Management of Sudden Onset Sensorineural Hearing Loss and the Role of Pentoxyphylline as an Add-on Therapy to Conventional Treatment
bAssistant Professor, Department of Otorhinolaryngology, Head and Neck Surgery, Sri Muthukumaran Medical College Hospital and Research Institute, Chennai, Tamil Nadu, India,
cAssistant Professor, Department of Otorhinolaryngology, Head and Neck SurgeryGovernment Omandurar Medical College, Chennai, Tamil Nadu, India,
dPost Graduate, Department of Otorhinolaryngology, Head and Neck Surgery, Sri Muthukumaran Medical College Hospital and Research Institute, Chennai, Tamil Nadu, India,
eJunior Resident, Department ofForensic MedicineSri Balaji Medical College, Chennai, Tamil Nadu, India
Keywords
Abstract
Background/Aims:
Sudden Sensorineural Hearing Loss (SSNHL) is a rapid-onset condition with varied etiologies, creating uncertainty in optimal treatment strategies. This study aimed to assess whether adding pentoxifylline to standard antiviral and steroid therapy could enhance hearing recovery in SSNHL patients.Methods:
Conducted as a randomized controlled trial from January 2021 to June 2023 in a private clinic within Chennai district, Tamil Nadu, the study enrolled 72 patients aged 20–70 years with SSNHL onset within 7 days. Participants were randomly assigned to receive either standard therapy (antiviral + steroids) or an intervention including pentoxifylline. Baseline assessments and serial pure-tone audiometry (PTA) was done to monitor outcomes.Results:
Among the 72 participants, the majority were male (58.3%) with a mean age of 43.4 years, with right-sided hearing loss predominantly. Comorbidities such as diabetes and hypertension were commonly seen among the study population. The intervention group exhibited significantly greater hearing recovery, particularly among those treated within 72 hours of onset. Non-responders underwent MRI brain, identifying CP angle pathology in some cases. Statistical analysis confirmed significantly improved outcomes for the intervention group (p < 0.05).Conclusion:
The findings suggest that pentoxifylline, when added to antiviral and steroid therapy, may enhance hearing recovery in SSNHL, especially with prompt treatment. Intratympanic dexamethasone and MRI of the brain are recommended for non-responders to identify underlying pathologies.Introduction
SSNHL is a medical emergency characterized by a rapid onset of hearing impairment, typically developing over seconds to days [1]. It is typically defined as a sensorineural hearing loss of at least 30 dB across three contiguous audiometric frequencies within a 72-hour period [2–8]. Globally, the incidence of SSNHL varies, with estimates ranging from 5 to 20 cases per 100, 000 persons per year [9]. The condition predominantly affects individuals in the fifth and sixth decades of life, as demonstrated by studies such as that by Byl et al., which reported a peak incidence in this age group [10]. Patients with SSNHL may present with additional symptoms including tinnitus, ear blockage, and vertigo which further complicate diagnosis and management [11, 12].
The etiopathogenesis of SSNHL remains unclear due to its multifactorial nature [9]. Specific causes, such as viral infections, vascular impairments, autoimmune disorders, and central nervous system pathologies, have been implicated, but in many cases, the precise mechanisms remain unidentified [13]. Comorbid conditions such as hypertension and cardiovascular disease, are often observed, complicating the clinical picture and contributing to increased morbidity [14, 15]. With over 100 potential etiological factors proposed, there is considerable debate over the most appropriate management strategies, often leaving clinicians in a difficult position regarding treatment decisions.
Management of SSNHL is challenging and lacks consensus due to its diverse etiological factors and unpredictable clinical course [16]. High-dose corticosteroids, administered systemically or intratympanically, are the cornerstone of treatment, aimed at reducing inflammation and preserving cochlear function [17]. However, no universally accepted treatment protocol exists, and responses vary widely among patients. The timing of treatment initiation plays a critical role, as earlier intervention is associated with better hearing recovery [18]. Despite the availability of numerous therapies, including antiviral agents, vasodilators, and hyperbaric oxygen, no single approach has demonstrated consistent efficacy, highlighting the need for a more structured treatment algorithm.
This study aimed to evaluate the efficacy of a structured treatment protocol for SSNHL, emphasizing the addition of pentoxifylline as an adjunct to conventional therapy and focusing on patient outcomes. The study also assessed the importance of treatment timing relative to symptom onset. By incorporating our experience, this study builds upon existing literature and provides practical insights to optimize the management of SSNHL, with a particular focus on the role of conservative management and targeted interventions for non-responders.
Materials and Methods
Study Design and Setting
This study was a randomized controlled trial with participants recruited from a private clinic in Chennai District, Tamil Nadu, between January 2021 and June 2023. The trial was approved by the Human Ethics Committee (IHEC) of the nearest tertiary care hospital. Each participant received a Participant Information Sheet (PIS) in their native language, with verbal explanations provided to ensure understanding. Written informed consent was obtained from all participants before enrollment.
Randomization and Sample Size
100 participants were screened, and 28 were excluded for reasons such as not meeting the eligibility criteria (Fig. 1). A total of 72 participants were enrolled and randomly allocated to one of two groups in a 1:1 ratio to ensure balance. The control group received standard treatment [Antiviral (Acyclovir) + Steroid (Prednisolone)], while the intervention group received standard treatment plus Pentoxifylline [Antiviral (Acyclovir) + Steroid (Prednisolone) + Pentoxifylline]. Randomization was conducted to minimize selection bias, ensuring that each group contained 36 participants. Sample size determination was based on power analysis to detect statistically significant differences between groups in hearing outcomes between the two groups.
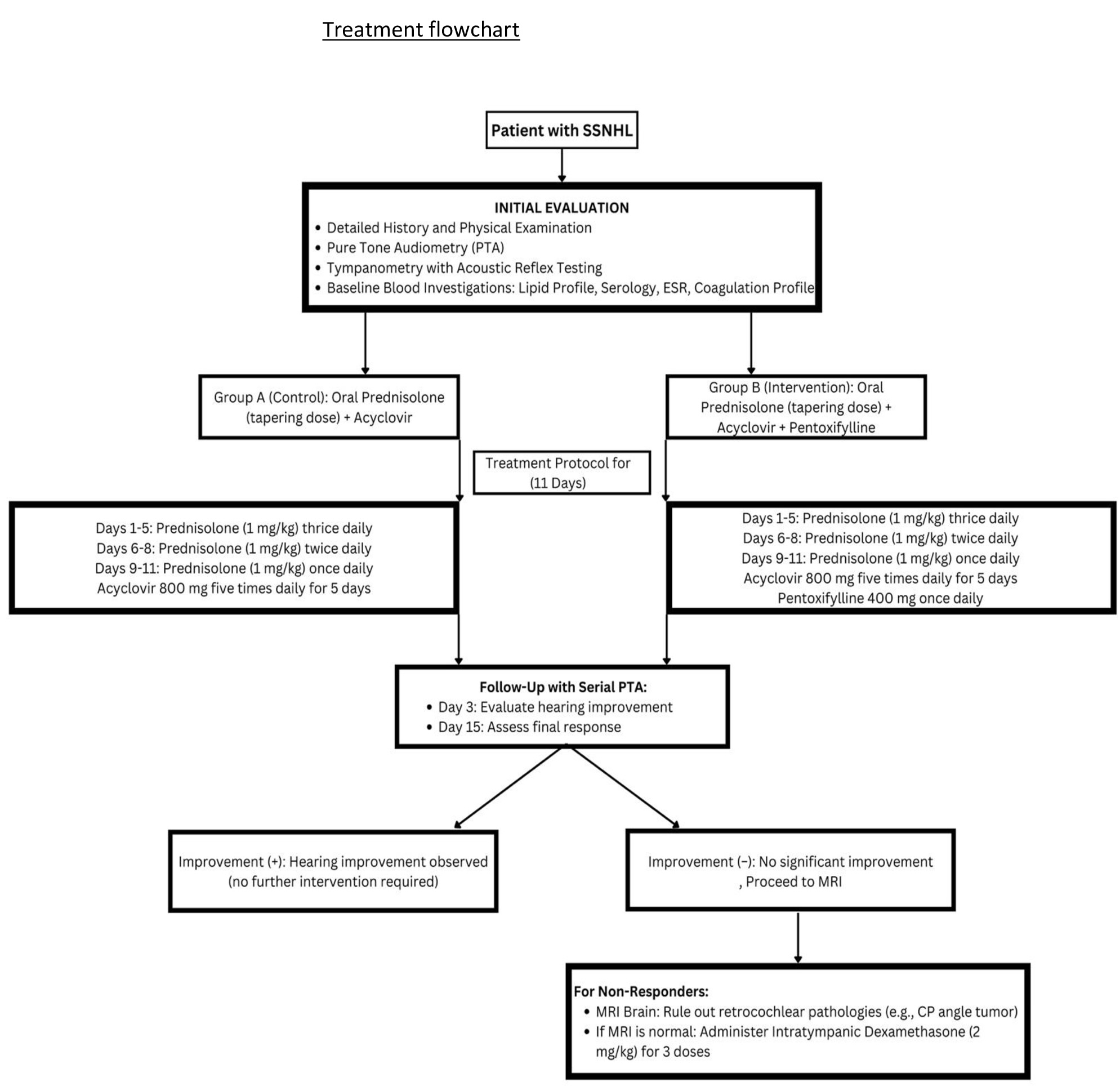
Fig. 1: Treatment flow chart.
Participant Selection
Patients presenting with sudden unilateral sensorineural hearing loss within seven days of onset were initially screened for eligibility. The inclusion criteria ensured participants were within this time frame to maximize treatment effectiveness. Exclusion criteria included patients presenting after seven days, those without three consecutive PTA records, patients unable to complete follow-up, and individuals intolerant to antiviral medications, steroids, or xanthine derivatives. After applying these criteria, 72 participants were retained for analysis, with 36 in each treatment group (Fig. 1).
Interventions
All participants underwent baseline assessments, including a detailed medical history, physical examination, and initial investigations with pure-tone audiometry, tympanometry with acoustic reflex testing, and blood tests (lipid profile, serology, erythrocyte sedimentation rate (ESR), and coagulation profile). Both groups received oral Prednisolone (1 mg/kg body weight) in a tapering dose over 11 days (three times daily for the first 5 days, twice daily for the next 3 days, and once daily for the final 3 days) and Acyclovir 800 mg five times daily for 5 days. The intervention group additionally received Pentoxifylline 400 mg once daily (Fig. 2).
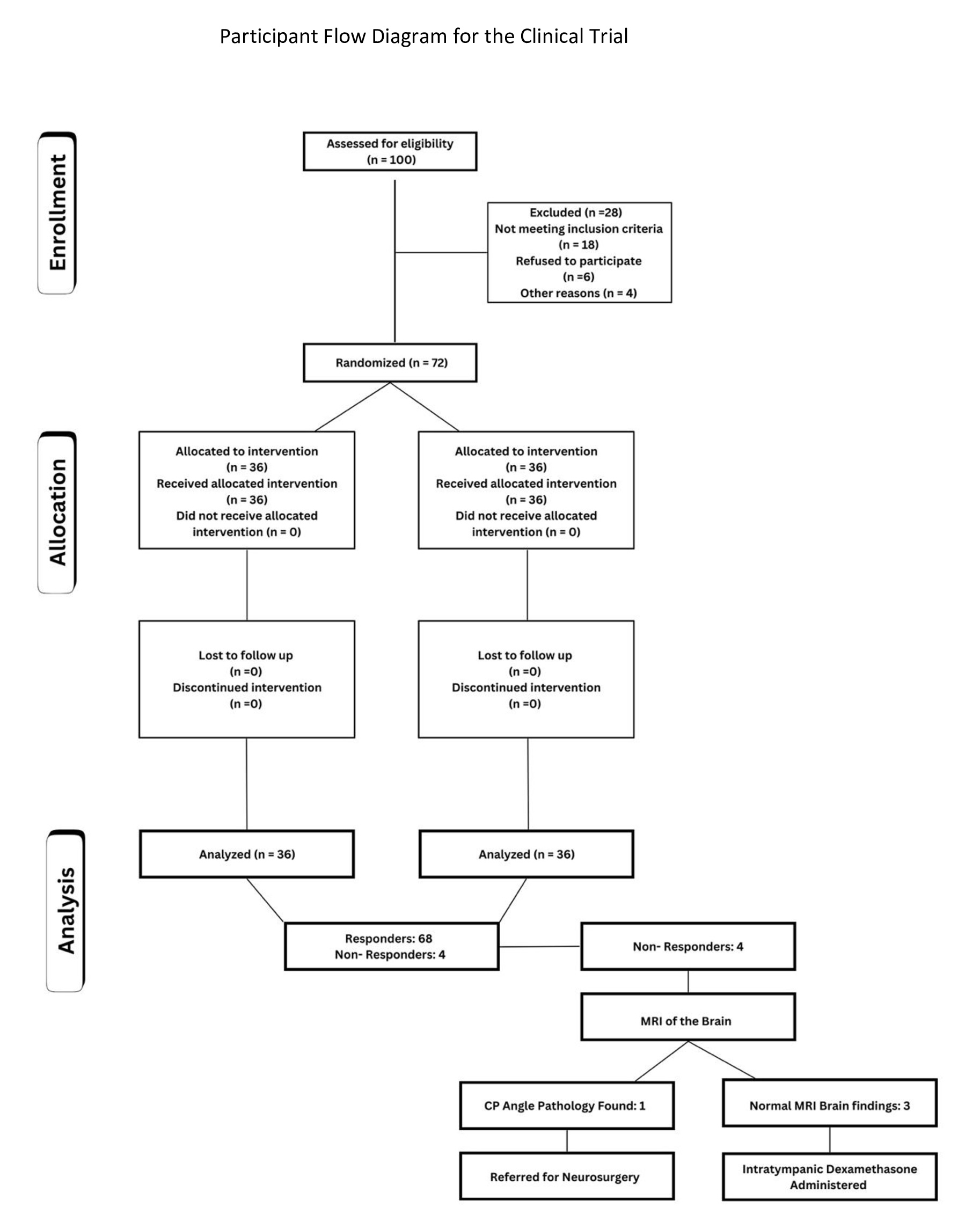
Fig. 2: Participant flow diagram for the clinical trial.
Outcome Measures and Follow-up
The primary outcome measure was an improvement in hearing as assessed by PTA. Serial audiograms were conducted daily for three consecutive days to monitor the response. Non-responders who showed no significant improvement within 7–10 days of conservative treatment underwent further evaluation (Fig. 2, Fig. 1). For these non-responders, intratympanic Dexamethasone (2 mg/kg) was administered as a secondary intervention in three doses, injected into the posteroinferior quadrant of the tympanic membrane using a 22G spinal needle, all within 15 days of symptom onset. Additionally, MRI of the brain was performed on non-responders to exclude retrocochlear pathologies, such as cerebellopontine angle tumors.
Data Collection and Statistical Analysis
Data were systematically recorded and organized in Microsoft Excel. Statistical analysis was performed using SPSS software (version 23). Categorical variables were summarized as frequencies and percentages, while continuous variables were reported as means ± standard deviations or medians with interquartile ranges, based on data normality (assessed by Kolmogorov–Smirnov and Shapiro–Wilk tests). To compare the efficacy of the interventions between groups, appropriate statistical tests were applied, with significance set at p < 0.05.
Results
A total of 72 participants were enrolled in the study, randomly assigned to either the control group (Antiviral + Steroid) or the intervention group (Antiviral + Steroid + Pentoxifylline), with 36 participants in each group. Among the entire study population, there were 40 males (55.6%) and 32 females (44.4%), with a mean age of 43.4 ± 5 years and a median age of 41 years. Age distribution revealed that 10 participants (13.9%) were under 30 years, 28 (38.9%) were between 31 and 40 years, 12 (16.7%) were between 41 and 50 years, 14 (19.4%) were between 51 and 60 years, and 8 (11.1%) were over 60 years (Table 1 and Fig. 3).
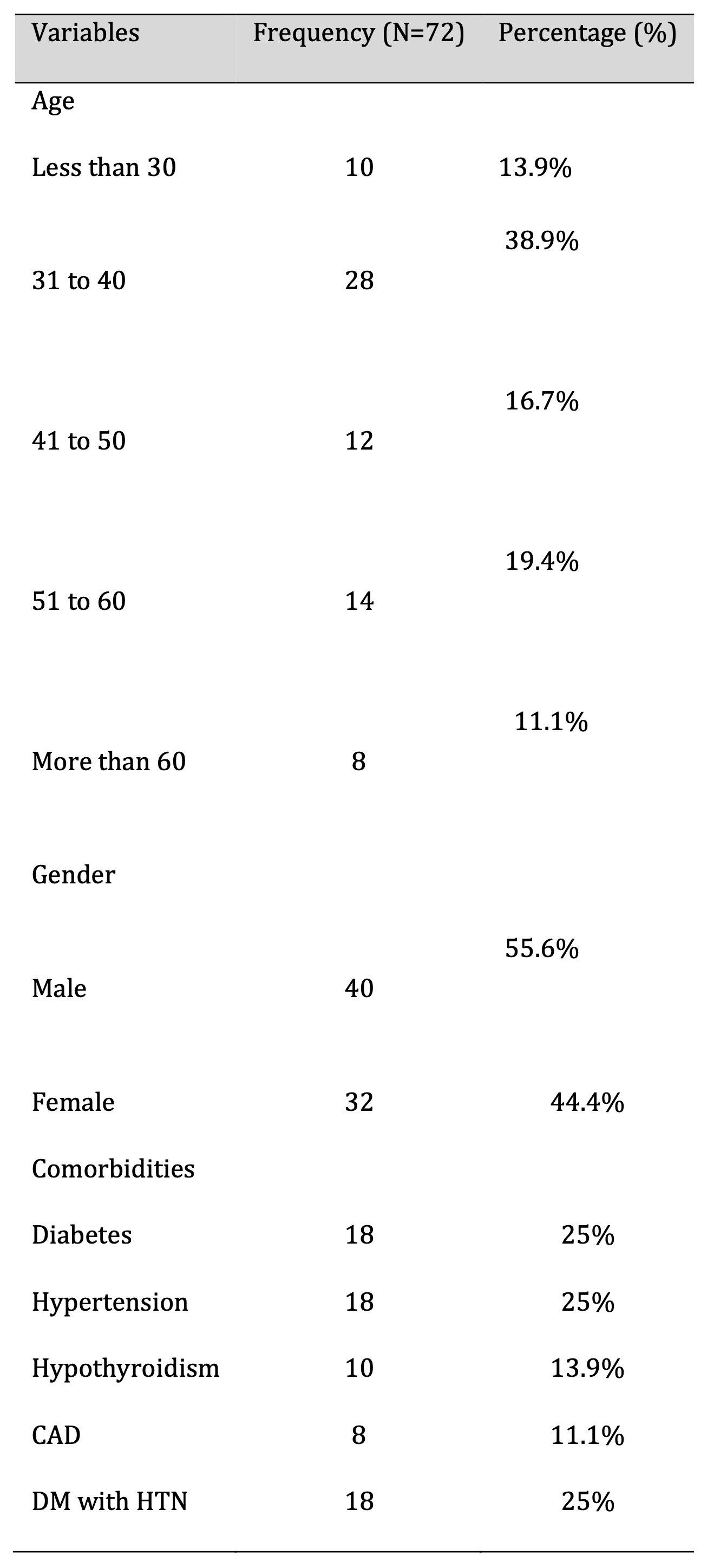
Table 1:
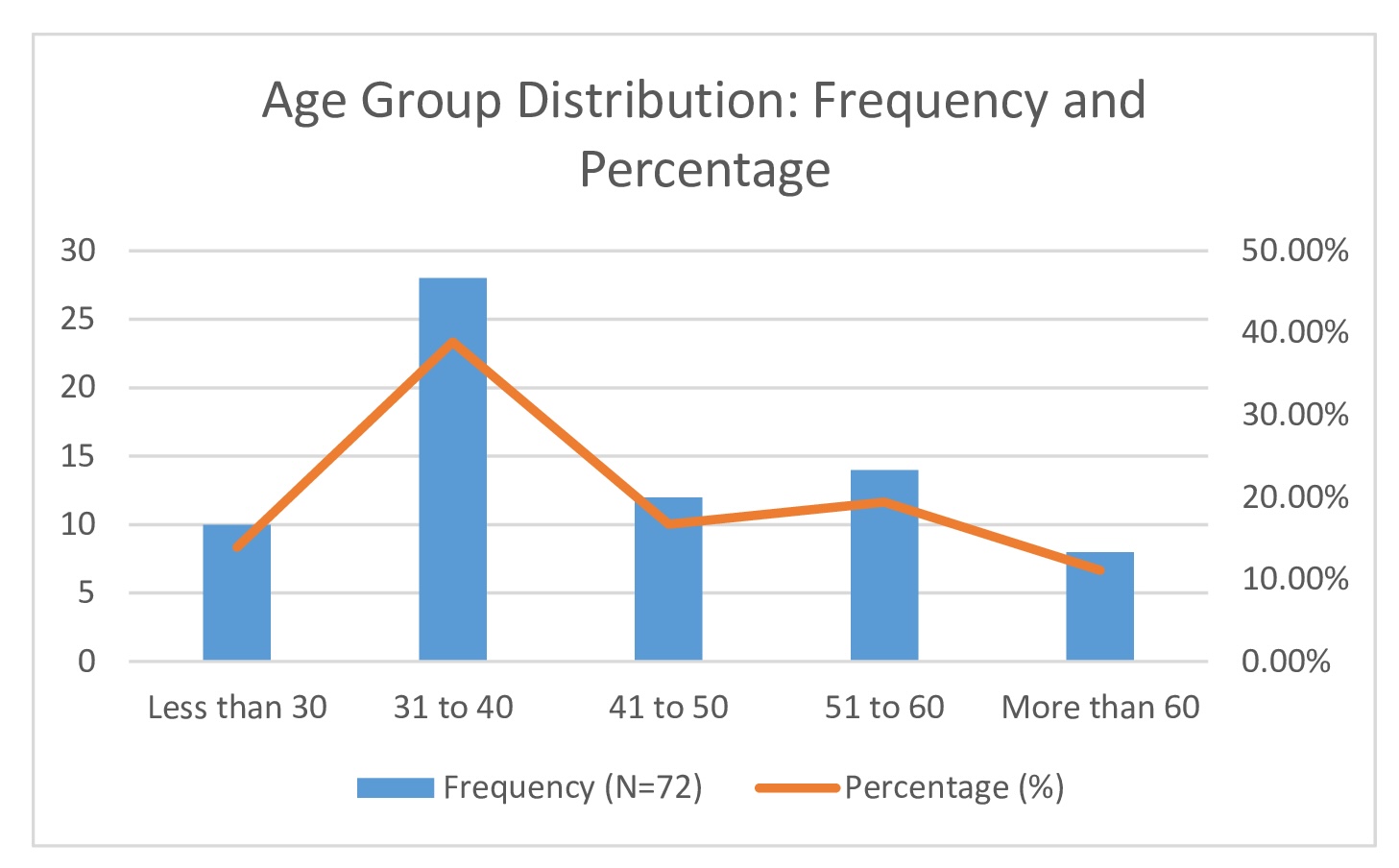
Fig. 3: Age Group Distribution: Frequency and Percentage.
Common comorbidities observed among participants included diabetes mellitus (25%), hypertension (25%), hypothyroidism (13.9%), and coronary artery disease (CAD) (11.1%) (Fig. 4). All participants presented with sudden hearing loss (100%). Additional symptoms included tinnitus in 18 patients (25%), ear blockage in 10 patients (13.9%), and dizziness in 5 patients (6.9%) (Table 2, Fig. 5). Right-sided hearing loss was present in 40 patients (55.6%), and 32 patients (44.4%) had left-sided hearing loss. The etiology was idiopathic in 42 cases (58.3%), with other causes including Meniere’s disease (13.9%), viral infection (11.1%), cerebellopontine (CP) angle pathology (8.3%), and trauma (8.3%) (Table 2)(Fig. 6).
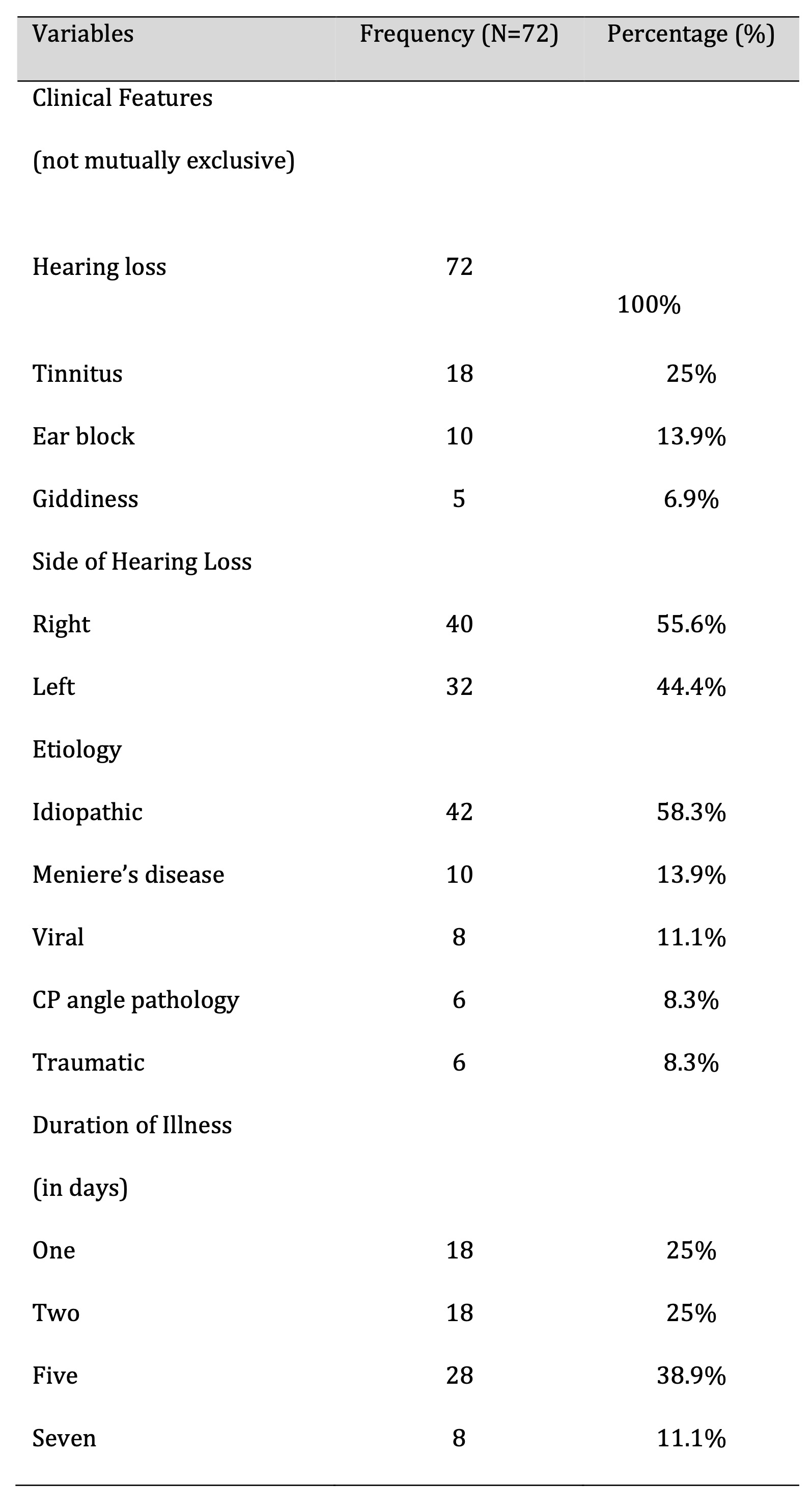
Table 2:
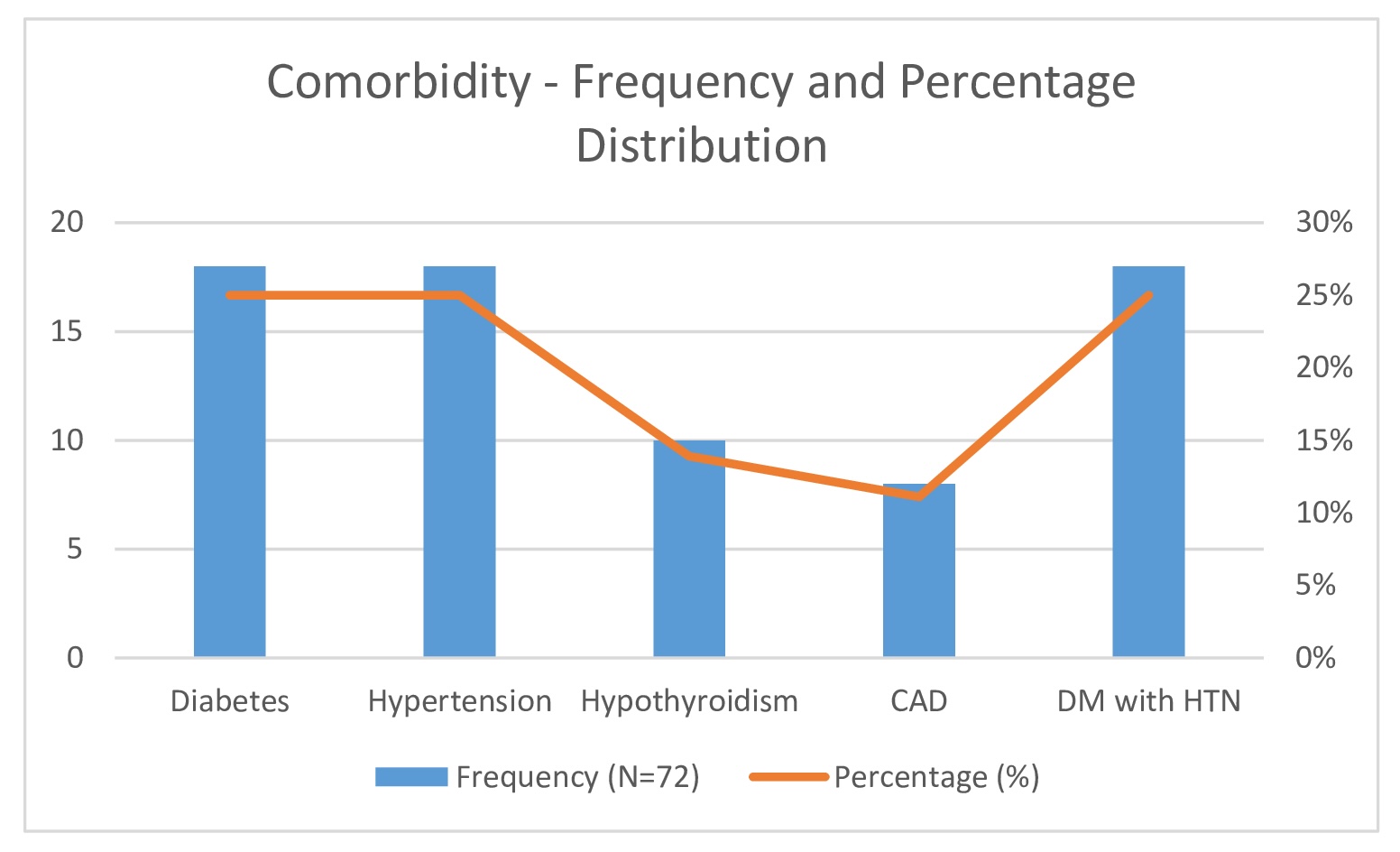
Fig. 4: Comorbidity - Frequency and Percentage Distribution.
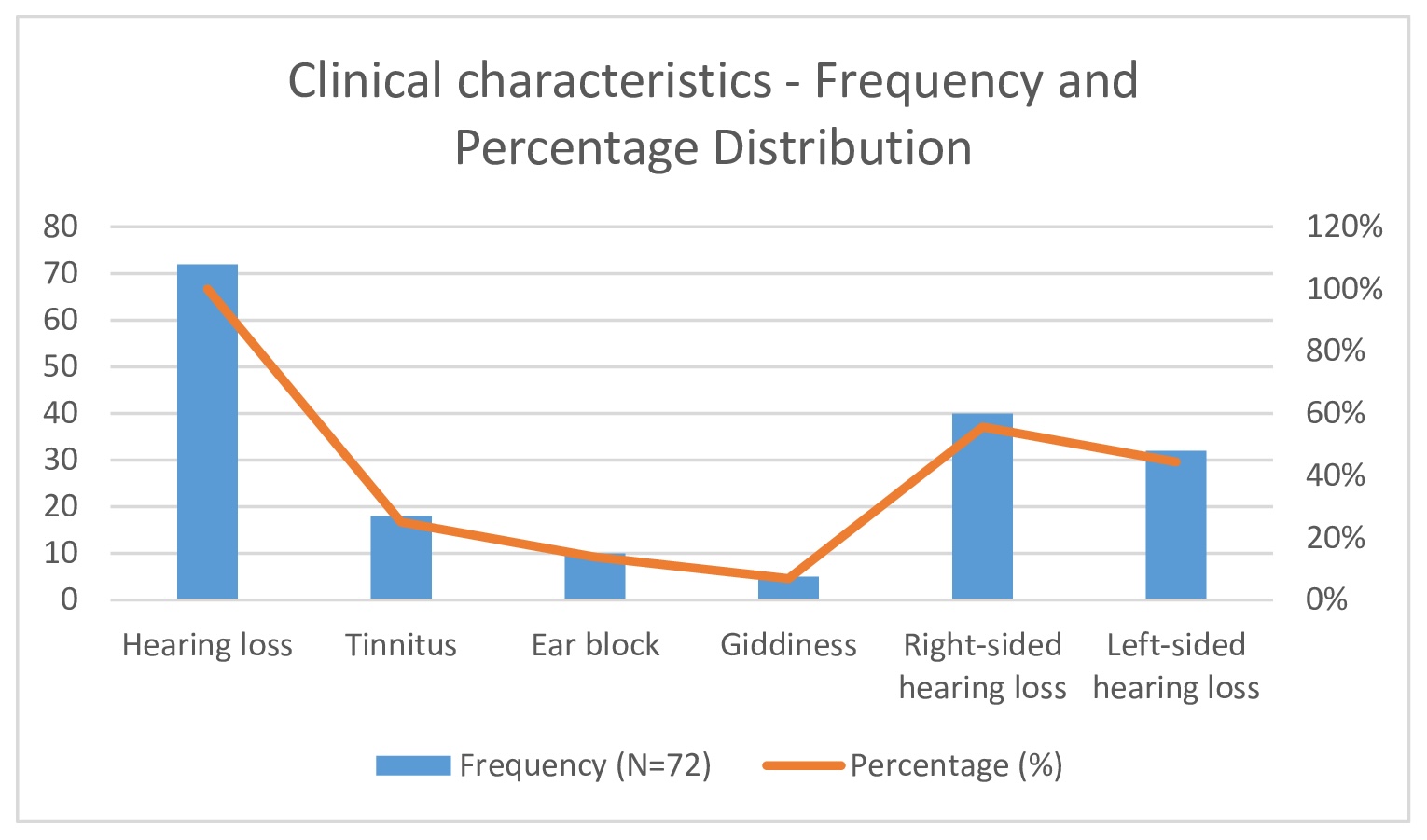
Fig. 5: Duration of illness - Frequency and Percentage Distribution.
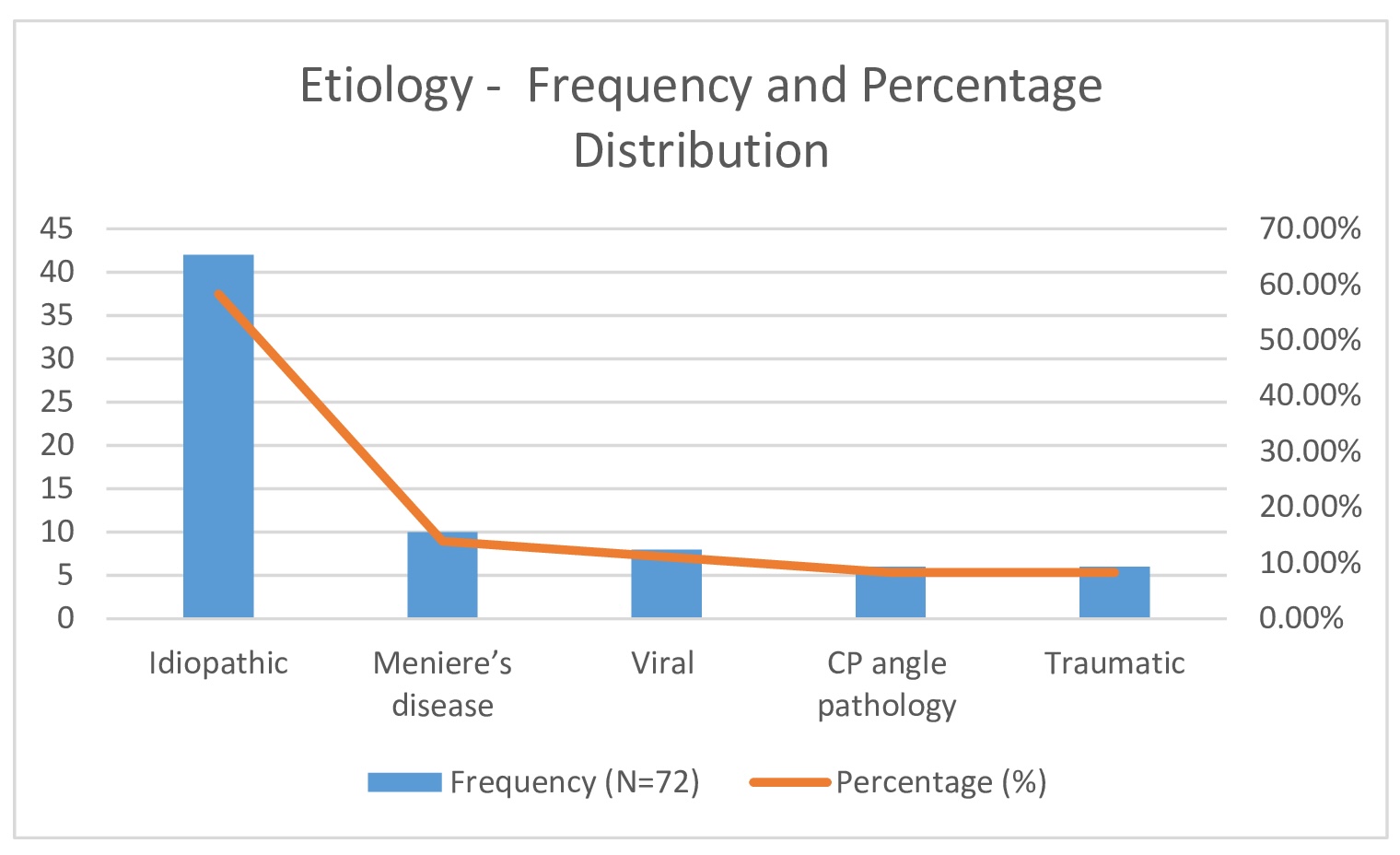
Fig. 6: Etiology- Frequency and Percentage Distribution.
The duration of illness at presentation varied: 25% of participants presented within one day, another 25% within two days, 38.9% within five days, and 11.1% within seven days (Table 2)(Fig. 7). All participants underwent three consecutive PTA tests (Table 3) to evaluate hearing improvement before and after treatment. Overall, 83.3% of participants showed hearing improvement, with those presenting within 72 hours demonstrating better outcomes compared to those who presented later.
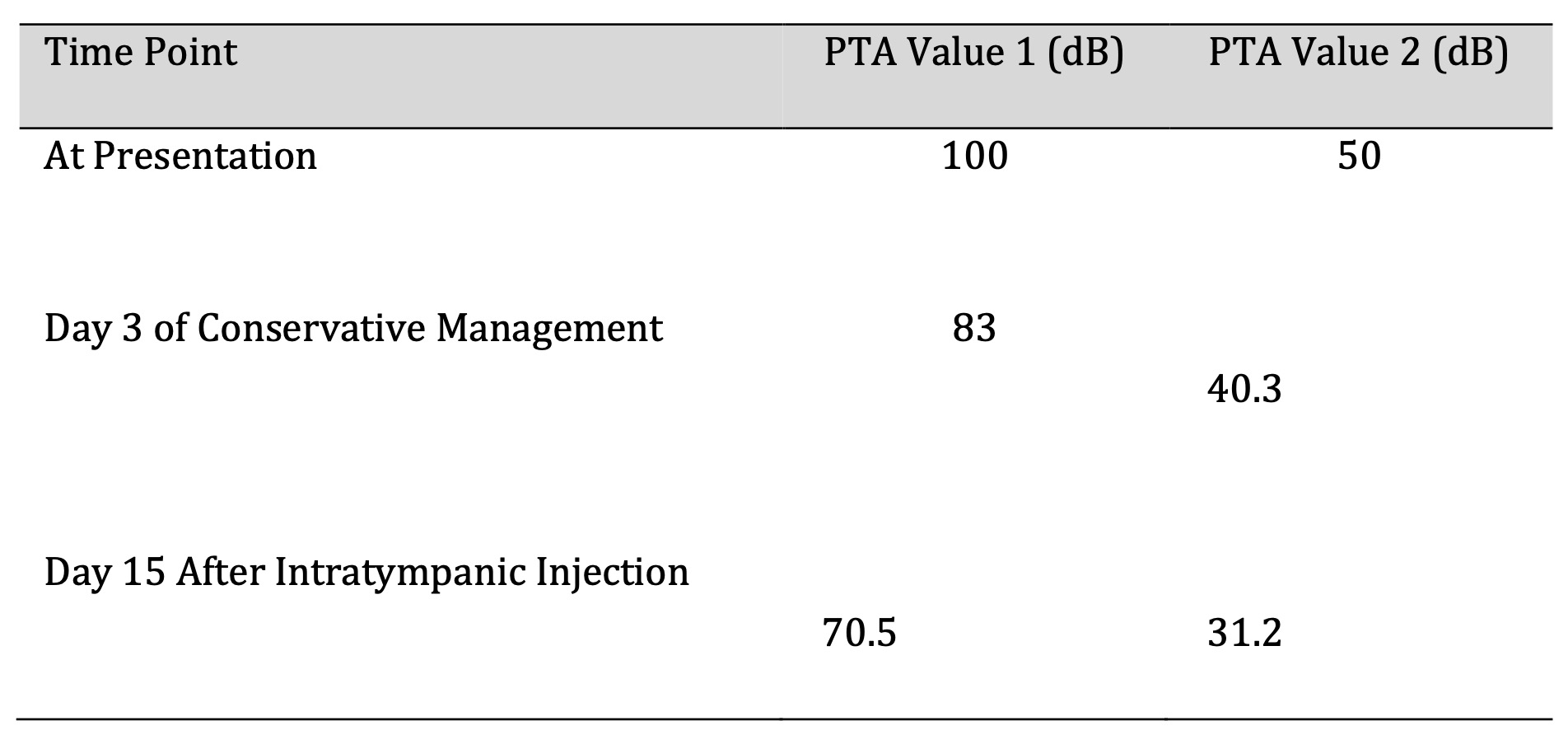
Table 3:
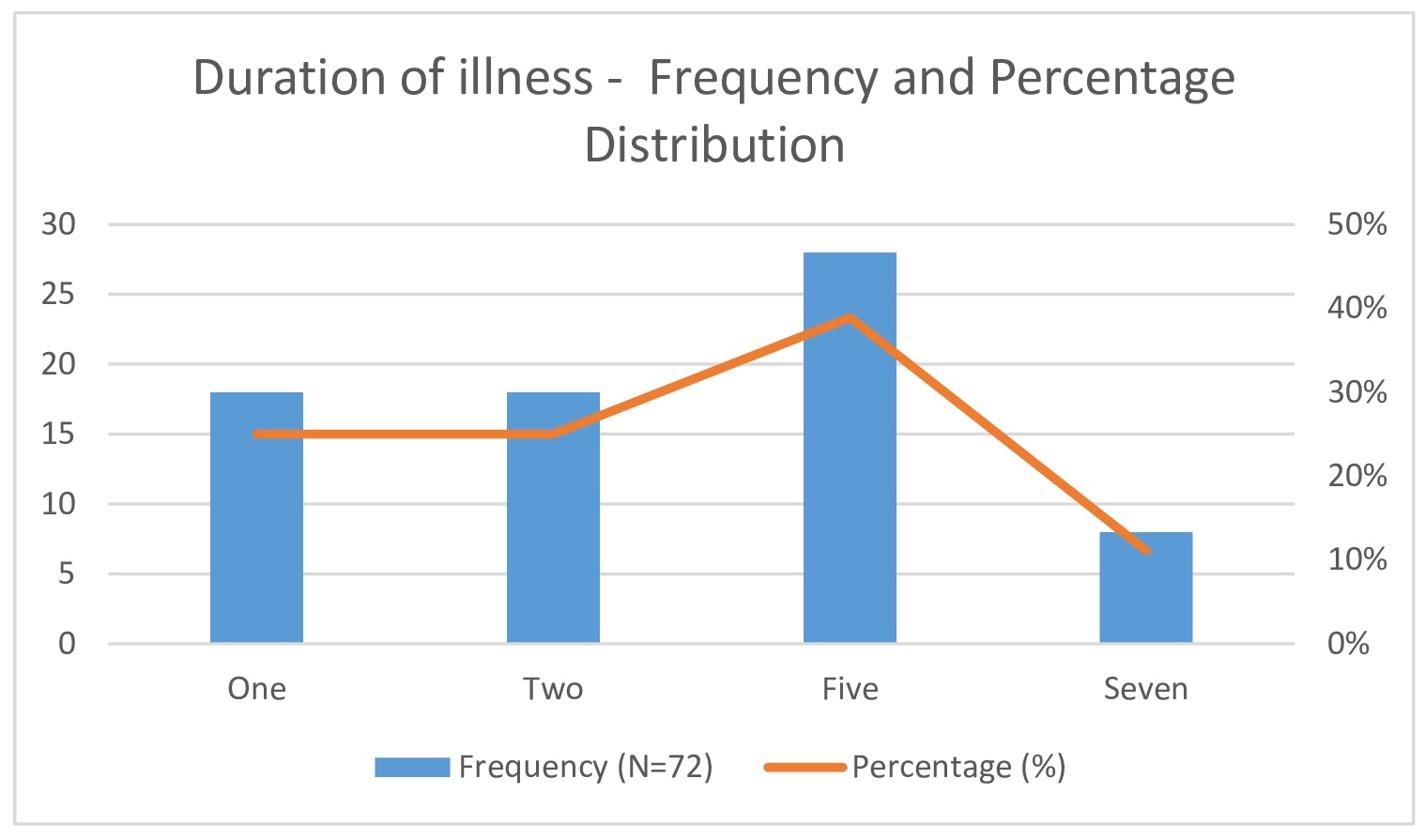
Fig. 7: Duration of illness - Frequency and Percentage Distribution.
Out of 72 patients, 4 patients did not respond to the medical intervention; hence, they underwent MRI imaging of the brain (Fig. 1). MRI imaging was performed to rule out retrocochlear pathologies. Secondary intervention with intratympanic dexamethasone injections (2 mg/kg) was administered only if MRI findings were normal. Imaging identified a vascular loop in the CP angle involving the 7th and 8th nerve complexes in one patient, who was subsequently referred for further neurosurgical evaluation. Among other non-responders, underlying etiologies such as viral infection or CP angle pathology were identified.
Statistical analysis demonstrated a significant improvement in hearing across the entire study cohort, with a p-value of 0.010. The observed improvement was particularly notable in patients who received treatment within 72 hours of symptom onset.
Two participants who presented with hearing loss persisting for over two months were excluded from the primary analysis due to delayed presentation. However, these individuals later received the study treatment protocol and achieved a 20 dB improvement in hearing within one month of treatment initiation.
Discussion
SSNHL is a critical otolaryngologic emergency characterized by a rapid onset of hearing loss, often indicative of inner ear damage. Advances in molecular biology have elucidated mechanisms of hair cell damage, significantly contributing to the pathology of SSNHL [19]. Typically affecting individuals aged 40–60, as highlighted in studies by Chau et al [9]., Byl [1], and Harish et al [20]., our cohort demonstrated peak incidence among those aged 31–40, with a male-to-female distribution of 55.6% to 44.4%. This aligns with existing literature suggesting no strong gender predisposition, although SSNHL predominantly affects middle-aged adults.
The prevalence of diabetes mellitus and hypertension in 25% of participants underscores their relevance in SSNHL. Previous research by Chau et al [9]., Cadoni et al [2]., and Capaccio et al [6]. supports an association between SSNHL and cardiovascular or autoimmune comorbidities. Our findings indicated more severe hearing impairment in patients with both conditions, suggesting that systemic disorders may exacerbate SSNHL due to compromised cochlear microvasculature.
Idiopathic cases comprised 58% of our cohort, consistent with literature identifying idiopathic SSNHL as the most common type [21, 22]. While the pathophysiology remains unclear, potential mechanisms include vascular compromise [16], cochlear membrane rupture [23], and viral infections [9]. Although Yamada et al [24]. suggested that up to 45% of SSNHL cases have an infectious origin, our findings align with a predominance of idiopathic cases.
Our data emphasize early intervention, showing significantly better hearing outcomes in patients treated within three days of symptom onset. This finding corroborates studies linking timely treatment to improved recovery. Notably, patients treated within 72 hours to one-week post-onset still demonstrated a 10–15 dB improvement, underscoring the importance of prompt intervention. MRI brain was used to evaluate non-responders, identifying CP angle pathology in some cases, which warranted neurosurgical referral. Comprehensive workups, including lipid profiles, ESR, and serologic testing, were conducted to exclude vascular, autoimmune, and syphilitic causes.
The debate over SSNHL treatment remains ongoing, with evidence suggesting spontaneous recovery in 45–65% of cases [25]. Corticosteroids, administered in tapering doses over 3–4 weeks, have shown promise, as noted by Wilson et al [17, 26, 27].. Intratympanic steroid injections have further demonstrated benefits by enhancing cochlear vascular permeability and reducing inflammation, as noted by Silverstein et al [28].
Unlike the broader “shotgun therapy” approach described by Wilkins et al [29]., which involves combining multiple treatment modalities such as vasodilators, colloids, plasma expanders, and xanthine derivatives, our study adopted a more focused treatment strategy. Specifically, we utilized two distinct groups: a control group treated with antiviral therapy (Acyclovir) and Steroid therapy (Prednisolone) and an intervention group that additionally received Pentoxifylline alongside Antiviral (Acyclovir) and Steroid (Prednisolone) therapies. This combination led to hearing improvement in 83.3% of participants. For those who did not respond to initial conservative treatment, intratympanic dexamethasone injections proved beneficial, consistent with findings reported by Xenelis et al. [30]. Two patients who presented with hearing loss persisting for over two months were excluded from the primary analysis; however, they later demonstrated a 20 dB improvement in hearing within one month of receiving the treatment protocol with Pentoxifylline, suggesting potential benefit even in chronic cases.
Limitations
Our study may limit generalizability due to regional healthcare practices and demographic variations. Sample size constraints limit the precision of subgroup analysis. As the study was carried out in a single geographic location, the results may not represent the diversity of patient populations in other regions, where clinical setup and practices may differ. Future studies involving multicenter trials with diverse patient populations are needed to enhance external validity and ensure that these results can be reliably applied to a broader range of clinical settings. Excluding patients with hearing loss beyond two months may introduce selection bias, affecting findings for chronic cases. The absence of a placebo control also introduces potential bias.
Generalisability
Our findings are most applicable to populations with similar demographic and clinical profiles. While our results align with existing SSNHL studies in middle-aged adults, comorbidities like diabetes and hypertension may influence outcomes in unique ways, suggesting the need for multicenter trials to enhance external validity.
Interpretation and Implications
This study highlights the potential of a focused treatment strategy employing antiviral therapy, steroids, and the addition of Pentoxifylline to improve hearing outcomes in patients with SSNHL. While spontaneous recovery has been documented [28], our findings emphasize the benefit of a structured, targeted therapeutic approach. Future studies should further explore long-term outcomes and the individual contributions of each treatment modality for optimized SSNHL management.
Conclusion
SSNHL is a multifactorial condition that can benefit from a targeted treatment approach. Our findings indicate that early intervention with a focused regimen of high-dose steroids and antiviral therapy, supplemented with Pentoxifylline, can significantly improve hearing outcomes, especially when initiated within 72 hours of symptom onset. The presence of additional symptoms such as ear blockage, tinnitus, and dizziness did not notably alter recovery, emphasizing the critical importance of prompt treatment regardless of symptom complexity. For patients who did not respond to the initial conservative regimen, intratympanic dexamethasone injections served as a valuable secondary measure, leading to further improvements in hearing thresholds.
MRI brain imaging proved essential for identifying retrocochlear and central nervous system pathologies, such as the detection of a CP angle vascular loop in one non-responder. Our study supports a structured, stepwise treatment strategy for SSNHL, advocating for early noninvasive management as the primary intervention. Intratympanic steroids should be reserved for cases resistant to initial treatment, maximizing patient outcomes and minimizing the need for invasive interventions.
Future studies should focus on conducting large-scale, multi-center trials across diverse clinical settings and populations to validate the effectiveness of the findings and proposed treatment strategy. These studies should aim to explore long-term outcomes of the proposed treatment plan and determine its sustainability and effectiveness over time. By involving multiple centers with a broader patient base, future studies can provide more effective and reliable evidence. This will help establish an evidence based, standardized management protocol for SSNHL, optimizing patient care and outcome.
Acknowledgements
We would like to acknowledge that this study was carried out at a three private clinics at Chennai district. No funding obtained. Ethical clearance was obtained from institutional ethical committee from the nearest tertiary care hospital. All patients provided written informed consent.
Author Contributions
Dr. Aishwarya Gajendran,1 - methadology, resources, data collection, manuscript writing, data analysis. Dr. Saranya Chithra Cheruvu,2*- conceptualisation, methodology, data collection, manuscript writing Dr. Aishwarya Pradeep,3 - data collection. Dr. Ramprasath Athikesavan,4 - manuscript writing. Dr. Xavier Ryon Washington Ramesh5 - manuscript revision
Statement of Ethics
Subjects (or their parents or guardians) have given their written informed consent.
The study protocol has been approved by the research institute’s committee on human research.
Disclosure Statement
No financial interests are involved. The authors have no conflicts of interest to declare.
References
| 1 | Byl FM, Jr. Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope. 1984;94(5 Pt 1):647-61.
https://doi.org/10.1288/00005537-198405000-00014 |
| 2 | Cadoni G, Agostino S, Scipione S, Ippolito S, Caselli A, Marchese R, et al. Sudden sensorineural hearing loss: our experience in diagnosis, treatment, and outcome. J Otolaryngol. 2005;34(6):395-401.
https://doi.org/10.2310/7070.2005.34606 |
| 3 | Salahaldin AH, Bener A, ElHakeem AA, Abdulhadi K. Management of idiopathic sudden sensorineural hearing loss: experience in newly developing Qatar. Int Tinnitus J. 2004;10(2):165-9.
|
| 4 | Cadoni G, Fetoni AR, Agostino S, De Santis A, Manna R, Ottaviani F, et al. Autoimmunity in sudden sensorineural hearing loss: possible role of anti-endothelial cell autoantibodies. Acta Otolaryngol Suppl. 2002(548):30-3.
https://doi.org/10.1080/00016480260094947 |
| 5 | Einer H, Tengborn L, Axelsson A, Edström S. Sudden sensorineural hearing loss and hemostatic mechanisms. Arch Otolaryngol Head Neck Surg. 1994;120(5):536-40.
https://doi.org/10.1001/archotol.1994.01880290046008 |
| 6 | Capaccio P, Ottaviani F, Cuccarini V, Bottero A, Schindler A, Cesana BM, et al. Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope. 2007;117(3):547-51.
https://doi.org/10.1097/MLG.0b013e31802f3c6a |
| 7 | Penido Nde O, Ramos HV, Barros FA, Cruz OL, Toledo RN. Clinical, etiological and progression factors of hearing in sudden deafness. Braz J Otorhinolaryngol. 2005;71(5):633-8.
https://doi.org/10.1016/S1808-8694(15)31268-4 |
| 8 | Gross M, Wolf DG, Elidan J, Eliashar R. Enterovirus, cytomegalovirus, and Epstein-Barr virus infection screening in idiopathic sudden sensorineural hearing loss. Audiol Neurootol. 2007;12(3):179-82.
https://doi.org/10.1159/000099021 |
| 9 | Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope. 2010;120(5):1011-21.
https://doi.org/10.1002/lary.20873 |
| 10 | Byl FM. Seventy-six cases of presumed sudden hearing loss occurring in 1973: prognosis and incidence. Laryngoscope. 1977;87(5 Pt 1):817-25.
https://doi.org/10.1002/lary.5540870515 |
| 11 | Li J, Ding L. Effectiveness of steroid treatment for sudden sensorineural hearing loss: a meta-analysis of randomized controlled trials. Annals of Pharmacotherapy. 2020 Oct;54(10):949-57.
https://doi.org/10.1177/1060028020908067 |
| 12 | Schwam Z, Wanna G. Update on the Management of Idiopathic Sudden Sensorineural Hearing Loss. Current Otorhinolaryngology Reports. 2022;10:1-7.
https://doi.org/10.1007/s40136-022-00414-5 |
| 13 | Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 Suppl):S1-35.
https://doi.org/10.1177/0194599812436449 |
| 14 | Merchant SN, Durand ML, Adams JC. Sudden deafness: is it viral? ORL J Otorhinolaryngol Relat Spec. 2008;70(1):52-60; discussion -2.
https://doi.org/10.1159/000111048 |
| 15 | Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359(8):833-40.
https://doi.org/10.1056/NEJMcp0802129 |
| 16 | Lee HA, Chung JH. Contemporary Review of Idiopathic Sudden Sensorineural Hearing Loss: Management and Prognosis. J Audiol Otol. 2024;28(1):10-7.
https://doi.org/10.7874/jao.2024.00024 |
| 17 | Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980;106(12):772-6.
https://doi.org/10.1001/archotol.1980.00790360050013 |
| 18 | Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. A Meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133(6):582-6.
https://doi.org/10.1001/archotol.133.6.582 |
| 19 | Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif. 2011;15(3):91-105.
https://doi.org/10.1177/1084713811408349 |
| 20 | Harish Chandra R, Muneeruddin A. UNILATERAL SENSORINEURAL DEAFNESS IN SCHOOL CHILDREN; A CLINICAL AND AUDIOLOGICAL EVALUATION AT A TERTIARY HOSPITAL OF TELANGANA. Journal of Evidence Based Medicine and Healthcare. 2016;3(1):6-10.
https://doi.org/10.18410/jebmh/2016/2 |
| 21 | Nosrati-Zarenoe R, Hansson M, Hultcrantz E. Assessment of diagnostic approaches to idiopathic sudden sensorineural hearing loss and their influence on treatment and outcome. Acta Otolaryngol. 2010;130(3):384-91.
https://doi.org/10.3109/00016480903161541 |
| 22 | Sirkeci BK. Idiopathic sudden sensorineural hearing loss, but not compatible with the classical definition. Cureus. 2023 Oct;15(10).
|
| 23 | Simmons FB. Sudden idiopathic sensori-neural hearing loss: some observations. Laryngoscope. 1973;83(8):1221-7.
https://doi.org/10.1288/00005537-197308000-00005 |
| 24 | Yamada S, Kita J, Shinmura D, Nakamura Y, Sahara S, Misawa K, et al. Update on Findings about Sudden Sensorineural Hearing Loss and Insight into Its Pathogenesis. J Clin Med. 2022;11(21).
https://doi.org/10.3390/jcm11216387 |
| 25 | Mattox DE, Lyles CA. Idiopathic sudden sensorineural hearing loss. Am J Otol. 1989;10(3):242-7.
|
| 26 | Arellano B, García Berrocal JR, Górriz C, González FM, Vicente J, Ramírez Camacho R [Treatment protocol for sudden deafness].. Acta Otorrinolaringol Esp. 1997;48(7):513-6.
|
| 27 | Khadav S, Arya P, Gupta G, Chand D, Bishnoi R, Chawra DS. Combined Intratympanic and Systemic Steroid Therapy in Sudden Sensorineural Hearing Loss. Indian J Otolaryngol Head Neck Surg. 2022;74(Suppl 1):293-8.
https://doi.org/10.1007/s12070-020-02056-9 |
| 28 | Silverstein H, Choo D, Rosenberg SI, Kuhn J, Seidman M, Stein I. Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report). Ear Nose Throat J. 1996;75(8):468-71, 74, 76 passim.
https://doi.org/10.1177/014556139607500806 |
| 29 | Wilkins SA, Jr., Mattox DE, Lyles A. Evaluation of a "shotgun" regimen for sudden hearing loss. Otolaryngol Head Neck Surg. 1987;97(5):474-80.
https://doi.org/10.1177/019459988709700508 |
| 30 | Xenellis J, Papadimitriou N, Nikolopoulos T, Maragoudakis P, Segas J, Tzagaroulakis A, et al. Intratympanic steroid treatment in idiopathic sudden sensorineural hearing loss: a control study. Otolaryngol Head Neck Surg. 2006;134(6):940-5.
https://doi.org/10.1016/j.otohns.2005.03.081 |