ACE2 Expression in the Kidneys of Pregnant and Postpartum Rats: Physiological and Pathological Significance During Pregnancy
Keywords
Abstract
Background/Aims:
Pregnancy is associated with changes in renal hemodynamics, such as increases in renal blood flow and the glomerular filtration rate (GFR). Angiotensin-converting enzyme 2 (ACE2), a transmembrane glycoprotein involved in vasodilation, also acts as a receptor for the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during coronavirus disease 2019 (COVID-19).Methods:
Using rats on pregnancy day 16 and postpartum day 5, we examined the histopathological changes in rat kidneys during and after pregnancy. The expressional changes in renal angiotensin-converting enzyme 2 (ACE2) and angiotensin (1-7) (Ang (1-7)) were examined, together with those of transmembrane protease serine 2 (TMPRSS2).Results:
In pregnant rats, the renal arterioles and venules as well as the glomerular capillaries were markedly dilated, indicating renal vasodilation. Immunohistochemistry demonstrated increased ACE2 and Ang (1-7) expression within the proximal renal tubules during pregnancy, which then returned to the virgin levels in the postpartum period. Additionally, the proximal tubular expression of ACE2 and TMPRSS2 was similarly enhanced during pregnancy.Conclusion:
As ACE2 and Ang (1-7) exert vasodilatory properties, they were considered responsible for renal vasodilation and the subsequent increase in GFR. Further, the similar distribution and enhanced expression of ACE2 and TMPRSS2 in the proximal renal tubules during pregnancy suggested their roles in the development of acute kidney injury (AKI) following COVID-19 in pregnancy. This study highlights the physiological and pathological significance of ACE2 during pregnancy.Introduction
Pregnancy is associated with changes in systemic hemodynamics, such as an increase in plasma volume and cardiac output but a decrease in blood pressure as a result of peripheral vasodilation [1]. Peripheral vasodilation during pregnancy is primarily caused by sympathetic nerve-related tonus changes in systemic vascular resistance and the influence of multiple vasodilatory factors, including nitric oxide, relaxin and progesterone [1-3]. During pregnancy, renal hemodynamic parameters, such as renal blood flow and glomerular filtration rate (GFR), also increase because of plasma volume expansion [4, 5]. However, the gestational increase in the GFR is usually much greater than that in the plasma volume or cardiac output [6, 7], suggesting the involvement of additional mechanisms underlying the renal hemodynamic changes during pregnancy. In our previous study, renal accumulation of prostaglandin E2 (PGE2) during pregnancy was found to directly dilate the afferent arterioles in the glomeruli, thus increasing the GFR [8]. Additionally, independent of the systemic renin-angiotensin system (RAS), which is responsible for systemic hemodynamic changes [9], a local or intrarenal RAS, directly affects renal function or causes renal damage under certain pathological conditions [10]. In the RAS, angiotensin converting-enzyme 2 (ACE2), a transmembrane glycoprotein, converts vasoconstrictive angiotensin II (Ang II) into vasodilative angiotensin (1-7) (Ang (1-7)), thereby exerting vasodilatory properties [11]. ACE2 and Ang (1-7) are within the interactive network of vasodilator factors in pregnancy, participating in the systemic and local hemodynamic adaptations [12].
On the other hand, during coronavirus disease 2019 (COVID-19), ACE2 acts as a host cell surface receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [13]. Further, in diseases such as heart failure, chronic kidney disease and chronic obstructive pulmonary disease, enhanced ACE2 expression in damaged organs is strongly associated with the development of severe COVID-19 [14-16]. Since pregnancy is a risk factor for COVID-19 and is associated with the development of kidney injury after infection [17-19], renal ACE2 expression may be involved in the pathogenesis. In the present study, we elucidated the physiological and pathological significance of ACE2 during pregnancy by examining the histopathological features of the kidneys in pregnant and postpartum rats.
Materials and Methods
Animal Preparation
Eleven-
to 12-week-old female Wistar rats (Japan SLC Inc., Shizuoka, Japan)
were mated with fertile male rats. The day when vaginal plugs were
observed was designated as pregnancy day 0. Pregnant rats and those
prior to becoming pregnant had free access to standard rat chow and
water throughout the experiment and were maintained in a humidity-
and temperature-controlled room on a 12-hour light-dark cycle. On
pregnancy days 16 and postpartum days 5, the rats were deeply
anaesthetized with isoflurane, and then killed by cervical
dislocation (n=3,
respectively). In rats, since GFR increases most prominently at the
later stage of pregnancy and restores by postpartum day 5 [6,
8], we examined the rats on
these gestational days. Age-matched virgin female rats were used as
controls (n=6).
Kidneys were harvested for histological examination. All experimental
protocols described in the present study were performed in accordance
with the Guide for the Care and Use of Laboratory Animals of Miyagi
University. Animal protocols were approved by the Animal Care and Use
Committee of Miyagi University (No. 2024-03). The animal study was
reported in accordance with ARRIVE guidelines
(https://arriveguidelines.org).
Histological Analyses
Renal
cross sections were fixed in 4% paraformaldehyde, embedded in
paraffin, deparaffinized in xylene, and then 3-μm sections were
stained with hematoxylin-eosin (H&E). Staining for CD31 was
conducted at the laboratory of Morpho Technology Co. Ltd (Sapporo,
Japan).
Immunohistochemistry
The
3-μm
paraffin sections of 4% paraformaldehyde-fixed kidneys were placed in
citrate-buffered solution (pH 6.0) and then boiled for 30 min for
antigen retrieval. Endogenous peroxidase was blocked with 3% hydrogen
peroxide, and nonspecific binding was blocked with 10% BSA. Primary
antibodies were as follows: Mouse anti-ACE2 (1:50; Santa Cruz
Biotechnology, Inc., Dallas, TX, U.S.A.), anti- transmembrane
protease serine 2 (TMPRSS2) (1:50; Santa Cruz Biotechnology, Inc) and
rabbit anti-Ang (1-7) (1:50; Cloud-Clone Corp., Katy, TX, U.S.A.).
Diaminobenzidine substrate (Sigma Chemical Co., St. Louis, MO, USA)
was used for the color reaction. At the end of the staining, the
sections were counterstained with hematoxylin.
The secondary antibody alone was
consistently negative on all sections. As we described in our
previous studies [20,
21], bright-field
images were acquired from randomly selected, nonoverlapping
high-power fields of view (more than 10 views from six virgin rats,
three pregnant rats and three postpartum rats, respectively). The
ACE2, Ang (1-7) or TMPRSS2 deposition, expressed as percentages of
each protein-positive areas relative to the total areas, was averaged
and quantified.
Results
Histological features of the kidneys in pregnant rats
Previous
studies have revealed an increase in the glomerular volume and
capillary wall surface area during pregnancy [22,
23]. However, these studies
did not provide clear morphological evidence for these changes. In
the present study, to reveal the influence of pregnancy on renal
morphology, we carefully examined the histological features of rat
kidneys on pregnancy day 16 and compared them with those in virgin
rats and on postpartum day 5 (Fig. 1). Sections of kidneys from
virgin rats showed normal vascular structures such as arterioles and
venules, which were localized in parallel within the cortical
tubulointerstitium (Fig. 1Aa). However, in rats on pregnancy day 16,
both the arterioles and venules were markedly dilated within the
intact tubulointerstitium (Fig. 1Ab). On postpartum day 5, the
dilated blood vessels had returned to a size comparable to that in
the virgin rats (Fig. 1Abc). Further, compared with the glomeruli
from the kidneys of virgin or postpartum rats (Fig. 1Ad and f), those
from pregnant rats were mostly enlarged (Fig. 1Ae). In these
glomeruli, the capillaries were markedly dilated and the Bowman’s
capsule was enlarged, indicating renal vasodilation and a subsequent
increase in GFR. However, immunohistochemistry for CD31, which is a
marker for endothelial cells, demonstrated an intact glomerular
endothelium in the kidneys of pregnant rats (Fig. 1Bb vs. a, c),
indicating that renal vasodilation during pregnancy was reversible
without any accompanying structural damages.
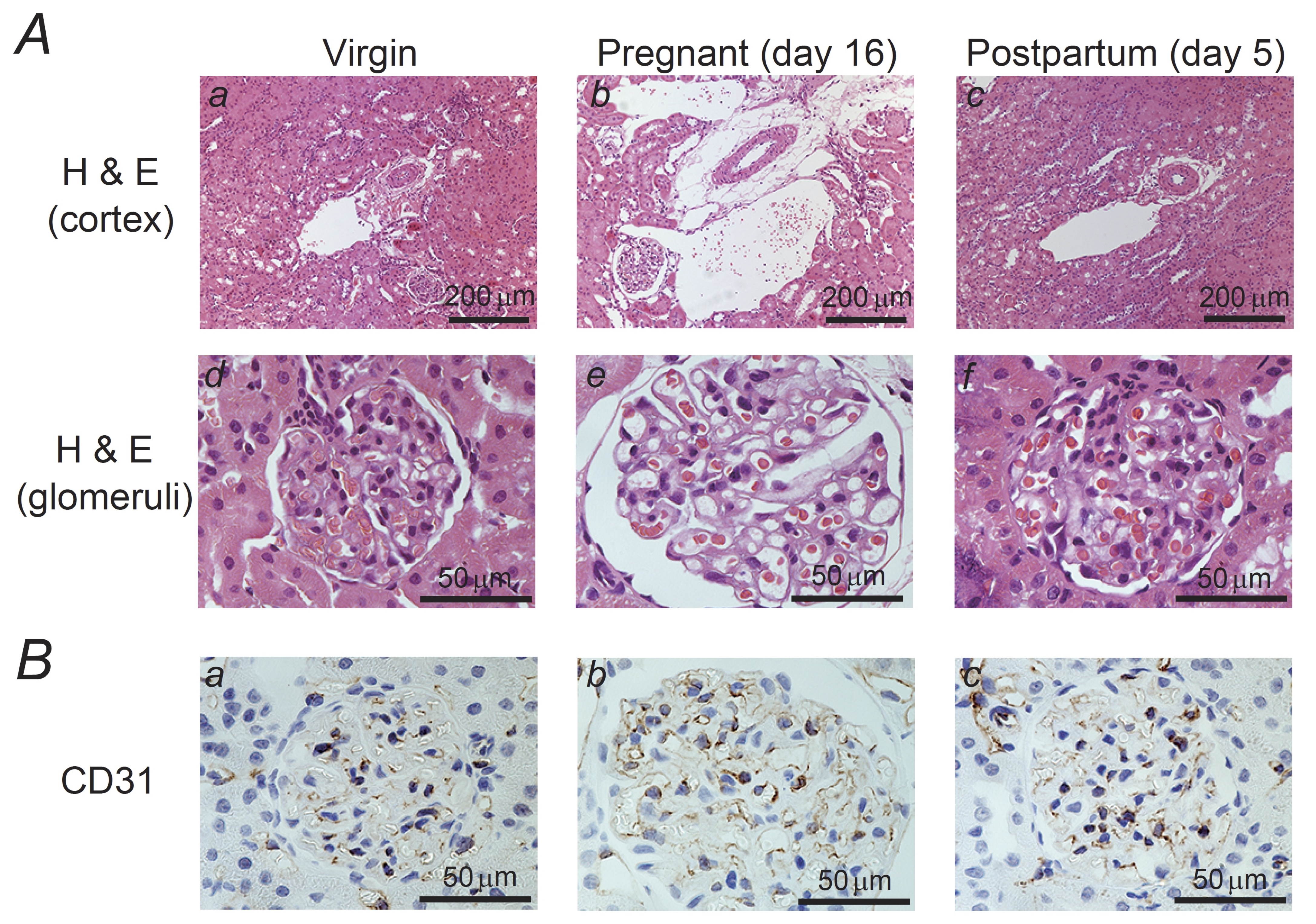
Fig. 1: Histological features and CD31 expression in the kidneys of pregnant and postpartum rats. (A) Hematoxylin and eosin (H&E) staining of the cortex (a to c; magnification, X10) and glomeruli (d to f; magnification, X60) in the kidneys of virgin (a, d), pregnant day 16 (b, e), and postpartum day 5 (c, f) rats. In rat kidneys on pregnancy day 16, in addition to the renal arterioles and venules, the glomerular capillaries were markedly dilated, indicating renal vasodilation. (B) Immunohistochemistry using antibodies against CD31 (brown) in the kidneys from virgin (a), pregnant day 16 (b), and postpartum day 5 (c) rats. An intact glomerular endothelium is seen in the sections from pregnant rats Magnification, X60.
ACE2 and Ang (1-7) expression in the kidneys of pregnant rats
As
ACE2 and Ang (1-7) exert vasodilatory properties [11],
we examined the renal expression of these proteins in pregnant and
postpartum rats (Fig. 2 and 3). In the kidneys of virgin rats,
consistent with previous findings [16,
24, 25],
immunohistochemistry for ACE2 demonstrated positive expression in the
brush border or apical membrane of the proximal tubules (Fig. 2Aa).
In the kidneys on pregnancy day 16, ACE2 protein expression was also
observed in the cytoplasm of proximal tubular cells (Fig. 2Ab). By
postpartum day 5, the protein expression returned to a localization
comparable to that in the kidneys of virgin rats (Fig. 2Ac vs. a).
Quantitatively, the percentages of ACE2-positive areas relative to
the total areas significantly
increased during pregnancy
(virgin,
12.0
± 0.79
% vs. pregnancy,
26.6
± 0.95
%; n=12,
p-value
=
1.29326022005154E-11
<0.05), which returned
to the virgin value by postpartum
day 5 (Fig. 2B). Further, as previously demonstrated [26-28],
the expression of Ang (1-7) was widely but weakly distributed within
the cytoplasm of proximal tubular cells in the kidneys of virgin rats
(Fig. 3Aa). However, in the kidneys on pregnancy day 16, cytoplasmic
expression became more intense (Fig. 3Ab), which by postpartum day 5,
returned to a level similar to that in the virgin rats (Fig. 3Ac).
There were statistically
significant differences in the Ang (1-7) expression during pregnancy
and postpartum (pregnancy,
34.3
± 0.87
% vs. postpartum,
15.8
± 0.85
%; n=15,
p-value
=
2.29238286339473E-15
<0.05) (Fig. 3B).
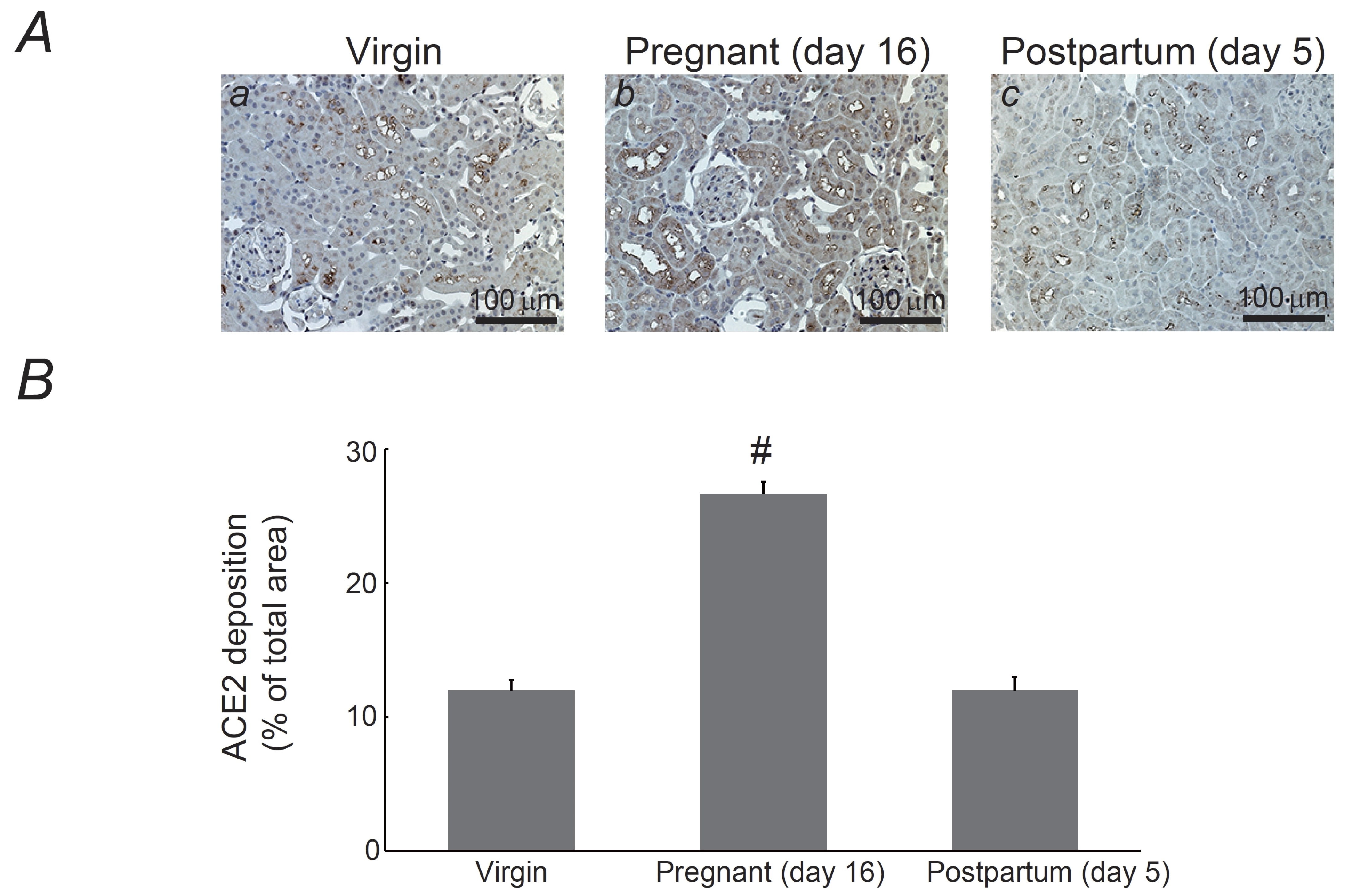
Fig. 2: Expression of angiotensin-converting enzyme 2 (ACE2) in the kidneys of pregnant and postpartum rats. (A) Immunohistochemistry for ACE2 (brown) in kidneys from virgin (a), pregnant day 16 (b), and postpartum day 5 (c) rats. Magnification, X20. (B) ACE2 deposition was quantified and expressed as percentages of ACE2-positive areas relative to the total areas. #P <0.05 vs. virgin rats. Values are means ± SEM (n=12). Differences were analyzed by ANOVA followed by Dunnett’s or Student’s t test.
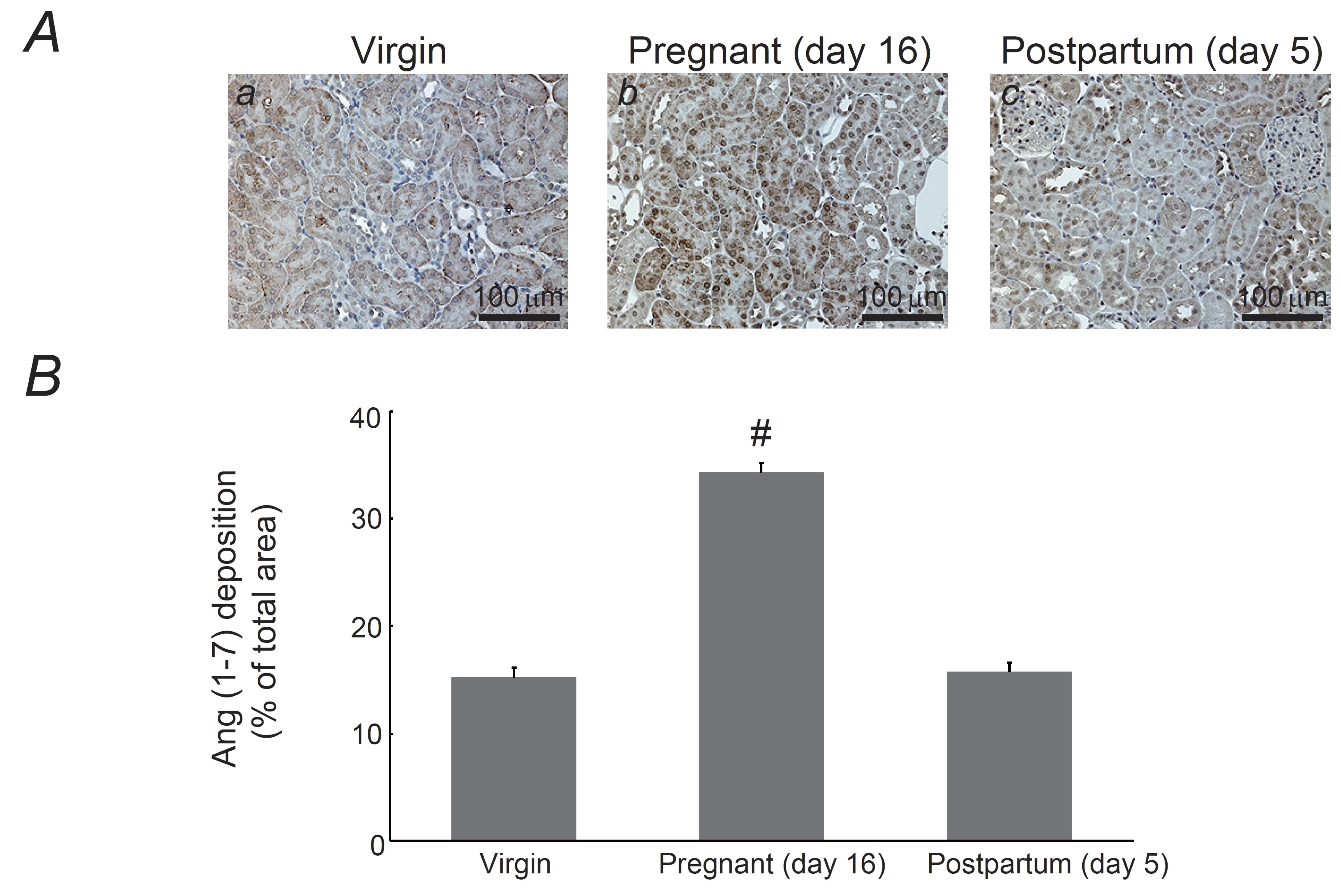
Fig. 3: Expression of angiotensin (1-7) (Ang (1-7)) in the kidneys of pregnant and postpartum rats. (A) Immunohistochemistry for Ang (1-7) (brown) in kidneys from virgin (a), pregnant day 16 (b), and postpartum day 5 (c) rats. Magnification, X20. (B) Ang (1-7) deposition was quantified and expressed as percentages of Ang (1-7)-positive areas relative to the total areas. #P <0.05 vs. virgin rats. Values are means ± SEM (n=15). Differences were analyzed by ANOVA followed by Dunnett’s or Student’s t test.
Renal TMPRSS2 expression in pregnant rats
In
COVID-19, ACE2 acts as a host cell surface receptor for SARS-CoV-2
[13].
When the virus enters host cells, its spike protein binds to ACE2,
which is predominantly expressed in the respiratory tract, lungs,
heart, and kidneys [29].
One of the transmembrane proteases of host cells, TMPRSS2, cleaves
viral spike proteins, thus activating and facilitating viral entry
[29].
In the present study, consistent with our previous findings [16],
immunohistochemistry for TMPRSS2 demonstrated positive staining in
the brush border or apical membrane of proximal tubules of the
kidneys from virgin rats (Fig. 4Aa), showing localization pattern
similar to that of ACE2 (Fig. 2Aa). In the kidneys of pregnant rats,
TMPRSS2 expression was observed in the cytoplasm of proximal tubular
cells (Fig. 4Ab). However, in the kidneys of postpartum rats, protein
localization returned to the brush border (Fig. 4Ac), showing an
expression pattern almost identical to that of ACE2 (Fig. 4A vs. 2A).
This was also quantitatively confirmed,
as the percentages of TMPRSS2-positive areas relative to the total
areas significantly
increased during pregnancy
(virgin,
15.3
± 1.56
% vs. pregnancy
26.7
± 0.68
%; n=14,
p-value
=
2.53090205408338E-6
<0.05)
and returned
to the virgin value by postpartum
day 5 (Fig. 4B).
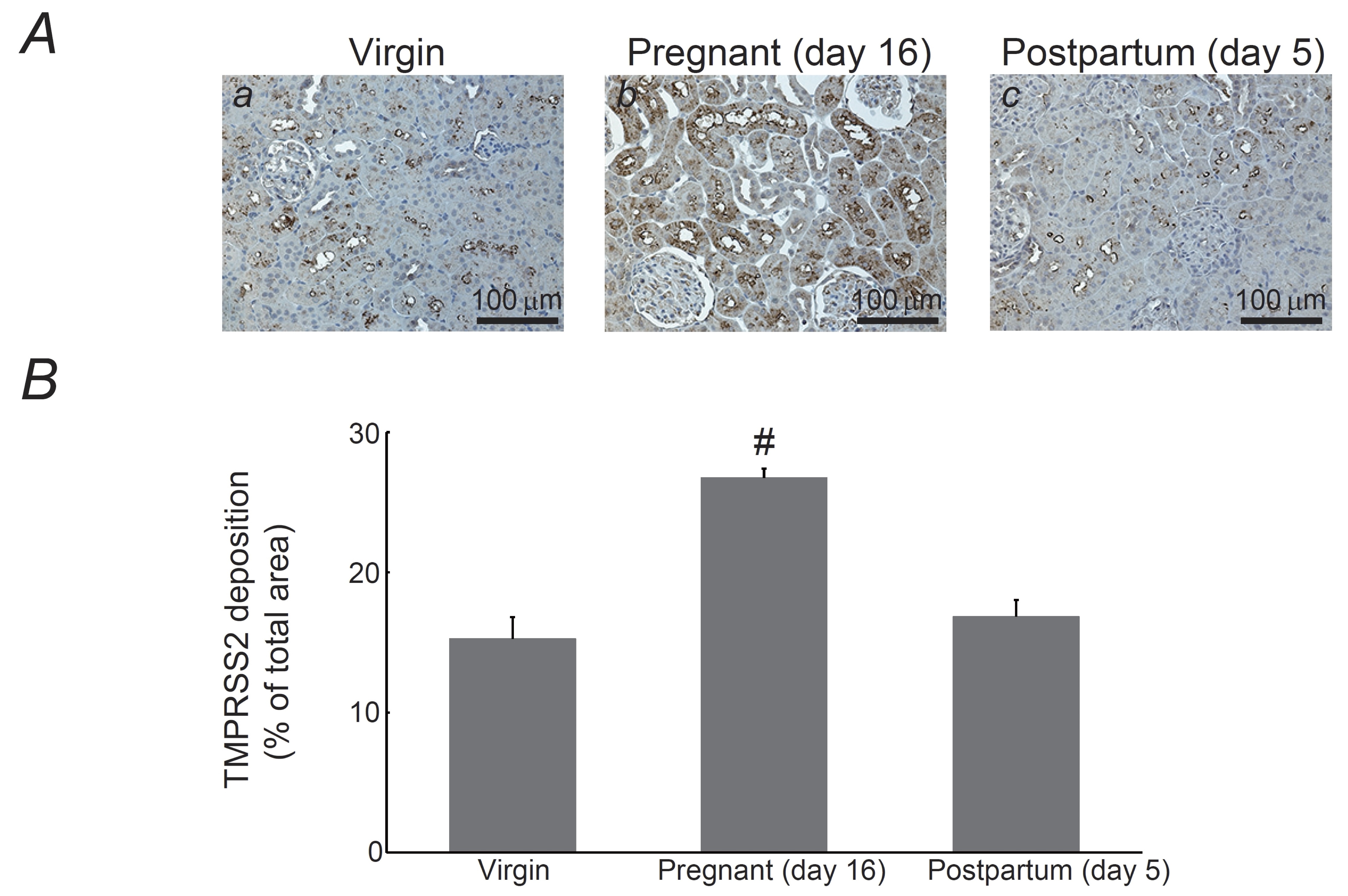
Fig. 4: Expression of transmembrane protease serine 2 (TMPRSS2) in the kidneys of pregnant and postpartum rats. (A) Immunohistochemistry for TMPRSS2 (brown) in kidneys from virgin (a), pregnant day 16 (b), and postpartum day 5 (c) rats. Magnification, X20. (B) TMPRSS2 deposition was quantified and expressed as percentages of TMPRSS2-positive areas relative to the total areas. #P <0.05 vs. virgin rats. Values are means ± SEM (n=14). Differences were analyzed by ANOVA followed by Dunnett’s or Student’s t test.
Discussion
In normal pregnancy, increased activities of the systemic RAS and sympathetic nerves result in sodium and water retention throughout the body, leading to plasma volume expansion [1]. In the RAS, ACE2 degrades and converts vasoconstrictive Ang II into vasodilatory Ang (1-7), thus exerting vasodilatory properties [11]. According to recent human and animal studies, in addition to serum concentrations [30], placental and renal concentrations of ACE2 are also elevated during pregnancy [27, 31], suggesting their direct effects on organ function. Previous studies have shown the increased renal expression of ACE2 and Ang (1-7) during pregnancy [26, 27, 32]. In this context, the present study further revealed that these protein expression during pregnancy specifically increased within the proximal tubular cells, which then returned to the virgin levels in the postpartum period (Fig. 2 and 3). Additionally, our study actually revealed that these changes were well synchronized with those in vascular tone observed during pregnancy (Fig. 1). Based on these results, the increased renal expression of ACE2 and Ang (1-7) in pregnancy was thought to be responsible for renal vasodilation, subsequently causing an increase in renal blood flow and the GFR (Fig. 5). Besides, as Ang (1-7) directly dilates the afferent arterioles in the glomeruli [33], it would bring about an additional increase in the GFR during pregnancy. In previous animal studies using rodents, such as rats or mice, short term or chronic infusion of Ang II decreased the renal expression of ACE2 [34-36]. In conditions such as renal hypoperfusion, Ang II more preferentially constricts the efferent arterioles than afferent arterioles in the glomeruli and thus maintains the GFR [37, 38]. In this context, the decreased ACE2 caused by Ang II infusion may be a negative feedback mechanism to modulate the excessive increase in GFR by reducing the renal blood flow.
Patients with severe COVID-19 sometimes develop acute kidney injury (AKI) [39, 40]. Recent studies have reported the involvement of direct viral invasion, renal medullary hypoxia due to hypoperfusion, rhabdomyolysis, and microangiopathy secondary to a cytokine storm in this pathogenesis. Thus, the pathological features of AKI following COVID-19 are typically characterized by proximal tubular damage due to acute tubular necrosis or interstitial inflammation [39-41]. Pregnancy is a risk factor for severe COVID-19 [17], and pregnant women are prone to develop AKI following COVID-19 [18, 19]. Our results showed that the proximal tubular expression of ACE2 and TMPRSS2 was similarly enhanced during pregnancy, which then returned to the virgin levels in the postpartum period (Fig. 2 and 4); notably, their distribution was almost overlapped with that of AKI lesions following COVID-19 [39-41]. ACE2 and TMPRSS2 are transmembrane proteins that facilitate SARS-CoV-2 entry into cells, allowing the virus to stimulate pro-inflammatory cytokine production and induce organ injury [29]. In chronic diseases, such as heart failure, chronic kidney disease, and chronic obstructive pulmonary disease, enhanced expression of these proteins in damaged organs results in the development of severe COVID-19 [14-16]. Therefore, enhanced proximal tubular expression of ACE2 and TMPRSS2 during pregnancy may be responsible for the development of AKI following COVID-19 in this condition (Fig. 5). Given this pathogenesis, the use of ACE2 inhibitors or soluble forms of ACE2 protein, which directly block the cellular entry of SARS-CoV-2 [42-44], as well as the use of natural products that directly or indirectly modulate ACE2 activity [45] would be beneficial in preventing AKI in pregnant women. However, since our observations are still hypothesis-generating, functional validation would additionally be required to confirm the causality. The possible approaches to address this issue in the future would include ACE2 inhibition studies, SARS-CoV-2 spike protein challenges or using SARS-CoV-2 infection models in pregnant animals. Because of pregnancy-related hemodynamic changes involving the kidneys, pregnant women are vulnerable to develop AKI independently from COVID-19 [46]. Although the histopathology of kidneys in pregnant animals with AKI has not been examined, previous studies clearly demonstrated the decreased renal tubular expression of ACE2 in animal models with ischemic AKI [47-49]. Therefore, in cases of AKI following COVID-19, ACE2 expression may become downregulated after ACE2 mediates SARS-CoV-2 entry into cells [47]. This can be the phylactic mechanism against the further progression of AKI during pregnancy by halting the additional entry of SARS-CoV-2.
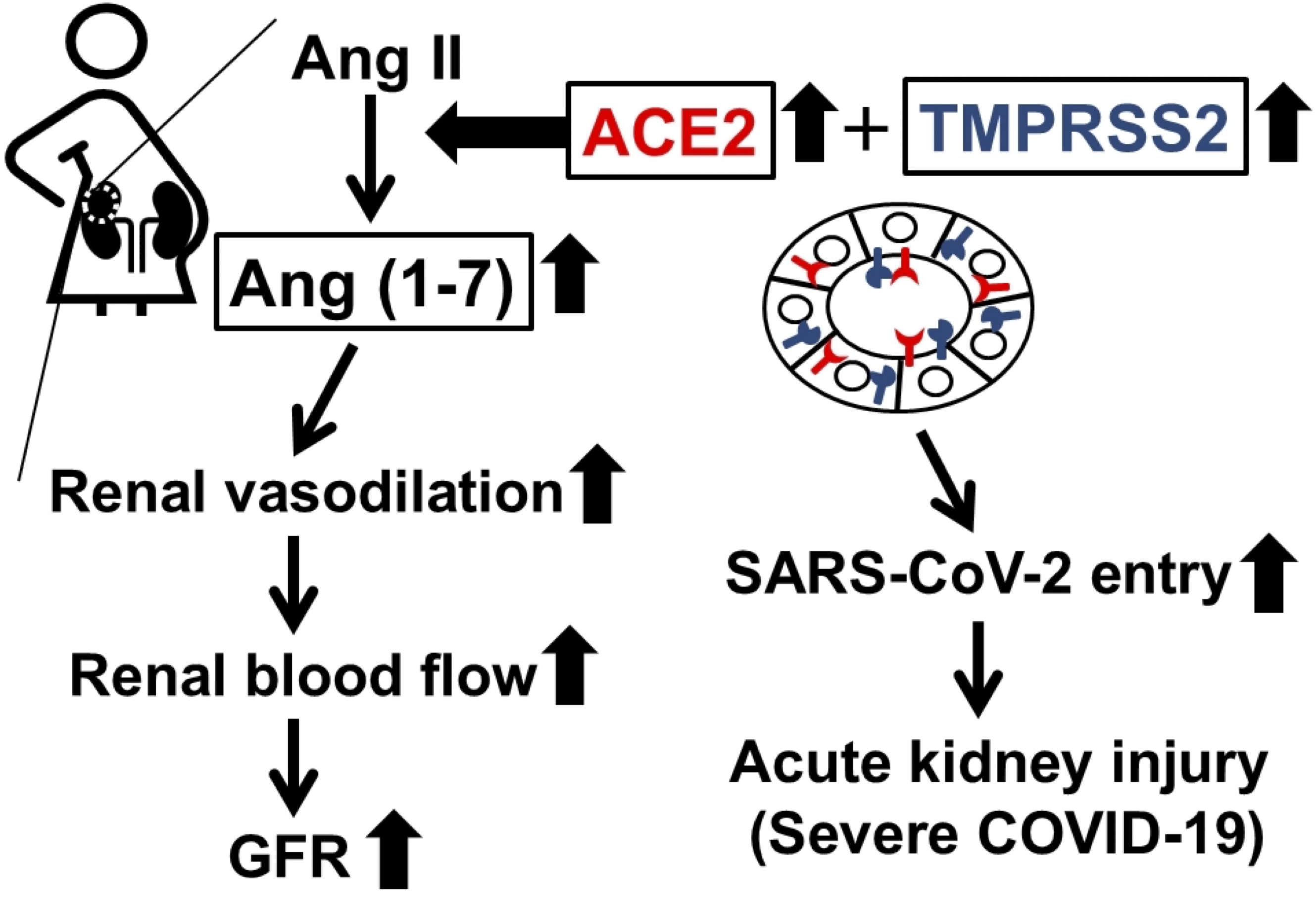
Fig. 5: Physiological and pathological mechanisms by which ACE2 increases the glomerular filtration rate (GFR) during pregnancy and causes acute kidney injury following coronavirus disease 2019 (COVID-19). ACE2 degrades vasoconstrictive angiotensin II and instead produces vasodilative Ang (1-7). Therefore, the increase in ACE2 and Ang (1-7) in the kidneys during pregnancy is considered responsible for renal vasodilation, which subsequently causes an increase in renal blood flow and the glomerular filtration rate (GFR). However, ACE2 also acts as a host cell surface receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during COVID-19. TMPRSS2 activates and facilitates viral entry by cleaving its spike proteins. Therefore, enhanced expression of these proteins in the proximal tubules during pregnancy may be responsible for the development of acute kidney injury following COVID-19 in pregnancy.
Limitations of our study include the semi-quantitative nature of immunohistochemistry and the lack of biochemical validation, such as protein quantification by Western blotting or enzyme-linked immunosorbent assay (ELISA). Additionally, the sample size was relatively small in our study, with n=3 for the pregnant and postpartum group of rats. To overcome these limitations, we carefully executed immunohistochemical analyses and confirmed the consistency and reproducibility of our results in each group of kidney samples. Besides, the percentages of each protein-positive areas, which were averaged from randomly selected, nonoverlapping high-power fields of view (more than 10 views from six virgin rats, three pregnant rats and three postpartum rats, respectively), demonstrated a small statistical variability in each group. However, more quantitative analyses of ACE2, Ang (1-7) and TMPRSS2 expression would be required in the future to strengthen the significance of our findings. Additionally, since sex hormones, such as estrogen, progesterone and androgen [50-53], and systemic inflammatory mediators, such as interleukins, interferons and tumor necrosis factor-a (TNF-a) [54-56], positively or negatively influence ACE2 or TMPRSS2 expression in the serum, lung and placenta during pregnancy, these factors may also be quantitatively analyzed in pregnant kidneys.
Conclusion
In the kidneys of pregnant rats, in addition to renal arterioles and venules, glomerular capillaries are markedly dilated and the Bowman’s capsule is enlarged, indicating renal vasodilation. Immunohistochemistry demonstrated increased ACE2 and Ang (1-7) expression in the proximal renal tubules of pregnant rats. As these proteins exert vasodilatory properties, they are considered responsible for renal vasodilation and the subsequent increase in GFR during pregnancy.
Acknowledgements
We thank the people at Morpho Technology Co. Ltd (Sapporo, Japan) for their technical support.
Author contributions
YK
performed the experiments and analyzed the data. IK designed the
experiments, interpreted the results, and wrote the manuscript. All
the authors have read and approved the final version of the
manuscript.
Funding
This
study was supported by the Tojuro Iijima Foundation for Food Science
and Technology, No. 2023-46, and Miyagi Kidney Foundation Grant of
IK.
Availability of data and materials
The data used to support the findings of this study are available from
the corresponding author upon request.
Ethics approval and consent to participate
This
study was performed in accordance
with the Guide
for the Care
and Use
of Laboratory
Animals
of Miyagi University, which included
ethical considerations.
Consent for publication
Not applicable.
Disclosure Statement
The authors have no competing interests to declare.
References
| 1 | Sanghavi M, Rutherford JD: Cardiovascular physiology of pregnancy. Circulation 2014;130:1003-1008.
https://doi.org/10.1161/CIRCULATIONAHA.114.009029 |
| 2 | Danielson LA, Sherwood OD, Conrad KP: Relaxin is a potent renal vasodilator in conscious rats. J Clin Invest 1999;103:525-533.
https://doi.org/10.1172/JCI5630 |
| 3 | Barbagallo M, Dominguez LJ, Licata G, Shan J, Bing L, Karpinski E et al.: Vascular Effects of Progesterone : Role of Cellular Calcium Regulation. Hypertension 2001;37:142-147.
https://doi.org/10.1161/01.HYP.37.1.142 |
| 4 | Conrad KP: Renal hemodynamics during pregnancy in chronically catheterized, conscious rats. Kidney Int 1984;26:24-29.
https://doi.org/10.1038/ki.1984.129 |
| 5 | Igarashi M, Miyake H, Suzuki S: Hemodynamic changes in maternal renal arteries in twin pregnancy. Gynecol Obstet Invest 2010;69:88-92.
https://doi.org/10.1159/000260331 |
| 6 | Baylis C: Glomerular filtration rate in normal and abnormal pregnancies. Semin Nephrol 1999;19:133-139.
|
| 7 | Baylis C: Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin Obstet Gynaecol 1994;8:235-264.
https://doi.org/10.1016/S0950-3552(05)80320-7 |
| 8 | Kazama I, Matsubara M, Kanai Y, Hatano R, Asano S, Endo Y et al.: Decreased expression of a novel prostaglandin transporter, OAT-PG, facilitates renocortical PGE2 accumulation during rat pregnancy. Gynecol Obstet Invest 2013;76:163-170.
https://doi.org/10.1159/000353977 |
| 9 | Lumbers ER, Pringle KG: Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol 2014;306:R91-101.
https://doi.org/10.1152/ajpregu.00034.2013 |
| 10 | Lin H, Geurts F, Hassler L, Batlle D, Mirabito Colafella KM, Denton KM et al.: Kidney Angiotensin in Cardiovascular Disease: Formation and Drug Targeting. Pharmacol Rev 2022;74:462-505.
https://doi.org/10.1124/pharmrev.120.000236 |
| 11 | Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M et al.: The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol Rev 2018;98:505-553.
https://doi.org/10.1152/physrev.00023.2016 |
| 12 | Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J: Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol 2009;7:79.
https://doi.org/10.1186/1477-7827-7-79 |
| 13 | Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ et al.: Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res 2020;126:1456-1474.
https://doi.org/10.1161/CIRCRESAHA.120.317015 |
| 14 | Khoury EE, Knaney Y, Fokra A, Kinaneh S, Azzam Z, Heyman SN et al.: Pulmonary, cardiac and renal distribution of ACE2, furin, TMPRSS2 and ADAM17 in rats with heart failure: Potential implication for COVID-19 disease. J Cell Mol Med 2021;25:3840-3855.
https://doi.org/10.1111/jcmm.16310 |
| 15 | Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK et al.: ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J 2020;55:
https://doi.org/10.1101/2020.03.18.20038455 |
| 16 | Kazama I: Targeting ACE2 as a potential prophylactic strategy against COVID-19-induced exacerbation of chronic kidney disease. Inflamm Res 2022;71:1123-1126.
https://doi.org/10.1007/s00011-022-01619-6 |
| 17 | Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA et al.: Pregnancy and COVID-19. Physiol Rev 2021;101:303-318.
https://doi.org/10.1152/physrev.00024.2020 |
| 18 | Bajpai D, Shah S: COVID-19 Pandemic and Pregnancy in Kidney Disease. Adv Chronic Kidney Dis 2020;27:397-403.
https://doi.org/10.1053/j.ackd.2020.08.005 |
| 19 | Chopra S, Syal A, Arya Y: Acute kidney injury in COVID-19: Considerations in pregnancy. Tzu Chi Med J 2022;34:29-34.
https://doi.org/10.4103/tcmj.tcmj_290_20 |
| 20 | Kazama I, Baba A, Endo Y, Toyama H, Ejima Y, Matsubara M et al.: Mast cell involvement in the progression of peritoneal fibrosis in rats with chronic renal failure. Nephrology (Carlton) 2015;20:609-616.
https://doi.org/10.1111/nep.12489 |
| 21 | Baba A, Tachi M, Ejima Y, Endo Y, Toyama H, Saito K et al.: Less contribution of mast cells to the progression of renal fibrosis in Rat kidneys with chronic renal failure. Nephrology (Carlton) 2017;22:159-167.
https://doi.org/10.1111/nep.12733 |
| 22 | Rasch R, Lauszus F, Thomsen JS, Flyvbjerg A: Glomerular structural changes in pregnant, diabetic, and pregnant-diabetic rats. APMIS 2005;113:465-472.
https://doi.org/10.1111/j.1600-0463.2005.apm_587.x |
| 23 | Baylis C, Rennke HG: Renal hemodynamics and glomerular morphology in repetitively pregnant aging rats. Kidney Int 1985;28:140-145.
https://doi.org/10.1038/ki.1985.133 |
| 24 | Mitani S, Yabuki A, Sawa M, Chang HS, Yamato O: Intrarenal distributions and changes of Angiotensin-converting enzyme and Angiotensin-converting enzyme 2 in feline and canine chronic kidney disease. J Vet Med Sci 2014;76:45-50.
https://doi.org/10.1292/jvms.13-0314 |
| 25 | Chueh TI, Zheng CM, Hou YC, Lu KC: Novel Evidence of Acute Kidney Injury in COVID-19. J Clin Med 2020;9:
https://doi.org/10.3390/jcm9113547 |
| 26 | Joyner J, Neves LA, Granger JP, Alexander BT, Merrill DC, Chappell MC et al.: Temporal-spatial expression of ANG-(1-7) and angiotensin-converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol 2007;293:R169-177.
https://doi.org/10.1152/ajpregu.00387.2006 |
| 27 | Brosnihan KB, Neves LA, Anton L, Joyner J, Valdes G, Merrill DC: Enhanced expression of Ang-(1-7) during pregnancy. Braz J Med Biol Res 2004;37:1255-1262.
https://doi.org/10.1590/S0100-879X2004000800017 |
| 28 | Munoz MC, Burghi V, Miquet JG, Giani JF, Banegas RD, Toblli JE et al.: Downregulation of the ACE2/Ang-(1-7)/Mas axis in transgenic mice overexpressing GH. J Endocrinol 2014;221:215-227.
https://doi.org/10.1530/JOE-13-0497 |
| 29 | Dong M, Zhang J, Ma X, Tan J, Chen L, Liu S et al.: ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother 2020;131:110678.
https://doi.org/10.1016/j.biopha.2020.110678 |
| 30 | Tamanna S, Lumbers ER, Morosin SK, Delforce SJ, Pringle KG: ACE2: a key modulator of the renin-angiotensin system and pregnancy. Am J Physiol Regul Integr Comp Physiol 2021;321:R833-R843.
https://doi.org/10.1152/ajpregu.00211.2021 |
| 31 | Levy A, Yagil Y, Bursztyn M, Barkalifa R, Scharf S, Yagil C: ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol 2008;295:R1953-1961.
https://doi.org/10.1152/ajpregu.90592.2008 |
| 32 | Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R et al.: Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension 2003;42:749-753.
https://doi.org/10.1161/01.HYP.0000085220.53285.11 |
| 33 | Simoes e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM: ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol 2013;169:477-492.
https://doi.org/10.1111/bph.12159 |
| 34 | Prieto MC, Gonzalez-Villalobos RA, Botros FT, Martin VL, Pagan J, Satou R et al.: Reciprocal changes in renal ACE/ANG II and ACE2/ANG 1-7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol 2011;300:F749-755.
https://doi.org/10.1152/ajprenal.00383.2009 |
| 35 | Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H et al.: Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension 2011;57:314-322.
https://doi.org/10.1161/HYPERTENSIONAHA.110.164244 |
| 36 | Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, Burrell LM: From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol 2014;5:227.
https://doi.org/10.3389/fphys.2014.00227 |
| 37 | Loutzenhiser K, Loutzenhiser R: Angiotensin II-induced Ca(2+) influx in renal afferent and efferent arterioles: differing roles of voltage-gated and store-operated Ca(2+) entry. Circ Res 2000;87:551-557.
https://doi.org/10.1161/01.RES.87.7.551 |
| 38 | Ichikawi I, Harris RC: Angiotensin actions in the kidney: renewed insight into the old hormone. Kidney Int 1991;40:583-596.
https://doi.org/10.1038/ki.1991.249 |
| 39 | Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K et al.: Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol 2021;17:751-764.
https://doi.org/10.1038/s41581-021-00452-0 |
| 40 | Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA et al.: Acute Kidney Injury in COVID-19: Emerging Evidence of a Distinct Pathophysiology. J Am Soc Nephrol 2020;31:1380-1383.
https://doi.org/10.1681/ASN.2020040419 |
| 41 | Hassanein M, Radhakrishnan Y, Sedor J, Vachharajani T, Vachharajani VT, Augustine J et al.: COVID-19 and the kidney. Cleve Clin J Med 2020;87:619-631.
https://doi.org/10.3949/ccjm.87a.20072 |
| 42 | Krishnamurthy S, Lockey RF, Kolliputi N: Soluble ACE2 as a potential therapy for COVID-19. Am J Physiol Cell Physiol 2021;320:C279-C281.
https://doi.org/10.1152/ajpcell.00478.2020 |
| 43 | Alhenc-Gelas F, Drueke TB: Blockade of SARS-CoV-2 infection by recombinant soluble ACE2. Kidney Int 2020;97:1091-1093.
https://doi.org/10.1016/j.kint.2020.04.009 |
| 44 | Ahmad I, Pawara R, Surana S, Patel H: The Repurposed ACE2 Inhibitors: SARS-CoV-2 Entry Blockers of Covid-19. Top Curr Chem (Cham) 2021;379:40.
https://doi.org/10.1007/s41061-021-00353-7 |
| 45 | Abubakar MB, Usman D, El-Saber Batiha G, Cruz-Martins N, Malami I, Ibrahim KG et al.: Natural Products Modulating Angiotensin Converting Enzyme 2 (ACE2) as Potential COVID-19 Therapies. Front Pharmacol 2021;12:629935.
https://doi.org/10.3389/fphar.2021.629935 |
| 46 | Taber-Hight E, Shah S: Acute Kidney Injury in Pregnancy. Adv Chronic Kidney Dis 2020;27:455-460.
https://doi.org/10.1053/j.ackd.2020.06.002 |
| 47 | Nath KA, Singh RD, Grande JP, Garovic VD, Croatt AJ, Ackerman AW et al.: Expression of ACE2 in the Intact and Acutely Injured Kidney. Kidney360 2021;2:1095-1106.
https://doi.org/10.34067/KID.0001562021 |
| 48 | Shirazi M, Cianfarini C, Ismail A, Wysocki J, Wang JJ, Ye M et al.: Altered kidney distribution and loss of ACE2 into the urine in acute kidney injury. Am J Physiol Renal Physiol 2024;327:F412-F425.
https://doi.org/10.1152/ajprenal.00237.2023 |
| 49 | Kale A, Shelke V, Sankrityayan H, Dagar N, Gaikwad AB: Klotho restoration via ACE2 activation: A potential therapeutic strategy against acute kidney injury-diabetes comorbidity. Biochim Biophys Acta Mol Basis Dis 2022;1868:166532.
https://doi.org/10.1016/j.bbadis.2022.166532 |
| 50 | Shook LL, Bordt EA, Meinsohn MC, Pepin D, De Guzman RM, Brigida S et al.: Placental Expression of ACE2 and TMPRSS2 in Maternal Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Are Placental Defenses Mediated by Fetal Sex? J Infect Dis 2021;224:S647-S659.
https://doi.org/10.1093/infdis/jiab335 |
| 51 | Stelzig KE, Canepa-Escaro F, Schiliro M, Berdnikovs S, Prakash YS, Chiarella SE: Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2020;318:L1280-L1281.
https://doi.org/10.1152/ajplung.00153.2020 |
| 52 | Wang ZP, Hua M, Jiu T, Ge RL, Bai Z: Biofunctional roles of estrogen in coronavirus disease 2019: Beyond a steroid hormone. Front Pharmacol 2022;13:1003469.
https://doi.org/10.3389/fphar.2022.1003469 |
| 53 | Baratchian M, McManus JM, Berk MP, Nakamura F, Mukhopadhyay S, Xu W et al.: Androgen regulation of pulmonary AR, TMPRSS2 and ACE2 with implications for sex-discordant COVID-19 outcomes. Sci Rep 2021;11:11130.
https://doi.org/10.1038/s41598-021-90491-1 |
| 54 | Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN et al.: SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020;181:1016-1035 e1019.
|
| 55 | Sajuthi SP, DeFord P, Li Y, Jackson ND, Montgomery MT, Everman JL et al.: Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun 2020;11:5139.
https://doi.org/10.1038/s41467-020-18781-2 |
| 56 | Gkogkou E, Barnasas G, Vougas K, Trougakos IP: Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol 2020;36:101615.
https://doi.org/10.1016/j.redox.2020.101615 |