Post-Acute COVID-19 Pathophysiology: Cellular Stress Responses, Immune Dysregulation, and Biochemical Signatures in Recovery Phase
bDepartment of Microbiology, Faculty of Science, Omar Al-Mukhtar University AL-Bayda, Libya,
cK.R.T. Arts, B.H. Commerce & A.M. Science (KTHM) College, Nashik, Maharashtra, India
Keywords
Abstract
Background/Aims:
Post-acute COVID-19 syndrome (PACS) presents with persistent symptoms such as fatigue, dyspnea, and cognitive impairment, even after apparent clinical recovery. Although widely reported, the biological basis of these symptoms remains unclear. This study aimed to investigate the underlying cellular, immunological, oxidative, and biochemical disturbances during the recovery phase of COVID-19 and evaluate their association with clinical symptomatology.Methods:
A cross-sectional observational study was conducted involving 120 participants who were previously SARS-CoV-2 positive, recruited ≥30 days post-recovery. Peripheral blood samples were analyzed for ER stress markers (HSP70, CHOP, GRP78), Pro- and anti-inflammatory cytokines (IL-6, TNF-α, IFN-γ, IL-10), oxidative biomarkers (MDA, SOD, GSH), and biochemical parameters (ALT, AST, CRP, ferritin). T cell subsets were evaluated via flow cytometry. Statistical comparisons and correlation analyses were performed using SPSS v22.0.Results:
Significant elevations were observed in all stress and inflammatory markers (p < 0.05). IL-6, CRP, and MDA showed strong positive correlations with fatigue and dyspnea scores. Treg percentages were reduced, and males exhibited higher biomarker levels than females. Persistent immune and oxidative activation was evident in the recovery phase.Conclusion:
Post-acute COVID-19 is associated with quantifiable cellular and molecular disturbances. This integrated analysis of ER stress, immune dysregulation, and oxidative imbalance provides a novel and comprehensive view of long COVID pathophysiology.Introduction
The coronavirus disease 2019 (COVID-19), induced by the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2), has surfaced as an unprecedented global health crisis. Although the majority of people recover from the infection’s acute phase, there is mounting evidence that shows the persistence of various clinical manifestations after viral clearance. Collectively, these symptoms, which are usually referred to as post-acute COVID-19 syndrome or long COVID, are characterized by fatigue, dyspnea, myalgia, cognitive impairment, and a spectrum of systemic abnormalities, which can last for weeks or even months [1]. The post-recovery is increasingly recognized not as a period of returning to the physiological baseline, but as one of immunological, biochemical, and cellular changes. In order to effectively clinically manage and therapeutically intervene in this process, it is necessary to understand the underlying mechanisms of this transition from acute illness into persistent dysfunction. SARS-CoV-2 pathophysiological studies suggest that damage to cells throughout the body is triggered through direct cytopathic effects, hyperinflammation, and oxidative stress [2]. An intense host immune response, which may be referred to as a “cytokine storm” during the acute phase, may result in multiorgan injury. The inflammatory markers remain elevated, and there is a disruption in immune cell populations, even after the viral clearance and resolution of acute symptoms [3]. Oxidative stress that occurs in the acute phase may not fully disappear, and could further contribute to cellular damage and dysfunction, mitochondrial imbalance, and malfunction of tissue repair [4].
“Cellular SARS-CoV-2 infection triggers stress mechanisms that include the Endoplasmic Reticulum (ER) stress pathway and the Unfolded Protein Response (UPR). Key molecular chaperones such as 70 (HSP70), C/EBP homologous protein (CHOP), and Glucose-Related Protein 78 (GRP78) are upregulated during viral invasion and cellular insult, serving as markers of prolonged ER stress and ongoing cellular distress in convalescent individuals [5, 6]. Another hallmark of the post-acute COVID-19 state is immune dysregulation. Severe sepsis is associated with persistent elevation of proinflammatory cytokines like interleukin 6 (IL-6), interleukin 10 (IL-10), tumor necrosis factor alpha (TNF-α), interleukin 2 (IL-2), and interleukin 4 (IL-4) and interferon gamma (IFN-γ), whereas regulatory cytokines like IL-10, fail to be normalized [7]. These cytokines are key to maintaining the immune balance and regulating inflammatory reactions. Their continued elevation during the recovery phase suggests ongoing immune activation to the extent that it may lead to long-term immune exhaustion or autoimmunity [8]. Disruption of T cell subsets, involving a lower proportion of regulatory T cells (Treg) compared to effector cells, can affect the immune tolerance and play an important part in the pathogenesis of the post-COVID complications [9].”
The post-infection phase is further illustrate by biochemical markers of the disease. Liver enzymes, C-reactive protein (CRP), ferritin, and D-dimer are elevated even in those patients in whom overt clinical symptoms have subsided [10]. These indicate subclinical organ stress and systemic inflammation, which may cumulatively affect long-term health outcomes. Liver enzyme elevation is associated with persistent hepatocellular injury, and elevated ferritin and CRP indicate unresolved inflammation [11]. Concerns have especially been raised that individual who are recovered still have ongoing endothelial dysfunction and thrombotic risks, which result in D-dimer elevations in these individuals [12]. Oxidative stress has become a key player in the pathophysiology of both acute and post-acute COVID-19. During viral infection, ROS produced contribute to lipid peroxidation, protein oxidation, and DNA damage, which can interfere with cellular signaling and induce apoptosis [13].
“Normally, markers such as Superoxide Dismutase (SOD), Malondialdehyde (MDA), and reduced glutathione (GSH) are used to assess oxidative status. According to studies, MDA and SOD levels increase, and GSH decreases, even in the convalescent phase, indicating that oxidative stress may be involved in the maintenance of a pro‐inflammatory state and tissue injury [14]. Oxidative imbalances may be detrimental to recovery and may aggravate any existing comorbid conditions. Use of relevant mouse models has led to significant progress in understanding the acute phase of COVID-19, however, there has been a paucity of clues on the molecular and cellular events that play out beyond the resolution of the acute illness. These data, in particular, are of great importance for designing post-discharge and long-term follow-up strategies for such patients. The concept of COVID-19 as a multi-system disease with long-term effects emerging necessitates such a comprehensive assessment [15].”
“While long COVID literature is expanding, few studies simultaneously evaluate cellular stress markers, immune profiles, and biochemical indicators within the same cohort. Most prior research has examined these domains in isolation, limiting integrated pathophysiological conclusions. Regional and demographic variability may influence the nature and severity of post-acute manifestations, highlighting the need for local investigations. Additionally, factors such as age, sex, and comorbidities may modulate immune and biochemical responses, suggesting that subgroup analyses could provide more precise insights [16]. With this knowledge gap, this study sought to bridge it by studying the interactions between cellular stress response, immune dysregulation, and biochemical signatures in people recovering from COVID-19. It evaluates key markers, including HSP70, CHOP and GRP78, pro and anti-inflammatory cytokines (IL 6, TNF-α, IFN-γ, and IL-10) and clinical biochemical indicators, CRP, ferritin, liver enzymes, and oxidative stress biomarkers (MDA, SOD, and GSH). This study uniquely integrates multiple biological domains, cellular stress, immune dysregulation, and oxidative stress into a unified analysis of post-acute COVID-19 recovery.
“The main goal of this study is to investigate the pathophysiological changes during the post-acute recovery phase of COVID-19. In particular, it is designed to evaluate the levels and relationships of cellular stress markers, pro- and anti-inflammatory cytokines, and key biochemical parameters in convalescent patients. Integrating these parameters will be used in the study to uncover the mechanisms causing persistent inflammation and dysfunction during the recovery period, with the aim of improving post-COVID clinical care and monitoring strategies.”
Materials and Methods
Study Design and Ethical Compliance
The study examined the pathophysiological changes in the recovery phase of COVID-19 through cellular
stress markers, immune dysregulation, and biochemical signatures, and followed a cross-sectional
observational design. The Institutional Ethics Committee reviewed and approved the study protocol as per
the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines. Participants gave written
informed consent before sample collection, and the confidentiality and anonymity of all participants were
ensured. To outline the participant selection process, a cohort flow diagram containing the recruitment,
inclusion, and exclusion criteria is illustrated in Fig.1.
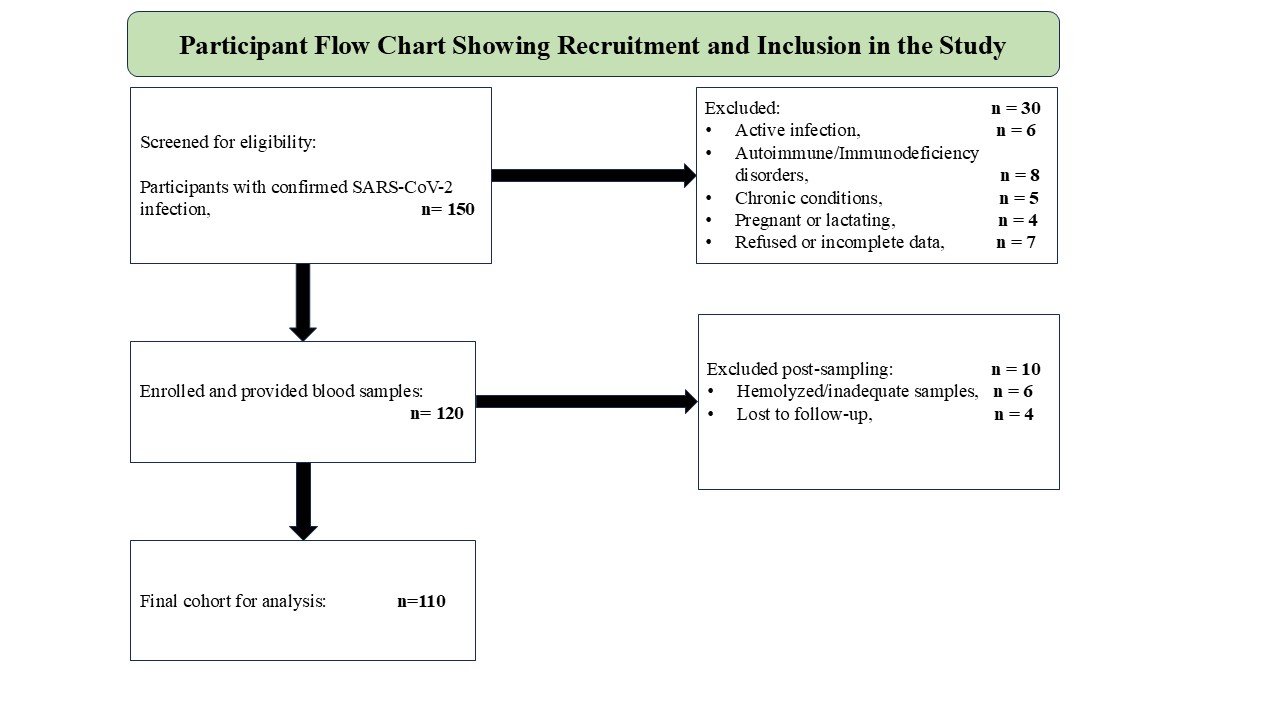
Fig. 1: Cohort Selection and Participant Recruitment Process in the Study of Post-Acute COVID-19 Pathophysiology.
Study Population and Participant Recruitment
Inclusion Criteria. Between January 2023 and June 2023, a total of 120 participants were recruited
from the Post-COVID Recovery Clinic, New Delhi, India. All participants were laboratory confirmed by
RT-PCR for SARS-CoV-2 and had completed at least 30 days since clinical recovery. The included
participants were between 25 to 60 years of age, had no acute reinfection, positive COVID-19 status, and
agreed to provide blood samples for immunological and biochemical evaluation during the recovery phase.
Exclusion Criteria. Exclusion criteria were active infection or fever at the time of sampling,
history of autoimmune disease, malignancy, immunodeficiency, or chronic inflammatory or metabolic
disorders. Also excluded were pregnant or lactating women, as well as individuals receiving
immunosuppressive or corticosteroid therapy. To be eligible and eliminate confounding variables by
post-COVID cohort study protocols, comprehensive clinical histories were obtained using a structured
questionnaire and verified in the electronic medical record [17].
Sample Collection and Biospecimen Processing
“10 milliliters of peripheral venous blood was collected from each participant aseptically between
8:00 AM and 10:00 AM to avoid circadian variability in blood parameters. Ficoll-Paque™ PLUS density
gradient centrifugation (GE Healthcare) was used to isolate Peripheral Blood Mononuclear Cells (PBMCs)
[18]. 1:1 dilution of whole blood with PBS, layering over Ficoll in a 15 mL conical tube and
centrifugation at 400 × g, 30 min, 20°C without brake. PBMCs were collected, washed, and
resuspended in RPMI 1640 medium containing 10% fetal bovine serum, 1% penicillin, streptomycin, and 1%
glutamine. Serum and plasma were aliquoted and stored at -80 °C. The remaining blood was centrifuged
at 2, 000 rpm for 15 minutes at 4°C. Analysis was limited to samples not been hemolyzed.”
Assessment of Cellular Stress Markers (Western Blot)
“Western blotting was used to determine the amount of cellular stress markers, including HSP70,
CHOP, and GRP78, in PBMC lysates. RIPA buffer (Thermo Scientific) containing protease and phosphatase
inhibitors was used to lyse PBMCs. The Bradford assay was used to quantify total protein concentration
[19]. SDS-PAGE (10-12%) of equal protein amounts (30 µg) was performed, and the proteins were
transferred to PVDF membranes. The membranes were blocked with 5% BSA in TBS, TBS-T overnight at 4°C
with primary antibodies (HSP70, CHOP, GRP78 1:1000, β-actin 1:5000) and subsequently with
HRP-conjugated secondary antibodies. ECL substrate was used to visualize bands on a ChemiDoc MP system,
and bands were quantified with ImageJ software [20].”
Serum Cytokine Analysis (ELISA)
“IL-6, TNF-α, IFN-γ and IL-10 serum concentrations were measured using high-sensitivity
ELISA kits (R&D Systems) according to the manufacturer’s protocol. Duplicate analysis of all
samples and standards was performed in order to achieve accuracy. Cytokine concentrations were determined
by standard curves, and absorbance was read at 450 nm using a Biosok microplate reader [21].”
T-Cell Subpopulation Analysis (Flow Cytometry)
T cell subsets, including CD4+, CD8+, and
CD4+CD25+FoxP3+ regulatory T cells (Tregs), were analyzed by flow
cytometry. PBMCs freshly isolated (1 × 106 cells) were stained with
fluorochrome-conjugated antibodies including CD3-FITC, CD4-PE, CD8-APC, CD25-PerCP, and FoxP3-PE-Cy7.
Cells were incubated on ice for 30 min in the dark and washed in FACS buffer before fixation with 1%
paraformaldehyde. FoxP3 was detected intracellularly using a fixation and permeabilization buffer set
(eBioscience). BD FACSCanto™ II cytometer was used to acquire data, and FlowJo v10 software was used
to analyze.
Clinical Biochemistry
“Organs’ function and systemic inflammations were evaluated by routine clinical biochemistry
findings using automated analyzers (Roche Cobas 6000). Liver enzymes studied, Alanine Aminotransferase
(ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), renal function markers, creatinine,
and Blood Urea Nitrogen (BUN), and inflammatory markers, C-reactive protein (CRP), ferritin, and D-dimer
were examined. The standard operating procedures were all followed in conducting all the tests to ensure
accuracy and reproducibility.”
Oxidative Stress Biomarkers
“Standard colorimetric spectrophotometric assays were performed to see the serum oxidative stress
biomarkers. The level of malondialdehyde (MDA) was determined by the thiobarbituric acid reactive
substances (TBARS) method with absorbance read at 532 nm. The activity of superoxide dismutase (SOD) was
measured by epinephrine autoxidation method and reduced glutathione (GSH) levels were determined using
Ellmann’s reagent method [22]. Triple assays were carried out to ensure reproducibility and
analytical accuracy.”
Statistical Analysis
Data were analysed statistically using SPSS version 22.0. The Shapiro–Wilk test was used to
determine the normality of data distribution. The Student’s t-test was used for comparison of groups
with normally distributed variables, while for comparison of groups with non-normally distributed
variables, the Mann-Whitney U test was applied. Pearson’s or Spearman’s correlation
coefficients were calculated to conduct correlation analyses between cellular stress markers, cytokines,
and biochemical parameters. All analyses were two-tailed and used a p-value of less than 0.05 as
significant. The results were described as the mean ± standard deviation or median with interquartile
range wherever appropriate.”
Results
Expression of Cellular Stress Markers
In PBMCs of post-acute COVID-19 participants, the levels of cellular stress markers were found to be
increased significantly. Levels of HSP70 (1.85 ± 0.25 AU), CHOP (2.32 ± 0.30 AU), and GRP78
(2.91 ± 0.28 AU) were elevated by an order of magnitude higher than control values. Unpaired t tests
on statistical comparison showed p values of 0.002, 0.001, and 0.0005, respectively, supporting that these
increases are significant mentioned in Table 1. These results indicate that there was persistence of ER
stress and cellular homeostatic disruption in the post-recovery phase.
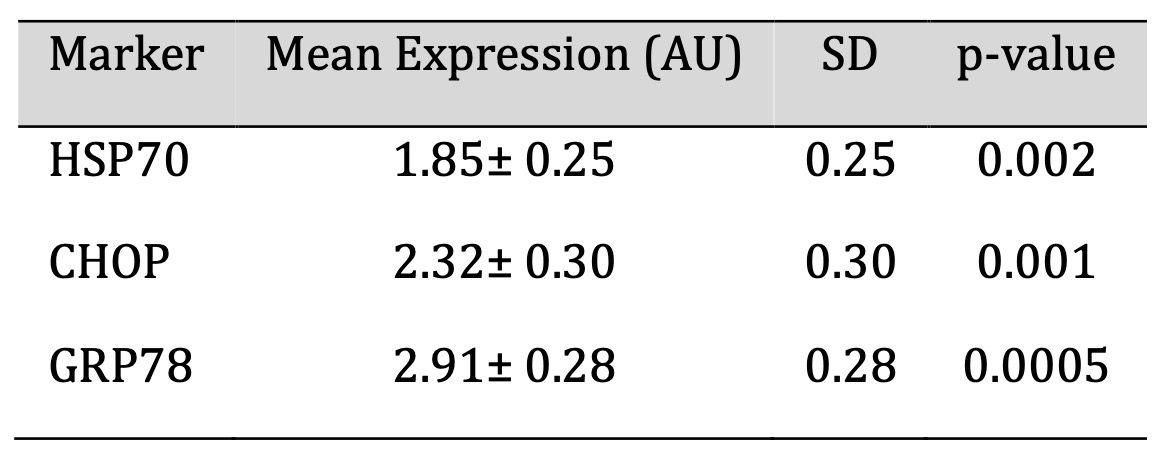
Table 1: Cellular Stress Marker Expression in PBMCs of Post-Acute COVID-19 Individuals
Cytokine Profiling and Immune Dysregulation
The recovered individuals showed a pro-inflammatory skew in the immune profile and were analyzed for
cytokines. IL-10 (21.8 ± 3.9 pg/mL) modestly increased, as did IL-6 (38.5 ± 5.2 pg/mL),
TNF-α (52.1 ± 6.1 pg/mL), and IFN-γ (47.2 ± 4.8 pg/mL). Statistically significant
increases (p < 0.05) were seen in all cytokines. The elevations of these elevations may be sustained
immune activation and could contribute to long-lasting post-COVID symptoms such as fatigue and systemic
inflammation. The serum cytokine concentrations are mentioned in Table 2.
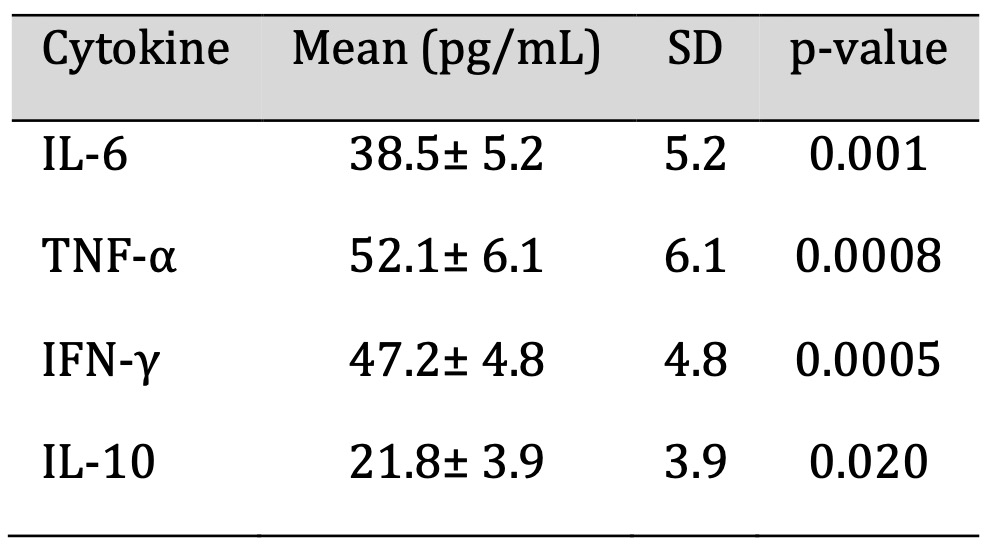
Table 2: Serum Cytokine Concentrations
Biochemical Marker Profiles in Post-Acute Phase
Biochemical testing demonstrated mildly elevated ALT (45.6 U/L) and AST (39.2 U/L) values, which were
statistically significant (p = 0.04 and p = 0.03) and above the upper reference range, but within range.
The clinical threshold for ferritin (312 ng/mL) was exceeded, and for CRP (14.7 mg/L), indicative of
persistent inflammatory activity. Although some of these abnormalities are subclinical, they may indicate
ongoing physiological stress and ultimate susceptibility to secondary complications in the recovery phase.
The biochemical markers profile is mentioned in Table 3.
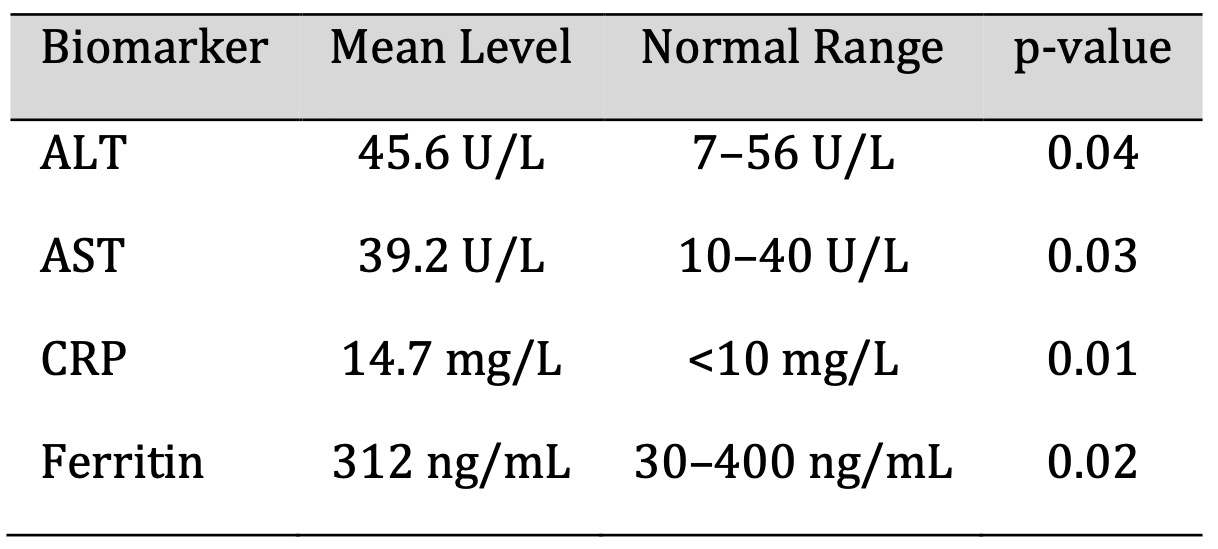
Table 3: Biochemical Marker Profiles
Oxidative Stress Marker Levels
Evaluations of oxidative stress was significantly elevated malondialdehyde (MDA: 5.8 units), superoxide
dismutase (SOD: 120.4 units) and reduced glutathione (GSH: 4.3 units) depicted in Fig. 2. This pattern
suggested the lipoperoxidation, mitochondrial stress, and a compensatory response. Endothelial
dysfunction, fatigue, and other lingering symptoms in long COVID patients may be due to persistent
oxidative imbalance.
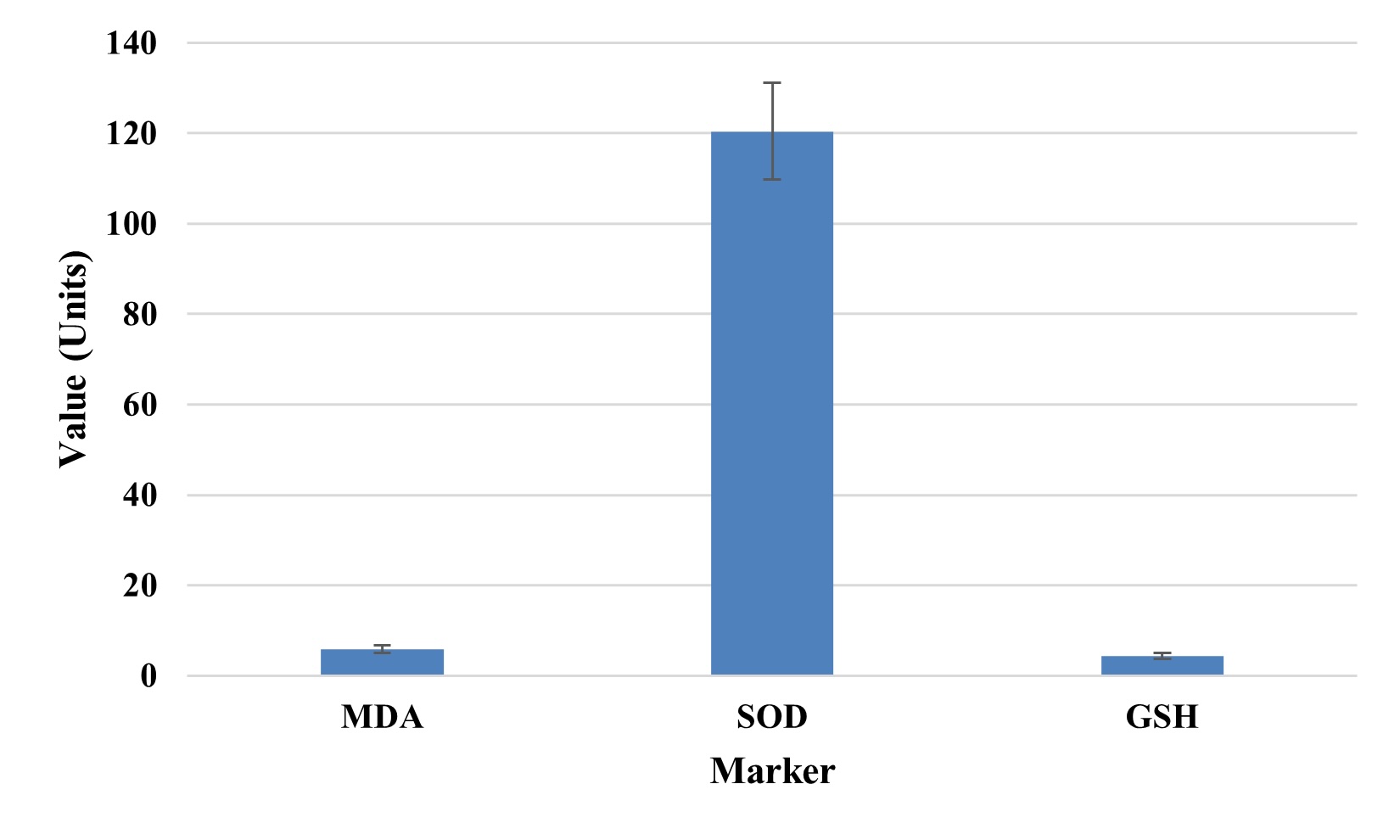
Fig. 2: Oxidative Stress Marker Levels.
Cytokine Inter-Relationship Analysis
Serum cytokines were found to be strongly coordinated in a pro-inflammatory response in post-acute
COVID-19 individuals. IL-6 also had a high positive correlation with TNF-α (r = 0.88) and IFN-γ
(r = 0.84) and may be acting synergistically with them to sustain inflammation. The correlation was also
demonstrated (r = 0.76) for TNF-α and IFN-γ. IL10, an anti-inflammatory cytokine, had a low
positive correlation with all the proinflammatory markers, suggesting an imbalance between inflammatory
activation and regulatory control, and thus may reflect protracted systemic inflammation during a recovery
phase.
T Cell Subset Distribution
T cell subsets profiling by flow cytometric was also done, which showed distribution of T cells as
CD4+ T cells (41.2%), CD8+ T cells (30.5%), and regulatory T cells (Tregs: 8.9%).
This skewed ratio suggested that this has a proinflammatory immune signature along with a relative
deficiency in immunoregulatory components. The reduced Treg percentage might contribute to the failure to
resolve the infection and facilitate lingering inflammation during convalescence. The distribution of T
cell subsets is illustrated in Fig. 3.
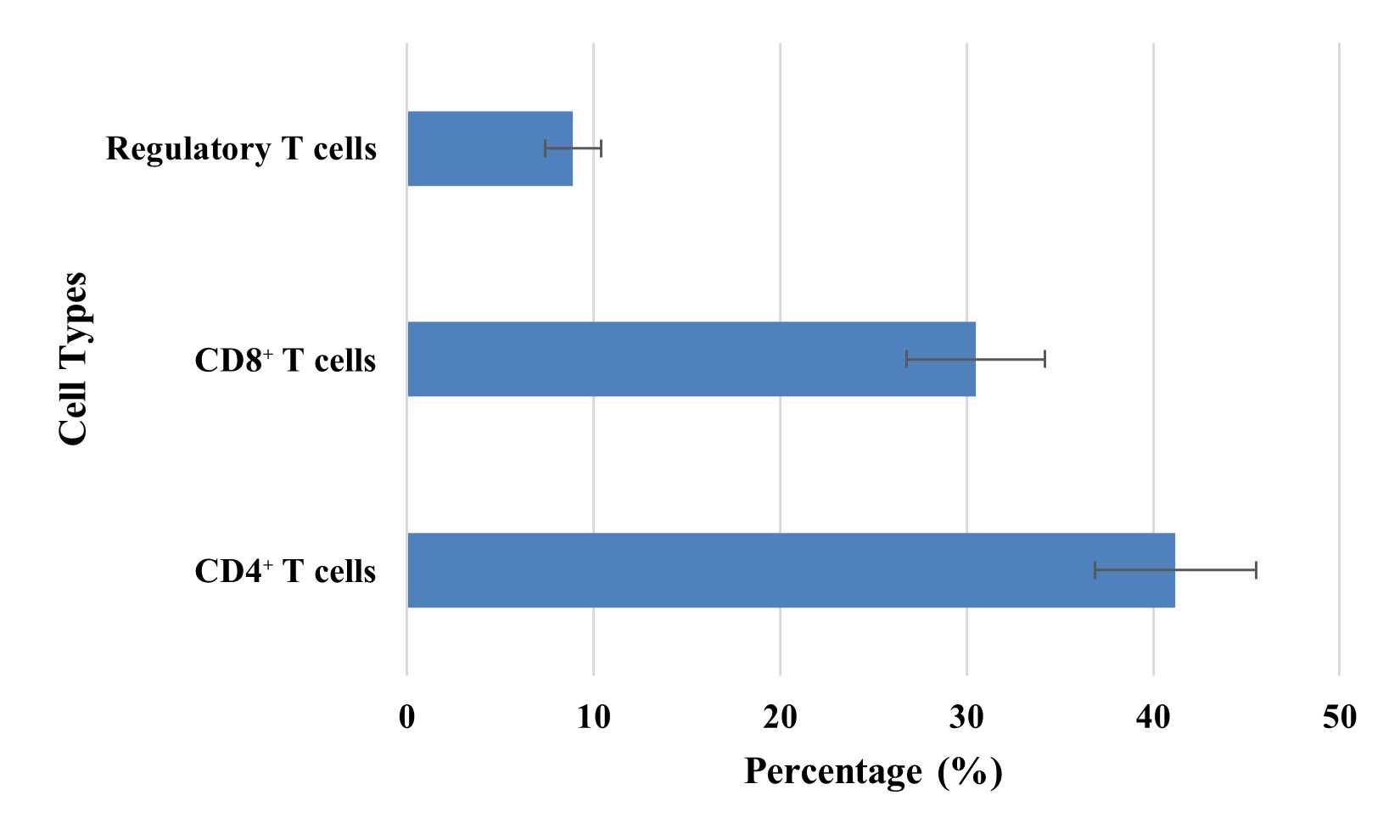
Fig. 3: Distribution of T Cell Subsets in Recovered Individuals.
Sex-Based Differences in Biomarker Expression
Biomarker expression was significant, and sex-based subgroups were found. Compared to females, males were
higher on IL-6 (40.1 ± 4.5 pg/mL), TNF-α (54.3 ± 5.8 pg/mL), MDA (6.0 ± 0.8 units) and
SOD (125.2 ± 9.6 units). Up to 46% higher ALT levels were observed in females (44.1 ± 5.2 U/L)
compared to males (46.9 ± 4.3 U/L). Statistical (p < 0.05) differences were found for these
traits. Males in post-acute recovery were at a greater inflammatory and oxidative burden than females
mentioned in Table 4.
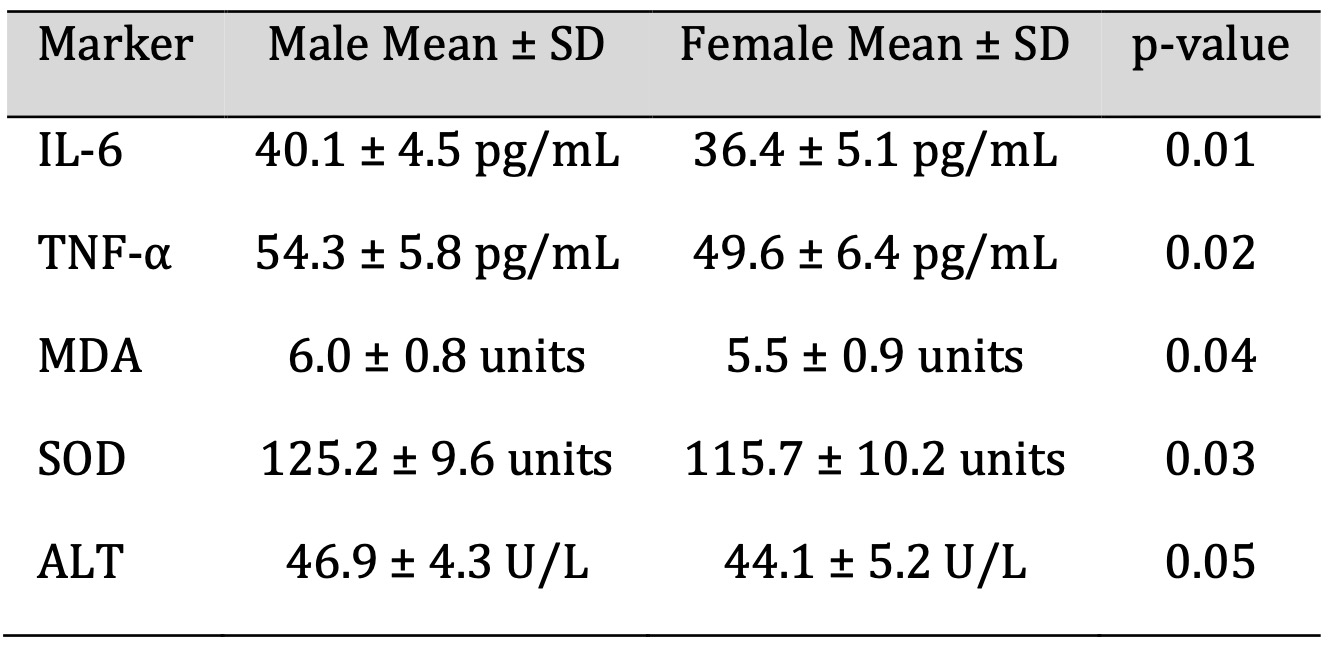
Table 4: Sex-Based Differences in Biomarker Expression
Correlation Between Clinical Symptoms and Biomarkers
Correlational analysis revealed the significant correlation between fatigue scores and several biomarkers,
i.e., IL-6 (r = 0.69, p = 0.001), CRP (r = 0.65, p = 0.002), and MDA (r = 0.61, p = 0.005). Biomarkers
were also found to moderately correlate with the same biomarkers and dyspnea scores. This highlights the
clinical significance of systemic inflammation and oxidative stress to generate patient-reported symptoms
in the post-acute care of COVID-19 and indicates the utility of biomarker tracking in this setting. Fig. 4
illustrates the correlation between the clinical symptoms and biomarkers.”
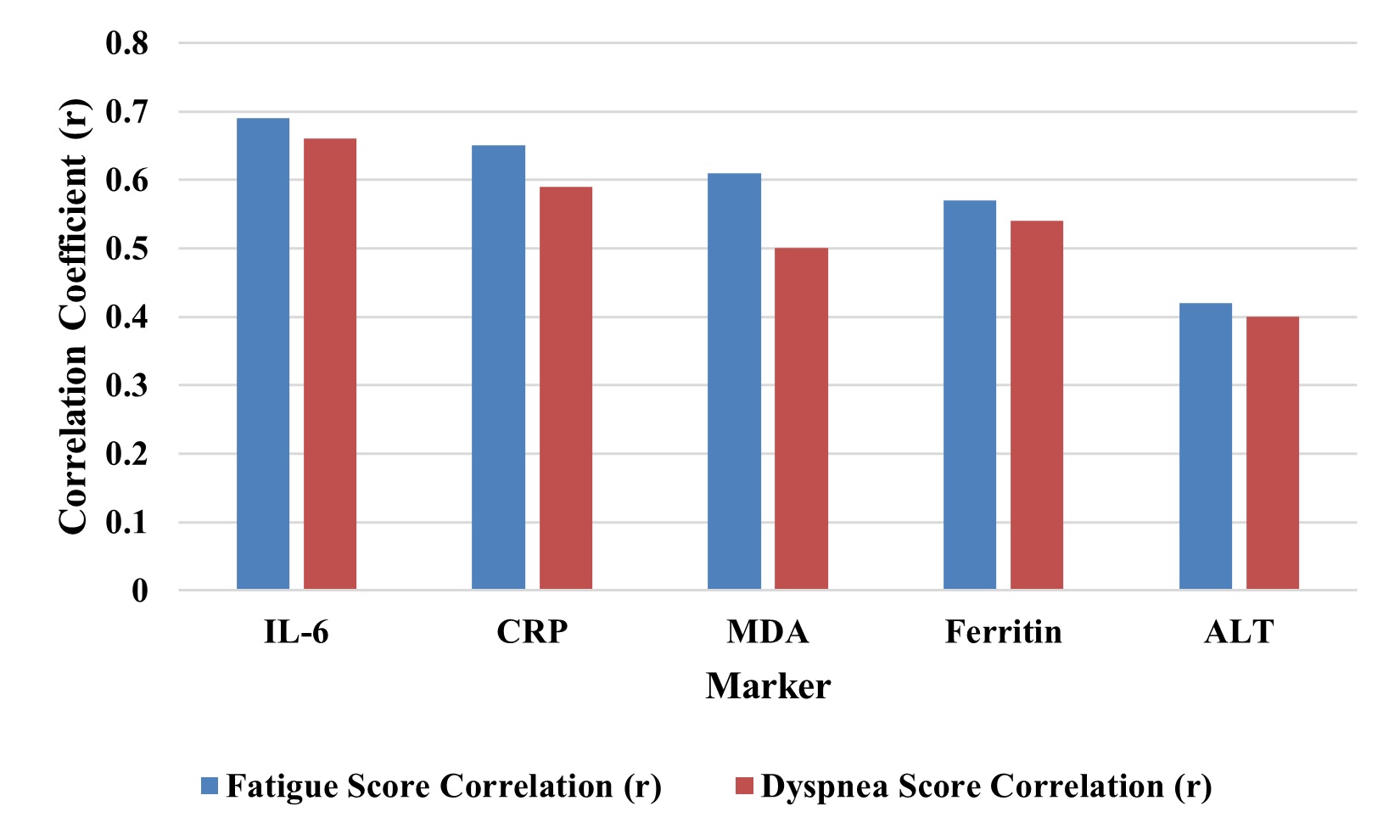
Fig. 4: Correlation Between Clinical Symptoms and Biomarkers.
Discussion
In this study post-acute COVID pathophysiology was examined by a multi-parameter analysis of cellular stress responses, immune dysfunctions, oxidative damage, and biochemical alterations. The results showed continuous biological disturbance in many pathways in people who had clinically recovered from SARS-CoV-2 infection. These disturbances were statistically significant, and they were correlated with common post-COVID symptoms, providing a compelling framework for explaining the long clinical manifestations of long COVID or post-acute COVID-19 syndrome (PACS).
One notable finding were that cellular stress markers, HSP70, CHOP, and GRP78, were also significantly elevated in PBMCs in a sustained manner, indicating a prolonged ER stress response pathway. This is consistent with previous studies that have demonstrated ER stress’s association with chronic immune dysfunction or inflammation after viral infection [23]. As shown in Table 1, these markers may be elevated as a result of continued cell attempts to resolve misfolded proteins and maintain homeostasis in the absence of viral exposure. GRP78 has also been shown to be a host factor that the coronaviruses exploit to gain cell entry and to contribute to ongoing subclinical cellular reactivity. This included a pro-inflammatory marked increase in cytokine signature, with marked elevations of IL-6, TNF-α, and IFN-γ, but only modest elevations in the anti-inflammatory cytokine IL-10. This imbalance is a failure to reestablish immune homeostasis after viral clearance, as is reported in previous studies on long COVID [24]. The inter-cytokine correlations of IL6 with TNFα (r = 0.88), IL6 with IFNg (r = 0.84), reflects the tightly orchestrated pro-inflammatory network. This weak correlation between IL10 and the proinflammatory cytokines indicates that the anti-inflammatory regulation was insufficient, hence prolonging systemic inflammation.”
This hypothesis was further supported by clinical biochemistry. ALT and AST levels, although at the upper reference range, were markedly elevated and could represent possible hepatocellular strain. With weeks of recovery, it was observed that residual inflammation remained present, and an elevated CRP and ferritin level seemed to point to this, too. Table 3 also matches with global post-COVID reports of persistent biochemical abnormalities in cohorts who were not hospitalized during acute illness [25]. Elevations in CRP and ferritin may indicate macrophage activation and endothelial stress, both of which are part of the disease process of COVID-19. Both damage and compensation at the level of oxidative stress markers were found. The results indicated lipid peroxidation and oxidative damage as indicated by elevated MDA levels, and the increased SOD and GSH levels were reflective of the body’s attempt to counteract free radicals. This combination is typical for chronic oxidative stress, as in post-viral fatigue syndromes and neuroinflammatory conditions [26]. The increase in oxidative markers between injury and antioxidant defense systems is depicted in Fig. 2. A plausible mechanism for fatigue, muscle weakness, and neurocognitive symptoms that are commonly seen in long COVID may be persistent oxidative stress.
T cell subsets were analysed using flow cytometry. The recovered individuals showed a skewed immune phenotype. As shown in Fig. 3, the majority of cells were CD4+ T cells (41.2%), followed by CD8+ T cells (30.5%) and a significantly lower percentage of regulatory T cells (Tregs, 8.9%). A relative deficiency of Tregs may also contribute to the failure of immune resolution, and continued activation of effector T cells and persistent inflammation. This is similar to post-infectious syndromes, and has been suggested as the basis of autoimmune sequelae [27]. Detailed in Table 4 is sex-based subgroup analysis of significantly higher IL-6, TNF α, MDA, and SOD levels in males compared to females. This suggests that men are more prone to prolonged inflammatory and oxidative responses after being infected with COVID. The findings are consistent with epidemiological data that show that males have more acute disease severity and greater mortality, and slower immune resolution in the post-recovery phase [28]. Along with it, slightly higher ALT levels in males also help support a pattern of sex-based vulnerability to organ-based inflammation.
A strong biological basis for subjective post-COVID complaints was set up through correlations with clinical symptom scores and biomarkers. Significant correlation was seen between IL-6 (r = 0.69), CRP (r = 0.65), and MDA (r = 0.61) with fatigue scores, as well as dyspnea showed moderate correlation with the same markers. Fig. 4 validates the use of objective biomarkers in quantifying symptom burden. The clinical importance of these findings lies in a context where subjective reports of vasomotor symptoms may not be taken seriously or their degree underappreciated if laboratory evidence is not available to support them. These results are compared to previous post-viral syndrome research, and they provide a unified biomarker-based model of long COVID. Previous studies have also reported similar patterns of persistent inflammation and symptomatology, such as in terms of fatigue and breathlessness [29]. In contrast, the presented study combines cellular stress markers, cytokine dynamics, oxidative stress profiles, and clinical biochemistry into a single post-COVID population.
The biomarker-symptom associations constitute the basis for the development of diagnostic algorithms utilizing IL-6, CRP, and MDA levels. They are available and measurable in routine laboratory settings and thus facilitate their feasibility in outpatient monitoring and risk stratification for long COVID management. Being a cross-sectional study, it prevents drawing any causal inference from the results presented here, while the study is reinforced by the fact that biomarkers need to be monitored to follow up on COVID-19 survivors. These markers need to be directly evaluated in longitudinal studies, which will confirm their prognostic value over time, evaluate intervention efficacy, and find out if there are other markers of greater significance. Such therapies that would reduce inflammation (e.g., IL-6 inhibitors), decrease the oxidative stress (e.g., antioxidants like N-acetylcysteine), or rebalance the autoimmune response (e.g., strategies to increase regulatory T cells, Tregs) may be useful in managing persistent post-COVID symptoms [30].
Hence, the study provides a constellation of biological disturbances that linger beyond the early stage of COVID-19. However, being a cross-sectional observational study, causality or progression of these biomarkers cannot be established. Longitudinal studies are needed to confirm these associations and assess their prognostic value in long COVID management. Additionally, the study’s findings are based on a single-center cohort from India, which may limit the generalizability of the results. Demographic, genetic, and environmental factors could influence biomarker levels and post-COVID outcomes, highlighting the need for multicenter and geographically diverse studies to confirm the broader applicability of these observations. It describes a syndrome of cellular stress, immune activation, oxidative damage, and residual biochemical abnormalities. While prior studies have addressed individual pathways of post-COVID disturbances, this study simultaneously analyze ER stress, immune imbalances, and oxidative markers in a single cohort, providing a holistic understanding of long COVID pathophysiology. Disruptions to these outcomes during SARS-CoV-2 infection may provide opportunities to achieve a deeper mechanistic understanding that might lead to personalized therapeutic approaches aimed at preventing the long-term consequences of the SARS-CoV-2 infection.
Conclusion
The study reports the complete molecular and immunological disturbances that lingering in the recovery phase of COVID-19. The results show that despite clinical resolution, patients remain stressed at the cellular level, have immune dysregulation, exhibit oxidative imbalance, and mild biochemical abnormalities. A syndrome of unresolved physiological stress is characterized by elevated expression of Endoplasmic Reticulum (ER) stress markers (HSP70, CHOP, GRP78), sustained pro-inflammatory cytokine profile (IL-6, TNF-a, IFN-gamma), oxidative stress markers (MDA, SOD, GSH), modest, though statistically significant, elevations of CRP, ferritin, and liver enzymes.These biomarkers show a good correlation with fatigue and dyspnea scores and are, thus, clinically relevant and useful to monitor symptom burden. In addition to the sex-based differences observed in biomarker expression, stratified follow-up care in post-COVID patients is needed. These results argue that the post-acute COVID is not just a subjective syndrome, but it is supported by quantifiable biological disruptions. This integration could allow clinicians to identify, monitor, and tailor treatment for individuals at risk of prolonged recovery from acute lung injury earlier. These biomarkers need to be validated in longitudinal studies to determine whether they can be used and, if so, what targeted therapeutic interventions can reduce the long-term effects of SARS-CoV-2 infection.
Acknowledgements
The authors would like to thank Dr. Mohamed Boshnaf at OSF Little Company of Mary Medical Center, Illinois, USA for the interpretation and reading of the HRCT scan.
Author Contributions
Inas M. Alhudiri: conceptualization, experimental design, data curation, investigation, formal analysis,
visualization, writing–original draft, writing–review and editing. Mahmoud F. Gaballa:
experimental assays, ELISA validation, data collection, visualization. Fawzi O. Ebrahim:
conceptualization, methodology development, project supervision, formal analysis, validation,
writing–original draft, and writing–review and editing. K. D. Ahire: statistical analysis,
data interpretation, software, writing–review and editing. Farag I. Eltaib: animal handling, sample
collection, biochemical processing. Idress H. Attitalla: project administration, lab logistics, ethical
approval coordination, manuscript review. Adam I. Elzagheid: formatting, figure preparation, referencing,
submission preparation, final manuscript review. All authors have read and agreed to the submitted version
of the manuscript.
Disclosure of AI Assistance
The authors declare that no AI tools or software were used in the preparation, writing, or editing of this
manuscript.
Statement of Ethics
Ethical approval was obtained between Dr. Harry and Libyan Biotechnology Research Centre, Ethical
Committee (BEC-BTRC 14-2020). The patient had signed an Informed Consent form.
Disclosure Statement
The authors declare no conflicts of interest.
References
| 1 | Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: An overview. Diabetes Metab Syndr. 2021
May-Jun;15(3):869-875. doi: 10.1016/j.dsx.2021.04.007. Epub 2021 Apr 20. Erratum in: Diabetes Metab
Syndr. 2022 May;16(5):102504. doi: 10.1016/j.dsx.2022.102504. Erratum in: Diabetes Metab Syndr. 2022
Dec;16(12):102660. doi: 10.1016/j.dsx.2022.102660.
https://doi.org/10.1016/j.dsx.2022.102660 |
| 2 | Georgieva E, Ananiev J, Yovchev Y, Arabadzhiev G, Abrashev H, Abrasheva D, Atanasov V, Kostandieva
R, Mitev M, Petkova-Parlapanska K, Karamalakova Y, Koleva-Korkelia I, Tsoneva V, Nikolova G.
COVID-19 Complications: Oxidative Stress, Inflammation, and Mitochondrial and Endothelial
Dysfunction. Int J Mol Sci. 2023 Oct 4;24(19):14876. doi: 10.3390/ijms241914876.
https://doi.org/10.3390/ijms241914876 |
| 3 | Zanza C, Romenskaya T, Manetti AC, Franceschi F, La Russa R, Bertozzi G, Maiese A, Savioli G,
Volonnino G, Longhitano Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina
(Kaunas). 2022 Jan 18;58(2):144. doi: 10.3390/medicina58020144.
https://doi.org/10.3390/medicina58020144 |
| 4 | Low RN, Low RJ, Akrami A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front
Med (Lausanne). 2023 Mar 31;10:1011936. doi: 10.3389/fmed.2023.1011936.
https://doi.org/10.3389/fmed.2023.1011936 |
| 5 | da Fonseca FG, Serufo ÂV, Leão TL, Lourenço KL. Viral Infections and Their Ability to Modulate
Endoplasmic Reticulum Stress Response Pathways. Viruses. 2024 Sep 30;16(10):1555. doi:
10.3390/v16101555.
https://doi.org/10.3390/v16101555 |
| 6 | Silva MJA, Ribeiro LR, Lima KVB, Lima LNGC. Adaptive immunity to SARS-CoV-2 infection: A
systematic review. Front Immunol. 2022 Oct 10;13:1001198. doi: 10.3389/fimmu.2022.1001198.
https://doi.org/10.3389/fimmu.2022.1001198 |
| 7 | Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS, Bosurgi L, Dutzmann J,
Sedding D, Frese T, Girndt M, Höll JI, Gekle M, Mikolajczyk R, Binder M. The IL-1β, IL-6, and TNF
cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022 Jun
21;3(6):100663. doi: 10.1016/j.xcrm.2022.100663.
https://doi.org/10.1016/j.xcrm.2022.100663 |
| 8 | Megha KB, Joseph X, Akhil V, Mohanan PV. Cascade of immune mechanism and consequences of
inflammatory disorders. Phytomedicine. 2021 Oct;91:153712. doi: 10.1016/j.phymed.2021.153712.
https://doi.org/10.1016/j.phymed.2021.153712 |
| 9 | Dhawan M, Rabaan AA, Alwarthan S, Alhajri M, Halwani MA, Alshengeti A, Najim MA, Alwashmi ASS,
Alshehri AA, Alshamrani SA, AlShehail BM, Garout M, Al-Abdulhadi S, Al-Ahmed SH, Thakur N, Verma G.
Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities
with a Special Focus on Long COVID. Vaccines (Basel). 2023 Mar 19;11(3):699. doi:
10.3390/vaccines11030699.
https://doi.org/10.3390/vaccines11030699 |
| 10 | Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer,
and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020
Jan-Dec;14:1753466620937175. doi: 10.1177/1753466620937175.
https://doi.org/10.1177/1753466620937175 |
| 11 | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front
Immunol. 2018 Apr 13;9:754. doi: 10.3389/fimmu.2018.00754.
https://doi.org/10.3389/fimmu.2018.00754 |
| 12 | Chen AT, Wang CY, Zhu WL, Chen W. Coagulation Disorders and Thrombosis in COVID-19 Patients and a
Possible Mechanism Involving Endothelial Cells: A Review. Aging Dis. 2022 Feb 1;13(1):144-156. doi:
10.14336/AD.2021.0704.
https://doi.org/10.14336/AD.2021.0704 |
| 13 | Gain C, Song S, Angtuaco T, Satta S, Kelesidis T. The role of oxidative stress in the pathogenesis
of infections with coronaviruses. Front Microbiol. 2023 Jan 13;13:1111930. doi:
10.3389/fmicb.2022.1111930.
https://doi.org/10.3389/fmicb.2022.1111930 |
| 14 | Golabi S, Ghasemi S, Adelipour M, Bagheri R, Suzuki K, Wong A, Seyedtabib M, Naghashpour M.
Oxidative Stress and Inflammatory Status in COVID-19 Outpatients: A Health Center-Based Analytical
Cross-Sectional Study. Antioxidants (Basel). 2022 Mar 22;11(4):606. doi: 10.3390/antiox11040606.
https://doi.org/10.3390/antiox11040606 |
| 15 | Negrut N, Menegas G, Kampioti S, Bourelou M, Kopanyi F, Hassan FD, Asowed A, Taleouine FZ,
Ferician A, Marian P. The Multisystem Impact of Long COVID: A Comprehensive Review. Diagnostics
(Basel). 2024 Jan 24;14(3):244. doi: 10.3390/diagnostics14030244.
https://doi.org/10.3390/diagnostics14030244 |
| 16 | Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, Reeves WB; RECOVER Mechanistic Pathway Task Force.
Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023 Mar
22;12:e86002. doi: 10.7554/eLife.86002.
https://doi.org/10.7554/eLife.86002 |
| 17 | Nalbandian, A., Sehgal, K., Gupta, A., Madhavan, M. V., McGroder, C., Stevens, J. S., Cook, J. R.,
Nordvig, A. S., Shalev, D., Sehrawat, T. S., Ahluwalia, N., Bikdeli, B., Dietz, D.,
Der-Nigoghossian, C., Liyanage-Don, N., Rosner, G. F., Bernstein, E. J., Mohan, S., Beckley, A. A.,
Seres, D. S., … Wan, E. Y. (2021). Post-acute COVID-19 syndrome. Nature medicine, 27(4), 601-615.
doi: 10.1038/s41591-021-01283-z
https://doi.org/10.1038/s41591-021-01283-z |
| 18 | Böyum A. (1968). Isolation of mononuclear cells and granulocytes from human blood. Isolation of
monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and
sedimentation at 1 g. Scandinavian journal of clinical and laboratory investigation. Supplementum,
97, 77-89.
|
| 19 | Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities
of protein utilizing the principle of protein-dye binding. Analytical biochemistry, 72, 248-254.
doi: 10.1016/0003-2697(76)90527-3
https://doi.org/10.1016/0003-2697(76)90527-3 |
| 20 | Lee, J., Giordano, S., & Zhang, J. (2012). Autophagy, mitochondria and oxidative stress:
cross-talk and redox signalling. The Biochemical journal, 441(2), 523-540. doi: 10.1042/BJ20111451
https://doi.org/10.1042/BJ20111451 |
| 21 | Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb
Perspect Biol. 2014 Sep 4;6(10):a016295. doi: 10.1101/cshperspect.a016295.
https://doi.org/10.1101/cshperspect.a016295 |
| 22 | BEUTLER, E., DURON, O., & KELLY, B. M. (1963). Improved method for the determination of blood
glutathione. The Journal of laboratory and clinical medicine, 61, 882-888.
|
| 23 | Salminen A, Kaarniranta K, Kauppinen A. ER stress activates immunosuppressive network:
implications for aging and Alzheimer's disease. J Mol Med (Berl). 2020 May;98(5):633-650. doi:
10.1007/s00109-020-01904-z.
https://doi.org/10.1007/s00109-020-01904-z |
| 24 | Nunn AVW, Guy GW, Brysch W, Bell JD. Understanding Long COVID; Mitochondrial Health and
Adaptation-Old Pathways, New Problems. Biomedicines. 2022 Dec 2;10(12):3113. doi:
10.3390/biomedicines10123113.
https://doi.org/10.3390/biomedicines10123113 |
| 25 | Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, Blyuss O, El-Taravi Y,
DunnGalvin A, Comberiati P, Peroni DG, Apfelbacher C, Genuneit J, Mazankova L, Miroshina A,
Chistyakova E, Samitova E, Borzakova S, Bondarenko E, Korsunskiy AA, Konova I, Hanson SW, Carson G,
Sigfrid L, Scott JT, Greenhawt M, Whittaker EA, Garralda E, Swann OV, Buonsenso D, Nicholls DE,
Simpson F, Jones C, Semple MG, Warner JO, Vos T, Olliaro P, Munblit D; and the Sechenov StopCOVID
Research Team. Risk factors for post-COVID-19 condition in previously hospitalised children using
the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J. 2022 Feb
3;59(2):2101341. doi: 10.1183/13993003.01341-2021.
https://doi.org/10.1183/13993003.01341-2021 |
| 26 | Solleiro-Villavicencio H, Rivas-Arancibia S. Effect of Chronic Oxidative Stress on
Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front Cell
Neurosci. 2018 Apr 27;12:114. doi: 10.3389/fncel.2018.00114.
https://doi.org/10.3389/fncel.2018.00114 |
| 27 | Dhawan M, Rabaan AA, Alwarthan S, Alhajri M, Halwani MA, Alshengeti A, Najim MA, Alwashmi ASS,
Alshehri AA, Alshamrani SA, AlShehail BM, Garout M, Al-Abdulhadi S, Al-Ahmed SH, Thakur N, Verma G.
Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities
with a Special Focus on Long COVID. Vaccines (Basel). 2023 Mar 19;11(3):699. doi:
10.3390/vaccines11030699.
https://doi.org/10.3390/vaccines11030699 |
| 28 | Sapir T, Averch Z, Lerman B, Bodzin A, Fishman Y, Maitra R. COVID-19 and the Immune Response: A
Multi-Phasic Approach to the Treatment of COVID-19. Int J Mol Sci. 2022 Aug 3;23(15):8606. doi:
10.3390/ijms23158606.
https://doi.org/10.3390/ijms23158606 |
| 29 | Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, Razani B, Mwangi J,
Asadi-Pooya AA, Malekmakan L, Bastani B. Long COVID, a comprehensive systematic scoping review.
Infection. 2021 Dec;49(6):1163-1186. doi: 10.1007/s15010-021-01666-x.
https://doi.org/10.1007/s15010-021-01666-x |
| 30 | Du P, Geng J, Wang F, Chen X, Huang Z, Wang Y. Role of IL-6 inhibitor in treatment of
COVID-19-related cytokine release syndrome. Int J Med Sci. 2021 Jan 21;18(6):1356-1362. doi:
10.7150/ijms.53564.
https://doi.org/10.7150/ijms.53564 |