Migrasomes in Health and Disease: Insights into Mechanisms, Pathogenesis, and Therapeutic Opportunities
bDepartment of Human Physiology and Pathophysiology, School of Medicine, Collegium Medicum, University of Warmia and Mazury in Olsztyn, Warszawska 30, 10-082 Olsztyn, Poland,
cDepartment of Morphological and Physiological Sciences, Faculty of Medicine, Collegium Medicum, Mazovian Academy in Płock, 09-402 Płock, Poland
Keywords
Abstract
Migrasomes are newly discovered, migration-dependent organelles that mediate the release of cellular contents into the extracellular environment through a process known as migracytosis. Since their identification in 2014, growing evidence has highlighted their critical roles in intercellular communication, organ development, mitochondrial quality control, and disease pathogenesis. Migrasome biogenesis is a complex, multi-step process tightly regulated by lipid composition, tetraspanin-enriched microdomains, and molecular pathways involving sphingomyelin synthase 2, Rab35, and integrins. Unlike exosomes, migrasomes possess distinct structural and functional characteristics, which position them as novel organelles rather than classic extracellular vesicles. Recent studies have revealed their involvement in diverse pathological contexts, including kidney disease, cancer progression, proliferative vitreoretinopathy, viral infections, and myocardial infarction. Notably, migrasomes hold promise as diagnostic biomarkers, especially in early podocyte injury, and as therapeutic targets in oncology and regenerative medicine. This review summarizes the current understanding of migrasome biology, and their implications in health and disease, and explores emerging perspectives on harnessing migrasomes for diagnostic and therapeutic applications.
Introduction
Migrasomes and the process of migracytosis were first identified in 2014 by Liang Ma et al [1]. Using transmission electron microscopy (TEM) on rat kidney cells, they observed that migrasomes originate from retraction fibers and can either be taken up by neighboring cells or break down, releasing their contents into the surrounding microenvironment. Migracytosis, a migration-dependent mechanism, facilitates the release of cellular components, including migrasomes, which are essential for this process. Liang Ma et al. described these organelles as having a diameter between 0.5 and 3 μm and a distinctive pomegranate-like morphology [2, 3]. Migrasomes have been identified in diverse cell types, tissues, and physiological fluids, where they serve an essential role in intercellular communication [4]. This communication is critical for coordinating cellular responses in multicellular organisms. Emerging evidence suggests that migrasome activity is linked to cancer cell behavior, as they may contribute to processes such as migration and tumor progression [5]. If cell migration contributes to cancer development, it prompts the question of whether it could be utilised to regulate disease progression.
Although research on the role of migrasomes in health and disease remains in its early stages, several studies have highlighted their involvement in diverse biological processes. These include mitochondrial quality control, stroke, soft tissue regeneration through stem cell recruitment, pancreatic cancer progression, and their potential as diagnostic markers for kidney disease. Additionally, migrasomes have been implicated in hepatocellular carcinoma (HCC) progression, angiogenesis, viral infections, proliferative vitreoretinopathy, and leukemia. Given their presence in physiological and pathological conditions, understanding migrasomes may provide novel insights into disease mechanisms. This review aims to consolidate the latest advancements in migrasome research and to identify critical areas requiring further investigation, to unlock their potential for therapeutic applications.
Migrasome biogenesis
Overview
Migrasomes are large vesicular structures that form on retraction fibers, which extend from the trailing
edge of
migrating cells. Their biogenesis is inherently linked to cell migration, with multiple molecular factors
playing critical roles in their formation. At the molecular level, migrasome development occurs in two
distinct
phases: the growth phase and the stabilization phase. The growth phase can be further divided into three
successive stages: nucleation, maturation, and expansion. Each of these stages involves specific molecular
mechanisms that regulate the complex process of migrasome formation. Prior to their biogenesis, Liang et
al.
observed significant biochemical activity at migrasome formation sites (MFSs), suggesting a preparatory
phase
that occurs prior to migrasome biogenesis [6].
Nucleation
The growth phase consists of a dynamic 3-stage process which involves nucleation, maturation and expansion
facilitated by protein recruitment and cell migration [4, 7]. There are several factors which play crucial
roles
within these stages. Numerous elements significantly influence these stages. Sphingomyelin (SM), as
explained in
Fig. 1A, is a specialized sphingolipid associated with organelles, playing crucial roles in cell signaling
and
the formation of nanodomains with cholesterol and ceramide [8]. Sphingomyelin (SM) is characterized as a
membrane-stabilizing lipid and exhibits greater biochemical stability compared to phospholipids, as it may
be
hydrolysed solely by a particular phospholipase known as sphingomyelinase, which is considerably rarer in
mammals. A study by Felix M. Goñi stated that SM and other phospholipases are distinguished by their
organelle-specificity. In contrast to most phospholipids, such as the structurally similar
phosphatidylcholine,
SM is not evenly distributed across organelle membranes, but it is predominantly found in the plasma
membrane,
the Golgi network, and the lysosome [8, 9]. During the nucleation stage, on the migrating cells, fixed
sphingomyelin synthase 2 (SMS2) foci are formed on the basal membranes of migrating cells located on
retraction
fibers. SMS2 is an enzyme primarily located on the plasma membrane and Golgi apparatus that acts as the
essential protein converting ceramide to sphingomyelin [10]. The anchored SMS2 foci act as starting points
for
the formation of migrasomes known as migrasome formation sites (MFSs) [11]. Liang et al. demonstrated the
essential role of SMS2 in migrasome formation by creating an SMS2 mutant, lacking the ability to form foci
along
the basal membranes. As a result of this, there was a significant decrease in the number of migrasomes
formed,
despite introducing exogenous SM [11]. Zhai et al. suggested that the de novo synthesis of SM is crucial
to
migrasome formation by maintaining structural integrity [7]. Similarly, Ding et al. demonstrated that when
the
crucial protein SMS2 was mutated, a decrease in migrasome numbers was observed, whereas, by blocking
fosfatydylo-D-mio-inozytolo-4, 5-bisfosforan (PI(4, 5)P2), the entire migrasome formation process was
inhibited,
emphasizing the integral role of PI(4, 5)P2 and Rab35 as a PI(4, 5)P2-binding protein within this process
[12].
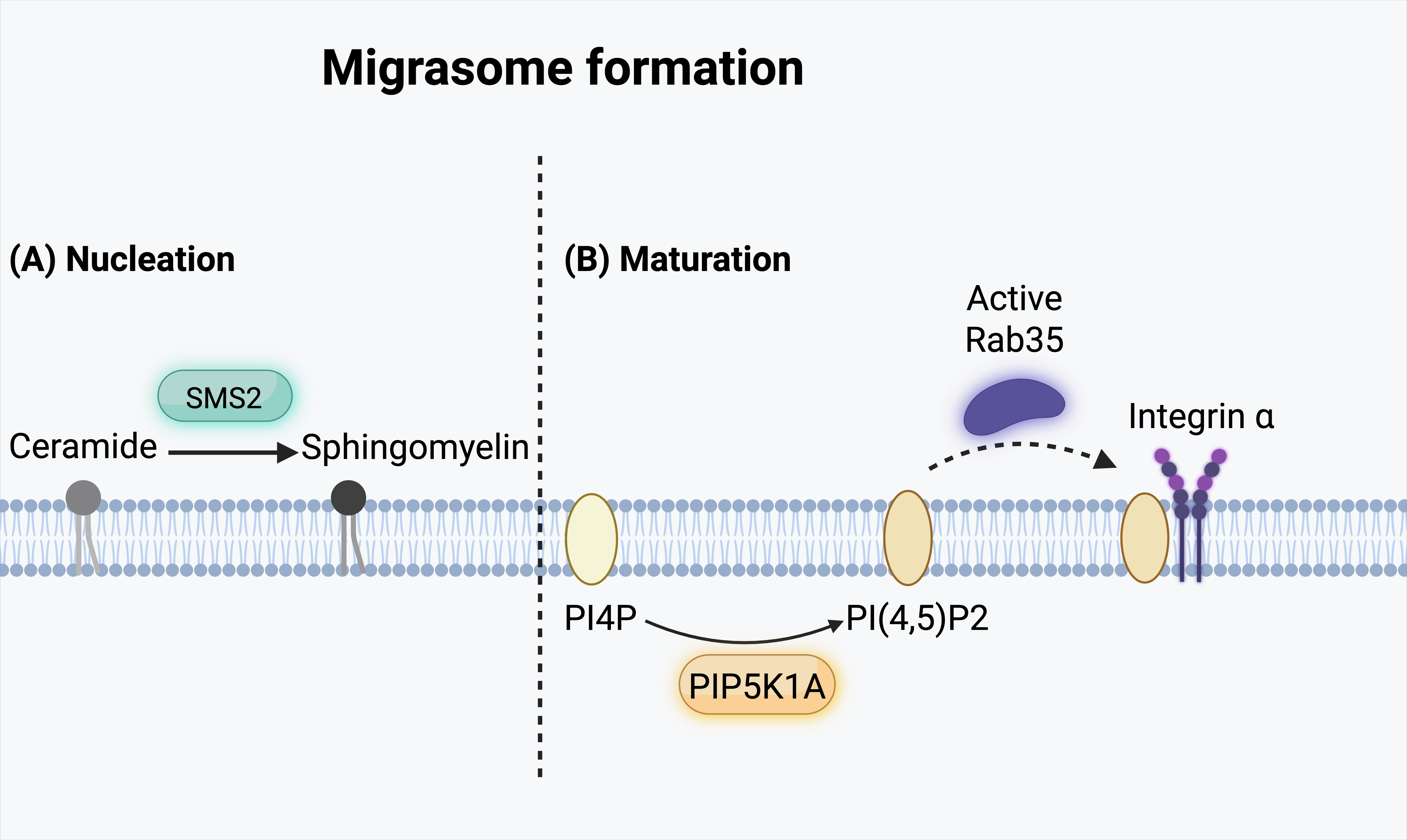
Fig. 1: Migrasome formation process. The formation of migrasomes involves two key steps: (A) Nucleation and (B) Maturation. (A) During the nucleation phase, sphingomyelin synthase 2 (SMS2) catalyzes the conversion of ceramide into sphingomyelin in the plasma membrane. (B) In the maturation phase, phosphatidylinositol 4-phosphate (PI4P) is phosphorylated by phosphatidylinositol-4-phosphate 5-kinase type I alpha (PIP5K1A) to generate phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P₂]. The recruitment of active Rab35 further promotes migrasome maturation, while the accumulation of α integrin at the migrasome membrane supports its structural organization.
Maturation
Marking the initiation of the maturation stage, a lipid signaling molecule called
phosphatidylinositol-4-phosphate 5-kinase type 1 alpha (PIP5K1A), is recruited to the retraction
filaments,
facilitating the conversion of phosphatidylinositol-4-phosphate (PI4P) to the multi-functional
phosphatidylinositol (4, 5)-bisphosphate (PI(4, 5)P2). Through the use of an anti-PI(4, 5)P2 antibody to
label
cells, Ding et al. demonstrated that migrasomes were enriched with PI(4, 5)P2. Furthermore, by comparing
its
recruitment to that of integrin and Tetraspanin-4 (TSPAN4) using time-lapse imaging and probes, Ding et
al.
proved that PI(4, 5)P2 plays an important role in the primary steps of migrasome formation. The image
indicated
that the recruitment of the pleckstrin homology domain of phospholipase C gamma fused to green fluorescent
protein (PLCγ-PH-GFP), a probe for phosphatidylinositol 4, 5-bisphosphate (PI(4, 5)P₂), occurred prior to
that
of TSPAN4. Employing the same methodology, they demonstrated that PLC𝛾-PH-TagBFP exhibited a marginally
superior speed to integrin a5. Through merging these images, Ding et al. established that the generation
of
PI(4, 5)P2 preceded the recruitment of integrin and TSPAN4, thus occuring before migrasome formation [13].
An important protein in organelle biogenesis is Rab35, which, when activated, binds to Integrin ⍺5 (Itga5)
and
facilitates its recruitment to MFSs. Ding et al. demonstrated that Rab35 localises to punctate structures
along
retraction fibers and that its recruitment precedes migrasome biogenesis, as shown by time-lapse imaging
of
mCherry-Rab35. To illustrate the cascade effect of proteins implicated in migrasome formation, cells were
administered the PIP5K1A inhibitor ISA2011B to evaluate Rab35's reliance on PI(4, 5)P2; results indicated
a
compromised recruitment of Rab35 to the MFSs. These results merely demonstrated that Rab35 depended on
PI(4,
5)P2, based on accurately describing migrasome biogenesis [13]. Additionally, Rab35 knockout cells
demonstrated
a marked decrease in migrasome formation, highlighting Rab35's essential role in this process. The
relationship
between Rab35 and integrin α5β1 was explored in Ding et al.'s study, revealing that integrin α5 is highly
enriched on migrasomes, particularly when cells are cultured on fibronectin-coated substrates. When
discussing
whether these factors are required for this process, it is necessary to assemble a clear picture of the
molecular sequence of events as described in Fig. 1B [13]. Wu et al.'s study corroborated these findings
by
comparing the production of migrasomes on Normal Rat Kidney (NRK) cells expressing TSPAN4-GFP between
fibronectin, laminin 511 and collagen. The research confirmed that migrasome production is enhanced on
fibronectin compared to other extracellular matrix components, with integrin α5 displaying the highest
expression compared to integrins α1 and α2 (which bind to collagen), α3 and α6 (which bind to
laminin-511), and
αV (which binds to vitronectin), as well as α5 (which binds to fibronectin). Similarly, Ding et al. and Wu
et
al. demonstrated that the α5β1 integrin was abundant on migrasomes using MGC803 cells expressing
tetraspanin 4
(TSPAN4) and antibodies against integrins α5 and β1. Additionally, using normal rat kidney (NRK) cells
transfected with integrin α5-GFP, it was found that integrin α5 was enriched on migrasomes. At the same
time,
its presence on retraction fibers was less prominent [14]. Wu et al. further investigated the relationship
between migrasomes, integrins, and the extracellular matrix (ECM), and found that migrasomes were anchored
to
the ECM via their basal surface. By comparing the 3D distribution of integrin α5 and TSPAN4 on migrasomes,
Wu et
al. demonstrated that TSPAN4 localized to the upper side facing away from the ECM, whereas integrin α5 was
concentrated at the base in direct contact with the ECM. The importance of integrin α5 was emphasised, as
it
plays a key role in maintaining migrasome stability [14].
Expansion
As integrins recruitment marks the completion of the maturation stage, the expansion stage is marked by
the
growth and expansion of migrasomes along RFs. TSPAN4 was identified as a migrasome marker while screening
for
proteins enriched in migrasomes [1, 15]. Tetraspanins constitute a family of proteins firmly integrated
into the
cell membrane. These membrane proteins consist of 4 transmembrane 𝛼-helical transmembrane domains. What
sets
TSPANS apart is their ability to leverage different areas of their structure to facilitate the formation
of
extensive protein complexes in the cell membranes, such as the formation of specialized
tetraspanin-enriched
microdomains (TEMS) highly associated with cholesterol [16]. It is worth noting, 14 of the 33 members of
tetraspanin family can contribute to migrasome formation. Huang et al. demonstrated that 14 of these TSPAN
proteins, when induced, were able to promote migrososme formation; moreover, they demonstrated that
migrasome
formation without TSPAN4 in NRK and MGC803 cells was significantly impaired [17]. In a similar study, a
considerable reduction in migrasome formation was observed in zebrafish embryos, knockout of TSPAN4 and
TSPAN7
[18]. While it was proven that TSPAN4 played a significant role in migrasome biogenesis, its clear role
was
undefined until Huang et al. investigated the relationship between TSPAN4 expression and migrasome
development.
Through studying the process of migrasome biogenesis, the expression of TSPAN4 corresponded with the
growth
pattern of migrasomes. This was shown through detecting the gradually increasing signals of TSPAN4,
consistent
with the rate of migrasome growth, and the TSPAN4 signals plateau once migrasomes reached their maximum
size; if
we were to describe this relationship, it would show a positive linear correlation [19]. By identifying
this
relationship, we can highlight the potential harm that endogenous TSPAN4 deficiency may cause to migrasome
development. Huang et al. underscored this by demonstrating the essential role of TSPANS by preparing
proteoliposomes with purified TSPAN4 embedded in the membrane, followed by the generation of giant
unilamellar
vesicles with or without TSPAN4 and adhering the samples to the bottom of a flow chamber, creating leading
edges
of a cell. During this process, migrasome-like structures were formed only in the presence of TSPAN4 [17,
19].
Within the membrane, tetraspanins organize to form tetraspanin-enriched microdomains (TEMs). These
microdomains
are formed through the interaction of tetraspanins along with a wide variation of transmembrane proteins
and a
limited number of lipids [19]. A lipid playing a central role in migrasome formation is cholesterol. Huang
et
al. underscored this integral function by treating live cells with methyl-B-cyclodextrin (MBCD), which is
a
cholesterol-depleting agent, leading to the reduction in migrasome numbers. This finding supported the
importance of cholesterols' indirect impact on migrasome formation through its relationship with TSPANs
[17]. As
described in Fig. 2, in the final stages of migrasome formation, TEMs on retraction fibers coalesce to
form
large organizational hubs within the membrane, known as tetraspanin-enriched macrodomains (TEMAs),
enabling
migrasomes to reach their maximum size. These specialized macrodomains then develop into migrasomes. The
overall
formation of migrasomes mimics a cascade-like mechanism with a factor-to-factor dependency successfully
demonstrated by multiple studies by using various techniques, including introducing antibodies,
inhibitors, and
knockout cell lines.
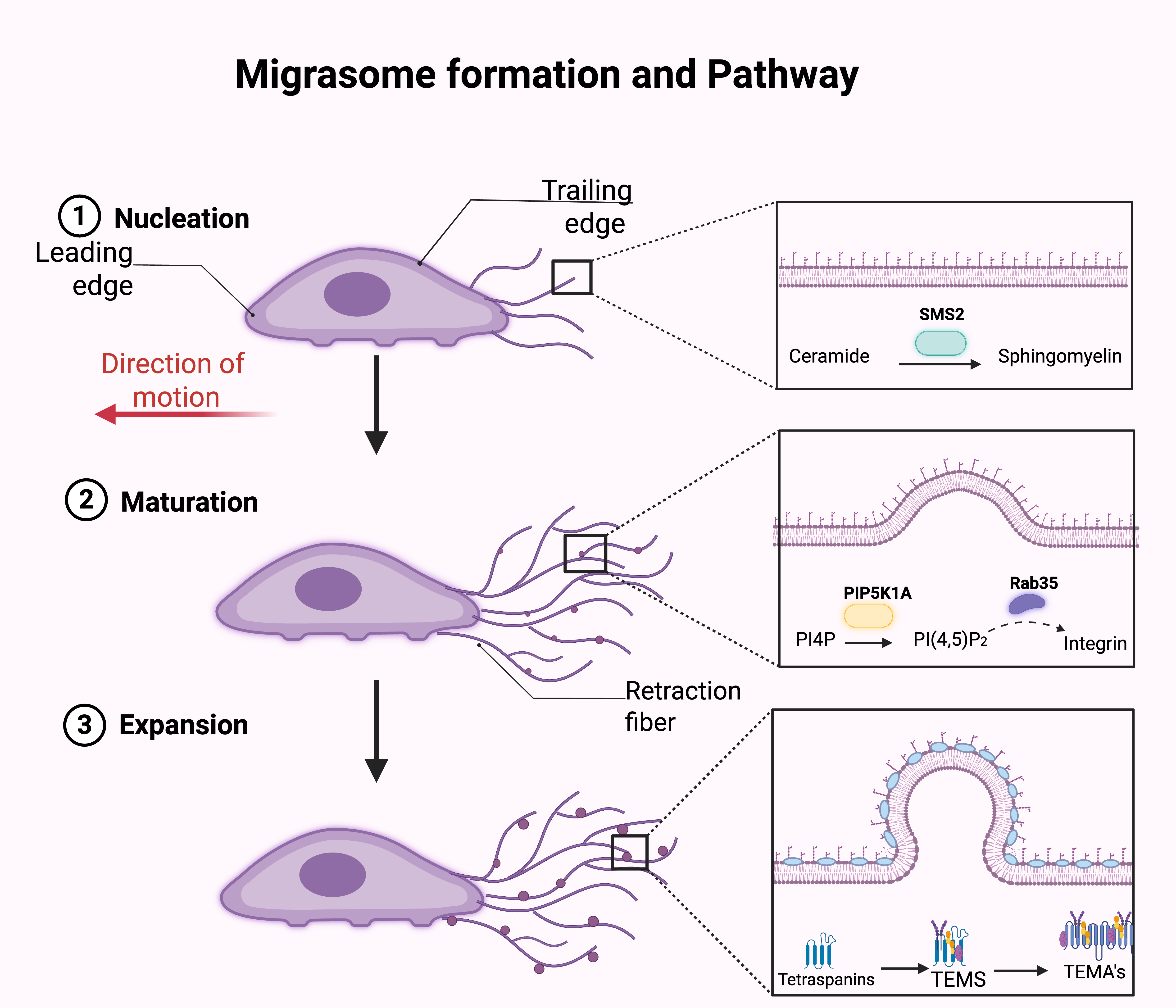
Fig. 2: Migrasome formation and pathway. Migrasome formation involves three sequential stages: (1) Nucleation, (2) Maturation, and (3) Expansion. During nucleation, as the cell migrates, ceramide is converted into sphingomyelin by sphingomyelin synthase 2 (SMS2) at the trailing edge of the plasma membrane. This lipid modification initiates migrasome formation along retraction fibers extending from the cell body. In the maturation stage, phosphatidylinositol 4-phosphate (PI4P) is phosphorylated by phosphatidylinositol-4-phosphate 5-kinase type I alpha (PIP5K1A) to produce phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P₂]. The active form of Rab35 is then recruited, which facilitates the accumulation of integrins at the developing migrasome membrane, supporting further growth and structural stabilization. During the expansion phase, fully matured migrasomes continue to grow and become enriched in tetraspanins and specialized tetraspanin-enriched microdomains (TEMs), which can further evolve into more complex membrane-associated structures (TEMAs), reflecting advanced compartmentalization and functionality. The side panels illustrate the molecular events at each stage: SMS2 converts ceramide to sphingomyelin (top), PIP5K1A generates PI(4,5)P₂ and recruits Rab35, aiding integrin accumulation (middle), and TEMs/TEMAs enriched in tetraspanins organize the expanding migrasome membrane (bottom), contributing to cargo sorting and intercellular communication.
Migrasome pathway
Examining the developmental trajectory of migrasomes offers crucial insights into the distinctive
properties and
singular nature of these newly discovered cellular structures. As previously mentioned, migrasomes formed
on the
rear end of migrating cells along retraction fibers, as the cells migrate forward, migrasomes detach from
the
cell body and ultimately are left behind. Ma et al. named this cell migration-dependent process
‘migracytosis’
and this process facilitates cell-to-cell communication by releasing cellular contents that are taken up
by
other cells [1, 20]. Through time-lapse microscopy, researchers observed that as cells move forward, they
create
a trail of retraction fibers. Following a delay of roughly 40 minutes after cell movement, migrasomes
began to
appear at various points along these fibers. No spatial relationship between the distance of migrasomes to
the
cell periphery was found, as the position of these structures along RFs showed significant variation [1].
Based
on the results showing a lack of spatial relationship between migrasomes and the migrating cells, it is
important to explore and underscore which factors influenced the position along the cell to which
migrasomes
were formed and their subsequent release. In the same study, researchers suggested that direction and
speed
influenced migrasome formation and release. Ma et al. employed fibronectin-coated culture dishes, known to
increase cell migration [1]. The results of this study showed that cells cultured on fibronectin-coated
surfaces
not only exhibited accelerated movement but also produced substantially higher numbers of migrasomes.
Researchers also compared time-lapse imaging over 160 minutes of 2 straight moving TSPAN4-GFP expressing
cells
at different speeds, slow migrating at 19µm/h and fast migrating at 33.9µm/h. Results showed the formation
of 3
and 7 migrasomes on the slow and fast-moving cells, respectively [1]. Fan et al. conducted a similar study
to
test whether vimentin affected cell migration patterns [21]. Researchers used three separate cell lines
with
CRISPR/Cas9 vimentin knockout lines in TSPAN4-GFP L929 cells. Rescue lines were created by reintroducing
full-length vimentin into the knockout lines. From each condition, one line was chosen, and the effects of
vimentin depletion were assessed by carrying out cell random migration and wound healing assays [21]. The
results of this study showed that vimentin depletion significantly reduced straightness and the speed of
cell
migration. These studies confirmed that proteins such as fibronectin and vimentin regulate cell migration
patterns. To further investigate the characteristics of migrasomes using cultured TSPAN4-GFP L929 cells,
they
investigated the relationship between velocity and migrasome formation using live-cell imaging to track
the
behavior of retraction fibers and migrasomes. During cell migration, the pathway is not always running in
a
straight direction but may alternate direction; the altering patterns may indirectly impact migrasome
formation
[22, 23]. Fan et al. divided the cell migratory process into a straight and turning
phase; the
turning phase was further categorized into sharp turning, mild turning, and continuous turning cells. The
results of this study highlighted the reduced number of retraction filaments and the reduced number of
migrasome
formation in cells that underwent extensive turning in the migration process. These results underscored
the
correlation between migrasome formation and straight migrating cells. Fan et al. demonstrated that speed
plays a
crucial role in migrasome formation, with TSPAN4-GFP-expressing L929 cells migrating in a straight line at
an
average speed of 30.5 µm/h. By comparing the speed of cell migration and migrasomes produced, a linear
relationship was displayed. Through comparison of cell migration speed and the length of RFs, results also
showed that the cells that migrated faster were able to generate longer RFs. To demonstrate the influence
of
persistency in migrasome formation, experiments with Human gastric carcinoma cell line mGC803 and normal
rat
kidney cell line NRK were analysed. Through live-cell imaging, results showed that there was an increased
production of migrasomes when cells migrated more persistently and faster [21]. The migrasome formation
undergoes a complex process involving multiple factors that play a central role in determining the level
of
migrasome formation. While microcellular components determine the qualitative aspect of migrasome
formation,
parameters such as velocity, direction and persistency can quantitatively assess migrasome formation [21].
Differences between Migrasomes and Exosomes
Migrasomes are novel organelles, discovered by Ma et al. which have been compared to exosomes, which are smaller extracellular vesicles [1, 24]. Although migrasomes and exosomes have some similarities, the nature of migrasomes have been found to differ from those of exosomes despite their shared role in cell signaling. It has been suggested that migrasomes should not be considered extracellular vesicles [15, 28, 25, 26] hence, various literature refers to them as novel organelles [24, 27, 28]. Through comparing their biogenesis, structure, composition, and function, the differences become unmistakably evident. Exosomes and migrasomes both undergo a general 3-step formation. The first step includes early endosome formation, which is formed by the inward folding of the plasma membrane, invagination of the late endosomal membrane occurs and a multivesicular body (MVB) consisting of intraluminal vesicles (ILVs) is formed [24, 29]. The multivesicular body then fuses with the plasma membrane, and ILVs are released into the extracellular space. These are known as “exosomes”. Unique to exosomes is the endosomal sorting complex required for transport (which is a complex protein central to exosome formation). The endosomal sorting complex required for transport (ESCRT) consists of four protein complexes, classified from 0 to III, that act in a cascade-like manner during exosome formation. Exosomes are 40–160 nm in diameter, while migrasomes range from 1–3 µm in diameter [30]. Through proteomic studies, it was found that exosomes consist of a specific subset of proteins including tetraspanins CD81, CD63, CD9, heat shock proteins HSP70, HSP90, exosome release proteins Alix and TSG101, membrane transport and fusion proteins annexins and Rab. Proteomic analysis of migrasomes underscored the presence of unique protein markers that are absent in exosomes. These include N-deacetylase/N-sulfotransferase 1 (NDST1), phosphatidylinositol glycan anchor biosynthesis class K protein (PIGK), G-protein coupled receptor Q (GPQ), and EGF domain-specific O-linked N-acetylglucosamine transferase (EOGT) [32]. Lipid studies highlighted the similarities between migrasomes and exosomes, showing they are enriched with cholesterol, sphingomyelin, ceramide and phosphatidylserine [11, 17, 33]. Exosomes have been defined as “lipid bound vesicles secreted by cells into the extracellular space” [35].
Although migrasomes and exosomes exhibit several overlapping functions, exosomes have been extensively studied and validated. In contrast, much about migrasomes remains unknown, with their functions still under active investigation as researchers work to bridge the gap between current findings and potential therapeutic uses. Exosomes consist of bioactive molecules that play central roles in intercellular communication with surrounding cells. These bioactive molecules are responsible for physiological changes in surrounding cells. Exosomes' role in immunoregulation has been extensively studied [29]. Mature dendritic cells produce exosomes that carry antigens inducing antigen-specific immune responses by dendritic cells. Exosomes produced by dendritic cells also display immunomodulatory properties [29, 34]. Much like exosomes, migrasomes primary function is cellular communication.
Migrasomes mediate the lateral and horizontal transfer of RNAs and proteins. They are information carriers, with a target destination [3]. As described in Fig. 3 the distinctive features of a migrasomes membrane include TSPAN4, TSPAN7, integrin ⍺1, ⍺3, ⍺5 and 𝛽1 [1, 15, 35]. Gubbergsen argued that migrasomes should not be classified as extracellular vesicles due to their mode of formation and release. Using electron microscopy, Ma et al. demonstrated that migrasomes remain attached to segments of retraction fibres upon release, indicating a shared membrane [1, 27]. Gubergson argues that we cannot classify migrasomes so distinctly since migrasomes are not released as strictly separate vesicles but as a microsome-retraction fiber complex, once again highlighting its distinction from exosomes.
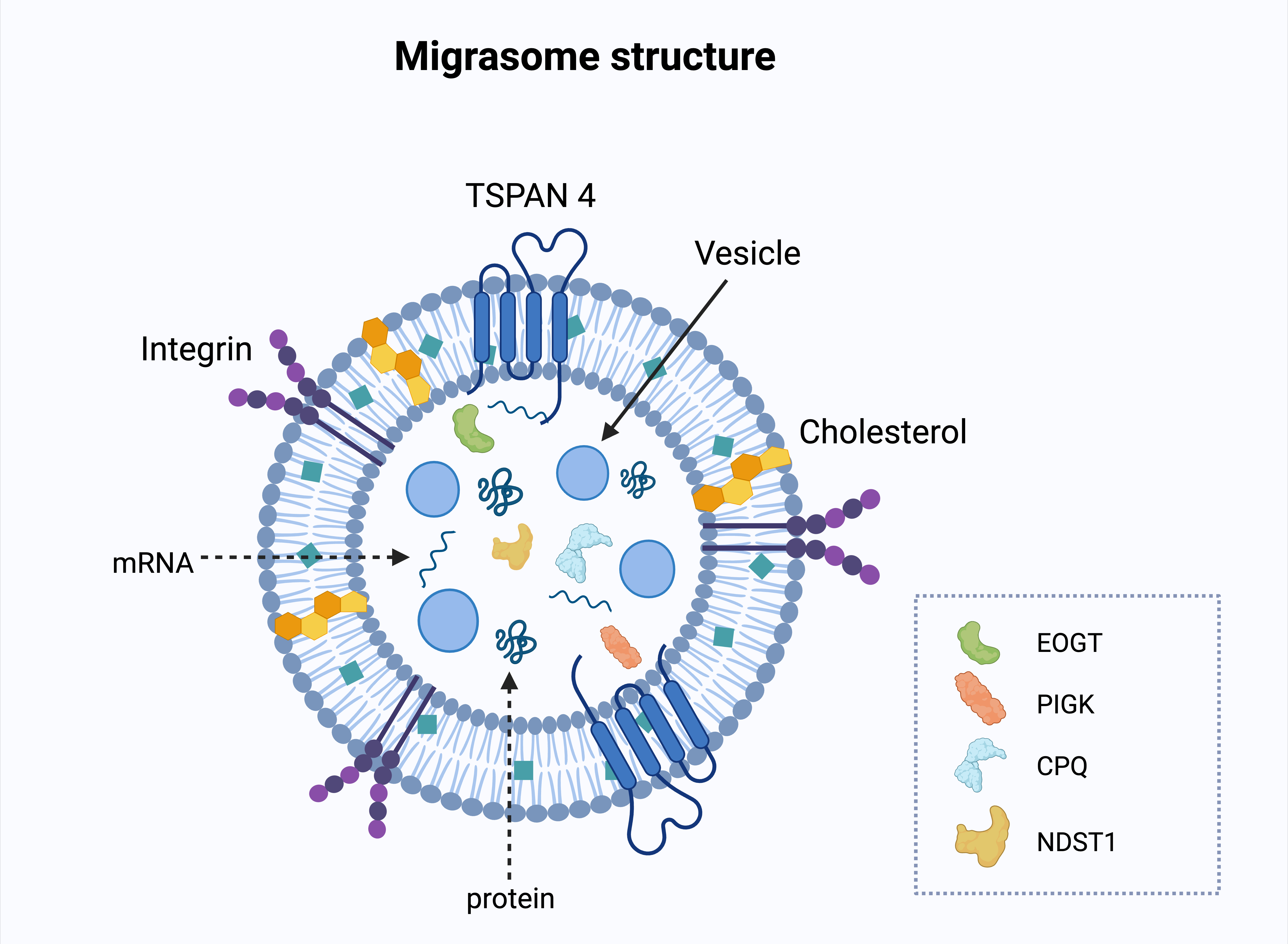
Fig. 3: Structure of a migrasome. The migrasome membrane is enriched with tetraspanin 4 (TSPAN4), integrins, and cholesterol, which contribute to its structural stability and function. Inside the migrasome, vesicles, mRNA, and proteins are encapsulated, allowing the transport of molecular cargo to recipient cells. Specific migrasomal protein markers - EOGT (EGF domain-specific O-linked N-acetylglucosamine transferase), PIGK (Phosphatidylinositol glycan anchor biosynthesis class K), CPQ (Carboxypeptidase Q), and NDST1 (N-deacetylase/N-sulfotransferase 1) - are depicted, reflecting the unique protein profile of migrasomes. These molecules are involved in post-translational modifications and protein processing, indicating specialized biochemical roles of migrasomes in intercellular communication
Applications of migrasomes
Novel applications of migrasomes have emerged in the contexts of health and disease. These findings, particularly those highlighting the role of migrasomes in disease, represent a significant advancement in understanding pathological mechanisms and improving early screening for common diseases [25, 26, 36, 37]. The possibility of reverse engineering the migrasome-mediated disease pathway has been proposed and could significantly transform disease management. Migrasomes have been found to be widely distributed among various organisms and tissues. Human, mouse and rat species were found to contain migrasomes in brain, eye, lung, kidney, bone marrow, and gut tissue [1, 25, 26, 36-38]. Migrasomes have also been found in chicken embryo and zebrafish species [18, 39]. Despite the relatively recent nature of migrasome research, significant breakthroughs have identified these unique organelles' diagnostic and therapeutic potential. In the following sections, we explore the potential applications of migrasomes through an analysis of their structural and functional characteristics.
Investigation of Podocyte-Derived Migrasomes as Early Diagnostic Biomarkers
Podocytes are integral to the glomerular filtration process. Their intricate architecture, characterized
by foot
processes that connect neighboring podocytes, contributes to the formation of the cytoskeleton, thereby
maintaining an effective filtration barrier that ensures selective permeability [42]. Podocyte injury can
occur
due to a number of processes, including inflammation, autoimmune diseases, and metabolic disorders [36,
37].
These processes may alter podocyte structure, leading to foot process effacement and/or detachment from
the
glomerular basement membrane. A study found that activation of RhoA and Rac-1, members of the Rho-family
GTPases, has been linked to podocyte dysfunction. These GTPases regulate cell signaling and control
inflammation
[43]. Once GTPases are activated, the glomerular filtration membrane is disrupted, leaving the membrane
susceptible to increased permeability to larger molecules such as proteins, which under physiologic
conditions
would not cross the glomerular filtration barrier. Among these proteins, albumin is the most commonly
detected.
Elevated levels of albumins found in the urine can indicate a pathologic process within the glomerular
filtration system, suggesting membrane disruption [36]. Proteinuria is a highly and extensively recognized
diagnostic tool; however, like many other conditions, there is usually an upper limit of normal used to
qualify
a diagnosis. As a result, underlying pathological processes may remain undetected, leading to potential
delays
in diagnosis for patients who do not fulfil the established criteria. Investigating cellular changes
before the
onset of proteinuria may enable the development of more sensitive and accurate diagnostic tools,
facilitating
earlier detection and intervention [44]. As previously discussed, migrasomes are cells released during
cell
migration, indicating a potential correlation between migrasome release and highly motile cells,
particularly
podocytes [23].
Due to their motility, Liu et al. hypothesized that podocytes could release a significant amount of
migrasomes.
Their study aimed to determine whether migrasomes could serve as non-invasive biomarkers for early
podocyte
injury, enabling the detection of oncoming proteinuria and the progression of nephropathy. To explore this
hypothesis, Liu et al. conducted a study on patients with diabetic nephropathy and mild podocyte injury
with low
levels of proteinuria. Human podocyte cells (HPCs) were cultured and exposed to inflammatory (LPS), toxic
(puromycin aminonucleoside; PAN), or metabolic (high-concentration glucose; HG) stressors for varying
durations
to model relevant disease processes. A control group of HPCs were cultured but exposed to no inflammatory
or
metabolic stressors. Parallel experiments were performed on murine podocytes, with PAN-induced nephropathy
serving as the model of injury and healthy animals as controls [26].
Migrasome formation and release were assessed using a combination of immunofluorescence, electron
microscopy,
sequential centrifugation, and nanoparticle tracking. The use of LPS, PAN, and HG effectively simulated
inflammatory, toxic, and diabetic injuries, which are known contributors to podocyte damage. The study
also
focused on Rac-1, a key regulator frequently upregulated during podocyte injury, to evaluate its
involvement in
migrasome release. Application of Rac-1 inhibitors resulted in a marked reduction in migrasome production,
establishing a functional link between Rac-1 activity, podocyte injury, and migrasome secretion [26].
Similarly
the results from the murine models showed significantly increased levels of podocyte-released migrasomes
in
urine in the group with PAN-nephropathy compared with the control mice. Liu et al's results also showed an
increased number of urinary migrasomes compared to the control group. Importantly, elevated urinary
migrasome
levels were detected before the onset of proteinuria, highlighting their potential as sensitive and early
indicators of podocyte injury [26]. In a similar study, conducted in 2024, Yang et al. examined the levels
of
migrasomes in the urine of 288 participants with kidney disease and podocyte injury with a group of 110
healthy
volunteers. Using similar methods to those described by Liu et al., migrasomes and exosomes were isolated
using
centrifugation, followed by analysis using Nanosight. This study involved PAN-induced kidney injured mice.
Urine, serum, and kidney samples were collected and analyzed by TEM. Yang et al. found a substantial
increase in
urinary podocyte-derived migrasome concentration among individuals with kidney disease [40]. These
findings were
similar to Liu et al.'s, showing consistency with their hypothesis. These results highlight the promise of
urinary migrasome quantification as a novel, non-invasive diagnostic approach for the early detection of
glomerular diseases.
Tumor microenvironment and tumor progression
The tumor microenvironment (TME) is an extensive cellular and acellular environment consisting of
components
that promote cancer progression and resistance to therapeutic interventions [45]. Recent studies found
that
migrasomes can carry signaling factors, including cytokines and extracellular matrix proteins, possibly
influencing the tumor microenvironment and modulating processes like angiogenesis [56, 57]. The process of
tumor
progression is typically indicated when neovascularization is observed, due to migrasomes chemotactic and
angiogenic factors. These factors create the ideal environment for tumor growth by providing more pathways
for
tumor metastasis. We can hypothesize that these migrasome properties may play a role in neovascularization
and
therefore have a direct role in tumor progression [46].
Targeting Pancreatic Cancer Progression Through Migrasome-Associated Pathways. According to the Centers for Disease Control and Prevention (CDC), pancreatic cancer is among the four leading causes of cancer-related death in the United States [47, 48]. Pancreatic adenocarcinoma is known globally for its poor prognosis and is characterized by its advanced metastasis and aggressive progression. The rising incidence of this cancer is a growing concern for researchers and healthcare professionals. Despite this, few new screening tests or therapeutic interventions have successfully been developed. Ongoing research focuses on exploring novel strategies to reduce the morbidity associated with this malignancy [48]. In a study investigating the role of pancreatic cancer cell-derived migrasomes in tumor progression, Zhang et al. employed in-vivo and ex-vivo methods by using cultured murine pancreatic cancer cell line PAN02 and human pancreatic cancer cell line, Mia PaCa-2. Cell lines were cultured with fibronectin and live-cell imaging with confocal microscopy was used. Through TEM of ultra-thin cell sections and migrasome purification, researchers observed the migrasome generation progress in human and murine pancreatic cancer cell lines and assessed the morphology and distribution under TEM. The results showed that both cell lines were highly enriched with proteins including migrasome markers PIGK, TSPAN superfamily, and integrin proteins. Concluding that pancreatic cancer cells can generate migrasomes [40]. Researchers simultaneously investigated the role of immune cells under these conditions. Mice were divided into groups and injected intraperitoneally with polycaprolactone-based degradable microspheres (PCDMS) or phosphate-buffered saline (PBS), followed by injections with PAN02-cherry pancreatic cancer cells. These cells were observed using live imaging. Results showed that the immune cells collected via peritoneal lavage had a higher tumor burden; it was also found that the group injected with PCDMs had higher levels of M2 macrophages. Further investigations were carried out on the effect of PCDMs on macrophages, which showed that PCDMs were able to phagocytose migrasomes. The profiles of PCDMs were analyzed and a significant enrichment of chemokines CX3CL1, CXCL5, CCL1, CCL27 and CCL21 was observed. The protein profiles of these migrasomes were further analyzed, and it was found they were enriched with numerous chemokines, cytokines, and signaling molecules, CXCL5 and dTGF-B1 [41]. CXCL5 is a proinflammatory factor with angiogenic properties [49, 50] and some research has shown that CXCL5 plays a significant role in carcinogenesis in a number of cancers such as prostate, colorectal and osteosarcoma among others [50-54]. In a previous study, researchers found that elevated CXCL5 is correlated with a poor prognosis in pancreatic ductal adenocarcinoma and is linked to increased immune cell infiltration [54]. The results of this study underscored the role of migrasomes in tumor progression, migrasomal enrichment of carcinogenic chemokines highlights the potential role of utilizing migrasomes in the modification of tumor progression.
Potential role of Role of Migrasome Marker TSPAN4 and CD151 in Hepatocellular Carcinoma. Hepatocellular carcinoma (HCC) is one of the most common solid tumors worldwide and accounts for approximately 90% of all primary liver malignancies [55]. In a study investigating the role of migrasomes in the mediation of hepatocellular carcinoma invasion and promotion of angiogenesis it was found that there was a significant correlation between CD151 expression and migrasome TSPAN4 marker. This correlation suggests that CD151 is involved in signalling pathways that promote angiogenesis via migrasomes, thereby accelerating the progression of HCC [17]. Through a series of experiments, including western blot analysis, total proteins were extracted using a whole protein extraction kit, and their concentrations were subsequently determined using bicinchoninic acid (BCA) protein assay kits. For live images, the cells were cultured in fibronectin-precoated confocal dishes, followed by TEM analysis. After 48 hours of incubation, non-invasive cells on the upper membrane surface were removed, and invasive cells on the lower surface were fixed and stained. Images were then captured and migrated cells were quantified using an inverted microscope. Further analyses, including chemotaxis assays, transmission electron microscopy (TEM), field emission scanning electron microscopy (FE-SEM), and enzyme-linked immunosorbent assay (ELISA), were conducted. Zhang et al. concluded that CD151 expression in liver cancer cells plays a crucial role in migrasome generation [56]. This was proven by investigating the mRNA expression data of the tetraspanin daily genes in various data sets such as LIHC, GSE1898, LIRI and DepMap (HCC cell lines). The correlation analysis demonstrated a significant association between CD151 and TSPAN4 across all previously mentioned data sets, with a correlation coefficient exceeding 0.4, indicating a strong correlation in liver cancer. Moreover, the analysis of differential expression and survival of CD151 in TCGA-LIHC, ICGC-LIRI and GSE1898 proved that its elevated expression in tumor samples is associated with poor prognosis. The findings indicate that TSPANA4, previously recognised as a marker for migrasomes, may play a role in liver cancer metastasis due to its association with CD151 [57]. The experiments indicate that CD151 expression in liver cancer plays a crucial role in migrasome generation, with CD151 serving as a marker in these organelles. The upcoming challenge will be to either inhibit or regulate this expression to enhance prognosis. Further investigation is necessary; however, we remain optimistic regarding the potential application of migrasome in this area.
Migrasomes as Emerging Therapeutic Targets in Neuroblastoma. Neuroblastoma is one of the most common extracranial solid tumors found in children; it is associated with a high level of metastatic disease at diagnosis [58]. Neuroblastoma accounts for 8% to 10% of childhood cancers with an approximate 15% association with cancer deaths in children [59]. Studies have demonstrated a correlation between the amplification of the v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog (MYCN), DNA ploidy, and chromosomal loss with the clinical presentation and progression of neuroblastoma [59, 60]. Li et al. investigated the prognostic significance of migrasomes in neuroblastoma through a comprehensive bioinformatics and machine learning approach. They extracted RNA sequencing data from public databases (GSE62564, GSE181669, TARGET, and FANTOM5). This data was classified using migrasome-specific markers to identify differential gene expression patterns and infer potential biological processes related to migrasomes. With this information, a novel machine learning model was developed to analyze complex gene expression profiles and other relevant molecular markers. This was used to predict immune cell infiltration within tumors, providing insights into the tumor microenvironment (TME) and potential drug compatibility. By focusing on 283 intersecting genes, 50 were shown to be correlated with neuroblastoma prognosis. This study highlights the potential for developing targeted cancer therapeutics by providing vital information into migrasome-related pathways in neuroblastoma, although further research is required before any significant conclusions can be made [61].
Migrasomes and Their Emerging Role in Glioblastoma Multiforme Progression and Therapeutics. Glioblastoma multiforme (GBM) is an aggressive brain cancer involving the brain and brainstem. It is classified as a grade IV highly malignant tumor. Multiple studies have investigated GBM and its response to different treatment strategies, however, the brain's limited self-repairing function acts as a barrier to forging new paths and advancements in curative treatment of GBM. Kötürk et al. conducted a study to investigate the effects of adipokinetic hormone (AKH) on brain-derived neurotrophic factor (BDNF) and the formation of migrasomes. AKH release causes hyperglycemia, therefore promoting proliferation, which has been previously linked with cancer cell migration and is a favorable factor in the TME [63-65]. Similarly, BDNF is expressed in numerous human cancers and has been associated with tumor growth and metastasis [65, 66]. Using rat C6 glioma cells treated with varying AKH concentrations, transmission electron microscopy revealed that AKH treatment significantly increased BDNF expression and migrasome formation, while control cells showed no migrasomes structures [62]. The results of this study further emphasised the indicative role of migrasomes in tumor progression. When factors benefiting the tumor microenvironment (TME) are present, the number of migrasomes closely correlates with the development of metastatic processes, once again highlighting their potential use as a prognostic marker and therapeutic tool.
Exploring Migrasomes as Diagnostic and Therapeutic Tools in Proliferative Vitreoretinopathy
Proliferative vitreoretinopathy (PVR) is characterised by the formation of fibrovascular membranes in the
vitreous cavity, complicating and frequently leading to the failure of rhegmatogenous retinal detachment
(RD)
repair. With an incidence of 5-10% of all retinal detachment cases, within 1 month roughly 77%
postoperative
forms of PVR appear [67, 68].
Wu et al. used ex vivo in vitro and in vivo models to investigate the characteristics and
functions of migrasomes in the retinal pigmented epithelium activation and the proliferative
vitreoretinopathy
development. They found that human proliferative vitreoretinopathy samples expressed the migrasome marker
TSPAN4
[25]. Moreover, the retinal pigmented epithelium can internalize migrasomes and enhances their migration
and
proliferation. This is relevant because the retinal pigmented epithelium activated by cytokines can lead
to the
proliferative vitreoretinopathy, which can cause retinal detachment and blindness [69, 70]. Through
various
experiments such as western blot and immunofluorescence, they also proved that the migrasome markers were
expressed in the extracellular vesicles under the proliferative vitreoretinopathy microenvironment. In
addition,
transmission electron microscope identified externally deposited extracellular vesicles to be reminiscent
of
migrasomes. Wu et al. also concluded that the extracellular vesicles in the proliferative
vitreoretinopathy
membrane fell within the size requirements for migrasomes, which aided in their identification of said
migrasomes. In addition, they were also of similar morphology to migrasomes as described by Ma L et al
[1].
Further experiments included inducing migrasome formation using transforming growth factor beta 1 (TGF-β1)
treatment, followed by observation of migrasome formation using transmission electron microscopy (TEM) and
scanning electron microscopy (SEM). Furthermore, they examined the expression of migrasome marker TSPAN4
in
RPE-Bruch’s membrane-choriocapillaris complex using immunofluorescence and found that the TBF-β1-treated
group
displayed higher labeling for TSPAN4 compared to the control group [25]. It was through a combination of
these
findings that they concluded that migrasomes play a large role in the retinal pigmented epithelium
activation
and in the proliferative vitreoretinopathy progression. The next task is to identify methods that block
the
migrasome formation, possibly through the associated TSPANA4 marker, to slow the progression or completely
inhibit the proliferative vitreoretinopathy. Some headway into this has already been made, as in the same
experiment, Wu et al. discovered in the rabbit model that the inhibition of TSPANA4 expression in the
retinal
vitreoretinopathy significantly abrogates its ability to initiate the proliferative vitreoretinopathy
[25].
Mesenchymal Stromal Cell-Derived Migrasomes in Leukemia Progression
Mesenchymal stromal cells (MSCs) have the ability to differentiate into various cell lineages. These
progenitor
cells are found in bone marrow, adipose tissue, umbilical cord tissue and the placenta [71]. A study by
Deniz et
al. found that mesenchymal stromal cells (MSCs) produce migrasomes that can be taken up by migrating
leukemic
cells, potentially playing a crucial role in leukemic cell survival and progression. Blocking this uptake
may be
key to halting disease advancement [70]. To conduct this experiment, Deniz first obtained primary
mesenchymal
stromal cells from the bone marrow of healthy donors. They were then cultured. Alternatively, they were
grown on
uncoated glass-bottom 35mm dishes. The mobilized peripheral blood was collected from healthy donors. The
mobilization was achieved by subcutaneous injection of granulocyte colony-stimulating factor. The
mesenchymal
stromal cells were pre-cultured for 24 hours before adding hematopoietic cells. The mesenchymal stromal
cells
were then transiently transfected. The cells were then incubated at 37℃ and cultured for 24 hours prior to
being
processed for downstream experiments. The growth medium was replaced with a fresh medium with or without
desired
treatments before live-cell imaging. The time-lapse video-microscopy revealed the dynamic maturation of
migrasomes i.e. from the early stage to fully formed migrasomes along the retraction fibers and
corresponding
insets. The time-lapse video-microscopy also allowed Deniz et al. to observe that migrating KG-1a cells
can
absorb both retraction fiber-attached or cell-free migrasomes during their movement. The results obtained
ultimately revealed that 25-40% of mesenchymal stromal cells harbored migrasomes regardless of the time in
culture i.e. from 24-72 hours [72]. Deniz et al. concluded that migrating mesenchymal stromal cells leave
behind
a trail of migrasomes that may guide other cells within the bone marrow microenvironment. However, whether
these
migrasomes influence the migration or retention of metastatic cancer cells in the bone marrow remains
unclear.
If so, this pathway could represent a novel target for cancer therapy.
Targeting Migrasome Formation as a Potential Strategy Against Poxvirus Infections
Under the extensive Poxviridae family, Vaccinia virus is a complex virus that has provided huge success in
the
creation of the smallpox vaccine, hugely contributing to the elimination of the once prevalent virus.
Vaccinia
virus is widely studied in the scope of infectious diseases and preventative methods in certain cancers
[73].
Zhang et al. conducted a study with the aim to explore whether poxvirus could evade treatment with
tecovirimat
(ST-246), an antiviral drug, with the use of migrasomes [74]. To our knowledge, this is the first study to
demonstrate that vaccinia virions (VACV), a prototype poxvirus, can be located within migrasomes and may
induce
migrasome formation during the late stages of infection. We believe this finding suggests that inhibiting
migrasome formation may contribute to developing an anti-poxvirus agent. However, there is limited
research on
this subject, indicating that further investigation could be advantageous [74].
Migrasomes in Myocardial Infarction: Advancing Diagnostics and Therapeutics
Acute myocardial infarction (AMI), which affects around 3 million people annually, is a severe form of
cardiovascular disease [75]. Since the discovery of migrasomes, whose role provides mitochondrial quality
control, increased research has explored their potential applications in the occurrence, progression, and
diagnosis of myocardial infarction [3, 76]. Cardiac troponins are currently recognised as the gold
standard
biomarker in the diagnosis of AMI; however, research has shown that false-positive results can be seen in
cases
of sepsis, chronic kidney disease and heart failure. Zhu et al. conducted an original study on the
potential use
of migrasomes as biomarkers in AMI [77]. This study aimed to develop a predictive migrasome-related
signature
for AMI patients. As mentioned previously, TSPANs are essential structural components in migrasomes, and
in this
case, nine microsome-related genes were expressed: BMP1, ITGB1, NDST1, TSPAN1, TSPAN18, TSPAN2, TSPAN4,
TSPAN7,
TSPAN9, and WNT8A. RNA sequencing on CD45+ cells was isolated from murine models with induced AMI to
achieve
this. The analysis of cell-specific expression patterns of microsome-related genes at a single-cell
resolution
determined whether genes were up or downregulated in these CD45+ cells. Furthermore, Mendelian
Randomization
(MR) analysis explored the causal relationship between the expression levels of these multiple sclerosis
(MS)
genes and the risk of AMI. Zhu et al. concluded that the nine gene-based signatures were promising
biomarkers
laboratories could use to diagnose AMI [77]. This finding is consistent with the original study by Zheng
et al.,
which reported that TSPAN4 expression is significantly associated with atherosclerosis and upregulated in
cases
of spontaneous myocardial infarction [78].
Similarly, Sun et al. examined the use of low-intensity pulse ultrasound to improve myocardial ischaemia
reperfusion injury via migrasome mediated mitocytosis [79]. Their original study analysed the effects of
low-intensity pulsed ultrasound (LIPUS) on mitochondrial quality control through migrasome-dependent
mitocytosis. Sun et al. identified that LIPUS effectively facilitated the selective removal of damaged
mitochondria by promoting the formation of migrasomes, through increased cell displacement. This research
highlights the potential use of LIPUS as a non-invasive therapeutic strategy for mitigating the effects of
myocardial ischemia-reperfusion injury by enhancing the mitochondrial health and function [79].
The continuously increasing research on the migrasomes in AMI offers promising new diagnostic and
treatment
options. The therapeutic potential of migrasomes is highlighted by Sun et al. 's work demonstrating the
effectiveness of LIPUS in promoting the migrasome-mediated mitocytosis in the treatment of myocardial
ischemia-reperfusion injury [79]. Similarly, despite cardiac troponins being the gold standard for
diagnosing
MI, research like that done by Zhu et al., identifying a nine-gene microsome-related signature, raises the
possibility of more precise and targeted biomarkers to overcome the limitations of the current methods
[77].
The role of migrasomes in soft tissue regeneration
Soft tissue defects can vary from minor lacerations requiring sutures to extensive injuries requiring stem
cell-based therapy. Through stem cell pluripotency, self-renewal and regenerative cytokine secretion, stem
cell
therapy aims to rejuvenate or replace dysfunctional tissue and organs [80]. Finding suitable cell sources
to use
as regenerative agents is the first difficulty in stem cell-based treatment. Research has shown that
adipose-derived stem cells (ASCs) have proven efficiency in regenerating damaged soft tissue, the
recruitment of
ASCs is mediated by chemokine CXCL12. However, the mechanism at which CXCL12 is generated remains unclear
[80-82]. Chen et al. conducted an original study analyzing the use of migrasomes enriched with CXLXL12 and
whether migrasomes could aid in ASC-mediated tissue regeneration [82]. Their study found migrasomes
stimulated
ASC migrations by activating CXCL12-mediated CXCR3/RhoA signaling both in vitro and in vivo.
Through analyzing TSPAN4, Chan et al. demonstrated that the pattern of migrasomes during adipose tissue
regeneration corresponded with CXCL12 expression and ASC infiltration. Thus, uncovering a previously
unknown
function of migrasomes in tissue regeneration. To our knowledge, this study is the first to explore the
role of
migrasomes in soft tissue regeneration; thus, additional research is necessary to confirm and elaborate on
these
preliminary results.
The potential function of migrasomes in Osteoclastogenesis
Osteoclastogenesis is the process by which precursor cells develop into osteoclasts and is essential for
maintaining bone health. Mature osteoclast development depends on the creation of multinucleated cells,
which
occurs when mononuclear pre-osteoclasts move close to one another to fuse [83]. The dysfunction of this
process
is related to multiple skeletal diseases such as osteoporosis and rheumatoid arthritis [84]. Lampiasi et
al.
conducted an original study examining the mechanisms, such as migration and fusion, that regulate the
initial
processes of osteoclastogenesis [85]. By assessing morphological changes, cytoskeleton structure, and the
subcellular distribution of specific proteins, their study examined the timing and behavior of osteoclasts
during their in vitro development. Lampiasi et al. observed that morphological changes and
cytoskeleton
organization were related to RANKL stimulation. Interestingly, they observed unknown vesicles along the
filopodia of 3-day RANKL-positive cells, which Lampiasi et al. believe resemble migrasomes. While it
remains
challenging to determine the exact function of migrasomes during osteoclastogenesis, Lampaidi et al.
speculate
the migrasomes could serve as migration mediators. Although further investigation is necessary to fully
understand the roles of migrasomes, this initial finding by Lampiasi et al. indicates that they may play
an
important role in osteogenesis [86]. We believe these early findings are promising and may lead to the
development of novel treatment approaches for diseases due to osteoclast dysfunction such as rheumatoid
arthritis and osteoporosis. Their possible contributions to bone health and disease management will become
more
evident as research into migrasome activity continues.
New perspectives and further research directions
As previously mentioned, significant advancements have been made in migrasome research; however, much of this work remains in the early stages of investigation. Despite the novelty of migrasomes, our review's most profound takeaway is the transformative potential migrasomes hold for medicine and disease management. While our analysis of the applications of migrasomes is promising, Additional research is crucial, particularly regarding disease precipitation and the mechanisms involved, to identify strategies for inhibiting the progression of severe disease or, ideally, halting it entirely.
A promising future direction for migrasomes is their potential use as a novel osteoporosis marker. Pekkinen et al. previously reported that SMS2 variants are associated with osteoporosis and skeletal dysplasia [86]. The relationship between SMS2 and migrasome formation as a marker of osteoporosis was then further investigated by Sokoya et al [10]. Their research revealed that the accumulation of sphingomyelin (SM) in the endoplasmic reticulum (ER) affects the lipid composition and organization of cellular membranes. Notably, there is a disruption in the trans-bilayer asymmetry of SM, meaning the usual distribution of SM across the lipid bilayer is altered. These changes have been observed in patient-derived fibroblasts and are accompanied by imbalances in cholesterol distribution, glycerophospholipid profiles, and overall membrane lipid order within the secretory pathway. The disruption of these lipid landscapes is believed to impair the function of osteogenic cells, which are essential for bone formation and maintenance. They further deduced that the inability to maintain proper lipid distributions may compromise membrane properties critical for the activity of these bone-forming cells, thereby contributing to skeletal abnormalities. Through the investigation of lipid homeostasis this study has uncovered new connections between lipid homeostasis and genetic disorders. This allows us to investigate if there are any other currently undiscovered inherited disorders with a similar mode of action. These findings also highlight a new mode of sphingomyelin function that expands beyond its role within the plasma membrane. Novel therapeutic effects that may emerge as a result of this study include possibly targeting lipid imbalances that cause osteoporosis with Calvarial Doughnut Lesions and other disorders associated with membrane disorder defects.
Another promising future direction in migrasome research involves investigating migrasome activation in cerebral amyloid angiopathy (CAA). CAA is a primary cause of intracerebral haemorrhage (ICH) and cognitive impairment in the elderly [89]. Despite its high prevalence and poor prognosis, there is currently no viable therapy for CAA due to the limited understanding of its pathogenesis. An original study by Hu et al. analysed migrasome activation in a cerebral amyloid angiopathy mouse model [90]. An original study by Hu et al. analysed migrasome activation in a cerebral amyloid angiopathy mouse. Their research revealed that macrophage lineage cells stimulated by amyloid proteins produce migrasomes excessively, contributing to complement-dependent vascular damage. This suggests that complement inhibition could serve as a therapeutic treatment for cerebral amyloid angiopathy (CAA), with C5b-9 identified as a potential biomarker for the disease [90]. Despite the encouraging preliminary results, further research is needed to analyze the molecular mechanisms mediating interactions among CD5L, migrasomes, and migrasome recipient cells before any conclusive findings can be drawn.
In a letter to editors, Liu et al. hypothesised a novel mechanism of HSV-2 cell-to-cell spread mediated by migrasomes [92]. Their initial study suggests that HSV-2-GFP spread to cells likely via migrasomes , could lead to infection. While more research is needed into the potential role of migrasomes in viral infections, this initial research by Liu et al. could lead to developing a possible target for intervention against HSV-2 transmission. During the formation of this study, it became evident that, despite the growing research into migrasomes, many fundamental questions remain unresolved, such as a detailed mechanism of migrasome recognition and uptake, their role in mitochondrial quality control and their role in immune modulation, to name a few. Additionally, advancements in the technologies employed for visualizing migrasomes could significantly enhance the understanding and validation of the proposed hypotheses. This is a positive reflection of the inherent characteristics of science and research. These advancements in understanding this newly discovered organelle is significant.
Conclusion
As highlighted previously, Ma et al. introduced the term migracytosis to describe the migration-dependent release of cellular contents via migrasomes, enabling direct cell-to-cell communication. In this review, we have outlined various ways migrasomes, through migracytosis, influence both physiological and pathological processes. This mechanism has been associated with numerous conditions, including cancer, acute, and chronic diseases. Notably, our review of migrasomes and tumor microenvironments studies suggests a link between migrasomes and tumor progression, highlighting the potential to alter migrasome formation. Ding et al. identified phosphoinositide kinase (PIP5K1A) and PIP2 production as key factors determining migrasome biogenesis sites and proposed the phosphatidylinositol Rab35 axis as a regulator of migrasome formation [13, 87, 88]. We believe these findings offer a promising foundation for developing migrasome formation inhibitors, allowing future research to explore this potential further.
Similarly, given that many essential biological processes such as neuronal network formation and immune responses rely on precise, localized cell communication, it is likely that future research will explore ways to harness migracytosis as a novel strategy for disease treatment. While promising, this line of research is currently limited by the scarcity of comprehensive studies on migrasomes. Notably, no conflicting data have been published to date. As a newly identified organelle, we believe that, over time, further research will continue to uncover its complex functions and potential applications.
Acknowledgements
Lauryn Akeme and Pollyanna Sibanda contributed equally to the conceptualization of the manuscript and drafted the sections on migrasome biogenesis and comparisons between migrasomes and exosomes. Aisling Fitzgerald prepared the section on podocyte injury and kidney disease, including the figures, and completed the edits required for publication. Agnieszka Bossowska contributed to the sections on myocardial infarction and osteoclastogenesis, and participated in critical revision of the text. Klaudia Bonowicz was responsible for the conceptual development and substantive content of the section on proliferative vitreoretinopathy, and co-authored the summary and future perspectives section.
Dominika Jerka was responsible for the content development of the section on the tumor microenvironment, with a particular focus on pancreatic and liver cancers. Maciej Gagat supervised the overall structure of the review, edited the entire manuscript, and prepared the final version for submission. All authors read and approved the final version of the manuscript.
Disclosure Statement
The authors have no conflicts of interest to declare.
Disclosure of AI Assistance
In the process of writing, we utilized artificial intelligence tools, specifically language models, to
assist
with text development, formulating precise statements, and improving grammar and style. AI was used
exclusively
for the editorial process and did not influence the substantive aspects of the research, data analysis, or
interpretation of results
References
| 1 | Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, et al. Discovery of the migrasome, an organelle mediating
release of cytoplasmic contents during cell migration. Cell Res. 2015 Jan;25(1):24-38.
https://doi.org/10.1038/cr.2014.135 |
| 2 | Mei J, Cao X, Zhou B, Zhu W, Wang M. Migrasomes: Emerging organelles for unveiling physiopathology
and advancing clinical implications. Life Sci. 2024 Dec 1;358:123152.
https://doi.org/10.1016/j.lfs.2024.123152 |
| 3 | Yu S, Yu L. Migrasome biogenesis and functions. FEBS J. 2022 Nov;289(22):7246-54.
https://doi.org/10.1111/febs.16183 |
| 4 | Jiang D, He J, Yu L. The migrasome, an organelle for cell-cell communication. Trends Cell Biol.
2025 Mar;35(3):205-16.
https://doi.org/10.1016/j.tcb.2024.05.003 |
| 5 | Zanotelli MR, Zhang J, Reinhart-King CA. Mechanoresponsive metabolism in cancer cell migration and
metastasis. Cell Metab. 2021 Jul 6;33(7):1307-21.
https://doi.org/10.1016/j.cmet.2021.04.002 |
| 6 | Dharan R, Huang Y, Cheppali SK, Goren S, Shendrik P, Wang W, et al. Tetraspanin 4 stabilizes
membrane swellings and facilitates their maturation into migrasomes. Nat Commun. 2023 Feb
23;14(1):1037.
https://doi.org/10.1038/s41467-023-36596-9 |
| 7 | Zhai Z, Liu B, Yu L. The roles of migrasome in development. Cell Insight. 2024 Feb;3(1):100142.
https://doi.org/10.1016/j.cellin.2023.100142 |
| 8 | Goñi FM. Sphingomyelin: What is it good for? Biochem Biophys Res Commun. 2022 Dec 10;633:23-5.
https://doi.org/10.1016/j.bbrc.2022.08.074 |
| 9 | Steinbauer B, Mehnert T, Beyer K. Hydration and lateral organization in phospholipid bilayers
containing sphingomyelin: a 2H-NMR study. Biophys J. 2003 Aug;85(2):1013-24.
https://doi.org/10.1016/S0006-3495(03)74540-8 |
| 10 | Sokoya T, Parolek J, Foged MM, Danylchuk DI, Bozan M, Sarkar B, et al. Pathogenic variants of
sphingomyelin synthase SMS2 disrupt lipid landscapes in the secretory pathway. eLife. 2022 Sep
14;11:e79278.
https://doi.org/10.7554/eLife.79278 |
| 11 | Liang H, Ma X, Zhang Y, Liu Y, Liu N, Zhang W, et al. The formation of migrasomes is initiated by
the assembly of sphingomyelin synthase 2 foci at the leading edge of migrating cells. Nat Cell Biol.
2023 Aug;25(8):1173-84.
https://doi.org/10.1038/s41556-023-01188-8 |
| 12 | Zhai Z, Liu B, Yu L. The roles of migrasome in development. Cell Insight. 2023 Nov 28;3(1):100142.
https://doi.org/10.1016/j.cellin.2023.100142 |
| 13 | Ding T, Ji J, Zhang W, Liu Y, Liu B, Han Y, et al. The phosphatidylinositol (4,
5)-bisphosphate-Rab35 axis regulates migrasome formation. Cell Res. 2023 Aug;33(8):617-27.
https://doi.org/10.1038/s41422-023-00811-5 |
| 14 | Wu D, Xu Y, Ding T, Zu Y, Yang C, Yu L. Pairing of integrins with ECM proteins determines
migrasome formation. Cell Res. 2017 Nov;27(11):1397-400.
https://doi.org/10.1038/cr.2017.108 |
| 15 | Zhang X, Yao L, Meng Y, Li B, Yang Y, Gao F. Migrasome: a new functional extracellular vesicle.
Cell Death Discov. 2023 Oct 18;9(1):381.
https://doi.org/10.1038/s41420-023-01673-x |
| 16 | Kummer D, Steinbacher T, Schwietzer MF, Thölmann S, Ebnet K. Tetraspanins: integrating cell
surface receptors to functional microdomains in homeostasis and disease. Med Microbiol Immunol
(Berl). 2020 Aug;209(4):397-405.
https://doi.org/10.1007/s00430-020-00673-3 |
| 17 | Huang Y, Zucker B, Zhang S, Elias S, Zhu Y, Chen H, et al. Migrasome formation is mediated by
assembly of micron-scale tetraspanin macrodomains. Nat Cell Biol. 2019 Aug;21(8):991-1002.
https://doi.org/10.1038/s41556-019-0367-5 |
| 18 | Jiang D, Jiang Z, Lu D, Wang X, Liang H, Zhang J, et al. Migrasomes provide regional cues for
organ morphogenesis during zebrafish gastrulation. Nat Cell Biol. 2019 Aug;21(8):966-77.
https://doi.org/10.1038/s41556-019-0358-6 |
| 19 | Huang Y, Yu L. Tetraspanin-enriched microdomains: The building blocks of migrasomes. Cell Insight.
2022 Feb;1(1):100003.
https://doi.org/10.1016/j.cellin.2021.100003 |
| 20 | Jiao H, Yu L. Migrasomes: Biogenesis, physiological roles, and therapeutic potentials. J Cell
Biol. 2024 Nov 4;223(11):e202403051.
https://doi.org/10.1083/jcb.202403051 |
| 21 | Fan C, Shi X, Zhao K, Wang L, Shi K, Liu YJ, et al. Cell migration orchestrates migrasome
formation by shaping retraction fibers. J Cell Biol. 2022 Apr 4;221(4):e202109168.
https://doi.org/10.1083/jcb.202109168 |
| 22 | Stoker M, Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta
BBA - Rev Cancer. 1991 Apr 16;1072(1):81-102.
https://doi.org/10.1016/0304-419X(91)90008-9 |
| 23 | Zhang F, Liu W, Mao Y, Yang Y, Ling C, Liu Y, et al. Migrasome, a migration-dependent organelle.
Front Cell Dev Biol. 2024;12:1417242.
https://doi.org/10.3389/fcell.2024.1417242 |
| 24 | Xu X, Wu T, Lin R, Zhu S, Ji J, Jin D, et al. Differences between migrasome, a 'new organelle',
and exosome. J Cell Mol Med. 2023 Dec;27(23):3672-80.
https://doi.org/10.1111/jcmm.17942 |
| 25 | Wu L, Yang S, Li H, Zhang Y, Feng L, Zhang C, et al. TSPAN4-positive migrasome derived from
retinal pigmented epithelium cells contributes to the development of proliferative
vitreoretinopathy. J Nanobiotechnology. 2022 Dec 9;20(1):519.
https://doi.org/10.1186/s12951-022-01732-y |
| 26 | Liu Y, Li S, Rong W, Zeng C, Zhu X, Chen Q, et al. Podocyte-Released Migrasomes in Urine Serve as
an Indicator for Early Podocyte Injury. Kidney Dis Basel Switz. 2020 Nov;6(6):422-33.
https://doi.org/10.1159/000511504 |
| 27 | Gudbergsson JM, Etzerodt A. Migrasomes should not be classified as extracellular vesicles. J Cell
Mol Med. 2024 May;28(9):e18337.
https://doi.org/10.1111/jcmm.18337 |
| 28 | Tan X, He S, Wang F, Li L, Wang W. Migrasome, a novel organelle, differs from exosomes. Biochem
Biophys Rep. 2023 Sep;35:101500.
https://doi.org/10.1016/j.bbrep.2023.101500 |
| 29 | Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential.
Cell Biosci. 2019;9:19.
https://doi.org/10.1186/s13578-019-0282-2 |
| 30 | Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic
potential. Expert Rev Proteomics. 2009 Jun;6(3):267-83.
https://doi.org/10.1586/epr.09.17 |
| 31 | Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their
composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta.
2012 Jul;1820(7):940-8.
https://doi.org/10.1016/j.bbagen.2012.03.017 |
| 32 | Zhao X, Lei Y, Zheng J, Peng J, Li Y, Yu L, et al. Identification of markers for migrasome
detection. Cell Discov. 2019 May 21;5(1):1-4.
https://doi.org/10.1038/s41421-019-0093-y |
| 33 | Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014
Aug;29:116-25.
https://doi.org/10.1016/j.ceb.2014.05.004 |
| 34 | Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011 Jul
1;3:15.
https://doi.org/10.3410/B3-15 |
| 35 | Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and
Methods for Exosome Isolation and Analysis. Cells. 2019 Jul 15;8(7):727.
https://doi.org/10.3390/cells8070727 |
| 36 | Maeda K, Abdi R, Tsokos GC. The Role of Podocytes in Lupus Pathology. Curr Rheumatol Rep. 2024 Dec
28;27(1):10.
https://doi.org/10.1007/s11926-024-01175-4 |
| 37 | Yang C, Zhang Z, Liu J, Chen P, Li J, Shu H, et al. Research progress on multiple cell death
pathways of podocytes in diabetic kidney disease. Mol Med Camb Mass. 2023 Oct 12;29(1):135.
https://doi.org/10.1186/s10020-023-00732-4 |
| 38 | Schmidt-Pogoda A, Strecker JK, Liebmann M, Massoth C, Beuker C, Hansen U, et al. Dietary salt
promotes ischemic brain injury and is associated with parenchymal migrasome formation. PloS One.
2018;13(12):e0209871.
https://doi.org/10.1371/journal.pone.0209871 |
| 39 | Zhang C, Li T, Yin S, Gao M, He H, Li Y, et al. Monocytes deposit migrasomes to promote embryonic
angiogenesis. Nat Cell Biol. 2022 Dec;24(12):1726-38.
https://doi.org/10.1038/s41556-022-01026-3 |
| 40 | Yang R, Zhang H, Chen S, Lou K, Zhou M, Zhang M, et al. Quantification of urinary podocyte-derived
migrasomes for the diagnosis of kidney disease. J Extracell Vesicles. 2024 Jun;13(6):e12460.
https://doi.org/10.1002/jev2.12460 |
| 41 | Zhang R, Peng J, Zhang Y, Zheng K, Chen Y, Liu L, et al. Pancreatic cancer cell-derived migrasomes
promote cancer progression by fostering an immunosuppressive tumor microenvironment. Cancer Lett.
2024 Nov 28;605:217289.
https://doi.org/10.1016/j.canlet.2024.217289 |
| 42 | Mathieson PW. The podocyte cytoskeleton in health and in disease. Clin Kidney J. 2012
Dec;5(6):498-501.
https://doi.org/10.1093/ckj/sfs153 |
| 43 | Babelova A, Jansen F, Sander K, Löhn M, Schäfer L, Fork C, et al. Activation of Rac-1 and RhoA
contributes to podocyte injury in chronic kidney disease. PloS One. 2013;8(11):e80328.
https://doi.org/10.1371/journal.pone.0080328 |
| 44 | Asanuma K, Yanagida-Asanuma E, Takagi M, Kodama F, Tomino Y. The role of podocytes in proteinuria.
Nephrology. 2007;12(s3):S15-20.
https://doi.org/10.1111/j.1440-1797.2007.00876.x |
| 45 | Arneth B. Tumor Microenvironment. Med Kaunas Lith. 2019 Dec 30;56(1):15.
https://doi.org/10.3390/medicina56010015 |
| 46 | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during
tumorigenesis. Cell. 1996 Aug 9;86(3):353-64.
https://doi.org/10.1016/S0092-8674(00)80108-7 |
| 47 | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010 Apr 29;362(17):1605-17.
https://doi.org/10.1056/NEJMra0901557 |
| 48 | Bugazia D, Al-Najjar E, Esmail A, Abdelrahim S, Abboud K, Abdelrahim A, et al. Pancreatic ductal
adenocarcinoma: the latest on diagnosis, molecular profiling, and systemic treatments. Front Oncol.
2024;14:1386699.
https://doi.org/10.3389/fonc.2024.1386699 |
| 49 | Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J, et al. Overexpression of CXCL5 Is Associated
With Poor Survival in Patients With Pancreatic Cancer. Am J Pathol. 2011 Mar;178(3):1340-9.
https://doi.org/10.1016/j.ajpath.2010.11.058 |
| 50 | Xia J, Xu X, Huang P, He M, Wang X. The potential of CXCL5 as a target for liver cancer - what do
we know so far? Expert Opin Ther Targets. 2015 Feb;19(2):141-6.
https://doi.org/10.1517/14728222.2014.993317 |
| 51 | Wu K, Yu S, Liu Q, Bai X, Zheng X, Wu K. The clinical significance of CXCL5 in non-small cell lung
cancer. OncoTargets Ther. 2017;10:5561-73.
https://doi.org/10.2147/OTT.S148772 |
| 52 | Dang H, Wu W, Wang B, Cui C, Niu J, Chen J, et al. CXCL5 Plays a Promoting Role in Osteosarcoma
Cell Migration and Invasion in Autocrine- and Paracrine-Dependent Manners. Oncol Res. 2017 Jan
26;25(2):177-86.
https://doi.org/10.3727/096504016X14732772150343 |
| 53 | Roca H, Jones JD, Purica MC, Weidner S, Koh AJ, Kuo R, et al. Apoptosis-induced CXCL5 accelerates
inflammation and growth of prostate tumor metastases in bone. J Clin Invest. 2018 Jan
2;128(1):248-66.
https://doi.org/10.1172/JCI92466 |
| 54 | Zhang R, Liu Q, Peng J, Wang M, Li T, Liu J, et al. CXCL5 overexpression predicts a poor prognosis
in pancreatic ductal adenocarcinoma and is correlated with immune cell infiltration. J Cancer. 2020
Feb 10;11(9):2371-81.
https://doi.org/10.7150/jca.40517 |
| 55 | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular
carcinoma. Nat Rev Dis Primer. 2021 Jan 21;7(1):1-28.
https://doi.org/10.1038/s41572-020-00240-3 |
| 56 | Zhang K, Zhu Z, Jia R, Wang NA, Shi M, Wang Y, et al. CD151-enriched migrasomes mediate
hepatocellular carcinoma invasion by conditioning cancer cells and promoting angiogenesis. J Exp
Clin Cancer Res CR. 2024 Jun 6;43(1):160.
https://doi.org/10.1186/s13046-024-03082-z |
| 57 | Jiang Y, Liu X, Ye J, Ma Y, Mao J, Feng D, et al. Migrasomes, a new mode of intercellular
communication. Cell Commun Signal. 2023 May 8;21(1):105.
https://doi.org/10.1186/s12964-023-01121-4 |
| 58 | Rivera Z, Escutia C, Madonna MB, Gupta KH. Biological Insight and Recent Advancement in the
Treatment of Neuroblastoma. Int J Mol Sci. 2023 May 9;24(10):8470.
https://doi.org/10.3390/ijms24108470 |
| 59 | Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin
North Am. 2010 Feb;24(1):65-86.
https://doi.org/10.1016/j.hoc.2009.11.011 |
| 60 | Riley RD, Heney D, Jones DR, Sutton AJ, Lambert PC, Abrams KR, et al. A systematic review of
molecular and biological tumor markers in neuroblastoma. Clin Cancer Res Off J Am Assoc Cancer Res.
2004 Jan 1;10(1 Pt 1):4-12.
https://doi.org/10.1158/1078-0432.CCR-1051-2 |
| 61 | Li W, Xia Y, Wang J, Jin H, Li X. Prognostic significance of migrasomes in neuroblastoma through
machine learning and multi-omics. Sci Rep. 2024 Jul 18;14(1):16629.
https://doi.org/10.1038/s41598-024-67682-7 |
| 62 | Köktürk S, Doğan S, Yılmaz CE, Cetinkol Y, Mutlu O. Expression of brain-derived neurotrophic
factor and formation of migrasome increases in the glioma cells induced by the adipokinetic hormone.
Rev Assoc Médica Bras. 70(5):e20231337.
https://doi.org/10.1590/1806-9282.20231337 |
| 63 | Han J, Zhang L, Guo H, Wysham WZ, Roque DR, Willson AK, et al. Glucose promotes cell
proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK
signaling. Gynecol Oncol. 2015 Sep;138(3):668-75.
https://doi.org/10.1016/j.ygyno.2015.06.036 |
| 64 | Phoomak C, Vaeteewoottacharn K, Silsirivanit A, Saengboonmee C, Seubwai W, Sawanyawisuth K, et al.
High glucose levels boost the aggressiveness of highly metastatic cholangiocarcinoma cells via
O-GlcNAcylation. Sci Rep. 2017 Mar 6;7(1):43842.
https://doi.org/10.1038/srep43842 |
| 65 | Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, et al. Metabolic determinants
of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014 Apr
3;508(7494):108-12.
https://doi.org/10.1038/nature13110 |
| 66 | Malekan M, Nezamabadi SS, Samami E, Mohebalizadeh M, Saghazadeh A, Rezaei N. BDNF and its
signaling in cancer. J Cancer Res Clin Oncol. 2023 Jun 1;149(6):2621-36.
https://doi.org/10.1007/s00432-022-04365-8 |
| 67 | Carpineto P, Licata AM, Ciancaglini M. Proliferative Vitreoretinopathy: A Reappraisal. J Clin Med.
2023 Aug 14;12(16):5287.
https://doi.org/10.3390/jcm12165287 |
| 68 | Idrees S, Sridhar J, Kuriyan AE. Proliferative Vitreoretinopathy: A Review. Int Ophthalmol Clin.
2019;59(1):221-40.
https://doi.org/10.1097/IIO.0000000000000258 |
| 69 | Garweg JG, Tappeiner C, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in
retinal detachment. Surv Ophthalmol. 2013;58(4):321-9.
https://doi.org/10.1016/j.survophthal.2012.12.004 |
| 70 | Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM. An updated classification of
retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991 Aug 15;112(2):159-65.
https://doi.org/10.1016/S0002-9394(14)76695-4 |
| 71 | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell
perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22.
https://doi.org/10.1038/s41536-019-0083-6 |
| 72 | Deniz IA, Karbanová J, Wobus M, Bornhäuser M, Wimberger P, Kuhlmann JD, et al. Mesenchymal stromal
cell-associated migrasomes: a new source of chemoattractant for cells of hematopoietic origin. Cell
Commun Signal CCS. 2023 Feb 14;21(1):36.
https://doi.org/10.1186/s12964-022-01028-6 |
| 73 | Liu L, Cooper T, Howley PM, Hayball JD. From Crescent to Mature Virion: Vaccinia Virus Assembly
and Maturation. Viruses. 2014 Oct 7;6(10):3787-808.
https://doi.org/10.3390/v6103787 |
| 74 | Lv L, Zhang L. Identification of poxvirus inside migrasomes suggests a novel mode of mpox virus
spread. J Infect. 2023 Aug;87(2):160-2.
https://doi.org/10.1016/j.jinf.2023.05.024 |
| 75 | Mechanic OJ, Gavin M, Grossman SA: Acute Myocardial Infarction; in StatPearls. Treasure Island
(FL), StatPearls Publishing, 2023 [Cited 2025 Mar 22]..
|
| 76 | Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y, et al. Mitocytosis, a migrasome-mediated mitochondrial
quality-control process. Cell. 2021 May 27;184(11):2896-2910.e13.
https://doi.org/10.1016/j.cell.2021.04.027 |
| 77 | Zhu Y, Chen Y, Xu J, Zu Y. Unveiling the Potential of Migrasomes: A Machine-Learning-Driven
Signature for Diagnosing Acute Myocardial Infarction. Biomedicines. 2024 Jul 22;12(7):1626.
https://doi.org/10.3390/biomedicines12071626 |
| 78 | Zheng Y, Lang Y, Qi B, Li T. TSPAN4 and migrasomes in atherosclerosis regression correlated to
myocardial infarction and pan-cancer progression. Cell Adhes Migr. 17(1):14-9.
https://doi.org/10.1080/19336918.2022.2155337 |
| 79 | Sun P, Li Y, Yu W, Chen J, Wan P, Wang Z, et al. Low-intensity pulsed ultrasound improves
myocardial ischaemia-reperfusion injury via migrasome-mediated mitocytosis. Clin Transl Med. 2024
Jul;14(7):e1749.
https://doi.org/10.1002/ctm2.1749 |
| 80 | Qin Y, Ge G, Yang P, Wang L, Qiao Y, Pan G, et al. An Update on Adipose-Derived Stem Cells for
Regenerative Medicine: Where Challenge Meets Opportunity. Adv Sci Weinh Baden-Wurtt Ger. 2023
Jul;10(20):e2207334.
https://doi.org/10.1002/advs.202207334 |
| 81 | Coulange Zavarro A, Velier M, Arcani R, Abellan Lopez M, Simoncini S, Benyamine A, et al. Adipose
Tissue and Adipose-Tissue-Derived Cell Therapies for the Treatment of the Face and Hands of Patients
Suffering from Systemic Sclerosis. Biomedicines. 2023 Jan 26;11(2):348.
https://doi.org/10.3390/biomedicines11020348 |
| 82 | Chen Y, Li Y, Li B, Hu D, Dong Z, Lu F. Migrasomes from adipose derived stem cells enrich CXCL12
to recruit stem cells via CXCR4/RhoA for a positive feedback loop mediating soft tissue
regeneration. J Nanobiotechnology. 2024 May 3;22(1):219.
https://doi.org/10.1186/s12951-024-02482-9 |
| 83 | Blackman MR, Boyce A, Chrousos G, et al. (eds): Endotext. South Dartmouth (MA), MDText.com, Inc.,
2000 [Cited 2025 Mar 22].
|
| 84 | Allard-Chamard H, Carrier N, Dufort P, Durand M, de Brum-Fernandes AJ, Boire G, et al. Osteoclasts
and their circulating precursors in rheumatoid arthritis: Relationships with disease activity and
bone erosions. Bone Rep. 2020 Jun;12:100282.
https://doi.org/10.1016/j.bonr.2020.100282 |
| 85 | Lampiasi N, Russo R, Kireev I, Strelkova O, Zhironkina O, Zito F. Osteoclasts Differentiation from
Murine RAW 264.7 Cells Stimulated by RANKL: Timing and Behavior. Biology. 2021 Feb 4;10(2):117.
https://doi.org/10.3390/biology10020117 |
| 86 | Pekkinen M, Terhal PA, Botto LD, Henning P, Mäkitie RE, Roschger P, et al. Osteoporosis and
skeletal dysplasia caused by pathogenic variants in SGMS2 JCI Insight. 2019 Apr 4;4(7):e126180,
126180.
https://doi.org/10.1172/jci.insight.126180 |
| 87 | Zhen Y, Stenmark H. A phosphoinositide kinase triggers migrasome formation. Cell Res. 2023
Aug;33(8):577-8.
https://doi.org/10.1038/s41422-023-00822-2 |
| 88 | Tang H, Huang Z, Wang M, Luan X, Deng Z, Xu J, et al. Research progress of migrasomes: from
genesis to formation, physiology to pathology. Front Cell Dev Biol. 2024;12:1420413.
https://doi.org/10.3389/fcell.2024.1420413 |
| 89 | Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol Seoul
Korea. 2011 Mar;7(1):1-9.
https://doi.org/10.3988/jcn.2011.7.1.1 |
| 90 | Hu M, Li T, Ma X, Liu S, Li C, Huang Z, et al. Macrophage lineage cells-derived migrasomes
activate complement-dependent blood-brain barrier damage in cerebral amyloid angiopathy mouse model.
Nat Commun. 2023 Jul 4;14(1):3945.
https://doi.org/10.1038/s41467-023-39693-x |
| 91 | GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney
disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 Lancet Lond
Engl. 2020 Feb 29;395(10225):709-33.
https://doi.org/10.1016/S0140-6736(19)32977-0 |
| 92 | Liu Y, Zhu Z, Li Y, Yang M, Hu Q. Migrasomes released by HSV-2-infected cells serve as a
conveyance for virus spread. Virol Sin. 2023 Aug;38(4):643-5.
https://doi.org/10.1016/j.virs.2023.06.001 |