Association Between DRD4 rs747302 and VNTR Polymorphisms and Drug Addiction in An Iraqi Population
bZoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt,
cCentre of Biotechnology Research, University of Al Nahrin, Baghdad, Iraq
Keywords
Abstract
Background/Aims:
Drug addiction is a neuropsychiatric disorder characterised by compulsive drug-seeking behaviour notwithstanding adverse consequences. This work seeks to address a deficiency in the literature by comparing drug-addicted and non-addicted individuals within an Iraqi population through the analysis of a 1000-base pair variable number of tandem repeats (VNTRs) polymorphism of the dopamine receptor gene DRD4. The association of this novel polymorphism with drug addiction has not yet been examined.Methods:
A total of 270 people were registered between May 2022 and June 2023. Of these, 180 had drug addictions and 90 were healthy controls. DNA was extracted from the participants’ blood samples. Restriction Fragment Length Polymorphism was used to investigate genetic polymorphisms in the DRD4 and VNTR genes to identify differences.Results:
The genotype frequencies differed markedly between the control group (GC, 3% frequency) and the patient group (GC, 37% frequency). The control group had more of the genotype that was more common among addicts. The C allele was present in 60% of the patients but in only 1% of the controls. The results showed that the CC genotype is more common in the patient group than in the control group. A comparison of repetitions between the control and patient groups was made based on the distribution of genotypes of SNP rs747302. Patients with the GG genotype had an average of 17 repetitions, whereas those with the GC genotype had 18, and those with the CC genotype had 18.3. The results showed that people in the CC genotype group had a lot more repetitions.Conclusion:
The results of our study indicated that the CC, GC, and VNTR genotypes significantly contribute to heroin addiction risk in Iraqis.Introduction
Drug addiction is a chronic, relapsing brain disease that is defined by an obsessive need to get drugs and use them even when they have detrimental effects on behaviour and neurobiology [1]. The main way that psychomotor stimulants like cocaine, methamphetamine, and amphetamine function is by raising the amounts of extracellular dopamine in the brain [2]. So, genes that are involved in dopaminergic neurotransmission are very important in the causes of drug addiction [1]. Dopamine is necessary for long-term neural plasticity, which in turn makes reward-predictive cues important [3]. The human brain has five different types of dopamine receptors: D1, D2, D3, D4, and D5 [4]. These receptors are encoded by the genes DRD1, DRD2, DRD3, DRD4, and DRD5 [5], controlling several physiological processes that are affected by dopamine. People have recently become quite interested in the gene DRD4, which is located on chromosome 11p15.5 [7]. This gene may be a risk factor for disorders that make people more likely to acquire addictive habits [8].
A Variable Number of Tandem Repeats locus (VNTR) shows how a short nucleotide motif is arranged as a series of repeated sequences in a DNA sequence [9]. These polymorphism zones are spread out over numerous chromosomes and are defined by differences in the number of copies of the repeating unit [9]. Minisatellites are VNTRs that have repeat units that are between 10 and 100 base pairs long. Microsatellites, on the other hand, have repeat units that are less than 10 base pairs long. VNTRs are useful identifiers for forensic research, genetic linkage analysis, DNA fingerprinting, and other similar domains since they are so different from each other [9]. The potential of VNTR markers for linkage mapping was first recognised when researchers observed that the number of tandem repeat units in certain genomic regions varied greatly between individuals [10].
The DRD4 exon III VNTR polymorphism is a possible genetic factor that makes people more likely to develop substance use disorders in several populations. It has gotten a lot of interest in psychiatry and behavioural genetics [11]. In most human groups, this polymorphism in DRD4 exon 3 has common length variants of 2, 4, and 7 repeats [12]. There is conflicting evidence about whether the DRD4 VNTR polymorphism is a direct genetic risk factor for substance abuse or dependence. However, some studies have shown that it may be involved in the development of intermediate phenotypes or personality traits that make people more likely to use drugs and become addicted [14].
Variable number tandem repeat polymorphisms (VNTRs) have been studied in many different populations since they change a lot and have higher mutation rates than single-nucleotide polymorphisms (SNPs). VNTRs are very beneficial for DNA fingerprinting and genetic studies that look at an entire population since they are naturally different from each other [15]. Dopaminergic, opioid, and serotonergic pathways have been the primary targets of candidate gene studies in the context of substance use disorders. Chinese groups have done research that shows there are big genetic differences between white people and Chinese people that are linked to drug addiction. Scientists have also found inherited risk factors that are only present in the Chinese people [16]. Changes in the DRD4 gene, namely in the exon III VNTR, have been regularly related to heroin addiction. This gene is involved in regulating dopamine production. One way that these VNTR mutations may change the likelihood of substance abuse is by changing how dopamine signals [17]. Functional investigations have made it clearer how DRD4 VNTR allelic variation affects dopamine action in the central nervous system. People with the long-repeat allele of DRD4 exon III VNTR seem to respond differently to drug-related signals than people with the short-repeat allele. For example, individuals carrying the long-repeat allele showed stronger cravings for heroin when exposed to drug-related cues [18]. Some researchers have revealed no link between substance use disorders and polymorphisms in DRD2 TaqI and DRD4 exon III VNTR in male Han Chinese people who are addicted to methamphetamine. This makes it possible that there are genetic differences that are unique to this community [19]. There is proof that DRD4 VNTR polymorphisms make people more likely to get addicted. Specifically, people who carry the DRD4 long-repeat allele (DRD4L) report much higher rates of substance use and more frequent use compared to control groups [20, 14].
A recent study has connected polymorphisms in the dopamine receptor D4 gene (DRD4) to substance use disorders (SUDs), especially in people who use drugs heavily. People with seven or more repetitions of the DRD4 exon III VNTR alleles were more likely to have used hard drugs in the recent six months than people with fewer repetitions [21]. There were statistically significant differences in the frequency of DRD4 genotypes between healthy controls and people with SUD in comparative analyses. This suggests that people who are addicted may be genetically more likely to become addicted [22, 23].
A 48-base pair VNTR variation in the DRD4 gene was connected to a higher risk of substance use disorder in an Arab-majority community in Jordan [24]. In the past, research in the US and Israel indicated a similar strong association between long alleles (≥6 repeats) and a higher risk of addiction [20, 25]. Also, the distribution of DRD4 exon III genotypes was very different between control subjects and those with behavioural addictions or amphetamine dependence. This is further evidence that DRD4 VNTR variation has a role in addiction phenotypes [26].
This study investigated a sample of Iraqis who had never been studied before to see if there was a link between drug addiction and the DRD4 rs747302 SNP. It also looked at how the distribution of DRD4 1000-base pair VNTR polymorphisms was different between people who were addicted and those who weren't. The aim of the study was to investigate how genetic variations in the DRD4 gene might contribute to increased susceptibility to addiction among individuals from Middle Eastern populations.
Materials and Methods
Study Design and Participants
This case-control study took place at Al-Hillah, Babylon Governorate, Iraq, from May 2022 to June 2023.
The
biological samples were obtained from two key sources: a nearby prison and a hospital. There were 185
people in
the study group who had a history of drug abuse and 90 healthy people in the control group. People in both
groups were between 25 and 45 years old. To verify a diagnosis of substance use disorder based on clinical
symptoms, the patients in the addiction group had to complete a urine test designed to screen for multiple
drugs. Everyone in Al-Hillah who was a member of the general population was considered a potential member
of the
control group. They also had to be drug-free, have no known health problems, and have never taken drugs
previously.
Blood Collection
Each participant underwent a standard phlebotomy procedure to acquire two millilitres of venous blood in
an
aseptic manner. The samples were promptly transferred to EDTA tubes to prevent clotting and facilitate DNA
extraction. To preserve the samples and ensure the accuracy of the molecular study, they were all kept at
-20 °C
until they were processed.
Single Nucleotide Polymorphisms (SNP) Genotyping
The gReliaPrep™ Blood DNA Extraction Kit (Promega, USA) was used to separate genomic DNA from whole blood
samples, following the manufacturer's instructions. A NanoDrop spectrophotometer (Nas-9, China) was used
to
measure the absorbance at 260/280 nm to determine DNA purity. DNA samples were kept at –20 °C until they
were
ready for genotyping analysis.
The polymorphism investigation was conducted at the DRD4 gene, paying special attention to the
single-nucleotide
polymorphism (SNP) rs747302 and a variable number tandem repeat (VNTR) region. Table 1 illustrates the
acquisition of primer sequences from the Integrated DNA Technologies (IDT) website and the National Centre
for
Biotechnology Information (NCBI). The custom primers were produced by AccuOlig Bioneer in Korea. The total
amount of each polymerase chain reaction (PCR) was 25 μL. It had 12.5 μL of Master Mix, 2.5 μL of each
primer, 2
μL of genomic DNA, and water that didn't have any nucleases in it. A Bio-Rad thermal cycler was used for
the
amplification. The cycling conditions were as follows: 4 minutes of initial denaturation at 95 °C; 40
cycles of
denaturation at 94 °C for 30 seconds, annealing at 70 °C for 45 seconds for DRD4 and 66 °C for VNTR, and
extension at 72 °C for 30 seconds; and lastly, a final extension at 72 °C for 10 minutes. Ethidium bromide
was
used to stain a 1.5% agarose gel, which allowed us to see the PCR results under UV light. A 1000 bp DNA
ladder
was used to determine fragment sizes and ensure the amplification of target gene fragments.
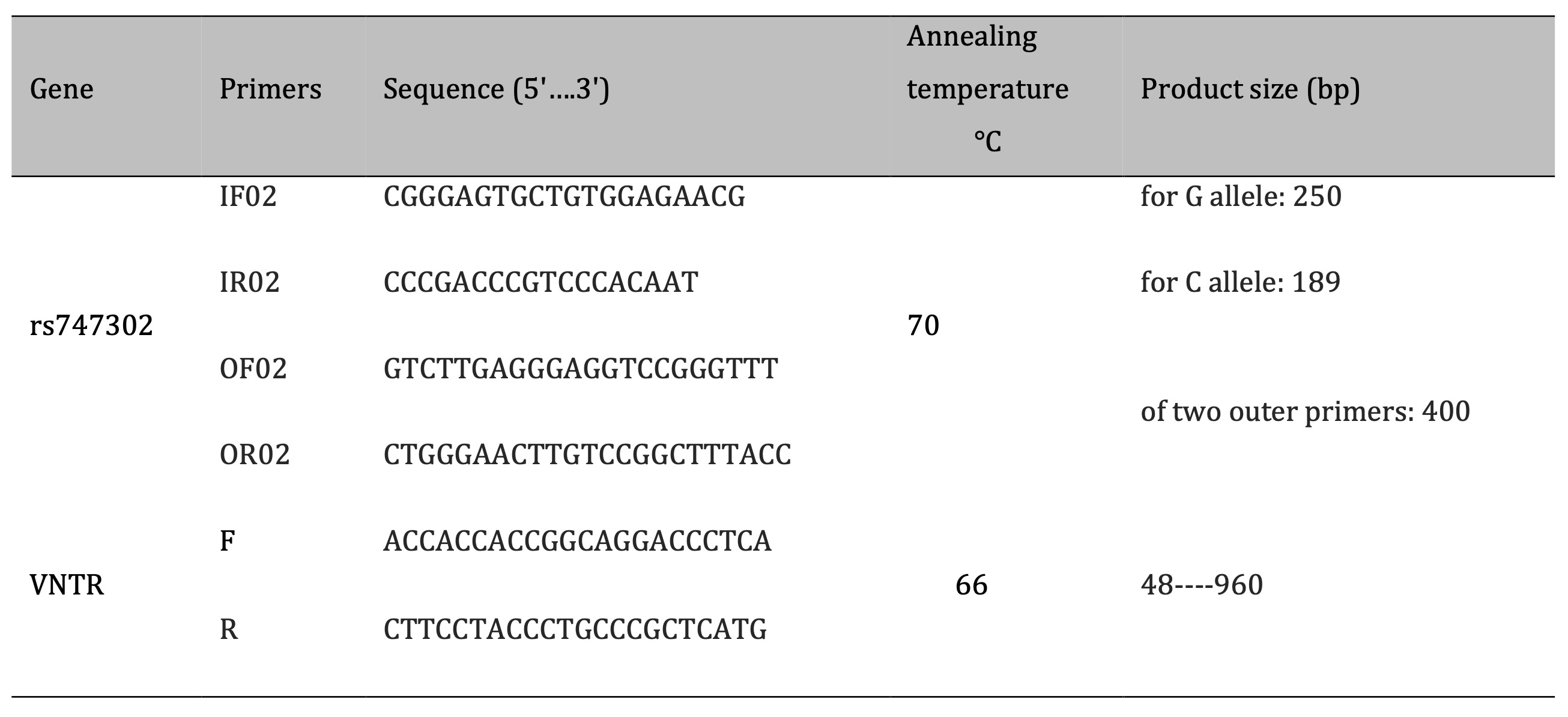
Table 1: The sequence of the primers used in the study
Statistical Analyses
The data were analysed using SPSS software (version 26) (IBM Corp., Armonk, NY, USA). The data's normality
was
evaluated before conducting statistical tests. One-way ANOVA was employed to compare group means, whilst
Student's t-test was utilised for comparisons between two groups. Genotype and allele frequencies were
determined using direct counting, and departures from Hardy–Weinberg equilibrium (HWE) were assessed using
Pearson's chi-squared test [27]. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to
assess
the strength of the associations. VNTR data were expressed as mean ± standard deviation (SD). A two-way
ANOVA
was performed to investigate the relationship between genotype distribution and VNTR patterns in patient
and
control groups. Statistical significance was established as p ≤ 0.05 (*) and p ≤ 0.01 (**).
Results
Genotypic analysis of DRD4 rs747302
Genotypic analyses of the control and patient groups examined the distribution of GG, GC, and CC genotypes
for
the rs747302 variant. Genotypic and allelic frequencies were analysed to investigate if there was a link
between
the DRD4 rs747302 polymorphism and a higher risk of drug addiction. The genotype distribution and allele
frequency among healthy controls were in line with the Hardy-Weinberg equilibrium (P < 0.05), as shown
in
Table 2. The patient group had a lot more GC (37%) and CC (43%) genotypes than the control group (20% and
3%).
This was especially true for individuals with addictions. Participants in the study had a 40% probability
of
harbouring the G allele, while those participating in the control group had a 90% probability. On the
other
hand, the C allele was found in 60% of the patients and only 1% of the controls (Table 2). The p-value of
0.0001
is well below the significance threshold of 0.05, indicating a highly significant association. This study
found
that the CC genotype is more prevalent in the patient group than in the control group (Table 2).
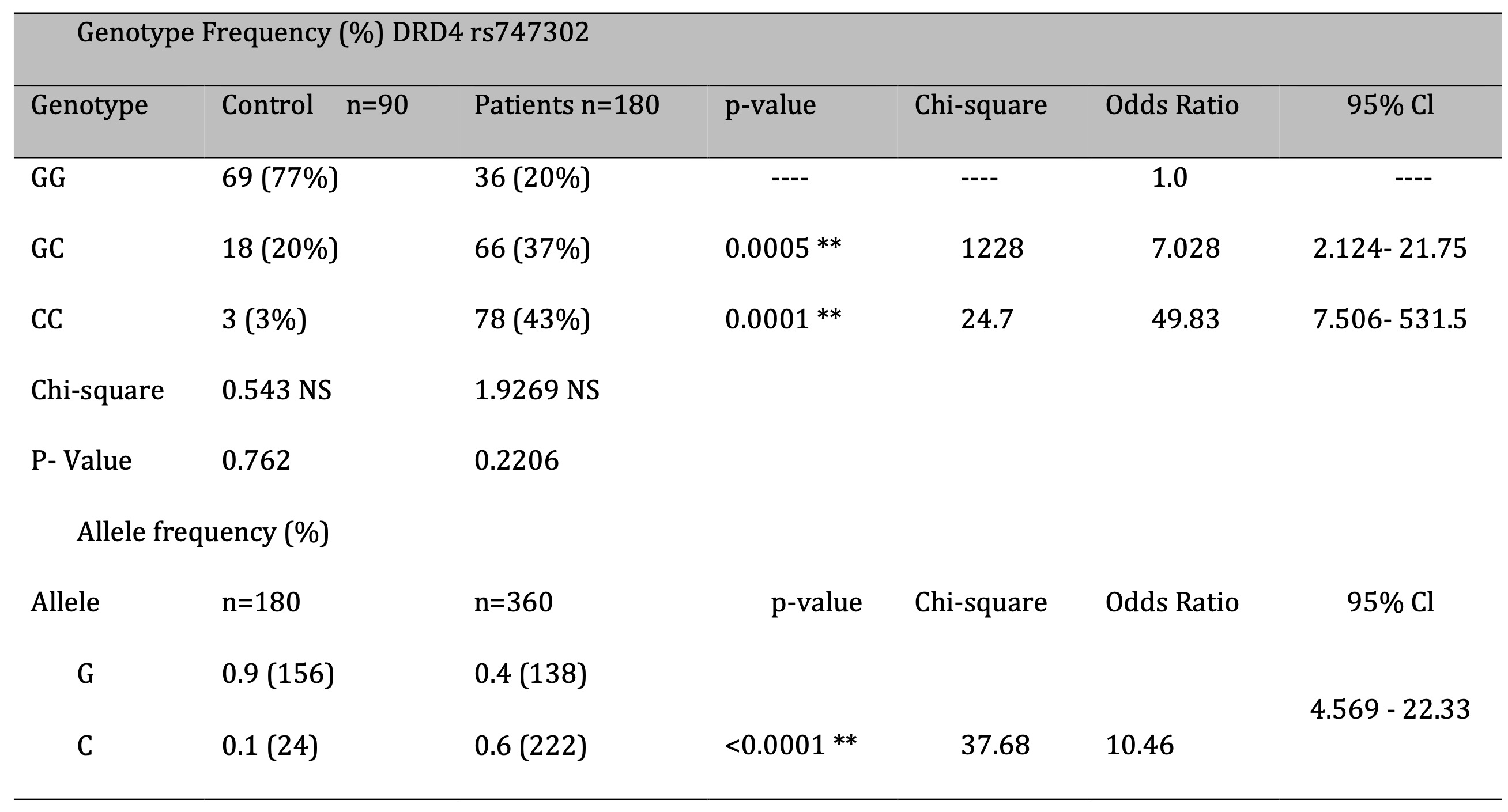
Table 2: Genotypes and alleles frequency (%) of dopamine receptor gene DRD4 rs7473; 02
Statistical analysis of VNTR
A comparison was performed between the control group and the patient group within the Iraqi population
about the
DRD4 exon III VNTR. Independent samples t-test and standard deviation were computed to evaluate the VNTR
between
the two groups. The patient group demonstrated considerably elevated levels, showing a strong correlation
between the existence of repetitions and the patient group (Table 3).
A two-way ANOVA was used to compare the VNTR repeat numbers in the control and patient groups. The DRD4
SNP
rs747302 genotypes (GG, GC, and CC) were then used to classify the participants into groups. On average,
patients with the GG genotype had 17 repetitions, those with the GC genotype had 18, and the highest
repeat
count was 18.3 for the CC genotype. Based on these results, it appears that the patient group has a
substantially higher number of repeats when the CC genotype is present. Fig. 1 also shows that within the
CC
genotype subgroup; there was a more noticeable variation in repetition numbers between the control and
patient
groups.
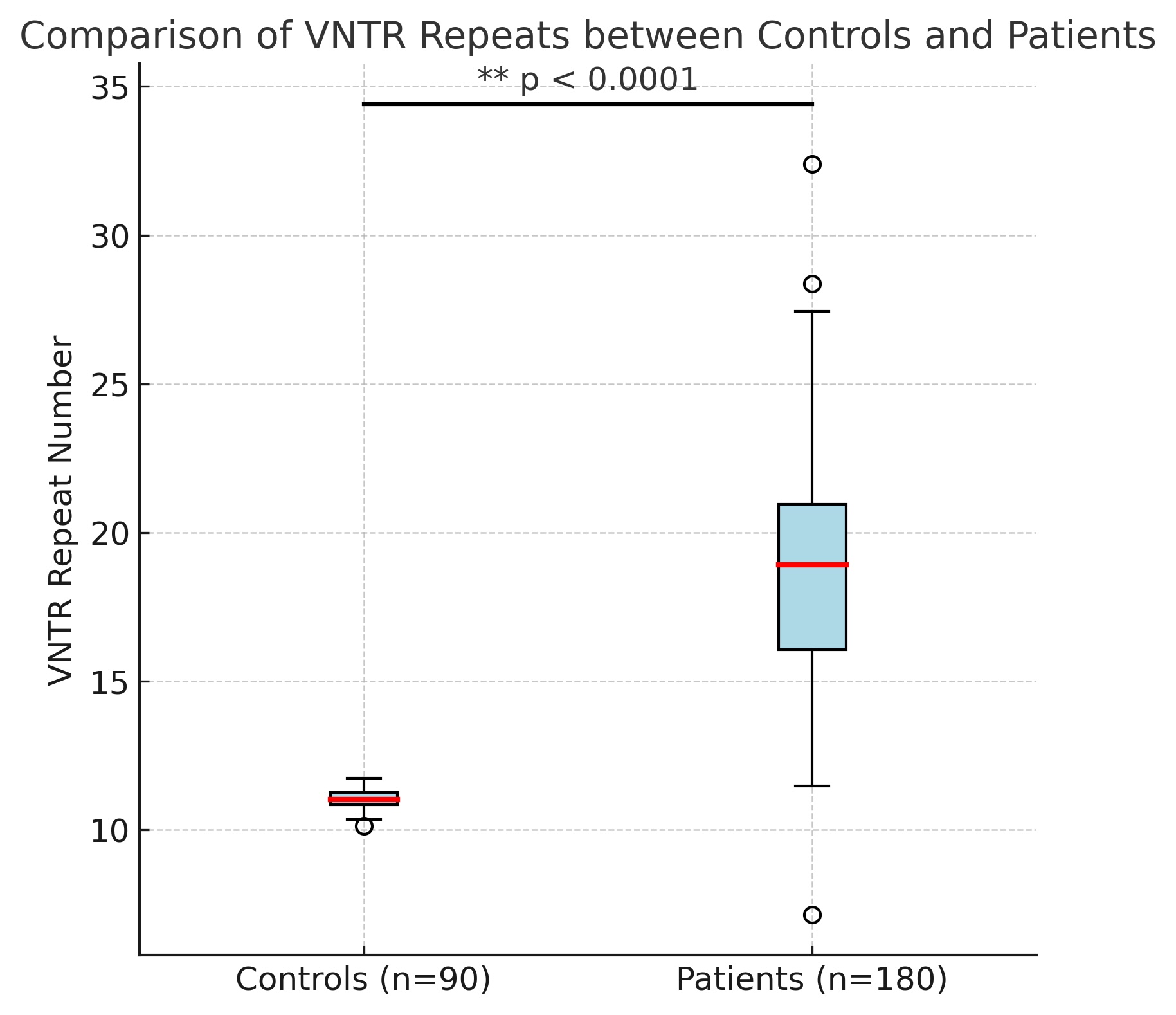
Fig. 1: A highly significant difference between the control and the patient groups of (GG, GC, and CC).
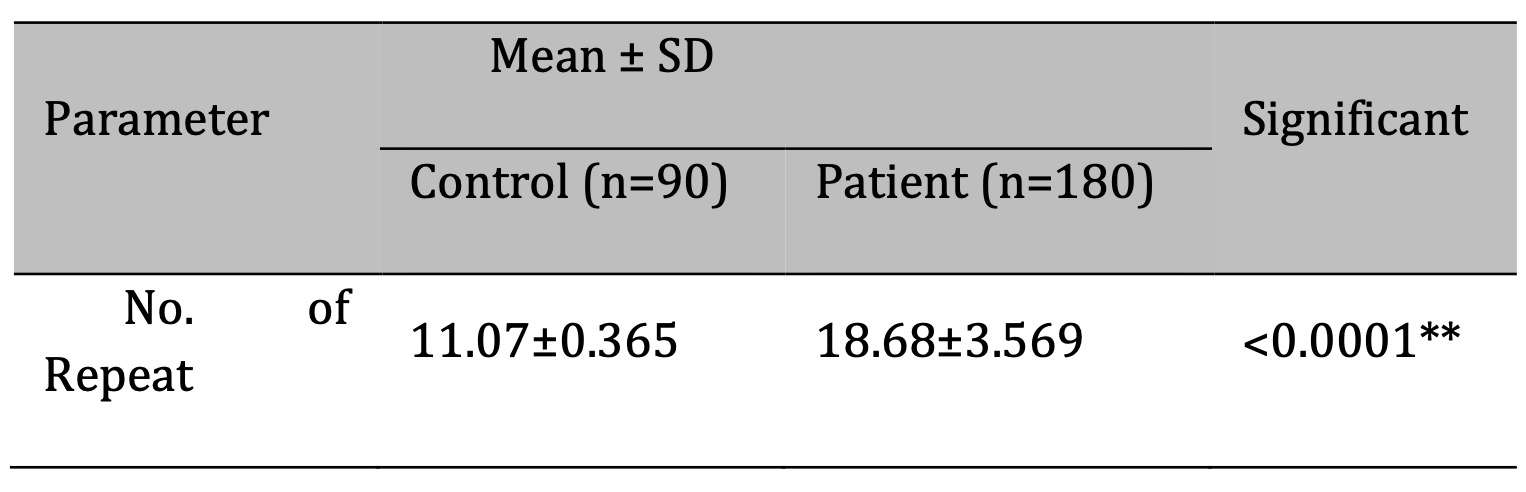
Table 3: A comparison of the VNTR between patients and control groups.
Discussion
Drug addiction is a neuropsychiatric disorder that is caused by several genetic and environmental factors. Researchers have extensively studied polymorphisms in dopamine-related genes because they may influence susceptibility to addictive behaviours [30]. The SNP rs747302 and the VNTR region inside the dopamine receptor D4 (DRD4) gene may play a role in controlling behaviour and processing rewards [31].
The coding region of the DRD4 gene has four exons and three introns, based on an analysis of its structure [5]. Our findings demonstrate a strong association between the GC and CC genotypes and VNTRs in exon 3 of the DRD4 gene. The results are consistent with recent studies done in the US and Israel that indicated a strong connection between lengthy alleles (≥6 repeats) and the risk of drug addiction [20, 27]. The 48 bp VNTR polymorphism in exon 3 of DRD4 is a key genetic difference linked to substance use disorders (SUD). It is strongly linked to SUD in Arab communities, such as the Jordanian group, where the 7-repeat allele was strongly linked to SUD [11].
Long alleles (≥7 repeats) have been connected to behavioural and addictive disorders such as delinquency, smoking, and being more sensitive to heroin, alcohol-related stimuli, and nicotine [28]. The length polymorphism of the DRD4 VNTR also sets people with long-type genotypes apart from those with shorter alleles. This polymorphism has been connected to aggressive, delinquent, thrill-seeking, impulsive, insecure, and angry behaviour [29].
This novel case-control study examined the association between drug addiction risk and polymorphisms in the DRD4 gene, namely SNP rs747302 and the adjacent VNTR region, among a cohort of Iraqis. The findings indicated that individuals possessing the C allele of rs747302, specifically those with CC and GC genotypes, exhibited a higher propensity for drug addiction. It indicates that there may be a genetic link regarding how well dopamine works.
Analysis of the SNP rs747302 showed that the genotype distribution of heroin users and healthy controls was very different. Thirty-seven per cent of people with the GC genotype were in the patient group, but just 3% of people in the control group had it. In the same way, 60% of the patients had the C allele, whereas just 1% of the controls did. Patients also had a much higher rate of the CC genotype. The average number of VNTR repetitions also changed with genotype. The CC genotype had the most repeats (18.3), followed by the GG genotype (17 repeats) and the GC genotype (18 repeats). The results of this study reveal that Iraqis with the CC and GC genotypes and more VNTR repeats are more likely to become addicted to heroin. This is the first study to report a significant association between the DRD4 rs747302 polymorphism, VNTR repeat number, and heroin addiction risk in an Iraqi population, demonstrating that carriers of the CC and GC genotypes—with higher VNTR repeat counts—are substantially more susceptible to heroin dependence.
Szantai et al. (2005) discovered a 27-base pair deletion at the -521C>T SNP, making it difficult to accurately genotype the -616C>G SNP using the Sau96I RFLP method. This deletion could change the DRD4 gene's function and is rare. Szantai et al.'s (2005) study highlights the complexity of DRD4 genetic variation and its potential impact on gene expression and dopaminergic transmission. Understanding these differences across groups like the Iraqi cohort is crucial for drug addiction research [32]. The dopaminergic system, particularly the mesolimbic pathway, is crucial for repetitive drug-seeking behaviour, with the polymorphic DRD4 gene primarily found in the receptor's third cytoplasmic loop [33]. Together, these findings highlight how changes in DRD4—even outside the protein-coding regions—can shape dopamine signalling, and our results extend this understanding to a Middle Eastern population with clear implications for addiction risk.
The DRD4 receptor, one of the most polymorphic functional proteins, has varied pharmacodynamic properties and has been shown to work with atypical antipsychotic drugs like clozapine [34]. Our findings are in line with past studies that found a relationship between certain changes in dopaminergic genes and addiction [35]. Researchers discovered receptor variants on chromosome 11p15.5 are linked to impulsivity, novelty seeking, and reward sensitivity, making individuals more susceptible to substance use disorders. The research focuses on variable number tandem repeats in exon III [36].. There hasn’t been much research that looked at the rs747302 variant about addiction yet. Our study expands this scope by examining the DRD4 rs747302 SNP in a non-coding region, alongside VNTR repeat counts, in a previously unstudied Middle Eastern cohort.
Gervasini et al. (2018) revealed that several intronic SNPs in DRD4 and other dopaminergic genes are linked to psychiatric and behavioural disorders. This indicates that non-coding regions play a crucial role in function [37]. Du et al. (2010) found that individuals without the 7-repeat allele were more likely to drink. They also found a 48-base pair VNTR mutation in exon 3 of the DRD4 gene, indicating a higher drinking likelihood [38].
Our findings support earlier research that found a link between the 7-repeat allele and impulsivity, risk-taking, and a yearning for new experiences—all of which are strongly associated with a tendency to seek out drugs [35]. The study suggests that the rs747302 risk allele and long VNTR repeats may increase the likelihood of addictive behaviour. DRD4 polymorphisms may play a role in addiction and other personality traits. The 7-repeat (7R) allele is more common in ADHD patients, indicating that DRD4 changes may be linked to behavioural disorders. Dopaminergic genetic differences may alter an individual's initial enjoyment of a new substance [39].
McGeary et al. (2006) revealed that the DRD4 VNTR is linked to alcohol cue-reactivity. People with the long allele are more likely to feel cravings and physical arousal when they see cues that are linked to alcohol [40]. This gives us more proof that DRD4 is involved in conditioned responses, which are a huge challenge for those who want to stop using drugs. There are certain intronic SNPs, including rs1800955 (C-521T), that are close by or functionally like rs747302 and have been linked to changes in transcriptional activity and traits related to addiction. However, rs747302 has not been investigated much for addiction [41].
Kreek et al. (2005) highlighted the role of gene-environment interactions in addiction, highlighting the importance of multifactorial models that consider both biological and environmental factors. They found that adolescents with the long allele were more likely to develop substance use disorders [42]. Mallard et al.'s 2016 study found that DRD4 VNTR polymorphism in 51 adolescents with disruptive behaviour disorders increased their likelihood of drug abuse, with DRD4L carriers using marijuana and heavy drugs more frequently than DRD4S homozygotes [24]..
Al-Eitan et al. (2020) found a strong link between substance use disorder (SUD) and DRD4 VNTR polymorphisms in Jordanian Arabs and heroin addiction in Iraq. However, there was no link with SLC6A4 polymorphisms. The study suggests unique genetic patterns in each population, supporting the idea that the gene plays a role in Arab populations [43].
A meta-analysis by Chen et al. (2011) found that DRD4 exon III VNTR alleles, particularly the 7-repeat and long alleles, are linked to a higher risk of opioid addiction. Recent Iraqi research also linked two DRD4 genotypes, CC and GC, to heroin addiction [44]..
When it comes to addiction genetics, Iraqis are significantly different from the groups examined in the West and East Asia, both in terms of their genes and their environment. There may be a lot of differences between ethnic groups in how often alleles occur and how genes interact with their environments. Our results highlight the need for comprehensive genetic studies across diverse populations to better elucidate individual risk factors and inform more effective strategies for prevention and treatment. A combination of genetic predisposition and unique cultural, economic, and post-conflict pressures may make people in Iraq more likely to become addicted. When researchers and politicians plan future initiatives, they should keep in mind how genetic predisposition and environmental pressures affect each other.
The findings of this study are the first to show a relationship between drug addiction and certain genetic variants in the Iraqi population, namely DRD4 rs747302 and VNTR polymorphisms. The results show how important it is to include populations that aren't well represented in genetic studies. This will help us better understand the biology of addiction and the impact that differences in dopaminergic genes play in making people more likely to become addicted. To make the results more useful for a wider range of people, future studies should use larger, multi-centre samples from all around Iraq and the neighbouring territories. It is also crucial to employ epigenetic and transcriptome studies to figure out how DRD4 expression is controlled and to investigate how genes and the environment interact with one another to change the risk of addiction. Researchers may be able to figure out the best ways to stop and treat substance use problems in people who are more likely to develop them in the long run.
While this study provides valuable and novel insights into the genetic factors associated with drug addiction and strongly suggests a role for DRD4 rs747302 genotypes and VNTR repeat number in heroin addiction among Iraqis, several limitations should be acknowledged. Firstly, the the modest sample size (180 patients, 90 controls) may have limited the statistical power of the findings (180 patients and 90 controls), which may reduce sensitivity for detecting smaller genetic effects. Secondly, the study did not examine additional genetic variants within the dopaminergic and serotonergic systems, which are also known to play significant roles in addiction vulnerability. Thirdly, the absence of control for important environmental and psychosocial factors—such as trauma, socioeconomic status, and peer influence—may have introduced confounding variables, reducing the reliability of the observed gene-behaviour associations. Furthermore, the cross-sectional design of the study prevents any conclusions about causal relationships. Additionally, potential confounders should be acknowledged: environmental influences such as socioeconomic status, stress exposure, and peer networks were not controlled for; participants were recruited from specific treatment and community settings, introducing possible recruitment source bias; and, as our sample represents a single regional population, unaccounted-for population stratification could influence allele frequency differences. These factors highlight the need for larger, multi-centre studies with careful adjustment for environmental variables to validate and generalise our results. Future research should aim to address these limitations by employing larger, more diverse samples, incorporating longitudinal designs, and exploring gene–environment interactions to better understand the complex aetiology of addiction.
Conclusion
Our research shows that the DRD4 gene, which codes for a dopamine receptor that is part of the brain's reward system, has a big effect on the neurobiological processes that lead to drug addiction. Dopamine is a key part of pleasure, motivation, and reinforcement; however, substance use disorders are often linked to problems with dopamine. It looks like several VNTR mutations in the DRD4 gene, along with the CC and GC genotypes, make Iraqis more likely to become addicted. Changes in genes may modify how receptors work and how dopamine is supplied. This, in turn, alters individuals’ responses to addiction-related cues and increases their desire to use drugs.
Acknowledgements
The authors would like to acknowledge all professionals and collaborators, and all participant volunteers whose efforts and support contributed to the successful completion of this research.
Author Contributions
All authors significantly contributed to the study's conception and design, data collection and analysis,
article composition, critical revision, and final approval.
Financial Support
The authors declare that no financial support was received from any organisation for this study.
Ethical Approval
The Research Ethical Committee at the scientific research requires ethical approval from both the MOH in
Iraq
and the Babylon Health Directorate. This study received ethical approval from the Babylon Health
Directorate
(Approval No.1260, on 19/12/2021) and the Adult Health Centre (Approval No.30/2998, on 10/3/2022). Verbal
and
written informed consent were obtained from each participant (personal permission).
Disclosure Statement
The authors declare no conflicts of interest related to this work. The content of this paper has been written without the assistance of AI-based services.
References
| 1 | Ponce G, Jimenez-Arriero MA, Rubio G, Hoenicka J, Ampuero I, Ramos JA, Palomo T: The A1 allele of
the DRD2 gene (TaqI A polymorphisms) is associated with antisocial personality in a sample of
alcohol-dependent patients. Eur Psychiatry 2003; 18:356-360.
https://doi.org/10.1016/j.eurpsy.2003.06.006 |
| 2 | Gough B, Pereira FC, Fontes Ribeiro CA, Ali SF, Binienda ZK: Propentophylline increases striatal
dopamine release but dampens methamphetamine-induced dopamine dynamics: a microdialysis study.
Neurochem Int 2014; 76:109-113.
https://doi.org/10.1016/j.neuint.2014.07.003 |
| 3 | Wise RA, Robble MA: Dopamine and addiction. Annu Rev Psychol 2020; 71:79-106.
https://doi.org/10.1146/annurev-psych-010418-103337 |
| 4 | Zhuo Y, Li Y: New imaging methods for monitoring dopaminergic neurotransmission. Sci China Life
Sci 2022; 65:838-841.
https://doi.org/10.1007/s11427-021-2041-4 |
| 5 | Ma P, Ou Y: Correlation between the dopaminergic system and inflammatory disease: a review. Mol
Biol Rep 2023; 50:7043-7053.
https://doi.org/10.1007/s11033-023-08610-2 |
| 6 | Li M, Zhou L, Sun X, Yang Y, Zhang C, Wang T, Fu F: Dopamine, a co-regulatory component, bridges
the central nervous system and the immune system. Biomed Pharmacother 2022; 145:1124; 58.
https://doi.org/10.1016/j.biopha.2021.112458 |
| 7 | Martel MM, Nikolas M, Jernigan K, Friderici K, Waldman I, Nigg JT: The dopamine receptor D4 gene
(DRD4) moderates family environmental effects on ADHD. J Abnorm Child Psychol 2011; 39:1-10.
https://doi.org/10.1007/s10802-010-9439-5 |
| 8 | Bonvicini C, Cortese S, Maj C, Baune BT, Faraone SV, Scassellati C: DRD4 48 bp multiallelic
variants as age-population-specific biomarkers in attention-deficit/hyperactivity disorder. Transl
Psychiatry 2020; 10:70.
https://doi.org/10.1038/s41398-020-0755-4 |
| 9 | Krynetskiy E: Beyond SNPs and CNV: pharmacogenomics of polymorphic tandem repeats. J
Pharmacogenomics Pharmacoproteomics 2017; 8:170.
https://doi.org/10.4172/2153-0645.1000170 |
| 10 | Nakamura Y, Koyama K, Matsushima M: VNTR (variable number of tandem repeat) sequences as
transcriptional, translational, or functional regulators. J Hum Genet 1998; 48:149-152.
https://doi.org/10.1007/s100380050059 |
| 11 | Al-Eitan LN, Alshudaifat KM, Anani JY: Association of the DRD4 exon III and 5-HTTLPR VNTR
polymorphisms with substance abuse in the Jordanian Arab population. Gene 2020; 733:1442; 67.
https://doi.org/10.1016/j.gene.2019.144267 |
| 12 | Creswell KG, Sayette MA, Manuck SB, Ferrell RE, Hill SY, Dimoff JD: DRD4 polymorphism moderates
the effect of alcohol consumption on social bonding. PLoS One 2012; 7:e2891; 4.
https://doi.org/10.1371/journal.pone.0028914 |
| 13 | Franke P, Nöthen MM, Wang T, Knapp M, Lichtermann D, Neidt H, Sander T, Propping P, Maier W: DRD4
exon III VNTR polymorphism-susceptibility factor for heroin dependence? Results of a case-control
and a family-based association approach. Mol Psychiatry 2000; 5:101-104.
https://doi.org/10.1038/sj.mp.4000583 |
| 14 | McGeary J: The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacol
Biochem Behav 2009; 93:222-229.
https://doi.org/10.1016/j.pbb.2009.03.010 |
| 15 | Rasekh ME, Hernández Y, Drinan SD, Fuxman Bass JI, Benson G: Genome-wide characterisation of human
minisatellite VNTRs: population-specific alleles and gene expression differences. Nucleic Acids Res
2021; 49:4308-4324.
https://doi.org/10.1093/nar/gkab224 |
| 16 | Sun Y, Meng S, Li J, Shi J, Lu L: Advances in genetic studies of substance abuse in China.
Shanghai Arch Psychiatry 2013; 25:199-211.
|
| 17 | Chen D, Liu F, Shang Q, Song X, Miao X, Wang Z: Association between polymorphisms of DRD2 and DRD4
and opioid dependence: evidence from the current studies. Am J Med Genet B Neuropsychiatr Genet
2011; 156B:661-670.
https://doi.org/10.1002/ajmg.b.31208 |
| 18 | Shao C, Li Y, Jiang K, Zhang D, Xu Y, Lin L, Wang Q, Zhao M, Jin L: Dopamine D4 receptor
polymorphism modulates cue-elicited heroin craving in Chinese. Psychopharmacology (Berl) 2006;
186:185-190.
https://doi.org/10.1007/s00213-006-0375-6 |
| 19 | Tsai SJ, Cheng CY, Shu LR, Yang CY, Pan CW, Liou YJ, Hong CJ: No association for D2 and D4
dopamine receptor polymorphisms and methamphetamine abuse in Chinese males. Psychiatr Genet 2002;
12:29-33.
https://doi.org/10.1097/00041444-200203000-00004 |
| 20 | Vandenbergh DJ, Rodriguez LA, Hivert E, Schiller JH, Villareal G, Pugh EW, Lachman H, Uhl GR: Long
forms of the dopamine receptor (DRD4) gene VNTR are more prevalent in substance abusers: no
interaction with functional alleles of the catechol-O-methyltransferase (COMT) gene. Am J Med Genet
2000; 96:678-683.
https://doi.org/10.1002/1096-8628(20001009)96:5<678::AID-AJMG15>3.0.CO;2-8 |
| 21 | McGeary JE, Esposito-Smythers C, Spirito A, Monti PM: Associations of the dopamine D4 receptor
gene VNTR polymorphism with drug use in adolescent psychiatric inpatients. Pharmacol Biochem Behav
2007; 86:401-406.
https://doi.org/10.1016/j.pbb.2006.11.001 |
| 22 | Stolf AR, Cupertino RB, Müller D, Sanvicente-Vieira B, Roman T, Vitola ES, Grevet EH, von Diemen
L, Kessler FHP, Grassi-Oliveira R, Bau CHD, Rovaris DL, Pechansky F, Schuch JB: Effects of DRD2
splicing-regulatory polymorphism and DRD4 48 bp VNTR on crack cocaine addiction. J Neural Transm
(Vienna) 2019; 126:193-199.
https://doi.org/10.1007/s00702-018-1946-5 |
| 23 | Chmielowiec J, Chmielowiec K, Suchanecka A, Trybek G, Mroczek B, Małecka I, Grzywacz A:
Associations between the dopamine D4 receptor and DAT1 dopamine transporter genes polymorphisms and
personality traits in addicted patients. Int J Environ Res Public Health 2018; 15:2076.
https://doi.org/10.3390/ijerph15102076 |
| 24 | Mallard TT, Doorley J, Esposito-Smythers CL, McGeary JE: Dopamine D4 receptor VNTR polymorphism
associated with greater risk for substance abuse among adolescents with disruptive behaviour
disorders: preliminary results. Am J Addict 2016; 25:56-61.
https://doi.org/10.1111/ajad.12320 |
| 25 | Boroń A, Remigiusz R, Chmielowiec K, Chmielowiec J, Strońska-Pluta A, Kowalski M, Masiak J,
Gibas-Dorna M, Trybek G, Grzywacz A: Association analysis of the Ex3 VNTR polymorphism of the DRD4
dopamine receptor gene with personality traits in patients with a behavioural addiction. 2024; 1-25.
https://doi.org/10.21203/rs.3.rs-4409644/v1 |
| 26 | IBM Corp: IBM SPSS Statistics for Windows, Version 25.0 Armonk, NY: IBM Corp; 2017.
|
| 27 | Szilágyi G, Nagy Z, Balkay L, Boros I, Emri M, Lehel S, Márián T, Molnár T, Szakáll S, Trón L,
Bereczki D, Csiba L, Fekete I, Kerényi L, Galuska L, Varga J, Bönöczk P, Vas A, Gulyás B: Effects of
vinpocetine on the redistribution of cerebral blood flow and glucose metabolism in chronic ischemic
stroke patients: a PET study. J Neurol Sci 2005; 229-230:275-284.
https://doi.org/10.1016/j.jns.2004.11.053 |
| 28 | Uysal MA, Sever Ü, Nursal AF, Yedikule Smoking Cessation Study Group, Pehlivan S: Dopamine D4
receptor gene exon III VNTR variant influences smoking status in Turkish population. Noro Psikiyatr
Ars 2019; 56:248-252.
https://doi.org/10.29399/npa.23408 |
| 29 | Dmitrieva J, Chen C, Greenberger E, Ogunseitan O, Ding YC: Gender-specific expression of the DRD4
gene on adolescent delinquency, anger and thrill seeking. Soc Cogn Affect Neurosci 2011; 6:82-89.
https://doi.org/10.1093/scan/nsq020 |
| 30 | Blum K, Oscar-Berman M, Barh D, Giordano J, Gold M: Dopamine genetics and function in food and
substance abuse. J Genet Syndr Gene Ther 2013; 4:1000; 121.
|
| 31 | Simpson J, Vetuz G, Wilson M, Brookes KJ, Kent L: The DRD4 receptor exon 3 VNTR and 5' SNP
variants and mRNA expression in human post-mortem brain tissue. Am J Med Genet B Neuropsychiatr
Genet 2010; 153B:1228-1233.
https://doi.org/10.1002/ajmg.b.31084 |
| 32 | Szantai E, Szmola R, Sasvári-Székely M, Guttman A, Rónai Z: The polymorphic nature of the human
dopamine D4 receptor gene: a comparative analysis of known variants and a novel 27 bp deletion in
the promoter region. BMC Genet 2005; 6:39.
https://doi.org/10.1186/1471-2156-6-39 |
| 33 | Franken IHA, Booij J, van den Brink W: The role of dopamine in human addiction: from reward to
motivated attention. Eur J Pharmacol 2005; 526:199-206.
https://doi.org/10.1016/j.ejphar.2005.09.025 |
| 34 | Lichter JB, Barr CL, Kennedy JL, Van Tol HHM, Kidd KK, Livak KJ: A hypervariable segment in the
human dopamine receptor D4 (DRD4) gene. Hum Mol Genet 1993; 2:767-773.
https://doi.org/10.1093/hmg/2.6.767 |
| 35 | Munafò MR, Yalcin B, Willis-Owen SA, Flint J: Association of the dopamine D4 receptor (DRD4) gene
and approach-related personality traits: meta-analysis and new data. Biol Psychiatry 2008;
63:197-206.
https://doi.org/10.1016/j.biopsych.2007.04.006 |
| 36 | Botticelli L, Micioni Di Bonaventura E, Del Bello F, Giorgioni G, Piergentili A, Romano A, Quaglia
W, Cifani C, Micioni Di Bonaventura MV: Underlying susceptibility to eating disorders and drug
abuse: genetic and pharmacological aspects of dopamine D4 receptors. Nutrients 2020; 12:2288.
https://doi.org/10.3390/nu12082288 |
| 37 | Gervasini G, González LM, Gamero-Villarroel C, Mota-Zamorano S, Carrillo JA, Flores I,
García-Herráiz A: Effect of dopamine receptor D4 (DRD4) haplotypes on general psychopathology in
patients with eating disorders. Gene 2018; 654:43-48.
https://doi.org/10.1016/j.gene.2018.02.035 |
| 38 | Du Y, Yang M, Yeh HW, Wan YJ: The association of exon 3 VNTR polymorphism of the dopamine receptor
D4 (DRD4) gene with alcoholism in Mexican Americans. Psychiatry Res 2010; 177:358-360.
https://doi.org/10.1016/j.psychres.2010.02.021 |
| 39 | LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, Kennedy J: Dopamine D4 receptor gene
polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry 1996;
1:121-124.
|
| 40 | McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R Jr: Genetic moderators of
naltrexone's effects on alcohol cue reactivity. Alcohol Clin Exp Res 2006; 30:1288-1296.
https://doi.org/10.1111/j.1530-0277.2006.00156.x |
| 41 | de Rubira A, Georges L, Fehren-Schmitz L: Ancient DNA reveals that the variability of the DRD4-521
C/T SNP associated with novelty seeking behaviour is influenced by selection in western South
American populations. Adapt Hum Behav Physiol 2016; 2:77-91.
https://doi.org/10.1007/s40750-015-0033-5 |
| 42 | Kreek MJ, Nielsen DA, Butelman ER, LaForge KS: Genetic influences on impulsivity, risk taking,
stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci 2005; 8:1450-1457.
https://doi.org/10.1038/nn1583 |
| 43 | Al-Eitan LN, Alshudaifat KM, Anani JY: Association of the DRD4 exon III and 5-HTTLPR VNTR
polymorphisms with substance abuse in the Jordanian Arab population. Gene 2020; 733:1442; 67.
https://doi.org/10.1016/j.gene.2019.144267 |
| 44 | Chen D, Liu F, Shang Q, Song X, Miao X, Wang Z: Association between polymorphisms of DRD2 and DRD4
and opioid dependence: evidence from the current studies. Am J Med Genet B Neuropsychiatr Genet
2011; 156B:661-670.
https://doi.org/10.1002/ajmg.b.31208 |