Original Article - DOI:10.33594/000000748
Accepted 20 December 2024 - Published online 28 December 2024
Exploration the Effects of Mesenchymal Stem Cells and Olive Leaf Extract on Physiological and Histopathological Changes on the Kidney of Diabetes Rats
bZoology Department, Faculty of Science (Boys), Al-Azhar University, Cairo 11884, Egypt,
cBiology Department, College of Science, University of Jeddah, Jeddah 23218, Saudi Arabia,
dOlive Research Center, Common First Year Dean’s Office, Jouf University, El Jouf, Saudi Arabia,
eDepartment of Biology, College of Science, Princess Nourah bint Abdulrahman University, 13225 Riyadh, Saudi Arabia,
fDepartment of Biological Sciences, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
Keywords
Abstract
Background/Aims:
Gestational Diabetes Mellitus (GDM) is a common complication during pregnancy, defined as diabetes diagnosed in the second or third trimester, often asymptomatic. This study investigates the therapeutic potential of olive leaf extracts and stem cells in mitigating GDM-induced complications, particularly focusing on renal function, oxidative stress, and pancreatic cell regeneration.Methods:
Measurements were made in gravid female rats with or without intraperitoneal administration of Streptozotocin (35 mg/kg body weight). Biochemical analyses were conducted to evaluate renal function markers (urea, uric acid, creatinine) and oxidative stress parameters (malondialdehyde, glutathione, and superoxide dismutase levels). Histopathological and immunohistopathological evaluations of kidney tissues were performed using hematoxylin and eosin staining and specific markers (p53, Insulin, and PCNA) to assess cellular changes.Results:
The diabetic group exhibited significantly elevated levels of urea, uric acid, and creatinine (p<0.01) compared to the control group. Treatment with stem cells and olive leaf extracts significantly reduced these levels. Malondialdehyde levels were elevated in the diabetic group (p<0.01) but showed marked improvement in the treatment groups. Additionally, glutathione and superoxide dismutase activities were diminished in the diabetic rats (p<0.05) but increased following treatment. Histopathological and immunohistopathological analyses revealed cellular regeneration and improved tissue morphology in the treatment groups compared to the diabetic group.Conclusion:
Stem cells and olive leaf extracts exhibit significant therapeutic potential in ameliorating renal dysfunction, oxidative stress, and tissue damage associated with GDM, highlighting their role in enhancing pancreatic cell regeneration.Introduction
Hyperglycemia and poor metabolism of proteins, fats, and carbohydrates are hallmarks of diabetes mellitus (DM), a chronic metabolic illness. It currently affects 382 million individuals, and by 2035, that figure may rise to 592 million (Martiniakova et al., 2024). One major issue affecting public health worldwide is diabetes (Liu et al., 2021). By 2045, there will likely be over 783.2 million diabetic patients worldwide, up from 536.6 million in 2021 (Du et al., 2022). One of the main clinical risk factors for a few conditions, such as retinopathy, poor blood flow, cardiovascular and renal illness, is hyperglycemia in people with type 2 diabetes mellitus (T2DM) (Huang et al., 2024; Ríos et al., 2015). The most prevalent causes of T2DM pathophysiology are believed to be disruptions in insulin secretion, sensitivity to tissue insulin effects, or both (Chaudhury et al., 2017). Type 2 diabetes mellitus (T2DM) is predominantly characterized by tissue insulin resistance, ultimately progressing to a complete loss of secretory activity in pancreatic cells. It is crucial to address these global health challenges by identifying more efficacious treatments and state-of-the-art preventive measures. Notably, there is a growing demand from patients for the utilization of natural products, driven by the adverse effects associated with oral hypoglycemic medications and insulin (Archana & Sudheesh, 2024; Mir, 2024). Gestational diabetes mellitus (GDM), a prevalent complication occurring during pregnancy, is characterized by the American Diabetes Association as diabetes that manifests not in the early stages but rather in the second or third trimester (Maor-Sagie et al., 2024). As we strive for advancements in therapeutic strategies, the exploration of innovative approaches becomes paramount in mitigating the impact of T2DM and GDM on public health. To meet the fetus’s energy needs, moms go through a few metabolic changes during pregnancy (Marshall et al., 2022). To provide the fetus with more glucose, resistance to insulin increases. A normoglycemic condition is therefore maintained because of pancreatic beta cells compensating for the increased glucose demand. On the other hand, women with GDM experience deficiencies in the responsiveness of their beta cells, which results in inadequate insulin secretion and hyperglycemia (Eng et al., 2024). Previous research has shown that inflammation resolution failure may be the cause of diabetes and its related consequences (Alharbi, 2024). Because normal functioning parenchyma is packed with extracellular matrix (ECM) and the kidneys’ capacity for regeneration and repair is disordered, the development of renal fibrosis also implies a failure of resolution (Yin et al., 2024). Diabetic nephropathy (DN), a hazardous consequence of diabetes mellitus, is the primary cause of end-stage renal disease with a fatality rate of 30–40%. (Varra et al., 2024). Renal fibrosis and increasing renal dysfunction are the hallmarks of diabetic kidney disease (DN) (Wetzel et al., 2020). Considering that the present treatments, like blood pressure and glycemic management, may only partially slow the progression of DN, a novel therapeutic approach to battle DN is critically needed. Many anti-obesity and anti-diabetic medications, including rimonabant, sibutramine, and orlistat, have been produced. Numerous alternatives are put out considering their grave negative effects (Rahman et al., 2022).
Owing to the extensive botanical richness and diversity of plant species endowed by nature, medicinal plants constitute a significant reservoir of global economic value (Ciocan et al., 2023; Shukla et al., 2021). Alkaloids, flavonoids, tannins, terpenoids, and phenolics emerge as prominent chemical constituents exerting specific physiological effects on the human body, underpinning the medicinal properties of plants (El-Beltagi & Badawi, 2013). These compounds exhibit potential anti-inflammatory, hypocholesterolemic, antihypertensive, hypoglycemic, and antioxidant attributes.
The olive tree (Olea europaea) stands out as a preeminent botanical entity employed in the treatment of diabetes. Across European and Mediterranean regions, the leaves of the olive tree (Olea europaea L.) have enjoyed a longstanding tradition as natural remedies (Wainstein et al., 2012). Rich in various potentially advantageous components, these leaves have been incorporated into the human diet in the form of extracts, powders, and herbal teas (Ronca et al., 2024; Wainstein et al., 2012). The biological activity of byproduct extracts from the olive tree can be ascribed to phenolic and antioxidant elements, notably tyrosol, hydroxytyrosol, oleuropeinaglycone, and oleuropein (Mir-Cerdà et al., 2024; Totaro et al., 2024). Olive and oil production emanates from the Mediterranean species Olea europaea L. (Oleaceae). Traditional applications of olive leaves encompass the treatment of flatulence, diarrhea, and diabetes, with their phenolic content being pivotal to their medicinal efficacy (Gonçalves et al., 2024).
The physiological impact on the human body is evident, with alkaloids, flavonoids, tannins, terpenoids, and phenolics emerging as the most noteworthy constituents (Ronca et al., 2024). These compounds exhibit diverse properties, including anti-inflammatory, hypocholesterolemic, antihypertensive, hypoglycemic, and antioxidant effects (Pandey et al., 2024). In investigations involving alloxan and streptozotocin-induced diabetic rats, the Olive Leaf Extract (OLE) and its active components—oleuropein, hydroxytyrosol, apigenin, luteolin, and luteolin-7-O-glucoside—have been explored for their potential as diabetes-preventive agents (Acar-Tek & Ağagündüz, 2020; Soliman et al., 2019). Previous research has probed the impact of oleuropein on glucose absorption in muscle cells and its potential to enhance insulin sensitivity (Hadrich et al., 2016). Oleuropein, notably found in olive leaves, has been associated with various pharmacological benefits, such as reduced risk of coronary heart disease (Fitó et al., 2008), anti-inflammatory (Laaboudi, Ghanam, Ghoumari, et al., 2016), antitumor, anti-proliferative (Ghanam et al., 2015), antidiabetic (Bouallagui et al., 2011), antibacterial, and antifungal properties (Medina et al., 2006). These effects, supported by numerous studies, are often attributed to the antioxidant characteristics of polyphenols, including oleuropein, which has been linked to improved glucose metabolism and normalized cardiovascular indicators. Thus, olive leaf extract emerges as a promising candidate for managing diabetes and mitigating associated cardiovascular complications.
Olive leaf extracts are recognized for their pharmacological properties, particularly in relation to antidiabetic and anti-obesity effects (Medina et al., 2006). The initial discovery of Mesenchymal Stem Cells (MSCs), a subset of pluripotent stem cells, was credited to FriedenStein et al. (Friedenstein et al., 1966; Wu et al., 2016). The term “mesenchymal” denotes cells originating in the embryonic stage. Initially known as bone marrow stromal cells or fibroblast colony-forming units, “mesenchymal stem cells” possess the ability to differentiate into various mesodermal tissues (Caplan, 1991).
The differentiation potential of mesenchymal stem cells (MSCs) is influenced by the cultural environment, amplification conditions, and the origin of the stem cells. The induction of differentiation can be facilitated by specific hormones, growth factors, or differentiation agents (Almalki & Agrawal, 2016). This process is intricately regulated by a dynamic interplay between genetic and epigenetic factors. Epigenetic elements include histone modification, DNA methylation, and altered expression of non-coding RNA, while genetic factors involve specific transcription factors and signaling molecules (Yang et al., 2021).
Stem cells are primarily characterized by their ability to undergo multi-differentiation and self-renewal, originating from various sources. Furthermore, MSCs contribute to tissue regeneration by secreting cytokines and growth factors, thereby attracting other cells to the site of injury (Guillamat-Prats, 2021). Notably, mesenchymal stem cells exhibit anti-inflammatory properties, promote angiogenesis and re-epithelialization, and play a role in immune modulation, collectively contributing to their therapeutic potential (Yu et al., 2023).
Recent research has been done on MSCs in the treatment of DM. Although it has demonstrated its attractive qualities to enhance renal function and prevent fibrosis in animal models, the underlying mechanisms are still unknown (Yamashita & Kramann, 2024).
Materials and Methods
Animal
Eight- to nine-week-old female albino rats, weighing between 150 and 170 g, were obtained from VACSERA Co., Egypt. The rats were individually housed in a temperature-controlled environment at 25°C, maintaining a 12-hour light-dark cycle (Kumar et al., 2016). The light-dark cycle was structured to consist of 12 hours of light exposure for the control group and 18 hours of light followed by 4 hours of darkness for the gestational diabetes mellitus (GDM) group—a condition known to be associated with oxidative stress (Abdel-Reheim et al., 2014). Stringent ethical and welfare standards were observed, as sanctioned by the Institutional Animal Care and Use Committee (IACUC) at Fayoum University, Egypt, in accordance with the guidelines set forth by the National Institute of Health (NIH).
Olive Leaf Extract (OLE) Preparation
The Olive Leaf Extract (OLE) was methodically synthesized from samples of olive tree leaves, following the procedures delineated by Giacometti et al. (Giacometti et al., 2018)The resulting dry residue was precisely quantified and subsequently dissolved in water. The resultant solution was then stored at -20°C, awaiting utilization as a therapeutic agent.
Isolation and Characterization of Mesenchymal Stem Cells from Bone Marrow (BM-MSCs)
Mesenchymal stem cells (BM-MSCs) were obtained from the bone marrow of four-week-old rats following established protocols. Briefly, the rats were euthanized in accordance with ethical guidelines, and their femurs and tibiae were extracted and thoroughly cleaned to eliminate extraneous muscle and connective tissue. Subsequent to rinsing, the bone marrow was cultured in a low-glucose DMEM solution supplemented with 10% fetal bovine serum (FBS, Invitrogen Australia Pty Ltd., Mount Waverley, Victoria, Australia), and 1% penicillin/streptomycin. Following epiphysis removal, the bone marrow was incubated in a humidified environment with 5% CO2 at 37°C. The culture medium was renewed biweekly until cells reached an approximate confluence of 80%. BM-MSCs from the third and fourth passages were utilized for all experiments.BM-MSCs in their third passage underwent a meticulous series of procedures, including washing, purification, and characterization based on specific cell markers assessed using a flow cytometer. To ensure the exclusive examination of viable cells, forward and side scatter measurements were employed to exclude deceased cells and debris. Each sample underwent gating to encompass over 10, 000 events (El-Sayed et al., 2023; Wang Ying-hui, 2014).
Experimental Design
The experimental protocol encompassed the utilization of 50 gravid female rats subjected to investigation through vaginal smear examination to ascertain the phases of the estrous cycle. Rats displaying deviations from the typical 4-day estrous cycle pattern were excluded from the study. Subsequently, the remaining rats were randomly allocated into five groups, each comprising ten individuals: The normal pregnant group. The gestationally diabetic rats, denoted as “GD,” underwent a singular intraperitoneal (ip) administration of Streptozotocin (STZ) at a concentration of 35 mg/kg body weight on day 0. Evaluation of blood glucose levels occurred 72 hours post-STZ administration to identify rats manifesting stable hyperglycemia [50]. The gestationally diabetic assembly treated with mesenchymal stem cells, designated as “GD + MSCs,” involved the injection of (1x106) bone marrow-derived mesenchymal stem cells (BM-MSCs) suspended in 500 µL phosphate-buffered saline (PBS) via caudal vein administration in pregnant rats. The group of pregnant individuals with diabetes subjected to olive leaf extract intervention, hereinafter referred to as “GD + OLE,” received a dose of 200 mg extract per kilogram of body weight.The gestationally diabetic group treated with mesenchymal stem cells and olive leaf extract (referred to as “GD + OLE + MSCs”).
Blood Sample Collection
On the 21st day of the experiment, blood samples were obtained through jugular plexus veins and cardiac puncture for subsequent biochemical analyses. Serum was collected using standard tubes, whereas plasma was acquired using tubes containing heparin. Coagulation in the standard tubes was allowed to proceed for 30 minutes. Subsequently, both types of tubes underwent centrifugation at 2500 rpm for 12 minutes at a temperature of -4°C. The resultant serum and plasma were then carefully transferred to microcentrifuge tubes and stored at -80°C for subsequent analyses.
Histopathological Investigation
Paraffin sections of kidney tissue 4–5 μm thickness were obtained and stained using hematoxylin and eosin (Suvarna et al., 2018). The stained sections were meticulously examined for circulatory disturbances, inflammation, degenerations, apoptosis, necrosis, and any other pathological changes present in the examined tissues.
Immunohistochemical Investigation
The tissue sections underwent microwave treatment, and a two-step immunostaining procedure was implemented to validate the presence of antigens within the tissues. Initially, the primary antibody was conjugated to the respective antigen, followed by visualization utilizing a biotin-streptavidin (BSA) system (Hirsch et al., 2002). Hematoxylin was employed for counterstaining, and the permanent preparation utilized diamethylbenzidine (DAB). Paraffin slices, with a thickness of five microns (Biogenex, Fremont, CA, USA), were affixed to positively charged glass slides. Subsequently, the paraffin sections underwent sequential immersion in Xylene overnight and a series of ethanol concentrations (50%, 75%, 95%, and 100%). Slides were dried post-buffer removal. A singular drop of primary monoclonal antibodies targeting insulin, PCNA, and P53 antigens with heightened sensitivity was administered onto the sections. After a 60-minute incubation, slides were rinsed for 5 minutes in PBS. Two drops of DAKO EnVision were applied for 20 minutes, followed by PBS rinsing. Diaminobenzidine (DAB) chromogen was applied for 10–20 minutes until achieving a desirable brown color, and slides were washed to eliminate excess DAB. Mayer’s hematoxylin (Hx) facilitated nuclear counterstaining, with sections placed in Hx solution for 3–5 minutes, washed in tap water, and differentiated in acid-alcohol before a final tap water wash. Air-dried slides were mounted with Canada balsam. For myeloperoxidase immunohistochemistry, antigen extraction involved applying heat to the slides in a pressure cooker containing Tris-buffered saline with 0.075% Tween-20 (pH 7.6) for 10 minutes (Suvarna et al., 2018). The samples then underwent a 20-minute incubation in 0.3% v/v H2O2 in methanol at room temperature to inhibit endogenous peroxidase activity (Hsu et al., 1981). The sections were incubated at room temperature for 30 minutes with a polyclonal rabbit anti-human myeloperoxidase antibody (diluted 1:1500) and subsequently stained. Immunostaining was conducted using an avidin-biotin-horseradish peroxidase system (Vector Laboratories, Burlingame, CA) with 3-amino-9-ethyl carbazole as the chromogen for myeloperoxidase and diaminobenzidine for CD68 (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
Statistical analysis
The data underwent analysis through SPSS Statistics 23.0. Descriptive statistics were employed for data summarization. Paired t-tests facilitated two-group comparisons, while the ANOVA test was applied for comparing multiple groups in the case of normally distributed data. Conversely, related sample Wilcoxon signed-rank tests were utilized for non-normally distributed data.
Results
The Effect of OLE , BM-MSC and their combination on renal functions markers
The diabetic rats exhibited a significant elevation (p < 0.05) in the renal function, including urea, uric acid, and creatinine compared to the control groups . Conversely, the diabetic group subjected to interventions involving the administration of olive oil, stem cells, or a combination thereof demonstrated a significant reduction in these parameters, indicating a mitigating effect on renal function (Table 1 & Fig. 1).
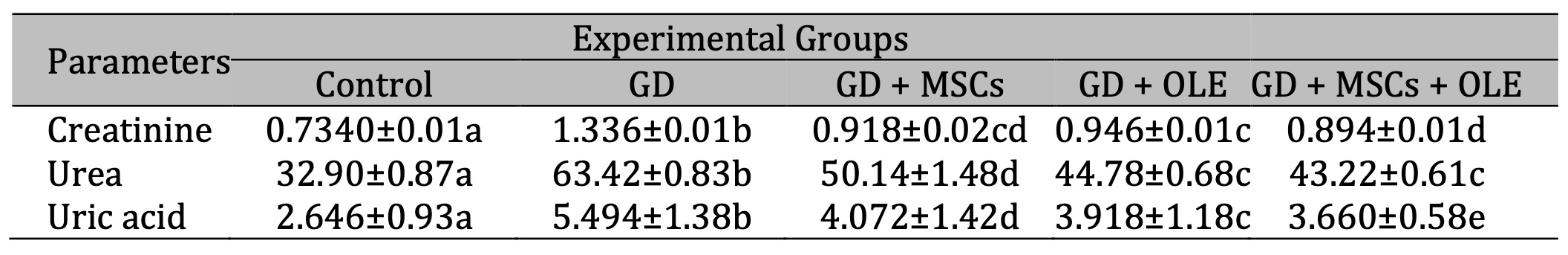
Table 1: Effects of olive leaf extract (OLE), mesenchymal stem cells (MSC) and their combination on kidney functions of gestational diabetic rats. Each value depicted signifies the mean of five records with a standard error of measurement. The use of identical letters (a, b, c, d, e) to represent groups signifies their lack of statistically significant variance. Conversely, different letters signify a significant variation
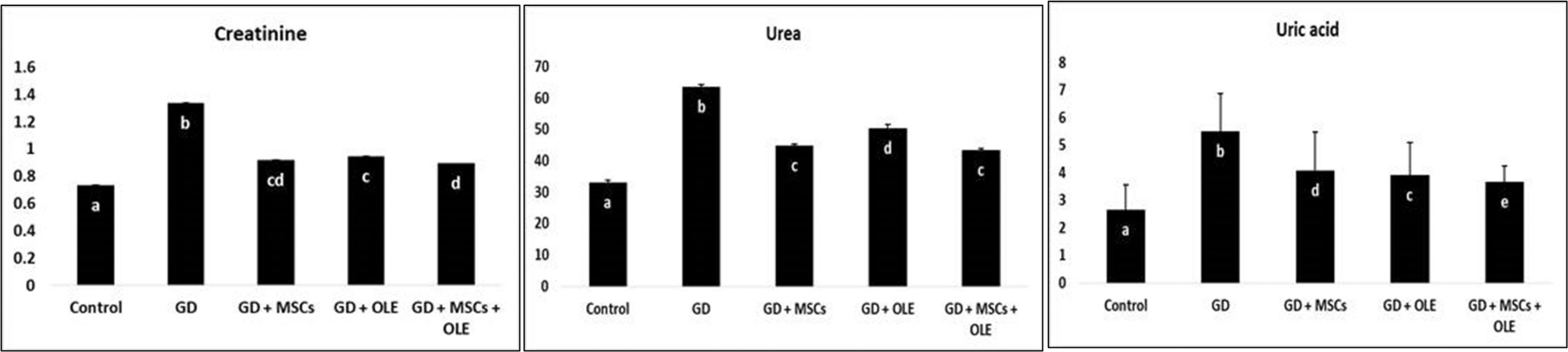
Fig. 1: Effects of olive leaf extract (OLE), mesenchymal stem cells (MSC) and their combination on kidney functions of gestational diabetic rats.
The Effect of OLE , BM-MSC and their combination on renal oxidative markers
A significant statistically increase (p < 0.05) were noted in malondialdehyde levels within the group treated by STZ alone. In contrast, the administration of a combined treatment comprising olive extract and stem cells led to a considerable reduction in malondialdehyde levels in diabetic rats. Furthermore, a comparative analysis between the diabetic rats and the control group unveiled a noteworthy decrease in renal glutathione (GSH) and superoxide dismutase (SOD) levels among the diabetic rats. The assessment of GSH and SOD activity was conducted post-treatment and juxtaposed against the diabetes group for evaluation (Table 2 & Fig. 2).
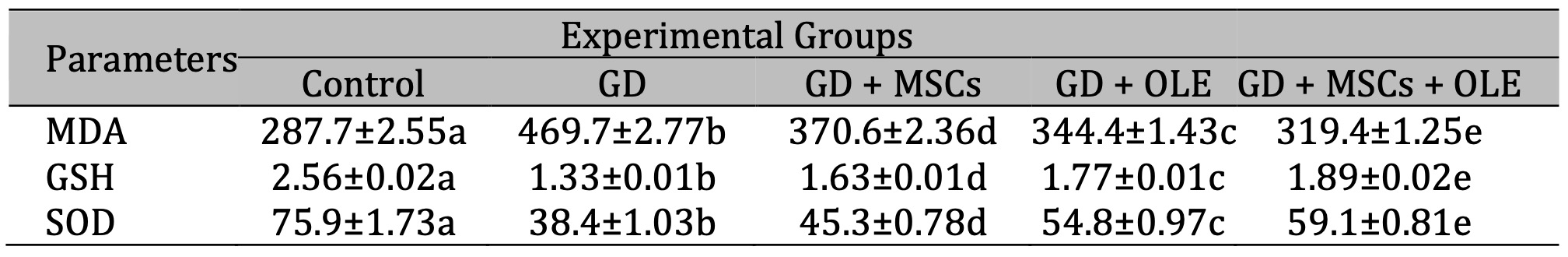
Table 2: Effects of OLE, BM-MSC and their combination on renal oxidative markers of gestational diabetic rats. Each value depicted signifies the mean of five records with a standard error of measurement. The use of identical letters (a, b, c, d, e) to represent groups signifies their lack of statistically significant variance. Conversely, different letters signify a significant variation
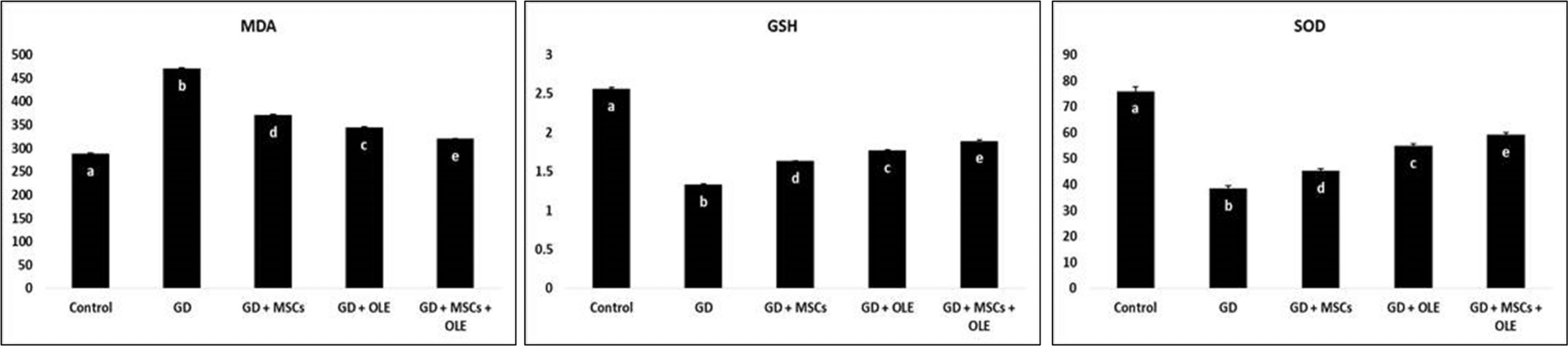
Fig. 2: Effects of OLE, BM-MSC and their combination on renal oxidative markers of gestational diabetic rats.
Histopathological and Immunohistochemical assesments of renal sections
The histological structures of the filtering units of the kidney (renal corpuscles) are crucial for this function. The renal corpuscles are located only in the kidney cortex, with about 1 million per kidney with variation due to race. This unique filtration barrier contains three histological structures: the capillary endothelium of the glomeruli, specialized cells called podocytes, and the fused basement membranes of both of these cells (Fig.3A). This filtration barrier allows for the filtration of small molecules such as water, ions, creatinine and glucose, and small proteins (less than 90 kDa). This structure must prevent the filtration of large proteins present in the blood, such as albumin and immunoglobulins. No pathologic lesions could be recorded in any of the control sections. Pathologic data obtained by routine HE staining, showed that rat’s kidney of diabetic group suffered obvious pathological changes characterized by glomerular atrophy, extension of the renal glomerulus capsular space, serious renal tubular epithelial cells degeneration and necrosis, abundant protein exudation in the renal tubular lumen and occasional hyaline casts formation, beside renal perivascular, interstitial, lymphocytic aggregations, edema and hemorrhage (Fig.3B). In group GD+MSCs showed pathological changes of milder degrees, although glomerular atrophy, renal perivascular, interstitial round cells aggregation, edema and hemorrhage tubular epithelial cells degeneration and necrosis with focal cystic tubular dilatation were also sporadically observed (Fig.3C). Sections from kidney of diabetic rats treated OLE and OLE +MSCs revealed the apparently normal histo-morphology of nephron unites with a keeping normal feature of glomeruli, tubules, papillae, pelvis, vasculature and stroma (Figs.3D&E).
Immune stained examined renal tissue sections for P53 revealed weak positive cytoplasmic reactivity in a few bowmen capsular cells of the control rats (Fig.4A). A weak to moderate staining reaction was detected in some tubular epithelia and glomerular tuft endothelial respectively in the diabetic rats (Fig.4B). An absolute negative stain-ability was seen in the 3 treatment groups post diabetes (Figs.4C, D&E).
Immunohistochemical investigation of renal tissue showed that the PCNA was distributed randomly throughout a variable number of the tubules of the kidney in the diabetic and treatment groups with a higher rate of compensatory proliferation in the diabetic ones (Fig.5B), while few PCNA- weak positive cells were found in the renal tubular epithelium in the control rats (Fig.5A). The renal glomerular endothelium was negatively reacted in all experimental animals; however, a few Bowmen’s partial and visceral cells were weakly reactive in the three treatment groups post diabetes (Figs.5C, D&E).
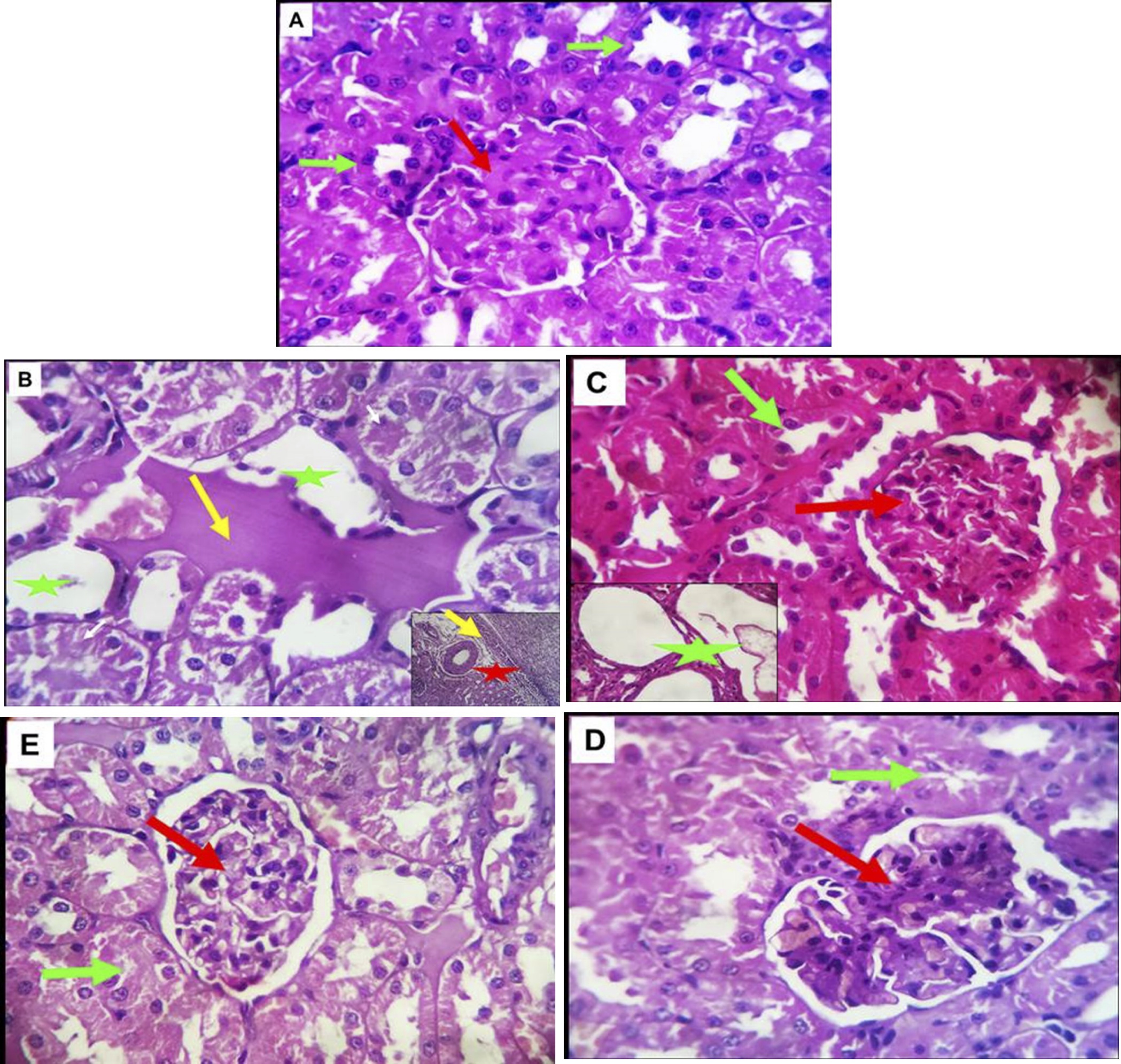
Fig. 3: (A-E). Photomicrographs of the kidney tissue of control pregnant rats and treated groups stained with Hematoxylin and eosin. A (control group): Normal renal glomerular (red arrow) and tubular structures (green arrow). B (GD group): Renal tubular epithelial cells degeneration and necrosis (yellow arrow) and abundant protein exudation in the renal tubular lumen (green asterisk), perivascular, interstitial, lymphocytic aggregations (red asterisk). C (GD+MSCs group): Normal renal glomerular (red arrow) and tubular structures (green arrow), but necrosis with focal cystic tubular dilatation (green asterisk).D&E (GD+OLE&GD+OLE+MSCs groups): Normal nephron units (red and green arrows) with no obvious light microscopic pathologic changes. H&E X 400.
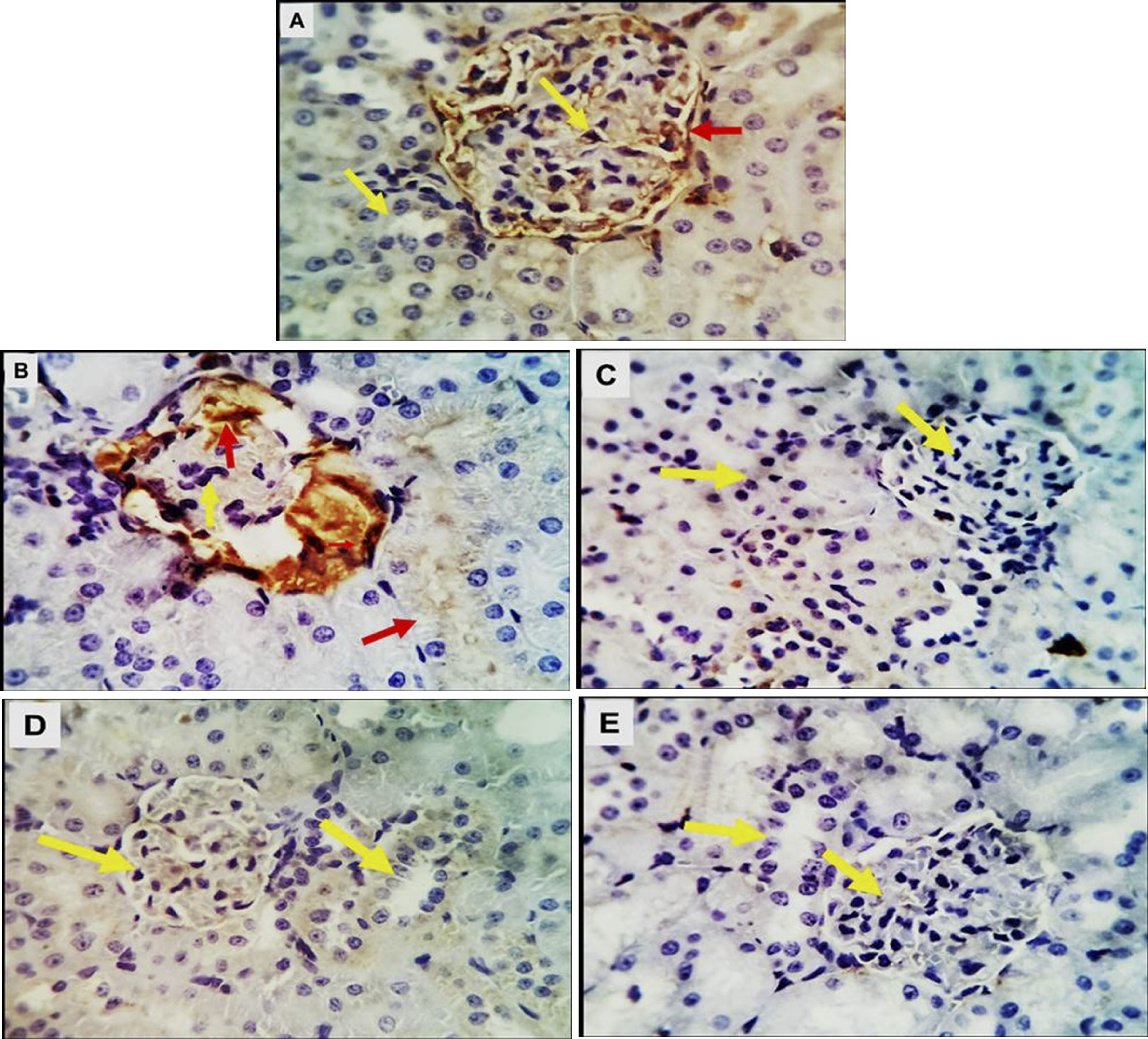
Fig. 4: (A-E). Photomicrographs of kidney tissue of control pregnant rats and treated groups immune-stained with the apoptotic marker P53 showing: A (Control group); weak positive cytoplasmic reactivity in a few bowmen capsular cells (red arrow). B(GD group); a weak to moderate staining reaction is detectable in some tubular epithelia and glomerular tuft endothelial respectively in the diabetic rats (red arrows).C,D&E(GD+MSCs, GD+OLE &GD+OLE+MSCs groups);An absolute negative satiability was seen in these groups (yellow arrows). X 400
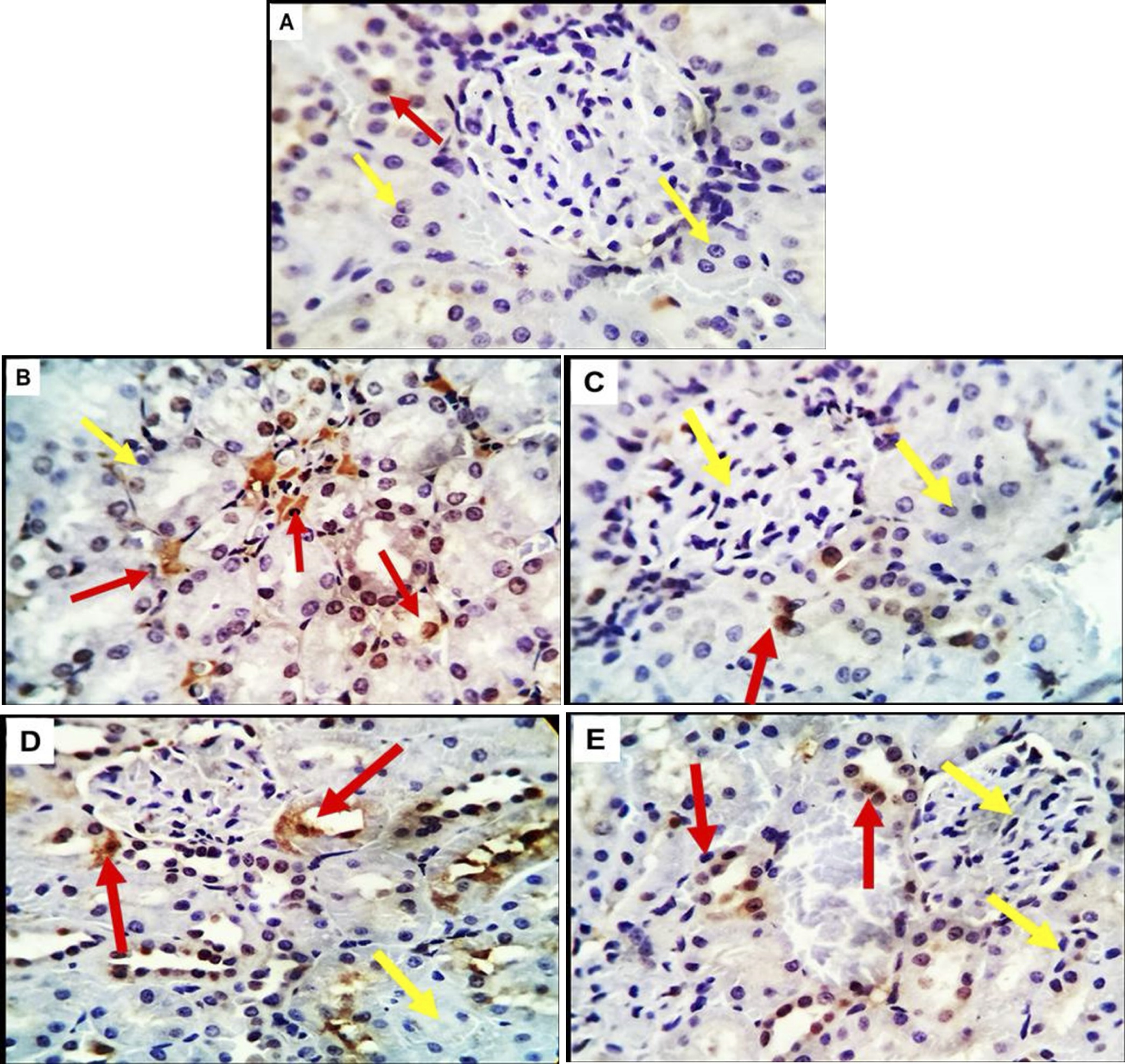
Fig. 5: (A-E). Photomicrographs of kidney tissue of control pregnant rats and treated groups immune-stained with the Proliferating cell nuclear antigen (PCNA) showing, A (control group);A few PCNA- weak positive cells are seen in the renal tubular epithelium were noted in the control, B, C,D&E (GD, GD+MSCs, GD+OLE &GD+OLE+MSCs groups ) showed a variable number positive cells in renal tubules of the diabetic and treatment groups with a higher rate of compensatory proliferation in the diabetic ones (red arrows). The renal glomerular endothelium appears negatively reacted in all experimental animals (yellow arrows) .X 400.
Discussion
Primary Findings
There exist three distinct forms of diabetes mellitus (DM): Type 1, characterized by autoimmune-mediated destruction of pancreatic cells; Type 2, marked by cell failure and insulin resistance in peripheral tissues; and Type 3, recognized as gestational diabetes mellitus (GDM) (Childs et al., 2017). DM is a complex and severe multifactorial disorder, where hyperglycemia, a prominent feature of GDM, ensues from diminished insulin action, production, or a combination thereof, posing a potential threat to maternal health (Elgazzaz et al., 2024). DM has the capacity to inflict substantial damage on various tissues, especially those within the reproductive system. Gestational diabetes mellitus (GDM), a perilous complication of pregnancy, arises due to an aberration in glucose metabolism throughout gestation (Goodman, 2023).
Over the last two decades, there has been a consistent upward trend in the incidence and prevalence of diabetes mellitus (DM), affecting an estimated 387 million individuals globally (Zheng et al., 2021). At present, oral hypoglycemic medications have emerged as the predominant therapeutic modality for managing diabetes mellitus, in addition to insulin. However, the clinical utilization of these medications is linked to adverse effects, including acute hypoglycemia, lactic acidosis, peripheral edema, and gastrointestinal discomfort (Aziz et al., 2015).
In the current investigation, untreated diabetic rats demonstrated a significant reduction in levels of glutathione (GSH) and superoxide dismutase (SOD), coupled with a substantial increase in levels of creatinine, blood urea nitrogen (BUN), uric acid, and malondialdehyde (MDA). Furthermore, histopathological examinations of the kidney unveiled various alterations, consistent with findings from previous experimental diabetes research (Akter et al., 2021).
The present study demonstrates a pronounced decline in renal function in untreated diabetic rats, substantiated by elevated levels of serum creatinine, blood urea nitrogen (BUN), and uric acid, along with observable histological changes. Blood urea nitrogen (BUN) is a derivative of protein breakdown, with approximately 90% of the generated urea being excreted by the kidneys (Hebi et al., 2017). In the meantime, creatinine is a waste product that is produced when muscles contract and utilize creatinine. As a common indicator of glomerular function, creatinine is measured (D’Elia & Weinrauch, 2024). Dehydration, antidiuretic medications, and nutrition can all raise BUN levels, whereas creatinine is more specific to the kidneys because renal injury is the only important factor that raises blood creatinine levels (Carswell & O’Neil, 2024). Furthermore, the kidney is the only organ through which creatinine is eliminated. As a result, renal impairment results in the kidney’s inability to effectively eliminate urea and creatinine, which builds up in the blood. Consequently, renal injury will be indicated by elevated blood urea and creatinine levels (Aktas et al., 2023). During the metabolic process, dietary protein undergoes conversion into urea and creatinine, both of which are exclusively excreted by the kidneys. Elevated concentrations of blood glucose in individuals diagnosed with diabetes can have adverse effects on renal vasculature, leading to the onset of renal failure and concurrent increases in blood urea and creatinine levels. Consequently, the assessment of blood urea and creatinine concentrations serves as a valuable metric for evaluating renal health. The illustrated results in Fig. 2 detail the analysis of urea, creatinine, and uric acid levels in the kidneys of rats induced with diabetes. Substantially elevated levels of urea, creatinine, and uric acid were observed in rats induced with diabetes using streptozotocin (STZ), in comparison to the negative control (NC) group. Treatment with 200 mg doses of olive leaf extract (OLE), stem cells, and their combination exhibited a significant mitigation in creatinine and urea levels, respectively (Dollah et al., 2013).
Contextualizing Findings
The present findings are in concordance with the observations made by Abdel Aziz et al. in 2014, wherein a substantial reduction in plasma concentrations of urea and creatinine, as well as arterial blood pressure values, were documented after mesenchymal stem cell (MSC) injection. Additionally, their investigation disclosed a noteworthy augmentation in the percentage of body weight gain and a partial amelioration in the histological presentation of renal parenchyma (Abdel Aziz et al., 2014). In the course of our inquiry, we have reaffirmed the impact of MSCs on the kidneys of diabetic rats. Specifically, kidney transplantation with MSCs exhibited a marked alleviation of renal dysfunction and structural impairment, concomitant with a diminution in localized inflammation and fibrosis within the renal tissue (Zhang et al., 2020).
Significantly, our study unveiled that the early administration of MSCs in diabetic rats led to a substantial reduction in renal cytokine levels and macrophage infiltration. This intervention impeded nephrocyte death and alleviated renal impairment, thereby averting glomerular abnormalities and kidney failure (Sun et al., 2018). An additional pivotal aspect is immunoregulation, encompassing anti-inflammatory, antiapoptotic, and antioxidant effects (Lin et al., 2021). MSCs present a highly promising prospect for the comprehensive therapy of diabetic nephropathy (DN).
Olive leaf extract (OLE) emerges as a pivotal agent, demonstrating a pronounced and efficacious influence on the restoration of creatinine and urea levels in the kidneys. This restorative effect potentially contributes to the reinstatement of antioxidant properties and the mitigation of oxidative stress, thereby preserving the integrity of renal blood vessels. Previous research has substantiated that OLE possesses the capacity to restore renal function and holds promise as a therapeutic agent (Dollah et al., 2013).
In accordance with the findings of this investigation, it becomes apparent that the administration of olive leaf extract to diabetic rats resulted in a significant reduction in elevated levels of blood creatinine, blood urea nitrogen (BUN), and uric acid. Al-Janabi et al. demonstrated that the use of olive leaf extract elevated urea and creatinine levels induced by streptozotocin (STZ) in diabetic rats. Moreover, in comparison to their diabetic counterparts, the administration of olive leaf extract to diabetic rats induced modifications in pancreatic β-cell regeneration (Al-Janabi et al., 2013). In male rats with STZ-induced diabetes, Laaboudi et al. showed that oral treatment with extracts from olive fruits and leaves increased HDL-C levels and decreased blood levels of creatinine, urea, and uric acid (Al-Attar & Alsalmi, 2019). In our current investigation, rats treated with STZ exhibited significantly reduced renal glutathione (GSH) and superoxide dismutase (SOD) levels but elevated malondialdehyde (MDA) levels. These results unequivocally illustrate that STZ induces oxidative damage in rats with experimental diabetes. Hyperglycemia-induced oxidative stress is closely associated with diabetic complications, overwhelming the body’s natural antioxidant defense system through glucose autoxidation, nonenzymatic glycosylation of macromolecules, and reactive oxygen species (ROS) production (Laaboudi, Ghanam, Aissam, et al., 2016). Elevated glucose levels, indicative of poor glycemic control, can lead to oxidative damage. Clinical data highlights the strong link between oxidative stress and diabetes mellitus (DM), resulting in a reduction in antioxidant defense systems or an increase in free radical generation (Ademiluyi & Oboh, 2012). Cells employ enzymatic or non-enzymatic processes to protect essential functions against free radicals, utilizing antioxidant enzymes such as catalase (CAT), SOD, and glutathione peroxidase (GPx). Glutathione (GSH), a potent non-enzymatic antioxidant, serves as a biomarker for cellular redox imbalance.
This investigation assesses the influence of olive leaf extract (OLE), mesenchymal stem cells (MSC), and their synergistic effects on indicators of oxidative stress and lipid peroxidation in homogenized rat kidney samples. In the diabetic control group, glutathione (GSH) levels and catalase (CAT) activities exhibited a statistically significant decrease compared to those observed in the non-diabetic control (NC) group. Increased lipid peroxidation response is correlated with heightened oxidative stress (Much et al., 2014), with endogenous antioxidants like CAT and SOD acting to scavenge superoxide anions and other free radicals. OLE demonstrated an elevation in SOD levels, suggesting its potential to alleviate oxidative stress and promote the body’s free radical elimination. Crocetin, observed to enhance insulin-sensitizing effects and endogenous antioxidant enzymes, substantially reduced ROS.
Microscopic examination of kidney tissues in healthy rats from the negative control group met normal histological criteria. Conversely, animals treated with MSCs and OLE exhibited nearly normal and active criteria, with defensive mononuclear cells infiltrating from periglomerular cells in the kidneys or portal regions in the liver. The kidneys of these treated animals displayed less tubular degeneration, cast formation, glomerular hypercellularity, and mononuclear cell aggregation. Additionally, the kidneys exhibited flattened uroepithelium, dilated medullary tubules with moderate degeneration, and glomerular hypercellularity. These observed alterations suggest that MSCs and OLE partially mitigate the toxicity induced by STZ. Microscopic analysis of diabetic rats treated with OLE alone revealed some modification, with most cortical and medullary tubules displaying nearly normal uroepithelium and fewer cellular glomeruli.
Implications
The present investigation unequivocally establishes a notable decrease in structural abnormalities within the kidneys of diabetic rats subjected to combined treatment with mesenchymal stem cells (MSCs) and olive leaf extract. Stem cells procured from bone marrow play a pivotal role in tissue repair and regeneration across diverse organs, including the kidneys (Cornacchia et al., 2001; Gupta et al., 2022). Considering the mesenchymal lineage of nephrons and the indispensable involvement of stromal cells in nephron and collecting duct development, MSCs emerge as highly promising candidates for facilitating renal repair (L. Li et al., 2007).
While the exact mechanism by which stem cells enhance kidney function remains undetermined in this study, both trans-differentiation and fusion were observed, indicating the sustained high population of cells following MSC injection throughout the investigation (Leonov et al., 2024). These findings highlight a connection between MSCs’ anti-inflammatory effects in the diabetic nephropathy (DN) rat model and macrophage regulation, demonstrating a decrease in infiltrating macrophages in target tissues (Su et al., 2024). Histological analysis confirms the capacity of both OLE and MSCs to restore renal tissues, bringing them close to normal status. These findings are supported by histopathological examination of renal tissue samples from diabetic female rats treated with MSCs and OLE groups (Mansour et al., 2023).
Limitations
- Mechanistic Uncertainty: The exact mechanisms through which MSCs and OLE exert their renoprotective effects remain unclear, specifically the roles of trans-differentiation versus paracrine signaling in MSC-mediated renal repair.
- Dosage and Safety: Further research is required to determine the optimal dosage and long-term safety of OLE to ensure its effective and safe use in clinical settings.
Future Perspectives
- Molecular Pathways: Future studies should delve into the specific molecular pathways involved in the Reno-protective effects of MSCs and OLE.
- Clinical Translation: It is essential to extend research to larger animal models or clinical trials to evaluate the therapeutic efficacy and safety of these treatments in humans.
Conclusion
This study underscores the significant therapeutic potential of Mesenchymal Stem Cells (MSCs) and Olive Leaf Extract (OLE) in ameliorating renal dysfunction, oxidative stress, and tissue damage associated with Gestational Diabetes Mellitus (GDM). The treatments notably improved renal function markers and oxidative stress parameters, as well as histopathological indicators of tissue regeneration.
Acknowledgements
The authors express gratitude to the Deanship of Scientific Research, in collaboration with the Olive Research Center at Jouf University, for their financial support of this study under research grant number DSR2022-RG-0165
Data Statement
Data is contained within the article.
Funding
This work was funded by the Deanship of Scientific Research in cooperation with the Olive Research Center at Jouf University, under Grant Number (DSR2022-RG-0165).
Disclosure Statement
All authors declare that they have no conflicts of interest.
Disclosure of AI usage
No AI tools were used in the creation of this work.
References
| 1 | Abdel-Reheim, E. S., Abd-Elmoneim, A. A., & Hosni, A. A. (2014). Fatty-sucrosed diet/minimal dose of streptozotocin-treated rat: a novel model of gestational diabetes mellitus, metabolic and inflammatory insight. Journal of Diabetes & Metabolism, 5(9).
|
| 2 | Abdel Aziz, M. T., Wassef, M. A., Ahmed, H. H., Rashed, L., Mahfouz, S., Aly, M. I., . . . Abdelaziz, M. (2014). The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr, 6(1), 34 doi:10.1186/1758-5996-6-34
https://doi.org/10.1186/1758-5996-6-34 |
| 3 | Acar-Tek, N., & Ağagündüz, D. (2020). Olive Leaf (Olea europaea L. folium): Potential Effects on Glycemia and Lipidemia. Ann Nutr Metab, 76(1), 10-15 doi:10.1159/000505508
https://doi.org/10.1159/000505508 |
| 4 | Ademiluyi, A. O., & Oboh, G. (2012). Attenuation of oxidative stress and hepatic damage by some fermented tropical legume condiment diets in streptozotocin-induced diabetes in rats. Asian Pac J Trop Med, 5(9), 692-697 doi:10.1016/s1995-7645(12)60108-4
https://doi.org/10.1016/S1995-7645(12)60108-4 |
| 5 | Aktas, G., Yilmaz, S., Kantarci, D. B., Duman, T. T., Bilgin, S., Balci, S. B., & Atak Tel, B. M. (2023). Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgraduate Medicine, 135(5), 519-523 doi:10.1080/00325481.2023.2214058
https://doi.org/10.1080/00325481.2023.2214058 |
| 6 | Akter, Y., Junaid, M., Afrose, S. S., Nahrin, A., Alam, M. S., Sharmin, T., . . . Hosen, S. M. Z. (2021). A Comprehensive Review on Linum usitatissimum Medicinal Plant: Its Phytochemistry, Pharmacology, and Ethnomedicinal Uses. Mini Reviews in Medicinal Chemistry, 21(18), 2801-2834 doi:10.2174/1389557521666210203153436
https://doi.org/10.2174/1389557521666210203153436 |
| 7 | Al-Attar, A. M., & Alsalmi, F. A. (2019). Influence of olive leaves extract on hepatorenal injury in streptozotocin diabetic rats. Saudi Journal of Biological Sciences, 26(7), 1865-1874 doi:https://doi.org/10.1016/j.sjbs.2017.02.005
https://doi.org/10.1016/j.sjbs.2017.02.005 |
| 8 | Al-Janabi, O. S. I., Amer, M. S., & Khayri, M. H. (2013). Effects of the extracts of olive and Morus alba leaves on experimentally STZ induced diabetes in male rats. International Journal of Science and Research, 4(3), 1526-1532.
|
| 9 | Alharbi, S. H. (2024). Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Therapeutic Advances in Endocrinology and Metabolism, 15, 20420188231222367 doi:10.1177/20420188231222367
https://doi.org/10.1177/20420188231222367 |
| 10 | Almalki, S. G., & Agrawal, D. K. (2016). Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation, 92(1-2), 41-51 doi:10.1016/j.diff.2016.02.005
https://doi.org/10.1016/j.diff.2016.02.005 |
| 11 | Archana, T. M., & Sudheesh, S. (2024). Natural Products as Nano-Antidiabetic Drugs. In M. Haridas, S. Abdulhameed, D. Francis, & S. S. Kumar (Eds.), Drugs from Nature: Targets, Assay Systems and Leads (pp. 531-552). Singapore: Springer Nature Singapore.
https://doi.org/10.1007/978-981-99-9183-9_19 |
| 12 | Aziz, Z., Absetz, P., Oldroyd, J., Pronk, N. P., & Oldenburg, B. (2015). A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Sci, 10, 172 doi:10.1186/s13012-015-0354-6
https://doi.org/10.1186/s13012-015-0354-6 |
| 13 | Beutler, E., Duron, O., & Kelly, B. M. (1963). Improved method for the determination of blood glutathione. J Lab Clin Med, 61, 882-888.
|
| 14 | Bouallagui, Z., Han, J., Isoda, H., & Sayadi, S. (2011). Hydroxytyrosol rich extract from olive leaves modulates cell cycle progression in MCF-7 human breast cancer cells. Food Chem Toxicol, 49(1), 179-184 doi:10.1016/j.fct.2010.10.014
https://doi.org/10.1016/j.fct.2010.10.014 |
| 15 | Caplan, A. I. (1991). Mesenchymal stem cells. J Orthop Res, 9(5), 641-650 doi:10.1002/jor.1100090504
https://doi.org/10.1002/jor.1100090504 |
| 16 | Carswell, C., & O'Neil, S. (2024). Applied Anatomy and Physiology, the Kidney Disease Process, and Kidney Investigations. In Renal Nursing (pp. 14-51).
https://doi.org/10.1002/9781394178797.ch2 |
| 17 | Chaudhury, A., Duvoor, C., Reddy Dendi, V. S., Kraleti, S., Chada, A., Ravilla, R., . . . Mirza, W. (2017). Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front Endocrinol (Lausanne), 8, 6 doi:10.3389/fendo.2017.00006
https://doi.org/10.3389/fendo.2017.00006 |
| 18 | Childs, B. B., Cypress, M., & Spollett, G. (2017). Complete nurse's guide to diabetes care: American Diabetes Association.
|
| 19 | Ciocan, A.-G., Maximilian, C., Mitoi, E. M., Moldovan, R.-C., Neguț, D., Iuga, C.-A., . . . Cogălniceanu, G. (2023). The Impact of Acute Low-Dose Gamma Irradiation on Biomass Accumulation and Secondary Metabolites Production in Cotinus coggygria Scop. and Fragaria × ananassa Duch. Red Callus Cultures. Metabolites, 13(8). Retrieved from doi:10.3390/metabo13080894
https://doi.org/10.3390/metabo13080894 |
| 20 | Cornacchia, F., Fornoni, A., Plati, A. R., Thomas, A., Wang, Y., Inverardi, L., . . . Striker, G. E. (2001). Glomerulosclerosis is transmitted by bone marrow-derived mesangial cell progenitors. J Clin Invest, 108(11), 1649-1656 doi:10.1172/jci12916
https://doi.org/10.1172/JCI12916 |
| 21 | D'Elia, J. A., & Weinrauch, L. A. (2024). Lipid Toxicity in the Cardiovascular-Kidney-Metabolic Syndrome (CKMS). Biomedicines, 12(5). Retrieved from doi:10.3390/biomedicines12050978
https://doi.org/10.3390/biomedicines12050978 |
| 22 | Dollah, M. A., Parhizkar, S., & Izwan, M. (2013). Effect of Nigella sativa on the kidney function in rats. Avicenna J Phytomed, 3(2), 152-158.
|
| 23 | Du, S., Zeugolis, D. I., & O'Brien, T. (2022). Scaffold-based delivery of mesenchymal stromal cells to diabetic wounds. Stem Cell Res Ther, 13(1), 426 doi:10.1186/s13287-022-03115-4
https://doi.org/10.1186/s13287-022-03115-4 |
| 24 | El-Beltagi, H. S., & Badawi, M. H. (2013). Comparison of antioxidant and antimicrobial properties for Ginkgo biloba and rosemary (Rosmarinus officinalis L.) from Egypt. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 41(1), 126-135.
https://doi.org/10.15835/nbha4118928 |
| 25 | El-Sayed, M. E., Atwa, A., Sofy, A. R., Helmy, Y. A., Amer, K., Seadawy, M. G., & Bakry, S. (2023). Mesenchymal stem cell transplantation in burn wound healing: uncovering the mechanisms of local regeneration and tissue repair. Histochemistry and Cell Biology. doi:10.1007/s00418-023-02244-y
https://doi.org/10.1007/s00418-023-02244-y |
| 26 | Elgazzaz, M., Woodham, P. C., Maher, J., & Faulkner, J. L. (2024). Implications of Pregnancy on Cardiometabolic Disease Risk: Preeclampsia and Gestational Diabetes. American Journal of Physiology-Cell Physiology. doi:10.1152/ajpcell.00293.2024
https://doi.org/10.1152/ajpcell.00293.2024 |
| 27 | Eng, P. C., Teo, A. E. D., Yew, T. W., & Khoo, C. M. (2024). Implementing care for women with gestational diabetes after delivery-the challenges ahead. Frontiers in Global Women's Health, 5.
https://doi.org/10.3389/fgwh.2024.1391213 |
| 28 | Fitó, M., Cladellas, M., de la Torre, R., Martí, J., Muñoz, D., Schröder, H., . . . Covas, M. I. (2008). Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur J Clin Nutr, 62(4), 570-574 doi:10.1038/sj.ejcn.1602724
https://doi.org/10.1038/sj.ejcn.1602724 |
| 29 | Friedenstein, A. J., Piatetzky, S., II, & Petrakova, K. V. (1966). Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol, 16(3), 381-390.
https://doi.org/10.1242/dev.16.3.381 |
| 30 | Furman, B. L. (2015). Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc Pharmacol, 70, 5.47.41-45.47.20 doi:10.1002/0471141755.ph0547s70
https://doi.org/10.1002/0471141755.ph0547s70 |
| 31 | Ghanam, J., Laaboudi, W., & Benlemlih, M. (2015). Effects of rich polyphenols olive tree extract on inflammation and pain in patients with rheumatoid arthritis: an 8-weeks randomized, double-blind, placebo-controlled clinical trial. Int J Biol Pharm Res, 2, 51-61.
https://doi.org/10.22159/ijpps.2016v8i12.14077 |
| 32 | Giacometti, J., Žauhar, G., & Žuvić, M. (2018). Optimization of Ultrasonic-Assisted Extraction of Major Phenolic Compounds from Olive Leaves (Olea europaea L.) Using Response Surface Methodology. Foods, 7(9). doi:10.3390/foods7090149
https://doi.org/10.3390/foods7090149 |
| 33 | Gonçalves, M., Aiello, A., Rodríguez-Pérez, M., Accardi, G., Burgos-Ramos, E., & Silva, P. J. N. (2024). Olive Oil Components as Novel Antioxidants in Neuroblastoma Treatment: Exploring the Therapeutic Potential of Oleuropein and Hydroxytyrosol. 16(6), 818.
https://doi.org/10.3390/nu16060818 |
| 34 | Goodman, J. R. (2023). Diabetes Mellitus in Pregnancy. NeoReviews, 24(3), e144-e157 doi:10.1542/neo.24-3-e144
https://doi.org/10.1542/neo.24-3-e144 |
| 35 | Guillamat-Prats, R. (2021). The Role of MSC in Wound Healing, Scarring and Regeneration. Cells, 10(7). doi:10.3390/cells10071729
https://doi.org/10.3390/cells10071729 |
| 36 | Gupta, S., Pinky, Vishal, Sharma, H., Soni, N., Rao, E. P., . . . Mohanty, S. (2022). Comparative Evaluation of Anti-Fibrotic Effect of Tissue Specific Mesenchymal Stem Cells Derived Extracellular Vesicles for the Amelioration of CCl4 Induced Chronic Liver Injury. Stem cell reviews and reports, 18(3), 1097-1112 doi:10.1007/s12015-021-10313-9
https://doi.org/10.1007/s12015-021-10313-9 |
| 37 | Hadrich, F., Garcia, M., Maalej, A., Moldes, M., Isoda, H., Feve, B., & Sayadi, S. (2016). Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci, 151, 167-173 doi:10.1016/j.lfs.2016.02.027
https://doi.org/10.1016/j.lfs.2016.02.027 |
| 38 | Harting, M., Jimenez, F., Pati, S., Baumgartner, J., & Cox, C., Jr. (2008). Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy, 10(3), 243-253 doi:10.1080/14653240801950000
https://doi.org/10.1080/14653240801950000 |
| 39 | Hebi, M., Farid, O., Ajebli, M., & Eddouks, M. (2017). Potent antihyperglycemic and hypoglycemic effect of Tamarix articulata Vahl. in normal and streptozotocin-induced diabetic rats. Biomedicine & Pharmacotherapy, 87, 230-239 doi:https://doi.org/10.1016/j.biopha.2016.12.111
https://doi.org/10.1016/j.biopha.2016.12.111 |
| 40 | Hirsch, J. D., Eslamizar, L., Filanoski, B. J., Malekzadeh, N., Haugland, R. P., Beechem, J. M., & Haugland, R. P. (2002). Easily reversible desthiobiotin binding to streptavidin, avidin, and other biotin-binding proteins: uses for protein labeling, detection, and isolation. Analytical Biochemistry, 308(2), 343-357 doi:https://doi.org/10.1016/S0003-2697(02)00201-4
https://doi.org/10.1016/S0003-2697(02)00201-4 |
| 41 | Hsu, S. M., Raine, L., & Fanger, H. (1981). Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem, 29(4), 577-580 doi:10.1177/29.4.6166661
https://doi.org/10.1177/29.4.6166661 |
| 42 | Huang, S.-T., Bair, P.-J., Chang, S.-S., Kao, Y.-N., Chen, S.-N., Wang, I. K., . . . Yu, T.-M. (2024). Risk of Diabetic Retinopathy in Patients With Type 2 Diabetes After SGLT-2 Inhibitors: A Nationwide Population Cohort Study. Clinical Pharmacology & Therapeutics, 115(1), 95-103 doi:https://doi.org/10.1002/cpt.3074
https://doi.org/10.1002/cpt.3074 |
| 43 | Kumar, V., Bhatt, P. C., Kaithwas, G., Rashid, M., Al-abbasi, F. A., Khan, J. A. J., . . . Verma, A. (2016). α-Mangostin Mediated Pharmacological Modulation of Hepatic Carbohydrate Metabolism in Diabetes Induced Wistar Rat. Beni-Suef University Journal of Basic and Applied Sciences, 5(3), 255-276 doi:https://doi.org/10.1016/j.bjbas.2016.07.001
https://doi.org/10.1016/j.bjbas.2016.07.001 |
| 44 | Laaboudi, W., Ghanam, J., Aissam, H., Merzouki, M., & Benlemlih, M. (2016). Anti-inflammatory and analgesic activities of olive tree extract. Int j pharm pharm sci, 8(7), 414-419.
https://doi.org/10.22159/ijpps.2016v8i12.14077 |
| 45 | Laaboudi, W., Ghanam, J., Ghoumari, O., Sounni, F., Merzouki, M., & Benlemlih, M. (2016). Hypoglycemic and hypolipidemic effects of phenolic olive tree extract in streptozotocin diabetic rats. International Journal of Pharmacy and Pharmaceutical Sciences, 8(12), 287-291.
https://doi.org/10.22159/ijpps.2016v8i12.14077 |
| 46 | Leonov, S., Dorfman, A., Pershikova, E., Inyang, O., Alhaddad, L., Wang, Y., . . . Merkher, Y. (2024). Extracellular Vesicle- and Mitochondria-Based Targeting of Non-Small Cell Lung Cancer Response to Radiation: Challenges and Perspectives. Cancers, 16(12). Retrieved from doi:10.3390/cancers16122235
https://doi.org/10.3390/cancers16122235 |
| 47 | Li, L., Truong, P., Igarashi, P., & Lin, F. (2007). Renal and bone marrow cells fuse after renal ischemic injury. J Am Soc Nephrol, 18(12), 3067-3077 doi:10.1681/asn.2007030284
https://doi.org/10.1681/ASN.2007030284 |
| 48 | Li, Q., Yang, Z., Lu, B., Wen, J., Ye, Z., Chen, L., . . . Hu, R. (2011). Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovascular Diabetology, 10(1), 72 doi:10.1186/1475-2840-10-72
https://doi.org/10.1186/1475-2840-10-72 |
| 49 | Lin, W., Li, H. Y., Yang, Q., Chen, G., Lin, S., Liao, C., & Zhou, T. (2021). Administration of mesenchymal stem cells in diabetic kidney disease: a systematic review and meta-analysis. Stem Cell Res Ther, 12(1), 43 doi:10.1186/s13287-020-02108-5
https://doi.org/10.1186/s13287-020-02108-5 |
| 50 | Liu, P., Zhang, Z., & Li, Y. (2021). Relevance of the Pyroptosis-Related Inflammasome Pathway in the Pathogenesis of Diabetic Kidney Disease. Frontiers in Immunology, 12.
https://doi.org/10.3389/fimmu.2021.603416 |
| 51 | Mansour, H. M. M., Zeitoun, A. A., Abd-Rabou, H. S., El Enshasy, H. A., Dailin, D. J., Zeitoun, M. A. A., & El-Sohaimy, S. A. (2023). Antioxidant and Anti-Diabetic Properties of Olive (Olea europaea) Leaf Extracts: In vitro and In vivo Evaluation. Antioxidants (Basel), 12(6). doi:10.3390/antiox12061275
https://doi.org/10.3390/antiox12061275 |
| 52 | Maor-Sagie, E., Hallak, M., Haggiag, N., Naeh, A., Toledano, Y., & Gabbay-Benziv, R. (2024). Timing of gestational diabetes diagnosis and progression to type 2 Diabetes: A comparative analysis. Diabetes Research and Clinical Practice, 214, 111782 doi:https://doi.org/10.1016/j.diabres.2024.111782
https://doi.org/10.1016/j.diabres.2024.111782 |
| 53 | Marklund, S., & Marklund, G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem, 47(3), 469-474 doi:10.1111/j.1432-1033.1974.tb03714.x
https://doi.org/10.1111/j.1432-1033.1974.tb03714.x |
| 54 | Marshall, N. E., Abrams, B., Barbour, L. A., Catalano, P., Christian, P., Friedman, J. E., . . . Thornburg, K. L. (2022). The importance of nutrition in pregnancy and lactation: lifelong consequences. Am J Obstet Gynecol, 226(5), 607-632 doi:10.1016/j.ajog.2021.12.035
https://doi.org/10.1016/j.ajog.2021.12.035 |
| 55 | Martiniakova, M., Biro, R., Penzes, N., Sarocka, A., Kovacova, V., Mondockova, V., & Omelka, R. (2024). Links among Obesity, Type 2 Diabetes Mellitus, and Osteoporosis: Bone as a Target. International Journal of Molecular Sciences, 25(9). Retrieved from doi:10.3390/ijms25094827
https://doi.org/10.3390/ijms25094827 |
| 56 | Medina, E., de Castro, A., Romero, C., & Brenes, M. (2006). Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: correlation with antimicrobial activity. J Agric Food Chem, 54(14), 4954-4961 doi:10.1021/jf0602267
https://doi.org/10.1021/jf0602267 |
| 57 | Mir-Cerdà, A., Granados, M., Saurina, J., & Sentellas, S. J. F. C. (2024). Olive tree leaves as A great source of phenolic compounds: Comprehensive profiling of NADES extracts. 140042.
https://doi.org/10.1016/j.foodchem.2024.140042 |
| 58 | Mir, M. A. (2024). Chapter 17 - Utilization of medicinally important plants as antidiabetic medicines in a sustainable manner. In B. K. Banik (Ed.), Green Approaches in Medicinal Chemistry for Sustainable Drug Design (Second Edition) (Vol. 1, pp. 397-410): Elsevier.
https://doi.org/10.1016/B978-0-443-16166-7.00017-7 |
| 59 | Much, D., Beyerlein, A., Roßbauer, M., Hummel, S., & Ziegler, A. G. (2014). Beneficial effects of breastfeeding in women with gestational diabetes mellitus. Mol Metab, 3(3), 284-292 doi:10.1016/j.molmet.2014.01.002
https://doi.org/10.1016/j.molmet.2014.01.002 |
| 60 | Pandey, A., Usmani, S., Ahmad, M., Khatoon, S., Wahab, S., & Prakash, O. (2024). Phytochemical and Pharmacological Attributes of Nerium oleander: A Review. Current Nutrition & Food Science, 20(5), 570-585 doi:10.2174/1573401319666230522160742
https://doi.org/10.2174/1573401319666230522160742 |
| 61 | Rahman, M. M., Islam, M. R., Shohag, S., Hossain, M. E., Rahaman, M. S., Islam, F., . . . Idris, A. M. (2022). The multifunctional role of herbal products in the management of diabetes and obesity: a comprehensive review. Molecules, 27(5), 1713.
https://doi.org/10.3390/molecules27051713 |
| 62 | Ríos, J. L., Francini, F., & Schinella, G. R. (2015). Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med, 81(12-13), 975-994 doi:10.1055/s-0035-1546131
https://doi.org/10.1055/s-0035-1546131 |
| 63 | Ronca, C. L., Duque-Soto, C., Samaniego-Sánchez, C., Morales-Hernández, M. E., Olalla-Herrera, M., Lozano-Sánchez, J., & Giménez Martínez, R. (2024). Exploring the Nutritional and Bioactive Potential of Olive Leaf Residues: A Focus on Minerals and Polyphenols in the Context of Spain's Olive Oil Production. Foods, 13(7), 1036.
https://doi.org/10.3390/foods13071036 |
| 64 | Ruiz-Larrea, M. B., Leal, A. M., Liza, M., Lacort, M., & de Groot, H. (1994). Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids, 59(6), 383-388 doi:10.1016/0039-128x(94)90006-x
https://doi.org/10.1016/0039-128X(94)90006-X |
| 65 | Shukla, L. I., Vardhan, P. V., Devika, T. K., Roy, S., & Bhatacharya, S. (2021). Generation of Plant Mutant Lines Using Gamma Radiation with Enhanced Secondary Metabolite Contents. In Biotechnological Approaches to Enhance Plant Secondary Metabolites (pp. 27-48): CRC Press.
https://doi.org/10.1201/9781003034957-2 |
| 66 | Soliman, G. A., Saeedan, A. S., Abdel-Rahman, R. F., Ogaly, H. A., Abd-Elsalam, R. M., & Abdel-Kader, M. S. (2019). Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: Molecular and biochemical study. Saudi Pharm J, 27(3), 326-340 doi:10.1016/j.jsps.2018.11.015
https://doi.org/10.1016/j.jsps.2018.11.015 |
| 67 | Su, W., Yin, Y., Zhao, J., Hu, R., Zhang, H., Hu, J., . . . Cheng, Y. (2024). Exosomes derived from umbilical cord-derived mesenchymal stem cells exposed to diabetic microenvironment enhance M2 macrophage polarization and protect against diabetic nephropathy. The FASEB Journal, 38(14), e23798 doi:https://doi.org/10.1096/fj.202400359R
https://doi.org/10.1096/fj.202400359R |
| 68 | Sun, J., Zhao, F., Zhang, W., Lv, J., Lv, J., & Yin, A. (2018). BMSCs and miR-124a ameliorated diabetic nephropathy via inhibiting notch signalling pathway. J Cell Mol Med, 22(10), 4840-4855 doi:10.1111/jcmm.13747
https://doi.org/10.1111/jcmm.13747 |
| 69 | Suvarna, K. S., Layton, C., & Bancroft, J. D. (2018). Bancroft's theory and practice of histological techniques: Elsevier health sciences.
|
| 70 | Totaro, M. P., Difonzo, G., Pasqualone, A., & Summo, C. (2024). Physicochemical properties and sensory features of ripened, industrially prepared sausages, enriched with olive leaf extract to replace nitrite and nitrate. LWT, 196, 115852.
https://doi.org/10.1016/j.lwt.2024.115852 |
| 71 | Varra, F.-N., Varras, M., Varra, V.-K., & Theodosis-Nobelos, P. (2024). Molecular and pathophysiological relationship between obesity and chronic inflammation in the manifestation of metabolic dysfunctions and their inflammation‑mediating treatment options (Review). Mol Med Rep, 29(6), 95 doi:10.3892/mmr.2024.13219
https://doi.org/10.3892/mmr.2024.13219 |
| 72 | Wainstein, J., Ganz, T., Boaz, M., Bar Dayan, Y., Dolev, E., Kerem, Z., & Madar, Z. (2012). Olive Leaf Extract as a Hypoglycemic Agent in Both Human Diabetic Subjects and in Rats. Journal of Medicinal Food, 15(7), 605-610 doi:10.1089/jmf.2011.0243
https://doi.org/10.1089/jmf.2011.0243 |
| 73 | Wang Ying-hui, Z. R. C. L. (2014). Isolation and culture of rat bone marrow mesenchymal stem cells using density gradient centrifugation and adherence separation screening. Chinese Journal of Tissue Engineering Research, 18(28), 4463-4468.
|
| 74 | Wetzel, M. D., Gao, T., Stanley, K., Cooper, T. K., Morris, S. M., Jr., & Awad, A. S. (2020). Enhancing kidney DDAH-1 expression by adenovirus delivery reduces ADMA and ameliorates diabetic nephropathy. Am J Physiol Renal Physiol, 318(2), F509-f517 doi:10.1152/ajprenal.00518.2019
https://doi.org/10.1152/ajprenal.00518.2019 |
| 75 | Wu, Q., Chen, B., & Liang, Z. (2016). Mesenchymal Stem Cells as a Prospective Therapy for the Diabetic Foot. Stem Cells Int, 2016, 4612167 doi:10.1155/2016/4612167
https://doi.org/10.1155/2016/4612167 |
| 76 | Yamashita, N., & Kramann, R. (2024). Mechanisms of kidney fibrosis and routes towards therapy. Trends in Endocrinology & Metabolism, 35(1), 31-48 doi:10.1016/j.tem.2023.09.001
https://doi.org/10.1016/j.tem.2023.09.001 |
| 77 | Yang, Y., Liu, S., He, C., Chen, Z., Lyu, T., Zeng, L., . . . Zhao, R. C. (2021). Long Non-coding RNA Regulation of Mesenchymal Stem Cell Homeostasis and Differentiation: Advances, Challenges, and Perspectives. Front Cell Dev Biol, 9, 711005 doi:10.3389/fcell.2021.711005
https://doi.org/10.3389/fcell.2021.711005 |
| 78 | Yin, J., Fu, X., Luo, Y., Leng, Y., Ao, L., & Xie, C. (2024). A Narrative Review of Diabetic Macroangiopathy: From Molecular Mechanism to Therapeutic Approaches. Diabetes Therapy, 15(3), 585-609 doi:10.1007/s13300-024-01532-7
https://doi.org/10.1007/s13300-024-01532-7 |
| 79 | Yu, X., Liu, P., Li, Z., & Zhang, Z. (2023). Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front Endocrinol (Lausanne), 14, 1099310 doi:10.3389/fendo.2023.1099310
https://doi.org/10.3389/fendo.2023.1099310 |
| 80 | Zhang, F., Wang, C., Wen, X., Chen, Y., Mao, R., Cui, D., . . . Lu, Y. (2020). Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103(+) DCs-mediated CD8(+) T cell responses. J Cell Mol Med, 24(10), 5817-5831 doi:10.1111/jcmm.15250
https://doi.org/10.1111/jcmm.15250 |
| 81 | Zheng, Y., Zhu, N., Wang, J., Zhao, N., & Yuan, C. (2021). Crocetin suppresses gestational diabetes in streptozotocin-induced diabetes mellitus rats via suppression of inflammatory reaction. J Food Biochem, 45(9), e13857 doi:10.1111/jfbc.13857
https://doi.org/10.1111/jfbc.13857 |