Mast Cell Response to Parasites: from Recognition and Activation to Host Defense Modulation
Keywords
Abstract
Parasites represent a diverse and widely distributed group of pathogens that cause diseases with significant global health implications. The interaction between parasite and host is characterized by a high degree of complexity, with both parties continuously adapting to changes in the other. The successful host invasion is largely attributable to the evasion strategies employed by parasites to ensure their survival in immunocompetent individuals. In turn, the host’s defense mechanisms utilize a variety of structures and processes, ranging from primary barriers to the most sophisticated ones, to counter the parasite attack. Acting as an early line of defense, the immune system includes a variety of cell types that are capable of recognizing, destroying, and eliminating infectious agents. Undoubtedly, the orchestration of first-line innate immune responses but also adaptive immunity processes during infection depends to a large extent on the involvement of tissue-resident mast cells (MCs). MCs are capable of supporting immune reactions to parasites through a broad spectrum of processes, including degranulation, synthesis and release of cytokines/chemokines and other mediators, and the generation of reactive oxygen species (ROS). They may also be involved in immune cell recruitment, phagocytosis, and the provision of extracellular DNA traps. Despite the well-documented association of MCs with antibacterial and antiviral defense, their role in host protection against parasites remains incompletely identified. This article provides an overview of the engagement of MCs in host defense mechanisms developed during parasitic infections. Furthermore, it considers the impact of parasites or parasite-derived molecules on the various aspects of MC activity.Introduction
Parasitic infections caused by protozoa or helminths are distributed virtually worldwide, but predominantly they constitute a significant health problem in tropical developing countries where they are often neglected. Among the parasitic diseases with important mortality or morbidity rates, are malaria, leishmaniasis, amoebiasis, trypanosomiasis, and schistosomiasis. Furthermore, toxoplasmosis, ascariasis, and taeniasis represent common human parasitic illnesses. The widespread prevalence of parasites is due to their diverse anatomical, physiological, and behavioral adaptations, which allow them to survive even in extreme conditions [1]. Despite the global influence of parasites, effective antiparasitic therapeutics are limited, and malaria is currently the only parasitic disease for which two vaccines are available for humans and recommended by the World Health Organization (WHO) [2]. Depending on the species, parasites may be transmitted to their suitable hosts in several ways, both vertically and horizontally. For example, protozoa and helminths can be spread orally by ingesting water or food contaminated with invasive forms. A defining characteristic of certain parasitic nematodes is their capacity to autoinfect and, as a consequence, self-replicate within their host. Other modes of parasitic infection include active penetration through the skin or mucous membranes or direct contact between hosts. Finally, many blood parasites are transmitted by arthropod representatives that serve as disease vectors [3]. Infection with parasites may result in a spectrum of symptoms, ranging from mild discomfort to severe illness. In some individuals, infection progresses to a chronic phase, which, depending on the type and quantity of parasites, can lead to weakness, severe anemia, and malnutrition with weight loss. It is important to note that asymptomatic carriers of parasites constitute a great challenge for public health, meaning they have a silent infection. Nevertheless, they contribute to the transmission of the infection to the others.
Despite the large number and diversity of antigens presented by the parasite to the host immune system and the response initiated against them, parasites try to survive within the body host by using multiple evasion strategies that have been acquired over millions of years of evolution [4]. In turn, the host's defense mechanisms range from primary barriers to more complex responses involving diverse immune cells and mediators capable of identifying and eliminating infectious agents [5]. Undoubtedly, the orchestration of first-line innate immune responses and adaptive immunity during infection relies on the coordinated activity of various immune cells, including macrophages, granulocytes, dendritic cells, natural killer (NK) cells, B lymphocytes, and T lymphocytes [6]. Among these, tissue-resident mast cells (MCs) occupy a strategic position at the host-environment interface, especially in barrier tissues such as skin, and the gastrointestinal and genitourinary mucosa, which are common entry sites for parasites. Due to their location and rapid response capacity, MCs are well positioned to act as important responders during parasitic infections. These cells recognize pathogen-associated molecular patterns (PAMPs)/microbe‐associated molecular patterns (MAMPs), release preformed and newly synthesized bioactive mediators, recruit and activate other immune cells, and participate in processes such as phagocytosis and extracellular DNA trap (MCETs) formation [7, 8]. Although numerous studies have addressed the role of MCs in anti-parasitic defense, their involvement remains incompletely characterized. Therefore, the aim of the present article is to provide an overview of MC engagement in host defense mechanisms developed during parasitic infections. Furthermore, the article considers the impact of parasites or parasite-derived molecules on the various aspects of MC activity.
Hallmarks of the host immune response to parasites
To survive until parasites reach maturity and complete their life cycle, these organisms have evolved a variety of complex strategies. Frequently, these strategies are stage-specific, allowing parasites to use, avoid, or modulate the host's immune response and metabolism [9, 10]. Conversely, physical and chemical barriers, as well as numerous innate and adaptive components of the host immune system, have been identified as either less or more crucial during a parasite attack. However, the direction of the host immune response, reflecting differences in infection strategies, tissue localization, and interactions with the immune system, is primarily determined by the type of parasitic invader.
The primary line of host defense against parasites, as in the case of bacteria, viruses, or fungi, consists of anatomical and structural barriers, particularly the skin and mucosal surfaces, which are further supported by chemical defenses. Nevertheless, as the common site of infection or route of access for the parasites, mucosal barriers are frequently unable to repel the attack. This phenomenon has been well-described in the context of gut protozoa, such as Entamoeba histolytica [11] and Giardia duodenalis [12] or Toxoplasma gondii [13], which may lead to the opening of intercellular tight junctions and the subsequent breakdown of the intestinal mucosal barrier. Mucins, as integral components of the mucus layer, can either block parasite colonization or facilitate their expulsion. Conversely, data indicate that G. duodenalis [14], E. histolytica [15], and Trichuris muris [16] can proteolytically cleave the major structural component of the mucus gel, i.e., mucin 2 (MUC2), and disrupt the host colonic mucus by breaking down the macromolecular structure and invading the underlying epithelium. Another crucial element of mucosal defense is secretory immunoglobulin A (sIgA), which neutralizes pathogens and prevents their adhesion to the epithelium. sIgA antibodies are important for the clearance of E. histolytica [17], Giardia sp [18, 19], and Clonorchis sinensis [20]. In addition, some authors have proposed that IgA could function as a biomarker for worm infections [21, 22].
Recently there has been renewed interest in the interaction between the host gut microbiota and invading parasites. It is well established that intestinal parasites have a profound impact on the composition and diversity of the host microbiota, which in turn has significant implications for the efficacy of host defense mechanisms [23, 24]. Conversely, an increasing body of evidence suggests that the intestinal microbiota may be firmly involved in the defense against parasites [25, 26]. Nevertheless, the precise function of the gut microbiota in this process is still not well understood. Some reports indicate that the expulsion of parasites may be strongly dependent on the composition of the microbiota. For example, Li and colleagues [27] demonstrated that early Trichinella spiralis infection reduces gut microbiota diversity and alters its composition, leading to a predominance of bacteria that produce pro-inflammatory metabolites, such as ceramides. In the subsequent phase of the infection, the same authors observed an increase in the number of representatives belonging to the Lactobacillaceae family that have anti-inflammatory properties through the production of short-chain fatty acids (SCFAs). The presence of G. duodenalis was found to be reduced by Lactobacillus sp., while the bacteriocins produced prevented parasite adhesion [28–30]. It has been demonstrated that SCFAs, the principal microbiota-derived metabolites, exhibit inhibitory activities against protozoa [31, 32]. Microbiota-derived SCFAs are well-known anti-inflammatory mediators and regulatory T cell (Treg) inducers for host intestinal immunity, as was documented in the case of Echinococcus multilocularis-infected mice [33].
Following the breach of anatomical and chemical barriers by invaders, the host immune system must first recognize the threat, after which most, if not all, immune cell types are mobilized in antiparasitic defense. The identification of pathogens is achieved by expressing a set of pattern-recognition receptors (PRRs) by host immune cells. PRRs are able to recognize PAMPs/ MAMPs derived from microbes or parasites, including proteins, lipoproteins, lipids, and nucleic acids. PRRs also detect endogenous danger-associated molecular patterns (DAMPs) released upon cellular stress or tissue injury. These receptors include Toll-like receptors (TLRs), NOD-like receptors (NLRs), RIG-I-like receptors (RLRs), and C-type lectin receptors (CLRs) [34]. Some of these have been documented to detect components derived from parasites (Fig. 1). For instance, TLR1/2 and TLR2/6 heterodimers are capable of recognizing glycosylphosphatidylinositol (GPI) anchors of Plasmodium sp. [35] and/or Trypanosoma sp. [36]. Lipopeptidophosphoglycan (LPPG) of E. histolytica and Taenia crassiceps carbohydrates are the most common ligands for TLR4 [37, 38]. TLR11 and TLR12 are localized to endosomes to recognize T. gondii-derived profilin [39]. Other significant TLRs include TLR3, TLR7, and TLR9, which may collaborate with TLR11 and TLR12 in the host response to T. gondii [40, 41]. In turn, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), a known CLR, has been demonstrated to bind to Leishmania sp. or Schistosoma mansoni egg antigens [42, 43].
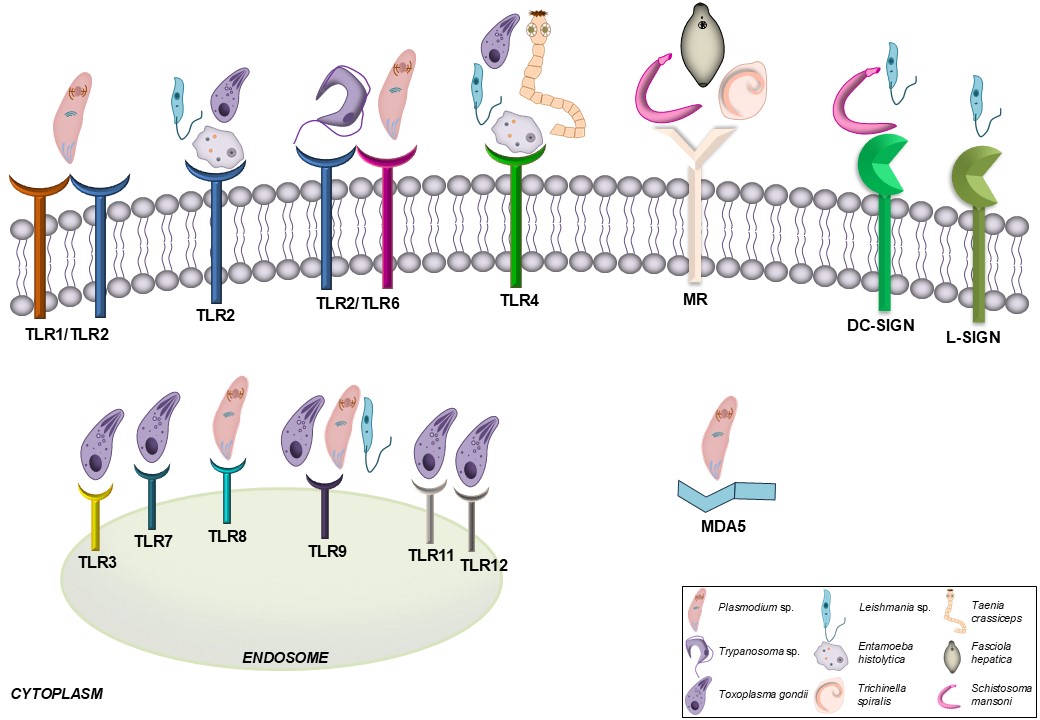
Fig. 1: PRRs representatives involved in the recognition of parasites. DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin; L-SIGN, liver/lymph node- specific intercellular adhesion molecule-3-grabbing nonintegrin; MDA5, melanoma differentiation-associated protein 5; MR, mannose receptor; TLR, Toll-like receptor.
Each type of innate immune cell occupies a specific role within the host's defense system, collaborating with others to form interconnected networks regulated by cytokines and other molecules. Macrophages and neutrophils are among the most crucial players in the immune response due to their multifaceted functions. Concerning their capacity to phagocytose parasites, these cells can do so with some protozoa, smaller helminth larvae, and eggs. However, the adult forms are typically too large to be engulfed. Instead, macrophages and neutrophils contribute to the immune response against helminths by migrating to the infection site, secreting effector molecules such as reactive oxygen species (ROS), and recruiting other immune cells to attack the parasite. Of note is the observation that macrophages and neutrophils release extracellular DNA traps in response to protozoa and helminths [44–46]. Regarding antiparasitic properties of eosinophils, they are primarily based on their increase in the circulation and affected tissues during helminth infection. Eosinophils bind to the worm larvae through antibodies or complement, after which they release intracellular granules containing toxic substances for parasites, such as major basic protein (MBP), eosinophil cationic protein (ECP), and many others. Also, NK cells control parasitic infections by contributing to parasite lysis and producing significant amounts of interferon (IFN)-γ, a cytokine that is vital in the context of infection with intracellular protozoan parasites [47]. Nevertheless, the most characteristic feature of the immune response to parasite attack is the binding of immunoglobulin E (IgE) to the high-affinity IgE Fc receptor (FcεRI) on basophil and MC surfaces, which triggers degranulation and the release of numerous mediators, including interleukin (IL)-4 and IL-13. These, in turn, trigger a T-helper type 2 (Th2) response [48].
The innate immune defense system is supported by its specific soluble components. Among these, the complement system plays a pivotal role in the elimination of pathogens by forming the membrane attack complex (MAC) and promoting an inflammatory reaction on the surface of invaders. The available data regarding the effectiveness of the complement system in host defense against parasites appears to be largely ambiguous. Although the host complement system has been demonstrated to play a beneficial role during malaria infection [49], an increasing number of studies point to the effective evasion of this mechanism by many other parasites, such as Leishmania sp. [50], Trypanosoma sp. [51], and Fasciola hepatica [52]. Parasites avoid complement attacks using strategies including the expression of proteins that are homologous to host regulators to inhibit complement activation or the expression of proteins that target different complement components, to inhibit complement function and final formation of the MAC [53].
The role of numerous other effector molecules produced by the host organism for the elimination of parasites has been documented. Substances with a recognized role in anti-parasitic defense are antimicrobial peptides (AMPs). Among the AMPs, the defensins are the most extensively researched family, with several members demonstrating antiparasitic activities. The direct parasiticidal activity of β-defensin 130 (DEFB130) against Plasmodium falciparum [54], defensin α-1 against Trypanosoma cruzi [55], α-defensin-5 against T. gondii [56], and β-defensin-1 and -2 against Cryptosporidium parvum [57] has been observed. Furthermore, the potential of cathelicidins to damage E. histolytica or Leishmania sp. has also been revealed [58, 59].
The ability of parasites to evade innate immune mechanisms highlights the importance of adaptive immunity, which exhibits certain general patterns in its response. Typically, extracellular parasites (mainly helminths) induce a Th2-type response. The main drivers of this response are CD4+ Th2 cells, which release type-2 cytokines such as IL-4, IL-5, and IL-13, with contributions from group 2 innate lymphoid cells (ILC2s) that serve as an additional source of these cytokines [60]. Type 2 immune responses can be accompanied by IL-10, produced by Th2 cells as well as other T cell subsets and innate immune cells, which primarily exerts regulatory and immunosuppressive functions [61]. In turn, IL-4, mainly produced by Th2 cells, is important for Th2 cell differentiation, as well as for the activation of the class switching mechanism in B cells that enables IgE synthesis [62]. Conversely, intracellular protozoan infections rely on a Th1-type response to resolve infection and results in a significant increase of Th1 cytokines, including IL-1β, IL-12, tumor necrosis factor (TNF), and most importantly, IFN-γ. Some data suggests that IFN-γ-producing CD4+ Th1 cells are important for the infection caused by Leishmania sp. [63] and Plasmodium sp. [64]. However, it should be stressed that many intracellular protozoa have evolved sophisticated egress mechanisms that allow them to evade immune detection and destruction [65].
Although the aforementioned mechanisms and components each contribute in a parasite-specific manner, one of the central characteristics of anti-parasitic immunity is the activation of IgE-mediated type 2 responses. In this context, MCs emerge as pivotal effector cells acting at barrier sites, capable of bridging innate and adaptive immunity, and shaping the outcome of parasitic infections.
MCs and their role in host defense
The presence of MCs at surfaces in contact with the external environment, a common site of microbe attack,
indicates that these cells constitute a potent arm of the immune response against external invaders. MCs
are
typically located in the subepithelial layers of the skin, the respiratory system, or the gastrointestinal
and
genitourinary tracts, but they are also found in the adipose tissue or around the blood vessels or nerves
[66].
Furthermore, a number of additional factors contribute to the crucial role of MCs in host defense. MCs
represent
a dominant source of bioactive compounds that may affect all stages of microbial-induced inflammation,
from its
initiation, maintenance, and modulation, to resolution [67]. They include pre-formed mediators present
within
cytoplasmic granules (for example, histamine and proteases), lipid-derived molecules synthesized from
arachidonic acids (for example, prostaglandins (PGs) and leukotrienes (LTs)), and a wide array of
cytokines and
chemokines [68]. The direct antimicrobial activity of MCs may be mediated through the release of
antimicrobial
peptides (AMPs) with multidirectional mechanisms of action [69]. Also, the formation of ROS or reactive
nitrogen
species (RNS), such as nitric oxide (NO), represents an important approach of MCs that contributes to
pathogen
eradication [70]. Crucial to the MC involvement in the host defense is their capacity to efficiently
destroy
microorganisms through phagocytosis and kill them through oxidative and non-oxidative pathways. Following
phagocytosis, MCs can process pathogen-derived antigens for presentation mediated by class I and II major
histocompatibility complex (MHC) molecules, thereby initiating adaptive antimicrobial immunity [71]. Also,
MCs
are able to release their nuclear DNA to become MCETs, which consequently trap and eliminate a variety of
pathogens [72, 73]. Additionally, MCs may indirectly regulate host defense mechanisms through the
activation of
other immune cells and the release of chemoattractants, which recruit, for example, other phagocytic cells
to
the site of infection [74].
As mediators of host defense, MCs express multiple classes of PRRs that detect various MAMPs/PAMPs or
endogenous
DAMPs. Expression of TLRs, RLRs, NLRs, and CLRs has been confirmed in a wide range of MC types and MC cell
lines
[75, 76]. The selective activation of PRRs represents an essential mechanism in regulating the type of MC
antimicrobial response. For example, PAMPs associated with bacteria are mainly recognized by TLR or NLR
representatives. RLRs and some TLRs can detect viral double-stranded RNA (dsRNA), single-stranded RNA
(ssRNA),
or envelope proteins. In turn, MCs express CLRs, which are essential for the sensing of fungal antigens
[75].
However, limited data are available concerning the detection of parasite components by MCs. In a
study
conducted by Furuta and colleagues [77], it was demonstrated that MCs can produce TNF in response to the
binding
of TLR4 or FcεRI/IgE to malaria parasite-derived peroxiredoxin. A recent report indicates that
5ʹ-methylthioinosine, a P. falciparum-specific intermediate of the purine salvage pathway, is an
agonist
for TLR8, which has been documented to be expressed in MCs [78]. Besides, some studies suggest that
parasites or
parasite-derived constituents may influence the expression of certain PRRs in MCs. For example,
stimulation of a
hybrid rat mast cell line (HRMC) with either F2 or the total soluble extract of G. duodenalis
resulted in
an increase (but non-significant) in TLR2 and TLR4 expression in these cells [79]. Further,
lipophosphoglycan
(LPG) from Leishmania mexicana was observed to enhance the expression of TLR2 in bone
marrow-derived MCs
(BMMCs) [80].
Parasites affect MC activity
Modes of MC response to Protozoa
Evidence indicates that various species of parasitic protozoa may directly affect MC activity in
vitro.
Many available data concern their impact on the generation and/or release of bioactive molecules from MCs.
Firstly, it has been documented that protozoa have different effects on the MC degranulation and the
secretion
of preformed mediators, including those known for their potent proinflammatory properties. The
trophozoites of
G. duodenalis and their total soluble extract have been observed to increase the expression of
tryptase
and the secretion of histamine from rat peritoneal MCs (PMCs) [79, 81]. Conversely, it has been
demonstrated
that the stimulation of BMMCs with an extract containing soluble proteins of Giardia activates
those
cells, resulting in the release of tryptase, but not degranulation [82]. A more pronounced stimulatory
effect
observed with whole Giardia trophozoites compared to the extract alone indicates the presence of
additional factors that enhance MC degranulation and preformed mediator release. In turn, unambiguous data
indicate that another common flagellated protozoan parasite, i.e., Trichomonas vaginalis, is
capable of
triggering MC degranulation. It has been reported that T. vaginalis-derived excretory-secretory
product
(ESP) or live trichomonads activate rat PMCs and human MC (HMC-1) line to degranulate [83–85]. It is
worthy of
note that when HMC-1 cells were exposed to live trichomonads, there was a notable increase in
β-hexosaminidase
release in proportion to the number of trichomonads present. Moreover, the addition of supernatants
derived from
human vaginal epithelial cells incubated with live T. vaginalis to HMC-1 cell cultures led to an
increase
in β-hexosaminidase secretion [86]. Also, parasites belonging to the Trypanosoma genus, i.e.,
Leishmania donovani and Leishmania tropica as well as Leishmania major and
Leishmania infantum have been observed to activate the rat-derived basophilic leukemia
cell
(RBL-2H3) line or murine BMMCs, thus resulting in degranulation and β-hexosaminidase release
[87,
88]. More detailed studies have confirmed that LPG, a surface protein derived from L. mexicana
stimulates
BMMCs from BALB/c but not C57BL/6 mice to degranulate, suggesting that susceptibility to LPG-induced MC
activation is strain-dependent and may be attributed to the genetic background of the experimental model
[80].
In addition, the exposure of primary MC types, such as BMMCs or rat PMCs to tachyzoites of the Apicomplexa
protozoan T. gondii has been demonstrated to stimulate the secretion of histamine and serotonin by
these
cells [89, 90]. On the other hand, Smith and co-workers [91] documented that T. gondii inhibits
RBL-2H3
cell degranulation and β-hexosaminidase release by suppressing the mobilization of intracellular
Ca2+
by phospholipase C (PLC), a pivotal and well-known aspect of IgE/FcεRI-mediated signal transduction in
MCs.
Same as central indicators of MC degranulation (histamine, β-hexosaminidase), other MC-derived mediators
are
synthesized in response to protozoa in vitro. Mounting evidence indicates that these organisms
promote
the production of a variety of factors, cytokines, and chemokines, including those with potent
pro-inflammatory
and antiparasitic properties. It was observed that the RH strain of T. gondii activates rat PMCs to
release LTs, an important group of robust pro-inflammatory mediators known for their antiparasitic
activities
[90, 92]. Also, T. gondii lysates trigger the release of certain cytokines (TNF and IL-4) and
chemokines
(CCL2 and CXCL8) from HMC-1 cells, but not from murine BMMCs [89, 93]. Both G. duodenalis
live
trophozoites and total soluble extract (TSE) of trophozoites induce mRNA expression and production of the
pro-inflammatory cytokines IL-6 and TNF by the hybrid rat MC line (HRMC) [81]. Further detailed
studies
demonstrated that distinct protein fractions derived from Giardia trophozoites and designated as
F1-F3
exhibited slight variations in their impact on cytokine synthesis by MCs. The findings of this study
showed that
fraction F2, which contains molecules with important biological activities such as enolase and arginine
deiminase (ADI), has the greatest capacity to activate HRMC cells to produce TNF and IL-6 [79]. Also,
G.
duodenalis has been observed to induce the release of IL-6 from murine BMMCs [82]. The stimulation
of
HMC-1 cells with the secretory products of another intestinal protozoan, namely E. histolytica,
resulted
in an increase in CXCL8 mRNA and protein expression in these cells [94]. Living L. major or L.
infantum promastigotes enhance the release of TNF from BMMCs [88], whereas LPG from L. mexicana
activates those cells to synthesize pro-inflammatory cytokines, including TNF and CCL3, but also
anti-inflammatory IL-10 [80]. The ESP and/or live trichomonads of T. vaginalis stimulate rat PMCs
and
HMC-1 cells to produce, for example, TNF, CXCL8, and CCL2 [83, 85, 86]. Notwithstanding the observed
disparities
in cytokine and chemokine release, likely attributable to the various parasite species and distinct
characteristics of the MC source, the aforementioned data strongly indicate that MCs function as
regulators or
even initiators of inflammation during protozoan infections. Additional support for this hypothesis comes
from
some in vivo studies. The idea that MC-derived IL-6 plays an important role in the control of
Giardia
infection was confirmed by a study conducted by Li et al. [95], which demonstrated that
intestinal
tissue IL-6 mRNA levels are reduced in infected mice treated with MC blocking antibody compared to
infected mice
treated with control IgG or not treated with antibody. It was also noted that PMCs obtained from
Plasmodium
berghei ANKA-infected C57BL/6 mice released elevated amounts of TNF in comparison to MCs derived
from
control mice [96].
Furthermore, a substantial body of evidence from in vivo and in situ studies substantiates
the
influence of protozoa on the accumulation of MCs in tissues and/or their local degranulation. One of the
initial
findings in this context was performed by Im and co-workers [97]. They observed a higher number of MCs and
their
degranulation in murine mesenteric tissues of the E. histolytica-infected group than the control
mice
[97]. Further, Rose and colleagues [98] reported an augmented number of MCs in the intestinal lamina
propria of
chickens after Eimeria sp. oocyst inoculation; however, there was no evidence of MC degranulation.
Infections with other enteric protozoa, such as C. parvum and G. duodenalis, further
underscore
this trend. The increase in the number of MCs within the intestinal mucosa and histamine level in the
serum and
intestinal contents of calves following an oral challenge with C. parvum oocysts compared to
the
animals from the control group was documented [99]. Likewise, G. duodenalis infection in mice was
associated not only with higher MC counts in the small intestine but also with degranulation [100].
Malaria parasites induce similarly responses. Elevated MC number in ileal tissues and plasma histamine
levels
were noted in Rhesus macaques infected with the malaria parasite Plasmodium fragile in
comparison
to control animals [101]. Wilainam and colleagues [102] present an interesting in vivo study
dealing with MCs in patients with Plasmodium infection. They showed that MC degranulation was
significantly higher in the skin of patients with the complicated P. falciparum group in comparison
to
the uncomplicated P. falciparum and control groups. In that case, it has also been proposed that
the
percentage of MC degranulation correlates significantly with the degree of parasitaemia [102]. An
additional
study found that MCs promote Plasmodium spreading and that P. berghei infection of mice
caused
massive MC degranulation in the skin and draining of lymph nodes [103]. Also, a higher number of MCs and
degree
of MC degranulation were observed in the skin, cervical lymph node, and brain of mice with experimental
cerebral
malaria induced by the P. berghei ANKA strain than in uninfected mice [104]. It was also shown that
MCs
are recruited to the ileum in mice infected with Plasmodium yoelii, accompanied by elevated plasma
histamine levels in that animal model [105]. It has recently been demonstrated that the activation of MCs
and
the subsequent release of MC protease-4 (MCPT-4) serve to suppress the host immune response to P.
yoelii
[106]. Although the aforementioned data suggest that Plasmodium species may induce MC degranulation
in
vivo, a recent study has demonstrated that the different stages of malarial infection exert varying
effects on the murine PMC degranulation mechanism [107].
In vivo studies have also indicated the potential for MCs to play a significant role in the immune
response to toxoplasmosis. The use of toluidine blue staining and immunofluorescence staining of tryptase
revealed a high number of degranulated/total MCs in the spleen and mesentery tissues from mice infected
with
tachyzoites of the highly pathogenic T. gondii RH strain [108]. Similar findings were obtained by
Ferreira et al. [109] who performed a morphological analysis of rodent PMCs following
intraperitoneal
injection with the same strain of T. gondii and observed a higher number of degranulated MCs
obtained
from peritoneal cavities compared to uninfected animals. Simultaneously, they showed the changed size and
shape
of MCs as well as exhibited lower numbers of granules, with a fusion of their membranes and the formation
of
intracytoplasmic channels [109]. There is also strong evidence that MCs are required for host survival
following
oral infection with T. gondii. In studies using MC-deficient (W/Wv) mice orally infected
with
a low-virulent ME49 T. gondii strain, Cruz and colleagues [110] observed a rapid lethality and
decreased
serum IFN-γ and IL-12 levels compared to control mice (control +/+ counterparts).
Trypanosoma infections similarly engage MCs, contributing to modulation of host immune responses. In
murine
experimental trypanosomosis induced by T. brucei or T. cruzi, there have been descriptions
of an
increase in the number and/or degranulation of MCs in the jejunum and cardiac lesions. This may be
associated
with a worse prognosis, possibly implying ongoing inflammation and fibrotic processes involving MCs
[111–113].
During the T. brucei infection, the levels of histamine in the mucosal tissues of the jejunum of
the
infected mice were found to be significantly elevated [111]. In an experimental model of Chagas' disease,
mice
infected with T. cruzi show increased histamine levels in the heart tissues compared to control
animals
[114] and histological examination revealed the presence of MCs in these mice in regions of fibrosis
[115].
Martins et al. [116] found an elevated numbers of MCs and elevated tryptase levels in the colons of
Trypanosoma cruzi-infected patients, suggesting MC activation and a potential role in the
recruitment and
activation of eosinophils.
During Leishmania infection, alterations in MC numbers and degranulation have been also observed.
Cutaneous infection with L. major in C57BL/6 and BALB/c mice resulted in a decrease in the number
of
dermal MCs, but these cells exhibited extensive degranulation [117]. Interestingly, MC degranulation may
inhibit
leishmaniasis. The intraperitoneal and intrafootpad administration of a well-known MC degranulating agent,
compound 48/80, to mice before infection with L. major resulted in a reduction in the incidence of
infection, an increase in the popliteal lymph nodes' levels of IFN-γ, CCL2, CCL5, iNOS and a decrease in
IL-4
levels [118]. Recently, Sánchez-García and co-workers [119] have conducted fascinating studies regarding
the
effect of male sex hormones on the MC-mediated response to Leishmania infection. They found that
MCs
showed a retarded activation pattern associated with slower degranulation and weaker histamine and
tryptase
staining in response to the infection with L. mexicana combined with vector-salivary proteins, as
compared to sham mice in orchiectomized mice [119].
Very little was found in the literature on the question of the exact effects of protozoa on MC
proliferation and/or survival. For instance, L. major or L. infantum notably reduced BMMC
viability [88], yet no proliferation of dermal MCs from L. major-infected C57BL/6 and BALB/c mice
was
observed [117]. In turn, analysis of cardiac tissue samples obtained from T. cruzi-infected mice
revealed
enhanced MC proliferation [115]. Other authors revealed that during the T. cruzi infection, murine
cardiac MCs exhibited increased expression of molecules involved in cell death, namely the P2X7
receptor and Fas [120]. The viability of BM-MMCs remains unchanged in response to soluble Giardia
proteins (sGPs) [82].
There is also some data regarding the mechanisms of various killing strategies of parasitic protozoa
exerted by
MCs. Noteworthy, Naqvi et al. [87] reported that RBL-2H3 cells phagocytose the promastigotes
of
L. tropica but not of L. donovani. This finding may suggest species-specific interactions
between
the parasites and the MCs, possibly involving distinct surface molecules or immune evasion strategies. The
same
authors have demonstrated that RBL-2H3 cells release extracellular structures upon stimulation with
promastigotes of L. tropica and L. donovani. These structures are MCETs, which are capable
of
ensnaring pathogens [87]. Considering that ROS are highly toxic to pathogenic microorganisms, the
information
that MCs generate free radicals in response to parasites is highly relevant. It has been established that
L.
tropica and L. donovani stimulate ROS generation by RBL-2H3 cells [87]. Additionally, T.
vaginalis-derived ESP has been observed to induce a significant increase in ROS production by HMC-1
cells [84]. HMC-1 cells treated with T. gondii lysate produce greater amounts of NO, which
is
known to be involved in anti-microbicidal activity [93]. In contrast, Henderson and Chi [90] documented
that ROS
are not implicated in the rat PMC-mediated toxoplasmacidal activity. Similarly, the production of ROS was
not
observed in HMC-1 cells stimulated with secretory products derived from E. histolytica [94].
It is well established that the recruitment of MCs represents a crucial aspect of the immune response to
infection. However, up to date, the subject that parasitic protozoa-derived substances promote MC
chemotactic
activity has received minimal attention. T. vaginalis-derived ESP has been demonstrated to
function as a potent chemoattractant for rat PMCs and HMC-1 cells [83, 86]. It has been also observed
indirect
effect on the promotion of MC migration in response to T. vaginalis-secreted cysLTs [85].
Activities of MCs regarding Platyhelminthes
The data indicate that the number of MCs increases in host tissues infected with parasitic flatworms,
suggesting
that MC infiltration is a common immune response across different hosts and plays a role in the host's
defense
against parasites from this phylum. Birck and colleagues [121] showed that the extent of MC infiltration
was
higher in the liver tissues of pigs infected with Schistosoma japonicum compared to the unexposed
control
group. In turn, a mild to moderate degree of MC infiltration was observed in the majority of hepatic
granulomas.
Also, it was documented that the number of MCs increased markedly in the peritoneal cavity and liver of
mice
infected with F. hepatica metacercariae and mice that had been injected with the tegumental coat
antigen
of F. hepatica [122]. A considerable number of MCs were observed in the cardiac tissues of fish
infected
with Ichthyocotylurus erraticus metacercariae [123]. The number of MCs was found to be higher in
the
duodenum and bile duct tissues of mice infected with Hymenolepis microstoma than in uninfected
animals
[124]. Interestingly, immunostaining of tissues collected during liver biopsies from children with
echinococcosis revealed an abundance of tryptase-positive MCs within the cyst capsules and the portal
tracts
surrounding the cyst [125]. Likewise, an increased number of MCs was observed in the intestinal mucosa of
mice
infected with Echinostoma hortense, with the most visible rise in the duodenum [126]. Observational
studies in individuals vocationally exposed to S. mansoni demonstrated a negative correlation
between the
number of circulating MC precursors and resistance to reinfection. However, there is a lack of mechanistic
explanation of this phenomenon [127].
Studies on host immunity to parasitic flatworms also looked at the in vitro and in vivo
processes
of MC degranulation. However, the results in this context are ambiguous and dependent on the flatworm
species,
possibly due to variations in their molecular components and specific mechanisms by which each parasite
interacts with MCs. The molecule obtained from the adult worm of S. mansoni (i.e., S.
mansoni
incubation product, SIP) strongly inhibited rat MC degranulation in both in vitro and in vivo
contexts [128]. In contrast, Coelho-Castelo and co-workers [129] evaluated rat peritoneal MC
degranulation
by exposing these cells to S. mansoni-derived mannose-binding protein, termed Sm60. They reported a
high
number of degranulated cells determined by their counting using a Neubauer chamber. Also, the stimulation
of rat
peritoneal MCs with synthetic peptides based on sequences identified in F. hepatica resulted in the
degranulation of these cells as evidenced by the release of histamine [130]. In contrast, bone marrow- and
peritoneum-derived murine MCs do not degranulate in the presence of F. hepatica tegumental coat
antigen
[122]. It has been also found that T. crassiceps metacestode-secreted products from the peritoneal
cavity
of infected mice inhibit the in vitro degranulation of murine and rat MCs, as well as in
vivo
degranulation in rats [131]. Dezfuli et al. [123] observed a considerable number of MCs and a high
rate
of degranulation in regions in close proximity to the site of Eubothrium crassum attachment in the
caecum
of infected fish.
There is no evidence to suggest that molecules derived from flatworms directly promote the migration of
MCs.
However, Vukman et al. [122] have demonstrated that the tegumental coat antigen of F.
hepatica
indirectly induces BMMC migration through the action of dendritic cell-derived chemokines, including CCL3
and
CXCL2. Furthermore, E. multilocularis-obtained calreticulin, which is known to regulate the host
immune
system through binding to complement C1q, was found to suppress the chemotactic effect of C1q on HMC-1
cells
[132]. These findings imply that E. multilocularis can use calreticulin to interfere with the host
immune
system's attack mechanisms, potentially representing a strategy of immune evasion.
There is a lack of data concerning the synthesis and function of MC-derived mediators during infection
with
parasitic flatworms. Only Vukman et al. [133] demonstrated that F. hepatica tegumental coat
antigen suppresses TNF and IL-6 expression in LPS- or Bordetella pertussis-stimulated BMMCs and
PCMCs.
The use of a murine model of Hymenolepis diminuta infection has demonstrated that mice lacking MCs
require a longer time to expel the invading parasites entirely [134]. Furthermore, it appears that
MC-derived
proteases play a role in this process [135].
Effect of Nematode species on MC actions
According to available literature, molecules derived from parasitic nematodes may have the capacity to
influence
MC degranulation in vitro, potentially exerting either a stimulatory or an inhibitory effect on
this
process. Stimulation of rat PMCs with antigens derived from the muscle larval stage of T. spiralis
(TSL-1
antigens) and a total extract of its adult forms resulted in the release of histamine but not
β-hexosaminidase
from these cells [136, 137]. On the contrary, synthetically obtained recombinant nematode galectin reduced
the
secretion of β-hexosaminidase from RBL-2H3 cells [138]. In addition, ES-62, a molecule secreted by
filarial nematodes, has been shown to inhibit human BMMC degranulation and β-hexosaminidase release by
forming a
complex with TLR4 at the plasma membrane [139]. The process of MC degranulation during nematode infections
has
also been studied in vivo. The earliest study demonstrating that MC numbers in host tissues
increase and
degranulate during nematode infection was presented by Wells in 1962 [140]. A further report on this
phenomenon
was presented by Befus and colleagues in 1979 [141]. They observed increased histamine content in the
intestinal
tissues of rats infected with Nippostrongylus brasiliensis, which correlated with the number of
intestinal MCs [141]. Additionally, serum levels of mMCPT-1 were higher in N. brasiliensis-infected
mice
than in healthy controls [142]. Increased MC density in the murine thoracic cavity and circulating levels
of MC
protease-1 (MCPT-1) have also been observed during infection with Litomosoides sigmodontis, a
parasite
that is widely used as a study model for human filarial infections [143]. Furthermore, the accumulation of
MCs
in infected tissues and/or elevated levels of MC degranulation markers have been reported in animal models
infected with T. spiralis, Strongyloides stercoralis, Strongyloides venezuelensis,
Ascaridia galli, Toxocara canis, and Heligmosomoides polygyrus [144–151]. The
findings obtained by Patrizi et al. [152] indicate that infection with Enterobius
vermicularis may
act as a trigger for the onset of general cutaneous mastocytosis symptoms or may exacerbate cutaneous
mastocytosis due to massive MC degranulation. Although the degranulation of MCs and the release of
preformed mediators are generally regarded as crucial for the host's defense against parasites, some in
vivo studies indicate that this process may enhance vascular permeability and, consequently,
facilitate
larval migration. Indeed, it has been demonstrated that the blockade of MC degranulation with MC
stabilizers
results in a reduction in the burden of L. sigmodontis in mice. This suggests that the release of
MC-derived mediators during degranulation improves larval migration through the host skin [153, 154].
Conversely, MCs may promote the expulsion of T. spiralis by increasing the permeability of the gut
via mouse MC protease-1 (mMCP-1)-mediated breakdown of epithelial tight junction proteins [144].
The
interest of researchers has been also generated in the context of the activity of other MC-derived
mediators' in
host defense against parasitic nematodes. The stimulation of the rat HRMC-1 line with TSL-1 antigens has
been
found to increase mRNA expression of TNF and IL-4, while simultaneously reducing the expression of IFN-γ
and
IL-10 in these cells [155]. It has also been demonstrated that nematode galectin reduces the release of
leukotriene C4 (LTC4) from RBL-2H3 cells [138]. Conversely, Carlos and colleagues
[151]
observed elevated circulating LTB4 levels in rats following T. canis infection, which
correlated with the accumulation of MCs and eosinophils. Shimokawa et al. [156] have pointed out
the
significance of IL-33 produced by MCs in eradicating Heligmosomoides polygyrus in the early stage
of
infection in mice. It has been established that MC-derived IL-33, following the recognition of tissue
damage-derived ATP by the P2X7 receptor, activates ILC2s, which have been shown to contribute to the
expulsion
of a variety of helminths [156, 157].
There is currently no direct evidence to suggest that parasitic nematodes impact the survival of MCs.
However,
Knight et al. [145] reported that the expression of stem cell factor (SCF), a well-known MC growth
factor, was significantly up-regulated in the epithelium of T. spiralis-infected mice. Very little
is
known about MC proliferation in response to parasitic nematodes or their molecules in vitro. Only
Donskow-Łysoniewska et al. [138] demonstrated that synthetically obtained nematode galectin
dose-dependently decreases the proliferation of RBL-2H3 cells.
Conclusion
The current state of knowledge regarding the role of MCs in the host's defense against parasites is not yet clearly defined, due to at least two major reasons. The heterogeneity of MCs employed in in vitro studies does not always yield consistent conclusions as different MC types display various characteristics, including their phenotype, activity, and lifespan. Furthermore, it is important to consider that various parasites possess a vast array of antigens, and there is presently limited information regarding the parasite-derived specific molecules that are responsible for triggering the MC response. Further studies, which consider these variables, will need to be undertaken. Nevertheless, several strong assumptions concerning the role of MCs in anti-parasitic defense mechanisms can be deduced from an analysis of the available research literature. The majority of studies indicate that MCs exert a protective immune response during parasitic infection. The first substantial evidence to support this premise is the observation that MCs accumulate at the site of parasite presence in the host organism. Furthermore, the control of parasitic infections is achieved through the multifaceted actions of MCs (Fig. 2), which range from the elimination of invaders by phagocytosis or MCET formation to the synthesis and/or release of various mediators with direct anti-parasitic activity or which are necessary for the recruitment of other immune cells and their activation. The significance of preformed mediators such as histamine and MC-specific proteases in the context of parasite defense is of particular interest, as evidenced by several in vivo studies (Table 1). Finally, crucial to host defense during parasitic infection is the involvement of MCs in triggering a Th2-associated response [158]. The evidence presented above unequivocally indicates that MCs either initiate or modulate the inflammatory response during parasitic infections. Additional support for this hypothesis can be found in studies on human tissue samples from infected individuals and findings in various animal models. Nevertheless, to gain a comprehensive understanding of the role of MCs in defense mechanisms that develop during parasitic infection, additional studies will be invaluable. Further research should focus on the mechanisms by which MCs recognize parasites and how parasite-derived immunomodulatory factors act on these cells. Such an approach may be used in developing strategies to protect the host against infection and/or the pathological consequences of infection.
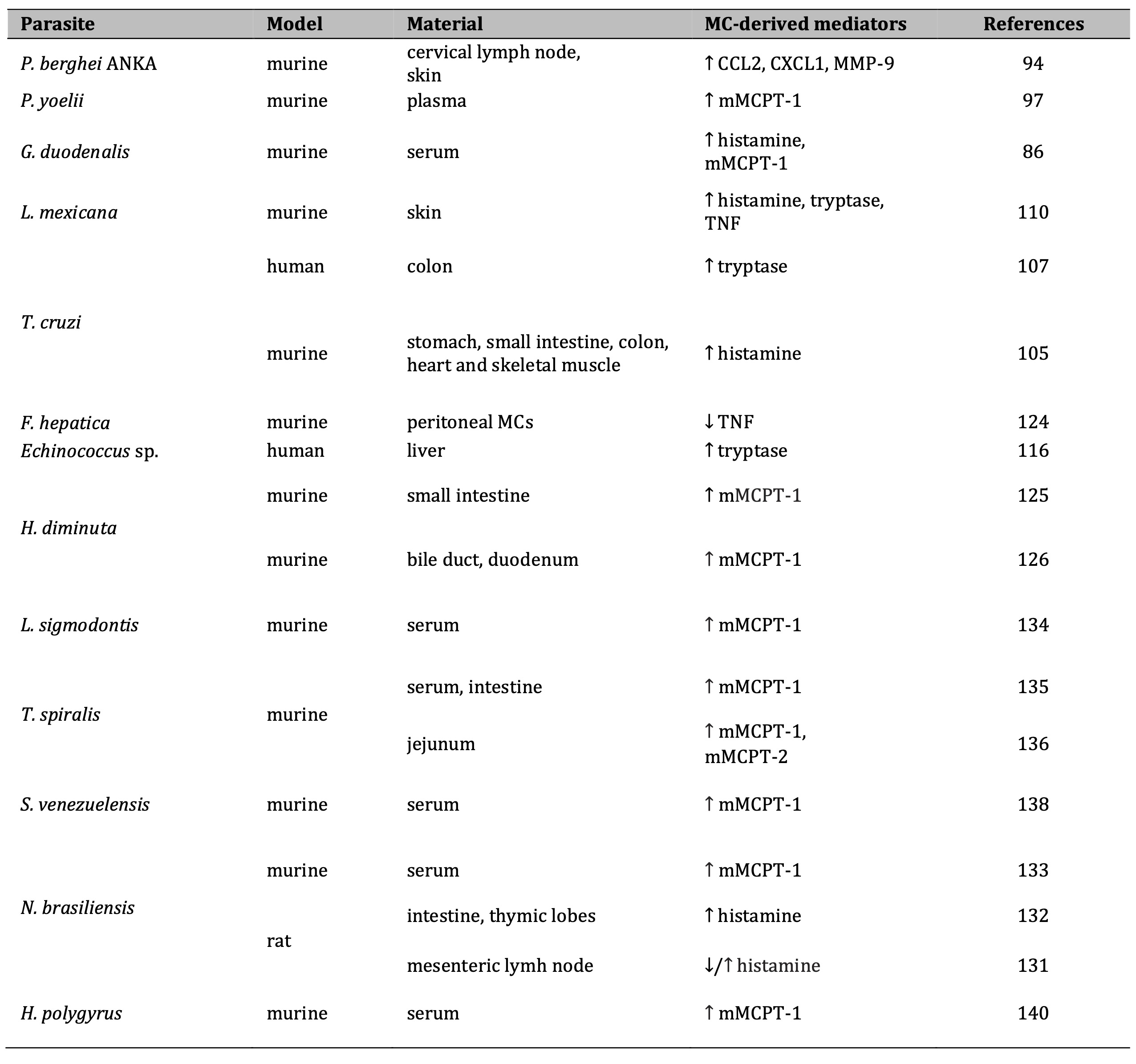
Table 1: Changes in MC-derived mediators' mRNA/protein expression during parasitic infection. Data from in vivo studies. Note: ↑, increased; ↓, decreased. Abbreviations: CCL2, CC chemokine ligand 2; CXCL1, C-X-C motif chemokine ligand 1; mMCPT-1, mouse mast cell protease-1; MMP-9, matrix metalloproteinase-9; TNF, tumor necrosis factor
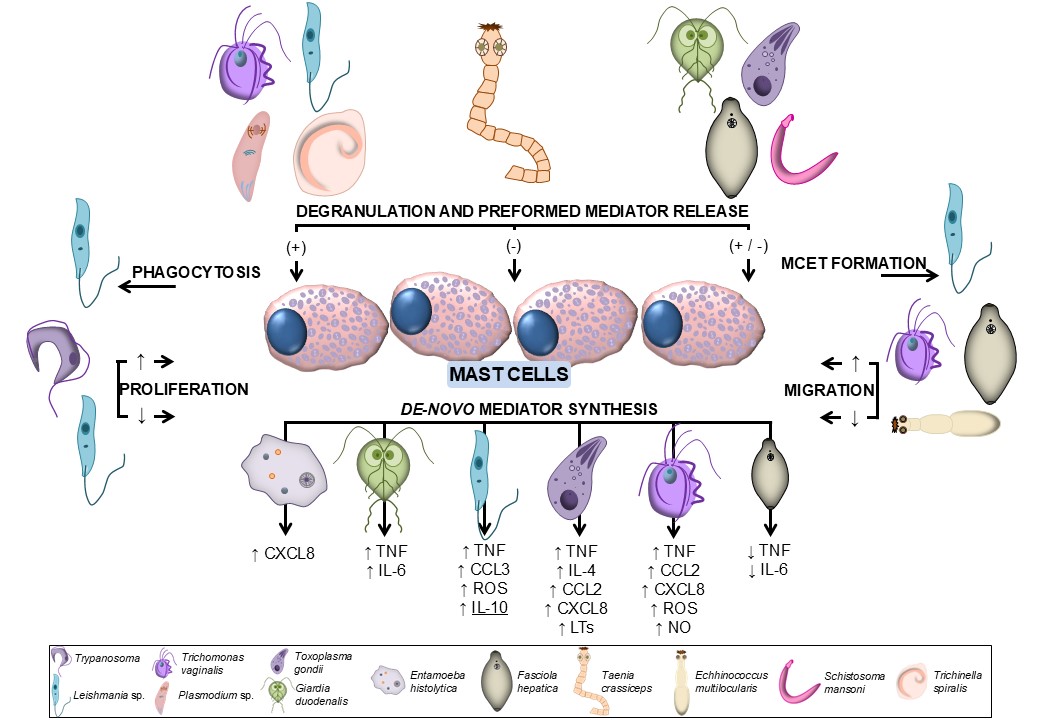
Fig. 2: MC activities in response to different parasite species. CCL, CC chemokine ligand; CXCL, C-X-C motif chemokine ligand; IL, interleukin; LT, leukotriene; NO, nitric oxide; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Acknowledgements
Funding
This work was supported by grant from the Medical University of Lodz, Poland (Grant No.
503/6-127-07/503-61-001).
Author contributions
Paulina Żelechowska, Conceptualization, Writing – original draft, Writing – review and editing ǀ
Aleksandra
Góralczyk-Bińkowska, Writing – original draft, Writing – review and editing
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Wong LW, Ong KS, Khoo JR, Goh CBS, Hor JW, Lee SM: Human intestinal parasitic infection: a
narrative review on global prevalence and epidemiological insights on preventive, therapeutic and
diagnostic strategies for future perspectives. Expert Rev Gastroenterol Hepatol 2020;14:1093-1105.
https://doi.org/10.1080/17474124.2020.1806711 |
| 2 | Feehan J, Plebanski M, Apostolopoulos V: Recent perspectives in clinical development of malaria
vaccines. Nat Commun. 2025;16:3565.
https://doi.org/10.1038/s41467-025-58963-4 |
| 3 | Cholewiński M, Derda M, Hadaś E: Parasitic diseases in humans transmitted by vectors. Ann
Parasitol 2015;61:137-157.
|
| 4 | Chulanetra M, Chaicumpa W: Revisiting the mechanisms of immune evasion employed by human
parasites. Front Cell Infect Microbiol 2021;11:702125.
https://doi.org/10.3389/fcimb.2021.702125 |
| 5 | Yasmin H, Datta P, Deb A, Willingham AL, Kishore U: Innate immune response to helminth infections.
Adv Exp Med Biol 2025;1476:251-273.
https://doi.org/10.1007/978-3-031-85340-1_10 |
| 6 | Wang R, Lan C, Benlagha K, Camara NOS, Miller H, Kubo M, Heegaard S, Lee P, Yang L, Forsman H, Li
X, Zhai Z, Liu C: The interaction of innate immune and adaptive immune system. MedComm (2020)
2024;5(10):e714.
https://doi.org/10.1002/mco2.714 |
| 7 | Li NS, Yeh YW, Li L, Xiang Z: Mast cells: key players in host defence against infection. Scand J
Immunol 2025;102(2):e70046.
https://doi.org/10.1111/sji.70046 |
| 8 | Suárez Vázquez TA, López López N, Salinas Carmona MC: MASTer cell: chief immune modulator and
inductor of antimicrobial immune response. Front Immunol 2024;15:1360296.
https://doi.org/10.3389/fimmu.2024.1360296 |
| 9 | Wiedemann M, Voehringer D: Immunomodulation and immune escape strategies of gastrointestinal
helminths and schistosomes. Front Immunol 2020;11:572865.
https://doi.org/10.3389/fimmu.2020.572865 |
| 10 | Ewald S, Nasuhidehnavi A, Feng T, Lesani M, McCall L: The intersection of host in vivo metabolism
and immune responses to infection with kinetoplastid and apicomplexan parasites. Microbiol Mol Biol
Rev 2024;88:e00164-22.
https://doi.org/10.1128/mmbr.00164-22 |
| 11 | Cuellar P, Hernández-Nava E, García-Rivera G, Chávez-Munguía B, Schnoor M, Betanzos A, Orozco E:
Entamoeba histolytica EhCP112 dislocates and degrades claudin-1 and claudin-2 at tight junctions of
the intestinal epithelium. Front Cell Infect Microbiol 2017;7:372.
https://doi.org/10.3389/fcimb.2017.00372 |
| 12 | Maia-Brigagão C, Morgado-Díaz JA, de Souza W: Giardia disrupts the arrangement of tight, adherens
and desmosomal junction proteins of intestinal cells. Parasitol Int 2012;61:280-287.
https://doi.org/10.1016/j.parint.2011.11.002 |
| 13 | Briceño MP, Nascimento LA, Nogueira NP, Barenco PV, Ferro EA, Rezende-Oliveira K, Goulart LR,
Alves PT, Barbosa Bde F, Lima WR, Silva NM: Toxoplasma gondii infection promotes epithelial barrier
dysfunction of Caco-2 cells. J Histochem Cytochem 2016;64:459-469.
https://doi.org/10.1369/0022155416656349 |
| 14 | Amat CB, Motta JP, Fekete E, Moreau F, Chadee K, Buret AG: Cysteine protease-dependent mucous
disruptions and differential mucin gene expression in Giardia duodenalis infection. Am J Pathol
2017;187:2486-2498.
https://doi.org/10.1016/j.ajpath.2017.07.009 |
| 15 | Lidell ME, Moncada DM, Chadee K, Hansson GC: Entamoeba histolytica cysteine proteases cleave the
MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad
Sci U S A 2006;103:9298-9303.
https://doi.org/10.1073/pnas.0600623103 |
| 16 | Hasnain SZ, McGuckin MA, Grencis RK, Thornton DJ: Serine protease(s) secreted by the nematode
Trichuris muris degrade the mucus barrier. PLoS Negl Trop Dis 2012;6:e1856.
https://doi.org/10.1371/journal.pntd.0001856 |
| 17 | Carrero JC, Cervantes-Rebolledo C, Aguilar-Díaz H, Díaz-Gallardo MY, Laclette JP, Morales-Montor
J: The role of the secretory immune response in the infection by Entamoeba histolytica. Parasite
Immunol 2007;29:331-338.
https://doi.org/10.1111/j.1365-3024.2007.00955.x |
| 18 | Langford TD, Housley MP, Boes M, Chen J, Kagnoff MF, Gillin FD, Eckmann L: Central importance of
immunoglobulin A in host defense against Giardia spp. Infect Immun 2002;70:11-18.
https://doi.org/10.1128/IAI.70.1.11-18.2002 |
| 19 | Davids BJ, Palm JE, Housley MP, Smith JR, Andersen YS, Martin MG, Hendrickson BA, Johansen FE,
Svärd SG, Gillin FD, Eckmann L: Polymeric immunoglobulin receptor in intestinal immune defense
against the lumen-dwelling protozoan parasite Giardia. J Immunol 2006;177:6281-6290.
https://doi.org/10.4049/jimmunol.177.9.6281 |
| 20 | Chu KB, Kim SS, Lee SH, Lee HS, Joo KH, Lee JH, Lee YS, Zheng S, Quan FS: Enhanced protection
against Clonorchis sinensis induced by co-infection with Trichinella spiralis in rats. Parasite
Immunol 2014;36:522-530.
https://doi.org/10.1111/pim.12125 |
| 21 | Kaneva E, Harizanov R, Velcheva D, Tsvetkova N, Pavlova M, Alexiev I, Dimitrova R, Videnova M,
Borisova R, Ivanova A: Studies on the significance of secretory IgA antibodies in the pathogenesis
and clinical course of enterobiasis in infected persons from Bulgaria: preliminary findings.
Helminthologia 2024;61:277-285.
https://doi.org/10.2478/helm-2024-0034 |
| 22 | Castilla Gómez de Agüero V, Valderas-García E, González Del Palacio L, Giráldez FJ, Balaña-Fouce
R, Martínez-Valladares M: Secretory IgA as biomarker for gastrointestinal nematodes natural
infection in different breed sheep. Animals (Basel) 2023;13:2189.
https://doi.org/10.3390/ani13132189 |
| 23 | Jenkins TP, Rathnayaka Y, Perera PK, Peachey LE, Nolan MJ, Krause L, Rajakaruna RS, Cantacessi C:
Infections by human gastrointestinal helminths are associated with changes in faecal microbiota
diversity and composition. PLoS One 2017;12(9):e0184719.
https://doi.org/10.1371/journal.pone.0184719 |
| 24 | Grondin JA, Jamal A, Mowna S, Seto T, Khan WI: Interaction between intestinal parasites and the
gut microbiota: Implications for the intestinal immune response and host defence. Pathogens
2024;13(8):608.
https://doi.org/10.3390/pathogens13080608 |
| 25 | Beyhan YE, Yildiz MR: Microbiota and parasite relationship. Diagn Microbiol Infect Dis
2023;106:115954.
https://doi.org/10.1016/j.diagmicrobio.2023.115954 |
| 26 | Leung JM, Graham AL, Knowles SCL: Parasite-microbiota interactions with the vertebrate gut:
synthesis through an ecological lens. Front Microbiol 2018;9:843.
https://doi.org/10.3389/fmicb.2018.00843 |
| 27 | Li C, Liu Y, Liu X, Bai X, Jin X, Xu F, Chen H, Zhang Y, Vallee I, Liu M, Yang Y: The gut
microbiota contributes to changes in the host immune response induced by Trichinella spiralis. PLOS
Negl Trop Dis 2023;17:e0011479.
https://doi.org/10.1371/journal.pntd.0011479 |
| 28 | Boucard AS, Kulakauskas S, Alazzaz J, Chaouch S, Mammeri M, Millan-Oropeza A, Machado C, Henry C,
Péchoux C, Richly H, Gassel M, Langella P, Polack B, Florent I, Bermúdez-Humarán LG: Isolation of
derivatives from the food-grade probiotic Lactobacillus johnsonii CNCM I-4884 with enhanced
anti-Giardia activity. Gut Microbes 2025;17(1):2474149.
https://doi.org/10.1080/19490976.2025.2474149 |
| 29 | Pérez PF, Minnaard J, Rouvet M, Knabenhans C, Brassart D, De Antoni GL, Schiffrin EJ: Inhibition
of Giardia intestinalis by extracellular factors from Lactobacilli: an in vitro study. Appl Environ
Microbiol 2001;67:5037-5042.
https://doi.org/10.1128/AEM.67.11.5037-5042.2001 |
| 30 | Amer EI, Mossallam SF, Mahrous H: Therapeutic enhancement of newly derived bacteriocins against
Giardia lamblia. Exp Parasitol 2014;146:52-63.
https://doi.org/10.1016/j.exppara.2014.09.005 |
| 31 | Keelaghan AP, Charania R, Mead JR: The effect of short-chain fatty acids on growth of
Cryptosporidium parvum in vitro. Microorganisms 2022;10:1822.
https://doi.org/10.3390/microorganisms10091822 |
| 32 | Byers J, Faigle W, Eichinger D: Colonic short-chain fatty acids inhibit encystation of Entamoeba
invadens. Cell Microbiol 2005;7:269-279.
https://doi.org/10.1111/j.1462-5822.2004.00457.x |
| 33 | Wang Y, Guo A, Zou Y, Mu W, Zhang S, Shi Z, Liu Z, Cai X, Zhu XQ, Wang S: Interaction between
tissue-dwelling helminth and the gut microbiota drives mucosal immunoregulation. NPJ Biofilms
Microbiomes 2023;9:43.
https://doi.org/10.1038/s41522-023-00410-7 |
| 34 | Li D, Wu M: Pattern recognition receptors in health and diseases. Signal Transduct Target Ther
2021;6:291.
https://doi.org/10.1038/s41392-021-00687-0 |
| 35 | Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC: Induction
of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium
falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and
regulation of GPI activity. J Biol Chem 2005;280:8606-8616.
https://doi.org/10.1074/jbc.M413541200 |
| 36 | Oliveira AC, Peixoto JR, de Arruda LB, Campos MA, Gazzinelli RT, Golenbock DT, Akira S, Previato
JO, Mendonça-Previato L, Nobrega A, Bellio M: Expression of functional TLR4 confers proinflammatory
responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection
with T. cruzi. J Immunol 2004;173:5688-5696.
https://doi.org/10.4049/jimmunol.173.9.5688 |
| 37 | Maldonado-Bernal C, Kirschning CJ, Rosenstein Y, Rocha LM, Rios-Sarabia N, Espinosa-Cantellano M,
Becker I, Estrada I, Salazar-González RM, López-Macías C, Wagner H, Sánchez J, Isibasi A: The innate
immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors
2 and 4. Parasite Immunol 2005;27:127-137.
https://doi.org/10.1111/j.1365-3024.2005.00754.x |
| 38 | Dissanayake S, Amith RS, Shahin A: Taenia crassiceps carbohydrates stimulate IL-6 expression in
naïve murine macrophages via Toll-like receptors (TLRs). Mol Immunol 2004;41:391-398.
https://doi.org/10.1016/j.molimm.2004.03.020 |
| 39 | Raetz M, Kibardin A, Sturge CR, Pifer R, Li H, Burstein E, Ozato K, Larin S, Yarovinsky F:
Cooperation of TLR12 and TLR11 in the IRF8-dependent IL-12 response to Toxoplasma gondii profilin. J
Immunol 2013;191:4818-4827.
https://doi.org/10.4049/jimmunol.1301301 |
| 40 | Andrade WA, Souza Mdo C, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S,
Golenbock DT, Gazzinelli RT: Combined action of nucleic acid-sensing Toll-like receptors and
TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe
2013;13:42-53.
https://doi.org/10.1016/j.chom.2012.12.003 |
| 41 | Melo MB, Kasperkovitz P, Cerny A, Könen-Waisman S, Kurt-Jones EA, Lien E, Beutler B, Howard JC,
Golenbock DT, Gazzinelli RT: UNC93B1 mediates host resistance to infection with Toxoplasma gondii.
PLoS Pathog 2010;6:e1001071.
https://doi.org/10.1371/journal.ppat.1001071 |
| 42 | Caparrós E, Serrano D, Puig-Kröger A, Riol L, Lasala F, Martinez I, Vidal-Vanaclocha F, Delgado R,
Rodríguez-Fernández JL, Rivas L, Corbí AL, Colmenares M: Role of the C-type lectins DC-SIGN and
L-SIGN in Leishmania interaction with host phagocytes. Immunobiol 2005;210:185-193.
https://doi.org/10.1016/j.imbio.2005.05.013 |
| 43 | van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CM, Appelmelk B, Geijtenbeek TB, van Kooyk Y:
The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens
and recognizes the glycan antigen Lewis x. Glycobiol 2003;13:471-478.
https://doi.org/10.1093/glycob/cwg052 |
| 44 | Bonne-Année S, Kerepesi LA, Hess JA, Wesolowski J, Paumet F, Lok JB, Nolan TJ, Abraham D:
Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing
of larval Strongyloides stercoralis. Microbes Infect 2014;16:502-511.
https://doi.org/10.1016/j.micinf.2014.02.012 |
| 45 | Ramírez-Ledesma MG, Romero-Contreras YJ, Rodríguez MC, Reyes-Cortes R, Cuéllar-Mata P, Avila EE:
Trichomonas vaginalis triggers neutrophil extracellular traps reducing parasite integrity and
growth. Parasitol Res 2022;121(5):1355-1367.
https://doi.org/10.1007/s00436-022-07475-x |
| 46 | Omar M, Abdelal H: NETosis in parasitic infections: A puzzle that remains unsolved. Int J Mol Sci
2023;24:8975.
https://doi.org/10.3390/ijms24108975 |
| 47 | Brillantes M, Beaulieu AM: Memory and memory-like NK cell responses to microbial pathogens. Front
Cell Infect Microbiol 2020;10:102.
https://doi.org/10.3389/fcimb.2020.00102 |
| 48 | Peng J, Siracusa MC: Basophils in antihelminth immunity. Semin Immunol 2021;53:101529.
https://doi.org/10.1016/j.smim.2021.101529 |
| 49 | Reiss T, Müller F, Pradel G: The impact of human complement on the clinical outcome of malaria
infection. Mol Immunol 2022;151:19-28.
https://doi.org/10.1016/j.molimm.2022.08.017 |
| 50 | Filho AAP, Nascimento AAS, Saab NAA, Fugiwara RT, D'Ávila Pessoa GC, Koerich LB, Pereira MH,
Araújo RN, Sant'Anna MRV, Gontijo NF: Evasion of the complement system by Leishmania through the
uptake of factor H, a complement regulatory protein. Acta Trop 2021;224:106152.
https://doi.org/10.1016/j.actatropica.2021.106152 |
| 51 | Menon SS, Ramirez-Toloza G, Wycoff KL, Ehinger S, Shaughnessy J, Ram S, Ferreira VP: Mechanisms by
which factor H protects Trypanosoma cruzi from the alternative pathway of complement. Front Immunol
2024;15:1152000.
https://doi.org/10.3389/fimmu.2024.1152000 |
| 52 | De Marco Verissimo C, Jewhurst HL, Dobó J, Gál P, Dalton JP, Cwiklinski K: Fasciola hepatica is
refractory to complement killing by preventing attachment of mannose binding lectin (MBL) and
inhibiting MBL-associated serine proteases (MASPs) with serpins. PLoS Pathog 2022;18:e1010226.
https://doi.org/10.1371/journal.ppat.1010226 |
| 53 | Shao S, Sun X, Chen Y, Zhan B, Zhu X: Complement evasion: an effective strategy that parasites
utilize to survive in the host. Front Microbiol 2019;10:532.
https://doi.org/10.3389/fmicb.2019.00532 |
| 54 | Terkawi MA, Takano R, Furukawa A, Murakoshi F, Kato K: Involvement of β-defensin 130 (DEFB130) in
the macrophage microbicidal mechanisms for killing Plasmodium falciparum. Sci Rep 2017;7:41772.
https://doi.org/10.1038/srep41772 |
| 55 | Johnson CA, Rachakonda G, Kleshchenko YY, Nde PN, Madison MN, Pratap S, Cardenas TC, Taylor C,
Lima MF, Villalta F: Cellular response to Trypanosoma cruzi infection induces secretion of defensin
α-1, which damages the flagellum, neutralizes trypanosome motility, and inhibits infection. Infect
Immun 2013;81:4139-4148.
https://doi.org/10.1128/IAI.01459-12 |
| 56 | Tanaka T, Rahman MM, Battur B, Boldbaatar D, Liao M, Umemiya-Shirafuji R, Xuan X, Fujisaki K:
Parasiticidal activity of human alpha-defensin-5 against Toxoplasma gondii. In vitro Cell Dev Biol
2010;46:560-565.
https://doi.org/10.1007/s11626-009-9271-9 |
| 57 | Carryn S, Schaefer DA, Imboden M, Homan EJ, Bremel RD, Riggs MW: Phospholipases and cationic
peptides inhibit Cryptosporidium parvum sporozoite infectivity by parasiticidal and
non-parasiticidal mechanisms. J Parasitol 2012;98:199-204.
https://doi.org/10.1645/GE-2822.1 |
| 58 | Rico-Mata R, De Leon-Rodriguez LM, Avila EE: Effect of antimicrobial peptides derived from human
cathelicidin LL-37 on Entamoeba histolytica trophozoites. Exp Parasitol 2013;133:300-306.
https://doi.org/10.1016/j.exppara.2012.12.009 |
| 59 | Crauwels P, Bank E, Walber B, Wenzel UA, Agerberth B, Chanyalew M, Abebe M, König R, Ritter U,
Reiling N, van Zandbergen G: Cathelicidin contributes to the restriction of Leishmania in human host
macrophages. Front Immunol 2019;10:2697.
https://doi.org/10.3389/fimmu.2019.02697 |
| 60 | Zaiss DMW, Pearce EJ, Artis D, McKenzie ANJ, Klose CSN: Cooperation of ILC2s and TH2 cells in the
expulsion of intestinal helminth parasites. Nat Rev Immunol 2024;24(4):294-302.
https://doi.org/10.1038/s41577-023-00942-1 |
| 61 | Rasquinha MT, Sur M, Lasrado N, Reddy J: IL-10 as a Th2 cytokine: Differences between mice and
humans. J Immunol 2021;207(9):2205-2215.
https://doi.org/10.4049/jimmunol.2100565 |
| 62 | Nutman TB: Looking beyond the induction of Th2 responses to explain immunomodulation by helminths.
Parasite Immunol 2015;37:304-313.
https://doi.org/10.1111/pim.12194 |
| 63 | Kima PE, Soong L: Interferon gamma in leishmaniasis. Front Immunol 2013;4:156.
https://doi.org/10.3389/fimmu.2013.00156 |
| 64 | Stephens R, Langhorne J: Effector memory Th1 CD4 T cells are maintained in a mouse model of
chronic malaria. PLoS Pathog 2010;6:e1001208.
https://doi.org/10.1371/journal.ppat.1001208 |
| 65 | Friedrich N, Hagedorn M, Soldati-Favre D, Soldati T: Prison break: pathogens' strategies to egress
from host cells. Microbiol Mol Biol Rev 2012;76:707-720.
https://doi.org/10.1128/MMBR.00024-12 |
| 66 | Ribatti D: Mast cells are at the interface between the external environment and the inner
organism. Front Med (Lausanne) 2024;10:1332047.
https://doi.org/10.3389/fmed.2023.1332047 |
| 67 | Pastwińska J, Agier J, Dastych J, Brzezińska-Błaszczyk E: Mast cells as the strength of the
inflammatory process. Pol J Pathol 2017;68(3):187-196.
https://doi.org/10.5114/pjp.2017.71526 |
| 68 | Xu H, Bin NR, Sugita S: Diverse exocytic pathways for mast cell mediators. Biochem Soc Trans
2018;46(2):235-247.
https://doi.org/10.1042/BST20170450 |
| 69 | Agier J, Brzezińska-Błaszczyk E: Cathelicidins and defensins regulate mast cell antimicrobial
activity. Postepy Hig Med Dosw (Online) 2016;70:618-636.
https://doi.org/10.5604/17322693.1205357 |
| 70 | Swindle EJ, Metcalfe DD: The role of reactive oxygen species and nitric oxide in mast
cell-dependent inflammatory processes. Immunol Rev 2007;217:186-205.
https://doi.org/10.1111/j.1600-065X.2007.00513.x |
| 71 | Katsoulis-Dimitriou K, Kotrba J, Voss M, Dudeck J, Dudeck A: Mast cell functions linking innate
sensing to adaptive immunity. Cells 2020;9(12):2538.
https://doi.org/10.3390/cells9122538 |
| 72 | Elieh Ali Komi D, Kuebler WM: Significance of mast cell formed extracellular traps in microbial
defense. Clin Rev Allergy Immunol 2022;62(1):160-179.
https://doi.org/10.1007/s12016-021-08861-6 |
| 73 | Möllerherm H, Von Köckritz-Blickwede M, Branitzki-Heinemann K: Antimicrobial activity of mast
cells: role and relevance of extracellular DNA traps. Front Immunol 2016;7:265.
https://doi.org/10.3389/fimmu.2016.00265 |
| 74 | Jiménez M, Cervantes-García D, Córdoba-Dávalos LE, Pérez-Rodríguez MJ, Gonzalez-Espinosa C,
Salinas E: Responses of mast cells to pathogens: Beneficial and detrimental roles. Front Immunol
2021;12:685865.
https://doi.org/10.3389/fimmu.2021.685865 |
| 75 | Agier J, Pastwińska J, Brzezińska-Błaszczyk E: An overview of mast cell pattern recognition
receptors. Inflamm Res 2018;67:737-746.
https://doi.org/10.1007/s00011-018-1164-5 |
| 76 | Xie G, Wang F, Peng X, Liang Y, Yang H, Li L: Modulation of mast cell Toll-Like receptor 3
expression and cytokines release by histamine. Cell Physiol Biochem 2018;46:2401-2411.
https://doi.org/10.1159/000489646 |
| 77 | Furuta T, Imajo-Ohmi S, Fukuda H, Kano S, Miyake K, Watanabe N: Mast cell-mediated immune
responses through IgE antibody and Toll-like receptor 4 by malarial peroxiredoxin. Eur J Immunol
2008;38:1341-1350.
https://doi.org/10.1002/eji.200738059 |
| 78 | Köllisch G, Solis FV, Obermann HL, Eckert J, Müller T, Vierbuchen T, Rickmeyer T, Muche S,
Przyborski JM, Heine H, Kaufmann A, Baumeister S, Lingelbach K, Bauer S: TLR8 is activated by
5'-methylthioinosine, a Plasmodium falciparum-derived intermediate of the purine salvage pathway.
Cell Rep 2022;39:110691.
https://doi.org/10.1016/j.celrep.2022.110691 |
| 79 | Muñoz-Cruz S, Gomez-García A, Matadamas-Martínez F, Alvarado-Torres JA, Meza-Cervantez P,
Arriaga-Pizano L, Yépez-Mulia L: Giardia lamblia: identification of molecules that contribute to
direct mast cell activation. Parasitol Res 2018;117:2555-2567.
https://doi.org/10.1007/s00436-018-5944-1 |
| 80 | Villaseñor-Cardoso MI, Salaiza N, Delgado J, Gutiérrez-Kobeh L, Pérez-Torres A, Becker I: Mast
cells are activated by Leishmania mexicana LPG and regulate the disease outcome depending on the
genetic background of the host. Parasite Immunol 2008;30:425-434.
https://doi.org/10.1111/j.1365-3024.2008.01042.x |
| 81 | Muñoz-Cruz S, Gómez-García A, Millán-Ibarra J, Giono-Cerezo S, Yépez-Mulia L: Giardia lamblia:
interleukin 6 and tumor necrosis factor-alpha release from mast cells induced through an
Ig-independent pathway. Exp Parasitol 2010;126:298-303.
https://doi.org/10.1016/j.exppara.2010.06.013 |
| 82 | Li Z, Peirasmaki D, Svärd S, Åbrink M: Giardia excretory-secretory proteins modulate the enzymatic
activities of mast cell chymase and tryptase. Mol Immunol 2019;114:535-544.
https://doi.org/10.1016/j.molimm.2019.07.024 |
| 83 | Im SJ, Ahn MH, Han IH, Song HO, Kim YS, Kim HM, Ryu JS: Histamine and TNF-α release by rat
peritoneal mast cells stimulated with Trichomonas vaginalis. Parasite 2011;18:49-55.
https://doi.org/10.1051/parasite/2011181049 |
| 84 | Narantsogt G, Min A, Nam YH, Lee YA, Kim KA, Agvaandaram G, Dorjsuren T, El-Benna J, Shin MH:
Activation of MAPK is required for ROS generation and exocytosis in HMC-1 cells induced by
Trichomonas vaginalis-derived secretory products. Korean J Parasitol 2015;53:597-603.
https://doi.org/10.3347/kjp.2015.53.5.597 |
| 85 | Lee YA, Nam YH, Min A, Shin MH: Trichomonas vaginalis-secreted cysteinyl leukotrienes promote
migration, degranulation and MCP-1 production in mast cells. Parasite Immunol 2020;42:e12789.
https://doi.org/10.1111/pim.12789 |
| 86 | Han IH, Park SJ, Ahn MH, Ryu JS: Involvement of mast cells in inflammation induced by Trichomonas
vaginalis via crosstalk with vaginal epithelial cells. Parasite Immunol 2012;34:8-14.
https://doi.org/10.1111/j.1365-3024.2011.01338.x |
| 87 | Naqvi N, Ahuja K, Selvapandiyan A, Dey R, Nakhasi H, Puri N: Role of mast cells in clearance of
Leishmania through extracellular trap formation. Sci Rep 2017;7:13240.
https://doi.org/10.1038/s41598-017-12753-1 |
| 88 | Bidri M, Vouldoukis I, Mossalayi MD, Debré P, Guillosson JJ, Mazier D, Arock M: Evidence for
direct interaction between mast cells and Leishmania parasites. Parasite Immunol 1997;19:475-483.
https://doi.org/10.1046/j.1365-3024.1997.d01-153.x |
| 89 | Bidri M, Conti M, Hanoun N, Kuen RL, Feger F, Taoufiq B, Arock M, Mazier D, Vouldoukis I: c-Kit
ligand promotes mast cell infection by Toxoplasma gondii. Open Parasitol J 2008;2:43-50.
https://doi.org/10.2174/1874421400802010043 |
| 90 | Henderson WR Jr, Chi EY: The importance of leukotrienes in mast cell-mediated Toxoplasma gondii
cytotoxicity. J Infect Dis 1998;177:1437-1443.
https://doi.org/10.1086/517833 |
| 91 | Smith NL, Abi Abdallah DS, Butcher BA, Denkers EY, Baird B, Holowka D: Toxoplasma gondii inhibits
mast cell degranulation by suppressing phospholipase Cγ-mediated Ca2+ mobilization. Front Microbiol
2013;4:179.
https://doi.org/10.3389/fmicb.2013.00179 |
| 92 | Rogerio AP, Anibal FF: Role of leukotrienes on protozoan and helminth infections. Mediators
Inflamm 2012;2012:595694.
https://doi.org/10.1155/2012/595694 |
| 93 | Park EA, Han IH, Kim JH, Park SJ, Ryu JS, Ahn MH: Production of inflammatory cytokines and nitric
oxide by human mast cells incubated with Toxoplasma gondii lysate. Korean J Parasitol
2019;57:201-206.
https://doi.org/10.3347/kjp.2019.57.2.201 |
| 94 | Lee YA, Nam YH, Min A, Kim KA, Nozaki T, Saito-Nakano Y, Mirelman D, Shin MH: Entamoeba
histolytica-secreted cysteine proteases induce IL-8 production in human mast cells via a
PAR2-independent mechanism. Parasite 2014;21:1.
https://doi.org/10.1051/parasite/2014001 |
| 95 | Li E, Zhou P, Petrin Z, Singer SM: Mast cell-dependent control of Giardia lamblia infections in
mice. Infect Immun 2004;72:6642-6649.
https://doi.org/10.1128/IAI.72.11.6642-6649.2004 |
| 96 | Furuta T, Kikuchi T, Iwakura Y, Watanabe N: Protective roles of mast cells and mast cell-derived
TNF in murine malaria. J Immunol 2006;177:3294-3302.
https://doi.org/10.4049/jimmunol.177.5.3294 |
| 97 | Im KI, Hwang HK, Soh CT: Behaviour of mast cells in mice in the course of Entamoeba histolytica
infection by strains. Kisaengchunghak Chapchi 1975;13:115-122.
https://doi.org/10.3347/kjp.1975.13.2.115 |
| 98 | Rose ME, Ogilvie BM, Bradley JW: Intestinal mast cell response in rats and chickens to
coccidiosis, with some properties of chicken mast cells. Int Arch Allergy Immunol 1980;63:21-29.
https://doi.org/10.1159/000232606 |
| 99 | Li S, Li W, Yang Z, Song S, Yang J, Gong P, Zhang W, Liu K, Li J, Zhang G, Zhang X: Infection of
cattle with Cryptosporidium parvum: mast cell accumulation in small intestine mucosa. Vet Pathol
2013;50:842-848.
https://doi.org/10.1177/0300985813476055 |
| 100 | Li Z, Peirasmaki D, Svärd S, Åbrink M: The chymase mouse mast cell protease-4 regulates intestinal
cytokine expression in mature adult mice infected with Giardia intestinalis. Cells 2020;9:925.
https://doi.org/10.3390/cells9040925 |
| 101 | Potts RA, Tiffany CM, Pakpour N, Lokken KL, Tiffany CR, Cheung K, Tsolis RM, Luckhart S: Mast
cells and histamine alter intestinal permeability during malaria parasite infection. Immunobiology
2016;221:468-474.
https://doi.org/10.1016/j.imbio.2015.11.003 |
| 102 | Wilainam P, Nintasen R, Viriyavejakul P: Mast cell activation in the skin of Plasmodium falciparum
malaria patients. Malar J 2015;14:67.
https://doi.org/10.1186/s12936-015-0568-8 |
| 103 | Huang B, Huang S, Chen X, Liu XB, Wu Q, Wang Y, Li X, Li K, Gao H, Cen S, Lin R, Liu Z, Jin X:
Activation of mast cells promote Plasmodium berghei ANKA infection in murine model. Front Cell
Infect Microbiol 2019;9:322.
https://doi.org/10.3389/fcimb.2019.00322 |
| 104 | Huang K, Huang L, Zhang X, Zhang M, Wang Q, Lin H, Yu Z, Li X, Liu XB, Wu Q, Wang Y, Wang J, Jin
X, Gao H, Han X, Lin R, Cen S, Liu Z, Huang B: Mast cells-derived exosomes worsen the development of
experimental cerebral malaria. Acta Trop 2021;224:106145.
https://doi.org/10.1016/j.actatropica.2021.106145 |
| 105 | Chau JY, Tiffany CM, Nimishakavi S, Lawrence JA, Pakpour N, Mooney JP, Lokken KL, Caughey GH,
Tsolis RM, Luckhart S: Malaria-associated L-arginine deficiency induces mast cell-associated
disruption to intestinal barrier defenses against nontyphoidal Salmonella bacteremia. Infect Immun
2013;81:3515-3526.
https://doi.org/10.1128/IAI.00380-13 |
| 106 | Céspedes N, Donnelly EL, Lowder C, Hansten G, Wagers D, Briggs AM, Schauer J, Haapanen L, Åbrink
M, Van de Water J, Luckhart S: Mast cell chymase/Mcpt4 suppresses the host immune response to
Plasmodium yoelii, limits malaria-associated disruption of intestinal barrier integrity and reduces
parasite transmission to Anopheles stephensi. Front Immunol 2022;13:801120.
https://doi.org/10.3389/fimmu.2022.801120 |
| 107 | Xiong-Hang K, Haynes CL: Plasmodium chabaudi affects mast cell degranulation as measured by
carbon-fiber microelectrode amperometry. ACS Infect Dis 2021;7:1650-1656.
https://doi.org/10.1021/acsinfecdis.0c00820 |
| 108 | Huang B, Huang S, Chen Y, Zheng H, Shen J, Lun ZR, Wang Y, Kasper LH, Lu F: Mast cells modulate
acute toxoplasmosis in murine models. PLoS One 2013;8:e77327.
https://doi.org/10.1371/journal.pone.0077327 |
| 109 | Ferreira GLS, Mineo JR, Oliveira JG, Ferro EA, Souza MA, Santos AA: Toxoplasma gondii and mast
cell interactions in vivo and in vitro: experimental infection approaches in Calomys callosus
(Rodentia, Cricetidae). Microbes Infect 2004;6:172-181.
https://doi.org/10.1016/j.micinf.2003.11.007 |
| 110 | Cruz A, Mendes ÉA, de Andrade MV, do Nascimento VC, Cartelle CT, Arantes RM, Melo JR, Gazzinelli
RT, Ropert C: Mast cells are crucial in the resistance against Toxoplasma gondii oral infection. Eur
J Immunol 2014;44:2949-2954.
https://doi.org/10.1002/eji.201344185 |
| 111 | Ben-Rashed M, Ingram GA, Pentreath VW: Mast cells, histamine and the pathogenesis of intestinal
damage in experimental Trypanosoma brucei brucei infections. Ann Trop Med Parasitol 2003;97:803-809.
https://doi.org/10.1179/000349803225002444 |
| 112 | Meuser-Batista M, Correa JR, Soares MJ, Henriques-Pons A: Isolation of cardiac mast cells in
experimental Trypanosoma cruzi infection. Tissue Cell 2008;40:309-316.
https://doi.org/10.1016/j.tice.2008.02.006 |
| 113 | Kannen V, Sakita JY, Carneiro ZA, Bader M, Alenina N, Teixeira RR, de Oliveira EC, Brunaldi MO,
Gasparotto B, Sartori DC, Fernandes CR, Silva JS, Andrade MV, Silva WA Jr, Uyemura SA, Garcia SB:
Mast cells and serotonin synthesis modulate Chagas disease in the colon: Clinical and experimental
evidence. Dig Dis Sci 2018;63:1473-1484.
https://doi.org/10.1007/s10620-018-5015-6 |
| 114 | Pires JG, Milanez MC, Pereira FE: Histamine levels in tissues of Trypanosoma cruzi-infected mice.
Agents Actions Suppl 1992;36:96-98.
|
| 115 | Postan M, Correa R, Ferrans VJ, Tarleton RL: In vitro culture of cardiac mast cells from mice
experimentally infected with Trypanosoma cruzi. Int Arch Allergy Immunol 1994;105:251-257.
https://doi.org/10.1159/000236765 |
| 116 | Martins PR, Nascimento RD, Lopes JG, Santos MM, de Oliveira CA, de Oliveira EC, Martinelli PM,
d'Ávila Reis D: Mast cells in the colon of Trypanosoma cruzi-infected patients: are they involved in
the recruitment, survival and/or activation of eosinophils? Parasitol Res 2015;114:1847-1856.
https://doi.org/10.1007/s00436-015-4371-9 |
| 117 | Wershil BK, Theodos CM, Galli SJ, Titus RG: Mast cells augment lesion size and persistence during
experimental Leishmania major infection in the mouse. J Immunol 1994;152:4563-4571.
https://doi.org/10.4049/jimmunol.152.9.4563 |
| 118 | Romão PR, Da Costa Santiago H, Ramos CD, De Oliveira CF, Monteiro MC, De Queiroz Cunha F, Vieira
LQ: Mast cell degranulation contributes to susceptibility to Leishmania major. Parasite Immunol
2009;31:140-146.
https://doi.org/10.1111/j.1365-3024.2008.01084.x |
| 119 | Sánchez-García L, Pérez-Torres A, Muñoz-Cruz S, Salaiza-Suazo N, Morales-Montor J, Becker I:
Mast-cell response to Leishmania mexicana and sand-fly salivary proteins is modulated by
orchiectomy. Pathogens 2022;11:398.
https://doi.org/10.3390/pathogens11040398 |
| 120 | Meuser-Batista M, Corrêa JR, Carvalho VF, de Carvalho Britto CF, Moreira OC, Batista MM, Soares
MJ, Filho FA, E Silva PM, Lannes-Vieira J, Silva RC, Henriques-Pons A: Mast cell function and death
in Trypanosoma cruzi infection. Am J Pathol 2011;179:1894-1904.
https://doi.org/10.1016/j.ajpath.2011.06.014 |
| 121 | Birck MM, Pors S, Johansen MV, Iburg T: Distribution of mast cells in relation to Schistosoma
japonicum induced lesions in pigs. Southeast Asian J Trop Med Public Health 2006;37:630-640.
|
| 122 | Vukman KV, Adams PN, Dowling D, Metz M, Maurer M, O'Neill SM: The effects of Fasciola hepatica
tegumental antigens on mast cell function. Int J Parasitol 2013;43:531-539.
https://doi.org/10.1016/j.ijpara.2013.01.011 |
| 123 | Dezfuli BS, Lui A, Boldrini P, Pironi F, Giari L: The inflammatory response of fish to helminth
parasites. Parasite 2008;15:426-433.
https://doi.org/10.1051/parasite/2008153426 |
| 124 | Novak M, Buchanan LG, Howlader H: Effects of cyclophosphamide and dexamethasone on mast cell
populations in Hymenolepis microstoma-infected mice. Parasitology 1990;100 Pt 2:337-343.
https://doi.org/10.1017/S0031182000061357 |
| 125 | Gulubova M, Vlaykova T, Kalinova K, Vodenicharov A, Vasilev I, Velev V: Liver tryptase-positive
mast cells and fibrosis in children with hepatic echinococcosis. J Indian Assoc Pediatr Surg
2005;10:237-243.
https://doi.org/10.4103/0971-9261.19273 |
| 126 | Park KY, Lee KJ, Kim IS, Yang EJ, Lim SJ, Lim BH, Ryang YS: Reaction of mast cells and goblet
cells in the small intestine of C57BL/6 and C3H/HeN mice infected with Echinostoma hortense. Korean
J Biomed Lab Sci 2005;11:259-266.
|
| 127 | Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, Andove J, Hightower AW, Karanja DM, Colley DG, Secor
WE: Higher percentages of circulating mast cell precursors correlate with susceptibility to
reinfection with Schistosoma mansoni. Am J Trop Med Hyg 2006;75:1053-1057.
https://doi.org/10.4269/ajtmh.2006.75.1053 |
| 128 | Mazingue C, Camus D, Dessaint JP, Capron M, Capron A: In vitro and in vivo inhibition of mast cell
degranulation by a factor from Schistosoma mansoni. Int Arch Allergy Immunol 1980;63:178-189.
https://doi.org/10.1159/000232624 |
| 129 | Coelho-Castelo AA, Panunto-Castelo A, Moreno AN, Dias-Baruffi M, Jamur MC, Oliver C,
Roque-Barreira MC, Rodrigues V: Sm60, a mannose-binding protein from Schistosoma mansoni with
inflammatory property. Int J Parasitol 2002;32:1747-1754.
https://doi.org/10.1016/S0020-7519(02)00211-4 |
| 130 | Trudgett A, Watt AP, Harriott P, Ennis M: Liver fluke (Fasciola hepatica)-derived peptides
activate rat peritoneal mast cells. Int Immunopharmacol 2003;3:775-781.
https://doi.org/10.1016/S1567-5769(03)00079-1 |
| 131 | Seifert B, Geyer E: Inhibition of in vitro and in vivo mast cell degranulation by Taenia
crassiceps metacestodes in vitro incubation products. Zentralbl Bakteriol 1989;271:521-531.
https://doi.org/10.1016/S0934-8840(89)80114-8 |
| 132 | Xian S, Chen L, Yan Y, Chen J, Yu G, Shao Y, Zhan B, Wang Y, Zhao L: Echinococcus multilocularis
calreticulin interferes with C1q-mediated complement activation. Trop Med Infect Dis 2023;8:47.
https://doi.org/10.3390/tropicalmed8010047 |
| 133 | Vukman KV, Adams PN, Metz M, Maurer M, O'Neill SM: Fasciola hepatica tegumental coat impairs mast
cells' ability to drive Th1 immune responses. J Immunol 2013;190:2873-2879.
https://doi.org/10.4049/jimmunol.1203011 |
| 134 | González MI, Lopes F, McKay DM, Reyes JL: Mast cell deficiency in mice results in biomass
overgrowth and delayed expulsion of the rat tapeworm Hymenolepis diminuta. Biosci Rep
2018;38:BSR20180687.
https://doi.org/10.1042/BSR20180687 |
| 135 | McLauchlan PE, Roberts HC, Loxton NJ, Wastling JM, Newlands GF, Chappell LH: Mucosal mast cell
responses and release of mast cell protease-I in infections of mice with Hymenolepis diminuta and H.
microstoma: modulation by cyclosporin A. Parasite Immunol 1999;21:151-161.
https://doi.org/10.1046/j.1365-3024.1999.00214.x |
| 136 | Yépez-Mulía L, Montaño-Escalona C, Fonseca-Liñán R, Muñoz-Cruz S, Arizmendi-Puga N, Boireau P,
Ortega-Pierres G: Differential activation of mast cells by antigens from Trichinella spiralis muscle
larvae, adults, and newborn larvae. Vet Parasitol 2009;159:253-257.
https://doi.org/10.1016/j.vetpar.2008.10.039 |
| 137 | Arizmendi-Puga NG, Enciso JA, Ortega-Pierres G, Zhao Z, Duszyk M, Ulanova M, Befus AD, Yépez-Mulia
L: Trichinella spiralis: histamine secretion induced by TSL-1 antigens from unsensitized mast cells.
Exp Parasitol 2006;114:67-76.
https://doi.org/10.1016/j.exppara.2006.02.016 |
| 138 | Donskow-Łysoniewska K, Maruszewska-Cheruiyot M, Krawczak-Wójcik K, Gonzalez JF, Hernández JN,
Stear MJ: Nematode galectin binds IgE and modulates mast cell activity. Vet Parasitol
2022;311:109807.
https://doi.org/10.1016/j.vetpar.2022.109807 |
| 139 | Melendez AJ, Harnett MM, Pushparaj PN, Wong WS, Tay HK, McSharry CP, Harnett W: Inhibition of
FcɛRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med
2007;13:1375-1381.
https://doi.org/10.1038/nm1654 |
| 140 | Wells PD: Mast cell, eosinophil and histamine levels in Nippostrongylus brasiliensis infected
rats. Exp Parasitol 1962;12:82-101.
https://doi.org/10.1016/S0014-4894(62)80002-2 |
| 141 | Befus AD, Johnston N, Bienenstock J: Nippostrongylus brasiliensis: mast cells and histamine levels
in tissues of infected and normal rats. Exp Parasitol 1979;48:1-8.
https://doi.org/10.1016/0014-4894(79)90048-1 |
| 142 | Ohnmacht C, Voehringer D: Basophils protect against reinfection with hookworms independently of
mast cells and memory Th2 cells. J Immunol 2010;184:344-350.
https://doi.org/10.4049/jimmunol.0901841 |
| 143 | Linnemann LC, Reitz M, Feyerabend TB, Breloer M, Hartmann W: Limited role of mast cells during
infection with the parasitic nematode Litomosoides sigmodontis. PLoS Negl Trop Dis 2020;14:e0008534.
https://doi.org/10.1371/journal.pntd.0008534 |
| 144 | McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK: Mast cells disrupt
epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A
2003;100:7761-7766.
https://doi.org/10.1073/pnas.1231488100 |
| 145 | Knight PA, Brown JK, Wright SH, Thornton EM, Pate JA, Miller HR: Aberrant mucosal mast cell
protease expression in the enteric epithelium of nematode-infected mice lacking the integrin αvβ6, a
transforming growth factor-β1 activator. Am J Pathol 2007;171:1237-1248.
https://doi.org/10.2353/ajpath.2007.061245 |
| 146 | Barrett KE, Neva FA, Gam AA, Cicmanec J, London WT, Phillips JM, Metcalfe DD: The immune response
to nematode parasites: modulation of mast cell numbers and function during Strongyloides stercoralis
infections in nonhuman primates. Am J Trop Med Hyg 1988;38:574-581.
https://doi.org/10.4269/ajtmh.1988.38.574 |
| 147 | Sasaki Y, Yoshimoto T, Maruyama H, Tegoshi T, Ohta N, Arizono N, Nakanishi K: IL-18 with IL-2
protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent
type 2 innate immunity. J Exp Med 2005;202:607-616.
https://doi.org/10.1084/jem.20042202 |
| 148 | Darmawi DBU, Hambal M, Frengki F, Priosoeryanto BP: Mucosal mast cells response in the jejunum of
Ascaridia galli-infected laying hens. Media Peternakan 2013;36:113-119.
https://doi.org/10.5398/medpet.2013.36.2.113 |
| 149 | Hepworth MR, Daniłowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M: Hartmann S: Mast cells
orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl
Acad Sci U S A 2012;109:6644-6649.
https://doi.org/10.1073/pnas.1112268109 |
| 150 | Alizadeh H, Murrell KD: The intestinal mast cell response to Trichinella spiralis infection in
mast cell-deficient w/wv mice. J Parasitol 1984;70:767-773.
https://doi.org/10.2307/3281760 |
| 151 | Carlos D, Machado ER, De Paula L, Sá-Nunes A, Sorgi CA, Jamur MC, Oliver C, Lima WT, Faccioli LH:
Evidence for eosinophil recruitment, leukotriene B4 production and mast cell hyperplasia following
Toxocara canis infection in rats. Braz J Med Biol Res 2011;44:319-326.
https://doi.org/10.1590/S0100-879X2011007500027 |
| 152 | Patrizi A, Virdi A, Neri I: Cutaneous mastocytosis exacerbated by pinworms in a young boy. Pediatr
Dermatol 2012;29:229-230.
https://doi.org/10.1111/j.1525-1470.2011.01530.x |
| 153 | Muhsin M, Ajendra J, Gentil K, Berbudi A, Neumann AL, Klaas L, Schmidt KE, Hoerauf A, Hübner MP:
IL-6 is required for protective immune responses against early filarial infection. Int J Parasitol
2018;48:925-935.
https://doi.org/10.1016/j.ijpara.2018.05.011 |
| 154 | Specht S, Frank JK, Alferink J, Dubben B, Layland LE, Denece G, Bain O, Förster I, Kirschning CJ,
Martin C, Hoerauf A: CCL17 controls mast cells for the defense against filarial larval entry. J
Immunol 2011;186:4845-4852.
https://doi.org/10.4049/jimmunol.1000612 |
| 155 | Arizmendi N, Yépez-Mulia L, Cedillo-Rivera R, Ortega-Pierres MG, Muñoz O, Befus D, Enciso-Moreno
JA: Interleukin mRNA changes in mast cells stimulated by TSL-1 antigens. Parasite 2001;8(2
Suppl):S114-S116.
https://doi.org/10.1051/parasite/200108s2114 |
| 156 | Shimokawa C, Kanaya T, Hachisuka M, Ishiwata K, Hisaeda H, Kurashima Y, Kiyono H, Yoshimoto T,
Kaisho T, Ohno H: Mast cells are crucial for induction of group 2 innate lymphoid cells and
clearance of helminth infections. Immunity 2017;46:863-874.e4.
https://doi.org/10.1016/j.immuni.2017.04.017 |
| 157 | Herbert DR, Douglas B, Zullo K: Group 2 innate lymphoid cells (ILC2): Type 2 immunity and helminth
immunity. Int J Mol Sci 2019;20:2276.
https://doi.org/10.3390/ijms20092276 |
| 158 | Ryan NM, Oghumu S: Role of mast cells in the generation of a T-helper type 2 dominated
anti-helminthic immune response. Biosci Rep 2019;39:BSR20181771.
https://doi.org/10.1042/BSR20181771 |