Oxidative Stress, Antioxidants, Gut Microbiota and Male Fertility
bDepartment of Medical Biology and Biochemistry, Division of Ecology and Environmental Protection, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń, M. Skłodowska-Curie Str. 9, 85-094 Bydgoszcz, Poland,
cUniversity of Zielona Góra, Institute of Biological Sciences, Department of Biotechnology, Prof. Z. Szafran Str. 1, 65-516 Zielona Góra, Poland
Keywords
Abstract
It is imperative to comprehend the multifactorial causes of male infertility and to identify effective treatment methods, to enhance male reproductive health, and to develop more personalised and effective therapeutic interventions. This review discusses the multifactorial aspects contributing to male infertility, focusing on oxidative stress (OS), sperm quality, gut microbiota, and the potential role of adaptogens. A comprehensive literature search was conducted across several major databases, including the Cochrane Library, Medline, Embase, SciSearch, PubMed, Web of Science, Scopus, and Google Scholar. The findings from the studies included in the databases highlight the significant role of oxidative stress in male infertility, with reactive oxygen species (ROS) contributing to sperm DNA fragmentation and impairment of spermatogenesis. The review further elucidates the influence of both endogenous and exogenous sources of ROS, including lifestyle factors and environmental exposures, on male reproductive health. Emerging research also highlights the involvement of key molecular pathways, such as Nrf2, AMPK/PGC-1α, and NF-κB, in regulating OS within the male reproductive system. Additionally, the review outlines the relationship between endothelial dysfunction, cardiovascular health, and male infertility, identifying OS as a common underlying factor. In addition to the OS, the gut microbiota has been identified as a pivotal factor in male fertility, influencing inflammation and hormonal regulation. This review underscores the potential merits of a synergistic strategy that integrates dietary interventions, antioxidants, gut microbiota modulation, and adaptogens to enhance fertility outcomes. Adaptogens, recognised for their capacity to assist the body in coping with stress and re-establishing equilibrium, may confer protective effects against OS and improve reproductive health. The review under discussion emphasises the importance of a holistic approach to male infertility, integrating molecular, clinical, and lifestyle factors to optimise reproductive health.
Introduction
In approximately 50% of cases of infertility in couples, male factors are either the sole or contributing cause, thus highlighting their significant role in the aetiology of infertility [1, 2]. This emphasises the necessity to prioritise male reproductive health in both diagnostic assessments and therapeutic strategies to address the issue of infertility [3] effectively. A global prevalence of erectile dysfunction (ED) is reported, reflecting the widespread impact of this mechanism [3]. It is estimated that over 72 million people worldwide are affected by this condition [4]. The rising prevalence of ED underscores the pressing need to address the underlying OS through targeted interventions, including antioxidant therapy and lifestyle modifications, to mitigate its effects on endothelial function and male reproductive health [3, 5].
The incidence of marital infertility is estimated at approximately 15%, with male factor infertility accounting for approximately 50% of these cases [6, 7, 8]. Male factor infertility is characterised by such factors as poor sperm quality, low sperm count, and impaired sperm motility, which significantly reduce the chances of successful fertilisation [9]. Various biological, environmental, and lifestyle factors can cause these problems. In approximately 30-50% of cases of male infertility, the underlying causes remain unexplained and are classified as idiopathic [10]. This signifies that, despite extensive testing and medical evaluation, no specific cause of infertility can be identified. Idiopathic infertility can be particularly challenging for healthcare providers, as it makes it difficult to develop targeted treatments. The absence of definitive diagnostic markers often necessitates a more comprehensive approach to treatment, emphasising the enhancement of general reproductive health as opposed to the specific targeting of identifiable conditions [11].
Evidence presented in the literature has highlighted that oxidative stress (OS) is emerging as a critical factor in male infertility, driven by the overproduction of reactive oxygen species (ROS) - toxic byproducts of oxygen metabolism [12]. While ROS are harmful in excessive amounts, at physiological levels they play a central role in regulating intracellular signalling pathways essential for reproductive processes, according to the findings reported by [13]. These include sperm maturation, hyperactivation, capacitation, acrosome reaction, and successful fertilisation [14]. ROS levels in cells are tightly regulated by a balance between their production and neutralisation by antioxidant defence mechanisms. Perturbation of this balance by increased ROS generation or decreased antioxidant activity leads to redox dysfunction, significantly impairing cellular functions [15].
Previous studies suggest that there is a significant relationship between male infertility and a multitude of factors, including hormonal imbalances, genetic abnormalities, varicocele, infections, age, obesity, smoking, alcohol consumption, environmental toxins, and exposure to heat or radiation [16, 17]. Notwithstanding, idiopathic male infertility is the most prevalent category, as its aetiology is frequently unexplained despite rigorous diagnostic procedures and the absence of identifiable underlying factors [18]. The unexplained nature of idiopathic male infertility may be attributable to intricate genetic and molecular factors, microbial infections in the genital tract, or environmental interactions that remain to be fully elucidated, as evidenced by the observations reported by Wang et al [19].. Recent research has explored several potential mechanisms, including subtle genetic mutations, environmental exposures to toxins (such as endocrine-disrupting chemicals), or immune-related issues that may affect sperm function or quality without being immediately apparent [20, 21]. Despite the challenges posed by idiopathic male infertility, advancements in assisted reproductive technologies, such as in vitro fertilisation and intracytoplasmic sperm injection, have offered couples renewed hope. These technologies can circumvent many problems associated with male infertility by enabling sperm with minimal motility or abnormal morphology to directly fertilise an egg, as shown in the experiments by Zheng et al [22]. and Ribeiro et al [23]..
It has previously been demonstrated that spermatozoa are uniquely susceptible to reactive oxygen species (ROS) owing to their limited capacity for repair and the high content of polyunsaturated fatty acids (PUFA) in their plasma membranes [24]. The oxidation of these lipids has been shown to compromise membrane integrity, resulting in ATP leakage and impaired flagellar motility [25]. In addition to this, it has further been demonstrated that OS can cause direct DNA damage in spermatozoa, which results in the disruption of chromatin integrity and the consequent reduction in fertilisation potential [13, 26]. Moreover, OS has been observed to affect the intracellular metabolism of carbohydrates, proteins, and nucleic acids [25], exacerbating its deleterious effects on sperm functionality. In the context of male infertility, the antioxidant defence mechanisms present in semen have been shown to be significantly impaired. In the context of infertile males exhibiting elevated levels of ROS, a decline in antioxidant capacity has been observed, leading to a range of sperm dysfunctions [13]. In conjunction with diminished enzymatic scavenging systems, the combination of excessive ROS production by immature germ cells and leukocytes may lead to oxidative imbalance. This imbalance can result in substantial oxidative damage to crucial biomolecules, such as lipids, proteins, and DNA, thereby reducing sperm viability and fertility potential [15].
In the context of the growing interest in the topic of male infertility, particular attention has been paid to the analysis of sperm quality in men worldwide, leading to concerns regarding reproductive health [27]. This decline has been attributed to environmental pollutants and lifestyle factors, such as poor diet and lack of exercise [28]. Recent decades have seen a marked decrease in the molecular mechanisms involving increased reactive oxygen species overload and sperm DNA damage, as shown in the experiments by various research teams [26, 29, 30], and impaired mitochondrial function, which collectively reduce fertility potential [31]. The relationship between lifestyle factors and sperm quality remains under-explored in the scientific literature, leaving critical gaps in our understanding of how specific behaviours and environmental exposures interact to influence male fertility [28]. While some studies address individual factors like diet, exercise, and smoking, there is a lack of comprehensive understanding of their combined impact, underlying mechanisms, and long-term consequences [32].
This underscores the necessity for more integrative and multidisciplinary research to comprehensively elucidate the intricate interrelationships between lifestyle factors and reproductive health and develop effective interventions and public health strategies. The present study hypothesises that a synergistic approach involving the gut microbiota and nutritional interventions for male fertility is crucial due to the increasing prevalence of male infertility and the complex multifactorial nature of its causes [33]. Given the significant influence of male fertility on such factors as redox imbalance, hormonal imbalances, and systemic inflammation, it is crucial to understand how these factors interact with gut health and nutrition. This understanding is essential for developing more effective treatments, as previously demonstrated by some authors [34]. Conventional approaches tend to address these factors individually. However, combining probiotics, prebiotics, antioxidants, amino acids, omega-3 fatty acids, and adaptogens holds promise for tackling multiple underlying causes concurrently.
Previous studies suggest a significant relationship between the gut microbiota composition and overall health, including reproductive function, which has become a subject of mounting research interest [35]. The gut microbiome plays a significant role in regulating inflammation, metabolic processes, and hormone levels, which are critical to male fertility [36]. Consequently, it is imperative to comprehend how dietary and microbiota-based interventions may augment fertility outcomes. This presents novel prospects for both the prevention and treatment of male reproductive health, adopting a more holistic and sustainable approach. Given the increasing prevalence of lifestyle-related infertility, the present research domain is not only timely but also harbours considerable potential in enhancing the quality of life for individuals grappling with infertility, as evidenced in a recent study [37].
The innovative character of the present studies is evidenced by their exploration of the multifactorial nature of male infertility, with attention not only to well-established factors, including OS and sperm quality, but also to emerging considerations such as the gut microbiota and synergistic dietary interactions. Integrating molecular pathways, such as Nrf2, AMPK/PGC-1α, and NF-κB, with novel approaches that combine antioxidants, probiotics, and adaptogens, provides a holistic and novel framework for addressing male reproductive health. This multidisciplinary approach offers new insights into the prevention and treatment of male infertility, paving the way for more personalised and effective therapeutic strategies.
The objective of this research was threefold: first, to identify reliable biomarkers of OS in seminal fluid; second, to evaluate the efficacy of antioxidant therapies in mitigating OS and restoring fertility, particularly about gut microbiota parameters; and third, to explore the synergistic interplay between gut microbiota modulation and dietary interventions in addressing lifestyle factors, environmental exposures, and oxidative damage to develop comprehensive preventive strategies. A comprehensive literature review was conducted, encompassing several databases, including the Cochrane Library, Medline, Embase, SciSearch, and conference proceedings, along with additional sources, such as PubMed, Web of Science, Scopus, and Google Scholar.
The search criteria encompassed studies that focused on male infertility, OS, sperm quality, the role of gut microbiota, and the relationship between lifestyle factors and male reproductive health. Peer-reviewed scientific journals, including clinical trials, systematic reviews, and meta-analyses, were considered. Search terms included “male infertility”, “oxidative stress”, “sperm quality”, “gut microbiota", and "lifestyle factors and male reproductive health". Studies published between 2014 and 2024 were included to ensure data relevance. Research on molecular mechanisms, dietary interventions, and microbiome-related topics was also included. To enhance the reliability of the results, studies with high methodological quality, such as randomised controlled trials, were given preference. In the course of the present review, some articles were excluded based on specific criteria, including publications not available in full text, not peer-reviewed (conference abstracts, articles from non-peer-reviewed journals), focusing on populations, interventions, or outcomes outside the scope of the study, or based solely on expert opinion without empirical data.
Our review addresses critical gaps in the existing literature by providing a comprehensive and integrated analysis of male reproductive health, focusing on the molecular mechanisms and lifestyle factors that influence sperm quality. A vital gap we address is the interplay between oxidative stress and antioxidant defence mechanisms in male infertility, particularly the role of redox imbalance in sperm DNA fragmentation. By examining key molecular pathways such as Nrf2, AMPK/PGC-1α, and NF-κB, we can better understand how these pathways regulate sperm function and contribute to infertility. While previous studies have examined individual components, our review synthesises these mechanisms into a cohesive framework, providing a more holistic view of the factors involved in male infertility.
In addition, our work provides new perspectives on the therapeutic potential of phytochemicals, micronutrients, and gut microbiota modulation in improving male fertility. While phytomedicinal therapeutics such as ginsenosides and anthocyanins have been studied in isolation, our review highlights the synergistic effects of combining these interventions with dietary approaches to optimise sperm quality. We also explore the under-researched relationship between gut microbiota and male fertility, particularly how gut health may influence reproductive outcomes through mechanisms such as the gut-brain axis and immune system regulation. By highlighting these emerging areas, we provide a new avenue for future research and intervention strategies, filling essential gaps and advancing the understanding of male reproductive health.
The present study analysed synergistic approaches to improving male fertility by integrating gut microbiota modulation and dietary interventions, investigating the combined effects of probiotics, prebiotics, antioxidants, amino acids, omega-3 fatty acids, and adaptogens, to identify effective strategies for the improvement of reproductive health.
Markers for male infertility
As sperm quality has declined worldwide in recent decades, global trends and environmental factors in male fertility represent a critical area of research [38]. A complex interplay of factors, including pollution, endocrine-disrupting chemicals, climate change, and lifestyle changes, has been implicated in this phenomenon [39]. As male fertility is a key indicator of reproductive health and a broader marker of population health and environmental sustainability, it is vital to understand these influences. Developing a comprehensive framework for studying and managing these trends is essential for designing interventions that can reduce risks, improve reproductive outcomes, and inform global health policy, a perspective extensively discussed by Knapke et al [40]..
In the context of the growing interest in male infertility, particular attention has been paid to the analysis of such conditions as oligospermia and asthenozoospermia, such markers as sperm concentration, motility analysis (progressive motility), and semen analysis that are essential to assess sperm quality [41, 42, 43]. These dependencies are shown in Fig.1. Hormonal markers, such as testosterone, follicle-stimulating hormone (FSH), and luteinising hormone (LH), are essential in assessing hormonal imbalances, as shown by Oduwole et al [44].. In cases of azoospermia, genetic testing (e.g., Y chromosome microdeletions) and scrotal ultrasound are commonly used to assess anatomical blockages, as shown in the experiments conducted by Cioppi et al [45].. In addition, reactive oxygen species overload markers, such as ROS and antioxidant levels in seminal plasma, can provide insights into sperm quality. In the context of teratozoospermia, the analysis of sperm morphology (per Kruger's criteria) is imperative for evaluating aberrant sperm morphologies [46, 47, 48].
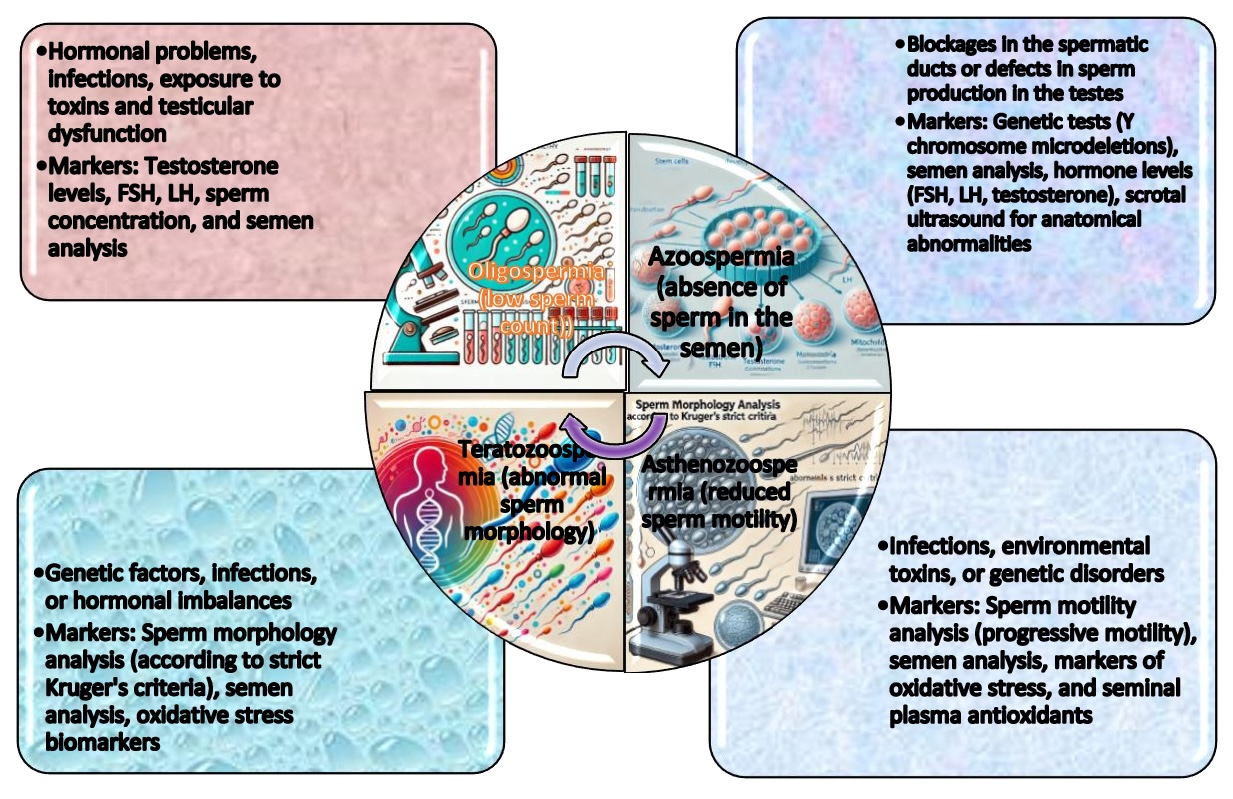
Fig. 1: Sperm quality assessment in such conditions as oligospermia, asthenozoospermia, teratozoospermia, and azoospermia relies on four key markers: sperm concentration, progressive motility, semen analysis, and hormonal evaluation. Abbreviations: FSH – Follicle-Stimulating Hormone; LH – Luteinizing Hormone.
Research in this area has suggested that scrotal and Doppler ultrasound are used to assess varicocele, and semen analysis is performed to determine sperm count and quality [49]. For such conditions as hypo- and hypergonadism, key markers include serum testosterone, FSH, LH, oestradiol, and inhibin B, along with semen analysis to assess sperm production [50]. Kumanov's study [50] indicates that inhibin B is a more accurate marker of fertility status than FSH and LH. Furthermore, inhibin B levels in infertile patients may provide valuable insights into spermatogenesis, potentially serving as a more direct indicator of spermatogenic function than FSH [50].
Findings from prior investigations have indicated that genetic testing (e.g., karyotyping and Y chromosome microdeletion testing) is essential for identifying genetic disorders, such as Klinefelter's syndrome, which may result in infertility [51]. In the context of infection and inflammation, such as epididymitis and prostatitis, prostate-specific antigen (PSA), inflammatory cytokines (IL-6, TNF-alpha), and semen culture have been identified as valuable diagnostic markers [52]. The assessment of environmental and lifestyle factors can be facilitated by the measurement of biomarkers of OS (e.g., malondialdehyde, an end product of lipid peroxidation processes), the analysis of sperm quality, and the testing for toxin exposure (e.g., pesticide or plastic levels), as demonstrated previously [20, 21, 25]. These markers provide crucial insights into the causes of male infertility and help to determine appropriate interventions (Fig. 2).
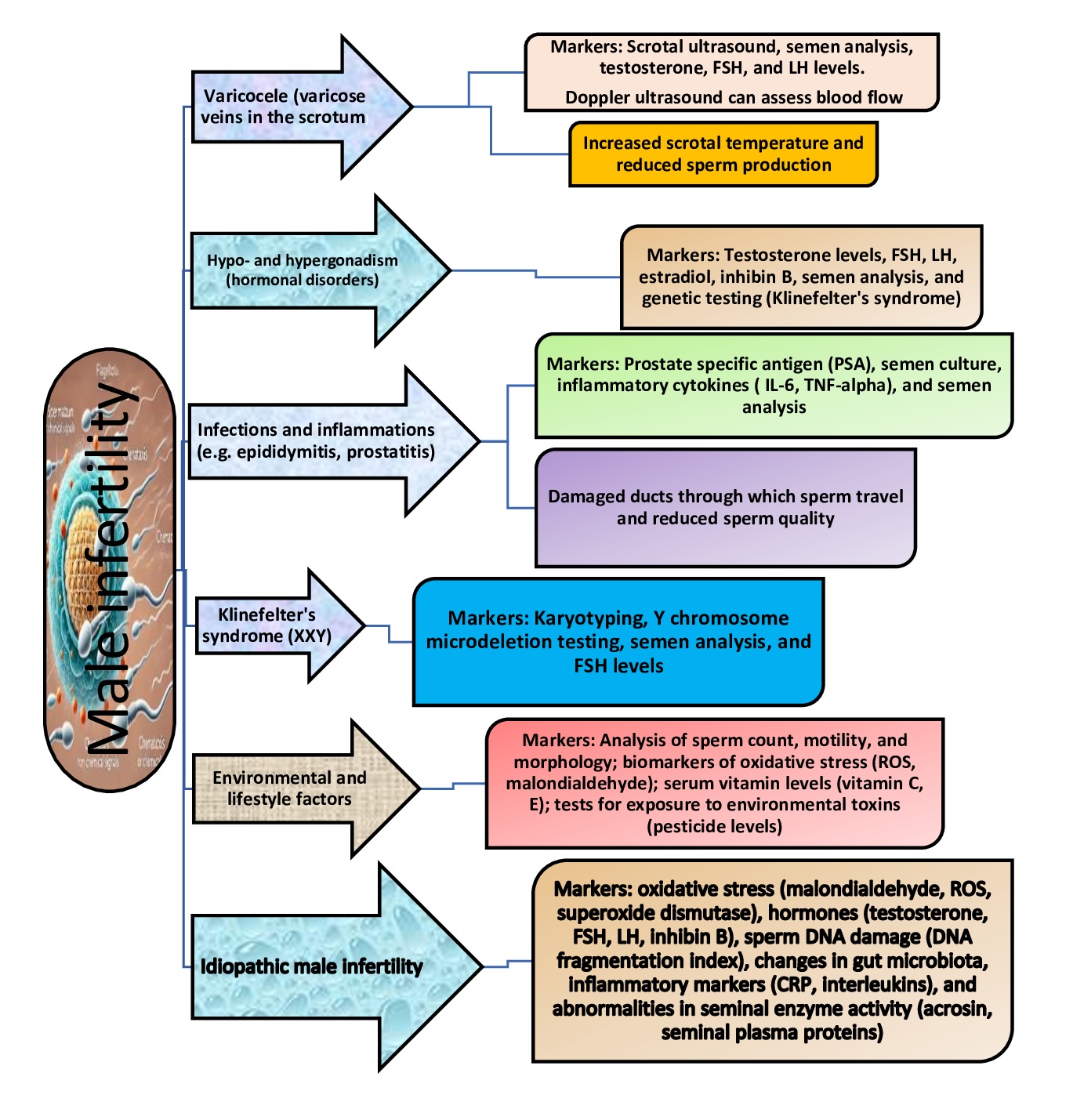
Fig. 2: Common pathological conditions associated with male infertility, conditions and markers. Abbreviations: L-6 - interleukin-6; TNF-alpha - tumour necrosis factor-alpha; FSH - follicle stimulating hormone; ROS - reactive oxygen species; LH - luteinising hormone; CRP - C-reactive protein.
Relationship between lifestyle and sperm quality
The subsequent section delves into lifestyle factors and male reproductive health to further clarify these observations. It was previously understood that sperm quality is a critical determinant of male fertility, and lifestyle factors play a significant role in influencing fertility; numerous studies have demonstrated the profound impact of diet, physical activity, substance use, and environmental exposures on various parameters of sperm health, such as motility, concentration, morphology, and DNA integrity [53, 54]. Understanding these factors provides actionable insights to improve male reproductive health. These dependencies are shown in Fig. 3.
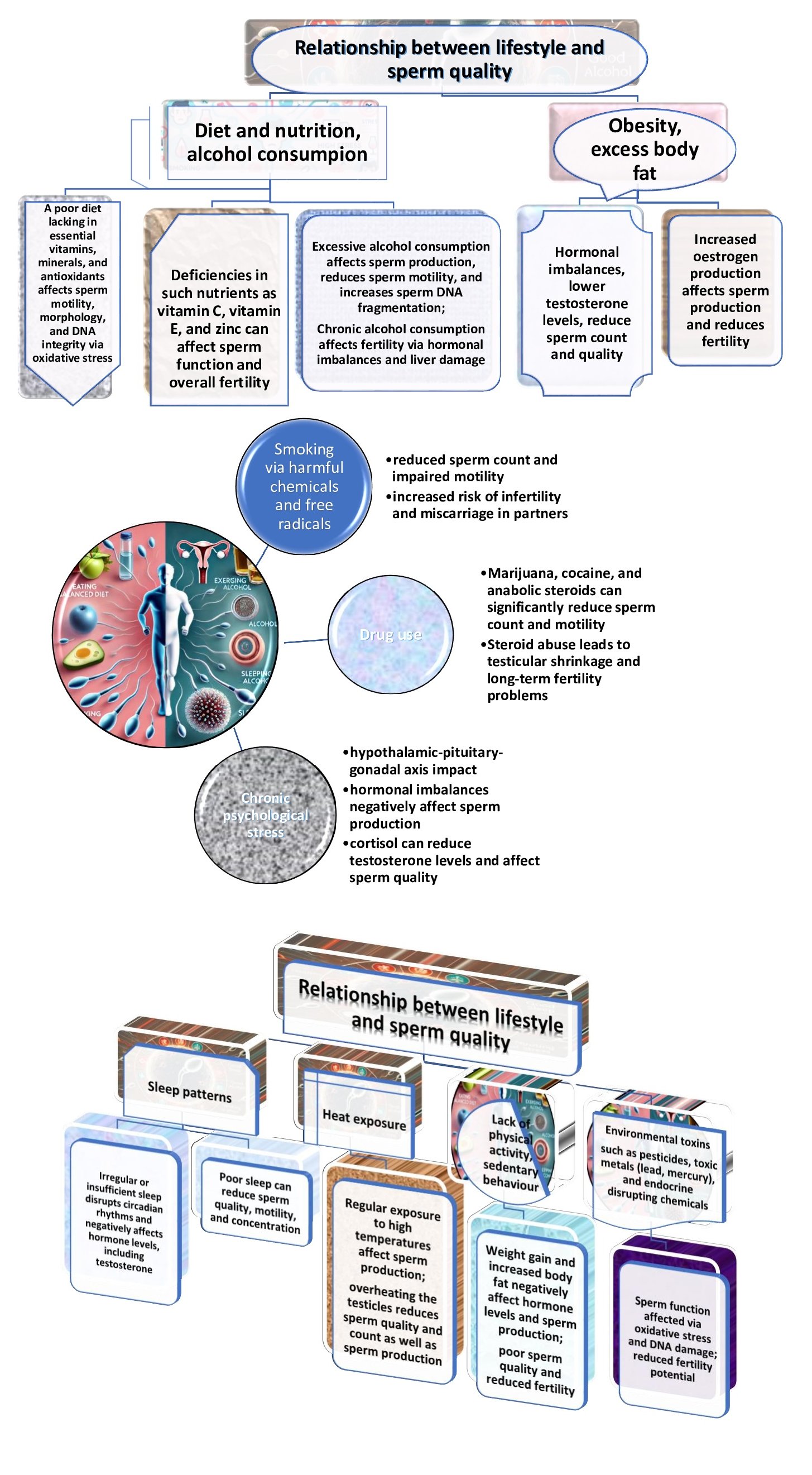
Fig. 3: Factors influencing the relationship between lifestyle and sperm quality and their negative effects.
It has been established that psychological stress and lifestyle factors are strictly related. Indeed, chronic psychological and emotional stress leads to activation of the hypothalamic-pituitary-adrenal (HPA) axis, which increases the production of cortisol [56, 57]. While cortisol is essential for the stress response, it can also increase ROS production. This imbalance leads to oxidative damage to lipids, proteins, and DNA, affecting various cellular functions [58]. In addition, unhealthy lifestyle choices, such as smoking, alcohol consumption, and poor diet, contribute to the generation of ROS [11]. Smoking, for example, introduces free radicals into the body, which directly damage cellular structures, as shown by Messner and Bernhard [58].
Environmental pollutants, such as toxic metals and industrial toxins, have been demonstrated to increase OS by disrupting the body's antioxidant systems [7, 9]. Once absorbed into the body, these toxins have been shown to promote the production of ROS and interfere with the function of antioxidant enzymes [59]. Current findings demonstrate that elevated levels of arsenic, cadmium, and lead in the urine are significantly associated with increased OS markers, including 8-OHdG and 8-isoPGF2α, which correlate with impaired semen quality. Mediation analysis by He et al [59]. revealed that OS markers partially mediate the relationship between exposure to selected heavy metals and reduced sperm motility and morphology, highlighting their potential role as key mediators of environmental toxins.
Another study conducted by Anyanwu and Orisakwe [60] highlights the mechanisms by which toxic metals contribute to male reproductive toxicity, including ion mimicry, disruption of cell signalling pathways, oxidative imbalance, alterations in gene expression, epigenetic regulation, apoptosis, blood-testis barrier disruption, inflammation processes, and endocrine disruption. Their findings also highlight the central role of non-coding RNAs (ncRNAs) in mediating paternal intergenerational epigenetic inheritance, underscoring their functional importance and potential as novel biomarkers in the context of male reproductive toxicity.
Toxic metals contribute to male infertility through a variety of molecular mechanisms. First, ion mimicry occurs when these metals disrupt the balance of essential metal ions in the body, as shown previously [61], interfering with protein folding, enzyme activity, and ion channel regulation critical for spermatogenesis. In addition, toxic metals affect key cell signalling pathways, such as MAPK, Nrf2, NF-kB, and PI3K, by altering kinase activation, receptor binding, and activation of downstream transcription factors, thereby leading to impaired cellular responses vital for reproductive function [62, 63]. In addition, these metals induce oxidative burden by generating ROS that cause lipid peroxidation, DNA damage, and protein oxidation, ultimately triggering apoptosis and cellular senescence [29, 64].
Toxic metals also affect gene expression by interfering with transcription factor binding and epigenetic regulation, including changes in DNA methylation and histone acetylation, leading to altered gene expression profiles critical for reproductive cell function, as shown by [65]. In addition, metals activate mitochondrial pathways and unfolded protein response, inducing apoptotic cell death in germ cells and reducing sperm count and quality, as shown in the experiments carried out by Park and Pang [66]. In addition, toxic metals disrupt the blood-testis barrier by affecting the integrity of tight junctions and adhesion molecules, allowing harmful substances to enter the testes and impair spermatogenesis [67]. Inflammatory pathways, including activation of NF-kB and release of pro-inflammatory cytokines, further exacerbate testicular damage and reduce sperm quality, as shown by Hasan et al [68].. Thus, toxic metals interfere with endocrine regulation by disrupting the hypothalamic-pituitary-gonadal axis, affecting the synthesis, release, and receptor binding of key reproductive hormones, further compromising male fertility.
Research conducted in this area focuses on the interaction between toxic metals (aluminium, boron, cadmium, arsenic), genetic polymorphisms (MTHFRv.C677T (rs1801133) (chromosome-1) and IL-4v.C589T (rs2243250) (chromosome-5)), and oxidative damage (lipoperoxidation level estimated via MDA concentration), which was analysed in men from central Poland. A study conducted by Baszyński et al [69]. highlights that concentrations of toxic metals (aluminium, boron, cadmium, arsenic), lipid peroxidation (malondialdehyde, MDA levels), and genetic polymorphisms (MTHFRv.C677T and IL-4v.C589T) should be measured. The results suggest that, while toxic metals and reactive oxygen species overload influence male reproductive potential, genetic polymorphisms indirectly influence these processes without directly causing fertility disorders. The research also suggests that, while MTHFR and IL-4 polymorphisms indirectly influence metal concentrations and OS biomarkers, cadmium exposure significantly increases the risk of infertility by predisposing men to reproductive disorders. These findings highlight the importance of identifying and mitigating environmental stressors to improve diagnostic accuracy, reduce idiopathic infertility, and develop targeted therapeutic strategies [69].
Another study carried out by Baszyński et al [70]. highlighted the significant impact of environmental factors on male reproductive potential through their influence on OS and antioxidant defence mechanisms, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) as well as the levels of chemical elements (sodium, barium, aluminium, and boron) and the level of malondialdehyde (MDA), a marker of oxidative imbalance. The results showed that environmental exposure to specific elements significantly modulates antioxidant defences, with higher GPx activity observed in infertile men, suggesting an enhanced oxidative response. Furthermore, Na, Ba, Al, and B were identified as key environmental modulators of male fertility in this study, highlighting the importance of mitigating ecological stressors to maintain reproductive health.
A growing body of work has demonstrated that nutrition is an essential factor in preventing male infertility, as it plays a vital role in regulating various physiological processes that impact reproductive health [71]. Many studies demonstrated that a balanced diet rich in antioxidants, vitamins, and essential nutrients supports optimal sperm quality, i.e., such antioxidants as vitamin C, vitamin E, zinc, and selenium combat oxidative imbalance, which is a major cause of DNA damage in sperm [29, 72]. Diets high in processed foods, trans fats, and sugars have been linked to reduced sperm motility and concentration [73]. Recent research suggests that the Mediterranean diet, which incorporates the traditional healthy lifestyles of people living around the Mediterranean Sea, has many health benefits, including improvement of male reproductive health by increasing the number and quality of sperm, because omega-3 fatty acids and a plant-based diet rich in polyphenols may have a protective effect on sperm quality, as previously indicated by Montano et al [74]..
The study highlights the evidence provided by the authors, showing that moderate physical activity positively affects sperm parameters by improving hormonal balance, reducing redox imbalance, and improving blood flow to the testes [75]. However, excessive physical activity, especially endurance exercise, can have an adverse effect due to increased reactive oxygen species overload and hormonal imbalances, such as reduced testosterone levels, and can provide compelling evidence for the significance of this phenomenon [76]. Conversely, a sedentary lifestyle and obesity are associated with decreased sperm concentration and increased DNA fragmentation [77].
As Zhang et al [78]. demonstrated, the core action pathways emphasise stress and mental health in relation to sperm quality. Indeed, chronic stress has been shown to increase cortisol levels, which can in turn suppress testosterone production and impair spermatogenesis [79]. Furthermore, mental stress is associated with increased oxidative damage, which further exacerbates sperm damage [80]. However, mindfulness and stress management have shown promise in mitigating these effects. The results reported by Kim et al [81]. and Potter et al [82]. demonstrate that sleep and circadian rhythms are significant factors since inadequate sleep quality and disrupted circadian rhythms harm reproductive hormones, consequently leading to diminished sperm quality. Furthermore, a study by Lateef and Akintubosun [83] indicated that sleep deprivation is associated with reduced testosterone levels and augmented oxidative damage in sperm cells.
Therefore, a healthy lifestyle is essential for maintaining optimal sperm quality and overall reproductive health. Addressing modifiable factors, such as diet, physical activity, substance use, and environmental exposures, offers a practical approach to reducing the risk of infertility and improving outcomes with assisted reproductive technologies. However, relevant studies were unable to establish any definitive positive or negative correlations between a vegetarian diet and semen quality, sex hormone levels, and infertility [84].
In their study, Karayiannis et al [85]. examined the association between adherence to the Mediterranean diet (MD) and semen quality in men from subfertile couples seeking fertility treatment. The findings indicated that higher adherence to the MD was significantly associated with improved semen parameters, including sperm concentration, total sperm count, and sperm motility. Specifically, men in the highest tertile of the MD score exhibited a significantly lower prevalence of abnormal sperm characteristics, such as lower sperm concentration and motility, than those in the lowest tertile. However, the study's cross-sectional design precludes the ability to establish causality between MD adherence and semen quality. Nevertheless, the results are consistent with previous research suggesting that dietary patterns rich in fruits, vegetables, legumes, and whole grains, characteristic of the MD, may positively influence semen quality [85]. Findings reported by another scientific team [86] imply a potentially beneficial effect of traditional Mediterranean dietary patterns on male reproductive potential.
Thus, preventing and treating ROS overload and antioxidant deficiency requires a multifaceted approach that includes lifestyle modifications, environmental management, and dietary interventions. Addressing the causes of ROS production and boosting the body’s antioxidant capabilities are key steps towards reducing the harmful effects of OS. Targeting these mechanisms makes it possible to mitigate the detrimental effects of oxidative damage on male reproductive health and overall well-being.
Stress reaction to testicular hyperthermia
In the following section, the application of these insights to the realm of thermal and environmental stressors impacting sperm integrity is examined. Research in this field has indicated a critical interdependence between hyperthermia and temperature regulation in human health. Specifically, Durairajanayagam et al [87]. and Mieusset and Bujan [88] have demonstrated that testicular function is maintained at approximately 2°C lower than the body's core temperature. Furthermore, elevated scrotal temperatures resulting from such factors as physical inactivity, obesity, occupational heat exposure, and laptop use have been shown to exert several detrimental effects on testicular health [89]. These include germ cell apoptosis and autophagy, DNA damage, testicular atrophy, spermatogenic arrest, reduced inhibin B levels, and increased production of reactive oxygen species, all contributing to impaired male reproductive function. Specifically, elevated scrotal temperatures, which are defined as temperatures higher than the body's core temperature, can increase ROS production [90].
As demonstrated in studies conducted by Jeng et al [91, 92, 93]., individuals occupationally exposed to polycyclic aromatic hydrocarbons exhibited increased bulky DNA adducts and 8-oxo-dGuo levels, suggesting DNA damage. Additionally, the study indicates that oxidative imbalance, possibly exacerbated by thermal stress, contributes to sperm DNA fragmentation. It is crucial to note that the presence of bulky DNA adducts does not necessarily indicate the existence of oxidative damage and fragmentation. This underscores the deleterious impact of thermal and environmental stressors on maintaining the integrity of spermatozoa.
Findings from prior investigations have indicated that the scrotum, being outside the body cavity, is highly sensitive to temperature changes and prolonged exposure to heat (through such activities as hot baths, saunas, and prolonged sitting), which can increase OS, especially in testicular cells [94]. It was shown that hyperthermia inhibits spermatogenesis (sperm production) and can also cause lipid peroxidation, which disrupts sperm membrane integrity and motility [95]. Paul et al [90]. investigated the stress response in mouse testes following a mild transient scrotal heat exposure (40°C or 42°C for 30 minutes). The results showed an increase in the expression of hypoxia-inducible factor 1 alpha (Hif1a) mRNA and translocation of HIF1A protein to the germ cell nucleus, indicating hypoxic stress. In addition, upregulation of haem oxygenase 1 (Hmox1) and antioxidant enzymes, including glutathione peroxidase 1 (GPX1) and glutathione S-transferase alpha (GSTA), confirmed the activation of redox dysfunction pathways. Germ cell death, as evidenced by increased expression of cleaved caspase-3 and decreased levels of caspase-activated DNase inhibitor (ICAD), was associated with DNA fragmentation observed by TUNEL staining. These findings confirm that transient mild testicular hyperthermia induces temperature-dependent germ cell death and a complex stress response involving hypoxia and redox dysfunction pathways [90, 94].
Next, elevated temperatures affect mitochondrial function in sperm cells, which is essential for ATP production, leading to the generation of greater amounts of ROS. In a study conducted by Kopalli et al [96]., it was demonstrated that Panax ginseng Meyer, more commonly known as Korean Red Ginseng (KRG), is a traditional herb that has been used to enhance libido and male fertility. The study evaluated the effects of an Rg3-enriched KRG extract (KGC04P) on heat stress-induced testicular damage in rats. The results significantly improved parameters, including sperm kinematics and testicular enzyme expression. The findings suggest that KGC04P can effectively prevent heat stress-induced damage, offering potential as a therapeutic agent for hyperthermia-related male infertility [96].
Physiological role of reactive oxygen species in spermatogenesis
A study conducted by Dutta et al [97]. demonstrated that ROS play a critical role in sperm capacitation, maturation, hyperactivation, acrosome reaction, and fertilisation. The authors elucidated that, in physiological conditions, ROS are indispensable for regulating cellular signalling and activating molecular mechanisms that support the proper function and reproductive capacity of sperm [8]. Spermatozoa generate ROS via two primary mechanisms: the plasma membrane and the mitochondria [98]. Aitken [99] demonstrated that reactive oxygen species overload is a critical factor in the life and function of mammalian spermatozoa, with ROS generated in three ways involving sperm mitochondria, cytosolic L-amino acid oxidases, and plasma membrane NADPH oxidases. These ROS have been shown to drive physiological changes during sperm capacitation by activating the cAMP/PKA phosphorylation cascade, upregulating tyrosine phosphorylation in the sperm tail, and inducing sterol oxidation, all of which are essential for sperm function [99].
Research conducted in this area has suggested that mitochondrial respiration is the primary biological source of ROS in normal physiological conditions [100]. However, mitochondrial dysfunction can significantly exacerbate electron leakage within the electron transport chain, leading to overproduction of ROS that can reach toxic levels, as shown by Koppers et al [101].. This disrupts redox homeostasis and triggers oxidative damage. High levels of ROS can induce lipid peroxidation, resulting in the formation of reactive aldehydes that further propagate cellular damage. At the plasma membrane, ROS are generated via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system [102]. In mitochondria, ROS generation occurs predominantly through NAD-dependent redox reactions, driven by the high metabolic activity of these organelles. Thus, mitochondrial functioning plays a critical role in energy production for sperm motility, making them a significant site of ROS generation [103].
Studies conducted by Perl et al [104]. and Perl [105] emphasise the pivotal function of transaldolase (TAL) in signalling pathways that are indispensable for sperm function. In particular, it was highlighted that the pentose phosphate pathway plays a role in male sterility, and TAL is particularly important in this regard. Earlier research by the same authors had already demonstrated that TAL deficiency disrupts the balance of NAD(P)H and reactive oxygen intermediates, resulting in mitochondrial dysfunction in sperm cells. This structural and functional damage ultimately leads to male infertility [105]. TAL deficiency (TAL⁻/⁻) in animals has been shown to disrupt the mitochondrial transmembrane potential by impairing the redox balance involving reactive oxygen intermediates, NADPH, NADH, and glutathione (GSH). This results in mitochondrial dysfunction, characterised by diminished Ca2+ flux, reduced mitochondrial ROS production, and intracellular acidosis, all contributing to the impairment of forward motility and fertility. Furthermore, compensatory changes in signalling molecules, such as the downregulation of carbonic anhydrase IV and the upregulation of CD38 and gamma-glutamyl transferase, further reflect the disruption of key metabolic and signalling pathways. Microarray analysis has revealed the predominant impact of TAL deficiency on the latter stages of sperm cell development, affecting the electron transport chain and GSH metabolism, thereby underscoring the essential role of TAL in maintaining mitochondrial function and critical sperm signalling pathways for fertility [104].
Building on prior research, Sotolongo and Ward [106] provided evidence that spermatozoa reach the epididymis and contain a less compact DNA strand, making them inert and unable to perform their functions effectively. However, under the influence of hydrogen peroxide, nuclear proteins are oxidised, leading to DNA condensation, making the sperm more motile; this aligns with the conclusions reached by other authors [107, 108]. It is widely acknowledged that this process is essential for sperm to swim towards the egg [109] and penetrate the egg [110]. Notably, hydrogen peroxide at low concentrations also induces tyrosine phosphorylation, which increases the area on the sperm membrane that interacts with the egg, ultimately increasing the chances of fertilisation; this is essential for sperm to capacitate and successfully fertilise an egg [111, 112].
There is also evidence that hydrogen peroxide is necessary for the acrosomal reaction, although the exact mechanism by which this ROS influences this process remains unclear [109]. The acrosomal reaction is a crucial event in fertilisation, as it allows the sperm to penetrate the egg's outer layers. While ROS, including hydrogen peroxide, can play critical physiological roles, their accumulation must be tightly regulated, as excessive levels of ROS can damage sperm and other cells.
It is imperative to comprehend the intricate interplay between redox imbalance and antioxidant defence systems in a physiological milieu that fosters normal spermatogenesis. The importance of such research stems from its ability to show that seminal plasma and sperm exhibit a notable abundance of protective antioxidants [113, 114]. These antioxidants are instrumental in ensuring that ROS, indispensable for crucial functions, such as antimicrobial defence and intracellular signalling, do not inflict harm upon the body's cells. Antioxidants, e.g., SOD, catalase, and glutathione peroxidase, neutralise excess ROS, thereby preventing oxidative damage to sperm DNA and membrane integrity [115]. This balance is essential for preserving sperm quality and function and maintaining male fertility [116].
It has been established in the scientific literature that both spermatozoa and seminal plasma contain natural antioxidants that protect against OS, particularly after testicular production, which is crucial for maintaining sperm quality [15]. Several high-molecular-weight enzymatic antioxidants in seminal plasma are fascinating, as they are essential in neutralising ROS [116]. This field of research provides valuable insights and shows that, during spermatogenesis and epididymal maturation, spermatozoa acquire key antioxidant enzymes, including SOD, catalase, GPx, peroxiredoxins, glutathione-S-transferases, thioredoxins, and thioredoxin reductase, working collectively to prevent oxidative damage [79]. The absence of any of these enzymes, as evidenced by studies utilising knockout models, has been demonstrated to have a detrimental effect on sperm quality, resulting in compromised motility, fertilisation ability, and DNA integrity due to increased redox imbalance [79]. Another vital aspect is connected with the activity of SOD, which catalyzes the conversion of superoxide anion into less harmful compounds, while catalase functions by breaking down hydrogen peroxide into water and oxygen. Notably, the seminal plasma and the sperm itself contain SOD, with the former protecting the sperm from oxidative damage [99], thus illustrating its crucial role in safeguarding sperm from the potentially deleterious effects of OS [79].
As a vital component of a protective antioxidant triad, GPx plays a pivotal role in preventing oxidative damage to sperm DNA and membranes [118, 115] through interaction with hydroperoxides using glutathione as an electron donor and reduction of peroxides using glutathione as a substrate. A seminal study conducted by this scientific team underscored the pivotal function of selenium-containing GPxs, particularly GPx4, in preserving male fertility. This is achieved by forming the mitochondrial sheath of spermatozoa, which is imperative for their structure and function. Furthermore, GPx4 has been identified as a pivotal regulator of oxidative imbalance, functioning by reducing lipid hydroperoxides and silencing lipoxygenases. This process is vital in preventing oxidative damage, which has the potential to compromise sperm integrity and viability, as shown in the experiments carried out by [118].
Seminal plasma contains non-enzymatic antioxidants as well as enzymatic antioxidants [119]. Examples include ascorbic acid (vitamin C), alpha-tocopherol (vitamin E), pyruvate, glutathione, L-carnitine, taurine, and hypotaurine. These non-enzymatic antioxidants directly affect free radicals, scavenging them and preventing cell damage. For instance, ascorbic acid is a highly effective free radical scavenger, capable of directly neutralising ROS. At the same time, alpha-tocopherol is a potent antioxidant that protects the sperm membrane from lipid peroxidation. L-carnitine and taurine maintain sperm motility and function by stabilising the mitochondrial membrane and protecting against oxidative damage [120]. In addition, seminal plasma contains other antioxidant components, including urate, pyruvate, albumin, β-carotene, and ubiquinol [121]. Urate, for example, acts as a powerful antioxidant, scavenging peroxynitrite and other reactive species [122]. Pyruvate helps maintain mitochondrial function by buffering excess ROS production. Albumin has also been demonstrated to bind and neutralise potentially damaging free radicals, while the role of β-carotene and ubiquinol in the overall antioxidant defence system is to protect against lipid peroxidation and antioxidant deficiency [123, 124].
The antioxidant capacity of seminal plasma has been shown to be significantly greater than that of blood plasma [125], being approximately ten times higher. In a study employing the Total Radical Trapping Antioxidant Potential (TRAP) method, Rhemrev and colleagues demonstrated that the antioxidant properties of seminal plasma are primarily attributed to the presence of vitamin C, uric acid, and tyrosine, which together protect sperm cells from oxidative damage. While other known antioxidants, such as glutathione and taurine, demonstrate some antioxidant activity, their contribution is limited due to their relatively low concentrations [125]. These findings suggest that the fast and slow TRAPs are valuable infertility biomarkers and potential targets for antioxidant-based therapeutic interventions. However, it is essential to note that, during spermatogenesis and in the epididymis, spermatozoa are not directly exposed to the antioxidants present in the seminal plasma [79]. Instead, protection against oxidative damage is provided by the antioxidants produced in the testes and epididymis and by the intrinsic antioxidant capacity of the sperm. Consequently, spermatozoa exhibit heightened vulnerability to OS as they traverse the epididymis, particularly in inflammatory conditions.
Another study [126] demonstrated that tyrosine, with its strong antioxidant capacity and high plasma concentration, plays a significant role in the total antioxidant capacity of seminal plasma. The post-addition method using ABTS radical scavenging revealed a distinctive antioxidant profile in seminal plasma, which quenches radicals continuously and slowly. Some compounds, such as ascorbic acid, alpha-tocopherol, and uric acid, have been shown to exhibit rapid radical scavenging properties. In contrast, others, including hypotaurine and tyrosine, have contributed to the same slow radical scavenging pattern as in the case of seminal plasma, as demonstrated by Pérez-Pé et al [127]..
Thus, spermatozoa rely on a delicate balance of enzymatic and non-enzymatic antioxidants to protect them from oxidative damage. Depletion or dysfunction of these antioxidant systems can lead to irreversible damage to sperm, including chromosome fragmentation and loss of fertility. The complex interplay of these antioxidants highlights the importance of managing redox imbalance in maintaining male fertility and reproductive health.
Implications of ROS production and male fertility
It is well-documented in the literature that, in men, controlled production of ROS plays a critical role in essential reproductive processes, such as sperm hyperactivation, capacitation, acrosome reaction, and natural fertilisation, as revealed earlier by Aitken [99] and Aitken et al [100].. Spermatozoa exhibit heightened vulnerability to redox imbalance, as evidenced by the capacity of ROS to inflict damage to the sperm membrane and DNA, culminating in lipid peroxidation and sperm DNA fragmentation (SDF), as elucidated in their studies by Cannarella et al [128]. and Jeng et al [92].. These functions rely on moderate levels of ROS to facilitate key signalling pathways required for successful reproduction. However, excessive ROS production under OS, particularly by immature germ cells and leukocytes, has detrimental effects. These include increased lipid peroxidation, reduced sperm motility, and cellular damage, all of which contribute to male infertility. The imbalance between ROS production and antioxidant defences is a central mechanism in the pathogenesis of OS-induced reproductive dysfunction [96, 101].
ROS induce oxidative DNA damage in sperm through single- and double-strand breaks and base modifications, such as 8-hydroxy-2’-deoxyguanosine (8-OHdG), as demonstrated by Jeng et al [92].. These alterations compromise genetic integrity, potentially affecting fertilisation outcomes and increasing the risk of inherited defects. A study carried out by authors [92] found that coke oven workers exposed to polycyclic aromatic hydrocarbons (PAHs) showed a significant increase in bulky DNA adducts and 8-oxo-dGuo, compared to the control group, indicating DNA damage. However, no significant differences were observed in sperm DNA fragmentation or denaturation between the exposed and control groups, despite a positive correlation between 8-oxo-dGuo levels and DNA fragmentation. The findings of this study suggest that reactive oxygen species overload may contribute to sperm DNA fragmentation. In contrast, bulky DNA adducts appear independent of oxidative DNA damage and fragmentation in sperm integrity [93].
Lipid peroxidation, another critical process, targets the polyunsaturated fatty acids (PUFAs) of the sperm membrane, resulting in MDA production [25]. This process leads to membrane remodelling, dissipation of mitochondrial membrane potential, electron leakage via increased ROS production and reduced energy production, accumulation of cytotoxic byproducts like 4-hydroxynonenal, dysregulation of bioenergetic pathways, and disruption of structural and signalling components of the motility apparatus, all of which contribute to sperm dysfunction and genomic lesions. This toxic byproduct also weakens membrane integrity, reduces fluidity, and impairs sperm motility, which is critical for successful fertilisation [116]. In addition, ROS-induced mitochondrial dysfunction exacerbates oxidative damage and disrupts ATP production, which is essential for sperm energy and motility [129].
ROS are integral to sperm function via redox signalling, which modulates protein phosphorylation critical for capacitation and the acrosome reaction. The mechanisms described above are consistent with the findings reported by de Lamirande and O'Flaherty [130], who demonstrated that ROS, such as superoxide anion, hydrogen peroxide, and nitric oxide, act as second messengers regulating sperm capacitation, and this process is essential for sperm-oocyte interaction, acrosome reaction, and fertilisation. Their research highlighted that ROS modulate key signalling events during capacitation, including the increase in cAMP, PKA activation, phosphorylation of specific substrates, MEK-like protein phosphorylation, and late tyrosine phosphorylation of fibrous sheath proteins, with these processes being regulated by various kinases, including protein kinase C, PKA, and protein tyrosine kinases. Furthermore, the role of ROS in regulating sperm motility and the acrosome reaction was elucidated, with these effects likely being achieved through sulfhydryl/disulfide interactions on sperm proteins. The proper timing and function of spermatozoa is ensured by redundancies and cross-talk [130].
Phosphorylation of tyrosine residues is a redox-sensitive process for sperm to acquire fertilising capacity. One of the critical molecular mechanisms is the effect of OS on Leydig cells, which are responsible for testosterone production. OS can disrupt the steroidogenic pathway in these cells by inhibiting enzymes responsible for testosterone synthesis, such as 17β-hydroxysteroid dehydrogenase and cytochrome P450 side chain cleavage enzyme (P450scc), as demonstrated by Miller and Auchus [131]. This results in reduced testosterone levels, directly affecting sperm production and motility. In addition, a decrease in testosterone levels further exacerbates infertility by impairing spermatogenesis [4]. A comprehensive elucidation of the mechanisms of steroidogenesis is imperative for comprehending a broad spectrum of physiological functions, as it governs the synthesis of biologically active steroid hormones indispensable for sexual differentiation, reproduction, fertility, and overall homeostasis. A more profound comprehension of the enzymes, cofactors, and genetic factors implicated in steroidogenesis provides valuable insights into various disorders, including reproduction, metabolic conditions such as obesity, and hypertension [131].
In obese men, elevated ROS levels also cause damage to sperm DNA, which can significantly reduce sperm fertility. ROS-induced DNA damage can lead to mutations, chromosome fragmentation, and overall loss of sperm quality, making it difficult to achieve a successful pregnancy [26, 29, 30]. Furthermore, the adverse effects of obesity on sperm DNA integrity have been shown to reduce the success rates of assisted reproductive technologies, such as in vitro fertilisation, where sperm quality is critical for achieving fertilisation and subsequent embryo development [132].
Infertile men often have reduced activity of enzymatic antioxidants, such as GPx, SOD, and catalase (CAT). The depletion of non-enzymatic antioxidants, including reduced GSH, ascorbic acid (vitamin C), and tocopherol (vitamin E), further exacerbates oxidative damage. As demonstrated by Kopalli et al [96]., exposure to heat stress elicits substantial alterations in diverse parameters, encompassing body and organ weight, sperm motility, and lipid metabolism markers in both serum and testicular tissue. Concurrently, this exposure affects the expression of testicular antioxidant enzymes, inflammatory cytokines, sex hormone receptors, and genes associated with spermatogenesis [91-93]. Together, these deficiencies weaken the ability of sperm to maintain redox balance, resulting in increased susceptibility to oxidative damage. Therapeutic approaches with antioxidants are an essential way to combat male infertility. Therefore, given the role of ROS in sperm dysfunction, antioxidant therapy offers a promising approach to the treatment of male infertility. Antioxidants work by neutralising ROS, increasing the activity of enzymatic defence systems, stabilising sperm membranes, and protecting DNA from oxidative damage. Supplementation with key antioxidants, such as vitamins C and E, selenium, and coenzyme Q10, can help restore redox balance, reduce lipid peroxidation, and improve sperm quality. By lowering oxidative imbalance, these interventions improve fertility potential and the likelihood of successful conception [121, 123, 124].
Endogenous and exogenous sources of ROS in the male reproductive system
The primary ROS in human spermatozoa is superoxide anion (O2-), which can undergo enzymatic or spontaneous dismutation to form hydrogen peroxide (H2O2) [133]. In the presence of transition metals, such as iron or copper, H2O2 can participate in Fenton or Haber-Weiss reactions, forming the highly reactive hydroxyl radical (OH-). These hydroxyl radicals are among the most destructive ROS, initiating lipid peroxidation (LPO) cascades [134]. LPO significantly compromises the integrity and fluidity of the sperm plasma membrane, affecting critical functions, such as motility, capacitation, and the acrosome reaction [135, 136].
In a study conducted by Gil-Guzmán et al [137]., significant variations in ROS production among subsets of spermatozoa at different stages of maturation were observed, and it was hypothesised that oxidative damage to mature spermatozoa by ROS-producing immature spermatozoa during their migration from the seminiferous tubules to the epididymis may be a significant cause of male infertility. In addition to membrane damage, ROS also causes oxidative DNA damage, contributing to fragmentation in sperm nuclei [138]. A study conducted by authors [138] demonstrated that the chromosomal architecture within the mouse sperm nucleus exhibits a distinct and organised arrangement, where certain chromosomes preferentially occupy specific positions. Employing a combination of the fluorescence in situ hybridisation method, confocal microscopy, and three-dimensional reconstruction techniques, the authors conclusively demonstrated that chromosome positioning is non-random. Their investigation of a transgenic mouse model (Gpx5-/-) revealed that oxidative DNA damage does not disrupt the organization of chromosomes but significantly alters specific 3D nuclear parameters. The findings of this study suggest that oxidative DNA damage, which was previously underappreciated, may have a profound impact on chromatin quality and potentially influence post-fertilisation processes [138].
As Cassina et al [8]. suggested, mitochondria are likely the primary sources of ROS in sperm cells. Their study employed high-resolution respirometry, ROS production analysis, and the evaluation of oxidative and nitrative stress markers in intact human sperm cells. The findings demonstrated a correlation between mitochondrial dysfunction, as indicated by a reduced respiratory control ratio, and decreased sperm motility. ROS also cause oxidative DNA damage that can compromise genetic integrity and reduce fertilisation potential. Furthermore, mitochondrial dysfunction exacerbates ROS production, creating a vicious cycle that amplifies oxidative burden. This cascade disrupts the electron transport chain, further increasing ROS generation and impairing ATP production, which is essential for sperm motility, as demonstrated earlier by Koppers et al [101]..
The primary endogenous sources of ROS in the male reproductive system are leukocytes and immature spermatozoa. This is confirmed by the direct correlation between the production of ROS and cytokines in seminal fluid, such as interleukin IL-6, IL-8, and tumour necrosis factor (TNF), as shown by Jiang et al [139]. and Collodel et al [140].. Among leukocytes, polymorphonuclear neutrophils and macrophages are the major contributors to ROS production [141], particularly during infections or inflammatory processes in the prostate and seminal vesicles. A study by Rosales [142] highlights neutrophils' diverse and critical roles, i.e., the most abundant leukocytes in circulation. These cells are rapidly mobilised to sites of inflammation or infection, where they perform essential antimicrobial functions, including degranulation, ROS production, phagocytosis, and the formation of neutrophil extracellular traps. While the traditional view regards these cells as short-lived cells primarily tasked with pathogen elimination, recent findings have revealed their capacity to modulate adaptive immune responses through cytokine production and interactions with lymphocytes and dendritic cells, as shown earlier by the author [142].
Furthermore, neutrophil heterogeneity, with distinct functional phenotypes, has been observed in pathological conditions, such as cancer and chronic inflammation. These findings align with studies on sperm cells, where ROS also play a dual role, being crucial for physiological processes yet harmful when produced excessively, contributing to cellular dysfunction and pathologies [142]. In these conditions, leukocytes can produce up to 100 times more ROS than normally as part of the immune defence response [143]. This increased ROS production is exacerbated by activation of the NADPH oxidase system and increased NADPH levels through the hexose monophosphate shunt, which supports ROS generation [144].
In addition to leukocytes, immature spermatozoa contribute to ROS production [145], which is essential for sperm maturation. However, excessive ROS in spermatozoa can lead to oxidative damage, particularly to the sperm plasma membrane, DNA, and mitochondria, impairing sperm motility and fertilisation potential [137]. Furthermore, inflammation in the reproductive tract increases pro-inflammatory mediators, such as cytokines and prostaglandins, while simultaneously depleting antioxidant defences, as shown by the authors [140] in a rabbit model.
In summary, the impaired function of antioxidant systems in the context of chronic inflammation is a crucial factor in the maintenance of a persistent OS environment. This imbalance between the production of ROS and antioxidant defence mechanisms can severely impact male fertility, leading to disruptions in sperm motility, DNA integrity, and overall reproductive health. A deeper understanding of the endogenous sources of ROS emphasises the necessity of managing inflammation effectively and maintaining optimal antioxidant status in the male reproductive system. Therefore, interventions such as antioxidant supplementation and regulation of inflammatory processes may prove vital in mitigating the adverse effects of ROS on male fertility and reproductive health.
Role of sperm DNA fragmentation in male infertility
As demonstrated in a seminal study by Krausz et al [146]., a significant proportion of the male population, approximately 7%, is afflicted by male infertility. The condition, marked by its intricate genetic underpinnings, encompasses considerable heterogeneity in semen parameters and testicular histological phenotypes, with at least 2, 000 genes implicated in the complicated process of spermatogenesis. As highlighted by authors [147], the most prevalent genetic factors contributing to male infertility, amounting to 25% of cases, are observed in azoospermia. Baszyński et al [69]. emphasise that male infertility is a complex multifactorial condition influenced by both environmental and genetic factors, accounting for 20-50% of infertility cases worldwide. Nevertheless, the identification of genetic abnormalities in other semen parameters and aetiological categories continues to increase. The findings of these studies emphasise the increasing complexity of male infertility, with the continued discovery of genetic variations playing a crucial role in its diagnosis and treatment.
The role of the process of sperm DNA fragmentation (SDF) in the development of male infertility is well documented in the research literature [148, 149]. DNA fragmentation represents the final stage of intracellular changes leading to apoptosis, a programmed cell death process primarily responsible for eliminating damaged cells [150]. A seminal study conducted by Collodel et al [140]. and Jiang et al [139]. demonstrated that OS is a major trigger of this process. SDF is increasingly being recognised as a critical factor in male infertility [151]. SDF represents the culmination of intracellular damage that precedes apoptosis, a biological process essential for the elimination of defective cells [148], and OS is a primary driver of this programmed cell death. Elevated ROS levels in sperm cells initiate oxidative damage that disrupts DNA integrity and triggers molecular pathways that lead to fragmentation. Muratori et al [152]. provided valuable insights into the mechanisms underlying SDF, highlighting apoptosis as the primary pathway responsible for DNA breaks in sperm cells. The findings indicate that defects in chromatin maturation, combined with redox dysfunction, contribute significantly to the fragmentation process, particularly during sperm transit through the male genital tract. The results of this study are of significant clinical importance, as they contribute to a more comprehensive understanding of the effects of drugs and OS on SDF in infertile men, and they inform the development of new therapeutic strategies that target these mechanisms [152].
SDF leads to intracellular damage and ultimately triggers apoptosis in sperm cells. ROS, particularly hydroxyl radicals, induce DNA breaks by oxidising nucleotide bases, resulting in single-strand and double-strand breaks, as Cannan and Pederson show [153]. Their study demonstrated that exposure to ionising radiation can induce double-strand breaks (DSBs) in DNA, and such DSBs may also arise when replication forks encounter DNA lesions or repair intermediates. Namely, the processing and repair of these breaks can lead to mutations. Such alterations can potentially result in cell death or cancer, with the most common repair pathway in metazoans, non-homologous DNA end joining, being more mutagenic than homologous recombination-mediated repair [153]. As emphasised by Dianov and Hübscher [154], base excision repair (BER) is paramount in maintaining genome integrity, a concept directly relevant to sperm DNA repair processes. BER plays a crucial role in mitigating DNA damage caused by endogenous and exogenous mutagens, ensuring the maintenance of sperm quality and preventing premature aging, cancer, and infertility. A study by [154] highlights the necessity of precise coordination between BER and cell cycle progression to avoid the replication of damaged DNA, a process particularly significant in sperm cells, where reactive oxygen species overload and DNA fragmentation are prevalent.
As demonstrated by Stinson and Loparo [155], non-homologous DNA end joining (NHEJ) has been identified as the predominant repair pathway for DSBs in mammalian cells during the cell cycle. This repair pathway is particularly crucial in the S and G2 phases when DNA replication is active and homologous recombination is less feasible due to the absence of an undamaged template. It is therefore vital to understand the role of NHEJ in maintaining genomic stability by facilitating the rapid repair of DSBs, a function that 8-oxo-7, 8-dihydroguanine, especially in such cells as sperm, is especially important in such cells as sperm, which are highly susceptible to redox imbalance and DNA damage [155].
Redox imbalance also forms a specific DNA lesion that leads to mutagenesis, as Fleming and Burrows show [156]. Their study highlights the mutagenic potential of oxidative DNA damage, particularly the formation of 8-oxo-7, 8-dihydroguanine, which can lead to G→T transversion mutations, a process relevant to sperm cells exposed to oxidative imbalance. In sperm, oxidative modifications of DNA, including OG formation, can result in polymerase misreading, leading to mutations that affect sperm DNA integrity and contribute to infertility. Additionally, the involvement of base excision repair in removing 8-oxo-7, 8-dihydroguanine and its potential regulatory role in gene activation underscores the complex balance between DNA repair and the epigenetic functions of oxidative DNA damage in maintaining reproductive health. As a result of this damage, chromatin remodelling is impaired, and incomplete protamination of sperm DNA increases susceptibility to oxidative damage, making DNA more prone to fragmentation [148, 154].
Redox dysfunction has been demonstrated to activate intrinsic apoptotic pathways in sperm cells, as evidenced by experimental studies [150]. This process is characterised by the release of cytochrome c from the mitochondria and the subsequent activation of caspases (particularly caspase-3 and caspase-9). Another study [157] has shown that the process of apoptosis induced by the administration of staurosporine leads to the fragmentation of nuclear DNA mediated by endonucleases, such as caspase-activated DNase. The analysed molecular processes lead to sperm DNA fragmentation, which is a key factor in male infertility.
Agarwal et al [149]. emphasise the significance of SDF tests as clinical markers in the assessment of male fertility, as SDF serves as a critical indicator of sperm quality and potential infertility. It is known that SDF is associated with early embryonic arrest and miscarriage and reduces the success of assisted reproductive technologies by impairing sperm fertility and embryo development [158]. These tests are categorised into two main types: direct and indirect. Direct assays operate probes and dyes to measure SDF directly. In contrast, indirect assays evaluate existing DNA breaks and the susceptibility of DNA to denaturation, a characteristic commonly observed in fragmented DNA. The most widely used methods for assessing SDF include TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labelling), which detects DNA strand breaks by labelling exposed DNA ends with nucleotides. The SCSA test (Sperm Chromatin Structure Assay), which analyses sperm DNA integrity by examining the structural configuration of sperm chromatin, is also commonly used. The Sperm Chromatin Dispersion Test measures the dispersion of sperm chromatin, with fragmented DNA displaying a broader dispersion pattern. These tests are of top importance in the identification of sperm with compromised DNA integrity, thus providing valuable information to guide infertility treatment strategies and clinical interventions [149].
Researchers have been actively investigating the relationship between sperm DNA integrity and male fertility. In approximately 20% of cases of idiopathic male infertility, the inability of a sexually active couple to achieve pregnancy can be attributed to single- and/or double-stranded DNA breaks in the sperm head [159]. These DNA breaks often correlate with the severity of pathozoospermia [160, 161]. In addition, in many cases, SDF is responsible for the arrest of embryonic development and subsequent elimination of the embryo during the early stages of embryogenesis, resulting in missed pregnancies, as shown by Sahin et al [161].. Importantly, this condition significantly reduces the effectiveness of assisted reproductive technologies in treating infertile couples [163]. The persistence of DNA fragmentation in sperm cells highlights the importance of targeted interventions to improve DNA integrity, thereby improving reproductive outcomes and supporting successful pregnancies [161].
Thus, SDF has been identified as a pivotal factor in male infertility, given its direct impact on sperm quality and fertilisation capacity. Assessment of sperm DNA fragmentation using such methods as terminal deoxynucleotidyl transferase dUTP nick end labelling, sperm chromatin structure assay, and sperm chromatin dispersion provides valuable insights into the extent of DNA damage. It is therefore a reliable means of assessing sperm integrity. The identification of elevated levels of DNA fragmentation is paramount for the diagnosis of infertility, the guidance of clinical interventions, and the development of targeted therapeutic strategies aimed at the enhancement of male reproductive health.
Role of Nrf2 in male infertility via ROS
The significance of this research lies in its potential to elucidate phenomena that, in normal physiological conditions, demonstrate the vital role of ROS as secondary messengers in diverse signalling pathways within cells [164]. However, it is essential to note that their excessive accumulation can lead to oxidative damage to cellular components, including lipids, proteins, and DNA [165]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a pivotal regulator of antioxidant and cytoprotective genes, playing a crucial role in mitigating redox imbalances by orchestrating cellular defence mechanisms [62] and particularly impacting reproductive success [166]. Studies highlight factors contributing to sperm membrane and DNA damage, dysregulated RNA processing, and telomere destruction via Nrf2 [163, 166]. The studies emphasise the regulatory function of Nrf2 in maintaining the balance between oxidants and antioxidants, thereby protecting male fertility. The authors established a link between Nrf2 signalling pathways and the regulation of spermatogenesis and sperm quality. This provides valuable insights into potential therapeutic strategies for modulating oxidative imbalance and inflammation to improve male reproductive health [163].
A study highlighting the central role of Nrf2 in male fertility, particularly in the context of oligospermia, was conducted by Han et al [167].. It elucidated the protection offered by Nrf2 against ferroptosis, emphasising its importance in maintaining sperm quality and overall male reproductive health. The research findings indicate that Nrf2 and GPX4 protein levels are significantly reduced in sperm from patients diagnosed with oligospermia. Furthermore, Nrf2 knockout mice exhibit reduced sperm concentrations, motility, and fertility. The study further demonstrated the presence of ferroptosis-related biomarkers, such as elevated malondialdehyde (MDA) levels, and altered expression of ferroptosis-related genes, including GPX4 and ferroportin 1 (FPN1), in Nrf2 knockout mice. Treatment with a ferroptosis inhibitor was found to reverse these effects, proving that Nrf2 deletion induces ferroptosis in spermatogenic cells, thereby contributing to oligospermia pathogenesis. This finding underscores the critical role of Nrf2 in maintaining sperm quality, as demonstrated by many authors [167] and shown in Fig. 4.
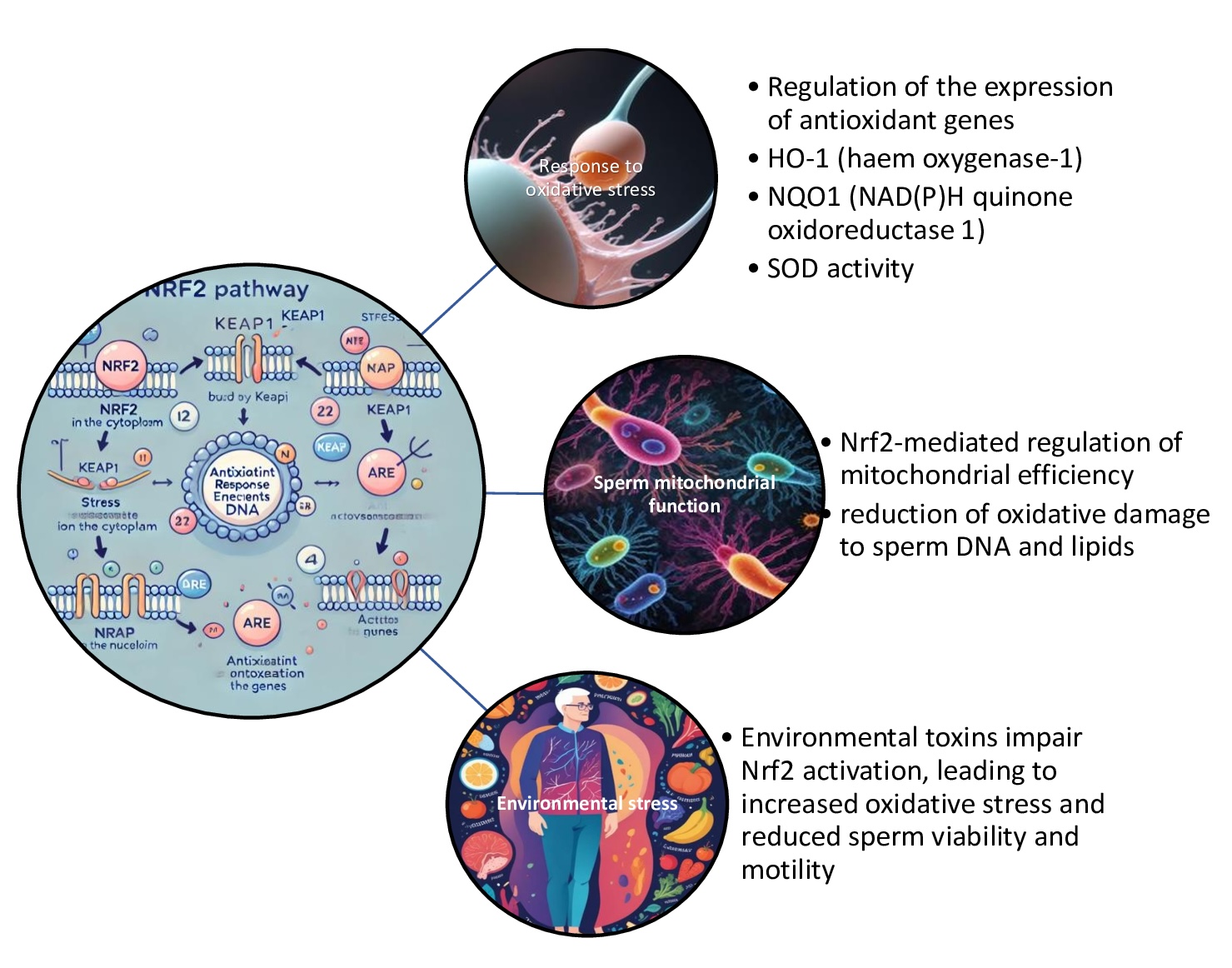
Fig. 4: Nrf2 (nuclear factor erythroid 2-related factor 2) pathway and male infertility. The Nrf2 pathway plays a significant role in male infertility by protecting sperm cells from oxidative stress, which is a major contributor to male reproductive dysfunction. Oxidative damage can impair sperm motility, DNA integrity, and overall sperm function, leading to infertility. Activation of the Nrf2 pathway can enhance antioxidant defence mechanisms in the testes and improve sperm quality, suggesting that targeting Nrf2 signalling may offer therapeutic potential in treating male infertility related to oxidative stress.
A study conducted by Akino et al [168]. demonstrated the potential of dimethylfumarate (DMF) in mitigating age-related infertility by activating the Nrf2/Keap1-antioxidant response element (ARE) signalling pathway. The authors showed that DMF plays a key role in reducing OS and is a significant factor contributing to ovarian aging and infertility by upregulating antioxidant defences and reducing DNA damage. The study's findings indicate that DMF administration enhances Nrf2 expression, increases antioxidant activity, and preserves the ovarian reserve. This process is characterised by improved oocyte quality and maintenance of primordial follicles. The study results suggest that DMF could be a therapeutic approach with great potential in combating age-related infertility. The mechanism of action appears to involve protection of ovarian function from oxidative damage and enhancement of cellular resilience, both of which are crucial for successful fertilisation [168].
Valipour et al [169]. explained the mechanisms underlying OS and its impact on infertility, underscoring the pivotal role of Nrf2 in cellular defence mechanisms. Specifically, their findings demonstrate that, upon release from its inhibitor, Kelch-like ECH-associated protein 1 (Keap1), Nrf2 translocates to the nucleus, activating the expression of genes involved in antioxidant production and detoxification processes. The experimental models applied in this study have demonstrated that Nrf2-mediated pathways can mitigate oxidative damage in reproductive tissues, offering protection against such conditions as sperm DNA fragmentation, ovarian follicle development disruption, and testicular tissue damage. This emphasises the therapeutic potential of targeting the Nrf2 signalling pathway in treating OS-related infertility.
The research conducted by Falvo et al [170]. offers a compelling perspective on the deleterious impact of short-term high-fat diets on male reproductive function. Their findings underscore an association between the consumption of high-fat diets and a range of adverse outcomes, including the impairment of steroidogenesis, the induction of spermatogenic cell apoptosis, and the disruption of spermatogenesis. The mechanisms by which these effects occur are elucidated in the study, which finds that there is compromised blood-testis barrier integrity due to the downregulation of structural proteins (N-cadherin, ZO-1, occludin, connexin 43, and VANGL2) and altered phosphorylation of regulatory kinases, such as Src and FAK. Furthermore, the mitochondrial dynamics, including fission, fusion, and biogenesis of spermatogenesis, is disrupted, as are the SIRT1/NRF2/MAPKs signaling pathways. This finding derived from experiments conducted on 10-week-old male Wistar rats is particularly concerning in the context of the WHO estimates that 50% of adults and 30% of children and adolescents are overweight or obese [170]. Concurrently, a global decline in sperm quality and male fertility has been observed [171], underscoring the imperative to investigate the molecular underpinnings connecting metabolic disorders and reproductive health, to identify potential therapeutic interventions.
Therefore, the results highlight the critical role of oxidative balance in male fertility, where excessive reactive oxygen species can lead to cellular damage, particularly in sperm cells via Nrf2, which plays a key role in maintaining redox homeostasis by regulating antioxidant defense mechanisms, thereby protecting sperm quality. Disruption of Nrf2 signalling, e.g., by ferroptosis, has been associated with impaired spermatogenesis and reduced sperm quality, particularly in such conditions as oligospermia. These findings highlight the potential for therapeutic strategies targeting Nrf2 pathways to alleviate OS and improve male reproductive health.
Antioxidant-rich diets, male fertility, and Nrf2, AMPK/PGC-1α, and NF-κB pathways
Numerous studies have shown that antioxidants are crucial in protecting cells from OS, which has been identified as a significant contributing factor to male infertility [12]. The Nrf2 pathway (nuclear factor erythroid 2-related factor 2) is the central regulator of the body's antioxidant response [172]. In normal conditions, Nrf2 is bound to a molecule known as Keap1 (Kelch-like ECH-associated protein 1), and this binding results in the degradation of Nrf2 [173]. However, during periods of redox dysfunction or following the intake of antioxidants, Nrf2 is released and transported to the nucleus, where it activates the expression of antioxidant-related genes, including SOD, CAT, and GPx [173]. The function of these genes is to neutralize ROS. This, as demonstrated by Valipour et al [169]., results in a reduction of OS, improvement in sperm motility, and, consequently, enhancement of DNA integrity and overall fertilising capacity.
Recent studies have indicated that the enhancement of mitochondrial function via the AMPK/PGC-1α pathway is another significant factor in this process [174]. It has been established that antioxidants also influence other metabolic pathways via AMPK (AMP-activated protein kinase) and PGC-1α (peroxisome proliferator-activated receptor- gamma coactivator 1-alpha), both of which are crucial for mitochondrial function, as demonstrated by [174]. This is related to the fact that mitochondria present in spermatozoa are critical for generating the energy required for motility [175]. AMPK has been reported to be activated by resveratrol [176] and quercetin [177], which enhanced mitochondrial biogenesis and improved mitochondrial function via PGC-1α. The improved mitochondrial performance resulting from this process has been shown to reduce the production of ROS in sperm cells, which aligns with the conclusions reached by other authors [165]. In turn, this, in synergy with the NRF2 pathway, has been demonstrated to mitigate OS, as shown in Fig. 5.
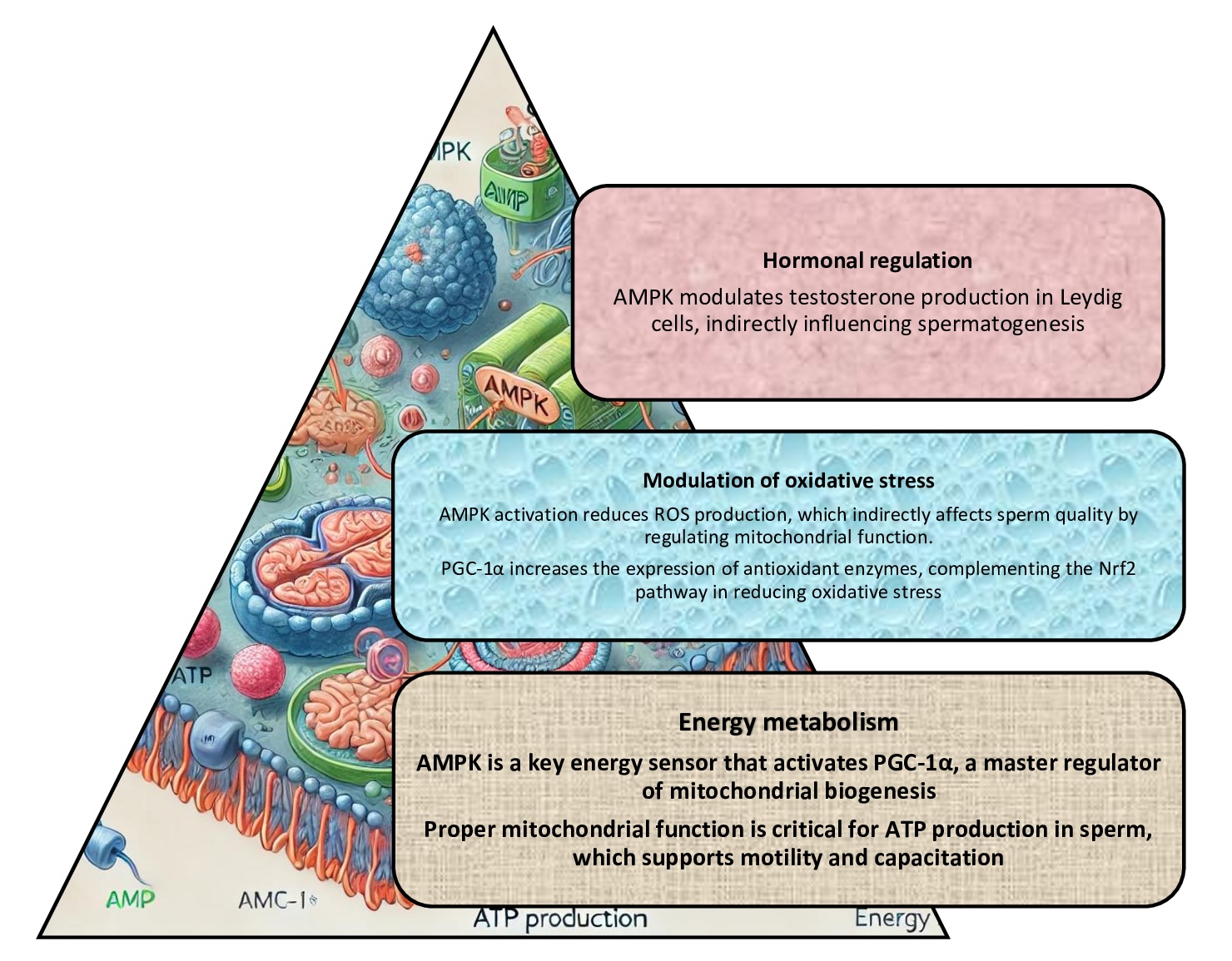
Fig. 5: AMPK/PGC-1α pathway (AMP-activated protein kinase and peroxisome proliferator-activated receptor gamma coactivator-1α) and male infertility. The AMPK/PGC-1α pathway has been demonstrated to play a crucial role in regulating cellular energy balance and metabolic processes; these processes are essential for the proper function of reproductive cells; dysregulation of this pathway impairs spermatogenesis and mitochondrial function, which can contribute to male infertility.
Studies investigate the potential of inflammation reduction, focusing on NF-κB pathway regulation, as previously demonstrated by authors [178]. It was previously understood that the NF-κB (nuclear factor kappa B) pathway plays an instrumental role in regulating inflammatory responses in the body. Excessive activation of NF-κB has been linked to chronic inflammation, exerting detrimental effects on testicular function, spermatogenesis, and semen quality [179], as shown in Fig.6. Some investigations have provided valuable insights into such antioxidants as curcumin, vitamin E, and polyphenols from green tea, which have been shown to inhibit NF-κB activation, thereby reducing inflammation in reproductive tissues [180]. Consequently, the combined modulation of the NRF2, AMPK, and NF-κB pathways by an antioxidant-rich diet triggers an integrated protective mechanism that improves semen quality, reduces sperm DNA fragmentation, and enhances sperm fertilising ability, thus supporting the treatment of male infertility.
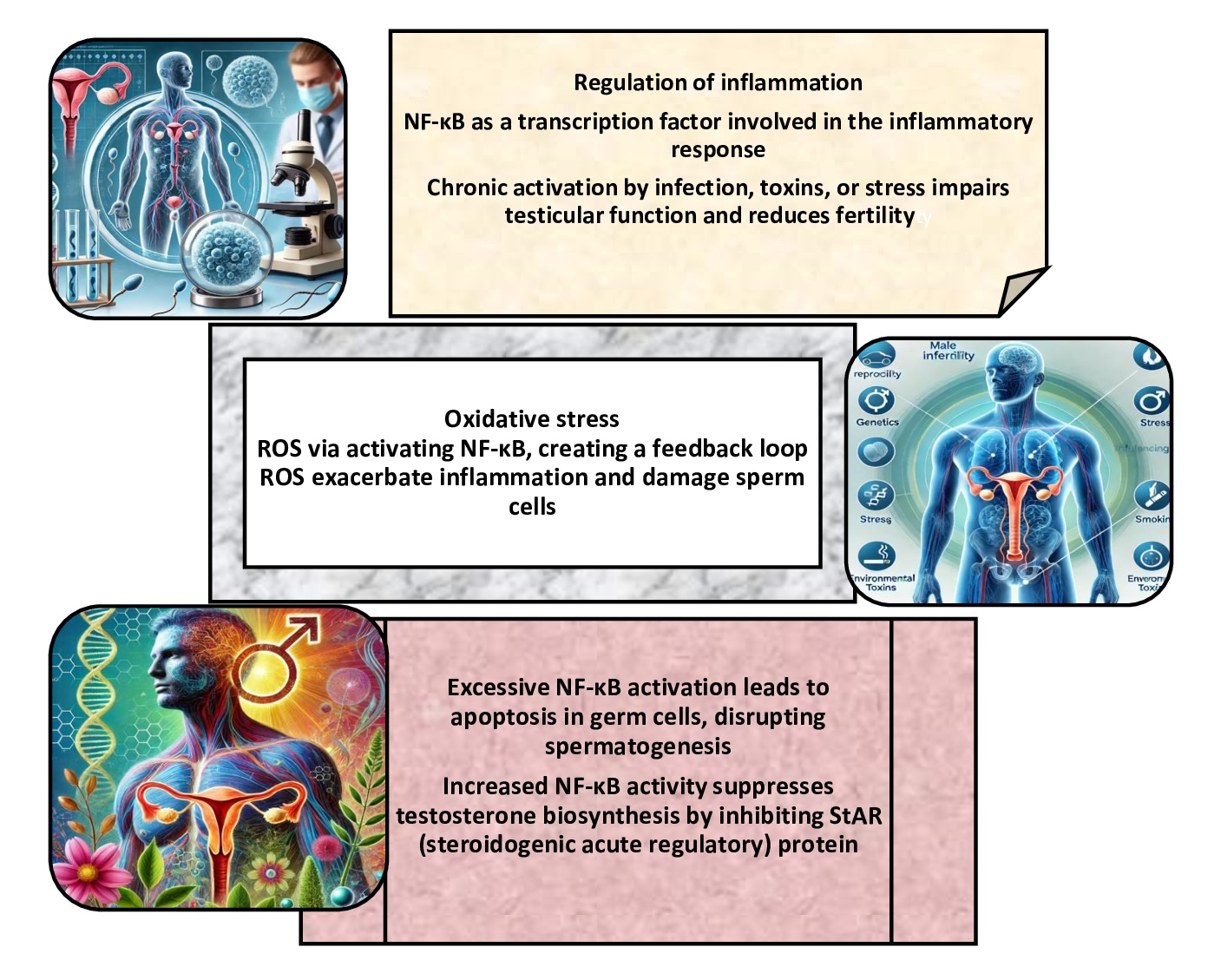
Fig. 6: Nuclear factor kappa B (NF-κB) signalling pathway and male infertility. The NF-κB signalling pathway plays a pivotal role in the regulation of immune responses, inflammation, and cell survival, which are critical processes for maintaining normal spermatogenesis. Dysregulated NF-κB activity can lead to chronic inflammation, oxidative stress, and impaired testicular function, contributing to male infertility.
Thus, by improving the function of key regulatory pathways, such as Nrf2, AMPK/PGC-1α, and NF-κB, an antioxidant-rich diet plays a crucial role in supporting male fertility. Nrf2, in particular, is essential for maintaining redox balance and protecting sperm quality by activating antioxidant defences. Disruption of these pathways, including OS-induced ferroptosis, can impair spermatogenesis and fertility. Therefore, dietary interventions rich in antioxidants may offer a promising strategy to mitigate oxidative damage and support male reproductive health by modulating these critical pathways.
Prevention and treatment of oxidative stress in the reproductive system
The human body has highly effective mechanisms to combat OS, with particular emphasis on protecting the reproductive system [181]. In addition to sperm, the ejaculate of a healthy adult male contains many essential components, including natural antioxidants, such as vitamin C, sodium, calcium, zinc, citric acid, fructose, and various proteins [182]. These components help neutralise free radicals and prevent cell damage. A deficiency of these compounds can be replenished through the diet. Research suggests that fruit and vegetables, especially those with yellow, orange, and red pigments, e.g., carrots and tomatoes, can improve male fertility significantly [183].
The findings presented by Britton and Khachik [184] categorise carotenoid patterns based on the colour of plant tissues into five distinct groups characterised by 1) high levels of the acyclic carotene lycopene, resulting in a red colour, as seen in tomatoes; 2) high levels of β-carotene and/or its hydroxyl derivatives, such as β-cryptoxanthin and zeaxanthin, contributing to an orange colour; 3) a combination of β-carotene with α-carotene and/or lutein, resulting in a yellow-orange colour; 4) a predominance of carotenoid epoxides, leading to a yellow colour; and 5) carotenoids unique to specific species, such as capsanthin and capsorubin in red peppers or crocetin in saffron, contributing to yellow, orange, or red hues. Notably, lycopene, a key component of the first pattern, is typically accompanied by colourless carotenoids like phytoene and phytofluene, as discussed by Dias et al [185].. These foods are rich in antioxidants, such as beta-carotene (provitamin A) and lycopene, essential in reducing oxidative damage, especially in idiopathic oligoasthenoteratozoospermia [186].
As reported by Ramgir et al [187]., the mechanisms of action of phytomedicinal therapeutics exert an impact on the testosterone pathway that is involved in the stimulation of spermatogenesis, the reduction of redox imbalance, the inhibition of inflammation, and the activation of signalling pathways in the testes via the extracellular-regulated kinase (ERK)/protein kinase B (PKB)/transformation of growth factor-beta 1 (TGF-β1)/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signalling pathways and mediation of sexual behaviour. It has been demonstrated that ashwagandha can inhibit lipid peroxidation, improve sperm count and motility, and regulate reproductive hormone levels [188]. Selected studies of these effects and proposed mechanisms of action are presented in Table 1.
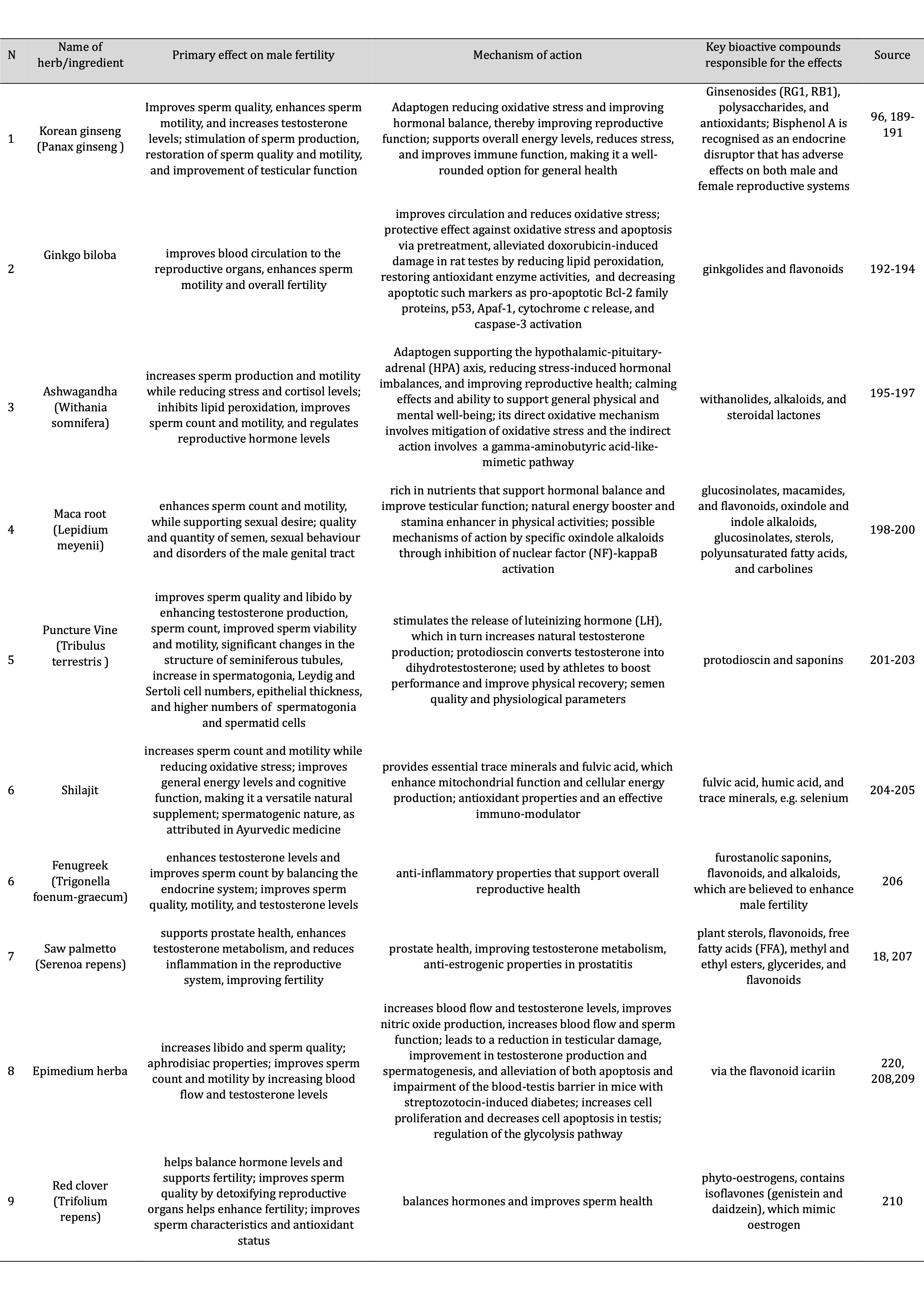
Table 1: Effects, mechanisms, and active ingredients of natural herbs and other ingredients used to support male fertility
Micronutrients and energy boosters further support male fertility by enhancing key biological processes. Zinc and folic acid are essential for spermatogenesis, DNA synthesis, and testosterone production, while selenium contributes to sperm structural integrity, as shown by [211]. Supplements, such as L-carnitine and omega-3 fatty acids, promote energy metabolism in sperm cells, improving their motility and vitality [212]. A study conducted by authors [212] through a comprehensive literature search has underscored the advantageous effects of various nutritional supplements on sperm parameters. Selenium, zinc, omega-3 fatty acids, coenzyme Q10, and carnitines have been observed to positively influence such parameters as total sperm concentration, sperm count, motility, and morphology. Specifically, omega-3 fatty acids and coenzyme Q10 significantly enhanced sperm count and motility, while carnitines were particularly effective in improving sperm progressive motility. Additionally, selenium, omega-3 fatty acids, coenzyme Q10, and carnitines contributed to improvements in sperm morphology, demonstrating the potential of these supplements in supporting male reproductive health [212].
The microbiome and its effect on male fertility
The human microbiome is a diverse ecosystem comprising bacteria, fungi, viruses, archaea, and protozoa [19, 36]. Bacteria are taxonomically classified into phyla, classes, orders, families, genera, and species, with the gut microbiota dominated by a limited number of phyla, as shown by Laterza et al [213]., comprising over 160 species. Firmicutes and Bacteroidetes account for approximately 90% of the gut microbiota [214], with Firmicutes including over 200 genera, such as Lactobacillus, Bacillus, and Clostridium. In contrast, Bacteroidetes and Actinobacteria are comprised of genera such as Bacteroides, Prevotella, and Bifidobacterium. These findings, supported by Rinninella et al [215]., highlight the diversity and dominance of these phyla within the gut microbial community. These dependencies are shown in Fig. 7.
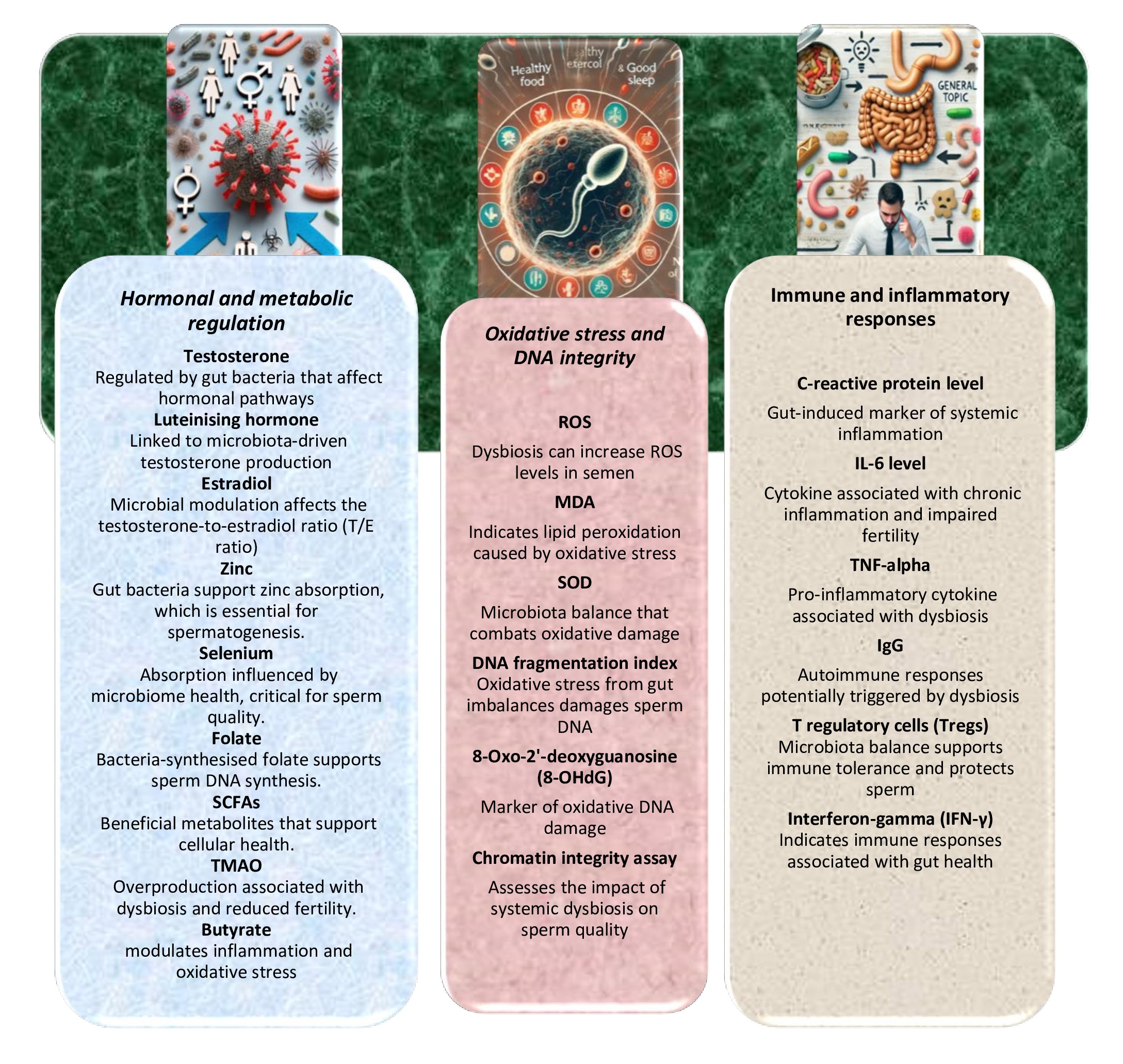
Fig. 7: Mechanisms and biomarkers in multifaceted links between the microbiome and male fertility. The relationship between the microbiome and male fertility involves a dynamic interplay of hormonal regulation, oxidative stress modulation, immune response control, nutrient absorption, and the gut-brain axis, with dysbiosis and microbial imbalances emerging as critical factors influencing sperm quality, DNA integrity, and overall reproductive health. Abbreviations: SCFAs - short-chain fatty acids; TMAO - trimethylamine-N-oxide; ROS - reactive oxygen species; MDA - malondialdehyde; SOD - superoxide dismutase; IL-6 - interleukin-6; TNF-alpha - tumour necrosis factor-alpha; IgG - Immunoglobulin G.
These bacteria contribute to vital functions, such as nutrient metabolism, immune modulation, and the production of metabolites, e.g., short-chain fatty acids (SCFAs) [33, 34, 37, 216]. The fungal mycobiome, comprising such genera as Candida, Saccharomyces, and Malassezia, influences digestive processes and immune function [217]. The virome, primarily consisting of bacteriophages, regulates bacterial populations and maintains microbial diversity. Archaea, including Methanobrevibacter, play a role in fermentation and methane production, while protozoa, such as Blastocystis hominis, interact with other microbes and influence immune responses [218, 219].
This intricate community functions collectively to maintain homeostasis [33, 34, 37]. The contribution of bacteria and fungi to nutrient absorption and inflammation regulation, the role of viruses in shaping microbial dynamics, the assistance of archaea in metabolic processes, and the interaction of protozoa with the immune system are all pivotal in this regard. Collectively, these elements constitute a balanced ecosystem, which is imperative for overall health. However, disruptions in this composition can lead to dysbiosis and associated systemic conditions (Fig. 8).

Fig. 8: Complex relationships between the microbiome and male fertility.
The microbiome plays a significant role in male fertility through its direct influence on the gut-brain axis, immune system, hormonal regulation, and inflammation. The vagus nerve is a key component in the gut-brain axis [35, 220]. An imbalance in the microbiome can contribute to hormonal disruptions, increased inflammation, and altered brain function, negatively affecting reproductive health. Bonaz et al [220]. reported that the vagus nerve serves as a crucial mediator in the bidirectional communication between the gut and brain. It plays a critical role in maintaining homeostasis by monitoring the "milieu intérieur" and coordinating nervous and endocrine responses to support gastrointestinal health. Its anti-inflammatory functions are achieved through activation of the hypothalamic-pituitary-adrenal (HPA) axis, leading to cortisol release, and through the vagovagal reflex, which exerts an anti-tumour necrosis factor (TNF) effect, known as the cholinergic anti-inflammatory pathway. As a result, vagus nerve stimulation is emerging as a promising non-drug therapeutic strategy for treating gastrointestinal disorders characterised by impaired brain-gut communication, such as inflammatory bowel disease and related conditions [220].
Recent research highlights the importance of a healthy gut microbiome in male fertility. In their study, Lv et al [216]. emphasised the critical role of the gut microbiota in improving sperm quality, testicular structure, and sexual behaviour, primarily through the production of metabolites and the regulation of host metabolism. Endotoxemia, linked to gut microbiota imbalance, is identified as a key factor in testicular damage and disruption of the blood-testis barrier, which can lead to orchitis. The gut microbiota has also been demonstrated to influence sex hormone levels by modulating sex hormone-related enzymes and enterohepatic circulation, impacting the hypothalamic-pituitary-testicular axis and promoting sexual arousal and behaviour [216].
Diets high in fibre and probiotics improve reproductive health via the gut-testis axis, as shown by authors [221]. Beneficial gut flora can reduce systemic inflammation, while microbial metabolites influence hormone levels and sperm quality [222]. Molecular pathways include modulation of inflammatory markers and hormone synthesis by short-chain fatty acids (SCFAs) and other microbial products, as demonstrated earlier by Tan et al [223].. Moreover, dietary and lifestyle choices that promote a healthy microbiome can support male fertility by optimising the hypothalamic-pituitary-gonadal axis function, reducing systemic inflammation, and enhancing sperm production [224]. A comprehensive understanding of the intricate relationship between the microbiome, brain, behaviour, and fertility can yield novel therapeutic interventions for male infertility.
The microbiome's influence on male fertility is attributable to its effects on systemic inflammation, OS, and hormonal balance [225]. Corral-Vazquez et al [226]. demonstrated that a healthy gut microbiome can promote anti-inflammatory states and support testosterone production by regulating the hypothalamic-pituitary-gonadal (HPG) axis. Bacteria, such as Lactobacillus and Bifidobacterium, can produce SCFAs and other metabolites that enhance gut barrier integrity and modulate immune responses, thereby creating an environment conducive to healthy spermatogenesis [227]. Conversely, dysbiosis, defined as an overgrowth of pathogens like Escherichia coli and Enterococcus, has increased systemic inflammation and OS, resulting in damaged sperm DNA and impaired motility [228]. In addition to inflammation, disruptions in the microbiome have been demonstrated to impair hormone regulation (Fig.9). The overproduction of inflammatory cytokines and endotoxins, such as lipopolysaccharides, has been shown to disrupt the HPG axis, reducing testosterone levels and hindering sperm production [97]. The presence of pathogens and a concomitant reduction in the abundance of beneficial bacteria in the seminal microbiome directly impact sperm quality and viability. Therapeutic interventions addressing microbial imbalances through probiotics, prebiotics, and dietary modifications can mitigate infertility [222, 225].
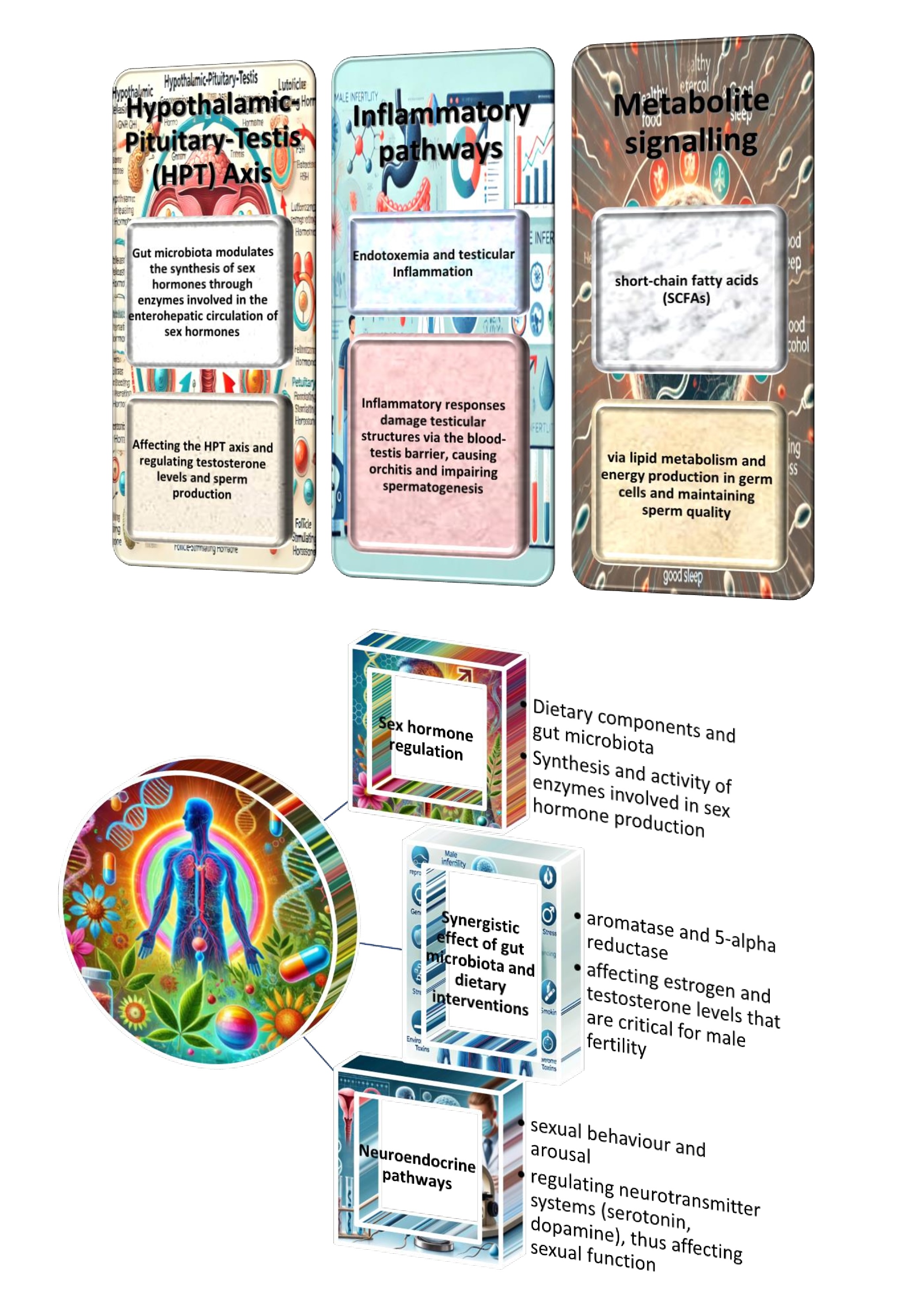
Fig. 9: Pathways demonstrating the synergistic effect of the gut microbiota and dietary interventions in maintaining and enhancing male fertility through the modulation of both physiological and hormonal mechanisms.
Synergistic approaches to gut microbiota and dietary interventions
Developing supplements targeting the gut microbiota and male fertility involves combining ingredients with proven benefits for both gut health and reproductive function. Probiotics play a central role, with Lactobacillus rhamnosus, Lactobacillus reuteri, Bifidobacterium longum, and Bifidobacterium bifidum strains being particularly effective in improving gut health, reducing systemic inflammation, and positively influencing testosterone levels and sperm quality, as reported by Raheem et al [229].. Prebiotics, including inulin, fructooligosaccharides (FOS), and galactooligosaccharides (GOS), also promote the growth of beneficial bacteria, which indirectly supports fertility by reducing OS and balancing hormones [230].
In addition to gut-focused ingredients, fertility-specific ingredients are essential [26, 29, 30]. Antioxidants, e.g., selenium, vitamin E, CoQ10, and N-acetylcysteine, protect sperm from oxidative damage, while amino acids, such as L-carnitine and L-arginine, also improve motility and overall sperm function [9]. Omega-3 fatty acids reduce inflammation and improve the structural integrity of sperm membranes, providing a further layer of support. Adaptogens, such as ashwagandha, maca root, and ginseng, reduce stress while boosting fertility, making them a valuable addition to a comprehensive formula as shown earlier in this review.
In the context of male fertility, the influence of the gut microbiota and dietary interventions on various molecular pathways has been demonstrated. These pathways illustrate the synergistic effect of the gut microbiota and nutritional interventions in maintaining and enhancing male fertility by modulating physiological and hormonal mechanisms (see Fig. 8). Such mechanisms include the regulation of the hypothalamic-pituitary-testicular axis, which controls testosterone and sperm production, and the modulation of inflammatory pathways, where gut imbalances can cause endotoxemia and disrupt the blood-testis barrier, as shown by authors [231]. Furthermore, microbiota-derived metabolites have been shown to impact metabolic processes vital for sperm quality. At the same time, gut bacteria have also been demonstrated to regulate sex hormone synthesis and influence brain areas that are responsible for sexual behaviour and arousal [232]. Together, these findings underscore the pivotal role of gut health in male reproductive function (Fig. 9).
When designing supplements to improve male fertility, it is crucial to consider synergistic effects and precise delivery mechanisms, as highlighted by Sánchez et al [233].. For example, combining probiotics with antioxidants has been shown to reduce inflammation in both the gut and reproductive organs, while combining prebiotics with adaptogens addresses stress regulation and gut health [190]. A comprehensive formulation, i.e., a capsule or powder integrating probiotics, prebiotics, antioxidants, amino acids, omega-3 fatty acids, and adaptogens, offers a dual-action strategy to improve the gut microbiota and male fertility [189, 191]. Ensuring proper dosing and clinical validation enhances the efficacy of such supplements, providing a science-based approach to male infertility [96].
When developing male fertility supplements, it is essential to consider the synergistic effects of combining different ingredients that target gut health and reproductive function [234]. Probiotics and prebiotics work together to improve the gut microbiota, which can improve overall systemic health. Probiotics, such as Lactobacillus and Bifidobacterium strains, help to balance the gut microbiota, reduce inflammation, and support hormonal regulation, especially in mood health, as demonstrated previously [235]. Prebiotics, such as inulin and FOS, feed beneficial gut bacteria, promoting a healthy gut environment that can improve metabolic and reproductive health. Combined, these ingredients can provide a dual benefit by addressing gut and fertility issues [230].
Oxidative stress is a key factor in male infertility, leading to impaired sperm function and DNA damage [236, 237], and is closely linked to gut microbiota dysbiosis. Excessive ROS not only damages sperm function but also disrupts the gut microbiome, exacerbating systemic inflammation and oxidative imbalance [238]. Environmental exposures, such as heavy metals, further impair the composition of the gut microbiota, which amplifies oxidative damage [238, 239]. On the other hand, intense physical activity has similarly altered microbial diversity, affecting systemic oxidative stress and sperm health [240, 241]. It is known that, in normal conditions, leukocytes produce 1000 times more free radicals than spermatozoa, making leukocytes the dominant producers of free radicals in semen [241]. The level of activity of leukocytes plays an essential role in forming free radicals [241]. Therapies that target oxidative stress, including antioxidants and adaptogens such as Withania somnifera, have shown promise in improving sperm quality and fertility outcomes [242, 243]. Therefore, antioxidant supplementation and adaptogens may improve gut microbiota and reproductive outcomes by mitigating oxidative stress.
The use of antioxidants and amino acids is another synergistic combination. Antioxidants, such as CoQ10, selenium, and N-acetylcysteine, protect sperm from oxidative damage, a significant cause of male infertility [9, 205, 219]. By helping to reduce oxidative stress in the body, these antioxidants also help to support the functioning of the reproductive organs. Combined with amino acids such as L-carnitine and L-arginine, they further improve sperm motility and function. L-carnitine helps enhance mitochondrial energy production in sperm cells, while L-arginine supports nitric oxide production, which promotes better blood flow to reproductive organs [72, 120, 125]. Together, these ingredients provide a comprehensive approach to combating oxidative damage and supporting sperm function. Complementary effects are also seen when omega-3 fatty acids are combined with such adaptogens as ashwagandha, maca root, and Tribulus terrestris. Omega-3 fatty acids reduce inflammation and support sperm membrane integrity, improving sperm quality and motility, as shown by Montano et al [74].. Adaptogens help the body manage stress and regulate hormones, critical factors in maintaining optimal fertility. These herbs and supplements can help restore hormonal balance, reduce cortisol levels, and support ovulation [244].
Emerging systemic and lifestyle factors in male infertility: future research directions
In addition to direct testicular or oxidative mechanisms, there is an increasing recognition of several systemic and lifestyle-related factors as significant contributors to male infertility. These include metabolic dysfunctions such as lipid imbalance and obesity, socioemotional stress and unhealthy lifestyle patterns, as well as mitochondrial genetic polymorphisms that may compromise sperm function. This section provides a comprehensive overview of the multifactorial impact of these emerging determinants on male reproductive health, to inform future studies in this area. It is evident from the extant literature that lipid metabolism disorders and obesity represent well-documented risk factors for male infertility.
Firstly, excess adipose tissue, particularly visceral fat, has been demonstrated to contribute to a pro-inflammatory and hormonally imbalanced state that has a detrimental effect on testicular function. Obesity has been demonstrated to be associated with hypogonadism, reduced testosterone levels, and elevated aromatisation of androgens into oestrogens, thereby disrupting the HPG axis. Excess body fat has been demonstrated to exert a substantial influence on the reproductive health of males, through its capacity to disrupt hormonal regulation, to engender increased oxidative stress, and to impair testicular function. Obesity-related changes, including elevated estrogenic levels resulting from aromatase activity in adipose tissue, have been demonstrated to induce hypogonadotropic hypogonadism and diminished fertility potential in males [245]. Furthermore, dyslipidaemia has been demonstrated to compromise the structural integrity of sperm membranes by modifying their lipid composition, thereby impacting motility and fertilisation capacity. In men diagnosed with non-obstructive azoospermia (NOA), both basal and stimulated levels of inhibin B and anti-Müllerian hormone (AMH) are significantly lower than in fertile control subjects. However, dynamic testing using exogenous FSH (EFSERT) does not provide additional diagnostic value beyond baseline measurements of these hormones [246]. Emerging evidence also links obesity-related oxidative stress and insulin resistance with reduced sperm quality and DNA fragmentation. Given the intricacy of these mechanisms, further research is indicated to elucidate precise pathways and therapeutic interventions targeting lipid metabolism in male infertility.
It was shown that mitochondrial DNA (mtDNA) polymorphisms and mutations have garnered increasing attention as potential contributors to male infertility. Mitochondria have been demonstrated to play a pivotal role in sperm bioenergetics, particularly in the generation of ATP, which is essential for flagellar movement. A single nucleotide polymorphism (rs527236194) in the mitochondrial cytochrome B gene (MT-CYB) has been associated with impaired sperm parameters, particularly in men diagnosed with asthenoteratozoospermia. This finding highlights the potential role of mitochondrial genetic variation in the pathophysiology of male infertility and underscores the need for further investigation in broader populations [247]. Variations in mtDNA have been demonstrated to impair mitochondrial respiratory chain function, resulting in diminished ATP availability, augmented generation of ROS, and, in turn, oxidative damage to both nuclear and mitochondrial genomes. Specific mtDNA haplogroups or point mutations have been associated with impaired asthenozoospermia, abnormal morphology, and reduced fertilisation potential. Although research in this area is still evolving, future studies focusing on mitochondrial genetics show great promise in advancing diagnostics and targeted therapies in the field of male reproductive health.
The role of chronic socioemotional stress and adverse lifestyle behaviours in the development of male reproductive dysfunction is a significant yet often underestimated factor. Psychological stress activates the HPA axis, leading to elevated cortisol levels, which have been demonstrated to suppress gonadotropin release and impair spermatogenesis. Furthermore, several studies have shown a link between certain lifestyle factors and sub-optimal male reproductive health. These factors, which include a lack of physical activity, inadequate sleep, smoking, alcohol consumption, and unhealthy dietary choices, have been shown to contribute to oxidative stress, endocrine function imbalances, and reduced sperm quality. While the associations are increasingly recognised, further detailed studies are required to define causal relationships and develop effective interventions to mitigate lifestyle-related risks in infertile men.
Throughout the manuscript, we have outlined several mechanisms by which the gut microbiota may influence male infertility, including the gut-brain axis, modulation of the immune system, and hormonal regulation. While these mechanisms highlight potential interaction pathways, we have been careful to present them as possible contributors rather than definitive causal factors, emphasising the need for further research to validate these relationships. Thus, future directions in lifestyle interventions for male infertility connected with innovative approaches include wearable technology to monitor physical activity, stress, health parameters, and personalised dietary plans. Epigenetic therapies, which use lifestyle modifications to alter gene expression associated with fertility, represent an innovative avenue for intervention. Integrating dietary strategies, fitness programmes, and psychological support offers a holistic and effective solution to improve male fertility outcomes.
Conclusion
The identification of reliable biomarkers of male infertility is crucial for both early diagnosis and personalised treatment. The assessment of sperm quality, including such parameters as motility, morphology, and DNA integrity, remains essential for evaluating male reproductive health. Advances in molecular biology have provided insights into how epigenetics and lifestyle interact to influence sperm quality.
Common pathological conditions, such as varicocele, infections, and testicular hyperthermia, significantly contribute to male infertility. Additionally, stress responses, particularly due to testicular hyperthermia, further exacerbate oxidative stress, leading to sperm damage and impaired fertility. The role of ROS in spermatogenesis is discussed in this study, and it is demonstrated that ROS play a dual role in spermatogenesis, being necessary for normal sperm function but resulting in sperm DNA fragmentation and diminished fertility potential through excessive ROS production from both endogenous and exogenous sources. Understanding the balance between ROS production and antioxidant defences is crucial for male infertility management.
Antioxidants and pathways involved in male infertility are discussed. An analysis of antioxidant-rich diets and key molecular pathways, including Nrf2, AMPK/PGC-1α, and NF-κB, reveals their vital role in mitigating oxidative stress and improving sperm quality. Targeting these pathways could offer novel therapeutic options for improving male fertility. Male infertility is often associated with endothelial dysfunction and cardiovascular diseases, suggesting a systemic impact on reproductive health. Addressing these comorbidities through lifestyle changes and interventions may enhance male fertility outcomes, as the gut microbiota and synergistic dietary approaches require further investigation to understand this complex relationship better.
The findings of this review highlight the complexity of male infertility and underscore the need for further research to develop effective therapeutic interventions and improve reproductive outcomes. Consequently, a multifaceted therapeutic approach holds considerable promise for enhancing fertility outcomes.
Disclosure Statement
The authors have no competing interests to declare.
References
| 1 | Pescatori ES. Diagnosis of infertility. In: Cavallini G, Beretta G, editors. Clinical Management
of Male Infertility. Springer, Cham; 2015.
https://doi.org/10.1007/978-3-319-08503-6_4 |
| 2 | World Health Organization (WHO). WHO laboratory manual for the examination and processing of human
semen. 6th ed. WHO; 2021.
|
| 3 | WHO. Manual for the Standardised Investigation and Diagnosis of the Infertile Couple. Cambridge:
Cambridge University Press; 2000 p. 353.
|
| 4 | Wang Y, Chen F, Ye L, Zirkin B, Chen H. Steroidogenesis in Leydig cells: effects of aging and
environmental factors. Reproduction. 2017;154:111-22.
https://doi.org/10.1530/REP-17-0064 |
| 5 | WHO. Laboratory Manual for the Exam of Human Semen and Spermcervical Mucus Interaction. 4th ed.
Cambridge: Cambridge University Press; 1999 p. 450.
|
| 6 | Assidi M. Infertility in men: Advances towards a comprehensive and integrative strategy for
precision theranostics. Cells. 2022;11:1711.
https://doi.org/10.3390/cells11101711 |
| 7 | Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe.
Reprod Biol Endocrinol. 2015;13:37.
https://doi.org/10.1186/s12958-015-0032-1 |
| 8 | Cassina A, Silveira P, Cantu L, Montes JM, Radi R, Sapiro R. Defective human sperm cells are
associated with mitochondrial dysfunction and oxidant production. Biol Reprod. 2015;93:119.
https://doi.org/10.1095/biolreprod.115.130989 |
| 9 | Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, et al. Male infertility. Lancet.
2021;397:319-33.
https://doi.org/10.1016/S0140-6736(20)32667-2 |
| 10 | Duvuru R, Halabi M, Omolaoye TS, Du Plessis SS. The genetic causes of male infertility: a Middle
East and North Africa perspective. F1000Res. 2022;11:125.
https://doi.org/10.12688/f1000research.106950.2 |
| 11 | Gül M, Russo GI, Kandil H, Boitrelle F, Saleh R, Chung E, et al. Male infertility: New
developments, current challenges, and future directions. World J Mens Health. 2024;42:502-17.
https://doi.org/10.5534/wjmh.230232 |
| 12 | Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol.
2017;14:470-85.
https://doi.org/10.1038/nrurol.2017.69 |
| 13 | Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:12.
https://doi.org/10.1186/1477-7827-2-12 |
| 14 | Takalani NB, Monageng EM, Mohlala K, Monsees TK, Henkel R, Opuwari CS. Role of oxidative stress in
male infertility. Reprod Fertil. 2023;4:e230024.
https://doi.org/10.1530/RAF-23-0024 |
| 15 | Kaltsas A. Oxidative stress and male infertility: The protective role of antioxidants. Medicina.
2023;59:1769.
https://doi.org/10.3390/medicina59101769 |
| 16 | Eisenberg ML, Esteves SC, Lamb DJ, Hotaling JM, Giwercman A, Hwang K, et al. Male infertility. Nat
Rev Dis Primers. 2023;9:49.
https://doi.org/10.1038/s41572-023-00459-w |
| 17 | Agarwal A, Leisegang K, Majzoub A, Henkel R, Finelli R, Panner Selvam MK, et al. Utility of
antioxidants in the treatment of male infertility: Clinical guidelines based on a systematic review
and analysis of evidence. World J Mens Health. 2021;39:233-90.
https://doi.org/10.5534/wjmh.200196 |
| 18 | Cannarella R, Condorelli RA, Mongioì LM, La Vignera S, Calogero AE. Molecular biology of
spermatogenesis: Novel targets of apparently idiopathic male infertility. Int J Mol Sci.
2020;21:1728.
https://doi.org/10.3390/ijms21051728 |
| 19 | Wang Y, Xie Z. Exploring the role of gut microbiome in male reproduction. Andrology.
2022;10:441-50.
https://doi.org/10.1111/andr.13143 |
| 20 | Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al.
Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev.
2009;30:293-342.
https://doi.org/10.1210/er.2009-0002 |
| 21 | Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, Prakash A, et al. Environmental
Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front Public Health.
2020;8:553850.
https://doi.org/10.3389/fpubh.2020.553850 |
| 22 | Zheng D, Zeng L, Yang R, Lian Y, Zhu YM, Liang X, et al. Intracytoplasmic sperm injection (ICSI)
versus conventional in vitro fertilisation (IVF) in couples with non-severe male infertility
(NSMI-ICSI): protocol for a multicentre randomised controlled trial. BMJ Open. 2019;9: e030366.
https://doi.org/10.1136/bmjopen-2019-030366 |
| 23 | Ribeiro S, Sousa M. In vitro Fertilisation and Intracytoplasmic Sperm Injection predictive
factors: A review of the effect of female age, ovarian reserve, male age, and male factor on
IVF/ICSI treatment outcomes. JBRA Assist Reprod. 2023;27:97-111.
https://doi.org/10.5935/1518-0557.20220000 |
| 24 | Gualtieri R, Kalthur G, Barbato V, Longobardi S, Di Rella F, Adiga SK, et al. Sperm Oxidative
Stress during In vitro Manipulation and Its Effects on Sperm Function and Embryo Development.
Antioxidants (Basel). 2021;10:1025.
https://doi.org/10.3390/antiox10071025 |
| 25 | Nowicka-Bauer K, Nixon B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm
Motility. Antioxidants (Basel). 2020;9:134.
https://doi.org/10.3390/antiox9020134 |
| 26 | Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between
oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic
criteria. Hum Reprod. 2010;25:2415-26.
https://doi.org/10.1093/humrep/deq214 |
| 27 | Rotimi DE, Singh SK. Implications of lifestyle factors on male reproductive health. JBRA Assist
Reprod. 2024;28:320-330.
https://doi.org/10.5935/1518-0557.20240007 |
| 28 | Ilacqua A, Izzo G, Emerenziani GP, Baldari C, Aversa A. Lifestyle and fertility: the influence of
stress and quality of life on male fertility. Reprod Biol Endocrinol. 2018;16:115.
https://doi.org/10.1186/s12958-018-0436-9 |
| 29 | Oliveira JB, Massaro FC, Baruffi RL, Mauri AL, Petersen CG, Silva LF, et al. Correlation between
semen analysis by motile sperm organelle morphology examination and sperm DNA damage. Fertil Steril.
2010;94:1937-40.
https://doi.org/10.1016/j.fertnstert.2010.01.042 |
| 30 | Belloc S, Benkhalifa M, Cohen-Bacrie M, Dalleac A, Chahine H, Amar E, et al. Which isolated sperm
abnormality is most related to sperm DNA damage in men presenting for infertility evaluation. J
Assist Reprod Genet. 2014;31:527-32.
https://doi.org/10.1007/s10815-014-0194-3 |
| 31 | Merzenich H, Zeeb H, Blettner M. Decreasing sperm quality: a global problem?. BMC Public Health.
2010;10:24.
https://doi.org/10.1186/1471-2458-10-24 |
| 32 | Onor IO, Stirling DL, Williams SR, Bediako D, Borghol A, Harris MB, et al. Clinical Effects of
Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. Int J Environ Res
Public Health. 2017;14:1147.
https://doi.org/10.3390/ijerph14101147 |
| 33 | Kamel M, Aleya S, Alsubih M, Aleya L. Microbiome Dynamics: A Paradigm Shift in Combatting
Infectious Diseases. J Pers Med. 2024;14:217.
https://doi.org/10.3390/jpm14020217 |
| 34 | Ma Z, Zuo T, Frey N, Rangrez AY. A systematic framework for understanding the microbiome in human
health and disease: from basic principles to clinical translation. Signal Transduct Target Ther.
2024;9:237.
https://doi.org/10.1038/s41392-024-01946-6 |
| 35 | Wang Y, Duan C, Du X, Zhu Y, Wang L, Hu J, et al. Vagus Nerve and Gut-Brain Communication.
Neuroscientist. 2024;10738584241259702.
https://doi.org/10.1177/10738584241259702 |
| 36 | Shehata E, Parker A, Suzuki T, Swann JR, Suez J, Kroon PA, et al. Microbiomes in physiology:
insights into 21st-century global medical challenges. Exp Physiol. 2022;107:257-264.
https://doi.org/10.1113/EP090226 |
| 37 | Magill RG, MacDonald SM. Male infertility and the human microbiome. Front Reprod Health.
2023;5:1166201.
https://doi.org/10.3389/frph.2023.1166201 |
| 38 | Sciorio R, Tramontano L, Adel M, Fleming S. Decrease in Sperm Parameters in the 21st Century:
Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. J Pers Med. 2024;14:198.
https://doi.org/10.3390/jpm14020198 |
| 39 | Daniels D, Berger Eberhardt A. Climate change, microplastics, and male infertility. Curr Opin
Urol. 2024;34:366-370.
https://doi.org/10.1097/MOU.0000000000001201 |
| 40 | Knapke ET, Magalhaes DP, Dalvie MA, Mandrioli D, Perry MJ. Environmental and occupational
pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology.
2022;465:153017.
https://doi.org/10.1016/j.tox.2021.153017 |
| 41 | Chao HH, Zhang Y, Dong PY, Gurunathan S, Zhang XF. Comprehensive review on the positive and
negative effects of various important regulators on male spermatogenesis and fertility. Front Nutr.
2023;9:1063510.
https://doi.org/10.3389/fnut.2022.1063510 |
| 42 | Dcunha R, Hussein RS, Ananda H, Kumari S, Adiga SK, Kannan N, et al. Current Insights and Latest
Updates in Sperm Motility and Associated Applications in Assisted Reproduction. Reprod Sci.
2022;29(1):7-25.
https://doi.org/10.1007/s43032-020-00408-y |
| 43 | Zou C, Xu S, Geng H, Li E, Sun W, Yu D. Bioinformatics analysis identifies potential hub genes and
crucial pathways in the pathogenesis of asthenozoospermia. BMC Med Genomics. 2022;15(1):252.
https://doi.org/10.1186/s12920-022-01407-5 |
| 44 | Oduwole OO, Huhtaniemi IT, Misrahi M. The Roles of Luteinizing Hormone, Follicle-Stimulating
Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int J Mol Sci.
2021;22(23):12735.
https://doi.org/10.3390/ijms222312735 |
| 45 | Cioppi F, Rosta V, Krausz C. Genetics of Azoospermia. Int J Mol Sci. 2021;22(6):3264.
https://doi.org/10.3390/ijms22063264 |
| 46 | Høst E, Lindenberg S, Ernst E, Christensen F. Sperm morphology and IVF: embryo quality in relation
to sperm morphology following the WHO and Krüger's strict criteria. Acta Obstet Gynecol Scand.
1999;78(6):526-9 PMID: 10376863.
https://doi.org/10.1034/j.1600-0412.1999.780609.x |
| 47 | Sariibrahim B, Cogendez E, Kayatas S, Asoglu MR, Koleli I, Bakir L. Does Kruger's strict criteria
have prognostic value in predicting ICSI clinical results? Clin Exp Obstet Gynecol.
2013;40(2):257-60 PMID: 23971254.
|
| 48 | D'Andrea S, Micillo A, Barbonetti A, Giordano AV, Carducci S, Mancini A, Necozione S, Francavilla
F, Francavilla S. Determination of spermatic vein reflux after varicocele repair helps to define the
efficacy of treatment in improving sperm parameters of subfertile men. J Endocrinol Invest.
2017;40(10):1145-1153.
https://doi.org/10.1007/s40618-017-0695-x |
| 49 | Shomarufov AB, Bozhedomov VA, Sorokin NI, Matyukhov IP, Fozilov AA, Abbosov SA, Kamalov AA.
Predictors of microsurgical varicocelectomy efficacy in male infertility treatment: critical
assessment and systematization. Asian J Androl. 2023;25(1):21-28.
https://doi.org/10.4103/aja2021125 |
| 50 | Kumanov P, Nandipati K, Tomova A, Agarwal A. Inhibin B is a better marker of spermatogenesis than
other hormones in the evaluation of male factor infertility. Fertil Steril. 2006;86(2):332-8.
https://doi.org/10.1016/j.fertnstert.2006.01.022 |
| 51 | Sá R, Ferraz L, Barros A, Sousa M. The Klinefelter Syndrome and Testicular Sperm Retrieval
Outcomes. Genes (Basel). 2023;14(3):647.
https://doi.org/10.3390/genes14030647 |
| 52 | Pelzman DL, Hwang K. Genetic testing for men with infertility: techniques and indications. Transl
Androl Urol. 2021;10(3):1354-1364.
https://doi.org/10.21037/tau-19-725 |
| 53 | Verón GL, Tissera AD, Bello R, Beltramone F, Estofan G, Molina RI, Vazquez-Levin MH. Impact of
age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil
Steril. 2018;110(1):68-75.e4.
https://doi.org/10.1016/j.fertnstert.2018.03.016 |
| 54 | Balawender K, Orkisz S. The impact of selected modifiable lifestyle factors on male fertility in
the modern world. Cent European J Urol. 2020;73(4):563-568.
|
| 55 | Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation
of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016;6(2):603-21.
https://doi.org/10.1002/j.2040-4603.2016.tb00694.x |
| 56 | Knezevic E, Nenic K, Milanovic V, Knezevic NN. The Role of Cortisol in Chronic Stress,
Neurodegenerative Diseases, and Psychological Disorders. Cells. 2023;12:2726.
https://doi.org/10.3390/cells12232726 |
| 57 | Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto
A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763.
https://doi.org/10.1155/2017/8416763 |
| 58 | Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction
and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509-15.
https://doi.org/10.1161/ATVBAHA.113.300156 |
| 59 | He Y, Zou L, Luo W, Yi Z, Yang P, Yu S, Liu N, Ji J, Guo Y, Liu P, He X, Lv Z, Huang S. Heavy
metal exposure, oxidative stress and semen quality: Exploring associations and mediation effects in
reproductive-aged men. Chemosphere. 2020;244:125498.
https://doi.org/10.1016/j.chemosphere.2019.125498 |
| 60 | Anyanwu BO, Orisakwe OE. Current mechanistic perspectives on male reproductive toxicity induced by
heavy metals. J Environ Sci Health C Toxicol Carcinog. 2020;38(3):204-244.
https://doi.org/10.1080/26896583.2020.1782116 |
| 61 | Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl
Pharmacol. 2005;204(3):274-308.
https://doi.org/10.1016/j.taap.2004.09.007 |
| 62 | Haidar Z, Fatema K, Shoily SS, Sajib AA. Disease-associated metabolic pathways affected by heavy
metals and metalloid. Toxicol Rep. 2023;10:554-570.
https://doi.org/10.1016/j.toxrep.2023.04.010 |
| 63 | Hammad M, Raftari M, Cesário R, Salma R, Godoy P, Emami SN, et al. Roles of oxidative stress and
Nrf2 signaling in pathogenic and non-pathogenic cells: A possible general mechanism of resistance to
therapy. Antioxidants. 2023;12:1371.
https://doi.org/10.3390/antiox12071371 |
| 64 | Calogero AE, Fiore M, Giacone F, Altomare M, Asero P, Ledda C, et al. Exposure to multiple
metals/metalloids and human semen quality: A cross-sectional study. Ecotoxicol Environ Saf.
2021;215:112165.
https://doi.org/10.1016/j.ecoenv.2021.112165 |
| 65 | Kim N, Filipovic D, Bhattacharya S, Cuddapah S. Epigenetic toxicity of heavy metals - implications
for embryonic stem cells. Environ Int. 2024;193:109084.
https://doi.org/10.1016/j.envint.2024.109084 |
| 66 | Park YJ, Pang MG. Mitochondrial functionality in male fertility: From spermatogenesis to
fertilization. Antioxidants (Basel). 2021;10(1):98.
https://doi.org/10.3390/antiox10010098 |
| 67 | Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol.
2009;238(3):240-9.
https://doi.org/10.1016/j.taap.2009.01.028 |
| 68 | Hasan H, Bhushan S, Fijak M, Meinhardt A. Mechanism of inflammatory-associated impairment of sperm
function, spermatogenesis, and steroidogenesis. Front Endocrinol (Lausanne). 2022;13:897029.
https://doi.org/10.3389/fendo.2022.897029 |
| 69 | Baszyński J, Kamiński P, Mroczkowski S, Szymański M, Wasilow K, Stuczyński T, et al. Do aluminum,
boron, arsenic, cadmium, lipoperoxidation, and genetic polymorphism determine male fertility?
Ecotoxicol Environ Saf. 2024;284:116919.
https://doi.org/10.1016/j.ecoenv.2024.116919 |
| 70 | Baszyński J, Kamiński P, Bogdzińska M, Mroczkowski S, Szymański M, Wasilow K, et al. Enzymatic
antioxidant defense and polymorphic changes in male infertility. Antioxidants (Basel).
2022;11(5):817.
https://doi.org/10.3390/antiox11050817 |
| 71 | Pecora G, Sciarra F, Gangitano E, Venneri MA. How food choices impact male fertility. Curr Nutr
Rep. 2023;12(4):864-876.
https://doi.org/10.1007/s13668-023-00503-x |
| 72 | Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods, and nutrients in male fertility
parameters and fecundability: A systematic review of observational studies. Hum Reprod Update.
2017;23(4):371-389.
https://doi.org/10.1093/humupd/dmx006 |
| 73 | Ferramosca A, Zara V. Diet and male fertility: The impact of nutrients and antioxidants on sperm
energetic metabolism. Int J Mol Sci. 2022;23(5):2542.
https://doi.org/10.3390/ijms23052542 |
| 74 | Montano L, Maugeri A, Volpe MG, Micali S, Mirone V, Mantovani A, et al. Mediterranean diet as a
shield against male infertility and cancer risk induced by environmental pollutants: A focus on
flavonoids. Int J Mol Sci. 2022;23(3):1568.
https://doi.org/10.3390/ijms23031568 |
| 75 | Adelowo OE, Akindele BM, Adegbola CA, Oyedokun PA, Akhigbe TM, Akhigbe RE. Unraveling the
complexity of the impact of physical exercise on male reproductive functions: A review of both sides
of a coin. Front Physiol. 2024;15:1492771.
https://doi.org/10.3389/fphys.2024.1492771 |
| 76 | Vaamonde D, Garcia-Manso JM, Hackney AC. Impact of physical activity and exercise on male
reproductive potential: A new assessment questionnaire. Rev Andal Med Deport. 2017;10(2):79-93.
https://doi.org/10.1016/j.ramd.2016.11.017 |
| 77 | Gill K, Jakubik J, Kups M, Rosiak-Gill A, Kurzawa R, Kurpisz M, et al. The impact of sedentary
work on sperm nuclear DNA integrity. Folia Histochem Cytobiol. 2019;57(1):15-22.
|
| 78 | Zhang Y, Chen B, Wang Y, Liu C, Sun J, Zhang Z, et al. Association between mental health and male
fertility: Depression, rather than anxiety, is linked to decreased semen quality. Front Endocrinol
(Lausanne). 2024;15:1478848.
https://doi.org/10.3389/fendo.2024.1478848 |
| 79 | O'Flaherty C, Scarlata E. Oxidative stress and reproductive function: The protection of mammalian
spermatozoa against oxidative stress. Reproduction. 2022;164(6):F67-F78.
https://doi.org/10.1530/REP-22-0200 |
| 80 | Kaltsas A, Markou E, Kyrgiafini MA, Zikopoulos A, Symeonidis EN, Dimitriadis F, et al.
Oxidative-stress-mediated epigenetic dysregulation in spermatogenesis: Implications for male
infertility and offspring health. Genes. 2025;16:93.
https://doi.org/10.3390/genes16010093 |
| 81 | Kim TW, Jeong JH, Hong SC. The impact of sleep and circadian disturbance on hormones and
metabolism. Int J Endocrinol. 2015;2015:591729.
https://doi.org/10.1155/2015/591729 |
| 82 | Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ. Circadian Rhythm and Sleep
Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr Rev. 2016;37(6):584-608.
https://doi.org/10.1210/er.2016-1083 |
| 83 | Lateef OM, Akintubosun MO. Sleep and Reproductive Health. J Circadian Rhythms. 2020;18:1.
https://doi.org/10.5334/jcr.190 |
| 84 | Samimisedeh P, Afshar EJ, Ejtahed HS, Qorbani M. The impact of vegetarian diet on sperm quality,
sex hormone levels and fertility: a systematic review and meta-analysis. J Hum Nutr Diet.
2024;37(1):57-78.
https://doi.org/10.1111/jhn.13230 |
| 85 | Karayiannis D, Kontogianni MD, Mendorou C, Douka L, Mastrominas M, Yiannakouris N. Association
between adherence to the Mediterranean diet and semen quality parameters in male partners of couples
attempting fertility. Hum Reprod. 2017;32(1):215-222.
https://doi.org/10.1093/humrep/dew288 |
| 86 | Cutillas-Tolín A, Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Jørgensen N, Navarrete-Muñoz EM,
et al. Mediterranean and western dietary patterns are related to markers of testicular function
among healthy men. Hum Reprod. 2015;30(12):2945-55.
https://doi.org/10.1093/humrep/dev236 |
| 87 | Durairajanayagam D, Agarwal A, Ong C. Causes, effects and molecular mechanisms of testicular heat
stress. Reprod Biomed Online. 2015;30(1):14-27.
https://doi.org/10.1016/j.rbmo.2014.09.018 |
| 88 | Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a
review. Int J Androl. 1995;18(4):169-84.
https://doi.org/10.1111/j.1365-2605.1995.tb00408.x |
| 89 | Sheynkin Y, Jung M, Yoo P, Schulsinger D, Komaroff E. Increase in scrotal temperature in laptop
computer users. Hum Reprod. 2005;20(2):452-5.
https://doi.org/10.1093/humrep/deh616 |
| 90 | Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and
oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80(5):913-9.
https://doi.org/10.1095/biolreprod.108.071779 |
| 91 | Jeng HA, Pan CH, Chao MR, Chiu CC, Zhou G, Chou CK, Lin WY. Sperm quality and DNA integrity of
coke oven workers exposed to polycyclic aromatic hydrocarbons. Int J Occup Med Environ Health.
2016;29(6):915-926.
https://doi.org/10.13075/ijomeh.1896.00598 |
| 92 | Jeng HA, Pan CH, Chao MR, Lin WY. Sperm DNA oxidative damage and DNA adducts. Mutat Res Genet
Toxicol Environ Mutagen. 2015;794:75-82.
https://doi.org/10.1016/j.mrgentox.2015.09.002 |
| 93 | Jeng HAC, Lin WY, Chao MR, Lin WY, Pan CH. Semen quality and sperm DNA damage associated with
oxidative stress in relation to exposure to polycyclic aromatic hydrocarbons. J Environ Sci Health A
Tox Hazard Subst Environ Eng. 2018;53(14):1221-1228.
https://doi.org/10.1080/10934529.2018.1528035 |
| 94 | Zhang MH, Shi ZD, Yu JC, Zhang YP, Wang LG, Qiu Y. Scrotal heat stress causes sperm chromatin
damage and cysteinyl aspartate-specific proteinases 3 changes in fertile men. J Assist Reprod Genet.
2015;32(5):747-55.
https://doi.org/10.1007/s10815-015-0451-0 |
| 95 | Zhang MH, Zhai LP, Fang ZY, Li AN, Xiao W, Qiu Y. Effect of scrotal heating on sperm quality,
seminal biochemical substances, and reproductive hormones in human fertile men. J Cell Biochem.
2018;119(12):10228-10238.
https://doi.org/10.1002/jcb.27365 |
| 96 | Kopalli SR, Cha KM, Hwang SY, Jeong MS, Kim SK. Korean Red Ginseng (Panax ginseng Meyer) with
enriched Rg3 ameliorates chronic intermittent heat stress-induced testicular damage in rats via
multifunctional approach. J Ginseng Res. 2019;43(1):135-142.
https://doi.org/10.1016/j.jgr.2018.06.004 |
| 97 | Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: A systematic review on
evaluation and management. Arab J Urol. 2019;17(2):87-97.
https://doi.org/10.1080/2090598X.2019.1599624 |
| 98 | Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J
Mens Health. 2014;32(1):1-17.
https://doi.org/10.5534/wjmh.2014.32.1.1 |
| 99 | Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol
Reprod Dev. 2017;84(10):1039-1052.
https://doi.org/10.1002/mrd.22871 |
| 100 | Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function--in sickness and in
health. J Androl. 2012;33(6):1096-106.
https://doi.org/10.2164/jandrol.112.016535 |
| 101 | Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial
reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol
Metab. 2008;93(8):3199-207.
https://doi.org/10.1210/jc.2007-2616 |
| 102 | Samland AK, Sprenger GA. Transaldolase: from biochemistry to human disease. Int J Biochem Cell
Biol. 2009;41(7):1482-94.
https://doi.org/10.1016/j.biocel.2009.02.001 |
| 103 | Kuznetsov AV, Margreiter R, Ausserlechner MJ, Hagenbuchner J. The Complex Interplay between
Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants (Basel). 2022;11(10):1995.
https://doi.org/10.3390/antiox11101995 |
| 104 | Perl A, Qian Y, Chohan KR, Shirley CR, Amidon W, Banerjee S, et al. Transaldolase is essential for
maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc Natl
Acad Sci U S A. 2006;103(40):14813-8.
https://doi.org/10.1073/pnas.0602678103 |
| 105 | Perl A. The pathogenesis of transaldolase deficiency. IUBMB Life. 2007;59(6):365-73.
https://doi.org/10.1080/15216540701387188 |
| 106 | Sotolongo B, Ward WS. DNA loop domain organization: the three-dimensional genomic code. J Cell
Biochem Suppl. 2000;Suppl 35:23-6.
https://doi.org/10.1002/1097-4644(2000)79:35+<23::AID-JCB1122>3.0.CO;2-N |
| 107 | Aitken RJ, Vernet P. Maturation of redox regulatory mechanisms in the epididymis. J Reprod Fertil
Suppl. 1998;53:109-18.
|
| 108 | Aitken RJ. Possible redox regulation of sperm motility activation. J Androl. 2000;21(4):491-6.
https://doi.org/10.1002/j.1939-4640.2000.tb02113.x |
| 109 | Aitken RJ, Baker HW, Irvine DS. On the nature of semen quality and infertility. Hum Reprod.
1995;10(2):248-9.
https://doi.org/10.1093/oxfordjournals.humrep.a135922 |
| 110 | Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, et al. Relative impact of oxidative
stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod.
1998;59(5):1037-46.
https://doi.org/10.1095/biolreprod59.5.1037 |
| 111 | Tremellen K. Oxidative stress and male infertility - a clinical perspective. Hum Reprod Update.
2008;14(3):243-58.
https://doi.org/10.1093/humupd/dmn004 |
| 112 | El-Taieb MA, Herwig R, Nada EA, Greilberger J, Marberger M. Oxidative stress and epididymal sperm
transport, motility and morphological defects. Eur J Obstet Gynecol Reprod Biol. 2009;144 Suppl
1:S199-203.
https://doi.org/10.1016/j.ejogrb.2009.02.018 |
| 113 | Fujii J, Iuchi Y, Matsuki S, Ishii T. Cooperative function of antioxidant and redox systems
against oxidative stress in male reproductive tissues. Asian J Androl. 2003;5(3):231-42.
|
| 114 | Garrido N, Meseguer M, Simon C, Pellicer A, Remohi J. Pro-oxidative and anti-oxidative imbalance
in human semen and its relation with male fertility. Asian J Androl. 2004;6(1):59-65.
|
| 115 | Gupta S, Finelli R, Agarwal A, Henkel R. Total antioxidant capacity-Relevance, methods and
clinical implications. Andrologia. 2021;53(2):e13624.
https://doi.org/10.1111/and.13624 |
| 116 | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in
normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44-84.
https://doi.org/10.1016/j.biocel.2006.07.001 |
| 117 | O'Flaherty C. Orchestrating the antioxidant defenses in the epididymis. Andrology.
2019;7(5):662-668.
https://doi.org/10.1111/andr.12630 |
| 118 | Brigelius-Flohé R, Flohé L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily.
Antioxid Redox Signal. 2020;33(7):498-516.
https://doi.org/10.1089/ars.2019.7905 |
| 119 | Yaris M, Akdogan N, Öztürk M, Bozkurt A, Karabakan M. The effects of two different antioxidant
combinations on sperm parameters. Urologia. 2022;89(4):629-635.
https://doi.org/10.1177/03915603211049888 |
| 120 | Sharma AP, Sharma G, Kumar R. Systematic Review and Meta-analysis on Effect of Carnitine, Coenzyme
Q10 and Selenium on Pregnancy and Semen Parameters in Couples With Idiopathic Male Infertility.
Urology. 2022;161:4-11.
https://doi.org/10.1016/j.urology.2021.10.041 |
| 121 | Zini A, Al-Hathal N. Antioxidant therapy in male infertility: fact or fiction? Asian J Androl.
2011;13(3):374-81.
https://doi.org/10.1038/aja.2010.182 |
| 122 | Lefer DJ, Scalia R, Campbell B, Nossuli T, Hayward R, Salamon M, et al. Peroxynitrite inhibits
leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J
Clin Invest. 1997;99(4):684-91.
https://doi.org/10.1172/JCI119212 |
| 123 | Ford WC, Whittington K. Antioxidant treatment for male subfertility: a promise that remains
unfulfilled. Hum Reprod. 1998;13(6):1416-9.
https://doi.org/10.1093/oxfordjournals.humrep.a019707 |
| 124 | Qamar AY, Naveed MI, Raza S, Fang X, Roy PK, Bang S, et al. Role of antioxidants in fertility
preservation of sperm - A narrative review. Anim Biosci. 2023;36(3):385-403.
https://doi.org/10.5713/ab.22.0325 |
| 125 | Rhemrev JP, van Overveld FW, Haenen GR, Teerlink T, Bast A, Vermeiden JP. Quantification of the
nonenzymatic fast and slow TRAP in a postaddition assay in human seminal plasma and the antioxidant
contributions of various seminal compounds. J Androl. 2000;21(6):913-20.
https://doi.org/10.1002/j.1939-4640.2000.tb03422.x |
| 126 | van Overveld FW, Haenen GR, Rhemrev J, Vermeiden JP, Bast A. Tyrosine as important contributor to
the antioxidant capacity of seminal plasma. Chem Biol Interact. 2000;127(2):151-61.
https://doi.org/10.1016/S0009-2797(00)00179-4 |
| 127 | Pérez-Pé R, Grasa P, Fernández-Juan M, Peleato ML, Cebrián-Pérez JA, Muiño-Blanco T. Seminal
plasma proteins reduce protein tyrosine phosphorylation in the plasma membrane of cold-shocked ram
spermatozoa. Mol Reprod Dev. 2002;61(2):226-33.
https://doi.org/10.1002/mrd.1152 |
| 128 | Cannarella R, Calogero AE, Condorelli RA, Giacone F, Mongio' LM, La Vignera S. Non-hormonal
treatment for male infertility: the potential role of Serenoa repens, selenium and lycopene. Eur Rev
Med Pharmacol Sci. 2019;23(7):3112-3120.
|
| 129 | Bromfield EG, Aitken RJ, Anderson AL, McLaughlin EA, Nixon B. The impact of oxidative stress on
chaperone-mediated human sperm-egg interaction. Hum Reprod. 2015;30(11):2597-613.
https://doi.org/10.1093/humrep/dev214 |
| 130 | de Lamirande E, O'Flaherty C. Sperm activation: role of reactive oxygen species and kinases.
Biochim Biophys Acta. 2008;1784(1):106-15.
https://doi.org/10.1016/j.bbapap.2007.08.024 |
| 131 | Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis
and its disorders. Endocr Rev. 2011;32(1):81-151.
https://doi.org/10.1210/er.2010-0013 |
| 132 | Xue Y, Cheng X, Xiong Y, Li K. Gene mutations associated with fertilization failure after in vitro
fertilization/intracytoplasmic sperm injection. Front Endocrinol (Lausanne). 2022;13:1086883.
https://doi.org/10.3389/fendo.2022.1086883 |
| 133 | Ofoedu CE, You L, Osuji CM, Iwouno JO, Kabuo NO, Ojukwu M, et al. Hydrogen Peroxide Effects on
Natural-Sourced Polysaccharides: Free Radical Formation/Production, Degradation Process, and
Reaction Mechanism-A Critical Synopsis. Foods. 2021;10(4):699.
https://doi.org/10.3390/foods10040699 |
| 134 | Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling
mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438.
https://doi.org/10.1155/2014/360438 |
| 135 | de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act
between beneficial and detrimental effects. Hum Reprod. 1995;10 Suppl 1:15-21.
https://doi.org/10.1093/humrep/10.suppl_1.15 |
| 136 | Almansa-Ordonez A, Bellido R, Vassena R, Barragan M, Zambelli F. Oxidative Stress in Reproduction:
A Mitochondrial Perspective. Biology (Basel). 2020;9(9):269.
https://doi.org/10.3390/biology9090269 |
| 137 | Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ Jr, Agarwal A. Differential
production of reactive oxygen species by subsets of human spermatozoa at different stages of
maturation. Hum Reprod. 2001;16(9):1922-30.
https://doi.org/10.1093/humrep/16.9.1922 |
| 138 | Champroux A, Damon-Soubeyrand C, Goubely C, Bravard S, Henry-Berger J, Guiton R, et al. Nuclear
Integrity but Not Topology of Mouse Sperm Chromosome is Affected by Oxidative DNA Damage. Genes
(Basel). 2018;9(10):501.
https://doi.org/10.3390/genes9100501 |
| 139 | Jiang L, Zheng T, Huang J, Mo J, Zhou H, Liu M, et al. Association of semen cytokines with
reactive oxygen species and histone transition abnormalities. J Assist Reprod Genet.
2016;33(9):1239-46.
https://doi.org/10.1007/s10815-016-0756-7 |
| 140 | Collodel G, Moretti E, Brecchia G, Kuželová L, Arruda J, Mourvaki E, et al. Cytokines release and
oxidative status in semen samples from rabbits treated with bacterial lipopolysaccharide.
Theriogenology. 2015;83(7):1233-40.
https://doi.org/10.1016/j.theriogenology.2015.01.008 |
| 141 | Wu TH, Hsieh SC, Li TH, Lu CH, Liao HT, Shen CY, Li KJ, Wu CH, Kuo YM, Tsai CY, Yu CL. Molecular
Basis for Paradoxical Activities of Polymorphonuclear Neutrophils in Inflammation/Anti-Inflammation,
Bactericide/Autoimmunity, Pro-Cancer/Anticancer, and Antiviral Infection/SARS-CoV-II-Induced
Immunothrombotic Dysregulation. Biomedicines. 2022;10(4):773.
https://doi.org/10.3390/biomedicines10040773 |
| 142 | Rosales C. Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol.
2020;108(1):377-396.
https://doi.org/10.1002/JLB.4MIR0220-574RR |
| 143 | Bassoy EY, Walch M, Martinvalet D. Reactive Oxygen Species: Do They Play a Role in Adaptive
Immunity? Front Immunol. 2021;12:755856.
https://doi.org/10.3389/fimmu.2021.755856 |
| 144 | Robinson JM. Reactive oxygen species in phagocytic leukocytes. Histochem Cell Biol.
2008;130(3):281-297.
https://doi.org/10.1007/s00418-008-0461-4 |
| 145 | Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress.
Int J Reprod Biomed. 2016;14(4):231-240 PMID: 27351024; PMCID: PMC4918773.
https://doi.org/10.29252/ijrm.14.4.231 |
| 146 | Krausz C, Cioppi F, Riera-Escamilla A. Testing for genetic contributions to infertility: potential
clinical impact. Expert Rev Mol Diagn. 2018;18(4):331-346.
https://doi.org/10.1080/14737159.2018.1453358 |
| 147 | Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15(6):369-384.
https://doi.org/10.1038/s41585-018-0003-3 |
| 148 | Stavros S, Potiris A, Molopodi E, Mavrogianni D, Zikopoulos A, Louis K, et al. Sperm DNA
Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence. Int.
J. Mol. Sci. 2024;25(18):10167.
https://doi.org/10.3390/ijms251810167 |
| 149 | Agarwal A, Cho CL, Majzoub A, Esteves SC. The Society for Translational Medicine: clinical
practice guidelines for sperm DNA fragmentation testing in male infertility. Transl Androl Urol.
2017;6(Suppl 4):S720-S733.
https://doi.org/10.21037/tau.2017.08.06 |
| 150 | Budzinska M, Kamieniczna M, Wojnar L, Gill K, Piasecka M, Kups M, et al. The role of the intrinsic
pathway of apoptosis in human ejaculated sperm damage under a state of scrotal heat stress. J Assist
Reprod Genet. 2024;41(1):99-108.
https://doi.org/10.1007/s10815-023-02992-9 |
| 151 | Cho CL, Agarwal A. Role of sperm DNA fragmentation in male factor infertility: A systematic
review. Arab J Urol. 2017;16(1):21-34.
https://doi.org/10.1016/j.aju.2017.11.002 |
| 152 | Muratori M, Tamburrino L, Marchiani S, Cambi M, Olivito B, Azzari C, et al. Investigation on the
Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol Med.
2015;21(1):109-122.
https://doi.org/10.2119/molmed.2014.00158 |
| 153 | Cannan WJ, Pederson DS. Mechanisms and Consequences of Double-Strand DNA Break Formation in
Chromatin. J Cell Physiol. 2016;231(1):3-14.
https://doi.org/10.1002/jcp.25048 |
| 154 | Dianov GL, Hübscher U. Mammalian base excision repair: the forgotten archangel. Nucleic Acids Res.
2013;41(6):3483-3490.
https://doi.org/10.1093/nar/gkt076 |
| 155 | Stinson BM, Loparo JJ. Repair of DNA Double-Strand Breaks by the Nonhomologous End Joining
Pathway. Annu Rev Biochem. 2021;90:137-164.
https://doi.org/10.1146/annurev-biochem-080320-110356 |
| 156 | Fleming AM, Burrows CJ. 8-Oxo-7, 8-dihydroguanine, friend and foe: Epigenetic-like regulator
versus initiator of mutagenesis. DNA Repair (Amst). 2017;56:75-83.
https://doi.org/10.1016/j.dnarep.2017.06.009 |
| 157 | Yuste VJ, Sánchez-López I, Solé C, Moubarak RS, Bayascas JR, Dolcet X, et al. The contribution of
apoptosis-inducing factor, caspase-activated DNase, and inhibitor of caspase-activated DNase to the
nuclear phenotype and DNA degradation during apoptosis. J Biol Chem. 2005;280(42):35670-35683.
https://doi.org/10.1074/jbc.M504015200 |
| 158 | Chua SC, Yovich SJ, Hinchliffe PM, Yovich JL. The Sperm DNA Fragmentation Assay with SDF Level
Less Than 15% Provides a Useful Prediction for Clinical Pregnancy and Live Birth for Women Aged
under 40 Years. J Pers Med. 2023;13(7):1079.
https://doi.org/10.3390/jpm13071079 |
| 159 | Leslie SW, Soon-Sutton TL, Khan MAB. Male Infertility [Updated 2024 Feb 25].. In: StatPearls
[Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK562258/.
|
| 160 | Aydos OS, Yükselten Y, Kaplan F, Sunguroğlu A, Aydos K. Analysis of the correlation between sperm
DNA integrity and conventional semen parameters in infertile men. Turk J Urol. 2015;41(4):191-197.
https://doi.org/10.5152/tud.2015.98475 |
| 161 | Gosálvez J, Johnston SD, Prado A, López-Fernández C, Contreras P, Bartolomé-Nebreda J,
González-Martínez M, Fernández JL, de la Vega CG, Góngora A. Strong Correlation Between
Double-Strand DNA Breaks and Total Sperm DNA Fragmentation in the Human Ejaculate. Arch Med Res.
2024;55(8):103122.
https://doi.org/10.1016/j.arcmed.2024.103122 |
| 162 | Sahin GN, Yildirim RM, Seli E. Embryonic arrest: causes and implications. Curr Opin Obstet
Gynecol. 2023;35(3):184-192.
https://doi.org/10.1097/GCO.0000000000000871 |
| 163 | Kaltsas A, Dimitriadis F, Zachariou D, Zikopoulos A, Symeonidis EN, Markou E, Tien DMB, Takenaka
A, Sofikitis N, Zachariou A. From Diagnosis to Treatment: Comprehensive Care by Reproductive
Urologists in Assisted Reproductive Technology. Medicina (Kaunas). 2023;59(10):1835.
https://doi.org/10.3390/medicina59101835 |
| 164 | Afzal S, Abdul Manap AS, Attiq A, Albokhadaim I, Kandeel M, Alhojaily SM. From imbalance to
impairment: the central role of reactive oxygen species in oxidative stress-induced disorders and
therapeutic exploration. Front Pharmacol. 2023;14:1269581.
https://doi.org/10.3389/fphar.2023.1269581 |
| 165 | Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol.
2014;24(10):R453-462.
https://doi.org/10.1016/j.cub.2014.03.034 |
| 166 | Tantengco OAG, de Castro Silva M, Shahin H, Bento GFC, Cursino GC, Cayenne S, da Silva MG, Menon
R. The role of nuclear factor erythroid 2-related factor 2 (NRF2) in normal and pathological
pregnancy: A systematic review. Am J Reprod Immunol. 2021;86(6):e13496.
https://doi.org/10.1111/aji.13496 |
| 167 | Han P, Wang X, Zhou T, Cheng J, Wang C, Sun F, Zhao X. Inhibition of ferroptosis attenuates
oligospermia in male Nrf2 knockout mice. Free Radic Biol Med. 2022;193(Pt 1):421-429.
https://doi.org/10.1016/j.freeradbiomed.2022.10.314 |
| 168 | Akino N, Wada-Hiraike O, Isono W, Terao H, Honjo H, Miyamoto Y, Tanikawa M, Sone K, Hirano M,
Harada M, Hirata T, Hirota Y, Koga K, Oda K, Fujii T, Osuga Y. Activation of Nrf2/Keap1 pathway by
oral Dimethylfumarate administration alleviates oxidative stress and age-associated infertility
might be delayed in the mouse ovary. Reprod Biol Endocrinol. 2019;17(1):23.
https://doi.org/10.1186/s12958-019-0466-y |
| 169 | Valipour J, Taghizadeh F, Esfahani R, Ramesh M, Rastegar T. Role of nuclear factor erythroid
2-related factor 2 (Nrf2) in female and male fertility. Heliyon. 2024;10(9):e29752.
https://doi.org/10.1016/j.heliyon.2024.e29752 |
| 170 | Falvo S, Minucci S, Santillo A, Senese R, Chieffi Baccari G, Venditti M. A short-term high-fat
diet alters rat testicular activity and blood-testis barrier integrity through the SIRT1/NRF2/MAPKs
signaling pathways. Front Endocrinol (Lausanne). 2023;14:1274035.
https://doi.org/10.3389/fendo.2023.1274035 |
| 171 | Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive
technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411-26.
https://doi.org/10.1093/humupd/dmv016 |
| 172 | Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 2010;690(1-2):12-23.
https://doi.org/10.1016/j.mrfmmm.2009.09.007 |
| 173 | Baird L, Swift S, Llères D, Dinkova-Kostova AT. Monitoring Keap1-Nrf2 interactions in single live
cells. Biotechnol Adv. 2014;32(6):1133-44.
https://doi.org/10.1016/j.biotechadv.2014.03.004 |
| 174 | Abu Shelbayeh O, Arroum T, Morris S, Busch KB. PGC-1α Is a Master Regulator of Mitochondrial
Lifecycle and ROS Stress Response. Antioxidants (Basel). 2023;12(5):1075.
https://doi.org/10.3390/antiox12051075 |
| 175 | Liu P, Shi J, Sheng D, Lu W, Guo J, Gao L, Wang X, Wu S, Feng Y, Dong D, Huang X, Tang H.
Mitopherogenesis, a form of mitochondria-specific ectocytosis, regulates sperm mitochondrial
quantity and fertility. Nat Cell Biol. 2023;25(11):1625-1636.
https://doi.org/10.1038/s41556-023-01264-z |
| 176 | Zhou J, Yang Z, Shen R, Zhong W, Zheng H, Chen Z, Tang J, Zhu J. Resveratrol Improves
Mitochondrial Biogenesis Function and Activates PGC-1α Pathway in a Preclinical Model of Early Brain
Injury Following Subarachnoid Hemorrhage. Front Mol Biosci. 2021;8:620683.
https://doi.org/10.3389/fmolb.2021.620683 |
| 177 | Koshinaka K, Honda A, Masuda H, Sato A. Effect of Quercetin Treatment on Mitochondrial Biogenesis
and Exercise-Induced AMP-Activated Protein Kinase Activation in Rat Skeletal Muscle. Nutrients.
2020;12(3):729.
https://doi.org/10.3390/nu12030729 |
| 178 | Anilkumar S, Wright-Jin E. NF-κB as an Inducible Regulator of Inflammation in the Central Nervous
System. Cells. 2024;13(6):485.
https://doi.org/10.3390/cells13060485 |
| 179 | Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol.
2009;1(6):a001651.
https://doi.org/10.1101/cshperspect.a001651 |
| 180 | Mamun AA, Shao C, Geng P, Wang S, Xiao J. Polyphenols Targeting NF-κB Pathway in Neurological
Disorders: What We Know So Far? Int J Biol Sci. 2024;20(4):1332-1355.
https://doi.org/10.7150/ijbs.90982 |
| 181 | Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters:
An evidence-based review. Int J Reprod Biomed. 2016;14(12):729-736.
https://doi.org/10.29252/ijrm.14.12.729 |
| 182 | Mason MM, Schuppe K, Weber A, Gurayah A, Muthigi A, Ramasamy R. Ejaculation: the Process and
Characteristics From Start to Finish. Curr Sex Health Rep. 2023;15(1):1-9.
https://doi.org/10.1007/s11930-022-00340-z |
| 183 | Durairajanayagam D, Agarwal A, Ong C, Prashast P. Lycopene and male infertility. Asian J Androl.
2014;16(3):420-425.
https://doi.org/10.4103/1008-682X.126384 |
| 184 | Britton G, Khachik F. Carotenoids in Food. In: Britton G, Pfander H, Liaaen-Jensen S (eds)
Carotenoids. Carotenoids, vol 5 Birkhäuser Basel. 2009 https://doi.org/10.1007/978-3-7643-7501-0_3.
https://doi.org/10.1007/978-3-7643-7501-0_3 |
| 185 | Dias MG, Olmedilla-Alonso B, Hornero-Méndez D, Mercadante AZ, Osorio C, Vargas-Murga L et al.
Comprehensive Database of Carotenoid Contents in Ibero-American Foods. J Agric Food Chem.
2018;66(20):5055-507.
https://doi.org/10.1021/acs.jafc.7b06148 |
| 186 | Alahmar AT. The effects of oral antioxidants on the semen of men with idiopathic
oligoasthenoteratozoospermia. Clin Exp Reprod Med. 2018;45(2):57-66.
https://doi.org/10.5653/cerm.2018.45.2.57 |
| 187 | Ramgir SS, Renu K, Vellingiri B, George A, Tirupapuliyur D, Thiagarajan P et al. Phytomedicinal
therapeutics for male infertility: critical insights and scientific updates. J Nat Med.
2022;76(3):546-573.
https://doi.org/10.1007/s11418-022-01619-0 |
| 188 | Sengupta P, Agarwal A, Pogrebetskaya M, Roychoudhury S, Durairajanayagam D, Henkel R. Role of
Withania somnifera (Ashwagandha) in the management of male infertility. Reprod Biomed Online.
2018;36(3):311-326.
https://doi.org/10.1016/j.rbmo.2017.11.007 |
| 189 | Abarikwu SO, Onuah CL, Singh SK. Plants in the management of male infertility. Andrologia.
2020;52(3):e13509.
https://doi.org/10.1111/and.13509 |
| 190 | El-Shimi BI, Mohareb RM, Ahmed HH, Abohashem RS, Mahmoud KF, Hanna DH. Mechanistic Insights into
Bisphenol A-Mediated Male Infertility: Potential Role of Panax Ginseng Extract. Chem Biodivers.
2024;21(9):e202400480.
https://doi.org/10.1002/cbdv.202400480 |
| 191 | Salvati G, Genovesi G, Marcellini L, Paolini P, De Nuccio I, Pepe M et al. Effects of Panax
Ginseng C.A. Meyer saponins on male fertility. Panminerva Med. 1996;38(4):249-254 PMID: 9063034.
|
| 192 | Essawy A, Matar S, Mohamed N, Abdel-Wahab W, Abdou H. Ginkgo biloba extract protects against
tartrazine-induced testicular toxicity in rats: involvement of antioxidant, anti-inflammatory, and
anti-apoptotic mechanisms. Environ Sci Pollut Res Int. 2024;31(10):15065-15077.
https://doi.org/10.1007/s11356-024-32047-0 |
| 193 | Yeh YC, Liu TJ, Wang LC, Lee HW, Ting CT, Lee WL et al. A standardized extract of Ginkgo biloba
suppresses doxorubicin-induced oxidative stress and p53-mediated mitochondrial apoptosis in rat
testes. Br J Pharmacol. 2009;156(1):48-61.
https://doi.org/10.1111/j.1476-5381.2008.00042.x |
| 194 | Amin A, Mahmoud-Ghoneim D, Syam MI, Daoud S. Neural network assessment of herbal protection
against chemotherapeutic-induced reproductive toxicity. Theor Biol Med Model. 2012;9:1.
https://doi.org/10.1186/1742-4682-9-1 |
| 195 | Sengupta P, Agarwal A, Pogrebetskaya M, Roychoudhury S, Durairajanayagam D, Henkel R. Role of
Withania somnifera (Ashwagandha) in the management of male infertility. Reprod Biomed Online.
2018;36(3):311-326.
https://doi.org/10.1016/j.rbmo.2017.11.007 |
| 196 | Boroujeni SN, Malamiri FA, Bossaghzadeh F, Esmaeili A, Moudi E. The most important medicinal
plants affecting sperm and testosterone production: a systematic review. JBRA Assist Reprod.
2022;26(3):522-530.
https://doi.org/10.5935/1518-0557.20210108 |
| 197 | Dutta S, Sengupta P, Slama P, Roychoudhury S. Oxidative Stress, Testicular Inflammatory Pathways,
and Male Reproduction. Int J Mol Sci. 2021;22(18):10043.
https://doi.org/10.3390/ijms221810043 |
| 198 | Tafuri S, Cocchia N, Vassetti A, Carotenuto D, Esposito L, Maruccio L, Avallone L, Ciani F.
Lepidium meyenii (Maca) in male reproduction. Nat Prod Res. 2021;35(22):4550-4559.
https://doi.org/10.1080/14786419.2019.1698572 |
| 199 | Valerio LG Jr, Gonzales GF. Toxicological aspects of the South American herbs cat's claw (Uncaria
tomentosa) and Maca (Lepidium meyenii) : a critical synopsis. Toxicol Rev. 2005;24(1):11-35.
https://doi.org/10.2165/00139709-200524010-00002 |
| 200 | Cicero AF, Piacente S, Plaza A, Sala E, Arletti R, Pizza C. Hexanic Maca extract improves rat
sexual performance more effectively than methanolic and chloroformic Maca extracts. Andrologia.
2002;34(3):177-9.
https://doi.org/10.1046/j.1439-0272.2002.00490.x |
| 201 | Haghmorad D, Mahmoudi MB, Haghighi P, Alidadiani P, Shahvazian E, Tavasolian P, et al. Improvement
of fertility parameters with Tribulus Terrestris and Anacyclus Pyrethrum treatment in male rats. Int
Braz J Urol. 2019;45(5):1043-1054.
https://doi.org/10.1590/s1677-5538.ibju.2018.0843 |
| 202 | Khaleghi S, Bakhtiari M, Asadmobini A, Esmaeili F. Tribulus terrestris Extract Improves Human
Sperm Parameters In vitro. J Evid Based Complementary Altern Med. 2017;22(3):407-412.
https://doi.org/10.1177/2156587216668110 |
| 203 | Salgado RM, Marques-Silva MH, Gonçalves E, Mathias AC, Aguiar JG, Wolff P. Effect of oral
administration of Tribulus terrestris extract on semen quality and body fat index of infertile men.
Andrologia. 2017;49(5).
https://doi.org/10.1111/and.12655 |
| 204 | Biswas TK, Pandit S, Mondal S, Biswas SK, Jana U, Ghosh T, et al. Clinical evaluation of
spermatogenic activity of processed Shilajit in oligospermia. Andrologia. 2010;42(1):48-56.
https://doi.org/10.1111/j.1439-0272.2009.00956.x |
| 205 | Wilson E, Rajamanickam GV, Dubey GP, Klose P, Musial F, Saha FJ, et al. Review on shilajit used in
traditional Indian medicine. J Ethnopharmacol. 2011;136(1):1-9.
https://doi.org/10.1016/j.jep.2011.04.033 |
| 206 | Sadogh A, Gorji N, Moeini R. Herbal foodstuffs in Avicenna's recommended diet to improve sperm
quality and increase male fertility; an evidence-based approach. J Complement Integr Med.
202;19(1):47-70.
https://doi.org/10.1515/jcim-2020-0254 |
| 207 | Morgia G, Mucciardi G, Galì A, Madonia M, Marchese F, Di Benedetto A, Romano G, Bonvissuto G,
Castelli T, Macchione L, Magno C. Treatment of chronic prostatitis/chronic pelvic pain syndrome
category IIIA with Serenoa repens plus selenium and lycopene (Profluss) versus S. repens alone: an
Italian randomized multicenter-controlled study. Urol Int. 2010;84(4):400-6.
https://doi.org/10.1159/000302716 |
| 208 | He W, Liu H, Hu L, Wang Y, Huang L, Liang A, et al. Icariin improves testicular dysfunction via
enhancing proliferation and inhibiting mitochondria-dependent apoptosis pathway in high-fat diet and
streptozotocin-induced diabetic rats. Reprod Biol Endocrinol. 2021;19(1):168.
https://doi.org/10.1186/s12958-021-00851-9 |
| 209 | Liu F, Liao B, Ling YL, Meng XZ, Wang JL, Hu LL, et al. Icariin protects testicular damage in
streptozotocin-induced diabetic rats through regulation of glycolysis pathway. Int J Immunopathol
Pharmacol. 2024;38:3946320241279525.
https://doi.org/10.1177/03946320241279525 |
| 210 | Khazaei MR, Gravandi E, Ghanbari E, Niromand E, Khazaei M. Trifolium pratense extract increases
testosterone and improves sperm characteristics and antioxidant status in diabetic rats. Biotech
Histochem. 2022;97(8):576-583.
https://doi.org/10.1080/10520295.2022.2039766 |
| 211 | Li X, Zeng YM, Luo YD, He J, Luo BW, et al. Effects of folic acid and folic acid plus zinc
supplements on the sperm characteristics and pregnancy outcomes of infertile men: A systematic
review and meta-analysis. Heliyon. 2023;9(7):e18224.
https://doi.org/10.1016/j.heliyon.2023.e18224 |
| 212 | Salas-Huetos A, Rosique-Esteban N, Becerra-Tomás N, Vizmanos B, Bulló M, Salas-Salvadó J. The
Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and
Meta-Analysis of Randomized Clinical Trials. Adv Nutr. 2018;9(6):833-848.
https://doi.org/10.1093/advances/nmy057 |
| 213 | Laterza L., Rizzatti G., Gaetani E., Chiusolo P., Gasbarrini A. The gut microbiota and immune
system relationship in human graft-versus-host disease. Mediterr. J. Hematol. Infect. Dis.
2016;8:e2016025.
https://doi.org/10.4084/mjhid.2016.025 |
| 214 | Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and
health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577-589.
https://doi.org/10.1038/nrgastro.2012.156 |
| 215 | Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the
Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and
Diseases. Microorganisms. 2019;7(1):14.
https://doi.org/10.3390/microorganisms7010014 |
| 216 | Lv S, Huang J, Luo Y, Wen Y, Chen B, Qiu H, et al. Gut microbiota is involved in male reproductive
function: a review. Front Microbiol. 2024;15:1371667.
https://doi.org/10.3389/fmicb.2024.1371667 |
| 217 | Madhogaria B, Bhowmik P, Kundu A. Correlation between human gut microbiome and diseases. Infect
Med (Beijing). 2022;1(3):180-191.
https://doi.org/10.1016/j.imj.2022.08.004 |
| 218 | Matijašić M, Meštrović T, Paljetak HČ, Perić M, Barešić A, Verbanac D. Gut Microbiota beyond
Bacteria-Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int J Mol Sci.
2020;21(8):2668.
https://doi.org/10.3390/ijms21082668 |
| 219 | Liang G, Bushman FD. The human virome: assembly, composition and host interactions. Nat Rev
Microbiol. 2021;19(8):514-527.
https://doi.org/10.1038/s41579-021-00536-5 |
| 220 | Bonaz B, Sinniger V, Pellissier S. Vagus Nerve Stimulation at the Interface of Brain-Gut
Interactions. Cold Spring Harb Perspect Med. 2019;9(8):a034199.
https://doi.org/10.1101/cshperspect.a034199 |
| 221 | Dubey I, K N, G V, Rohilla G, Lalruatmawii, Naxine P, P J, Rachamalla M, Kushwaha S. Exploring the
hypothetical links between environmental pollutants, diet, and the gut-testis axis: The potential
role of microbes in male reproductive health. Reprod Toxicol. 2024130:108732.
https://doi.org/10.1016/j.reprotox.2024.108732 |
| 222 | Basnet, J., Eissa, M.A.; Yanes Cardozo, L.L.; Romero, D.G.; Rezq, S. Impact of Probiotics and
Prebiotics on Gut Microbiome and Hormonal Regulation. Gastrointest. Disord. 2024; 6:801-815
https://doi.org/10.3390/gidisord6040056
https://doi.org/10.3390/gidisord6040056 |
| 223 | Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty
acids in health and disease. Adv Immunol. 2014;121:91-119.
https://doi.org/10.1016/B978-0-12-800100-4.00003-9 |
| 224 | Cano Sokoloff N, Misra M, Ackerman KE. Exercise, Training, and the Hypothalamic-Pituitary-Gonadal
Axis in Men and Women. Front Horm Res. 2016;47:27-43.
https://doi.org/10.1159/000445154 |
| 225 | Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation,
and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024; 13: 985
https://doi.org/10.3390/antiox13080985
https://doi.org/10.3390/antiox13080985 |
| 226 | Corral-Vazquez C, Blanco J, Sarrate Z, Anton E. Unraveling the Intricacies of the Seminal
Microbiome and Its Impact on Human Fertility. Biology (Basel). 2024;13(3):150.
https://doi.org/10.3390/biology13030150 |
| 227 | De Oliveira FL, Salgaço MK, de Oliveira MT, Mesa V, Sartoratto A, Peregrino AM, Ramos WS, Sivieri
K. Exploring the Potential of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 as
Promising Psychobiotics Using SHIME. Nutrients. 2023;15(6):1521.
https://doi.org/10.3390/nu15061521 |
| 228 | Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation,
and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024; 13: 985
https://doi.org/10.3390/antiox13080985
https://doi.org/10.3390/antiox13080985 |
| 229 | Raheem A, Liang L, Zhang G, Cui S. Modulatory Effects of Probiotics During Pathogenic Infections
With Emphasis on Immune Regulation. Front Immunol. 2021;12:616713.
https://doi.org/10.3389/fimmu.2021.616713 |
| 230 | Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-Inflammatory and
Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front Immunol.
2021;12:578386.
https://doi.org/10.3389/fimmu.2021.578386 |
| 231 | Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol
Rev. 2012;64(1):16-64.
https://doi.org/10.1124/pr.110.002790 |
| 232 | Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel
disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223-237.
https://doi.org/10.1038/s41575-019-0258-z |
| 233 | Sánchez IA, Cuchimba JA, Pineda MC, Argüello YP, Kočí J, Kreider RB, Petro JL, Bonilla DA.
Adaptogens on Depression-Related Outcomes: A Systematic Integrative Review and Rationale of
Synergism with Physical Activity. Int J Environ Res Public Health. 2023;20(7):5298.
https://doi.org/10.3390/ijerph20075298 |
| 234 | Simon E, Călinoiu LF, Mitrea L, Vodnar DC. Probiotics, Prebiotics, and Synbiotics: Implications
and Beneficial Effects against Irritable Bowel Syndrome. Nutrients. 2021;13(6):2112.
https://doi.org/10.3390/nu13062112 |
| 235 | Thangaleela S, Sivamaruthi BS, Kesika P, Chaiyasut C. Role of Probiotics and Diet in the
Management of Neurological Diseases and Mood States: A Review. Microorganisms. 2022;10(11):2268.
https://doi.org/10.3390/microorganisms10112268 |
| 236 | Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ Jr, et al. Differential
production of reactive oxygen species by subsets of human spermatozoa at different stages of
maturation. Hum Reprod. 2001;16(9):1922-30.
https://doi.org/10.1093/humrep/16.9.1922 |
| 237 | Greco F., Guarascio G., Giannetta E., Oranges F.P., Quinzi F., et al.The Influence of an Intense
Training Regime in Professional and Non-Professional Athletes on Semen Parameters: A Systematic
Review. J. Clin. Med. 2025; 14:201 https://doi.org/10.3390/jcm14010201
https://doi.org/10.3390/jcm14010201 |
| 238 | Jóźków P, Rossato M. The Impact of Intense Exercise on Semen Quality. Am J Mens Health.
2017;11(3):654-662.
https://doi.org/10.1177/1557988316669045 |
| 239 | Manouchehri A, Shokri S, Pirhadi M, Karimi M, Abbaszadeh S, Mirzaei G, Bahmani M. The Effects of
Toxic Heavy Metals Lead, Cadmium and Copper on the Epidemiology of Male and Female Infertility. JBRA
Assist Reprod. 2022;26(4):627-630.
https://doi.org/10.5935/1518-0557.20220013 |
| 240 | Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but
not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril.
1994;62(2):387-93.
https://doi.org/10.1016/S0015-0282(16)56895-2 |
| 241 | Dutta S, Gorain B, Choudhury H, Roychoudhury S, Sengupta P. Environmental and occupational
exposure of metals and female reproductive health. Environ Sci Pollut Res Int.
2022;29(41):62067-62092.
https://doi.org/10.1007/s11356-021-16581-9 |
| 242 | Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, et al. Leukocytospermia is
associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril.
2002;78(6):1215-24.
https://doi.org/10.1016/S0015-0282(02)04237-1 |
| 243 | Sengupta P, Pinggera GM, Calogero AE, Agarwal A. Oxidative stress affects sperm health and
fertility-Time to apply facts learned at the bench to help the patient: Lessons for busy clinicians.
Reprod Med Biol. 2024;23(1):e12598.
https://doi.org/10.1002/rmb2.12598 |
| 244 | Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, Oxidative
Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol.
2020;11:694 .
https://doi.org/10.3389/fphys.2020.00694 |
| 245 | Mintziori G, Nigdelis MP, Mathew H, Mousiolis A, Goulis DG, Mantzoros CS. The effect of excess
body fat on female and male reproduction. Metabolism. 2020;107:154193.
https://doi.org/10.1016/j.metabol.2020.154193 |
| 246 | Tsametis C, Mintziori G, Iliadou PK, Tarlatzis BC, Papadimas I, Goulis DG. Dynamic endocrine test
of inhibin B and anti-Müllerian hormone in men with non-obstructive azoospermia. Gynecol Endocrinol.
2011;27(9):661-5.
https://doi.org/10.3109/09513590.2010.521267 |
| 247 | Gromenko YY, Galimov KS, Gilyazova IR, Galimova EF, Bulygin KV, Ryagin SN, Galimov SN, Litvitskiy
PF, Piavchenko GA, Pavlov VN. Single nucleotide polymorphism rs527236194 of the cytochrome B gene
(MT-CYB) is associated with alterations in sperm parameters. Mol Biol Rep. 2023; 50(12):10131-10136.
https://doi.org/10.1007/s11033-023-08849-9 |