Original Article - DOI:10.33594/000000752
Accepted 16 December 2024 - Published online 10 January 2025
Bone Marrow Mesenchymal Stem Cells Promote Repairing the Bruised Tissue via Regulating mRNA Expression of Molecular Biomarkers and the Apoptotic Rate
cZoology Department–Faculty of Science–Beni-Suef University–Beni-Suef–Egypt,
dDepartment of Chemistry, College of Science, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, Dammam City, 31441, Saudi Arabia,
eBasic and Applied Scientific Research Center, University of Imam Abdulrahman Bin Faisal, P.O. Box 1982, Dammam, 31441, Saudi Arabia
Keywords
Abstract
Background/Aims:
Bruise is the extravasation of blood that may be mild or severe. Bone marrow mesenchymal stem cells (BM-MSCs) are one of the most promising cells used in regenerative medicine for treating many disorders. We aimed to evaluate the efficiency of BM-MSCs in treating cutaneous bruises.Methods:
78 male albino rats were equally divided into 3 groups, control group (G1), bruise wound group (G2) and Bruised animals treated with BM-MSCs group (G3). The sequences of color changes were recorded. Animals were sacrificed and skin samples were collected for histopathological examination and analyzing the mRNA expression rate of transforming growth factor- β (TGF-β), interleukin-6 (IL-6), tumor necrotic factor-α (TNF-α), Heat shock protein-90 α (HSP-90α), Metalloprotiens-9 (MMP-9), and microRNA-21 (miR-21), which incorporated in the healing process and the apoptotic rat.Results:
Subcutaneous injection of BM-MSCs reduced the color intensity of the bruised skin, with statistically significant upregulation of TGF-β, TNF-α, and HSP-90α, significant down-regulation of MMP-9 and miR-21 mRNA expression rate, and significant reduction of the apoptotic rate and the inflammatory cells.Conclusion:
BM-MSCs have a promising improvement in the healing process of bruises by regulating the expression rate of TGF-β- IL-6- TNF-α- HSP-90α- MMP-9- miR-21 and reducing the apoptotic rate and inflammatory cell infiltration.Introduction
Bruises, contusions, or ecchymoses are a common type of mechanical injury [1], in which blood extravasates in the surrounding cutaneous connective tissue or deeper in the underlying tissues [2]. Blood extravasation occurs due to shredding the walls of veins and small arteries [3]. It appears as a non-raised, irregular, or rounded patch with blue or purplish coloration [4].
Trauma, surgery, bleeding disorders, scurvy, and cosmetic procedures considering different insults to the skin resulting in ecchymosis, which is bleeding under the which may result in combination with proteins called clotting factors to form a clot and considered a sign of bleeding disorders as hemophilia, liver disease or medical treatment with Aspirin and antibiotics, Moreover, bruises, represent a later stage of various pathophysiological processes accompanied with vascular permeability adjacent to the venules of the skin or papillary dermis capillary kinks [5].
Disseminated intravascular coagulation, Ehlers-Danlos syndrome, Meningococcemia infections, inflammatory or autoimmune disorders such as Gardner-Diamond syndrome and Henoch-Schönlein purpura, neuroblastoma and leukemia malignancy, deficiency of vitamin C and K are medical complaints combined with bruising [6, 7]. During bruises evaluation the coagulative illnesses (inherited or acquired) should be taken into consideration, [8] like von Willebrand disease, hemophilia, and abnormalities of blood platelet [9], besides the non-traumatic skin diseases and other findings as phytophotodermatitis, eczema, erythema multiforme, striae, hemangiomas, and tattooing [6].
The forensic significance of bruises is its observation in cases of violence such as vulnerable adult abuse, sexual assault, and intimate partner violence [10]. It is identified to be the most prevalent injury of physical child abuse [11]. Elderly patients with minimal repair mechanisms and those with thin and delicate skin are more susceptible to bruising and a long period of recovery [4].
The presence of different colors on the body surface reflects the presence of multiple bruises in different stages of healing; this condition may refer to long-term physical abuse [12]. In some cases of child abuse, assault, and sexual assault, forensic experts are called up for bruises and age determination, in which their comments have a medico-legal consequence [13]. In which the determined time point by the expert, can confirm or exclude the presence of a suggested perpetrator [14].
Bruises could be treated through the application of local compression, [15] such as cold compress, topical application of vitamin K, arnica, or bromelain, or using laser therapy in case of persistent bruising [16].
MSCs act as foundational elements in the newly emerging discipline of regenerative therapy; in which they could be isolated from variable sources, [17] capable of fascinating cellular biology, [18] strongly possess stemness potency, and developed rapidly for clinical applications on a large scale with minimal ethical concerns rather than embryonic stem cells (ESCs) [17].
BM-MSCs are one of the most multipotent progenitor stem cells; they could be distinguished into variable cell types as the endothelial cells and epithelial cells, fibroblasts, myofibroblasts, neurons, adipocytes, chondrocytes, osteoblasts [19]. Besides their powerful ability to regenerate the whole structures of the cutaneous tissue [20], they are also, isolated from other marrow cells easily, and have a high propensity to attach to the plastic surface of the flask of culturing [21].
When they are administrated systemically to humans or animals from an exogenous source, they tend to migrate to the inflamed damaged tissues [22]. After their engraftment into cutaneous injuries, they are mingled with the sebaceous glands, blood vessels, and hair follicles [23], helping in wound bed reconstruction and regulation of tissue repair [24]. On the level of angiogenesis, they promote this process via elevating the proliferation capacity of the endothelial cell, the vascular permeability [25], the capillary density, formation of new blood vessels, wound strength [26], secretion of the soluble factors [27], as well as, formation of blood vessels in the smooth muscle that conjugating the endothelial layer of the vascular wall [28]. Transplantation of the dermis-derived multipotent cells systemically has the superiority for curing the rat of cutaneous injuries that resulted from irradiation than their topical applications [29].
Multilineage differentiation of MSCs into different cell types [30], cytoprotective and anti-apoptotic effects, angiogenic capacity [31], immunomodulatory, and low immunogenicity [32] are unique properties [33] making them the most promising cells for regenerative medicine, cell therapy, and tissue repairment [34]. They have been identified as a curative agent for skin regeneration and rejuvenation in recent years [35].
Therefore, the purpose of the present study was designed to evaluate the potency of BM-MSCS in the therapy of bruised cutaneous tissue as a type of regenerative medicine through analysis of the expression rate of certain genetic markers that regulate and control the healing process via quantitative polymerase chain reaction (qPCR) and determining the histopathological improvements and the apoptotic rate.
Materials and Methods
BM-MSCs isolation
In sterile Greiner cell culture flasks with a canted neck (T-25 cm2), 2.5× 106 cells were cultured at the cell density of (1×106 cells/ cm2 area), and incubated in a 5% CO2-humidified incubator (Biobase, Model: BJPX-C50; South Gongye Road, Jinan, Shandong Province, China) at 37°C. 4 days post-incubation, the dead, non-adherent, and floating cells were removed. Subsequently, through 7-10 days of culturing (the cells have spindle shape) (Fig. 1), the adherent cells were washed with pre-warmed sterile PBS at 37°C twice and trypsinized with 1-2 mL of pre-warmed trypsin (0.25%)/EDTA (1 mM) for 2-3 min. The detachment of the adherent cells was ensured via examination under an inverted biological microscope (Novel Model: NIB-100; Jiangsu, China). 3-5 ml complete culture medium was added to stop the trypsin action and the cells were harvested and centrifuged for 5 min at 3000 rpm. The obtained pellet was washed with incomplete DMEM and centrifugation, following 2 times of washing; the cells were resuspended in incomplete DMEM, counted, and checked for viability by adding an equal volume of trypan blue (0.2%).
The suspended BM-MSCs (viability > 95%) were injected S/C in the bruised rats quickly at a dose of (2 × 106 cells/mL) [37]. The procedures of isolation and culturing were conducted according to the guidelines of Chaudhary and Rath [38] with some modifications.
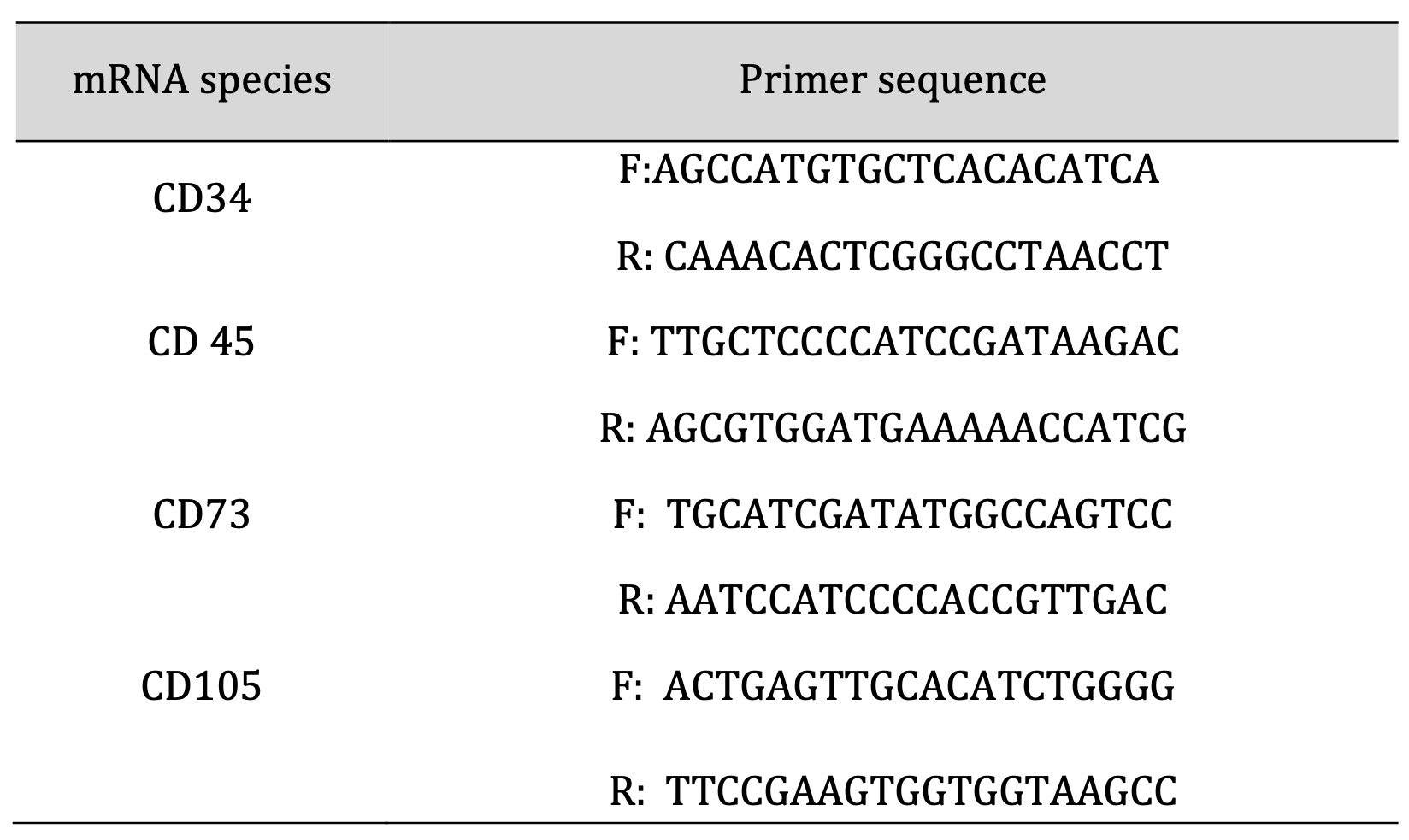
Table 1: Sequence of primers used in the characterization of BM-MSCs
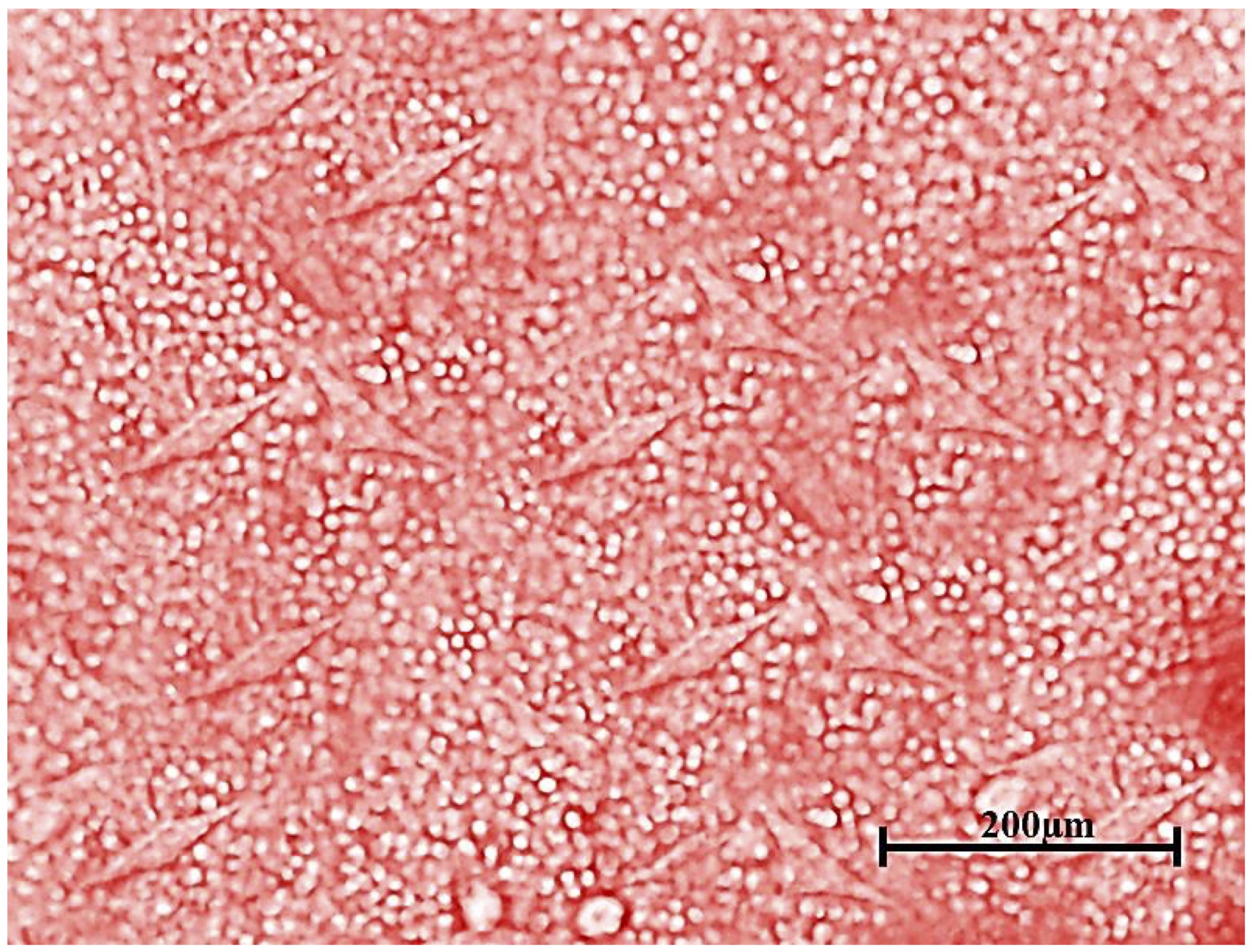
Fig. 1: Spindle-shaped plastic-adherent BM-MSCs.
Animals
The animals were divided into the following 3 groups (6 animals /time interval), Group I (control) was not subjected to injury. Group II (bruise injury) animals were anesthetized via Isoflurane (0.5-2 %) which was brought through an oxygen flow system. Then the abdominal hair was removed using hair removal cream and pinched by a small pair of Spencer-Well forceps lightly and actually traumatized [39]. Group III (bruised animals treated with BM-MSCs) the bruised animals were inoculated with BM-MSCs (2×106 cells/ mL) S/C [37]. Animals were observed for detecting the different colors of bruises throughout the experiment. After each interval (3, 6, 12, 24, 48, and 72 hr.), the animals underwent anesthesia via mild diethyl ether (ACE) mixture for scarification, which was followed by the collection of skin samples, which snapped frozen at once in liquid nitrogen and then well kept under -80 oC for analysis mRNA expression rate of variable genes and determining the rate of apoptosis, the other parts were preserved in neutral buffer formalin (10%) for the histopathological examination.
Histopathological examination
The fixed skin specimens in neutral buffer formalin were undergoing dehydration, embedded in paraffin wax, and sectioning at 5 μm for staining with hematoxylin and eosin stain (H&E) [40].
Quantifiable assays of the mRNA expression rate of TGF-β, IL-6, and TNF-α through PCR
The RNA Easy extraction Kit (Qiagen Inc.) was utilized for total RNA extraction from the skin samples from both traumatized and treated traumatized tissue, following the manufacturer’s instructions. Then the RNA yield and purity were assessed via Nanodrop ND-2000 spectrophotometer (Thermo Electron) at 260 and 280 nm correspondingly. Viva 2-step RT-PCR Kit was utilized for the synthesis of cDNA from a total amount of 1 ug RNA following its guidelines. Quantitative PCR using Thermo Scientific Verso 1-Step RT-PCR Ready-Mix kit (Applied Biosystems, Foster City, CA, USA) was performed to assess the mRNA expression rate of the studied genetic markers (Table 2).
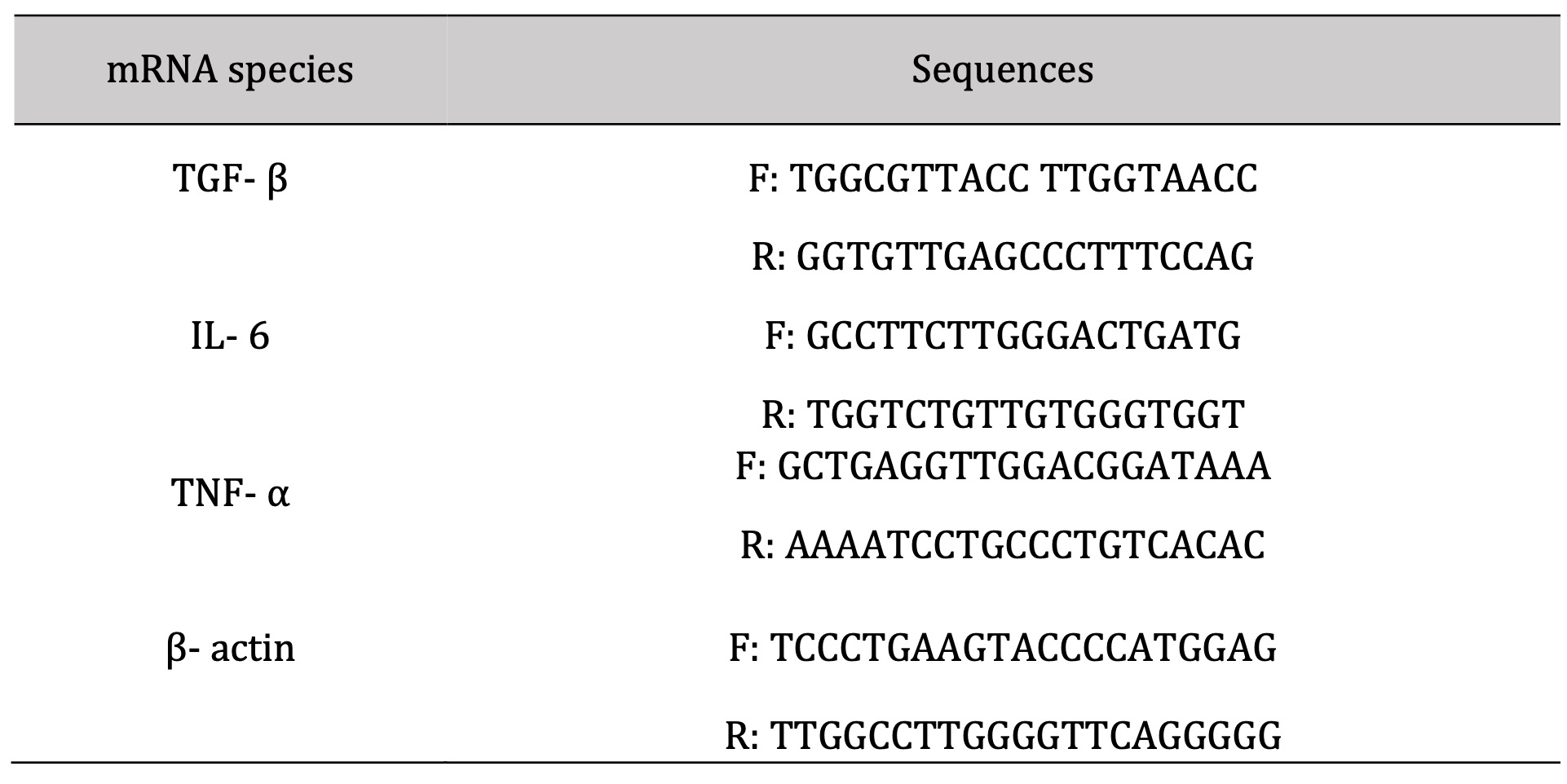
Table 2: Sequence of primers used in amplification
Quantifiable assays of the mRNA expression rate of HSP-90 α, MMP-9, and miR-21 genes via RT-qPCR
The nucleic acid extraction kit (NucleoSpin®) was used for the extraction of RNA obtained from Macherey- Nagel GmbH and Co. KG- Germany (REF 740955.50) following the producer’s guidelines. The spectrophotometry (dual Wavelength, Beckman Spectrophotometer, USA) was used for detecting the RNA purity (A260/A280 ratio) and concentration. Synthesis of the cDNA was done via Vivantis, ViPrimePLUS One Step Taq RT-qPCR Green Master Mix I with ROX (SYBR Green Dye) (cat no #QLMM14-R) kit following the manufacturer’s instructions. Samples of the prepared reaction mix were placed in real-time PCR (Step One, Applied Biosystem, Foster City, USA). ViPrime PLUS One Step Taq RT-qPCR Green Master Mix I with ROX Kit was compatible with three-step cycling (Table (3), performed for analyzing the mRNA expression rate of the target gene (Table 4).
The RQ of each studied gene is calculated according to the calculation of delta-delta Ct (ΔΔCt). We determined the RQ for each gene by taking 2-∆∆Ct.
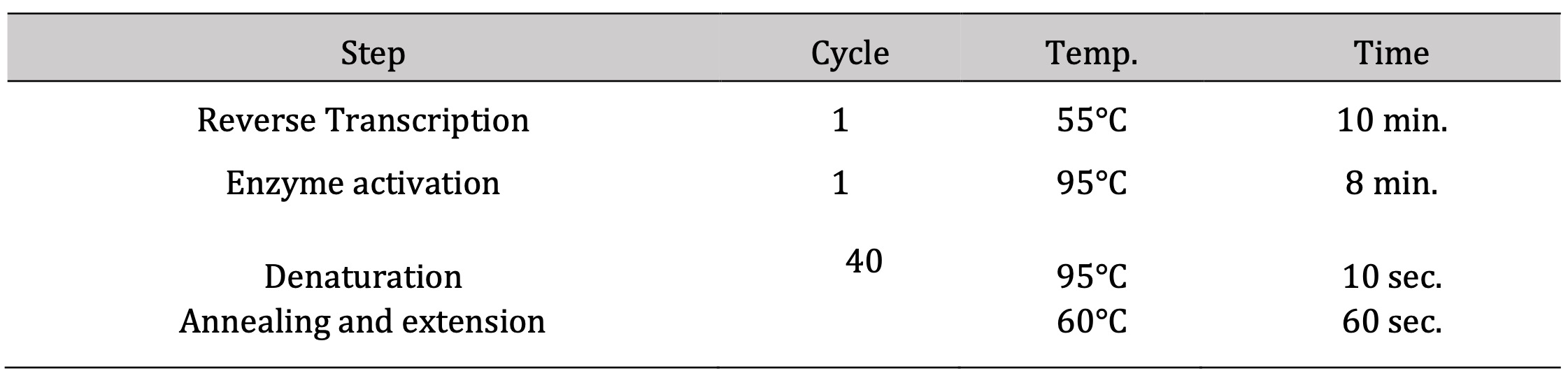
Table 3: The thermal profile cycling of RT-qPCR
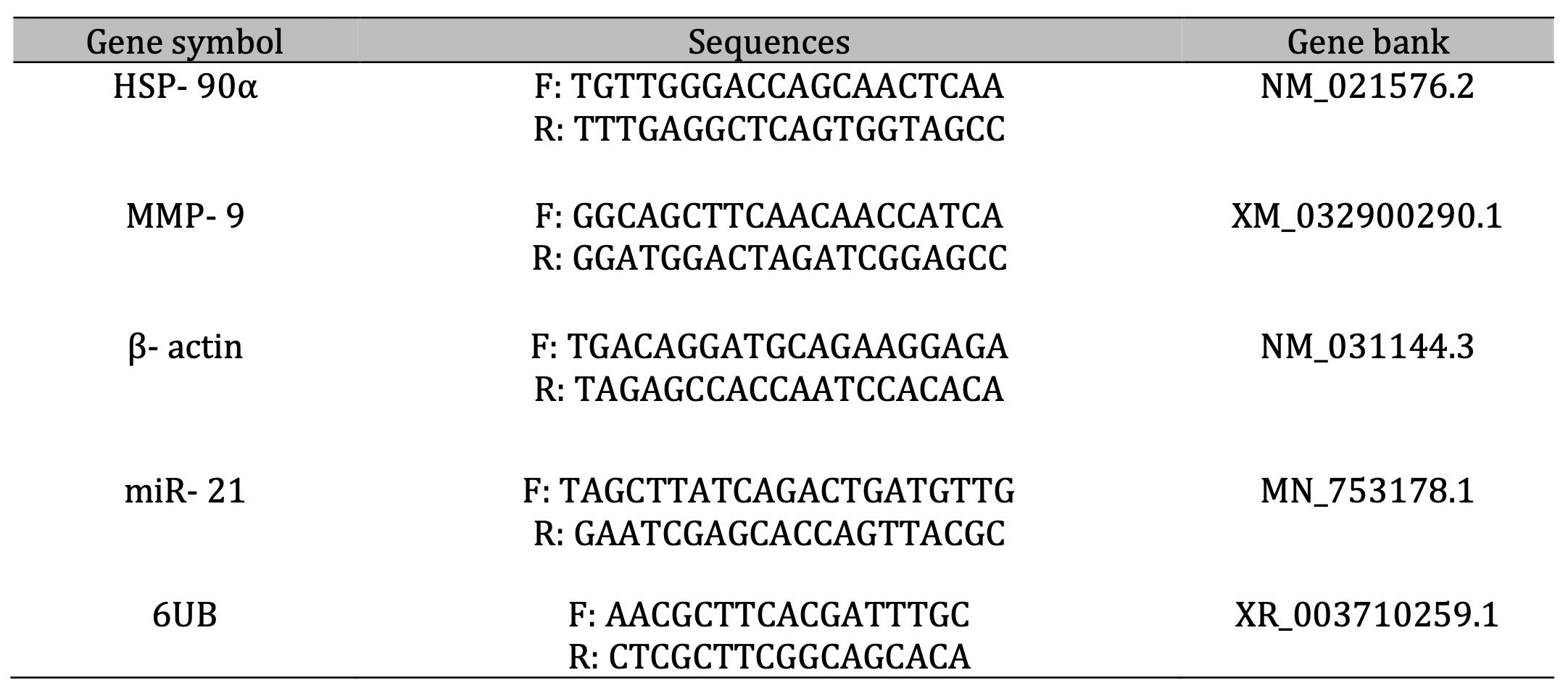
Table 4: The primer sets used for amplification
Determination rate of apoptosis
For determination of the cutaneous apoptotic rate, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) immunostaining was done using the Apo-BrdU In Situ DNA Fragmentation Assay Kit (Catalog #K401-60) in relation to its instructions.
The frozen tissue was sectioned and fixed with 4% formaldehyde/PBS in a Coplin jar followed by incubation and washing with PBS. Each section was covered with Proteinase K Solution followed by washing with PBS and fixation with 4% formaldehyde, then washing and drying the slide. About 50 μl of the DNA Labeling Solution was placed on the cells and covered with a plastic coverslip, and then incubated in a dark, humidified 37°C incubator for 60 minutes, the coverslip was removed and the slides were transferred to a fresh Coplin jar filled with PBS for rinsing then drying occur, and 100 μl of the Antibody solution was placed for incubation for 30 minutes at room temperature after that the solution was removed and 100 μl of Propidium Iodide/Rnase A solution was added with incubation, finally, the slides washed with ddH2O in a fresh Coplin jar and incubated at room temperature for 5 minutes. The slides were viewed using FITC and rhodamine filters. Apoptotic cells will exhibit strong nuclear green fluorescence. All cells should be stained with PI and exhibit strong red counterstaining.
Statistical analysis
The results are represented as the mean values ± the Standard Error (SE). Continuous variables were analyzed with the one-way analysis of variance (ANOVA), followed by the Duncan post hoc test. A P value of < 0.05 was regarded as statistically significant. All data analyses were performed via SPSS version 9 [41].
Results
Characterization of MSCs
Results illustrated in Table 5 and Figure 2 revealed marked-down regulation of the mRNA expression rate of CD 34 & CD 45, and significant up-regulation of CD 73 and CD 105.
The color changes of the bruised skin were observed as follows: Red color was developed after 3 hours of pinching the skin, and its intensity reduced with time and became slightly red at 6 and 12 hours. A slight bluish coloration appeared after 24 hours, while the yellow coloration was detected at 48 hours and became prominent at 72 hours. The chronology of the previous colors was noted following therapy, but their intensity markedly reduced, especially at 72 hours (Fig. 3).
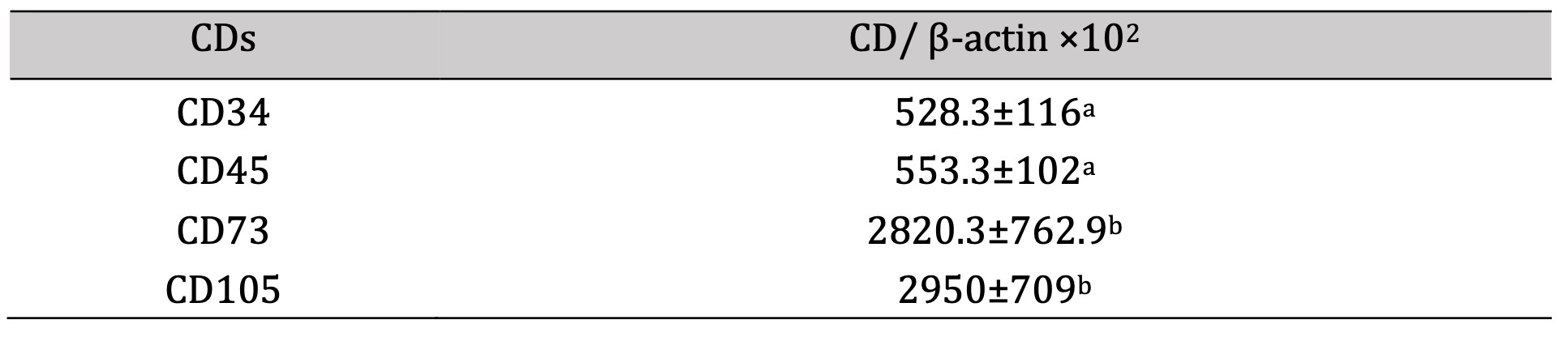
Table 5: mRNA expression rate of CDs compared to β-actin. *a-b, Means which have the same superscript symbol(s) are not significantly different. Data are expressed as mean ± SE (n=6) A P value of (< 0.05)
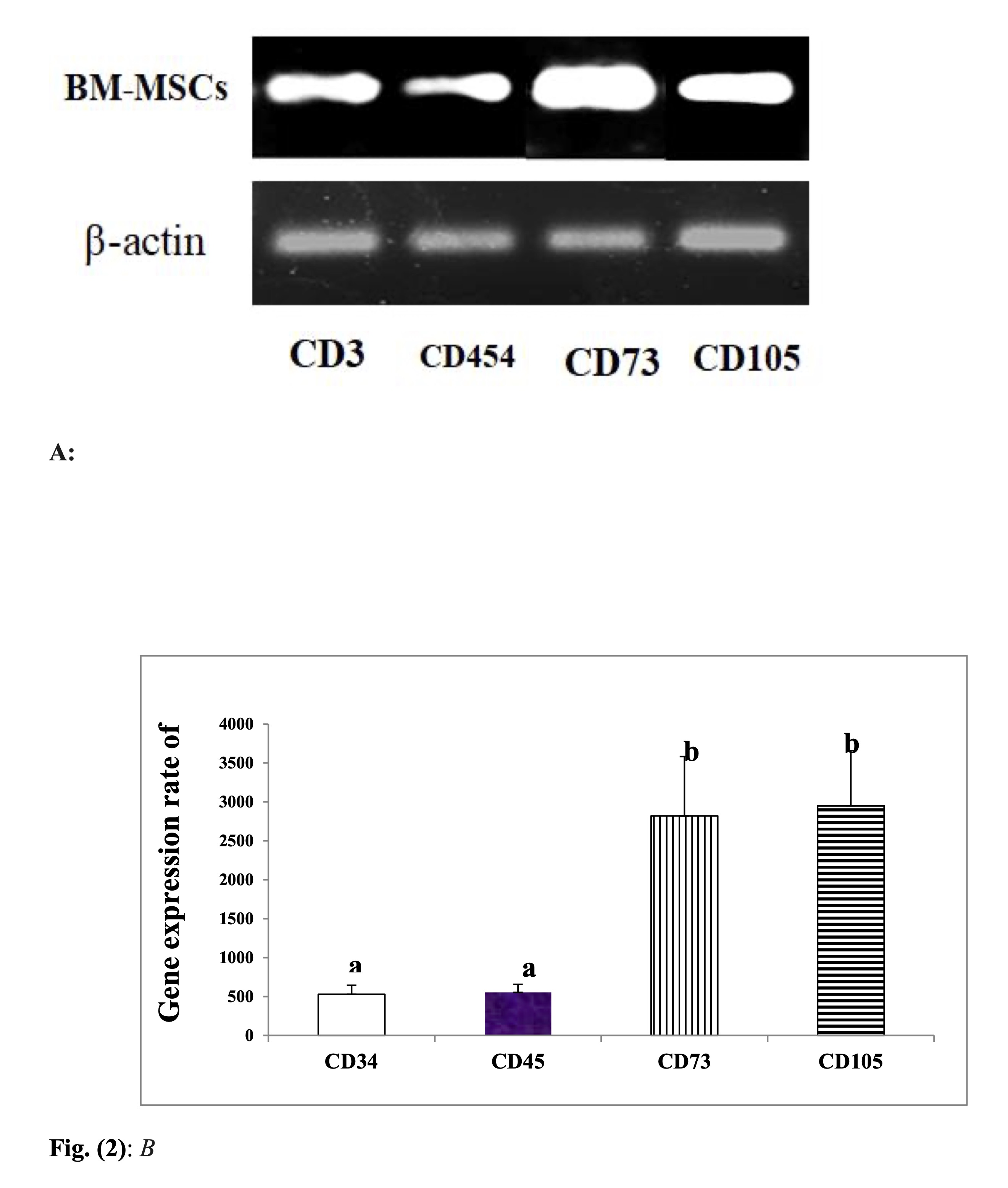
Fig. 2: mRNA expression rate of CDs of BM-MSCs. B: Desinometric analysis of PCR products of CDs A: Gel photograph showing PCR products of CDs in BM-MSCs.
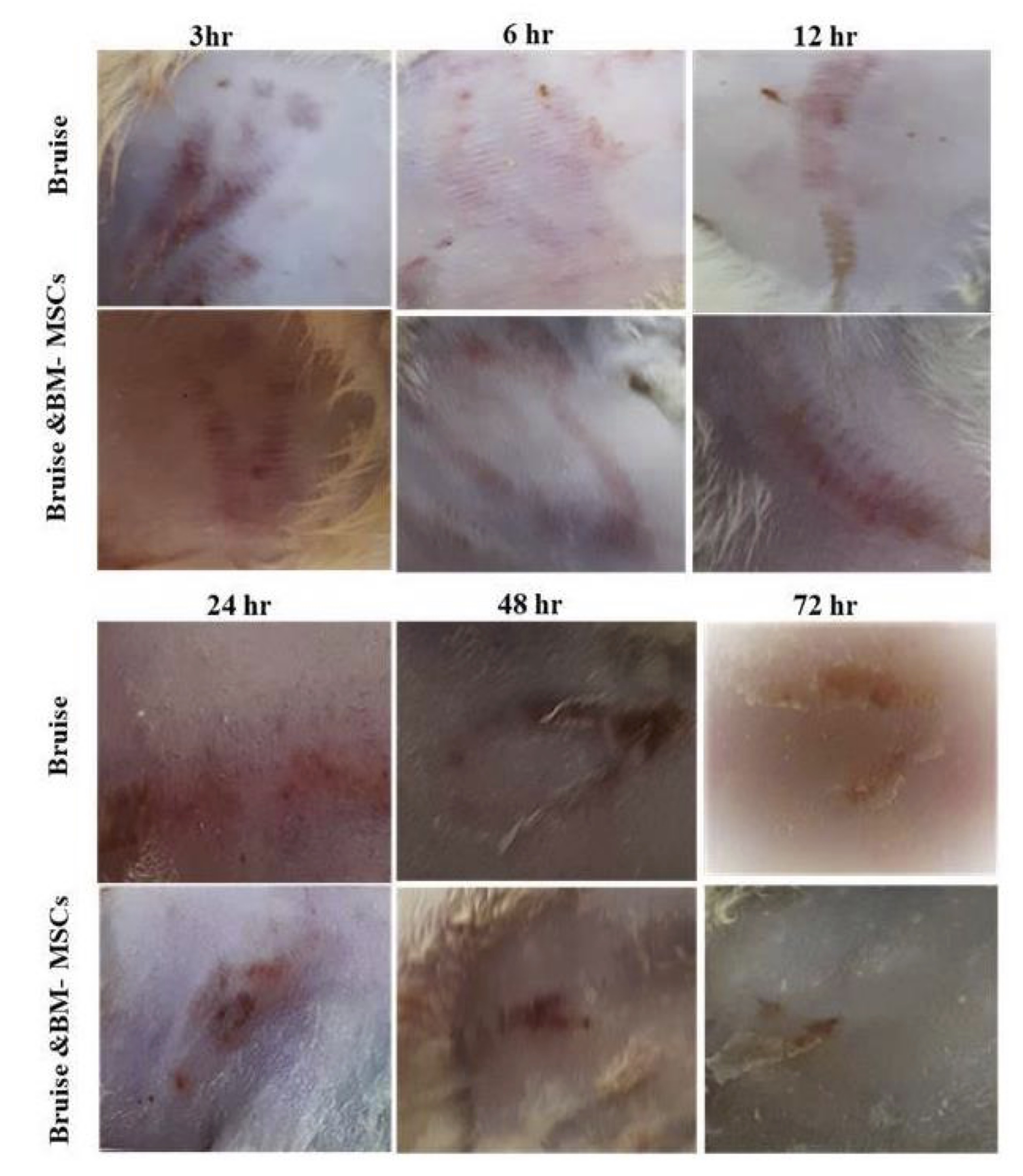
Fig. 3: Effect of BM-MSCs on the color changes of bruised tissue.
Histopathological findings
Histopathological examination of H&E-stained skin sections of the bruised animals (G2) and the treated animals (G3) revealed degenerative changes of the squamous epithelium lining, presence of leukocytic infiltrations mainly neutrophils in the dermal layer associated with congested blood vessels till 12 hr post-traumatic injury Fig. 4 (A, B, and C) and (a, b, and c). At 24 hr, the neutrophilic infiltration was extended to the muscular layer (Fig. 5A), which increased significantly at the treated corresponding hour (Fig. 5a). Then the leuckocytic infiltration of the dermal layer was reduced until 72 hr, Fig. 5 (B and C). Moreover, in the corresponding treated hours, minimal leuckocytic infiltration in the dermal layer can be found in Fig. 5 (b and c).
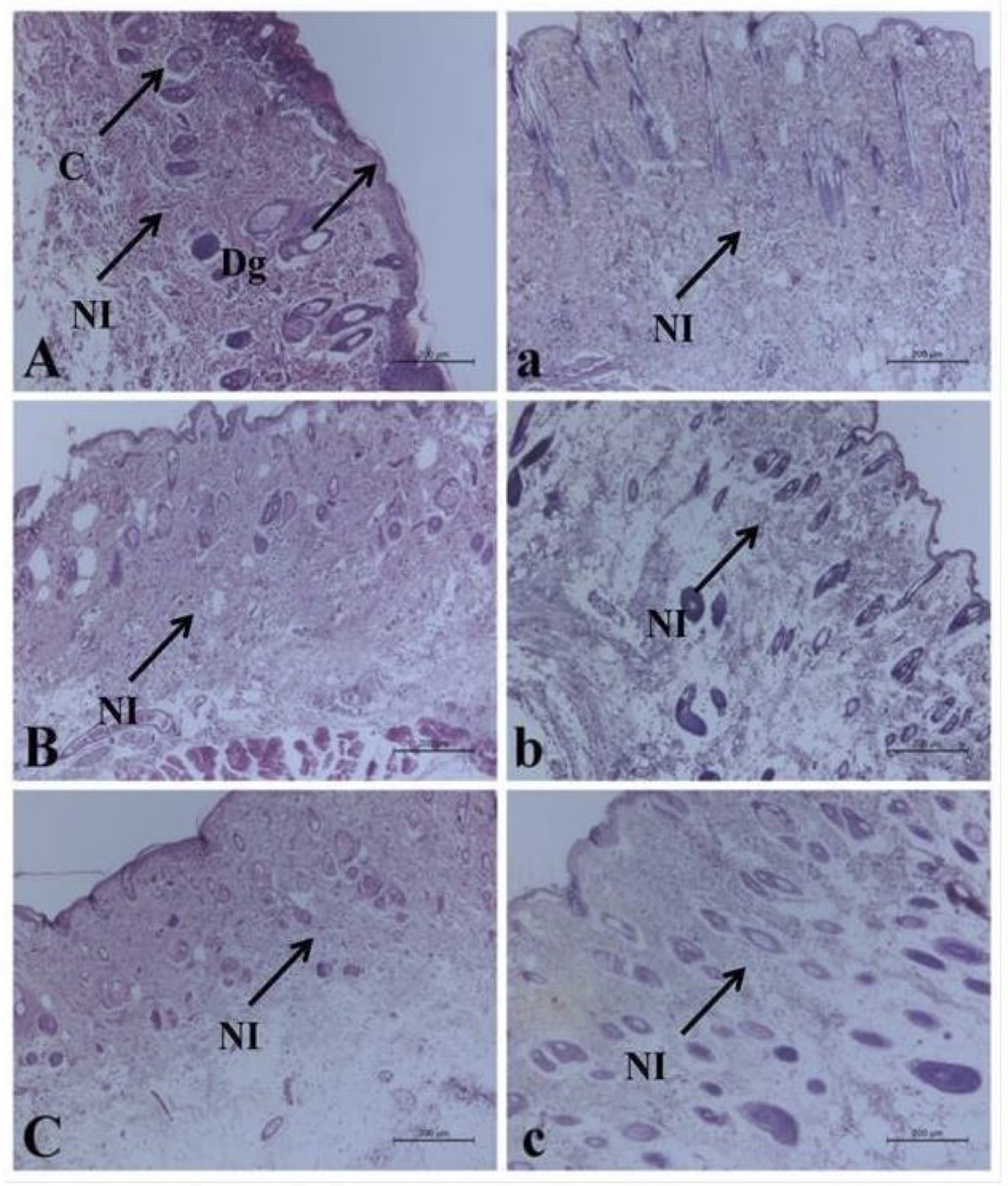
Fig. 4: Histopathological alterations of the bruise wound at (3, 6, and 12 hr) in A, B, and C respectively, and the treated wound in (a, b, and c) respectively, including degenerative changes (Dg), congestion (C) and neutrophilic infiltration (NI). (H&E).
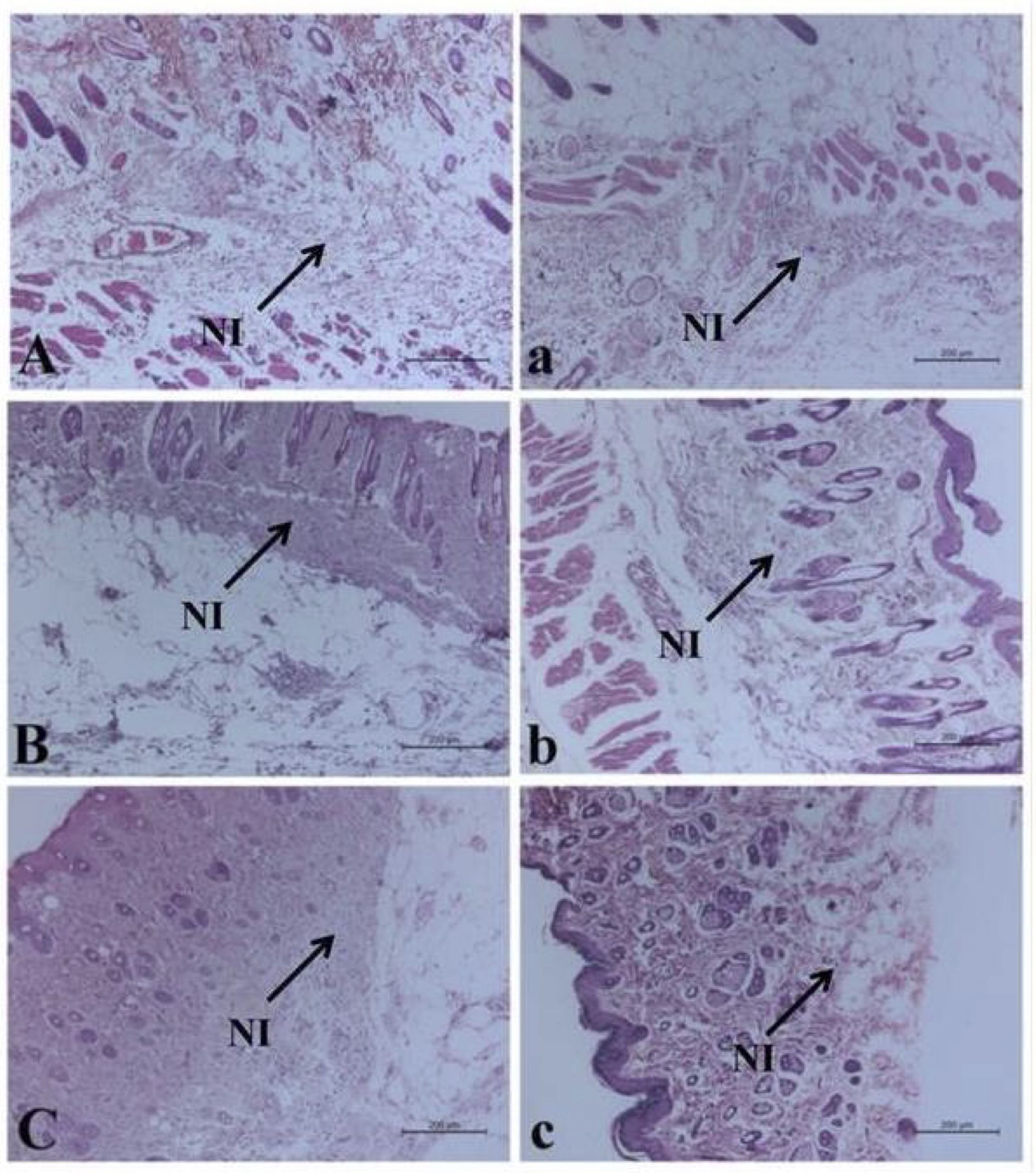
Fig. 5: Histopathological alterations of the bruise wound at (24, 48 and 72 hr) in (A, B, and C) respectively, and the treated wound in (a, b and c) respectively including neutrophilic infiltration (NI). (H&E).
The mRNA expression rate of TGF- β, IL- 6, and TNF- α
Injection of the bruised skin with BM-MSCs resulted in significant upregulation of TGF-β mRNA expression at 3, 6,12 and 48 hr. with marked reduction at 72 hr. (Fig. 6). In contrast, a significant downregulation in the IL-6 expression rate was determined at 6 and 24 hr., While a significant upregulation, was detected at 48 and 72 hr. following therapy with BM-MSCs (Fig. 7). Concerning the mRNA expression rate of the mRNA expression rate of TNF-α was upregulated significantly posttreatment with BM-MSCs (Fig. 8, Table 6).
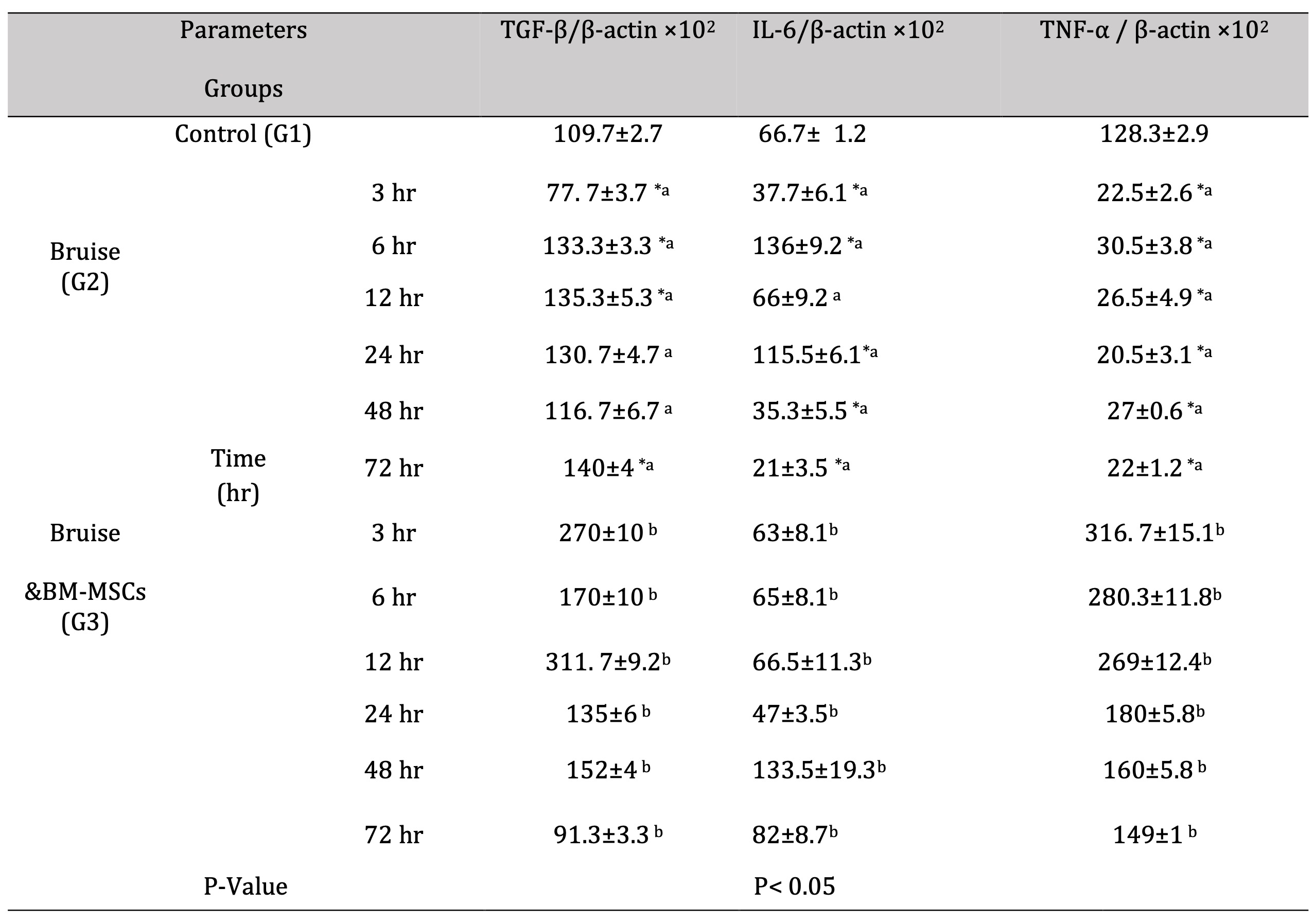
Table 6: mRNA expression rate of TGF-β and the pro-inflammatory cytokines (IL-6 &TNF-α). *a- b, Means which have the same superscript symbol(s) are not significantly different. Data are expressed as mean ± SE (n=6) A P value of (< 0.05). The different superscript letters indicated a significant difference between groups at level p< 0.05
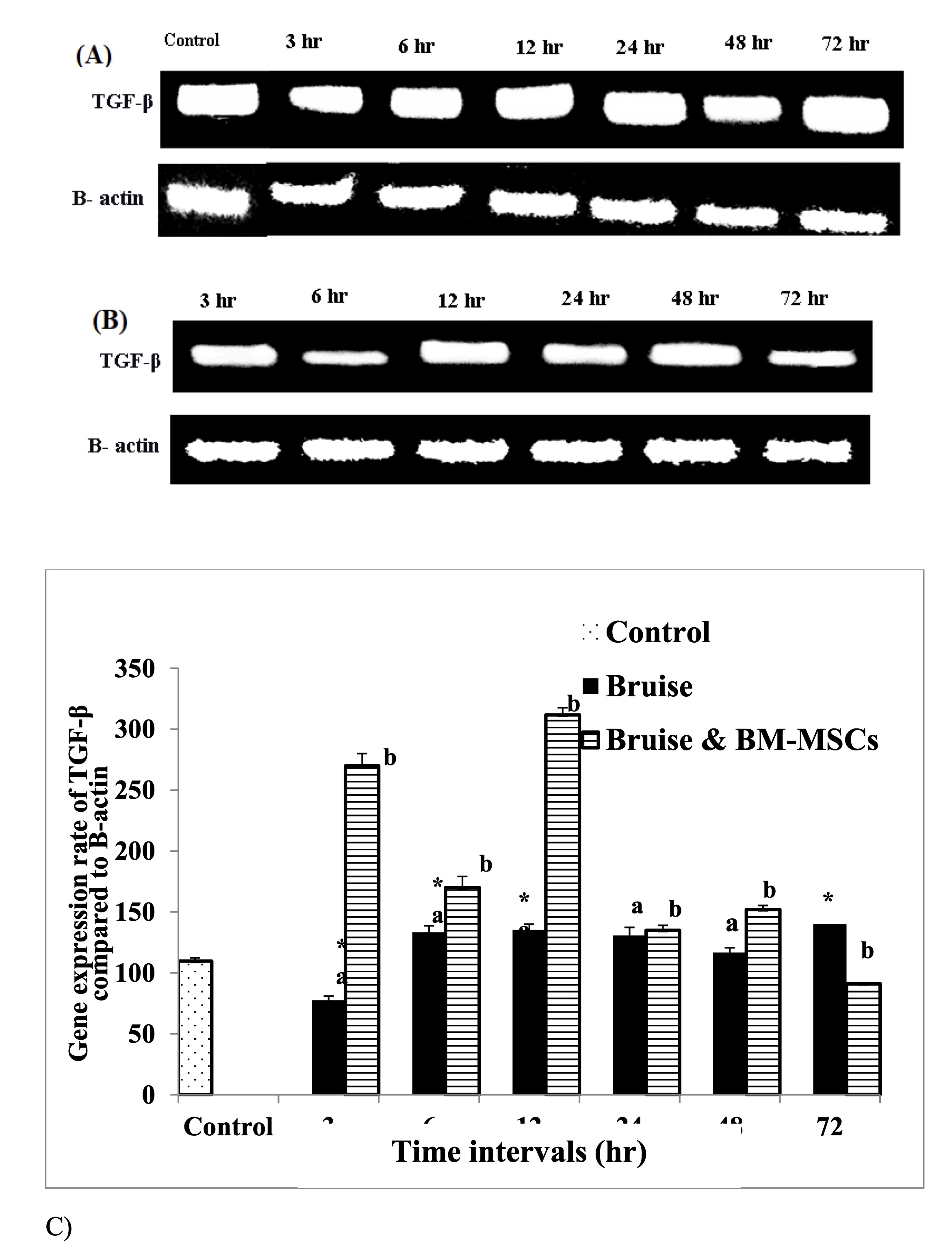
Fig. 6: mRNA expression rate of TGF-β. A: Gel photograph demonstrating PCR products of TGF-β in bruise, B: Gel photograph demonstrating PCR products of TGF-β in bruise and BM MSCs and C: Desinometric analysis of PCR products of TGF- β. * Means significance, data are expressed as mean ± SE (n=6) A P value of (< 0.05).
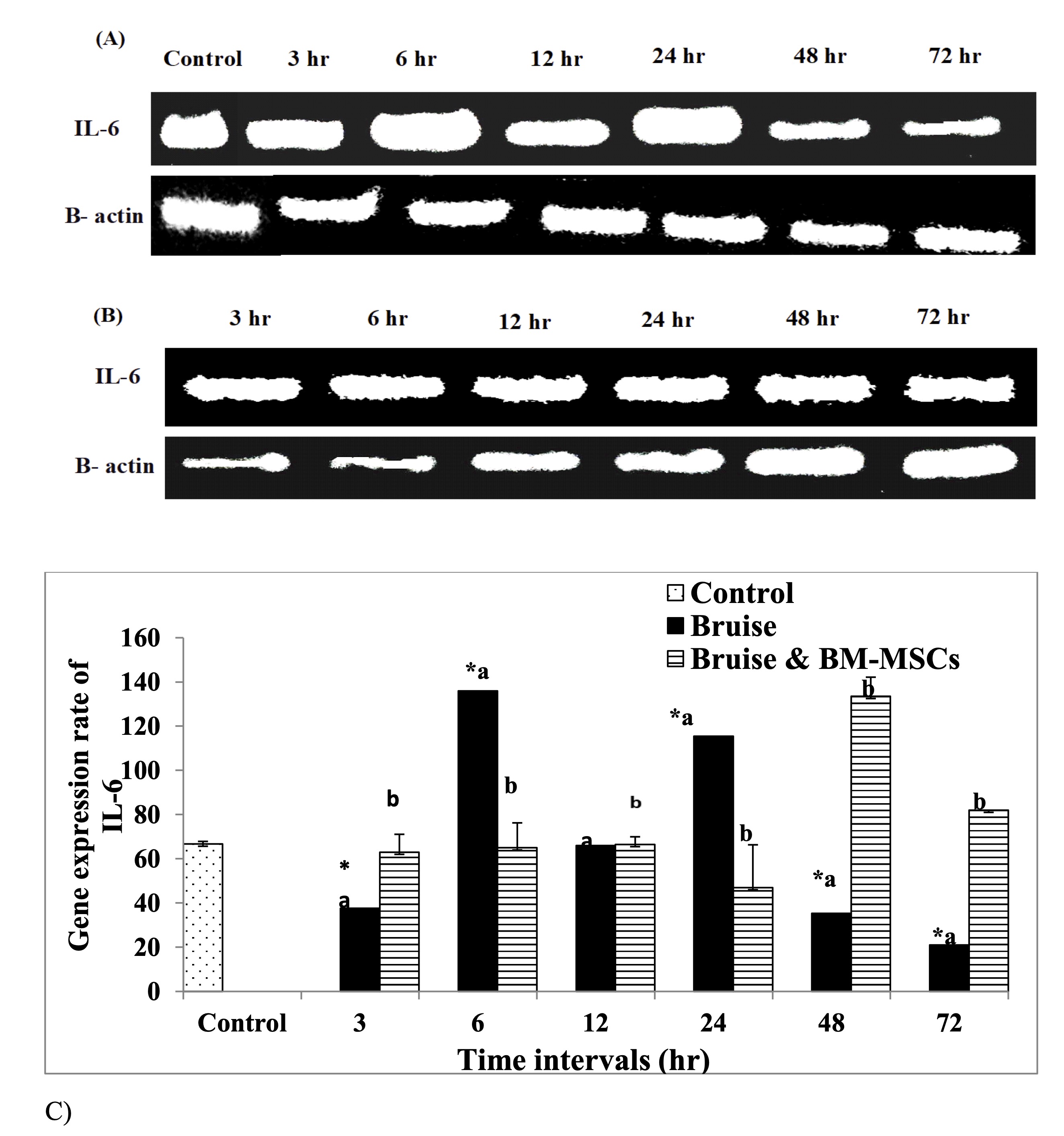
Fig. 7: mRNA expression rate of IL-6 pro-inflammatory cytokine A: Gel photograph demonstrating PCR products of IL-6 in bruise, B: Gel photograph demonstrating PCR products of IL-6 in bruise and BM MSCs and C: Desinometric analysis of PCR products of IL-6. * Means significance, data are expressed as mean ± SE (n=6) A P value of (< 0.05).
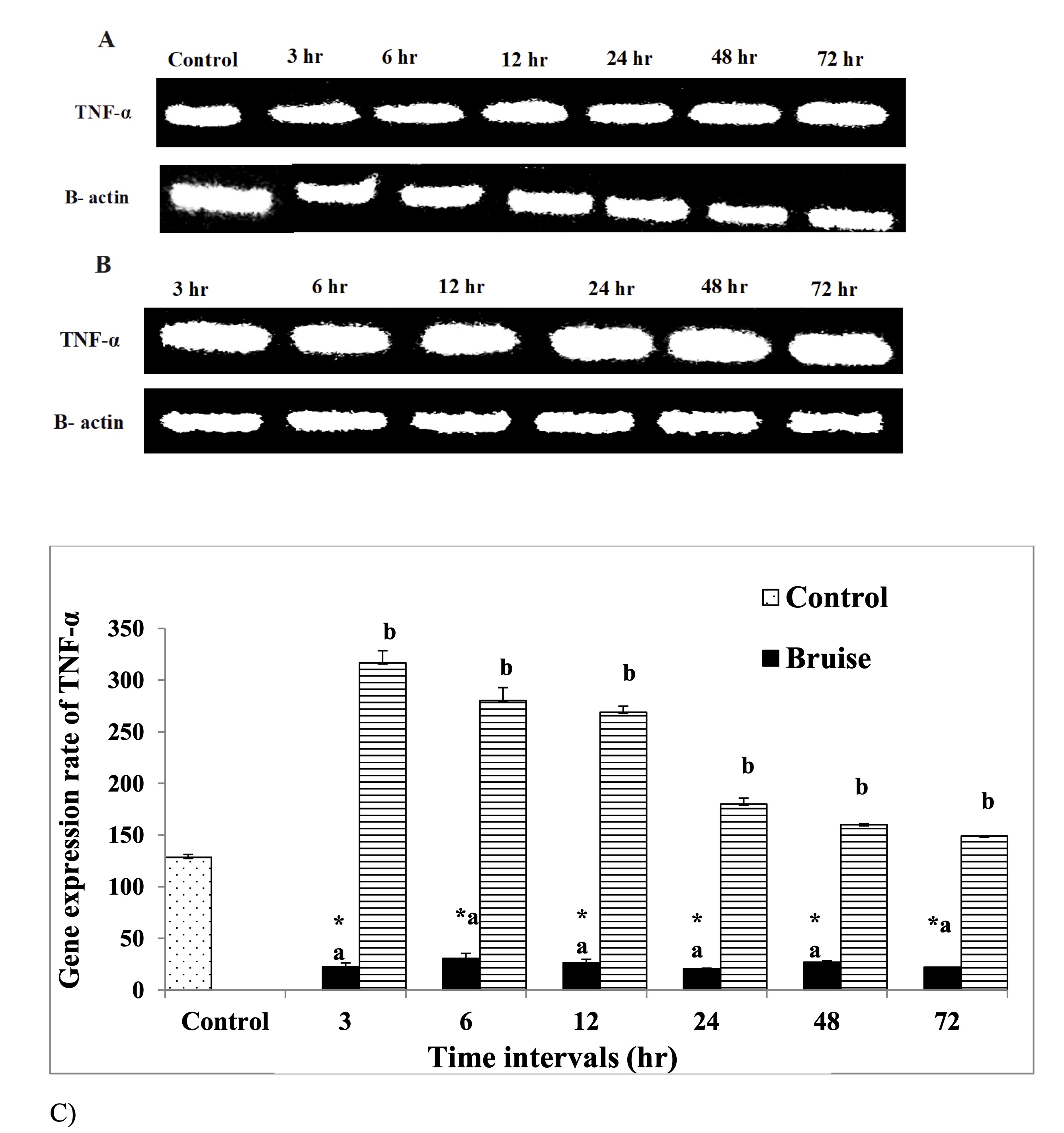
Fig. 8: mRNA expression rate of TNF-α pro-inflammatory cytokine A: Gel photograph demonstrating PCR products of TNF-α in bruise, B: Gel photograph demonstrating PCR products of TNF-α in bruise and BM MSCs and C: Desinometric analysis of PCR products of TNF-α.* Means significance, data are expressed as mean ± SE (n=6) A P value of (< 0.05).
The mRNA expression rate of HSP- 90 α, MMP- 9, and miR- 21 genes
The HSP-90α expression was markedly upregulated in the bruised animals treated with BM-MSCs, in all-time intervals (Fig. 9). Regarding MMP-9 and miR-21 expression rates, the treated animals with BM-MSCs showed significant down-regulation as illustrated in Fig. 10 and Fig. 11 respectively Table 7.
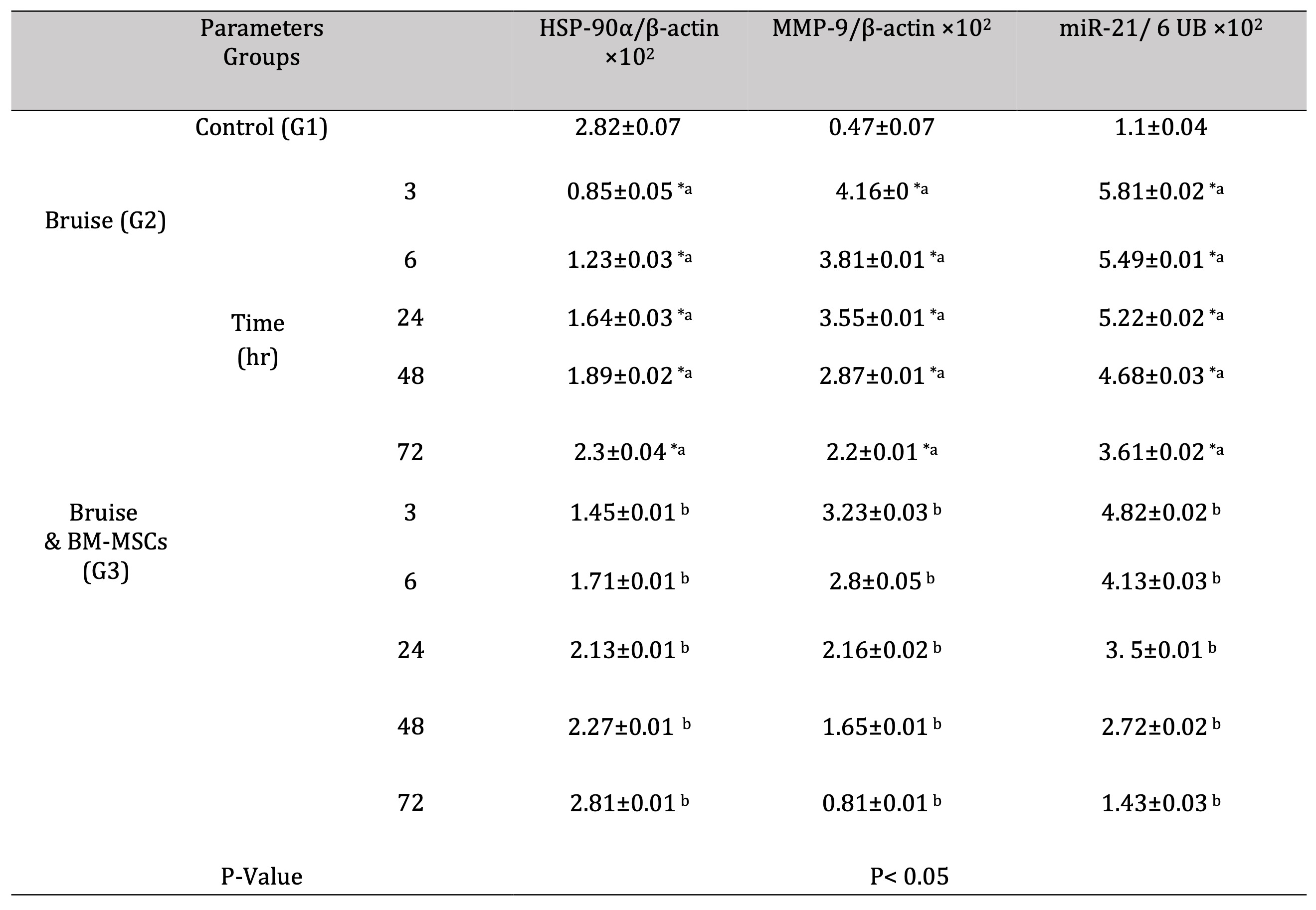
Table 7: mRNA expression rate of HSP-90α, MMP-9, and miR-21 genes in bruise wound and the treated wound with BM-MSCs. *a- b, Means which have the same superscript symbol(s) are not significantly different. Data are expressed as mean ± SE (n=6) A P value of (< 0.05)
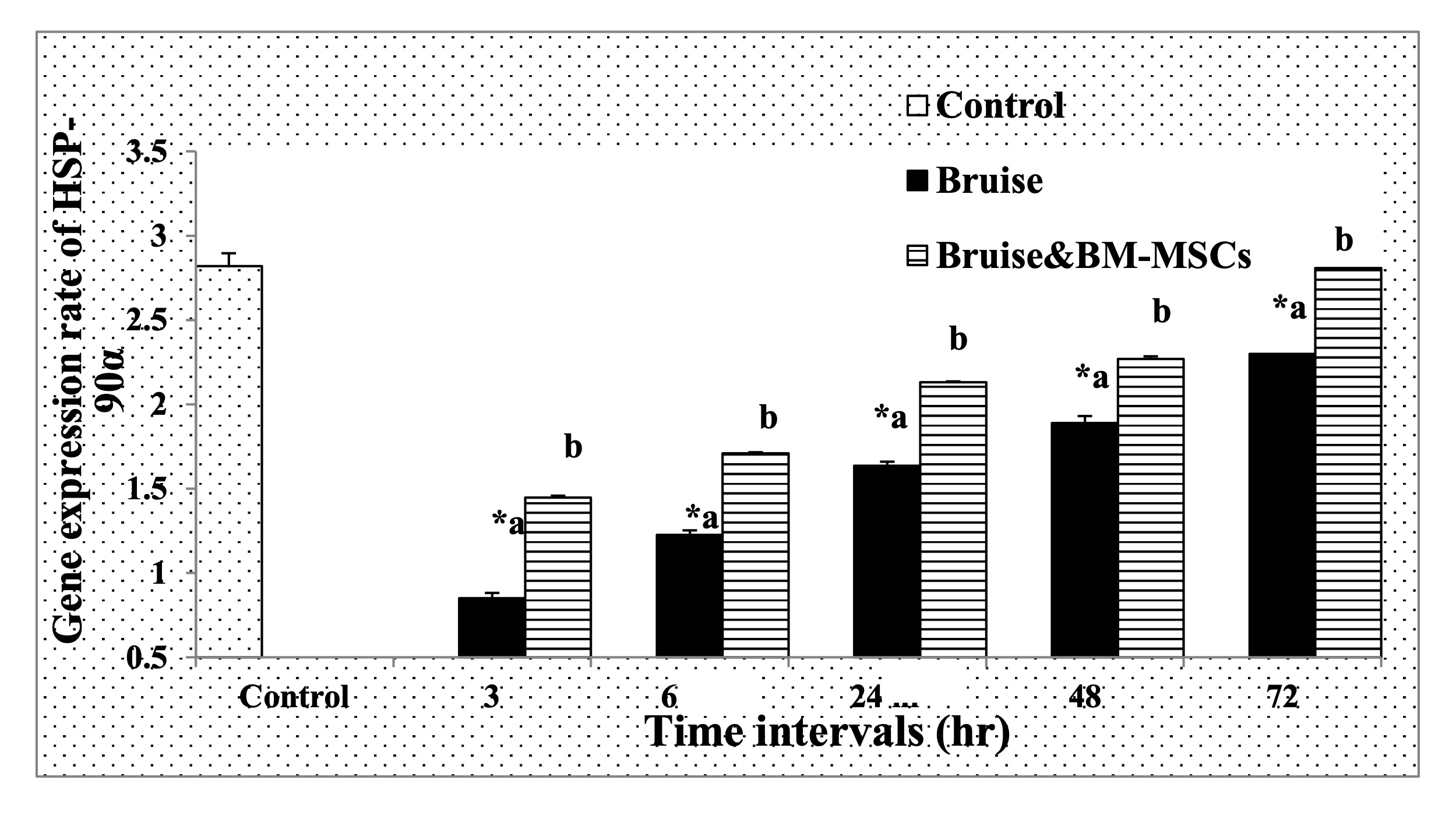
Fig. 9: The mRNA expression rate of HSP-90α. * Means significance, data are expressed as mean ± SE (n=6) A P value of (< 0.05).
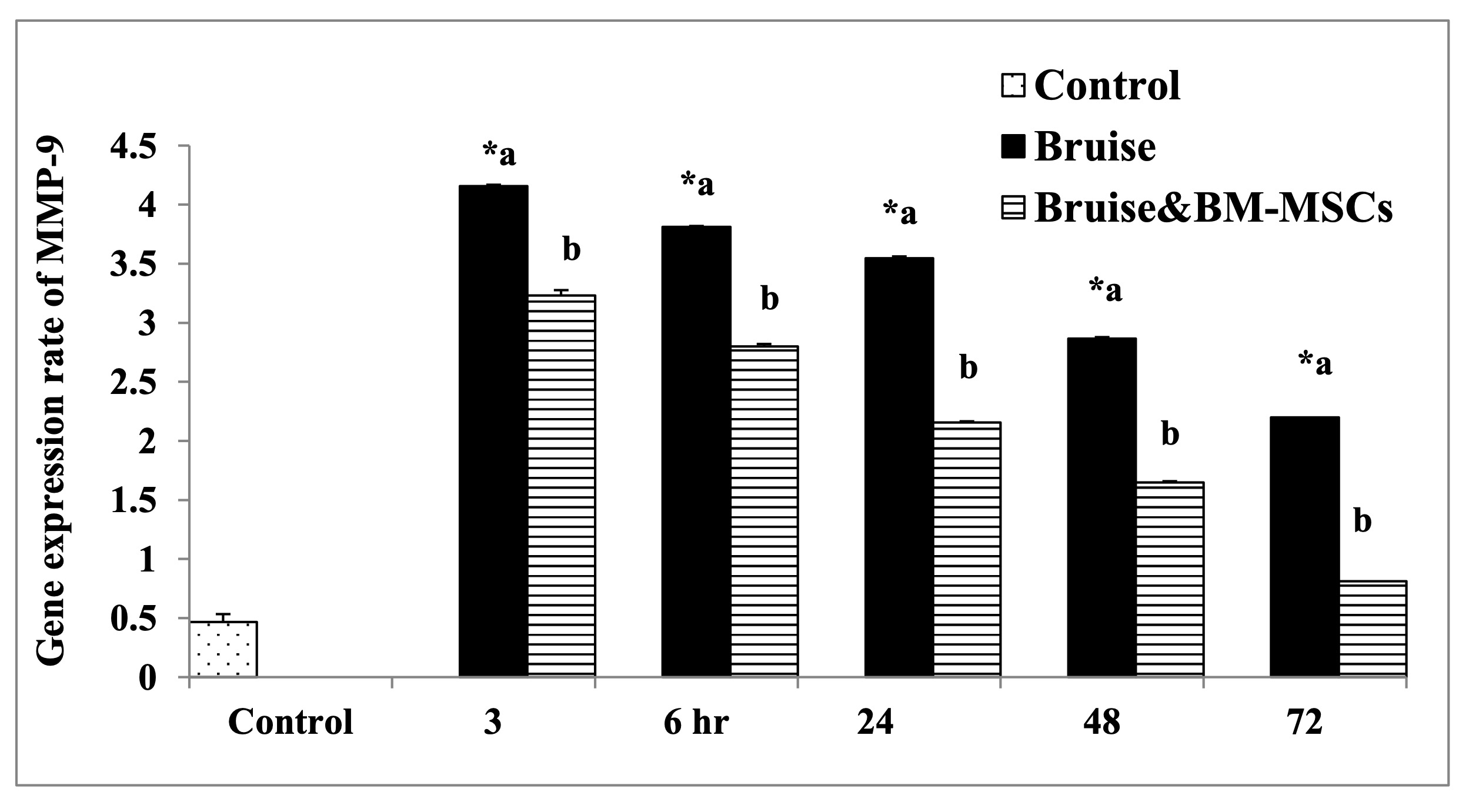
Fig. 10: The mRNA expression rate of MMP-9. * Means significance, data are expressed as mean ± SE (n=6) A P value of (< 0.05).
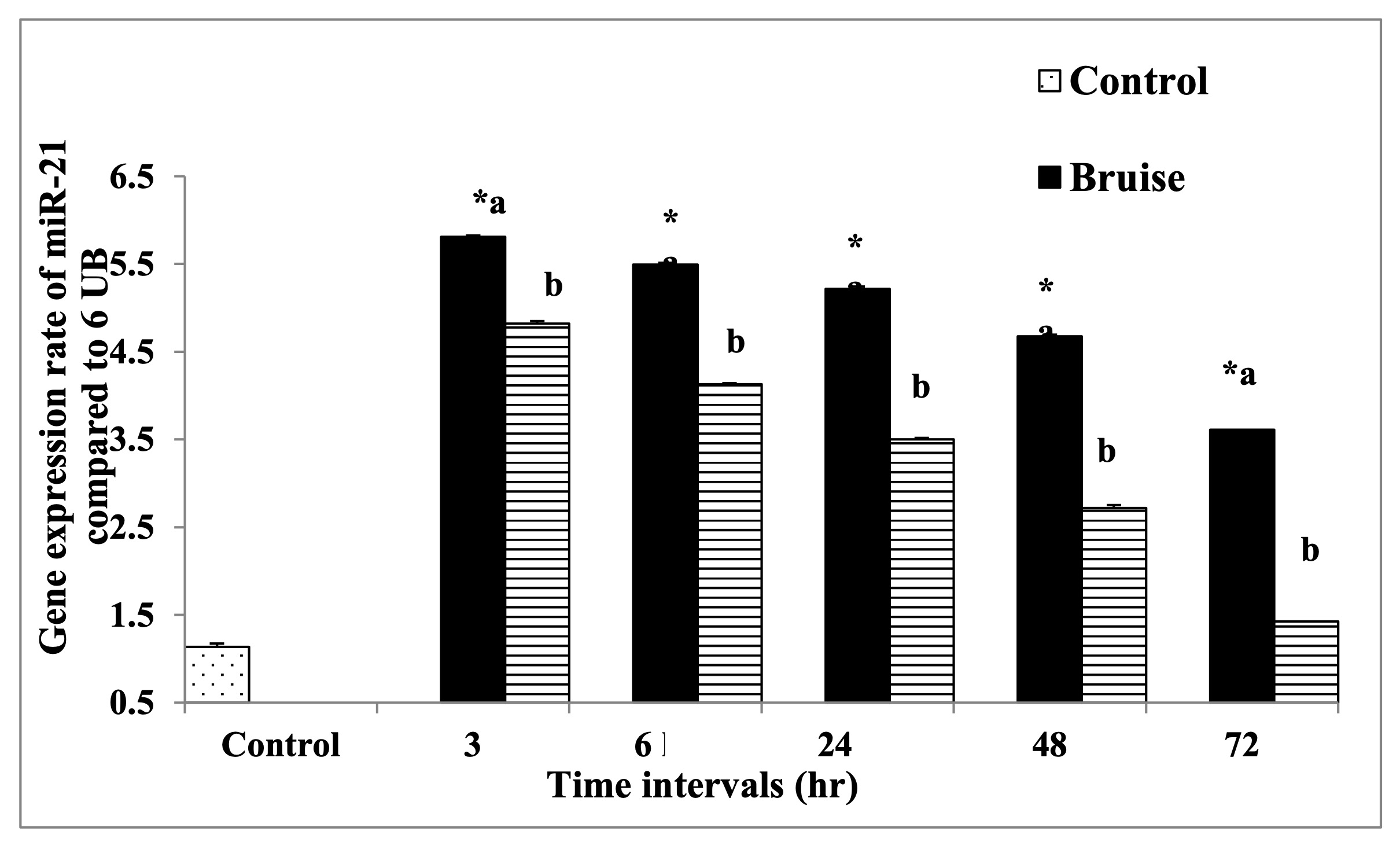
Fig. 11: The mRNA expression rate of miR-21 gene. * Means significance, data are expressed as mean ± SE (n=6) A P value of (< 0.05).
The rate of apoptosis
The obtained data in Figure (12) and Table (8) revealed that treating the traumatic animals with BM-MSCs causing decreased the apoptotic rate at all time intervals significantly compared to the traumatic ones.
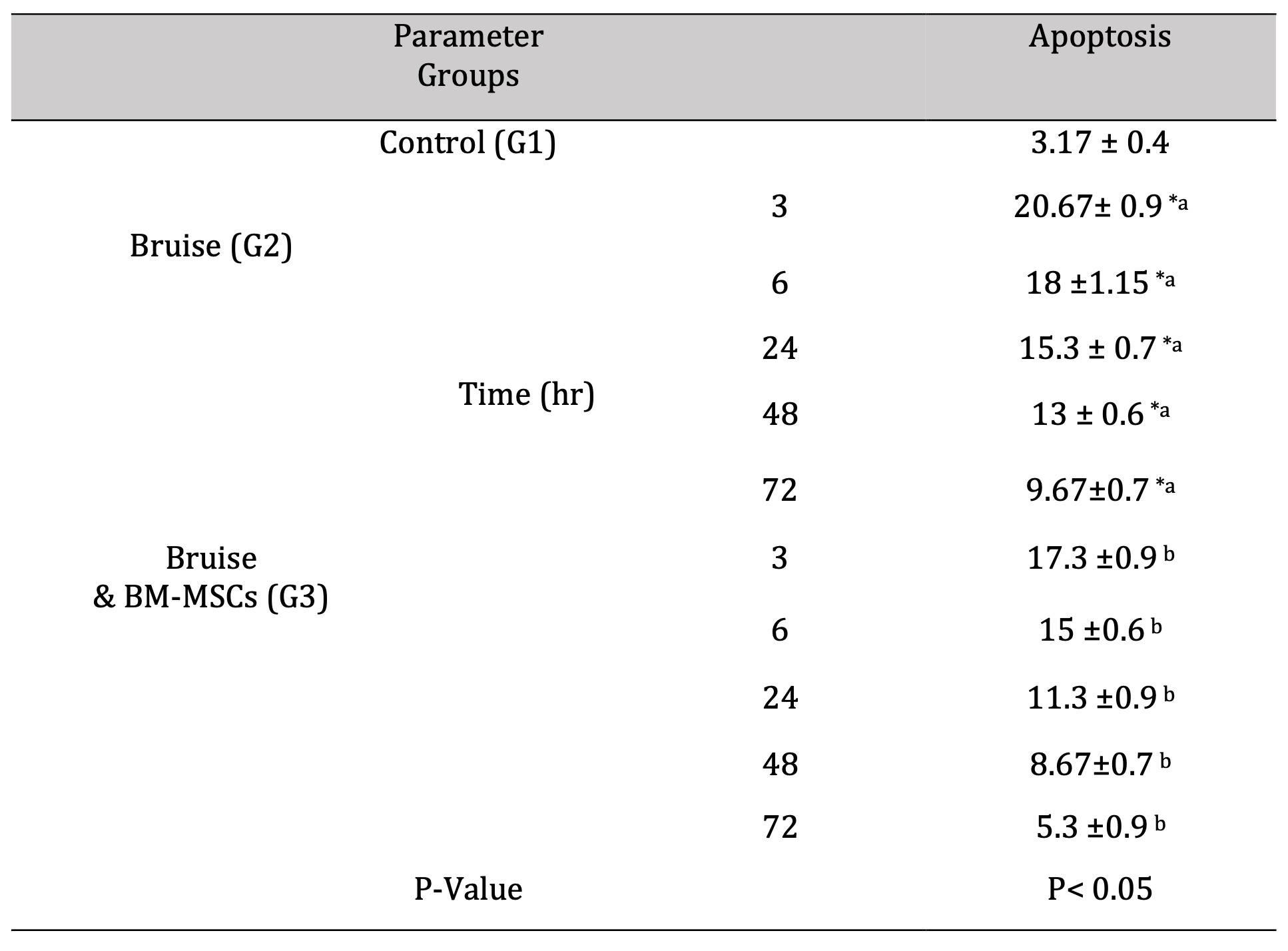
Table 8: The rate of apoptosis. *a- b, Means which have the same superscript symbol(s) are not significantly different. Data are expressed as mean ± SE (n=6) A P value of (< 0.05)
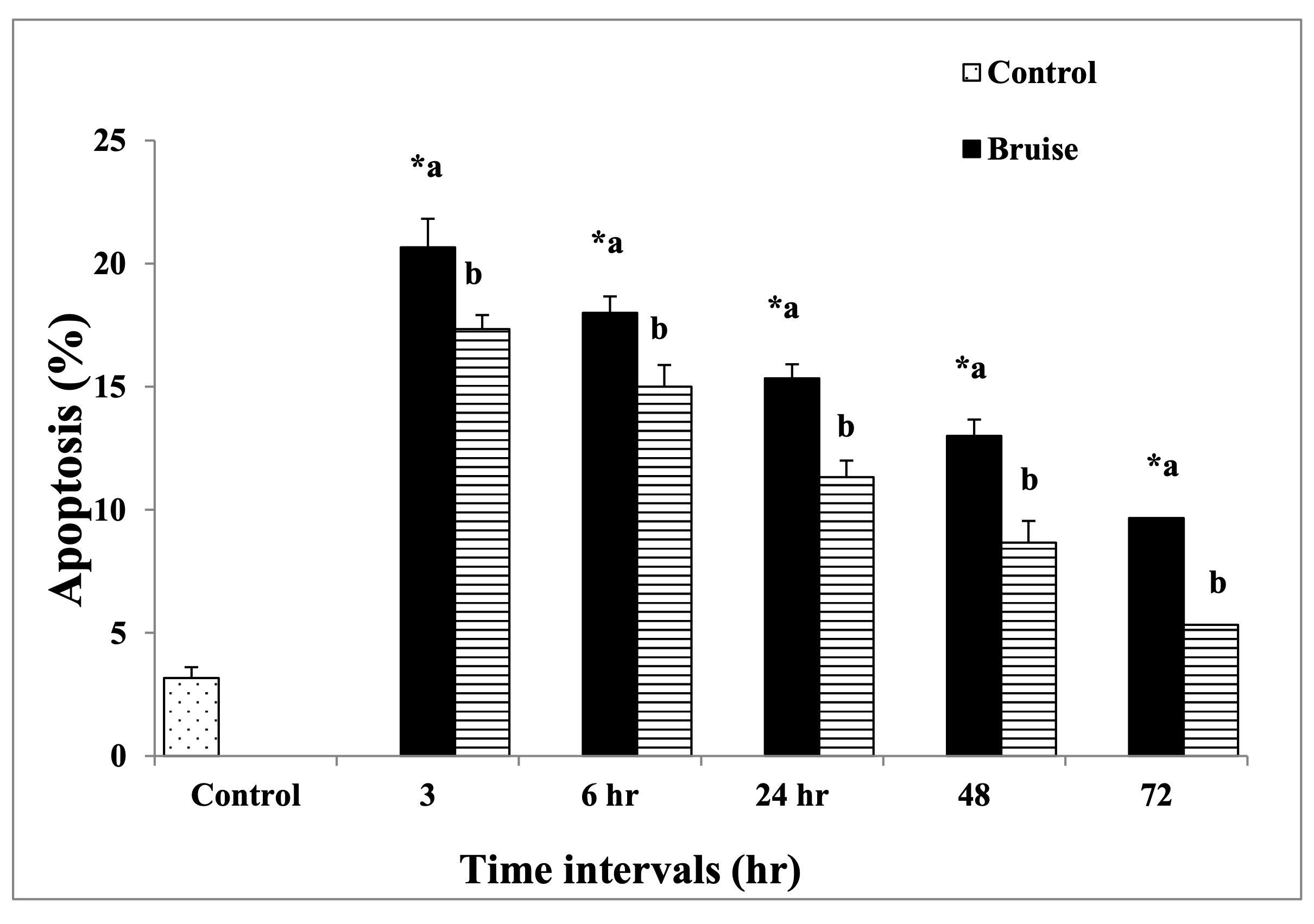
Fig. 12: The rate of apoptosis. * Means significance, data are expressed as mean ± SE (n=6) A P value of (< 0.05).
Discussion
Bruising is distinctly a source of pain, [42] it has resulted from an extreme mechanical force sufficient for crushing the small vessels within the skin and the underlying tissues [43]. After the blood leaked into the tissues, the inflammatory response was stimulated to break and remove it [44]. In which the activated macrophages phagocytose RBCs and hemoglobin undergo degradation, [45] through a biochemical process, which catalyzes via Haem-oxygenase generating an iron atom, carbon monoxide, and biliverdin (green) [46]. Biliverdin was metabolized rapidly to bilirubin (yellow) [44]. whilst the created iron constituting complex with ferritin to yield the hemosiderin (brown), so bruises assume variable colors through healing [44]. In the existing study, the color intensity of the bruised skin was markedly reduced after therapy with BM-MSCs especially at 72 hr., as MSCs cause up-regulation of haemoxygenase, [46] which has anti-apoptotic, anti-inflammatory, and antioxidant properties [47].
MSCs ameliorate the tissue repair process via paracrine signaling and differentiation mechanisms; whereas their differentiating capacity participates in regenerating damaged tissues and paracrine signaling orchestrates the local cellular responses to injury resulting in regression of inflammation, and promotion of angiogenesis by stimulating cell migration and proliferation [48]. Hence, the histopathological examination of the treated bruised tissues revealed that
the neutrophilic infiltration was increased significantly in the muscular layer at 24 hr., While, at 48 and 72 hr., minimal leukocytic infiltration in the dermal layer could be found.
MSCs’ paracrine function means that MSCs have the ability to modulate and incorporate in the inflammatory, proliferative, and remodeling wound healing process through their secretion of variable bioactive molecules as chemokines, growth factors, cytokines, and exosomes [49]. The excreted exosomes bind to the vascular endothelial cell receptors resulting in stimulation of the downstream signaling pathways as the EGFR, ERK1/2, PI3K/Akt and subsequently modulating their proliferation, migration, and differentiation with activation of neovascularization [50].
Healing of mechanical injury starts with platelet arrival for hemostasis, mediation, and secretion of various vasoactive substances and mediators such as prostaglandins, fibroblast growth factor (FGF) platelet-derived growth factor (PDGF), TGF-β, serotonin, b-thromboglobulin, epidermal growth factor (EGF), bradykinin, thromboxane, and histamine, which acts as a stimulator for the healing process [51]. TGF-β can trigger the early response of pro-inflammatory cytokines secretion, involving IL-6, IL-1b, and TNF-α [52]. Therefore, the level of IL-6 was markedly increased in the current study under the influence of TGF-β; also, MSCs secreted IL-6 as an immunomodulatory effect causing inhibition of lymphocyte apoptosis [53].
Modulation of the inflammatory microenvironment via BM-MSCs could be done under the effect of secreted TSG-6 that suppresses the stimulation of the TLR2/NF-κB signaling pathway [31].
Triggering of vigorous anti-inflammatory and immunosuppressive mechanisms has been recorded following apoptotic cell engulfment by activated macrophages at the wound site which secretes IL-10 (anti-inflammatory cytokine) and inhibits TNF-α secretion, [54] which may be the reason for the inhibited TNF-α expression rate, while the rate was up-regulated with BM-MSCs therapy in the current study, as MSCs could become pro-inflammatory to stimulate the early tissue repair [55].
The latest study by Jiang et al [56]. recorded that the activation of TGF-β receptors on MSCs may upregulate or suppress the expression level of miR-21, according to the concentration of TGF-b1 in the wound bed, while overactivation of TGF-β receptors on MSCs at extreme TGF-β1 concentrations down-regulates miR-21, so inhibition of Smad7 translation not happened via miR-21 and ultimately production of TGF-b1 from MSCs was suppressed. Thus, the signaling between the TGF-β receptor, Smad7, miR-21, and TGF-β1 regulates the production of MSC-derived TGF-b1 production according to the wound site needs [57]. These results were identified in the current study where the rate of both TGF-β and miR-21 were down-regulated after treatment with BM-MSCs.
The paracrine function of BM-MSCs causes upregulation of MMP- 9 and TGF-β3, leading to increasing granulation tissue formation with inhibition of extracellular matrix (ECM) deposition during the proliferation phase [58]. Furthermore, MSCs have the ability to inhibit the regression of collagenous matrix and maintain the matrix with the promotion of the regeneration of fibroblasts through downregulating the expression rate of MMPs [59] hence, they provide favorable conditions for ECM remodeling during the healing process [60], which reported in our study in which BM-MSCs causing significant down-regulation of MMP-9.
In addition, their paracrine actions downregulated nucleic acid and protein metabolism, apoptotic MSCs enhance the process of wound healing by suppressing inflammation, accelerating angiogenesis, and stimulating fibroblast migration and collagen production via their paracrine actions [61], and upregulated homeostasis and antiapoptotic genes, they can synthesize the granulocyte-macrophage colony-stimulating factor (GM-CSF), that suppress the cellular apoptosis and maintain the tissue homeostasis [62]. So the apoptotic rate was significantly reduced after therapy with BM-MSCs in the present study.
Multiple mechanisms have been reported to be incorporated in MSCs anti-apoptotic effects such as prevention of the dysfunctional MMPs [63], inhibition of cellular death signals as caspase-3 [64], or activation of survival signaling pathways such as phosphorylation of STAT3 and Akt [65], secretion of exosomes and growth factors [66].
A plenty amount of Hsp90α was preserved in all cell types of the skin and secreted into the extracellular space under stress conditions, such as tissue injury leading to disruption of the vessel integrity with rapid consumption of oxygen by the cells, resulting in a hypoxic microenvironment that stimulated Hsp90α production that influenced by HIF-1α and consequently enhanced skin cell migration through LRP-1 to initiate the wound healing [67]. In our study, a dramatic increase in the Hsp90α level has been observed following treatment by BM-MSCs till the level reached its level in normal state, which may be due to the production of Hsp-90α from cells in the wound bed or penetrated immune cells or increased Hsp90α expression intracellular [68].
One of the facts that helps in the scalability of BM-MSCs widely is their easy collection and isolation from the bone marrow, [69] but many issues faced the Conveying MSCs from the laboratory to clinical application such as quality control and discrepancies insufficiencies in many aspects as immunocompatibility, stability, heterogeneity, differentiation, and the noted migratory capacity in the clinical trials [70]. Despite these challenges, Wu et al. stated that MSCs will become the backbone of wound healing in the future. Based on that we suggest a flow up studies in the same concept of healing the bruised or contused wound either cutaneous type or other internal types that may threaten life or other studies regarding blood vascular disorders.
Conclusion
BM-MSCs proved to be efficient in therapy the bruised type of wounds through modulation of the wound healing mediators, which resulted in improvement in the healing process that reflected morphologically and restored the normal skin histological structure, this provided a great insight into the therapy of related conditions of skin destruction or disorders related to vascular conditions. Moreover, BM-MSCs may be promising therapeutic approaches for other mucosal healing and augment the integrity of the gut barrier, suggesting their potential to treat inflammatory bowel disease. However, extensive clinical trials and assessment of the long-term impact of cell therapy are required for additional work.
Acknowledgements
The authors are thankful to the Department of Chemistry, College of Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia for their cooperation in this study.
Statement of Ethics
All animal procedures followed the standards set 4th guideline for the care and use of experimental animals regarding their handling, housing, feeding, cleaning, temperature, humidity, anathesia, sacrification, and pain reduction by the Animal Ethics Committee of the Zoology Department, Faculty of Science, Beni – Suef University (Approval number is BSU/FS/020-110).
Author Contributions
DA, MA, RR, and WM conducted the laboratory studies, participated in the sequence alignment, and drafted the manuscript. DA, KA, WM, and DA carried out the assays. WM and KA performed the histopathological examination. KA, WM, SA, HM, and DA participated in the study design and performed the statistical analysis. DA, WM, RR, HA, SA, HM, and KA, contributed to the study design and coordination, supported the interpretation and discussion of the results, and assisted in drafting the manuscript. All authors read and approved the final manuscript.
Funding Sources
Not applicable.
Disclosure Statement
The authors have no conflicts of interest to declare.
Disclosure of AI Assistance
No AI tools or AI-generated content were used in the creation of this article text. The content was entirely developed, written, and edited by the authors.
References
| 1 | Ekstrand J, Hägglund M, Waldén M: Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med 2011;39:1226-32 https://Doi: 10.1177/0363546510395879.
https://doi.org/10.1177/0363546510395879 |
| 2 | Langlois N: The science behind the quest to determine the age of bruises - a review of the English language literature. Forensic Sci Med Pathol 2007;3:241-51 https://Doi: 10.1007/s12024-007-9019-3.
https://doi.org/10.1007/s12024-007-9019-3 |
| 3 | Verner I, Prag Naveh H, Bertossi D: Treatment of injection-induced ecchymoses with light/laser-assisted technology. Dermatol Ther 2019;32:2-5 https://Doi: 10.1111/dth.12861.
https://doi.org/10.1111/dth.12861 |
| 4 | Brennan C: Stop "cruising for a bruising": mitigating bruising in aesthetic medicine. Plast Surg Nurs 2014;34:75-79 https://Doi: 10.1097/PSN.0000000000000040.
https://doi.org/10.1097/PSN.0000000000000040 |
| 5 | Craig S, Kitchens, Craig M. Kessler, Barbara A Konkle, Michael B: Purpura and Other Hematovascular Disorders, Consultative Hemostasis and Thrombosis ed 4 Elsevier, 2019.
https://doi.org/10.1016/B978-0-323-46202-0.00010-8 |
| 6 | Carpenter SL, Abshire TC, Anderst JD: American Academy of Pediatrics, Section on Hematology/Oncology and Committee on Child Abuse and Neglect. Technical Report: Evaluating for suspected child abuse: Conditions that predispose to bleeding. Pediatria 2013;131(4):1357-73 https://Doi: 10.1542/peds.2013-0196.
https://doi.org/10.1542/peds.2013-0196 |
| 7 | Makoroff KL, McGraw ML: Skin conditions confused with child abuse. In: Jenny C, ed. Child Abuse and Neglect: Diagnosis, treatment, and evidence. St Louis: Elsevier-Saunders, 2011;252-9 https://Doi.org/10.1016/B978-1-4160-6393-3.00030-0.
https://doi.org/10.1016/B978-1-4160-6393-3.00030-0 |
| 8 | Khair K, Liesner R: Bruising and bleeding in infants and children - a practical approach. Br J Haematol 2006;133(3):221-31 https://Doi: 10.1111/j.1365-2141.2006.06016.x.
https://doi.org/10.1111/j.1365-2141.2006.06016.x |
| 9 | Jackson J, Carpenter S, Anderst J: Challenges in the evaluation for possible abuse: Presentation of congenital bleeding disorders in childhood. Child Abuse Negl 2012;36(2):127-34 https://Doi: 10.1016/j.chiabu.2011.09.009.
https://doi.org/10.1016/j.chiabu.2011.09.009 |
| 10 | Kemp AM, Maguire SA, Nuttall D, Collins P, Dunstan F: Bruising in children who are assessed for suspected physical abuse. Arch Dis Child 2014;99:108-13 https://Doi: 10.1136/archdischild-2013-304339.
https://doi.org/10.1136/archdischild-2013-304339 |
| 11 | Chen, Chia-Jung, CHEN, Yi-Wen, CHANG Hsin-Yi, FENG, Jui-Ying Screening Tools for Child Abuse Used by Healthcare Providers: A Systematic Review. J Nurs Res 2022;30: e193 https://Doi: 10.1097/JNR.0000000000000475.
https://doi.org/10.1097/JNR.0000000000000475 |
| 12 | Maguire S: Bruising as an indicator of child abuse: when should I be concerned? Paediatr Child Health 2008;18(12):545-549 https://Doi.org/10.1016/j.paed.2008.09.008.
https://doi.org/10.1016/j.paed.2008.09.008 |
| 13 | Tujillo O, Vanezis P, Cermignani M: Photometric assessment of skin colour and lightness using a tristimulus colorimeter: reliability of inter- and intrainvestigator observations in healthy adult volunteers. Forensic Sci Int 1996;81:1-10 https://Doi: 10.1016/0379-0738(96)01939-1.
https://doi.org/10.1016/0379-0738(96)01939-1 |
| 14 | Stam B, Martin JC, van Gemert MJC, van Leeuwen TG, Maurice CG, Aalders MC, G: 3D finite compartment modeling of formation and healing of bruises may identify methods for age determination of bruises. Med Biol Eng Comput 2010;48:911-921 https://doi.org/10.1007/s11517-010-0647-5.
https://doi.org/10.1007/s11517-010-0647-5 |
| 15 | Songül Karadağ, Ayşe Aydinli, Cansu Yilmaz, Nuri Tutar: Effect of cold application and compression on pain and bruising in subcutaneous heparin injection. J Vascular Nursing 2023;41: 22-26 https:// Doi: 10.1016/j.jvn.2023.01.002.
https://doi.org/10.1016/j.jvn.2023.01.002 |
| 16 | Funt D, Pavicic T: Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol 2013;6:295-316 https:// Doi: 10.2147/CCID.S50546.
https://doi.org/10.2147/CCID.S50546 |
| 17 | Zhidu S, Ying T, Rui J, Chao Z: Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. Stem Cell Res Ther 2024;15, 266 https://Doi.org/10.1186/s13287-024-03885-z.
https://doi.org/10.1186/s13287-024-03885-z |
| 18 | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI: Mesen¬chymal stem cell perspective: cell biology to clinical progress. NPJ Regenera¬tive Med 2019;4(1):22 https:// Doi: 10.1038/s41536-019-0083-6.
https://doi.org/10.1038/s41536-019-0083-6 |
| 19 | Rohban R, Pieber TR: Mesenchymal stem and progenitor cells in regeneration: Tissue specificity and regenerative potential. Stem Cells Int 2017:5173732 https:// Doi:10.1155/2017/5173732
https://doi.org/10.1155/2017/5173732 |
| 20 | Cerqueira MT, Pirraco RP, Marques AP: Stem cells in skin wound healing: Are we there yet? Adv Wound Care 2016;5:164-175 https:// Doi: 10.1089/wound.2014.0607.
https://doi.org/10.1089/wound.2014.0607 |
| 21 | Ali H, Kosar M, Sara S, Seyed MH: Mesenchymal stromal/stem cells spheroid culture effect on the therapeutic efficacy of these cells and their exosomes: A new strategy to overcome cell therapy limitations. Biomed Pharmacother 2022;152:113211. https://Doi.org/10.1016/j.biopha.2022.113211
https://doi.org/10.1016/j.biopha.2022.113211 |
| 22 | Mahmood A, Lu D, Lu M, Chopp M: Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurg 2003;53: 697-702 https:// Doi: 10.1227/01.neu.0000079333.61863.aa.
https://doi.org/10.1227/01.NEU.0000079333.61863.AA |
| 23 | Lewis CJ: Stem cell application in acute burn care and reconstruction. J Wound Care 2013;22(1):7-8:10:12-6 https:// Doi: 10.12968/jowc.2013.22.1.7.
https://doi.org/10.12968/jowc.2013.22.1.7 |
| 24 | Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC: Targeting non-healing ulcers of lower extremity in human through autologous bone marrow derived mesenchymal stem cells. Rejuvenation Res 2009:12(5):359-366 https://Doi: 10.1089/rej.2009.0872.
https://doi.org/10.1089/rej.2009.0872 |
| 25 | Salvolini E, Lucarini G, Zizzi A, Orciani M, Di Benedetto G, Di Primio R: Human skin-derived mesenchymal stem cells as a source of VEGF and nitric oxide. Arch Dermatol Res 2010;302: 367-374 https:// Doi: 10.1007/s00403-009-1018-7.
https://doi.org/10.1007/s00403-009-1018-7 |
| 26 | Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, Hocking AM: Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 2010;316: 48-54 https:// Doi: 10.1016/j.yexcr.2009.08.001.
https://doi.org/10.1016/j.yexcr.2009.08.001 |
| 27 | Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C: Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004;22:377-384 https:// Doi: 10.1634/stemcells.22-3-377.
https://doi.org/10.1634/stemcells.22-3-377 |
| 28 | Al-Khaldi A, Al-Sabti H, Galipeau J, Lachapelle K: Therapeutic angiogenesis using autologous bone marrow stromal cells: Improved blood flow in a chronic limb ischemia model. Ann Thorac Surg 2003;75: 204-209 https:// Doi: 10.1016/s0003-4975(02)04291-1.
https://doi.org/10.1016/S0003-4975(02)04291-1 |
| 29 | Chunmeng S, Tianmin C, Yongping S: Effects of dermal multipotent cell transplantation on skin wound healing. J Surg Res 2004: 121(1): 13-19, 204 https:// Doi: 10.1016/j.jss.2004.04.008.
https://doi.org/10.1016/j.jss.2004.04.008 |
| 30 | Fu S, Luan J, Xin M Q, Wang Q, Xiao R, Gao Y Z: Fate of adipose-derived stromal vascular fraction cells after co-implantation with fat grafts: evidence of cell survival and differentiation in ischemic adipose tissue. Plast Reconstr Surg 2013;132: 363-73 https:// Doi: 10.1097/PRS.0b013e31829588b3.
https://doi.org/10.1097/PRS.0b013e31829588b3 |
| 31 | Liu XB, Wang JA, Ji XY, Yu SP, Wei L: Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res Ther 2014;25; 5(5):111 https:// Doi: 10.1186/scrt499.
https://doi.org/10.1186/scrt499 |
| 32 | Amorin B, Alegretti AP, Valim V, Pezzi A, Laureano AM, da Silva MA: Mesenchymal stem cell therapy and acute graft-versus-host disease: a review. Hum Cell 2014;27:137-50 https:// Doi: 10.1007/s13577-014-0095-x
https://doi.org/10.1007/s13577-014-0095-x |
| 33 | Gutierrez-Fernandez M, Rodriguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdan S: Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther 2013;4:11 https:// Doi: 10.1186/scrt159.
https://doi.org/10.1186/scrt159 |
| 34 | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans R J, Krause DS, Keating A: Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005;7: 393-395 https:// Doi: 10.1080/14653240500319234.
https://doi.org/10.1080/14653240500319234 |
| 35 | Jo H, Brito S, Kwak BM, Park S, Lee M-G, Bin B-H: Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. Int J Mol Sci 2021;22: 2410 https:// Doi: 10.3390/ijms22052410.
https://doi.org/10.3390/ijms22052410 |
| 36 | Sun X, Jiang H, Yang H (2007) In vitro culture of bone marrow mesenchymal stem cells in rats and differentiation into retinal neural-like cells. Journal of Huazhong University of Science and Technology-Medical Sciences 2007; 27: 598-600 https:// Doi:10.1007/s11596-007-0531-1.
https://doi.org/10.1007/s11596-007-0531-1 |
| 37 | Revilla G, Afriani N, Rusnita D: Effects of Bone Marrow Mesenchymal Stem Cell to Transforming Grow Factor-β3 and Matrix Metalloproteinase-9 Expression in Burns. J Med Sci 2018;18:164-171 https:// Doi: 10.3923/jms.2018.164.171.
https://doi.org/10.3923/jms.2018.164.171 |
| 38 | Chaudhary JK, Rath PC: A simple method for isolation, propagation, characterization, and differentiation of adult mouse bone marrow-derived multipotent mesenchymal stem cells. J Cell Sci Therap 2017;8: 1-10 https:// Doi: 10.4172/2157-7013.1000261.
https://doi.org/10.4172/2157-7013.1000261 |
| 39 | Ross C, Byard RW, Langlois NEI: Does the intensity of the inflammatory reaction in a bruise depend on its proximity to the site of trauma? Forensic Sci Med Pathol 2013 ;9(3):358-62 Doi: 10.1007/s12024-013-9466-y.
https://doi.org/10.1007/s12024-013-9466-y |
| 40 | Bancroft JD, Gamble M: Theory and practice of histological techniques, ed 5, Edinburgh, Churchill Living stone Pub, 2002.
|
| 41 | SPSS Inc: SPSS Base 8.0 for Windows User's Guide. SPSS Inc, Chicago IL, 1998.
|
| 42 | Gregory N: Physiology and behaviour of animal suffering. UFAW Animal Welfare. Royal Veterinary College, London, UK, 2007.
|
| 43 | Vanezis P: Interpreting bruises at necropsy. J Clin Pathol 2001;54: 348-55.
https://doi.org/10.1136/jcp.54.5.348 |
| 44 | Hughes VK, Ellis PS, Langlois NEI: The perception of yellow in bruises. J Clin Forensic Med 2004;11: 257-9 https:// Doi: 10.1016/j.jcfm.2004.01.007.
https://doi.org/10.1016/j.jcfm.2004.01.007 |
| 45 | Soares MP, Hamza I: Macrophages and Iron Metabolism. Immunity 2016;44: 492-504 https:// Doi: 10.1016/j.immuni.2016.02.016.
https://doi.org/10.1016/j.immuni.2016.02.016 |
| 46 | Mahrouf-Yorgov M, Augeul L, Da Silva C: Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ 2017;24: 1224-1238 https:// Doi: 10.1038/cdd.2017.51.
https://doi.org/10.1038/cdd.2017.51 |
| 47 | Motterlini R, Foresti R: Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal 2014;10:20(11):1810-26 https:// Doi: 10.1089/ars.2013.5658.
https://doi.org/10.1089/ars.2013.5658 |
| 48 | Gnecchi M, Zhang Z, Ni A: Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008;21:103(11):1204-19 https:// Doi: 10.1161/CIRCRESAHA.108.176826.
https://doi.org/10.1161/CIRCRESAHA.108.176826 |
| 49 | Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L: Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol 2013; 191(11):5515-23 https:// Doi: 10.4049/jimmunol.1301885.
https://doi.org/10.4049/jimmunol.1301885 |
| 50 | Ren S, Chen J, Duscher D, Liu Y, Guo G, Kang Y, Xiong H, Zhan P, Wang Y, Wang C, Machens HG, Chen Z (2019) Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res Ther 10(1):47 https:// doi: 10.1186/s13287-019-1152-x.
https://doi.org/10.1186/s13287-019-1152-x |
| 51 | Scopelliti F, Cattani C, Dimartino V, Mirisola C, Cavani A: Platelet Derivatives and the Immunomodulation of Wound Healing. International J Molec Scie 2022;23:8370 https:// Doi: 10.3390/ijms23158370.
https://doi.org/10.3390/ijms23158370 |
| 52 | El-Sayed ME, Atwa A, Sofy AR, Helmy YA, Amer K, Seadawy MG, Bakry S: Mesenchymal stem cell transplantation in burn wound healing: uncovering the mechanisms of local regeneration and tissue repair. Histochem Cell Biol 2024;161(2):165-181 https:// Doi: 10.1007/s00418-023-02244-y.
https://doi.org/10.1007/s00418-023-02244-y |
| 53 | Weiss AR, Dahlke MH: Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol 2019;10:1191. https:// Doi: 10.3389/fimmu.2019.01191.
https://doi.org/10.3389/fimmu.2019.01191 |
| 54 | Steen EH, Wang X, Balaji S, Butte MJ, Bollyky PL, Keswani SG: The Role of the Anti-Inflammatory Cytokine Interleukin-10 in Tissue Fibrosis. Adv Wound Care (New Rochelle) 2020;9(4):184-198 https:// Doi: 10.1089/wound.2019.1032
https://doi.org/10.1089/wound.2019.1032 |
| 55 | Lee DE, Ayoub N, Agrawal DK: Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther 2016;7: 37 https:// Doi.org/10.1186/s13287-016-0303-6
https://doi.org/10.1186/s13287-016-0303-6 |
| 56 | Jiang D, Singh K, Muschhammer J, Schatz S, Sindrilaru A, Makrantonaki E: MSCs rescue impaired wound healing in a murine LAD1 model by adaptive responses to low TGF-beta1 levels. EMBO Rep 2020;21:49115 https:// Doi: 10.15252/embr.201949115
https://doi.org/10.15252/embr.201949115 |
| 57 | Jiang D, Scharffetter-Kochanek K: Mesenchymal Stem Cells Adaptively Respond to Environmental Cues Thereby Improving Granulation Tissue Formation and Wound Healing. Front. Cell Develop Biol 2020;8:697 https:// Doi: 10.3389/fcell.2020.00697
https://doi.org/10.3389/fcell.2020.00697 |
| 58 | Gilbert R, Vickaryous M, Viloria-Petit AM: Signaling by Transforming Growth Factor Beta Isoforms in Wound Healing and Tissue Regeneration. J Dev Biol 2016;4: 21 https:// Doi: 10.3390/jdb4020021.
https://doi.org/10.3390/jdb4020021 |
| 59 | Jeon YK, Jang YH, Yoo DR, Kim SN, Lee SK, Nam MJ: Mesenchymal stem cells' interaction with skin: wound-healing effect on fibroblast cells and skin tissue. Wound Rep Regen 2010:18:655-61 https:// Doi: 10.1111/j.1524-475X.2010.00636.x.
https://doi.org/10.1111/j.1524-475X.2010.00636.x |
| 60 | Lee SH, Jin SY, Song JS, Seo KK, Cho KH: Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol 2012;24:136-43 https:// Doi: 10.5021/ad.2012.24.2.136
https://doi.org/10.5021/ad.2012.24.2.136 |
| 61 | Basiouny HS, Salama NM, Maadawi ZME, Farag EA: Effect of Bone Marrow-Derived Mesenchymal Stem Cells on Healing of Induced Full-Thickness Skin Wounds in Albino Rat. IJSC 2013;6:12-25 https:// Doi: 10.15283/ijsc.2013.6.1.12.
https://doi.org/10.15283/ijsc.2013.6.1.12 |
| 62 | Kwon S, Ki SM, Park SE, Kim MJ, Hyung B, Lee NK, Shim S, Choi BO, Na DL, Lee JE, Chang JW: Anti-apoptotic Effects of Human Wharton's Jelly-derived Mesenchymal Stem Cells on Skeletal Muscle Cells Mediated via Secretion of XCL1 Mol Ther 2016;24(9):1550-https:// Doi: 10.1038/mt.2016.125.
https://doi.org/10.1038/mt.2016.125 |
| 63 | Chelluboina B, Nalamolu K, Mendez G, Klopfenstein J, Pinson D, Wang D, Veeravalli K (2017) Mesenchymal stem cell treatment prevents post-stroke dysregulation of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Cell Physiol Biochem 44:1360-1369 https:// doi: 10.1159/000485533.
https://doi.org/10.1159/000485533 |
| 64 | Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta S, Borlongan C: Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 2017;158:94-131 https:// Doi: 10.1016/j.pneurobio.2017.07.004.
https://doi.org/10.1016/j.pneurobio.2017.07.004 |
| 65 | Schiebe F, Klein O, Lose J, Priller J: Mesenchymal stromal cells rescue cortical neurons from apoptotic cell death in an in vitro model of cerebral ischemia. Cell Mol Neurobiol 2012;32:567-576 https:// Doi: 10.1007/s10571-012-9798-2.
https://doi.org/10.1007/s10571-012-9798-2 |
| 66 | Cunningham C, Redondo-Castro E, Allan S: The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab 2018;38:1276-1292 https:// Doi: 10.1177/0271678X18776802.
https://doi.org/10.1177/0271678X18776802 |
| 67 | Bhatia A, O'Brien K, Guo J, Lincoln V, Kajiwara C, Chen M, Woodley D T, Udono H, Li W: Extracellular and Non-Chaperone Function of Heat Shock Protein-90α Is Required for Skin Wound Healing. J Invest Dermatol 2018;138:423-433 https:// doi: 10.1016/j.jid.2017.08.043
https://doi.org/10.1016/j.jid.2017.08.043 |
| 68 | Du J, Liu L, Lay F, Wang Q, Dou C: Combination of HIF-1alphagene transfection and HIF-1-activated bone marrow-derived angiogenic cell infusion improves burn wound healing in aged mice. Gene Ther 2013;20: 1070-1076 https:// Doi: 10.1038/gt.2013.32.
https://doi.org/10.1038/gt.2013.32 |
| 69 | Wu S, Sun S, Fu W, Yang Z, Yao H, Zhang Z: The Role and Prospects of Mesenchymal Stem Cells in Skin Repair and Regeneration. Biomedicines 2024;12:743 https://Doi.org/10.3390/biomedicines12040743
https://doi.org/10.3390/biomedicines12040743 |
| 70 | Borakati A, Mafi R, Mafi P, Khan WS: A systematic review and meta-analysis of clinical trials of mesenchymal stem cell therapy for cartilage repair. Curr Stem Cell Res Ther 2018;13(3):215e25 . https://Doi: 10.2174/1574888X12666170915120620
https://doi.org/10.2174/1574888X12666170915120620 |