Original Article - DOI:10.33594/000000757
Accepted 27 January 2025 - Published online 7 February 2025
Can Metabolic Biomarkers of Oxygen-Dependent Processes Determine Health Status of Pigeon Columba Livia F. Urbana?
bDepartment of Medical Biology and Biochemistry, Department of Ecology and Environmental Protection, Faculty of Medicine, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń, Bydgoszcz, Poland,
cDepartment of Biotechnology, Institute of Biological Sciences, Faculty of Biological Sciences, University of Zielona Góra, Zielona Góra, Poland
Keywords
Abstract
Background/Aims:
Anthropogenic impact is irreversibly changing natural habitats of birds. Changes caused by the bioaccumulation of trace metals can lead to the development of oxidative stress and affect oxygen-dependent metabolic pathways in bird tissues, which can be used as effective bioindicators in these conditions. The objectives of our study were (a) to investigate the tissue-specific activity of key enzymes involved in metabolic changes and energy production, including Krebs cycle enzymes, as well as variations in metabolites associated with oxygen-dependent processes; and (b) to apply multivariate regression analysis, using beta and correlation coefficients, to elucidate the mechanisms of adaptive responses in pigeons to environmental changes in lead-contaminated areas.Methods:
This study investigates the ecotoxicological effects on feral pigeons (Columba livia f. urbana) in their natural habitats. It examines the influence of key environmental factors, sex, and biochemical alterations across five tissues (liver, kidney, heart, muscle, and brain). The analysis includes the combined effects of these variables on biochemical biomarkers related to energy metabolism, oxidative stress, and antioxidant defenses, considering the levels of chemical elements present in the pigeons. The analyses involved two groups of pigeons, namely, 7 females and 10 males (n = 17) in the group sampled in Słupsk and 7 females and 7 males, (n = 14) in Szpęgawa that living in two areas in central part of Northern Poland, which differed in the level of anthropopressure.Results:
We report significant values of lead bioaccumulation in feathers of pigeons and the impact of this metal on the activities of Krebs cycle enzymes (succinate dehydrogenase, pyruvate dehydrogenase, isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase), biomarkers of oxygen-dependent processes (lactate dehydrogenase activity, lactate and pyruvate levels, and their ratio), and aminotransferases in different tissues of pigeons.Conclusion:
Biomarkers of oxygen-dependent processes in five tissues of pigeons are depending on sex and environment. Pigeons from lead-exposed areas exhibited decreased antioxidant defence by biochemical alterations in tissues. Analytical model of oxidative stress biomarkers, Krebs cycle enzymes, and chemical elements is significant. Using multivariate regression analysis with beta- and correlative coefficients, relationships were shown for the optimal development of adaptation alterations in biochemical reactions in pigeons in response to the modification of their environments. Research on Columba livia f. urbana provides valuable insights into understanding the effects of anthropogenic pollution on bird physiology and offers practical methods for assessing environmental health.Introduction
Environmental pollutants, including toxic metals, pose significant risks to the physiological integrity of organisms and disrupt ecological balance globally [1]. Potential harm resulting from exposure to environmental contaminants has been largely demonstrated with collateral damage ranging from geno- and cytotoxicity to tissue dysfunction and alterations in body physiology [2]. Anthropogenic impact is irreversibly changing the state of natural environment. Many parts of the planet have become areas of ecological disaster and can serve as good bioindicators for environmental assessment. Not only regional but also global environmental problems, such as land degradation, water pollution, increased acidity of precipitation, greenhouse effect, desertification, etc., have emerged. As a result of anthropogenic transformations, the human and animal environment is changing, which can lead to the development of oxidative stress and affect the red-ox processes in living organisms [3, 4]. These effects are related to the presence of toxic elements in soils and animal feed, as well as to atmospheric pollution from exhaust gases and pesticides, which, together with other factors, are prerequisites for the activation of oxidative stress. This in turn can cause DNA damage, protein modification, lipid peroxidation, and disturbances in the functioning of the antioxidant system of living organisms [1].
Abnormal environmental factors contribute not only to advanced behavioral strategies in higher regulatory centers but also to the evaluation of possible morpho-functional and energetic transformations in the body. Animals, particularly birds, are the most sensitive to the presence of environmental pollution, as they spend all their time interacting with the environment. Anthropogenic stressors include contaminated soil and dust, as well as various sources of persistent trace metals and organic pollutants, such as car breakdowns, sewage and industrial discharges, domestic and industrial waste, agricultural fertilisers and manure run-off [5].Technological progress is accompanied by an increase in anthropogenic pollution by toxic metals, in particular by lead and its compounds. Lead is a global pollutant and a classic toxicant [6]. The data are constantly being updated, and their generalization requires an update, taking into considering changes in the climatic and environmental conditions, anthropogenic effects, and expansion of regional observations.
The effects of oxidative stress in animal tissues together with negative effects of anthropopressure prevent the development of effective adaptation mechanisms, including physiological processes of correction of hypoxic and hemostasis shifts versus accompanying destabilization of metabolic processes with increasing free-radical generation [7]. This tendency reduces the adaptive potential of animals and their survival in the environment, affecting the quality of the breeding process as well [4].
Given their sensitivity to environmental alterations, organisms can be used as indicators of environment conditions relative to the degree of environmental contamination or pollution [8-10]. It is believed that animal species, mainly birds, are most frequently used for determination of the concentration of toxic substances in laboratory and mainly environmental conditions, as their sensitivity is greater than that of even the best chemical indicators [11]. The urban pigeon (Columba livia f. urbana) is a synanthropic bird species that has become established in urban and suburban environments [12, 13]. It is therefore a suitable bioindicator for assessing pollution-affected urban environments [10, 14, 15]. In the region under study, environmental conditions are significantly influenced by the presence of the A1 highway, which contributes to increased atmospheric pollution through vehicular emissions. Additionally, household coal combustion is a common practice in the area, leading to the accumulation of combustion residues in the soil. These residues are characterized by a high lead content, as demonstrated in our previous studies [9, 10, 15].These birds accumulate various noxious substances in their bodies from certain urban or rural areas, most often near their breeding colonies [9, 10, 16]. Their attachment to foraging sites is an advantage for monitoring studies, as this makes it possible to determine the difference in the environmental pollution of specific city districts and villages of certain regions [17].
Activation of free radical-induced lipid peroxidation (LPO) and disruption of calcium homeostasis are considered the main mechanisms of the cytotoxic action of lead ions. Mechanisms of regulation of cellular metabolism include, on the one hand, changes in the intensity of free-radical LPO and, on the other, modification of membrane lipid composition [18]. Intensification of free-radical LPO under lead impact is caused not only by the generation of reactive oxygen species but also by a decrease in the activity of antioxidant enzymes such as superoxide dismutase and catalase. Changes in the composition of biological membranes affect the activity of membrane-bound proteins (enzymes, channel-forming proteins, receptors), which affects calcium homeostasis and cell function as a whole [19, 20]. Cytotoxic effects of lead ions involve mitochondria, which provide energy to cells [21, 22].
This study aims to bridge knowledge gaps by analysing differences in chemical element concentrations in soil and pigeon feathers across two Polish regions with varying anthropogenic pressures. Additionally, we evaluated stress-induced alterations in redox systems and energy metabolism across multiple pigeon tissues (liver, kidney, heart, muscles, brain). By employing X-ray structural analysis and multivariate regression techniques, we sought to elucidate tissue-specific adaptive responses to oxidative stress and environmental modifications. Our findings contribute to understanding the role of metabolic biomarkers in assessing health and environmental conditions.
Therefore, the aim of our study was (a) to characterize tissue-dependent functioning of the main enzymes related to metabolic alterations and energy supply, such as Krebs cycle enzymes, as well as changes in metabolites of oxygen-dependent processes; (b) to use multivariate regression analysis techniques with beta- and correlative coefficients to demonstrate the mechanisms of adaptation reactions in pigeons as a response to modification of environment conditions caused lead-exposed areas.
Materials and Methods
Sampling sites and pigeon groups
The
research was carried out in two towns located 120 km apart in
northern Poland. The first locality, Słupsk (54°27′57″N
17°01′45″E), is a medium-sized town with an area of 43 km2
and the current
population of 89, 000. Słupsk is located on the Koszalin Coast 16 km
south of the Baltic Sea coast. The city center has the Old Market,
which is an open square of approximately 0.3 hectares surrounded by
dense buildings with numerous shops, restaurants, and a church. The
Old Market Square has long been a recreational and tourist
destination intensively visited by tourists during the holiday
season. The image of the Old Market Square is constantly being
changed in order to restore the ancient architecture.
The
second study area is a farm (54°05'43.8″N 18°43'16.1″E) with
urban pigeon (Columba
livia
f. urbana)
breeding for scientific purposes started in 2007 [13]. The flock was
formed from urban pigeons brought from Słupsk. The farm is located
in the village of Szpęgawa, which is situated in the Gdańsk Coast
region approximately 30 km south of the Gulf of Gdańsk. This village
is situated about 1.5 km west of the town of Tczew, which is an
important traffic junction in northern Poland. The Silesia-Baltic and
Berlin transport routes intersect there. The pigeon colony from
Słupsk, presented earlier in our study as an uncontaminated area,
was taken for comparative analysis [9, 10, 15]. Adult pigeons (at
least 1-year-old) were the research material. The sex of birds was
investigated only after decapitation in the laboratory as described
in the paper [13]. The sex of birds was determined by the type of
gonads (presence of testes or ovaries).
The
same paper shows that sexual maturation in these birds begins already
at the age of 3-4 months. This method of determining sex of pigeons
by the type of gonads is the best method because sexual dimorphism in
this species is hardly visible and sex determination in live pigeons
requires familiarity and long observation of their behavior.
The
mean body weight of pigeons was (398.7 ± 28.10 g) (7 females and 10
males,
n = 17) in the group sampled in Słupsk and the
mean body weight of pigeons (409.8
± 27.76 g) (7 females and 7 males,
n = 14)
in Szpęgawa. This difference was not statistically significant
(p=0.281). Study
area and Study design was shown in Fig. 1.
The
pigeons were captured using specially designed nets for bird capture,
ensuring both efficiency and minimal harm. The transport time to the
laboratory varied, ranging from 20 minutes to 3 hours, depending on
the location of capture. During this period, the birds were kept in
special cages designed to minimize stress as much as possible. We
took extra care to provide optimal conditions to reduce any potential
discomfort. All birds were delivered to the laboratory alive. The
experiments were performed in accordance with the guidelines of the
Council of the European Union and the current legislation in Poland.
Animal
research was conducted in accordance with the Animal Welfare Act
(1986)
and EU Directive 2010/63/EU
(Directive
2010/63/EU of the European Parliament and of the Council on the
protection of animals used for scientific purposes.
Official Journal of the European Union, L 276, 33-79) [23].
The studies were performed with the approval of the Bioethics
Committee (University of Gdansk, Poland, Licence No 44/2012).
Fig. 1: Study area and study design of Wild Pigeon Columba livia f. urbana in the Pomeranian region, northern Poland; Słupsk (54°27′57″N 17°01′45″E) and Szpęgawa (54°05'43.8″N 18°43'16.1″E).
Tissue samples
The
study used the following five tissue types as heart, liver, brain,
skeletal muscle (musculus pectoralis), and kidney, which were
immediately extracted from the birds after decapitation. One pigeon
was used for each preparation.
Briefly, all tissues were excised and weighed on ice. Tissues were
also washed separately (to remove blood residues) with cold buffer for
subsequent homogenisation (pH 7.2). The composition of the
homogenisation medium consisted of 10 mM HEPES, 10 mM EGTA,10 mM
EGTA,180 mM KCl and 0.5% bovine serum albumin (pH 7.2). Homogenates
were then centrifuged for
5 min at 600g at 0◦C
(Centrifuge 5910 Ri, Eppendorf SE, Hamburg, Germany). The Bradford
method (1976) with bovine serum albumin as a standard was used for
quantification of proteins [24]. Absorbance was recorded at 595 nm
(VICTOR®
Nivo™ Multimode Plate Readers, PerkinElmer, Waltham, USA).
Enzymatic reactions were started by adding tissue homogenates.
Specific assay conditions are presented subsequently. Each sample was
analyzed in duplicate.
Chemical elements concentration
Concentration
of chemical elements was carried out in soil and feather samples with
an X-Ray fluorescence (XRF) technique using the XRF analyzer (model
Sci Sps X-200 from Sci Sps, Inc.). Determination of element
concentrations was described earlier [9, 10].
Oxidative stress biomarkers
2-Thiobarbituric acid reactive substance (TBARS) assay. Lipid
peroxidation was assessed by measurement of the level of
2-thiobarbituric acid reactive substances (TBARS) with the method
proposed by Buege and Aust (1978) [25]. This method is based on the
reaction of degradation of lipid peroxidation final product, i.e.
MDA, at high temperature and acidity. A colored pink adduct at 532 nm
was measured (VICTOR®
Nivo™ Multimode Plate Readers, PerkinElmer, Waltham, USA).
The results were presented in nmol MDA per mg of protein.
Protein carbonyl derivative assay.
Levels of
carbonyl derivatives of oxidatively modified proteins (OMP) were
determined by measuring the carbonyl groups of amino acids with the
method proposed by Levine et al. (1990) [26]. The assay was performed
based on spectrophotometric measurements of aldehydic (AD OMP) and
ketonic derivatives (KD OMP) in the tissue samples. In this method,
carbonyl groups react with 2, 4-dinitrophenylhydrazine and form 2,
4-dinitrophenylhydrazone derivatives, which can be measured
spectrophotometrically (VICTOR®
Nivo™ Multimode Plate Readers, PerkinElmer, Waltham, USA).
Antioxidant enzymes
The
following methods were used for assessment of antioxidant enzyme
activity and total antioxidant status using standardized biochemical
assay techniques and following the principle adopted in the
methodology described in Randox Assay Kit protocols with our
modification: superoxide dismutase (SOD) activity assay (RANSOD, Cat.
N SD 125, Randox Laboratories Limited, UK), glutathione reductase
(GR) activity assay (RX Monza, GR 2368, Randox Laboratories Limited,
UK), glutathione peroxidase (GPx) activity assay (RX Monza, RS 504,
Randox Laboratories Limited, UK), and total antioxidant status (TAS)
assay (Randox, Cat. N NX 2332, Randox Laboratories Limited, UK).
Catalase (CAT) activity was determined with the method proposed by
Koroliuk et al. (1988) by measuring the decrease in H2O2
level in the reaction mixture. One unit of CAT activity was defined
as the amount of enzyme required for decomposition of 1 μmol H2O2
per min per mg protein [27].
Energetic metabolism parameters
Isocitrate
dehydrogenase (ICDH) activity,
α-ketoglutarate dehydrogenase (KGDH) activity, pyruvate
dehydrogenase (PDH) activity, succinate dehydrogenase (SDH) activity
measured according to the methods proposed by authors Eschenko and
Volski (1982) [28].
Lactate dehydrogenase (LDH) activity and lactate and pyruvate
concentrations were measured spectrophotometrically according with
the method proposed by Sevela and Tovarek (1959) [29] and Herasimov
and Plaksina (2000) [30] accordingly.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity
Alanine
aminotransferase and aspartate aminotransferase activities were
analyzed spectrophotometrically with a standard enzymatic method
described by Reitman and Frankel (1957) [31]. One unit of AST or ALT
is defined as the liberation of 1 nmol of pyruvate per min at 37°C
incubation per mg of protein. Additionally, the De Ritis ratio (AST
to ALT activity) was calculated.
Statistical analysis
Data Preprocessing.
STATISTICA
v.13.3 package (TIBCO Software Inc., Palo Alto, California, USA) was
used for statistical analysis. The results were expressed as
arithmetic mean ± standard deviation (S.D.). Significant differences
between the means were measured using a multiple-range test at min. p
< 0.05. Data that did not have a normal distribution were
log-transformed.
Normality and homogeneity tests.
The
data were tested for homogeneity of variance using Levene’s test,
and normality was checked with Kolmogorov-Smirnov
test. Other
methods, such as the coefficients of multiple correlation analysis
(R) and the coefficient of determination (R2)
with its corrected form reduced by random errors (R2
adjusted) were used in the data analysis [32, 33].
Correlation and regression analysis.
Correlation
of parametric values was based on Pearson’s regression analysis
using the multiple regression module. In the context of this study,
the correlation coefficient and determination coefficient were used
to evaluate the strength and significance of the relationship between
the variables under investigation. The correlation coefficient (r)
serves to quantify the direction and strength of the linear
relationship between two variables, thereby providing insight into
whether changes in one variable are associated with changes in
another. Correlation and regression analysis comprised the
correlation coefficient (r), regression equation, and significance of
these dependencies (p). The determination coefficient (R²) is a
statistical measure of the proportion of variance in the dependent
variable that can be explained by the independent variable(s). The
combination of these coefficients provides a comprehensive
statistical evaluation of the relationships, thereby validating the
findings and ensuring the robustness of the study's conclusions. For
assessment of the multivariate dependencies of the influence of
analyzed predictors in the model of pigeon functioning in two types
of environment relative to sexes, we used standardized
beta-coefficients of regression to compare the overall effect of each
predictor on the dependent variable with each other. These
analyses were designed to identify key predictors of oxidative stress
and metabolic changes in pigeons exposed to varying environmental
conditions.
The list of variables is as follows: independent variables: environmental
types (polluted, non-polluted), sex (male, female), tissue type
(liver, brain, kidney, muscles, heart); dependent variables:
oxidative stress biomarkers (TBARS, AD OMP, KD OMP,
TAS), antioxidant enzymes (SOD, CAT, GPx, GR), biochemical indices of
oxygen-dependent metabolic processes (LDH, lactate, pyruvate,
lactate-to-pyruvate ratio), activities of Krebs cycle enzymes (IDH,
KGDH, PDH, SDH), biochemical indices (ALT, AST, de Ritis ratio),
concentration of elements in soil samples and in bird feathers.
Results
Analysis of the impact of the main factors
In
the current study, three main factors (environments, sex, type of
tissues) were chosen to estimate eight biomarkers of oxidative stress
(TBARS, AD OMP and KD OMP, TAS, and antioxidant enzymes SOD, CAT, GR,
and GPx) and eleven metabolic parameters of oxygen-dependent
metabolic processes (ALT, AST, AST/ALT ratio, LDH activity, lactate
and pyruvate levels and their ratio, SDH, PDH, ICDH, and KGDH
activities) in five selected tissues (muscles, heart, brain, kidney,
and liver) depending on the sex and environments of the pigeons. We
also analyzed the combinative effects of these factors. The results
of four tests of multivariate analysis and statistical significance
are presented in Table 1.
All
biochemical parameters showed significant differences, except when
considering the effect of sex in isolation and its combination with
all three factors. While sex alone did not show a significant effect,
its interaction with environmental factors underlines possible
sex-specific responses to lead exposure and oxidative stress. Four
tests of the multivariate analysis showed that primarily the
environmental factor (F = 58.71, p = 0.000) had the largest stable
effect in this model. The effect of tissue type in this analysis
using the four tests also showed a high variance of statistical
significance (F = 28.38-116.92, p = 0.000). It is important to note
that the combination of sex and environment factors (F = 2.496, p =
0.000) and tissue specificity and sex were also statistically
significant in two tests (F = 1.317-2.60, p = 0.048 and p = 0.001,
respectively). Thus, although the sex of the bird alone does not have
a clearly pronounced influence (lack of statistical regularity), it
plays a critical role in the model in combination with the effects of
the environment and the influence of lead (i.e. it becomes
statistically significant in combinations of influences).
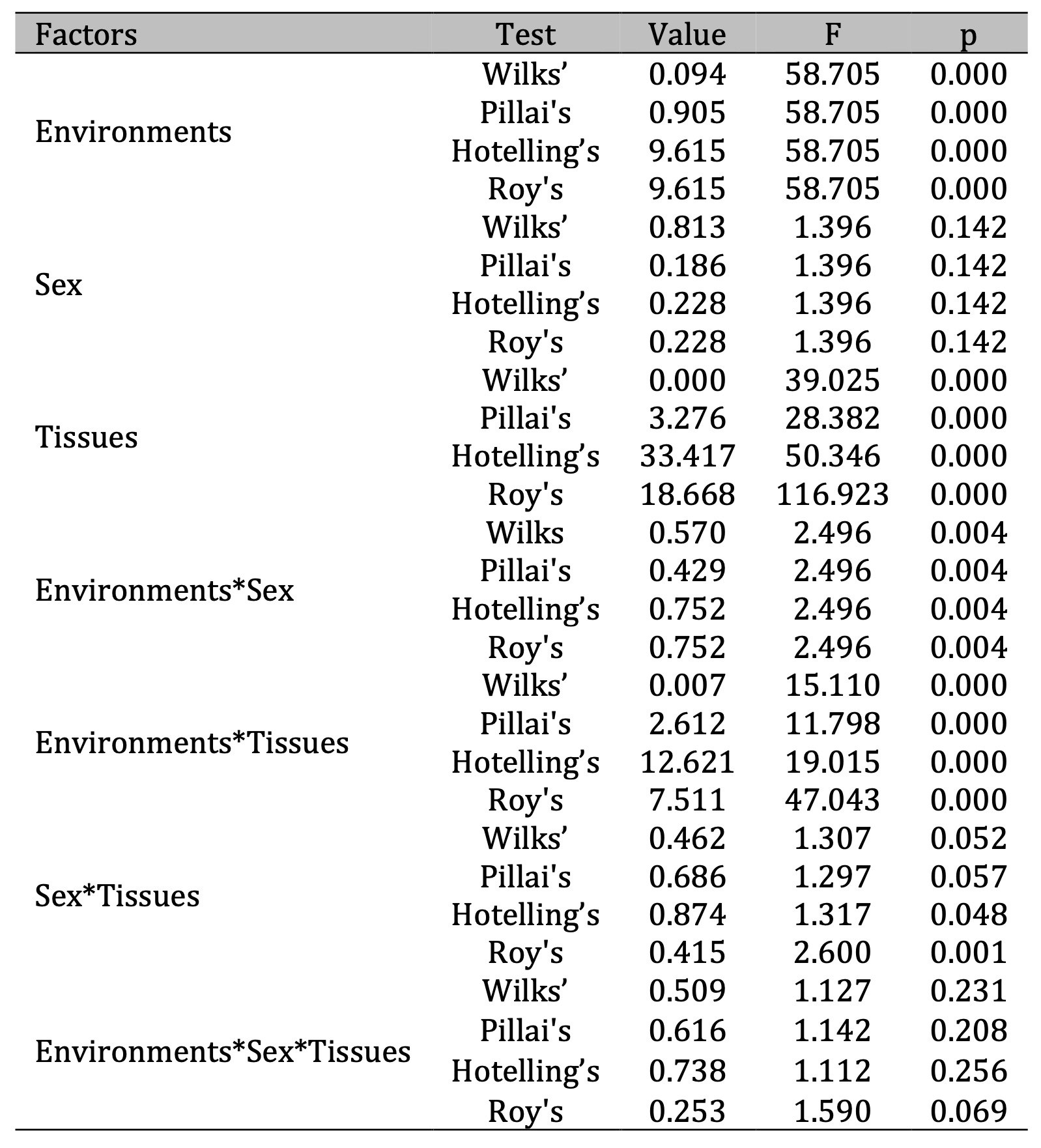
Table 1: Multivariate tests of significance with sigma-restricted parameterization and effective hypothesis decomposition for biomarkers of oxidative stress and biochemical alterations of metabolism in five tissues (muscles, heart, brain, liver and kidney) of pigeon Columba livia f. urbana from Pomeranian region, Northern Poland depending on their habitats and sex. F – Fisher’s test index, p – significance level
Analysis of oxidative stress biomarkers
Lipid peroxidation and oxidatively modified proteins.
Firstly, we determined lipid
peroxidation by assessing TBARS levels and oxidatively modified
proteins by assessing the amount of aldehyde and ketone derivatives
(Figures 2-4). Two important physiological aspects of these processes
should be noted. Firstly, each of the tissues differs in the baseline
level of functional activity of lipid peroxidation and protein damage
as well as the associated antioxidant defences in these tissues.
Various factors, such as sex, genetics, environmental conditions,
nutritional habits, etc., have an additional influence on these
processes. Therefore, along with presenting the baseline values of
the biochemical parameters determined for the selected tissues, we
also provided better visualization of the observed changes depending
on sex of individuals via recalculation of percentage changes of
oxidative stress biomarkers relative to the polluted vs.
unpolluted areas (Fig. 2).
The
considered
percentages of the effects of the polluted area on oxidative stress
biomarkers in five tissues of pigeons were associated with an
increase in the intensity of lipid peroxidation assessed by the level
of TBARS (Fig. 2A), an increase in oxidative modification of proteins
evaluated from the amount of aldehydic and ketonic derivatives (Figs
2B and 2C), and a significant decrease in the total antioxidant
status (TAS) in all tissues except kidneys. However, this percentage
varied according to sex of pigeons and the type of tissues. We found
a significant increase in lipid peroxidation with a difference
between two sexes of pigeons in case of brain and kidney tissues.
Significant increase in these processes was shown for cardiac tissue,
but these changes were not sex-dependent. The level of TBARS was
significantly higher in the brain and kidney tissues in the males
compared to the females.
Therefore,
the observed variations in lipid peroxidation levels are closely
related to the type of tissue and its physiological function. Brain
and kidney tissues, which are metabolically active and susceptible to
oxidative stress, showed higher levels of TBARS in males, suggesting
a sex-specific susceptibility. In contrast, cardiac tissue, which
relies on a steady oxidative metabolism for its function, showed
increased lipid peroxidation that was not influenced by sex. These
results highlight the interplay between tissue-specific roles and
susceptibility to oxidative damage in determining lipid peroxidation
levels.
Total antioxidant status.
This
study provides important insights into the functioning of cellular
redox systems. Firstly, the assessment of TAS in different tissue
types highlighted the deleterious effects of environmental conditions
and adaptive responses to oxidative stress. Secondly, a pronounced
sex-dependent decrease in TAS was observed in brain and heart
tissues, with a greater decrease in females compared to males.
Conversely, an increase in TAS was observed in kidney tissue, which
was more pronounced in females. These findings highlight the tissue-
and sex-specific differences in antioxidant defence mechanisms.
Antioxidant enzymes.
Since
we obtained pronounced results of intensification of lipid
peroxidation in the muscle and cardiac tissues with a simultaneous
increase in the oxidative modification of proteins (OMP), which
significantly affected the total antioxidant status, we present
values of antioxidant defence in various tissues of pigeons living in
the unpolluted and polluted areas. The values for the skeletal muscle
and cardiac tissues are shown in Fig. 3. In turn, Fig. 4 shows the
changes in the activity of main antioxidant enzymes, i.e. superoxide
dismutase (SOD, 4A) and catalase (CAT, 4B), and glutathione-dependent
enzymes, i.e. glutathione reductase (GR, 4C) and glutathione
peroxidase (GPO, 4D). It should be noted that the effects of the
environmental pollution had a pronounced component of the effect of
sex of pigeons on
CAT and GR activities in these types of muscle.
In
the current study, SS test was used to determine the next dependences
considering the role of each of investigated parameters in the
formation of full statistical model of the biomarkers of oxidative
stress and antioxidant defense: CAT > GPx > SOD > GR > KD
OMP > AD OMP > TBARS > TAS. It should be noted that
statistical analysis performed showed that, in this model, CAT played
the leading role in the analyzed antioxidant defense, since we
obtained maximum values of the multiple correlation coefficient
(R=0.92) as well as the coefficient of determination (R2
= 0.85) and its adjusted form (R2adj
= 0.83) in this case.
In
assessment of the multivariate dependencies of the influence of the
predictors in a sex-dependent functioning model of pigeons living in
two types of areas (polluted vs. unpolluted), we used standardized
regression coefficients (β-coefficients). This standardized
coefficient allowed us to compare the overall effect of each
predictor on the dependent variable. It helped to compare the effects
of each of the main factors and their combined effects on each of the
biochemical parameters studied in five tissues of pigeons. Table 2
presents significant values of beta-coefficients and their standard
errors as well as t-value for beta-coefficients and significance
level.
It
should be noted that we obtained maximum β-coefficient values among
analyzed parameters for such factors as the type of environment for
TBARS parameter (β = 0.567 ± 0.05; p = 0.000) and OMP AD (β =
0.457 ± 0.04; p = 0.000), as shown in Table 2. The level of
oxidative modification of proteins in the muscle tissue was shown to
be at its maximum level for aldehydic derivatives of OMP (β = 0.577
± 0.05; p = 0.000). High correlations for ketonic derivatives of OMP
in muscle tissue (β = 0.684 ± 0.05; p = 0.000) were shown. It is
important to note that we also obtained negative values of the beta
coefficient of the cumulative effects of Environment*Tissue factors
for TAS, which reflects the specificity of the decrease in the total
antioxidative status with the pressure on the functioning of red-ox
reactions under anthropogenic stress. These values are presented in
the following form: β = -0.643 ± 0.05 (p = 0.000).
Thus,
the regression multivariate analysis of impact of three factors, i.e.
environment, sex, and tissue types, showed that proteins and cell
membrane lipids in various tissues of pigeons living in polluted area
were the most significant target for oxidative stress.
It
is known that a number of metabolism-related enzymes participating in
red-ox system are characterized by a decrease in their catalytic
activity with excessive oxidative stress that is not neutralized by
antioxidants. These peculiarities of the influences and correlation
of the mutual separation of factors in the model for the final stages
of lipid peroxidation, oxidative modification of proteins, and main
antioxidant enzymes, and the integrity of this system in various
tissues of pigeons living in polluted and unpolluted areas were
presented in this part of studies.
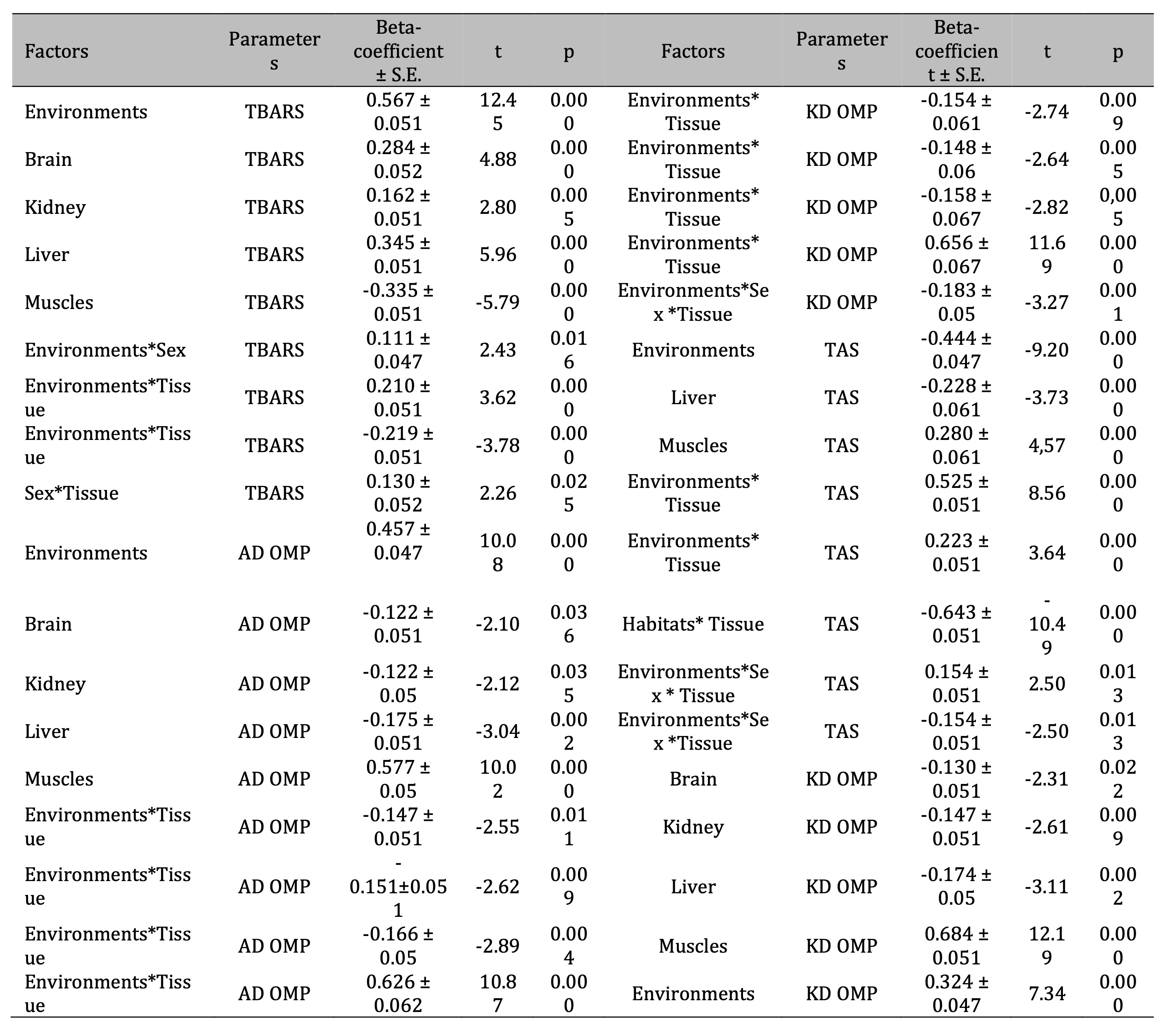
Table 2: Standardized β-coefficient of the levels of lipid peroxidation biomarkers (TBARS), the content of oxidatively modified proteins (aldehydic and ketonic derivatives of OMP: AD OMP and KD OMP), and total antioxidant status (TAS) according to the analyzed influence of such factors as habitats, bird sex, and type of tissues (brain, muscles, heart, liver, kidney) of pigeon Columba livia f. urbana from Pomeranian region, Northern Poland and t-value with its significance (p). Parameterization with sigma-constans
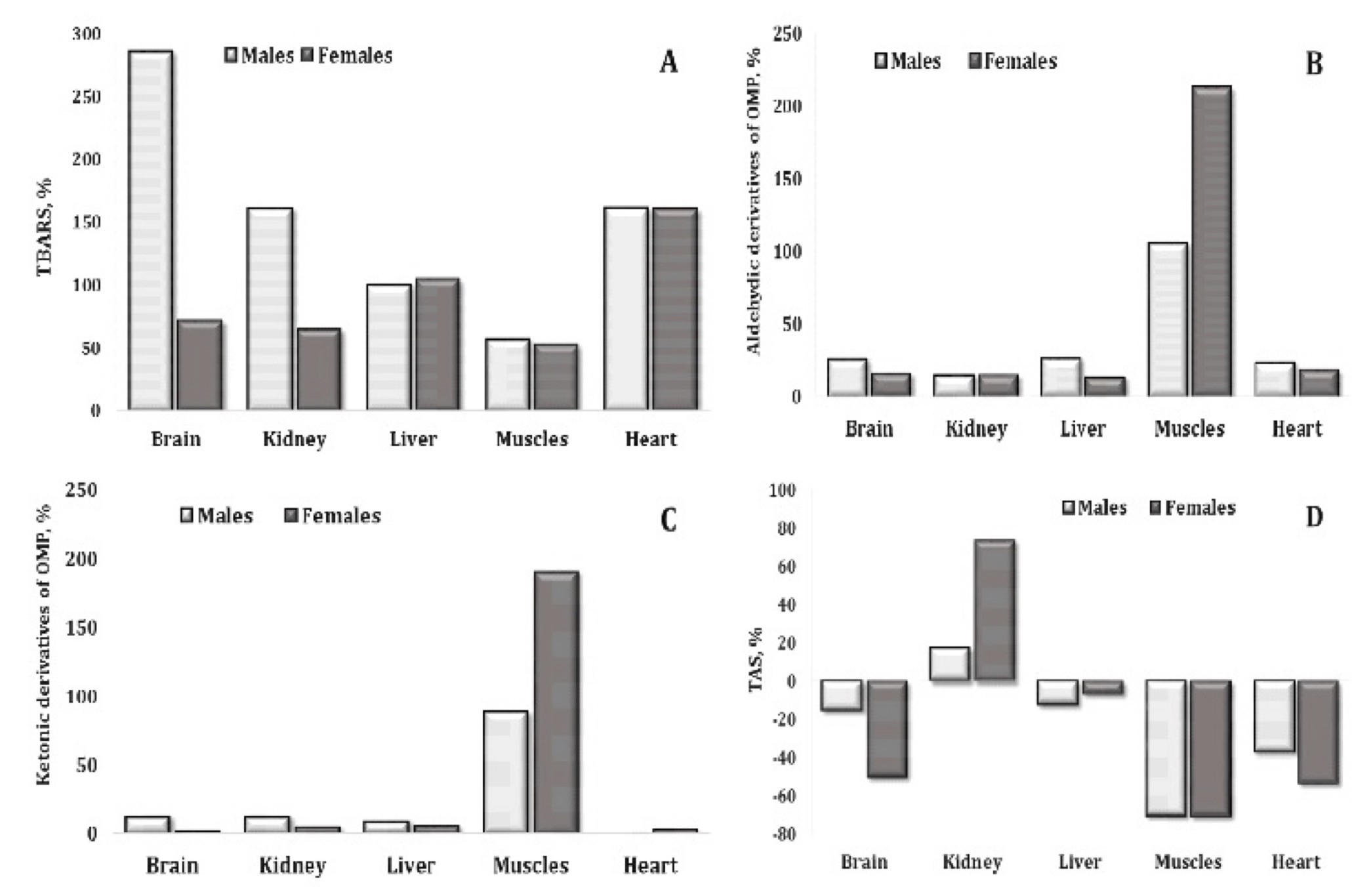
Fig. 2: Percentages of changes in TBARS (A), aldehydic derivatives of OMP (B), ketonic derivatives of OMP (C), and TAS (D) levels in relation to polluted vs. unpolluted areas and the sex of pigeon Columba livia f. urbana nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of Pomeranian region, Northern Poland.
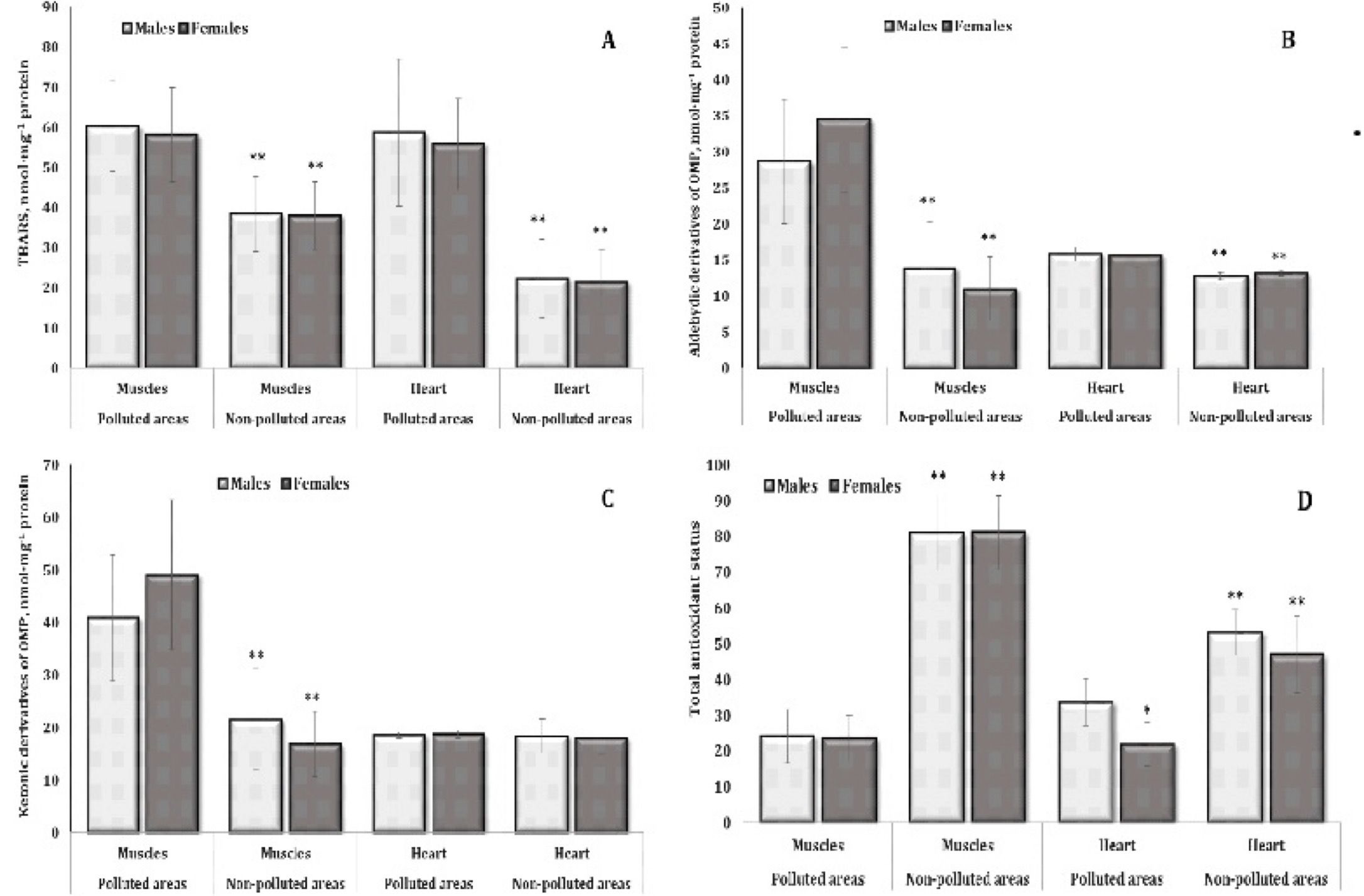
Fig. 3: Levels of TBARS (A, nmol∙mg-1 protein), aldehydic derivatives of OMP (B, nmol∙mg-1 protein), ketonic derivatives of OMP (C, nmol∙mg-1 protein), and TAS (D, %) in the muscle and cardiac tissues of different sexes of pigeon Columba livia f. urbana nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of Pomeranian region, Northern Poland. The results are presented as the mean and standard deviation (mean ± S.D.). The comparison of means between all groups was carried out using MANOVA followed by Tukey’s post-hoc test. * P < 0.05 – polluted vs. unpolluted areas in relation to the same sex; ** P < 0.05 – males vs. females in relation to the same type of habitats.
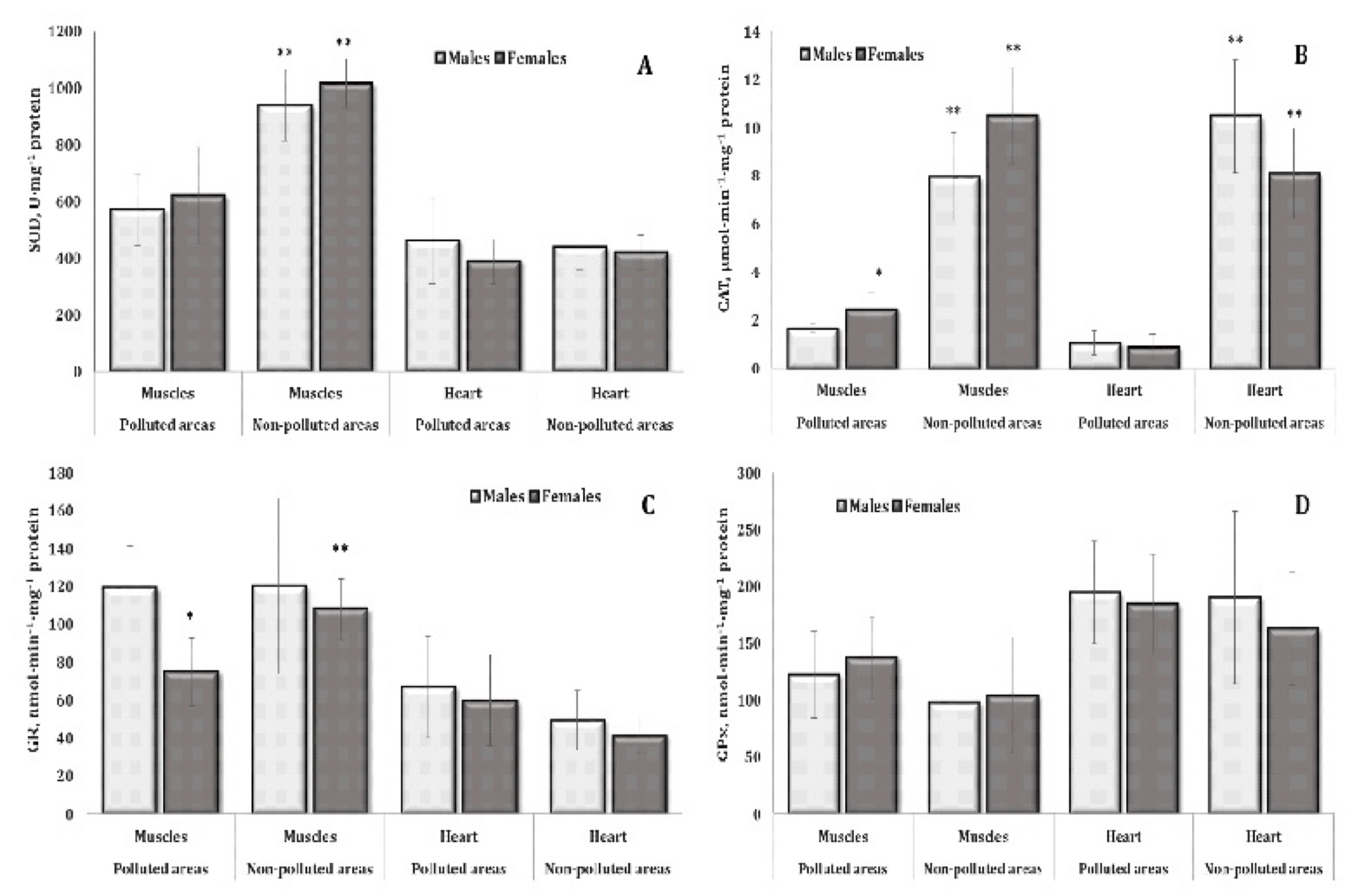
Fig. 4: Activities of superoxide dismutase (SOD, A, U∙mg-1 protein), catalase (CAT, B, μmol∙min-1∙mg-1 protein), glutathione reductase (GR, C, nmol∙min-1∙mg-1 protein), and glutathione peroxidase (GPx, D, nmol∙min-1∙mg-1 protein) in the muscle and cardiac tissues of different sexes of pigeon Columba livia f. urbana nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of Pomeranian region, Northern Poland. The results are presented as the mean and standard deviation (mean ± S.D.). The comparison of means between all groups was carried out using MANOVA followed by Tukey’s post-hoc test. * P < 0.05 – polluted vs. unpolluted areas in relation to the same sex; ** P < 0.05 – males vs. females in relation to the same type of habitats.
Biomarkers of oxygen-dependent and metabolic processes
Activity of aminotransferases.
Aminotransferases
are enzymes in the class of transferases that reversibly catalyze the
transfer of amino groups from alpha-amino acids to alpha-keto acids,
which are important in protein metabolism (Fig.
5). Transaminases, which are catalyzed by aminotransferases, have
been shown to be integral to protein and carbohydrate metabolism and
thus reflect key adaptive properties of animal tissues. The activity
of these enzymes was therefore analyzed in detail in five of the
tissues studied. The significance of the role of these enzymes in the
overall dependences was also confirmed by us in multivariate
regression analysis using the standardized beta-coefficient for
hepatic tissue, where significant highest values of beta-coefficient
were found in determining effects of tissue factor. The results of
this predictor effect analysis are presented in Table 3. ALT activity
in the brain tissue in male pigeons, muscle tissue in both sexes, and
cardiac tissue in males from polluted areas was increased (Fig. 5A).
AST activity was statistically changed only in the hepatic tissue, as
shown by the comparison of data obtained from birds from polluted and
unpolluted areas. These changes were significant, as shown by
comparison of values between females and males (namely, the levels
were higher in females) and between the groups of females from the
polluted and unpolluted areas (Fig. 5B). The values of the ratio of
activity of these enzymes described as de Ritis coefficient
(AST/ALT), which have important clinical significance for analytical
diagnostics, were recalculated by us for five tissues examined and
are presented in Fig. 6. It should be noted that the values of this
coefficient in the brain, kidney, muscle, and heart tissues in the
group of male birds living in polluted area were statistically
decreased (Fig. 6A).
Lactate dehydrogenase activity.
Lactate
dehydrogenase (LDH) is an intracellular carbohydrate-metabolizing
enzyme catalyzing the interconversion of pyruvic acid and lactic
acid, i.e. a reaction that completes the internal redox cycle of
glycolysis (Table 4). Therefore, studying the activity of this enzyme
and the levels of substrates of its conversion was the next step of
our studies. For both LDH and other metabolic indices in pigeons,
evident tissue specificity of the baseline activity of these
parameters was shown. However, we revealed differences in LDH
activity in the brain and kidney tissues of pigeons of different
sexes. General changes in LDH activity were manifested in a marked
increase in the enzyme activity in the brain, kidney, and hepatic
tissues of pigeons of both sexes living in polluted area and in
muscle tissue of male pigeons. These changes were transformed into a
pattern of notable acidosis in most tissues of pigeons of both sexes
accompanied by significant increase in lactate levels in these
conditions (Table 5). It should be noted that this statistically
significant increase in the brain tissue was 286.7% (p = 0.000) of
the males and 138.2% (p = 0.000) in the females, compared to the
values obtained for birds living in the unpolluted area. A two- to
three-fold increase in lactate levels was also found in the kidney,
hepatic, and cardiac tissues of pigeons of both sexes. The
preferential accumulation of lactate associated with LDH metabolism
in the tissues caused an increase in pyruvate metabolism, which is
shown in Tables 5. These changes were accompanied by statistically
significant increases in pyruvate levels in all tissue types analyzed
in both sexes of pigeons.
Krebs cycle enzymes.
Since
Krebs cycle acts as a link between chemical reactions of anabolism
and catabolism with the formation of active metabolites, we
investigated the activity of four main enzymes in this metabolic
process (Tables 6 and 7). Since the substrates of this cycle are the
basis for biochemical synthesis of new molecules capable of
conversion into succinyl-CoA, oxalic acid, a complex of amino acids,
and glucose, the study of these processes revealed the mechanisms of
energy supply depending on the induction of oxidative stress and
resulting intracellular acidosis in tissues of pigeons living in
differently polluted areas, depending on their sex.
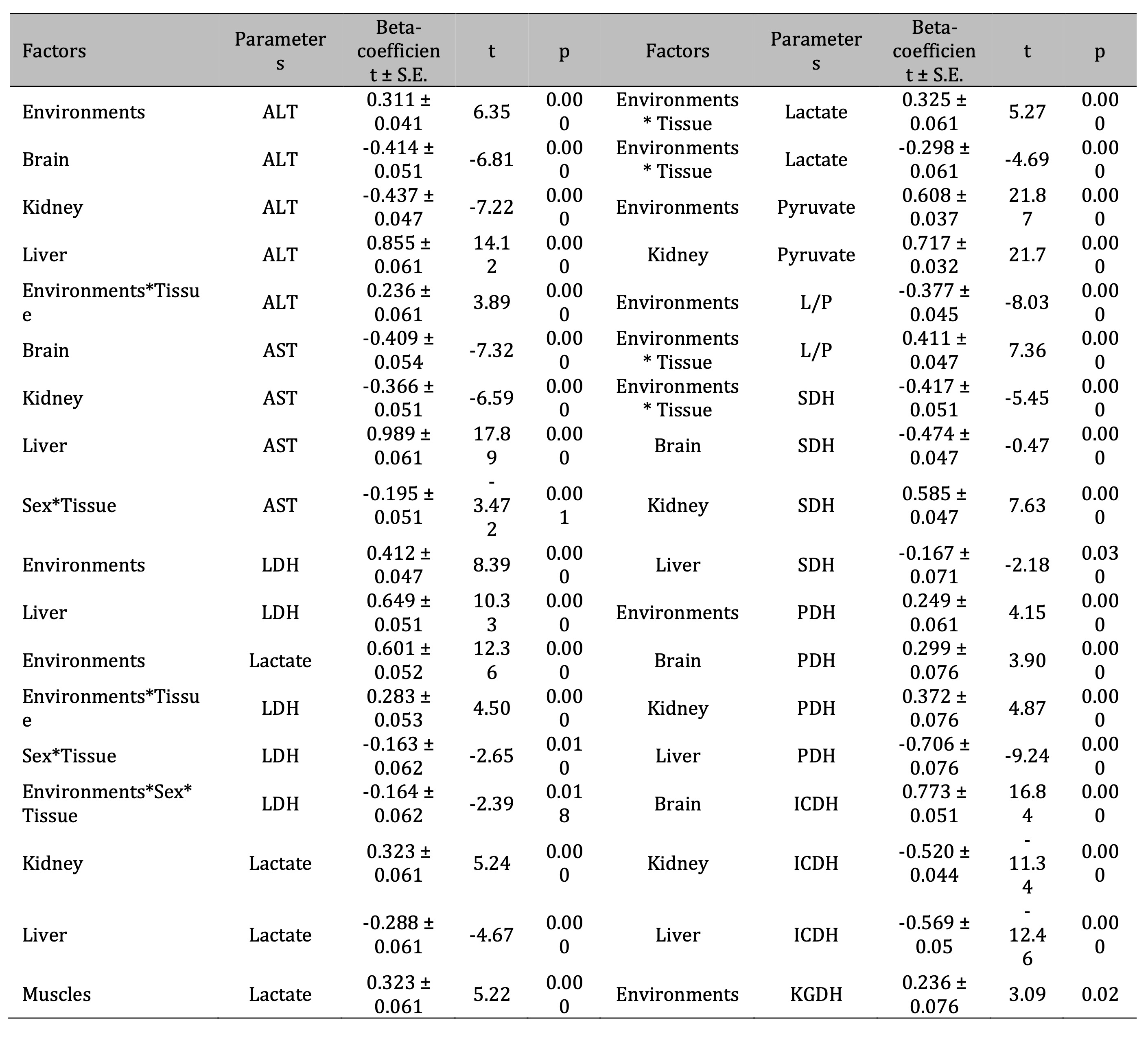
Table 3: Standardized β-coefficient of antioxidant enzymes activity [superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx)] according to the analyzed influence of such factors as habitats, bird sex, and type of tissues (brain, muscles, heart, liver, kidney) of pigeon Columba livia f. urbana from Pomeranian region, Northern Poland and t-value with its significance (p). Parameterization with sigma-constans
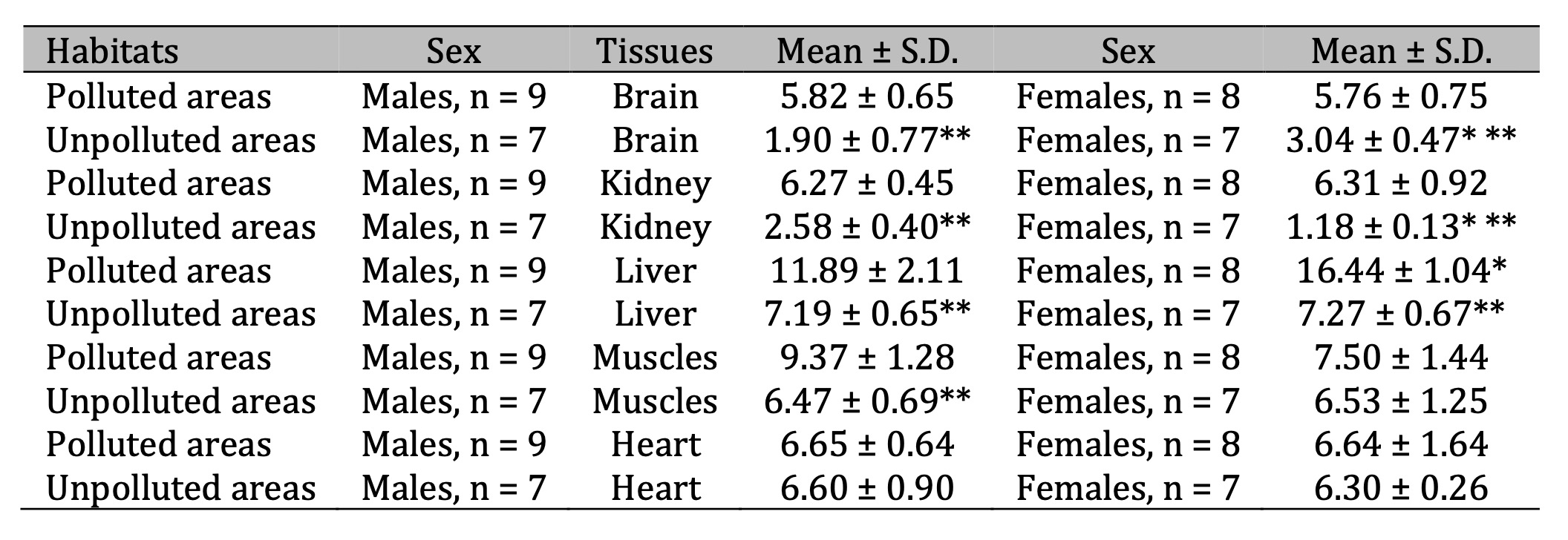
Table 4: Lactate dehydrogenase activity (LDH, nmol∙min-1 ∙mg-1 protein) in the brain, kidney, liver, muscle, and cardiac tissues of pigeon males and females nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of the Pomeranian region, northern Poland. Legend: * P < 0.05 in relations between sexes; ** P < 0.05 in relations between habitats
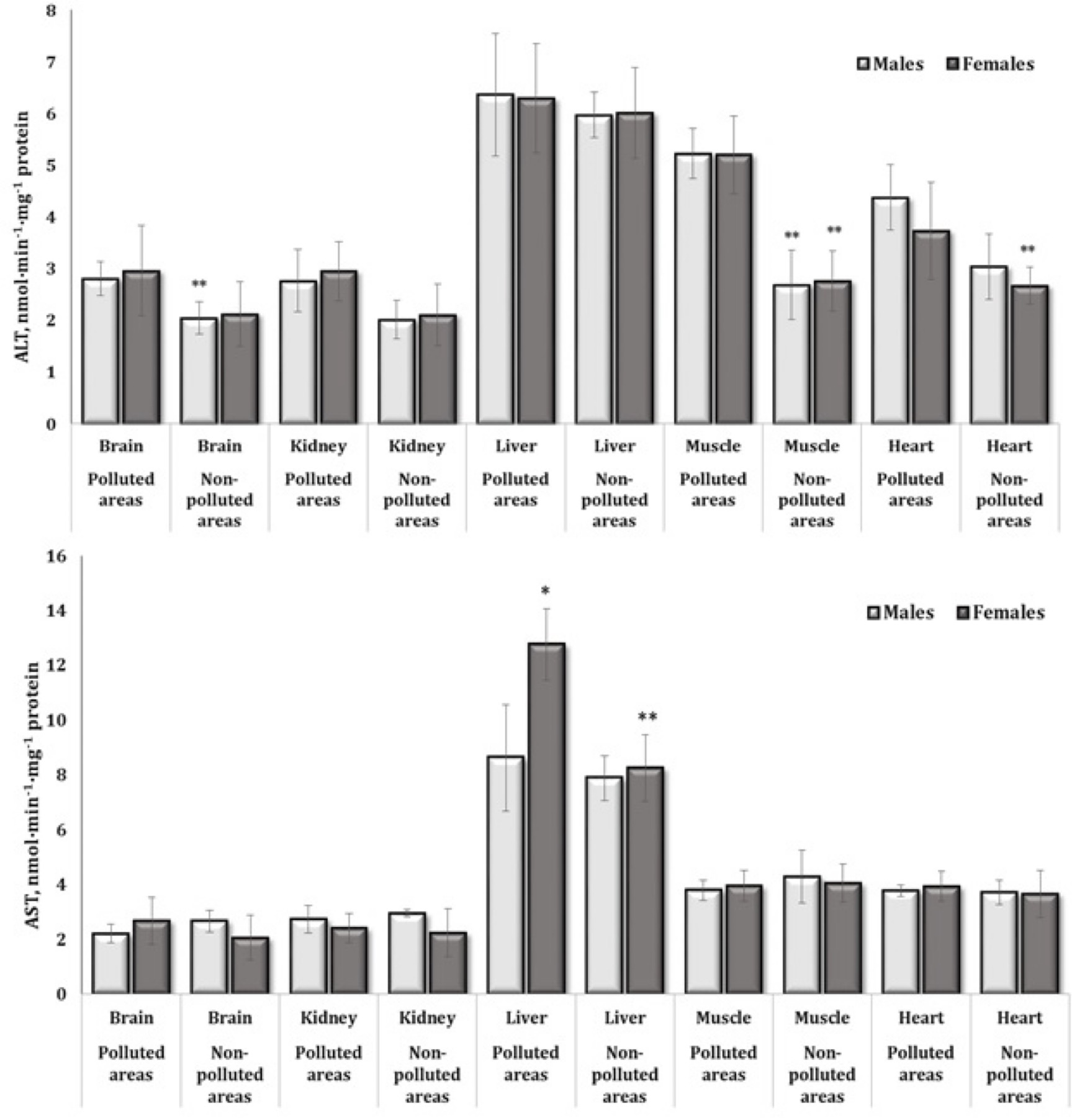
Fig. 5: Activities of alanine (nmol∙min-1∙mg-1 protein) and aspartate aminotransferase (nmol∙min-1∙mg-1 protein) in the brain, kidney, liver, muscle, and heart tissues of different sexes of pigeon Columba livia f. urbana nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of Pomeranian region, Northern Poland. The results are presented as the mean and standard deviation (mean ± S.D.). The comparison of means between all groups was carried out using MANOVA followed by Tukey’s post-hoc test. * P < 0.05 – polluted vs. unpolluted areas in relation to the same sex; ** P < 0.05 – males vs. females in relation to the same type of habitats.
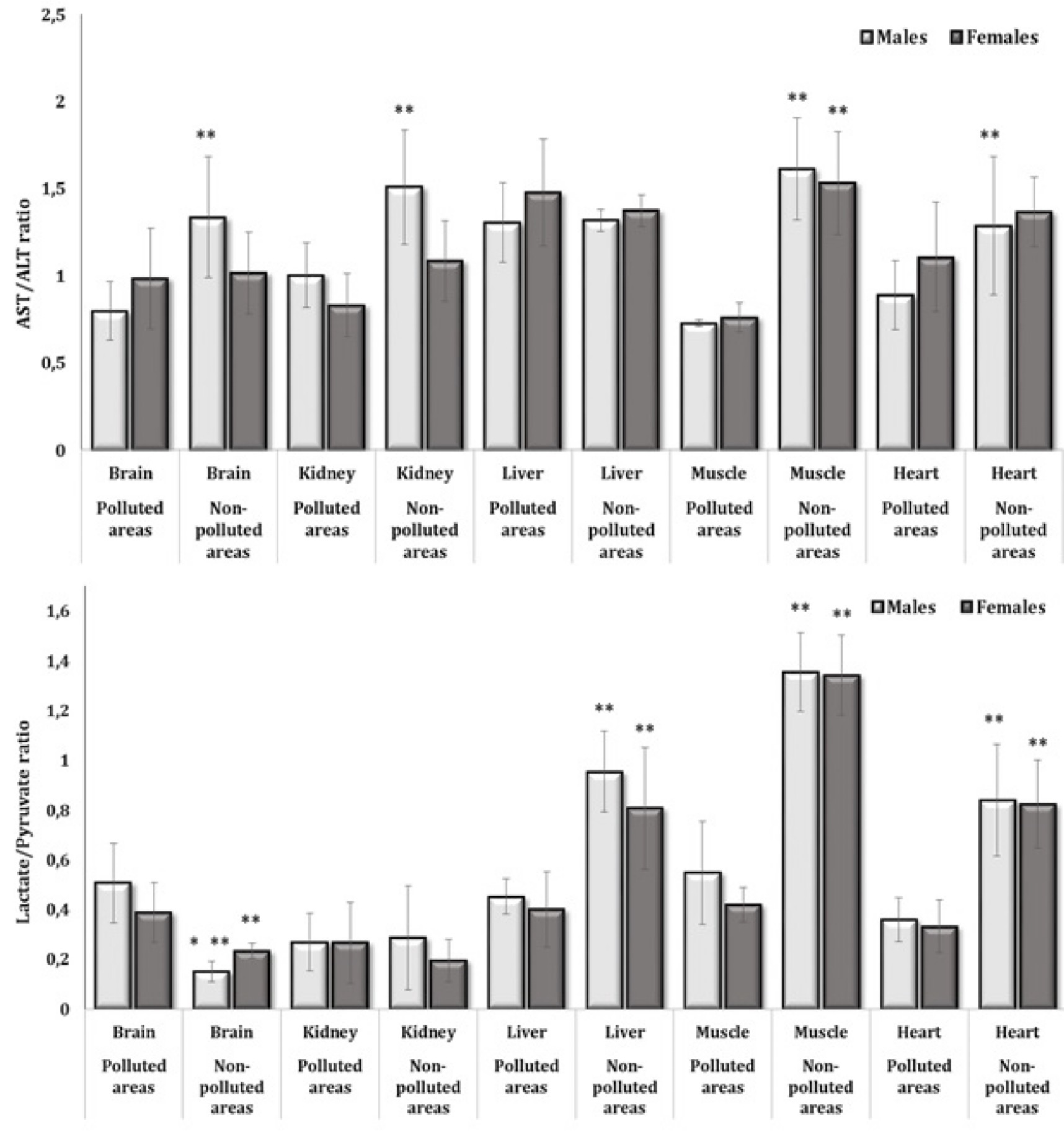
Fig. 6: De Ritis ratio (AST/ALT) and lactate-to-pyruvate ratio in the brain, kidney, liver, muscle, and heart tissues of different sexes of pigeon Columba livia f. urbana nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of Pomeranian region, Northern Poland. The results are presented as the mean and standard deviation (mean ± S.D.). The comparison of means between all groups was carried out using MANOVA followed by Tukey’s post-hoc test. * P < 0.05 – polluted vs. unpolluted areas in relation to the same sex; ** P < 0.05 – males vs. females in relation to the same type of habitats.
Our results regarding the activity of isocitrate dehydrogenase (ICDH) as one of the main enzymes of Krebs cycle, which is considered to be the speed-limiting enzyme of the whole cycle, are presented in Table 6. It should be noted that statistical analysis confirmed this concept, as a significant role was assigned to ICDH in this pigeon model; in this case, we obtained maximum values of the multiple correlation coefficients (R = 0.91) as well as the coefficient of determination (R2 = 0.83) and its adjusted form (R2adj = 0.80). The significance of this enzyme in the overall statistical analysis was also confirmed by us in multivariate regression analysis performed using standardized β-coefficient for the brain, kidney, and hepatic tissues, where statistically significant high values of β-coefficient were found in determining the role of tissue versus the effect factor (Table 3). Beta-coefficient had a value of β = 0.773 ± 0.051 (p = 0.000) for the brain tissue, β = -0.520 ± 0.044 (p = 0.000) for the kidney, and β = -0.569 ± 0.05 (p = 0.000) for the liver tissue (Table 2).
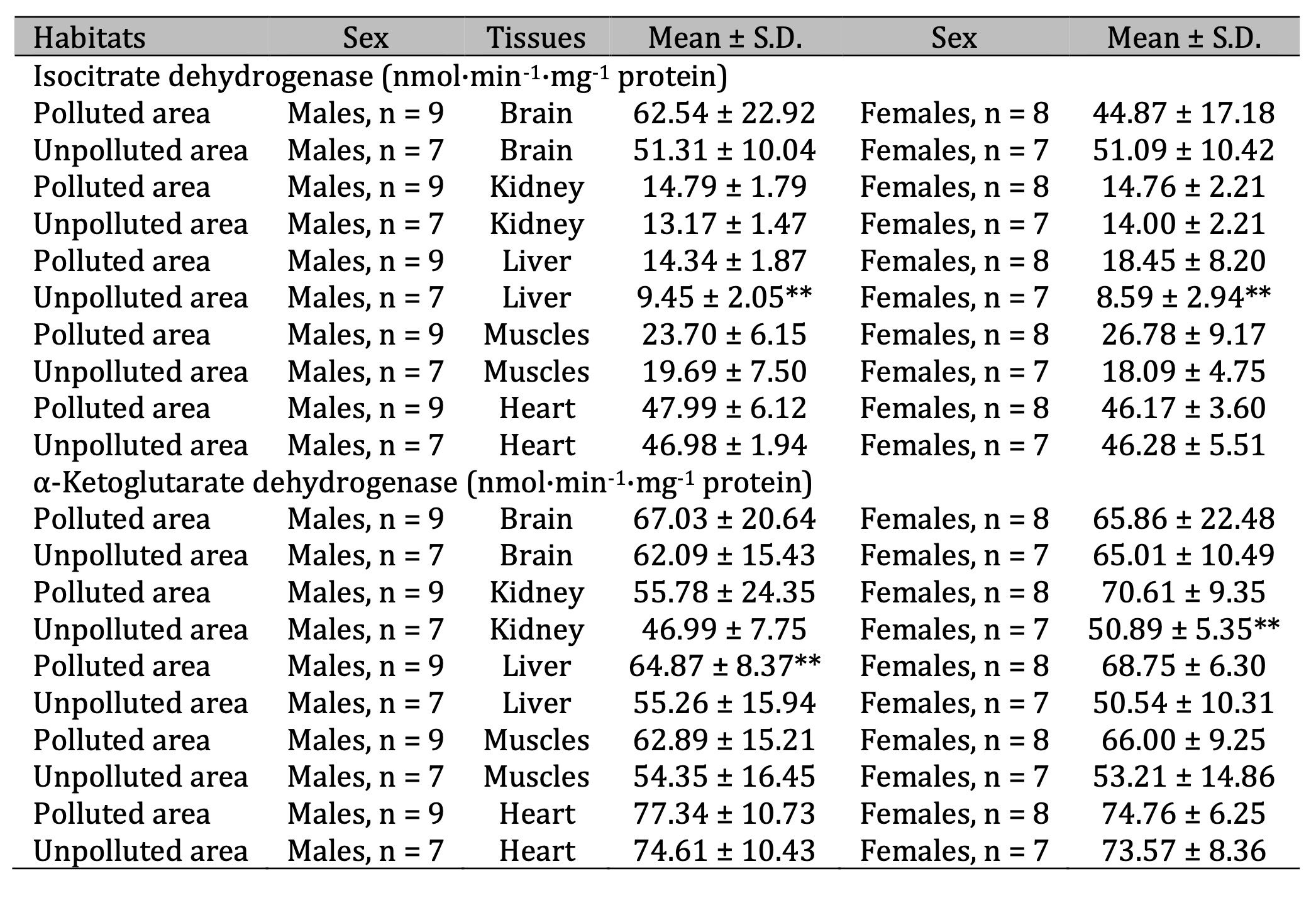
Table 6: Activities of isocitrate dehydrogenase (ICDH, nmol·min-1·mg-1 protein) and α-ketoglutarate dehydrogenase (KGDH, nmol·min-1·mg-1 protein) in the brain, kidney, liver, muscle, and cardiac tissues of pigeon males and females nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of the Pomeranian region, northern Poland. Legend: * P < 0.05 in relations between sexes; ** P < 0.05 in relations between habitats
We obtained statistically significant increase in the activity of this enzyme in the hepatic tissue of birds of both sexes living in polluted area, compared to those from unpolluted. KGDH activity increased significantly in the liver of males and showed an increasing trend in the kidneys of females from polluted areas (Table 6). Significant increases in the activity of succinate dehydrogenase (SDH) in three analyzed tissues (muscle, liver, kidney) were shown for both sexes of birds living in polluted area. The same trend of increased SDH was observed in the brain tissue of males. Pyruvate dehydrogenase (PDH) activity was significantly increased in the kidneys of males and cardiac tissue of females living in polluted area (Table 7).
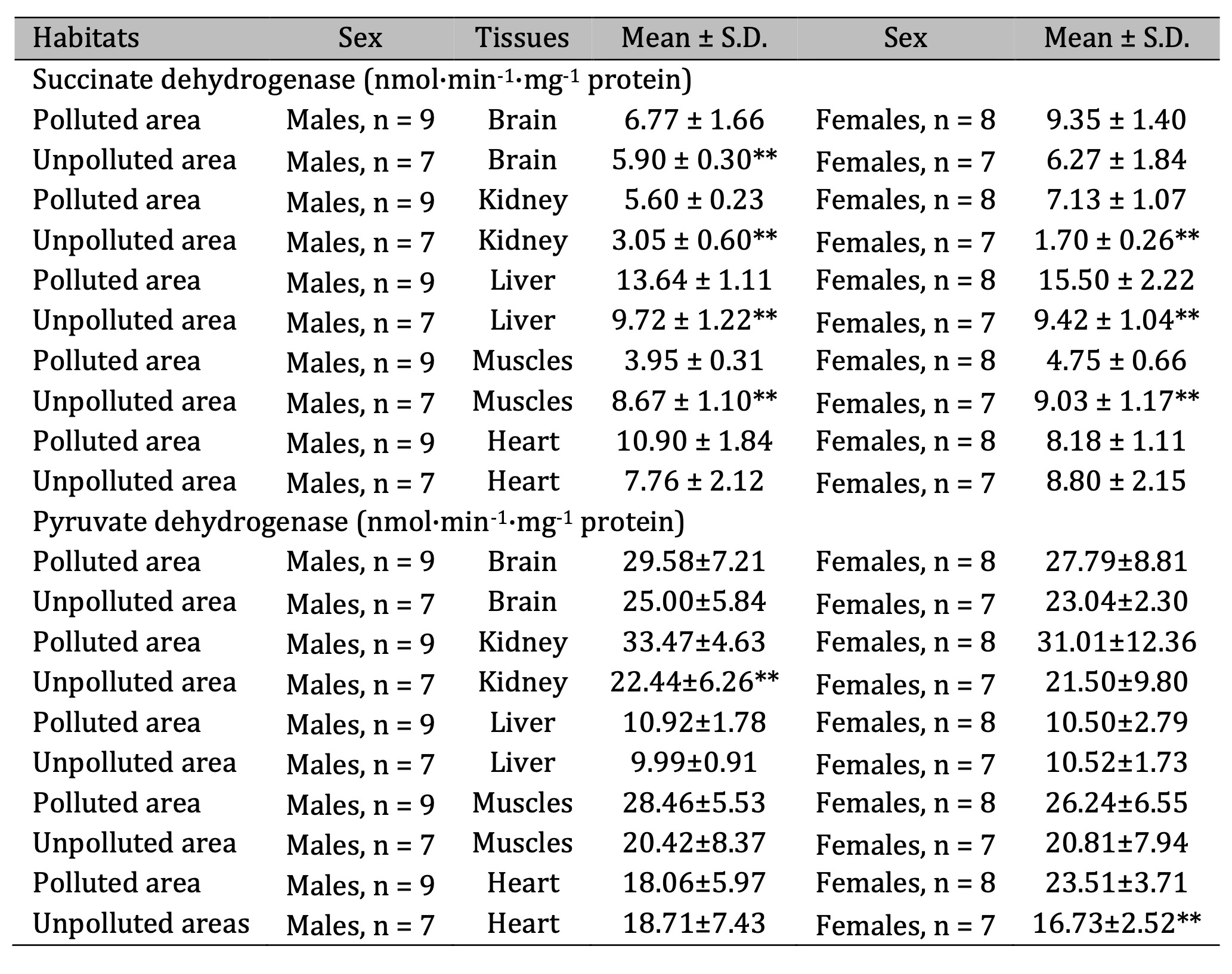
Table 7: Activities of succinate dehydrogenase (SDH, nmol·min-1·mg-1 protein) and pyruvate dehydrogenase (PDH, nmol·min-1·mg-1 protein) in the brain, kidney, liver, muscle, and cardiac tissues of pigeon males and females nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of the Pomeranian region, northern Poland. Legend: * P < 0.05 in relations between sexes; ** P < 0.05 in relations between habitats
The analysis of statistical dependencies between metabolic biomarkers identified pyruvate as the most influential factor, with ICDH, AST and ALT also playing key roles. ICDH, as a rate-limiting enzyme of the Krebs cycle, showed a significant function in the pigeon model, strongly influencing metabolic processes. Multivariate regression analysis highlighted its importance in brain, kidney and liver tissues, with distinct patterns of tissue-specific involvement. These findings highlight the critical role of ICDH in metabolic adaptation in different tissues. A complete analysis of the entire model of statistical dependencies in the value of metabolic processes is as follows Pyruvate > ICDH > AST > ALT > L/P ratio > Lactate > LDH > ALT/AST > PDH > SDH > KGDH.
Correlation analysis determined the quantitative measure of the relationship (joint variability) of parameters, and correlation coefficients were presented to identify relationships between the levels of biomarkers of oxidative stress and energy metabolism in different tissues as well as chemical elements in their feathers. It is equally important to determine the strength of these correlations. These correlation interactions (dependencies) are presented in Table 8. Data are presented for birds living in polluted area according to the tissue type, with subsequent interpretation between one pair or many pairs of traits to establish statistical relationships between them.
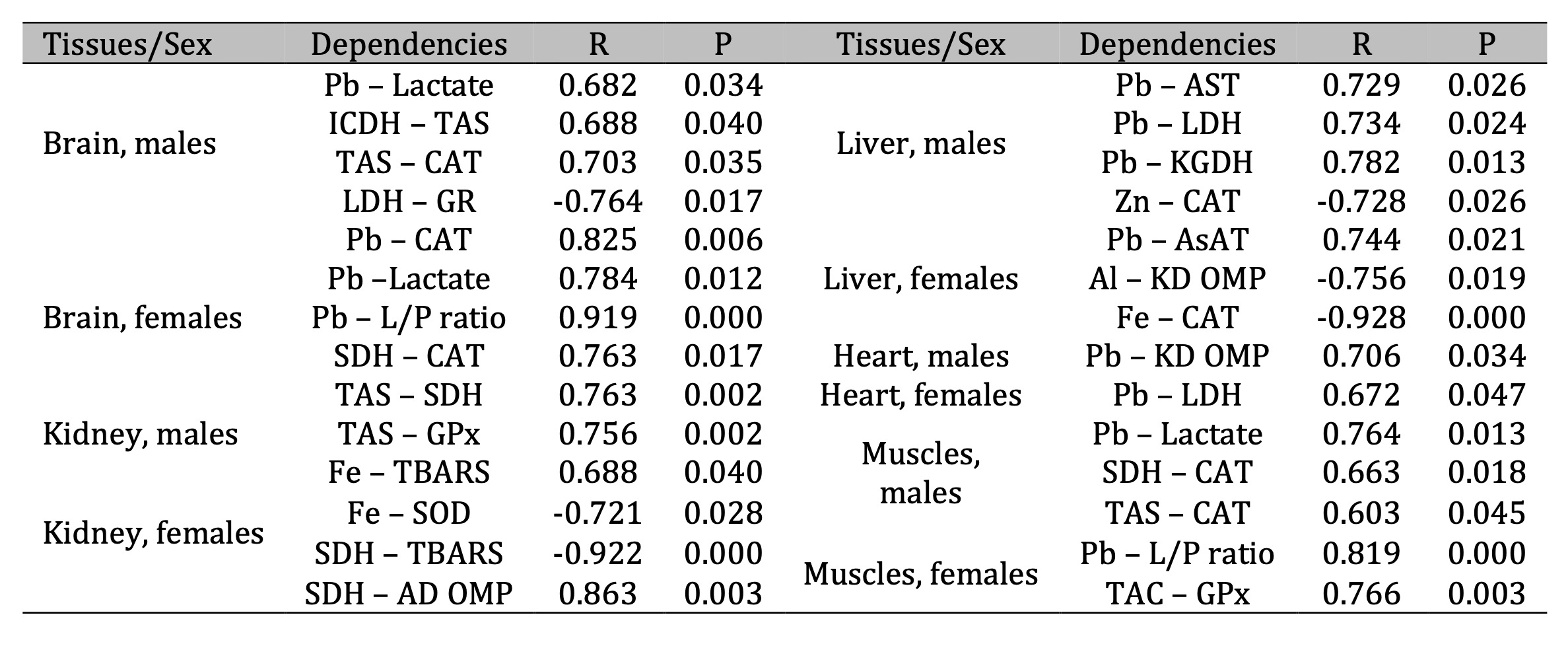
Table 8: Parameters of correlative dependencies between five types of tissues and oxidative stress biomarkers, activities of metabolic enzymes, and levels of substrates and elements in male and female pigeons nestling in polluted areas of Northern Poland. The significance of differences in different sexes and tissues was examined using ANOVA for correlation tests
Discussion
In our studies, we have presented ecotoxicological investigations of feral pigeons in their natural environments, assessing the impact of three factors (environments, sex, tissue types) on the alterations in oxidative stress biomarkers and biochemical parameters in selected tissues of pigeons and the levels of chemical elements in their feathers. Importantly, we considered these characteristics simultaneously for two environments (polluted and unpolluted areas), sexes of birds (males, females), and five different tissues (brain, kidney, liver, muscles, heart) of pigeons living in areas with different levels of lead exposure. Various authors have previously conducted systematic studies of only one or two of factors listed [8, 16, 17]. Therefore, our comprehensive study significantly extends physiological and biochemical analysis of this synanthropic bird species, i.e. pigeons for urban agglomerations [34].
The results of studies by various authors, including our own [9, 10, 15], show that lead exposure exerts a distinct effect on different tissues of pigeons, manifested by a range of molecular mechanisms [35-37]. The effects of lead on the brain have been shown to occur through disruption of calcium homeostasis, inhibition of neurotransmitter release and induction of oxidative stress [38]. Oxidative stress has been shown to impair neurotransmission, alter mitochondrial function, and modify gene expression, all of which contribute to cognitive and behavioural dysfunction, as demonstrated in younger subjects [39]. The liver, as the body's primary detoxification organ, is susceptible to oxidative damage and altered enzyme activity due to lead accumulation, with detrimental effects on metabolic homeostasis [40]. Lead affects the liver at the molecular level by generating reactive oxygen species (ROS), which in turn induce oxidative stress and damage cellular macromolecules such as lipids, proteins and DNA [41]. Lead has also been shown to disrupt mitochondrial function, impairing ATP production and inducing apoptosis by activating caspases [40]. Lead is also capable of interfering with the antioxidant defence system by reducing the activity of enzymes such as SOD and GPx, and it can alter the expression of key hepatic detoxifying enzymes such as cytochrome P450, thereby affecting the liver's ability to metabolize and eliminate toxins [42]. In addition, lead exposure has been shown to affect liver cell signalling, including activation of inflammatory pathways through the NF-κB pathway, contributing to liver inflammation and fibrosis [43]. As shown by Zhang et al. (2023), lead exposure has been shown to induce nephrotoxicity by disrupting ion transport and causing oxidative damage. This in turn leads to impaired renal filtration and excretory functions [44].
Relationship between energy metabolism and free radical production upon lead-induced exposure is related to changes in the functioning of mitochondria, which are the main energy centers of the cell [45]. The main stages of Krebs cycle take place in these organelles. Studies of tissue-specific activity of the main enzymes of this cycle, changed by the influence of some factors, such as environments, bird sex, and tissue types, to which these studies were devoted, are important link in determining the main pathways of cellular metabolism. This is important in view of Krebs cycle intermediates providing key reactions and substrates for biosynthesis (anabolism) of many important metabolites from amino-acids, purines, and pyrimidines to long-chain fatty acids and porphyrins [46, 47].
There are several important findings of current studies. Firstly, the basis of ecological model in our studies is based on preferential lead exposure in environments of pigeons, as the difference in soil contamination levels with other chemical elements was significantly pronounced in this case. The MANOVA tests, which allow clarification of the role of influence of environmental factors, demonstrated a dominant role of environment factor in the biochemical alterations in pigeon tissues. This may be related to subsequent important biochemical alterations after the accumulation and action of this toxic metal on physiological and biochemical processes in the organisms of wild pigeons in these breeding habitats and on survival and adaptive mechanisms (Fig. 7).
Fig. 7: Effects of environmental factors on biomarkers of oxidative stress and antioxidant defences, and biochemical changes in different tissues and sexes of pigeons.
Secondly, the presented changes in oxidative stress biomarkers are associated with a significant weakening of adaptive reactions in different tissues of birds to the conditions of polluted areas, with a pronounced component of the sex influence. This may have a significant effect on homeostasis maintenance, reproduction, and survival in general. This tendency in different pigeon tissues has a clear manifestation through intensification of oxidative reactions initiated in the lead-contaminated environments. The complex of antioxidant defense in each tissue under oxidative stress due to the intensification of free-radical production reflects the loading conditions of both functioning and adaptive reactions shown by us for the different sexes of pigeons first of all. We assessed these sex- and environment-related dependencies in relation to the functional specificities of metabolic processes and antioxidant defense for such tissues as the brain, kidney, muscles, and heart. We revealed significant increase in lipid peroxidation in the brain and kidney tissues differing between the two sexes of pigeons.
The concept of oxidative stress is now widely used to refer to a broad group of various interrelated phenomena, including increased production of ROS and oxidative damage to cellular molecular components [48]. Oxidative stress is currently used to describe the imbalance between pro-oxidants and antioxidants in favor of the former, leading to damage to biological molecules and cellular structures [49]. However, ROS are not the only factors that play a leading role in oxidative metabolism. Also, the reactive forms of nitrogen, carbon, chlorine, and sulfur play an important role in oxidative stress. These highly reactive molecules are involved in a fairly large number of reactions and in the regulation of various metabolic processes in the body. These reactions underlie many pathological processes and disorders [50].
It was noted that ROS mainly oxidize polyunsaturated fatty acid residues of a variety of lipids. Oxidation of proteins leads to the formation of glutathionylated or carbonylated derivatives, whereas non-enzymatic lipid oxidation produces, isoprostanes, malonic dialdehyde, and diene conjugates [51]. Oxidized protein and lipid derivatives can also damage other molecules, exacerbating the effects of developing oxidative stress [52]. E.g., 4-hydroxy-2-nonenal modifies proteins by interacting with amino group of lysine, cysteine, or histidine residues, resulting in the formation of adducts [53]. The adducts formed can cause damage to metabolically important proteins such as glucose and glutamate transporters, GTP-binding proteins, ion-dependent ATPases, etc., and can also initiate carbonylation of proteins. The effects of development of oxidative stress in animal tissues under negative effects of anthropopressure hinder the development of efficient energy supply mechanisms [54]. This is associated with an increase in the compensatory activation of aerobic glycolysis and a decrease in the inhibition of oxidative processes in Krebs cycle, resulting in an increase in ATP and creatine phosphate content and activation of the mitochondria energy-synthesizing functions [55].
Thirdly, in these current studies, the multivariate tests were used to determine the role of each of investigated parameters in the formation of full statistical model of biomarkers of oxidative stress and antioxidant defense, with the leading role of CAT and GPx. Regression multivariate analysis of the influence of three factors, i.e. environments, sex, and tissue types, showed cell membrane proteins and lipids as the most significant targets for ROS and their derivatives and the accompanying development of oxidative stress in tissues of birds exposed to anthropopressure conditions.
It was shown that toxic effects of lead in mammalian organs and tissues are characterized by a reduction in the number of viable cells, leading to disruption of physiological organ functions [56]. Activation of free radical lipid peroxidation and disruption of calcium homeostasis are considered the main mechanisms of cytotoxic effect of lead ions [9, 10, 15]. Correlation between the activities of Krebs cycle enzymes, lipid peroxidation processes, and the status of the antioxidant defense in various tissues shown in our studies (Table 8) may be related to the increased activity of antioxidant enzymes, i.e. a combination of antioxidant properties and inhibition of anti-hypoxic conditions during oxidative stress development. Correlations obtained in our studies between lead levels in feathers and the enzymes and metabolites of energy metabolism in the brain tissue in pigeon males and females confirm this finding. In case of brain tissue of males and females, the same values of relationships between lead levels and lactate concentration were as follows: Pb–lactate (R = 0.682, p = 0.034) for males and Pb–lactate (R = 0.784, p = 0.012) for females, respectively. It is important to note that the developing acidosis under lead exposure in males showed significant interactions with lactate and pyruvate levels Pb–L/P (R = 0.919, p = 0.000) and catalase activity Pb–CAT (R = 0.825, p = 0.006). These relationships may be important in determining interactions of the main parameters of energy metabolism and antioxidant defense against increasing role of toxic free radicals and tissue antioxidant defense system, as shown in the earlier studies [37, 57-61].
Correlations between the activities of enzymes shown in our studies (Table 8) are aimed at increasing antioxidative and anti-hypoxic processes in tissues and at enhancing the membrane-protective abilities of adaptation in lead exposure. This is confirmed by the correlations in the brain tissue of male pigeons: ICDH-TAS (R = 0.688, p = 0.040), TAS-CAT (R = 0.703, p = 0.035), and in the female group: SDH-CAT (R = 0.763, p = 0.017) and TAS-SDH (R = 0.763, p = 0.002). Physiological adaptative mechanisms modulating the activity of membrane-bound enzymes and receptor complexes are directed to the preservation of structural and functional organization of biomembranes, transport of neurotransmitters, and improvement of synaptic transmission in brain tissue [62]. E.g., an increase in the compensatory function of glycolysis, a decrease in the degree of inhibition of oxidative energy-synthesizing mitochondrial functions, and stabilization of cell membranes contribute to the effective preservation and maintenance of compensatory mechanisms of energy production under the lead exposure [37, 57].
Intensification of free-radical lipid peroxidation processes under lead exposure is caused not only by the generation of ROS but also by a decrease in the activity of the antioxidant enzymes, i.e. SOD and CAT [63]. Their inhibition is associated with high affinity of lead to the thiol groups and its ability to inhibit protein elements Cu2+, Zn2+, and Mg2+ ions in the catalytic sites of SOD. Cu2+, Zn2+, and Mg2+ ions are present in the catalytic sites of SOD and Fe2+ ions are present in CAT [64]. This study focused on pigeons, but the effects of lead exposure on other species, including humans, are highly relevant. Lead contamination, particularly in urban and industrial areas, poses a risk to several species, as similar molecular mechanisms of oxidative stress, toxicity and impaired metabolic processes are observed in different vertebrates. This research highlights the potential health risks to populations living in areas affected by lead contamination, as lead can bioaccumulate through the food chain, affecting both wildlife and human health [65]. The findings emphasize the need to assess lead exposure not only in birds, but also in other animals and human populations living in contaminated environments. The adverse effects of lead on the neurological, renal and cardiovascular systems are well documented [38, 66-68] and these studies are therefore crucial for the development of improved environmental policies and health risk assessments for populations in areas affected by lead contamination.
It is important to note the following important aspect of our studies related to determining antioxidant role of such glutathione-related enzymes as glutathione peroxidase (GPx) and glutathione reductase (GR). The statistical analysis in our studies highlighted the leading role of CAT and GPx in the functioning of antioxidant defense in five analyzed tissues (SS test of MANOVA). The main mechanism of the cytotoxic action of lead ions is based on Ca-binding protein, which is thought to promote absorption of not only of Ca2+ but also of Pb2+ from the gastrointestinal tract [22]. It should be noted that the activation of Ca-binding protein occurs with participation of vitamin D [69]. In excessive dietary intake, divalent Ca2+, Zn2+, and Fe2+ cations inhibit lead absorption by altering its ability to attach to the membrane. At the same time, in Fe2+ deficiency, the absorption of lead increases, which may be related to the function of Fe2+ transporter proteins [20]. This was also confirmed for correlations between lead and iron levels in feathers and the levels of total antioxidant status of tissues [70, 71]. This applies to the following relationships: Pb–CAT in the brain of females, Zn–CAT in the liver of males, Fe–CAT in the liver of females, TAS–CAT in the muscles of males, and TAS–GPx in the muscles of females as well as Fe–TBARS and Fe–SOD in the kidney of females. The role of iron in the induction of Fenton's reaction has been convincingly demonstrated when the ferrous and/or ferric cation decomposes catalytically hydrogen peroxide to generate powerful oxidizing agents [72].
Stable amounts of ROS in cells are maintained by both oxidative phosphorylation in mitochondria and antioxidant defense. The toxic effects of lead ions on renal tubule cells and epithelial cells are accompanied by changes in the shape, structure, and size of the mitochondria [73]. In addition, disruption of transmembrane ion transport leads to changes in calcium homeostasis. Moreover, lead ions inhibit the calcium flux into mitochondria and stimulate Ca2+ release from these organelles [74]. A decrease in the membrane potential and membrane swelling was observed at lead exposure [75, 76]. Changes in the membrane potential and mitochondrial swelling resulted in the opening of pores in the inner membrane of mitochondria [77]. It is suggested that lead ions bind directly to calcium sites in the pores of mitochondria. It is necessary to emphasize that the opening of pores is caused by an increase in the concentration of superoxide anion or its products, whereas its closure is caused by a decrease in its content. Prolonged opening of calcium pores leads to apoptosis and cell death due to mitochondrial damage [75].
Fourthly, the complete statistical analysis of all dependencies between the biomarkers of oxygen-dependent metabolic processes and Krebs cycle’s enzymes showed that pyruvate, ICDH, AST, and ALT play a key role in the analyzed model system [78]. It is known that, in certain species and in some tissues, specific mass of amino acids is important in catabolism carried out through citric acid cycle involving transamination. We studied these processes using the activities of ALT and AST. Our results regarding ICDH activity as one of leading enzymes of Krebs cycle involved in speed-limiting processes of the whole cycle [79] were confirmed by the statistical analysis, as a significant role of ICDH was shown by the multiple correlative coefficients as well as the coefficient of determination and its adjusted form. Significance of this enzyme in the overall statistical analysis was also confirmed by the multivariate regression analysis using standardized beta-coefficients for the brain, kidney, and liver tissues (Table 5).
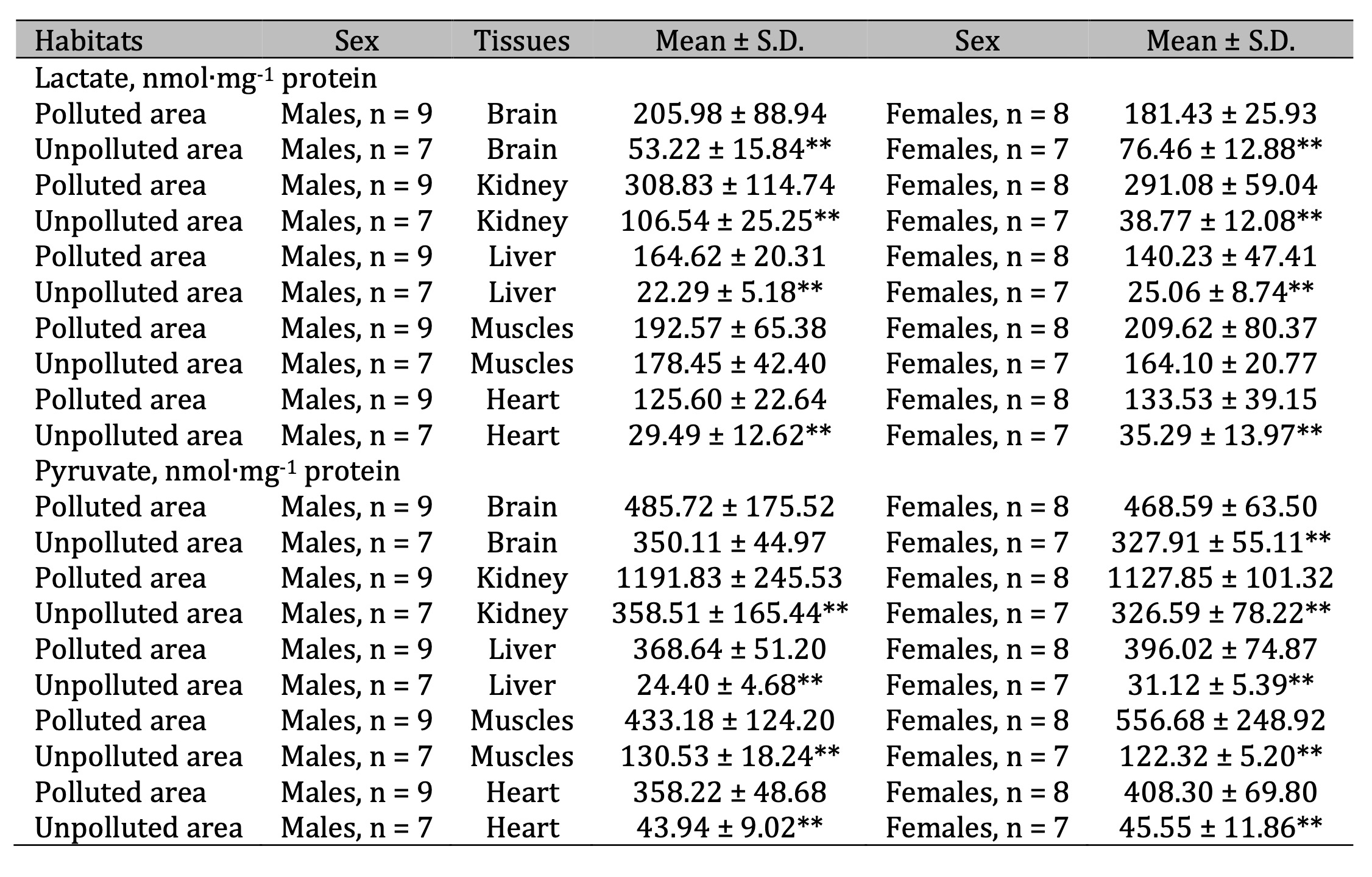
Table 5: Lactate (nmol∙mg-1 protein) and pyruvate levels (nmol∙mg-1 protein) in the brain, kidney, liver, muscle, and cardiac tissues of pigeon males and females nesting in polluted (Szpęgawa) and unpolluted areas (Słupsk) of the Pomeranian region, northern Poland. Legend: * P < 0.05 in relations between sexes; ** P < 0.05 in relations between habitats
Fifthly, activation of Krebs cycle enzymes, showing the leading role of investigated enzyme complexes in tissues of male and female pigeons nesting in areas with different levels of lead exposure, is associated with an increase in energy-dependent processes of macro-energy synthesis. These reactions, depending on the functional characteristics of each tissue, proceed at pronounced cellular acidosis caused by significant level of lactate as well as intensified oxidative stress, resulting in an imbalance of oxygen-dependent energy processes necessary for the repair of intracellular free-radical damage.
The protective role of thiol compounds, which include glutathione, is known in the context of direct antioxidant defence [62]. Our studies revealed high statistical correlations between Pb levels and oxidative stress data in selected five tissues. It is known that thiol compounds, which are able to accumulate in the brain and other tissues, have a pronounced antioxidant protective effect in hypoxia and ischemia conditions [80]. With their mechanism of action, they facilitate the conversion of lactic acid into pyruvic acid followed by its decarboxylation, i.e. they contribute to elimination of metabolic acidosis. These compounds contribute to the formation of coenzyme A involved in the mobilization of energy resources in conditions of ATP deficiency with developing hypoxia caused by acidosis [81]. In this study, for enzymes of metabolic rate and oxygen-dependent processes, similar particularities of metabolic changes were shown in the investigated five selected tissues of pigeons living in a polluted areas.
Our research has its limitations and strengths. The limitations are associated with the consent from the Bioethical Commission to collect a small number of birds from their habitats for research are always difficult to overcome. The strength of our research lies in the consideration of multivariate aspects of influence of such factors as environments, sex of birds, and specific tissue-related biochemical alterations. As shown in our study, we chose an appropriate way to analyze biomarkers of oxidative stress and antioxidant defense as well as markers of metabolic oxygen-dependent processes under lead exposure. It is possible that future studies will contribute to more detailed elucidation of other environmental factors that influence animal health (also considering lead content) such as noise, traffic, gas composition, and organic pollution near highways, etc. Our studies will serve as the basis for taking into considering such important factors in these analyses as bird sex and tissue specificity.
Conclusion
- In the present studies, multivariate tests were used to assess the role of each investigated parameter in constructing a comprehensive statistical model of biomarkers related to oxidative stress and antioxidant defence. The analysis revealed that CAT and GPx played the leading role in mitigating oxidative damage, highlighting their importance in the antioxidant defence system.
- Regression analysis was used to further investigate the effect of three critical factors - environment, gender and tissue type - on oxidative stress levels. The results indicated that cell membrane proteins and lipids were the primary targets for ROS and their derivatives, particularly in tissues from birds exposed to anthropogenic pressures. These findings highlight the complex interplay of environmental factors in driving oxidative stress and the susceptibility of specific tissues to damage in polluted environments.
- Multivariate regression analysis revealed that proteins and cell membrane lipids in different tissues of pigeons from polluted areas were the major targets of oxidative stress, with excessive oxidative damage affecting key metabolic enzymes and disrupting the redox balance in both polluted and unpolluted environments.
- Analysis of statistical dependencies between metabolic biomarkers highlighted pyruvate as the most influential factor, with ICDH playing a critical role in metabolic adaptation in various tissues, particularly brain, kidney and liver.
Acknowledgements
The study was supported by Pomeranian University in Słupsk, Słupsk, Poland.
Ethical Approval
The
research involving animals was carried out in line with the Animals
(Scientific Procedures) Act (1986)/EU Directive 2010/63/EU. The study
was conducted with the consent of the Bioethics Committee (Permission
License Number 44/2012).
Authors Contributions
NK
and HT conceived the ideas and designed the methodology; NK and HT
collected data; NK and HT analyzed and interpreted data; NK, HT, PK
and TH wrote the manuscript, drafted the article, and revised it
critically for important intellectual content.
Funding
The
study was supported by Pomeranian University in Słupsk, Słupsk,
Poland.
Disclosure Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosure of AI usage
The
authors declare that they did not use AI tools at any stage when
writing the work.
References
| 1 | Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int. 2016;23(9):8244-8259 https://doi.org/10.1007/s11356-016-6333-x.
https://doi.org/10.1007/s11356-016-6333-x |
| 2 | Martínez-García GG, Mariño G. Autophagy role in environmental pollutants exposure. Prog Mol Biol Transl Sci. 2020;172:257-291 https://doi.org/10.1016/bs.pmbts.2020.02.003.
https://doi.org/10.1016/bs.pmbts.2020.02.003 |
| 3 | Sies H. Oxidative stress: a concept in red-ox biology and medicine. Redox Biol. 2015;4:180-183 https://doi.org/10.1016/j.red-ox.2015.01.002.
https://doi.org/10.1016/j.redox.2015.01.002 |
| 4 | Pintus E, Ros-Santaella JL. Impact of Oxidative Stress on Male Reproduction in Domestic and Wild Animals. Antioxidants (Basel). 2021;10(7):1154 https://doi.org/10.3390/antiox10071154.
https://doi.org/10.3390/antiox10071154 |
| 5 | Canipari R, De Santis L, Cecconi S. Female Fertility and Environmental Pollution. Int J Environ Res Public Health. 2020;17(23):8802 https://doi.org/10.3390/ijerph17238802.
https://doi.org/10.3390/ijerph17238802 |
| 6 | Williams RJ, Holladay SD, Williams SM, Gogal RM Jr. Environmental Lead and Wild Birds: A Review. Rev Environ Contam Toxicol. 2018;245:157-180 https://doi.org/10.1007/398_2017_9.
https://doi.org/10.1007/398_2017_9 |
| 7 | Dasharathy S, Arjunan S, Maliyur Basavaraju A, Murugasen V, Ramachandran S, Keshav R, Murugan R. Mutagenic, Carcinogenic, and Teratogenic Effect of Heavy Metals. Evid. Based Complement. Alternat Med. 2022;2022:8011953 https://doi.org/10.1155/2022/8011953.
https://doi.org/10.1155/2022/8011953 |
| 8 | Frantz A, Federici P, Legoupi J, Jacquin L, Gasparini J. Sex-associated differences in trace metals concentrations in and on the plumage of a common urban bird species. Ecotoxicology. 2016;25(1):22-29 https://doi.org/10.1007/s10646-015-1562-1.
https://doi.org/10.1007/s10646-015-1562-1 |
| 9 | Tkachenko H, Kurhaluk N, Hetmański T, Włodarkiewicz A, Tomin V. Changes in energetic metabolism and lysosomal destruction in the skeletal muscle and cardiac tissues of pigeons (Columba livia f. urbana) from urban areas of the northern Pomeranian region (Poland). Ecotoxicology. 2021;30(6):1170-1185. https://doi.org/10.1007/s10646-021-02423-4.
https://doi.org/10.1007/s10646-021-02423-4 |
| 10 | Kurhaluk N, Tkachenko H, Hetmański T, Włodarkiewicz A, Tomin V. Profile of Heavy Metals and Antioxidant Defense in the Muscle Tissues of Pigeons (Columba livia f. urbana) from Anthropogenically Transformed Areas in the Pomeranian Region (Northern Poland). Arch Environ Contam Toxicol. 2021;80(3):601-614. https://doi.org/10.1007/s00244-021-00825-3.
https://doi.org/10.1007/s00244-021-00825-3 |
| 11 | Burger J, Gochfeld M. Effects of lead on birds (Laridae): a review of laboratory and field studies. J Toxicol Environ Health B Crit Rev. 2000;3(2):59-78 https://doi.org/10.1080/109374000281096.
https://doi.org/10.1080/109374000281096 |
| 12 | Hetmański T. Dispersion asymmetry within a Feral Pigeon Columba livia population. Acta Ornithol. 2007;42:23-31. https://doi. org/10.3161/000164507781646889.
https://doi.org/10.3161/068.042.0109 |
| 13 | Hetmański T. Różnice morfologiczne i behawioralne między płciami gołębia miejskiego Columba livia [Morphological and behavioral differences between the sexes of the Columba livia]. Scientific Publisher of the Pomeranian University in Słupsk, Słupsk, Poland, 2011 [in Polish].
|
| 14 | Hoff Brait CH, Antoniosi Filho NR. Use of feathers of feral pigeons (Columba livia) as a technique for metal quantification and environmental monitoring. Environ Monit Assess. 2011;179(1-4):457-467 https://doi.org/10.1007/s10661-010-1748-1.
https://doi.org/10.1007/s10661-010-1748-1 |
| 15 | Tkaczenko H, Hetmański T, Kamiński P, Kurhaluk N. Can blood morphology, oxidative stress, and cholinesterase activity determine health status of pigeon Columba livia f. urbana? Environ Sci Pollut Res Int. 2024;31(13):19927-19945 https://doi.org/10.1007/s11356-024-32296-z.
https://doi.org/10.1007/s11356-024-32296-z |
| 16 | Frantz A, Pottier MA, Karimi B, Corbel H, Aubry E, Haussy C, Gasparini J, Castrec-Rouelle M. Contrasting levels of heavy metals in the feathers of urban pigeons from close habitats suggest limited movements at a restricted scale. Environ Pollut. 2012;168:23-28 https://doi.org/10.1016/j.envpol.2012.04.003.
https://doi.org/10.1016/j.envpol.2012.04.003 |
| 17 | Nam DH, Lee DP, Koo TH. Monitoring for lead pollution using feathers of feral pigeons (Columba livia) from Korea. Environ Monit Assess. 2004;95(1-3):13-22 https://doi.org/10.1023/b:emas.0000029898.28393.30.
https://doi.org/10.1023/B:EMAS.0000029898.28393.30 |
| 18 | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44-84 https://doi.org/10.1016/j.biocel.2006.07.001.
https://doi.org/10.1016/j.biocel.2006.07.001 |
| 19 | Pounds JG. Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: a review. Neurotoxicology. 1984;5(3):295-331.
|
| 20 | Simons TJ. Lead-calcium interactions in cellular lead toxicity. Neurotoxicology 1993;14(2-3):77-85.
|
| 21 | Sun Q, Li Y, Shi L, Hussain R, Mehmood K, Tang Z, Zhang H. Heavy metals induced mitochondrial dysfunction in animals: Molecular mechanism of toxicity. Toxicology 2022;469:153136 https://doi.org/10.1016/j.tox.2022.153136.
https://doi.org/10.1016/j.tox.2022.153136 |
| 22 | Upadhyay K, Viramgami A, Bagepally BS, Balachandar R. Association between blood lead levels and markers of calcium homeostasis: a systematic review and meta-analysis. Sci Rep 2022;12:1850. https://doi.org/10.1038/s41598-022-05976-4.
https://doi.org/10.1038/s41598-022-05976-4 |
| 23 | EU Directive 2010/63/EU. (2010). Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. Official Journal of the European Union, L 276, 33-79.
|
| 24 | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-254. https://doi.org/10.1016/0003-2697.90527-90533.
https://doi.org/10.1006/abio.1976.9999 |
| 25 | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310 https://doi.org/10.1016/s0076-6879(78)52032-6.
https://doi.org/10.1016/S0076-6879(78)52032-6 |
| 26 | Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464-478. https://doi.org/10.1016/0076-6879(90)86141-h.
https://doi.org/10.1016/0076-6879(90)86141-H |
| 27 | Koroliuk MA, Ivanova LI, Maĭorova IG, Tokarev VE. Metod opredeleniia aktivnosti katalazy [A method of determining catalase activity]. Lab Delo 1988;:16-19. (Russian).
|
| 28 | Eschenko ND, Volski GG. (eds.) Methods of biochemical studies. Leningrad, Sankt-Petersburg National University, 1982 358 p.
|
| 29 | Sevela M, Tovarek J. Metoda stanovení laktikodehydrogenázy v télních tekutinách [Method for the estimation of lactic dehydrogenase]. Cas Lek Cesk 1959;98:844-848. (Czech).
|
| 30 | Herasimov I, Plaksina O. Non-enzymatic assessment of lactate and pyruvate concentrations in blood samples. Laboratorna Diagnostyka, 2000;2:46-48 Ukrainian.
|
| 31 | Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56-63 https://doi.org/10.1093/ajcp/28.1.56.
https://doi.org/10.1093/ajcp/28.1.56 |
| 32 | Zar JH. Biostatistical Analysis, 4th ed., Prentice Hall Inc., New Jersey, 1999.
|
| 33 | Stanisz A. An accessible course of statistics with the use of STATISTICA PL on examples from medicine (Przystępny kurs statystyki z zastosowaniem STATISTICA PL na przykładach z medycyny). Vol. 1-3, 3rd ed., Cracow, Poland, 2006, 2007.
|
| 34 | Hosseinian SA, Ansari S. Prophylactic effects of dietary ascorbic acid on oxidative stress indices, physiological and behavioural responses of domestic pigeons exposed to road transport stress. Vet Med Sci. 2021;7(6):2389-2398 https://doi.org/10.1002/vms3.609.
https://doi.org/10.1002/vms3.609 |
| 35 | Bannon DI, Parsons PJ, Centeno JA, Lal S, Xu H, Rosencrance AB, Dennis WE, Johnson MS. Lead and copper in pigeons (Columbia livia) exposed to a small arms-range soil. Arch Environ Contam Toxicol. 2011;60(2):351-360 https://doi.org/10.1007/s00244-010-9540-3.
https://doi.org/10.1007/s00244-010-9540-3 |
| 36 | Cai F, Calisi RM. Seasons and neighborhoods of high lead toxicity in New York City: The feral pigeon as a bioindicator. Chemosphere 2016;161:274-279 https://doi.org/10.1016/j.chemosphere.2016.07.002.
https://doi.org/10.1016/j.chemosphere.2016.07.002 |
| 37 | Levin R, Zilli Vieira CL, Rosenbaum MH, Bischoff K, Mordarski DC, Brown MJ. The urban lead (Pb) burden in humans, animals and the natural environment. Environ Res. 2021;193:110377 https://doi.org/10.1016/j.envres.2020.110377.
https://doi.org/10.1016/j.envres.2020.110377 |
| 38 | Sanders T, Liu Y, Buchner V, Tchounwou PB. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 2009;24(1):15-45 https://doi.org/10.1515/reveh.2009.24.1.15.
https://doi.org/10.1515/REVEH.2009.24.1.15 |
| 39 | Parithathvi A, Choudhari N, Dsouza HS. Prenatal and early life lead exposure induced neurotoxicity. Hum Exp Toxicol. 2024;43:9603271241285523 https://doi.org/10.1177/09603271241285523.
https://doi.org/10.1177/09603271241285523 |
| 40 | Schilderman PA, Hoogewerff JA, van Schooten FJ, Maas LM, Moonen EJ, van Os BJ, van Wijnen JH, Kleinjans JC. Possible relevance of pigeons as an indicator species for monitoring air pollution. Environ Health Perspect. 1997;105(3):322-330 https://doi.org/10.1289/ehp.97105322.
https://doi.org/10.1289/ehp.97105322 |
| 41 | Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front Pharmacol. 2021;12:643972 https://doi.org/10.3389/fphar.2021.643972.
https://doi.org/10.3389/fphar.2021.643972 |
| 42 | Akbar M, Essa MM, Daradkeh G, Abdelmegeed MA, Choi Y, Mahmood L, Song BJ. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. 2016;1637:34-55 https://doi.org/10.1016/j.brainres.2016.02.016.
https://doi.org/10.1016/j.brainres.2016.02.016 |
| 43 | Lu LL, Zhang YW, Li ZC, Fang YY, Wang LL, Zhao YS, Li SJ, Ou SY, Aschner M, Jiang YM. Therapeutic Effects of Sodium Para-Aminosalicylic Acid on Cognitive Deficits and Activated ERK1/2-p90RSK/NF-κB Inflammatory Pathway in Pb-Exposed Rats. Biol Trace Elem Res. 2022;200(6):2807-2815 https://doi.org/10.1007/s12011-021-02874-0.
https://doi.org/10.1007/s12011-021-02874-0 |
| 44 | Zhang G, Zhang Y, Jing L, Zhao H. Lead exposure induced developmental nephrotoxicity in Japanese quail (Coturnix japonica) via oxidative stress-based PI3K/AKT pathway inhibition and NF-κB pathway activation. Comp Biochem Physiol C Toxicol Pharmacol. 2023;268:109599 https://doi.org/10.1016/j.cbpc.2023.109599.
https://doi.org/10.1016/j.cbpc.2023.109599 |
| 45 | Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11(1):102 https://doi.org/10.1038/s41467-019-13668-3.
https://doi.org/10.1038/s41467-019-13668-3 |
| 46 | Ryan DG, Murphy MP, Frezza C, Prag HA, Chouchani ET, O'Neill LA, Mills EL. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat Metab. 2019;1:16-33 https://doi.org/10.1038/s42255-018-0014-7.
https://doi.org/10.1038/s42255-018-0014-7 |
| 47 | Jiménez-Uribe AP, Hernández-Cruz EY, Ramírez-Magaña KJ, Pedraza-Chaverri J. Involvement of Tricarboxylic Acid Cycle Metabolites in Kidney Diseases. Biomolecules 2021;11(9):1259 https://doi.org/10.3390/biom11091259.
https://doi.org/10.3390/biom11091259 |
| 48 | Aranda-Rivera AK, Cruz-Gregorio A, Aparicio-Trejo OE, Pedraza-Chaverri J. Mitochondrial Red-ox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules 2021;11:1144. https://doi.org/10.3390/biom11081144.
https://doi.org/10.3390/biom11081144 |
| 49 | Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008;295:C849-868. https://doi.org/10.1152/ajpcell.00283.2008.
https://doi.org/10.1152/ajpcell.00283.2008 |
| 50 | Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 2015;97:55-74. https://doi.org/10.1016/j.ejmech.2015.04.040.
https://doi.org/10.1016/j.ejmech.2015.04.040 |
| 51 | Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011;283:65-87. https://doi.org/10.1016/j.tox.2011.03.001.
https://doi.org/10.1016/j.tox.2011.03.001 |
| 52 | Hotz P, Hoet P, Lauwerys R. Peroxydation lipidique en pathologie humaine: évaluation des données de la littérature [Lipid peroxidation in human pathology: evaluation of data in literature]. Pathol Biol (Paris) 1987;35:1067-1073.
|
| 53 | Kato Y. The formation of lipid hydroperoxide-derived amide-type lysine adducts on proteins: a review of current knowledge. Subcell Biochem 2014;77:21-39. https://doi.org/10.1007/978-94-007-7920-4_2.
https://doi.org/10.1007/978-94-007-7920-4_2 |
| 54 | Sugiyama A, Sun J. Immunochemical detection of lipid hydroperoxide- and aldehyde-modified proteins in diseases. Subcell Biochem 2014;77:115-125. https://doi.org/10.1007/978-94-007-7920-4_10.
https://doi.org/10.1007/978-94-007-7920-4_10 |
| 55 | Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol 2020;37:101674. https://doi.org/10.1016/j.red-ox.2020.101674.
https://doi.org/10.1016/j.redox.2020.101674 |
| 56 | Huang H, Wang Y, An Y, Jiao W, Xu Y, Han Q, Teng X, Teng X. Selenium alleviates oxidative stress and autophagy in lead-treated chicken testes. Theriogenology 2019;131:146-152. https://doi.org/10.1016/j.theriogenology.2019.03.015.
https://doi.org/10.1016/j.theriogenology.2019.03.015 |
| 57 | Koivula MJ, Eeva T. Metal-related oxidative stress in birds. Environ Pollut 2010;158:2359-2370. https://doi.org/10.1016/j.envpol.2010.03.013.
https://doi.org/10.1016/j.envpol.2010.03.013 |
| 58 | Lismont C, Revenco I, Fransen M. Peroxisomal Hydrogen Peroxide Metabolism and Signaling in Health and Disease. Int J Mol Sci 2019;20:3673. https://doi.org/10.3390/ijms20153673.
https://doi.org/10.3390/ijms20153673 |
| 59 | Romero D, de José A, Theureau JM, Ferrer A, Raigón MD, Torregrosa JB. Lead in terrestrial game birds from Spain. Environ. Sci Pollut Res Int 2020;27:1585-1597. https://doi.org/10.1007/s11356-019-06827-y.
https://doi.org/10.1007/s11356-019-06827-y |
| 60 | Sharapov MG, Gudkov SV, Lankin VZ. Hydroperoxide-Reducing Enzymes in the Regulation of Free-Radical Processes. Biochemistry (Mosc) 2021;86:1256-1274. https://doi.org/10.1134/S0006297921100084.
https://doi.org/10.1134/S0006297921100084 |
| 61 | Kozák G, Janiga M, Solár J. Pollution of Feral Pigeon (Columba livia) Depends on Their Age and Their Health Status. Biol Trace Elem Res 2022;200:790-799. https://doi.org/10.1007/s12011-021-02689-z.
https://doi.org/10.1007/s12011-021-02689-z |
| 62 | Aoyama K. Glutathione in the Brain. Int J Mol Sci 2021;22:5010. https://doi.org/10.3390/ijms22095010.
https://doi.org/10.3390/ijms22095010 |
| 63 | Fan Y, Zhao X, Yu J, Xie J, Li C, Liu D, Tang C, Wang C. Lead-induced oxidative damage in rats/mice: A meta-analysis. J Trace Elem Med Biol 2020;58:126443. https://doi.org/10.1016/j.jtemb.2019.126443.
https://doi.org/10.1016/j.jtemb.2019.126443 |
| 64 | Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Rhodes CJ, Valko M. Essential metals in health and disease. Chem Biol Interact 2022;367:110173. https://doi.org/10.1016/j.cbi.2022.110173.
https://doi.org/10.1016/j.cbi.2022.110173 |
| 65 | Kumar A, Kumar A, Cabral-Pinto MMS, Chaturvedi AK, Shabnam AA, Subrahmanyam G, Mondal R, Gupta DK, Malyan SK, S Kumar S, A Khan S, Yadav KK. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int J Environ Res Public Health 2020;17:2179. https://doi.org/10.3390/ijerph17072179.
https://doi.org/10.3390/ijerph17072179 |
| 66 | Bernard BP, Becker CE. Environmental lead exposure and the kidney. J Toxicol Clin Toxicol 1988;26:1-34. https://doi.org/10.3109/15563658808995395.
https://doi.org/10.3109/15563658808995395 |
| 67 | Poręba R, Gać P, Poręba M, Andrzejak R. Environmental and occupational exposure to lead as a potential risk factor for cardiovascular disease. Environ Toxicol Pharmacol 2011;31:267-277. https://doi.org/10.1016/j.etap.2010.12.002.
https://doi.org/10.1016/j.etap.2010.12.002 |
| 68 | Boskabady M, Marefati N, Farkhondeh T, Shakeri F, Farshbaf A, Boskabady MH. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ Int 2018;120:404-420. https://doi.org/10.1016/j.envint.2018.08.013.
https://doi.org/10.1016/j.envint.2018.08.013 |
| 69 | Fullmer CS. Intestinal interactions of lead and calcium. Neurotoxicology 1992;13:799-807.
|
| 70 | Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim Biophys Acta Mol Cell Res 2019;1866:118535. https://doi.org/10.1016/j.bbamcr.2019.118535.
https://doi.org/10.1016/j.bbamcr.2019.118535 |
| 71 | Nakamura T, Naguro I, Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta Gen Subj 2019;1863:1398-1409. https://doi.org/10.1016/j.bbagen.2019.06.010.
https://doi.org/10.1016/j.bbagen.2019.06.010 |
| 72 | Carlos TD, Bezerra LB, Vieira MM, Sarmento RA, Pereira DH, Cavallini GS. Fenton-type process using peracetic acid: Efficiency, reaction elucidations and ecotoxicity. J Hazard Mater 2021;403:123949. https://doi.org/10.1016/j.jhazmat.2020.
https://doi.org/10.1016/j.jhazmat.2020.123949 |
| 73 | Han Q, Zhang W, Guo J, Zhu Q, Chen H, Xia Y, Zhu G. Mitochondrion: a sensitive target for Pb exposure. J Toxicol Sci 2021;46:345-358. https://doi.org/10.2131/jts.46.345.
https://doi.org/10.2131/jts.46.345 |
| 74 | Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L. Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 2016;90:1193-1209. https://doi.org/10.1007/s00204-015-1547-0.
https://doi.org/10.1007/s00204-015-1547-0 |
| 75 | Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007;12:835-840. https://doi.org/10.1007/s10495-006-0525-7.
https://doi.org/10.1007/s10495-006-0525-7 |
| 76 | Bertero E, Maack C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ Res 2018;122:1460-1478. https://doi.org/10.1161/CIRCRESAHA.118.310082.
https://doi.org/10.1161/CIRCRESAHA.118.310082 |
| 77 | Wang ZK, Zhou XL, Song XB, Zhuang DM, Wang ZY, Yang DB, Wang L. Alleviation of Lead-Induced Apoptosis by Puerarin via Inhibiting Mitochondrial Permeability Transition Pore Opening in Primary Cultures of Rat Proximal Tubular Cells. Biol Trace Elem Res 2016;174:166-176. https://doi.org/10.1007/s12011-016-0701-8.
https://doi.org/10.1007/s12011-016-0701-8 |
| 78 | Kang W, Suzuki M, Saito T, Miyado K. Emerging Role of TCA Cycle-Related Enzymes in Human Diseases. Int J Mol Sci 2021;22:13057. https://doi.org/10.3390/ijms222313057.
https://doi.org/10.3390/ijms222313057 |
| 79 | Sazanov LA, Jackson JB. Proton-translocating transhydrogenase and NAD- and NADP-linked isocitrate dehydrogenases operate in a substrate cycle which contributes to fine regulation of the tricarboxylic acid cycle activity in mitochondria. FEBS Lett 1994;344:109-116. https://doi.org/10.1016/0014-5793(94)00370-x.
https://doi.org/10.1016/0014-5793(94)00370-X |
| 80 | Liu S, Xin D, Wang L, Zhang T, Bai X, Li T, Xie Y, Xue H, Bo S, Liu D, Wang Z. Therapeutic effects of L-Cysteine in newborn mice subjected to hypoxia-ischemia brain injury via the CBS/H2S system: Role of oxidative stress and endoplasmic reticulum stress. Redox Biol. 2017;13:528-540 https://doi.org/10.1016/j.red-ox.2017.06.007.
https://doi.org/10.1016/j.redox.2017.06.007 |
| 81 | Gout I. Coenzyme A, protein CoAlation and red-ox regulation in mammalian cells. Biochem Soc Trans 2018;46:721-728. https://doi.org/10.1042/BST20170506.
https://doi.org/10.1042/BST20170506 |