Dapagliflozin, An SGLT2 Inhibitor, Improves Endothelial Cell Energy Metabolism Through Enhanced Mitochondrial Respiration
bDepartment of Clinical Physiology, The Centre of Postgraduate Medical Education, Warsaw, Poland,
cDepartment of Cardiac Diagnostics, Medical University of Gdansk, Gdańsk, Poland,
dCentre of Experimental Cardiooncology, Medical University of Gdansk, Gdańsk, Poland
Keywords
Abstract
Background/Aims:
Flozins (sodium-glucose cotransporter 2 inhibitors, SGLT2i) are a new class of antidiabetic drugs that reduce cardiovascular mortality and hospitalization rates in heart failure, regardless of type 2 diabetes status. Besides lowering glycemia by inhibiting renal glucose reabsorption, SGLT2 inhibitors may exert sodium-dependent hemodynamic effects and improve cardiomyocyte energy metabolism, substrate preference, and mitochondrial function. However, their impact on endothelial cells remains largely unknown. This study aimed to analyse the effects and mechanisms of SGLT2i on endothelial cell metabolism and function.Methods:
Mouse cardiac endothelial cells (H5V) were used to test the impact of dapagliflozin on endothelial cell metabolism and function in the presence of hypoxia-mimicking conditions. The concentration of intracellular nucleotides was measured using high-performance liquid chromatography. Mitochondrial and glycolytic activity were assessed using Seahorse XFp, while nitric oxide (NO) production was determined by 4-Amino-5-Methylamino-2’,7’-Difluorofluorescein (DAF-FM) fluorescence staining. The effects of dapagliflozin treatment on endothelial NO synthesis were also analysed in patients with chronic heart failure and left ventricular ejection fraction above 40% and C57Bl/6J mice.Results:
Dapagliflozin augmented adenosine triphosphate (ATP) levels and the ATP/ADP (adenosine diphosphate) ratio in cultured endothelial cells correlated to increased NO production. Dapagliflozin-treated endothelial cells produced ATP through both mitochondrial respiration and glycolysis. Interestingly, mitochondrial respiration was enhanced, while glycolysis was unaffected in endothelial cells after in vitro dapagliflozin treatment. In a murine model, dapagliflozin doubled the rate of coronary NO synthesis and tended to improve coronary capillary density. In humans with chronic heart failure, 3-month treatment with dapagliflozin revealed many metabolic effects, suggesting potential mechanisms related to nitric oxide homeostasis, mitochondrial function, and L-arginine metabolism.Conclusion:
This study demonstrated the beneficial effect of dapagliflozin on endothelial cell metabolism and function. Regulation of endothelial cell bioenergetics may be an undervalued mechanism of SGLT2i to delay heart failure progression and support cardiac regeneration. These may accelerate endothelial-targeted strategies to support heart failure treatment.Introduction
Cardiovascular diseases, particularly heart failure (HF), represent a significant global health burden, contributing to high rates of morbidity and mortality [1]. HF can result from a variety of underlying conditions, mainly coronary obstructive atherosclerosis and coronary microvascular dysfunction. Coronary obstructive atherosclerosis is characterized by the build-up of plaques within the large coronary arteries, leading to reduced blood flow to the myocardium and subsequent ischemic damage. In contrast, coronary microvascular dysfunction affects the smaller vessels that penetrate the heart muscle, impairing blood flow regulation at the microvascular level [2]. Given the prevalence of cardiovascular disease, the search for new treatments is important.
Sodium-glucose cotransporter 2 inhibitors (SGLT2i), which are antidiabetic drugs, such as dapagliflozin (DAPA), have emerged as promising cardioprotective agents, significantly reducing HF hospitalizations and cardiovascular mortality, even in non-diabetic patients [3, 4]. Beyond glucose-lowering effects, these drugs exhibit anti-inflammatory, hemodynamic, and metabolic benefits, but their exact molecular mechanisms remain incompletely understood [5]. In addition, the impact of SGLT2 inhibitors on the metabolism and function of endothelial cells is still largely unexplored.
In healthy conditions, endothelial cells produce nitric oxide (NO), a potent vasodilator that helps to maintain vessel elasticity and prevents platelet aggregation [6]. However, in the presence of risk factors such as hypertension, hyperlipidemia, and diabetes, endothelial function becomes impaired, leading to reduced NO production, increased oxidative stress, and a pro-inflammatory state [7, 8]. Research suggests that flozins can counteract these factors through multiple molecular pathways [9]. Endothelial dysfunction is a key contributor to both HF and atherosclerosis, by disrupting vascular homeostasis, blood flow regulation, and inflammation control [10].
Considering the central role of endothelial cells in cardiovascular health, it is crucial to understand how SGLT2 inhibitors like dapagliflozin directly affect the endothelium. This study aimed to analyse dapagliflozin’s effects and potential mechanisms on endothelial metabolism and function.
Materials and Methods
Human participants
This study enrolled 13 adult patients with symptomatic chronic HF (class NYHA II or III) with preserved ejection fraction (HFpEF) or mid-range ejection fraction (HFmrEF). All participants were recruited from the Cardiology Outpatient Clinic at the University Hospital in Gdansk. All patients received dapagliflozin at a dose of 10 mg per day, in addition to the therapy for HF. Blood samples for biochemical analysis to determine the concentration of amino acids and stable NO metabolites were obtained before and after three months of adding dapagliflozin. To obtain plasma, 4 ml of blood was drawn into tubes with sodium heparin. The tube contents were centrifuged at 1200 × g for 15 minutes at 21°C. The plasma was transferred and preserved at -80°C until analysis.
Table 1 summarizes the characteristics of the study group. Written informed consent was obtained from all human participants under the Declaration of Helsinki, and the study was performed following guidelines approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdansk, Poland (approval No NKBBN/208/2023).
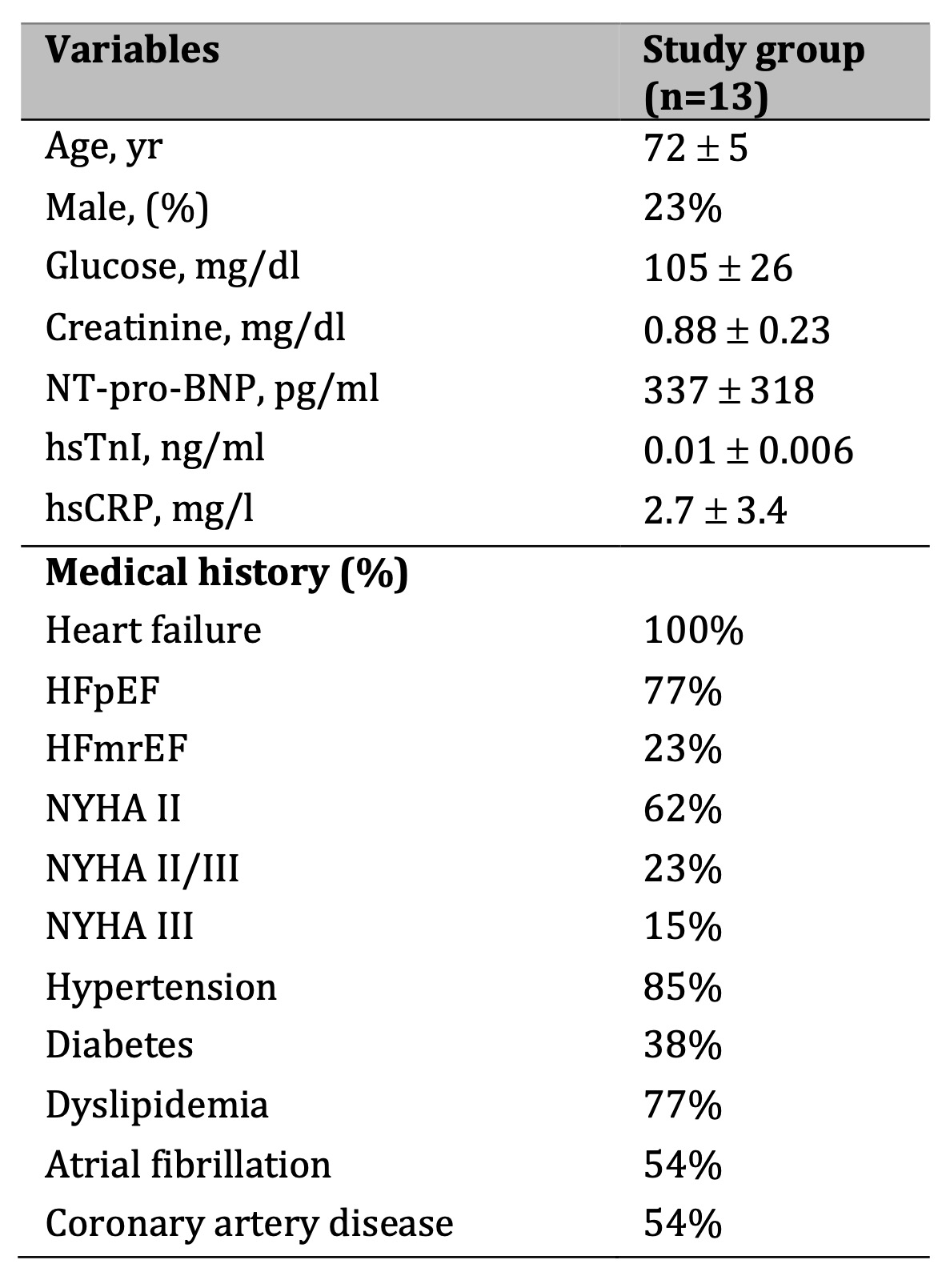
Table 1: Patient characteristics. Results are shown as mean ± SD. Abbreviations: BNP – B type natriuretic peptide, HFmrEF – heart failure with mid-range ejection fraction, HFpEF – heart failure with preserved ejection fraction, hsCRP – high sensitive C-reactive protein, hsTnI – High sensitive troponin I, NT-pro-BNP - N-terminal pro-B-type natriuretic peptide, NYHA - New York Heart Association Functional Classification for Heart Failure.
Animals
All experiments were performed in 8-12-week-old female C57BL/6 mice obtained from the Animal House of the Medical Research Center, Polish Academy of Sciences (Warsaw, Poland). A total of 12 mice were included. Animals were housed in controlled conventional environmental conditions at the animal facility of the Medical Centre for Postgraduate Education in Warsaw, with water and food provided ad libitum. The experiments were performed following the guidelines approved by the 2nd Local Ethics Committee in Warsaw (approvals No WAW2/003/2021 and WAW2/152/2023), and following the requirements of the EU (Directive 2010/63/EU) and Polish (Dz. U. poz. 266/15.01.2015) legislation.
Dapagliflozin (Bristol-Myers Squibb/AstraZeneca EEIG) was given at 35 mg/kg dissolved in tap water. Six mice were given pure drinking water, and six were given drinking water with dapagliflozin for six weeks. Mice in both groups drank the same volume of water (6.0 and 5.9 ml, respectively).
Intravital microscopy imaging of coronary arteries
Mice were anesthetized with ketamine and xylazine (100 mg and 5 mg/kg body weight, respectively, intraperitoneal). The trachea was exposed microsurgically, and animals were put on a ventilator and ventilated with a tidal volume of 250 µl. The chest was opened, and the heart was exposed; subsequently, the mouse was inoculated into the left ventricle (LV) with 5 µg of Alexa Fluor® 647 anti-mouse CD31 Antibody (BioLegend, cat. no. 102416) to outline the contours of blood vessels. A suction cardiac camera (with suction pressure of approximately 80 mmHg) was used to provide stabilization of a beating heart, and live imaging of epicardial coronary vessels was performed with an intravital microscope (IVM-CMS3, IVIM Technology, Seoul, Korea). Magnification ×20 was used. The conventional field of vision was 500 µm × 500 µm, and the depth of the signal was 20 µm. After confirmation of positive anti-CD31 staining (visibility of cardiac capillaries), arteriolar and venular diameters were recorded. Subsequently, capillaries were counted in at least 3 different (LV) sites, and their number was averaged. The final result was expressed as the number of capillaries (estimated in long axis view) per 500 µm (determined by the signal penetration depth, corresponding to a cross-section area of 10 mm2). Mice’s body temperature was monitored and maintained at 37ºC during the procedure. Then, diaminofluorescein-2 diacetate (DAF2-DA), a cell-permeable nitric oxide probe that is hydrolyzed to DAF2 by intracellular esterases, was injected into LV to visualize NO production (24 µmol in 0.5 ml PBS). Imaging was performed immediately at 3 different areas of LV. In selected experiments, N(gamma)-nitro-L-arginine methyl ester (L-NAME, Merck, Germany), 50 mg/kg dissolved in 1 ml PBS was injected over 5 minutes into the LV before DAF2-DA staining. NO production was measured as mean fluorescence intensity in capillaries, small vessels (<50 µm diameter), and intermediate vessels (>50 µm diameter) at depths 20 to 30 µm to minimize signal loss associated with the depth of imaging. Image analysis was performed using ImageJ software (Version 2.14, NIH, USA).
Cell culture and treatment
Mouse cardiac endothelial cells (H5V) were obtained from the Department of Cell Biology and Immunology, Institute of Medical Biotechnology and Experimental Oncology, Intercollegiate Faculty of Biotechnology UG and MUG. Cells were cultured at 37°C in 5% CO2 in DMEM medium (Merck, Germany) supplemented with 10% fetal bovine serum (FBS, ThermoFisher Scientific, USA). Before the experiments were performed, the cells were seeded onto the appropriate plates. After reaching 80% confluence, the plate’s culture medium was changed to medium with 1% fetal bovine serum 24 hours before the experiment. Cells in the respective wells were treated with dapagliflozin (cat. No. SML2804, Merck, Germany) stock solutions in dimethyl sulfoxide (DMSO, cat. No. 25-950-CQC, Corning, USA) to reach a final concentration of 100 nM or 1 μM. The vehicle (DMSO) was added to the remaining wells to reach a 0.5% (v/v) final concentration. In addition, to mimic hypoxia conditions, cobalt chloride (CoCl2) reconstituted in sterile water was added to a portion of the cell plates to reach a final concentration of 100 μM.
For specific experiments, antimycin A (cat. no. A8674, Merck, Germany), oligomycin (cat. no. O4876, Merck, Germany), iodoacetate (cat. no. I2512, Merck, Germany), and 2-deoxyglucose (cat. no. D6134, Merck, Germany) were administered to treated cells 2 hours before the experimental endpoint. Stock solutions of antimycin A and oligomycin were prepared in DMSO, while phosphate-buffered saline (PBS) was used as a solvent for iodoacetate and 2-deoxyglucose. The final working concentrations in the culture wells were as follows: antimycin A at 5 μM, oligomycin at 10 μg/mL, iodoacetate at 40 μM, and 2-deoxyglucose at 10 μM.
Quantification of Plasma Amino Acids Using LC-MS
Liquid chromatography/mass spectrometry (LC/MS) was used to analyze serum amino acid concentrations as previously described [11]. Sample preparation involved the extraction of 25 µL of plasma with 5 µL of internal standards using 70 µL of acetonitrile (ACN) for 20 minutes on ice, followed by centrifugation at 1400 rpm at 4°C for 15 minutes. The resulting supernatants were collected and evaporated to dryness. At the same time, the precipitate was reconstituted in 25 µL of distilled water, extracted with 75 µL of methanol, and centrifuged to obtain additional supernatants, which were subsequently dried. Before analysis, the dried residues were reconstituted in 25 µL of distilled water and subjected to ion-pair high-performance liquid chromatography (HPLC) with mass detection using positive-mode electrospray ionization. Following established methods, individual amino acids were identified based on their molecular weight, chromatographic retention time, and fragmentation pattern.
Quantification of Plasma Nitrites and Nitrates Concentration
Serum nitrites and nitrates concentrations were determined using the nitrate/nitrite assay kit (Cayman Chemicals, No. 780001) according to the manufacturer’s protocol. The nitrate/nitrite colorimetric assay kit provides a reliable and efficient method for quantifying total nitrate and nitrite concentrations through a two-step enzymatic and chemical reaction process utilizing a nitrite standard. Initially, nitrate is enzymatically reduced to nitrite by nitrate reductase. Subsequently, adding Griess reagents facilitates the conversion of nitrite into a stable azo compound with a distinct deep purple coloration. The concentration of nitrite (NO₂⁻) is then precisely quantified through photometric absorbance measurement of the azo chromophore at 550 nm, ensuring accurate determination of nitric oxide metabolites.
Quantification of Nucleotide and Their Catabolites Concentration Using RP-HPLC
After treatment, the medium was removed from above the cells. The cell monolayer was washed twice with PBS solution, and then 0.4 mol/L of pre-cooled perchloric acid (HClO4) (300 μL) was added. The plate was wrapped in parafilm and frozen at -80 °C. After 24 hours, the plate was thawed on ice and then refrozen. Upon thawing again, the supernatant was collected into Eppendorf tubes and centrifuged (20, 800 × g, 4 °C, 10 min). The precipitate was removed, and the supernatant was titrated to pH 6 with potassium phosphate 3 M (K3PO4). All samples were then centrifuged again (20, 800 × g, 4 °C, 10 min), and the supernatant was used to analyze nucleotide concentrations by reverse-phase high-performance liquid chromatography (HPLC) [12]. As adenosine 5′-diphosphoribose (ADPR) is the major product formed by acidic cleavage of NADH during the tissue extraction, the measured ADPR level reflected NADH concentration in our experimental setting [13]. The results were demonstrated as nmol/mg protein.
Mitochondrial Function Analysis
To assess mitochondrial function in H5V cells, OCR was measured using a Seahorse XFp analyzer (Agilent, USA). Cells were seeded in XFp 8-well microplates (5 × 104 cells/well) in DMEM complete medium and treated as described above. After 24h of treatment, the medium was removed from the microplates, and cells were washed and incubated in 180 μL of Seahorse DMEM medium (supplemented with 1 mM pyruvate, 2 mM glutamine, and 10 mM glucose) at 37°C for 45 min. Then, microplates were transferred to the analyzer and Oligomycin (inhibitor of ATP-synthase), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP; mitochondrial uncoupler), and a mix of rotenone (a complex I inhibitor) and antimycin A (a complex III inhibitor) were sequentially injected to a final concentration of 1.5 μM, 1.0 μM and 0.5 μM, respectively. Oxygen consumption rate (OCR) was recorded throughout the assay, and basal respiration, ATP production, proton leak, maximal respiration, spare capacity, and non-mitochondrial respiration were calculated. OCR values were normalized to protein concentration. All parameters were determined from the averages of the three OCR measurements following the injection of the relevant modulators of mitochondrial respiration. Non-mitochondrial oxygen consumption was shown as OCR after rotenone and antimycin A. Basal respiration was calculated as the difference between basal OCR before and after oligomycin injection. ATP production was determined as the difference between basal respiration and OCR after oligomycin injection, while proton leak was determined as the difference between OCR after oligomycin and non-mitochondrial oxygen consumption. The difference between OCR after FCCP injection and non-mitochondrial respiration was used to calculate maximal respiration. Spare capacity was determined as the difference between maximal respiration and basal respiration.
Glycolysis Stress Test
To assess glycolysis function in H5V cells, extracellular acidification rate (ECAR) was measured using a Seahorse XFp analyzer (Agilent, USA). Cells were seeded in XFp 8-well microplates (5 × 104 cells/well) in DMEM complete medium and treated as described above. After 24h of treatment, the medium was removed from the microplates, and cells were washed and incubated in 180 μL of Seahorse Dulbecco′s Modified Eagle′s (DMEM) medium (supplemented with 2 mM glutamine) at 37°C for 45 min. Then, microplates were transferred to the analyser, and glucose, oligomycin (inhibitor of ATP-synthase), and 2-deoxy-glucose (2-DG - a glucose analog that inhibits glycolysis through competitive binding to hexokinase) were sequentially injected to a final concentration of 10 mM, 1.0 μM, and 50 mM, respectively. ECAR was recorded throughout the assay, and glycolysis, glycolytic capacity, glycolytic reserve, and nonglycolytic acidification were calculated. ECAR values were normalized to protein concentration. All parameters were determined from the averages of the three ECAR measurements after injecting the relevant substances. Non-glycolytic acidification was shown as ECAR after 2-DG injection, while glycolysis was calculated as a difference in ECAR between glucose and 2-DG injections. Glycolytic capacity was calculated as the difference between ECAR following the injection of oligomycin and the basal ECAR reading. Glycolytic reserve was determined as the difference in ECAR between glucose and oligomycin injections.
Immunofluorescence staining
Immunofluorescent staining of real-time NO production in H5V cells plated in 96-well plates (Corning, NY, USA). Treated as described above, cells were washed twice with DMEM medium without FBS. Then, cells were incubated with 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI, 1:1000, cat. No. MBD0015, Merck, Germany) for 5 min They were washed twice with DMEM medium without FBS. It was followed by incubation with DAF-FM diacetate at 10 μM final concentration for 45 min. Then, cells were washed twice, and a fresh medium was added. After 15 minutes, images were captured and analysed using a fluorescence microscope (Zeiss, Dresden, Germany) and ZEN software v.3.3 blue edition (Zeiss, Dresden, Germany).
Quantification of Cell Protein Content
Following the experiments, cell protein from relevant plates was dissolved in 0.5 M sodium hydroxide (NaOH) and quantified using the Bradford method, following the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was conducted using InStat software (GraphPad, San Diego, CA, USA). Initially, normality tests were carried out to assess the distribution. Mean values between groups were compared using either an unpaired Student’s t-test or one-way ANOVA followed by the Holm-Sidak post-hoc test, where all p-values were adjusted for multiple comparisons, as suitable. Error bars represented the standard error of the mean (SEM). The exact value of (n) was specified for each experiment. Statistical significance was defined as p ≤ 0.05.
Results
apagliflozin improved nucleotide concentration in cardiac endothelial cells in vitro
Intending to investigate how dapagliflozin affects the energy metabolism of endothelial cells, we determined the intracellular nucleotide concentration in these cells in both control conditions and after stimulation with CoCl2 (Fig. 1). We noticed a dose-dependent growing trend in adenine nucleotide concentration in control conditions. Dapagliflozin also improved intracellular ATP and total adenine nucleotide pool (TAN) concentration in endothelial cells, which were additionally stimulated with hypoxia-mimetic CoCl2. Interestingly, dapagliflozin treatment has decreased the ATP/ADP ratio in control conditions, while after stimulation with CoCl2, an increase in this ratio was observed.
To determine the importance of mitochondrial respiration and glycolysis in dapagliflozin-treated endothelial cells, we assessed the intracellular concentrations of adenine nucleotides and nicotinamide dinucleotides after dapagliflozin treatment under hypoxia-mimicking conditions with the addition of antimycin A, oligomycin, iodoacetate, and 2’deoxyglucose. These results revealed that endothelial cells treated with dapagliflozin produced ATP through mitochondrial respiration and glycolysis (Fig. 2).
In addition, dapagliflozin-treated endothelial cells had a lower oxidized nicotinamide adenine dinucleotide/reduced nicotinamide adenine dinucleotide (NAD+/NADH) ratio (Fig. 3), but only when co-treated with mitochondrial respiration and ATP synthase inhibitors (antimycin A, oligomycin).
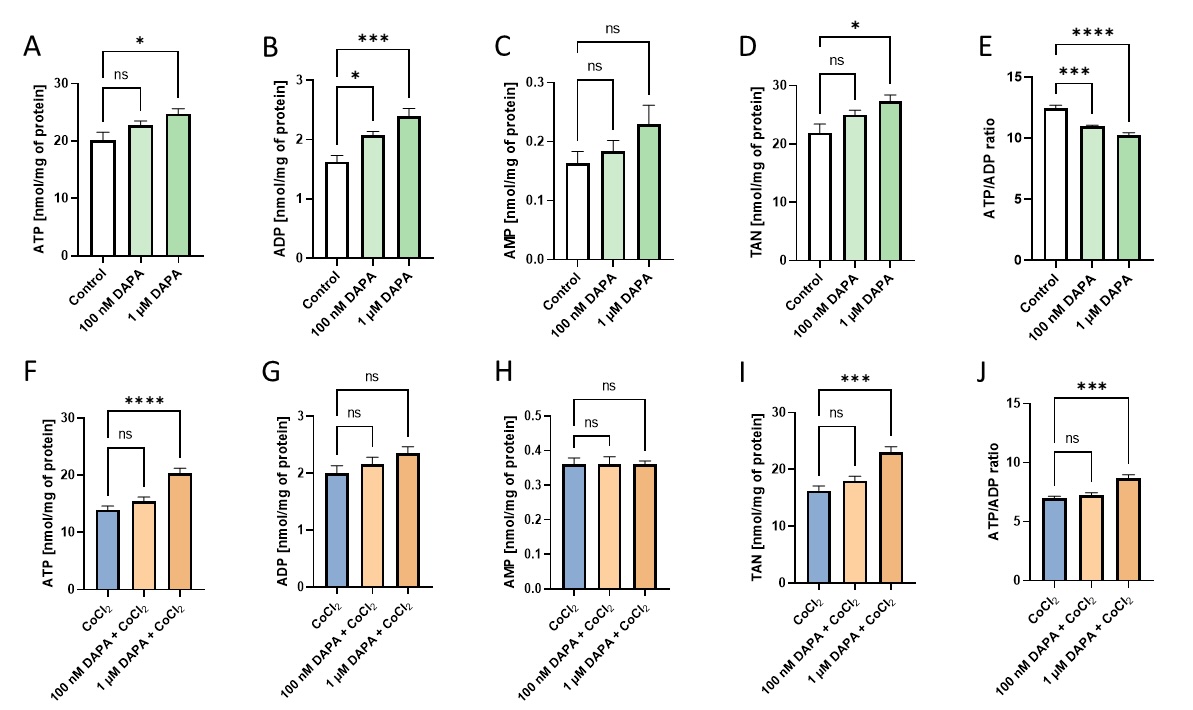
Fig. 1: Dapagliflozin treatment increased adenine nucleotides in cardiac endothelial cells. The intracellular concentration of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), total adenine nucleotide pool (TAN), and intracellular ATP/ADP ratio in mouse cardiac endothelial cells (H5V) after 24h dapagliflozin treatment in control conditions and after stimulation with hypoxia-mimetic CoCl2. Results are shown as mean ± SEM; n = 6; * p < 0.05; ** p < 0.01; *** p < 0.001, **** p < 0.0001; ns = not significant.
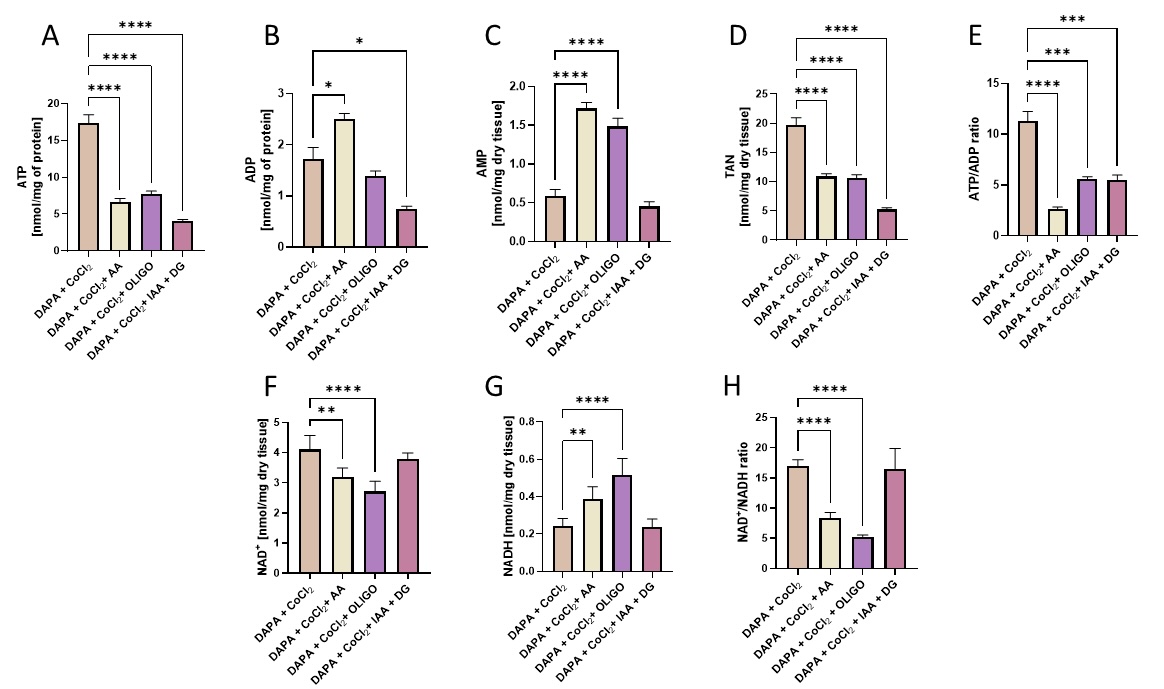
Fig. 2: Dapagliflozin-treated cardiac endothelial cells produced ATP via both mitochondrial oxidative phosphorylation and glycolysis and had a lower NAD+/NADH ratio when co-treated with mitochondrial respiration and ATP synthase inhibitors. The intracellular concentration of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), total adenine nucleotide pool (TAN), oxidized nicotinamide adenine dinucleotide (NAD+), reduced nicotinamide adenine dinucleotide (NADH), and intracellular ATP/ADP and NAD+/NADH ratios in mouse cardiac endothelial cells (H5V) after 24h dapagliflozin treatment in control conditions and after stimulation with hypoxia-mimetic CoCl2. Antimycin A (AA), oligomycin (OLIGO), and iodoacetate + 2’deoxyglucose (IAA+ DG) were added for the last 2h of incubation. Results are shown as mean ± SEM; n = 6-10; * p < 0.05; ** p < 0.01; *** p < 0.001, **** p < 0.0001.
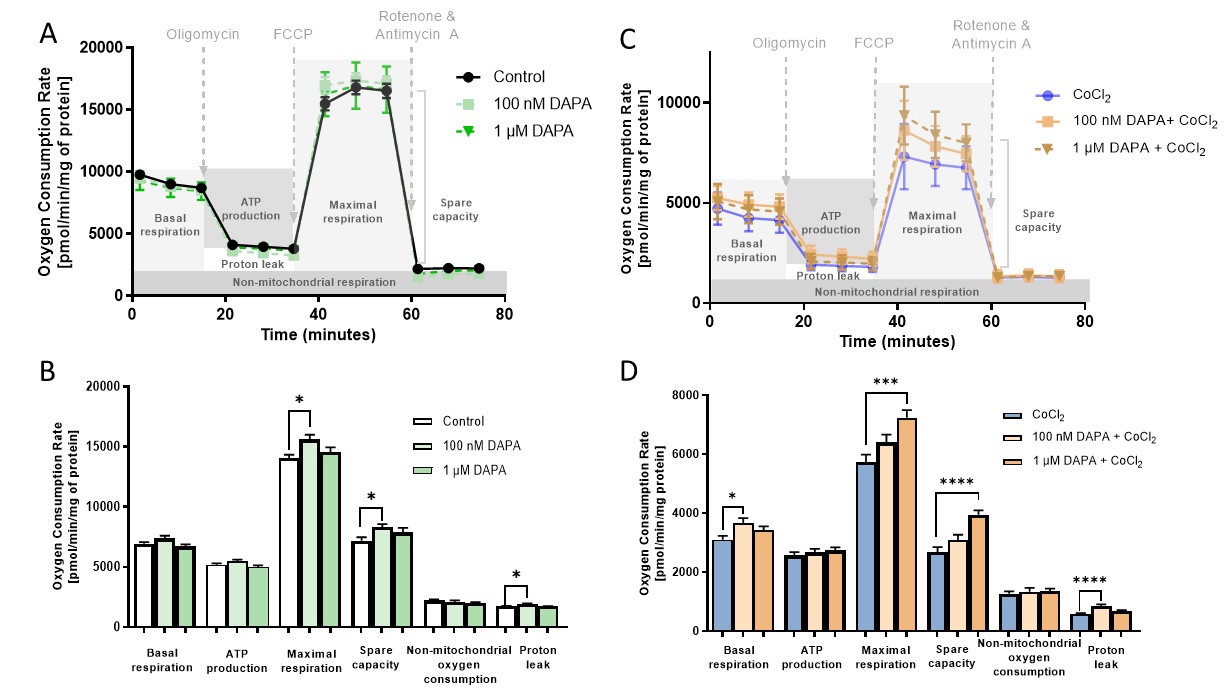
Fig. 3: Dapagliflozin treatment enhanced the mitochondrial respiration in cardiac endothelial cells. Oxygen consumption rate (OCR) assessed with Seahorse XFp Cell Mito Stress Test after sequential injection of oligomycin, FCCP (Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone), and rotenone with antimycin A in mouse cardiac endothelial cells (H5V) after 24h dapagliflozin treatment in control conditions after stimulation with hypoxia-mimetic CoCl2. Parameters of mitochondrial respiration represent basal respiration, ATP production, maximal respiration, spare capacity, non-mitochondrial oxygen production, and proton leak. Results are shown as mean ± SEM; n = 6; * p < 0.05; ** p < 0.01; *** p < 0.001, **** p < 0.0001; ns = not significant.
Dapagliflozin enhanced mitochondrial respiration but not glycolysis in cardiac endothelial cells in vitro
To elucidate the functional impact of dapagliflozin on the bioenergetics of endothelial cells, we examined mitochondrial respiration in H5V cells after treatment with dapagliflozin in control and hypoxia-mimicking conditions (Fig. 4). Endothelial cells treated with dapagliflozin in normal conditions revealed increased parameters of mitochondrial respiration such as maximal respiration, spare capacity, and proton leak compared to the control. In addition, dapagliflozin treatment under hypoxia-mimicking conditions improved basal and maximal respiration, spare capacity, and proton leak.
Then, we analysed the glycolytic parameters after treating H5V cells with dapagliflozin and demonstrated no changes in control conditions (Fig. 5). In turn, under hypoxia-mimetic conditions, a decreased dose-dependent trend was observed to decrease glycolysis and glycolytic capacity after dapagliflozin.
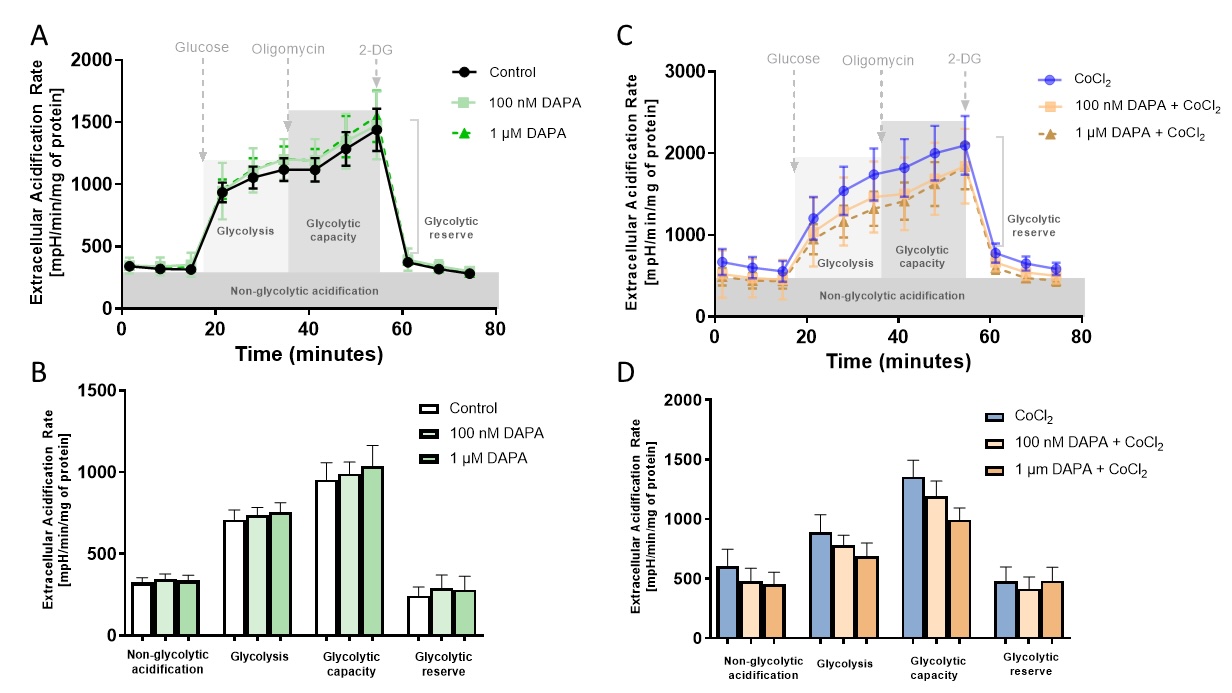
Fig. 4: Dapagliflozin treatment did not affect the rate of glycolysis in cardiac endothelial cells. Extracellular acidification (ECAR) was assessed with Seahorse XFp Glycolysis Stress Test after sequential injection of glucose, oligomycin, and 2-deoxyglucose in mouse cardiac endothelial cells (H5V) after 24h of dapagliflozin treatment in control conditions, after stimulation with hypoxia-mimetic CoCl2. The parameters of the glycolytic stress test represent non-glycolytic acidification, glycolysis, glycolytic capacity, and glycolytic reserve. Results are shown as mean ± SEM; n = 6; * p < 0.05; ** p < 0.01; **** p < 0.0001; ns = not significant.
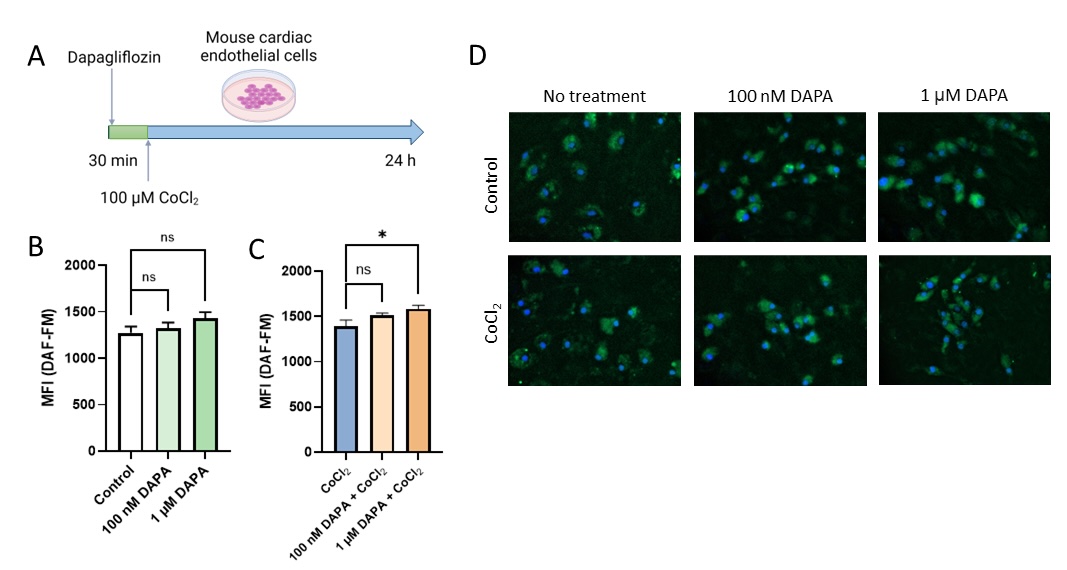
Fig. 5: Dapagliflozin treatment improved NO production in cardiac endothelial cells in vitro. Effects of dapagliflozin treatment on real-time NO production in mouse cardiac microvascular endothelial cells (H5V) in control conditions and after stimulation with hypoxia-mimetic CoCl2. (A) experiment scheme. (B) Representative images from fluorescence DAF-FM staining of H5V cells. (C) mean fluorescence intensity of DAF-FM in H5V cells after dapagliflozin treatment in control conditions and (D) stimulation with hypoxia-mimetic CoCl2. Results are shown as mean ± SEM; n = 6; * ns = not significant.
Dapagliflozin treatment improved endothelial NO production in vitro and in vivo
Since mitochondrial ATP production was proved to be detrimental to endothelial cell function and NO synthesis, we evaluated NO production in living H5V cells after dapagliflozin by fluorescence DAF-FM staining (Fig. 6). A growing trend in real-time NO production was observed in control conditions. In hypoxia-mimic conditions, these effects were significant.
Then, we aimed to verify the results of in vitro studies and investigated the effect of dapagliflozin treatment on NO production in vivo in the mouse coronary circulation (Fig. 7). After dapagliflozin treatment, NO signal was doubled in both small (50-100 µm in diameter) and intermediate vessels (<50 µm), but in the coronary capillaries, the difference was not significant. In addition, coronary capillary density tended to be higher in dapagliflozin-treated mice.
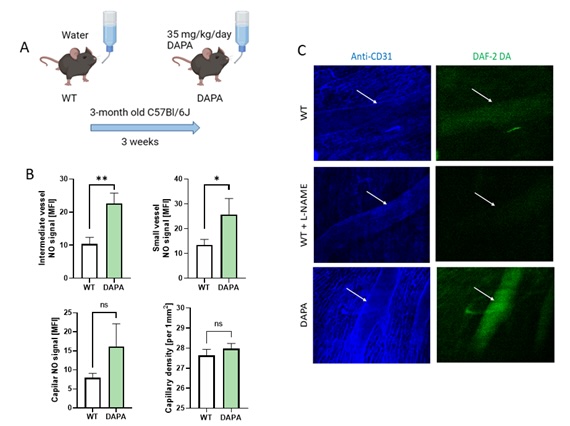
Fig. 6: Dapagliflozin treatment improved NO production in the coronary circulation in mice. A. Experiment scheme, B. Quantitative analysis of DAF-2DA staining intensity corresponded to real-time NO availability in subepicardial coronary intermediate blood vessels (arterioles and venules 50-100 μm in diameter), small blood vessels (10-50 μm in diameter), capillaries, and capillary density expressed per 1 mm2. Results are shown as mean ± SEM; n = 6; * p < 0.05; ** p < 0.01, ns = not significant.
Dapagliflozin treatment affected L-arginine metabolism in heart failure patients
This study also examined the effects of 3-month treatment with dapagliflozin (10 mg/day) on plasma concentration of stable NO products and amino acids related to NO metabolism in patients with heart failure with ejection fraction >40%. Our findings demonstrate that dapagliflozin leads to a significant decrease in plasma L-arginine concentration (Fig. 7). Concurrently, there was a tendency for increased plasma L-citrulline and L-ornithine levels, along with significantly increased ratios of L-ornithine/L-arginine and L-citrulline/L-arginine. Notably, the concentration of stable NO products (nitrites and nitrates) was slightly increased in patients after dapagliflozin. Similarly, a trend toward decreased asymmetric dimethylarginine (ADMA) and monomethylarginine (L-NMMA) concentrations was demonstrated. There were no significant changes in other measured amino acids (Table 2).
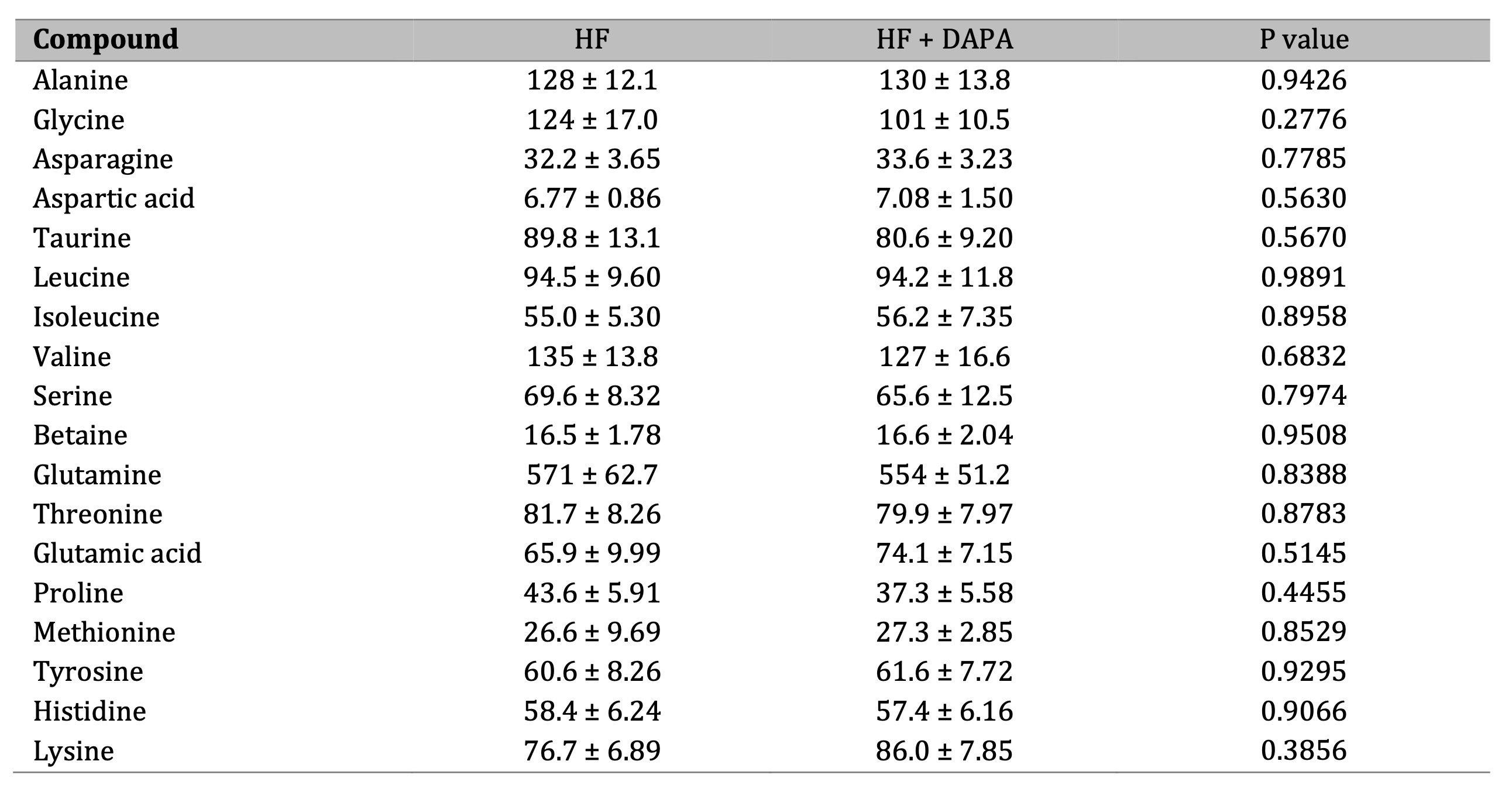
Table 2: Dapagliflozin treatment affected amino acid metabolism in heart failure patients. Plasma concentration of amino acids in heart failure patients (HF) before and after 3 months of treatment with dapagliflozin (10 mg/day) (HF + DAPA). Results are shown as mean ± SEM; n = 13; * p < 0.05; ns = not significant.
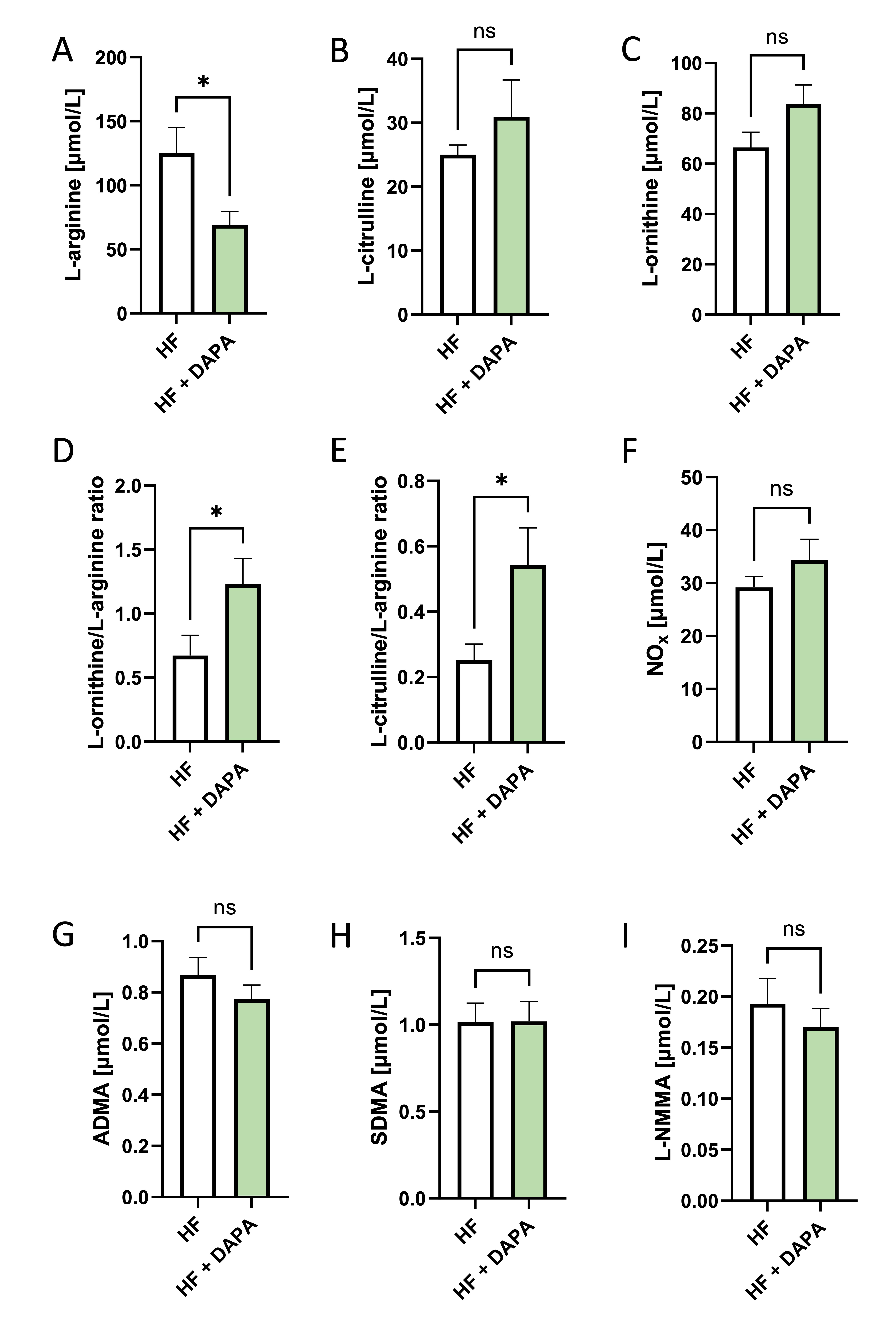
Fig. 7: Dapagliflozin treatment affected L-arginine metabolism in heart failure patients. A. Experiment scheme, B. Plasma concentration of L-arginine, L-citrulline, L-ornithine, asymmetric dimethylarginine, symmetric dimethylarginine N-monomethylarginine, and plasma nitrite and titrate pool heart failure patients (HF) before and after 3 months of treatment with dapagliflozin (10 mg/kg) (HF + DAPA). Results are shown as mean ± SEM; n = 13; * p < 0.05; ns = not significant.
Discussion
This study demonstrated that SGLT2i, dapagliflozin, improved the energy metabolism of endothelial cells by enhancing mitochondrial respiration, which correlated with higher NO production. It improved endothelial bioenergetics, indicated by higher levels of intracellular adenine nucleotides and a better ATP/ADP ratio, without affecting glycolysis. Dapagliflozin treatment in mice enhanced endothelial function, particularly in small and intermediate vessels. In vitro studies confirmed increased NO production in endothelial cells. In patients with heart failure and ejection fraction above 40%, dapagliflozin (10 mg/day) led to significant changes in biomarkers related to NO homeostasis, mitochondrial function, and L-arginine metabolism after 3 months.
Previously, it was shown that SGLT2i promoted mitochondrial biogenesis and reduced oxidative stress in endothelial cells [14]. However, how these drugs affect mitochondrial function in endothelial cells and the bioenergetics of the endothelium has not been studied. To our knowledge, this study is the first to show that dapagliflozin improved mitochondrial function and increased energy metabolism, which is closely associated with endothelial function and NO production.
Endothelial dysfunction and senescence are marked by reduced NO availability and heightened pro-inflammatory activity, all contributing to vascular stiffness, atherosclerosis, hypertension, and worsening microvascular function. Mitochondria play a pivotal role in these processes by modulating endothelial metabolism, maintaining redox balance, and regulating signaling pathways that impact cellular aging and vascular health. Dapagliflozin’s mitochondrial effects may play a crucial role in vascular aging resistance by enhancing mitochondrial function, reducing oxidative stress, and preserving endothelial health. These benefits could slow age-related vascular deterioration, reduce arterial stiffness, and maintain endothelial NO bioavailability, contributing to improved cardiovascular health in cardiovascular disorders mediated by endothelial senescence and dysfunction [15, 16].
Dapagliflozin may accelerate mitochondrial oxidative phosphorylation by supporting mitochondrial biogenesis, diminishing oxidative stress, and controlling autophagy. These processes are mediated by multiple pathways, including SIRT1-PGC1α (sirtuin 1, peroxisome proliferator-activated receptor gamma and coactivator 1 alpha) pathway, NADPH oxidase (NOX) inhibition, and AMP-activated protein kinase (AMPK) activation. Dapagliflozin upregulates SIRT1, which is an NAD+ dependent enzyme that, in turn, activates PGC-1α. This enhances mitochondria biogenesis and improves oxidative phosphorylation efficiency [17]. By inhibiting NOX2 and NOX4, dapagliflozin reduces mitochondrial ROS production and prevents mitochondrial respiratory chain complexes from oxidative damage [18]. AMPK is a key cellular energy sensor that is activated by dapagliflozin. AMPK enhances oxidative phosphorylation, increases ATP production, and regulates mitochondrial autophagy and quality control [19]. Moreover, by upregulating mitochondrial dynamin-like GTPase (OPA1), dapagliflozin promotes mitochondrial fusion, maintaining its integrity [20]. All these processes regulate mitochondrial protection and promote their proper function.
The elevated NO synthesis is mediated through endothelial nitric oxide synthase (eNOS, NOS3) activity, which dapagliflozin appears to upregulate. eNOS is a key NO producer in the endothelium, and its activity is closely regulated by protein phosphorylation [21]. It was shown that by activating the SIRT1 pathway, dapagliflozin can promote eNOS phosphorylation, enhancing its catalytic activity [22]. Moreover, dapagliflozin enhances NO production by activating AMPK, a key regulator of cellular energy homeostasis. The signaling cascade is triggered by inducing a negative caloric balance and a nutrient deprivation state, then upregulation of the energy deprivation sensor AMPK [23]. AMPK phosphorylates eNOS at Ser1177, leading to increased eNOS activity and enhanced NO synthesis from L-arginine [24] On the other hand, increased mitochondrial ATP production after dapagliflozin treatment can activate a calcium wave and IP3 signalling, directly influencing eNOS activity [25]. In addition to stimulating NO production, dapagliflozin, by reducing oxidative stress and inflammation, may increase NO stability and availability [26]. The increase in NO availability plays a crucial role in maintaining vascular homeostasis by promoting vasodilation and angiogenesis while reducing oxidative stress and inhibiting platelet aggregation. This may enhance endothelial function, preventing and treating both macro- and microcirculatory injury, delaying heart failure progression, and supporting cardiac regeneration [27].
Clinical data systematically confirm the beneficial effect of SGLT2i on the endothelium. Most studies evaluated endothelial function in groups of diabetic patients with high cardiovascular risk. The results of these studies confirm the outcomes obtained in preclinical studies [28]. In trials investigating the effect of dapagliflozin on endothelial function, it was evaluated by functional tests and/or plasma biomarker levels.
Flow Mediated Dilatation (FMD) and Reactive Hyperaemia Index (RHI) are functional tests used to assess endothelial function based on endothelial-dependent vasodilation after transient arterial occlusion [29]. FMD is a well-established noninvasive method that measures the dilation of the brachial artery using ultrasound before and after blood flow occlusion [30]. RHI, another noninvasive method, assesses peripheral vascular endothelial function by measuring digital reactive hyperemia, partially mediated by NO [31, 32]. Additionally, endothelial function can be evaluated through biomarkers like intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and endothelin 1 (ET-1).
Few studies examine the effect of dapagliflozin on endothelial function. Sposito et al. showed a significant increase in FMD in type 2 diabetes mellitus patients treated with dapagliflozin 10 mg/day plus metformin for 12 weeks, compared to those receiving glibenclamide. However, there were no significant changes in plasma levels of sVCAM-1 and sICAM-1. Similarly, Sugiyama et al. found FMD improvement in patients on dapagliflozin 5 mg/day plus metformin over 16 weeks versus metformin alone [33]. Solini et al. noted significant FMD changes after 2 days of dapagliflozin treatment in hypertensive T2DM patients compared to hydrochlorothiazide, but no changes after 4 weeks [34, 35]. Zainordin et al. reported no significant FMD change with dapagliflozin compared to placebo, but noted increased nitroglycerin-mediated dilatation and decreased ICAM-1 levels [36]. Studies on reactive RHI yield conflicting results. Sugiyama et al. found significant RHI improvement with dapagliflozin, while Kong et al. and Ilyas et al. reported no change [37, 38].
FMD and RHI assess endothelial function by inducing transient hypoxia through occlusion, reflecting NO-dependent vasodilation, which is often impaired in cardiovascular diseases. Hypoxia is a critical factor in the development and progression of cardiovascular diseases, often co-occurring with heart failure [39]. Experimentally, the hypoxic state can be induced in cells by reducing the concentration of oxygen in the culture medium or chemically using CoCl2 [40]. Cobalt inactivates prolyl hydroxylases, which degrade hypoxia-inducible factor 1 (HIF-1α), a key regulator in cellular adaptation to hypoxia. This stabilizes HIF-1α and promotes its further accumulation in the nucleus, activating hypoxia-responsive genes involved in angiogenesis, glycolysis, and cell survival and proliferation [41]. We previously demonstrated that CoCl₂ increases HIF-1α levels in mouse microvascular endothelial cells by inhibiting prolyl hydroxylases. This effect was linked to a reduction in intracellular ATP concentration due to impaired mitochondrial oxidative phosphorylation [42]. Although low oxygen culture conditions might be more physiologically relevant by mimicking tissue oxygen levels in vivo and allowing gradual cell adaptation to hypoxia, CoCl2 treatment is sufficient for short-time culture. Thus, in our in vitro model, we triggered hypoxia-activated pathways without manipulating the O2 level and analysed the effects of SGLT2i on endothelial cell bioenergetics.
In addition to its numerous effects dependent on glycaemic regulation, dapagliflozin shows effects independent of glycaemic reduction. A large body of evidence based on preclinical trials indicates that SGLT2i improve endothelial function via a combination of mechanisms that appear to act independently of glucose-lowering benefits. They appear to be mediated through reduced oxidative stress, improved mitochondrial function, and anti-inflammatory effects as well as are observed in non-diabetic patients. DAPA-HF trial demonstrated the cardiovascular benefits of an SGLT2i in patients without diabetes by reducing the total number of hospitalizations for heart failure and deaths from cardiovascular causes, as an outcome of worsening heart failure symptoms [43]. In the DELIVER trial, dapagliflozin also reduced the combined risk of worsening heart failure or cardiovascular death among patients with heart failure and a mildly reduced or preserved ejection fraction, and the results were similar in patients with or without diabetes [44]. In EMPEROR-Preserved and EMPEROR-Reduced, empagliflozin lowered the overall risk of cardiovascular-related death or hospitalization due to heart failure in individuals with heart failure and preserved ejection fraction, irrespective of their diabetic status [45, 46]. However, large-scale clinical studies investigating the effects of SGLT2i on endothelial function in patients with HF are lacking.
This study revealed that patients with heart failure treated with dapagliflozin (10 mg/day) for 3 months demonstrated a tendency towards higher plasma levels of stable NO products correlated with significant changes in plasma L-arginine and its derivatives. We demonstrated decreased circulating L-arginine concentration with increased ratios of L-ornithine/L-arginine and L-citrulline/L-arginine. Notably, a trend toward diminished levels of methylated L-arginine derivatives (ADMA and L-NMMA), well-known endogenous inhibitors of eNOS, was demonstrated. This supports a potential enhancement of NO production, which could benefit vascular function. On the other hand, the results may suggest an upregulation of arginase activity, which converts arginine into ornithine and urea. Increased arginase activity has been linked to endothelial dysfunction in heart failure, and its activation by SGLT2 inhibitors may reflect an adaptive or compensatory response. In line with that, the increased L-citrulline/L-arginine and L-ornithine/L-arginine ratios indicate a shift toward urea cycle activation, potentially enhancing ammonia clearance, which is crucial in the metabolic dysregulation observed in heart failure. In addition, given that L-ornithine and L-citrulline are intermediates in both the urea cycle and mitochondrial metabolism, these changes may reflect an SGLT2 inhibitor-induced modulation of mitochondrial function, improving energy efficiency in endothelial cells and failing myocardium.
Conclusion
This study demonstrated that dapagliflozin, the SGLT2i, enhances endothelial energy metabolism by improving mitochondrial respiration, which correlates with increased nitric oxide production. Dapagliflozin’s ability to enhance mitochondrial function, as evidenced by higher intracellular adenine nucleotide levels and an elevated ATP/ADP ratio, highlights its pivotal role in bioenergetic regulation. Notably, these effects were achieved without altering glycolytic pathways, suggesting that dapagliflozin specifically targets mitochondrial function.
Despite promising preclinical findings, clinical studies have shown varying results regarding the effects of SGLT2i on macro- and microvascular function. Our findings also provide novel insights into the metabolic effects of SGLT2i in heart failure and suggest potential mechanisms related to nitric oxide homeostasis, mitochondrial function, and L-arginine metabolism. However, further long-term studies on a larger cohort of patients are necessary to evaluate the specific effects and mechanisms of SGLT2i on endothelial function.
Acknowledgements
Mouse cardiac endothelial cells (H5V) were obtained courtesy of Dr. hab. Patrycja Koszałka from the Department of Cell Biology and Immunology, Institute of Medical Biotechnology and Experimental Oncology, Intercollegiate Faculty of Biotechnology, University of Gdańsk and Medical University of Gdańsk.
Author Contributions
Conceptualization: Barbara Kutryb-Zając, Michał Mączewski, Marcin Hellmann, Ryszard T. Smoleński. Data curation: Iga Walczak, Maria Tarnawska, Aleksandra Parzuchowska, Marcin Hellmann, Michał Mączewski, Barbara Kutryb-Zając. Formal analysis: Iga Walczak, Alicja Braczko, Aleksandra Paterek, Filip Rolski, Krzysztof Urbanowicz, Maria Tarnawska, Aleksandra Parzuchowska, Michał Mączewski, Barbara Kutryb-Zając. Investigation: Iga Walczak, Alicja Braczko, Aleksandra Paterek, Filip Rolski, Krzysztof Urbanowicz, Maria Tarnawska, Aleksandra Parzuchowska, Marcin Hellmann, Michał Mączewski, Barbara Kutryb-Zając. Project administration: Barbara Kutryb-Zając, Michał Mączewski, Marcin Hellmann. Validation: Barbara Kutryb-Zając, Michał Mączewski, Marcin Hellmann. Writing – original draft: Iga Walczak, Maria Tarnawska, Aleksandra Parzuchowska, Michał Mączewski, Barbara Kutryb-Zając. Writing – review & editing: Iga Walczak, Alicja Braczko, Aleksandra Paterek, Filip Rolski, Krzysztof Urbanowicz, Maria Tarnawska, Ryszard T. Smoleński, Marcin Hellmann, Michał Mączewski, Barbara Kutryb-Zając
Funding Sources
The National Science Centre of Poland supported this study (grant no. 2023/51/B/NZ4/03017).
Statement of Ethics
All animal experiments complied with internationally accepted standards and were approved by the appropriate institutional review bodies. Specifically, the study was conducted following the guidelines approved by the 2nd Local Ethics Committee in Warsaw (approvals No WAW2/003/2021 and WAW2/152/2023) and following EU Directive 2010/63/EU and Polish legislation (Dz. U. poz. 266/15.01.2015).
Data availability statement
The data that support the findings of this study are available from the corresponding author,
B. K-Z., upon reasonable request.
Disclosure Statement
The authors have no conflicts of interest to declare.
Disclosure of AI Assistance
The authors utilized ChatGPT during the preparation of this work to enhance language clarity and readability. All content was subsequently reviewed and edited as needed by the authors, who take full responsibility for the final version of the manuscript.
References
| 1 | Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3). DOI: 10.1016/J.IJCARD.2013.12.028
https://doi.org/10.1016/j.ijcard.2013.12.028 |
| 2 | Vancheri F, Longo G, Vancheri S, Henein M. Coronary Microvascular Dysfunction. J Clin Med. 2020 Sep;9(9):2880.
https://doi.org/10.3390/jcm9092880 |
| 3 | Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020 Jun;5(6):632-44.
https://doi.org/10.1016/j.jacbts.2020.02.004 |
| 4 | Correale M, Mazzeo P, Mallardi A, Leopizzi A, Tricarico L, Fortunato M, et al. Switch to SGLT2 Inhibitors and Improved Endothelial Function in Diabetic Patients with Chronic Heart Failure. Cardiovasc Drugs Ther. 2022 Dec;36(6):1157-64.
https://doi.org/10.1007/s10557-021-07254-3 |
| 5 | Salvatore T, Caturano A, Galiero R, Di Martino A, Albanese G, Vetrano E, et al. Cardiovascular Benefits from Gliflozins: Effects on Endothelial Function. Biomedicines. 2021 Sep;9(10):1356.
https://doi.org/10.3390/biomedicines9101356 |
| 6 | Cyr AR, Huckaby L V, Shiva SS, Zuckerbraun BS. Nitric Oxide and Endothelial Dysfunction. Crit Care Clin. 2020 Apr;36(2):307-21.
https://doi.org/10.1016/j.ccc.2019.12.009 |
| 7 | Kutryb-Zajac B, Zukowska P, Toczek M, Zabielska M, Lipinski M, Rybakowska I, et al. Extracellular Nucleotide Catabolism in Aortoiliac Bifurcation of Atherosclerotic ApoE/LDLr Double Knock Out Mice. Nucleosides Nucleotides Nucleic Acids. 2014;33(4-6):323-8.
https://doi.org/10.1080/15257770.2014.880478 |
| 8 | Klar A, Lin Y, Immanuel J, Yun S. Vascular Inflammatory Diseases and Endothelial Phenotypes. Cells 2023, Vol 12, Page 1640. 2023 Jun;12(12):1640.
https://doi.org/10.3390/cells12121640 |
| 9 | Dimitriadis K, Adamopoulou E, Pyrpyris N, Sakalidis A, Leontsinis I, Manta E, et al. The effect of SGLT2 inhibitors on the endothelium and the microcirculation: from bench to bedside and beyond. Eur Heart J Cardiovasc Pharmacother. 2023 Dec;9(8):741-57.
https://doi.org/10.1093/ehjcvp/pvad053 |
| 10 | Alexander Y, Osto E, Schmidt-Trucksäss A, Shechter M, Trifunovic D, Duncker DJ, et al. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc Res. 2021 Jan;117(1):29-42.
https://doi.org/10.1093/cvr/cvaa085 |
| 11 | Olkowicz M, Debski J, Jablonska P, Dadlez M, Smolenski RT. Application of a new procedure for liquid chromatography/mass spectrometry profiling of plasma amino acid-related metabolites and untargeted shotgun proteomics to identify mechanisms and biomarkers of calcific aortic stenosis. J Chromatogr A. 2017 Sep;1517:66-78.
https://doi.org/10.1016/j.chroma.2017.08.024 |
| 12 | Kutryb-Zajac B, Jablonska P, Serocki M, Bulinska A, Mierzejewska P, Friebe D, et al. Nucleotide ecto-enzyme metabolic pattern and spatial distribution in calcific aortic valve disease; its relation to pathological changes and clinical presentation. Clinical Research in Cardiology. 2019 May DOI: 10.1007/s00392-019-01495-x
https://doi.org/10.1007/s00392-019-01495-x |
| 13 | Karaś A, Bar A, Pandian K, Jasztal A, Kuryłowicz Z, Kutryb-Zając B, et al. Functional deterioration of vascular mitochondrial and glycolytic capacity in the aortic rings of aged mice. Geroscience. 2024 Feb;46(4):3831-44.
https://doi.org/10.1007/s11357-024-01091-6 |
| 14 | Kowalska K, Wilczopolski P, Buławska D, Młynarska E, Rysz J, Franczyk B. The Importance of SGLT-2 Inhibitors as Both the Prevention and the Treatment of Diabetic Cardiomyopathy. Antioxidants. 2022 Dec;11(12):2500.
https://doi.org/10.3390/antiox11122500 |
| 15 | Katsuumi G, Shimizu I, Suda M, Yoshida Y, Furihata T, Joki Y, et al. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat Aging. 2024 May;4(7):926-38.
https://doi.org/10.1038/s43587-024-00642-y |
| 16 | Tai S, Zhou Y, Fu L, Ding H, Zhou Y, Yin Z, et al. Dapagliflozin impedes endothelial cell senescence by activating the SIRT1 signaling pathway in type 2 diabetes. Heliyon. 2023 Aug;9(8):e19152.
https://doi.org/10.1016/j.heliyon.2023.e19152 |
| 17 | He L, Li Y, Di Z, Hongjie S, Dan X, Zchanchun S. Dapagliflozin improves endothelial cell dysfunction by regulating mitochondrial production via the SIRT1/PGC-1α pathway in obese mice. Biochem Biophys Res Commun. 2022;615. DOI: 10.1016/J.BBRC.2022.05.022
https://doi.org/10.1016/j.bbrc.2022.05.022 |
| 18 | Zügner E, Yang H-C, Kotzbeck P, Boulgaropoulos B, Sourij H, Hagvall S, et al. Differential In vitro Effects of SGLT2 Inhibitors on Mitochondrial Oxidative Phosphorylation, Glucose Uptake and Cell Metabolism. Int J Mol Sci. 2022 Jul;23(14). DOI: 10.3390/ijms23147966
https://doi.org/10.3390/ijms23147966 |
| 19 | Tsai K-L, Hsieh P-L, Chou W-C, Cheng H-C, Huang Y-T, Chan S-H. Dapagliflozin attenuates hypoxia/reoxygenation-caused cardiac dysfunction and oxidative damage through modulation of AMPK. Cell Biosci. 2021 Feb;11(1):44.
https://doi.org/10.1186/s13578-021-00547-y |
| 20 | Tu W, Li L, Yi M, Chen J, Wang X, Sun Y. Dapagliflozin attenuates high glucose-and hypoxia/reoxygenation-induced injury via activating AMPK/mTOR-OPA1-mediated mitochondrial autophagy in H9c2 cardiomyocytes. Arch Physiol Biochem. 2024 Dec;130(6):649-59.
https://doi.org/10.1080/13813455.2023.2252200 |
| 21 | Zhou Y, Tai S, Zhang N, Fu L, Wang Y. Dapagliflozin prevents oxidative stress-induced endothelial dysfunction via sirtuin 1 activation. Biomed Pharmacother. 2023 Sep;165:115213.
https://doi.org/10.1016/j.biopha.2023.115213 |
| 22 | Tai S, Zhou Y, Fu L, Ding H, Zhou Y, Yin Z, et al. Dapagliflozin impedes endothelial cell senescence by activating the SIRT1 signaling pathway in type 2 diabetes. Heliyon. 2023 Aug;9(8):e19152.
https://doi.org/10.1016/j.heliyon.2023.e19152 |
| 23 | Hoong CWS, Chua MWJ. SGLT2 Inhibitors as Calorie Restriction Mimetics: Insights on Longevity Pathways and Age-Related Diseases. Endocrinology. 2021 Aug;162(8). DOI: 10.1210/endocr/bqab079
https://doi.org/10.1210/endocr/bqab079 |
| 24 | Okayasu T, Tomizawa A, Suzuki K, Manaka K, Hattori Y. PPARalpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci. 2008 Apr;82(15-16):884-91.
https://doi.org/10.1016/j.lfs.2008.02.002 |
| 25 | Wilson C, Lee MD, Buckley C, Zhang X, McCarron JG. Mitochondrial ATP Production is Required for Endothelial Cell Control of Vascular Tone. Function (Oxf). 2022;4(2). DOI: 10.1093/FUNCTION/ZQAC063
https://doi.org/10.1093/function/zqac063 |
| 26 | Alsereidi FR, Khashim Z, Marzook H, Al-Rawi AM, Salomon T, Almansoori MK, et al. Dapagliflozin mitigates cellular stress and inflammation through PI3K/AKT pathway modulation in cardiomyocytes, aortic endothelial cells, and stem cell-derived β cells. Cardiovascular Diabetology 2024 23:1. 2024 Oct;23(1):1-22.
https://doi.org/10.1186/s12933-024-02481-y |
| 27 | Tousoulis D, Kampoli A-M, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012 Jan;10(1):4-18.
https://doi.org/10.2174/157016112798829760 |
| 28 | Dimitriadis K, Adamopoulou E, Pyrpyris N, Sakalidis A, Leontsinis I, Manta E, et al. The effect of SGLT2 inhibitors on the endothelium and the microcirculation: from bench to bedside and beyond. Eur Heart J Cardiovasc Pharmacother. 2023 Dec;9(8):741-57.
https://doi.org/10.1093/ehjcvp/pvad053 |
| 29 | Green D. Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol (1985). 2005 Sep;99(3):1233-4; discussion 1237-8.
https://doi.org/10.1152/japplphysiol.00601.2005 |
| 30 | Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992 Nov;340(8828):1111-5.
https://doi.org/10.1016/0140-6736(92)93147-F |
| 31 | Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003 Jul;146(1):168-74.
https://doi.org/10.1016/S0002-8703(03)00094-2 |
| 32 | Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985). 2006 Aug;101(2):545-8.
https://doi.org/10.1152/japplphysiol.01285.2005 |
| 33 | Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017 Dec;16(1):84.
https://doi.org/10.1186/s12933-017-0564-0 |
| 34 | Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017 Dec;16(1):138.
https://doi.org/10.1186/s12933-017-0621-8 |
| 35 | Solini A, Seghieri M, Giannini L, Biancalana E, Parolini F, Rossi C, et al. The Effects of Dapagliflozin on Systemic and Renal Vascular Function Display an Epigenetic Signature. J Clin Endocrinol Metab. 2019 Oct;104(10):4253-63.
https://doi.org/10.1210/jc.2019-00706 |
| 36 | Zainordin NA, Hatta SFWM, Mohamed Shah FZ, Rahman TA, Ismail N, Ismail Z, et al. Effects of Dapagliflozin on Endothelial Dysfunction in Type 2 Diabetes With Established Ischemic Heart Disease (EDIFIED). J Endocr Soc. 2020 Jan;4(1):bvz017.
https://doi.org/10.1210/jendso/bvz017 |
| 37 | Kong SH, Koo BK, Moon MK. Effects of Dapagliflozin on Endothelial Function, Renal Injury Markers, and Glycemic Control in Drug-Naïve Patients with Type 2 Diabetes Mellitus. Diabetes Metab J. 2019 Oct;43(5):711-7.
https://doi.org/10.4093/dmj.2018.0208 |
| 38 | Ilyas F, Jones L, Tee SL, Horsfall M, Swan A, Wollaston F, et al. Acute pleiotropic effects of dapagliflozin in type 2 diabetic patients with heart failure with reduced ejection fraction: a crossover trial. ESC Heart Fail. 2021 Oct;8(5):4346-52.
https://doi.org/10.1002/ehf2.13553 |
| 39 | Abe H, Semba H, Takeda N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J Atheroscler Thromb. 2017 Sep;24(9):884-94.
https://doi.org/10.5551/jat.RV17009 |
| 40 | Muñoz-Sánchez J, Chánez-Cárdenas ME. The use of cobalt chloride as a chemical hypoxia model. J Appl Toxicol. 2019 Apr;39(4):556-70.
https://doi.org/10.1002/jat.3749 |
| 41 | Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt Inhibits the Interaction between Hypoxia-inducible Factor-α and von Hippel-Lindau Protein by Direct Binding to Hypoxia-inducible Factor-α*. Journal of Biological Chemistry. 2003;278:15911-6.
https://doi.org/10.1074/jbc.M300463200 |
| 42 | Kutryb-Zajac B, Kawecka A, Braczko A, Franczak M, Slominska EM, Giovannoni R, et al. CoCl2-Mimicked Endothelial Cell Hypoxia Induces Nucleotide Depletion and Functional Impairment That Is Reversed by Nucleotide Precursors. Biomedicines. 2022;10(7). DOI: 10.3390/BIOMEDICINES10071540
https://doi.org/10.3390/biomedicines10071540 |
| 43 | McMurray JJ V, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019 Nov;381(21):1995-2008.
|
| 44 | Solomon SD, McMurray JJ V, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022 Sep;387(12):1089-98.
|
| 45 | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021 Oct;385(16):1451-61.
|