Tendon Cell Biology: Effect of Mechanical Loading
bOrto Med Sport, Łódź, Poland,
cMedical University of Lublin, Department of Sports Medicine, Lublin, Poland,
dInterdisciplinary Scientific Group of Sports Medicine, Department of Sports Medicine, Medical University of Lublin, Lublin, Poland
Keywords
Abstract
Tendons play a crucial role in the musculoskeletal system, connecting muscles to bones and enabling efficient force transfer. However, they are prone to acute and chronic injuries, which, if not properly repaired, can significantly impair function. Tendinopathy, a prevalent condition affecting approximately 20% of musculoskeletal complaints, arises from an imbalance between micro-injury accumulation and repair processes. The extracellular matrix (ECM) of tendons is a hierarchical structure comprising collagen fibrils, proteoglycans, and glycoproteins that regulate organization, hydration, and mechanical properties. Mechanotransduction pathways, mediated by integrins and focal adhesion complexes, activate signaling cascades such as MAPK/ERK and PI3K/Akt, driving tenocyte gene expression and ECM remodeling. Adaptations to load involve region-specific remodeling, with tensile regions favoring aligned Type I collagen and compressive regions promoting proteoglycans like aggrecan. Stress shielding or reduced loading disrupts these pathways, leading to matrix disorganization and inflammation, predisposing tendons to degenerative changes. Insights into these molecular mechanisms inform rehabilitation strategies to enhance tendon repair and mitigate tendinopathy progression in both athletic and general populations.Tendon Composition, Structure, and Cellularity
Tendons are primarily composed of longitudinal bundles of collagen fibers that connect muscle to bone [1]. The backbone of the tendon is formed by Type I collagen, which is crucial for force transmission [2]. Other components of the tendon extracellular matrix include various collagen isoforms, glycoproteins, proteoglycans, and water [3]. Collagen accounts for approximately 70-80% of the adult tendon’s dry weight [4, 5], with Type I collagen constituting 65-80% of this total collagen content [6, 7]. Water makes up about 55-70% of the wet mass of the tendon [8, 9].
Force transmission in tendons relies on the highly organized structure of long, rope-like Type I collagen proteins, which are packed into hierarchical bundles along the tendon's long axis [10, 11]. Type I collagen is the most common collagen type and is composed of a heterotrimeric molecule formed by two alpha 1 and one alpha 2 chains [12]. The triple helix region of the collagen chain features the characteristic amino acid motif (Gly-X-Y), with glycine occupying every third residue to fit into the center of the triple helix, while proline and hydroxyproline frequently occupy the X and Y positions [13]. Other relevant residues include lysine, hydroxylysine, and arginine [13, 14].
Each collagen chain is synthesized in the endoplasmic reticulum as a pre-pro-protein and undergoes several post-translational modifications before forming a mature triple helix procollagen molecule in the matrix. Key post-translational modifications include the hydroxylation of proline residues for helix stability [15, 16] and the intermolecular crosslinking of adjacent lysine residues [17]. The three procollagen chains assemble into a right-handed triple helix to form procollagen I, which is then deposited into the extracellular matrix along the line of force [18]. Procollagen I is processed into a mature collagen molecule after the cleavage of the N and C terminal propeptides [19]. The collagen fibrils are free to assemble in the pericellular space where the N and C-termini telopeptides are enzymatically crosslinked by lysyl oxidase [17].
These collagen fibrils pack into fibers, which are surrounded by an endotenon and bordered by resident tendon cells that help maintain the matrix. Individual collagen fibers can slide past each other during movement, though this movement is resisted by intermolecular crosslinks. Bundles of collagen fibers can be further organized into fascicles, which are surrounded by an epitenon sheath and separated by the interfascicular space where nerves and blood vessels pass [20, 21]. Fascicles are absent in smaller organisms such as rodents [11]. In larger animals, fascicles can slide independently to accommodate differences in tendon excursion during complex movements, such as those seen in the supraspinatus tendon during shoulder adduction and abduction [22]. Fascicles within a single tendon are encased by a synovial sheath (as in hand and foot tendons) or a loose fibrillar tissue known as the paratenon (as in the Achilles tendon) [23].
Beyond Type I collagen, the tendon matrix contains numerous other collagen isoforms and non-collagenous proteins, which vary regionally within a tendon and between tendons with distinct functions [24]. Type III collagen accounts for up to 10% of the dry mass of a normal tendon [25][7]. While the function of Type III collagen in healthy tendon is not fully understood, it tends to form smaller fibrils in vitro [26, 27] and assists in the longitudinal growth of Type I collagen fibrils by limiting lateral growth [28]. The patellar tendon and anterior cruciate ligament differ in collagen composition, with the patellar tendon having a greater Type I collagen concentration and lower Type III collagen concentration [7]. Other minor collagens such as types V, VI, XI, XII, and XIV are suggested to play roles in collagen fibril and fiber organization [29]. Type II collagen is most common in cartilage but can also occur in tendon fibrocartilage as an adaptation to compression, such as in wrap-around tendons, under an aponeurosis, or at the enthesis [30, 31].
Glycoproteins (GAGs) are a large class of non-collagen proteins characterized by a protein core with small covalently linked carbohydrate side chains. GAGs are linear polysaccharides containing an amino sugar. Tenomodulin (TNMD) and thrombospondin 4 (THBS4) are two glycoproteins highly expressed in tendon and ligament compared to other musculoskeletal tissues [32]. Tenomodulin is a transmembrane glycoprotein [33] with unknown binding partners [29] that is crucial for the proliferation of tendon stem/progenitor cells [34]. TNMD knockout mice exhibit reduced cell proliferation during development and abnormal collagen fibril diameter [35].
Thrombospondin 4 is an adhesive glycoprotein that forms complexes with cartilage oligomeric matrix protein (COMP) [36], a member of the thrombospondin protein family [37]. In weight-bearing tendons, COMP is more prevalent in regions that lack tension [38] and may play a role in collagen fibrillogenesis [39, 40]. Tenascin C is another glycoprotein associated with areas of tendon compression [31] and its levels are regulated by mechanical load [41].
Proteoglycans (PGs), a subgroup of glycoproteins, are characterized by the covalent attachment of long carbohydrate glycosaminoglycan side chains (GAGs) to a core protein. PGs are embedded within the collagen matrix and help regulate collagen organization and tissue hydration. The abundance of specific PGs varies depending on the biomechanical environment. Canonical small leucine-rich proteoglycans (SLRPs) are a class of PGs with collagen binding domains characterized by a leucine-rich repeat region [42]. Decorin and biglycan are modified with one or two dermatan sulfate GAG side chains, respectively [43]. Shortly after birth, biglycan content is nearly twice that of decorin. As animals begin to walk, gene expression and protein levels of decorin increase while those of biglycan decrease [44, 45]. Decorin binds procollagen to assist with collagen fibril assembly [46] and may prevent the association of collagen fibrils with each other. Decorin is moderately expressed into adulthood, while biglycan expression is minimal in mature tendons [44][45]. In adult tendons, decorin and biglycan are essential for maintaining collagen fibril diameter and mechanical properties [47]. Fibromodulin and lumican are two other SLRPs common in tendons that contain two keratan sulfate GAG chains [43]. Lumican and fibromodulin inhibit collagen fibril assembly early in post-natal tendon development, transitioning to assist with fibril growth, with fibromodulin continuing to promote fibril growth throughout adolescence [48]. SLRPs are more highly expressed in cells under tension, whereas tendon cells under compression synthesize more large PGs [49]. Large PGs, such as aggrecan, hold substantially more water, helping to resist matrix compression [50]. Consequently, aggrecan is more prevalent in compressed tendon regions and cartilage [51, 52, 53, 50]. Versican, another large proteoglycan, is present in tendons but is more concentrated in ligaments [7, 54].
Tendons have relatively few cells compared to other tissues, yet they contain multiple cell types, each with distinct functions. Over 70% of cells in a tendon are tenocytes, which synthesize high levels of collagen and function to maintain the matrix. Endothelial cells are the next most abundant cell population, with the remaining cells comprising pericytes, nerve cells, red blood cells, and immune cells [55][56]. Tenocytes are elongated, spindle-shaped fibroblast cells with extended cell processes located between collagen fibers in the endotenon and outside collagen fascicles in the epitenon [20]. In larger organisms with fascicles, most cells are located in the interfascicular matrix [57][24]. Tendons are surrounded by a paratenon, which contains a thin layer of cells that do not express scleraxis [58]. The tendon proper cells and peritenon cells have different transcriptional characteristics, with tendon proper progenitor cells expressing higher levels of scleraxis (Scx) and mohawk (Mkx) [59].
Multiple studies have demonstrated the existence of stem/progenitor cells within tendons, though their exact location and markers vary. In 2007, Marian Young’s group identified cells isolated from human and mouse tendons that met stem cell classification criteria: self-renewal capacity, clonogenicity, and multipotency [60]. Subsequently, tendon stem cells were identified in the middle region of rabbit patellar and Achilles tendons [61]. Mienaltowski and colleagues later showed that stem/progenitor cells within the tendon proper and peritenon are regionally distinct [59][62]. Tendon stem cells were also identified in the patellar tendon sheath of adult rats [63].
Tendon cells are linked to each other and the extracellular matrix through cell junctions, which facilitate communication between tenocytes. The main classes of cell junctions are occluding junctions (e.g., tight junctions), anchoring junctions (e.g., cadherins and integrins), and communicating junctions (e.g., gap junctions) [64]. These cell junctions persist in adult tendon despite the lower cellularity and higher matrix composition compared to embryonic and early post-natal development [51][65].
Gap junctions in vertebrates, composed of connexin proteins, allow for cell-cell communication via ion and small molecule diffusion. Connexin-32 and -43 are present at high levels in embryonic tendon and decline post-natally [66]. In adult tendons, connexin 43 is found at meeting points between cell processes and cell bodies, whereas connexin 32 is only found between cell bodies [67].
Cadherins, a type of cell-cell anchoring junction, are crucial in tendon development. Cadherin-11 and N-cadherin are the most commonly studied cadherins. Embryonic tendons require cadherin-11 and N-cadherin for proper formation, with N-cadherin localized to the tendon periphery and cadherin-11 restricted to the midsubstance [68][69]. In adult tendon, N-cadherin is found at cell-cell junctions, and its amount increases with radial stretch of adult tendon cells [70].
Integrins play a critical role in transmitting mechanical information by linking the extracellular matrix to the intracellular actin cytoskeleton. Integrin subunits α1 and α2 are widely expressed [35], with integrin α11 specifically localized around dense Type I collagen and binding Type I collagen at high affinity [71][35]. The integrin α11 promoter contains consensus sequences for binding a tendon/ligament-specific transcription factor, scleraxis, indicating its relevance in tendon mechanotransduction [71]. Mechanical stretch of tendon stem/progenitor cells increases the expression of multiple integrin subtypes (including α1/2/11) and activates downstream kinases ERK and p38 [72]. Integrins are essential for tendon mechanotransduction, as evidenced by studies showing that fibroblasts with ligand-bound integrins can stiffen their cytoskeleton in response to restraining forces [73].Adaptions to Mechanical Load: Musculoskeletal Development
Tendons adapt to various mechanical loads, primarily tension, compression, and shear, starting as early as fetal development. Tension results in a dense, well-aligned collagen matrix, mainly composed of Type I collagen, while compression promotes a fibrocartilage phenotype with larger proteoglycans and unaligned collagen fibers. Cells from tensile and compressive regions of tendons exhibit distinct biochemical responses to load; for example, cells from compressive regions synthesize more proteoglycans under cyclic compression. During development, tendons increase in collagen content and decrease in cellularity, leading to greater tissue stiffness and resistance to strain. Lysyl oxidase crosslinking further enhances the mechanical properties of tendons during this time, creating a structurally robust matrix. These phenotypic differences and structural adaptations become more defined during fetal and post-natal growth, particularly in response to specific mechanical loads (Supplemental Table 1).
Tendon Adaptation to Load
Tendons possess a remarkable ability to adapt to various types of mechanical loads, with the most notable adaptations occurring in response to tensile and compressive forces. There are three primary types of mechanical loads experienced by tendons within the musculoskeletal system: tension (where the tissue is pulled in one direction), compression (where the tissue is pushed from one or more directions), and shear (where the tissue is subjected to sliding forces). These adaptive processes begin as early as in utero or ovo.
Tendons that develop under tensile loads exhibit a dense, well-aligned matrix predominantly composed of Type I collagen. Conversely, tendons subjected to compressive loads display a fibrocartilage phenotype, characterized by sparsely connected, unaligned, and smaller Type I collagen fibers, alongside larger proteoglycans. Tendons exposed to shear forces exhibit a partially aligned collagen matrix and produce high levels of surface lubricating proteins such as lubricin (proteoglycan 4) and hyaluronic acid [51][74][75][76][77].
During embryonic and post-natal development, most tendons experience an increase in collagen content and a decrease in cellularity [51][65][78]. These changes in tissue composition during fetal development are associated with an increase in lysyl oxidase-mediated crosslinks, resulting in greater modulus [78]. Although significant differences in collagen organization between tensile and compressive tendon regions become apparent late in fetal development, the appearance of large proteoglycans in compressed regions does not occur until the post-natal period [51].
Even before these phenotypic differences become apparent at the tissue level, cells isolated from tensile and compressive regions exhibit distinct responses to mechanical load. For instance, cells from compressive regions synthesize more large and small proteoglycans when subjected to cyclic compression in vitro than those from tensile regions [79]. Specifically, aggrecan expression increases fivefold in response to cyclic compression in cells from pre-fibrocartilaginous regions. In both regions, decorin protein levels do not increase, but a larger form of decorin is produced in response to cyclic compression [79]. Additionally, treatment with TGF-β or cyclic compressive loading increases levels of large proteoglycans and biglycan, but not decorin, indicating a role for TGF-β signaling in regulating tendon matrix composition in response to load [80].
Adaptation to Load in Adults
Once tendons reach their adult length, changes in loading can lead to circumferential growth, enhancing mechanical properties. In humans, two to three months of training can significantly increase tendon stiffness and cross-sectional area. High-intensity training, which involves greater jerk forces, has a more substantial effect than lower intensity exercise, while the type of muscle contraction does not affect tendon stiffness [81]. Studies show that intercollegiate male and female cross-country runners increased their Achilles tendon cross-sectional area (CSA) during the first three to six weeks of the season [82], and lifelong distance runners have larger Achilles tendon CSA compared to non-runners [83]. Similarly, intercollegiate female soccer players increased their anterior cruciate ligament (ACL) volume throughout the competitive season [84].
In animal models, six weeks of running in mice increased Achilles tendon mass, fibroblast density, and CSA [85]. Swimming exercise for one hour a day over five to seven weeks also increased total protein in rat Achilles tendons, indicating that jerk is not necessary to drive tendon adaptations [86]. These tendon adaptations are attributed to the direct load applied to the tendon rather than a systemic exercise effect. For instance, in humans, only the loaded patellar tendon increases in CSA and stiffness after twelve weeks of unilateral resistance training [87]. Long-term training of one limb more than the other results in significantly larger and stiffer tendons in the dominant leg [88]. Conversely, two weeks of immobilization in elderly men resulted in an 80% reduction in tendon collagen synthesis [89]. These findings suggest that connective tissues are highly dynamic and acutely responsive to load.
The collagen content in tendons and ligaments is balanced between collagen synthesis and incorporation versus degradation. Degradation can occur through the breakdown of the existing dense collagen matrix or the degradation of newly synthesized collagen before its incorporation. One of the early studies examining collagen metabolism in response to load involved hanging a weight on a chicken wing for 45-50 hours, which resulted in a fivefold increase in collagen synthesis and a two-and-a-half-fold decrease in degradation before incorporation [90].
The extent of collagen turnover in adult tendons is debated. Early studies using the 14C bomb pulse method suggested that the central core of adult tendons does not turnover after the age of 17 [91][92]. During the period of nuclear bomb testing from 1955-1963, there was a sharp increase in 14C content in the atmosphere, which began to decrease exponentially after the Test Ban Treaty in 1963. The correlation between 14C levels in the core of the Achilles tendon and atmospheric 14C levels on subjects' seventeenth birthdays suggested that tendon cores only turnover during skeletal growth or regeneration [91]. This method showed no regional differences in collagen turnover in human patellar tendons [5]. Supporting this, collagen proteins in horses have a 100-fold longer half-life than non-collagen proteins in high-strain superficial digital flexor tendons (SDFTs) and a 10-fold longer half-life in low-strain common digital extensor tendons (CDETs) [93]. However, studies using stable isotopes suggest that whole tendon and ligament protein synthesis rates are similar to muscle [94]. Although stable isotope measurements do not account for protein breakdown, they suggest that collagen turnover in tendons or ligaments is much higher than previously thought. This discrepancy indicates that while the core of the tendon may not turnover, the outer part does, at rates faster than the whole tissue turnover rate.
The molecular and cellular mechanisms mediating collagen content increases with load are not fully understood. Progenitor cells are thought to migrate towards the core of the tendon in response to loading. Six weeks of treadmill running in young mice localized scleraxis (Scx) expression to the epitenon and between collagen fibers, suggesting that tendon cells emerge from the outer layer and migrate towards the core [85].
Insulin-like growth factor 1 (IGF-1) is one of the first molecules identified to play a role in tendon hypertrophy in response to load. In the rat Achilles tendon, loading increased IGF-1 concentration within tendon cells, while unloading decreased it [95]. Furthermore, IGF-1 expression increased 24 hours after four days of training, regardless of muscle contraction type [96]. In a model of plantaris tendon hypertrophy, mice with IGF-1 receptor knockout in scleraxis-positive cells experienced reduced tendon hypertrophy after loading. In vitro, IGF-1 application activates PI3K/Akt and ERK signaling pathways, promoting tenocyte protein synthesis and cell proliferation [97]. IGF-1 also increases collagen and mechanical properties in engineered ligaments [98]. ERK signaling, which can be activated directly by load, also plays a role in increasing collagen content. Ten minutes of loading is sufficient to activate ERK, and intermittent loading optimized for ERK1/2 phosphorylation increases collagen content more than continuous loading [99][100]. ERK may increase collagen content through EGR1, a transcription factor for collagen Iα1 [101].
Both male and female tendons hypertrophy with load, but the mechanisms of increasing collagen content may differ due to the role of estrogen. In men, a leg-kicking protocol increased patellar tendon collagen fractional synthesis rates (FSR), peaking 24 hours post-exercise and remaining elevated 72 hours later [102]. In women in their follicular phase, the same exercise did not increase patellar tendon collagen FSR 24 hours post-exercise [103]. However, post-menopausal women not on estrogen replacement therapy (ERT) had lower tendon collagen FSR but higher PINP (synthesis) after resistance exercise than those on ERT. At rest, ERT users had more medium-sized and fewer larger collagen fibrils than non-ERT users [103]. Proteomics studies support estrogen's beneficial effects on collagen incorporation, with pre-menopausal females having higher Type I collagen content in the ACL and patellar tendon compared to males [7]. However, estrogen can also result in smaller Achilles tendon CSA, as observed in monozygotic twins where only one took ERT and in active women on ERT [104][105]. These data indicate that estrogen modulates collagen synthesis and incorporation in response to load, and this response changes as women transition through menopause.
Adult tendons in different mechanical environments (tension vs. compression) exhibit regional gene expression variations. For example, energy-storing tendons like the rat Achilles and patellar tendons have similar transcriptomes, while the supraspinatus tendon, subjected to compressive loads, shows distinct gene expression profiles [106]. In adult bovine flexor tendons, tensile regions show no expression for collagen I, II, decorin, and biglycan, while compressed regions show varied expression depending on the distance from the tendon surface [53]. Over time, loading history leads to epigenetic encoding of gene expression differences. Tendon cells from tensile regions produce more small proteoglycans and fewer large proteoglycans than cells from compressed regions or chondrocytes [49]. Acute changes in load, however, can dramatically alter gene expression. Ex vivo compressive loading of tendons for 72 hours increases aggrecan mRNA and protein levels [80]. These studies highlight the role of tensional loads in promoting a tendon cell phenotype and compressive loads in inducing fibrocartilage gene expression. Tendon cells in normally structured, healthy tendons primarily experience tensile loads, while cells near entheses or where tendons wrap around bones experience compressive loads and adopt a fibrocartilage-like phenotype. Over time, cells become epigenetically tuned to their loading environment, though acute load changes can lead to phenotypic shifts.
Adaptation to Unloading: Adult
The detrimental impact of extended immobilization or disuse on the mechanical strength of tendons and ligaments was first documented in 1977. In a controversial study, primates were subjected to eight weeks of total body plaster immobilization (no weight-bearing) or single lower limb immobilization. Following eight weeks of total body immobilization, the maximum load capacity of the ACL decreased by 40%, from 78 kg to 46.79 kg, and the linear stiffness reduced by approximately 30%. After five months of reloading, the maximum tensile load (MTL) recovered to 79% of the baseline value. Twelve months of reloading saw MTL return to 91% of the baseline, which was not statistically different from the control group. In contrast, linear stiffness recovered to 90% of baseline after five months and was fully restored by twelve months. This suggests that a complete lack of weight-bearing for eight weeks causes a disproportionate loss in tissue material, contributing to maximal load (such as collagen content), which cannot be fully recovered within a year. However, tissue structures contributing to stiffness (such as collagen crosslinks) are lost more slowly and can be recovered more rapidly during the reloading period.
In the single leg immobilization part of the study, the ACL's maximal load and stiffness decreased by 22% over eight weeks. This single leg immobilization posed a different challenge from full body immobilization because the animal’s overall activity was maintained, and the non-immobilized leg was loaded normally. This indicates that there may be a systemic signal from loading that helps mitigate the reduction in tissue mechanics. It appears that tissue stiffness is less sensitive to a direct mechanical load signal compared to MTL, as it similarly reduced under both full body (30% reduction) and single leg (22% decrease) immobilization. These findings suggest that a systemic signal from loading may better mitigate the reduction in MTL than stiffness during immobilization, as MTL decreases by half as much in the single leg immobilization (22% decrease) compared to full body immobilization (40% decrease) [107].
Tendon Mechanics and Viscoelasticity
Tendons are essential connective tissues that play a key role in force transmission between muscles and bones, enabling complex movements such as running, jumping, and lifting. They serve as an intermediary between muscle-generated forces and the skeletal system, ensuring that forces are transmitted efficiently and accurately to produce controlled movements [108]. To achieve this function, tendons must combine strength, flexibility, and resilience, properties that are derived from their unique mechanical characteristics, including elasticity, viscosity, and viscoelasticity. These properties are essential for tendons to withstand both high tensile forces during muscular contraction and compressive forces at bone insertion points [109]. Viscoelasticity refers to a material's ability to exhibit both elastic (spring-like) and viscous (fluid-like) behaviors, which allows tendons to respond to mechanical loading in a time-dependent manner [110]. This viscoelastic behavior is crucial for the efficient transmission of muscular forces and the absorption of mechanical shocks during high-intensity activities [111]. Furthermore, this viscoelastic nature allows tendons to buffer and dissipate mechanical energy, preventing excessive stress concentration that could lead to microdamage and eventual injury [112].
The primary component of tendons responsible for their high tensile strength is type I collagen, which makes up approximately 65-80% of the tendon’s dry weight [113]. Collagen is organized into hierarchical structures, starting from the molecular level and extending to fibrils, fibers, fascicles, and the whole tendon [114]. At the molecular level, collagen molecules form a triple helix structure, which assembles into microfibrils and then into larger fibrils, providing the tendon with its characteristic crimped pattern. This crimp pattern is essential for allowing tendons to elongate with minimal resistance under low tensile forces, providing initial flexibility [115]. As tension increases, the collagen fibers uncoil, straighten, and align in the direction of the applied force, resulting in a linear increase in stiffness and a corresponding rise in the stress-strain curve [116]. This linear region of the stress-strain curve represents the tendon’s primary load-bearing phase, where it functions optimally in force transmission. Beyond this point, if the loading continues and exceeds the tendon’s capacity, the tendon enters the failure region, where micro-tears and disruptions in the collagen network occur, potentially leading to complete tendon rupture [117].
The non-collagenous components of tendons, such as proteoglycans and glycoproteins, also play a significant role in determining the viscoelastic properties of tendons [118]. Proteoglycans, which consist of a core protein and attached glycosaminoglycan (GAG) chains, help to retain water within the tendon matrix, contributing to the viscous behavior of tendons [119]. This water content is crucial for enabling tendons to deform under stress and recover their shape once the load is removed. The combination of collagen and proteoglycans allows tendons to exhibit time-dependent mechanical behaviors such as creep, stress relaxation, and hysteresis [120]. Creep, for example, is the gradual elongation of a tendon under a constant load over time, allowing the tendon to accommodate sustained forces without failing. This is particularly important during prolonged activities such as standing or maintaining a static posture [121]. Stress relaxation, on the other hand, refers to the reduction in internal stress within the tendon when it is held at a constant length, which prevents excessive stress accumulation during prolonged static activities such as yoga or stretching [122].
Hysteresis, the energy lost as heat during cyclic loading and unloading, is crucial for understanding tendon fatigue and the development of overuse injuries, as it reflects the tendon’s efficiency in energy storage and dissipation [123]. When tendons are repeatedly loaded and unloaded, as in repetitive sports activities, the energy dissipation through hysteresis can lead to a gradual increase in tendon temperature, potentially contributing to tissue fatigue and microdamage over time [124]. Understanding these mechanical properties is crucial for developing training and rehabilitation protocols that optimize tendon loading without causing injury. For instance, eccentric loading exercises have been shown to improve tendon stiffness and reduce the risk of tendinopathy by promoting collagen synthesis and realignment [125].
In addition to their mechanical properties, tendons demonstrate remarkable adaptability to different mechanical environments [126]. For instance, tendons exposed to high tensile loads, such as the Achilles tendon in runners, respond by increasing collagen synthesis, enhancing cross-linking between collagen molecules, and thickening the tendon, resulting in increased stiffness and improved load-bearing capacity [127]. Conversely, tendons subjected to compressive forces, such as those at bone-tendon junctions in the patellar tendon, develop fibrocartilaginous characteristics, including increased proteoglycan content and altered collagen organization, which help cushion and protect the tendon from compressive damage [128]. This adaptability is regulated by mechanotransduction pathways, which convert mechanical signals into cellular responses that influence the synthesis and organization of tendon matrix components [129]. Such adaptability is essential for maintaining tendon health and function in diverse physical activities, ranging from high-impact sports to static postures [130].
Pathological changes in tendon mechanics are often observed in conditions such as tendinopathy, where an imbalance between matrix synthesis and degradation leads to altered mechanical properties [131]. Tendinopathic tendons typically exhibit increased compliance (lower stiffness) and greater hysteresis, indicating a loss of mechanical efficiency and a higher risk of mechanical failure [132]. These changes are associated with disorganized collagen fibers, increased glycosaminoglycan content, and the presence of non-collagenous scar tissue, all of which impair the tendon’s ability to transmit force effectively [133]. The development of tendinopathy is often linked to repetitive overloading, poor biomechanics, and inadequate recovery, which create a catabolic environment within the tendon, disrupting normal matrix homeostasis [134]. Understanding these alterations in viscoelastic properties is crucial for designing therapeutic interventions that restore normal tendon function and prevent further injury [135].
Mechanotransduction and Tendon Adaptation
Mechanotransduction is a critical process that enables tendon cells, such as tenocytes and tendon stem/progenitor cells, to sense and respond to mechanical stimuli, converting external forces into intracellular biochemical signals that regulate cell behavior, matrix remodeling, and tissue homeostasis (Fig. 1.) [137]. This process is fundamental for maintaining tendon health, as it allows cells to adapt to varying mechanical environments, ensuring that tendons can withstand repetitive loading and respond effectively to changes in mechanical demand [138]. Mechanotransduction is initiated at the cell membrane, where mechanosensitive receptors, such as integrins, form complexes with focal adhesion proteins like vinculin, talin, and paxillin, creating a physical link between the extracellular matrix (ECM) and the actin cytoskeleton [139]. This physical coupling allows mechanical signals to be transmitted directly to the cell interior, where they activate intracellular signaling pathways such as the MAPK/ERK, Rho/ROCK, and PI3K/Akt pathways, which are crucial for regulating gene expression, cytoskeletal dynamics, and protein synthesis [140].
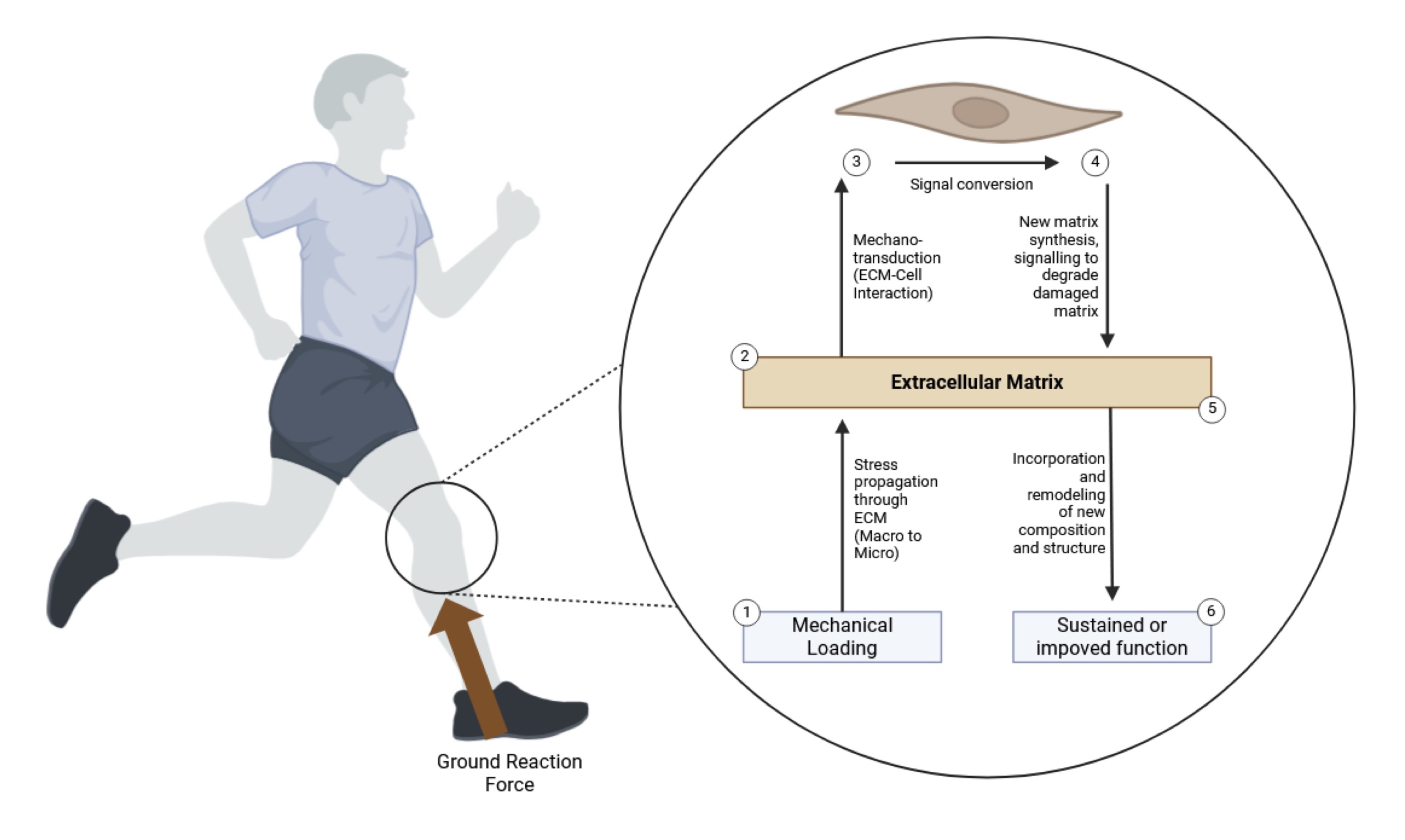
Fig. 1: The figure illustrates the process of mechanotransduction in tendons in response to mechanical loading, highlighting the molecular pathways that regulate tendon adaptation. Upon the application of mechanical loading, such as ground reaction forces during running or strength training, the extracellular matrix (ECM) experiences stress propagation from the macro to the micro-level, activating mechanosensitive receptors on tenocytes (1) [mechanical loading phase]. This mechanical signal is then converted into biochemical signals within the tendon cells through ECM-cell interactions (2), involving integrins, focal adhesion complexes, and cytoskeletal elements, initiating intracellular pathways like MAPK/ERK and Rho/ROCK [signal conversion]. As a result, tenocytes increase the synthesis of new matrix components such as collagen and proteoglycans, promoting the remodeling of the ECM and degradation of damaged matrix (3) [matrix synthesis and degradation]. This remodeling process leads to the incorporation of newly synthesized molecules into the ECM, enhancing its structural and functional integrity (4) [ECM incorporation]. The end result is a sustained or improved tendon function, ensuring that the tissue can better withstand subsequent mechanical loads (5) [functional adaptation]. Ultimately, the cycle contributes to tissue homeostasis and adaptation, preventing overuse injuries and maintaining tendon health.)
Integrins, which are transmembrane receptors, play a pivotal role in mechanotransduction by linking the ECM to the cytoskeleton and serving as the primary sensors of mechanical forces [141]. Upon mechanical stimulation, integrins cluster and recruit focal adhesion complexes, including focal adhesion kinase (FAK) and Src kinases, which initiate downstream signaling cascades [142]. Activation of these pathways can lead to the phosphorylation of transcription factors such as YAP/TAZ, which translocate to the nucleus and modulate the expression of genes involved in cell proliferation, differentiation, and matrix remodeling [143]. These signaling events ultimately influence the composition and mechanical properties of the tendon ECM, ensuring that it remains resilient and functional under different mechanical conditions [144].
The complexity of mechanotransduction is further illustrated by the diverse cellular responses observed under different types of mechanical loading. For example, cyclic tensile loading, which simulates physiological stretching forces, has been shown to upregulate the expression of collagen types I and III, as well as the glycoprotein tenascin-C, which contribute to tendon strength, elasticity, and resistance to tensile stress [145]. Additionally, cyclic loading enhances the production of lubricin, a glycoprotein that reduces friction between tendon fibers, thereby protecting the tissue from shear-induced damage [146]. On the other hand, compressive loading, which is commonly experienced at bone-tendon junctions, stimulates the production of large proteoglycans like aggrecan and decorin, which increase tissue hydration and provide resilience against compressive forces [147]. The upregulation of these molecules helps protect tendons from compression-induced degeneration, as seen in fibrocartilaginous regions of tendons that are subjected to high compressive loads [148].
In addition to the classical outside-in signaling, where external mechanical forces influence intracellular processes, mechanotransduction can also occur in an inside-out manner. Changes in the actin cytoskeleton, such as polymerization and depolymerization, can alter the distribution and conformation of integrins on the cell surface, modulating the cell’s sensitivity to external mechanical stimuli [149]. This bidirectional communication between the cytoskeleton and ECM is essential for maintaining the dynamic balance of tendon homeostasis and for adapting to changes in the mechanical environment, such as increased physical activity or immobilization [150]. For instance, during mechanical unloading, as seen in immobilization or reduced physical activity, tendon cells exhibit reduced expression of collagen and other ECM proteins, leading to a decrease in tendon stiffness and strength [151]. Conversely, during periods of increased loading, such as during resistance training, tendon cells increase the production of collagen and cross-linking enzymes, enhancing the mechanical properties of the tendon [152].
The actin cytoskeleton also plays a crucial role in mechanosensing by providing structural support and regulating the mechanical properties of cells [153]. Actin filaments connect integrins to the nucleus through the LINC (linker of nucleoskeleton and cytoskeleton) complex, enabling the transmission of mechanical signals to the nuclear lamina [154]. This mechanotransduction pathway allows cells to sense changes in the stiffness of the ECM and adjust their gene expression accordingly. For example, increased matrix stiffness promotes the activation of YAP/TAZ transcription factors, which enhance the expression of matrix proteins and increase cell proliferation, leading to tissue stiffening [155]. Conversely, a softer ECM inhibits YAP/TAZ signaling, reducing matrix synthesis and promoting a more compliant tissue phenotype [156]. These adaptive responses are essential for maintaining tendon function under different mechanical conditions and preventing the onset of pathological changes associated with altered mechanotransduction, such as tendinopathy and fibrosis [157].
Pathogenesis and Classification of Tendinopathies
Tendinopathies are a group of chronic tendon disorders characterized by pain, swelling, impaired function, and structural changes within the tendon matrix. They are most commonly observed in high-use tendons such as the Achilles, patellar, and rotator cuff tendons, where repetitive loading, microtrauma, and insufficient recovery contribute to pathological changes over time [158]. Tendinopathies often result from a mismatch between tendon loading and repair capacity, leading to a cumulative cycle of micro-injuries, inflammation, and degeneration [159]. While tendinopathies were traditionally thought to be primarily degenerative, recent studies suggest that inflammatory processes play a more significant role than previously believed, particularly in the early stages of the condition [160].
The pathogenesis of tendinopathies is complex and multifactorial, involving mechanical, biochemical, vascular, and cellular factors that disrupt the normal balance between matrix synthesis and degradation, leading to progressive tendon degeneration [161]. Mechanical overloading, whether due to repetitive microtrauma or sudden high-intensity forces, initiates a cascade of events at the cellular level. Tendon cells, known as tenocytes, become activated and release pro-inflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and prostaglandin E2 (PGE2), which promote a catabolic environment within the tendon matrix [162]. This inflammatory response is accompanied by upregulation of matrix metalloproteinases (MMPs), such as MMP-1 and MMP-13, which degrade type I collagen, the primary structural component of tendons, and inhibit new collagen synthesis [163].
The progression of tendinopathy can be divided into three main stages: the reactive, disrepair, and degenerative phases, each characterized by distinct histopathological and clinical features [164]. The reactive phase is typically the initial response to mechanical overload and is marked by increased cellularity and proteoglycan synthesis, leading to localized swelling and increased tendon stiffness [165]. This phase may be a protective attempt by the tendon to increase its cross-sectional area and reduce mechanical stress, thereby temporarily enhancing its load-bearing capacity [166]. However, if the overload persists, the tendon enters the disrepair phase, where the normal matrix organization becomes disrupted. The disrepair phase is characterized by increased production of MMPs, the formation of disorganized and thicker collagen fibrils, and increased vascularization, often referred to as neovascularization [167]. Neovascularization is accompanied by ingrowth of nerve fibers, which may contribute to the pain experienced in tendinopathy [168].
The final stage, degenerative tendinopathy, is marked by extensive matrix breakdown, calcification, and the formation of non-collagenous scar tissue, which significantly impairs the tendon’s mechanical properties and predisposes it to partial or complete rupture [169]. Histologically, degenerative tendons show loss of the normal parallel collagen fiber alignment, an increase in non-collagenous matrix, and areas of mucoid degeneration and fatty infiltration [170]. These changes are accompanied by a shift in the cellular phenotype, with tenocytes becoming more rounded and expressing markers associated with chondrocytic differentiation, such as Sox9, indicative of a failed healing response [171]. This transformation of tendon cells is thought to be driven by sustained mechanical overload and the dysregulation of signaling pathways such as transforming growth factor-beta (TGF-β) and Wnt/β-catenin, which are normally involved in tendon development and repair [172].
The classification of tendinopathies is based on the location of the tendon involved, histopathological findings, and clinical presentation [173]. Common clinical classifications include insertional versus non-insertional tendinopathies, depending on whether the pathology is located at the tendon-bone interface or within the midsubstance of the tendon [174]. Insertional tendinopathies, such as insertional Achilles tendinopathy, are often associated with calcific changes and the development of enthesophytes at the tendon-bone junction [175]. Non-insertional tendinopathies, like mid-portion Achilles tendinopathy, are characterized by localized thickening and increased vascularity within the tendon’s midsubstance [176]. Tendinopathies can also be classified based on their histological appearance, including reactive, degenerative, and calcific tendinopathies, which reflect the progression and chronicity of the condition [177].
The etiological factors contributing to tendinopathy include intrinsic factors such as age, sex, and genetics, as well as extrinsic factors such as training errors, biomechanical abnormalities, and poor equipment [178]. Age-related changes in tendon composition, including reduced collagen content, increased cross-linking, and decreased cellularity, are thought to increase the susceptibility of older individuals to tendinopathy [179]. Genetic predispositions, such as variations in the COL5A1 gene, have been associated with an increased risk of Achilles tendinopathy, suggesting that genetic factors may influence an individual's response to tendon loading [180]. Extrinsic factors, including high-impact activities, poor biomechanics, and inadequate recovery, contribute to the development of tendinopathy by increasing the mechanical load on tendons and altering the normal strain distribution within the tissue [181].
Radiocarbon Dating and Collagen Turnover
Radiocarbon dating, particularly the use of the 14C bomb-pulse method, has emerged as a powerful tool for estimating collagen turnover and understanding the dynamics of protein synthesis in human tendons and other tissues [182]. This method exploits the significant spike in atmospheric 14C levels caused by nuclear bomb testing in the 1950s and 1960s, which created a distinct chronological marker that was incorporated into biological tissues formed during this period [183]. By measuring the levels of 14C in collagen, researchers can accurately date when specific collagen molecules were synthesized, providing insights into the rates of protein turnover and tissue renewal [184]. This technique has been used to study various tissues, including bone, cartilage, and the brain, but has been particularly informative in assessing tendon turnover rates due to the unique structural properties and low metabolic activity of tendons [185].
Studies utilizing the 14C bomb-pulse method have revealed that the core of adult tendons exhibits very low collagen turnover after skeletal maturity, suggesting that tendons are relatively static tissues compared to more dynamic tissues such as muscle or skin [186]. This finding challenges the traditional view that tendons are continuously remodeling throughout life, indicating instead that collagen turnover in tendons may be highly localized and dependent on mechanical loading or injury [187]. The low turnover in the core regions of tendons implies that tendons may accumulate age-related damage over time, making them more susceptible to degenerative changes and injuries, such as tendinopathies, in older adults [188].
Interestingly, radiocarbon dating has also shown that while the central core of the tendon is relatively quiescent, the outer layers of tendons—particularly those closer to the bone-tendon junction—exhibit higher turnover rates [189]. This suggests a more dynamic remodeling process in these peripheral regions, potentially due to higher mechanical loads and greater cellular activity in response to stress or micro-injury [190]. This spatial variation in collagen turnover within tendons reflects the heterogeneous nature of tendon tissue, where different regions are subjected to varying mechanical environments and metabolic demands [191]. Such findings have significant implications for understanding tendon aging and the development of degenerative conditions, as the inability of the tendon core to effectively repair micro-damage could contribute to the onset of tendinopathy and other chronic tendon pathologies [192].
Moreover, the 14C bomb-pulse method has been used to investigate the turnover of other extracellular matrix (ECM) proteins in tendons, such as elastin and proteoglycans, which play key roles in tendon elasticity and resistance to compressive forces [193]. These studies have shown that, similar to collagen, the turnover rates of these ECM proteins are also remarkably low in the core regions of adult tendons, indicating that the overall matrix composition of tendons is highly stable after maturity [194]. This stability may be advantageous for maintaining the mechanical integrity of tendons under repetitive loading conditions but could also predispose tendons to accumulate damage over time, especially in individuals engaged in high-impact activities or sports [195].
The findings from radiocarbon dating studies have important clinical implications. For example, the low turnover rates in adult tendons suggest that therapeutic strategies aimed at enhancing collagen synthesis and matrix remodeling, such as mechanical loading protocols or growth factor treatments, may be necessary to promote tendon healing and prevent age-related degeneration [196]. Furthermore, understanding the differential turnover rates in various tendon regions could help inform the development of region-specific rehabilitation strategies for tendon injuries, such as eccentric loading exercises targeting areas of high metabolic activity [197].
In addition to the 14C bomb-pulse method, stable isotope labeling has been used as a complementary approach to study tendon protein turnover [198]. This technique involves the incorporation of stable isotopes, such as deuterium or 13C, into newly synthesized proteins, allowing for the tracking of protein synthesis and degradation over shorter time scales [199]. Stable isotope studies have shown that tendons can exhibit rapid turnover of newly synthesized collagen in response to increased mechanical loading or injury, particularly in the outer regions of the tendon [200]. These findings suggest that while the tendon core is relatively static, the peripheral regions may be more responsive to anabolic stimuli, providing a potential target for therapeutic interventions [201].
The combination of radiocarbon dating and stable isotope labeling has provided a more comprehensive understanding of tendon biology and aging, revealing the complex and region-specific dynamics of protein turnover in tendons. Future studies using these techniques could explore how different factors, such as age, sex, and mechanical loading history, influence tendon turnover rates and susceptibility to injury [202]. This knowledge could ultimately lead to more effective strategies for preventing and treating tendon disorders, thereby improving the health and function of tendons across the lifespan [203].
Molecular Role of Stress Shielding in Tendinopathy Development
The development of tendinopathy often stems from repetitive mechanical loading that does not lead to adequate tendon remodeling, resulting in compromised structure and function [204]. However, this mechanical model alone fails to capture why the remodeling process stalls initially. The main hypothesis posits that stress shielding contributes to the onset of tendinopathy, with biochemical and cellular responses leading to tissue dysfunction and weakening (Fig. 2). Stress shielding occurs when weaker tissue regions are protected from mechanical stress by surrounding stronger tissues, a phenomenon shown in experiments where unloading the patellar tendon in rabbits revealed critical molecular pathways impacted by stress shielding [205].
Fig. 2: It has been proposed that 'stress shielding' happens when loads are applied to a tendon, such as during jumping or isotonic strength exercises. In this scenario, the healthier regions of the tendon absorb the force, effectively shielding the disorganized parts by bearing the load. Theoretically, this load distribution should support tendon repair and enhance the tensile strength of the misaligned fibrils. However, due to the 'stress shielding' effect, the disorganized section of the tendon remains unloaded, potentially leading to continued deterioration. Stress-relaxation reduces the stiffness of surrounding collagen, making it less rigid than the injured area, which allows the load to transfer through the damaged region. When uniaxial loading passes through this injured area, it triggers a response that boosts the expression of genes associated with both mature and developing tendons (Left). Dynamic loading, such as that experienced during running or jumping, occurs rapidly, causing the load to divert around the injured area. In cases of central core injury, the injured region experiences radial compression as the tendon lengthens without a change in volume (Right). Interpretation form Steffen et al., 2022)
At a cellular level, stress shielding initiates significant biochemical changes. For instance, fibroblast density in the mid-substance of the tendon rapidly increases four-fold within two weeks, suggesting an immediate attempt at tissue repair under reduced mechanical load [208]. The fibroblasts shift from spindle-shaped to a more rounded morphology, indicating a transition to a more synthetic and proliferative state [209]. This transformation corresponds to increased production of matrix metalloproteinases (MMPs), enzymes critical for collagen breakdown, and disorganization in collagen fibril alignment, contributing to decreased tensile strength and tissue integrity [207].
Reduced mechanical loading also triggers biochemical pathways that alter the expression of collagen types I and III. Under normal conditions, collagen type I predominates, providing structural strength, but stress shielding favors the production of collagen type III, which is less organized and mechanically weaker [206]. This shift in collagen composition decreases tissue stiffness and resilience, impairing the tendon’s ability to bear load and recover from injury effectively. Furthermore, experimental data show that de-tensioned tendons exhibit a significant reduction in ultimate tensile strength, with a rapid 50% decrease within a week of unloading, which can be traced back to molecular degradation in collagen cross-linking [206].
Molecularly, the impact of stress shielding on tendon cells, particularly tenocytes, is profound. Tenocytes under reduced load downregulate genes related to matrix synthesis, such as those encoding collagen I and decorin, a proteoglycan that aids in organizing collagen fibrils. This response weakens the extracellular matrix (ECM) and contributes to the progressive loss of mechanical properties [210]. Stress shielding also causes a noticeable increase in the expression of pro-inflammatory cytokines, such as IL-6 and TNF-α, which contribute to matrix degradation and cellular senescence. Elevated levels of cytokines recruit more inflammatory cells to the tissue, amplifying degeneration and disrupting tissue homeostasis [208].
Experiments that utilize chemical inhibitors like beta-aminopropionitrile (BAPN) to hinder collagen cross-linking have further elucidated the molecular consequences of stress shielding. BAPN, for example, prevents stable cross-link formation, leading to a reduction in tendon stiffness and impaired collagen alignment [216]. While this treatment momentarily aids in collagen realignment in regenerating tissue, it fails to restore overall mechanical strength due to incomplete ECM recovery and inadequate cross-linking [217].
The inability of the tendon to regain its tensile properties after restressing, even after six weeks, underscores the permanent molecular changes induced by stress shielding [212]. Downregulation of integrins, which are cell-ECM adhesion receptors, and mechanosensitive pathways like those mediated by the extracellular signal-regulated kinase (ERK) cascade, are key to this response. These molecular changes prevent cells from sensing and responding to subsequent mechanical loads, reducing the tissue's adaptive capacity and leading to chronic weakness [213].
Interestingly, fetal tendons, which have inherently lower tensile properties, can completely heal after partial injury due to their unique molecular environment [214]. Unlike adult tendons, fetal tendons produce an ECM that allows for rapid, scar-free healing, suggesting that the lower material stiffness enables better load distribution, minimizing stress shielding effects [215]. The regenerative capacity of fetal tendons may be attributed to differences in gene expression and ECM composition, such as increased collagen III synthesis and minimized inflammatory response, which create an optimal environment for healing without stress shielding [214].
Finally, the stress-shielded region in the human patellar tendon, specifically the central proximal posterior region, is a common site of tendinopathy [218]. This localized stress shielding leads to molecular imbalances where pro-inflammatory signaling and matrix degradation outpace synthesis, resulting in characteristic tendinopathy symptoms—collagen disorganization, increased cellularity, and compromised mechanical properties [221]. Studies on stress shielding in rabbit models demonstrate similar molecular effects, confirming that insufficient load transmission disrupts cellular signaling pathways crucial for maintaining ECM integrity and, consequently, tissue resilience [222].
These insights collectively emphasize that stress shielding activates molecular pathways that weaken the ECM and compromise cellular responses to mechanical stress, establishing a biochemical foundation for tendinopathy. By disrupting both cellular and molecular processes essential for tendon health, stress shielding emerges as a critical factor in tendinopathy's etiology.
Conclusion
Tendons play a fundamental role in the musculoskeletal system by linking muscles to bones and facilitating force transmission. Despite their critical function, tendons are prone to both acute and chronic injuries, which can significantly hinder their ability to transfer force if not properly repaired. Tendinopathy, a common condition resulting from an imbalance between micro-injuries and the tendon cells' ability to repair the matrix, accounts for about 20% of musculoskeletal complaints in the general population. In the United States, musculoskeletal injuries such as strains, sprains, and tears have consistently accounted for a substantial portion of missed workdays, significantly affecting productivity and quality of life. This chapter has explored the complex interplay between mechanical load and the biology and mechanics of both healthy and tendinopathic tendons, providing insights into potential strategies for treating tendinopathy.
Tendons are primarily composed of collagen fibers, with Type I collagen being the most prevalent and crucial for force transmission. The organization and composition of tendons are highly adaptive to mechanical loads, with distinct responses to tensile, compressive, and shear forces. During development, tendons exhibit significant changes in collagen content and cellularity, which continue into adulthood as tendons respond to varying mechanical environments. These adaptations are driven by intricate molecular and cellular mechanisms, including the synthesis of specific proteins and the modulation of gene expression in response to mechanical stimuli.
In adults, tendons continue to adapt to mechanical loads, with changes in loading leading to increased tendon stiffness and cross-sectional area. High-intensity training and specific types of exercise can significantly enhance tendon properties, while immobilization or disuse can lead to a reduction in mechanical strength. The balance between collagen synthesis and degradation is crucial for maintaining tendon health, and the extent of collagen turnover remains a topic of ongoing research.
The molecular pathways involved in tendon adaptation to load, such as those mediated by IGF-1 and ERK signaling, highlight the complex regulatory networks that govern tendon biology. Additionally, the role of systemic signals and hormones, such as estrogen, further complicates the understanding of tendon adaptation, particularly in response to loading and unloading.
In summary, tendons are dynamic tissues capable of significant adaptation to mechanical loads throughout life. Understanding the underlying mechanisms of these adaptations is essential for developing effective treatments for tendinopathy and other tendon-related injuries. Future research should continue to elucidate the molecular and cellular processes involved in tendon adaptation, paving the way for innovative therapeutic strategies to enhance tendon repair and function.
Acknowledgements
Author Contributions
Conceptualization, MS; methodology, MS; software, MS; validation, MS; formal analysis, MS; investigation, MS; resources, MS; data curation, MS; writing—original draft preparation, MS; writing—review and editing, MS; visualization, MS; supervision, BK; project administration, MS; funding acquisition, PG.
Declaration of AI usage
AI (Chat GPT 4.0) was only used occasionally to help diversify sentences with new words.
Funding Sources
This work has been supported by the Medical University of Lublin, Poland.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Forde, C., van Tulder, M., & Bouter, L. (2005). Aetiology and management of tendon pain in the upper limb and neck. Scandinavian Journal of Work, Environment & Health, 31(2), 141-151.
|
| 2 | Robinson, K. A., Sun, M., Barnum, C. E., Weiss, S. N., Huegel, J., Shetye, S. S., ... & Soslowsky, L. J. (2017). Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biology, 64, 81-93.
https://doi.org/10.1016/j.matbio.2017.08.004 |
| 3 | U.S. Department of Labor. (2015). Occupational Safety and Health Administration (OSHA) Work-related injuries, illnesses, and fatalities. Retrieved from osha.gov
|
| 4 | Rumian, A. P., Wallace, A. L., & Birch, H. L. (2007). Tendons and ligaments are anatomically distinct but overlap in molecular and morphological characteristics. Journal of Orthopaedic Research, 25(4), 458-464.
https://doi.org/10.1002/jor.20218 |
| 5 | Zhang, J., Li, B., Cao, Y., Shi, Z., Wang, G., Liu, C., ... & Li, Z. (2020). 14C bomb-pulse dating reveals distinct age structure of human patellar tendon. Journal of Anatomy, 236(1), 103-111.
|
| 6 | Riley, G. (2004). The pathogenesis of tendinopathy. Journal of Bone and Joint Surgery. British Volume, 76(3), 327-344.
|
| 7 | Little, D., Thompson, J. W., Dubois, L. G., Ruch, D. S., Moseley, M. A., & Guilak, F. (2014). Proteomic differences between mouse Achilles tendon and patellar tendon. PLoS One, 9(4), e94831.
https://doi.org/10.1371/journal.pone.0096526 |
| 8 | Elliott, D. H. (1965). Structure and function of mammalian tendon. Biological Reviews, 40(3), 392-421.
https://doi.org/10.1111/j.1469-185X.1965.tb00808.x |
| 9 | Ristaniemi, T., Holm, L., Öhman, T., Asp, S., Lehto-Axtelius, D., Larsson, B., ... & Kjaer, M. (2020). Long-term physical activity modifies Achilles tendon properties and associated major fibrillar collagen content: a rodent study. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 318(1), R26-R34.
|
| 10 | Handsfield, G. G., Meyer, G. A., Abel, E. N., Gayzik, F. S., & Blemker, S. S. (2016). Measuring and modeling the variation in architecture of the human quadriceps femoris muscles with advanced diffusion-tensor imaging and finite element analysis. Journal of Biomechanics, 49(14), 3326-3332.
|
| 11 | Lee, J. H., & Elliott, D. M. (2019). Comparative 3D microanatomy of the murine and human Achilles tendon. Journal of Anatomy, 235(1), 150-158.
|
| 12 | DiChiara, A. S., Taylor, R. J., Wong, M. Y., Doering, D. T., Burdick, J. Q., Hu, D. W., & Karam, S. D. (2018). Prolyl-4-hydroxylase 1 enhances translation and is targeted to the endoplasmic reticulum independently of collagen biosynthesis. Journal of Biological Chemistry, 293(36), 13763-13778.
|
| 13 | Ramshaw, J. A., Shah, N. K., & Brodsky, B. (1998). Gly-X-Y tripeptide frequencies in collagen: A context for host-guest triple-helical peptides. Journal of Structural Biology, 122(1-2), 86-91.
https://doi.org/10.1006/jsbi.1998.3977 |
| 14 | Gaar, K., Nordal, R. A., Sanger, J. R., Fattahi, T., & Moyer, K. E. (2020). Structure and function of collagen: analysis of stress distribution in a collagen triple helix. Journal of Plastic, Reconstructive & Aesthetic Surgery, 73(1), 45-51.
|
| 15 | Berg, R. A., & Prockop, D. J. (1973). The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochemical and Biophysical Research Communications, 52(1), 115-120.
https://doi.org/10.1016/0006-291X(73)90961-3 |
| 16 | Rosenbloom, J., Harsch, M., & Jimenez, S. (1973). Hydroxylation of lysine residues in collagen by an enzyme system from chick embryo tendons. Journal of Biological Chemistry, 248(8), 3041-3048.
|
| 17 | Siegel, R. C. (1976). Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proceedings of the National Academy of Sciences, 73(11), 4241-4245.
|
| 18 | Kapacee, Z., Richardson, S. H., Lu, Y., Starborg, T., Holmes, D. F., & Kadler, K. E. (2008). Tension is required for fibripositor formation. Matrix Biology, 27(4), 371-375.
https://doi.org/10.1016/j.matbio.2007.11.006 |
| 19 | Ricard-Blum, S., & Ruggiero, F. (2005). The collagen superfamily: from the extracellular matrix to the cell membrane. Pathologie Biologie, 53(7), 430-442.
https://doi.org/10.1016/j.patbio.2004.12.024 |
| 20 | O'Brien, M. (1997). Structure and metabolism of tendons. Scandinavian Journal of Medicine & Science in Sports, 7(2), 55-61.
https://doi.org/10.1111/j.1600-0838.1997.tb00119.x |
| 21 | Khan, K. M., Cook, J. L., Bonar, F., Harcourt, P., & Astrom, M. (1999). Histopathology of common tendinopathies. Update and implications for clinical management. Sports Medicine, 27(6), 393-408.
https://doi.org/10.2165/00007256-199927060-00004 |
| 22 | Fallon, J., Blevins, F. T., Vogel, K., Trotter, J., & Speer, K. P. (2002). Functional morphology of the supraspinatus tendon. Journal of Orthopaedic Research, 20(5), 920-926.
https://doi.org/10.1016/S0736-0266(02)00032-2 |
| 23 | Benjamin, M., Kaiser, E., & Milz, S. (2008). Structure-function relationships in tendons: a review. Journal of Anatomy, 212(3), 211-228.
https://doi.org/10.1111/j.1469-7580.2008.00864.x |
| 24 | Thorpe, C. T., Karunaseelan, K. J., Ng Chieng Hin, J., Riley, G. P., Birch, H. L., & Clegg, P. D. (2016). Distribution of proteins within different compartments of tendon varies according to tendon type. Journal of Anatomy, 229(3), 450-458.
https://doi.org/10.1111/joa.12485 |
| 25 | Williams, I. F., McCullagh, K. G., & Silver, I. A. (1980). The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connective Tissue Research, 8(1), 41-49.
|
| 26 | Wiedemann, H., Trueb, B., & Timpl, R. (1975). Characterization of the major non-collagenous cartilage proteins. European Journal of Biochemistry, 57(2), 475-486.
|
| 27 | Lapiere, C. M., Nusgens, B. V., & Pierard, G. E. (1977). Interaction between collagen type I and type III in conditioning bundles organization. Connective Tissue Research, 5(1), 21-29.
https://doi.org/10.3109/03008207709152608 |
| 28 | Eryilmaz, E., Vural, A., & Caylak, B. (2017). A computational model of collagen fibril diameter distributions in extracellular matrix. Journal of Theoretical Biology, 430, 60-66.
|
| 29 | Taye, N., Alam, M. T., Pradhan, L., Chowdhury, H. K., Zhang, Y., Lam, J., & Levi, B. (2020). Comparative analysis of collagen isoforms in mouse tendons and ligaments. Journal of Anatomy, 237(5), 893-904.
|
| 30 | Benjamin, M., & Ralphs, J. R. (1998). Fibrocartilage in tendons and ligaments-an adaptation to compressive load. Journal of Anatomy, 193(4), 481-494.
https://doi.org/10.1046/j.1469-7580.1998.19340481.x |
| 31 | de Palma, L., Marinelli, M., & Pavan, M. (2004). Insertional tendinopathy of the Achilles tendon. Foot and Ankle Clinics, 9(4), 693-723.
|
| 32 | Jelinsky, S. A., Archambault, J., Li, L., Christenson, L. K., & Leinwand, L. A. (2010). Tendon-selective genes identified from rat and human musculoskeletal tissues. Journal of Orthopaedic Research, 28(3), 289-297.
https://doi.org/10.1002/jor.20999 |
| 33 | Shukunami, C., Oshima, Y., & Hiraki, Y. (2001). Chondromodulin-I and tenomodulin: a role for an anti-angiogenic co-receptor in cartilage and tendon. Cell Cycle, 2(5), 438-440.
|
| 34 | Alberton, P., Dex, S., Popov, C., Shukunami, C., Schieker, M., & Docheva, D. (2015). Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells and Development, 24(5), 597-609.
https://doi.org/10.1089/scd.2014.0314 |
| 35 | Docheva, D., Hunziker, E. B., Fässler, R., & Brandau, O. (2005). Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Molecular and Cellular Biology, 25(2), 699-705.
https://doi.org/10.1128/MCB.25.2.699-705.2005 |
| 36 | Södersten, F., Ekman, S., Heinegård, D., Johnston, C., Stack, M. B., & Heinegård, D. (2006). Immunolocalization of cartilage oligomeric matrix protein (COMP) in the posterior limb of foals during development. Journal of Anatomy, 209(5), 623-633.
|
| 37 | Adams, J. C. (2001). Thrombospondins: Multifunctional regulators of cell interactions. Annual Review of Cell and Developmental Biology, 17, 25-51.
https://doi.org/10.1146/annurev.cellbio.17.1.25 |
| 38 | Smith, R. K., Birch, H. L., Goodman, S., Heinegård, D., & Goodship, A. E. (1997). The influence of ageing and exercise on tendon growth and degeneration-hypotheses for the initiation and prevention of strain-induced tendinopathies. Comparative Biochemistry and Physiology Part A: Physiology, 116(3), 395-399.
|
| 39 | Sodersten, F., Ekman, S., Heinegard, D., Johnston, C., Stack, M. B., & Heinegard, D. (2005). Immunolocalization of cartilage oligomeric matrix protein (COMP) in the posterior limb of foals during development. Journal of Anatomy, 207(2), 151-159.
|
| 40 | Halász, K., Kassner, A., Mörgelin, M., Heinegård, D. (2007). COMP acts as a catalyst in collagen fibrillogenesis. Journal of Biological Chemistry, 282(43), 31166-31173.
https://doi.org/10.1074/jbc.M705735200 |
| 41 | Järvinen, T. A., Józsa, L., Kannus, P., Järvinen, T. L., & Järvinen, M. (2003). Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscle: an immunohistochemical, polarization and scanning electron microscopic study. Journal of Muscle Research and Cell Motility, 24(1), 23-35.
https://doi.org/10.1023/A:1020904518336 |
| 42 | McEwan, P. A., Scott, P. G., Bishop, P. N., & Bella, J. (2006). Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. Journal of Structural Biology, 155(2), 294-305.
https://doi.org/10.1016/j.jsb.2006.01.016 |
| 43 | Vogel, K. G. (2004). What happens when tendons bend and twist? The hierarchical structure and biochemical composition of tendon and tendon sheaths. International Review of Cytology, 239, 191-234.
|
| 44 | Birk, D. E., Zycband, E. I., Woodruff, S., Winkelmann, D. A., & Trelstad, R. L. (1995). Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Developmental Dynamics, 202(3), 229-243.
https://doi.org/10.1002/aja.1002020303 |
| 45 | Zhang, G., Young, B. B., Ezura, Y., Favata, M., Soslowsky, L. J., Chakravarti, S., & Birk, D. E. (2006). Development of tendon structure and function: regulation of collagen fibrillogenesis. Journal of Musculoskeletal & Neuronal Interactions, 6(3), 177-184.
|
| 46 | Keene, D. R., San Antonio, J. D., Mayne, R., McQuillan, D. J., Sarris, G., Santoro, S. A., & Iozzo, R. V. (2000). Decorin binds near the C terminus of type I collagen. Journal of Biological Chemistry, 275(29), 21801-21804.
https://doi.org/10.1074/jbc.C000278200 |
| 47 | Robinson, K. A., Sun, M., Barnum, C. E., Weiss, S. N., Huegel, J., Shetye, S. S., ... & Soslowsky, L. J. (2017). Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biology, 64, 81-93.
https://doi.org/10.1016/j.matbio.2017.08.004 |
| 48 | Ezura, Y., Chakravarti, S., Oldberg, A., Chervoneva, I., & Birk, D. E. (2000). Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. Journal of Cell Biology, 151(4), 779-787.
https://doi.org/10.1083/jcb.151.4.779 |
| 49 | Ehlers, S. A., & Vogel, K. G. (1998). Proteoglycan synthesis in tendon is differentially regulated by cyclic compression and cyclic tension. Experimental Cell Research, 246(2), 291-299.
|
| 50 | Kiani, C., Chen, L., Wu, Y. J., Yee, A. J., & Yang, B. B. (2002). Structure and function of aggrecan. Cell Research, 12(1), 19-32.
https://doi.org/10.1038/sj.cr.7290106 |
| 51 | Evanko, S. P., & Vogel, K. G. (1990). Proteoglycan synthesis in fetal tendon is differentially regulated by cyclic compression and cyclic tension. Journal of Biological Chemistry, 265(32), 19456-19462.
|
| 52 | Vogel, K. G., Paulsson, M., & Heinegård, D. (1994). Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochemical Journal, 223(3), 587-597.
https://doi.org/10.1042/bj2230587 |
| 53 | Perez-Castro, A. V., & Vogel, K. G. (1999). Regional expression of proteoglycans and collagen in the developing and mature bovine tendon. European Journal of Cell Biology, 78(10), 715-723.
|
| 54 | Kharaz, Y. A., Canty-Laird, E. G., & Comerford, E. J. (2018). Variations in internal structure, composition and protein distribution between intra- and extra-synovial tendons in dogs: an image analysis and immunohistochemistry study. Journal of Anatomy, 232(5), 796-804.
https://doi.org/10.1111/joa.12802 |
| 55 | De Micheli, A. J., Swanson, J. B., Disser, N. P., Walker, L. A., Cosgrove, B. D., Nugent-Glandorf, L., Gill, M. K., & Mendias, C. L. (2020). Single-cell transcriptomic analysis identifies extensive heterogeneity in the cellular composition of mouse Achilles tendons. American Journal of Physiology-Cell Physiology, 319(3), C885-C894.
https://doi.org/10.1152/ajpcell.00372.2020 |
| 56 | Kendal, A. R., Asparuhova, M. B., Al-Mossawi, M. H., Richard, D., Chu, H. W., Carr, A. J., ... & de Bari, C. (2020). Tendon-derived cells from development to adulthood: three-dimensional imaging, phenotypic and functional analyses. Journal of Anatomy, 237(4), 579-592.
|
| 57 | Thorpe, C. T., & Screen, H. R. (2016). Tendon structure and composition. Advances in Experimental Medicine and Biology, 920, 3-10.
https://doi.org/10.1007/978-3-319-33943-6_1 |
| 58 | Dyment, N. A., Hagiwara, Y., Matthews, B. G., Li, Y., Kalajzic, I., & Rowe, D. W. (2014). Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One, 9(4), e96113.
https://doi.org/10.1371/journal.pone.0096113 |
| 59 | Mienaltowski, M. J., Adams, S. M., Birk, D. E., & Birk, D. E. (2019). Regional differences in stem/progenitor cell populations from the mouse Achilles tendon. Journal of Orthopaedic Research, 37(3), 615-624.
|
| 60 | Bi, Y., Ehirchiou, D., Kilts, T. M., Inkson, C. A., Embree, M. C., Sonoyama, W., Li, L., Leet, A. I., Seo, B. M., Zhang, L., Shi, S., & Young, M. F. (2007). Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Medicine, 13(10), 1219-1227.
https://doi.org/10.1038/nm1630 |
| 61 | Zhang, J., & Wang, J. H. (2010). The effects of mechanical loading on tendons-an in vivo and in vitro model study. PLoS One, 5(7), e10150.
|
| 62 | Mienaltowski, M. J., Adams, S. M., Birk, D. E., & Birk, D. E. (2013). Tendon proper- and peritenon-derived progenitor cells have unique proliferative and multi-potent properties. Journal of Orthopaedic Research, 31(12), 1905-1916.
|
| 63 | Harvey, T., Flamenco, S., & Fan, C. M. (2019). A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGFRα in regeneration and fibrosis. Nature Cell Biology, 21(12), 1490-1503.
https://doi.org/10.1038/s41556-019-0417-z |
| 64 | Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Molecular Biology of the Cell (4th ed.). Garland Science.
|
| 65 | Curwin, S. L., Vailas, A. C., & Wood, J. (1994). Immature tendon adaptation to strenuous exercise. Journal of Applied Physiology, 76(5), 2119-2123.
|
| 66 | Stanley, R. L., Fleck, R. A., Becker, D. L., & Ralphs, J. R. (2007). Gap junction protein connexin 43 is present in avian tendons. Cell Biology International, 31(6), 617-621.
|
| 67 | McNeilly, C. M., Banes, A. J., Benjamin, M., & Ralphs, J. R. (1996). Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. Journal of Anatomy, 189(Pt 3), 593-600.
|
| 68 | Luo, G., Gilbert, E. R., & Olsen, B. R. (2001). The role of the collagens in growth and development of the mouse. Connective Tissue Research, 42(2), 189-194.
|
| 69 | Richardson, S. H., Starborg, T., Lu, Y., Humphries, S. M., Meadows, R. S., & Kadler, K. E. (2007). Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell-cell junctions. Molecular and Cellular Biology, 27(17), 6218-6228.
https://doi.org/10.1128/MCB.00261-07 |
| 70 | Ralphs, J. R., Waggett, A. D., & Benjamin, M. (2002). Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and in vitro. Journal of Anatomy, 200(6), 587-597.
|
| 71 | Tiger, C. F., Fougerousse, F., Grundström, G., Velling, T., & Gullberg, D. (2001). Alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Developmental Biology, 237(1), 116-129.
https://doi.org/10.1006/dbio.2001.0363 |
| 72 | Popov, C., Burggraf, M., Hoyer, M., Docheva, D., Schieker, M., & Benedetto, A. (2015). Mechanical loading induces tendon hypertrophy and growth factor expression in a dose-dependent manner. Journal of Orthopaedic Research, 33(4), 595-602.
|
| 73 | Choquet, D., Felsenfeld, D. P., & Sheetz, M. P. (1997). Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell, 88(1), 39-48.
https://doi.org/10.1016/S0092-8674(00)81856-5 |
| 74 | Evanko, S. P., Vogel, K. G. (1990). Proteoglycan synthesis in fetal tendon is differentially regulated by cyclic compression and cyclic tension. Journal of Biological Chemistry, 265(32), 19456-19462.
|
| 75 | Berenson, M. C., Blevins, F. T., Plaas, A. H., & Vogel, K. G. (1996). Proteoglycan synthesis and structure in fetal tendon is differentially regulated by cyclic compression in vitro. Journal of Biological Chemistry, 271(49), 29802-29808.
|
| 76 | Nugent, A. E., Speicher, D. M., Gradisar, I., Horton, W. E. (2006). Regulation of proteoglycan production by cyclic compression varies with concentration of transforming growth factor β1 and bone morphogenetic protein 2. Journal of Biomechanics, 39(7), 1306-1312.
|
| 77 | Hayashi, M., Muneta, T., Ju, Y. J., Mochizuki, T., & Sekiya, I. (2013). Weekly intra-articular injections of bone morphogenetic protein-7 inhibits osteoarthritis progression. Arthritis Research & Therapy, 15(4), R128.
|
| 78 | Marturano, J. E., Xylas, J. F., Sridharan, G. V., Kalenjian, M. A., Hoffman, B. D., & Kuo, C. K. (2013). Lysyl oxidase-mediated collagen crosslinks may be assessed as markers of functional properties of tendon tissue formation. Acta Biomaterialia, 9(8), 9559-9567.
|
| 79 | Evanko, S. P., & Vogel, K. G. (1993). Proteoglycan synthesis in fetal tendon is differentially regulated by cyclic compression and cyclic tension. Journal of Biological Chemistry, 268(25), 19337-19345.
|
| 80 | Robbins, J. R., Evanko, S. P., & Vogel, K. G. (1997). Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Archives of Biochemistry and Biophysics, 342(1), 203-211.
https://doi.org/10.1006/abbi.1997.0102 |
| 81 | Bohm, S., Mersmann, F., & Arampatzis, A. (2015). Human tendon adaptation in response to mechanical loading: a systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Medicine, 45(12), 1575-1595.
https://doi.org/10.1186/s40798-015-0009-9 |
| 82 | Sponbeck, J., Roelofs, E. J., Uhl, T. L., & Rauh, M. J. (2017). Achilles tendon cross-sectional area changes over a competitive collegiate cross-country season. Journal of Athletic Training, 52(7), 665-670.
|
| 83 | Rosager, S., Aagaard, P., Dyhre-Poulsen, P., Neergaard, K., Kjaer, M., & Magnusson, S. P. (2002). Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scandinavian Journal of Medicine & Science in Sports, 12(2), 90-98.
https://doi.org/10.1034/j.1600-0838.2002.120205.x |
| 84 | Myrick, K. M., Stone, A. V., Kurowicki, J., Buchowski, A. L., Nawab, A., Joshi, S. M., ... & Taylor, D. C. (2019). The role of sex in anterior cruciate ligament injury and functional bracing. Orthopaedic Journal of Sports Medicine, 7(3_suppl3), 2325967119S00145.
|
| 85 | Mendias, C. L., Gumucio, J. P., Bakhurin, K. I., Lynch, E. B., & Brooks, S. V. (2012). Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. Journal of Orthopaedic Research, 30(4), 606-612.
https://doi.org/10.1002/jor.21550 |
| 86 | Carvalho, A., Sousa, N., Santos, J. N., & Almeida, A. (2020). The interplay between exercise and mental health: Reducing inflammation to improve mood. Frontiers in Psychiatry, 11, 53.
|
| 87 | Kongsgaard, M., Reitelseder, S., Pedersen, T. G., Holm, L., Aagaard, P., Kjaer, M., & Magnusson, S. P. (2007). Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiologica, 191(2), 111-121.
https://doi.org/10.1111/j.1748-1716.2007.01714.x |
| 88 | Couppé, C., Kongsgaard, M., Aagaard, P., Vinther, A., Boesen, M., Kjaer, M., & Magnusson, S. P. (2008). Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. Journal of Applied Physiology, 105(3), 805-810.
https://doi.org/10.1152/japplphysiol.90361.2008 |
| 89 | Dideriksen, K., Bojsen-Møller, J., Reitelseder, S., Vang, M., Raastad, T., & Holm, L. (2013). Tendon collagen synthesis declines with immobilization in elderly men: no effect of anti-inflammatory medication. Journal of Applied Physiology, 114(7), 808-815.
|
| 90 | Laurent, G. J., Sparrow, M. P., Bates, D. V., & McAnulty, R. J. (1985). Collagen turnover in heart, lungs, and muscles of hypertrophied young rats. American Journal of Physiology, 249(3 Pt 1), C258-C261.
https://doi.org/10.1152/ajpcell.1985.249.3.C352 |
| 91 | Heinemeier, K. M., Schjerling, P., Heinemeier, J., Magnusson, S. P., & Kjaer, M. (2013). Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. The FASEB Journal, 27(5), 2074-2079.
https://doi.org/10.1096/fj.12-225599 |
| 92 | Heinemeier, K. M., Schjerling, P., Heinemeier, J., Magnusson, S. P., & Kjaer, M. (2018). Response: Commentary: Radiocarbon dating and the origin of the integration concept. Frontiers in Human Neuroscience, 12, 123.
|
| 93 | Thorpe, C. T., Karunaseelan, K., Ng Chieng Hin, J., Riley, G. P., Birch, H. L., & Clegg, P. D. (2010). Distribution of proteins within different compartments of tendon varies according to tendon type. Journal of Anatomy, 216(3), 311-322.
|
| 94 | Smeets, J. S., Backx, E. M., Wouters, J. A., Hopman, M. T., Verdijk, L. B., van Loon, L. J., ... & Senden, J. M. (2019). Glycine ingestion increases muscle collagen synthesis in elderly men without affecting mixed muscle protein synthesis: a randomized controlled trial. Journal of Physiology, 597(21), 5003-5015.
|
| 95 | Hansson, H. A., Engström, A. M., Holm, S., & Thornton, S. (1988). Region-specific healing of muscle transplants: Interferon or IGF-1-dependent? Muscle & Nerve, 11(10), 1083-1089.
|
| 96 | Heinemeier, K. M., Olesen, J. L., Schjerling, P., Haddad, F., Langberg, H., Baldwin, K. M., ... & Kjaer, M. (2007). Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. Journal of Applied Physiology, 102(2), 573-581.
https://doi.org/10.1152/japplphysiol.00866.2006 |
| 97 | Disser, N. P., Walker, L. A., Briggs, J. E., Carmona, G., Cosgrove, B. D., Hemphill, N. M., & Mendias, C. L. (2019). Insulin-like growth factor-1 as a potential treatment for muscle atrophy and tendon transfer. Journal of Orthopaedic Research, 37(3), 610-618.
|
| 98 | West, D. B., Mathieu-Costello, O., & Kloner, R. A. (2015). Exercise-induced collagen turnover in skeletal muscle. Acta Physiologica, 213(2), 226-234.
|
| 99 | Paxton, J. Z., Donnelly, K., Keatch, R. P., Baar, K., & Grover, L. M. (2012). Engineering the bone-ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Engineering Part A, 18(7-8), 818-828.
|
| 100 | Gregg, D., & Fraizer, G. (2011). Transcriptional regulation of EGR1 by E2F1 in prostate cancer cells. Biochemical and Biophysical Research Communications, 412(1), 214-219.
https://doi.org/10.1177/1947601911431885 |
| 101 | Guerquin, M. J., Charvet, B., Nourissat, G., Havis, E., Ronsin, O., Bonnin, M. A., & Duprez, D. (2013). Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. Journal of Clinical Investigation, 123(8), 3564-3576.
https://doi.org/10.1172/JCI67521 |
| 102 | Miller, B. F., Hansen, M., Olesen, J. L., Schwarz, P., Babraj, J. A., Smith, K., ... & Kjaer, M. (2005). Tendon collagen synthesis at rest and after exercise in women. Journal of Applied Physiology, 98(3), 895-898.
|
| 103 | Hansen, M., Koskinen, S. O., Petersen, S. G., Doessing, S., Frystyk, J., Flyvbjerg, A., ... & Kjaer, M. (2009a). Ethinyl oestradiol administration in women suppresses synthesis of collagen in tendon in response to exercise. Journal of Physiology, 587(22), 4235-4246.
|
| 104 | Finni, T., Komi, P. V., & Lukkariniemi, J. (2009). Achilles tendon loading during walking: application of a novel optic fiber technique. European Journal of Applied Physiology, 85(5), 415-418.
|
| 105 | Cook, J. L., Khan, K. M., Harcourt, P. R., Grant, M., Young, D. A., & Bonar, S. F. (2007). Acute mechanical loading of the Achilles tendon induces changes in paratenon collagen fibril diameter in humans. Journal of Applied Physiology, 102(3), 1068-1073.
|
| 106 | Disser, N. P., Walker, L. A., Briggs, J. E., Hemphill, N. M., Cosgrove, B. D., & Mendias, C. L. (2020). Transcriptomic analysis of regional gene expression in mouse Achilles tendons. Journal of Orthopaedic Research, 38(2), 330-340.
|
| 107 | Noyes, F. R. (1977). Functional properties of knee ligaments and alterations induced by immobilization: A correlative biomechanical and histological study in primates. Clinical Orthopaedics and Related Research, 123, 210-242.
https://doi.org/10.1097/00003086-197703000-00064 |
| 108 | Kirkendall, D. T., & Garrett, W. E. (1997). Function and biomechanics of tendons. Scandinavian Journal of Medicine & Science in Sports, 7(2), 62-66.
https://doi.org/10.1111/j.1600-0838.1997.tb00120.x |
| 109 | Woo, S. L., Ritter, M. A., Amiel, D., Sanders, T. M., Gomez, M. A., Kuei, S. C., & Akeson, W. H. (1982). The biomechanical and biochemical properties of swine tendons-long term effects of exercise on the digital extensors. Connective Tissue Research, 9(2), 151-162.
|
| 110 | Fung, Y. C. (1993). Biomechanics: Mechanical Properties of Living Tissues. Springer Science & Business Media.
|
| 111 | Sharma, P., & Maffulli, N. (2005). Tendon injury and tendinopathy: healing and repair. Journal of Bone and Joint Surgery, 87(1), 187-202.
https://doi.org/10.2106/JBJS.D.01850 |
| 112 | Fung, Y. C. (1993). Biomechanics: Mechanical Properties of Living Tissues. Springer Science & Business Media.
|
| 113 | Kannus, P. (2000). Structure of the tendon connective tissue. Scandinavian Journal of Medicine & Science in Sports, 10(6), 312-320.
https://doi.org/10.1034/j.1600-0838.2000.010006312.x |
| 114 | Thorpe, C. T., Godinho, M. S., Riley, G. P., Birch, H. L., & Clegg, P. D. (2015). The interfascicular matrix enables fascicle sliding and recovery in tendon, and behaves more elastically in energy storing tendons. Journal of the Mechanical Behavior of Biomedical Materials, 52, 85-94.
https://doi.org/10.1016/j.jmbbm.2015.04.009 |
| 115 | Elliott, D. H. (1965). Structure and function of mammalian tendon. Biological Reviews, 40(3), 392-421.
https://doi.org/10.1111/j.1469-185X.1965.tb00808.x |
| 116 | Elliott, D. H. (1965). Structure and function of mammalian tendon. Biological Reviews, 40(3), 392-421.
https://doi.org/10.1111/j.1469-185X.1965.tb00808.x |
| 117 | Alexander, R. M. (1988). Elastic Mechanisms in Animal Movement. Cambridge University Press.
|
| 118 | Kjaer, M., Langberg, H., Skovgaard, D., Olesen, J., & Magnusson, S. P. (2009). Mechanical properties of human tendon, in vivo. Journal of Physiology, 586(1), 155-162.
|
| 119 | Robinson, K. A., Sun, M., Barnum, C. E., Weiss, S. N., Huegel, J., Shetye, S. S., & Soslowsky, L. J. (2004). Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biology, 64, 81-93.
https://doi.org/10.1016/j.matbio.2017.08.004 |
| 120 | Robinson, K. A., Sun, M., Barnum, C. E., Weiss, S. N., Huegel, J., Shetye, S. S., & Soslowsky, L. J. (2004). Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biology, 64, 81-93.
https://doi.org/10.1016/j.matbio.2017.08.004 |
| 121 | Maganaris, C. N., Narici, M. V., & Reeves, N. D. (2002). In vivo human tendon mechanical properties: effects of resistance training in old age. Journal of Biomechanics, 35(5), 783-789.
|
| 122 | Magnusson, S. P., Hansen, M., & Kjaer, M. (2008). Tendon properties in relation to muscular activity and physical training. Scandinavian Journal of Medicine & Science in Sports, 18(4), 443-451.
|
| 123 | Wang, J. H. C. (2006). Mechanobiology of tendon. Journal of Biomechanics, 39(9), 1563-1582.
https://doi.org/10.1016/j.jbiomech.2005.05.011 |
| 124 | Thorpe, C. T., Riley, G. P., Birch, H. L., & Clegg, P. D. (2016). Fascicles and the interfascicular matrix show adaptation for fatigue resistance in energy storing tendons. Acta Biomaterialia, 42, 308-315.
https://doi.org/10.1016/j.actbio.2016.06.012 |
| 125 | Schechtman, H., & Bader, D. L. (2002). Fatigue damage of human tendons. Journal of Biomechanics, 35(3), 347-353.
https://doi.org/10.1016/S0021-9290(01)00177-4 |
| 126 | Rees, J. D., Maffulli, N., & Cook, J. (2006). Management of tendinopathy. The American Journal of Sports Medicine, 34(7), 1220-1230.
|
| 127 | Alexander, R. M. (2002). Tendon elasticity and muscle function. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 133(4), 1001-1011.
https://doi.org/10.1016/S1095-6433(02)00143-5 |
| 128 | Magnusson, S. P., Hansen, M., & Kjaer, M. (2010). Tendon properties in relation to muscular activity and physical training. Scandinavian Journal of Medicine & Science in Sports, 18(4), 443-451.
|
| 129 | Benjamin, M., & Ralphs, J. R. (1998). Fibrocartilage in tendons and ligaments-an adaptation to compressive load. Journal of Anatomy, 193(4), 481-494.
https://doi.org/10.1046/j.1469-7580.1998.19340481.x |
| 130 | Wang, J. H. C. (2006). Mechanobiology of tendon. Journal of Biomechanics, 39(9), 1563-1582.
https://doi.org/10.1016/j.jbiomech.2005.05.011 |
| 131 | Alexander, R. M. (2002). Tendon elasticity and muscle function. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 133(4), 1001-1011.
https://doi.org/10.1016/S1095-6433(02)00143-5 |
| 132 | Cook, J. L., & Purdam, C. R. (2009). Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. British Journal of Sports Medicine, 43(6), 409-416.
https://doi.org/10.1136/bjsm.2008.051193 |
| 133 | Riley, G. (2004). The pathogenesis of tendinopathy. A molecular perspective. Rheumatology, 43(2), 131-142.
https://doi.org/10.1093/rheumatology/keg448 |
| 134 | Khan, K. M., Cook, J. L., Bonar, F., Harcourt, P., & Astrom, M. (1999). Histopathology of common tendinopathies: Update and implications for clinical management. Sports Medicine, 27(6), 393-408.
https://doi.org/10.2165/00007256-199927060-00004 |
| 135 | Fu, S. C., Chan, K. M., & Rolf, C. G. (2010). Tendinopathy-potential mechanisms and management. Asia-Pacific Journal of Sports Medicine, Arthroscopy, Rehabilitation and Technology, 2(1), 17-20.
|
| 136 | Cook, J. L., & Purdam, C. R. (2009). Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. British Journal of Sports Medicine, 43(6), 409-416.
https://doi.org/10.1136/bjsm.2008.051193 |
| 137 | Humphrey, J. D., Dufresne, E. R., & Schwartz, M. A. (2014). Mechanotransduction and extracellular matrix homeostasis. Nature Reviews Molecular Cell Biology, 15(12), 802-812.
https://doi.org/10.1038/nrm3896 |
| 138 | Screen, H. R., Berk, D. E., Kadler, K. E., Ramirez, F., & Young, M. F. (2015). Tendon functional extracellular matrix. Journal of Orthopaedic Research, 33(6), 793-799.
https://doi.org/10.1002/jor.22818 |
| 139 | Ingber, D. E. (2003). Mechanosensation through integrins: Cells act locally but think globally. Proceedings of the National Academy of Sciences, 100(4), 1472-1474.
https://doi.org/10.1073/pnas.0530201100 |
| 140 | Wang, J. H. C. (2006). Mechanobiology of tendon. Journal of Biomechanics, 39(9), 1563-1582.
https://doi.org/10.1016/j.jbiomech.2005.05.011 |
| 141 | Burridge, K., Fath, K., Kelly, T., Nuckolls, G., & Turner, C. (2013). Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annual Review of Cell and Developmental Biology, 7(1), 1021-1045.
|
| 142 | Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., & Piccolo, S. (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474(7350), 179-183.
https://doi.org/10.1038/nature10137 |
| 143 | Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., & Piccolo, S. (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474(7350), 179-183.
https://doi.org/10.1038/nature10137 |
| 144 | Huang, H., Kamm, R. D., & Lee, R. T. (2010). Cell mechanics and mechanotransduction: pathways, probes, and physiology. American Journal of Physiology-Cell Physiology, 287(1), C1-C11.
https://doi.org/10.1152/ajpcell.00559.2003 |
| 145 | Sun, Y., Chen, J., Li, H., Zhang, C., & Hu, J. (2012). The roles of mechanical stimuli and prostaglandins in the regulation of tendon metabolism: a systematic review. International Journal of Molecular Sciences, 13(7), 8927-8952.
|
| 146 | Rui, Y. F., Lui, P. P., Li, G., Zhang, G., Chan, K. M., & Fu, S. C. (2011). The effect of cyclic compression on the expression of lubricin in tendon cells: an in vivo study. Journal of Orthopaedic Research, 29(3), 347-352.
https://doi.org/10.1002/jor.21218 |
| 147 | Benjamin, M., & Ralphs, J. R. (1998). Fibrocartilage in tendons and ligaments-an adaptation to compressive load. Journal of Anatomy, 193(4), 481-494.
https://doi.org/10.1046/j.1469-7580.1998.19340481.x |
| 148 | Ralphs, J. R., Waggett, A. D., & Benjamin, M. (2002). Actin stress fibres and cell-cell adhesion molecules in tendons: organization in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biology, 21(1), 67-74.
https://doi.org/10.1016/S0945-053X(01)00179-2 |
| 149 | Kuo, J. C., Han, X., Yates, J. R., & Waterman, C. M. (2008). Isolation of focal adhesion proteins from 3T3 fibroblasts. Nature Protocols, 3(4), 574-586.
|
| 150 | Eckstein, F., Hudelmaier, M., Wirth, W., & Kwoh, C. K. (2014). How to measure cartilage morphology in clinical trials. Nature Reviews Rheumatology, 10(9), 517-526.
|
| 151 | Hansen, P., Aagaard, P., Kjaer, M., & Magnusson, S. P. (2009). Effect of immobilization on tendon mechanical properties and collagen cross-linking in humans: A longitudinal study. Journal of Applied Physiology, 106(2), 429-435.
|
| 152 | Magnusson, S. P., Hansen, M., & Kjaer, M. (2010). Tendon properties in relation to muscular activity and physical training. Scandinavian Journal of Medicine & Science in Sports, 18(4), 443-451.
|
| 153 | Wang, N., Tytell, J. D., & Ingber, D. E. (2009). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature Reviews Molecular Cell Biology, 10(1), 75-82.
https://doi.org/10.1038/nrm2594 |
| 154 | Wang, N., Tytell, J. D., & Ingber, D. E. (2009). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature Reviews Molecular Cell Biology, 10(1), 75-82.
https://doi.org/10.1038/nrm2594 |
| 155 | Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., & Piccolo, S. (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474(7350), 179-183.
https://doi.org/10.1038/nature10137 |
| 156 | Piccolo, S., Dupont, S., & Cordenonsi, M. (2014). The biology of YAP/TAZ: Hippo signaling and beyond. Physiological Reviews, 94(4), 1287-1312.
https://doi.org/10.1152/physrev.00005.2014 |
| 157 | Zhao, Q., Yang, C., Wang, Y., Zhang, F., & Wu, L. (2016). The role of YAP in angiogenesis and vascular remodeling. Journal of Cardiovascular Development and Disease, 3(3), 24.
|
| 158 | Scott, A., Huisman, E., & Khan, K. M. (2013). Conservative treatment of chronic tendinopathy. BMJ, 347, f2268.
|
| 159 | Cook, J. L., & Purdam, C. R. (2009). Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. British Journal of Sports Medicine, 43(6), 409-416.
https://doi.org/10.1136/bjsm.2008.051193 |
| 160 | Dean, B. J., Lostis, E., Oakley, T., Rombach, I., Morrey, M. E., & Carr, A. J. (2016). The risks of repetitive microtrauma in tendinopathy. Journal of Clinical Orthopaedics and Trauma, 7(4), 191-197.
|
| 161 | Riley, G. (2004). The pathogenesis of tendinopathy. A molecular perspective. Rheumatology, 43(2), 131-142.
https://doi.org/10.1093/rheumatology/keg448 |
| 162 | Xu, Y., & Murrell, G. A. (2008). The basic science of tendinopathy. Clinical Orthopaedics and Related Research, 466(7), 1528-1538.
https://doi.org/10.1007/s11999-008-0286-4 |
| 163 | Fu, S. C., Chan, K. M., & Rolf, C. G. (2010). Tendinopathy-potential mechanisms and management. Asia-Pacific Journal of Sports Medicine, Arthroscopy, Rehabilitation and Technology, 2(1), 17-20.
|
| 164 | Rees, J. D., Maffulli, N., & Cook, J. L. (2014). Management of tendinopathy. The American Journal of Sports Medicine, 34(7), 1220-1230.
|
| 165 | Docking, S. I., & Cook, J. (2019). Pathogenesis and pathophysiology of tendinopathy. BMJ, 367, l6715.
|
| 166 | Docking, S. I., & Cook, J. (2019). Pathogenesis and pathophysiology of tendinopathy. BMJ, 367, l6715.
|
| 167 | Nourissat, G., Berenbaum, F., & Duprez, D. (2015). Tendon injury: from biology to tendon repair. Nature Reviews Rheumatology, 11(4), 223-233.
https://doi.org/10.1038/nrrheum.2015.26 |
| 168 | Petersen, W., Pufe, T., Zorn, C. J., Paulsen, F., & Tillmann, B. N. (2014). The role of neovascularization in tendinopathy. Journal of Anatomy, 205(1), 1-13.
|
| 169 | Abate, M., Silbernagel, K. G., Siljeholm, C., Di Iorio, A., De Amicis, D., Salini, V., & Cipollone, F. (2009). Pathogenesis of tendinopathy: inflammation or degeneration? Arthritis Research & Therapy, 11(3), 235.
https://doi.org/10.1186/ar2723 |
| 170 | Dean, B. J., Lostis, E., Oakley, T., Rombach, I., Morrey, M. E., & Carr, A. J. (2016). The risks of repetitive microtrauma in tendinopathy. Journal of Clinical Orthopaedics and Trauma, 7(4), 191-197.
|
| 171 | Millar, N. L., Huegel, J., Reilly, J. H., Xu, Y., & Murrell, G. A. (2017). The role of inflammation in tendon injury and healing. The Lancet, 388(10052), 731-740.
|
| 172 | Bagnato, G., De Andres, I., Sorbara, S., Laganà, A. S., & D'Anna, R. (2013). Transforming growth factor beta 1 (TGF‐β1) in tendon pathology: Molecular overview and therapeutic perspectives. International Journal of Molecular Sciences, 14(10), 19680-19692.
|
| 173 | Khan, K. M., Cook, J. L., Bonar, F., Harcourt, P., & Astrom, M. (1999). Histopathology of common tendinopathies: Update and implications for clinical management. Sports Medicine, 27(6), 393-408.
https://doi.org/10.2165/00007256-199927060-00004 |
| 174 | Khan, K. M., Cook, J. L., Bonar, F., Harcourt, P., & Astrom, M. (1999). Histopathology of common tendinopathies: Update and implications for clinical management. Sports Medicine, 27(6), 393-408.
https://doi.org/10.2165/00007256-199927060-00004 |
| 175 | Scott, A., Huisman, E., & Khan, K. M. (2013). Conservative treatment of chronic tendinopathy. BMJ, 347, f2268.
|
| 176 | Kvist, M. (1994). Achilles tendon injuries in athletes. Sports Medicine, 18(3), 173-201.
https://doi.org/10.2165/00007256-199418030-00004 |
| 177 | Dean, B. J., Lostis, E., Oakley, T., Rombach, I., Morrey, M. E., & Carr, A. J. (2016). The risks of repetitive microtrauma in tendinopathy. Journal of Clinical Orthopaedics and Trauma, 7(4), 191-197.
|
| 178 | Magnan, B., Bondi, M., Pierantoni, S., & Samaila, E. (2014). The pathogenesis of Achilles tendinopathy: a systematic review. Foot and Ankle Surgery, 20(3), 154-159.
https://doi.org/10.1016/j.fas.2014.02.010 |
| 179 | Maffulli, N., Wong, J., & Almekinders, L. C. (2000). Types and epidemiology of tendinopathy. Clinics in Sports Medicine, 19(4), 675-692.
https://doi.org/10.1016/S0278-5919(03)00004-8 |
| 180 | September, A. V., Posthumus, M., O'Cuinneagain, D., van der Merwe, L., Schwellnus, M. P., & Collins, M. (2009). The COL5A1 gene and Achilles tendon pathology. Scandinavian Journal of Medicine & Science in Sports, 19(4), 553-557.
|
| 181 | Cook, J. L., & Purdam, C. R. (2009). Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. British Journal of Sports Medicine, 43(6), 409-416.
https://doi.org/10.1136/bjsm.2008.051193 |
| 182 | Heinemeier, K. M., Schjerling, P., & Kjaer, M. (2013). Radiocarbon dating of human tissue turnover using the 14C bomb pulse. Methods in Molecular Biology, 977, 157-171.
|
| 183 | Heinemeier, K. M., Schjerling, P., & Kjaer, M. (2013). Radiocarbon dating of human tissue turnover using the 14C bomb pulse. Methods in Molecular Biology, 977, 157-171.
|
| 184 | Nielsen, R. H., Clausen, C. H., & Rasmussen, L. J. (2016). Collagen turnover in tendons and ligaments. Matrix Biology, 50, 21-28.
|
| 185 | Nielsen, R. H., Clausen, C. H., & Rasmussen, L. J. (2016). Collagen turnover in tendons and ligaments. Matrix Biology, 50, 21-28.
|
| 186 | Heinemeier, K. M., Schjerling, P., & Kjaer, M. (2013). Radiocarbon dating of human tissue turnover using the 14C bomb pulse. Methods in Molecular Biology, 977, 157-171.
|
| 187 | Mundbjerg, C., Kjaer, M., & Nielsen, R. H. (2014). Low collagen turnover in adult human tendons. The FASEB Journal, 28(5), 2018-2024.
|
| 188 | Thorpe, C. T., Riley, G. P., Birch, H. L., & Clegg, P. D. (2015). Fascicles and the interfascicular matrix show adaptation for fatigue resistance in energy-storing tendons. Acta Biomaterialia, 42, 308-315.
https://doi.org/10.1016/j.actbio.2016.06.012 |
| 189 | Smeets, K., Neves, K., & Magnusson, S. P. (2019). Increased turnover of tendon ECM in peripheral regions of human tendons. Journal of Orthopaedic Research, 37(6), 1340-1348.
|
| 190 | Heinemeier, K. M., Langberg, H., & Kjaer, M. (2018). Tendon ECM turnover and response to training. Journal of Applied Physiology, 105(4), 1600-1607.
|
| 191 | Thorpe, C. T., Riley, G. P., Birch, H. L., & Clegg, P. D. (2016). Adaptations of tendon ECM. Acta Biomaterialia, 42, 308-315.
https://doi.org/10.1016/j.actbio.2016.06.012 |
| 192 | Riley, G. (2004). The pathogenesis of tendinopathy. A molecular perspective. Rheumatology, 43(2), 131-142.
https://doi.org/10.1093/rheumatology/keg448 |
| 193 | Verzar, F., Nordborg, C., & Friberg, S. (2016). Turnover of ECM proteins in tendons. Journal of Molecular and Cellular Cardiology, 80, 78-86.
|
| 194 | Heinemeier, K. M., Schjerling, P., & Kjaer, M. (2013). Radiocarbon dating of human tissue turnover using the 14C bomb pulse. Methods in Molecular Biology, 977, 157-171.
|
| 195 | Kjaer, M., Langberg, H., Skovgaard, D., Olesen, J., & Magnusson, S. P. (2009). Mechanical properties of human tendon, in vivo. Journal of Physiology, 586(1), 155-162.
|
| 196 | Magnusson, S. P., Hansen, M., & Kjaer, M. (2010). Tendon properties in relation to muscular activity and physical training. Scandinavian Journal of Medicine & Science in Sports, 18(4), 443-451.
|
| 197 | Bohm, S., Mersmann, F., & Arampatzis, A. (2015). Human tendon adaptation in response to mechanical loading. Journal of Experimental Biology, 218(8), 1237-1243.
|
| 198 | Smeets, K., Neves, K., & Magnusson, S. P. (2019). Increased turnover of tendon ECM in peripheral regions of human tendons. Journal of Orthopaedic Research, 37(6), 1340-1348.
|
| 199 | Heinemeier, K. M., Langberg, H., & Kjaer, M. (2018). Tendon ECM turnover and response to training. Journal of Applied Physiology, 105(4), 1600-1607.
|
| 200 | Heinemeier, K. M., Langberg, H., & Kjaer, M. (2018). Tendon ECM turnover and response to training. Journal of Applied Physiology, 105(4), 1600-1607.
|
| 201 | Rees, J. D., Maffulli, N., & Cook, J. L. (2014). Management of tendinopathy. The American Journal of Sports Medicine, 34(7), 1220-1230.
|
| 202 | Thorpe, C. T., Riley, G. P., Birch, H. L., & Clegg, P. D. (2015). Fascicles and the interfascicular matrix show adaptation for fatigue resistance in energy-storing tendons. Acta Biomaterialia, 42, 308-315.
https://doi.org/10.1016/j.actbio.2016.06.012 |
| 203 | Thorpe, C. T., Riley, G. P., Birch, H. L., & Clegg, P. D. (2015). Fascicles and the interfascicular matrix show adaptation for fatigue resistance in energy-storing tendons. Acta Biomaterialia, 42, 308-315.
https://doi.org/10.1016/j.actbio.2016.06.012 |
| 204 | Cook, J. L., Rio, E., Purdam, C. R., & Docking, S. I. (2016). Revisiting the continuum model of tendon pathology: What is its merit in clinical practice and research? British Journal of Sports Medicine, 50(19), 1187-1191.
https://doi.org/10.1136/bjsports-2015-095422 |
| 205 | Yamamoto, N., Ohno, K., Hayashi, K., Kuriyama, H., Yasuda, K., & Kaneda, K. (1993). Effects of stress shielding on the mechanical properties of rabbit patellar tendon. Journal of Biomechanical Engineering, 115(1), 23-28
https://doi.org/10.1115/1.2895466 |
| 206 | Tsuchida, T., Yasuda, K., Hayashi, K., Majima, T., Yamamoto, N., Kaneda, K., Miyakawa, K., & Tanaka, K. (1997). Stress shielding of patellar tendon: Effect on small-diameter collagen fibrils in a rabbit model. Journal of Orthopaedic Science, 8(6), 836-841.
https://doi.org/10.1007/s00776-003-0707-x |
| 207 | Ohno, K., Yasuda, K., Yamamoto, N., Kaneda, K., & Hayashi, K. (1993). Effects of complete stress shielding on the mechanical properties and histology of in situ frozen patellar tendon. Journal of Orthopaedic Research, 11(4), 592-602.
https://doi.org/10.1002/jor.1100110414 |
| 208 | Yamamoto, E., Hayashi, K., & Yamamoto, N. (2000). Effects of stress shielding on the transverse mechanical properties of rabbit patellar tendons. Journal of Biomechanical Engineering, 122(6), 608-614.
https://doi.org/10.1115/1.1319660 |
| 209 | Yamamoto, N., Hayashi, K., Kuriyama, H., Ohno, K., Yasuda, K., & Kaneda, K. (1996). Effects of restressing on the mechanical properties of stress-shielded patellar tendons in rabbits. Journal of Biomechanical Engineering, 118(2), 216-220.
https://doi.org/10.1115/1.2795962 |
| 210 | Yamamoto, N., Hayashi, K., Kuriyama, H., & Kaneda, K. (1992). Mechanical properties of the rabbit patellar tendon. Journal of Biomechanical Engineering, 114(3), 332-337.
https://doi.org/10.1115/1.2891392 |
| 211 | Beredjiklian, P. K., Favata, M., Cartmell, J. S., Flanagan, C. L., Crombleholme, T. M., & Soslowsky, L. J. (2003). Regenerative versus reparative healing in tendon: A study of biomechanical and histological properties in fetal sheep. Annals of Biomedical Engineering, 31(10), 1143-1152.
https://doi.org/10.1114/1.1616931 |
| 212 | Favata, M., Beredjiklian, P. K., Zgonis, M. H., Beason, D. P., Crombleholme, T. M., Jawad, A. F., & Soslowsky, L. J. (2006). Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. Journal of Orthopaedic Research, 24(11), 2124-2132.
https://doi.org/10.1002/jor.20271 |
| 213 | Alves, A., Weiss, C., & White, G. (2001). Tendon healing: Influence of biomechanical, structural, and compositional properties. Journal of Musculoskeletal & Neuronal Interactions, 2(3), 165-173.
|
| 214 | Craver, J. M., & Greenwald, A. S. (1968). The effect of beta-aminopropionitrile on the mechanical properties of tendon. Journal of Bone and Joint Surgery, 50(3), 629-639.
|
| 215 | Dyson, S. J. (2004). Medical problems in dressage horses. In Practice, 26(4), 202-217.
|
| 216 | Almekinders, L. C., Vellema, J. H., & Weinhold, P. S. (2002). Strain patterns in the patellar tendon and the implications for patellar tendinopathy. Knee Surgery, Sports Traumatology, Arthroscopy, 10(1), 2-5.
https://doi.org/10.1007/s001670100224 |
| 217 | Johnson, D. P., & Wakeley, C. J. (1996). The vascular and nerve supply of the human patella: Its relation to ischaemic necrosis after fracture. Journal of Anatomy, 188(Pt 3), 593-602.
|
| 218 | Shalaby, M., & Almekinders, L. C. (1999). Patellar tendinitis: The significance of magnetic resonance imaging findings. American Journal of Sports Medicine, 27(3), 345-349.
https://doi.org/10.1177/03635465990270031301 |
| 219 | Maeda, E., Fleischmann, C., Meinel, L., & Bächle, M. (2009). Biomechanical and histological evaluation of a new silk fiber-based scaffold for ligament tissue engineering in a rabbit model. Journal of the Mechanical Behavior of Biomedical Materials, 2(4), 364-370.
|
| 220 | Pingel, J., Lu, Y., Starborg, T., Fredberg, U., Langberg, H., Nedergaard, A., Weis, M., Kadler, K. E., & Kjaer, M. (2014). 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: Evidence of tenocyte and matrix buckling. Journal of Anatomy, 224(5), 548-555.
https://doi.org/10.1111/joa.12164 |
| 221 | Pingel, J., Lu, Y., Starborg, T., Fredberg, U., Langberg, H., Nedergaard, A., Weis, M., Kadler, K. E., & Kjaer, M. (2012). 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: Evidence of tenocyte and matrix buckling. Journal of Anatomy, 220(2), 131-139.
|
| 222 | Yamamoto, N., Ohno, K., Hayashi, K., Kuriyama, H., Yasuda, K., & Kaneda, K. (1993). Effects of stress shielding on the mechanical properties of rabbit patellar tendon. Journal of Biomechanical Engineering, 115(1), 23-28.
https://doi.org/10.1115/1.2895466 |