Non-Enzymatic Antioxidant Defense and Polymorphic Changes in Male Infertility
Keywords
Abstract
Background/Aims:
Male infertility is conditioned in up to 25% genetically, but environmental factors are equally important. Dependencies analyzed here in this area have not been studied using such an approach so far. Therefore, they are innovative and constitute an important aspect of multi-range interdependencies. That is why we analyzed factors shaping male reproductive condition: glutathione, bilirubin, uric acid, chemical elements (Ca, Na, Mn, Fe, Mo, Li, V, Co, Ag, Ba, Tl, Al, Ni, Sn, B, Pb, Be), and genetic polymorphism (genotypes CC and TT of IL–4v.C589T(rs2243250). We studied infertile men from polluted Poland region with semen perturbations and healthy with normozoospermia.Methods:
We described semen abnormalities according to standard criteria. The population of patients with infertility consisted of 76 men with different fertility disorders. The control group consisted of 87 men with normozoospermia. The majority of infertile men came from Central Poland. The collection of biological samples and seminological tests were conducted by qualified medicians from the andrology clinic and by the authors of this paper (semen morphological parameters). Seminological analyses were based on macro- and microscopic analysis of ejaculate to verify semen volume, time of liquefaction, sperm density, motility, presence of agglutination, presence of leukocytes, and percentage of pathological forms. Concentrations of chemical elements in the blood were analyzed (ICP-MS). In serum, non-enzymatic antioxidants (glutathione GSH, bilirubin, uric acid) and lipid peroxidation intensity were qualified (Cayman Chemicals Co.). In researching gene polymorphisms connected with male infertility, molecular analysis was conducted (PCR–RFLP) and applied to chromosome 5: gene IL–4v.C589T.Results:
We found poorer antioxidative defense in infertile men, whilst the higher levels of uric acid, compared to healthy, may act as a deteriorating factor. High correlations between glutathione and uric acid in the infertile and healthy implicated that non–enzymatic antioxidants undergo mutual regulation. It also applies to patients with IL–4v.C589T polymorphism. Interactions between non–enzymatic antioxidants and chemical elements were particularly noticeable in men with CC genotype. The most important modulator appeared to be sodium, while boron was the most meaningful in the interactions. Higher concentration of bilirubin, uric acid, and GSH in men with TT (0.687 mg·dL−1, 6.097 mg·dL−1, 6.345 µM), compared to CC genotype (0.652 mg·dL−1, 4.980 mg·dL−1, 4.630 µM) suggest a better functionality of antioxidative barrier. Estimating the importance of unfavorable changes arising from oxidative stress about the functionality of non-enzymatic antioxidants and correlations with MDA in men's serum allows a complete look at the determinants of male infertility. Among genetic polymorphisms, genotypes TT and CC of IL–4v.C589T gene show their influence on generating fertility perturbations. They had an indirect but differentiated effect on antioxidant mechanisms involving bilirubin, uric acid, and glutathione. Therefore, we conclude that IL–4v.C589T polymorphism differentiated the body's response to environmental stressors. The results presented in our paper on IL–4v.C589T polymorphism and conclusions formulated on their basis are consistent with literature data, indicating the lack of a direct relationship between polymorphism studied and male infertility. However, the primary intention of this paper was, to a lesser extent, to exclude or confirm a direct relationship between studied polymorphism and male infertility. We wanted a broader approach to the subject and to establish relationships between genetic aspects and antioxidant parameters of defense mechanisms. Therefore, we were more interested in the status of antioxidant defense and its relationships to the genetic factor in groups of people with a fixed genotype. We obtained a more detailed picture of the sum of genetic aspects and parameters related to antioxidant defense.Conclusion:
Non–enzymatic defense, chemical elements, and genetic polymorphisms are related to and shape male reproductive potential. Our results may be helpful in the diagnosis of male infertility; they will enable the reduction of idiopathic cases and the implementation of targeted and more effective treatment. Identification of environmental stressors and their correlations with fertility disorders can help eliminate or reduce the impact of factors unfavorable to fertility. This shows the new importance of environmental and immunogenetic factors, oxidative stress, and genetic polymorphisms in male fertility.Introduction
The problem of male infertility is conditioned in up to 25% genetically, but environmental factors are equally important. Genetic and environmental impact, radiation, certain drugs, or immunologic conditions are the most important male factors. At the same time, the hormonal category encompasses imbalances in hormone levels and hypoandrogenism as well as possible psychological factors [1]. Certain types or causes of male infertility are still not sufficiently described or cannot be categorized [1]. One of the essential factors in the etiology of male infertility is oxidative stress since 30–80% of infertile men demonstrate increased levels of seminal reactive oxygen species (ROS). This fact encouraged the constitutional term "male oxidative stress infertility", which is particularly important for men with idiopathic infertility as a possible decisive factor [2]. However, low ROS levels are necessary to maintain sperm functionality and trigger processes that guarantee reproductive success. However, the uncontrolled increment in their level harms semen quality. Among them, lipoperoxidation of the sperm plasma membrane, sperm DNA fragmentation, and oxidation of sperm proteins occur [2]. The fact that spermatozoa are generally rich in polyunsaturated fatty acids propels lipid peroxidation and subsequently leads to the generation of damaging aldehydes like 4-hydroxynonenal or malondialdehyde (MDA). The accumulation of these lipid peroxidation products also lessens spermatozoa's protective abilities [3].
A large pool of environmental factors additionally stimulates ROS production in spermatozoa. Among them, estrogens are considered particularly important since extensive environmental expositions mediate in the decrease of testosterone levels, and this phenomenon confirms a link between environmental pollution and a general decrease in the male reproductive potential [3]. Another serious modulator of sperm quality is electromagnetic energy. Chronic exposure to this factor is connected with a decrease in sperm quality parameters such as vitality and motility, accompanied by an increment in DNA oxidation and fragmentation and escalated ROS production in the mitochondria [4]. Also, sudden changes in temperature or osmotic balance create a risk of declining sperm quality [5]. Lifestyle factors may modulate sperm quality and subsequent reproductive potential. Similarly, ethanol metabolization generates acetaldehyde and simultaneously contributes to the ROS increment [6]. Even psychological strain appears engaged in the worsening of sperm motility and concentration and the reduction of antioxidants [6].
The antioxidative system provides the counteraction against such multifactorial threats, which generally aims to scavenge and neutralize free radicals and repair damaged ones. Thus, both enzymatic and non-enzymatic antioxidants guarantee the regulation of free radical reactions and restoration of cellular integrity. Although the cooperative character of the entire system is crucial for its effectiveness, enzymatic antioxidants convert oxidized metabolic products to H2O2 and water. In contrast, non-enzymatic ones act through interruption and termination of free radical chain reactions [7]. These actions take place on the particular lines of defense and antioxidants, e.g., uric acid, bilirubin, glutathione (GSH), as well as vitamin C, alpha-tocopherol, ubiquinol, widely represents the second line preventing initiation of chain reactions and interrupting their propagation [7].
It is well established that in spermatozoa, there are two primary sources of ROS, namely NADH-dependent oxidoreductase at the mitochondrial level and NADPH oxidase in the sperm plasma membrane. On the other hand, spermatozoa generally contain little cytoplasm sequestering antioxidants, constitute seminal plasma as a significant source of antioxidants, and besides antioxidative enzymes also, relatively high levels of non-enzymatic antioxidants like ascorbate or thiol groups are present in this microenvironment. Therefore, the efficiency of antioxidative defense is crucial since harmful changes caused by oxidative stress, like a downgrade in the membrane integrity, enzyme inactivation, or cell death, are connected with the reduction of sperm quality parameters, e.g., count and motility [8].
One of the cytoprotective adaptations against oxidative stress engages biliverdin, which is generated as a byproduct of heme breakdown by heme oxygenase and subsequently, through the participation of biliverdin reductase, undergoes reduction to bilirubin. This process is a beneficial evolutionary mechanism that augments cytoprotection since bilirubin exhibits potent antioxidative and anti-inflammatory activity [9]. Thus, a synthesis of bilirubin is rapidly magnified in response to oxidative stress. At the same time, animal models widely confirm that its deficiency is connected with the increment in susceptibility of cells to oxidative damage. Fibroblasts from mice devoid of biliverdin reductase (BVR−/−) appear generally more sensitive to superoxide donors, hydrogen peroxide, or 4–hydroxynonenal, as well as highly subjected to oxidative death, since their potential to eliminate ROS is seriously handicapped. Oppositely, the addition of bilirubin to BVR−/− fibroblasts causes a reduction in the O2*− level, suggesting the engagement of antioxidants in scavenging this radical [10].
Furthermore, the other heme metabolites, namely ditaurated bilirubin and biliverdin, also scavenge O2*−. The first has almost comparable efficiency, and the latter has a relatively minor capacity. The fact that mitochondrial complexes II and III generate O2*− at membranous structures and these compartments are generally more available to bilirubin compared to superoxide dismutase (SOD) makes bilirubin a crucial O2*− scavenger in the membranous environment [10].
The animal models confirmed a positive correlation between the plasma's antioxidative efficiency and the total bilirubin level in the plasma. It is also connected with successive increases in the effectiveness of eliminating peroxyl radicals from plasma, demonstrating the scavenging activity of bilirubin [11]. When bilirubin is bound to albumin, it protects from oxidation of linolenic acid associated with this protein. On the other hand, bilirubin inhibits the activity of NADPH oxidase (an enzyme with the potential to generate superoxide anion) and counteracts oxidative DNA injuries. It may also deactivate singlet oxygen [12, 13]. Takeda et al. (2015) affirm that uninterrupted cellular generation of heme and its conversion to biliverdin and subsequently into bilirubin play an essential role in cytoprotection against widely considered cellular damages [14].
Similarly, glutathione is essential in the non-enzymatic antioxidant defense, scavenging various free radicals. At the same time, its deficiency contributes to perturbations in spermatozoa midpieces and affects morphology and motility. Oppositely, GSH supplementation improves testicular histology and sperm motility [15]. GSH concentration tends to be lower in infertile men in comparison to fertile. Meanwhile, semen GSH levels appear positively connected with essential seminogram parameters like total sperm cell count, progressive motility, concentration, plasma membrane integrity, viability, and correct morphology.
On the other hand, the GSH level in seminal plasma correlates negatively with the period of trying to have a child, suggesting that an increment in this parameter favors the achievement of reproductive success [16]. Sekhar et al. (2011) confirmed that reduced synthesis of glutathione due to aging processes leads to an intracellular deficit of GSH and an increment of oxidative stress in cells [17]. Gadea et al. (2011) demonstrated that freezing and thawing of human sperm cause an escalation in oxidative stress conditions, and it is connected with a significant disturbance in the antioxidative GSH efficiency [18].
Uric acid (2, 6,8–trihydroxypurine) constitutes the final product of purine nucleotides adenosine and guanosine catabolism. It plays the role of an important antioxidant, whose activity is somewhat restricted to semen but on a smaller scale, and is also noticeable in the epididymis. About 37% of antioxidant activity in semen applies to urate, ascorbate, and tyrosine. Furthermore, urate concentration in the seminal fluid of fertile men tends to be higher than that of infertile. In contrast, a diminished urate level indicates rising perturbations in the antioxidative protection or increment of oxidative damage in seminal plasma, testis, or epididymis [19]. Finally, uric acid exerts an evident improving effect on sperm quality parameters, such as motility, morphology, concentration, and membrane integrity, while simultaneously negatively connected with the intensity of lipid peroxidation and sperm DNA damage [19].
On the other hand, a high serum uric acid concentration (>420 µmol*L−1) called hyperuricemia may cause opposite harmful effects on semen parameters. Therefore, semen volume, total sperm count, sperm concentration, or percentage of proactive sperms are significantly or moderately diminished in hyperuricemia. This fact suggests that serum uric acid, in a concentration-dependent manner, may also worsen semen quality, demonstrating its ambiguous nature. It can be accompanied by some perturbations in the balance of serum reproductive hormones manifested in the decrease of testosterone and the increment of luteinizing hormone or even destabilization in the antioxidative potential [20]. Therefore, the role of uric acid is equivocal; it is a potent antioxidant in the extracellular environment, while inside cells, it is committed to the rapid escalation of oxidative stress [21, 22].
During the generation of uric acid, a certain pool of ROS is produced since the xanthine oxidoreductase, besides the generation of uric acid, may also catalyze the production of nitric oxide or other ROS potentially harmful to DNA or enzymes (uric acid as a marker of oxidative damage). However, the other side of uric acid activity makes it a potent free radical scavenger and chelator of transitional metal ions. It is also engaged in the increase of the level of glutathione (in the hippocampus) [21]. To describe this remarkably diverse activity of uric acid in human organisms, Sautin and Johnson (2008) use the term "oxidant-antioxidant paradox". From one side, this hydrophilic antioxidant scavenges, among others, peroxyl radicals or singlet oxygen. On the other, a high burden of uric acid accompanies pathological states like dyslipidemia, insulin resistance, and kidney disease. Therefore, the environment of occurrence is a decisive factor for the pro– or antioxidative properties of uric acid. In contrast, in the hydrophobic environment, it cannot scavenge lipophilic radicals or interrupt chain reactions of propagation within lipid membranes. On the contrary, it turns into a proinflammatory factor that stimulates intracellular production of oxidants on the way conditioned by NADPH oxidase [23].
IL–4 may influence male fertility since infertile patients tend to exhibit lower levels of this modulator compared to fertile in seminal plasma. Also, chronic toxic damage to the testes usually are connected with a downgrade in the level of IL–4. Oppositely, the increment of IL–4 concentration in semen causes specific inhibition of inflammatory factors and processes and participates in the maintenance of undisturbed spermatogenesis [24]. Even recent analysis of reproductive impairments due to COVID–19 indicates that decreased levels of testosterone, diminished sperm count, and generally disturbed spermatogenesis may be connected with disturbances among cytokines (TNF–α, IL–6, IL–4, IL–12). Furthermore, IL–4 contributes to the inhibition of Th1-mediated response. At the same time, during pregnancy, it undergoes increased generation and cooperates with progesterone, which indicates its anti-inflammatory nature and generally important role in successful reproduction [24].
IL–4 is considered one of the fundamental immunoregulatory cytokines since its expression is upregulated in basophils, lymphocytes, and mast cells, particularly those exposed to toxins. This fact implies that induction of IL–4 may constitute a specific defensive mechanism against different types of stress. Finally, it can also be engaged in DNA repair, attenuating DNA damage and mobilizing important mechanisms that favor the removement of injuries [25]. Therefore, antioxidative defense is ensured by various mutually regulated factors. However, only some low molecular mass antioxidants are generated in the organism. This group encompasses GSH, uric acid, bilirubin, ubiquinone, metallothioneins, and albumin. However, exogenous compounds appear even more numerous, including phenolic acids, flavonoids, carotenoids, coumarins, stilbenes, lignans, vitamins, and certain chemical elements (Zn, Se, Mn). This fact implicates not only that, in relatively large part, the efficiency of the antioxidative barrier depends on the individual diet or lifestyle but also encourages the search for the relationships between the components of this antioxidative mechanism [12, 26, 27].
In our previous paper [28] we considered relationships between concentrations of Li, Be, B, Na, Mg, Al, P, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Sr, Mo, Ag, Cd, Sn, Sb, Ba, Hg, Tl, Pb, and enzymatic antioxidants SOD, CAT, GPx, and GR in men with fertility disorders and healthy men. However, this paper aims to estimate the important aspects of the impact of unfavorable changes that arise from oxidative stress on the functionality of non-enzymatic antioxidants (bilirubin, uric acid, glutathione) and analysis of correlations with MDA (a marker of lipoperoxidation) in serum of men. These analyses allow a more complete look at the determinants of male infertility and the establishment of relationships between perturbations in the antioxidative defense and male reproductive condition. Molecular analysis was conducted and applied to the polymorphism of gene IL–4v.C589T (rs2243250) (chromosome 5). TT and CC genotypes were analyzed to notice their influence on the generation of fertility perturbations in the studied groups of men. Therefore, some methodological aspects of analyses used in the paper by Baszyński et al. (2022) [28] are identical to the research presented in this work. Therefore, they have been included in this work as Supplementary Materials (1–7) (Material and methods).
The relationship between the destabilization of the concentration of chemical elements, disturbances in the functionality of non-enzymatic antioxidative barriers, and the commitment to genetic polymorphism are considered in this paper. On this basis, the importance of environmental factors and oxidative stress in the shaping of male reproductive potential was established. The dependencies analyzed in the framework of the research included in this paper have not been studied using such an approach so far. Therefore, they are innovative and constitute an important aspect of the multi-range interdependencies.
Materials and Methods
Seminological studies
The population of patients with infertility consisted of 76 men with different fertility disorders, as
confirmed
via seminological tests [29]. In this paper, we used methodological details of seminological studies
presented
entirely in the paper by Baszyński et al. (2022) [28]. They are identical to the research presented in
this
work. Therefore, they have been included here as Supplementary Materials 1-7 downloadable from the articles website (1:
Seminological studies). We described semen abnormalities according to standard criteria [29]. The control
group
consisted of 87 men with normozoospermia [29]. The majority of infertile men came from Central Poland
(Cuyavian
region) [30]. The collection of biological samples and seminological tests were conducted by qualified
medicians
from the andrology clinic and by the authors of this paper (semen morphological parameters).
Normozoospermia or
specific seminological abnormalities were diagnosed, which allowed the division of patients into the
infertile or
control group.
Methods used for seminological analysis
In this paper, we used methodological details of methods applied for seminological analysis presented
entirely in
the paper by Baszyński et al. (2022) [28]. They are identical to the research presented in this paper.
Therefore, they have been included here as Supplementary Materials 1-7 downloadable from the articles website (2:
Methods used
for seminological analysis). Seminological analyses were based on macro- and microscopic analysis of
ejaculate to
verify semen volume, time of liquefaction, sperm density, motility, presence of agglutination, presence of
leukocytes, and percentage of pathological forms. Patients were assigned to the infertile or control group
after
comparing the results with those of the WHO (2010) [29]. Therefore, the classification of patients into
particular groups (healthy control or infertile) in our study was based on comparing the seminological
results
obtained with WHO guidelines. Patients who fulfilled the criteria of normozoospermic were included in
healthy
control, whilst men with diverse seminological disorders (that field normozoospermic classification) were
included in the infertile group.
Standard semen evaluation
All of the tested parameters were determined immediately after liquefaction or within one hour of
ejaculation at
room temperature. In this paper, we used methodological details of methods characterized for standard
semen
evaluation presented entirely in the paper by Baszyński et al. (2022) [28]. They are identical to the
research
presented in this work. Therefore, they have been included here as Supplementary Materials 1-7 downloadable from the articles website
(3: Standard semen evaluation, Macroscopic evaluation of semen, Microscopic evaluation of
semen).
These parameters were determined by the WHO criteria (2010) [29]. We consider the criteria included in
the
classification by Kruger [31] after staining with the Schorr method. Sperm were considered normal if they
exhibited specified characteristics.
Chemical elements analysis
Concentrations of Li, Be, B, Na, Mg, Al, P, K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Sr, Mo, Ag,
Cd,
Sn, Sb, Ba, Hg, Tl, and Pb in the blood of infertile and healthy men were analyzed (ICP-MS). Here, we used
methodological details of methods applied for chemical elements analysis presented entirely in the paper
by
Baszyński et al. (2022) [28]. They are identical to the research presented in this work. Therefore, they
have
been included here as Supplementary Materials 1-7 downloadable from the articles website (4: Chemical element analysis,
Digestion, Quantitative analyses, Mineralization, Calibration, and reference materials).
Non–enzymatic antioxidants
The assessment of the concentration of glutathione (GSH) in serum was conducted
with the commercially available kit (Glutathione Assay Kit) from Cayman Chemical Co., Ann Arbor, MI, USA
(Item
No. 703002). The method was based on enzymatic processing that engages glutathione reductase for
quantitative
assessment of GSH. Sulfhydryl group of GSH reacts with 5, 5'–dithio–bis–(2–nitrobenzoic) acid (DTNB) and
crates
yellow–colored 5–thio–2–nitrobenzoic (TNB) acid. Simultaneously generated mixed disulfide (GSTNB)
undergoes
reduction through glutathione reductase to process GSH and generate a larger amount of TNB. The intensity
of the
generation of TNB is directly proportional to the reaction of processing, which is directly proportional
to the
concentration of GSH in the sample. Therefore, the measurement of absorbance of TNB in the wavelength of
405–414
nm equals the precise measurement of the concentration of GSH in the sample (paragraph of included
instruction
About This Assay).
Under the influence of vanadic acid salt and detergent, total bilirubin undergoes oxidation in an acidic
environment to colored biliverdin. As a result of this process, a change of color is noticeable, from
yellow
(characteristic of bilirubin) to green (characteristic of biliverdin). The color intensity of this
derivative
measured spectrophotometrically (in wavelength of 420 nm) is proportional to the total bilirubin
concentration
in the sample (PZ Cormay, No 2–245).
The assessment of uric acid in serum is based on the enzymatic conversion of uric acid into allantoin
under the
influence of uricase with the simultaneous liberation of hydrogen peroxide. In the proportional amount of
uric
acid hydrogen peroxide in the reaction mixture reaction, peroxidase/ADPS/4–aminoantipyrine creates
chinonoimin –
a colored product. The intensity of the color is directly proportional to uric acid content in the sample
(PZ
Cormay, No 2–208).
Lipid peroxidation intensity
In this presented paper, we used methodological details of methods used for lipid peroxidation intensity
presented entirely in the paper by Baszyński et al. (2022) [28]. They are identical to the research
presented in
this work. Therefore, they have been included here as Supplementary Materials 1-7 downloadable from the articles website
(5:
Lipid peroxidation intensity).
Molecular Analysis
In the studies presented here, we used methodological details of methods applied for molecular analysis
presented entirely in the paper by Baszyński et al. (2022) [28]. They are identical to the research
presented in
this work. Therefore, they have been included here as Supplementary Materials 1-7 downloadable from the articles website
(6:
Molecular Analysis). Values were established based on the optimization and literature [33–35]. Starters,
PCR
reaction conditions, and the conditions of digestion with restrictive enzymes for respective polymorphism
were
established based on the literature [36, 37].
Polymorphism of Gene IL–4v.C589T (rs2243250)
We used here methodological details of methods used for the analysis of polymorphism of gene
IL–4v.C589T (rs2243250) presented entirely in the paper by Baszyński et
al. (2022) [28] and after [38]. They are identical to the research presented in this work. Therefore, they
have
been included here as Supplementary Materials 1-7 downloadable from the articles website (7: Polymorphism of Gene
IL–4v.C589T (rs2243250).
Statistical analysis
Statistical analyses were performed with STATISTICA (v.13, StatSoft, Cracov,
Poland) and Microsoft Office Excel 2021 (v.2108, Microsoft, Redmond, WA, USA). The results of quantitative
parameters are displayed as minimum and maximum values, quartiles (Q1, Q3), medians, arithmetical
averages, and
standard deviations. Data were analyzed for normal distribution (Shapiro–Wilk test). Those that did not
exhibit
normal distribution were analyzed with non-parametric tests (U–Mann–Whitney test, Spearman rank test). The
chi-square test was used to compare the frequency of genotypes. Correlations were analyzed with Spearman
rank
correlation tests. The coefficient of significance was set at α<0.05 and statistical significance at
P<0.05 [39–41].
Results
Non–enzymatic antioxidants activity
None of the analyzed non–enzymatic antioxidants achieved a statistically significant difference between
the
infertile and healthy control groups (P>0.05). For uric acid, the statistical tendency was noted
(P<0.10).
The difference between average values of uric acid in the mentioned groups was 1.571
mg*dL−1 (P=0.071). The results of bilirubin and GSH were more similar among groups
(P=0.355 and 0.162). Therefore, although the fact that concentration of bilirubin, uric acid, and GSH did
not
show statistically significant differences between the infertile group and the control, it is evident that
the
level of uric acid is higher in the serum of men with fertility disorders (7.058 mg*dL−1)
when compared to the control (5.487 mg*dL−1). Inversely, results for GSH and bilirubin in
healthy control were higher (6.812 µM and 0.617 mg*dL−1) than in the infertile men (6.150
µM and 0.588 mg*dL−1); Table 1, Fig. 1. Surprisingly, MDA also appeared to be more
accumulated in control than in the infertile men (Table 1).
In the men with normozoospermia, GSH correlated with uric acid
(r=0.511), while in men with fertility disorders, a relatively strong relationship between these
parameters was
also noted (r=0.524); Table 1. Besides the positive correlation between bilirubin and uric acid in the
infertile
group (r=0.324), the rest of the comparisons did not show any relationships (Table 1). However, the
obtained
correlations suggest the existence of the cooperation between non-enzymatic components of antioxidative
defense
in the reduction of oxidative stress with the strongest interaction between GSH and uric acid noted both
for
infertile group and healthy control (accordingly r=0.524 and r=0.511; Table 1, Fig. 2). Boron and sodium
correlated with GSH (respectively positive correlation B–GSH r=0.387 and negative Na–GSH r=–0.308) in
healthy
control, while boron also influenced on the MDA, both in the infertile men and healthy control
(accordingly
r=0.338 and r=0.326). The results suggest the commitment of chemical elements in modulating non-enzymatic
antioxidative defense and indirect lipid peroxidation (Table 1).
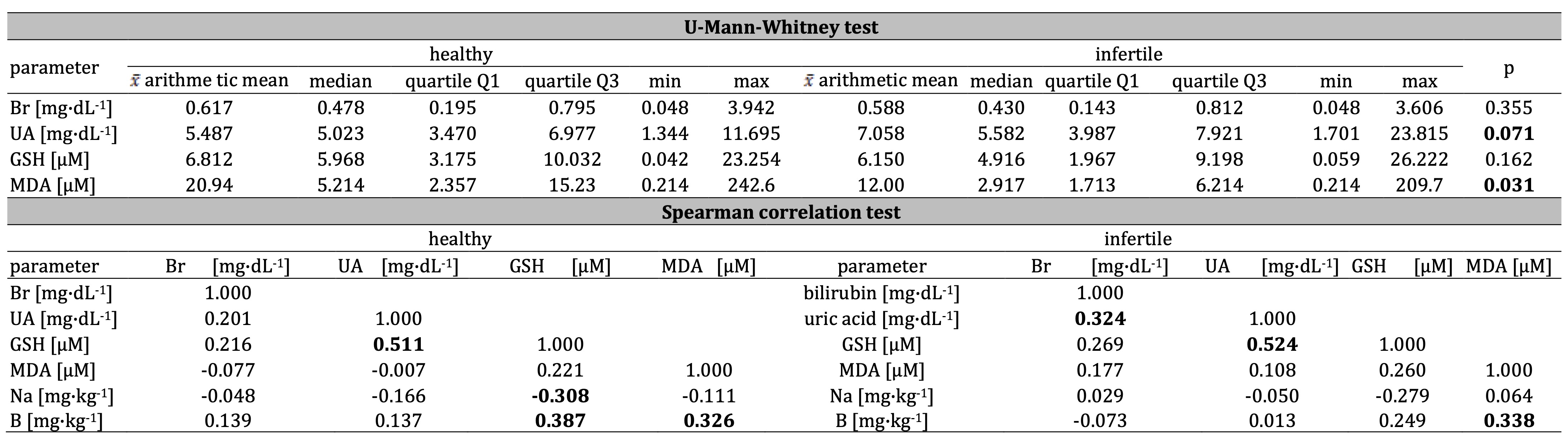
Table 1: The concentration of bilirubin Br, uric acid UA, glutathione GSH, and mutual interactions, including malondialdehyde MDA in infertile and healthy control men, as well as correlations between bilirubin, uric acid, GSH, MDA, and sodium Na, and boron B in healthy control and infertile men. Statistically significant differences and relationships are in bold
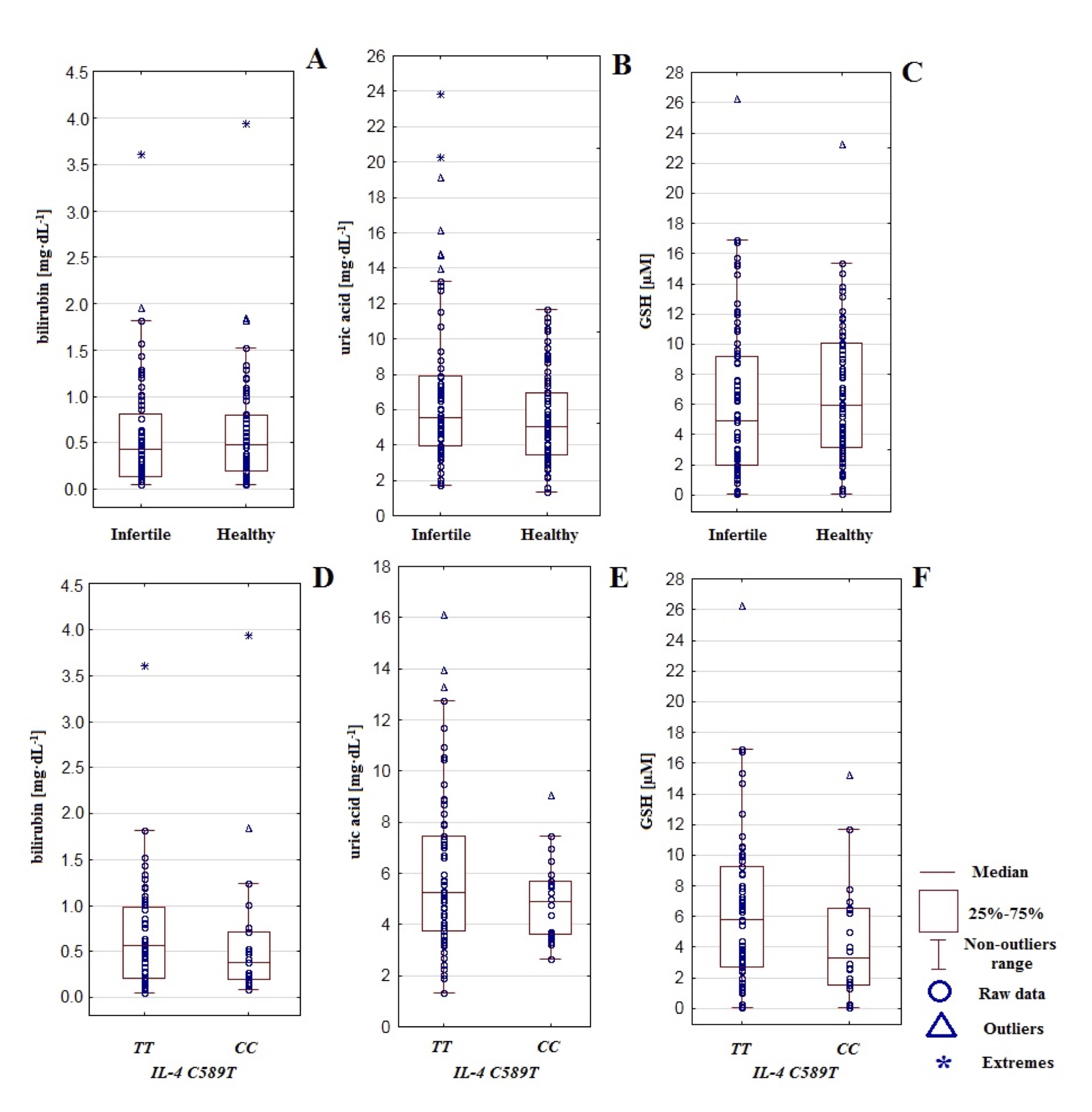
Fig. 1: The concentration of bilirubin, uric acid, and glutathione GSH in infertile and healthy control men (A-C) and regarding IL-4v.C589T (rs2243250) genotypes CC and TT (D-F).
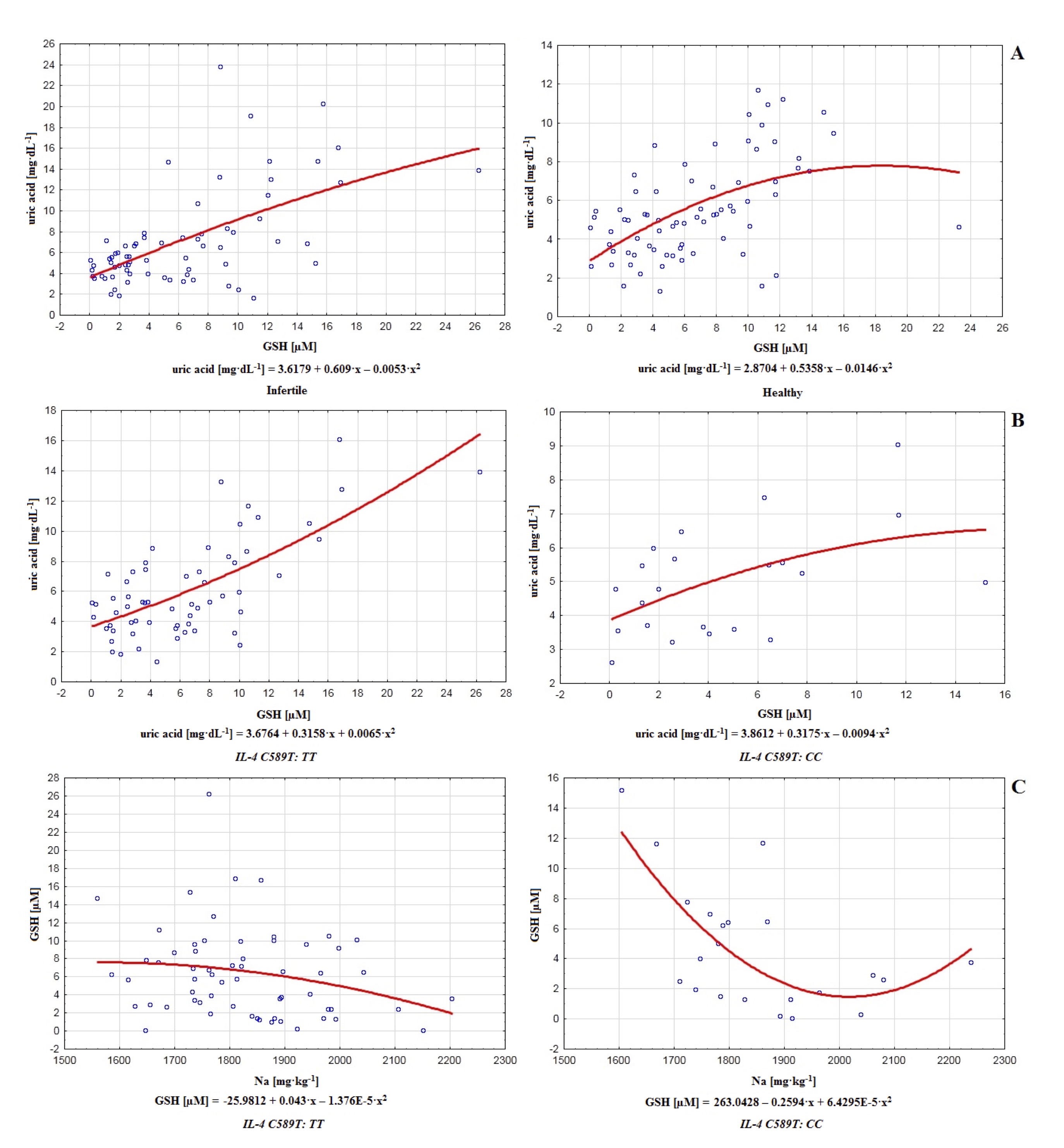
Fig. 2: Polynomial graphs of correlations between uric acid and glutathione GSH in infertile (in Spearman model r=0.524; in linear model r=0.590, r2= 0.349) and healthy control men (in Spearman model r=0.511; in linear model r=0.503, r2= 0.253) (A), as well as between uric acid and glutathione GSH regarding IL-4v.C589T (rs2243250) genotypes TT (in Spearman model r=0.522; in linear model r=0.684, r2= 0.468) and CC (in Spearman model r=0.419; in linear model r=0.495, r2= 0.245) (B) and between GSH and sodium Na (C) regarding IL-4v.C589T (rs2243250) genotypes TT (in Spearman model r=-0.225; in linear model r=-0.227, r2= 0.052) and CC (in Spearman model r=-0.506; in linear model r=-0.507, r2= 0.257).
Relationships with polymorphism of gene IL–4v.C589T (rs2243250)
The polymorphism of gene IL–4v.C589T (rs2243250) in the infertile group (n=76) was
determined for 43 men. Among them, 31 mutant-type homozygotes TT were noted (the frequency of
occurrence:
0.72) and 12 wild-type homozygotes CC (the frequency of occurrence: 0.27). There were not any
heterozygotes CT. In the control group (n=87), this polymorphism was determined for 44 men. Among
them,
34 mutant-type homozygotes TT were noted (the frequency of occurrence: 0.77), besides 10 wild-type
homozygotes CC (the frequency of occurrence: 0.22). In healthy control, similarly to the infertile
group,
no affirmed heterozygotes CT was found. During the electrophoretic section of the PCR product for a
variant of gene IL–4v.C589T (rs2243250), the stripe on the height of 195bp was
observed. Subsequently, after digestion with restrictive enzyme AvaII, the stripe was observed on
various
heights. The single stripe at the height of 195bp was characteristic of the mutant type of homozygote
TT,
while the single stripe at the height of 177bp informed about the wild type of homozygote CC. The
analysis of polymorphism of gene IL–4v.C589T (rs2243250) did not reveal significant
differences in the frequency of genotypes in the particular groups. Genotype TT is present in
72.09% of
men from the infertile group and 77.27% of men from healthy control. Genotype CC is present in
27.91% and
22.73% of men, respectively, from the infertile group and healthy control (Table 2). Based on the
frequency of
genotype occurrence in the infertile group and control, the direct impact of genetic factors on male
fertility
in the studied population may be excluded. The lack of significant difference in the frequency of
particular
genotypes in the infertile group and control (P=0.578) constitutes a decisive factor (Table 2). However,
for the
polymorphism of gene IL–4v.C589T (rs2243250), it is worth considering the
indirect
influence of genetic factors on male reproductive condition by comparison of studied parameters
(concentration
of non-enzymatic antioxidants and their relations with chemical elements according to the distinguished
genotypes TT and CC).
Significant differences between men with genotype TT compared to genotype CC in the level of
non–enzymatic antioxidants were not affirmed (bilirubin: P=0.267, uric acid: P=0.279, GSH: P=0.123).
However,
the concentration of bilirubin, uric acid, and GSH was slightly higher in men with genotype TT
(relatively 0.687 mg*dL−1, 6.097 mg*dL−1, and 6.345 µM) compared to
men with genotype CC (accordingly 0.652 mg*dL−1, 4.980
mg*dL−1, and 4.630 µM). The results may suggest that genotype TT is accompanied by
more intensive non-enzymatic antioxidative protection. This observation is additionally sustained by a
slightly
lower concentration of MDA in men with genotype TT (15.171 µM) compared to genotype CC
(18.094
µM). It suggests that in the case of genotype TT, more effective antioxidative defense is
accompanied by
reduced lipid peroxidation. In contrast, genotype CC may be connected with specific impairments in
the
non-enzymatic antioxidative protection and subsequently more escalated lipid peroxidation (Table 2, Fig.
1). The
analysis of correlations between non-enzymatic antioxidants and MDA regarding polymorphism of gene
IL–4v.C589T (rs2243250) revealed that in the group with genotype TT uric acid
correlates with GSH (r=0.522). A similar relationship was noted in the group with genotype CC (uric
acid–GSH: r=0.419). Some weaker interactions were restricted only to the genotype CC, namely
between
bilirubin and GSH (r=0.305) and between bilirubin and MDA (r=0.335), suggesting cooperation of
antioxidants,
their mutual regulation as well as modest influence on the lipid peroxidation. However, this positive
correlation between bilirubin and MDA may also be considered a symptom of destabilization of the
antioxidative
barrier in the group with genotype CC, compared to TT, where such interaction did not
appear (Table 2, Fig. 2).
However, there was not statistically significant relationship between genotype TT or CC
(polymorphism of gene IL–4v.C589T (rs2243250) and modulation of activity of
non-enzymatic antioxidants or overproduction of MDA in the studied groups (P>0.05). Still, the slightly
higher level of MDA that accompanies genotype CC (18.094 µM), compared to TT (15.171 µM),
suggests
that men with this genotype (CC) are more subjected to the lipid peroxidation and larger
destabilization
of antioxidative defense. Additionally, genotype TT was characterized by slightly higher
concentration of
bilirubin (0.687 mg*dL−1, CC: 0.652 mg*dL−1), uric acid
(6.097 mg*dL−1, CC: 4.980 mg*dL−1) and GSH (6.345 µM,
CC: 4.630 µM) (Table 2, Fig. 1). Therefore, non-enzymatic antioxidative defense is more effective
in the
case of genotype TT, while genetic factor is evidently committed in its modulation. This commitment
is
also visible in the interactions between particular antioxidants on the level of genotypes TT and
CC
(polymorphism of gene IL–4v.C589T (rs2243250). The relationships between uric
acid and
GSH (r=0.522 in the genotype TT and r=0.419 in the genotype CC), as well as correlations
restricted only to one genotype (weak interactions between bilirubin and MDA in the case of genotype
CC
r=0.335; Table 2), confirm that effectiveness of antioxidative mechanisms varies according to the genotype
(TT, CC). Correlations between antioxidants and MDA may even constitute a symptom of a
larger
destabilization of antioxidative protection in men with a particular genotype (CC). Certain
chemical
elements may be considered important modulators because they act on both analyzed levels correlating with
non-enzymatic antioxidants in the groups of men with normozoospermia and fertility disorders as well as in
the
groups connected with genotypes TT and CC (polymorphism of gene
IL–4v.C589T
(rs2243250). It applies to sodium, which interacts with GSH in healthy control (r=–0.308) and in men with
genotype CC (r=–0.506). Another prominent example is boron, which correlates with GSH in the
healthy
control group (r=0.387) and the group with genotype CC (r=0.401).
The obtained results in this matter even emphasize the role of genetic factors in strengthening
interactions
between chemical elements and non-enzymatic antioxidants. It is also important when it comes to
interactions
between toxic heavy metals and antioxidants, which appear to play a meaningful role but only in men with
genotype CC (Pb–bilirubin: r=0.416). Finally, it also concerns lipid peroxidation since the
interaction
between boron and MDA is slightly stronger in the group with genotype CC (r=0.428) compared to
analogous
relationships noted in men with fertility disorders (r=0.338) or healthy control (r=0.326). Therefore,
polymorphism of gene IL–4v.C589T (rs2243250), despite the lack of direct impact on
male
fertility in the studied population (the lack of significant difference in the frequency of genotypes;
P=0.578),
still may modulate status of non–enzymatic antioxidative barrier as well as the shape of interactions
between
chemical elements and antioxidants (Tables 2, 3, Figs. 1, 2).
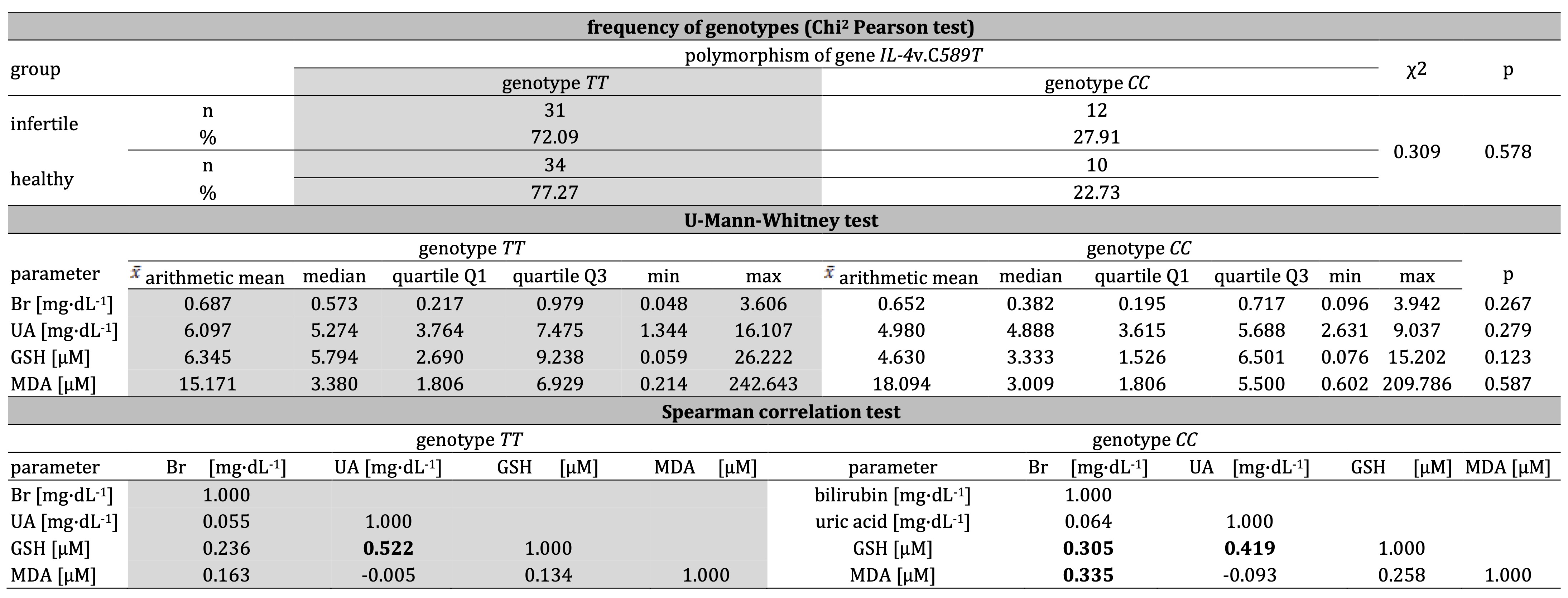
Table 2: Frequency of genotypes of IL-4v.C589T (rs2243250) in infertile and healthy control men as well as the concentration of bilirubin Br, uric acid UA, glutathione GSH and mutual interactions including malondialdehyde MDA regarding IL-4v.C589T (rs2243250) genotypes CC and TT. Statistically significant differences and correlations are in bold
Malondialdehyde concentration, non–enzymatic antioxidants and chemical elements
The analysis of correlations between the activity of non–enzymatic antioxidants, MDA, and chemical
elements
regarding polymorphism of gene IL–4v.C589T (rs2243250) revealed numerous
correlations, especially in the group with genotype CC. Correlations were noted between parameters:
Ca–bilirubin: r=–0.359, Ca-MDA: r=–0.447, Na–bilirubin: r=–0.399, Na–GSH: r=–0.506, Fe–uric acid:
r=–0.383,
Mo–bilirubin: r=0.408, Li–bilirubin: r=–0.398, V–bilirubin: r=–0.489, Co–bilirubin: r=–0.321, Co–GSH:
r=–0.445,
Ag–GSH: r=–0.398, Ba–GSH: r=–0.348, Ba–MDA: r=–0.465, Tl-bilirubin: r=–0.390, Tl–GSH: r=–0.415,
Al–bilirubin:
r=–0.320, Al–MDA: r=–0.365, Ni–GSH: r=0.415, B–bilirubin: r=0.355, B–uric acid: r=0.346, B–GSH: r=0.401,
B–MDA:
r=0.428, Pb–bilirubin: r=0.416, Be–bilirubin: r=0.333 (Table 3, Fig. 2). Therefore, the most important
modulators appear to be sodium (the strongest interaction with GSH: r=–0.506) and boron (most frequently
correlating trace element that interacted with analyzed antioxidants as well as influenced on the MDA
level).
However, many negative correlations between chemical elements and non-enzymatic antioxidants and ambiguous
relationships with MDA may constitute another symptom of greater destabilization or even inhibition of
non-enzymatic antioxidative defense in men with genotype CC. On the contrary, in the group with
genotype
TT, only a few negative correlations between elements and antioxidants were noted: Mn–GSH:
r=–0.326,
Ag–GSH: r=–0.305, Sn–uric acid: r=–0.313, Be–bilirubin: r=–0.317. In the group with genotype CC,
toxic
heavy metals play a larger role in the shaping of antioxidative response (Pb–bilirubin: r=0.416) compared
to
genotype TT (Table 3, Fig. 2). Interactions with MDA are also restricted only to group with
genotype
CC, where boron (B–MDA: r=0.428), barium (Ba–MDA: r=–0.465) and calcium (Ca–MDA: r=–0.447) play a
role as
modulators (Table 3).
It is worth considering the obtained results in the matter of uric acid; there was a statistical tendency
(P=0.071) of a higher level of this non-enzymatic antioxidant in the serum of men with fertility disorders
(7.058 mg*dL−1) compared to the control (5.487 mg*dL−1). It may be
disturbing since the admissible norm of uric acid is situated in the range of 3–7 mg*dL−1
[42]. When presuming that the optimum antioxidative potential of uric acid is also situated in this range
of
concentrations, it becomes noticeable that in men from the infertile group, the excess of uric acid may
intensify oxidative stress. Furthermore, concentrations of bilirubin and GSH in serum, although not
demonstrating significant difference (respectively P=0.355 and P=0.162), achieved higher levels in healthy
control (accordingly 0.617 mg*dL−1 and 6.812 µM), compared to the infertile group (0.588
mg*dL−1 and 6.150 µM). These two facts confirm better functionality of non–enzymatic
antioxidative defense in healthy normozoospermic men, compared to men with fertility disorders (Table 1,
Fig.
1). These interactions between GSH and uric acid inform about the cooperative character of the entire
antioxidative system with mutual regulations of particular components for optimal protection.
Also, the lipid peroxidation level is a reflecting factor of antioxidative defense. At the same time, the
interactions between chemical elements and MDA prove that lipid peroxidation itself undergoes modulation
by
relations with chemical elements. The correlations between B and MDA (infertile: r=0.338, control:
r=0.326)
suggest that the higher level of B favors escalation of lipid peroxidation. On the contrary, different
tendencies may be noticeable on the level of polymorphism of the gene IL–4v.C589T
(rs2243250), where negative correlations (Ca–MDA: r=–0.447, Ba–MDA: r=–0.465 and Al–MDA: r=–0.365)
suggest mitigating actions of these chemical elements about lipid peroxidation (Tables 1, 3). Therefore,
relationships with chemical elements, non–enzymatic antioxidative defense, and the intensity of lipid
peroxidation generally shape the defense mechanisms and responses of infertile men.
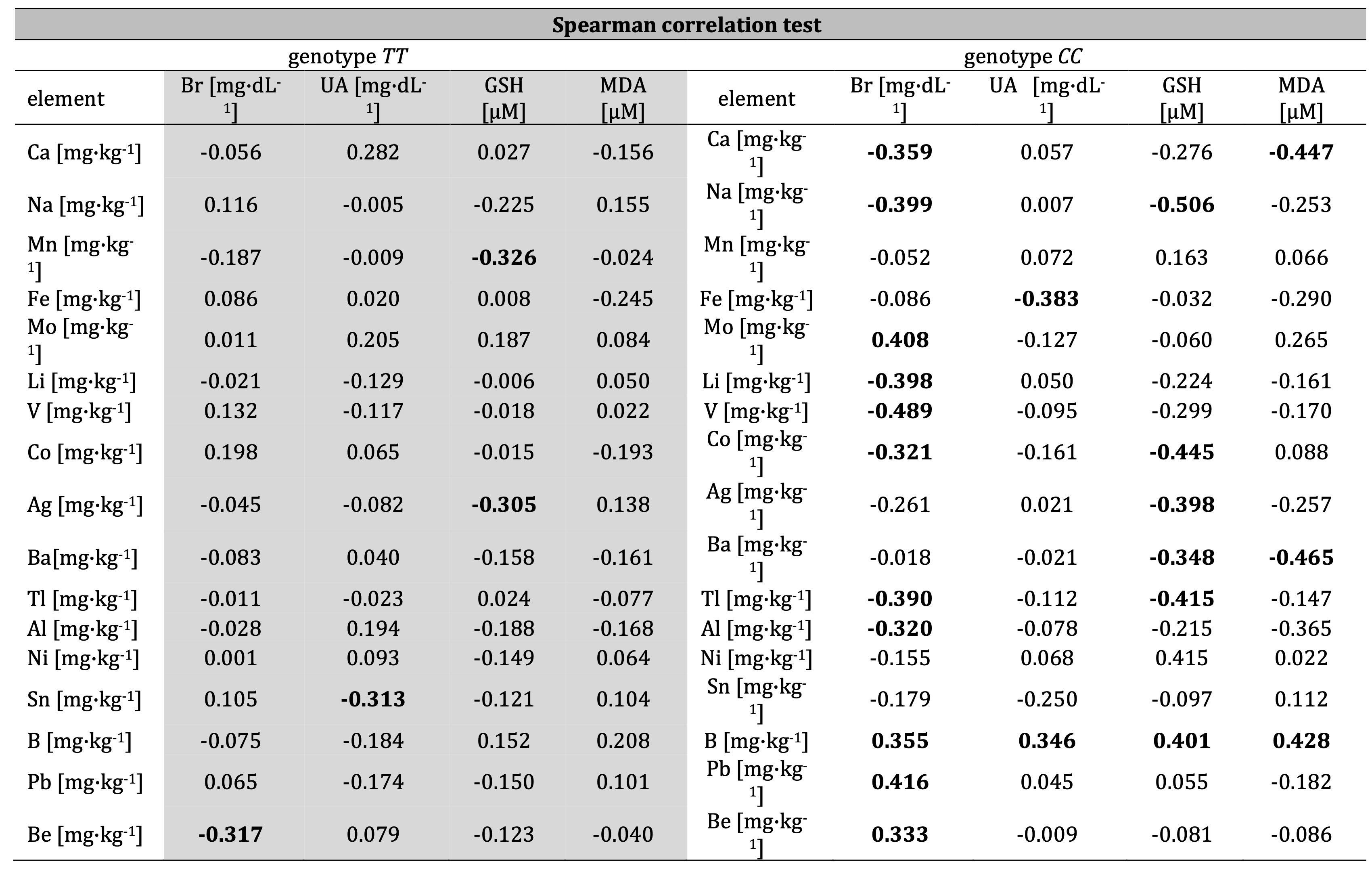
Table 3: Correlations (correlation coefficients; r) between concentration of bilirubin Br, uric acid UA, glutathione GSH, malondialdehyde MDA and Ca, Na, Mn, Fe, Mo, Li, V, Co, Ag, Ba, Tl, Al, Ni, Sn, B, Pb, and Be regarding IL-4v.C589T (rs2243250) genotypes CC and TT men. Statistically significant relationships are in bold
Discussion
The effectiveness of antioxidants in their participation in defense mechanisms and responses of infertile men strongly depends on their cooperation with other defense components. In that matter, glutathione is produced by cells that neutralize free radicals. However, it simultaneously ensures that certain exogenous antioxidants (vitamins E and C) remain in their reduced forms. GSH participates in the maintenance of intracellular redox status and constitutes a potent reservoir for reducing power. At the same time, its antioxidative functionality is also based on the cooperation with enzymes (i.e., peroxidase and reductase) committed to its conversions.
On the other hand, supplementation of exogenous antioxidants (vitamins C and E) is associated with the increment in GSH level [43]. Furthermore, supplementing certain antioxidants may positively affect the particular sperm quality parameters. Selenium increases the chance of conception and, according to the period of supplementation and dosage, also improves sperm motility, morphology, concentration, total sperm count, and semen volume. Similarly, vitamin E acts instead stimulatingly on sperm motility, while zinc causes improvement in the concentration and total motility. This confirms that antioxidative defense also undergoes crucial modulations by exogenous factors that influence overall systemic efficiency [44].
Uric acid shows equivocal character in its actions. It acts as a potent antioxidant in the plasma, contributing significantly to the total plasma antioxidant capacity. Peroxynitrite, hydroxyl, or iron-containing free radicals are selectively neutralized in this environment. Uric acid even preserves functionality and the structure of important enzymatic antioxidants, namely extracellular SOD, as well as prevents oxidative DNA injuries [45]. However, after entering the cell environment, uric acid alters into a strong pro-oxidative factor, augmenting oxidative stress by stimulating proinflammatory biomarkers, depletion of endothelial levels of nitric oxide, or provocation of peroxynitrite-mediated lipoperoxidation. Adding certain exogenous antioxidants, like polyphenols or flavonoids, may reduce uric acid by inhibiting xanthine oxidase. At the same time, vitamin C participates in the increment of urinary excretion [45].
On the other hand, the decrease in GSH level is connected with an increment of oxidative stress and mitochondrial dysfunctions. At the same time, a downgrade in the GSH/GSSG ratio accompanies the generation of ROS. Similarly, the depletion of serum levels of uric acid favors the progression of diseases that are driven by oxidative stress, e.g., neurodegenerative conditions [46]. We noted relatively high positive correlations between GSH and uric acid, both for men with fertility disorders and those from control (accordingly, r=0.524 and r=0.511). This observation was also sustained in analyzing groups assigned by polymorphism of gene IL–4v.C589T (rs2243250) in the particular genotypes (TT: r=0.522, CC: r=0.419). This result underlines the cooperative character of non-enzymatic antioxidants in general defense against oxidative stress (Tables 1, 2, Fig. 2).
Bilirubin is widely known for its anti-inflammatory activity, and plasma bilirubin is connected with a decreased risk of oxidative injuries in the disease process. Furthermore, bilirubin can participate in the modulation of heme oxidase-1 upregulation within specific cells. On the level of immune system responses, bilirubin lessens M1–macrophages, which release proinflammatory cytokines. Heme oxidase–1 also augments the anti-inflammatory response, while its inhibition causes significant growth of the cytokines (TNF-α, IL–1, IL–6) that generally favor inflammation. Physical activity exerts a positive effect on the expression of this modulator. It thus is also committed to incrementing plasma bilirubin, which mitigates oxidative stress [47].
Bilirubin's effectiveness in scavenging peroxyl radicals surpasses α-tocopherol, while after bonding with albumin, it prevents albumin-bound fatty acids from oxidation. Unconjugated bilirubin is engaged in the conservation of albumin from oxidation by O2−, OH, HO2, or ONOO−. It is also committed to inhibiting NADPH oxidase functionality, which implicates bilirubin's important role in lessening ROS production. Such beneficial effects may also arise from the cooperative action of bilirubin with other antioxidants [48]. Although the interactions between bilirubin and uric acid in the diseases caused by oxidative stress remain controversial, uric acid levels are associated with an increased risk of the disease [49].
In the matter of male fertility, the results of our work reflect an equivocal relation between the mentioned parameters. The positive correlation between bilirubin and uric acid was confirmed, but only for men with fertility disorders (r=0.324). Oppositely, the control group obtained coefficient did not attest to the relationship (r=0.201; Table 1). Besides, uric acid in excess may even provoke negative changes connected with oxidative stress, which appear detrimental for specific organs; e.g., high serum uric acid level is considered the leading risk factor for non-alcoholic fatty liver disease since it stimulates hepatic lipid accumulation. It may happen through induction of endoplasmic reticulum stress or mitochondrial oxidative stress [50]. Therefore, elevated serum uric acid may simultaneously lead to metabolic dysregulation and manifest in hyperlipidemia. This phenomenon undoubtedly results in intensified lipid peroxidation, which is closely connected with the increment of serum concentration of MDA [51]. Furthermore, the high level of uric acid tends to be accompanied by the increment in the inflammatory markers (C–reactive protein, white blood cells, neutrophils, interleukin–1 receptor antagonist, interleukin–6, interleukin–18, tumor necrosis factor-alpha).
On the contrary, the antioxidative potential of uric acid in physiological conditions is undisputed, and administration of uric acid causes an evident increase in the antioxidant capacity and the blocking of ROS generation [52]. Therefore, the ambiguous character of uric acid is mainly determined by its concentration. In this matter, the results obtained in our analysis show a higher concentration of this parameter in the case of men with fertility disorders (7.058 mg*dL−1) compared to control (5.487 mg*dL−1); p=0.071. Oppositely, other studied non–enzymatic antioxidants, namely bilirubin, and GSH, were more concentrated in healthy control (respectively 0.617 mg*dL−1 and 6.812 µM), compared to the infertile men (0.588 mg*dL−1 and 6.150 µM).
Although the differences in these two parameters between groups were not so evident (respectively p=0.355 and p=0.162; Table 1), still obtained results suggest more intensified antioxidative defense in healthy control since the higher concentration of uric acid in the infertile group may be recognized as a disturbing factor, for bilirubin concentrations those define borders of the norm are included in the range of 0.3–1.2 mg*dL−1 [53], when obtained concentrations of bilirubin measured in the infertile group and healthy control were confronted with admissible values of the norm, it became evident, that the higher percentage of results beyond norm was noted among men with fertility disorders (51.31% of results beyond range of norm), comparing to the control (34.48% of results beyond range of norm). In the uric acid concentration, the norm range is defined by 3–7 mg*dL−1 [42]. Similarly, like in the case of bilirubin, also for uric acid, a higher percentage of results beyond the norm was noted in the infertile group (39.47% of values beyond the range of the norm) compared to the control (32.18% of values beyond the range of the norm). This observation proves a larger destabilization of non-enzymatic mechanisms of antioxidative defense (bilirubin and uric acid) in the group of men with fertility disorders compared to healthy control. Furthermore, the obtained concentration of uric acid was also evidently higher in men with fertility disorders (7.058 mg*dL−1) compared to the control (5.487 mg*dL−1, P=0.071) (Table 1, Fig. 1) and exceeded the upper border of norm range (7.000 mg*dL−1)[42].
The detailed chemical elements are essential for maintaining male fertility. Therefore, perturbations in the level of macroelements, especially their deficiency, may exert a destructive impact on the level of testosterone and sperm count or manifest in the reduction of libido. For example, decreased seminal Mg is considered a marker of prostate infection and remains associated with specific vasoconstrictive actions, which in turn provoke premature ejaculation [54]. Similarly, Ca deficiency reduces general reproductive potential since it is necessary to initiate an acrosome reaction and condition the membrane alterations needed to achieve reproductive success. Ca also favors sperm motility, while Mg and Ca tend to be decreased in infertile men and may be connected with a downgrade in total antioxidant capacity [54].
On the other hand, studies revealed that certain Na compounds cause a significant improvement in antioxidant capacity in the conditions of intensive exposure to H2O2 [55]. However, the body of evidence conjoins extensive Na intake with impairments in endothelial functionality, while the increment in oxidative stress is often recognized as a decisive factor. In that matter, sodium may stimulate mRNA expression of NADPH oxidase subunits or generally participate in the augmentation of activity of NADPH oxidase and xanthine oxidase, which leads to the generation of O2− [56].
About male fertility, the results of our paper support mentioned equivocal reports with a negative correlation between Na and GSH in healthy control (r=–0.308), which is additionally strongly sustained in the genotype CC group of polymorphism of gene IL–4v.C589T (rs2243250), where negative correlation appear not only between Na and GSH (r=–0.506) but also between Na and bilirubin (r=–0.399). In the same group, Ca inversely correlated with bilirubin (r=–0.359) and MDA (r=–0.477; Table 3). Therefore, obtained results confirm the transitioning character of interactions between macroelements (Na, Ca) and non-enzymatic antioxidants, as well as suggest limiting influence on the MDA level (Ca), and this commitment of Ca in the reduction of MDA, in general, favors maintenance of fertility.
However, our analysis's most frequently correlating chemical element appears to be boron (Tables 1, 3). In humans, this microelement is present in an average amount of 18 mg [57, 58]. Still, its chemical nature distinguishes oneself with the ability to convert between particular forms instantly. Thus, boron in bioactive molecules tends to appear as a single atom or cluster and immediately change forms from natural trigonal planar to anionic tetrahedral in the single boron-containing compounds under physiological conditions [58]. Boron has a rather beneficial effect on the functioning of the body, and the reduction of the risk of changes is due to the anti-inflammatory properties of boron because its excretion is negatively related to inflammatory parameters (highly sensitive C–C-reactive protein, homocysteine, and the number of leukocytes) [59].
Our results agree with statements from the above references regarding non-enzymatic antioxidants. In healthy control men we noted a positive correlation between B and GSH (r=0.387), while in the group with genotype CC regarding polymorphism of gene IL–4v.C589T (rs2243250) all of studied antioxidants (bilirubin, uric acid, GSH) were positively correlated with B (respectively r=0.355, r=0.346 and r=0.401; Tables 1, 3). Therefore, these results implicate the beneficial impact of boron on the activity of studied non-enzymatic antioxidants.
Mn and Fe are the other microelements widely known for engagement in the modulation of oxidative stress. Mn maintains the activity of superoxide dismutase Mn-SOD in mitochondria and regulates immune response and developmental processes. However, in excess, it is committed to sperm damage and decreased libido accompanied by neurological pathologies. On the other hand, Mn deficiency may also cause a downgrade in the reproductive potential accompanied by growth impairments [60]. On the contrary, iron may catalyze the formation of free radicals and, in labile form, is recognized as a potent contributor to oxidative cellular damage. However, oxidative stress may also arise in Fe deficiency conditions that subsequently affect stored iron in the body. Therefore, ferritin plays the role of endogenous antioxidant through its engagement in sequestering potentially toxic labile Fe [61].
The results of our paper suggest the possible regulating influence of Mn and Fe on GSH (negative correlation between Mn and GSH (r=–0.326) in men with genotype TT regarding polymorphism of gene IL–4v.C589T (rs2243250) or uric acid (negative correlation between Fe and uric acid (r=–0.383) in men with genotype CC (Table 3). It is also worth mentioning that such less obvious microelements as Mo, Li, V, Co, Ag, Ba, Tl, Al, Ni, and Sn appear committed in the regulation of particular non–enzymatic antioxidants. However, in most cases, they exert relatively weak mitigating influence, which is restricted practically to men with genotype CC (polymorphism of gene IL–4v.C589T (rs2243250). It is noticeable in the correlations: Li–bilirubin (r=–0.398), V-bilirubin (r=–0.489), Co–bilirubin (r=–0.321), Co–GSH (r=–0.445), Ag–GSH (r=–0.398), Ba–GSH (r=–0.348), Tl–bilirubin (r=–0.390), Tl–GSH (r=–0.415), Al–bilirubin (r=–0.320); Table 3.
There are also some positive interactions in men with genotype CC (Mo-bilirubin: r=0.408 and Ni–GSH: r=0.415) as well as specific symptoms of mitigating modulation in the group with genotype TT: Ag–GSH (r=–0.305), Sn–uric acid (r=–0.313). Finally, Ba and Al correlate negatively with MDA (Ba–MDA: r=–0.465, Al–MDA: r=–0.365), implicating their commitment to the reduction of MDA, but it is also restricted only to men with genotype CC (Table 3). In this matter, boron appears to exert a larger and even stimulative effect on the level of MDA noticeable in the positive correlations both in the polymorphism of gene IL–4v.C589T (rs2243250) of the assigned group with genotype CC (B–MDA: r=0.428), as well as in the group of men with fertility disorders or healthy control (respectively r=0.338 and r=0.326) (Tables 1, 3).
Heavy metals constitute a considerable threat to reproductive potential since there is much evidence conjoining these chemical elements with a decrease in essential semen parameters. Lead contributes to the reduction of spermatic concentration, count, velocity, viability, and morphology. In contrast, greater concentrations in the blood and semen tend to be connected with lower sperm motility and provoke teratozoospermia. The exposition may also result in prolonged liquefaction time. Additionally, these metals stimulate increments in the seminal oxidative stress markers [62]. Beryllium can also induce the release of inflammatory cytokines, chemokines, and ROS from macrophages and dendritic cells. It may also be engaged in apoptosis, while Be–induced cell death is connected with the rapid release of alarmins, e.g., Il–1α [63]. The results obtained in our work in the matter of Be and Pb appear ambiguous and restricted only to the groups of men with genotype TT or CC regarding polymorphism of gene IL–4v.C589T (rs2243250), e.g., Be was inversely connected with bilirubin in men with genotype TT (r=–0.317). At the same time, it positively correlated with the same antioxidant in the case of men with genotype CC (r=0.333). Similarly, observations apply to Pb (Table 3).
In about 15% of infertile men, a genetic defect constitutes a decisive factor. Genetics is even considered to play a prominent role, particularly in the most serious spermatogenic perturbations (oligozoospermia or azoospermia). There is a need for the identification of novel genes associated with male infertility since the obtainment of an unambiguous causal diagnosis is still very problematic [64]. Our analysis of polymorphism of gene IL–4v.C589T (rs2243250) results in the exclusion of direct engagement of this factor in male infertility in the studied population is based on the lack of significant difference in the frequency of genotypes (P=0.578; Table 2). However, the construction of two groups that included men with particular genotypes (TT, CC) enabled further analysis of the possible indirect impact of this polymorphism on the status of non–enzymatic antioxidative defense.
The levels of non–enzymatic antioxidants (bilirubin, uric acid, GSH) were higher in men with genotype TT (respectively 0.687 mg*dL−1, 6.097 mg*dL−1, and 6.345 µM), compared to men with genotype CC (accordingly 0.652 mg*dL−1, 4.980 mg*dL−1 and 4.630 µM) (Table 2). Despite the lack of significance of these concentrations (P=0.267, 0.279, and 0.123), more intensive and balanced non-enzymatic antioxidative protection seems to be present in the group with genotype TT. Based on the mentioned results, we notice certain inhibitory potential in the relations between analyzed chemical elements and non–enzymatic antioxidants that manifest in evidently more numerous negative correlations in the case of genotype CC, compared to TT. Furthermore, the interactions between chemical elements and MDA in men with genotype CC are pretty ambiguous, while MDA concentration in this group is higher than in the group with genotype TT (respectively 18.094 µM and 15.171 µM; Tables 2, 3), which suggests more escalated lipid peroxidation accompanying genotype CC. Nowadays, a generally decreasing trend in total fertility rate has become noticeable, as well as a substantial decline in fertility, particularly in developed countries. Additionally, there is a growing tendency to delay childbearing [65]. Therefore, undertaking multifactorial research in this matter is so important.
There are still controversies about the effectiveness of therapy based on antioxidants. Despite suggestions that underscore improvements in fertility potential, the data may be unconvincing regarding inaccuracies in optimal dosage and combination, treatment duration, and difficulties with selecting specific patient populations most likely to benefit. Thus, there is a demand for high-quality research to clarify antioxidants' significance in infertility treatment. The most urgent challenge is a careful diagnostic assessment of oxidative stress indices as a fundamental pre-request before starting antioxidant-based therapy. Although there are numerous reports about beneficial outcomes of therapy in the context of sperm parameters, it is difficult to obtain firm conclusions due to the high heterogeneity among studies [66].
On the other hand, Mancini et al. (2023) [67] remind us that the dual role of ROS is increasingly becoming more evident, and exaggerated administration of antioxidants could also trigger the deterioration of seminal parameters. In such a situation, the phenomenon of reductive stress participates in the reduction instead of amelioration of male fertility potential. Therefore, as it was mentioned, the evaluation of the redox state of the sample should be applied before establishing a treatment. Finally, there are specific underestimated problems. For instance, during assisted reproduction procedures, some methods of sperm selection can evoke sperm damage via oxidative stress. Lifestyle factors may also be responsible for unexpected or incorrect effects of applied actions. Ultimately, there is a need to determine if particular dietetic or pharmacological therapy caused sperm amelioration, which is effectively linked to improving oxidative status [67].
In the case of polymorphism of IL–4v.C589T gene (rs2243250), a significant difference between the infertile group and control was not affirmed (chi2=0.309, P=0.578); Table 2. The direct connection between this polymorphism and the reproductive potential of men from the studied population was also excluded. However, considering the indirect influence of the mentioned polymorphism on the reproductive condition, important differences were made between genotype TT or CC and the functionality of non–enzymatic antioxidative defense in the studied men. There were correlations between antioxidants in the group with genotype TT or CC; in the case of TT, the strongest relationship appeared between uric acid and GSH (r=0.522), while in CC, the relation between these parameters was also confirmed (r=0.419). Only in the group with genotype CC correlation between antioxidative mechanism and MDA was noted (bilirubin–MDA: r=0.335), which may be a symptom of a larger destabilization of antioxidative protection in the group with genotype CC, compared to the TT (Table 2). The connection between genotype TT or CC (polymorphism of gene IL–4v.C589T (rs2243250) with interactions between chemical elements and antioxidants was also considered. Among men with genotype CC, the strongest correlation appeared between Na and GSH (r=–0.506).
Furthermore, some other chemical elements appear to be engaged in this group's modulation of antioxidative defense. Ca, Fe, Li, V, Co, Ag, Ba, Tl, and Al constituted a relatively large pool of elements that interacted inversely with non–enzymatic antioxidants (negative correlations). Positive interactions with antioxidants were noted only for Mo, Ni, B, Pb, and Be. The interactions with evidently toxic elements (Be, Pb) were quite ambiguous; positive in the group with genotype CC (Pb–bilirubin: r=0.416, Be–bilirubin: r=0.333) and neutral or negative in the group with genotype TT (Pb–bilirubin: r=0.065, Be–bilirubin: r=–0.317); Table 3. Nevertheless, in the group with genotype TT, negative correlations between non–enzymatic antioxidants and chemical elements were uncomparably less numerous (only Mn, Ag, Sn, and Be correlated negatively with particular non-enzymatic antioxidants) than in the CC genotype. This suggests mitigating or limiting the impact of certain chemical elements on the activity of non-enzymatic antioxidants, which is more noticeable in men with genotype CC than men with genotype TT. This observation is sustained by a higher concentration of MDA, which accompanied genotype CC (18.094 µM), suggesting more escalated lipid peroxidation compared to genotype TT (15.171 µM). Finally, the genotype TT was connected with higher concentrations of non–enzymatic antioxidants: bilirubin (0.687 mg*dL−1, CC: 0.652 mg*dL−1), uric acid (6.097 mg*dL−1, CC: 4.980 mg*dL−1), GSH (6.345 µM, CC: 4.630 µM), suggesting more effective antioxidative defense, comparing to genotype CC (Tables 2, 3, Figs. 1, 2).
All these facts confirm the indirect influence of genetic factors on antioxidative protection and the relationship between non–enzymatic antioxidants and chemical elements in the studied population. In the matter of GSH, the level in serum of men with fertility disorders was lower (6.150 µM) compared to normozoospermic men (6.812 µM; Table 1, Fig. 1). Despite that, the difference was not significant (P=0.162). Still, it might suggest more effective antioxidative defense in the group of men with normozoospermia, which was additionally sustained by a higher concentration of bilirubin in this group (0.617 mg*dL−1), comparing to men with fertility disorders (0.588 mg*dL−1; Table 1, Fig. 1). Again, components of non-enzymatic antioxidative protection underwent mutual regulation since important correlations between uric acid and GSH were noted both in the group with fertility disorders and healthy control (respectively r=0.524 and r=0.511; Table 1, Fig. 2). This result is in agreement with specific suggestions from available literature that emphasize commitment of uric acid in GSH increment [21].
Finally, the level of uric acid in men with fertility disorders was higher (7.058 mg*dL−1) compared to the control (5.487 mg*dL−1; P=0.071), but it might be considered an excessive burden, which causes instead an adverse effect in the infertile group. Despite the lack of direct influence of polymorphism of gene IL–4v.C589T (rs2243250) on male fertility, it indirectly shaped antioxidative status and relations between chemical elements and non-enzymatic antioxidants. TT genotype was connected with higher concentrations of bilirubin (0.687 mg*dL−1, CC: 0.652 mg*dL−1), uric acid (6.097 mg*dL−1, CC: 4.980 mg*dL−1), and GSH (6.345 µM, CC: 4.630 µM). The genotype also modulates or even strengthens some interactions between chemical elements and non–enzymatic antioxidants since correlations between Na, B, and GSH in men with genotype CC were stronger (Na–GSH: r=–0.506, B–GSH: r=0.401), than in the case of healthy control (Na–GSH: r=–0.308, B–GSH: r=0.387). It also applied to the interactions between certain chemical elements and MDA (in the group with genotype CC B–MDA: r=0.428, while in the infertile men B–MDA: r=0.338 and in the control B–MDA: r=0.326; Tables 1, 3).
The proteins encoded by IL–4 gene are cytokines produced by activated T cells. It is a ligand for IL–4 receptor. It shares its functionality with Interleukin 13. Interleukin 4 plays a role in tissue repair, balancing the effects of type 1 pro-inflammatory cytokines, regulating the body's antiparasitic response, and healing wounds. However, Interleukin 4 itself may show a proinflammatory nature, promoting, inter alia, allergic inflammation (mediating the production of allergen-specific immunoglobulin E) or participating in the course of acute lung injury (recent reports have confirmed the increased level of IL–4 in the course of COVID–19) [38, 68].
The importance of the IL–4 gene for male fertility is evidenced by its relatively high tissue-specific expression in the testes and the average level of expression in the prostate (compared to other analyzed tissues) [38]. The information provided corresponds to the results and conclusions presented by us in our paper and testifies to the legitimacy and relevance of the study of this issue about the male reproductive condition. This applies especially to the connection with oxidative stress, which is a series of reactions that can trigger or intensify the widely understood inflammatory processes in the body), which allowed us to combine this genetic aspect with the topic of oxidative stress, which also has a modulating effect on the course of broadly understood inflammatory processes and the mobilization of various defense mechanisms. Therefore, their level was an important research element within the undertaken project.
The high expression of IL–4 gene in the testicular tissue suggests that the processes that are the object of our research interest may be important for male fertility. Indeed, the C589T polymorphism did not show a direct relationship with infertility (which is consistent with the state of knowledge contained in the current literature on the subject).
Generally, -589C/T polymorphism of the IL-4 gene tends to increase the risk of asthma across various genetic models (dominant, recessive, allelic, TT vs. CC). Furthermore, this tendency concerns both pediatrics and adults [69]. Finally, an ethnicity analysis conducted by Kousha et al. (2020) [69] shows significant associations among Asians, Americans, and Europeans. In this context, polymorphism (recognized upstream of the transcription initiation site) is connected with specific enhancements in the process of binding a transcription factor caused by the appearance of the polymorphic T allele. Subsequently, it may lead to over-expression of the IL-4 gene and trigger escalations of immunological responses [69]. On the other hand, Razaghian et al. (2023) [70] focused on the Iranian pediatric population. They noted that the probability of asthma in children with the C allele was 3.33 times higher than that of children with the T allele, and this observation was statistically significant. However, comparing the serum IgE level between various IL-4 genotype groups (v.C589T; CC, CT, TT) in asthmatic and atopic and non-atopic asthmatic patients demonstrated no significant difference (similar level of IgE).
Ultimately, the authors stated that IL-4 rs2243250 gene polymorphism was certainly connected with asthma risk in the studied population. However, atopy and severity appear more problematic and diverse among various populations [70]. Quite recently, Shi et al. (2023) [71] analyzed the association of IL-4 gene polymorphisms with the susceptibility of atopic dermatitis (AD) since interleukin-4 is thought to be involved in the pathogenesis of this inflammatory skin disease.
Finally, researchers found no significant differences in genotype or allele frequencies between patients with AD and the control group in the studied population of Chinese Han children. However, additional analysis of haplotypes in the context of such polymorphisms as IL-4 rs2243283, rs2243250, and rs2243248 (located in the same haplotype block with linkage disequilibrium) showed that compared to the haplotype CCT, the frequency of haplotype GTT in patients with AD was significantly lower than that in controls. Accordingly, there was a connection between the IL-4/GTT haplotype and a lower disease risk. However, the mentioned team suggests that the study of the exact role of polymorphism rs2243250 in AD should be tested on a larger sample size [71]. Certain interleukins (among others, IL-4, IL-6, IL-8, IL-10) are considered key modulators in vulnerability to the hepatitis B virus and course of infection. Therefore, due to the significant role of the mentioned factors in immunomodulation, some studies also analyze the role of polymorphisms in influencing the activities of interleukins and other modulating agents that can affect even the process of liver scarring [72].
However, the indirect effects of C589T polymorphism in the context of male reproductive condition, which we demonstrated, combined with antioxidant defense status, prove the importance and innovation of our research project and the innovative conclusions obtained therein.
Non-enzymatic antioxidants, chemical elements, and genetic factors jointly shaped male reproductive conditions in the studied population. Genetic polymorphism of gene IL–4v.C589T (rs2243250) despite the lack of direct connection with male fertility disorders, indirectly influence non–enzymatic antioxidants, their relations with chemical elements and exert influence lipid peroxidation [73]. Therefore, the functionality of antioxidative defense was modulated by genotype, and the presence of genotype CC was connected with the inevitable destabilization of non–enzymatic antioxidative defense noticeable in numerous negative correlations between chemical elements and antioxidants, as well as lower activity of particular non–enzymatic mechanisms.
Another important issue is the meaning of inflammation in infertility. This process can lead to unfavorable effects in males, such as leukocytospermia, due to the production of reactive oxygen free radicals and chronic testicular inflammation, which threaten the male reproductive condition [74]. However, this problem needs to be considered multispectral, linking causative factors of male reproductive tract inflammation, induction of oxidative stress, and oxidant-sensitive cellular cascades leading to worsening reproductive potential. The presence of inflammatory states and the recognition of semen markers of inflammation are fundamental for the overall diagnostic-therapeutic management of male infertility [74]. Generally, in the context of treatment possibilities for male infertility, as it was mentioned, the empirical medical treatment strategies gain interest, particularly in cases of idiopathic male infertility. Empirical therapies, such as selective estrogen receptor modulators (SERMs), hormones, or antioxidants in general, offer a possibility of improvement of sperm parameters. However, probably the most serious disadvantage is that their effectiveness in enhancing pregnancy rates is still inconclusive.
On the other hand, alternative therapies like acupuncture or pharmacopuncture also exhibited particular clinical effectiveness in improving sperm parameters. Understanding the biological mechanisms underlying male infertility may also be improved thanks to the conceptions that differentiate human pluripotent stem cells into advanced spermatogenic cells [75]. The mentioned aspects demonstrate that the future of this discipline will undoubtedly be interesting and very promising.
The results presented in our paper on the IL–4v.C589T polymorphism and conclusions formulated on their basis are consistent with literature data, indicating the lack of a direct relationship between the polymorphism studied and male infertility. However, the primary intention of this paper was, to a lesser extent, to exclude or confirm a direct relationship between the studied polymorphism and male infertility. We wanted a broader approach to the subject and to establish relationships between genetic aspects and selected antioxidant parameters of defense mechanisms. Therefore, we were more interested in the status of antioxidant defense and its relationships to the genetic factor (in groups of people with a fixed genotype). On this basis, we obtained a more detailed picture of the topic – the sum of analyzed genetic aspects and parameters related to antioxidant defense (response to oxidative stress). Our results in no way challenge current data in this area. They only represent a broader perspective focused on finding the indirect impact of analyzed polymorphism. Our research focused on the polymorphism of IL–4v.C589T gene. The central premise was the involvement of the IL-4 gene in various inflammatory processes in the body [38].
The obtained results may be helpful in the diagnostics of male infertility (reduction of idiopathic cases, more effective treatment), decrement of idiopathic cases, and introduction of more directed and effective treatment. At the same time, identifying relationships between environmental stressors and fertility disorders may help eliminate or reduce the impact of factors detrimental to fertility and the perception of the importance of immunogenetic factors in shaping the defensive abilities of the organisms against the destruction of reproductive material. This allows us to infer the role of oxidative stress in the induction of genetic polymorphisms threatening male infertility. Subsequently, it may enlarge consciousness about factors harmful to male infertility (environmental and immunogenetic) and ways of their elimination.
Study perspectives and limitations
Current evidence invariably underlines the multispectral nature of male infertility in the context of its
connection with the presence of specific accompanying health concerns, which jointly result in reduced
life
expectancy [76]. Cohort studies even demonstrate that men with diminished semen parameters may be
subjected to a
greater risk of all-cause mortality compared to fertile controls. Thus, possible genetic, hormonal, or
lifestyle
causes of infertility may also escalate threats of other harmful conditions. Decreased testosterone, for
example, may be connected with testicular dysfunction but, on the other hand, engaged in obesity,
cardiovascular
disease, or insulin resistance. Klinefelter syndrome may be considered a cause of azoospermia but also as
a
factor predisposing to diabetes, metabolic syndrome, or malignancies. Finally, psychological stress or
unfavorable environmental exposures may further undermine reproductive conditions and intensify other
health-compromising events [76]. Therefore, Kaltsas et al. (2025) [76] postulate to consider male
infertility as
an early indicator of other important health vulnerabilities and notice a need to broaden the evaluation
of
infertile men beyond standard fertility assessments to include searching for chronic diseases, mental
issues,
specific cancers or hormonal imbalances [76]. Sciorio et al. (2025) [77] underscore the necessity of
improving
diagnostics to emphasize the significance of immunopathological mechanisms, infection, inflammation, and
autoimmune responses in male infertility.
Furthermore, the mentioned processes may affect sperm quality through anti-sperm antibodies and oxidative
stress, which worsen the reproductive condition and again reveal the multifactorial nature of this
phenomenon
[77]. Since anti-sperm antibodies interfere negatively with fertility by inhibiting sperm migration
through the
female reproductive tract or exerting an inhibitory effect during sperm-egg interaction and fertilization,
modern assisted reproductive treatments may be generally considered adequate for resolving this issue.
However,
an important diagnostic limitation of inadequate association of epididymal and testicular
immunopathologies with
male infertility is posed by the tendency that immunological conditions often manifest well before actual
fertility evaluation. In some instances, experimental data from animal models may constitute an effective
alternative to increase knowledge in this area and extend the possibilities of identifying new biomarkers
and
therapeutic options [77].
Nevertheless, there are still many fertility research gaps. Standard semen analysis has only a limited
ability
to correctly distinguish between men who struggle with infertility and men who do not. Recently, the pool
of
diagnostic tools has been willingly enriched with sperm DNA fragmentation studies, reactive oxygen species
measurements, and leukocyte detection tests. Furthermore, the nature of interactions between oocyte and
sperm
may be examined with the zone penetration assay, the vitality and hypo-osmotic swelling test, or the
mitochondrial activity index test [78]. Artificial intelligence (AI) achievements may improve diagnostics,
prevention, treatment, and management of diverse health conditions. It can potentially develop more
patient-centered precision in medicine in general, but in andrology, applying AI is still relatively new.
Additionally, using AI algorithms could, for instance, enable massive combinations of the body of
parameters
evaluated in the pre-intervention diagnostic analysis of varicocele patients, allowing the exact selection
of
the patients to undergo surgery [78]. In the field of terminology, there is also a recent descriptor for
men
with infertility with abnormal semen parameters and oxidative stress, namely Male Oxidative Stress
Infertility
(MOSI). This category includes many patients previously described as heaving idiopathic male infertility.
Undeniably, measuring the levels of oxidants and antioxidants can serve as a crucial clinical indicator
for
classifying MOSI. However, the therapy that utilizes antioxidants to treat male infertility still suffers
from a
lack of solid scientific backing. Therefore, besides the existence of such assays as MiOXSYS®
(male
infertility oxidative system) and OxiSperm® II, which constitute a diagnostic tool to assess
oxidative stress in an accurate and repeatable way, there is a need to develop new evidence-based
treatment
guidelines and algorithms that enable to qualify men with idiopathic or unexplained infertility into
MOSI-positive or MOSI-negative groups [78]. The mentioned MiOXSYS® technology is an innovative
method
to evaluate the oxidation-reduction potential (ORP) level in whole seminal fluid. Measurement of this
parameter
and the relationship between oxidants and antioxidants in the context of the potential for electrons to
move
from one species to another allows for the versatile measurement of oxidative stress. Despite some
limitations,
incorrect ORP levels can identify altered sperm functionality, which appears to be particularly important
in
cases of idiopathic infertility [79].
Another representation of an innovative approach is omics studies, which encompass such areas of research
as
genomics (genes), transcriptomics (transcription products), proteomics (proteins), and metabolomics
(metabolites). Successful development of this discipline may be improved thanks to the existence of
numerous
databases that combine data from the fields of molecular biology, genetics, statistics, and computational
biologists. However, because of the modernity of such approaches, there are still many challenges and
imbalances
in the development of particular omics fields. Nevertheless, it is generally very promising, among others,
in
identifying unknown genetic factors of male infertility or accomplishing new diagnostic tools for this
condition. Undoubtedly, the effective conjunction of altered levels of oxidative stress with fields like
glycomics or lipidomics may also deliver new knowledge about male reproductive potential [79].
An interesting issue is the linkage between oxidative stress and the effectiveness of assisted
reproductive
techniques. There are reports confirming that seminal oxidative stress level may be regarded as a
predictive
factor in the context of fertilization and blastulation rates in patients undergoing intracytoplasmic
sperm
injection. Since oxidative stress tends to be correlated with poor outcomes of in vitro
fertilization
procedure, there are propositions in which, thanks to a combination of antioxidants, the redox state of
ART
culture media may be adjusted to ensure the optimal effect of blastocyst formation and pregnancy rates
[80].
Antioxidant treatment is also willingly applied to decrease sperm DNA fragmentation. In this context,
there are
suggestions that testicular sperm presents a lower level of DNA fragmentation than ejaculated sperm.
Therefore,
the usage of testicular sperm for intracytoplasmic sperm injection may appear to be beneficial if the male
partner struggles with high sperm DNA fragmentation rate in the ejaculated sperm. Undeniably, in this
issue,
antioxidant therapy before ICSI (Intracytoplasmic Sperm Injection) and artificial oocyte activation may
also
deliver favorable outcomes [80].
Another aspect that may affect the male reproductive condition and simultaneously has the potential to
gain
interest shortly is microbial dysbiosis of the human genital tract. The fundamental mechanism linking
microbial
dysbiosis (among others, altered bacterial diversity and richness, the dominance of pathogenic species or
imbalances in the genital microbiome) to infertility engages oxidative stress, inflammation, and possible
sperm
structural degradation. In this context, such tactics as targeted antimicrobial therapies, prebiotics,
probiotics, fecal microbiota transplantation, and others can potentially improve male fertility by
adjusting the
genital microbiota [81]. However, there are limitations to the decreased strength of the research evidence
due
to unclear randomization methods, the lack of adequate controls, and the deficiency of double-masked
clinical
studies. Additionally, there are lots of disturbing factors like body mass index, age, lifestyle habits,
usage
of medications, and more, which can interfere with both the microbiome and specific reproductive
parameters,
obfuscating the reliability of research. Finally, the implementation of microbial-based interventions in
male
infertility generates some ethical and safety challenges. Selection of antimicrobial agents, probiotics or
prebiotics, possible interactions with the immune system, optimization of treatment route and duration, as
well
as the need for long-term monitoring of outcomes, may also constitute a serious issue that must be
answered in
the process of development of this innovative discipline [81].
Furthermore, couple fertility may also be disturbed by the microbiota, which is transferred between
partners
during intercourse. Therefore, detailed fertility evaluation should consider both partners' microbiota.
Certain
bacteria may be recognized as factors responsible for altering semen quality. For instance, Pseudomonas
spp. tend to be expressed in greater abundance in the semen of infertile men and probably exert
influence on
sperm motility. Moreover, some Prevotella species may lessen semen quality and be engaged in
impaired
spermatogenesis.
On the other hand, reduced Lactobacillus abundance may be associated with abnormal sperm morphology
[82].
Nevertheless, the validation of results of future investigations undoubtedly will require substantial
researches, that will combine data from various fields and institutions. It will not only focus on the
description of microbiota but also on the interactions between bacteria, microorganisms with human cells,
or the
production of biofilms [82].
Nowadays, the application of next-generation sequencing
(NGS) technologies offers a chance to better understand the genetic basis of infertility in cases where
conventional diagnostic tools appear ineffective. This approach also enables discoveries that enrich
existing
infertility treatment and prevention strategies. It is imperative since unexplained infertility may be
caused by
various unidentified complex genetic or polygenic factors, subtle genetic interactions, and additionally
be
affected by environmental influences. Achieving optimal results requires integrating genomic research,
including
broader gene panels, and engagement of transcriptomics and epigenetics [83]. However, there is a need to
constitute uniform standards for using NGS in infertility, allowing for more consistent variant analysis
and
credible cross-studies. Ultimately, longitudinal studies encompassing infertility in different populations
and
environments could deliver new knowledge about region-specific genetic variants and gene-environment
interactions essential for male reproductive conditions [83]. Generally, all of the mentioned reports
prove that
multi-aspectual investigations in male infertility remain in the phase of lively development. Undoubtedly,
cooperation between many innovative disciplines will be decisive in achieving success, both in the near
and more
distant future (Fig. 3).
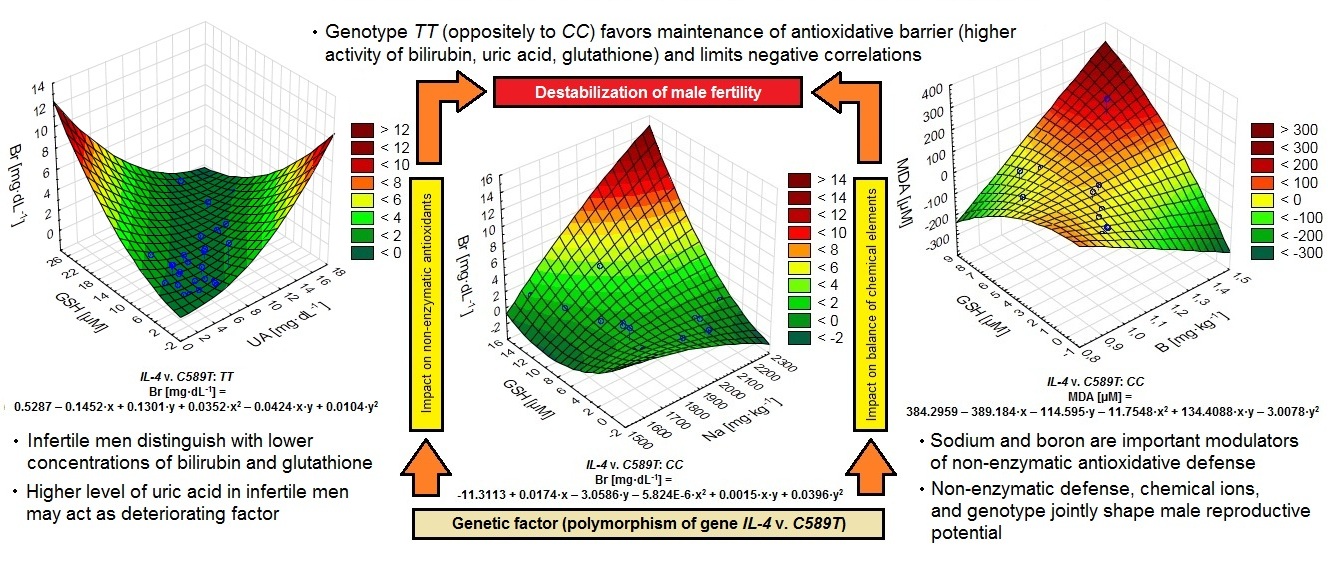
Fig. 3: Three-dimensional plots illustrating the influence of genetic polymorphism (IL-4v.C589T), nonenzymatic antioxidants, and chemical elements on male fertility parameters. Left: In individuals with the TT genotype, higher levels of bilirubin (Br), uric acid (UA), and glutathione (GSH) are associated with maintenance of the antioxidative barrier. Middle: In individuals with the CC genotype, a strong interaction between bilirubin, glutathione (GSH), and sodium (Na) levels indicates an impact on the balance of chemical elements and non-enzymatic antioxidants. Right: The combined effect of boron (B), glutathione (GSH), and malondialdehyde (MDA) levels, an indicator of oxidative stress, highlights the role of trace elements in modulating oxidative balance. Overall, the data suggest that genotype-dependent antioxidant defense mechanisms, together with chemical element interactions, influence male fertility. Infertile men show reduced levels of bilirubin and GSH, and increased uric acid concentrations may act as a deteriorating factor.
Conclusion
We estimate the importance of unfavorable changes arising from oxidative stress about the functionality of non-enzymatic antioxidants (bilirubin, uric acid, glutathione) and correlations with MDA in the serum of men, which allow a complete look at the determinants of male infertility. Molecular analysis was conducted and applied to the polymorphism of gene IL–4v.C589T (rs2243250). Particular genotypes TT and CC were analyzed to notice their influence on the generation of fertility perturbations. They had an indirect but differentiated effect on antioxidant mechanisms involving bilirubin, uric acid, and glutathione. Therefore, we conclude that IL–4v.C589T (rs2243250) polymorphism differentiated the body's response to environmental stressors.
1. The activity of non-enzymatic antioxidant mechanisms differs in men with fertility disorders and the control group. Numerous correlations between these mechanisms prove the cooperation of antioxidants in ensuring defense against oxidative stress. The concentration of glutathione and bilirubin suggests more effective antioxidative defense in the control group than in infertile men. Compared to the control, uric acid excessively burdens infertile men, and non-enzymatic mechanisms appear correlated, confirming their mutual regulation and cooperation in adequate antioxidative protection.
2. Genetic polymorphisms of IL–4v.C589T (rs2243250) do not directly correlate with conditioning of male reproductive potential in the study population (the lack of significant difference in the frequency of genotypes; P=0.5). However, men with TT or CC genotype (IL–4v.C589T (rs2243250) showed different antioxidant defenses. TT genotype was associated with a higher concentration of non–enzymatic antioxidants, which confirmed the cooperative character of non-enzymatic antioxidative mechanisms.
3. Due to the number of correlations, B should be considered an important modulator of non-enzymatic antioxidant defense. In contrast, due to the strength of correlation, Na is important. In the group with the CC genotype, Pb and Be play a greater role in shaping antioxidant defense and lipoperoxidation. Thus, in the influence of chemical elements on the non-enzymatic antioxidants, the most important modulators of non-enzymatic antioxidative defense appeared to be boron and sodium. In the group with CC genotype (IL–4v.C589T (rs2243250), the larger pool of chemical elements correlated negatively with antioxidants, which might signal destabilization.
4. Non-enzymatic defense, chemical elements, and genetic factors shape the male reproductive condition of the study population. Genetic polymorphism of IL–4v.C589T (rs2243250), although not directly related to impaired male fertility (no differences in the frequency of genotypes between groups), may indirectly shape the elemental balance and influence non-enzymatic antioxidants and their relations with chemical elements and exert influence lipid peroxidation. The CC genotype was associated with the inevitable destabilization of antioxidant defense, noticeable in numerous negative correlations between elements and antioxidants and lower activity of non-enzymatic antioxidants. This indicates the differentiation of functioning of antioxidant defense, depending on the genotype.
5. The dependencies analyzed in the framework of the research included in this research have not been studied in such an approach so far. Therefore, they are innovative and constitute an important aspect of multi-range interdependencies considered in this work.
Acknowledgements
Author Contribution Statement
All authors (Jędrzej Baszyński, Piotr Kamiński, Marek Szymański, Karolina Wasilow, Emilia Stanek, Sylwia
Brodzka, Renata Grochowalska, Tomasz Stuczyński, Rafał Bilski, Martin Hromada, Natalia Kurhaluk, Halina
Tkaczenko) jointly participated in the experimental studies on the environmental relationships between
nonenzymatic antioxidant defense and polymorphic changes in male infertility. They developed and
participated in
the development of the research problem and participated in the design of this article. All authors
discussed
the main theses of this work and revised the draft MS. They co-edited and revised the final version of the
manuscript, conceived every part of this original article and participated in its design and coordination,
and
helped with every part of the MS project. Piotr Kamiński edited the entire text. All authors read and
approved
the final version of MS.
Research authorization statement
All authors of this paper reporting experiments on humans confirm that all experiments were performed in
accordance with relevant guidelines and regulations by Bioethical Commission of the Collegium Medicum of
Nicolaus Copernicus University in Toruń, Poland (numbers of permissions as below). All experimental
protocols
were approved by institutional and licensing committee, i.e. Bioethical Commission of the Collegium
Medicum of
Nicolaus Copernicus University in Toruń, Poland. In this paper informed consent was obtained from all
participants of experiments.
The research was undertaken following to the Guidelines of the European Union Council and the current laws
in
Poland, according to the Bioethical Commission of the Collegium Medicum of Nicolaus Copernicus University
in
Toruń, Poland. Samples were collected under permit No KB 427/2014, and No KB 365/2015).
Disclosure Statement
The authors declare no conflicts of interest.
All authors have read and approved the MS of this paper and declare that it has not been published previously nor it is being considered by any other peer-reviewed journal. The authors confirm that neither the manuscript nor any parts of its content are currently under consideration or published in another journal. The authors gave written informed consent for publish this paper in its current form. The authors have not any relevant financial or non-financial or other potential Disclosure Statement and personal relationships with other people or organizations that could inappropriately influence (bias) this work. All authors have not any relationships or affiliations that may be perceived by readers to have influenced, or give the appearance of potentially influencing, what we wrote in submitted paper. The authors declare that there is no any conflict of interest that could be perceived as prejudicing the impartiality of the research reported. All authors have contributed significantly, and all authors are in agreement with the content of the manuscript.
References
| 1 | Pandruvada S, Royfman R, Shah TA, Sindhwani P, Dupree JM, Schon S, Avidor-Reiss T: Lack of trusted
diagnostic tools for undetermined male infertility. J Assist Reprod Genet 2021; 38, 2: 265-276.
https://doi.org/10.1007/s10815-020-02037-5 |
| 2 | Arafa M, Agarwal A, Majzoub A, Selvam MKP, Baskaran S, Henkel R, Elbardisi H: Efficacy of
Antioxidant Supplementation on Conventional and Advanced Sperm Function Tests in Patients with
Idiopathic Male Infertility. Antioxidants (Basel) 2020; 9, 3: 219.
https://doi.org/10.3390/antiox9030219 |
| 3 | Aitken RJ, Drevet JR, Moazamian A, Gharagozloo P: Male Infertility and Oxidative Stress: A Focus
on the Underlying Mechanisms. Antioxidants (Basel) 2022; 11, 2: 306.
https://doi.org/10.3390/antiox11020306 |
| 4 | Houston BJ, Nixon B, McEwan KE, Martin JH, King BV, Aitken RJ, De Iuliis GN: Whole-body exposures
to radiofrequency-electromagnetic energy can cause DNA damage in mouse spermatozoa via an oxidative
mechanism. Sci Rep 2019; 9: e17478.
https://doi.org/10.1038/s41598-019-53983-9 |
| 5 | Perez-Patiño C, Barranco I, Li J, Padilla L, Martinez EA, Rodriguez-Martinez H, Roca J, Parrilla
I: Cryopreservation Differentially Alters the Proteome of Epididymal and Ejaculated Pig Spermatozoa.
Int J Mol Sci 2019; 20, 7: e1791.
https://doi.org/10.3390/ijms20071791 |
| 6 | Evans EPP, Scholten JTM, Mzyk A, Reyes-San-Martin C, Llumbet AE, Hamoh T, Arts EGJM, Schirhagl R,
Cantineau AEP: Male subfertility and oxidative stress. Redox Biol 2021; 46: e102071.
https://doi.org/10.1016/j.redox.2021.102071 |
| 7 | Amjad S, Rahman MS, Pang M-G: Role of Antioxidants in Alleviating Bisphenol A Toxicity.
Biomolecules 2020; 10, 8: e1105.
https://doi.org/10.3390/biom10081105 |
| 8 | Chyra-Jach D, Kaletka Z, Dobrakowski M, Machoń-Grecka A, Kasperczyk S, Birkner E, Kasperczyk A:
The Associations between Infertility and Antioxidants, Proinflammatory Cytokines, and Chemokines.
Oxid Med Cell Longev 2018; e8354747.
https://doi.org/10.1155/2018/8354747 |
| 9 | Luckring EJ, Parker PD, Hani H, Grace MH, Lila MA, Pierce JG, Adin ChA: In vitro Evaluation of a
Novel Synthetic Bilirubin Analog as an Antioxidant and Cytoprotective Agent for Pancreatic Islet
Transplantation. Cell Transplant 2020; 29: e0963689720906417.
https://doi.org/10.1177/0963689720906417 |
| 10 | Vasavda Ch, Kothari R, Malla AP, Tokhunts R, Lin A, Ji M, Ricco C, Xu R, Saavedra HG, Sbodio JI,
Snowman AM, Albacarys L, Hester L, Sedlak TW, Paul BD, Snyder SH: Bilirubin links heme metabolism to
neuroprotection by scavenging superoxide. Cell Chem Biol 2019; 26, 10: 1450-1460.e7.
https://doi.org/10.1016/j.chembiol.2019.07.006 |
| 11 | Muchova L, Vanova K, Zelenka J, Lenicek M, Petr T, Vejrazka M, Sticova E, Vreman HJ, Wong RJ,
Vitek L: Bile acids decrease intracellular bilirubin levels in the cholestatic liver: implications
for bile acid-mediated oxidative stress. J Cell Mol Med. 2011; 15, 5: 1156-1165.
https://doi.org/10.1111/j.1582-4934.2010.01098.x |
| 12 | Bartosz G: Druga twarz tlenu. Wolne rodniki w przyrodzie. The other face of oxygen. Free radicals
in nature. 2009. PWN, PWN-Pol. Sci. Publ., Warsaw, Poland, 448 p.
|
| 13 | Oh SW, Lee ES, Kim S, Na KY, Chae DW, Kim S, Chin HJ: Bilirubin attenuates the renal tubular
injury by inhibition of oxidative stress and apoptosis. BMC Nephrol 2013; 14: 105.
https://doi.org/10.1186/1471-2369-14-105 |
| 14 | Takeda T, Mu A, Tai TT, Kitajima S, Taketani S: Continuous de novo biosynthesis of haem and its
rapid turnover to bilirubin are necessary for cytoprotection against cell damage. Sci Rep 2015; 5:
e10488.
https://doi.org/10.1038/srep10488 |
| 15 | Abdullah F, Nor-Ashikin MNK, Agarwal R, Kamsani YS, Malek MA, Bakar NS, Kamal A-AM, Sarbandi M-S,
Rahman N-SA, Musa NH: Glutathione (GSH) improves sperm quality and testicular morphology in
streptozotocin-induced diabetic mice. Asian J Androl 2021; 23, 3: 281-287.
https://doi.org/10.4103/aja.aja_81_20 |
| 16 | Krzyściak W, Papież M, Bąk E, Morava E, Krzyściak P, Ligęzka A, Gniadek A, Vyhouskaya P, Janeczko
J: Sperm Antioxidant Biomarkers and Their Correlation with Clinical Condition and Lifestyle with
Regard to Male Reproductive Potential. J Clin Med 2020; 9, 6: e1785.
https://doi.org/10.3390/jcm9061785 |
| 17 | Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, Jahoor F: Deficient
synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary
cysteine and glycine supplementation. Am J Clin Nutr 2011; 94, 3: 847-853.
https://doi.org/10.3945/ajcn.110.003483 |
| 18 | Gadea J, Molla M, Selles E, Marco MA, Garcia-Vazquez FA, Gardon JC: Reduced glutathione content in
human sperm is decreased after cryopreservation: Effect of the addition of reduced glutathione to
the freezing and thawing extenders. Cryobiol 2011; 62, 1: 40-6.
https://doi.org/10.1016/j.cryobiol.2010.12.001 |
| 19 | Banihani SA: Role of Uric Acid in Semen. Biomolecules 2018; 8, 3: 65.
https://doi.org/10.3390/biom8030065 |
| 20 | Ma J, Han R, Cui T, Yang Ch, Wang S: Effects of high serum uric acid levels on oxidative stress
levels and semen parameters in male infertile patients. Medicine (Baltimore) 2022; 101, 3: e28442.
https://doi.org/10.1097/MD.0000000000028442 |
| 21 | Pasalic D, Marinkovic N, Feher-Turkovic L: Uric acid as one of the important factors in
multifactorial disorders - facts and controversies. Biochem Med. (Zagreb) 2012; 22, 1: 63-75.
https://doi.org/10.11613/BM.2012.007 |
| 22 | Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY,
Lanaspa MA: Sugar, Uric Acid, and the Etiology of Diabetes and Obesity. Diabetes 2013; 62, 10:
3307-3315.
https://doi.org/10.2337/db12-1814 |
| 23 | Sautin YY, Johnson RJ: Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic
Acids 2008; 27, 6: 608-619.
https://doi.org/10.1080/15257770802138558 |
| 24 | Renu K, Subramaniam MD, Chakraborty R, Myakala H, Iyer M, Bharathi G, Siva K, Vellingiri B,
Gopalakrishnan AV: The role of Interleukin-4 in COVID-19 associated male infertility - A hypothesis.
J Reprod Immunol 2020; 142: e103213.
https://doi.org/10.1016/j.jri.2020.103213 |
| 25 | Ciszewski WM, Wagner W, Kania KD, Dastych J: Interleukin-4 Enhances PARP-Dependent DNA Repair
Activity In vitro. J Interferon Cytokine Res 2014; 34, 9: 734-740.
https://doi.org/10.1089/jir.2014.0029 |
| 26 | Pisoschi AM, Pop A, Cimpeanu C, Predoi G: Antioxidant Capacity Determination in Plants and
Plant-Derived Products: A Review. Oxid Med Cell Longev; 2016: e9130976.
https://doi.org/10.1155/2016/9130976 |
| 27 | Flieger J, Flieger W, Baj J, Maciejewski R: Antioxidants: Classification, Natural Sources,
Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials (Basel)
2021; 14, 15: e4135.
https://doi.org/10.3390/ma14154135 |
| 28 | Baszyński J, Kamiński P, Bogdzińska M, Mroczkowski S, Szymański M, Wasilow K, Stanek E,
Hołderna-Bona K, Brodzka S, Bilski R, Tkachenko H, Kurhaluk N, Stuczyński T, Lorek M, Woźniak A:
Enzymatic Antioxidant Defense and Polymorphic Changes in Male Infertility. Antioxidants 2022; 11, 5,
817: 1-29.
https://doi.org/10.3390/antiox11050817 |
| 29 | WHO laboratory manual for the examination and processing of human semen. 5th Ed. WHO Library
Cataloguing-in-Publication Data; 2010. ISBN 978 92 4 154778 9.
|
| 30 | Krechniak J: Toksykologia środowiskowa. Environmental toxicology [In:]. Toksykologia współczesna.
Contemporary toxicology. (Ed.: Seńczuk W.) 2006; PZWL-Medical Publ., Warsaw, Poland, 993 p.
|
| 31 | Radwan J: Badanie nasienia. Ocena seminologiczna. Seminological assessment [In:]. Andrologia,
Andrology. (Eds.: Semczuk M., Kurpisz M.) 2006; PZWL-Pol. Med. Publ., Warsaw, Poland, 494 p.
|
| 32 | Rosain RM, Wai CM: The rate of loss of mercury from aqueous solution when stored in various
containers. Analyt Chimica Acta 1973; 65, 2: 279-284.
https://www.sciencedirect.com/science/article/abs/pii/S0003267001824934
https://doi.org/10.1016/S0003-2670(01)82493-4 |
| 33 | Ahmad NN, Cu-Unjieng AB, Donoso LA: Modification of standard proteinase K/phenol method for DNA
isolation to improve yield and purity from frozen blood. J Med Genet 1995; 32, 2: 129-130.
https://doi.org/10.1136/jmg.32.2.129 |
| 34 | Rajatileka S, Luyt K, El-Bokle M, Williams M, Kemp H, Molnár E, Váradi A: Isolation of human
genomic DNA for genetic analysis from premature neonates: a comparison between newborn dried blood
spots, whole blood and umbilical cord tissue. BMC Genet 2013; 14: 105.
https://doi.org/10.1186/1471-2156-14-105 |
| 35 | Słomski R (Ed.), Bigos A, Bochenek M, Broszkiewicz A, Bryła M, Brzeziński D, Celczyńska-Bajew L,
Cieślik K., Drwęska N, Dzieduszycki AM, Frąckowiak H, Gabriel M, Giefing M, Golińska B, Gronek P,
Gumna E, Hoppe-Gołębiewska J, Horst-Sikorska W, Jałoszyński P, Juzwa W, Kaczmarek M, Kalak R,
Kątska-Książkiewicz L, Korcz A, Kręgielczak A, Króliczak J, Kujawski M, Lehmann J, Lipiński D,
Lorenz A, Mały E, Marcinkowska M, Mądrzak CJ, Mikołajczyk-Stecyna J, Napierała D, Narożna D,
Naskręt-Barciszewska MZ, Nawrocki M, Nuc K, Opiela J, Oszkinis G, Pawlaczyk K, Pawlak A, Piławski A,
Podralska M, Przewoźna J, Ryba MS, Samiec M, Schneider J, Siemieniako B, Skrzyszowska M, Słomska M,
Smorąg Z, Stopa J, Strauss E, Szalata M, Trzcińska M, Waliszewski K, Walkowiak B, Waszak M,
Wawrzyniak A, Wielgus K, Witucka-Wall H, Wolko Ł, Woźniak A, Zeyland J, Zowczak-Drabarczyk M:
Analiza DNA. Teoria i praktyka. 2008; Wyd. Uniw. Przyr. Poznań, DNA analysis. Theory and practice.
Univ Life Sci. Publ., Poznań, Poland, 573 p.
|
| 36 | Micheal S, Minhas K, Ishaque M, Ahmed F, Ahmed A: IL-4 Gene Polymorphisms and Their Association
With Atopic Asthma and Allergic Rhinitis in Pakistani Patients. J Invest Alergol Clin Immunol 2013;
23, 2: 107-111.
|
| 37 | https://github.com/WillChen2020/TMB-analysis [access: 13.12.2022]
|
| 38 | https://www.ncbi.nlm.nih.gov/gene?linkname=omim_gene&from_uid=147780 [access: 13.12.2022]
|
| 39 | Zar JH: Biostatistical Analysis. 1999; 4th Ed. Prentice-Hall Inc., Englewood Cliffs, N. Jersey,
662 p.
|
| 40 | Hill T, Lewicki P: Statistics. Methods and Applications. A Comprehensive Reference for Science,
Industry, and Data Mining. 2006; StatSoft Inc., USA, 832 p.
|
| 41 | Stanisz A: Przystępny kurs statystyki z zastosowaniem STATISTICA PL na przykładach z medycyny, T.
1. Statystyki podstawowe. An accessible course in statistics with the use of STATISTICA PL on
examples from medicine, v. 1. Basic statistics. 2006; StatSoft Polska, Sp. z o.o., Cracov, Poland,
532 p.
|
| 42 | Kapturkiewicz K: Kwas moczowy. 2017. https://www.mp.pl/pacjent/badania_zabiegi/172475,kwas-moczowy
[access: 17.03.2022].
|
| 43 | Adeoye O, Olawumi J, Opeyemi A, Christiania O: Review on the role of glutathione on oxidative
stress and infertility. JBRA Assist Reprod 2018; 22, 1: 61-66.
https://doi.org/10.5935/1518-0557.20180003 |
| 44 | Salas-Huetos A, Rosique-Esteban N, Becerra-Tomás N, Vizmanos B, Bulló M, Salas-Salvadó J: The
Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and
Meta-Analysis of Randomized Clinical Trials. Adv Nutr 2018; 9, 6: 833-848.
https://doi.org/10.1093/advances/nmy057 |
| 45 | Roumeliotis S, Roumeliotis A, Dounousi E, Eleftheriadis T, Liakopoulos V: Dietary Antioxidant
Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients 2019; 11, 8: 1911.
https://doi.org/10.3390/nu11081911 |
| 46 | Percário S, da Silva Barbosa A, Varela ELP, Gomes ARQ, Ferreira MES, de Nazaré Araújo Moreira T,
Dolabela MF: Oxidative Stress in Parkinson's Disease: Potential Benefits of Antioxidant
Supplementation. Oxid Med Cell Longev; 2020: e2360872.
https://doi.org/10.1155/2020/2360872 |
| 47 | Thomas DT, DelCimmuto NR, Flack KD, Stec DE, Hinds, Jr. TD: Reactive Oxygen Species (ROS) and
Antioxidants as Immunomodulators in Exercise: Implications for Heme Oxygenase and Bilirubin.
Antioxidants (Basel) 2022; 11, 2: e179.
https://doi.org/10.3390/antiox11020179 |
| 48 | Maruhashi T, Kihara Y, Higashi Y: Bilirubin and Endothelial Function. J Atheroscler Thromb 2019;
26, 8: 688-696.
https://doi.org/10.5551/jat.RV17035 |
| 49 | Kühn T, Sookthai D, Graf ME, Schübel R, Freisling H, Johnson T, Katzke V, Kaaks R: 2017. Albumin,
bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer
2020; 117, 10: 1572-1579.
https://doi.org/10.1038/bjc.2017.313 |
| 50 | Chen S, Chen D, Yang H, Wang X, Wang J, Xu Ch: Uric acid induced hepatocytes lipid accumulation
through regulation of miR-149-5p/FGF21 axis. BMC Gastroenterol 2020; 20: e39.
https://doi.org/10.1186/s12876-020-01189-z |
| 51 | Wu S-S, Kor Ch-T, Chen T-Y, Liu K-H, Shih K-L, Su W-W, Wu H-M: Relationships between Serum Uric
Acid, Malondialdehyde Levels, and Carotid Intima-Media Thickness in the Patients with Metabolic
Syndrome. Oxid Med Cell Longev 2019: e6859757.
https://doi.org/10.1155/2019/6859757 |
| 52 | Tanaka T, Milaneschi Y, Zhang Y, Becker KG, Zukley L, Ferrucci L: A double blind placebo
controlled randomized trial of the effect of acute uric acid changes on inflammatory markers in
humans: A pilot study. PLoS One 2017; 12, 8: e0181100.
https://doi.org/10.1371/journal.pone.0181100 |
| 53 | Paciorek K, Chmielewska-Walas K (Ed.): Bilirubina całkowita - badanie. Total bilirubin - the
study. 2015; https://wylecz.to/badania-laboratoryjne/bilirubina-calkowita-badanie/ [access:
17.03.2022].
|
| 54 | Bassey I., Isong IKP, Esiere KUS, Essien OE, Udoh AE, Akpan UO: Seminal Oxidative Stress Markers,
Calcium, Magnesium, and Semen Profile of Infertile Diabetic and Nondiabetic Nigerian Men. Int J Appl
Basic Med Res 2019; 9, 3: 159-164.
https://doi.org/10.4103/ijabmr.IJABMR_152_18 |
| 55 | Zhao J, Hu J, Ma X: Sodium Decanoate Improves Intestinal Epithelial Barrier and Antioxidation via
Activating G Protein-Coupled Receptor-43. Nutrients 2021; 13, 8: 2756.
https://doi.org/10.3390/nu13082756 |
| 56 | Patik JC, Lennon SL, Farquhar WB, Edwards DG: Mechanisms of Dietary Sodium-Induced Impairments in
Endothelial Function and Potential Countermeasures. Nutrients 2021; 13, 1: 270.
https://doi.org/10.3390/nu13010270 |
| 57 | Kabata-Pendias A, Szteke B: Trace Elements in Abiotic and Biotic Environments. 2019; CRC Press,
Taylor & Francis LLC, Boca Raton, 1st Ed., 469 p.
|
| 58 | Ali F, Hosmane NS, Zhu Y: Boron Chemistry for Medical Applications. Molecules 2020;, 25, 4: 828.
https://doi.org/10.3390/molecules25040828 |
| 59 | Kremer D, Post A, Seidel U, Huebbe P, van der Veen Y, Groothof D, Gomes-Neto AW, Knobbe TJ,
Lüersen K, Eisenga MF, Navis GJ, Rimbach G, Bakker SJL. Boron Intake and decreased risk of mortality
in kidney transplant recipients. Eur J Nutr 2022; 61, 2: 973-984.
|
| 60 | Miah MR., Ijomone OM, Okoh COA, Ijomone OK, Akingbade GT, Ke T, Krum B, da Cunha Martins, Jr. A,
Akinyemi A, Aranoff N, Soares FAA, Bowman AB, Aschner M: The Effects of Manganese Overexposure On
Brain Health. Neurochem Int 2020; 135: e104688.
https://doi.org/10.1016/j.neuint.2020.104688 |
| 61 | Imam MU, Zhang S, Ma J, Wang H, Wang F: Antioxidants Mediate Both Iron Homeostasis and Oxidative
Stress. Nutrients 2017; 9, 7: e671.
https://doi.org/10.3390/nu9070671 |
| 62 | López-Botella A, Velasco I, Acién M, Sáez-Espinosa P, Todolí-Torró J-L, Sánchez-Romero R,
Gómez-Torres MJ: Impact of Heavy Metals on Human Male Fertility - An Overview. Antioxidants (Basel)
2021; 10, 9: e1473.
https://doi.org/10.3390/antiox10091473 |
| 63 | Fontenot AP: Immunologic Effects of Beryllium Exposure. Ann Am Thorac Soc 2018; 15, Suppl. 2:
S81-S85.
https://doi.org/10.1513/AnnalsATS.201707-573MG |
| 64 | Xavier MJ, Salas-Huetos A, Oud MS, Aston KI, Veltman JA: Disease gene discovery in male
infertility: past, present and future. Human Genet 2021; 140, 1: 7-19.
https://doi.org/10.1007/s00439-020-02202-x |
| 65 | Maeda E, Nakamura F, Kobayashi Y, Boivin J, Sugimori H, Murata K, Saito H: Effects of fertility
education on knowledge, desires and anxiety among the reproductive-aged population: findings from a
randomized controlled trial. Human Reprod 2016; 31, 9: 2051-2060.
https://doi.org/10.1093/humrep/dew133 |
| 66 | Saleh R, Sallam H, Elsuity MA, Dutta S, Sengupta P, Nasr A: Antioxidant therapy for infertile
couples: comprehensive review of the current status and consideration of future prospects. Front
Endocrinol Lausanne) 2025; 15: e1503905.
https://doi.org/10.3389/fendo.2024.1503905 |
| 67 | Mancini A, Oliva A, Vergani E, Festa R, Silvestrini A: The Dual Role of Oxidants in Male
(In)fertility: Every ROSe Has a Thorn. Int J Mol Sci 2023; 24, 5: e4994.
https://doi.org/10.3390/ijms24054994 |
| 68 | Iwaszko M, Biały S, Bogunia-Kubik K: Significance of Interleukin (IL)-4 and IL-13 in Inflammatory
Arthritis. Cells 2021; 10, 11: e3000.
https://doi.org/10.3390/cells10113000 |
| 69 | Kousha A, Mahdavi Gorabi A, Forouzesh M, Hosseini M, Alexander M, Imani D, Razi B, Javad Mousavi
M, Aslani S, Mikaeili H: Interleukin 4 gene polymorphism (−589C/T) and the risk of asthma: a
meta-analysis and met-regression based on 55 studies. BMC Immunol 2020; 21: e55.
https://doi.org/10.1186/s12865-020-00384-7 |
| 70 | Razaghian A, Parvaneh N, Amirzargar AA, Nirouei M, Gharagozlou M: Association between IL-10 (at
position -592) and IL-4 (at position -589) genotype polymorphism with atopic and non-atopic asthma
in children. Am J Clin Exp Immunol 2023; 12 , 5: 98-106.
|
| 71 | Shi J, He L, Zheng H, Li W, Huang S, Li Y, Tao R: Association of IL-4 and IL-18 genetic
polymorphisms with atopic dermatitis in Chinese children. Front Pediatr 2023; 11: e1202100.
https://doi.org/10.3389/fped.2023.1202100 |
| 72 | Adu F, Aniakwaa-Bonsu E, Nyarko SB, Obeng AS, Ateko RO, Anyanful A, Thomford NE: Host cytokine
genetic polymorphisms in a selected population of persons living with hepatitis B virus infection in
the central region of Ghana. BMC Gastroenterol 2024; 24: e374.
https://doi.org/10.1186/s12876-024-03456-9 |
| 73 | Morabbi A, Karimian M: Trace and essential elements as vital components to improve the performance
of the male reproductive system: implications in cell signaling pathways. J Trace El Med Biol 2024:
e127403.
https://doi.org/10.1016/j.jtemb.2024.127403 |
| 74 | Chen P, Ni S, Ou-Yang L: Causal inference of inflammatory proteins in infertility: a Mendelian
andomization study. Front Endocrinol (Lausanne) 2025; 16: e1448530.
https://doi.org/10.3389/fendo.2025.1448530 |
| 75 | Kaltsas A, Zachariou A, Dimitriadis F, Chrisofos M, Sofikitis N: Empirical Treatments for Male
Infertility: A Focus on Lifestyle Modifications and Medicines. Diseases 2024; 12, 9: e209.
https://doi.org/10.3390/diseases12090209 |
| 76 | Kaltsas A, Koumenis A, Stavropoulos M, Kratiras Z, Deligiannis D, Adamos K, Chrisofos M: Male
Infertility and Reduced Life Expectancy: Epidemiology, Mechanisms, and Clinical Implications. J Clin
Med 2025; 14, 11: e3930.
https://doi.org/10.3390/jcm14113930 |
| 77 | Sciorio R, De Paola L, Notari T, Ganduscio S, Amato P, Crifasi L, Marotto D, Billone V, Cucinella
G, Perino A, Tramontano L, Marinelli S, Gullo G: Decoding the Puzzle of Male Infertility: The Role
of Infection, Inflammation, and Autoimmunity. Diagnostics (Basel) 2025; 15, 5: e547.
https://doi.org/10.3390/diagnostics15050547 |
| 78 | Motawi A, Crafa A, Hamoda T, Shah R, Agarwal A: The Andrological Landscape in the Twenty-First
Century: Making Sense of the Advances in Male Infertility Management for the Busy Clinicians. Int J
Environ Res Public Health 2025; 21, 9: e1222.
https://doi.org/10.3390/ijerph21091222 |
| 79 | Mottola F, Palmieri I, Carannante M, Barretta A, Roychoudhury S, Rocco L: Oxidative Stress
Biomarkers in Male Infertility: Established Methodologies and Future Perspectives. Genes (Basel)
2025; 15, 5: e539.
https://doi.org/10.3390/genes15050539 |
| 80 | Pavuluri H, Bakhtiary Z, Kumar Panner Selvam M, Hellstrom WJG: Oxidative Stress-Associated Male
Infertility: Current Diagnostic and Therapeutic Approaches. Medicina (Kaunas) 2024; 60, 6: e1008.
https://doi.org/10.3390/medicina60061008 |
| 81 | Kaltsas A, Zachariou A, Markou E, Dimitriadis F, Sofikitis N, Pournaras S: Microbial Dysbiosis and
Male Infertility: Understanding the Impact and Exploring Therapeutic Interventions. J Pers Med 2023;
13, 10: e1491.
https://doi.org/10.3390/jpm13101491 |
| 82 | Elahi Z, Mokhtaryan M, Mahmoodi S, Shahroodian S, Darbandi T, Ghasemi F, Ghanavati R, Darbandi A:
All Properties of Infertility Microbiome in a Review Article. J Clin Lab Anal 2025; 39, 6: e25158.
https://doi.org/10.1002/jcla.25158 |
| 83 | Jašinskienė E, Sniečkutė I, Galminas I, Žemaitis L, Simutis M, Čaplinskienė M: Evaluation of Risk
Factors and a Gene Panel as a Tool for Unexplained Infertility Diagnosis by Next-Generation
Sequencing. Medicina (Kaunas) 2025; 61, 2: e271.
https://doi.org/10.3390/medicina61020271 |