ACAN and VCAN Inhibition Through Downregulation of SOX9 and Induction of Neurogenesis in Rat Using Ischemic Stroke Model Due to Simvastatin Administration
bDepartment of Physiology, Faculty of Medicine, Universitas Brawijaya/Universitas Brawijaya Academic Hospital, Malang, Indonesia,
cResearch Center for Vaccine Technology and Development, Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia,
dVirology and Immunology Laboratory, Department of Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia,
eDepartment of Neurology, Faculty of Medicine, Universitas Airlangga/Dr. Soetomo General Hospital, Surabaya, Indonesia,
fPost Graduate Doctoral Program, Faculty of Medicine, Universitas Airlangga/Universitas Airlangga General Hospital, Surabaya, Indonesia,
gDepartment of Biomolecular Biochemistry, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia
Keywords
Abstract
Background/Aims:
Simvastatin is recognized for its pleiotropic properties. Beyond its role in lowering lipid, it also affects brain function, especially under pathological conditions like ischemic stroke. Neurogenesis and the increased expression of ACAN and VCAN in glial scar are linked to ischemic stroke, with both ACAN and VCAN being regulated by the transcription factor Sox9.This study aimed to investigate the mechanism by which simvastatin inhibits ACAN and VCAN expression and promotes neurogenesis.Methods:
Twenty male Wistar rats were randomly assigned to four groups: sham, P1, P2, and P3. The sham group consisted of rats with ischemic stroke that did not receive simvastatin, while groups P1, P2, and P3 are rats with ischemic stroke that received simvastatin at doses of 5 mg, 10 mg, and 15 mg/kg/day, respectively, for 7 consecutive days. Motor function was assessed using the LRWT test. The expression levels of ACAN, VCAN, BDNF, and SOX9 were analyzed using immunohistochemistry (IHC), while BDNF and SOX9 mRNA expression was measured via RT-PCR. Glial scar density was evaluated through hematoxylin and eosin staining.Results:
The findings revealed that simvastatin administration led to improved motor function, along with increased levels of BDNF, BDNF mRNA, and a higher number of neurons. Additionally, there was a reduction in ACAN, VCAN, and glial scar density, which corresponded with decreased expression of SOX9 and SOX9 mRNA.Conclusion:
These results suggest that simvastatin can suppress ACAN and VCAN expression and reduce glial scar formation by inhibiting SOX9. Moreover, simvastatin appears to promote neurogenesis, as indicated by elevated BDNF levels and an increased neuronal count.Introduction
Ischemic stroke is a devastating condition caused by an interruption in the blood supply to the brain due to a blood clot or embolism, which then causes neurological impairment [1]. Stroke remains the second leading cause of death worldwide, accounting for 11.6% of all deaths in 2019 [2]. Neurogenesis is the formation of new neurons, which can be triggered by an ischemic stroke. It can take place in both the damaged brain area and the surrounding tissue [1]. Today, neurogenesis is widely seen as a key factor in supporting brain plasticity. While it helps with recovery after a stroke, the endogenous neurogenesis is often not enough for full healing [3]. Therefore, boosting this process is important to support better recovery. One of the key proteins involved in neurogenesis is BDNF [4, 5].
Unlike neurogenesis, the formation of glial scar hinders the regeneration of neurons and axons. A key factor in this process is the presence of chondroitin sulfate proteoglycans (CSPGs) within the scar tissue [6, 7]. CSPGs are believed to block neuron regeneration by activating specific receptors, such as receptor protein tyrosine phosphatase (RPTP), while axon regeneration is inhibited through another receptor called leukocyte common antigen-related phosphatase (LAR) [7]. In the central nervous system (CNS), most CSPG proteins belong to the lectican family, which includes aggrecan (ACAN), brevican (BCAN), neurocan (NCAN), versican (VCAN), and phosphocan [8]. Among the lectican proteins, ACAN and VCAN are primarily expressed in the brain, especially during the embryonic stage [9]. Although neurocan is also highly expressed in the brain, it is not essential for overall brain development or function [10]. One of the key transcription factors involved in CSPG production is SOX9 (SRY-box transcription factor 9 ). SOX9 plays a role in increasing the expression of enzymes such as XT-I, XT-II, and C4ST [11], which are part of the CSPG biosynthesis gene group (CBG) [12]. Therefore, reducing ACAN and VCAN production by suppressing SOX9 expression could help limit glial scar formation and potentially lead to better recovery outcomes in stroke patients.
Numerous studies have explored ways to accelerate neurogenesis, including the use of antidepressant medications and treatments that alleviate depression, such as electroshock therapy and physical exercise [13]. Statins, a class of drugs known as HMG-CoA reductase inhibitors, are widely used to lower low-density lipoprotein (LDL) cholesterol in the blood. However, their benefits extend beyond cholesterol reduction. Statins also exhibit anti-inflammatory effects by reducing inflammatory mediators like TNFα, IL-1β, and iNOS [14]. Simvastatin, a type of statin with lipophilic properties, can cross the blood-brain barrier (BBB) more efficiently than other statins. This ability allows it to directly affect molecular processes within the brain, particularly under pathological conditions [15]. The exact role of simvastatin in the synthesis of ACAN and VCAN, which are key components of CSPGs, is still not fully understood. Likewise, further investigation is needed to clarify how simvastatin influences neurogenesis, particularly in relation to the appropriate dosage required to promote neurogenesis while reducing glial scar formation. According to a study by García-Bonilla L et al., 2012, simvastatin doses of 10 and 20 mg/kg/day were administered orally in a stroke animal model (tMCAO) [16], with 10 mg/kg/day being one of the selected doses in this study. A dose of 5 mg/kg/day was chosen based on human usage, where the standard dose for moderate LDL-C reduction is 40 mg/day. When adjusted using the animal equivalent dose formula, this corresponds to 5 mg/kg/day in rats [17, 18]. Meanwhile, a dose of 15 mg/kg/day was included to identify the most effective dose that enhances brain function without causing toxicity. The 20 mg/kg/day dose from García-Bonilla’s study, when converted to a human equivalent, is about 160 mg/day [18]. However, this dosage has been linked to side effects in humans, with 0.7% developing myopathy and 2.1% showing a threefold increase in liver transaminase levels [19].
Thus, this study aimed to explore how simvastatin reduces ACAN and VCAN expression and promotes neurogenesis in the context of ischemic stroke. The novelty of this research lies in demonstrating simvastatin’s potential to prevent glial scar formation by specifically inhibiting ACAN and VCAN, as well as its role in stimulating neurogenesis under ischemic conditions. The findings are expected to offer a new therapeutic approach for ischemic stroke by targeting ACAN and VCAN suppression, which may lead to reduced glial scar density and enhanced neurogenesis. Additionally, this study challenges the conventional view of simvastatin as solely a secondary prevention agent, suggesting it may also be beneficial during the acute phase of ischemic stroke treatment.
Materials and Methods
Study Design and Setting
The research design in this study was a laboratory experiment using an ischemic stroke model in rats. The
samples received simvastatin orally for seven days and then stopped. The motoric function was evaluated
using LRWT (Ladder Rung Walking Test) before administration of simvastatin and before discontinuation on
the seventh day. Several brain biomarkers such as BDNF, SOX9, ACAN and VCAN were assessed using IHC
techniques. RT-PCR was done to evaluate expression of BDNF mRNA and SOX9 mRNA.And to evaluate the glial
scar density was used H and E staining.
Animal and Intervention
Twenty male Wistar rats aged 3-4 months weighing 200-280 grams were obtained from the Department of Food
Security and Agriculture, Bandung, West Java, Indonesia. The determination of the number of samples used
the Lameshow formula. For the sake of the animal welfare, all rats were placed in a special rat room with
a 12-hour light-dark cycle, with the availability of food and water ad libitum, a temperature of
22-24°C and a humidity level of 55 ± 5%. The intervention carried out was the administration of
simvastatin orally in three doses, namely dose 1: 5 mg/kg/day, dose 2: 10 mg/kg/day, dose 3: 15 mg/kg/day
given since 3 hours after ischemic stroke until 7 days.
Group Devision
Twenty rats were randomly divided using the random table method into four groups where this procedure is
performed blindly. The first group was sham, namely five rats that experienced ischemic stroke without
simvastatin and were followed for 7 days and then terminated. The second group was group P1, namely five
rats that experienced ischemic stroke with simvastatin dose 1 for 7 days and then terminated. The third
group was group P2, namely five rats that experienced ischemic stroke with simvastatin dose 2 for 7 days
and then terminated. The fourth group was group P3, namely five rats that experienced ischemic stroke with
simvastatin dose 3 for 7 days and then terminated.
Ischemic Stroke Model
Ischemic stroke model was created by the left unilateral common carotid artery (CCA) occlusion for 180
minutes. After anesthesia, the rats were turned over to the supine position and fixed to the operating
table. Then a small incision was made in the midline of the neck about 3-4 cm. Next, exploration was
carried out slowly until the trachea was visible. Next, a search was carried out for the left CAA which
was seen running along the vagus nerve. The next step was to slowly isolate the left CAA from the vague
nerve and connective tissue. After that, the artery was blocked using a small bulldog clamp for 180
minutes. After 180 minutes, the bulldog clamp was removed and the neck incision was closed. (2) The
experiment was conducted in the pharmacology laboratory of the Faculty of Medicine, Brawijaya University.
The ethical protocol of the experiment has been approved by the animal commission of the Faculty of
Veterinary Medicine, Brawijaya University with protocol number 219-KEP-UB-2023.
Evaluation of Motoric Function
Motor function was assessed using the ladder rung walking test (LRWT) [20]. The LRWT provides qualitative
and quantitative measurements of motor function [21].
The ladder walking test apparatus consists of two side walls made of two clear plexiglasses and a metal
rungs. The diameter of each metal rung is 3 mm, and can be inserted to create a floor. The two clear
plexiglasses are 100 cm long and 20 cm high. The distance between the rung varies, ranging from 1 to 5 cm
for rats. The ladder is elevated 30 cm above the ground horizontally, with the neutral cage placed in the
starting position and the animal’s home cage placed at the opposite end of the ladder. In the LRWT,
walking ability is assessed using the foot fault score (FFS) which reflects the rat’s walking
pattern, forelimb and hindlimb kinematics. The foot fault scoring system (FFS) is used to assess walking
skills in rats forelimb and hindlimb kinematics [22].
The foot fault scoring system (FFS) is used to assess walking skills in mice [22]. Foot fault scoring is
assessed using 7 categories, namely (1) Score 0/Total miss: 0: the limb does not touch the rung at all and
the mouse falls (falling means posture and balance are disturbed). (2) Score 1/Deep slip: The limb was
initially placed on the rung and then detached from the step and a fall occurred. (3) Score 2/Slight slip:
The limb was placed on the rung and detached while bearing the weight, but did not result in a fall and
could maintain balance and continue coordinated gait. (4) Score 3/Replacement: The limb was placed on the
rung, but before bearing the weight of the limb on the rung, the mouse was quickly lifted and placed on
another rung. (5) Score 4/Correction: The limb approached for one rung, but then removes on another rung
without touching the first one. A score of 4 was also given when the limb was placed on a rung, but the
animal lifted the foot and repositioned it on the same rung. (6) Score 5/Partial placement: The foot is
placed on the rung with the wrist or toes of the front foot or the heel or toes of the back foot. (7)
Score 6/Correct placement: The center of the sole of the foot is placed on the rung with full weight
bearing. Error scores from five trials were averaged for analysis [21, 22].
Examination of ACAN, VCAN, and SOX9 Expression Exert Immunohistochemistry Technique
In this study, the expression of ACAN, VCAN, BDNF and SOX9 was assessed using immunohistochemistry
techniques. Paraffin blocks of rat brains were cut using a microtome along 1.5 cm in front of bregma, with
a thickness of 4 μm on coronal sections. Deparaffinization and rehydration of brain slices were
performed using xylene twice for 3 minutes each, followed by immersion in 100% ethanol for 3 minutes, then
washed in PBS for 5 minutes, and the next slices were wiped and immersed in 3% hydrogen peroxide solution
(v/v absolute methanol) for 15 minutes at room temperature. After the slices were washed in PBS for 5
minutes three times, the slides were left overnight at 4 °C with primary antibodies. Primary
antibodies include Anti-Aggrecan antibody Catalog #ab3778 Abcam, Anti-Versican antibody Catalog #MA5-42721
thermo scientific, BDNF antibody (Cat. No. Sc-65514; Santa Cruz Biotechnology). SOX9 antibody (Cat.
No.ab-185230). After the slides were washed in PBS for 5 minutes, three times of Avidin-Biotin Complex
reagent (Sc-516, 216, Santa Cruz Biotechnology) was applied and incubated at room temperature for 30
minutes, followed by washing in PBS for 5 minutes, three times. Next, chromogen 3,
3’-diaminobenzidine (DAB) (Nichirei Biosciences) was added and incubated at room temperature for 10
minutes then the slides were washed in distilled water for 5 minutes three times. After that the slides
were immersed in a counterstain. Finally, the sections were mounted with permanent mounting medium. The
experiment was conducted in the biochemistry laboratory of the Faculty of the Medical Faculty, Brawijaya
University.
Examination of Glial Scar Density Using Hematoxylin and Eosin Staining (H and E Staining)
The brain tissue was fixed in formalin 4%, then fixed with 4% paraformaldehyde in phosphate buffered
saline (PBS) (pH 7.4) and encapsulated in paraffin. The paraffin block was cut at 4 μm thickness in
coronal sections. Then, the slides were stained using Hematoxylin eosin.
Real-time RT-PCR Analysis for The Measurement of BDNF mRNA and SOX9 mRNA Expression
The parameter to depict expression of BDNF gene and SOX9 gene were BDNF mRNA and
SOX9 mRNA which were assessed using RT-PCR. Total RNA was extracted using RNA simple Total RNA Kit
(Cat. No. 4992858; Tiangen, Beijing, China) suitable with the manufacture’s instruction. Reverse
transcription reactions were conducted on 2 μg of RNA of nucleic acids from each sample using RT-PCR
Product Series (Cat. No. 4992226/4992227/4992251; Tiangen). Real-time PCR reactions conducted using
Forget- Me-Not™ EvaGreen® qPCR Master Mix (Low ROX) (Catalog No. 31045-1mL, 31045-5mL,
31045-20mL; Biotium, Fremont, CA, USA). Primer pairs for BDNF real-time PCR was BDNF Forward:
5’- CAA AAG GCC AAC TGA AGC- 3’; BDNF Reverse: 5’-CGC CAG CCA ATT CTC TTT-
3’. Primer pairs used for SOX9 real-time PCR was SOX9 Forward:5’-CGG AGG AAG TCG GTG
AAG A-3’, SOX9 reverse: 5’GTC GGT TTTGGG AGT GGT G -3’.
For PCR reaction, the RNA template was thawed on ice, and the FastKing-RT SuperMix and RNaseFree
ddH2O were also thawed for 5 times at room temperature (15-30°C). Cycling step: Enzyme
activation at 95°C holding time 2 minutes, number of cycle was 1. Denaturation at 95°C, for 2-5
seconds. Annealing/extension at 60°C for 20-30 seconds, with number of cycles was 40 [2]. All
procedures RT-PCR perform in Research Center for Vaccine Technology and Development, Universitas
Airlangga, Surabaya.
Statistical Analysis
Statistical analysis was performed using the software package SPSS (version 24.0). Statistical analysis
for data normality and data homogeneity was done using the shapiro-wilk test and levene test. Further, to
analyze the difference between four independent groups, test was administered through ANOVA Test.
Meanwhile, to analyze the different of FFS result between before and after simvastatin administration, the
T-test analysis was used. Data were presented as mean ± standard deviation.
Results
Analysis of Motoric Function
Evidence of cerebral ischemia is proved by the presence of motor deficits. To assess motor deficits, FFS
was assessed from the Ladder Rung Walking Test (LRWT). Motor deficit assessment was performed on
the right extremity due to left CCA occlusion. Table 1 shows that FFS in all groups decreased in both RHL
and RFL after left CCA occlusion, which means that the method used in this research has caused a brain
insult. Moreover, after simvastatin administration for 7 days, FFS in all groups increased in both RHL and
RFL compared to before simvastatin administration. The result shows that the higher the dose of
simvastatin, the higher the FFS value. Then, the higher the FFS value, the better the motor function is.
This fact illustrates that simvastatin can improve the motoric function for the ischemic stroke.
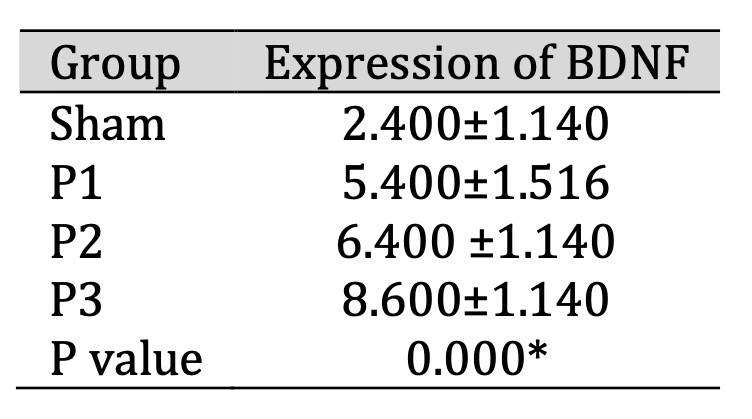
Table 1: Analysis of motoric function before and after simvastatin administration. Data presented in Mean±SD. *p<0.05 is considered to be significant.The difference between groups was analyzed with ANOVA test.The differenece between before and after simvastatin administration was analyzed with T-test
Analysis of ACAN and VCAN Expression in Brain Tissue After Simvastatin Administration
ACAN and VCAN expression in the glial scar area was assessed by immunohistochemistry (IHC). Table 2
shows
that ACAN and VCAN expression decreased after simvastatin administration in all groups, especially in
the
P3 group. This means that simvastatin administration can reduce ACAN and VCAN proteins in the ischemic
area. From Fig. 1, it can be seen that ACAN positive neurons was represented by brown cell experience a
decrease in distribution along with the increase in the dose of simvastatin. In Fig. 2 also shows that
VCAN positive neuron was represented by brown cells experience a decrease in distribution along with the
increase in the dose of simvastatin.
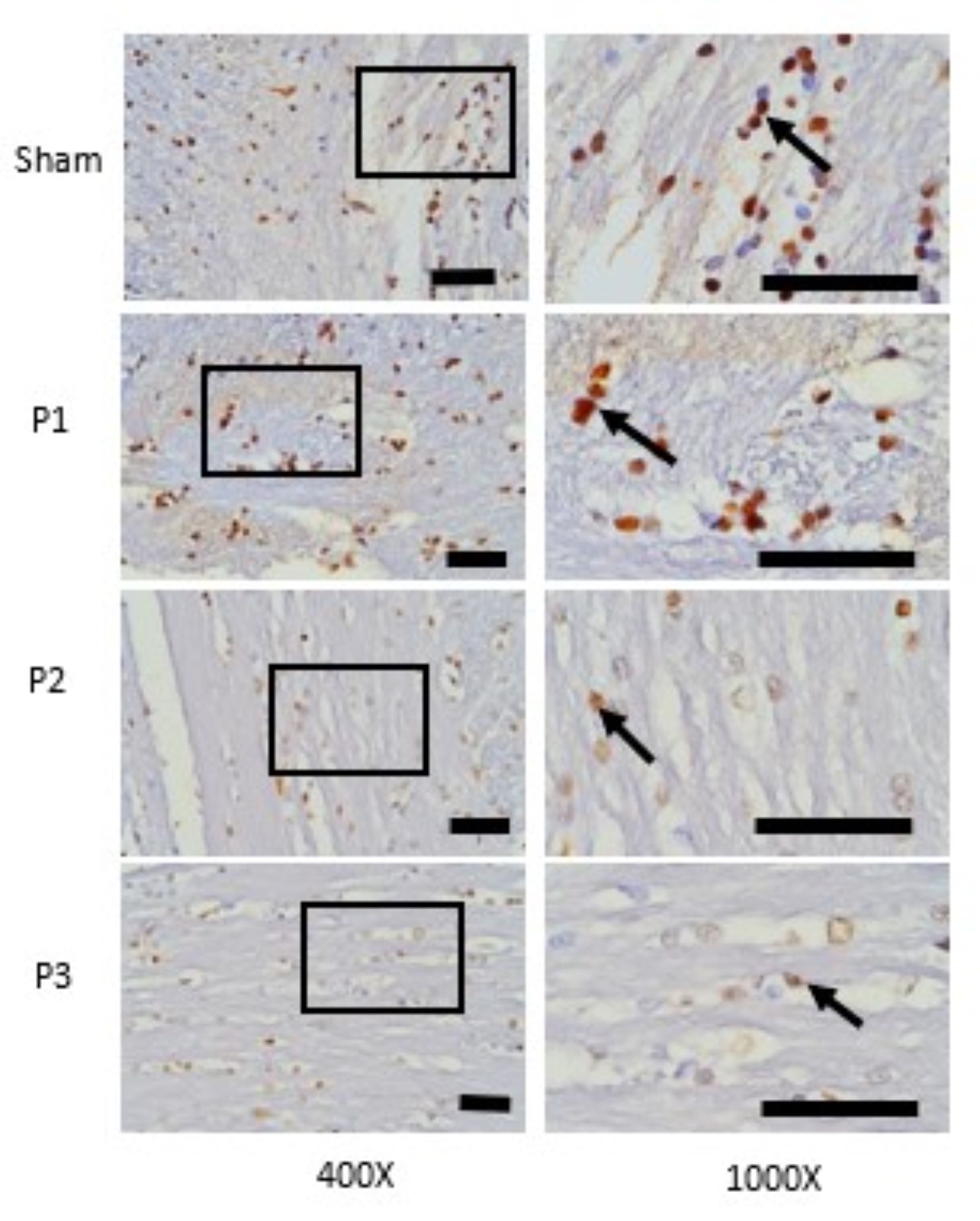
Fig. 1: Expression of ACAN in ischemic area was assessed with immunohistochemistry technique. At 400x magnifications showed the distributions of ACAN positive neuron which was represented by brown color cells were decrease in group P1, P2, P3 than sham. At 1000x magnifications showed the number of ACAN positive neuron was decrease in group P1, P2, P3 than sham. Black box: spread of ACAN positive neuron in ischemic area, black arrow: ACAN positive neuron. Black bar: 0.025mm.
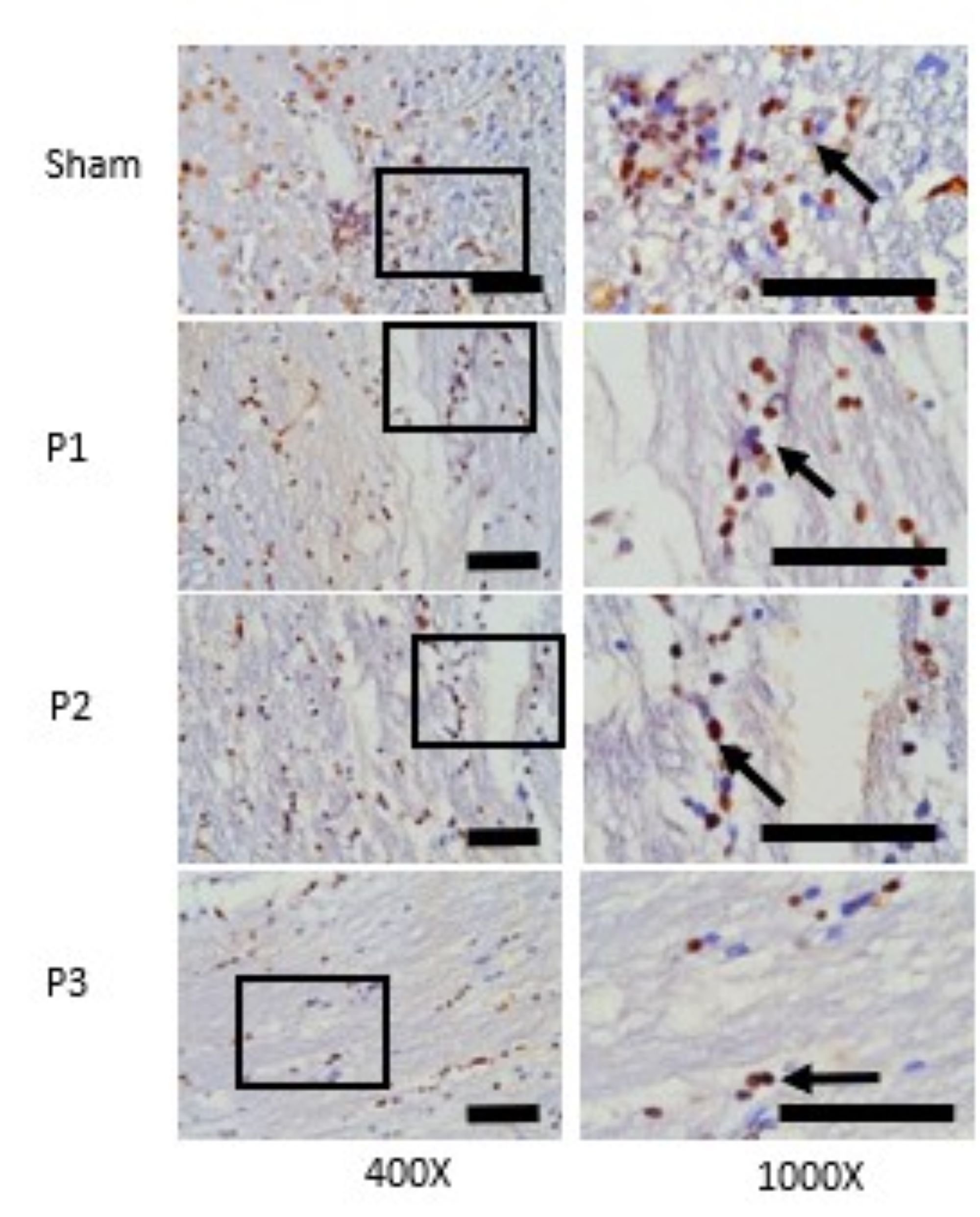
Fig. 2: Expression of VCAN in ischemic area was assessed with immunohistochemistry technique. At 400x magnifications showed the distributions of VCAN positive neuron which was represented by brown color cells were decrease in group P1, P2, P3 than sham. At 1000x magnifications showed the number of VCAN positive neuron was decrease in group P1, P2, P3 than sham. Black box: spread of VCAN positive neuron in ischemic area, black arrow: VCAN positive neuron. Black bar: 0.025mm.
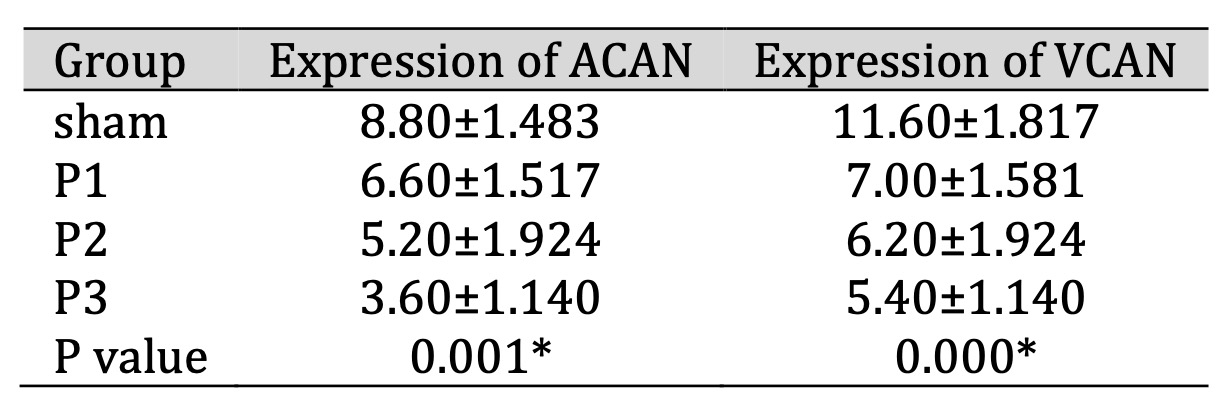
Table 2: Expression of ACAN and VCAN in brain tissue after administration of simvastatin. Data presented in Mean±SD. *p<0.05 is considered to be significant, analyzed with ANOVA test
Analysis of SOX9 Expression in Ischemia Area
SOX9 expression in the ischemic area was assessed using immunohistochemistry (IHC). Table 3 shows that
SOX9 expression decreased in groups P1, P2, P3 compared to the sham group. From Fig. 3 also shows that
the
distribution of SOX9 positive neuron which is represented by brown color cell was decrease in group
P1,
P2, P3 than sham, especially in group P3. This depicts shows that simvastatin has the ability to
reduce
the expression of the SOX9 transcription protein, especially at a dose of 15 mg/kg bw/day.
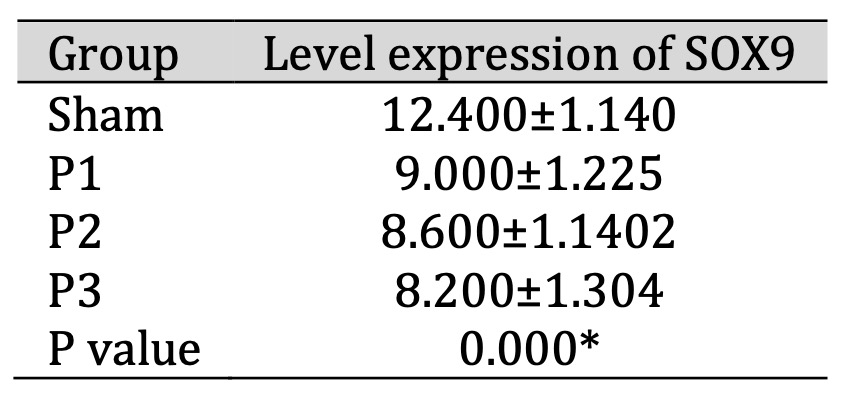
Table 3: Expression of SOX9 in ischemia area after administration of simvastatin. Data presented in Mean±SD. *p<0.05 is considered to be significant, analyzed with ANOVA test
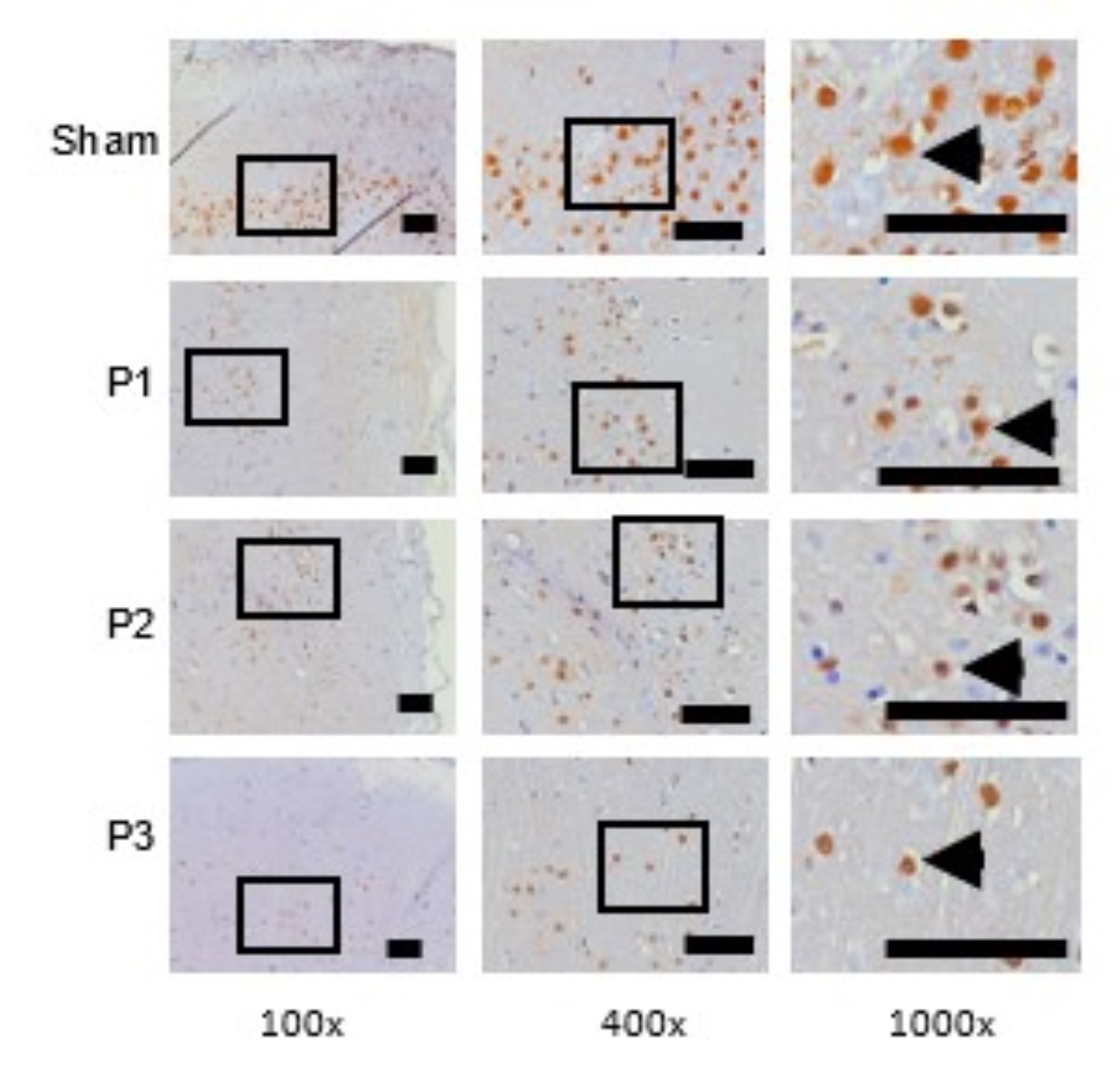
Fig. 3: SOX9 expression was assessed use immunohistochemistry technique. At 100x and 400x magnification showed the distribution of SOX9 positive neuron which was represented by brown color cells were decrease in group P1, P2, P3 than sham. At 1000x magnifications showed the number of SOX9 positive neuron was decrease in group P1, P2, P3 than sham. Black box: spread of SOX9 positive neuron in ischemic area, black arrow head: SOX9 positive neuron. Black bar: 0.025mm.
The Increase of BDNF Expression in Ischemia Area
BDNF expression in the ischemic area was assessed using immunohistochemistry (IHC). Table 4 shows
that
BDNF expression increased in groups P1, P2, P3 compared to the sham group, especially in dose 3.
From Fig.
4 shows that the distribution of BDNF positive neuron which is represented by brown color
cell was
increase in group P1, P2, P3 compare than sham. These results indicate that simvastatin can increase
BDNF
protein expression in the lesion area, and the most effective dose is 15mg/kg bw/d.
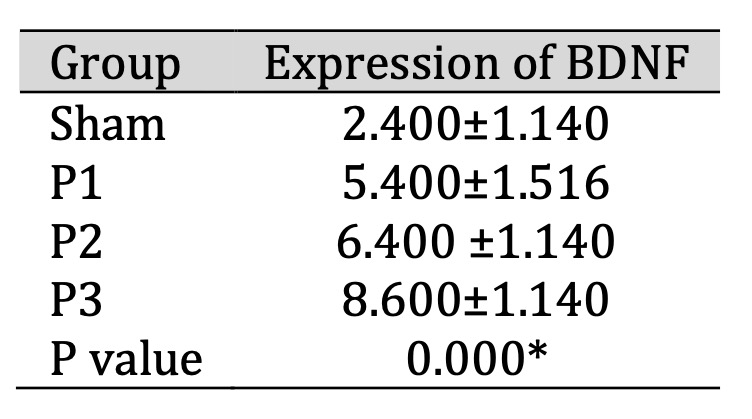
Table 4: Expression of BDNF in ischemic area after administration of simvastatin. Data presented in Mean±SD. *p<0.05 is considered to be significant, analyzed with ANOVA test
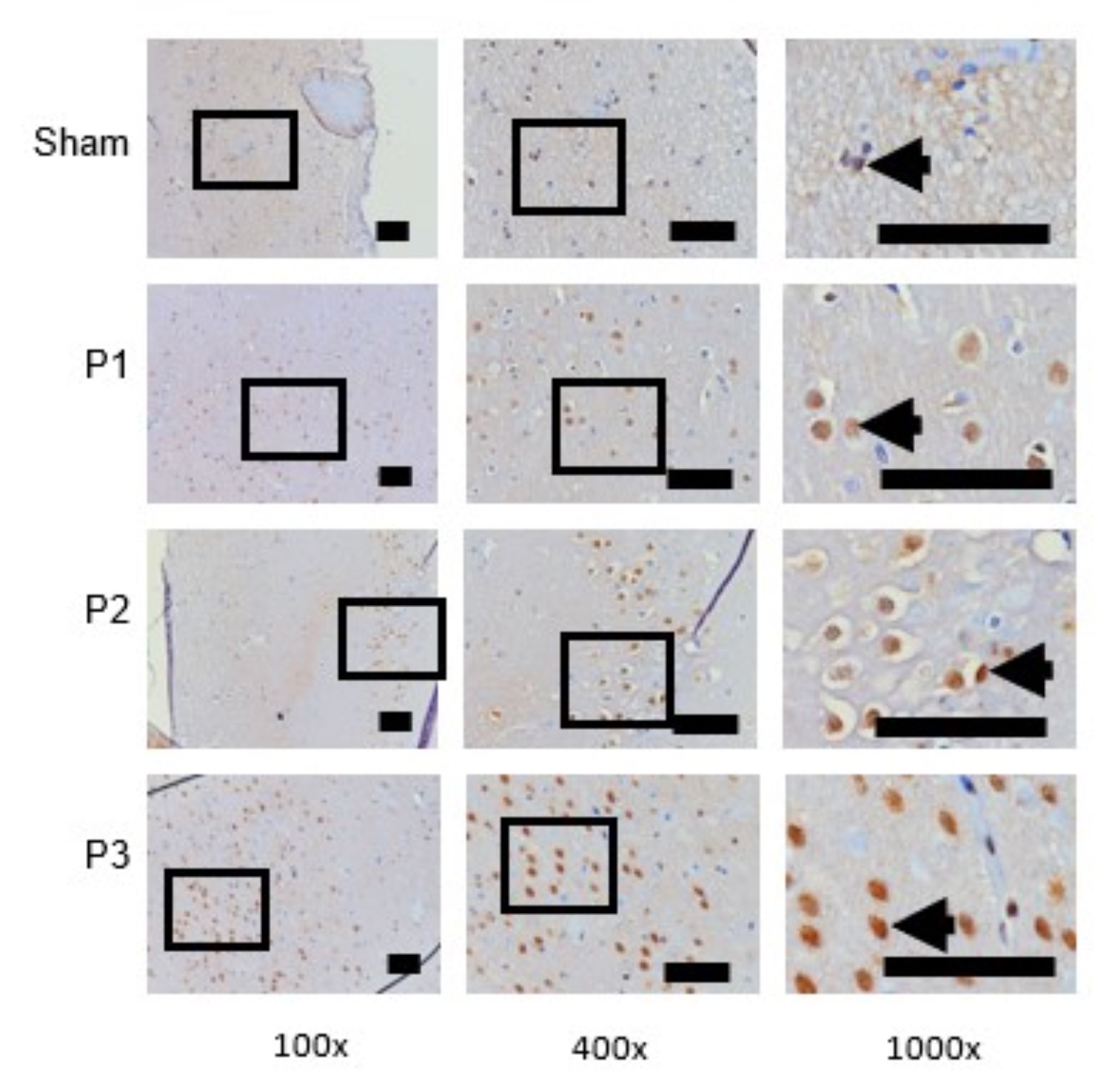
Fig. 4: BDNF expression was assessed use immunohistochemistry technique. At 100x and 400x magnification showed the distribution of BDNF positive neuron which was represented by brown color cells were increase in group P1, P2, P3 than sham. At 1000x magnifications showed the number of BDNF positive neuron was increase in group P1, P2, P3 than sham. Black box: spread of BDNF positive neuron in ischemic area, black arrow head: BDNF positive neuron. Black bar: 0.025mm.
Analysis of BDNF mRNA and SOX9 mRNA Expression
Level expression of SOX9 and BDNF gene were assessed using RT-PCR. From Fig. 5 shows that BDNF
mRNA
expression was found to increase in group P1 and P2 but unidentified in sham and P3. These results
indicate that simvastatin can growth BDNF gene expression, especially at a dose of 15 mg/kg/day.
From Fig.
6 shows that level expression of SOX9 mRNA was found to decrease in group P2 dan P3. In other
words, the
results implicitly means that simvastatin can possibly decrease SOX9 gene expression, especially
at a dose
of 15 mg/kg/day.
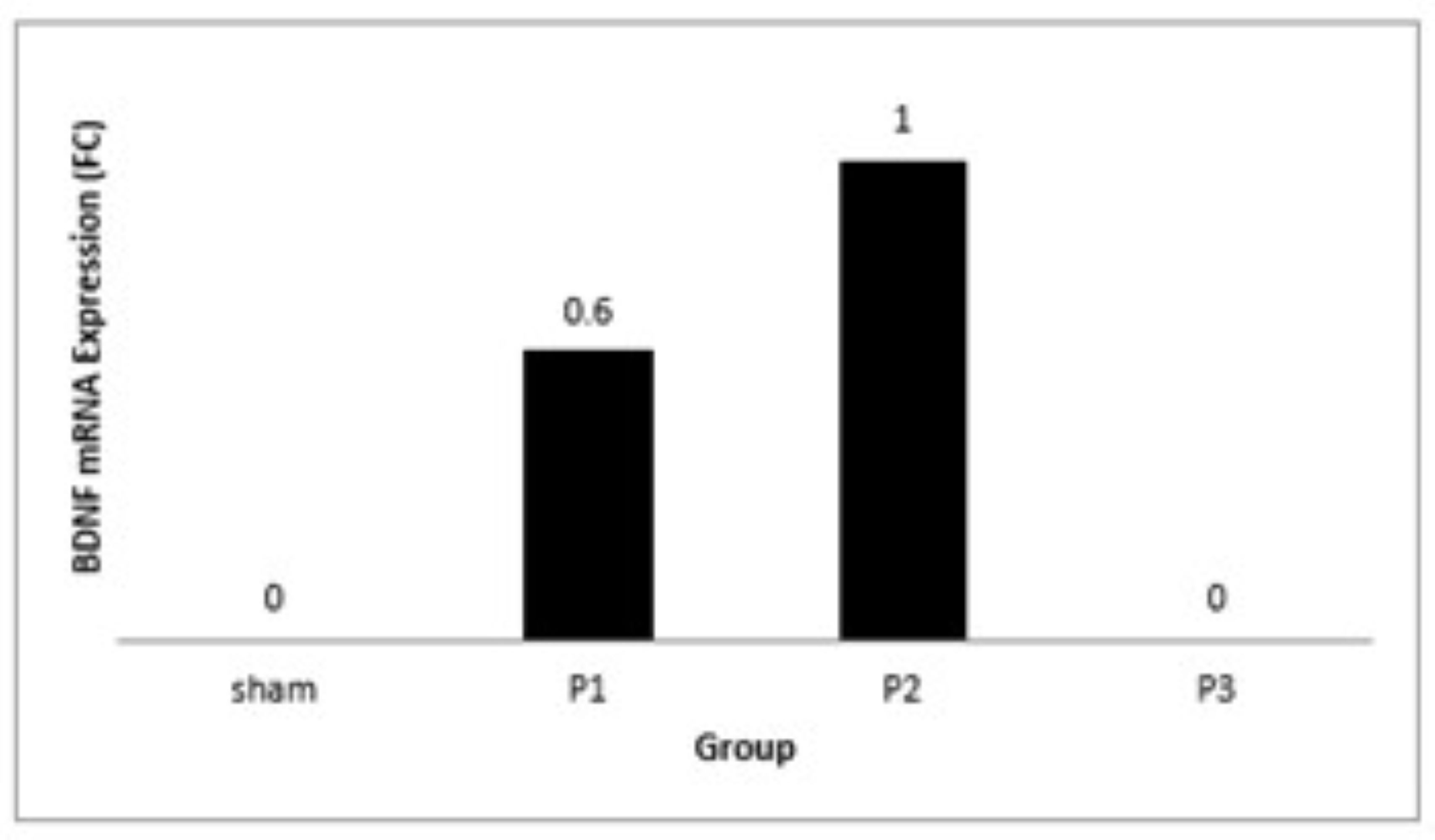
Fig. 5: Bar graph showed the BDNF mRNA expression (unit in fold change /FC) after administration of simvastatin dose 1, 2 and 3 for seven days.
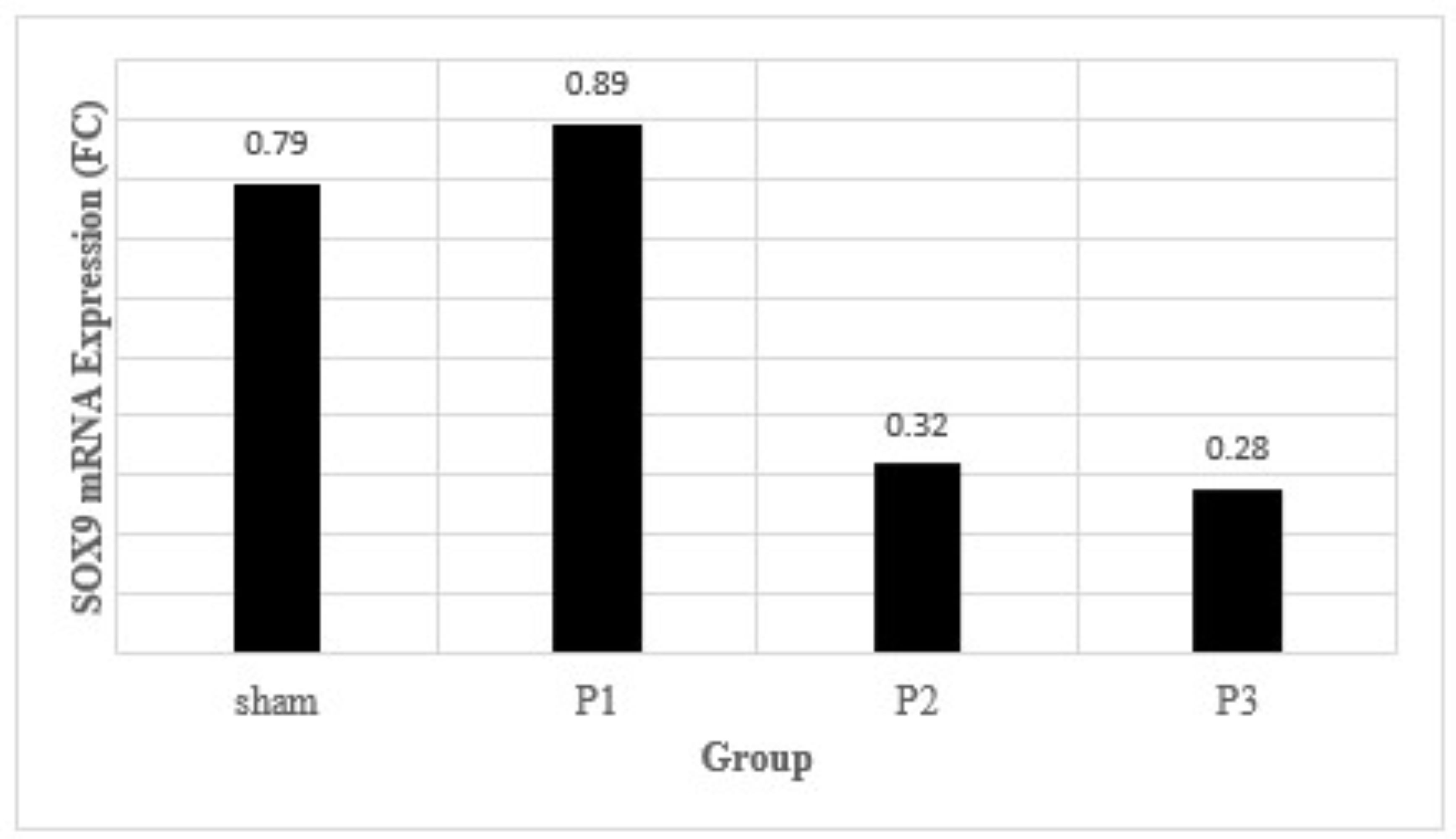
Fig. 6: The density of glial scar after 7 days of simvastatin administration. Data presented in Mean±SD. *p<0.05 is considered to be significant, analyzed with ANOVA test
Analysis The Number of Neuron in The Ischemic Area
Evaluation of the number of neurons in the ischemic area was assessed by H and E staining. Table
5
shows
that the number of neurons increased in groups P1, P2, P3 compared to sham. From Fig. 7 also
shows
the
number and distribution of neuron were increase in group P1, P2 and P3 especially in group P3.
This means
that simvastatin can induce the formation the new neuron in the ischemic area and the most
effective dose
to increase the number of neurons is 15mg/kg bb/day.
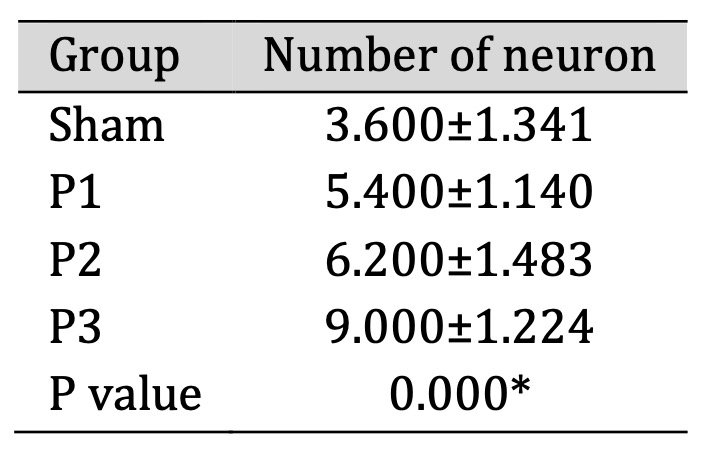
Table 5: The number of neuron in ischemic area after administration of simvastatin. Data presented in Mean±SD. *p<0.05 is considered to be significant, analyzed with ANOVA test
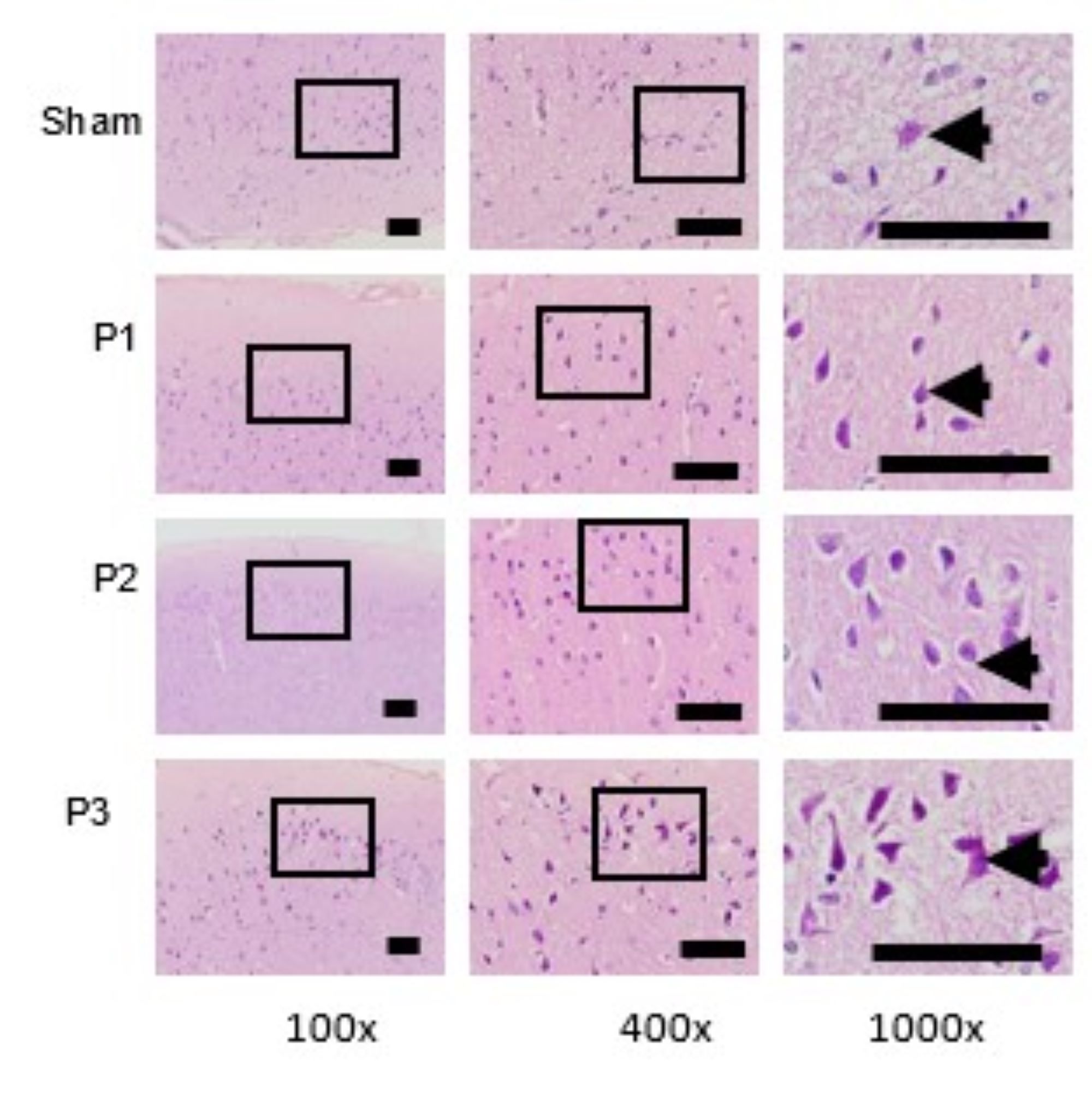
Fig. 7: Evaluation the number and distribution of neuron use H and E staining. At 100x and 400x magnification showed the distribution of neuron in ischemic area. At 1000x magnification depict the number of neuron. The distribution and number of neuron were found to increase in group P1, P2 and P3. The highest number of neuron was found in Group P3. Black box: spread of neuron in ischemic area, black arrow: neuron. Black bar: 0.025mm.
Decrease of Glial Scar Density in Ischemic Area
Examination of glial scar density in ischemic area was performed use H and E staining. From
table 6, its
can be seen that the density of glial scar was reduce in group P1, P2, P3 than sham. From Fig.
8. H and E
staining showed there was glial scar thinning which marked by reducing distribution and number
of glial
cells (purple-colored cells). From magnification 1000x was observed that the lowest
distribution
glial
cells was found in dose 3. This means that simvastatin can reduce density of glial scar in
ischemic area
and the most effective dose to reduce glial scar density was15mg/kgbw/d.
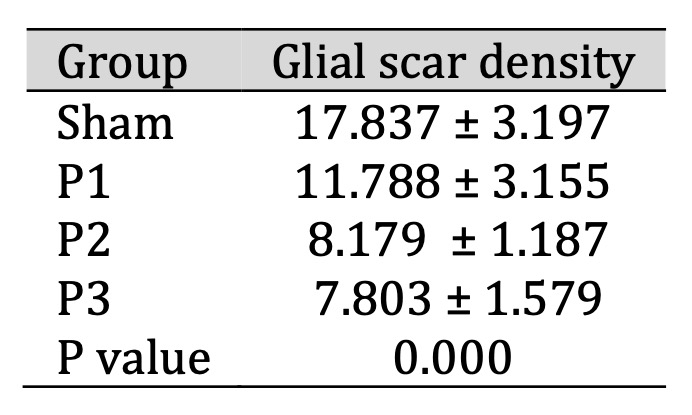
Table 6: The density of glial scar after 7 days of simvastatin administration. Data presented in Mean±SD. *p<0.05 is considered to be significant, analyzed with ANOVA test
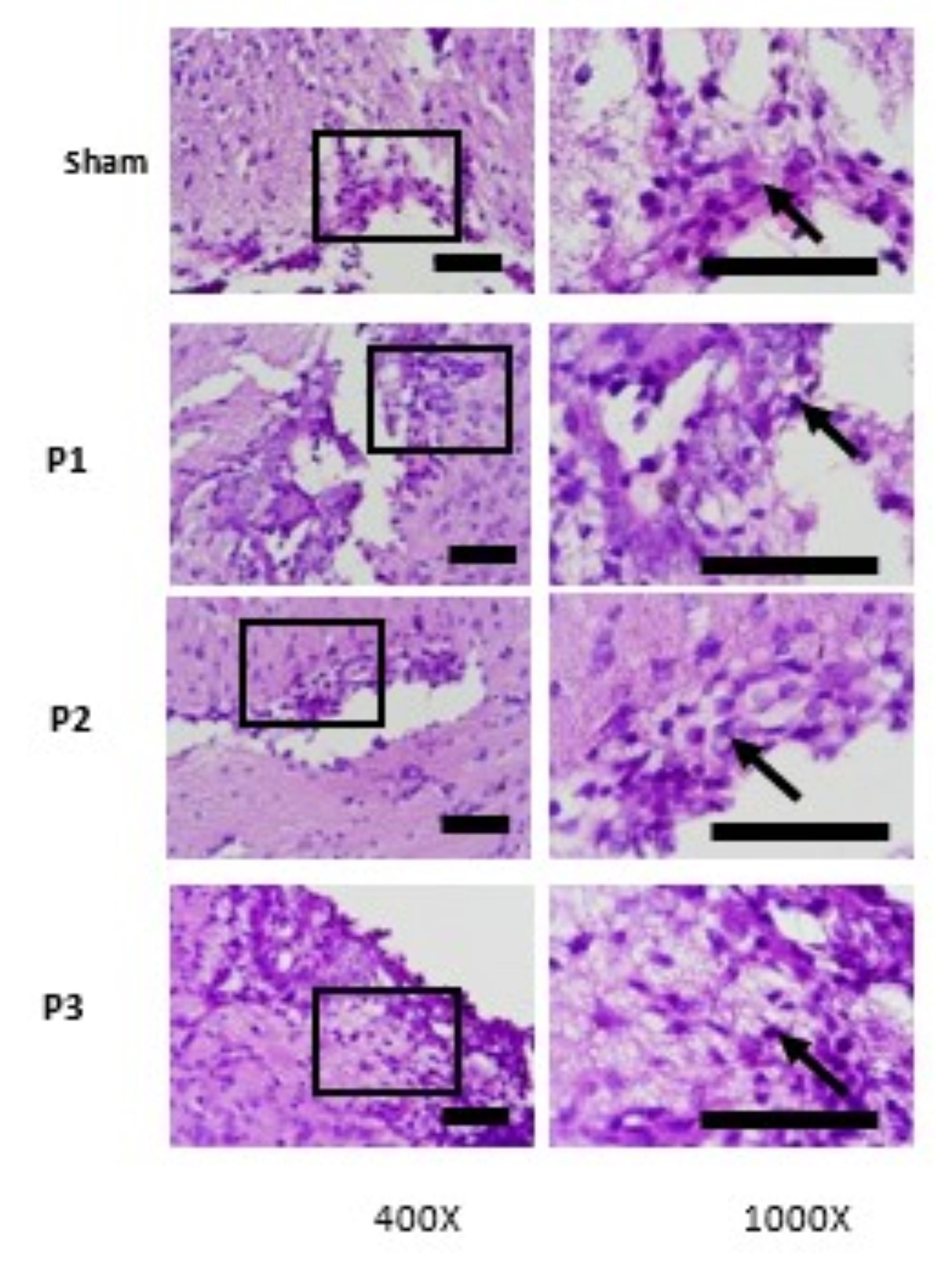
Fig. 8: Hematoxylin and eosin (HE) staining of brain tissue. Evaluation of glial scar density was done by evaluation of distribution and numerous of glial cells (purple-colored cells). At the magnifications 400x seem spread of glial cells, at 1000x magnification to identify glial cells. The Lowest distribution of glia cells was found in P3. Black box: spread of glial cells, black arrow: Glial cells. Black bar: 0.025mm.
Evalution of motoric disfunction
Motoric disfunction was assessed using LRWT (Ladder runk walking test), through evaluation
of
foot fault
scoring (FFS) of RHL and RFL.15 Motor deficit assessment was performed on the
right
extremities
as effect of left CCA occlusion. Table 1 shows that FFS in all groups were increase in both
RHL and RFL
after administration of simvastatin compare than before administration of simvastatin. From
table.1 was
also observed that the FFS after administration of simvastatin was increase in all groups
compare than
sham, especially in group D3. This means that the administration of simvastatin promotes the
improvement
of motoric function especially in dose 3.
Evaluation of CSPG constituent proteins (ACAN, BCAN, VCAN, NCAN and Phosphacan) in brain
tissue
Evaluation of ACAN, BCAN, VCAN, NCAN, and Phosphacan expressions were assessed using
immunohistochemistry
(IHC). In this study showed that all doses of simvastatin promote decrease of ACAN, BCAN,
VCAN, NCAN, and
phosphacan expression in brain tissue. Although the result were not statistically
significant
but there
were a tendency for decrease of CSPG constituent proteins along with the increase the dose
of
simvastatin.
The lowest expression of CSPG constituent protein was found in group D3. From Fig. 1 its
also
can be seen
that numerous and distribution of ACAN, BCAN, VCAN, NCAN, Phosphacan positive neuron (brown
colored cells)
were decrease after administration of simvastatin in all doses. And the dose 3 was most
effective to
decrease distribution and number of brown colored cells compare than dose 1 and 2.
Evaluation of EGR1 expression in brain tissue
Transcription Protein EGR1 is extensively expressed in reactive astrocyte. Reactive
astrocyte
is initial
step for glial scar formation. EGR1 has role to regulate activation of
astrocyte.18
In this
study, expression of EGR1 was found to decrease in all groups compare than sham (table 3).
In
table 3 was
also observed that the most effective dose to reduce expression of EGR1 was dose 3, namely
15mg/kgbw/day.
From table 3 also revealed that the decrease of EGR1 along with the increase in dose of
simvastatin. This
means that the higher dose of simvastatin the more effective it is in downregulate of EGR1.
From Fig. 2.
It’s also can be seen that distribution and numerous EGR1 positive neuron (brown
colored
cells) was
decrease in all groups, particularly in dose 3.
Evaluation of glial scar density
Glial scar density was assessed using H and E staining. In this study glial scar density was
found to
decrease significantly in all groups compare than sham (table 4). Based on this data, dose 3
was most
effective dose in reducing glial scar density. In this study also was observed that dose 2
more effective
in reducing glial scar density compare than dose1. Its means the higher dose of simvastatin,
the more
effective it is in reducing glial scar density. From Fig. 3 HE staining showed that there
was
glial scar
thinning which marked by reducing distribution and number of glial cells (purple-colored
cells). From
magnification 1000x was observed that the lowest distribution glial cells was found in dose
3
(or D3
group).
Analysis of BDNF mRNA and TrkB mRNA Expression after administration of simvastatin
Level expression of BDNF and TrkB gene were assessed using RT-PCR. From Fig. 4 showed that
TrkB mRNA
expression was found to increase greatly in group D1 meanwhile in group D2 and D3 were very
low amounts.
In Fig. 5 demonstrated that BDNF mRNA expression was found to increase in group D2 and D3,
with the
highest expression was found in group D2. Meanwhile in sham and group D1 were unidentified.
From these
data revealed that after administration of simvastatin for 14 days there was a difference
between BDNFmRNA
expression and TrkB mRNA. The increase of BDNF mRNA was not followed by the increase of TrkB
mRNA
expression.
Discussion
Simvastatin, one of the statin drugs, has a mechanism as an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), the rate-limiting enzyme in the mevalonate-cholesterol biosynthesis. In addition to its primary function in lowering the lipids, statins possess pleiotropic effects such as on brain cells. One compelling reason to focus on the brain is the possibility that cholesterol plays an important role in the brain, and that disturbances in this homeostatic balance can lead to disease conditions [23]. In obesity with ischemic stroke experience has been shown to worsen brain damage and also worsen astrogliosis which increases ECM deposits such as chondroitin sulphate proteo-glycans (CSPGs) which contribute to an inhibitory environment due to the presence of glial scar. CSPGs have been shown to be the main compound inhibiting axon growth in the ischemic core [24] and CSPG is main component of glial scar [25, 26]. The modulation of the glial scar would be a promising therapy to improve neuroplasticity and limit subsequent disability [24].In the Central Nervous System (CNS), most of the CSPG components are lectican proteins consisting of ACAN, VCAN, NCAN, and BCAN [8, 27]. In the embryonic period, VCAN and ACAN present exclusively in the nervous tissue. Moreover, they are shown in the marginal zone of the cerebral cortex, the amygdala, internal capsule, and lateral olfactory tracts at embryonic day 16. VCAN was also expressed in the hippocampus and dentate gyrus by E19. In the postnatal period, both aggrecan and versican are found in the granular cell layer of the cerebellum [9]. Therefore, this study focused on ACAN and VCAN, as these two proteins are found in large amounts in the brain. This study showed that simvastatin decreased the expression levels of ACAN and VCAN as CSPG components in glial scar tissue. The decrease in ACAN and VCAN expression was also accompanied by a decrease in SOX9 protein and also SOX9 gene expression. The SOX9 mRNA expression was decraease particularly in dose 10 mg/kgbw/d and 15 mg/kgbw/d.This result is most likely due to the anti-inflammatory effect of simvastatin. This finding is supported by a study stating that pro-inflammatory cytokines (IL-6, TGF-β2, and PDGF) increase SOX9 expression [28], while simvastatin has the effect in decreasing the pro-inflammatory cytokine interleukin (IL)-6 [29]. The decrease in ACAN and VCAN expression is thought to be due to a decrease in SOX9 regulation as an effect of simvastatin administration. This assumption is supported by the fact that Sox9 is a transcription factor involved in the regulation of genes required for scar tissue formation. Sox9 directly binds to XT-I, XT-II and C4ST, enzymes that synthesize chondroitin sulfate side chains in CSPG. Therefore, downregulation of sox9 will attenuate the activity of XT-I, XT-II and C4ST which play a significant role in regulating the biosynthesis of CSPG constituent proteins [28].
A study conducted by Holmberg, E et al, 2008 revealed that statins have the ability to directly inhibit the RhoA/ROCK pathway and reduce CSPG expression in several models of CNS injury. However, the attenuation of statin-induced CSPG is most likely not due to inhibition of the RhoA/ROCK pathway because direct inhibition of RhoA or ROCK will increase CSPG levels [14]. This enables making as assumption that the mechanism of simvastatin in reducing ACAN and VCAN occurs at the transcriptional stage involving downregulation of SOX 9 as the transcription protein of ACAN and VCAN, not at the CSPG receptor. This occurs because when simvastatin works as a CSPG receptor by blocking or inhibiting RhoA/ROCK signaling, it will increase CSPG expression due to inhibition of CSPG degradation. The results of this study also illustrates that the density of glial scar decreased after simvastatin administration. This evidence depicts that simvastatin can reduce the thickness of glial scar through inhibition of ACAN and VCAN biosynthesis by downregulating the SOX9 transcription protein.
Moreover, this study also represents that BDNF expression increase after simvastatin administration. BDNF, one of the neurotrophic factors, is expressed excessively in the brain. BDNF has beneficial effects in the development, survival, and maintenance of neurons in the central nervous system (CNS). The binding BDNF with the receptor TrkB mediated intracellular signals are involved in neurogenesis process especially in hippocampal region [30]. The increase in BDNF expression indicates clinical improvement in ischemic stroke patients.
Study conducted by Chaturvedi, P et al, 2020 said that BDNF was significantly lower in patients following acute stroke. Decrease of BDNF level was probably due to an induction altered neuronal excitability with downstream signal in excitatory neurotransmitters [4]. Nevertheless, the findings of this study show that administering simvastatin elevates BDNF expression levels, which are typically reduced in stroke cases. This suggests that simvastatin can promote neurogenesis under ischemic stroke conditions. Simvastatin is believed to enhance BDNF expression through the PPARα pathway, which activates the transcription of cAMP response element-binding protein (CREB), particularly at the Leu331 and Tyr334 sites, functioning as transcription factors involved in BDNF production [31]. Another potential mechanism is that simvastatin promotes neurogenesis by activating the Akt signaling pathway, which subsequently enhances the expression of growth factors [32]. Although this study observed a decrease in ACAN and VCAN expression, it is believed that simvastatin does not promote neurogenesis by suppressing ACAN and VCAN. This assumption aligns with findings by Kurihara D and Yamashita T (2011), which state that CSPG interaction with its receptor, PTPσ, leads to dephosphorylation of TrkB, a key BDNF receptor [33]. Therefore, the simvastatin-induced reduction in CSPG components may interfere with CSPG-PTPσ binding, preventing TrkB dephosphorylation. This inhibition enhances BDNF activity at TrkB, which in turn leads to a decrease in BDNF expression in the brain.
However, this study found that the BDNF gene expression was unidentified in dose 3 of simvastatin meanwhile in this dose the BDNF protein expression was increase. In addition, in the dose 2 the expression of SOX9 gene was increase but expression of SOX9 protein was decrease. This result may be caused by several mechanisms, such as feedback loops mechanism, where the BDNF protein levels are high, it might inhibit BDNF gene expression or when the SOX9 protein levels are low, it might induce SOX9 gene expression. As demonstrated in the study by Müller-McNicoll M et al., 2019, negative feedback autoregulation serves to control and limit protein production within tissues. When protein levels are low, RNA-binding proteins (RBPs) involved in feedback regulation become inactive, allowing protein synthesis to proceed. Conversely, when protein levels are elevated, the negative feedback mechanism is activated, enabling the protein to bind to regulatory sequences on its own mRNA, thereby initiating autoregulation and reducing further protein production [34]. This negative feedback mechanism was also described in a study by Zou M et al., 2005, which found that alterations in the expression of target genes occurred before changes in the expression of their known regulatory genes. This suggests that target genes may also influence the expression of their regulators through a negative feedback loop [33]. Another factor contributing to the differences between regulatory and target gene expression is the variation in mRNA half-life. When the mRNA of a regulatory gene has a shorter half-life than that of its target gene, it may take longer for the regulatory mRNA to achieve a stable expression level, whereas the target mRNA can reach its steady-state level more quickly [35]. This can result in differing expression patterns between the regulatory gene and its corresponding target protein.
More than that, this study also found that there was an increase in the number of neurons after the administration of simvastatin. The findings of this study suggest that simvastatin administration promotes neurogenesis, as evidenced by a rise in both the number of neurons and BDNF expression. The increase in neuron count is likely driven by the elevated BDNF levels. This assumption is supported by research conducted by Waterhouse, E.G., et al., 2012, which revealed that the local release of BDNF from the dendrites of mature granule cells can stimulate TrkB receptors on GABAergic interneurons, leading to an increase in GABA input to neural precursors, which induces the differentiation and maturation of precursor cells into neurons [36]. In addition to the BDNF induction effect, the increase in the number of neurons is also likely due to the inhibition of the change from neurons to glia due to the downregulation of SOX9. Sox9, a SoxE member, is expressed in neural stem cells (NSCs). Sox9 was required for maintenance of NSCs during development. SOX9 has been reported as a vital molecular in triggering the switch from neurogenic to gliogenic process in the developing mouse spinal cord. However, the main function of SOX9 in cerebellum was to suppress neurogenesis, rather than to trigger the initiation of gliogenesis [37]. Besides that, based on the study conducted by Stevanovic M, et al,2021, the increase in the number of neurons may occur due to the loss of inhibitors of neurogenesis suppression or the absence of induction that changes the neurogenic process to gliogenic, due to the downregulation of SOX9 [36].
Though this study has generated several findings, two limitations exist in this study. They are the measurement of IHC result which was done manually and the evaluation of neuron without exert specific biomarker for neuron. The process of accounting the neurons in IHC examination which was done manually unfortunately can result to a bias. Therefore, for future research, it is recommended to use bioimage analysis applications such as QuPath, to calculate the IHC result. Finally, for better result in evaluation of neuron, it is also suggested to use a specific neuronal biomarker which can assess the use of the immunofluorescence techniques.
Conclusion
Simvastatin is capable of lowering the expression of ACAN, VCAN, and the density of glial scar tissue in ischemic regions by downregulating the transcription factor SOX9. Additionally, simvastatin has been shown to stimulate neurogenesis, indicated by elevated BDNF expression and a greater number of functional neurons in the brain tissue of rats with ischemic stroke, along with improvements in motor function
Acknowledgements
Gratitude is extende to the pharmacology laboratory of Brawijaya University which has provided a place for conducting this research.
Statement of Ethics
The ethical protocol of the experiment has been approved by the animal commission of the
Faculty of
Veterinary Medicine, Brawijaya University with protocol number 219-KEP-UB-2023.
Author Contributions
AW, FAR, AM, are responsible for study concepts, study design, study analysis, and
manuscript
review. WR
are responsible for analysis of immunohistochemistry, study analysis, and statistical
analysis. All
authors took parts in giving critical revision of the manuscript and all authors approved
the
final
version of the manuscript.
Funding Sources
A sincere gratitude is also delivered to Higher Education Financing Center (BPPT) and the
Education Fund
Management Institute (LPDP) for funding this study and the research through the Indonesian
Education
Scholarship program (BPI) (Grant Number: 02492/J5.2.3./ BPI.06/9/2022).
Disclosure Statement
The authors have no conflicts of interest to declare.
Disclosure of AI Assistance
No AI tools or AI-generated content were used in the creation of this article text. The
content
was entirely
developed, written, and edited by the authors.
References
| 1 | Rahman AA, Amruta N, Pinteaux E, Bix GJ: Neurogenesis after stroke: a therapeutic
perspective.
Transl Stroke Res 2021;12(1):1–4
https://doi.org/10.1007/s12975-020-00841-w |
| 2 | Widayati A, Rantam FA, Machin A, Riawan W: Inhibition of neurogenesis and induction
of
glial scar
formation by neuroinflammation following ischemic stroke: evaluation of BDNF, GFAP,
HMGB1 and
TNF-α expressions. Indones Biomed J 2025;17(1):99–108
https://doi.org/10.18585/inabj.v17i1.3439 |
| 3 | Nagase T, Kin K, Yasuhara T: Targeting neurogenesis in seeking novel treatments for
ischemic
stroke. Biomedicines 2023;11(10):2773
https://doi.org/10.3390/biomedicines11102773 |
| 4 | Shen XY, Gao ZK, Han Y, Yuan M, Guo YS, Bi X: Activation and role of astrocytes in
ischemic
stroke. Front Cell Neurosci 2021;15:755955
https://doi.org/10.3389/fncel.2021.755955 |
| 5 | Chaturvedi P, Singh AK, Tiwari V, Thacker AK: Brain-derived neurotrophic factor
levels
in acute
stroke and its clinical implications. Brain Circ 2020;6(3):185–90
https://doi.org/10.4103/bc.bc_23_20 |
| 6 | Moeendarbary E, Weber IP, Sheridan GK, Koser DE, Soleman S, Haenzi B, et al.: The
soft
mechanical
signature of glial scars in the central nervous system. Nat Commun 2017;8(1):14787.
https://doi.org/10.1038/ncomms14787 |
| 7 | Volpato FZ, Führmann T, Migliaresi C, Hutmacher DW, Dalton PD: Using
extracellular matrix for
regenerative medicine in the spinal cord. Biomaterials 2013;34(21):4945–55.
https://doi.org/10.1016/j.biomaterials.2013.03.057 |
| 8 | Sami A, Selzer ME, Li S: Advances in the signaling pathways downstream of glial-scar
axon growth
inhibitors. Front Cell Neurosci 2020;14:174.
https://doi.org/10.3389/fncel.2020.00174 |
| 9 | Popp S, Andersen JS, Maurel P, Margolis RU: Localization of aggrecan and versican in
the
developing rat central nervous system. Dev Dyn 2003;227(1):143–9.
https://doi.org/10.1002/dvdy.10282 |
| 10 | Zhou XH, Brakebusch C, Matthies H, Oohashi T, Hirsch E, Moser M, et al.: Neurocan is
dispensable
for brain development. Mol Cell Biol 2001;21(17):5970–8.
https://doi.org/10.1128/MCB.21.17.5970-5978.2001 |
| 11 | Bass BR: Investigation of Sox9 ablation on neuroplasticity and recovery after
ischemic
stroke
[Dissertation].. Canada: The University of Western Ontario; 2014.
|
| 12 | Gris P, Tighe A, Levin D, Sharma R, Brown A: Transcriptional regulation of scar gene
expression in
primary astrocytes. Glia 2007;55(11):1145–55.
https://doi.org/10.1002/glia.20537 |
| 13 | Pechnick RN, Chesnokova V: Adult neurogenesis, cell cycle and drug discovery in
psychiatry.
Neuropsychopharmacology 2009;34(1)
https://doi.org/10.1038/npp.2008.164 |
| 14 | Holmberg E, Zhang SX, Sarmiere PD, Kluge BR, White JT, Doolen S: Statins decrease
chondroitin
sulfate proteoglycan expression and acute astrocyte activation in central nervous
system
injury. Exp
Neurol 2008;214(1):78–86.
https://doi.org/10.1016/j.expneurol.2008.07.020 |
| 15 | Huang L, Wu ZB, ZhuGe Q, Zheng W, Shao B, Wang B, et al.: Glial scar formation
occurs
in the human
brain after ischemic stroke. Int J Med Sci 2014;11(4):344.
https://doi.org/10.7150/ijms.8140 |
| 16 | Antonow-Schlorke I, Ehrhardt J, Knieling M: Modification of the ladder rung walking
task—new
options for analysis of skilled movements. Stroke Res Treat 2013;2013:418627.
https://doi.org/10.1155/2013/418627 |
| 17 | García-Bonilla L, Campos M, Giralt D, Salat D, Chacón P,
Hernández-Guillamon M, et
al.: Evidence for the efficacy of statins in animal stroke models: a meta-analysis. J
Neurochem
2012;122(2):233–43.
https://doi.org/10.1111/j.1471-4159.2012.07773.x |
| 18 | Rossini E, Biscetti F, Rando MM, Nardella E, Cecchini AL, Nicolazzi MA, et al.:
Statins in high
cardiovascular risk patients: do comorbidities and characteristics matter?. Int J Mol
Sci
2022;23(16):9326.
https://doi.org/10.3390/ijms23169326 |
| 19 | Nair AB, Jacob S: A simple practice guide for dose conversion between animals and
human. J Basic
Clin Pharm 2016;7:27–31.
https://doi.org/10.4103/0976-0105.177703 |
| 20 | Davidson MH, Stein EA, Dujovne CA, Hunninghake DB, Weiss SR, Knopp RH, et al.: The
efficacy and
six-week tolerability of simvastatin 80 and 160 mg/day. Am J Cardiol
1997;79(1):38–42.
https://doi.org/10.1016/S0002-9149(96)00742-4 |
| 21 | Metz GA, Whishaw IQ: The ladder rung walking task: a scoring system and its
practical
application.
J Vis Exp 2009;(28):1204.
https://doi.org/10.3791/1204-v |
| 22 | Martins LA, Schiavo A, Xavier LL, Mestriner RG: The foot fault scoring system to
assess skilled
walking in rodents: a reliability study. Front Behav Neurosci 2022;16:892010.
https://doi.org/10.3389/fnbeh.2022.892010 |
| 23 | Sodero AO, Barrantes FJ: Pleiotropic effects of statins on brain cells. Biochim
Biophys Acta
Biomembr 2020;1862(9):183340.
https://doi.org/10.1016/j.bbamem.2020.183340 |
| 24 | Clain J, Couret D, Bringart M, Lecadieu A, Meilhac O, Lefebvre d’Hellencourt
C,
et al.:
Metabolic disorders exacerbate the formation of glial scar after stroke. Eur J
Neurosci
2024;59(11):3009–29.
https://doi.org/10.1111/ejn.16325 |
| 25 | Yang T, Dai Y, Chen G, Cui S: Dissecting the dual role of the glial scar and scar
forming
astrocytes in spinal cord injury. Front Cell Neurosci 2020;14:78.
https://doi.org/10.3389/fncel.2020.00078 |
| 26 | Avram S, Shaposhnikov S, Buiu C, Mernea M: Chondroitin sulfate proteoglycans:
structure-function
relationship with implication in neural development and brain disorders. Biomed Res
Int
2014;
https://doi.org/10.1155/2014/642798 |
| 27 | McKillop WM: Sox9 conditional knockdown reduces chondroitin sulphate proteoglycan
expression,
increases neuroplasticity, and improves motor function in a mouse model of spinal cord
injury
[Dissertation].. Canada: The University of Western Ontario; 2014.
|
| 28 | Rezaie-Majd A, Maca T, Bucek RA, Valent P, Müller MR, Husslein P, et al.:
Simvastatin reduces
expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant
protein-1 in
circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc
Biol
2002;22(7):1194–9.
https://doi.org/10.1161/01.ATV.0000022694.16328.CC |
| 29 | Numakawa T, Odaka H, Adachi N: Actions of brain-derived neurotrophin factor in the
neurogenesis
and neuronal function, and its involvement in the pathophysiology of brain diseases.
Int
J Mol Sci
2018;19(11):3650.
https://doi.org/10.3390/ijms19113650 |
| 30 | Roy A, Jana M, Kundu M, Corbett GT, Rangaswamy SB, Mishra RK, et al.: HMG-CoA
reductase inhibitors
bind to PPARα to upregulate neurotrophin expression in the brain and improve
memory
in mice.
Cell Metab 2015;22(2):253–65.
https://doi.org/10.1016/j.cmet.2015.05.022 |
| 31 | Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, et al.: Simvastatin-mediated upregulation
of
VEGF and
BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated
with
therapeutic improvement after traumatic brain injury. J Neurotrauma
2008;25(2):130–9.
https://doi.org/10.1089/neu.2007.0369 |
| 32 | Kurihara D, Yamashita T: Chondroitin sulfate proteoglycans down-regulate spine
formation in
cortical neurons by targeting tropomyosin-related kinase B (TrkB) protein. J Biol Chem
2012;287(17):13822–8.
https://doi.org/10.1074/jbc.M111.314070 |
| 33 | Zou M, Conzen SD: A new dynamic Bayesian network (DBN) approach for identifying gene
regulatory
networks from time course microarray data. Bioinformatics 2005;21(1):71–9.
https://doi.org/10.1093/bioinformatics/bth463 |
| 34 | Müller-McNicoll M, Rossbach O, Hui J, Medenbach J: Auto-regulatory feedback by
RNA-binding
proteins. J Mol Cell Biol 2019;11(10):930–9.
https://doi.org/10.1093/jmcb/mjz043 |
| 35 | Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, et al.: BDNF promotes
differentiation and maturation of adult-born neurons through GABAergic transmission. J
Neurosci
2012;32(41):14318–30.
https://doi.org/10.1523/JNEUROSCI.0709-12.2012 |
| 36 | Stevanovic M, Drakulic D, Lazic A, Ninkovic DS, Schwirtlich M, Mojsin M: SOX
transcription factors
as important regulators of neuronal and glial differentiation during nervous system
development and
adult neurogenesis. Front Mol Neurosci 2021;14:654031.
https://doi.org/10.3389/fnmol.2021.654031 |
| 37 | Vong KI, Leung CK, Behringer RR, Kwan KM: Sox9 is critical for suppression of
neurogenesis but not
initiation of gliogenesis in the cerebellum. Mol Brain 2015;8:1–8
https://doi.org/10.1186/s13041-015-0115-0 |