Original Article - DOI:10.33594/000000753
Accepted 25 November 2024 - Published online 17 January 2025
Effect of Ph on the Physicochemical Properties of a Cassava Peel Starch Biopolymer
bCarrera Ingenieria Industrial. Facultad Ciencia e Ingenieria. Universidad Estatal de Milagro,
cDepartamento de Química. Facultad de Ciencias Básicas. Universidad Técnica de Manabí,
dFacultad de Agrociencias. Departamento de procesos Agroindustriales. Universidad Técnica de Manabí,
eCarrera de Ingeniería Industrial. Facultad de Ingeniería y Ciencias Aplicadas. Universidad Técnica de Manabí. Portoviejo Ecuador,
fCarrera de Ingeniería Química, Grupo de Investigación: Seguridad, Conservación e Innovación de Alimentos y Procesos. Facultad de Ingeniería y Ciencias Aplicadas. Universidad Técnica de Manabí. Portoviejo Ecuador,
gCarrera de Agroindustria, Escuela Superior Politécnica Agropecuaria de Manabí Manuel Félix López, ESPAM-MFL, Calceta. 130250, Ecuador,
hFacultad de Ciencias de la Salud y Bienestar Humano, Carrera de Enfermería, Universidad Indoamérica, 170102, Quito-Ecuador
Keywords
Abstract
Background/Aims:
This study investigates how pH levels affect the characteristics of biopolymer films manufactured from cassava peel starch. Cassava peel starch‘s abundance and biodegradability make it a promising candidate for sustainable packaging. The study seeks to improve film qualities such as thickness, density, moisture content, solubility, and optical properties by altering pH levels. Understanding these effects is critical for increasing the acceptability of cassava peel starch biopolymers in a variety of industrial applications, notably environmentally friendly packaging solutions.Methods:
Starch extracted from cassava peel was used to produce films using the casting method at specified pH levels. The films were evaluated for thickness and density using classical methods. Moisture content was determined following the AOAC 930.15 (2000) protocol. Color analysis was conducted using the CIELab color space technique. Water solubility and solubility in acidic (HCl) and alkaline (NaOH) solutions were assessed through chemical solubility tests performed by gravimetry.Results:
The study investigated how pH impacts biopolymer films manufactured from cassava peel starch. The film thickness varied greatly across pH levels, with pH 10.5 creating the thickest films (0.158 ± 0.012 mm) and pH 6.5 providing the thinnest (0.118 ± 0.015 mm). Density varied slightly, from 1.393 ± 0.122 g/cc to 1.551 ± 0.153 g/cc. Moisture content fluctuated significantly, affecting biodegradability. Color study indicated pH-dependent variations in transparency and opacity, with higher pH values resulting in larger color deviations (∆E). Water solubility remained constant, but NaOH solubility dropped with increasing pH, peaking at pH 7.5 (23.44 ± 2.82%).Conclusion:
This work investigates the use of cassava peel starch for biopolymer synthesis at controlled pH levels. The findings demonstrate the material‘s practicality and provide critical insights for enhancing film qualities, particularly in a variety of industrial applications and environmentally friendly packaging solutions.Introduction
The reduction of single-use plastics presents a significant challenge for the food industry. Each year, 37.3 million tons of plastic products are used for food packaging. Other industries are also major consumers of this material; for example, the agricultural and livestock sectors collectively utilize 10.2 million tons annually. By 2015, the total production of plastics for various uses reached 6.3 billion tons, of which only about 20% were properly managed [1]. It is estimated that by 2050, there will be approximately 12 billion tons of plastic waste in oceans and landfills [2]. For these reasons, the search for alternatives to replace single-use plastics has gained momentum.
In recent years, the food industry has focused on the study of biopolymers as an alternative to traditional packaging materials. Several studies have been conducted utilizing polysaccharides, proteins, and lipids [3, 4]. Among these, the use of polysaccharides, particularly starches, stands out as the primary raw material for biopolymer production due to their non-toxicity, low cost, abundance, and degradability. Additionally, antioxidant and antimicrobial compounds can be incorporated into these materials to extend the shelf life of packaged foods).
However, starch-based biopolymers exhibit poor mechanical properties compared to synthetic polymers, limiting their application in food packaging [5]. On the other hand, modifications in the pH of biopolymer films can enhance their mechanical properties [6]. In addition to mechanical properties, further studies are needed to examine the behavior of the films at different pH levels, particularly concerning their physicochemical properties. In this context, we hypothesize that if pH modifies the mechanical properties of a biopolymer, then it will also influence its physicochemical properties.
A new trend in food packaging is the use of food by-products as raw materials for biopolymer production. Among these, the use of starch derived from cassava peels emerges as a viable alternative [7]. Eight publications have reported the use of cassava peel starch in combination with various components, such as enzymes, whey, fruits, and carboxymethyl cellulose, to produce biopolymers [8]. However, there is no research reported about the effect of pH on the physicochemical properties of the biopolymer.
Thus, a new research initiative proposes utilizing cassava peel by converting it into starch, followed by creating a biofilm, with the assurance of no negative impact on food security. This initiative aligns with the objectives of the Organic Law for the Rationalization and Reduction of Single-Use Plastics in Commerce, which aims to tackle the issue of pollution caused by single-use polymers in Ecuador.
This study aims to evaluate the changes in the physicochemical characteristics and biodegradability of a biopolymer developed from cassava peel starch and glycerol as a plasticizer, through systematic modifications of pH values. By investigating the impact of varying pH levels on these properties, the research seeks to provide valuable insights for optimizing the performance and sustainability of cassava peel starch-based biopolymers for potential applications in food packaging and other relevant industries.
Materials and Methods
Isolation of Cassava Starch
The process for producing cassava peel starch commences with the selection of raw materials, cassava peel was obtained from local producers in Portoviejo, Ecuador, in 2023, the variety INIAP 650 was used, ensuring their freedom from decomposition and observable microorganisms, such as molds and yeasts. Tuber with lacerations that may impair quality are avoided. Washing, peeling, and cutting follow the inspection of the cassava tuber, which separates the peel from the pulp. The cassava peel represents the raw material. Typically, the pulp is used for producing starch, while the peel is considered a byproduct utilized in animal feed, compost, or discarded. The cassava peel is either manually grated or processed through industrial mills. The resulting mass undergoes extensive washing, stirring, and filtration for more than five cycles. The filtrate is then decanted and air-dried. Chemical additives are not utilized throughout the process. The resulting starch is preserved in an airtight container at room temperature until it is utilized.
Sieving
The cassava peel starch underwent two sieving processes to achieve a specific particle size distribution, ensuring product standardization prior to film manufacturing. Controls brand sieves were used. The initial round of sieving used a No. 40 sieve to separate larger particles. A second sieving process was then employed with a No. 200 sieve to obtain particles with a diameter of no more than 75 micrometers. The resulting starch was stored in an airtight plastic container prior to use.
Film Preparation
The film was prepared using the melt-casting technique. A measured amount of cassava peel starch was dissolved in distilled water (3:25) at room temperature with constant stirring at 400-500 rpm. The mixture‘s pH was then adjusted to alkaline values according to table 1. The temperature was increased to the gelatinization point (80 °C), followed by a decrease to induce starch retrogradation (40 °C). The process resulted in a modified rheological behavior, specifically regarding firmness and rigidity. Next, food-grade glycerol was added to the mixture (280:9) under controlled agitation and temperature to impart plasticity, allowing for proper molding. The molding process was executed on polymethylmethacrylate plates. Finally, the resulting film underwent a drying process at room temperature for 48 h. Fig. 1 shows the flowchart outlining the process of isolating starch from cassava peels to the obtention of the biofilm.
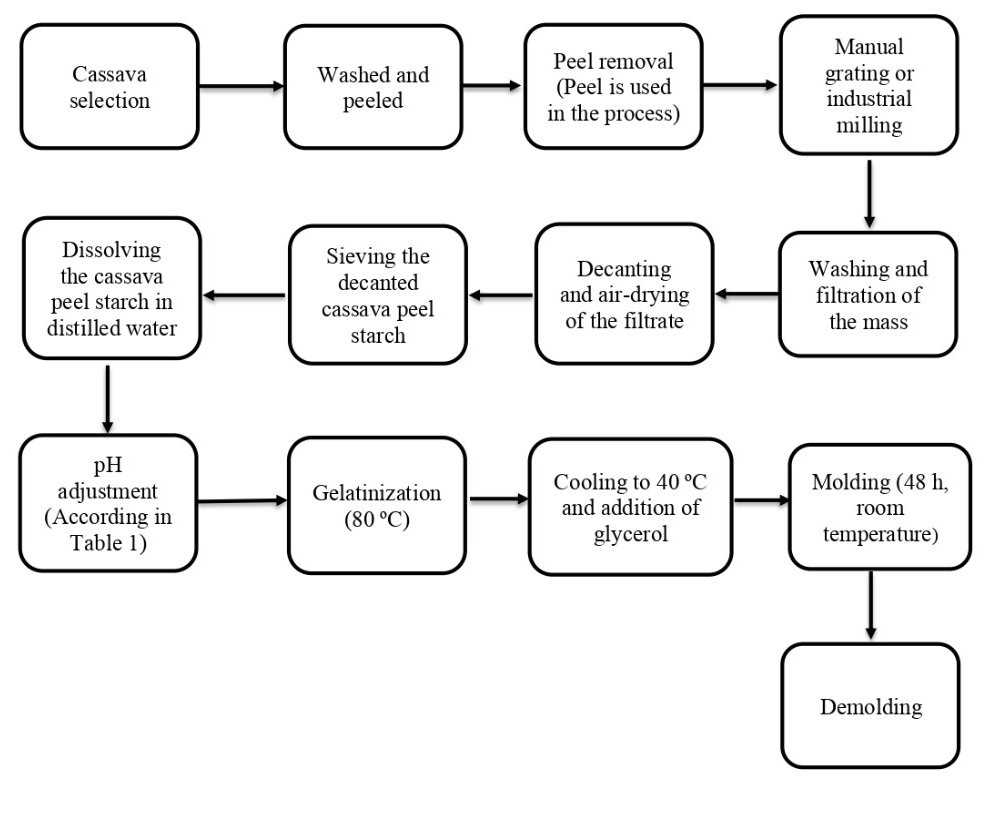
Fig. 1: Flowchart of cassava peel starch biofilm preparation
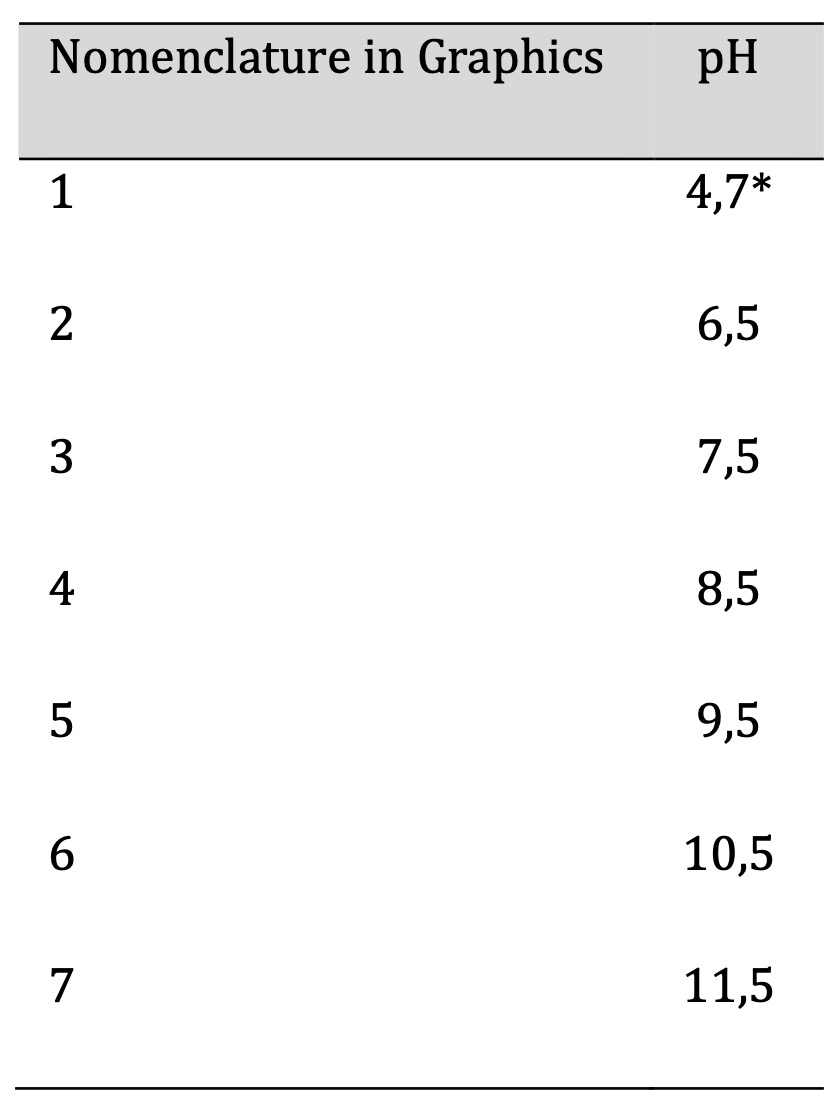
Table 1: pH Levels Studied. *Unaltered pH Levels
Characterization of the Biofilm
Physical-chemical and optical properties were examined to assess the effect of pH variations on biofilm production. The parameters analyzed included thickness, density, moisture content, water solubility, alkaline solubility, acid solubility, color, and biodegradability.
Thickness
The thickness of the film was measured using a Truper digital caliper, model No. CALDI 6MP, with a sensitivity of 0.01mm. Eight thickness measurements were taken for each sample (n=3) on all sides of the film (3x3 cm). The measurements were averaged to determine the final thickness. The standard deviation and coefficient of variation were evaluated for all data, with all cases showing a coefficient of variation ≤ 10, ensuring highly reliable and minimally variable data [9, 10].
Density
For the density, we cut films measuring 3x3 cm. Each film corresponded to a specific pH concentration (mass) and we weighed it on a digital scale manufactured by Boeco, Germany, which displays measurements to three decimal places. To calculate the volume, we multiplied the area of 9 cm² by the thickness. Using the density formula, which divides mass by volume, we obtained the necessary data [11, 12]. We calculated the average, standard deviation, and coefficient of variation from three repetitions (n=3). The following equation was used:
$${ \text{Density} = \frac{\text{Mass}}{\text{Volume}} \hspace{50px} (1)}$$
Moisture
For the moisture test, we cut films measuring 3x3 cm and placed them in a pre-tared crucible. Then, we recorded the initial weight (Mo) of each sample using a digital scale (Boeco, Germany). The crucible was placed in an oven at 105°C for 30 minutes (Memmert, Germany). After that time, the crucible was removed and placed in a desiccator containing silica gel to prevent moisture absorption [11]. Once cooled, we recorded the final weight (Mf) and calculated the moisture percentage using the following equation:
$${ \text{Moisture content} (\%) = \frac{M_0 - M_f}{M_0} \times 100 \hspace{50px} (2)}$$
Color
Color parameters were measured in samples of 9 cm² each one, using a Konica Minolta CR-400 colorimeter. The CIELAB color space in three dimensions (L*a*b*) was obtained by taking three measurements per sample. The L* value represents the variation of colors from black to white (lightness; from 0 = black to 100 = white), b* value from blue to yellow (+b = yellowness, −b = blueness), and a* value from green to red (+a = redness, −a = greenness). The calculations were conducted using the subsequent formulas, where ∆𝐸 refers to color difference, and 𝑊𝐼 denotes whiteness index [13, 14].
$${ \Delta E = \sqrt{((L-L_0)^2 + (a-a_0)^2 + (b-b_0)^2))} \hspace{50px} (3)}$$
$${ W_I= 100 - \sqrt{((100-L)^2 + a^2 + b^2)} \hspace{50px} (4)}$$
Water Solubility
To determine the water solubility of the film, samples of 9 cm² were used. The samples are weighed (W1) using a digital scale (Boeco, Germany) and placed in a beaker with 50 ml of distilled water. They are then allowed to sit at room temperature for 24 hours without stirring. Once this period is over, the sample is taken out, placed on absorbent paper to remove any residual water, and then weighed again to get the final weight (W2). With the data collected [15]. The percentage of water solubility is calculated through this formula:
$${ \text{Water solubility} (\%) = \frac{W_1-W_2}{W_1} \times 100 \hspace{50px} (5)}$$
Alkaline Solubility (NaOH)
To assess alkaline solubility, the pre-weighed film (3x3 cm) is placed in a beaker containing 50 ml of NaOH with a concentration of 1 N. It is left to stand for 24 hours at room temperature without agitation. Subsequently, the films are removed and placed in an oven for 30 minutes to evaporate any remaining NaOH residues. Finally, they are weighed on an analytical scale once cooled (Boeco, Germany). The respective calculation is performed using the following formula:
$${\text{Base solubility} (\%) = \frac{W_1-W_2}{W_1} \times 100 \hspace{50px} (6)}$$
Where W1 represents the initial weight of the film, and W2 represents the weight of the dried film after 24 hours of exposure [11].
Acid Solubility
To assess acid solubility, 9 cm² samples are placed in 20 ml of 1 N HCl for 24 hours at room temperature without agitation. Subsequently, they are removed and exposed for 30 minutes in an oven to evaporate the remaining HCl before being weighed on the balance once cooled [11]. The percentage weight variation is obtained using the following formula:
$${\text{Acid solubility} (\%) = \frac{W_1-W_2}{W_1} \times 100 \hspace{50px} (7)}$$
Biodegradability
The biodegradability test was conducted following the approach recommended by [16] with slight modifications. The film samples (n=3), after being cut into 9 cm² sections, were buried at a depth of 1-2 cm within a humus-based commercially obtained soil mass with a moisture content of 9.67%. The test was performed at room temperature, and the average temperature (27.75°C) and humidity levels (61.167%) were recorded. Rainfall did not affect the analysis as the samples were enclosed. The films were taken out and weighed at 7, 14, and 30-day intervals to establish the percentage of biodegradability relative to the initial weight. The following formula was employed for result computation:
$${\text{Biodegradability} (\%) = \frac{W_1-W_2}{W_1} \times 100 \hspace{50px} (8)}$$
Where W1 stands for the initial weight of the film, and W2 stands for the weight of the film after the exposure period [17].
Statistical Analysis
The study used a one-way analysis of variance (ANOVA) with multiple post hoc comparisons through the Tukey HSD test performed on the SigmaPlot version 14.5 software to carry out the statistical analysis. Significance levels were set at p < 0.05, with tests conducted at least three times, and results were regarded as valid when a coefficient of variation ≤ 10 was achieved.
Results
Physicochemical, optical, and biodegradable characteristics of the film have been evaluated; the results obtained have been discussed.
Table 1 displays the various pH levels that underwent testing. The study commenced by measuring the pH of the mixture that consisted of cassava peel starch and distilled water, devoid of other components, which was found to be pH 4.7. Further pH measurements were conducted after modifying this parameter to achieve alkalinity and consequently, the values reached up to 12.5. However, the value for this pH level was not included in Table 1 due to laboratory experimentation showing that the dried sample show low elasticity, resulting in it being challenging to demold from the polymethyl methacrylate plate for subsequent analyses. According to the properties of the matrix employed as the raw material and the mixing conditions of all components utilized in the film, it is impossible to operate with pH values exceeding 11.5. Thus, we can conclude that higher pH values cannot be used.
Values below 4.7 on the pH scale were not included in this research as the study was based on a prior investigation of biopolymers derived from cassava starch, which established optimal values for each component necessary for biofilm production (patent in progress). The previous study identified alkaline pH levels as both suitable and optimal; consequently, the present research focused on modifying pH towards these alkaline levels. Reactive-grade NaOH 1N (Merck, Germany) was used for pH modification of the biofilms.
Thickness
The procedure for measuring thickness is provided in section 2.6. Table 2 displays the thicknesses of samples at various pH levels, which range from 0.118±0.015mm to 0.158±0.012mm for films featuring pH values of 6.5 and 10.5, respectively. The statistical analysis indicates a marked distinction between the film with a pH of 10.5 compared to the films with pH levels of 6.5 and 11.5. Nonetheless, there are no significant differences among all the other films with varying pH levels. The substantial variation observed in the samples may be attributed to the manual molding procedure employed in the laboratory, which may introduce inconsistencies due to factors such as human error, variability in technique, and environmental conditions. These factors can affect the uniformity and quality. However, it is anticipated that the utilization of a mechanized process will reduce these significant differences when transitioning to an industrial scale. Mechanized processes provide greater precision and consistency, minimizing the impact of human error and allowing for more controlled environmental conditions.
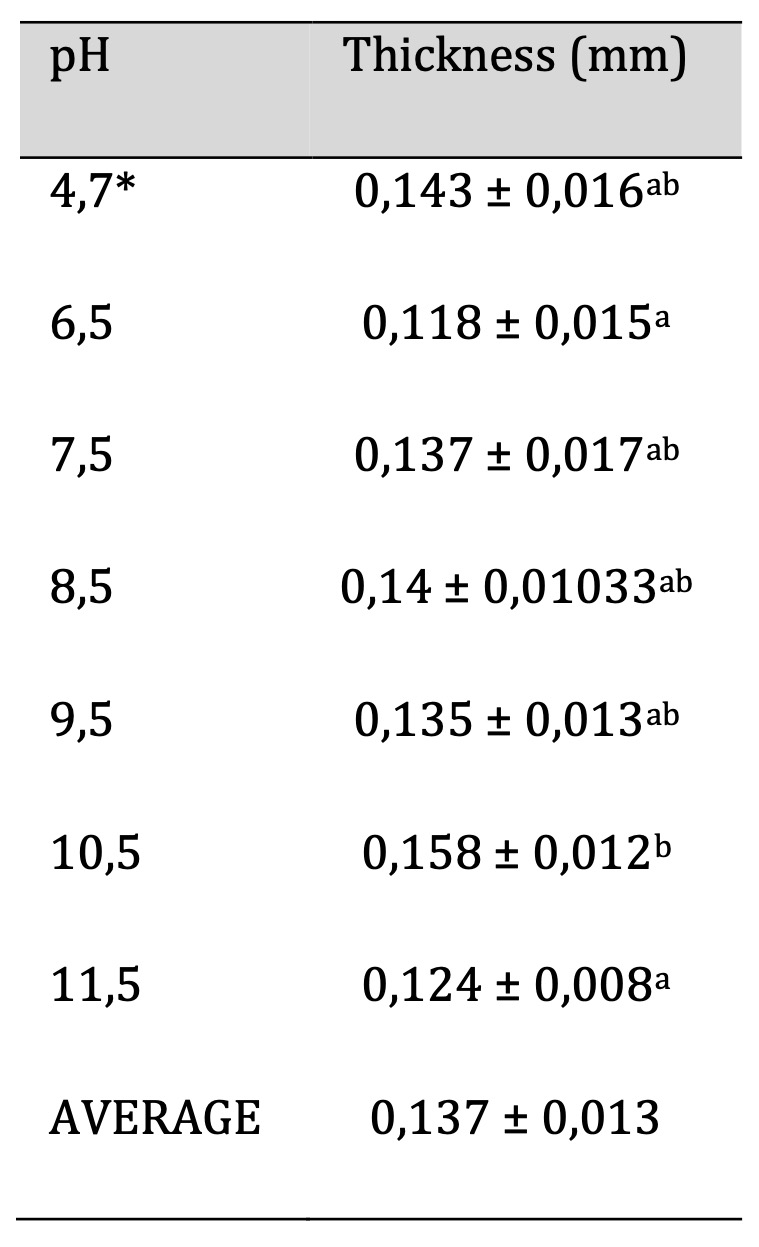
Table 2: Biofilm Thickness. Similar letters indicate no significant differences, p < 0.05. * Unaltered pH
Measuring the thickness is crucial as it directly affects other analyses, including density, water vapor permeability, solubility, degradability, and more.
Density
The method for determining density is explained in section 2.7. Table 3 presents the density values and their corresponding standard deviations for each film at various pH levels. The recorded densities range from 1.393±0.122 g/cc to 1.551±0.153 g/cc, corresponding to pH values of 7.5 and 4.7, respectively. There were no significant differences observed among the groups (p < 0.05). Therefore, modifying pH values does not affect the film structure, resulting in similar densities for all films.
Studies on biopolymers made from sorghum starch with yucca extract and glycerol as a plasticizer have reported density values of approximately 2.5 g/cc [18]. This study found that the density of biopolymers made from cassava peel starch is 1.484 g/cc This suggests that one of the characteristics of biopolymers made from starch is their high density. Density is a crucial parameter in the industry, particularly in polymers. This parameter affects characteristics such as toughness and rigidity.
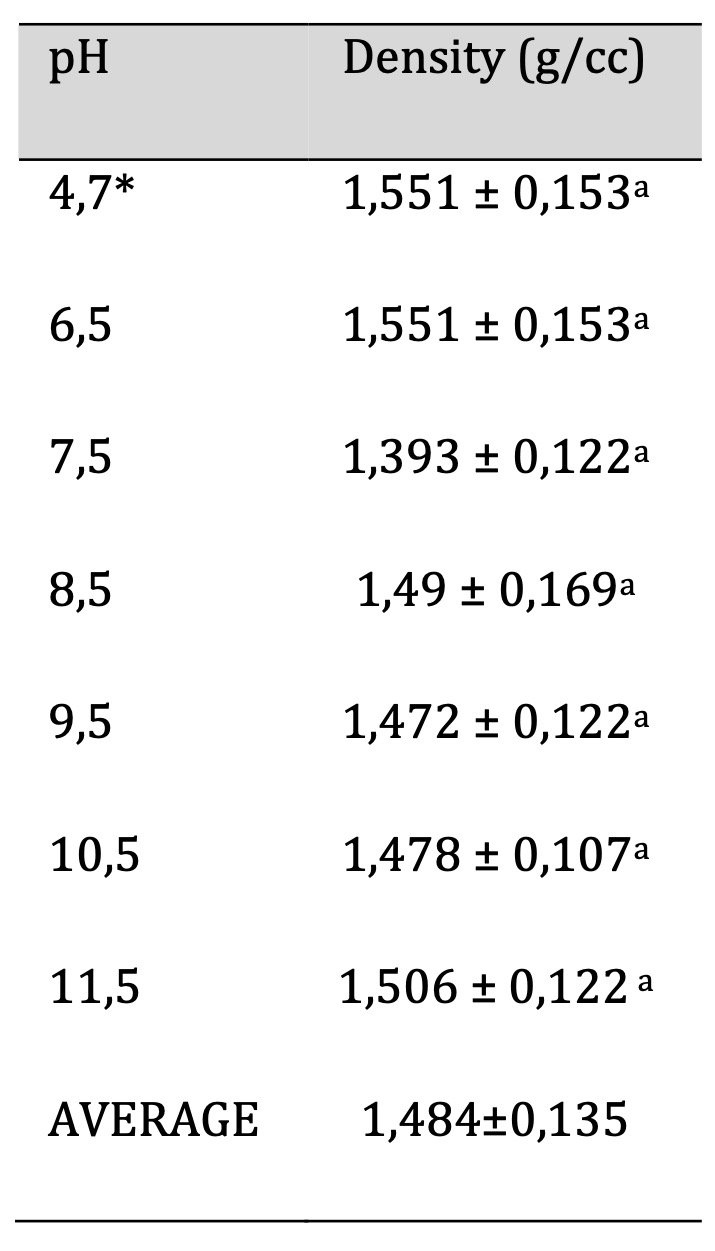
Table 3: Biofilm Density. Similar letters indicate no significant differences, p < 0.05. *Unaltered pH
Moisture
The technique for obtaining the percent moisture is described in section 2.8. Table 4 shows the moisture values obtained for films with different pH values. The highest value corresponds to 26.345±3.847 for the film with pH 7.5 and the lowest value corresponds to the film with pH 6.5 with a value of 13.039±1.978. The data show significant differences between the samples
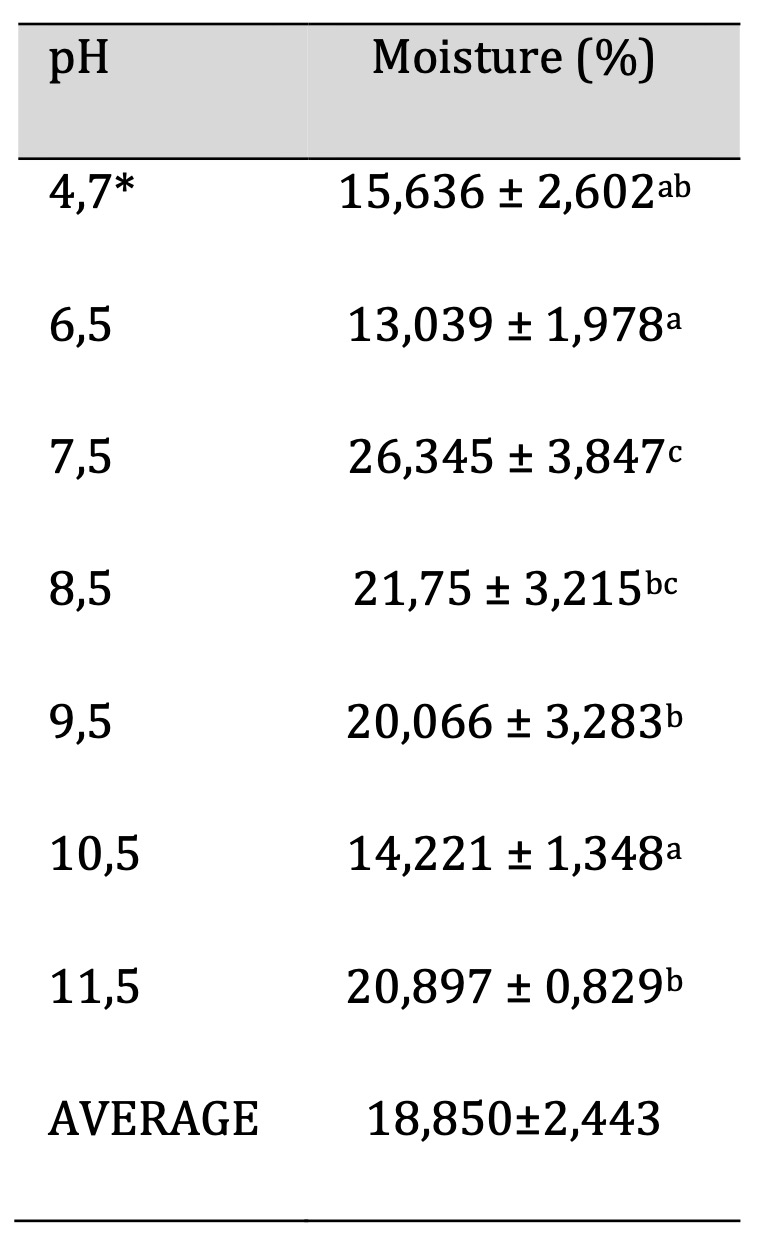
Table 4: Biofilm Moisture. Similar letters indicate no significant differences, p < 0.05. *Unaltered pH
Color
Color is an important parameter for consumers, who are often willing to pay a premium for visually appealing food products or may reject them [24]. The color and material of packaging significantly influence consumer acceptance, particularly among consumers who prioritize sustainability. This trend underscores the growing interest in sustainable practices within the food packaging sector [25].
The technique for obtaining color parameters is described in Section 2.9. Table 5 shows the values obtained for the color parameters: „L“, „a“ and „b“, where „L“ corresponds to brightness-luminosity, „a“ corresponds to red-green hues and „b“ corresponds to yellow-blue hues for films with different pH values. The total color difference (∆𝐸) and whiteness index (WI) were calculated using these parameters. The highest color difference value is 53.14 ± 3.73 for the pH 10.5 film, while the lowest value corresponds to the pH 4.7 film with a value of 31.93 ± 5.09. In general, it can be observed that the color difference increases as the pH increases. Conversely, WI decreases as pH increases. The highest WI value corresponds to a pH of 4.7, which coincides with the lowest color difference value at this pH. The lowest WI value coincides with the highest color difference value, both at pH 10.5.
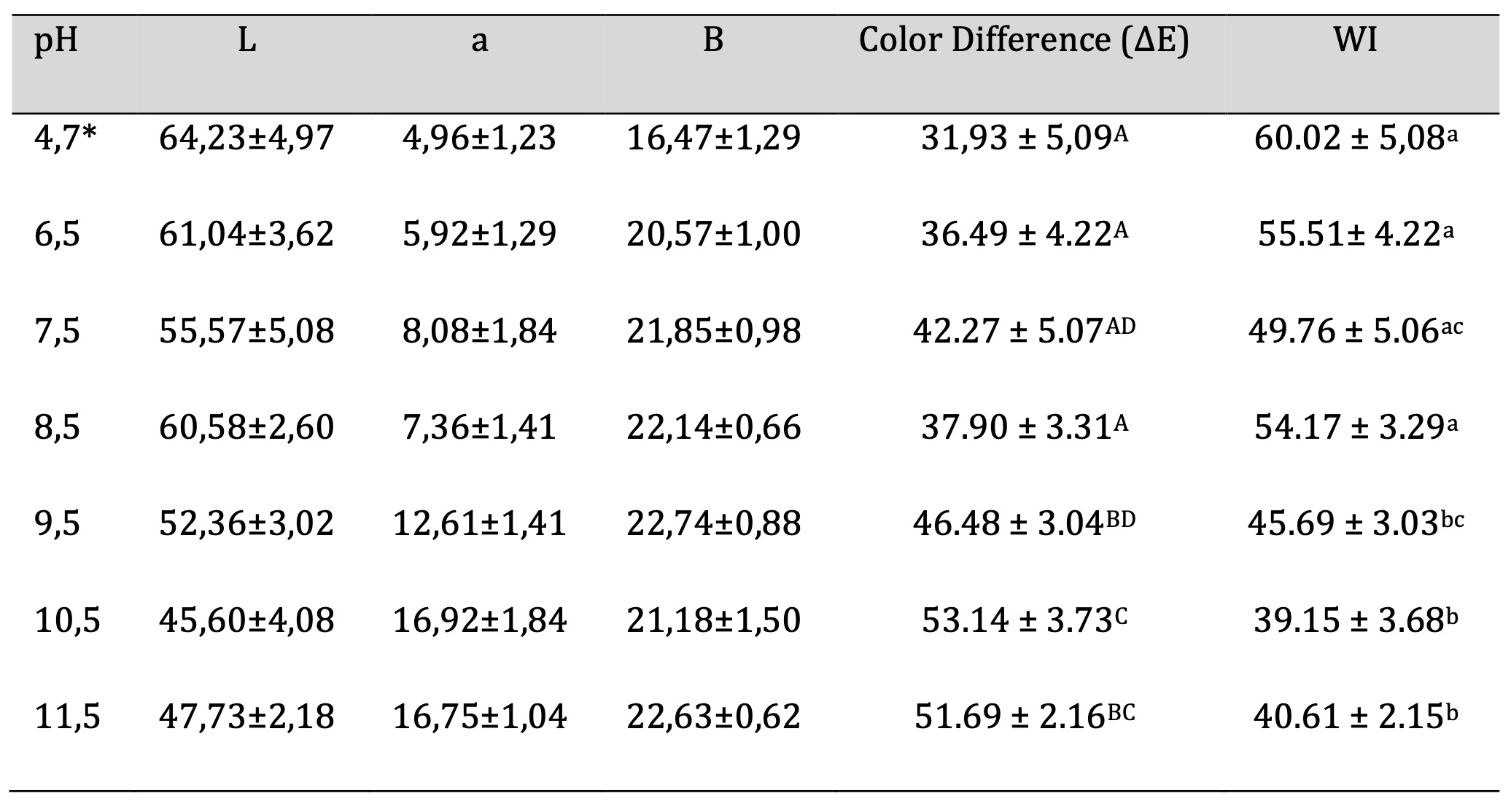
Table 5: Biofilm Color. Similar letters indicate no significant differences, p < 0.05. *Unaltered pH
Water Solubility
The methodology of the water solubility test is described in Section 2.10. Table 6 shows the values obtained for the percentage of water solubility at different pH values. The lowest value is 17.65 ± 0.00, corresponding to pH 9.5, while the highest solubility of 23.44 ± 2.82 is observed at pH 7.5.
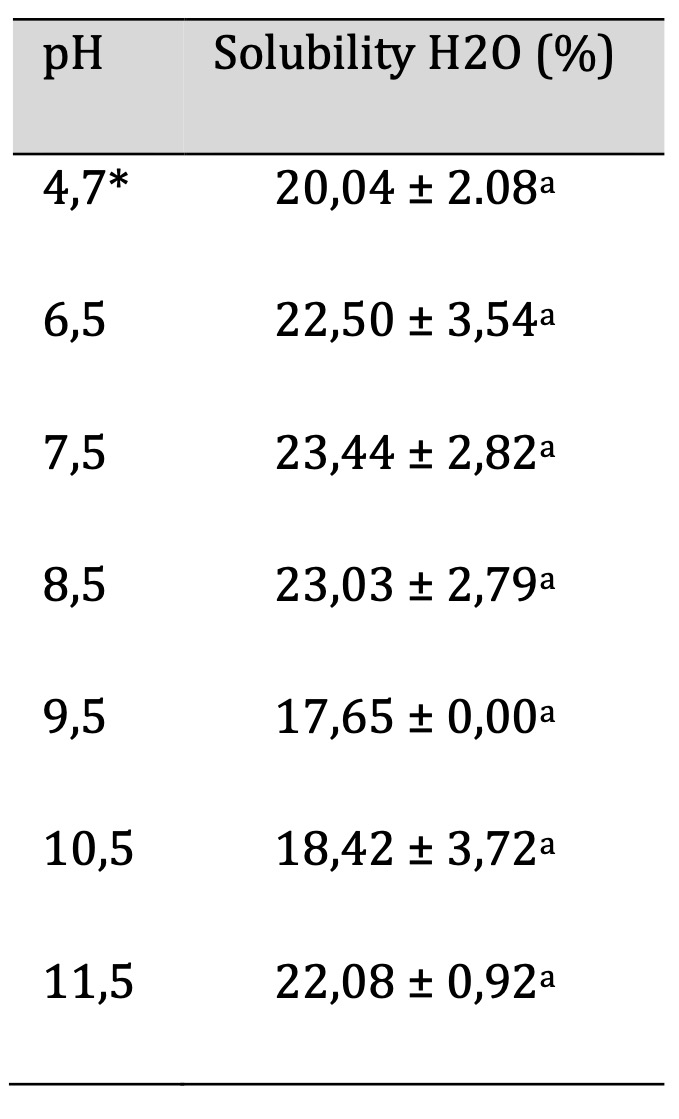
Table 6: Water Solubility of the Biofilm. Similar letters indicate no significant differences, p < 0.05. *Unaltered pH
Solubility in Na(OH) solutions
The technique for solubility in Na(OH) is described in detail in section 2.11. Table 7 shows the values obtained for the percentage of solubility in Na(OH) (concentration 1 N). The lowest value was 10.82 ± 0.41, corresponding to pH 10.5, in contrast, films with pH values of 4.7 and 6.5 were completely degraded.
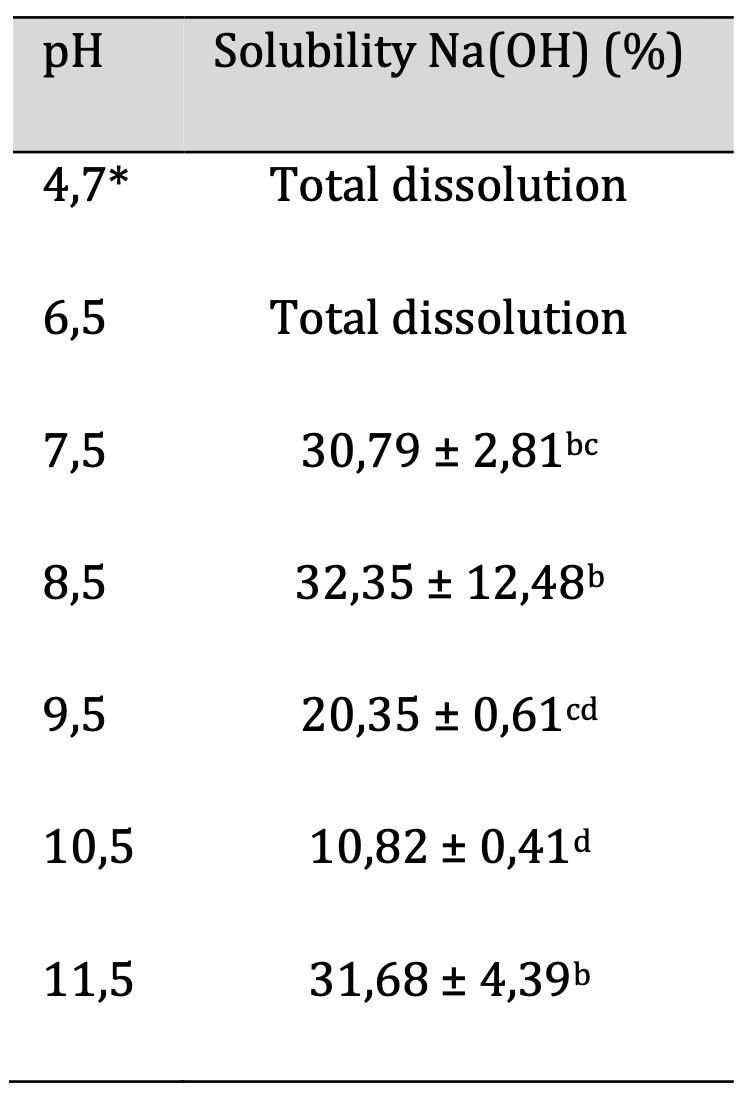
Table 7: Solubility to Na(OH) of the biofilm. Similar letters indicate no significant differences, p < 0.05. *Unaltered pH
Solubility in HCl solutions
The technique for obtaining the solubility in HCl is described in detail in section 2.12. Table 8 shows the values obtained for the percentage of solubility in HCl at different pH levels, with the lowest value being 15.79 ± 0.00 for pH 7.5 and the highest percentage of solubility at pH 11.5 being 28.41 ± 4.82. Contrary to the solubility in an alkaline solution, it is not observed that increasing the pH in the film affects the solubility percentage, as the film at pH 4.7 (acidic) does not show significant differences with pH 7.5 (near neutral) and pH 11.5 (alkaline). The findings indicate that these films maintain structural integrity across a range of pH conditions, making them effective for diverse applications in the food industry. Moreover, the ability to optimize biopolymer formulations based on solubility behavior enables customization to meet specific customer needs, ultimately enhancing food safety and shelf life. These results suggest that acidic pH foods can be packaged in any of the designed films without affect its degradation. Taking into account the results of section 3.7 on solubility in Na(OH), we could say that if a biopolymer is to be used for a range of foods with pH levels from acidic to alkaline, it should be produced with a pH ranging from neutral to 11.5, selecting different pH levels based on color according to consumer needs.
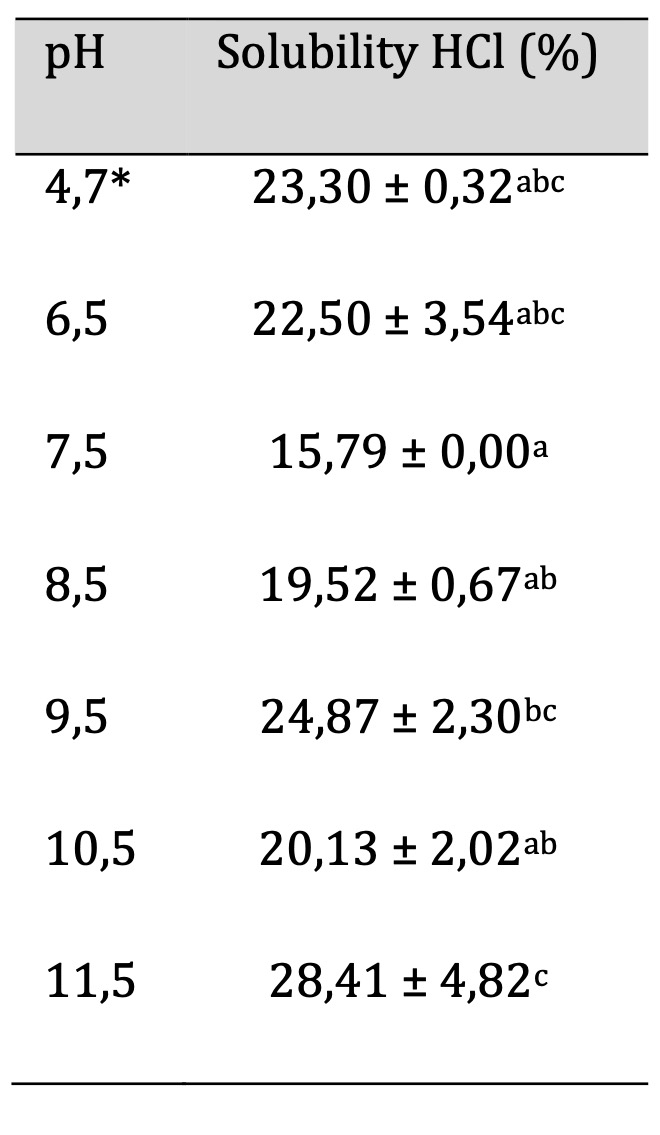
Table 8: Biofilm Solubility in HCl. Similar letters indicate no significant differences, p < 0.05. *Unaltered pH
Biodegradability
The biodegradability technique is described in section 2.13. Table 9 shows the values obtained for the percentage biodegradability of films at different pH levels after 7, 15 and 31 days. It can be seen from Table 9 that, in general, biodegradability increases with time. The highest percentage at 31 days corresponds to pH 7.5 with 30.56 ± 3.93% degradation, a pH value that coincides with the highest percentage of film moisture obtained, confirming that higher film moisture leads to greater biodegradability. Biodegradability values at 31 days ranged from 15.80 ± 2.65 to 30.56 ± 3.93% and it should be noted that obtaining accurate data was difficult due to the adherence of soil particles. This is in line with the Ecuadorian Organic Law Project for the Rationalization, Reuse, and Reduction of Single-Use Plastics in Commerce, which states on page 36 that to be considered biodegradable, a polymer‘s degradation time should not exceed 24 months [27].
However, from the obtained data, it is observed that the polymer‘s biodegradability does not exhibit a linear behavior. Therefore, to determine the exact biodegradation time under controlled environmental conditions, it is necessary to construct a curve that encompasses the phases of induction (microorganisms‘ adaptation to the substrate), accelerated biodegradation, deceleration, and plateau. This proposed approach serves as the basis for further research endeavors.
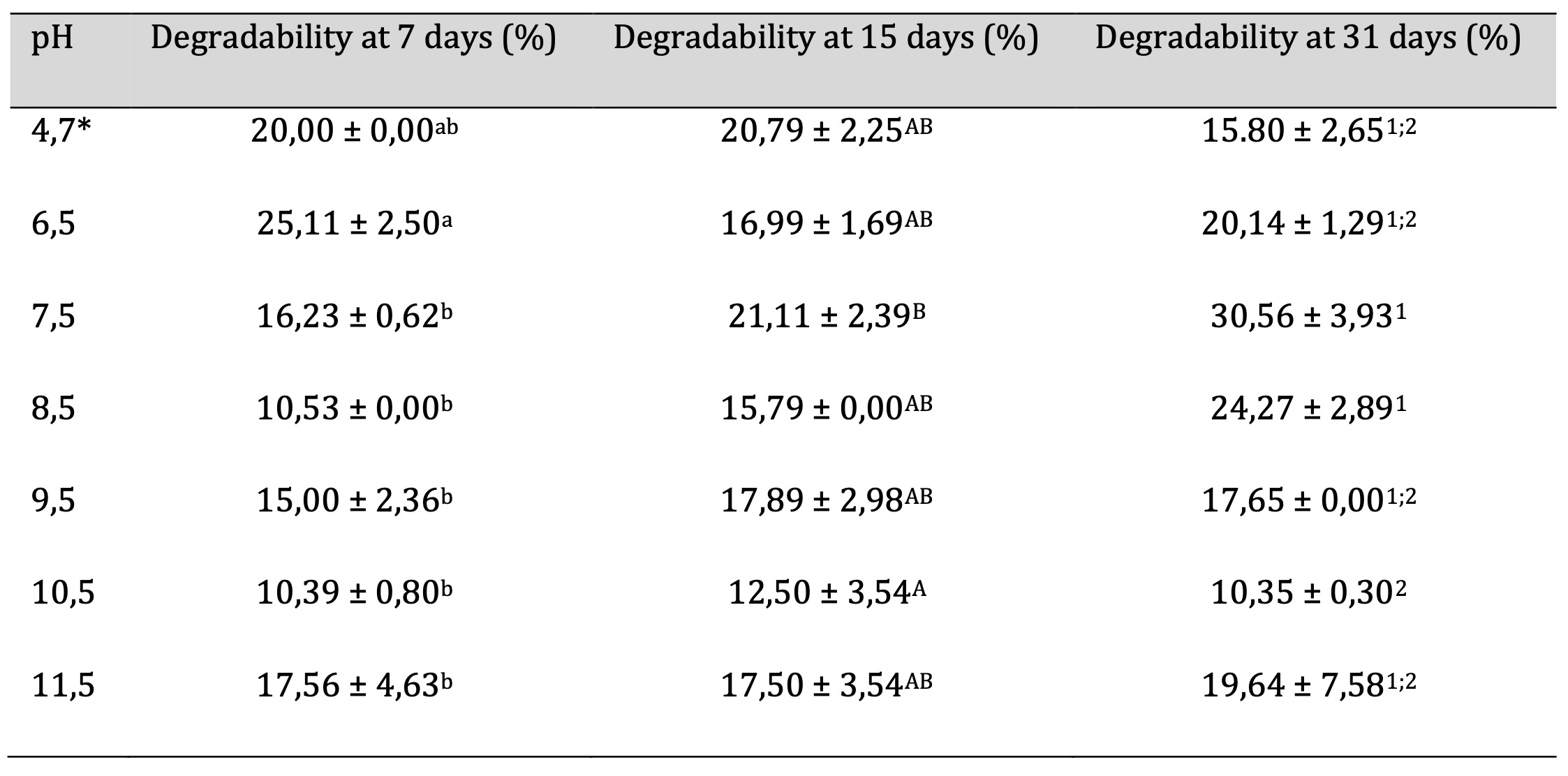
Table 9: Biodegradability of films based on cassava peel starch with different pH values. Equal letters and numbers indicate no significant differences, p < 0.05. *Unaltered pH
Discussion
Regarding pH levels used in the film the properties of the matrix employed as the raw material and the mixing conditions of all components utilized in the film, it is impossible to operate with pH values exceeding 11.5. Thus, we can conclude that higher pH values cannot be used. Values below 4.7 on the pH scale were not included in this research as the study was based on a prior investigation of biopolymers derived from cassava starch, which established optimal values for each component necessary for biofilm production (patent in progress). The previous study identified alkaline pH levels as both suitable and optimal; consequently, the present research focused on modifying pH towards these alkaline levels. Reactive-grade NaOH 1N (Merck, Germany) was used for pH modification of the biofilms.
In determining the thickness the statistical analysis indicates a marked distinction between the film with a pH of 10.5 compared to the films with pH levels of 6.5 and 11.5. Nonetheless, there are no significant differences among all the other films with varying pH levels
In the density análisis there were no significant differences observed among the groups (p < 0.05). Therefore, modifying pH values does not affect the film structure, resulting in similar densities for all films. Studies on biopolymers made from sorghum starch with yucca extract and glycerol as a plasticizer have reported density values of approximately 2.5 g/cc [18]. This study found that the density of biopolymers made from cassava peel starch is 1.484 g/cc This suggests that one of the characteristics of biopolymers made from starch is their high density. Density is a crucial parameter in the industry, particularly in polymers. This parameter affects characteristics such as toughness and rigidity.
For the moisture content, it was shown that this parameter is important because it directly affects biodegradability. A higher moisture content corresponds to a higher percentage of biodegradability over a given period. This is because the microorganisms that degrade the film require at least four factors for their growth and action, one of which is water, the others being nutrients, temperature, and time. Significant differences are observed, especially in the pH 7.5 sample compared to most of the rest of the set (except the pH 8.5 sample). The pH values presented do not show a clear relationship between moisture content and pH. However, similar data on moisture content variation ranging from 8.60±0.06 to 20.03±0.39 are reported in a research study on manioc starch film with beeswax and propolis [11] Similarly, other studies with potato starch (15.1%), corn starch (9%-16.5%) reported moisture percentage values within the range obtained in this research [19]. Higher moisture values of 52% for sweet potato starch film and 34% for cassava starch were also reported [20]. In our study, the average moisture content is 18.850±2.443.
The moisture content is influenced by both the quantity of glycerol utilized and the type of starch employed. An increased percentage of glycerol results in a higher moisture content within the biopolymer, as this plasticizer aids in water retention within the mixture [21]. According to Shafqat et al [22]. the hydroxyl groups present in glycerol exhibit a strong affinity for water molecules, facilitating the retention of moisture and the formation of hydrogen bonds within the film structure. Consequently, the humidity content of the biopolymer rises with the concentration of the plasticizer. The botanical origin of starch significantly influences the properties of the films, as evidenced by the substantial differences in moisture content observed among starch-based films derived from pumpkin, lentil, and quinoa, as reported by [23].
Regarding the color analysis, it was posible to determine that increase in pH is directly proportional to color difference and inversely proportional to whiteness index. This information is useful when designing a film for food applications, as color is an essential parameter that dominates the appearance of the product and, consequently, consumer acceptance [26]. Based on this, it is possible to design a film according to the desired commercial application. In some cases, the product inside the package is desired to be visible, while in other cases it is not. Thus, by modifying the pH, it is possible to meet the desired requirements of the industry and the end consumer
About the study of solubility in wáter to the statistical analysis determined, there are no significant differences between the different pH levels of the films studied. In the present study, the glycerol percentage was not modified. It has been established that the water solubility of a biopolymeric film increases with higher glycerol concentrations [21]. However, by maintaining a constant glycerol value, no significant modifications in solubility were observed. This observation is consistent with the results for solubility in acidic solutions (section 3.8) and indicates that the pH of the developed films can be used with any food, regardless of its pH. The only condition is that the pH of the film is affected by the alkalinity of the food, as discussed in the following section
For the solubility of the film in sodium hydroxide, the data indicate that the solubility of the film in the presence of Na(OH) decreases with increasing pH. Films at pH 4.7 and 6.5 show 100% solubility after 24 hours in an alkaline solution. This suggests that acidic pH films may not be suitable for packaging alkaline pH foods, as their life is likely to be shorter than the shelf life of the product. On the other hand, the table shows a significant decrease starting from a pH close to neutrality, with no significant change at pH 11.5. If we take in mind the section 3.5 (Color), this indicates that from pH 7.5, the color could be modified, without degrading the film when used for alkaline food storage. In conclusion, acidic pH films are not suitable for storing foods with pH values close to neutral and alkaline. These results highlight the importance of pH modification in the film, as it appears that a film with similar properties such as thickness and density, which would not be affected by the pH of the film, can significantly reduce its usefulness when used a pH under 7.5 with alkaline foods. In addition, it‘s worth mentioning that food grade magnesium hydroxide Mg(OH)2 could be used for pH modification to obtain an edible film.
Conclusion
A study was conducted to evaluate the effect of pH on a cassava peel starch biofilm with the aim of determining the optimal pH that would provide the best physicochemical, optical, and biodegradable properties in the film. The study evaluated the effect of pH on thickness, density, moisture, color, water solubility, alkaline solubility, acid solubility, and biodegradability.
The results show that the film without modification of pH (4.7) as well as pH 6.5 is not suitable for packaging alkaline or near neutral pH foods due to its rapid degradation. However, biopolymers with pH values of 7.5-11.5 are suitable for any type of food, whether alkaline, neutral or acidic. The study also revealed the effect of pH on the color of the film, indicating that as pH increases, whiteness index decreases and color difference increases. This visual effect enables a conscious selection of pH based on the desired color for the intended application, which should fall within the range of 7.5-11.5 for various foods. On the other hand, the study of biodegradability, the most important parameter from an environmental point of view, has shown that pH 7.5 exhibits the fastest biodegradation due to its high water content. However, all pH values studied fall within the time range (˂ 24 months) required to be considered biodegradable according to the Ecuadorian Organic Law Project for the Rationalization, Reuse, and Reduction of Single-Use Plastics in Commerce. Parameters such as thickness and density are not affected by pH variations. Any significant differences observed in thickness are attributed to manual processing, which is expected to be eliminated in an industrial process.
In conclusion, films with a pH of 7.5-11.5 are considered suitable for food packaging, allowing the selection of color and biodegradation time within this range. This study opens avenues for further research into biopolymers from food waste, providing opportunities to design packaging with specific colors.
Acknowledgements
We would like to express our gratitude to Domenik Saina Jadán Rovayo and Michael Jordan Párraga Sabando for their collaboration in the experimentation conducted in this research.
Disclosure Statement
The authors have no conflicts of interest to declare.
Disclosure of AI Assistance
No AI tools or AI-generated content were used in the creation of this article text. The content was entirely developed, written, and edited by the authors.
References
| 1 | Food and Agriculture Organization. Plásticos en los sistemas agroalimentarios: lo bueno, lo malo y lo feo. 2023 https://www.fao.org/newsroom/detail/plastics-in-agrifood-systems-the-good-the-bad-and-the-ugly/es
|
| 2 | United Nations. La contaminación por plásticos afecta a la salud y al medio ambiente. 2018 https://news.un.org/es/story/2018/06/1435111
|
| 3 | Mossavi MP, Kashiri M, Maghsoudlou Y, Khomiri M, Alami M. Development and characterization of a novel multifunctional film based on wheat filter flour incorporated with carvacrol: Antibacterial, antifungal, and insecticidal potentials. Food Sci Technol Int. 2022;28(7):603-612 https://doi.org/10.1177/10820132211041826
https://doi.org/10.1177/10820132211041826 |
| 4 | Palma M, Rojas E, Arriagada C. Biodegradable plastics: A comprehensive review. PubMed. 2022 https://pubmed.ncbi.nlm.nih.gov/35502832/
|
| 5 | Avella M, Bonifacio M, Puglia D. Biodegradable plastics: A review. Chemosphere. 2014;117:25-36 https://doi.org/10.1016/j.chemosphere.2014.01.051
https://doi.org/10.1016/j.chemosphere.2014.01.051 |
| 6 | Ertan K, Sahin S, Sumnu G. Effects of alkaline pH and gallic acid enrichment on the physicochemical properties of sesame protein and common vetch starch-based composite films. Int J Biol Macromol. 2024;257:128743 https://doi.org/10.1016/j.ijbiomac.2023.128743
https://doi.org/10.1016/j.ijbiomac.2023.128743 |
| 7 | Weligama Thuppahige VT, Moghaddam L, Welsh ZG, Wang T, Karim A. Investigation of critical properties of Cassava (Manihot esculenta) peel and bagasse as starch-rich fibrous agro-industrial wastes for biodegradable food packaging. Food Chem. 2023;422:136200 https://doi.org/10.1016/j.foodchem.2023.136200
https://doi.org/10.1016/j.foodchem.2023.136200 |
| 8 | Zhang Y, Xie J, Ellis WO, Li J, Appaw WO, Simpson BK. Bioplastic films from cassava peels: Enzymatic transformation and film properties. Ind Crops Prod. 2024;213:118427 https://doi.org/10.1016/j.indcrop.2024.118427
https://doi.org/10.1016/j.indcrop.2024.118427 |
| 9 | Shahrampour D, Khomeiri M, Razavi SMA, Kashiri M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45 LWT. 2020;118:108758 https://doi.org/10.1016/j.lwt.2019.108758
https://doi.org/10.1016/j.lwt.2019.108758 |
| 10 | Kanmani P, Lim ST. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013;141(2):1041-1049 https://doi.org/10.1016/j.foodchem.2013.03.103
https://doi.org/10.1016/j.foodchem.2013.03.103 |
| 11 | Pérez-Vergara LD, Cifuentes MT, Franco AP, Pérez-Cervera CE, Andrade-Pizarro RD. Development and characterization of edible films based on native cassava starch, beeswax, and propolis. NFS J. 2020;21:39-49 https://doi.org/10.1016/j.nfs.2020.09.002
https://doi.org/10.1016/j.nfs.2020.09.002 |
| 12 | Flores Fidelis JC, Marchi LB, Scapim MRS, et al. Development of biodegradable films containing pomegranate peel extract and potassium sorbate. LWT. 2022;160:113302 https://doi.org/10.1016/j.lwt.2022.113302
https://doi.org/10.1016/j.lwt.2022.113302 |
| 13 | Choque-Quispe D, Obregón Gonzales FH, Carranza-Oropeza MV, et al. Physicochemical and technofunctional properties of high Andean native potato starch. J Agric Food Res. 2024;15:100955 https://doi.org/10.1016/j.jafr.2023.100955
https://doi.org/10.1016/j.jafr.2023.100955 |
| 14 | Criollo-Feijoo J, Salas-Gomez V, Cornejo F, Auras R, Salazar R. Cassava bagasse starch and oregano essential oil as a potential active food packaging material: A physicochemical, thermal, mechanical, antioxidant, and antimicrobial study. Heliyon. 2024;10(16). https://doi.org/10.1016/j.heliyon.2024.e36150
https://doi.org/10.1016/j.heliyon.2024.e36150 |
| 15 | Vishnu Priya N, Vinitha UG, Meenakshi Sundaram M. Preparation of chitosan-based antimicrobial active food packaging film incorporated with Plectranthus amboinicus essential oil. Biocatal Agric Biotechnol. 2021;34:102021 https://doi.org/10.1016/j.bcab.2021.102021
https://doi.org/10.1016/j.bcab.2021.102021 |
| 16 | Mustapha FA, Jai J, Nik Raikhan NH, Sharif ZIM, Yusof NM. Response surface methodology analysis towards biodegradability and antimicrobial activity of biopolymer film containing turmeric oil against Aspergillus niger. Food Control. 2019;99:106-113 https://doi.org/10.1016/j.foodcont.2018.12.042
https://doi.org/10.1016/j.foodcont.2018.12.042 |
| 17 | Piñeros-Hernandez D, Medina-Jaramillo C, López-Córdoba A, Goyanes S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017;63:488-495 https://doi.org/10.1016/j.foodhyd.2016.09.034
https://doi.org/10.1016/j.foodhyd.2016.09.034 |
| 18 | Rodríguez-Castellanos W, Martínez-Bustos F, Jiménez-Arévalo O, González-Núñez R, Galicia-García T. Functional properties of extruded and tubular films of sorghum starch-based glycerol and Yucca Schidigera extract. Ind Crops Prod. 2013;44:405-412 https://doi.org/10.1016/j.indcrop.2012.11.027
https://doi.org/10.1016/j.indcrop.2012.11.027 |
| 19 | Nordin N, Othman SH, Rashid SA, Basha RK. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020;106:105884 https://doi.org/10.1016/j.foodhyd.2020.105884
https://doi.org/10.1016/j.foodhyd.2020.105884 |
| 20 | Gutiérrez TJ, Morales NJ, Pérez E, Tapia MS, Famá L. Physico-chemical properties of edible films derived from native and phosphated cush-cush yam and cassava starches. Food Packaging Shelf Life. 2015;3:1-8 https://doi.org/10.1016/j.fpsl.2014.09.002
https://doi.org/10.1016/j.fpsl.2014.09.002 |
| 21 | Hernando H, Marpongahtun, Julianti E, et al. Impact of glycerol on oil palm trunk starch bioplastics enhanced with citric-acid epoxidized palm oil oligomers. Case Stud Chem Environ Eng. 2024;10:100839 https://doi.org/10.1016/j.cscee.2024.100839
https://doi.org/10.1016/j.cscee.2024.100839 |
| 22 | Shafqat A, Al-Zaqri N, Tahir A, Alsalme A. Synthesis and characterization of starch based bioplastics using varying plant-based ingredients, plasticizers and natural fillers. Saudi J Biol Sci. 2021;28(3):1739-1749 https://doi.org/10.1016/j.sjbs.2020.12.015
https://doi.org/10.1016/j.sjbs.2020.12.015 |
| 23 | Pająk P, Przetaczek-Rożnowska I, Juszczak L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int J Biol Macromol. 2019;138:441-449 https://doi.org/10.1016/j.ijbiomac.2019.07.074
https://doi.org/10.1016/j.ijbiomac.2019.07.074 |
| 24 | Thies AJ, Altmann BA, Countryman AM, Smith C, Nair MN. Consumer willingness to pay (WTP) for beef based on color and price discounts. Meat Sci. 2024;217:109597 https://doi.org/10.1016/j.meatsci.2024.109597
https://doi.org/10.1016/j.meatsci.2024.109597 |
| 25 | Berthold A, Guion S, Siegrist M. The influence of material and color of food packaging on consumers' perception and consumption willingness. Food Hum. 2024;2:100265 https://doi.org/10.1016/j.foohum.2024.100265
https://doi.org/10.1016/j.foohum.2024.100265 |
| 26 | Califano G, Furno M, Caracciolo F. Beyond one-size-fits-all: Consumers react differently to packaging colors and names of cultured meat in Italy. Appetite. 2023;182:106434 https://doi.org/10.1016/j.appet.2022.106434
https://doi.org/10.1016/j.appet.2022.106434 |
| 27 | Food and Agriculture Organization. Ley sobre plásticos en la cadena alimentaria. FAOLEX. 2020 https://www.fao.org/faolex/results/details/es/c/LEX-FAOC200078/
|