Microbial Desalination Cells: Sustainable Water Desalination Application and Wastewater Management
Keywords
Abstract
Background/Aims:
Microbial desalination cells (MDCs) are bioelectrochemical systems using electroactive bacteria to generate energy simultaneously cleaning wastewater and desalinating water. This sustainable technology addresses pollution issues and water shortage using an environmentally friendly solution that aids in desalination as well as wastewater treatment. This research focuses on the effectiveness of microbial desalination cells (MDCs) in concurrently treating wastewater and removing salt from water. The study seeks to determine whether MDCs offer a viable, environmentally friendly method for purifying water while generating energy.Methods:
The MDC setup incorporated three distinct chambers: anode, desalination, and cathode. Wastewater samples were placed in the anode and cathode compartments, while the desalination chamber contained saline water. A digital multimeter was employed to regularly monitor and log the generated voltages. The microbial community was examined through 16S rRNA gene sequencing techniques. Organic matter elimination was quantified by measuring total organic carbon (TOC) levels. The MDC operated for 30 days continuously.Results:
The microbial desalination cell (MDC) produced bioelectricity, effectively desalinated water, and broke down organic molecules during its 30-day running. This suggests that since the voltage generation peaked at 638 mV and then stabilized at 460 mV, the electrochemical activity has been constant. From 46.2 mS/cm to 10.1 mS/cm, the desalination chamber’s electrical conductivity (EC) fell drastically, clearly removing the ions. A decline in sodium chloride (NaCl) concentration—from 29 mg/L to 7 mg/L—also proved a sign of effective desalination. Better organic degradation was shown by the cathode chamber reaching 99.9% while the anode chamber attained a total organic carbon (TOC) removal rate of 97.2%. Desalination mostly depends on selective ion exchange across cation and anion membranes; microbial biofilm adaptation helped in the slow development of voltage. These findings suggest that since they efficiently mix the processes of wastewater treatment, desalination, and power generation, MDCs are a reasonably sustainable technology. The Microbial Desalination Cell (MDC) effectively desalinated water and treated wastewater having a peak voltage of 638 mV and a drop in NaCl concentration from 29 mg/L to 7 mg/L. With TOC removal in the anode at 97.2% and the cathode at 99.9%, the system proved excellent in both desalination and organic matter degradation. Furthermore, found to be unique from NCBI-recognized species was the microbiome found in Iraqi municipal effluent.Conclusion:
Microbial Desalination Cells (MDCs) have many advantages over conventional desalination techniques like reverse osmosis, including being able to cleanse wastewater and simultaneously generate renewable electricity with far reduced energy usage. Constant challenges are improving ion exchange efficiency, honing interactions between microbial communities, and increasing technological scale. Improving MDC performance and incorporating it into whole energy and water management systems is the main emphasis of research nowadays. This could be a perfect choice for encouraging more environmentally friendly energy sources and lessening the consequences of world water shortage.Introduction
Water is essential to the life of all living entities (Gangaraju et al., 2021). It is used extensively in human hydration, agriculture, sanitation, food preparation, and a myriad of industrial purposes. Still, freshwater supplies are limited and declining annually, hence there is a global issue with obtaining clean water (Bejjanki et al., 2021). To address the rising water shortage, desalination of brackish and seawater has experienced a rapid rise recently (Elawwad & Ragab, 2019). Desalination is the method used to clean saltwater so that human consumption may be had (Hamza et al., 2023). The very concentrated saline byproduct this process generates—desalination brine—should be treated carefully if we are to minimize environmental consequences (Abushawish et al., 2024). The optimal desalination method should be selected considering several factors. These comprise the contaminants to be eliminated, the intended use of the water, the economic viability of the project, the energy needed, and the disposal of the waste (Al-Daraghi et al., 2019; Afolalu et al., 2022). Combining biological and electrochemical processes, a bioelectrochemical device called a Microbial Desalination Cell (MDC) desalinates water. Two areas that might profit from this innovative system’s capacity to permit new chemical reactions (Aber et al., 2023) are sustainable water desalination and environmental remediation. MDCs offer a practical means of desalination by using microbial activity to encourage ion removal. MDCs are structurally made of three separate compartments: anodic, desalination, and cathodic (Salman & Ismail, 2020) separated from one another by anion exchange membranes (AEM) and cation exchange membranes (CEM), which control the passage of ions. Wastewater-based organic compounds are oxidized by microorganisms in the anode chamber, which sends electrons over an external connection to the cathode (Husein et al., 2012.). When these electrons interact with the terminal electron acceptors at the cathode, Sadeq and Ismail (2024) say the process is deemed complete. Majumder et al. (2023) claim that an external wire and load move electrons from an anode to a cathode generating bioelectricity. Selective ion removal from the desalination chamber is made possible by anion exchange membranes (AEM) and cation exchange membranes (CEM); their strategic location mostly depends on the effectiveness of Microbial Desalination Cells (MDCs). The voltage differential between the anode and cathode drives the desalination process in MDCs, the same as in microbial fuel cells (MFCs). By oxidizing organic compounds, generating electrical energy, and simultaneously lowering salt concentrations in brine streams, Moruno et al. (2018) found that MDCs offer a sustainable and multifarious method of water treatment. This work highlights the potential of MDCs to provide effective water desalination, renewable electricity generation, and organic carbon bioremediation using the metabolic activity of local wastewater microbial populations as a biological driving force.
Materials and Methods
MDC manufacturing and system setup
Conceived and constructed in the Engineering Process Laboratory of the Institute of Genetic Engineering and Biotechnology for Postgraduate Studies, the Microbial Desalination Cell (MDC) was developed largely using locally obtained acrylic sheets. The three linked cubic chambers—the anode, the intermediate desalination unit, and the cathode—that comprised the MDC design are shown in Fig. 1. To split these compartments, Membranes International Inc. of Ringwood, NJ, USA provided cation and ion exchange membranes (CMI-7000), each measuring 10 x 10 cm. The anion-exchange membrane (AEM) linked the anode and desalination chambers; the cation-exchange membrane (CEM) split the desalination and cathode compartments. The desalination unit added 350 mL to the whole volume whereas the anode and cathode chambers of the MDC combined consumed 550 mL. To block any leaking, gaskets were placed between the sections; the entire thing was connected with strong screws. Seven mm thick and six x eight cm graphite plates designed for use as electrodes were fitted in the anode and cathode chambers. A digital multimeter might monitor the electrical output of the system and track voltage by wiring these electrodes to an electrical wire.
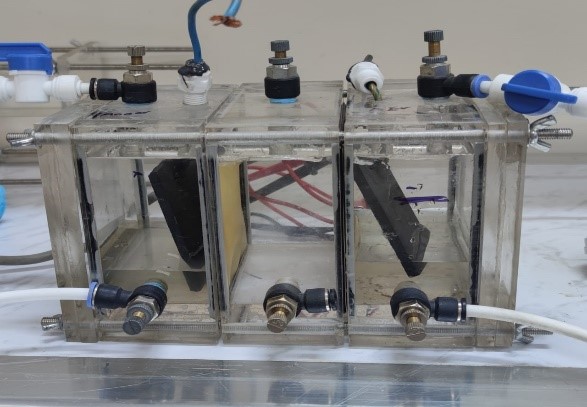
Fig. 1: Microbial desalination cell configuration.
Influent wastewater and saline water
Using wastewater as their feedstock—which supplied bacteria and nutrients for the anode and cathode chambers—the Microbial Desalination Cells (MDCs) were kept in an active state. Water samples were supplied by Baghdad, Iraq’s Al-Rustumia Wastewater Treatment Plant. Two different kinds of wastewater were used in the experiment: one from the aerobic digesters (dissolved oxygen (DO: 4 mg/L) and the other from the raw wastewater tank (0.2 mg/L), which were anaerobic and aerobic respectively. Additionally, collected from both sites and fed to the wastewater influent tank, five grammes of anaerobic and aerobic sludge were meant to enrich the microbiome. As Table 1 shows, the obtained samples were further characterized using general physicochemical criteria.
Using prepared saline water instead of seawater was meant to help regulate the experimental conditions and lessen the impact of several factors. Jaspal et al. (2023) claim that the place of sampling, geographical location, and seasonal fluctuations all considerably affect the makeup of saltwater. The inclusion of trace metals, organic compounds, nutrients, chloride and sodium ions in the study makes several possible causes of bias in it. Dissolving 20 g/L of NaCl in distilled water removed biological contaminants, heavy metals, dissolved organic molecules, and caused brackish water conditions. This approach guaranteed that the operation of the microbial desalination cell (MDC) rather than changes in the water composition was largely responsible for the observed effects, therefore enabling a more controlled assessment of the MDC’s efficiency in eliminating organic matter and salt. Typical issues in seawater desalination are membrane fouling and scaling; these could have damaged the experimental results; yet, using artificial saltwater helped eliminate these problems (Ahmed, 2013).
Running the microbial desalination cell (MDC) as a continuous-flow reactor helped this technology to show constant performance. Continuous sparged nitrogen gas was employed to maintain the anode chamber in anaerobic conditions using a 1-litre feeding tank, therefore excluding oxygen. Conversely, the cathode chamber was kept aerobically using a continually aerated 1 L feeding tank. Salt water was run to the intermediate membrane desalination plant to assist with ion transport and desalination. A peristaltic pump maintained the daily flow rate constant at 200 mL by regulating the influent and effluent flows of all chambers. The anode, cathode, and desalination chamber effluent were gathered in separate containers; samples were obtained routinely for further inspection. The MDC was pre-run for five days at room temperature before experimental measurements began to guarantee system integrity, prevent leakage, and provide the microbial colony time to adjust.
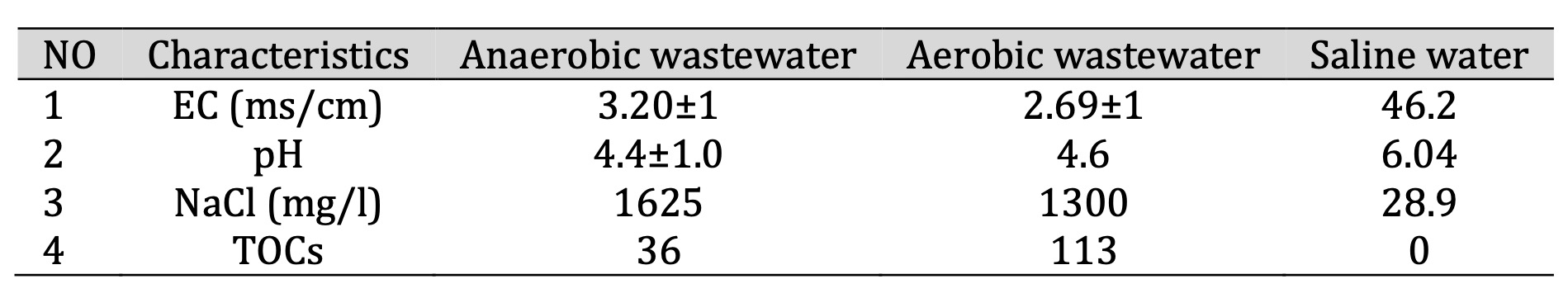
Table 1: Wastewater parameters. -EC (mS/cm) – Electrical Conductivity. -pH – Potential of Hydrogen. - NaCl (mg/L) – Sodium Chloride Concentration. - TOC (mg/L) – Total Organic Carbon, -Data were expressed as Mean ±SD
The experiment parameters
The voltages generated by the microbial desalination cell (MDC) were monitored regularly with a digital multimeter (DT-830D). Sodium chloride (NaCl) concentration was found using a sensionTM⁺ conductivity meter (HACH, UK). Conductivity and pH were measured using a professional benchtop pH meter to maintain strict control of the water chemistry. We measured the TOC concentration of the input and output water using a DR3900 spectrophotometer in line with the industry-standard Direct Method 1 (Test ‘N Tube procedure’). Every measurement was taken twice to ensure correct and dependable findings.
Microbial community DNA extraction and bacterial identification
DNA was isolated from samples drawn from the anode, cathode, and desalination cell (ion exchange membrane) in a triplicate study. Every one of the three parts of the experiment produced one gramme of biofilm, which served as a DNA extraction starting point. Following direct DNA extraction and PCR amplification using primers particular to 16S rRNA, amplicon sequencing was carried out. Enrichment agar-cultured bacteria underwent a second phase of DNA isolation to enter even further into the microbiome. Sequencing data were clipped (approximately 800 bp) to eliminate mistakes (Fig. 7) before being matched with the NCBI database for taxonomic identification. We considered both the forward and backward sequences for accuracy. Following the manufacturer of the QIAGEN DNA Mini Kit’s approach, the DNA was extracted from biofilm samples at the end of the experiment. After one month of continuous MDC operation, we investigated changes in bacterial population by using 16S rRNA polymerase chain reaction (PCR). Three separate sites—the anode, the cathode, and the desalination cell—provided samples. DNA extraction was done following the PrestoTM Mini gDNA Bacteria Kit’s technique to guarantee reliable and consistent results. Detailed bacterial composition and sequencing data are provided in the supplementary file (Supplementary Table S1).
By polymerase chain reaction (PCR), the 16S rRNA gene was amplified to investigate the microbiome in the Microbial Desalination Cell (MDC). The reaction mixture comprised a 1 µL forward primer (AGAGTTTGATCCTGGCTCAG) and a 1 µL reverse primer (TACCTTGTTACGACTT). A lyophilized premix helped to maintain the reaction components. Five microliters (5 µL) of extracted DNA was the template employed; thirteen microliters (µL) of deionized water completes the whole reaction volume. Put bacterial cells—up to 1 x 10⁴—in a 1.5 ml microcentrifuge tube and spin them for one minute at 14, 000–16, 000 x g to make Gram-positive bacteria ready. Discard the liquid on top. Arrange 200 μl of each sample into a 15 ml centrifuge tube to create the Gram+ Buffer. Combine the Gram+ Buffer with Lysozyme (0.8 mg/200 μl) then carefully swirl until dissolved. Add 200 μl of the Gram+ Buffer to the bacterial pellet in the 1.5 ml tube following confirmation of Lysozyme addition. Resuspender the mixture using either pipetting or vortexing. Turn the tube over every ten minutes while the sample is half an hour incubated at 37ºC. Before adding 20 μl of Proteinase K following incubation, be cautious about adding H₂O. Mix the concoction then vortexing. Then, invert the tube every three minutes as it incubates at 60ºC for a minimum of 10 minutes. Proceed then to Step 2: Lysis. Vortexing the sample with 200 μl of GB Buffer for 10 seconds will mix it once the first lysis is finished. Invert the tube every three minutes and incubate the sample at 70ºC for a minimum of ten minutes to achieve complete lysis. Bring the required elution buffer—200 μl for every sample—to a temperature of 70ºC while waiting for the DNA elution phase to start. For the optional RNA removal step, the clear lysate can be supplemented with 5 μl of RNase A (50 mg/ml) after incubation at 70ºC; then, vigorous shaking. Five minutes in a room temperature incubator will help the sample to come to. Quickly mix the sample lysate with 200 μl of 100% ethanol during the DNA binding stage. Should a precipitate develop, distribute it as much as possible with a pipette. Insert the whole mixture—including any insoluble precipitate—into a 2 ml collection tube before passing it to the GD Column. Remove the liquid-through after two minutes of centrifugation at 14, 000–16, 000 × g and slide the GD Column into a fresh two ml collection tube. Spin the mixture at 14, 000–16, 000 × g for 30 seconds following adding 400 μl of W1 Buffer to the GD Column for the wash phase, then remove the liquid that passes through. Add ethanol first then 600 μl of the Wash Buffer. Return the GD Column then to the 2 ml Collection Tube and spin it for 30 seconds at 14, 000–16, 000 × g. Return the GD Column to the 2 ml Collection Tube after you’ve thrown away the flow-through. Spin the column matrix in the centrifuge for an additional three minutes at 14, 000–16, 000 × g to guarantee complete drying.
Data analysis
The heat map plots were generated using the ClustVis web program (Metsalu and Vilo, 2015; https://biit.cs.ut.ee/clustvis/).
Results
Power production by Microbial Desalination Cell (MDC)
Regular tracking of the voltage generation of the microbial desalination cell (MDC) helped one evaluate its performance. As Fig. 2 shows, the voltage grew gradually throughout the first week until it peaked at 638 mV. Beyond this, though, the voltage began to drop and finally levelled off at roughly 460 mV. Fig. 2 also demonstrates that the pH of the desalination chamber changed across the experiment, ranging from 5 to 6.5. The peak voltage of this experiment, 638 mV, is in line with other field data. The 2014 Sabina et al. study, which again revealed a maximum voltage of 639 mV, is one instance of consistent MDC functioning. Cao et al. (2009) likewise noted a high voltage of 600 mV during MDC operation with an initial salt level of 20 g/L. In power-generating research by Faduos & Jwaid (2023), the output of 3.55 V and 9 mA was noted; this contrasts with this, illustrating how MDC performance could vary based on system configurations and operational situations.
In this experiment, the recorded voltage trendline showed that in combination with the desalination process, the microbial desalination cell (MDC) is a feasible method for producing energy. The technology reduced salt concentrations at the same time as efficiently using microbial activity to generate electrical energy. Furthermore, using wastewater as both a reservoir of electroactive bacteria and a nutritional source helps this method contribute to the creation of sustainable energy solutions. MDCs are a good solution for tackling renewable energy concerns and water shortages by combining wastewater treatment with bioelectricity generation. Fig. 3A shows that the adaptation of the microbial population and the progressive development of biofilm on the anode and cathode surfaces most likely led to the produced voltage progressively rising during the experiment. When a stable and mature biofilm develops, MDC performance is enhanced, hence raising electron transport’s efficiency. In an MDC, microorganisms oxidize organic molecules in the anode chamber to produce electrons that travel toward the cathode via an external circuit and generate power. As electrons pass a wire and load connected outside, electrical energy is created (Majumder et al., 2023). Over the initial part of the experiment, the voltage output of the microbial desalination cell (MDC) was less than 100 mV. This phenomenon can be explained by microorganisms gradually adjusting to the parameters of the system. Establishing a stable and efficient biofilm on the surface of the electrodes is necessary for an active electrochemical environment facilitating bacterial electron transfer. The results of Kokabian and Gude (2013), who discovered that extracellular electron transfer activities depend on the lag phase of microbial development, match the noted delay in voltage generation. Microbial experience during this period helps biofilm growth on electrode surfaces using metabolic and physiological changes. The biofilm enhances electron transport and microbial interactions, therefore enabling a progressive increase in voltage generation.
Research indicates that the power output of microbial desalination cells (MDCs), a major performance measure, directly influences the efficiency of microbial desalination systems (MDS). MDCs must grow biofilms on the anode if they are to progressively increase their power output. Since there aren’t many electroactive bacteria, electron transfer’s efficiency is first weak when microbial colonies grow on the surface of the electrode. The proportion of electroactive bacteria including Geobacter species increases as the biofilm ages, therefore enhancing the anode’s capacity to receive extracellular electron transfer (EET). Fig. 3 demonstrates how this slowly raises the power output. Apart from reducing internal resistance and raising energy generation, continuous biofilm development improves ion flow across chambers. Using a link between MDC voltage generation and the development of microbial communities, Dongre et al. (2024) and Jaroo et al. (2021) offer data supporting this. On the other hand, long-term stabilization or reduction of power generation can result from clogging and mass transfer limitations resulting from large biofilm development. Maintaining high energy output in MDCs hence calls for optimizing biofilm development.
Because the pH influences the metabolic activity of the microorganisms that use electrolysis for energy generation, microbial desalination cells (MDCs) and other bioelectrochemical systems are rather sensitive to pH (Mohammed & Ali, 2013). A main cause of the system-wide pH variation is the anode chamber proton accumulation resulting from microbial oxidation of organic substrates. The fundamental reason for this proton accumulation is the anion exchange membrane (AEM), which inhibits the migration of protons to the cathode chamber and generates a localized pH drop. On the other hand, the consumption of protons during the oxygen reduction process controls the pH dynamics of the cathode chamber, hence producing fluctuations in pH levels (Yang et al., 2014). The pH values of the anode and cathode chambers directly influence the voltage output of the MDC as, as Imoro et al. (2021) note, pH influences the metabolic efficiency of bacteria and, therefore, the general electrochemical performance of the system. Studies have indicated that voltage generation rose when the pH of the anode chamber dropped from 7 to 6.13. This implies that in microorganisms, somewhat acidic environments could enhance electron transport (Jaroo et al., 2021). Conversely, there have been cases when the pH in the anode chamber jumps. This could be the result of the materials used for the anode—activated carbon or layers of biofilm, for example. These components can be considered proton reservoirs, according to Ahmed (2013), which help to regulate or elevate the pH within the anode compartment and stop too much proton accumulation. Furthermore, underlined by Majumder et al. (2023) is the need for the buffering capability of anode materials for preserving bioelectrochemical activity and raising MDC performance by keeping an optimal electrochemical environment.
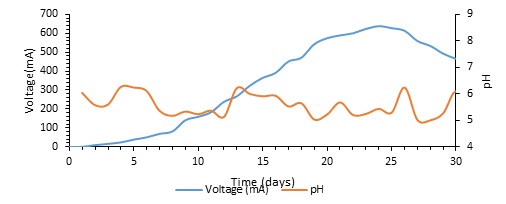
Fig. 2: Presents the generated voltage comparison with pH by microbial desalination unit.
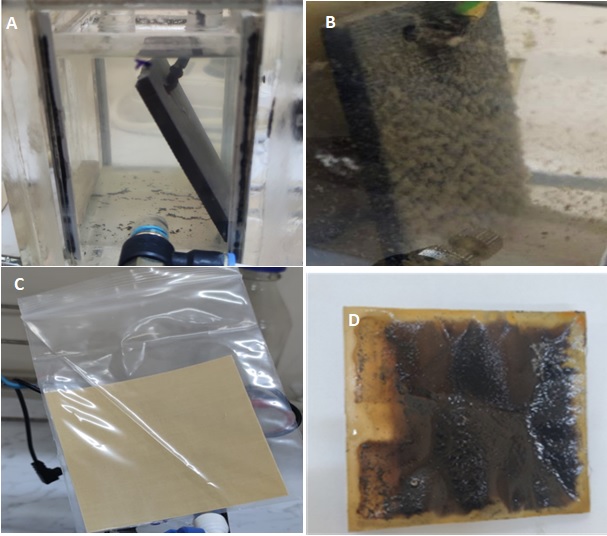
Fig. 3: A and B highlighted the biofilm on the graphite electrode before and after treatment, while C and D are the membranes before and after treatment.
Electrical conductivity (EC) and salinity removal in MDCs
Electrical conductivity (EC) was continuously tracked during the experiment to gauge the overall efficiency of the microbial desalination system. Fig. 4 illustrates a modest but consistent decline in the desalination chamber’s electrical conductivity (EC) from 46.2 mS/cm to 10.1 mS/cm following thirty days of continuous operation. This decrease in EC indicates the desalination performance of the system and the progressive elimination of ionic species. Moreover, EC values in the anode and cathode chambers changed inside specific ranges. Whereas cathode chamber values were 4 to 8.5 mS/cm, anode chamber EC values ranged from 3 to 4.5 mS/cm. These oscillations can be understood in the system using dynamic electrochemical reactions occurring there and ion movement across the chambers. Throughout the experiment, sodium chloride (NaCl) concentration was tracked constantly to evaluate membrane performance and spot probable leakage (Fig. 5). As seen in Fig. 6, from 29 mg/L to 7 mg/L, the consistent drop of the NaCl content in the desalination chamber proved effective ion removal. Simultaneously, the cathode chamber had values between 1.5 and 4.8 mg/L whereas the anode chamber had NaCl concentrations between 2.5 and 1.5 mg/L. These developments reveal that ion exchange activities are efficient in controlling the electrochemical balance of the system and that ions are transferred across membranes selectively.
Several connected elements can help to understand why the sodium chloride (NaCl) concentrations and the electrical conductivity (EC) of the anode and cathode chambers differ. These elements cover operational parameters, membrane characteristics, microbial activity, and components of the influent solution. Organic compounds broken down by microorganisms in the anode chamber release protons (H⁺) and electrons. Anions such as sulfate (SO₄²⁻) and chloride (Cl⁻) approach the anode to maintain the electrochemical equilibrium, hence reducing the concentration of NaCl and the electrochemical potential (EC). The approach sends the electrons to the cathode via an external circuit, where they participate in processes lowering oxygen (O₂ + 4e⁺ + 4H⁺ → 2H₂O). To preserve electroneutrality, sodium ions (Na⁺) move from the desalination chamber to the cathode, hence this chamber may observe a rise in EC and NaCl concentration. Between the anode and cathode, the desalination chamber is situated, and ions travel through ion exchange membranes. The concentration of NaCl and EC in this chamber is much lowered. We particularly detail the cation exchange membrane (CEM) migration of Na+ ions toward the cathode and the anion exchange membrane (AEM) migration of Cl⁻ ions toward the anode. Using this continuous ion transfer, the salt concentration in the desalination chamber is effectively lowered, therefore enhancing the general efficiency of the system.
Within the middle compartment, which functioned as the desalination unit, the electrical conductivity (EC) dropped progressively in this experiment. Selective ion movement across the chambers was made possible by the Cation exchange membrane (CEM) and anion exchange membrane (AEM), hence lowering the EC. Previous studies have also revealed a similar trend, with the conductivity of the desalination chamber declining drastically throughout the first day. The considerable improvement in ion transport efficiency brought about by the strong initial ion gradient across the membranes causes the fast drop. Desalination causes the ion gradient to drop, therefore slowing down the process (Santoro et al., 2017). The reduced availability of free ions in the desalination chamber stabilizes the conductivity of the system at lower levels by preventing additional ion migration. Concentration polarization accounts for this behaviour.
Apart from electricity generation and wastewater treatment, microbial desalination cells (MDCs) also help to remove salt from the centre area of the desalination chamber (Ebrahimi et al., 2017). The efficiency of salt removal in MDCs (Fatlawy et al., 2024; Kokabian & Gude, 2013) is determined by the hydraulic retention time (HRT), the surface area of the membrane, rates of microbial oxidation and oxygen reduction reactions, the volume of salt solution and wastewater, and other interrelated factors. These factors define the whole desalination performance and thereby influence the ion transport across the membranes. Although necessary for MDC operation, studies have indicated that microbial activity could not always directly affect desalination efficiency (Imoro et al., 2021). Conversely, ion exchange membranes allow ion migration dynamics, the main forces for salt removal. Operating under optimum conditions, MDCs offer considerable potential as a technology for ecologically friendly desalination (Carmalin Sophia et al., 2016). Their efficiency is 90%.
High salinity levels (AL-Amier & AL-Fatlawy, 2014) could significantly affect anaerobic bacteria in the anode chamber, which are vital for microbial oxidation and electron transfer. Excessive salt concentrations provide osmotic stress that can interfere with cellular function and stop microbiological activity. Consequently, there is a possibility that the entire electron transfer mechanism is compromised, thus lowering the cell potential and compromising the MDC’s efficiency (Liaquat et al., 2021). Luo et al. (2012) demonstrated how well MDCs remove salt by finding that, during 110 hours of operation, they could extract 90% of the salt from the desalination chamber. Conversely, dissolved salts and ionic species in the influent wastewater could influence system performance, which would in turn influence variations in electrical conductivity (EC) (Ishaq et al., 2024). MDCs have shown good desalination capacity in various conductivity environments independent of the challenges resulting from high EC levels. Dongre et al. (2024) reported that MDCs kept efficient desalination even at rising EC levels, hence highlighting their adaptability. Results of the Faduos and Jwaid (2023) study confirm this conclusion; the authors used MDCs to lower the sodium chloride concentration by 90%, therefore proving the potential of the technology for ecologically friendly desalination methods.
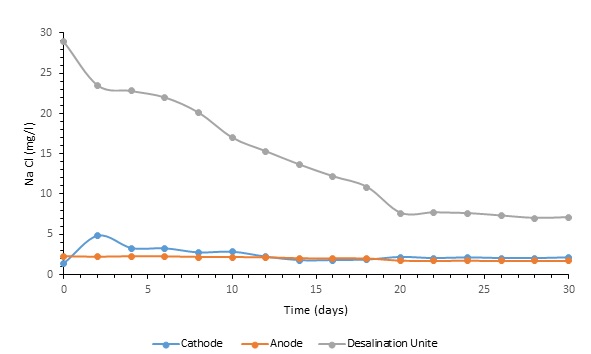
Fig. 4: The concentration of NaCl in the desalination unit, anode and cathode.
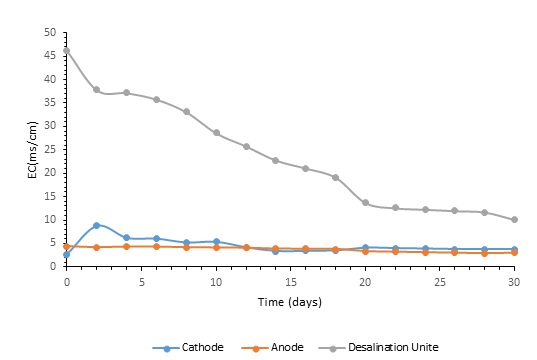
Fig. 5: The trend line of electrical conductivity and NaCl in MDC.
Total organic carbon (TOC) Removal
Fig. 6 illustrates the efficiency of the system’s organic degradation by showing the elimination of total organic carbon (TOC) in the anode and cathode effluent throughout a 30-day continuous operation period. The TOC concentration in the anode chamber fell sharply over the first four days, from 36 mg/L to 18 mg/L. This rapid decline suggests the simultaneous occurrence of microbial adaptability and active organic matter breakdown. TOC levels had reduced to a minimum of 1 mg/L at the end of the study, then continued a modest but consistent decrease beyond this point. Anode chamber TOC reduction over time reflects continuous microbial oxidation of organic substrates, a necessary process in bioelectrochemical energy generation. On the other hand, a different pattern was seen for the removal of TOC in the cathode chamber. Within just four days, total organic carbon contents dropped significantly from 119 mg/L to 96 mg/L. From day 4 to day 8, the total organic carbon levels showed a moderate but consistent decrease; thereafter, they fell abruptly until day 14, when they at last reached their lowest value at the end of the experiment. In an aerobic cathode environment, the elimination of TOC is more successful because oxygen is a good electron acceptor, which in turn accelerates microbial respiration and raises rates of organic matter breakdown. Moreover, aerobic surroundings promote microbial development, which in turn fosters a more varied population more suited for biodegrade. Furthermore, in an oxygen-rich environment, the synthesis of enzymes is improved, which facilitates a wider range of co-metabolic processes degrading intricate chemical compounds. The anode chamber runs under anaerobic conditions hence these advantages are absent. Oxygen deprivation lowers metabolic diversity among microorganisms, which slows down the decomposition of overall organic carbon (Zhou et al., 2024). Nevertheless, at the anode electron generation and biofilm formation—essential for microbial desalination—continue to occur.
Oxidizing carbon-containing compounds generates total organic carbon (TOC), which is then detected by CO₂ gas production. Microbial activity, electrochemical processes, and system operational conditions are among the several factors influencing the rate of TOC removal in microbial desalination cells (MDCs) (Atiya et al., 2020). Protons (Al Balushi et al., 2022), electrons, and carbon dioxide are among the anode chamber’s byproducts of organic matter oxidation. These electrons are driven to the cathode via an external circuit, where oxygen serves as the terminal electron acceptor to allow reduction reactions to occur. Using more complete oxidation of organic molecules, which in turn enhances the general performance of the system, using oxygen as an electron acceptor increases the efficiency of total organic carbon removal (Abd-Almohi et al., 2022).
Apart from quantifying organic molecules at every desalination stage, this approach offers valuable insights into the general system efficiency (Harvey et al., 2020). Remarkably good at removing total organic carbon (TOC) from the influent water was the microbial desalination cell (MDC). The cathode chamber attained an even better removal effectiveness of 99.9%; the anode chamber dropped 97.2% TOC. The TOC removing efficiency is computed using the following equation:
$${ \text{TOC}\% = (C_0 - C_T)/C_0 \times 100 \% }$$
C0 Initial total organic carbon concentration (mg/L)
CT total organic carbon concentration at time t (mg/L)
Su et al. (2023) observed that the catabolic metabolism of the bacteria in untreated wastewater helped break down organic materials, hence improving TOC elimination in this work. Ma et al. (2024) claimed that aerobic oxidation processes greatly raised microbial activity and accelerated the breakdown of organic compounds, thereby resulting in great removal efficiency. Rezk et al. (2023) underlined the need to control important parameters like pH, salt content, and applied voltage to maximize the performance of total organic carbon removal in microbial desalination systems. The cathode chamber in this experiment eliminated 99.9 per cent of TOC using an optimal pH range of 4.6 to 5.5 and a NaCl concentration of 20 g/L. Furthermore, proven by Naguib et al. (2023) is how strongly the beginning TOC concentration influences the treatment efficacy. Their results indicated that higher starting TOC levels enhanced TOC removal efficiency due to a decline in the rate of TOC reduction and an increase in the efficacy of the elimination of soluble TOC. This correlation could explain the distinct removal patterns observed in the cathode chamber and the anode chamber since the total organic carbon (TOC) level dropped considerably in the cathode chamber while it dropped more slowly in both chambers. Chi et al. (2023) also investigated how running settings affected TOC removal rates. Their results revealed that applied voltage in combination with the first pH and electrode spacing in combination with the starting pH greatly influenced the efficacy of TOC removal. The interaction of applied voltage and electrode spacing did not affect toxic off-gassing rates. These findings show that it is imperative to maximize specific parameter combinations if one is to increase the effectiveness of TOC removal in microbial desalination cells.
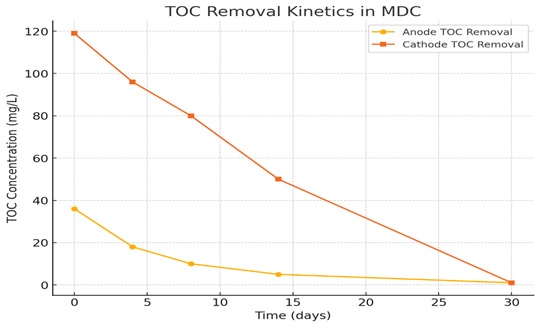
Fig. 6: The concentration of TOC in both the cathode and anode.
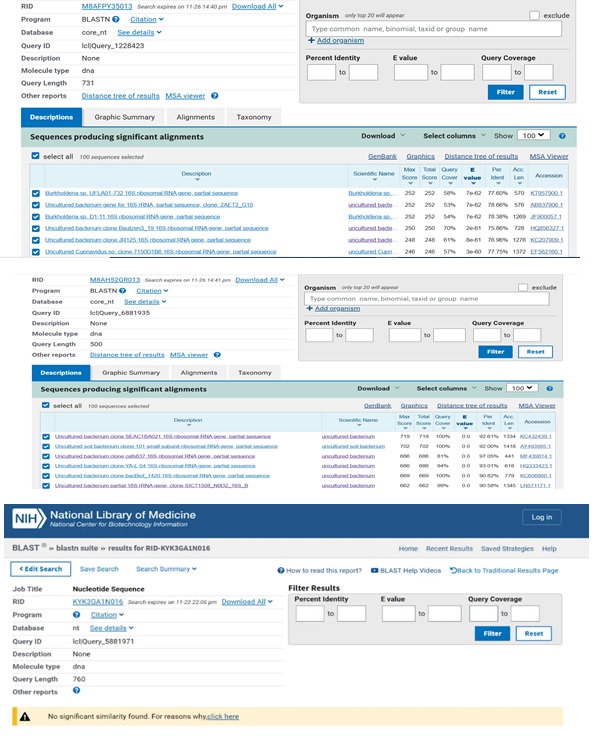
Fig. 7: NCBI blast data for the targeted samples where A is for the cathode, B anode and C is the desalination unit.
Bioinformatics Analysis
Fig. 7 shows how the sequencing data obtained from this study confirmed the unculturable species classification of the bacterial DNA. Still, a comparison of data from past studies on typically detected bacterial species in wastewater (Stewart et al., 2024) allowed one to delve deeper into the study and characterization of these microbial communities. We considered Salmonella, Shigella, Escherichia coli, Streptococcus, Pseudomonas, and Giardia among approximately a thousand distinct subspecies of bacteria from several genera and species. This work compared the bacterial sequences discovered here with those of the subspecies described in previous studies. The phylogenetic tree, which arranges recently identified bacterial species into groupings defined by genus and species, is shown in Fig. 8. Given their differences from all other species mentioned, the samples under examination could be new or hitherto unidentified species of bacteria. Further weight for this discovery came from the NCBI BLAST test’s results, which confirmed the discovered bacteria were uncultivated species. We grew the biomaterials taken from the biofilms developed in each of the three compartments using enrichment media to support these results. It is important to understand that this approach may be biased since the produced bacteria might not be a perfect replica of the natural microbial diversity in the biofilm. Notwithstanding this restriction, most of the identified bacterial species were once more classified as uncultured, as shown in Fig. 9, thereby confirming the sequencing results. The bioelectrochemical process occurs in the microbial desalination cell (MDC), and these results reveal the complexity and maybe novel nature of the microbial populations engaged in this process.
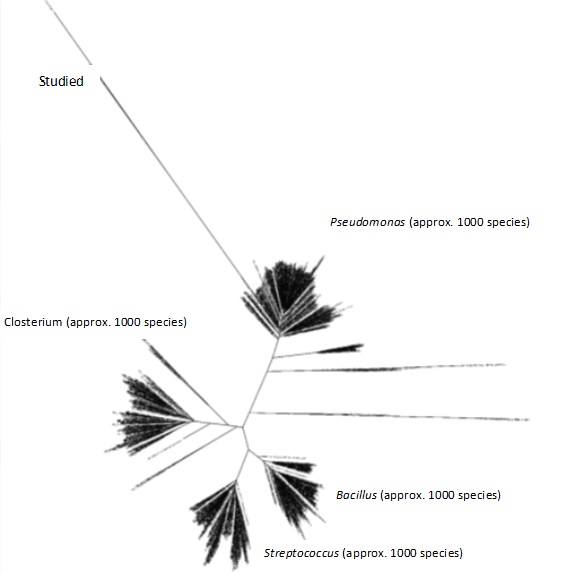
Fig. 8: Phylogenetic tree of the target sample (direct DNA sequencing) compared with common wastewater bacterial species of the 16SrRNA deposited in the NCBI database.
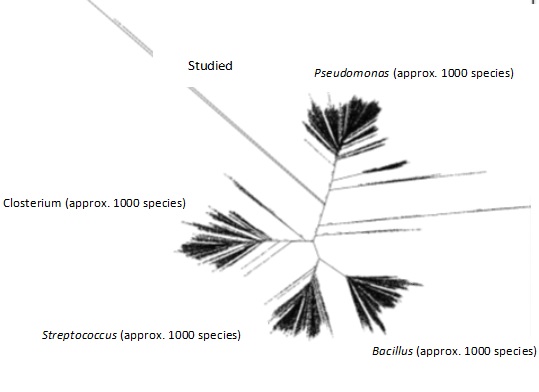
Fig. 9: Phylogenetic tree of the target sample (indirect DNA sequencing) compared with common bacterial wastewater species of the 16SrRNA deposited in the NCBI database.
Discussion
The results reveal that the bacterial species identified in the anode, cathode, and desalination chambers of the microbial desalination cell (MDC) experiment were vital in generating outstanding quantities of power and enabling efficient wastewater treatment. Notable to this research is the identification of novel types of bacteria; this suggests that these microorganisms flourished there and fit the particular operating parameters of the MDC. Their survival and spread most likely resulted from certain environmental elements inside the system. Both the continual aeration in the influent tank and the constant nitrogen gas supply in the anaerobic tank helped to maintain the conditions quite anaerobic.
Specialized settings are often home to unique microbial communities; one feature of modern study is the discovery of unculturable bacterial species. A similar thing occurred in the Bhatt et al. (2023) investigation, in which enhanced next-generation sequencing (NGS) techniques were particularly important in revealing uncultured bacterial species from wastewater sludge as a substrate. Taxonomic categorization based on 16S rRNA sequencing allowed most of the bacterial species found in a wastewater bioreactor study to be classified as uncultured bacteria (Niu et al., 2006). These findings underline the need to correctly identify and characterise non-culturable microorganisms using modern molecular techniques. Di Cesare et al. (2020) suggested that extra molecular and genomic studies be conducted to better grasp the ecological and functional roles of these unclassified bacteria, thereby improving bioelectrochemical and wastewater treatment applications.
Conclusion
Combining desalination, wastewater treatment, and renewable energy generation into one environmentally friendly system, modern green technology known as Microbial Desalination Cells (MDCs) is These systems operate at the anode by oxidizing organic molecules utilizing the metabolic activity of electroactive microorganisms, therefore generating electrons and protons. Produced electrical potential helps desalinate salt water by enabling salts to be transferred via ion-exchange membranes. Concurrent with this reduction process at the cathode, oxygen or another terminal electron acceptor enables the effective generation of energy. Reduced energy use and the possibility of resource recovery via simultaneous treatment of wastewater and generation of electricity define MDCs as advantages over conventional desalination methods. The primary topics of recent research are ways to scale up the technology for practical usage, enhance microbial consortiums for more electrochemical efficiency, and improve ion exchange membrane performance. Still causing disruptions to general acceptance are biofouling, membrane degradation, and expensive startup expenses. Notwithstanding these challenges, MDCs remain an interesting discovery, particularly for sustainable water management in areas with limited resources, where effective desalination technologies and access to clean water are top objectives.
Mostly feeding its microbial colony wastewater, the microbial desalination cell (MDC) used in this study was able to extract salt and create power. Long-term replacement of more conventional desalination and wastewater treatment methods by MDCs proved this new approach is feasible. Comparatively to the NCBI database, experimentally obtained results reveal unique bacterial species in the local wastewater microbial population in Iraq. These bacteria were quite good in eliminating salts and organic compounds as well as in turning wastewater and brackish water into energy. Moreover, the findings show that this system offers a greener and more energy-efficient means of desalinating and treating water, therefore substituting for conventional MDCs.
Disclosure Statement
The authors have nothing to disclose. No AI tools have been used to create this work.
References
| 1 | Abd-Almohi, H. H., Alismaeel, Z. T., & M-Ridha, M. J.: Study of Microbial Desalination Cell Performance; Power Generation and Desalination Efficiency using Pure Oxygen in a Cathode Chamber. Khwarizmi Engineering Journal 2022;18:37-47.
https://doi.org/10.22153/kej.2022.07.002 |
| 2 | Abd El-Ghany, N. A., Elella, M. H. A., Abdallah, H. M., Mostafa, M. S., & Samy, M.: Recent Advances in Various Starch Formulation for Wastewater Purification via Adsorption Technique: A Review. Journal of Polymers and the Environment 2023;31:2792-2825.
https://doi.org/10.1007/s10924-023-02798-x |
| 3 | Aber, S., Shi, Z., Xing, K., Rameezdeen, R., Chow, C. W. K., Hagare, D., & Jindal, T.: Microbial Desalination Cell for Sustainable Water Treatment: A Critical Review. Global Challenges 2023;7:1-15.
https://doi.org/10.1002/gch2.202300138 |
| 4 | Abushawish, A., Bouaziz, I., Almanassra, I. W., AL-Rajabi, M. M., Jaber, L., Khalil, A. K. A., Takriff, M. S., Laoui, T., Shanableh, A., Atieh, M. A., & Chatla, A. (2023). Desalination Pretreatment Technologies: Current Status and Future Developments. In Water (Switzerland) (Vol. 15, Issue 8).
https://doi.org/10.3390/w15081572 |
| 5 | Afolalu, S. A., Ikumapayi, O. M., Ogedengbe, T. S., Kazeem, R. A., & Ogundipe, A. T. (2022). Waste pollution, wastewater and effluent treatment methods - An overview. Materials Today: Proceedings, 62(April), 3282-3288.
https://doi.org/10.1016/j.matpr.2022.04.231 |
| 6 | Ahmed, A. S.:Alternative Chemical Treatments of Raw Water for Production of Drinking Water in Baghdad City. Iraqi Journal of Biotechnology 2013;12;77-90.
|
| 7 | AL-Amier. F., AL-Fatlawy, Y. F., R. K. A.: Study the Impact of the waste discharged from Al-Karama and Sharq-Dijla water treatment plants on water pollution of Tigris River water. Iraqi Journal of Science 2014;55:1006-1013.
|
| 8 | Al-Daraghi, W., Taha Lafta, I., & Al-Hamedi, F. H. (2019). Isolation and Identification of lipA Gene Producing Pseudomonas aeruginosa from Industrial Wastewater Introduction. Iraqi Journal of Biotechnology 2019;18:77-82.
|
| 9 | Atiya, M. A., M-Ridha, M. J., & Saheb, M. A.: Removal of aniline blue from textile wastewater using electrocoagulation with the application of the response surface approach. Iraqi Journal of Science 2020;61:2797-2811.
https://doi.org/10.24996/ijs.2020.61.11.4 |
| 10 | Bagheri Novair, S., Biglari Quchan Atigh, Z., Asgari Lajayer, B., Shu, W., & Price, G. W.: The role of sulphate-reducing bacteria (SRB) in bioremediation of sulphate-rich wastewater: Focus on the source of electron donors. Process Safety and Environmental Protection 2024;184:190-207.
https://doi.org/10.1016/j.psep.2024.01.103 |
| 11 | Bejjanki, D., Muthukumar, K., Radhakrishnan, T. K., Alagarsamy, A., Pugazhendhi, A., & Naina Mohamed, S.: Simultaneous bioelectricity generation and water desalination using Oscillatoria sp. as biocatalyst in photosynthetic microbial desalination cell. Science of the Total Environment;2021:754.
https://doi.org/10.1016/j.scitotenv.2020.142215 |
| 12 | Bhatt, P., Mathur, N., Singh, A., Sarkar, P., & Bhatnagar, P.: Hospital Wastewater Sludge: An Unaddressed Environmental Reservoir for Emerging and Rare Nosocomial Pathogens. Sustainability, Agri, Food and Environmental Research 2023 (DISCONTINUED);11:1-13.
https://doi.org/10.7770/safer.v11i1.2588 |
| 13 | Cao, X., Huang, X., Liang, P., Xiao, K., Zhou, Y., Zhang, X., & Logan, B. E.: A new method for water desalination using microbial desalination cells. Environmental Science and Technology 2009;43:7148-7152.
https://doi.org/10.1021/es901950j |
| 14 | Carmalin Sophia, A., Bhalambaal, V. M., Lima, E. C., & Thirunavoukkarasu, M.: Microbial desalination cell technology: Contribution to sustainable wastewater treatment process, current status and future applications. In Journal of Environmental Chemical Engineering 2016;4:3468-3478.
https://doi.org/10.1016/j.jece.2016.07.024 |
| 15 | Cheng, K. Y., Acuña, C. R., Kaksonen, A. H., Esslemont, G., & Douglas, G. B.: Sequential hydrotalcite precipitation, microbial sulfate reduction and in situ hydrogen sulfide removal for neutral mine drainage treatment. Science of the Total Environment 2024:926
https://doi.org/10.1016/j.scitotenv.2024.171537 |
| 16 | Chi, C., Zhou, X., Wang, Y., Gao, X., Bai, J., Guo, Y., & Ni, J.: Treatment of coking wastewater using a needle coke electro-Fenton cathode: optimizing of COD, NH4þ-N, and TOC removal and characterization of pollutants. Water Science and Technology 2023;88:106-122.
https://doi.org/10.2166/wst.2023.172 |
| 17 | Derwis, D., Al-Hazmi, H. E., Majtacz, J., Ciesielski, S., & Mąkinia, J.: Enhancing nitrogen removal in the partial denitrification/anammox processes for SO4− - Rich wastewater treatment: Insights into autotrophic and mixotrophic strategies. Journal of Environmental Management 2024:358.
https://doi.org/10.1016/j.jenvman.2024.120908 |
| 18 | Di Cesare, A., Corno, G., Manaia, C. M., & Rizzo, L.:Impact of disinfection processes on bacterial community in urban wastewater: Should we rethink microbial assessment methods? Journal of Environmental Chemical Engineering 2020;8.
https://doi.org/10.1016/j.jece.2020.104393 |
| 19 | Dongre, A., Kothari, S. L., Shami, A., Alsaad, M., Khan, S., Faizal, A., Razis, A., & Khan, S.: Environmental Technology & Innovation Development of microbial desalination cells for the treatment of reverse osmosis reject water : A new benchtop approach. Environmental Technology & Innovation 2024;35:103664.
https://doi.org/10.1016/j.eti.2024.103664 |
| 20 | Ebrahimi, A., Kebria, D. Y., & Darzi, G. N.: Enhancing biodegradation and energy generation via roughened surface graphite electrode in microbial desalination cell. Water Science and Technology 2017;76:1206-1214.
https://doi.org/10.2166/wst.2017.280 |
| 21 | El-Gawad, H. A., Ghaly, M. Y., El Hussieny, N. F., Abdel Kreem, M., & Reda, Y.: Novel collector design and optimized photo-fenton model for sustainable industry textile wastewater treatment. Scientific Reports 2024;14:1-18.
https://doi.org/10.1038/s41598-024-58610-w |
| 22 | Elawwad, A., & Ragab, M.: Simultaneous water purification and energy production in a microbial desalination cell. World Congress on Civil, Structural, and Environmental Engineering2019;1-4.
https://doi.org/10.11159/iceptp19.121 |
| 23 | Faduos, N. J., & Jwaid, A.: Microbial Desalination Cells for Water Desalination and Power Generation. Wasit Journal of Engineering Sciences 2023;11:84-92.
https://doi.org/10.31185/ejuow.Vol11.Iss1.408 |
| 24 | Fajri, N. R., & Mohammed, R. N.: Removal of Organic Pollutants from Wastewater Using Different Oxidation Strategies. Journal of Ecological Engineering 2023;24:351-359.
https://doi.org/10.12911/22998993/170897 |
| 25 | Fatlawy, Y. F. K. Al, Kadhim, F., & Mahdii, B. A.: A Study of Drinking Water Properties in Some Hospitals in Baghdad City. Iraqi Journal of Science 2024;65:43-54.
https://doi.org/10.24996/ijs.2024.65.1.5 |
| 26 | Gangaraju, G., Uma, R., & Shah, K. J.: Introduction to Conventional Wastewater Treatment Technologies: Limitations and Recent Advances 2021;91:1-36.
https://doi.org/10.21741/9781644901144-1 |
| 27 | Gurung, A., Thapa, B. Sen, Ko, S. Y., Ashun, E., Toor, U. A., & Oh, S. E.: Denitrification in Microbial Fuel Cells Using Granular Activated Carbon as an Effective Biocathode. Energies 2023;16.
https://doi.org/10.3390/en16020709 |
| 28 | Hamza, J. N., Ameen, A. M. S., & Mohammad, T. A.: The Effects of Intake Water Quality and Filtration Unit Performance on the Efficiency of the Al-Rasheed Water Treatment Plant in Baghdad, Iraq. Iraqi Journal of Science 2023;64:6158-6174.
https://doi.org/10.24996/ijs.2023.64.12.7 |
| 29 | Harvey, N. J., ur Rehman, Z., Leiknes, T. O., Ghaffour, N., Urakawa, H., & Missimer, T. M.: Organic compounds and microbial assessment of a seawater reverse osmosis facility at Tampa Bay Water, USA. Desalination 2020;496:114735.
https://doi.org/10.1016/j.desal.2020.114735 |
| 30 | Hong, Y., & Lin, J.: Efficient nitrogen removal via simultaneous ammonium assimilation and heterotrophic denitrification of Paracoccus denitrificans R-1. iScience 2024;27:1-14.
https://doi.org/10.21203/rs.3.rs-3890763/v1 |
| 31 | Husein, H. A., AL-Jumaily, E. F., & AL-Dulaimy, A. K.: Biological Treatment of Hydrocarbon Compounds in Oil Refinery Waste Water. Iraqi Journal of Biotechnology 2014; 1:77-89.
|
| 32 | Imoro, A. Z., Mensah, M., & Buamah, R.: Developments in the microbial desalination cell technology: A review. Water-Energy Nexus 2021;4:76-87.
https://doi.org/10.1016/j.wen.2021.04.002 |
| 33 | Ishaq, A., Said, M.I.M., Azman, S.B. et al. The influence of various chemical oxygen demands on microbial fuel cells performance using leachate as a substrate. Environ Sci Pollut Res 2024: https://doi.org/10.1007/s11356-024-32090-x
https://doi.org/10.1007/s11356-024-32090-x |
| 34 | Jaroo, S. S., Jumaah, G. F., & Abbas, T. R.: The operation characteristics of air cathode Microbial Desalination Cell to treat oil refinery wastewater. IOP Conference Series: Earth and Environmental Science 2021;877.
https://doi.org/10.1088/1755-1315/877/1/012002 |
| 35 | Jaspal, D., Malviya, A., El Allaoui, B., Zari, N., Bouhfid, R., El Kacem Qaiss, A., & Bhagwat, S.: Emerging advances of composite membranes for seawater pre-treatment: a review. In Water Science and Technology 2023;88:408-429).
https://doi.org/10.2166/wst.2023.220 |
| 36 | Karri, R. R., Ravindran, G., & Dehghani, M. H.: Wastewater-Sources, Toxicity, and Their Consequences to Human Health. In Soft Computing Techniques in Solid Waste and Wastewater Management 2021;3-33.
https://doi.org/10.1016/B978-0-12-824463-0.00001-X |
| 37 | Kasipandian, K., Sangeetha, S., Samrot, A. V., Abirami, S., Emilin Renitta, R., & Dhiva, S.: Bioelectricity production using microbial fuel cell-a review. Biointerface Research in Applied Chemistry 2021;11:9420-9431.
https://doi.org/10.33263/BRIAC112.94209431 |
| 38 | Kokabian, B., & Gude, V. G.: Photosynthetic microbial desalination cells (PMDCs) for clean energy, water and biomass production. Environmental Sciences: Processes and Impacts 2013;15:2178-2185.
https://doi.org/10.1039/c3em00415e |
| 39 | Kokabian, B., Gude, V. G., Smith, R., & Brooks, J. P.: Evaluation of anammox biocathode in microbial desalination and wastewater treatment. Chemical Engineering Journal 2017;342:410-419.
https://doi.org/10.1016/j.cej.2018.02.088 |
| 40 | Liaquat, R., Mehmood, T., Khoja, A. H., Iqbal, N., Ejaz, H., & Mumtaz, S.: Investigating the potential of locally sourced wastewater as a feedstock of microbial desalination cells (MDC) for bioenergy production. Bioprocess and Biosystems Engineering 2021;44:173-184.
https://doi.org/10.1007/s00449-020-02433-2 |
| 41 | Loh, Z. Z., Zaidi, N. S., Syafiuddin, A., Yong, E. L., Bahrodin, M. B., Aris, A., & Boopathy, R.: Current status and prospects of simultaneous nitrification and denitrification in wastewater treatment: A bibliometric review. Bioresource Technology Reports 2023;23:101505.
https://doi.org/10.1016/j.biteb.2023.101505 |
| 42 | Luo, H., Xu, P., Roane, T. M., Jenkins, P. E., & Ren, Z.:Microbial desalination cells for improved performance in wastewater treatment, electricity production, and desalination. Bioresource Technology 2012;105:60-66.
https://doi.org/10.1016/j.biortech.2011.11.098 |
| 43 | Ma, J., Wang, F., Fan, H., Li, E., Chu, H., Zhou, X., & Zhang, Y.: Metagenomic Insight Reveals the Microbial Structure and Function of the Full-Scale Coking Wastewater Treatment System: Gene-Based Nitrogen Removal. Engineering 2024;36:76-89.
https://doi.org/10.1016/j.eng.2023.06.019 |
| 44 | Madani, R. M., Liang, J., Cui, L., Zhang, D., Otitoju, T. A., Elsalahi, R. H., & Song, X.: Novel simultaneous anaerobic ammonium and sulfate removal process: A review. Environmental Technology and Innovation 2021;23:101661.
https://doi.org/10.1016/j.eti.2021.101661 |
| 45 | Majumder, S., Istalingamurthy, D., Murthy, S. B. M., & Prakash, B. M.: Impact of different electrodes, mediators, and microbial cultures on wastewater treatment and power generation in the microbial desalination cell (MDC). Water Science and Technology 2023;88:3194-3225.
https://doi.org/10.2166/wst.2023.406 |
| 46 | Metsalu T. and Vilo J.: ClustVis: a web tool for visualizing the clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res 2015;1:566-570.
https://doi.org/10.1093/nar/gkv468 |
| 47 | Mohammed, A. K., & Ali, S. A. A.-R.: Biotreatment of AL-KARAMA Teaching Hospital Wastewater Using Aerobic Packed Bed. Baghdad Science Journal 2013;10:144-151.
https://doi.org/10.21123/bsj.2013.10.1.144-151 |
| 48 | Moruno, F. L., Rubio, J. E., Atanassov, P., Cerrato, J. M., Arges, C. G., & Santoro, C.: Microbial desalination cells with sulfonated sodium (poly(ether ether ketone) as cation exchange membranes for enhancing power generation and salt reduction. Bioelectrochemistry 2018;121:176-184.
https://doi.org/10.1016/j.bioelechem.2018.02.004 |
| 49 | Naguib, A. M., Abdel-Gawad, S. A., & Mahmoud, A. S.: Using the Fenton reactions to eliminate Total Organic Carbon (TOC) from industrial effluents. Egyptian Journal of Petroleum 2023;32:36-41.
https://doi.org/10.1016/j.ejpe.2023.10.003 |
| 50 | Niu, S. Q., Fukushima, J., Jiang, Y., Ishikawa, Y., Ueda, T., & Matsumoto, S.: Analysis of bacterial community structure in the natural circulation system wastewater bioreactor by using a 16S rRNA gene clone library. Microbiology and Immunology 2006;50:937-950.
https://doi.org/10.1111/j.1348-0421.2006.tb03870.x |
| 51 | Kieu, T. Q. H., Nguyen, T. Y., & Do, C. L.: Treatment of Organic and Sulfate/Sulfide Contaminated Wastewater and Bioelectricity Generation by Sulfate-Reducing Bioreactor Coupling with Sulfide-Oxidizing Fuel Cell. Molecules 2023;28:6197.
https://doi.org/10.3390/molecules28176197 |
| 52 | Rezk, H., Olabi, A. G., Sayed, E. T., Alshathri, S. I., & Abdelkareem, M. A.: Optimized Artificial intelligence model to Boost the Efficiency of Saline Wastewater Treatment Based on the Hunger Games Search Algorithm and ANFIS. Sustainability 2023;15:4413.
https://doi.org/10.3390/su15054413 |
| 53 | Sabina, K., Fayidh, M. A., Archana, G., Sivarajan, M., Babuskin, S., Babu, P. A. S., Radha Krishnan, K., & Sukumar, M.: Microbial desalination cell for enhanced biodegradation of waste engine oil using a novel bacterial strain Bacillus subtilis moh3. Environmental Technology 2014;35:2194-2203.
https://doi.org/10.1080/09593330.2014.896951 |
| 54 | Sadeq, A. M., & Ismail, Z. Z.: Microalgae Growth in a Biocathode-Photosynthesis Microbial Desalination Cell: Molecular Characterization, Modeling Study, and Performance Evaluation. Iraqi Journal of Chemical and Petroleum Engineering 2024;25:1-12.
https://doi.org/10.31699/IJCPE.2024.1.1 |
| 55 | Salman, H. H., & Ismail, Z. Z.: Desalination of actual wetland saline water associated with biotreatment of real sewage and bioenergy production in microbial desalination cells. Separation and Purification Technology 2020;250:1-7.
https://doi.org/10.1016/j.seppur.2020.117110 |
| 56 | Santoro, C., Arbizzani, C., Erable, B., & Ieropoulos, I.: Microbial fuel cells: From fundamentals to applications. A review. Journal of Power Sources 2017; 356,225-244.
https://doi.org/10.1016/j.jpowsour.2017.03.109 |
| 57 | Smetana, G., & Grosser, A.: The Application of an Upflow Anaerobic Sludge Blanket Reactor in the Treatment of Brewery and Dairy Wastewater: A Critical Review. Energies 2024;17:1504.
https://doi.org/10.3390/en17061504 |
| 58 | Stewart, R. D., Oluwalana-Sanusi, A. E., Munzeiwa, W. A., Magoswana, L., & Chaukura, N.: Profiling the bacterial microbiome diversity and assessing the potential to detect antimicrobial resistance bacteria in wastewater in Kimberley, South Africa. Scientific Reports 2024;14:26867.
https://doi.org/10.1038/s41598-024-76466-y |
| 59 | Su, H., Wang, K., Lian, J., Wang, L., He, Y., Li, M., Han, D., & Hu, Q.: Advanced treatment and Resource recovery of brewery wastewater by Co-cultivation of filamentous microalga Tribonema aequale and autochthonous Bacteria. Journal of Environmental Management 2023;348:119285.
https://doi.org/10.1016/j.jenvman.2023.119285 |
| 60 | Yang, E., Choi, M.-J., Kim, K.-Y., & Kim, I. S.: Microbial desalination cell for concurrent hydrogen peroxide production and desalination. Journal of Environmental Engineering and Science 2014;9:197-206.
https://doi.org/10.1680/jees.14.00006 |
| 61 | Zhao, Z., Cheng, M., Li, Y., Song, X., Wang, Y., & Zhang, Y.: A Novel Constructed Wetland Combined with Microbial Desalination Cells and its Application. Microbial Ecology 2022;83:340-352.
https://doi.org/10.1007/s00248-021-01752-5 |