mg/kg, 30 min) before each IHT session (B), and in rats exposed to IHT and at 30, 60 and 180 days after the end of the IHT sessions (C).
Discussion
The results of the study presented in this paper show short- and long-term effects of the IHT method mediated by nitric oxide effects on different tissues of rats under the additional influence of the nitric oxide precursor, i.e. the amino acid L-arginine, and the NO synthase inhibitor Nω-nitro-L-arginine. In this work, we obtained a number of significant results that should be highlighted. The present study has complemented and extended our previous work (Kurhaluk et al., 2023) and studies conducted by other authors on the formation of hypoxia-induced bioenergetic effects (Korbecki et al., 2021), with special attention to the long-term effects of the IHT method (Serebrovska et al., 2019). The results of our previous study (Kurhaluk et al., 2023) revealed the effect of complex multifactorial dependencies and interactions between Krebs cycle intermediates (such as succinate and α-ketoglutarate oxidation) on oxygen-dependent processes in conditions of acute hypoxia. The study elucidated the biological role of nitric oxide (NO) with its metabolic changes and mechanism of action on tissues under hypoxia. In addition, our team investigated the effects of SC and KGL oxidation on mitochondrial oxygen consumption and oxidative phosphorylation parameters, microsomal oxidation, lipid peroxidation processes, and the antioxidant defence system. The study shed light on the reciprocal/competitive relationship between Krebs cycle intermediates and the nitric oxide system via intermediates associated with mediating the effects of the cholinergic regulatory system to preferentially oxidise KGL and adrenergic derivatives (dopamine, DOPA, noradrenaline, and epinephrine). This study has contributed to our understanding of the impact of Krebs cycle intermediates on oxygen-dependent processes during hypoxia and has provided insight into potential therapeutic interventions to help prevent the effects of acute hypoxia in many pathologies (Kurhaluk et al., 2023).
Firstly, the mechanisms underlying the IHT method are related to hypoxia-inducible factors (HIFs) through histone demethylation with activation of a family of transcription factors that play a crucial role in adaptation to low oxygen levels (Martinez et al., 2022). The use of acute hypoxia as a test tool in animals following IHT sessions in the processes of oxygen consumption in the oxidation of KGL and SC as substrates in the mitochondria has demonstrated the protective role of IHT in the immediate adaptation and long-term (remote) effects on oxygen-dependent processes in the liver and heart. Several studies have shown the important role of HIF factors in regulating the expression of genes involved in various processes (Yeo, 2019), including angiogenesis (formation of new blood vessels), erythropoiesis (red blood cell production), and glucose metabolism (Guan et al., 2023; Wiśniewska et al., 2020). IHT stimulates HIF activation, leading to adaptive responses that improve tissue oxygen delivery and utilisation.
Our studies have shown that acute hypoxia significantly reduced the nitrite and nitrate pools in the plasma, erythrocytes, and liver. In particular, the nitrite and nitrate pools in the plasma, erythrocytes, and liver decreased to the same extent. However, in the conditions of a sharp decrease in the oxygen content (7% O2 in N2) in the air inhaled by the rats, accompanied by signs of bioenergetic hypoxia, the ratio of nitrite to the sum of nitrite and nitrate anion increased, with the greatest changes observed in the blood plasma compared with the liver tissue. The acute hypoxic exposure was associated with a significant decrease in the sum of urea and polyamines in the blood and liver. In particular, a probable decrease in the intensity of the non-oxidative pathway associated with the functioning of the ornithine cycle and urea metabolism was studied in the blood plasma, erythrocytes, and liver (Figures 3-5, Table 4).
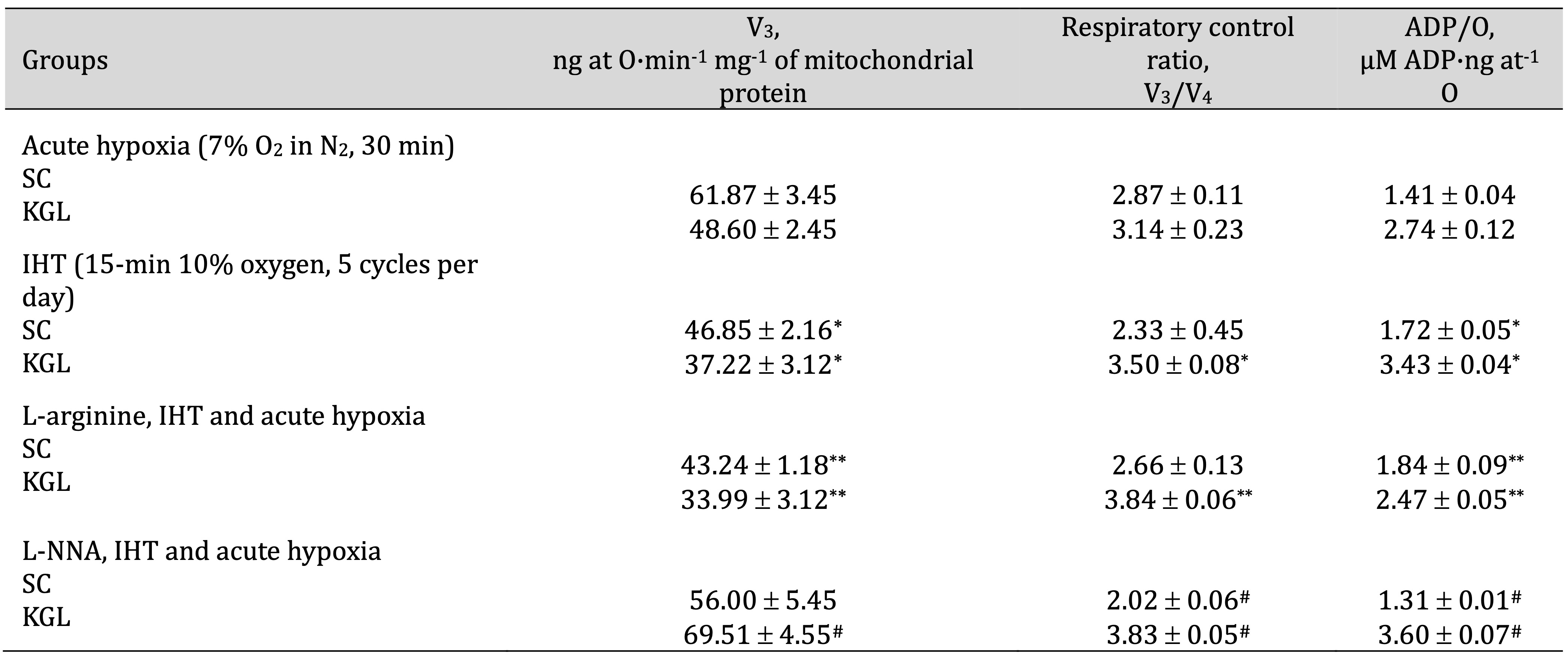
Table 4: Effects of L-arginine (600 mg/kg, 30 min) and L-NNA (35 mg/kg, 30 min) on parameters of ADP-stimulated oxidative phosphorylation processes in the hepatic mitochondria of rats (M ± m; n = 6) exposed to intermittent hypoxia (IHT, 15 min, 10% oxygen, 5 cycles per day) and acute hypoxia (7% O2 in N2, 30 min). Oxidation substrates – 0.35 mM succinate and 1 mM α-ketoglutarate. ADP was administered at a concentration of 0.2 mM as a phosphate acceptor. * – changes are statistically significant (p < 0.05) between the IHT-exposed group and the acute hypoxia group; **– changes are statistically significant (p < 0.05) between the L-arginine, IHT and acute hypoxia and IHT-exposed group; #– changes are statistically significant (p < 0.05) between the L-NNA, IHT and acute hypoxia and IHT-exposed group
Secondly, the results of our studies on mitochondrial function in terms of ATP production via oxidative phosphorylation in rats after IHT sessions are associated with significant redistribution of the metabolic pathways of energy supply during the oxidation of KGL and SC. These effects may be related to mitochondrial adaptations previously shown by others during IHT, such as increased mitochondrial density, improved respiratory chain function, and enhanced mitochondrial biogenesis (Kang et al., 2020; Adzigbli et al., 2022). Thus, these adaptive mechanisms may lead to an increase in cellular energy production and overall animal endurance, which we tested in our acute hypoxia experiments.
It is worth highlighting the third conclusion, which relates to the effects of the IHT method on erythrocyte membrane stabilisation, which we have also shown to reduce lipoperoxidation processes in various tissues (Figures 1, Table 6), changes in the activity of the main antioxidant enzymes (Table 6, Fig. 6,7), and stabilisation of erythrocyte membranes (Fig. 10). Thirdly, these effects of the IHT method are associated with an increase in erythropoietin (EPO) production when hypoxia causes the release of EPO from the kidneys (Wojan et al., 2023). EPO stimulates the production of red blood cells (erythropoiesis), resulting in their improved ability to transport oxygen. In addition, the IHT method can increase EPO levels, thereby increasing animals’ resistance to acute hypoxia and preventing its detrimental effects on the energy supply and oxygen-dependent processes, as we demonstrated in this study.
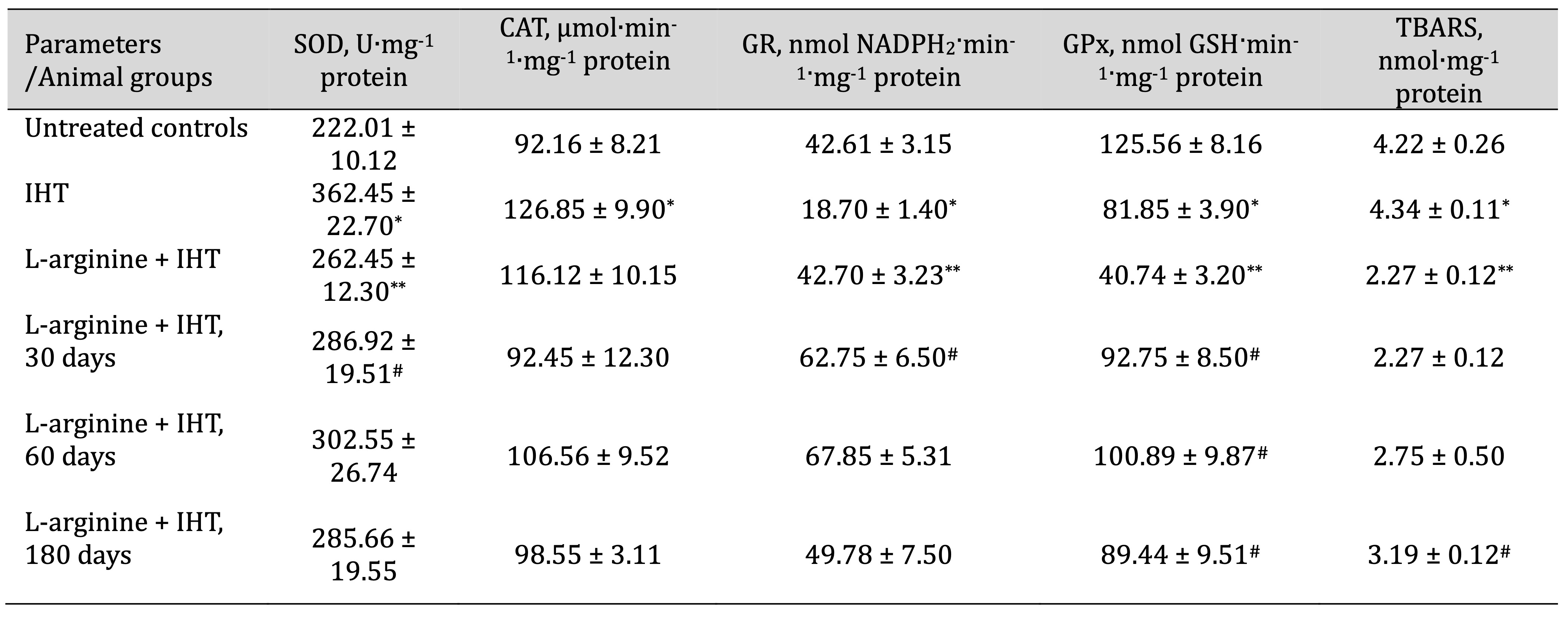
Table 6: Effects of L-arginine (600 mg/kg, 30 min) on activities of antioxidant enzymes such as superoxide dismutase (SOD, U∙mg-1 protein), catalase (CAT, μmol∙min-1∙mg-1 protein), glutathione reductase (GR, nmol NADPH2·min-1·mg-1 protein), glutathione peroxidase (GPx, nmol GSH·min-1·mg-1 protein) and intensity of lipid peroxidation (TBARS, nmol∙mg-1 protein) in the liver of rats (M ± m; n = 6) exposed to intermittent hypoxia training (IHT, 15 min, 10% oxygen, 5 cycles per day) and at 30, 60 and 180 days after the end of the IHT sessions. * – Changes are statistically significant (p < 0.05) between the control group and the IHT group; ** – Changes are statistically significant (p < 0.05) between the IHT exposed group and the L-arginine + IHT group; # – Changes are statistically significant (p < 0.05) between the L-arginine + IHT group and the IHT group at 30, 60 and 180 days after the end of the IHT sessions
Fourthly, the effects of the IHT method are related to the increased role of NO, a signalling molecule involved in vasodilation, as shown by our studies on the levels of the end metabolites of the nitric oxide system (nitrite and nitrate ions), urea, and total polyamines (Figures 3 and 4). The protective effects of the IHT method, which can increase NO production by improving blood flow and oxygen delivery to tissues, have previously been demonstrated in a number of studies (Lizamore et al., 2017; Fryer et al., 2019). Increased NO availability promotes metabolic shifts in cellular adaptation through the activation of stress response pathways, such as the AMP-activated protein kinase (AMPK) pathway (Yeo, 2019). These pathways enhance cellular resilience, antioxidant defence, and repair mechanisms.
The data presented in this study show an important role of nitric oxide in the formation of the effects of bioenergetic hypoxia, which we investigated in the animal model after the course of adaptation to hypoxia in an interval mode (IHT method). The study of oxygen-dependent processes (mitochondrial respiration, intensity of lipoperoxidation, and level of microsomal oxidation) in different tissues: blood and its separate components, i.e. plasma and erythrocytes, heart, and liver showed different ways of formation of these effects. It should be noted that additional stimulation with exogenous L-arginine as a precursor of nitric oxide synthesis showed important features of the formation of these dependencies, which may be important in therapeutic practice. The important role of this amino acid in the diet of modern man is well known, as it is conditionally essential, and its importance has been actively debated in the nutritional literature in recent years with regard to its role in the prevention of cardiovascular disease, especially in elderly patients (Kurhaluk, 2023a,b).
It is known that the strategy of adaptive changes in the conditions of acute hypoxia involves a significant increase in the production and effective accumulation of nitric oxide in the blood. A possible mechanism of NO deposition during adaptation to hypoxia is associated with an increase in free iron and haemoglobin levels (Weng et al., 2021), which stimulates the deposition of NO in the form of dinitrosyl iron complexes (Truzzi et al., 2021). Reoxygenation, which alternates with each hypoxic exposure during adaptation, stimulates free radical processes that promote the release of free iron from ferritin and the accumulation of dinitrosyl iron complexes (Lewandowska et al., 2015). However, it is possible that excess NO in the conditions of acute hypoxia can be sequestered by increasing the capacity of the NO depot during adaptation, preventing the negative and proapoptotic effects of excess nitric oxide (Vanin et al., 2017). Thus, when assessing the role of endogenous NO under acute hypoxia and with the IHT method, it is easy to see that nitric oxide plays an opposite role: in the first case, under acute hypoxia, it is involved in the development of cellular damage and the activation of ROS processes; on the contrary, during adaptation to hypoxia with the IHT method in the second case, it is involved in the mechanisms of reducing ROS levels and saving oxygen consumption (Soodaeva et al., 2020). This is particularly evident in our study after using the acute hypoxia test in the IHT-exposed rats.
However, the mechanism of switching between the directly opposite effects of the NO action during hypoxia is not yet known. Perhaps the dose dependence of the effects of nitric oxide is important in these cases: a periodic increase in NO production during adaptation has a pronounced protective effect, whereas its overproduction under acute hypoxia has a detrimental effect. This is consistent with the fact that severe hypoxia induces the expression of the iNOS gene (Zhao et al., 2019; Stachon et al., 2021), whereas adaptation to hypoxia does not affect this process, but increases the activity of eNOS in the brain and blood vessels (Kosutova et al., 2019; Wischmann et al., 2020). Thus, as shown in our studies, the IHT method triggers a cascade of adaptive responses involving mitochondrial energy supply, NO metabolism, and metabolic shifts in the production of reactive oxygen species and increased efficiency of antioxidant defences.
Conclusion
In conclusion, the development of the body’s responses to acute hypoxia and adaptive responses to hypoxia using the method of intermittent hypoxia training is mediated by the effect of NO-dependent metabolic mechanisms, assessed by changes in the levels of nitrates, nitrites, urea, and total polyamines. The activation of mitochondrial succinate oxidation by acute hypoxia is reduced in animals adapted to hypoxia with the IHT method. Intermittent hypoxia, which prevents the intensification of lipoperoxidation processes in the liver during acute hypoxia, increases the efficiency of liver and myocardial mitochondrial function by enhancing the role of NO-dependent regulatory mechanisms. The NADPH oxidase pathway is a limiting step in the early stages of hypoxia and largely determines the impairment of mitochondrial energy-producing function. The development of mechanisms of adaptation to oxygen deprivation is associated with an increase in the efficiency of the NADPH oxidase pathway, as revealed by the analysis of mitochondrial function inhibition with malonate and rotenone within 30, 60, and 180 days after the end of adaptation to hypoxia with the IHT method. The increasing role of NO-dependent effects enhances the role of NADPH oxidase oxidation, which contributes to the formation of long-term adaptive responses.
Acknowledgements
The authors would like to thank the Pomeranian University in Słupsk and T.H. Shevchenko National University “Chernihiv Colehium” for supporting this research.
Funding
The present study was financially supported by the Pomeranian University in Słupsk (Słupsk, Poland) and T.H. Shevchenko National University “Chernihiv Colehium” (Chernihiv, Ukraine).
Authors’ contributions
The authors contributed to the following aspects of the study Conceptualisation: NK, HT, OL, PK; Data curation: NK, HT; Formal analysis: NK, HT; Investigation: NK, HT, OL; Methodology: NK, HT; Supervision: NK, HT; Writing – original draft: NK, HT; Writing – revision and editing: NK, HT, PK.
Statement of Ethics
The experiments were conducted in accordance with the guidelines of the Council of the European Union, current legislation in Poland and Ukraine, and the recommendations of the Ethics Committee. Animal studies were performed in accordance with the guidelines for animal research: Reporting of In vivo Experiments (ARRIVE) developed by the National Centre for Replacement, Refinement and Reduction of Animals in Research (NC3R). They were approved by the Ethics Committee of the T.H. Shevchenko National University “Chernihiv Colehium” (05/02/2019). The experiment was conducted in accordance with both Directive 2010/63/EU on the protection of animals used for scientific purposes and the Polish Act of 15 January 2015 on the protection of animals used for scientific or educational purposes (Journal of Laws, 26 February 2015, item 266).
Disclosure Statement
The authors have no competing interests to declare.
References
| 1 | Lee JW, Ko J, Ju C, Eltzschig HK. Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med 2019;51:1-13.
https://doi.org/10.1038/s12276-019-0235-1 |
| 2 | Huang Q, Yang J, Goh RMW, You M, Wang L, Ma Z. Hypoxia-Induced circRNAs in Human Diseases: From Mechanisms to Potential Applications. Cells 2022;11:1381.
https://doi.org/10.3390/cells11091381 |
| 3 | Della Rocca Y, Fonticoli L, Rajan TS, Trubiani O, Caputi S, Diomede F, Pizzicannella J, Marconi G. Hypoxia: molecular pathophysiological mechanisms in human diseases. J Physiol Biochem 2022;78:739-752 .
https://doi.org/10.1007/s13105-022-00912-6 |
| 4 | Rybnikova EA, Nalivaeva NN, Zenko MY, Baranova KA. Intermittent Hypoxic Training as an Effective Tool for Increasing the Adaptive Potential, Endurance and Working Capacity of the Brain. Front Neurosci 2022;16:941740.
https://doi.org/10.3389/fnins.2022.941740 |
| 5 | Sales de Campos P, Olsen WL, Wymer JP, Smith BK. Respiratory therapies for Amyotrophic Lateral Sclerosis: A state of the art review. Chron Respir Dis 2023;20:14799731231175915.
https://doi.org/10.1177/14799731231175915 |
| 6 | Timón R, González-Custodio A, Gusi N, Olcina G. Effects of intermittent hypoxia and whole-body vibration training on health-related outcomes in older adults. Aging Clin Exp Res 2024;36:6.
https://doi.org/10.1007/s40520-023-02655-w |
| 7 | Zhao D, Yin CY, Ye XW, Wan ZF, Zhao DG, Zhang XY. Mitochondrial separation protein inhibitor inhibits cell apoptosis in rat lungs during intermittent hypoxia. Exp Ther Med 2019;17:2349-2358.
https://doi.org/10.3892/etm.2019.7201 |
| 8 | Urdampilleta Otegui A, Roche Collado E. Intermittent hypoxia in sport nutrition, performance, health status and body composition. Nutr Hosp 2024;41:224-229.
https://doi.org/10.20960/nh.04692 |
| 9 | Zhang Q, Zhao W, Li S, Ding Y, Wang Y, Ji X. Intermittent Hypoxia Conditioning: A Potential Multi-Organ Protective Therapeutic Strategy. Int J Med Sci 2023;20:1551-1561.
https://doi.org/10.7150/ijms.86622 |
| 10 | Song J, Sundar K, Gangaraju R, Prchal JT. Regulation of erythropoiesis after normoxic return from chronic sustained and intermittent hypoxia. J Appl Physiol 2017;123:1671-1675.
https://doi.org/10.1152/japplphysiol.00119.2017 |
| 11 | Sokolova I. Mitochondrial Adaptations to Variable Environments and Their Role in Animals' Stress Tolerance. Integr Comp Biol 2018;58:519-531.
https://doi.org/10.1093/icb/icy017 |
| 12 | Gong LJ, Wang XY, Gu WY, Wu X. Pinocembrin ameliorates intermittent hypoxia-induced neuroinflammation through BNIP3-dependent mitophagy in a murine model of sleep apnea. J Neuroinflammation 2020;17:337.
https://doi.org/10.1186/s12974-020-02014-w |
| 13 | Wang W, Ding W, Huang H, Zhu Y, Ding N, Feng G, Zhang X. The role of mitophagy in the mechanism of genioglossal dysfunction caused by chronic intermittent hypoxia and the protective effect of adiponectin. Sleep Breath 2021;25:931-940.
https://doi.org/10.1007/s11325-020-02211-0 |
| 14 | Wu X, Gong L, Xie L, Gu W, Wang X, Liu Z, Li S. NLRP3 Deficiency Protects Against Intermittent Hypoxia-Induced Neuroinflammation and Mitochondrial ROS by Promoting the PINK1-Parkin Pathway of Mitophagy in a Murine Model of Sleep Apnea. Front Immunol 2021;12:628168.
https://doi.org/10.3389/fimmu.2021.628168 |
| 15 | Iturriaga R, Moya EA, Del Rio R. Inflammation and oxidative stress during intermittent hypoxia: the impact on chemoreception. Exp Physiol. 2015;100(2):149-155 doi: 10.1113/expphysiol.2014.079525.
https://doi.org/10.1113/expphysiol.2014.079525 |
| 16 | Kondrashova MN, Doliba NM. Polarographic observation of substrate-level phosphorylation and its stimulation by acetylcholine. FEBS Lett 1989;243:153-155.
https://doi.org/10.1016/0014-5793(89)80119-X |
| 17 | Kurhaluk N, Lukash O, Tkaczenko H. Do the Effects of Krebs Cycle Intermediates on Oxygen-Dependent Processes in Hypoxia Mediated by the Nitric Oxide System Have Reciprocal or Competitive Relationships? Cell Physiol Biochem 2023;57:426-451.
https://doi.org/10.33594/000000669 |
| 18 | Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med 2006;231:343-65.
https://doi.org/10.1177/153537020623100401 |
| 19 | Liu T, Schroeder H, Power GG, Blood AB. A physiologically relevant role for NO stored in vascular smooth muscle cells: A novel theory of vascular NO signaling. Redox Biol 2022;53:102327.
https://doi.org/10.1016/j.redox.2022.102327 |
| 20 | Shobatake R, Ota H, Takahashi N, Ueno S, Sugie K, Takasawa S. The Impact of Intermittent Hypoxia on Metabolism and Cognition. Int J Mol Sci 2022;23:12957.
https://doi.org/10.3390/ijms232112957 |
| 21 | Prabhakar NR, Peng YJ, Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J Clin Invest 2020;130:5042-5051.
https://doi.org/10.1172/JCI137560 |
| 22 | Zhu J, Kang J, Li X, Wang M, Shang M, Luo Y, Xiong M, Hu K. Chronic intermittent hypoxia vs chronic continuous hypoxia: Effects on vascular endothelial function and myocardial contractility. Clin Hemorheol Microcirc 2020;74:417-427.
https://doi.org/10.3233/CH-190706 |
| 23 | Xiao F, Li X, Wang J, Cao J. Mechanisms of vascular endothelial cell injury in response to intermittent and/or continuous hypoxia exposure and protective effects of anti-inflammatory and anti-oxidant agents. Sleep Breath 2019;23:515-522.
https://doi.org/10.1007/s11325-019-01803-9 |
| 24 | González-Candia A, Candia AA, Paz A, Mobarec F, Urbina-Varela R, Campo AD, Herrera EA, Castillo RL. Cardioprotective Antioxidant and Anti-Inflammatory Mechanisms Induced by Intermittent Hypobaric Hypoxia. Antioxidants 2022;11:1043.
https://doi.org/10.3390/antiox11061043 |
| 25 | Wisniewski JA, Moody DE, Hammock BD, Shull LR. Interlobular distribution of hepatic xenobiotic-metabolizing enzyme activities in cattle, goats and sheep. J Anim Sci 1987;64:210-215.
https://doi.org/10.2527/jas1987.641210x |
| 26 | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-254
https://doi.org/10.1016/0003-2697(76)90527-3 |
| 27 | Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 1955;217:383-393.
https://doi.org/10.1016/S0021-9258(19)57189-7 |
| 28 | Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955b;217(1):409-427.
https://doi.org/10.1016/S0021-9258(19)57191-5 |
| 29 | Chance B. Reaction of oxygen with the respiratory chain in cells and tissues. J Gen Physiol 1965;49:163-195.
https://doi.org/10.1085/jgp.49.1.163 |
| 30 | Karuzina II, Archakov AI. Estimation of the microsomal fraction of the liver and characteristic of its oxidative systems. Modern methods in biochemistry. Ed. V.I. Orekhovich. Moscow: Medicine, 1977;49-52.
|
| 31 | Matsubara T, Touchi A, Tochino Y. Hepatic aminopyrine N-demethylase system: further studies of assay procedure. Jpn J Pharmacol 1977;27:127-136.
https://doi.org/10.1254/jjp.27.127 |
| 32 | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982;126:131-138.
https://doi.org/10.1016/0003-2697(82)90118-X |
| 33 | Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 1994;356:295-298.
https://doi.org/10.1016/0014-5793(94)01290-3 |
| 34 | Kolb VG, Kamyshnikov VS. A guide on clinical chemistry. Minsk: Republic of Belarus, 1982 336 p. Russian.
|
| 35 | Faber J, Menashe M, Bachrach U, Desser H. Formation of putrescine and acetylspermidine from spermidine by cultured human lymphocytes. FEBS Lett 1980;121:165-168.
https://doi.org/10.1016/0014-5793(80)81289-0 |
| 36 | Kamyshnikov VS. A reference work for clinical and biochemical research and laboratory diagnostics. Moscow, MEDpress-inform, 2004 911 p. Russian.
|
| 37 | Kostiuk VA, Potapovich AI, Kovaleva ZhV. Prostoĭ i chuvstvitel'nyĭ metod opredeleniia aktivnosti superoksid- dismutazy, osnovannyĭ na reaktsii okisleniia kvertsetina [A simple and sensitive method of determination of superoxide dismutase activity based on the reaction of quercetin oxidation]. Vopr Med Khim 1990;36:88-91.
|
| 38 | Koroliuk MA, Ivanova LI, Maĭorova IG, Tokarev VE. Metod opredeleniia aktivnosti katalazy [A method of determining catalase activity]. Lab Delo. 1988;1:16-19.
|
| 39 | Glatzle D, Vuilleumier JP, Weber F, Decker K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia 1974;30:665-667.
https://doi.org/10.1007/BF01921531 |
| 40 | Moin VM. Prostoĭ i spetsificheskiĭ metod opredeleniia aktivnosti glutationperoksidazy v éritrotsitakh [A simple and specific method for determining glutathione peroxidase activity in erythrocytes]. Lab Delo. 1986;(12):724-727.
|
| 41 | Ravin HA. An improved colorimetric enzymatic assay of ceruloplasmin. J Lab Clin Med 1961;58:161-168.
|
| 42 | Terskov IA, Gitelson II. Method of chemical (acid) erythrograms. Biofizika 1957;2:259-266.
|
| 43 | Zar JH. Biostatistical Analysis. 4th ed., Prentice-Hall Inc., Englewood Cliffs, New Jersey, 1999.
|
| 44 | Korbecki J, Simińska D, Gąssowska-Dobrowolska M, Listos J, Gutowska I, Chlubek D, Baranowska-Bosiacka I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int J Mol Sci 2021;22:10701.
https://doi.org/10.3390/ijms221910701 |
| 45 | Serebrovska TV, Portnychenko AG, Portnichenko VI, Xi L, Egorov E, Antoniuk-Shcheglova I, Naskalova S, Shatylo VB. Effects of intermittent hypoxia training on leukocyte pyruvate dehydrogenase kinase 1 (PDK-1) mRNA expression and blood insulin level in prediabetes patients. Eur J Appl Physiol 2019;119:813-823 .
https://doi.org/10.1007/s00421-019-04072-2 |
| 46 | Martinez CA, Jiramongkol Y, Bal N, Alwis I, Nedoboy PE, Farnham MMJ, White MD, Cistulli PA, Cook KM. Intermittent hypoxia enhances the expression of hypoxia inducible factor HIF1A through histone demethylation. J Biol Chem 2022;298:102536.
https://doi.org/10.1016/j.jbc.2022.102536 |
| 47 | Yeo EJ. Hypoxia and aging. Exp Mol Med 2019;51:1-15.
https://doi.org/10.1038/s12276-019-0233-3 |
| 48 | Guan Y, Liu J, Gu Y, Ji X. Effects of Hypoxia on Cerebral Microvascular Angiogenesis: Benefits or Damages? Aging Dis 2023;14:370-385.
https://doi.org/10.14336/AD.2022.0902 |
| 49 | Wiśniewska A, Płoszczyca K, Czuba M. Changes in erythropoietin and vascular endothelial growth factor following the use of different altitude training concepts. J Sports Med Phys Fitness 2020;60:677-684.
https://doi.org/10.23736/S0022-4707.20.10404-3 |
| 50 | Kang JJ, Fung ML, Zhang K, Lam CS, Wu SX, Huang XF, Yang SJ, Wong-Riley MTT, Liu YY. Chronic intermittent hypoxia alters the dendritic mitochondrial structure and activity in the pre-Bötzinger complex of rats. FASEB J 2020;34:14588-14601.
https://doi.org/10.1096/fj.201902141R |
| 51 | Adzigbli L, Sokolov EP, Wimmers K, Sokolova IM, Ponsuksili S. Effects of hypoxia and reoxygenation on mitochondrial functions and transcriptional profiles of isolated brain and muscle porcine cells. Sci Rep 2022;12:19881.
https://doi.org/10.1038/s41598-022-24386-0 |
| 52 | Wojan F, Stray-Gundersen S, Massoudian SD, Lalande S. Brief exposure to intermittent hypoxia increases erythropoietin levels in older adults. J Appl Physiol 2023;135:88-93.
https://doi.org/10.1152/japplphysiol.00172.2023 |
| 53 | Lizamore CA, Hamlin MJ. The Use of Simulated Altitude Techniques for Beneficial Cardiovascular Health Outcomes in Nonathletic, Sedentary, and Clinical Populations: A Literature Review. High Alt Med Biol 2017;18:305-321.
https://doi.org/10.1089/ham.2017.0050 |
| 54 | Fryer S, Stone K, Dickson T, Wilhelmsen A, Cowen D, Faulkner J, Lambrick D, Stoner L. The effects of 4 weeks normobaric hypoxia training on microvascular responses in the forearm flexor. J Sports Sci 2019;37:1235-1241
https://doi.org/10.1080/02640414.2018.1554177 |
| 55 | Kurhaluk N. Supplementation with L-arginine and nitrates vs age and individual physiological reactivity. Nutr Rev 2023, nuad131.
https://doi.org/10.1093/nutrit/nuad131 |
| 56 | Kurhaluk N. The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia. Int J Mol Sci 2023;24:8205
https://doi.org/10.3390/ijms24098205 |
| 57 | Weng X, Chen H, Yu Q, Xu G, Meng Y, Yan X, McConell G, Lin W. Intermittent Hypoxia Exposure Can Prevent Reductions in Hemoglobin Concentration After Intense Exercise Training in Rats. Front Physiol 2021;12:627708.
https://doi.org/10.3389/fphys.2021.627708 |
| 58 | Truzzi DR, Medeiros NM, Augusto O, Ford PC. Dinitrosyl Iron Complexes (DNICs). From Spontaneous Assembly to Biological Roles. Inorg Chem 2021;60:15835-15845.
https://doi.org/10.1021/acs.inorgchem.1c00823 |
| 59 | Lewandowska H, Sadło J, Męczyńska S, Stępkowski TM, Wójciuk G, Kruszewski M. Formation of glutathionyl dinitrosyl iron complexes protects against iron genotoxicity. Dalton Trans 2015;44:12640-12652.
https://doi.org/10.1039/C5DT00927H |
| 60 | Vanin AF, Borodulin RR, Mikoyan VD. Dinitrosyl iron complexes with natural thiol-containing ligands in aqueous solutions: Synthesis and some physico-chemical characteristics (A methodological review). Nitric Oxide 2017;66:1-9.
https://doi.org/10.1016/j.niox.2017.02.005 |
| 61 | Soodaeva S, Klimanov I, Kubysheva N, Popova N, Batyrshin I. The State of the Nitric Oxide Cycle in Respiratory Tract Diseases. Oxid Med Cell Longev 2020;2020:4859260.
https://doi.org/10.1155/2020/4859260 |
| 62 | Zhao N, Xu X, Jiang Y, Gao J, Wang F, Xu X, Wen Z, Xie Y, Li J, Li R, Lv Q, Liu Q, Dai Q, Liu X, Xu G. Lipocalin-2 may produce damaging effect after cerebral ischemia by inducing astrocytes classical activation. J Neuroinflammation 2019;16:168.
https://doi.org/10.1186/s12974-019-1556-7 |
| 63 | Stachon T, Latta L, Seitz B, Szentmáry N. Hypoxic stress increases NF-κB and iNOS mRNA expression in normal, but not in keratoconus corneal fibroblasts. Graefes Arch Clin Exp Ophthalmol 2021;259:449-458.
https://doi.org/10.1007/s00417-020-04900-8 |
| 64 | Kosutova M, Pechanova O, Barta A, Franova S, Cebova M. Different adaptive NO-dependent Mechanisms in Normal and Hypertensive Conditions. Molecules 2019;24:1682.
https://doi.org/10.3390/molecules24091682 |
| 65 | Wischmann P, Kuhn V, Suvorava T, Muessig JM, Fischer JW, Isakson BE, Haberkorn SM, Flögel U, Schrader J, Jung C, Cortese-Krott MM, Heusch G, Kelm M. Anaemia is associated with severe RBC dysfunction and a reduced circulating NO pool: vascular and cardiac eNOS are crucial for the adaptation to anaemia. Basic Res Cardiol 2020;115:43.
https://doi.org/10.1007/s00395-020-0799-x |