Magnesium and Zinc Dose-Dependently Stabilize Rat Peritoneal Mast Cells and Enhance the Effects of Adrenaline
bYamagata University, School of Nursing, Yamagata-shi, Yamagata, Japan
Keywords
Abstract
Background/Aims:
Magnesium and zinc are vital trace elements found in numerous foods and dietary supplements. In addition to their antioxidant, anticancer, antibacterial, and anti-inflammatory effects, clinical research has suggested that they possess anti-allergic properties.Methods:
Using differential-interference contrast (DIC) microscopy, we examined the effects of magnesium chloride (MgCl2) and zinc chloride (ZnCl2) on rat peritoneal mast cell degranulation. We also examined their effects in conjunction with adrenaline, the first-choice drug for anaphylaxis treatment.Results:
Both MgCl2 and ZnCl2 reduced the number of degranulating mast cells in a dose-dependent manner. MgCl2 significantly decreased the number of degranulating mast cells at concentrations of 50 mM or higher, whereas ZnCl2 achieved similar effects at much lower concentrations of 25 µM or more. These levels of MgCl2 or ZnCl2 enhanced the inhibitory effects of 1 mM adrenaline on mast cell degranulation. Additionally, pharmacological inhibition of the transient receptor potential cation channel subfamily M member 7 (TRPM7) by NS8593 reduced the number of degranulating mast cells in a dose-dependent manner.Conclusion:
This study is the first to provide in vitro evidence that magnesium and zinc stabilize mast cells in a dose-dependent manner and also enhance the effects of adrenaline. TRPM7, which has higher permeability to zinc ions than to magnesium ions, may contribute to the stronger mast cell-stabilizing properties of zinc.Introduction
Previous studies have revealed health-promoting properties of magnesium and zinc, including antioxidant, anti-cancer, antibacterial, and anti-inflammatory effects [1, 2]. Recently, in humans, magnesium and zinc have been reported to alleviate the symptoms of allergic rhinitis and allergic skin diseases [3-5], suggesting their potential as anti-allergic agents and opening new pharmacological possibilities. These anti-allergic effects have also been observed in animal models of allergic diseases such as atopic dermatitis, asthma, and anaphylaxis [6-8]. Previous studies have revealed the molecular mechanisms underlying the anti-inflammatory properties of magnesium and zinc using isolated lymphocytes or macrophages [1, 9]. In humans, deficiencies in these trace elements were found to be associated with the progression of inflammatory diseases [1, 9]. However, the exact mechanisms underlying the anti-allergic properties of magnesium and zinc remain largely unknown. During allergic reactions, mast cells have been shown to release secretory granules containing chemical mediators, such as histamine, serotonin, leukotrienes, and prostaglandins through exocytosis [10]. Using mast cells isolated from humans or animals, previous in vitro studies determined the anti-allergic properties of magnesium and zinc by showing their inhibitory effects on the release of histamine [11-13]. However, these past studies did not show the direct effects of magnesium or zinc on the process of exocytosis, nor did they show the effects of these trace elements at doses higher than physiological concentrations. Most anti-allergic medications work by blocking histamine H1 receptors in peripheral tissues [14]. However, some drugs or natural compounds exhibit strong anti-allergic effects by directly inhibiting exocytosis, thereby stabilizing mast cells [15]. Employing the standard patch-clamp whole-cell recording technique in rat peritoneal mast cells [16, 17], previous studies have shown that exocytosis is correlated with a gradual increase in the membrane [18-20]. Applying these techniques in our previous study, we showed through continuous monitoring of exocytosis in mast cells that substances such as adrenaline, macrolide antibiotics, corticosteroids, antihypertensives, and anti-allergic drugs have mast cell-stabilizing properties [21-26]. Recently, we demonstrated that food components such as caffeine, catechin, vitamins, and lemon constituents stabilize mast cells [27-29]. In this study, we aimed to assess the anti-allergic properties of magnesium and zinc and to uncover the physiological mechanisms involved by directly examining their impact on rat peritoneal mast cell degranulation. Here, we provide in vitro evidence for the first time that magnesium and zinc stabilize mast cells in a dose-dependent manner and enhance the effects of adrenaline. Transient receptor potential cation channel subfamily M member 7 (TRPM7), which is expressed in mast cells [30, 31] and is more permeable to zinc ions (Zn2+) than to magnesium ions (Mg2+) [32, 33], may play a role in the stronger mast cell-stabilizing properties of zinc.
Materials and Methods
Cell Sources and Preparation
Male Wistar
rats, which are outbred albino rats and are currently the most commonly used rats in laboratory research
[34],
were acquired from The Jackson Laboratory Japan, Inc. (Yokohama, Japan) at a minimum age of 25 weeks.
Historically, Wistar rats have been frequently used to isolate mast cells [35, 36]. Mast cells extracted
from
rats within this age bracket were sufficiently viable to undergo exocytosis when exposed to external
pharmacological stimuli [21-25, 28, 29, 37]. Due to variations in sex hormones [38], female mast cells are
generally more hypersensitive than male mast cells. Consequently, only male rats were used in the
experiments
performed in this study. The rats were anesthetized using isoflurane and euthanized by cervical
dislocation in
accordance with the euthanasia guidelines for adult laboratory rodents [39]. The Animal Care and Use
Committee
of Miyagi University approved the animal protocols (No. 2025-02). As previously outlined [21-28, 40], the
rat
peritoneum was rinsed with a standard external (bathing) solution, which included: NaCl, 145 mM; KCl, 4.0
mM;
CaCl2, 1.0 mM; MgCl2, 2.0 mM; HEPES, 5.0 mM; bovine serum albumin, 0.01 % (pH 7.2
adjusted
with NaOH); and mast cells were isolated from the peritoneal cavity. The isolated mast cells were kept in
the
external solution at room temperature (22-24°C) for about 8 hours until they were used. The mast cell
suspension, approximately 200/µL, was distributed in a chamber positioned at the head of an inverted
microscope
(Nikon, Tokyo, Japan). In the peritoneal cavity of rats, mast cells account for approximately 25% of all
the
cells [41, 42]. They are easily identifiable from other cell types owing to their distinctive
intracellular
secretory granules [21-29, 40, 43], which are smaller and more numerous than basophils [44]. The viability
of
mast cells was assessed based on their ability to release secretory granules in response to external
stimuli
(Fig. 1Ab vs. a) and their morphological integrity under differential-interference contrast (DIC)
microscopy, as
previously demonstrated [45, 46].
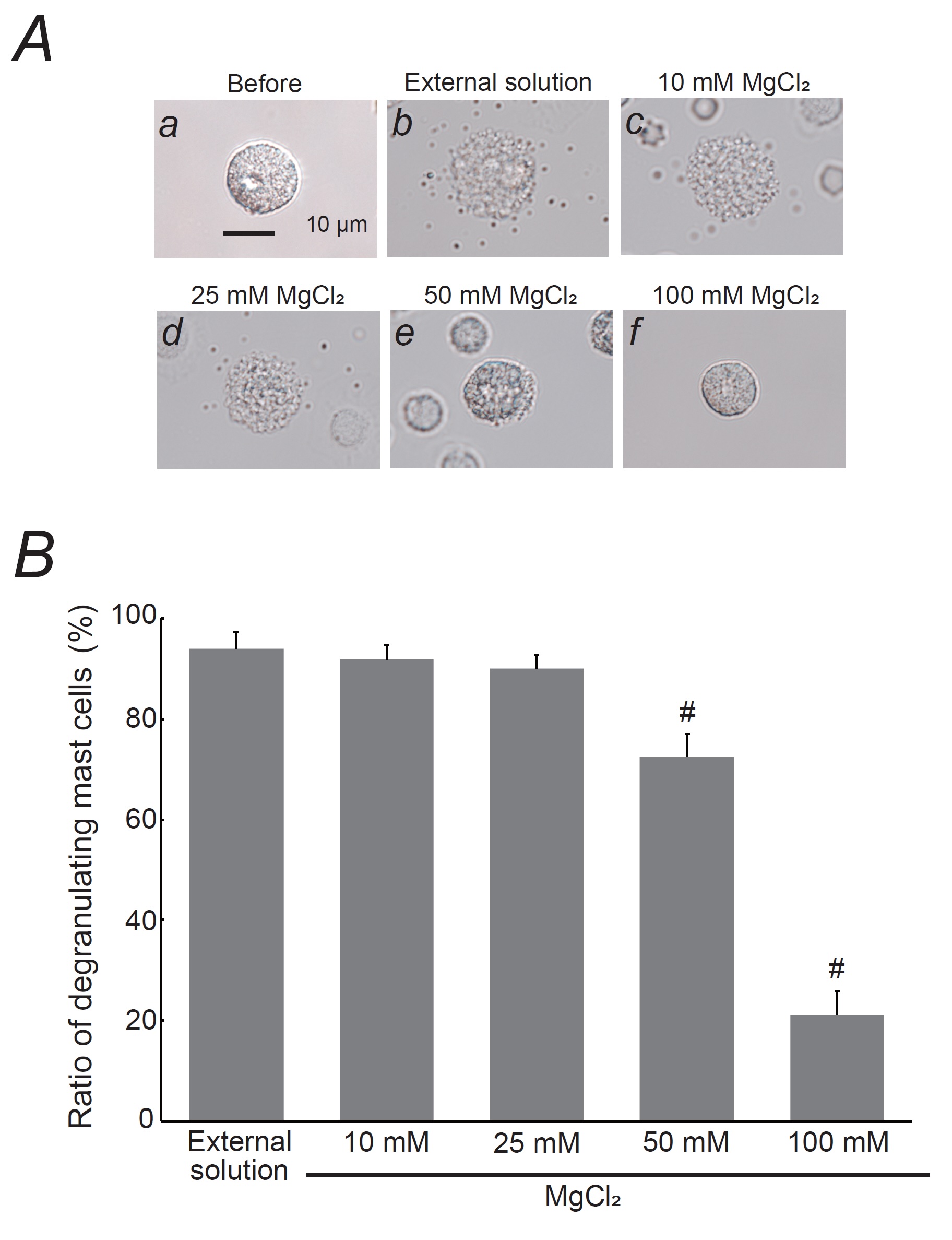
Fig. 1: Effects of magnesium chloride (MgCl2) on mast cell degranulation. A: Differential-interference contrast (DIC) microscopic images were taken before (a) and after exocytosis was externally induced by compound 48/80 in mast cells incubated in the external solutions containing no substance (b) or 10 mM MgCl2 (c), 25 mM MgCl2 (d), 50 mM MgCl2 (e), and 100 mM MgCl2 (f). B: After the mast cells were incubated in the external solutions containing no substance or different concentrations of MgCl2, exocytosis was induced by compound 48/80. From a single rat, several samples of mast cell suspension were obtained from the peritoneal cavity. The aliquot of the sample was spread in a chamber placed at the head stage of an inverted microscope. Then bright-field images were obtained from randomly chosen 0.1-mm2 fields of view, in which 30-40 mast cells were evenly observed per field. The degranulating mast cells were expressed as the average percentages of the total mast cells in the 10 bright fields. # p < 0.05 vs. incubation in the external solution alone. Values were presented as the means ± SEM. Differences were analyzed using ANOVA followed by Dunnett’s t-test. The experiments were repeated at least three times using three different rats to confirm the reproducibility of the data.
Quantification of Mast Cell Degranulation
MgCl2 and ZnCl2, obtained from Wako Pure Chemical Ind. (Osaka, Japan), were
separately
dissolved in the external solution at final concentrations of 10, 25, 50, and 100 mM and 10, 25, 50, and
100 µM,
respectively. In previous in vitro studies using cultured or primary human blood cells,
concentrations as
high as 50 -100 mM magnesium or 50 - 100 µM zinc were required to exert anti-inflammatory properties
[47-50].
Therefore, we used MgCl2 or ZnCl2 in this study, starting from these
concentrations.
MgCl2 and ZnCl2 were also dissolved in an external solution containing 1 mM
adrenaline
(Daiichi Sankyo, Inc., Tokyo, Japan) at final concentrations of 50, 100 mM and 50, 100 µM. NS8593 was
purchased
from Cayman Chemical (Ann Arbor, MI, USA) and dissolved to final concentrations of 10, 25, 50, and 100
µM.
After
incubating mast cells with these solutions or the external solution alone for 10 min, exocytosis was
externally
induced using compound 48/80 (Sigma-Aldrich Co., St. Louis, MO, USA; final concentration, 10 µg/mL)
[21-29, 40].
We utilized rat-derived mast cells in our experiments as they are more responsive to compound 48/80 than
those
isolated from the mouse peritoneal cavity [51]. Bright-field images were captured from randomly selected
0.1-mm2 fields of view (10 views from each condition), as previously described [21-29, 40,
43].
We
counted the number of degranulated mast cells (defined as cells surrounded by more than eight granules
outside
the cell membrane) and calculated their ratio to the total number of mast cells.
Immunohistochemistry
Parietal or
visceral peritoneal walls were removed from the rats for histological examination, as previously
described
[52].
The 3-µM paraffin sections of 4% paraformaldehyde-fixed peritoneal walls were placed in citrate-buffered
solution (pH 6.0) and then boiled for 30 min for antigen retrieval. Endogenous peroxidase was blocked
with
3%
hydrogen peroxide, and nonspecific binding was blocked with 10% BSA. Mouse anti-TRPM7 (1:50; StressMarq
Biosciences Inc., Vitoria, Canada) was used as the primary antibody. Diaminobenzidine substrate (Sigma
Chemical
Co., St. Louis, MO, USA) was used for the color reaction. Secondary antibodies alone were found to be
consistently negative in all of the sections. Toluidine blue staining was additionally performed by
immersing
sections in 0.1% toluidine blue (Muto Pure Chemical Co., Tokyo, Japan) for 30 min at room
temperature_ENREF_18.
Mast cells were identified based on their characteristic metachromasia [52].
Statistical Analysis
Data were
analyzed using Microsoft Excel (Microsoft Corporation, Redmond, WA., USA) and reported as means ± SEM.
Statistical significance was assessed using ANOVA. Statistical significance was set at p <
0.05.
Results
Effects of magnesium on mast cell degranulation
Mast cells
exposed only to the external solution or to lower concentrations of MgCl2 (10 and 25 mM)
exhibited
numerous surface wrinkles and secretory granule release via exocytosis (Fig. 1Ab-d vs. a). In contrast,
when
mast cells were incubated with higher MgCl2 concentrations (50 and 100 mM), signs of
exocytosis
were
partially or entirely absent (Fig. 1Ae, f). Lower MgCl2 concentrations (10 and 25 mM) did not
alter
the number of degranulating mast cells (Fig. 1B). However, 50 mM MgCl2 significantly reduced
the
number of degranulating mast cells (control, 94.0 ± 3.37 % vs. 50 mM MgCl2, 72.5 ± 4.53 %;
n=10, P<0.05), and 100 mM MgCl2 more markedly decreased the number of
degranulating
cells (21.0 ± 4.88 %; n=10, P<0.05; Fig. 1B). These findings therefore suggest that
MgCl2 inhibits exocytosis in a dose-dependent manner, thereby stabilizing mast cells.
Effects of zinc on mast cell degranulation
Similar to the
results obtained with MgCl2 (Fig. 1), a lower concentration of ZnCl2 (10 µM) did
not
affect mast cell degranulation (Fig. 2Ac vs. b), with degranulating cell numbers nearly matching those
in
the
external solution alone (Fig. 2B).
However, 25 µM ZnCl2 partially inhibited exocytosis (Fig. 2Ad) and significantly reduced the
number
of degranulating mast cells (control, 98.5 ± 0.77 % vs. 25 µM ZnCl2, 85.0 ± 1.28 %;
n=10,
P<0.05; Fig. 2B). Of note, 50 and 100 µM ZnCl2 almost completely halted exocytosis
(Fig.
2Ae, f) and further reduced the number of degranulating mast cells (50 µM ZnCl2, 37.3 ± 2.31
%;
100
µM ZnCl2, 32.6 ± 1.93 %; n=10, P<0.05; Fig. 2B). These results indicate
that,
similar to MgCl2, ZnCl2 inhibits exocytosis in a dose-dependent manner and
stabilizes mast
cells, but requires much lower doses than MgCl2 in order to achieve these effects (Fig. 2B
vs.
1B).
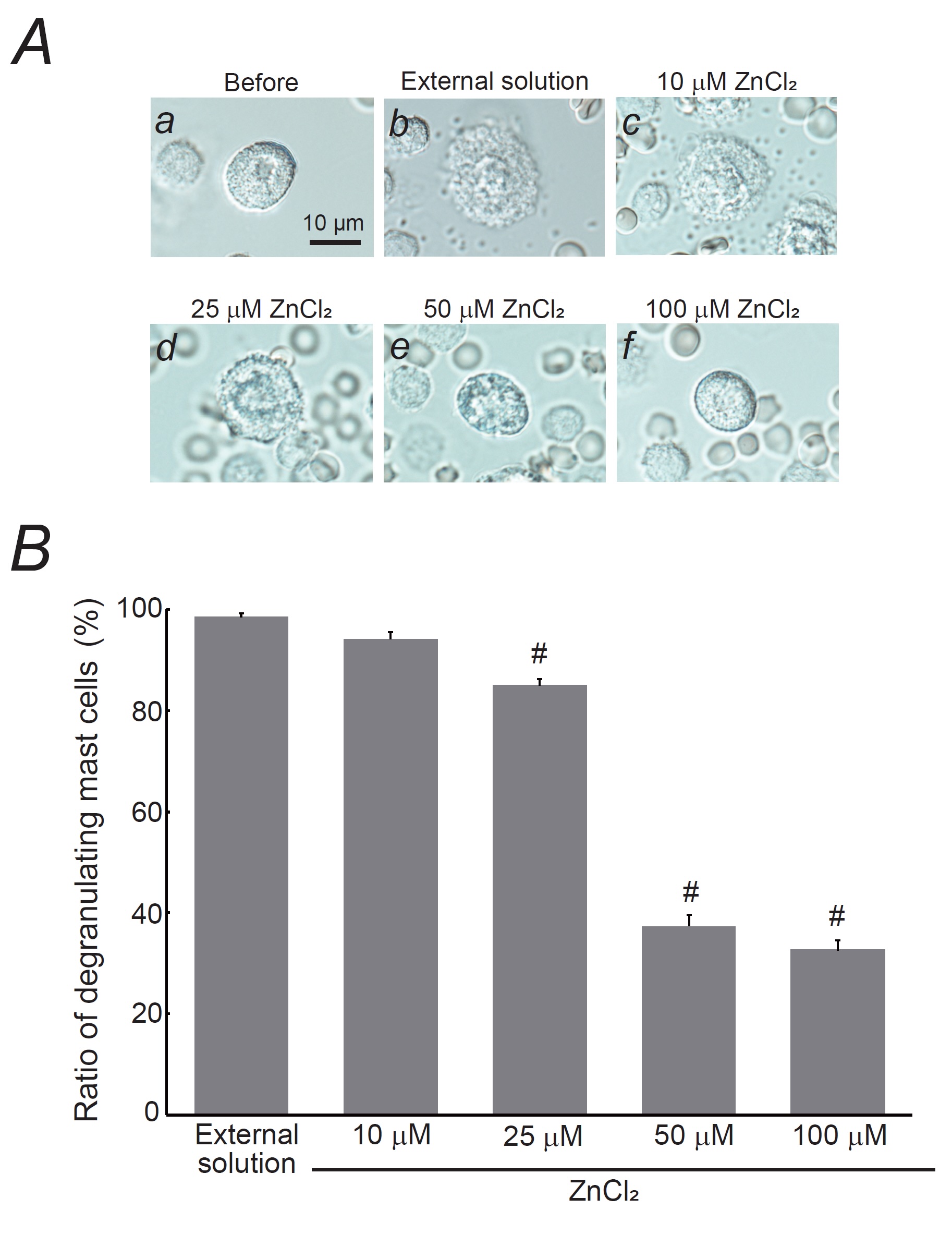
Fig. 2: Effects of zinc chloride (ZnCl2) on mast cell degranulation. A: Differential-interference contrast (DIC) microscopic images were taken before (a) and after exocytosis was externally induced by compound 48/80 in mast cells incubated in the external solutions containing no substance (b) or 10 mM ZnCl2 (c), 25 mM ZnCl2 (d), 50 mM ZnCl2 (e), and 100 mM ZnCl2 (f). B: After the mast cells were incubated in the external solutions containing no substance or different concentrations of ZnCl2, exocytosis was induced by compound 48/80. From a single rat, several samples of mast cell suspension were obtained from the peritoneal cavity. The aliquot of the sample was spread in a chamber placed at the head stage of an inverted microscope. Then bright-field images were obtained from randomly chosen 0.1-mm2 fields of view, in which 30-40 mast cells were evenly observed per field. The degranulating mast cells were expressed as the average percentages of the total mast cells in the 10 bright fields. # p < 0.05 vs. incubation in the external solution alone. Values were presented as the means ± SEM. Differences were analyzed using ANOVA followed by Dunnett’s t-test. The experiments were repeated at least three times using three different rats to confirm the reproducibility of the data.
Combined effects of magnesium or zinc with adrenaline on mast cell degranulation
In our
previous study, adrenaline, a first-line drug for anaphylaxis [53], was found to inhibit exocytosis in
mast
cells in a dose-dependent manner [25]. However, even at the highest concentration of 1 mM, the
suppressive
effect of adrenaline was found to be insufficient [25]. Given that higher concentrations of
MgCl2 and
ZnCl2 significantly suppressed mast cell degranulation (Fig. 1 and 2), we have examined
their
combined effects with adrenaline (Fig. 3). Consistent with our previous findings [25], 1 mM adrenaline
significantly reduced the number of degranulated mast cells (Fig. 3B and C). Notably, the presence of
MgCl2 (50 and 100 mM) or ZnCl2 (50 and 100 µM), which significantly decreased
the
number
of degranulating mast cells (Fig. 1B and 2B), effectively halted exocytosis process in these cells
(Fig.
3Ac-f
vs. b). Regarding the numbers of degranulating mast cells, there was a substantial decrease when
compared
to
those treated with 1 mM adrenaline alone (1 mM adrenaline, 53.3 ± 1.24 % vs. 1 mM adrenaline + 50 mM
MgCl2, 23.7 ± 3.86 %, n=10, P<0.05; 1 mM adrenaline + 100 mM
MgCl2,
3.34
± 1.08 %, n=10, P<0.05; Fig. 3B) (1 mM adrenaline, 43.9 ± 1.31 % vs. 1 mM adrenaline
+ 50
µM
ZnCl2, 25.4 ± 0.94 %, n=10, P<0.05; 1 mM adrenaline + 100 µM
ZnCl2,
18.9
± 0.80 %, n=10, P<0.05; Fig. 3C). These observations indicate that the inhibitory
effects
of
adrenaline on exocytosis were enhanced, with higher concentrations of MgCl2 or ZnCl2
additively potentiating the mast cell-stabilizing properties of adrenaline.
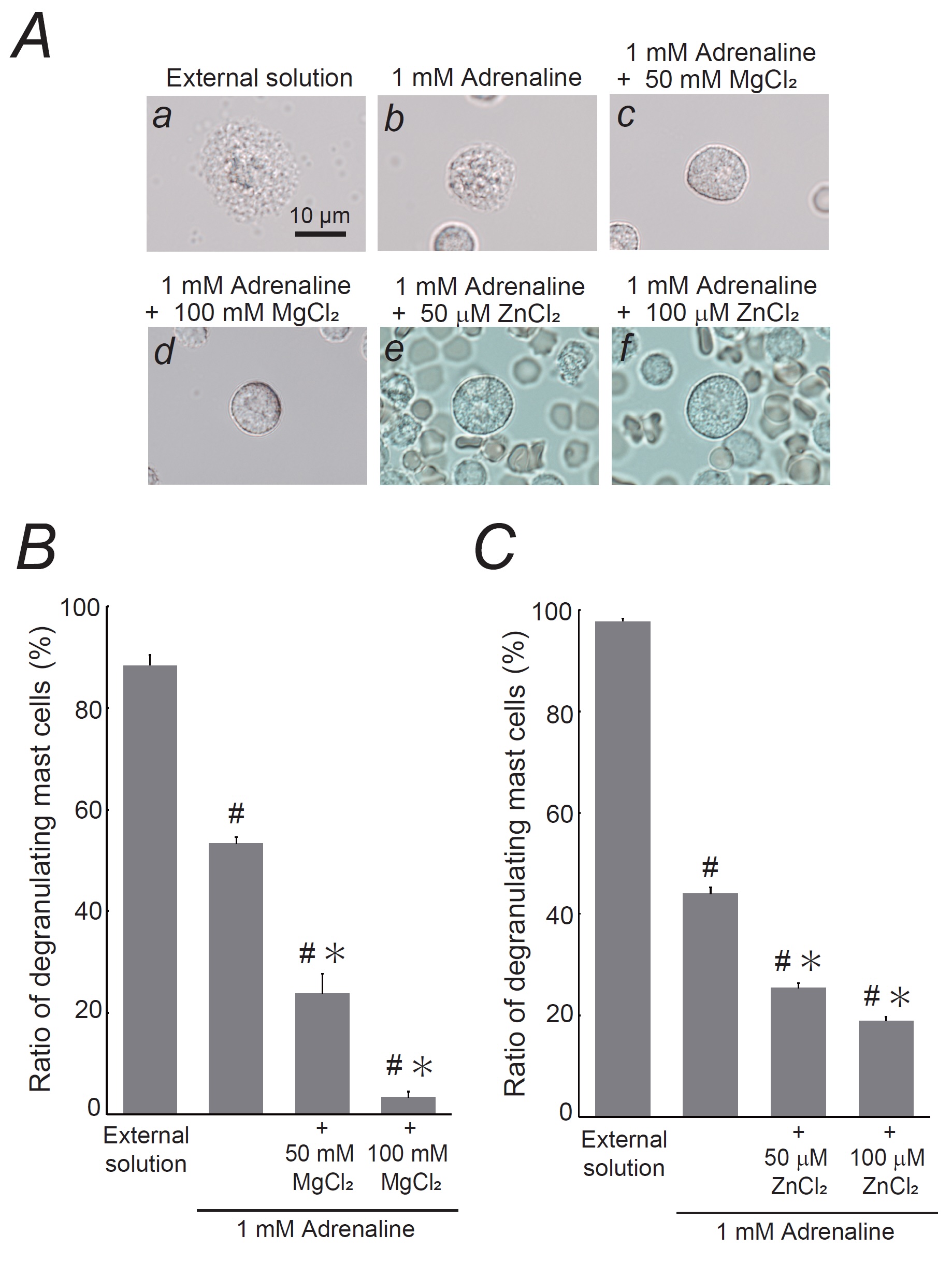
Fig. 3: Effects of magnesium chloride (MgCl2) or zinc chloride (ZnCl2) on adrenaline-induced inhibition of mast cell degranulation. A: Differential-interference contrast (DIC) microscopic images were taken after exocytosis was externally induced by compound 48/80 in mast cells incubated in the external solutions containing no substances (a), 1 mM adrenaline alone (b), 1 mM adrenaline in the presence of 50 mM MgCl2 (c), 1 mM adrenaline in the presence of 100 mM MgCl2 (d), 1 mM adrenaline in the presence of 50 mM ZnCl2 (e), and 1 mM adrenaline in the presence of 100 mM ZnCl2 (f). B: After exocytosis was induced in mast cells incubated in the external solutions containing no substance, 1 mM adrenaline alone, 1 mM adrenaline in the presence of 50 or 100 mM MgCl2, the numbers of degranulating mast cells were expressed as percentages of the total mast cell numbers in selected bright fields. C: After exocytosis was induced in mast cells incubated in the external solutions containing no substance, 1 mM adrenaline alone, 1 mM adrenaline in the presence of 50 or 100 mM ZnCl2, the numbers of degranulating mast cells were expressed as percentages of the total mast cell numbers in selected bright fields. From a single rat, several samples of mast cell suspension were obtained from the peritoneal cavity. The aliquot of the sample was spread in a chamber placed at the head stage of an inverted microscope. Then bright-field images were obtained from randomly chosen 0.1-mm2 fields of view, in which 30-40 mast cells were evenly observed per field. The degranulating mast cells were expressed as the average percentages of the total mast cells in the 10 bright fields. # p < 0.05 vs. incubation in the external solution alone. *p < 0.05 vs. incubation in the external solution containing 1 mM adrenaline. Values were presented as the means ± SEM. Differences were analyzed by ANOVA followed by Dunnett’s t-test. The experiments were repeated at least three times using three different rats to confirm the reproducibility of the data.
Role of TRPM7 in mast cell degranulation
TRPM7 is a
channel for divalent cations that also allow the passage of Mg2+ and Zn2+ [32,
33].
Since
TRPM7 is present in mast cells derived from humans and mice [30, 31], we examined its expression in
rat
peritoneal mast cells (Fig. 4A). In tissue sections of the rat peritoneum, mast cells were
identified
based on
their distinct metachromasia when stained with toluidine blue (Fig. 4A, arrows). Consistent with
previous
findings in murine mast cells [31], immunohistochemistry for TRPM7 showed positive expression in
both the
plasma
membrane, cytoplasm, and adipose tissue [54]. To reveal the involvement of TRPM7 in mast cell
degranulation,
exocytosis was triggered in the presence of its potent inhibitor, NS8593 [55, 56] (Fig. 4B and C).
Mast
cells
treated with 10 µM NS8593 showed no change in the number of degranulated cells (Fig. 4C). However,
25 µM
NS8593
was found to have significantly reduced the number of degranulating mast cells (control, 96.3 ± 0.80
% vs.
25 µM
NS8593, 60.7 ± 2.89 %; n=10, P<0.05; Fig. 4C), and even greater reductions were
observed
with
50 or 100 µM NS8593 (50 µM NS8593, 6.29 ± 1.13 %; n=10, P<0.05; 100 µM NS8593, 5.72
±
1.20 %;
n=10, P<0.05; Fig. 4C). These results suggest that the pharmacological inhibition
of
TRPM7
suppresses exocytosis in a dose-dependent manner, highlighting the crucial role of TRPM7 in mast
cell
degranulation.
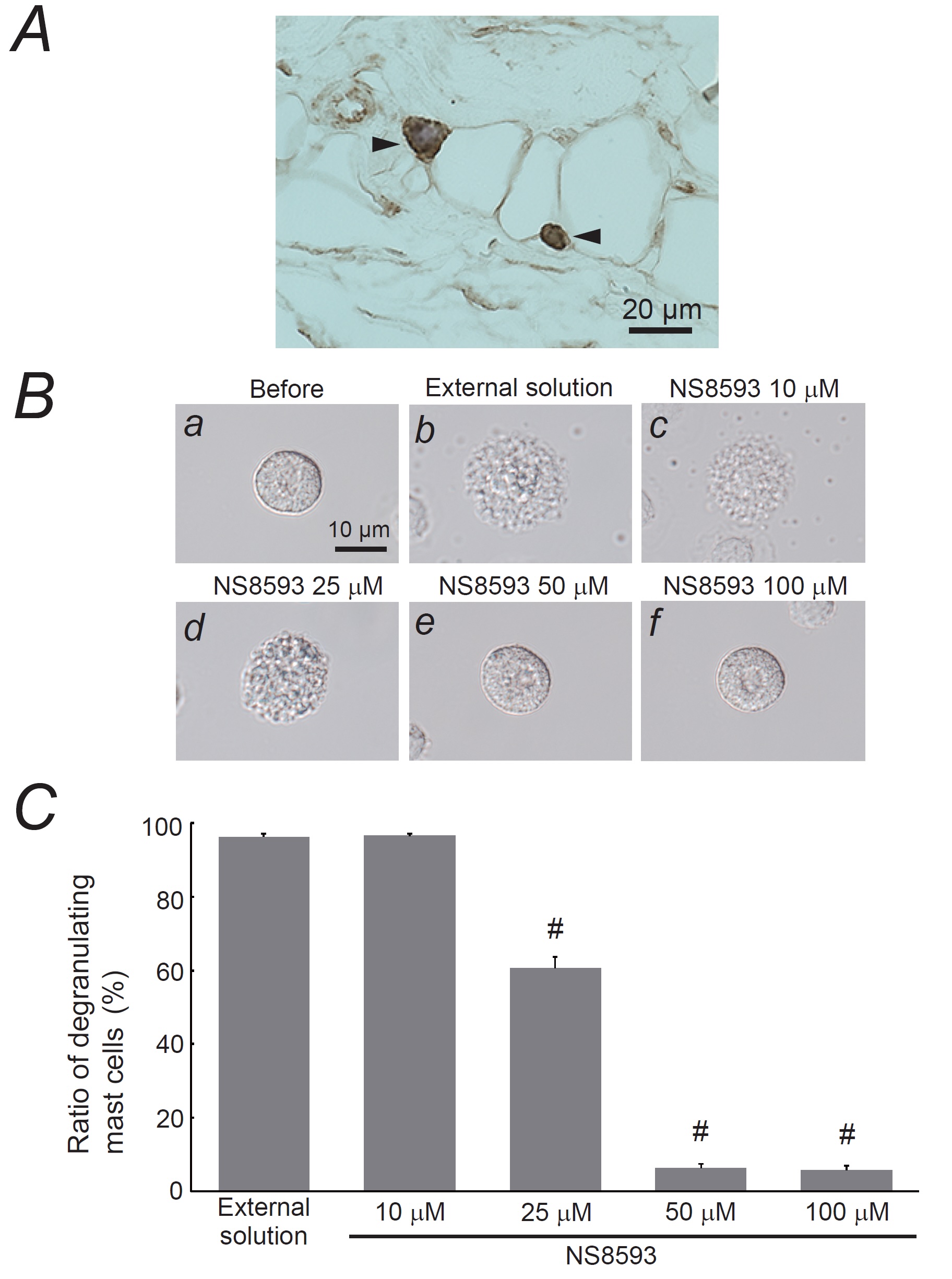
Fig. 4: Expression of transient receptor potential cation channel subfamily M member 7 (TRPM7) in mast cells and its involvement in mast cell degranulation. A: Immunohistochemistry using an antibody for anti-TRPM7 (brown) in rat peritoneal mast cells and adipose tissue, co-stained with 0.1% toluidine blue. Magnification X 60. B: Differential-interference contrast (DIC) microscopic images were taken before (a) and after exocytosis was externally induced by compound 48/80 in mast cells incubated in the external solutions containing no substance (b) or 10 mM NS8593 (c), 25 mM NS8593 (d), 50 mM NS8593 (e), and 100 mM NS8593 (f). C: After the mast cells were incubated in the external solutions containing no substance or different concentrations of NS8593, exocytosis was induced by compound 48/80. From a single rat, several samples of mast cell suspension were obtained from the peritoneal cavity. The aliquot of the sample was spread in a chamber placed at the head stage of an inverted microscope. Then bright-field images were obtained from randomly chosen 0.1-mm2 fields of view, in which 30-40 mast cells were evenly observed per field. The degranulating mast cells were expressed as the average percentages of the total mast cells in the 10 bright fields. # p < 0.05 vs. incubation in the external solution alone. Values were presented as the means ± SEM. Differences were analyzed using ANOVA followed by Dunnett’s t-test. The experiments were repeated at least three times using three different rats to confirm the reproducibility of the data.
Discussion
In addition to chemical mediators, such as histamine, serotonin, leukotrienes, and prostaglandins, mast cells release various cytokines and growth factors via exocytosis [10]. Therefore, to accurately assess the mast cell-stabilizing properties of drugs or substances, it is essential to directly observe exocytosis rather than indirectly measure the levels of chemical mediators released [18-22, 24, 43, 57]. In this study, we meticulously observed the entire exocytosis process under a microscope, defining it as the proportion of degranulating mast cells, to focus on the release of all chemical mediators [21-26, 29]. This method allowed us to demonstrate in vitro that adrenaline, macrolide antibiotics (clarithromycin), corticosteroids (dexamethasone and hydrocortisone), anti-hypertensives (prazosin), and anti-allergic drugs (tranilast, ketotifen, olopatadine, and cetirizine) possess mast cell-stabilizing properties [21-26]. Furthermore, we recently showed that food components such as caffeine, catechins, vitamins, and elements from lemon juice or peel (citric acid, hesperetin, and eriodictyol) stabilize mast cells and exert synergistic effects when combined [27-29]. In this study, using the same methodology, we provided direct evidence that essential trace elements such as magnesium and zinc inhibit exocytosis in a dose-dependent manner, thereby exhibiting mast cell-stabilizing properties (Fig. 1 and 2). In order to determine the effects of these trace elements, we examined the direct effects of MgCl2 and ZnCl2 on mast cell degranulation in the present study. However, as an electrolyte, chloride ions (Cl-) may affect the cell membrane potential and thus indirectly inhibit Ca2+ influx into mast cells [58]. Additionally, studies revealed that the pharmacological blockade of Cl- channels in mast cells modulate exocytosis [59, 60]. Therefore, caution should be exercised when interpreting these results.
Adrenaline is typically administered as a first-line treatment for anaphylaxis [53]. However, in our previous research, the ability of adrenaline to suppress mast cell degranulation was inadequate at the highest concentration of 1 mM [25]. Additionally, there have been cases of adrenaline-resistant refractory anaphylaxis in which patients did not respond well to adrenaline treatment [61-63]. Since adrenaline inhibits mast cell degranulation through the β2-adrenergic pathway [25], patients regularly taking β-adrenergic receptor blockers often show resistance to adrenaline [61]. Moreover, the use of perioperative drugs, such as muscle relaxants, certain antibiotics, and radiocontrast media, can increase the risk of adrenaline-resistant anaphylaxis, as these agents directly stimulate mast cells via the Mas-related G-protein coupled receptor member X2 (MRGPRX2), increasing the severity of the condition [62, 63]. In the present study, higher concentrations of MgCl2 and ZnCl2 enhanced the mast cell-stabilizing properties of adrenaline (Fig. 3). Thus, these trace elements may be beneficial in regard to augmenting the effects of adrenaline in cases of adrenaline-resistant refractory anaphylaxis.
In addition to their role in allergic reactions, mast cells are involved in the development of fibrosis in organs such as the lungs, liver, kidneys, and skin [64-66]. Under conditions, such as chronic inflammation, these cells release factors that activate fibroblasts, thereby worsening organ fibrosis [10]. Consequently, treatments that stabilize mast cells or inhibit chemokines that directly reduce mast cell activity have been shown to be effective against organ fibrosis [67-70]. Our previous study indicated that tranilast, a potent mast cell stabilizer, slowed the progression of peritoneal fibrosis in a rat model of chronic uremia [22]. In the current study, magnesium and zinc were identified as effective mast cell stabilizers (Fig. 1 and 2), suggesting their potential for treating or preventing organ fibrosis. Recent studies in humans and animals have linked magnesium and zinc deficiencies to the progression of organ fibrosis, including liver cirrhosis, renal fibrosis, and pulmonary fibrosis, and have shown that these trace elements can improve these conditions [71-75].
In this study, both MgCl2 and ZnCl2 demonstrated dose-dependent mast cell-stabilizing properties (Fig. 1 and 2) and they were shown to have enhanced the effects of adrenaline (Fig. 3). Notably, ZnCl2 appeared to be more potent than MgCl2 because much lower doses were required to achieve similar effects (Fig. 2 and 3). Our previous patch-clamp study showed that ethylene glycol tetra-acetic acid (EGTA), which chelates calcium ions (Ca2+) and blocks their intracellular transport, completely inhibits exocytosis [22]. Thus, consistent with earlier findings [16, 76], an increase in the intracellular Ca2+ concentration is considered the main trigger for mast cell exocytosis. Our results suggest that TRPM7, which is expressed in mast cells, plays a crucial role in mast cell degranulation (Fig. 4). TRPM7 facilitates Ca2+ entry into cells, contributing to an increase in the intracellular Ca2+ concentration in mast cells [30] (Fig. 5). As a channel for divalent cations, TRPM7 is also permeable to Mg2+ and Zn2+, which are thought to hinder Ca2+ entry into mast cells, thereby suppressing exocytosis. Importantly, since TRPM7 is more permeable to Zn2+ than to Mg2+ [32, 77], Zn2+ is believed to more effectively block Ca2+ entry than Mg2+ (Fig. 5), resulting in a stronger mast cell-stabilizing property of zinc when compared to magnesium. Additionally, once inside the cell, zinc modulates the p38 mitogen-activated protein kinase (p38MAPK) or nuclear factor-kappa B (NF-κB) signaling pathways, which are necessary for mast cell degranulation [78, 79]. This may provide zinc with additional mast cell-stabilizing properties.
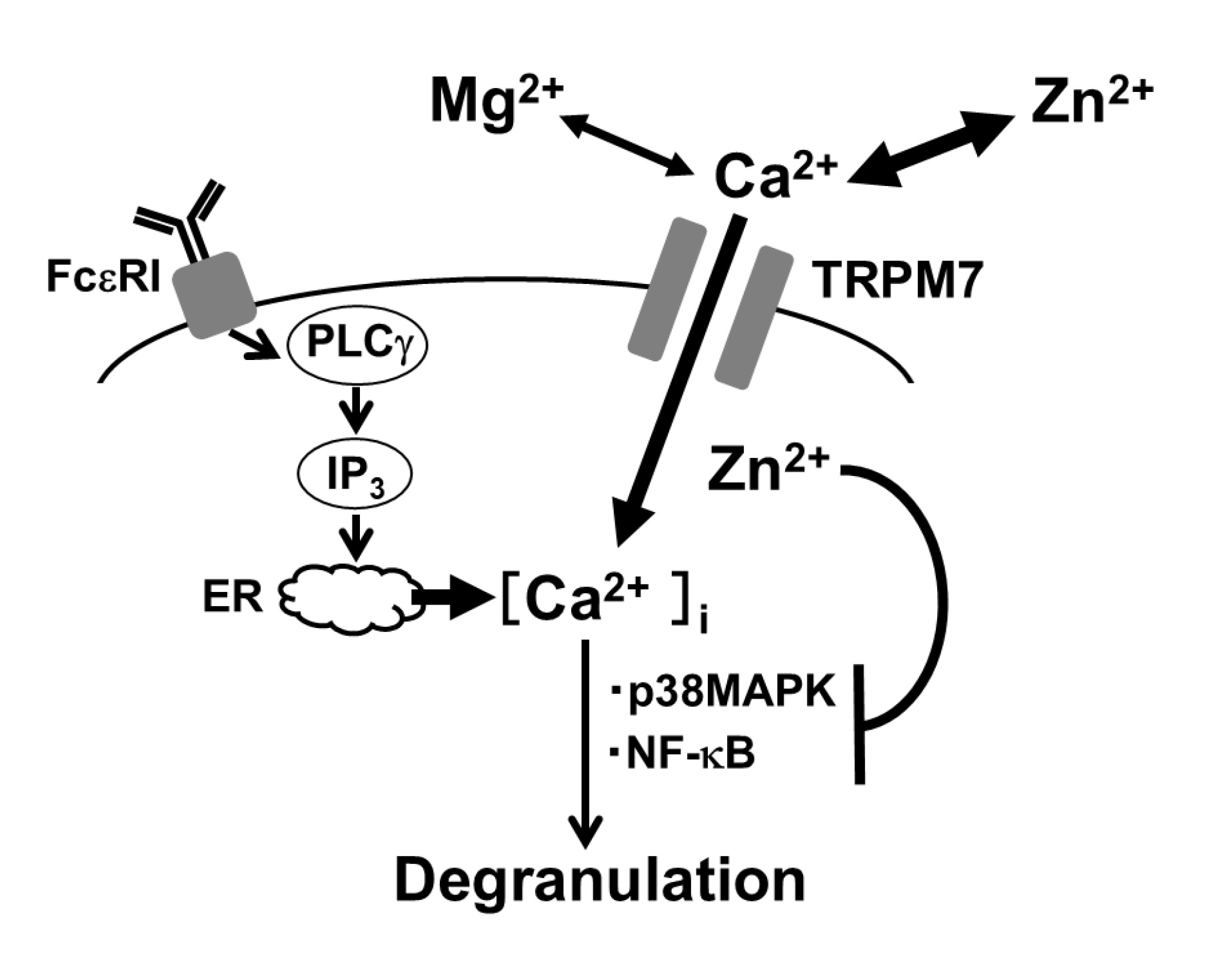
Fig. 5: Proposed mechanisms by which magnesium ions (Mg2+) and zinc ions (Zn2+) exert mast cell-stabilizing properties. Simulation of high-affinity IgE receptor (FceRI) results in the production of inositol triphosphate (IP3) by phospholipase C-g (PLC g) and release of calcium ions (Ca2+) through IP3 receptors from Ca2+ stores in the endoplasmic reticulum (ER). Such-induced rise in the intracellular Ca2+ concentration mediates a signal for mast cell degranulation. Transient receptor potential cation channel subfamily M member 7 (TRPM7), which allows Ca2+ entry into cells, contributes to the rise in intracellular Ca2+ concentration in mast cells. As a divalent cation channel, since TRPM7 is also permeable to Mg2+ and Zn2+, these cations interfere with Ca2+ entry into mast cells, causing the suppression of exocytosis. Because TRPM7 is much more permeable to Zn2+ than Mg2+, Zn2+ interferes with the Ca2+ entry more strongly than Mg2+. Additionally, once entering the cells, zinc modulates p38 mitogen-activated protein kinase (p38MAPK) or nuclear factor-kappa B (NF-κB) signaling pathways necessary for mast cell degranulation.
Conclusion
This study provides novel in vitro evidence for the first time that magnesium and zinc dose-dependently stabilize mast cells and additively potentiate the effects of adrenaline. TRPM7, which has higher permeability to Zn2+ than to Mg2+, may contribute to the stronger mast cell-stabilizing properties of zinc.
Acknowledgements
We thank Ms. Hiromi Yoshida at Institute of Development, Aging and Cancer, Tohoku University and people at the Biomedical Research Core of Tohoku University Graduate School of Medicine for their technical support and valuable advice.
Author contributions
IK and HS
performed the experiments and analyzed the data. IK designed the experiments, interpreted the
results, and
wrote
the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
This study was supported by the Tojuro Iijima Foundation for Food Science and
Technology, No. 2023-46, and the Salt Science Research Foundation, No. 2218, to IK and JSPS
KAKENHI Grant,
No.
22K11139, to JS.
Availability of data and materials
The data used
to support the findings of this study are available from the corresponding author upon request.
Ethics approval and consent to participate
This study was
performed in accordance with the Guide for the Care and Use of Laboratory Animals of Miyagi
University,
which
included ethical considerations.
Disclosure Statement
The authors have no competing interests to declare.
References
| 1 | Ashique S, Kumar S, Hussain A, Mishra N, Garg A, Gowda BHJ, Farid A, Gupta G, Dua K,
Taghizadeh-Hesary F: A narrative review on the role of magnesium in immune regulation,
inflammation, infectious diseases, and cancer. J Health Popul Nutr 2023;42:74.
https://doi.org/10.1186/s41043-023-00461-8 |
| 2 | Prasad AS: Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health.
Front Nutr 2014;1:14.
https://doi.org/10.3389/fnut.2014.00014 |
| 3 | Maarouf M, Vaughn AR, Shi VY: Topical micronutrients in atopic dermatitis-An
evidence-based review. Dermatol Ther 2018;31:e12659.
https://doi.org/10.1111/dth.12659 |
| 4 | Maywald M, Rink L: Zinc Deficiency and Zinc Supplementation in Allergic Diseases.
Biomolecules 2024;14
https://doi.org/10.3390/biom14070863 |
| 5 | Gray NA, Dhana A, Stein DJ, Khumalo NP: Zinc and atopic dermatitis: a systematic review
and meta-analysis. J Eur Acad Dermatol Venereol 2019;33:1042-1050.
https://doi.org/10.1111/jdv.15524 |
| 6 | Kusniec F, Fischer G, Sela BA, Ashkenazy Y, Feigel D, Moshonov S, Zor U: Magnesium
protects against anaphylactic shock and cardiac myolysis in guinea-pigs. J Basic Clin
Physiol Pharmacol 1994;5:45-58.
https://doi.org/10.1515/jbcpp-1994-050104 |
| 7 | Takahashi H, Nakazawa M, Takahashi K, Aihara M, Minami M, Hirasawa T, Ikezawa Z: Effects
of zinc deficient diet on development of atopic dermatitis-like eruptions in DS-Nh mice. J
Dermatol Sci 2008;50:31-39.
https://doi.org/10.1016/j.jdermsci.2007.11.002 |
| 8 | Lang C, Murgia C, Leong M, Tan LW, Perozzi G, Knight D, Ruffin R, Zalewski P:
Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models
of allergic inflammation. Am J Physiol Lung Cell Mol Physiol 2007;292:L577-584.
https://doi.org/10.1152/ajplung.00280.2006 |
| 9 | Shankar AH, Prasad AS: Zinc and immune function: the biological basis of altered
resistance to infection. Am J Clin Nutr 1998;68:447S-463S.
https://doi.org/10.1093/ajcn/68.2.447S |
| 10 | Gruber BL: Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep 2003;5:147-153.
https://doi.org/10.1007/s11926-003-0043-3 |
| 11 | Garland LG, Payne AN: The role of cell-fixed calcium in histamine release by compound
48/80. Br J Pharmacol 1979;65:609-613.
https://doi.org/10.1111/j.1476-5381.1979.tb07871.x |
| 12 | Cochrane DE, Douglas WW: Histamine release by exocytosis from rat mast cells on reduction
of extracellular sodium: a secretory response inhibited by calcium, strontium, barium or
magnesium. J Physiol 1976;257:433-448.
https://doi.org/10.1113/jphysiol.1976.sp011377 |
| 13 | Marone G, Columbo M, de Paulis A, Cirillo R, Giugliano R, Condorelli M: Physiological
concentrations of zinc inhibit the release of histamine from human basophils and lung mast
cells. Agents Actions 1986;18:103-106.
https://doi.org/10.1007/BF01987995 |
| 14 | Church DS, Church MK: Pharmacology of antihistamines. World Allergy Organ J 2011;4:S22-27.
https://doi.org/10.1186/1939-4551-4-S3-S22 |
| 15 | Finn DF, Walsh JJ: Twenty-first century mast cell stabilizers. Br J Pharmacol
2013;170:23-37.
https://doi.org/10.1111/bph.12138 |
| 16 | Neher E: The influence of intracellular calcium concentration on degranulation of dialysed
mast cells from rat peritoneum. J Physiol 1988;395:193-214.
https://doi.org/10.1113/jphysiol.1988.sp016914 |
| 17 | Penner R: Multiple signaling pathways control stimulus-secretion coupling in rat
peritoneal mast cells. Proc Natl Acad Sci U S A 1988;85:9856-9860.
https://doi.org/10.1073/pnas.85.24.9856 |
| 18 | Fernandez JM, Neher E, Gomperts BD: Capacitance measurements reveal stepwise fusion events
in degranulating mast cells. Nature 1984;312:453-455.
https://doi.org/10.1038/312453a0 |
| 19 | Lorenz D, Wiesner B, Zipper J, Winkler A, Krause E, Beyermann M, Lindau M, Bienert M:
Mechanism of peptide-induced mast cell degranulation. Translocation and patch-clamp studies.
J Gen Physiol 1998;112:577-591.
https://doi.org/10.1085/jgp.112.5.577 |
| 20 | Penner R, Neher E: The patch-clamp technique in the study of secretion. Trends Neurosci
1989;12:159-163.
https://doi.org/10.1016/0166-2236(89)90059-3 |
| 21 | Baba A, Tachi M, Maruyama Y, Kazama I: Olopatadine inhibits exocytosis in rat peritoneal
mast cells by counteracting membrane surface deformation. Cell Physiol Biochem
2015;35:386-396.
https://doi.org/10.1159/000369704 |
| 22 | Baba A, Tachi M, Ejima Y, Endo Y, Toyama H, Matsubara M, Saito K, Yamauchi M, Miura C,
Kazama I: Anti-Allergic Drugs Tranilast and Ketotifen Dose-dependently Exert Mast
Cell-Stabilizing Properties. Cell Physiol Biochem 2016;38:15-27.
https://doi.org/10.1159/000438605 |
| 23 | Mori T, Abe N, Saito K, Toyama H, Endo Y, Ejima Y, Yamauchi M, Goto M, Mushiake H, Kazama
I: Hydrocortisone and dexamethasone dose-dependently stabilize mast cells derived from rat
peritoneum. Pharmacol Rep 2016;68:1358-1365.
https://doi.org/10.1016/j.pharep.2016.09.005 |
| 24 | Kazama I, Saito K, Baba A, Mori T, Abe N, Endo Y, Toyama H, Ejima Y, Matsubara M, Yamauchi
M: Clarithromycin Dose-Dependently Stabilizes Rat Peritoneal Mast Cells. Chemotherapy
2016;61:295-303.
https://doi.org/10.1159/000445023 |
| 25 | Abe N, Toyama H, Ejima Y, Saito K, Tamada T, Yamauchi M, Kazama I: Prazosin Potentiates
Mast Cell-Stabilizing Property of Adrenaline. Cell Physiol Biochem 2024;58:212-225.
https://doi.org/10.33594/000000703 |
| 26 | Fujimura R, Asada A, Aizawa M, Kazama I: Cetirizine more potently exerts mast
cell-stabilizing property than diphenhydramine. Drug Discov Ther 2022;16:245-250.
https://doi.org/10.5582/ddt.2022.01067 |
| 27 | Yashima M, Sato Y, Kazama I: Catechin synergistically potentiates mast cell-stabilizing
property of caffeine. Allergy Asthma Clin Immunol 2021;17:1.
https://doi.org/10.1186/s13223-020-00502-5 |
| 28 | Kazama I, Sato Y, Tamada T: Pyridoxine Synergistically Potentiates Mast Cell-Stabilizing
Property of Ascorbic Acid. Cell Physiol Biochem 2022;56:282-292.
https://doi.org/10.33594/000000534 |
| 29 | Sato A, Kikuta Y, Kazama I: Lemon Juice and Peel Constituents Potently Stabilize Rat
Peritoneal Mast Cells. Cell Physiol Biochem 2024;58:445-457.
|
| 30 | Nam JH, Kim WK: The Role of TRP Channels in Allergic Inflammation and its Clinical
Relevance. Curr Med Chem 2020;27:1446-1468.
https://doi.org/10.2174/0929867326666181126113015 |
| 31 | Zierler S, Sumoza-Toledo A, Suzuki S, Duill FO, Ryazanova LV, Penner R, Ryazanov AG, Fleig
A: TRPM7 kinase activity regulates murine mast cell degranulation. J Physiol
2016;594:2957-2970.
https://doi.org/10.1113/JP271564 |
| 32 | Abiria SA, Krapivinsky G, Sah R, Santa-Cruz AG, Chaudhuri D, Zhang J, Adstamongkonkul P,
DeCaen PG, Clapham DE: TRPM7 senses oxidative stress to release Zn(2+) from unique
intracellular vesicles. Proc Natl Acad Sci U S A 2017;114:E6079-E6088.
https://doi.org/10.1073/pnas.1707380114 |
| 33 | Duan J, Li Z, Li J, Hulse RE, Santa-Cruz A, Valinsky WC, Abiria SA, Krapivinsky G, Zhang
J, Clapham DE: Structure of the mammalian TRPM7, a magnesium channel required during
embryonic development. Proc Natl Acad Sci U S A 2018;115:E8201-E8210.
https://doi.org/10.1073/pnas.1810719115 |
| 34 | Sudakov SK, Alekseeva EV, Nazarova GA, Bashkatova VG: Age-Related Individual Behavioural
Characteristics of Adult Wistar Rats. Animals (Basel) 2021;11.
https://doi.org/10.20944/preprints202106.0729.v1 |
| 35 | Magro AM, Brai M: Evidence for lipoxygenase activity in induction of histamine release
from rat peritoneal mast cells by chelated iron. Immunology 1983;49:1-8.
|
| 36 | Tasaka K, Endo K, Yamasaki H: Degranulation and histamine release in focal
antigen-antibody reaction by means of microelectrophoresis in a single rat mesentery mast
cell. Jpn J Pharmacol 1972;22:89-95.
https://doi.org/10.1016/S0021-5198(19)31711-1 |
| 37 | Zelechowska P, Agier J, Rozalska S, Wiktorska M, Brzezinska-Blaszczyk E: Leptin stimulates
tissue rat mast cell pro-inflammatory activity and migratory response. Inflamm Res
2018;67:789-799.
https://doi.org/10.1007/s00011-018-1171-6 |
| 38 | Mackey E, Moeser AJ: Sex Differences in Mast Cell-Associated Disorders: A Life Span
Perspective. Cold Spring Harb Perspect Biol 2022;14
https://doi.org/10.1101/cshperspect.a039172 |
| 39 | Clarkson JM, Martin JE, McKeegan DEF: A review of methods used to kill laboratory rodents:
issues and opportunities. Lab Anim 2022;56:419-436.
https://doi.org/10.1177/00236772221097472 |
| 40 | Kazama I, Maruyama Y, Takahashi S, Kokumai T: Amphipaths differentially modulate membrane
surface deformation in rat peritoneal mast cells during exocytosis. Cell Physiol Biochem
2013;31:592-600.
https://doi.org/10.1159/000350079 |
| 41 | Jamur MC, Grodzki AC, Moreno AN, Swaim WD, Siraganian RP, Oliver C: Immunomagnetic
isolation of rat bone marrow-derived and peritoneal mast cells. J Histochem Cytochem
1997;45:1715-1722.
https://doi.org/10.1177/002215549704501215 |
| 42 | Jamur MC, Moreno AN, Mello LF, Souza Junior DA, Campos MR, Pastor MV, Grodzki AC, Silva
DC, Oliver C: Mast cell repopulation of the peritoneal cavity: contribution of mast cell
progenitors versus bone marrow derived committed mast cell precursors. BMC Immunol
2010;11:32.
https://doi.org/10.1186/1471-2172-11-32 |
| 43 | Shimoda T, Liang Z, Suzuki H, Kawana S: Inhibitory effects of antipsychotic and anxiolytic
agents on stress-induced degranulation of mouse dermal mast cells. Clin Exp Dermatol
2010;35:531-536.
https://doi.org/10.1111/j.1365-2230.2009.03650.x |
| 44 | Stone KD, Prussin C, Metcalfe DD: IgE, mast cells, basophils, and eosinophils. J Allergy
Clin Immunol 2010;125:S73-80.
https://doi.org/10.1016/j.jaci.2009.11.017 |
| 45 | Caulfield JP, Lewis RA, Hein A, Austen KF: Secretion in dissociated human pulmonary mast
cells. Evidence for solubilization of granule contents before discharge. J Cell Biol
1980;85:299-312.
https://doi.org/10.1083/jcb.85.2.299 |
| 46 | Ye J, Piao H, Jiang J, Jin G, Zheng M, Yang J, Jin X, Sun T, Choi YH, Li L, Yan G:
Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK,
NF-kappaB and Nrf2/HO-1 pathways. Sci Rep 2017;7:11895.
https://doi.org/10.1038/s41598-017-12252-3 |
| 47 | Bessa-Goncalves M, Ribeiro-Machado C, Costa M, Ribeiro CC, Barbosa JN, Barbosa MA, Santos
SG: Magnesium incorporation in fibrinogen scaffolds promotes macrophage polarization towards
M2 phenotype. Acta Biomater 2023;155:667-683.
https://doi.org/10.1016/j.actbio.2022.10.046 |
| 48 | Mizrahi B, Shapira L, Domb AJ, Houri-Haddad Y: Citrus oil and MgCl2 as antibacterial and
anti-inflammatory agents. J Periodontol 2006;77:963-968.
https://doi.org/10.1902/jop.2006.050278 |
| 49 | von Bulow V, Rink L, Haase H: Zinc-mediated inhibition of cyclic nucleotide
phosphodiesterase activity and expression suppresses TNF-alpha and IL-1 beta production in
monocytes by elevation of guanosine 3',5'-cyclic monophosphate. J Immunol
2005;175:4697-4705.
https://doi.org/10.4049/jimmunol.175.7.4697 |
| 50 | Wellinghausen N, Martin M, Rink L: Zinc inhibits interleukin-1-dependent T cell
stimulation. Eur J Immunol 1997;27:2529-2535.
https://doi.org/10.1002/eji.1830271010 |
| 51 | Barrett KE, Pearce FL: A comparison of histamine secretion from isolated peritoneal mast
cells of the mouse and rat. Int Arch Allergy Appl Immunol 1983;72:234-238.
https://doi.org/10.1159/000234873 |
| 52 | Kazama I, Baba A, Endo Y, Toyama H, Ejima Y, Matsubara M, Tachi M: Mast cell involvement
in the progression of peritoneal fibrosis in rats with chronic renal failure. Nephrology
(Carlton) 2015;20:609-616.
https://doi.org/10.1111/nep.12489 |
| 53 | Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, Geller M,
Gonzalez-Estrada A, Greenberger PA, Sanchez Borges M, Senna G, Sheikh A, Tanno LK, Thong BY,
Turner PJ, Worm M: World allergy organization anaphylaxis guidance 2020. World Allergy Organ
J 2020;13:100472.
https://doi.org/10.1016/j.waojou.2020.100472 |
| 54 | Inoue H, Inazu M, Konishi M, Yokoyama U: Functional expression of TRPM7 as a Ca(2+) influx
pathway in adipocytes. Physiol Rep 2019;7:e14272.
https://doi.org/10.14814/phy2.14272 |
| 55 | Chubanov V, Mederos y Schnitzler M, Meissner M, Schafer S, Abstiens K, Hofmann T,
Gudermann T: Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit
magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol
2012;166:1357-1376.
https://doi.org/10.1111/j.1476-5381.2012.01855.x |
| 56 | Chubanov V, Gudermann T: Mapping TRPM7 Function by NS8593. Int J Mol Sci 2020;21
https://doi.org/10.3390/ijms21197017 |
| 57 | Yoo JM, Kim JH, Park SJ, Kang YJ, Kim TJ: Inhibitory effect of eriodictyol on
IgE/Ag-induced type I hypersensitivity. Biosci Biotechnol Biochem 2012;76:1285-1290.
https://doi.org/10.1271/bbb.110952 |
| 58 | Cao M, Gao Y: Mast cell stabilizers: from pathogenic roles to targeting therapies. Front
Immunol 2024;15:1418897.
https://doi.org/10.3389/fimmu.2024.1418897 |
| 59 | Aljameeli AM, Alsuwayt B, Bharati D, Gohri V, Mohite P, Singh S, Chidrawar V: Chloride
channels and mast cell function: pioneering new frontiers in IBD therapy. Mol Cell Biochem
2025.
https://doi.org/10.1007/s11010-025-05243-w |
| 60 | Alton EW, Norris AA: Chloride transport and the actions of nedocromil sodium and cromolyn
sodium in asthma. J Allergy Clin Immunol 1996;98:S102-105; discussion S105-106.
https://doi.org/10.1016/S0091-6749(96)80136-9 |
| 61 | Murakami Y, Kaneko S, Yokoyama H, Ishizaki H, Sekino M, Murata H, Hara T: Successful
treatment of severe adrenaline-resistant anaphylactic shock with glucagon in a patient
taking a beta-blocker: a case report. JA Clin Rep 2021;7:86.
https://doi.org/10.1186/s40981-021-00490-4 |
| 62 | Nakano T, Nakamura Y, Sato K, Izutani Y, Iyota H, Aoyagi M, Kitamura T, Hayashi T, Matsuo
K, Mishima K, Kamimura H, Ishikura H, Egawa T: Adrenaline-resistant anaphylactic shock
caused by contrast medium in a patient after risperidone overdose: a case report. J Pharm
Health Care Sci 2023;9:23.
https://doi.org/10.1186/s40780-023-00292-z |
| 63 | Francuzik W, Dolle-Bierke S, Knop M, Scherer Hofmeier K, Cichocka-Jarosz E, Garcia BE,
Lang R, Maris I, Renaudin JM, Worm M: Refractory Anaphylaxis: Data From the European
Anaphylaxis Registry. Front Immunol 2019;10:2482.
https://doi.org/10.3389/fimmu.2019.02482 |
| 64 | Gruber BL: Mast cells: accessory cells which potentiate fibrosis. Int Rev Immunol
1995;12:259-279.
https://doi.org/10.3109/08830189509056717 |
| 65 | Holdsworth SR, Summers SA: Role of mast cells in progressive renal diseases. J Am Soc
Nephrol 2008;19:2254-2261.
https://doi.org/10.1681/ASN.2008010015 |
| 66 | Blank U, Essig M, Scandiuzzi L, Benhamou M, Kanamaru Y: Mast cells and inflammatory kidney
disease. Immunol Rev 2007;217:79-95.
https://doi.org/10.1111/j.1600-065X.2007.00503.x |
| 67 | Miyajima A, Asano T, Yoshimura I, Seta K, Hayakawa M: Tranilast ameliorates renal tubular
damage in unilateral ureteral obstruction. J Urol 2001;165:1714-1718.
https://doi.org/10.1016/S0022-5347(05)66400-2 |
| 68 | Kelly DJ, Zhang Y, Gow R, Gilbert RE: Tranilast attenuates structural and functional
aspects of renal injury in the remnant kidney model. J Am Soc Nephrol 2004;15:2619-2629.
https://doi.org/10.1097/01.ASN.0000139066.77892.04 |
| 69 | Shiota N, Kakizoe E, Shimoura K, Tanaka T, Okunishi H: Effect of mast cell chymase
inhibitor on the development of scleroderma in tight-skin mice. Br J Pharmacol
2005;145:424-431.
https://doi.org/10.1038/sj.bjp.0706209 |
| 70 | Doggrell SA, Wanstall JC: Cardiac chymase: pathophysiological role and therapeutic
potential of chymase inhibitors. Can J Physiol Pharmacol 2005;83:123-130.
https://doi.org/10.1139/y04-136 |
| 71 | Tao MH, Fulda KG: Association of Magnesium Intake with Liver Fibrosis among Adults in the
United States. Nutrients 2021;13.
https://doi.org/10.3390/nu13010142 |
| 72 | Long M, Zhu X, Wei X, Zhao D, Jiang L, Li C, Jin D, Miao C, Du Y: Magnesium in renal
fibrosis. Int Urol Nephrol 2022;54:1881-1889.
https://doi.org/10.1007/s11255-022-03118-3 |
| 73 | Luo X, Deng Q, Xue Y, Zhang T, Wu Z, Peng H, Xuan L, Pan G: Anti-Fibrosis Effects of
Magnesium Lithospermate B in Experimental Pulmonary Fibrosis: By Inhibiting TGF-betaRI/Smad
Signaling. Molecules 2021;26
https://doi.org/10.3390/molecules26061715 |
| 74 | Aksoy-Ozer ZB, Bitirim CV, Turan B, Akcali KC: The Role of Zinc on Liver Fibrosis by
Modulating ZIP14 Expression Throughout Epigenetic Regulatory Mechanisms. Biol Trace Elem Res
2024;202:5094-5105.
https://doi.org/10.1007/s12011-023-04057-5 |
| 75 | Huang Z, Liao Y, Zheng Y, Ye S, Zhang Q, Yu X, Liu X, Li N: Zinc Deficiency Causes
Glomerulosclerosis and Renal Interstitial Fibrosis Through Oxidative Stress and Increased
Lactate Metabolism in Rats. Biol Trace Elem Res 2025;203:2084-2098.
https://doi.org/10.1007/s12011-024-04306-1 |
| 76 | Oshiro T, Kakuta Y, Maruyama N, Fushimi T, Okayama H, Tamura G, Shimura S, Shirato K:
Patch-clamp characterization of secretory process in human basophils. Int Arch Allergy
Immunol 1997;112:336-340.
https://doi.org/10.1159/000237477 |
| 77 | Inoue K, O'Bryant Z, Xiong ZG: Zinc-permeable ion channels: effects on intracellular zinc
dynamics and potential physiological/pathophysiological significance. Curr Med Chem
2015;22:1248-1257.
https://doi.org/10.2174/0929867322666150209153750 |
| 78 | Shi Q, Shen X, Long C, Mi Z, Li Y, Ma R: Zinc supplement reduces allergic responses
through modulating the p38 MAPK pathway activation in an allergic rhinitis mouse model. J
Trace Elem Med Biol 2023;75:127094.
https://doi.org/10.1016/j.jtemb.2022.127094 |
| 79 | Nishida K, Uchida R: Role of Zinc Signaling in the Regulation of Mast Cell-, Basophil-,
and T Cell-Mediated Allergic Responses. J Immunol Res 2018;2018:5749120.
https://doi.org/10.1155/2018/5749120 |