Is Salivary Α-Amylase a Reliable Indicator of Psychological Status and Quality of Life in Patients with Oral Lichen Planus: a Case-Control Study
Keywords
Abstract
Background/Aims:
The objectives of our study were to determine salivary α-amylase activity (stress biomarker) and its association with psychological status and quality of life (QoL), disease duration and intensity of symptoms (pain/burning) in patients with OLP.Methods:
A total of 50 subjects participated in this case-control study: 30 patients with oral lichen planus (OLP); 20 control subjects. Unstimulated whole saliva (UWS) was collected between 9 and 10 am to avoid diurnal fluctuations. Psychological status was assessed using the Croatian validated version of the original Depression, Anxiety and Stress Scale (DASS-21). The impact of oral health on QoL was assessed using the Croatian version of the Oral Health Impact Profile Questionnaire (OHIP-CRO14).Results:
There was no statistically significant difference in salivary α-amylase activity between patients with OLP (N=30) and control subjects (N=20) (133813.3 vs. 166815.5 U/L, p=0.314; t-test). Depression, anxiety and stress showed no statistically significant difference between patients with OLP and control subjects (p=0.076, p=0.111, p=0.209; t-test). The patients with OLP had statistically significantly poorer QoL (total) compared to control subjects (p=0.004, t-test). There was a moderate positive correlation between symptom intensity (pain/burning) and poor QoL (total) (r=0.584, p<0.001), the OHIP-CRO14 dimension “physical pain” (r=0.661, p<0.001), “psychological impossibility” (r=0.555, p<0.01), “handicap” (r=0.546, p<0.01).Conclusion:
Although salivary α-amylase showed no statistically significant difference between patients with OLP and control subjects, the patients with OLP had poorer psychological status (three times higher scores for depression and two times higher scores for anxiety) and poorer QoL compared to the control subjects. Recognising and treating mental disorders in patients with OLP is important in order to break the „vicious circle“ and achieve a better QoL in these patients.Introduction
Oral lichen planus (OLP) is a chronic autoimmune mucocutaneous disease. It is a disease of unknown aetiology in which genetic and environmental factors are involved. It is assumed that cellular immunity plays a key role in the development of OLP. The role of psychological stress is not clear [1]. Numerous studies show a dysregulated immune response in OLP, suggesting the possibility of autoimmunity [2]. Hepatitis C virus (HCV) is the only microorganism convincingly associated with OLP, and only in some geographical areas [3]. In patients with OLP, particularly with the reticular or erosive form of the disease, a type 1 immune response has been observed, resulting in damage to the epithelium of the oral mucosa [4]. This response involves plasmacytoid dendritic (pDC) and myeloid dendritic cells, CD4+ and CD8+ T-cells, natural killer (NK) cells and mast cells, and is dominated by soluble factors characteristic of the type 1 immune response [interferons, interleukin 12 (IL-12), tumour necrosis factor alpha (TNF-α)] [5]. Important studies investigating the oral microflora and the receptors that control the antimicrobial response are currently at an early stage [6, 7]. To date, it is not clear whether the destruction of keratinocytes in OLP is due to autoreactivity or a dysregulated response to an exogenous antigen.
The incidence of OLP is up to 2.2% worldwide [8]. The clinical picture of OLP is diverse and several clinical forms can occur in one patient, making an accurate diagnosis difficult. Despite the diverse clinical picture, the histopathological diagnosis of OLP is unique. Microscopic features of OLP include hyperparakeratosis, hyperorthokeratosis and their combination; cytoid (Civatte ) bodies; hydropic degeneration of the basal layer of the epithelium and, most importantly, a dense band-like inflammatory lymphocytic infiltrate in the lamina propria [9]. In case of uncertainty or doubt, an active discussion between an oral medicine specialist and a pathology specialist is always recommended. Reported rates of malignant transformation of OLP vary from 0.4% to 12.5%, with an average rate of 1.1% reported in a recent meta-analysis and systematic review of 7, 806 patients in 16 studies [10, 11]. OLP has a significant negative impact on quality of life (QoL) [8, 12].
Several studies have provided direct evidence that salivary α-amylase activity correlates with changes in blood catecholamine concentrations and thus serves as a surrogate marker of stress, indicating changes in acute psychosocial stress [13, 14]. Studies have shown that salivary α-amylase activity is associated with stressful conditions and that its activity increases during physical (treadmill training, running, cycling) and psychological stress (academic stress in students) [15, 16]. The effects of psychological and physical stress on the functions of the immune system are well known. By understanding these interactions, psychoneuroendocrinoimmunology (PNEI) contributes to a better understanding of the association between mental and physical health. Psychological factors can alter the course of various diseases by affecting different levels of the endocrine and immune systems [17]. Patients with OLP reported experiencing more stressful life events and scoring higher on anxiety and depression tests than control subjects [18]. Salivary biomarkers (such as α-amylase) represent an attempt to quantify and objectify stress mechanisms using simple and non-invasive diagnostic methods.
The objectives of our study were to determine salivary α-amylase activity, psychological status and QoL, disease duration and intensity of symptoms (pain/burning) in patients with OLP. The hypothesis of our study was that higher salivary α-amylase activity correlates with poorer mental health, poorer QoL, longer disease duration and higher intensity of symptoms (pain/burning) in patients with OLP.
Materials and Methods
Study Design and Subjects
A total of 50 subjects took part in this case-control study: 30 patients with a clinically and histopathologically confirmed diagnosis of OLP; 20 control subjects. The group of patients with OLP consisted of 15 patients with an erosive form and 15 patients with a non-erosive form of the disease. The control group consisted of randomly selected healthy patients who came to the Department of Diagnostic Radiology, Dental Clinic Split, Split, Croatia. Patients with diseases of the oral mucosa and/or systemic diseases, smokers and underage patients (<18 years) were excluded from the study.
Anamnestic data, a list of medications, the disease duration (months) and the topography of the lesions according to the WHO classification were collected from all subjects [19]. A visual analogue scale (VAS, from 0 to 100 mm) was used to assess the intensity of pain and/or burning (0 = no pain/burning, 100 = worst possible pain/burning) in patients with OLP. The same oral medicine specialist (A.G.) performed a clinical oral examination and an incisional/punch biopsy of the oral mucosa. The study protocol was explained to each subject, and after signing the informed consent form, they were enrolled in the study. The study lasted three months (from April 2023 to June 2023) and was conducted with the approval of the Ethics Committee of the School of Medicine, University of Split, Split, Croatia (study of Dental Medicine) (approved on 28 April 2023) (class: 003-08/23-03/0015; registration number: 2181-198-03-04-23-0025). The study was conducted in accordance with the principles of the Declaration of Helsinki (1964) and its subsequent amendments.
The inclusion criterion for OLP was:
- Patients with a clinically and histopathologically confirmed diagnosis of OLP according to the modified WHO criteria [20].
- Patients with a history of systemic and/or autoimmune diseases and/or cancer;
- Pregnant women;
- Medication (corticosteroid, immunosuppressants, psychoactive therapy within the last three months), hormone therapy;
- Harmful habits such as chewing betel nuts or tobacco or smoking;
- Cutaneous lichen planus (LP);
- High blood pressure;
- Patients with inflammatory diseases of the oral cavity (gingivitis, parodontitis);
- Patients who have not understood the purpose of the study and the content of the informed consent form.
Saliva Sampling
Unstimulated whole saliva (UWS) was collected between 9 and 10 am according to the following protocol:
- Patients were asked to refrain from intense physical activity and mental exhaustion for three days before sampling;
- Patients were asked to stop eating, drinking, smoking and brushing their teeth 90 minutes before sampling;
- In women of reproductive age, the UWS was taken during the follicular phase of the menstrual cycle;
- Approximately 2.00 to 2.50 mL of saliva was collected from each subject in graduated tubes (Salivette ) (ref. 51. 1534.500, SARSTEDT AG & Co. KG, Nümbrecht, Germany) using the “spit method” (subjects collected saliva in the mouth for 60 seconds and then spit it out into a graduated tube; the process was repeated for a further ten minutes).
Instruments
Depression, Anxiety and Stress Scale (DASS-21)
Psychological status was assessed using the Croatian validated version of the original Depression, Anxiety and Stress Scale, the DASS-21 scale (Lovibond & Lovibond, 1995a; Croatian adaptation Jokić-Begić, Jakšić, Ivezić and Surányi, 2012) [22]. The same researcher (A.G.) collected data through interviews. The three-factor structure scale (depression, anxiety, stress) consists of 21 statements, each of which contains seven statements. The depression subscale refers to symptoms such as dysphoria, hopelessness, self-deprecation, apathy and disinterest. The anxiety subscale refers to arousal of the autonomic system and situational anxiety. The stress scale includes indicators for chronic, non-specific arousal, relaxation difficulties, restlessness, impatience, etc. They are used to assess the level of depression, anxiety and stress in the last seven days. The DASS-21 scale proved to be an instrument with high internal reliability (reliability coefficients 0.90 vs. 0.89 vs. 0.91). The items are rated on a four-point Likert scale (0 - does not apply to me at all, to 3 - applies to me almost completely or mostly). The results obtained for the subscales must be multiplied by two. The maximum score for each subscale is 42. A higher score on each of these scales means poorer mental health [23].
Croatian version of the Oral Health Impact Profile Questionnaire (OHIP-CRO14)
The impact of oral health on QoL was assessed using the OHIP-CRO14. The OHIP-14 questionnaire consists of 14 questions, which can be divided into seven dimensions according to Locker’s theoretical model of oral health: Functional impairment, Physical pain, Psychological discomfort, Physical impossibility, Psychological impossibility, Social impossibility and Handicap. These seven dimensions can be divided into three categories, with functional impairment, physical pain and physical impossibility belonging to the physical factor, psychological discomfort and psychological impossibility to the psychological factor and social impossibility and handicap to the social factor. Subjects were asked how often they had experienced a particular problem in the last month. The answers were categorised using a Likert scale: 0 - never, 1 - almost never (rarely), 2 - occasionally, 3 - relatively often, 4 - very often. Lower scores on the scale indicate good oral health, while higher scores indicate poorer oral health, i.e. a poorer QoL [24, 25].
Statistical Analysis
Data were collected, coded and entered into Microsoft Excel, version 16.0 (Microsoft Corporation, Washington, USA). Data were analysed using IBM SPSS Statistics software, version 23.0 (IBM Corporation, New York, USA). We used an absolute number with a percentage to represent categorical variables and an arithmetic mean with standard deviation (AM±SD) to represent ordinal variables. The age of the subjects was expressed as median (M) with interquartile range (IQR). The chi-square (χ2) test was used to compare between groups for categorical variables, while the t-test was used to compare the AM of independent variables. We used the Pearson correlation test to examine a statistically significant linear relationship between two numerical variables. A three-way ANOVA with Wald correction was used to determine the influence of the different variables on the DASS-21 subscales (depression, anxiety, stress). The images (Figures 1 and 2) were processed using the Paint programme (Microsoft Corporation, Redmond, USA). The significance level was set at 0.05.
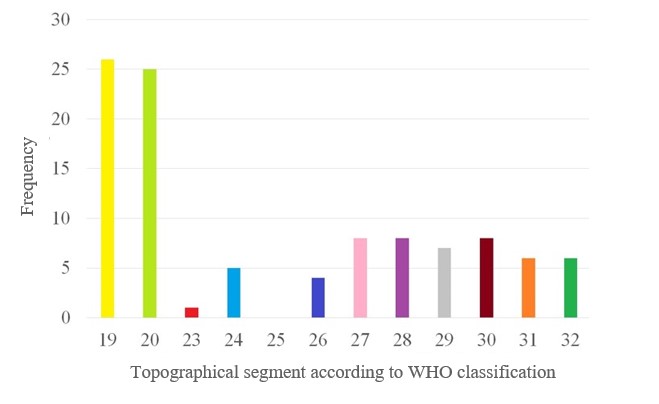
Fig. 1: Frequency of involvement of parts of the oral mucosa according to the WHO classification.
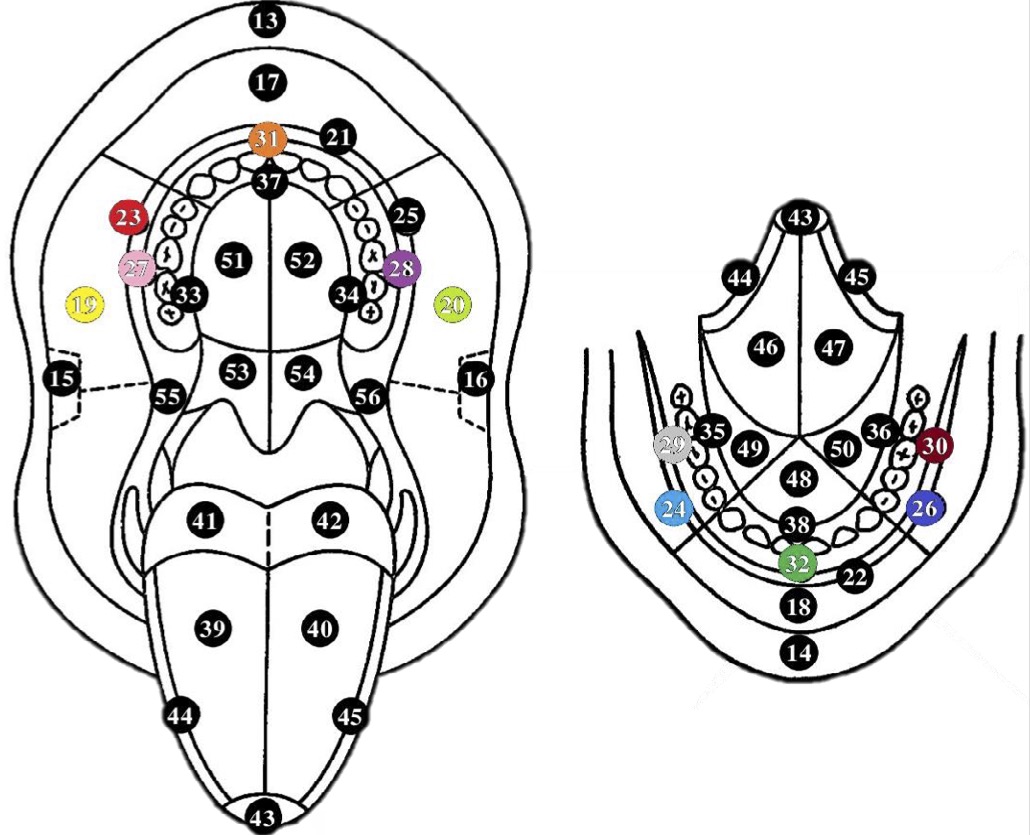
Fig. 2: The most common topographical localisations of OLP according to the WHO classification.
Results
Study Subjects
The sample of OLP patients consisted of 30 subjects aged 36 to 86 years, median 60.0 (IQR 50.8 to 75.0). The control group consisted of 20 subjects aged 41 to 80 years, median 61.0 (IQR 51.3 to 72.0). In both groups, the majority of subjects were women (66.7% vs. 65.0%). There was no statistically significant difference between the groups in terms of age and gender (p=0.945 and p=0.903, respectively) (Table 1).
The mean disease duration in patients with OLP was 38.0±59.2 and the mean VAS score was 1.9±2.6. The most common topographic localisations of OLP according to the WHO scheme were 19 and 20, i.e. the mucosa of the right cheek (86.7%) and the mucosa of the left cheek (83.3%). In a quarter of the patients with OLP, the attached gingiva of the maxilla and mandible on the right and left, the premolar and molar region (WHO 27, 28, 29, 30) was affected (Figures 1 and 2).
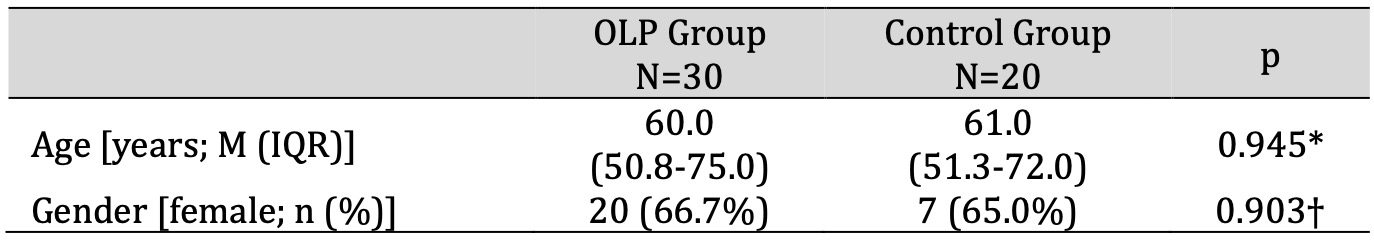
Table 1: Demographic data. * t-test for independent samples, † Chi-square test. Abbreviations: IQR, interquartile range; M, median; OLP, oral lichen planus
Salivary Stress Biomarker
There was no statistically significant difference in salivary α-amylase activity between patients with OLP (N=30) and control subjects (N=20) (133813.3 vs. 166815.5 U/L, p=0.314; t-test) . In both subject groups (OLP, control subjects), there was no statistically significant difference in salivary α-amylase activity between men and women (p=0.779 and p=0.794, respectively). There was also no statistically significant difference in salivary α-amylase activity between patients with erosive and non-erosive forms of OLP (142682.7 vs. 124944.0 U/L, p=0.560).
Psychological status and QoL
Depression, anxiety and stress showed no statistically significant difference between patients with OLP and control subjects (p=0.076, p=0.111, p=0.209; t-test). Stress was the leading mental disorder, i.e. it showed the highest scores in patients with OLP and control subjects. The patients with OLP had three times higher scores for depression and two times higher scores for anxiety than control subjects (Table 2). Women had statistically significantly higher scores for depression, anxiety and stress compared to men in patients with OLP (p=0.008, p=0.002, p=0.010) and the control group (p=0.015, p=0.033, p=0.034).
The patients with OLP had statistically significantly poorer QoL (total) compared to control subjects, as measured by the OHIP-CRO14 (p=0.004, t-test). The patients with OLP had statistically significantly higher scores on the dimensions (OHIP-CRO14) “physical pain”, “psychological discomfort”, “physical impossibility” and “handicap” compared to control subjects (p=0.002, p=0.043, p=0.040, p=0.011, t-test) (Table 3). Compared to men, women had statistically significantly poorer QoL (total) in patients with OLP (11.4 vs. 4.2, p=0.018; t-test).
There was a weak/moderate positive correlation between the disease duration and the intensity of symptoms (pain/burning) (r=0.409, p<0.05) and the OHIP-CRO14 dimension “psychological impossibility” (r=0.455, p<0.05). There was a moderate positive correlation between symptom intensity (pain/burning) and poor QoL (total) as measured by the OHIP-CRO14 (r=0.584, p<0.001), the OHIP-CRO14 dimension “physical pain” (r=0.661, p<0.001), “psychological impossibility” (r=0.555, p<0.01), “handicap” (r=0.546, p<0.01). There was a moderate positive correlation between poor QoL (total) and depression (r=0.651, p<0.001), anxiety (r=0.653, p<0.001), stress (r=0.587, p<0.001) (Table 4 ).
Multiple linear regression was performed for depression, anxiety and stress as dependent variables, while the form of OLP (erosive/non-erosive), age, gender, salivary α-amylase, disease duration and VAS were covariate factors in patients with OLP. There was a statistically significant effect of form of OLP (p=0.041) and VAS (p=0.015) on the level of depression and a statistically significant effect of gender (p=0.024) on the level of anxiety in patients with OLP (Table 5).
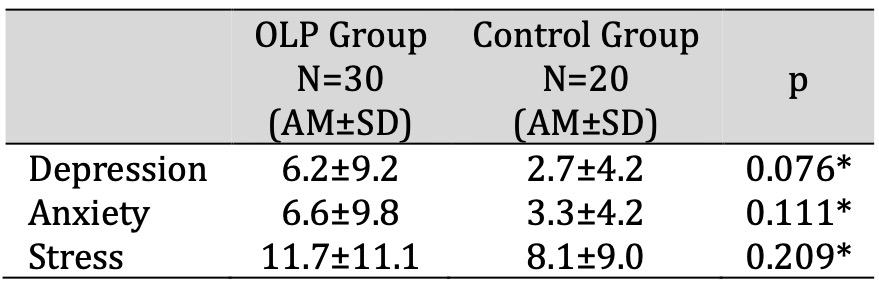
Table 2: Psychological status of patients with OLP and control subjects. * t-test for independent samples. Abbreviations: AM, arithmetic mean; OLP, oral lichen planus; SD, standard deviation
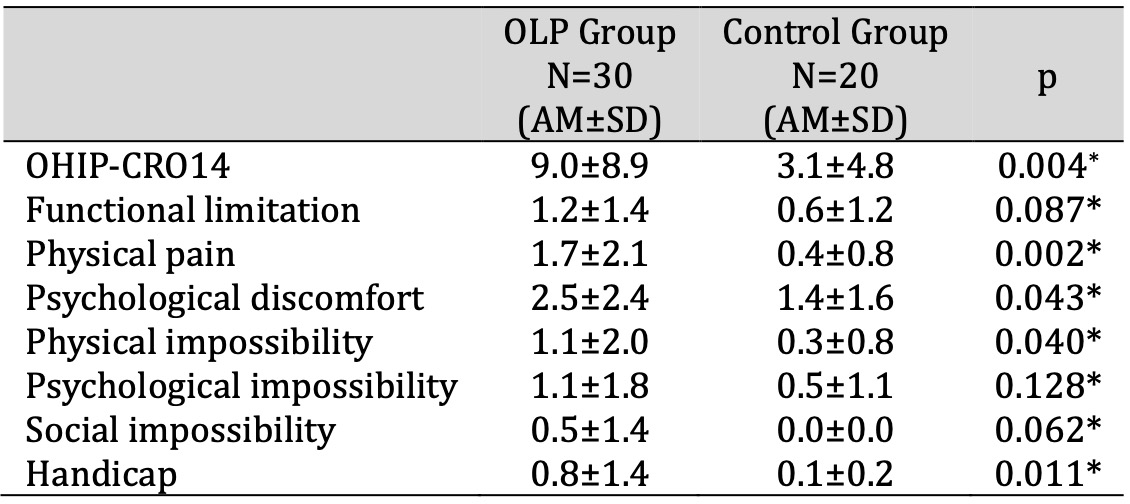
Table 3: QoL of patients with OLP and control subjects. * t-test for independent samples. Abbreviations: AM, arithmetic mean; OHIP-CRO14, oral health impact profile; OLP, oral lichen planus; QoL, quality of life; SD, standard deviation
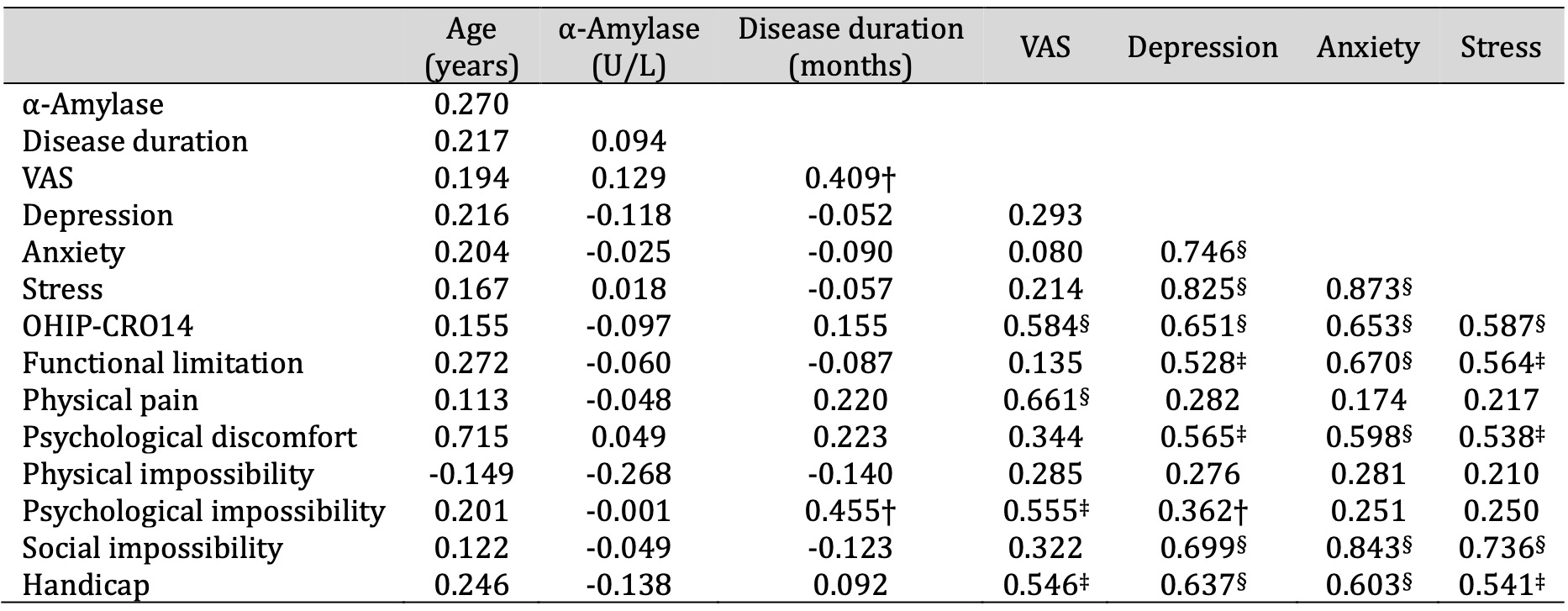
Table 4: Presentation of r values of Pearson's correlation of characteristics in patients with OLP. * Pearson correlation test † p < 0.05, ‡ p < 0.01, § p < 0.001. Abbreviations: OHIP-CRO14, oral health impact profile; OLP, oral lichen planus; VAS, visual analogue scale
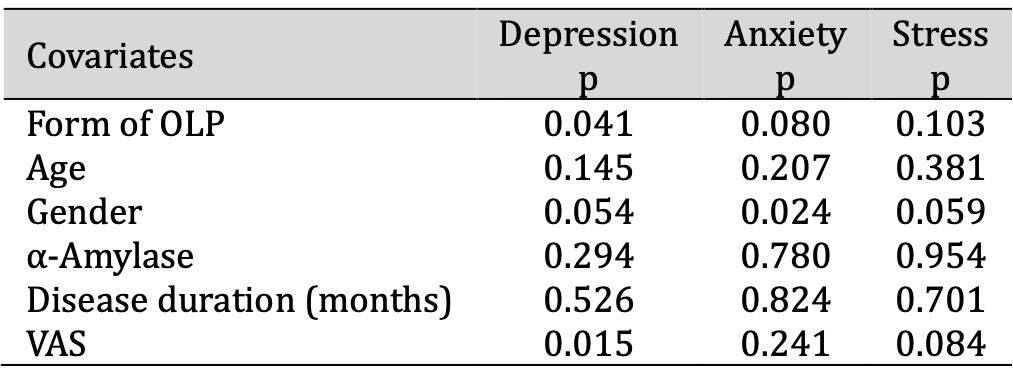
Table 5: Results of three-way ANOVA for depression, anxiety, stress as dependent variables. Abbreviations: ANOVA, analysis of variance; OLP, oral lichen planus; VAS, visual analogue scale
Erosive and Non-Erosive forms of OLP
There was a statistically significant difference in VAS between patients with erosive and non-erosive forms of OLP (3.5 vs. 0.3; p<0.001). Patients with an erosive form of OLP had statistically significantly higher scores on the OHIP-CRO14 dimension “physical pain” compared to patients with a non-erosive form of OLP (2.7 vs. 0.8; p=0.012) (Table 6).
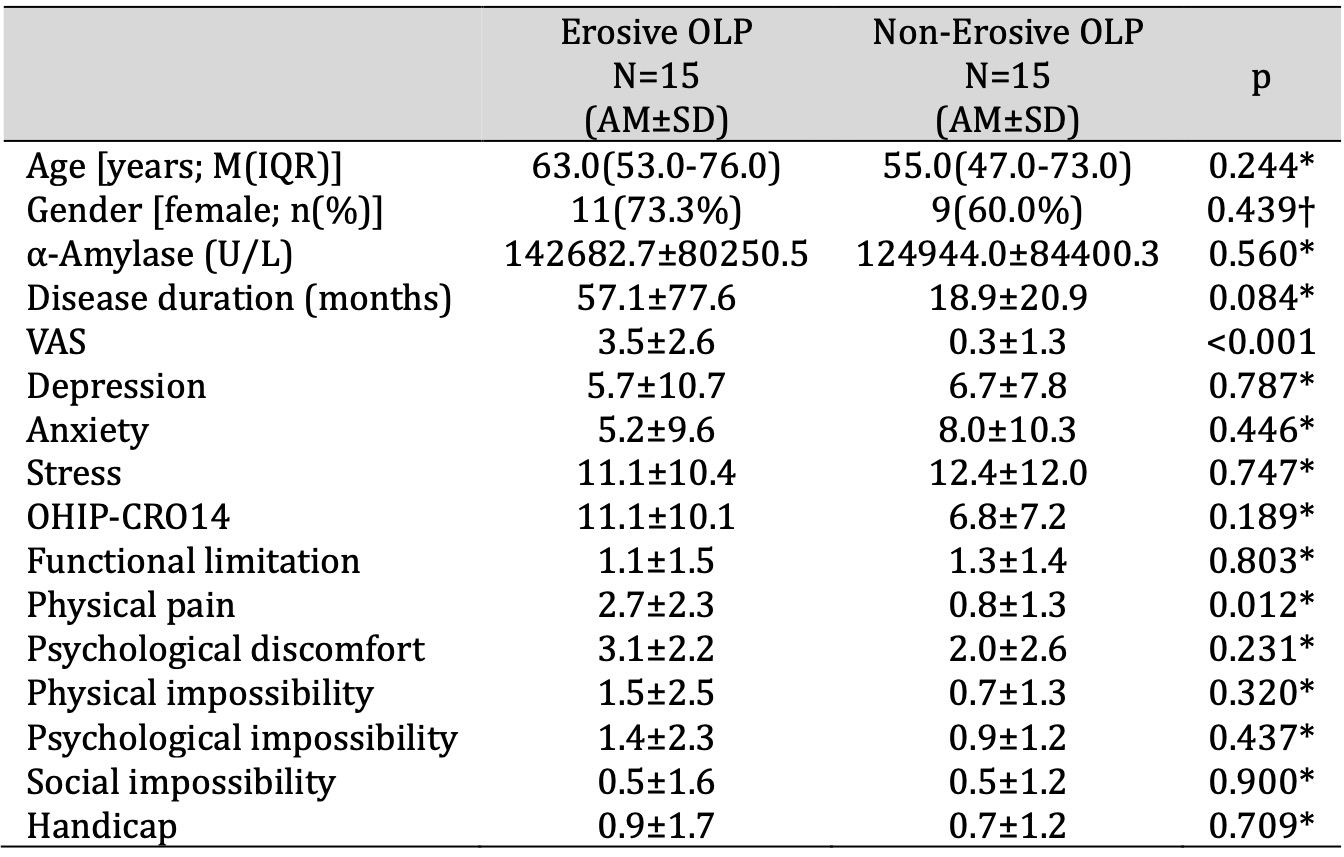
Table 6: Comparison of salivary α-amylase activity, psychological profile and QoL between patients with erosive and non-erosive forms of OLP. * t-test for independent samples, † Chi-square test. Abbreviations: AM, arithmetic mean; IQR, interquartile range; M, median; OHIP-CRO14, oral health impact profile; OLP, oral lichen planus; QoL, quality of life; SD, standard deviation; VAS, visual analogue scale
Discussion
OLP is a disease of unknown aetiology in which (among others) psychological difficulties play a certain role (cause or consequence). It is a disease of PNEI origin. Therefore, in this study we analysed salivary α-amylase activity as a stress biomarker and its association with disease duration, intensity of symptoms (pain/burning), psychological status and QoL.
In our study, there was no statistically significant difference in salivary α-amylase activity between patients with OLP and control subjects (p=0.314). There are few studies on their association, and most do not support the association between salivary α-amylase activity and OLP. The results of the study by Silva Simoura JA et al. showed increased salivary α-amylase activity [26]. Pippi R et al. and Glavina A et al. showed no statistically significant difference in salivary α-amylase activity between patients with OLP and control subjects [27, 28]. Their results are consistent with the results of our study.
The discrepancies in the study results on salivary α-amylase activity can be explained by the small number of studies performed, the heterogeneity of the diagnostic criteria for OLP, the lack of a comprehensive tool to distinguish between the different clinical forms of OLP, the different wake-up times of the subjects and the different times of sample collection. The inclusion and exclusion criteria for the control group were also not clearly defined in the studies. The studies by Pippi R et al. and Glavina A et al. define the inclusion criteria for the control group as „healthy“ so that the results can be compared with ours [27, 28]. The objective association between acute stress and salivary α-amylase activity has been confirmed in studies [13, 14]. The role of salivary α-amylase as a mediator of chronic stress needs to be clarified in future studies. Since OLP is a chronic disease, we did not demonstrate a relationship between α-amylase and chronic stress in our study.
When interpreting the results of different studies, it is necessary to know the daily fluctuations of salivary biomarkers. Salivary α-amylase has a distinct daily excretion profile. Salivary α-amylase activity decreases sharply in the first 30 minutes after waking and then increases steadily towards the afternoon and evening [29]. Studies on salivary α-amylase activity have used different units of measurement (i.e. U/ml, κU/L, ng/ml, IU/L) that could not be converted into a single unit of measurement. Therefore, no meta-analysis was performed [30]. Most studies that analysed salivary biomarkers used UWS, a smaller number of studies used stimulated whole saliva (SWS), while individual studies used both types of whole saliva. It is important to note that studies that collected both types of whole saliva obtained similar concentrations/activities of salivary biomarkers. Therefore, we can conclude that UWS is a reference for conducting future controlled longitudinal studies with standardised diagnostic criteria [30].
The patients with OLP did not have a poorer psychological status compared to the control subjects (p=0.076, p=0.111 and p=0.209 respectively). The results of the studies on the relationship between psychological status (depression, anxiety, stress) in patients with OLP are inconsistent. However, a large number of studies support the association between OLP and psychological disorders. The medical history of patients with OLP often indicates that the exacerbation of OLP lesions was preceded by a stressful event [18]. Shah B et al., Chaudhary S et al., Araya MS et al., Shetty S et al., Pires ALPV et al. showed higher scores for depression, anxiety and stress in patients with OLP compared to control subjects [31-35]. Their results are not consistent with the results of our study. Patients with OLP show discrepancies in depression, anxiety and stress scores. This could be due to the fact that no standardised diagnostic tool was used to identify mental disorders and that the inclusion and exclusion criteria of the control group were not defined. Most studies specified “healthy” as the inclusion criterion for the control group, while only one study by Araya MS et al. used a double control (positive and negative) [31-35]. The results of our study showed that patients with OLP had three times higher scores for depression and two times higher scores for anxiety compared to control subjects. Patients with OLP who have psychological problems and need medical help very often deny this condition. There are several reasons for this: shame, stigmatisation, fear of misunderstandings in the family, side effects of psychotropic drugs. Mental disorders have a significant impact on the poor QoL of these patients, which was initially affected by the diagnosis [33]. Psychological assessment and treatment of patients with OLP is as important as oral therapy to control the disease. It is necessary to involve psychiatric specialists and psychologists in the diagnosis of these comorbidities (depression, anxiety, stress).
The QoL (total), measured with the OHIP-CRO14, was statistically significantly different between patients with OLP and control subjects (p=0.004). Patients with OLP had statistically significantly poorer QoL compared to control subjects (9.0 vs. 3.1). The patients with OLP had statistically significantly higher scores in the OHIP-CRO14 dimensions “physical pain”, “psychological discomfort”, “physical impossibility” and “handicap” compared to the control subjects. The physical factor category was most strongly affected in patients with OLP. This could therefore indicate a secondary effect of depression, anxiety and stress rather than a causal effect (although this cannot be established in a case-control study). There was a moderate positive correlation between the disease duration and the psychological factor category (psychological impossibility, r=0.455, p<0.05). There was a moderate positive correlation between symptom intensity (pain/burning) and poor QoL (total) in patients with OLP (r=0.584, p<0.001). The symptom intensity (pain/burning) showed a moderate positive correlation with all three categories (physical, psychological, social) (physical pain, r=0.661, p<0.001; psychological impossibility, r=0.555, p<0.01; handicap, r=0.546, p<0.01). This indicates that all three factors (physical, psychological, social) are involved, i.e. that the QoL of patients with OLP who have symptoms (erosive form) is severely restricted. This leads to a “vicious circle ” in which poor QoL and social isolation become perpetuating factors that further and repeatedly exacerbate the clinical picture and the worsening of OLP symptoms. The OHIP-CRO14 dimension “physical pain” showed statistically significantly higher values in patients with erosive OLP compared to non-erosive OLP (p=0.012).
Our study has several limitations: small sample size; determination of salivary α-amylase activity at a single time point; different sensitivity of the kits in the different studies performed. The determination of haemoglobin or transferrin are more reliable methods for the detection of blood in saliva. Although they are much more sensitive than visual inspection, the accuracy of these methods can be affected by various factors such as age, hormones and salivary flow [29]. In our study, the exclusion criterion was drug therapy (corticosteroids, immunosuppressants, psychoactive therapy) three months before inclusion in the study. However, it is known that some medications can have a long-term effect [36].
Future longitudinal studies should use a standardised methodology: UWS and standardisation of the method for determining salivary α-amylase activity (international measure for assessing salivary α-amylase activity), use of agreed international diagnostic criteria for OLP, consideration of ethnicity and prevalence of clinical forms of OLP, consideration of sensitivity of kits and inclusion of a double control group (positive and negative).
Conclusion
The patients with OLP did not show higher salivary α-amylase activity or a correlation with psychological status and QoL. However, this does not mean that salivary α-amylase is not an indicator of chronic psychosocial stress when the characteristics of the stressed control group are taken into account. The patients with OLP had three times higher scores for depression and two times higher scores for anxiety, i.e. poorer psychological status. The patients with OLP had a poorer QoL (in terms of physical, psychological and social factors) compared to the control subjects. The DASS-21 can be a useful tool for the early detection of mental disorders, highlighting the need to involve psychiatric specialists (psychologists) in the therapeutic approach. In this way, the “vicious circle ” can be broken and a more successful treatment of these patients can be ensured.Acknowledgements
Author Contributions
Conceptualization, Supervision, Writing – original draft, Writing – review & editing, A.G.; Conceptualization, Funding acquisition, Supervision, L.L.M.;
Data curation, Funding acquisition, Methodology, L.C.; Data curation, Formal analysis, Methodology, D.Š.D.; Investigation, Writing – original draft, A.Z.; Conceptualization, Supervision, Visualization, A.T. All authors approved the final content for journal submission and publication.
Statement of Ethics
The study protocol has been approved by the research institute’s committee on human research.
The study was conducted with the approval of the Ethics Committee of the School of Medicine, University of Split, Split, Croatia (study of Dental Medicine) (approved on 28 April 2023) (class: 003-08/23- 03/0015; no: 2181-198-03-04-23-0025). The study was conducted in accordance with the principles of the Declaration of Helsinki (1964) and its subsequent amendments.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Eisen D: The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol 2022;46:207-214.
https://doi.org/10.1067/mjd.2002.120452 |
| 2 | Ebrahimi M, Nylander E, Bäcklund B, Wahlin Y-B, Coates PJ, Nylander K: The use of a novel ELISA method for detection of antibodies against p63 in sera from patients diagnosed with oral and/or genital and skin lichen planus. J Oral Pathol Med 2010;39:486-490.
https://doi.org/10.1111/j.1600-0714.2010.00890.x |
| 3 | Calvaruso V, Craxi A: Immunological alterations in hepatitis C virus infection. World J Gastroenterol 2013;19:8916-8923.
https://doi.org/10.3748/wjg.v19.i47.8916 |
| 4 | Lage D, Pimentel VN, Soares TCB, Souza EM, Metze K, Cintra ML: Perforin and granzyme B expression in oral and cutaneous lichen planus - a comparative study. J Cutan Pathol 2011;38:973-978 .
https://doi.org/10.1111/j.1600-0560.2011.01781.x |
| 5 | Hu J-Y, Zhang J, Cui J-L, Liang X-Y, Lu R, Du G-F, et al.: Increasing CCL5/CCR5 on CD4+ T cells in peripheral blood of oral lichen planus. Cytokine 2013;62:141-145.
https://doi.org/10.1016/j.cyto.2013.01.020 |
| 6 | Janardhanam SB, Prakasam S, Swaminathan VT, Kodumudi KN, Zunt SL, Srinivasan M: Differential expression of TLR-2 and TLR-4 in the epithelial cells in oral lichen planus. Arch Oral Biol 2012;57:495-502.
https://doi.org/10.1016/j.archoralbio.2011.10.013 |
| 7 | Kho H-S, Chang J-Y, Kim Y-Y, Kim Y: MUC1 and Toll-like receptor-2 expression in burning mouth syndrome and oral lichen planus. Arch Oral Biol 2013;58:837-842.
https://doi.org/10.1016/j.archoralbio.2013.01.008 |
| 8 | De Rossi SS, Ciarrocca K: Oral lichen planus and lichenoid mucositis. Dent Clin North Am 2014;58:299-313.
https://doi.org/10.1016/j.cden.2014.01.001 |
| 9 | Burgdorf WHC, Plewig G: Who described Civatte bodies? J Cutan Pathol 2014;41:340-346.
https://doi.org/10.1111/cup.12294 |
| 10 | Fitzpatrick SG, Hirsch SA, Gordon SC: The malignant transformation of oral lichen planus and oral lichenoid lesions: A systematic review. J Am Dent Assoc 2014;145:45-56.
https://doi.org/10.14219/jada.2013.10 |
| 11 | Landini G, Mylonas P, Shah IZ, Hamburger J: The reported rates of transformation of oral lichen planus. J Oral Maxillofac Surg Med Pathol 2014;26:213-220.
https://doi.org/10.1016/j.ajoms.2013.04.015 |
| 12 | Gorouhi F, Davari P, Fazel N: Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Scientific World J 2014;2014:742826.
https://doi.org/10.1155/2014/742826 |
| 13 | Van Stegeren A , Rohleder N, Everaerd W, Wolf OT: Salivary alpha amylase as marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology 2006;31:137-141.
https://doi.org/10.1016/j.psyneuen.2005.05.012 |
| 14 | Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, Stroud LR: Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann N Y Acad Sci 2007;1098:122-144.
https://doi.org/10.1196/annals.1384.008 |
| 15 | Unno K, Tanida N, Ishii N, Yamamoto H, Iguchi K, Hoshino M, et al.: Anti-stress effect of theanine on students during pharmacy practice: positive correlation among salivary alpha-amylase activity, trait anxiety and subjective stress. Pharmacol. Biochem Behav 2013;111:128-135.
https://doi.org/10.1016/j.pbb.2013.09.004 |
| 16 | Špiljak B, Vilibić M, Glavina A, Crnković M, Šešerko A, Lugović-Mihić L: A Review of psychological stress among students and its assessment using salivary biomarkers. Behav Sci (Basel) 2022;12:400.
https://doi.org/10.3390/bs12100400 |
| 17 | Par M, Tarle Z: Psychoneuro-immunology of oral diseases - a review. Stoma Edu J 2019;6:55-65.
https://doi.org/10.25241/stomaeduj.2019.6(1).art.7 |
| 18 | Valter K, Boras VV, Buljan D, Juras DV, Sušić M, Pandurić DG, et al.: The influence of psychological state on oral lichen planus. Acta Clin Croat 2013;52:145-149.
|
| 19 | Kramer IR, Pindborg JJ, Bezroukov V, Infirri JS: Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. World Health Organization. Communit Dent Oral Epidemiol. 1980;8:1-26.
https://doi.org/10.1111/j.1600-0528.1980.tb01249.x |
| 20 | Van der Meij EH, Van der Waal I: Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med 2003;32:507-512.
https://doi.org/10.1034/j.1600-0714.2003.00125.x |
| 21 | Kang J-H, Kho H-S: Blood contamination in salivary diagnostics: Current methods and their limitations. Clin Chem Lab Med 2019;57:1115-1124.
https://doi.org/10.1515/cclm-2018-0739 |
| 22 | Ivezić E, Jakšić N, Jokić-Begić N, Surányi Z: Validation of the Croatian adaptation of the Depression, Anxiety and Stress Scales (DASS-21) in a clinical sample, 18th Psychology Days in Zadar, Zadar, Croatia, May 2012.
|
| 23 | Pačić-Turk Lj, Ćepulić D-B, Haramina A, Bošnjaković J: The relationship of different psychological factors with the level of stress, anxiety and depression in health care workers during the COVID-19 pandemic in the Republic of Croatia. Suvremena psihologija 2020;23:35-53.
https://doi.org/10.21465/2020-SP-231-03 |
| 24 | Petricević N, Celebić A, Papić M, Rener-Sitar K: The Croatian vesrsion of the Oral Health Impact Profile questionnaire. Coll Antropol 2009;33(3):841-847.
|
| 25 | Rener-Sitar K, Petričević N, Čelebić A, Marion Lj: Psychometric properties of Croatian and Slovenian short form of Oral Health Impact Profile questionnaires. Croat Med J 2008;49:536-544.
https://doi.org/10.3325/cmj.2008.4.536 |
| 26 | Simoura JAdS, Pires ALPV, Alves LDB, Arsati F, Lima-Arsati YBdO, Dos Santos JN, et al.: Psychological profile and α-amylase levels in oral lichen planus patients: A case-control preliminary study. Oral Dis 2023;29:1242-1249.
https://doi.org/10.1111/odi.14081 |
| 27 | Pippi R, Patini R, Ghiciuc CM, Sandu RB, Pasquali V, Scaccianoce S, et al.: Diurnal trajectories of salivary cortisol, salivary α-amylase and psychological profiles in oral lichen planus patients. J Biol Regul Homeost Agents 2014;28:147-156.
|
| 28 | Glavina A, Lugović-Mihić L, Martinović D, Cigić L, Tandara L, Lukenda M, et al.: Association between Salivary Cortisol and α-Amylase with the Psychological Profile of Patients with Oral Lichen Planus and Burning Mouth Syndrome: A Case-Control Study. Biomedicines 2023;11:2182.
https://doi.org/10.3390/biomedicines11082182 |
| 29 | Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C: Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 2007;32:392-401.
https://doi.org/10.1016/j.psyneuen.2007.02.007 |
| 30 | Fernández-Agra M, González-Serrano J, De Pedro M, Virto L, Caponio VCA, Ibáñez-Prieto E, et al.: Salivary biomarkers in burning mouth syndrome: A systematic review and meta-analysis. Oral Dis 2022.
https://doi.org/10.1111/odi.14390 |
| 31 | Shah B, Ashok L, Sujatha GP: Evaluation of salivary cortisol and psychological factors in patients with oral lichen planus. Indian J Dent Res 2009;20:288-292.
https://doi.org/10.4103/0970-9290.57361 |
| 32 | Chaudhary S: Psychosocial stressors in oral lichen planus. Aust Dent J 2004;49:192-195.
https://doi.org/10.1111/j.1834-7819.2004.tb00072.x |
| 33 | Araya MS, Alcayaga GR, Esguep A: Association between psychological disorders and the presence of oral lichen planus, burning mouth syndrome and recurrent aphthous stomatitis. Med Oral 2004;9:1-7.
|
| 34 | Shetty S, Thomas P, Chatra L, Shenai P, Rao P, Babu S: An association between serum cortisol levels in erosive and nonerosive oral lichen planus patients. WebmedCentral DENTISTRY 2010;:WMC00560.
|
| 35 | Pires ALPV, Simoura JAdS, Cerqueira JDM, Lima-Arsati YBdO, Arsati F, Dos Santos JN, et al.: Relationship of psychological factors with salivary flow rate and cortisol levels in individuals with oral lichen planus: A case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol 2020;130:675-680.
https://doi.org/10.1016/j.oooo.2020.10.004 |
| 36 | Hershkovich O, Nagler RM: Biochemical analysis of saliva and taste acuity evaluation in patients with burning mouth syndrome, xerostomia and/or gustatory disturbances. Arch Oral Biol 2004;49:515-522.
https://doi.org/10.1016/j.archoralbio.2004.01.012 |