Knee Joint Response to Mechanical Loading: Bounding Mechanotransduction with Rehabilitation
bRehab Performance, Lublin, Poland,
cSports Research Center Nicolaus Copernicus University, Toruń, Poland,
dVolley Box, Gliwice, Poland,
eFaculty Medicine Ostrava University, Czech Republic,
fMedical Department of the Upper Silesian University in Katowice, Poland,
gChitomed, Poznań, Poland,
hFaculty of Medicine, Prince Mieszko I Poznan Medical University of Applied Sciences, Poznań, Poland
Keywords
Abstract
The knee joint is a weight-bearing structure that endures varied mechanical stresses in daily and athletic activities. Its cells convert these stresses into biochemical signals through mechanotransduction, prompting changes essential for joint health, repair, and adaptation. Understanding these processes is pivotal for developing rehabilitation strategies that address injuries and degenerative conditions like osteoarthritis. Different loading modalities—compression, tension, shear, and hydrostatic pressure—impact knee tissues (cartilage, synovium, ligaments, and tendons) and their resident cells (chondrocytes, synoviocytes, and fibroblasts). Chondrocytes adjust extracellular matrix synthesis to maintain cartilage integrity, while synoviocytes regulate synovial fluid components crucial for lubrication. Fibroblasts modulate collagen production, preserving ligament and tendon strength. Underlying these activities are key signaling pathways (e.g., MAPK, NF-κB, and Wnt) that regulate gene expression and cellular metabolism in response to mechanical stimuli. By linking basic mechanobiology insights to clinical practice, clinicians can tailor therapeutic interventions—such as controlled loading, exercise regimens, manual therapy, and orthotic devices—to optimize tissue repair, restore function, and prevent further degeneration. This mechanotransduction-focused approach offers a comprehensive framework for improving knee joint health and enhancing rehabilitation outcomes.
Introduction
This review aims to examine how mechanical loading affects the knee joint at the molecular and cellular levels, with particular emphasis on the pathways and factors regulating cartilage maintenance, synovial fluid composition, and structural integrity. By analyzing these mechanisms, the study seeks to establish a scientific foundation for developing precise rehabilitation programs that tailor loading conditions to individual patient needs. Consequently, the objective of this work extends beyond advancing knee joint biomechanics to translating research findings into clinical practice, accelerating treatment processes, preventing overuse injuries, and improving patient outcomes.
Review is based on an analysis of mechanotransduction mechanisms in various knee joint tissues, including cartilage, synovium, ligaments, and tendons. It explores different types of mechanical loading—compression, tension, shear, and hydrostatic pressure—and their structural and metabolic effects on joint tissues. The role of key mechanotransduction cells, such as chondrocytes in cartilage, synoviocytes in the synovium, and fibroblasts in ligaments and tendons, is discussed, highlighting their response to mechanical forces through receptors like integrins and ion channels. Furthermore, the study examines major signaling pathways, including MAPK, NF-κB, and Wnt, which regulate gene expression and cellular metabolism in response to mechanical stimuli.
The knee joint comprises the femur, tibia, and patella, along with cartilage, ligaments, tendons, and synovial fluid, each essential for movement, shock absorption, and weight bearing [1–3]. Articular cartilage coats the bone surfaces, reducing friction and distributing loads, while key ligaments (ACL, PCL, MCL, LCL) prevent excessive motion [4–5]. Tendons, such as the quadriceps and patellar, facilitate extension and flexion [6]. Meanwhile, synovial fluid—produced by the synovium—lubricates the joint, nourishes cartilage, and absorbs shock [7–8].
Mechanical loading involves compression, tension, shear, and hydrostatic forces acting on the knee during daily activities [9]. These forces stimulate tissue repair and regeneration, but excessive or abnormal loading may trigger damage, inflammation, and conditions like osteoarthritis [10–11]. Determining optimal loading conditions is thus critical for preserving knee function.
Mechanotransduction underlies how knee cells convert mechanical cues into biochemical responses [12]. Chondrocytes, synovial fibroblasts, and osteoblasts detect forces through mechanoreceptors such as integrins, primary cilia, and ion channels. These stimuli activate intracellular signaling cascades—including MAPK, NF-κB, and Wnt pathways—that regulate transcription factors like AP-1 and β-catenin [13–14]. In turn, this modulates genes for collagen, proteoglycans, and inflammatory mediators, orchestrating the remodeling of the extracellular matrix, controlling synovial fluid composition, and maintaining cartilage resilience. Conversely, aberrant loading escalates catabolic enzymes (e.g., matrix metalloproteinases), fueling cartilage breakdown and inflammation.
Harnessing mechanotransduction insights enables targeted rehabilitation to optimize tissue repair, minimize inflammation, and restore function [15–17]. Controlled loading exercises fine-tune mechanical stimuli, enhancing extracellular matrix synthesis without overloading the joint [18-20].
Incorporating these molecular and cellular principles into clinical practice supports personalized rehabilitation protocols that align with each patient’s unique mechanical environment [21–25]. Improved understanding of mechanotransduction can accelerate recovery, reduce chronic knee issues, and ultimately enhance quality of life for individuals with knee joint injuries or degenerative conditions.
A key focus of this work is bridging mechanobiology with clinical applications. The findings provide a basis for tailoring therapeutic interventions, such as controlled loading, exercise programs, manual therapy, and rehabilitation devices, to optimize tissue repair, restore function, and prevent further degeneration. By integrating cellular biology with biomechanics, this review establishes a comprehensive framework for rehabilitation strategies that enhance knee joint health and improve therapeutic outcomes.
Mechanotransduction in the Knee Joint
Mechanotransduction in the knee joint involves mechanoreceptors, ion channels, and signaling pathways [26, 27]. This process converts mechanical stimuli into biochemical signals essential for joint health, tissue repair, and load adaptation (Fig. 1) [28]. The primary cells involved are chondrocytes (in cartilage), synoviocytes (in the synovium), and fibroblasts (in ligaments and tendons). These cells detect mechanical cues largely through integrins and stretch-activated ion channels, which couple extracellular forces to intracellular cascades.
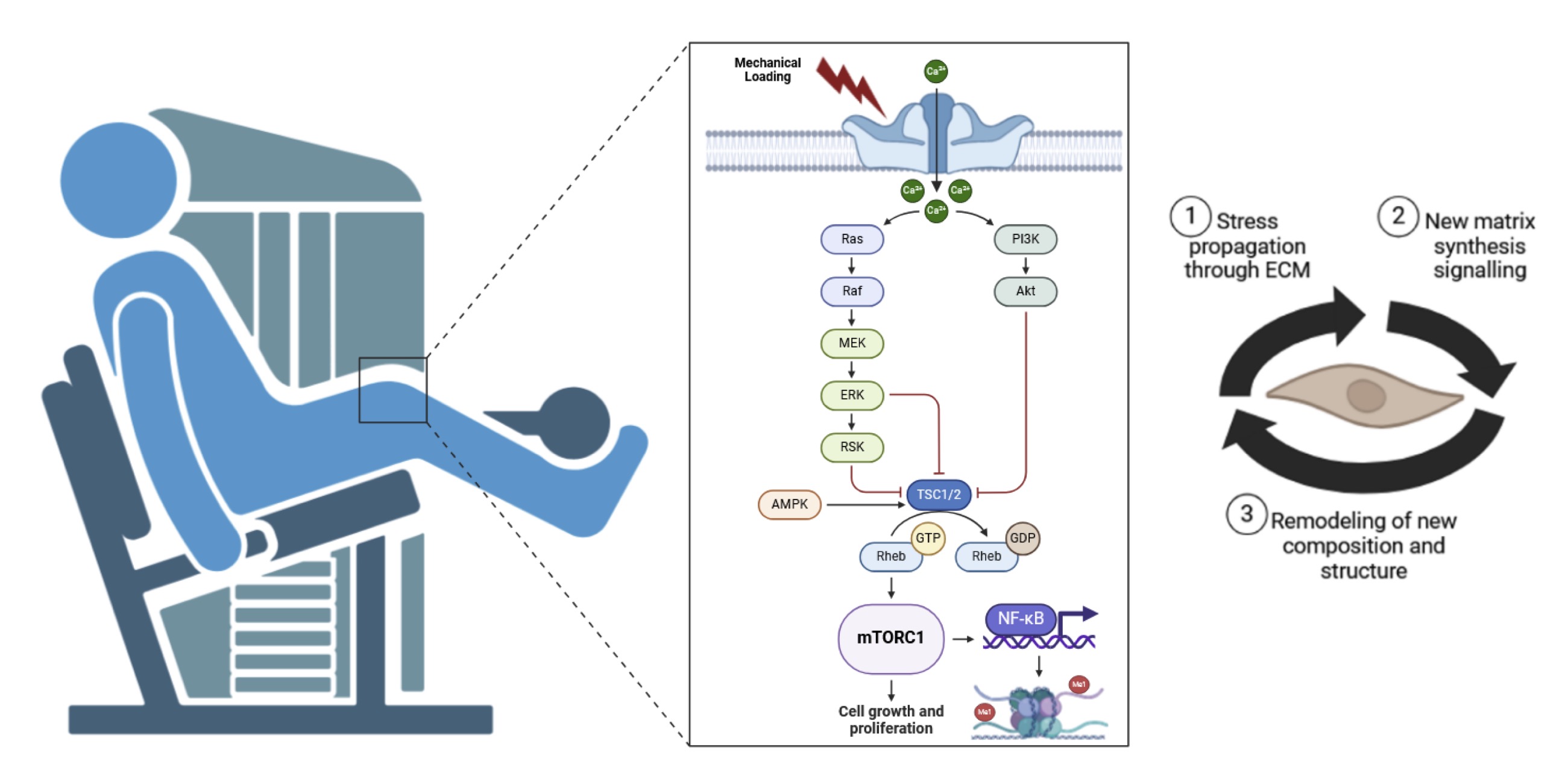
Fig. 1: The Fig. illustrates the process of mechanotransduction, depicting how mechanical loading leads to changes in the extracellular matrix (ECM) and ultimately results in sustained or improved function. The sequence begins with mechanical loading (1) due to mechanical loading in rehabilitation process. This mechanical load propagates stress through the ECM from macro to micro scales. The ECM then interacts with cells through mechanotransduction (2), converting the mechanical signals into cellular responses. These signals induce new matrix synthesis and the degradation of damaged matrix components. The ECM undergoes incorporation and remodeling of new composition and structure (3), leading to sustained or improved function of the tissue. The diagram highlights the dynamic interplay between mechanical forces and cellular responses in maintaining tissue health and function).
Chondrocytes reside in the avascular cartilage, where they depend on mechanical loading to facilitate nutrient diffusion and waste removal [29, 30]. Integrins on the chondrocyte surface bind ECM components (e.g., collagen, fibronectin), transmitting mechanical signals to the cytoskeleton and triggering mechanosensitive ion channel activation. This engagement launches several key intracellular pathways—most notably Mitogen-Activated Protein Kinase (MAPK), Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB), and Wnt signaling [31, 32]. MAPK includes the extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38, each controlling distinct aspects of gene expression and protein synthesis tied to cartilage maintenance [33].
On a molecular level, integrin clustering under load activates focal adhesion kinases (FAKs), which can
phosphorylate MAPK components, thus relaying mechanical signals to the nucleus [34]. ERK often promotes
anabolic
functions, such as collagen II or aggrecan synthesis, whereas p38 and JNK can accelerate catabolic
processes,
including matrix metalloproteinase (MMP) expression. NF-κB, central to inflammation and cell survival, is
typically held inactive by IκB proteins that sequester it in the cytoplasm; mechanical stress can activate
IκB
kinase (IKK), allowing NF-κB to translocate to the nucleus and regulate cytokine or MMP transcription
[36].
Simultaneously, canonical Wnt signaling involves the stabilization and nuclear translocation of β-catenin,
which
promotes genes critical for cartilage repair [37]. These three pathways show significant crosstalk: for
example,
p38 or JNK activity can enhance IKK-mediated NF-κB activation, while moderate ERK signaling can cooperate
with
Wnt/β-catenin to drive anabolic gene programs [38, 39]. Balancing these signals under physiologic loading
maintains tissue homeostasis; excessive or abnormal forces push the system toward degenerative
outcomes.
Synoviocytes line the knee’s synovial membrane, producing synovial fluid that lubricates the joint,
reduces
friction, and supplies nutrients to chondrocytes [40]. Mechanical loading activates integrins and
stretch-sensitive ion channels on synoviocytes, leading to increased synthesis of hyaluronic acid and
lubricin,
two critical components for joint lubrication [41]. On a molecular scale, hyaluronan synthase catalyzes
hyaluronic acid production and is upregulated by mechanically induced MAPK phosphorylation events. NF-κB
modulates the balance between pro- and anti-inflammatory signals; in mild or moderate activation states,
synoviocytes secrete anti-inflammatory cytokines that protect joint tissues, whereas excessive NF-κB
stimulation
drives inflammatory cascades [42].
Wnt/β-catenin signaling also influences synoviocyte behavior, potentially regulating cell proliferation and cytokine profiles. Dysregulation of Wnt may promote synovial hyperplasia or exacerbate inflammation. Another relevant pathway is the mechanistic target of rapamycin (mTOR), which can interact with Wnt and NF-κB to fine-tune lubricin production and immune modulation. Proper mechanical cues thus ensure adequate synovial fluid properties, preventing cartilage wear while minimizing pathological inflammation [43, 44].
Fibroblasts populate ligaments and tendons, providing structural support and transmitting muscular forces to bones. Mechanotransduction in fibroblasts involves integrin clustering at focal adhesions, actin cytoskeleton remodeling, and activation of MAPK and Transforming Growth Factor-beta (TGF-β) signaling [45]. Under normal loads, fibroblasts maintain collagen fiber alignment and ECM turnover, conferring the tensile strength and elasticity required for joint stability [46][47].
Research from Feng R. et al., [48] investigates how mechanical loading affects subchondral bone remodeling and its impact on cartilage degradation in knee osteoarthritis (OA). The authors identify RANKL (Receptor Activator of Nuclear Factor Kappa-Β Ligand) as a key mechanotransduction mediator that influences osteoclast activity under compression forces. The research reveals that hydrostatic pressure modulates Wnt/β-catenin signaling, which regulates bone metabolism and cartilage integrity. The study suggests that targeted modulation of RANKL signaling via controlled mechanical loading could serve as a novel therapy for knee OA.
Another study from Nims R. et al., [49] explores how mechanosensitive ion channels TRPV4 and PIEZO1 mediate chondrocyte mechanotransduction in the knee joint. The authors demonstrate that mechanical stimulation increases Ca²⁺ influx through TRPV4, which activates the MAPK/ERK1/2 pathway, leading to collagen type II synthesis—a crucial factor in cartilage maintenance. Meanwhile, PIEZO1 signaling triggers downstream YAP/TAZ activation, which influences chondrocyte proliferation and differentiation. The findings suggest that altering PIEZO1 and TRPV4 activity can enhance chondrocyte survival and cartilage regeneration, providing potential therapeutic targets for knee injuries.
Collectively, MAPK, NF-κB, and Wnt signaling converge at multiple checkpoints in chondrocytes, synoviocytes, and fibroblasts, shaping anabolic or catabolic responses based on the magnitude and duration of mechanical input. In moderate loading regimes, ERK and β-catenin support ECM synthesis, lubricin production, and balanced inflammatory responses. Under high or aberrant loads, p38/JNK and NF-κB activities predominate, enhancing inflammatory mediators and MMP-driven breakdown.
1. Chondrocytes and Cartilage
Cartilage is an avascular tissue, meaning it depends on mechanical loading to facilitate nutrient
diffusion and
waste removal [50]. Chondrocytes, the sole cellular component of healthy cartilage, detect mechanical cues
via
integrins and mechanosensitive ion channels, which connect extracellular forces to cytoskeletal changes
and
intracellular signaling cascades [51, 52]. Once activated, these receptors stimulate pathways including
MAPK
(Mitogen-Activated Protein Kinase), NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B
cells), and
Wnt, coordinating gene transcription that governs cell survival, differentiation, and matrix homeostasis
[53].
The MAPK pathway transmits signals from the cell surface to the nucleus through phosphorylation cascades
involving ERK, JNK, and p38 MAPKs, each responding to specific stress stimuli [54, 55]. ERK generally
supports
chondrocyte proliferation and differentiation, while JNK and p38 mediate stress and inflammatory responses
that
can trigger apoptosis if overactivated [56]. In chondrocytes, MAPK signaling enhances synthesis of
extracellular
matrix (ECM) proteins such as type II collagen and proteoglycans, including aggrecan, which confers
compressive
strength by binding water molecules [57–59].
On a deeper molecular level, integrin engagement activates focal adhesion kinases (FAKs), which
phosphorylate
intermediates like MEK (MAPK/ERK kinase). MEK then phosphorylates ERK, driving nuclear translocation of
transcription factors that upregulate cartilage-specific genes [34, 55]. In contrast, p38 and JNK often
increase
levels of matrix metalloproteinases (MMPs) or inflammatory mediators, tipping the balance toward
catabolism when
stress is excessive.
NF-κB orchestrates inflammatory and stress responses, regulating genes tied to matrix remodeling, cell
survival,
and apoptosis [60]. In chondrocytes, NF-κB activation boosts production of MMPs and aggrecanases that
degrade
the cartilage matrix, countered by tissue inhibitors of metalloproteinases (TIMPs) [61, 62]. An imbalance
favoring catabolic enzymes facilitates cartilage breakdown, a hallmark of osteoarthritis [63].
Mechanistically,
signals from integrins or toll-like receptors activate IκB kinase (IKK), phosphorylating IκB to liberate
NF-κB,
which then translocates to the nucleus to upregulate pro-inflammatory genes.
The Wnt pathway is another major regulator of chondrocyte function, modulating proliferation,
differentiation,
and ECM synthesis [64, 65]. Wnt ligands bind Frizzled receptors and LRP5/6 co-receptors, stabilizing
β-catenin
and promoting its nuclear accumulation. Once inside the nucleus, β-catenin forms transcriptional complexes
that
control anabolic gene expression [66]. Proper Wnt activity prevents premature chondrocyte hypertrophy,
which can
lead to calcification if unregulated [67]. TGF-β and BMP signaling often converge with Wnt, adding further
layers of control over cartilage growth and remodeling.
Type II collagen forms the tensile framework of cartilage, while large proteoglycans such as aggrecan
confer
resistance to compression by retaining water [68–70]. Minor collagens (e.g., types IX and XI) and
non-collagenous proteins (e.g., COMP) integrate into this network, ensuring biomechanical integrity [71,
72].
Balanced synthesis and degradation of these ECM components is key for homeostasis and repair [73, 74].
Excessive
or insufficient loading perturbs this equilibrium, driving degenerative changes characteristic of
osteoarthritis
[75, 76].
Besides MAPK, NF-κB, and Wnt, calcium signaling also underlies mechanotransduction in chondrocytes [77,
78].
Mechanical stretch or compression opens mechanosensitive channels, increasing intracellular Ca²⁺ levels
that
activate kinases (e.g., CaMKII) or phosphatases, further modulating transcription factor activity [79].
Cartilage’s low-oxygen milieu makes hypoxia-inducible factors (HIFs) pivotal for energy metabolism and ECM
production, particularly HIF-1α, which supports the chondrocyte phenotype under reduced oxygen tension
[78].
Growth factors within the ECM—such as TGF-β, BMPs, IGF-1, and FGFs—bind chondrocyte receptors to drive
collagen
and proteoglycan synthesis, maintaining cartilage stability.
Study from Matheson D. et al., [80] explores how PIEZO1, a mechanosensitive ion channel, mediates
chondrocyte
responses to mechanical loading in the human knee joint. Increased mechanical stress activates PIEZO1,
leading
to elevated Ca²⁺ influx in chondrocytes, which affects intracellular signaling cascades. This study finds
that
OA-associated PIEZO1 genetic variants exhibit altered conductance properties, resulting in hyperactivation
under
normal loading conditions. Overactivation of PIEZO1 leads to excessive calcium signaling, triggering
downstream
pathways such as YAP/TAZ, MAPK, and NF-κB, which promote cartilage matrix degradation and inflammation.
The
findings suggest that targeting PIEZO1 activity could be a potential strategy to modulate chondrocyte
mechanotransduction and slow osteoarthritis progression.
Mechanical loading is crucial for cartilage health [74]. Low-magnitude cyclic loading promotes ECM
synthesis and
chondrocyte activity, whereas excessive loading induces inflammation and degradation. Satic loading can
lead to
matrix breakdown by disrupting cellular homeostasis. Optimized rehabilitation strategies incorporating
controlled mechanical stimuli can enhance cartilage repair and prevent degenerative joint diseases (Table
1).
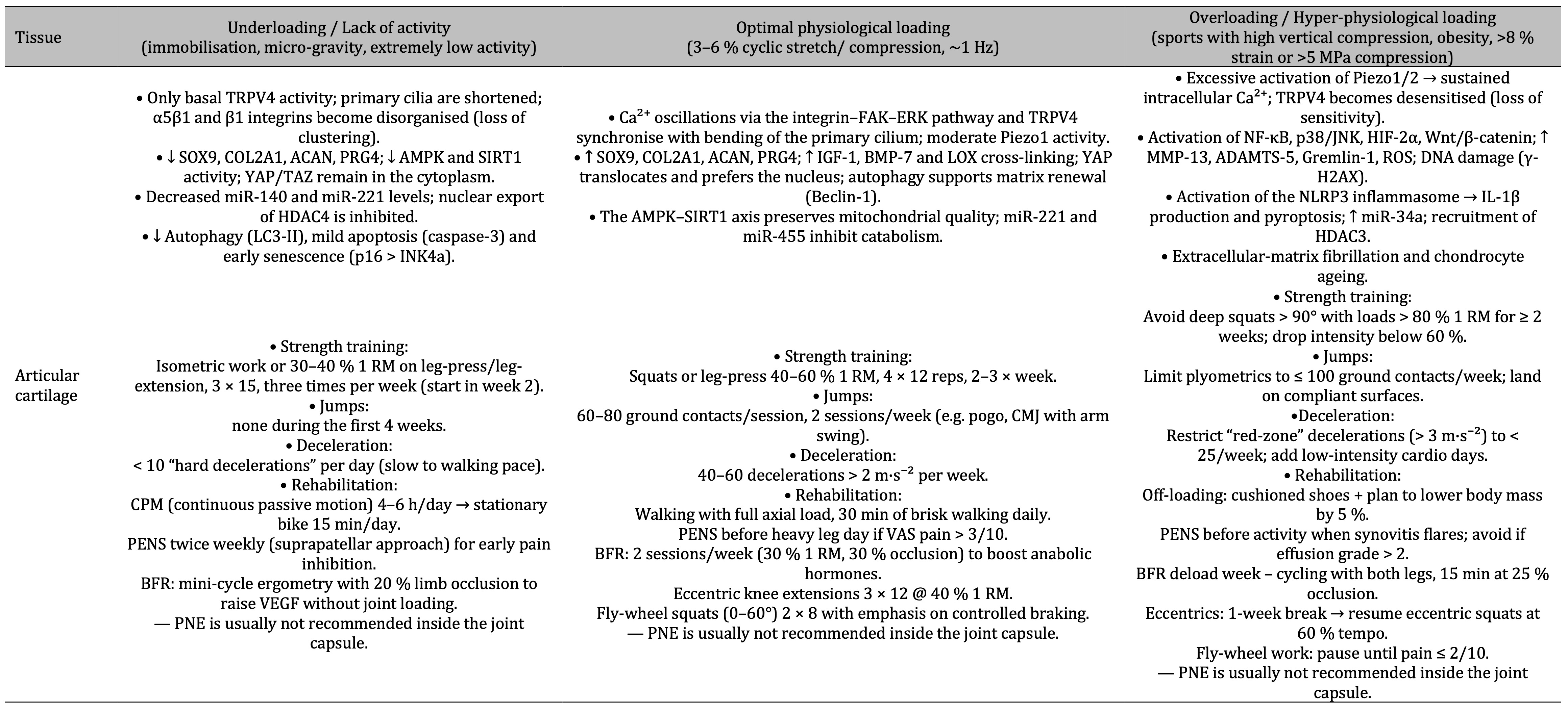
Table 1: The table shows that under-loading of articular cartilage induces catabolic signalling and early degeneration, optimal physiological loading engages coordinated Ca²⁺-integrin-TRPV4 pathways that foster anabolic matrix renewal, while over-loading hyperactivates Piezo1 and inflammatory NF-κB/MAPK cascades leading to tissue breakdown, with specific strength-training, plyometrics, deceleration and rehabilitation guidelines prescribed for each condition
2. Synoviocytes and Synovial Fluid
Synoviocytes are specialized cells in the synovium, a membrane lining the joint capsule that produces
synovial
fluid [81]. This fluid lubricates the joint, reduces friction, and provides nutrients to avascular
cartilage
[82]. Mechanical loading activates synoviocytes via mechanosensitive receptors like integrins and ion
channels,
stimulating the production of synovial fluid components, particularly hyaluronic acid and lubricin
[83].[84]
Hyaluronic acid, a high molecular weight glycosaminoglycan, enhances synovial fluid viscosity and forms a
viscoelastic network that absorbs mechanical shocks [85].[86] Its synthesis is regulated by cytokines and
growth
factors such as TGF-β and PDGF, while pro-inflammatory cytokines like IL-1 and TNF-α inhibit its
production,
reducing joint lubrication [87].[88] Lubricin, also known as PRG4, minimizes friction between cartilage
surfaces
by forming a slippery layer on the articular cartilage [89].[90] Its expression is upregulated by
mechanical
stimuli and biochemical signals, including TGF-β and IL-4 [91].[92]
Synoviocytes include fibroblast-like synoviocytes (FLS), responsible for synovial fluid production, and
macrophage-like synoviocytes (MLS), which regulate inflammation and tissue repair [93].[94] Mechanical
loading
activates intracellular pathways such as MAPK, NF-κB, and PI3K/Akt, governing cellular responses [95]. The
MAPK
pathway, through ERK, JNK, and p38 kinases, regulates synovial fluid component synthesis, while NF-κB
modulates
inflammatory responses, and PI3K/Akt influences cell survival and metabolism [96].[97]
Synoviocytes interact with joint microenvironment signals, including cytokines and extracellular matrix
(ECM)
components like collagen, fibronectin, and laminin [98]. Integrins mediate cell-ECM attachment,
transducing
signals for adhesion, migration, and differentiation. Disrupting these interactions alters synoviocyte
function
and contributes to joint pathology [99]. Epigenetic mechanisms, including DNA methylation, histone
modifications, and miRNAs, regulate genes involved in synovial fluid production and inflammation [100].
Extracellular vesicles (EVs) from synoviocytes transport bioactive molecules, modulating inflammatory
responses
and cartilage metabolism, making them potential therapeutic targets [101].
MLS play a key role in immune response, producing cytokines that recruit immune cells. While crucial for
infection defense and inflammation resolution, dysregulated responses contribute to chronic inflammation
and
joint damage in autoimmune conditions like rheumatoid arthritis [93]. Understanding synoviocyte signaling
and
molecular interactions provides insight into joint disease pathophysiology and therapeutic targets for
improving
lubrication, reducing inflammation, and promoting cartilage repair [94].
Study from Schröder A. et al., [102] investigates how mechanical loading influences synoviocyte behavior
and
synovial fluid composition in the knee joint. Synovial fibroblasts (SFs), a key component of the synovium,
respond to mechanical stress by activating mechanotransduction pathways, notably YAP/TAZ and NF-κB,
leading to
inflammatory signaling. This study found that excessive mechanical stress upregulates pro-inflammatory
cytokines
(IL-6, IL-8, TNF-α) in SFs, leading to synovial inflammation and cartilage degradation. In contrast,
moderate
mechanical loading promotes the secretion of lubricin (PRG4) and hyaluronic acid, enhancing synovial fluid
viscosity and joint lubrication. The results suggest that targeting SF activation through mechanical
modulation
could help balance synovial homeostasis and prevent osteoarthritis progression.
Mechanical loading regimes significantly affect synoviocyte activity. Cyclic compressive loading enhances
anabolic responses, stimulating synovial fluid production, while excessive static or shear loading
promotes
catabolic pathways, contributing to joint degradation. Optimizing mechanical loading strategies is
essential for
maintaining joint health and informing rehabilitation protocols (Table 2).
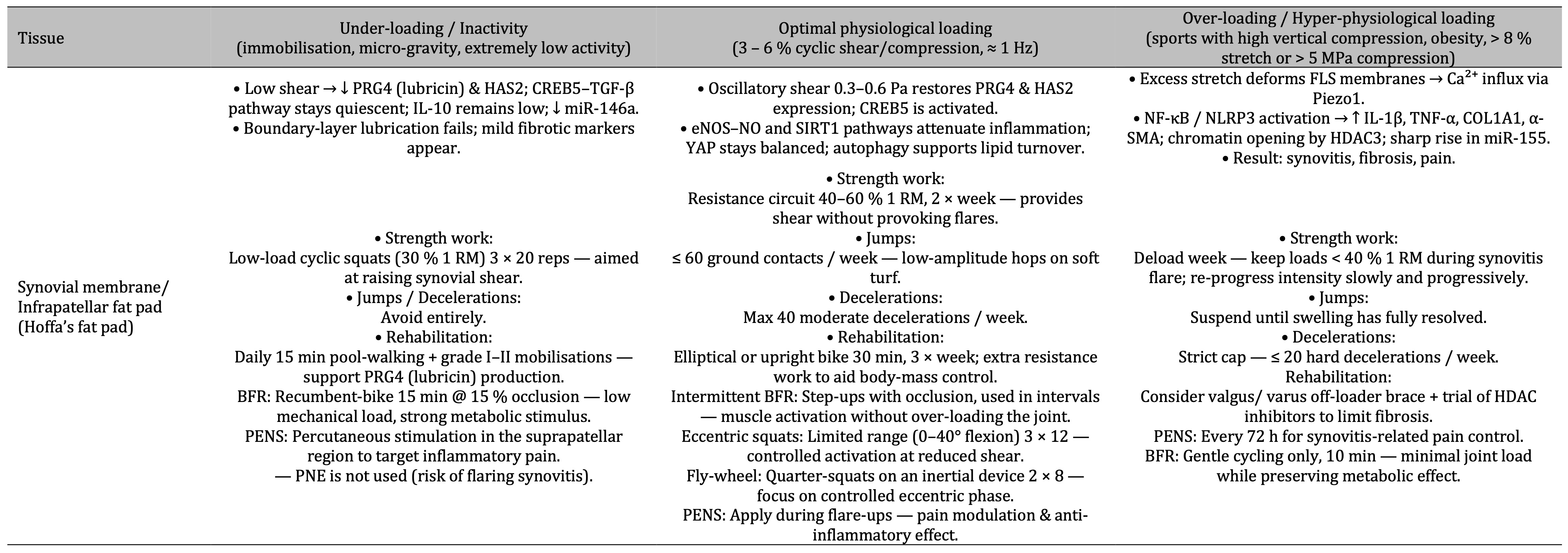
Table 2: The table shows that in the synovial membrane (Hoffa’s fat pad) low shear suppresses lubricin (PRG4) and HAS2, physiological cyclic shear restores these lubricating and anti-inflammatory pathways, while excessive stretch triggers Piezo1-driven Ca²⁺ influx and NF-κB/NLRP3 inflammation leading to synovitis, fibrosis and pain, with corresponding progressive guidelines for strength work, jumps/decelerations and rehabilitation at each loading level
3. Fibrochondrocytes and Meniscus
The menisci are fibrocartilaginous structures within the knee joint, essential for load distribution,
shock
absorption, and joint stability [102]. They consist of a dense extracellular matrix (ECM) primarily
composed of
collagen and proteoglycans, ensuring both strength and flexibility [103]. Fibrochondrocytes within the
meniscus
regulate ECM composition through mechanotransduction, responding to mechanical stimuli by modulating ECM
synthesis to adapt to varying mechanical stresses [104].[105]
The ECM mainly consists of type I collagen for tensile strength and type II collagen for compressive
resistance
[106]. Proteoglycans, particularly aggrecan, help retain water, enhancing shock absorption [107]. The ECM
exhibits an anisotropic organization, with collagen fibers arranged to resist multidirectional loads,
reflecting
the knee joint's complex mechanical environment [108].
Mechanotransduction in fibrochondrocytes relies on mechanoreceptors such as integrins and mechanosensitive
ion
channels [109]. These receptors activate intracellular signaling pathways, including MAPK, NF-κB, and Wnt,
influencing gene expression and ECM synthesis [110].[111] MAPK pathways—ERK, JNK, and p38—mediate
responses to
mechanical stimuli; ERK promotes cell proliferation, while JNK and p38 regulate stress responses and
apoptosis
[112].[113] In the meniscus, MAPK signaling modulates collagen and proteoglycan synthesis, maintaining
biomechanical integrity [114].
NF-κB signaling is primarily associated with inflammatory regulation in fibrochondrocytes [115].
Mechanical
loading modulates NF-κB activity, affecting cytokine and matrix metalloproteinase (MMP) expression, which
governs ECM remodeling [116].[117] A balanced NF-κB response ensures ECM homeostasis, crucial for meniscal
maintenance and repair [118].
Wnt signaling regulates fibrochondrocyte proliferation, differentiation, and ECM production [119].[120] It
interacts with TGF-β and BMP pathways to coordinate cellular responses to mechanical stimuli [121]. This
cross-talk ensures fibrochondrocytes adapt to mechanical stress, preserving meniscal functionality [122].
Intracellular calcium (Ca²⁺) signaling plays a vital role in mechanotransduction [123]. Mechanosensitive
ion
channels mediate Ca²⁺ influx, acting as secondary messengers that activate kinases and phosphatases,
further
influencing transcription factors involved in ECM organization [124].[125]
Meniscal vascularization impacts its healing capacity [126-128]. Peripheral regions contain blood vessels
and
nerves for nutrient supply and sensory feedback, whereas central regions are avascular, relying on
synovial
fluid diffusion [129].[130] Consequently, peripheral tears have better healing potential than central ones
[131].
Additional ECM components like fibronectin, elastin, and decorin contribute to meniscal biomechanics.
Fibronectin aids in cell adhesion and tissue repair, elastin enables shape recovery post-deformation, and
decorin regulates collagen fibrillogenesis and ECM assembly.
Meniscal degeneration, as seen in osteoarthritis, results from disrupted ECM synthesis and degradation
balance
[124]. Elevated catabolic enzyme activity, such as MMPs and aggrecanases, accelerates collagen and
proteoglycan
breakdown. Pro-inflammatory cytokines like IL-1β and TNF-α further enhance catabolic pathways while
suppressing
anabolic pathways, worsening degeneration [129].
Study from Ma Z. et al., [132] examines how fibrochondrocytes in the human meniscus respond to altered
mechanical loading conditions, particularly simulated microgravity, which mimics unloading stress similar
to
prolonged bed rest or space travel. The researchers found that mechanical unloading leads to
downregulation of
mechanotransduction pathways, including FAK (Focal Adhesion Kinase) and YAP/TAZ, which are essential for
maintaining meniscus homeostasis. A reduction in mechanical stimulation suppresses extracellular matrix
(ECM)
synthesis, particularly collagen type I, collagen type II, and aggrecan, which are critical for meniscus
integrity [131]. Unloading also increases oxidative stress and apoptosis in fibrochondrocytes, mediated by
the
activation of ROS (reactive oxygen species) and caspase-3/7 pathways, leading to cellular senescence and
tissue
degeneration [129]. The study highlights the importance of maintaining optimal mechanical loading to
prevent
meniscus degeneration, which is crucial for knee joint health.
Mechanical loading regimes significantly influence meniscal cell behavior and ECM maintenance. Cyclic
compressive loading promotes ECM synthesis and chondroprotective responses, whereas excessive static or
shear
loading induces catabolic activity and accelerates degeneration. Optimal loading strategies are crucial
for
rehabilitation and tissue engineering applications, aiming to balance anabolic and catabolic processes for
sustained meniscal health and functionality (Table 3).
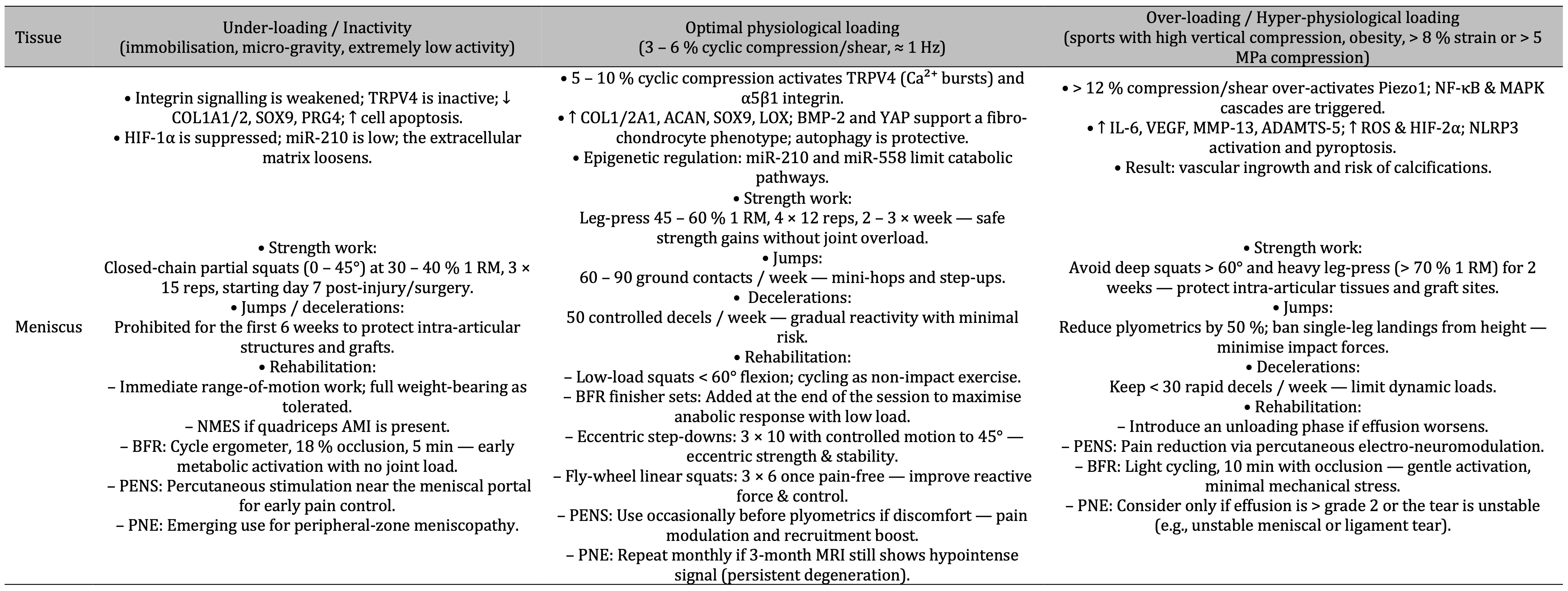
Table 3: Under-loading weakens integrin-TRPV4 signalling, suppresses matrix genes and loosens the meniscus, optimal 5–10 % cyclic compression re-engages TRPV4/α5β1 pathways for anabolic repair, while > 12 % compression overstimulates Piezo1 and NF-κB/MAPK inflammation that drives vascular ingrowth and calcification—each state paired with tailored strength, plyometric, deceleration and rehabilitation guidelines
4. Fibroblasts and Ligaments/Tendons
Fibroblasts are the primary cells in ligaments and tendons, tissues that connect bones and muscles,
playing
essential roles in knee joint stability and movement [133]. These cells regulate collagen synthesis and
matrix
remodeling in response to mechanical loading via mechanotransduction mechanisms involving integrins, focal
adhesion complexes, and mechanosensitive ion channels [134]. Activation of these structures initiates
intracellular signaling pathways such as MAPK and TGF-β, crucial for collagen fiber production and
organization,
ensuring tissue strength and elasticity [135].
Type I collagen, produced by fibroblasts, dominates the extracellular matrix (ECM) of ligaments and
tendons,
forming parallel bundles that provide tensile strength and resistance to mechanical forces [136]. Its
synthesis
is tightly regulated by mechanical stimuli, with integrins acting as transmembrane receptors that sense
stress
and initiate intracellular signaling cascades [137]. When fibroblasts undergo mechanical loading,
integrins
cluster into focal adhesions, sites for biochemical signal transduction involving focal adhesion kinase
(FAK)
and Src family kinases [138].
The MAPK pathway, comprising ERK, JNK, and p38 MAPK, plays key roles in fibroblast responses to mechanical
stimuli [139]. ERK promotes proliferation and collagen synthesis, while JNK and p38 MAPK mediate stress
responses and inflammation [140]. These pathways regulate transcription factors that control ECM
production and
remodeling [141]. TGF-β signaling further modulates fibroblast function by enhancing collagen synthesis
and
regulating matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs),
maintaining ECM
homeostasis [142].
Mechanosensitive ion channels, such as Piezo1 and Piezo2, contribute to mechanotransduction by mediating
calcium
(Ca²⁺) influx, which acts as a secondary messenger in kinase activation, including CaMK and PKC [143].
These
kinases influence transcription factors involved in collagen synthesis and fibroblast proliferation,
fine-tuning
cellular responses to mechanical stress [144-145].
Mechanical loading is essential for maintaining ligament and tendon function, ensuring joint stability and
efficient force transmission [146-148]. However, excessive or abnormal loading can lead to microtears,
inflammation, and tendinopathy, conditions associated with disrupted ECM balance [149]. Hypoxia-inducible
factors (HIFs), particularly HIF-1α, help fibroblasts adapt to the relatively avascular environment of
ligaments
and tendons by upregulating angiogenesis, ECM production, and metabolic adaptation genes [150].
ECM stiffness significantly influences fibroblast behavior, impacting differentiation, proliferation, and
apoptosis through integrin-mediated signaling cascades, including RhoA/ROCK and YAP/TAZ [151]. Fibroblasts
also
produce and respond to growth factors such as FGF, PDGF, and interleukins, which modulate cell
proliferation,
migration, and matrix synthesis, crucial for tissue repair and regeneration [152].[153]
Study from Stańczak M. et al., [154] explores how fibroblasts within knee ligaments and tendons respond to
mechanical loading through mechanotransduction pathways. Mechanical strain activates integrin-mediated FAK
(Focal Adhesion Kinase) signaling, leading to downstream activation of the MAPK/ERK and PI3K/Akt pathways,
which
regulate cell proliferation and extracellular matrix remodeling.The research identifies that cyclic
mechanical
loading enhances fibroblast alignment and increases collagen type I and III synthesis, which is crucial
for
tendon and ligament remodeling. However, excessive mechanical loading leads to an upregulation of MMP-1
and
MMP-13 (matrix metalloproteinases), promoting collagen degradation and increasing the risk of injury. The
findings emphasize that controlled mechanical loading is essential for maintaining the balance between
fibroblast-mediated collagen synthesis and degradation, supporting tendon and ligament repair.
Mechanical loading regimes are crucial for optimizing tissue repair and regeneration. Low-magnitude cyclic
loading enhances fibroblast proliferation and collagen deposition, while excessive or static loading can
induce
matrix degradation and inflammation [155]. Regulating loading parameters, including frequency, amplitude,
and
duration, is essential for promoting tissue adaptation and minimizing injury risk [156]. These insights
contribute to developing targeted rehabilitation strategies and regenerative therapies for ligament and
tendon
injuries [157] (Table 4) (Table 5).
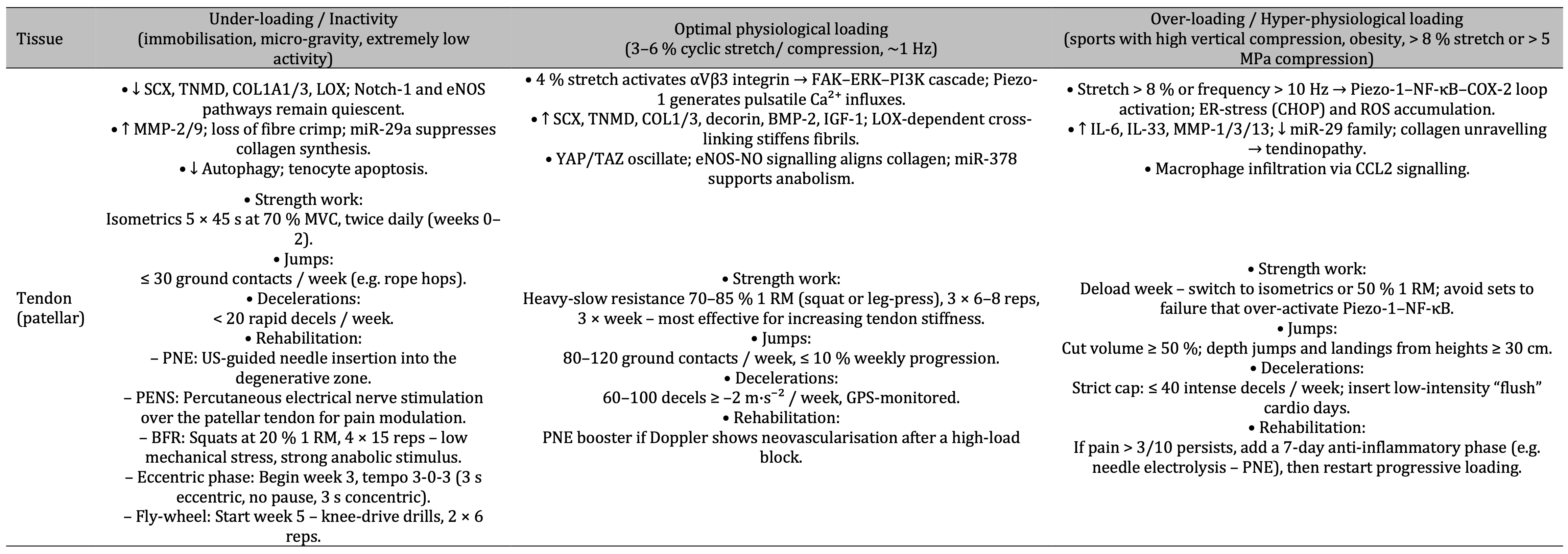
Table 4: Under-loading of the patellar tendon suppresses collagen-building genes and increases matrix breakdown, optimal 4 % cyclic stretch triggers αVβ3–FAK–ERK signalling that boosts anabolic collagen cross-linking, while > 8 % stretch or high-frequency loading hyperactivates Piezo1–NF-κB–COX-2 pathways causing inflammatory tendinopathy—each state matched to specific strength, jump, deceleration and rehab guidelines
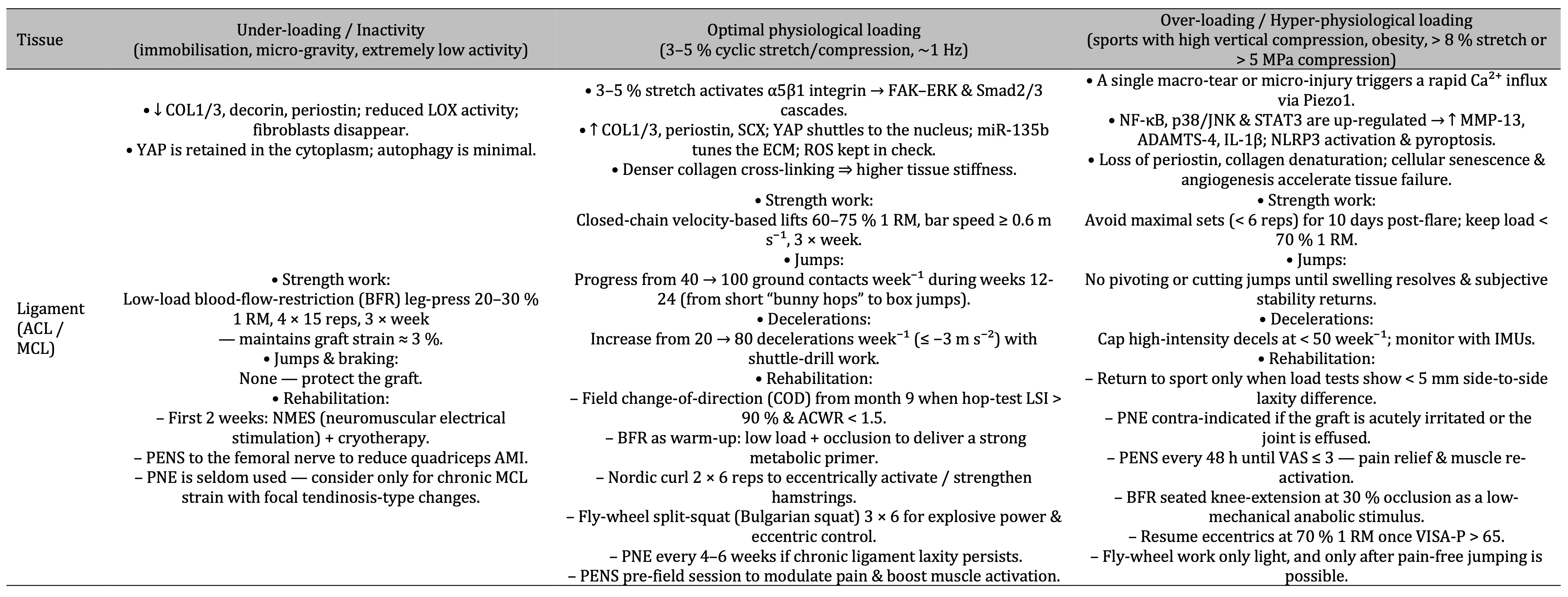
Table 5: Under-loading of ACL/MCL ligaments suppresses collagen synthesis and cell activity, optimal 3–5 % cyclic stretch activates α5β1-integrin FAK/ERK-Smad signalling that densifies collagen and increases stiffness, while over-loading triggers Piezo1-driven Ca²⁺ influx and NF-κB/p38/STAT3 inflammation that accelerates matrix breakdown—each state matched to distinct strength, plyometric, deceleration and rehab guidelines
Mechanical Loading Modalities and Their Effects in Molecular Biology Context
Different mechanical loading types—compression, tension, shear, and hydrostatic pressure—affect knee joint tissues at macroscopic and molecular levels [183]. Understanding these effects is essential for optimizing rehabilitation protocols.
On a molecular level, mechanical loading regulates gene expression, protein synthesis, and signaling pathways within knee joint tissues [184]. Compression loading stimulates chondrocytes to produce extracellular matrix components such as collagen and proteoglycans, vital for cartilage integrity [185]. This loading activates mechanotransduction pathways involving integrins and the cytoskeleton, leading to transcriptional regulation by NF-κB and AP-1 [186]. Additionally, compression enhances anabolic factors like IGF-1 and TGF-β, promoting cartilage repair [187].
Tension loading, prevalent in tendons and ligaments, enhances tensile strength by increasing collagen synthesis [188]. It activates mechanosensitive ion channels and MAPK signaling, upregulating structural proteins and ECM remodeling enzymes [189]. Tension also modulates MMPs and TIMPs, balancing matrix turnover [190].
Shear stress, occurring during knee joint movement, affects endothelial cells and nitric oxide production, influencing vascular tone and inflammatory responses [191]. Shear-responsive genes such as eNOS and COX-2 regulate inflammation and angiogenesis through VEGF expression, supporting blood supply to joint tissues [192].
Hydrostatic pressure regulates synoviocyte behavior and synovial fluid composition, affecting joint lubrication and nutrient supply [193]. This pressure modulates ion channels, aquaporins, and fluid homeostasis genes, maintaining osmotic balance in the joint cavity [194, 195].
Mechanical loading also influences inflammatory pathways [196]. Compression suppresses pro-inflammatory cytokines like IL-1β and TNF-α while upregulating anti-inflammatory cytokines such as IL-10, fostering a regenerative environment [197, 198]. Additionally, it regulates MMPs and TIMPs, ensuring ECM integrity and preventing excessive matrix degradation [199, 200].
Understanding these molecular mechanisms enables the development of targeted rehabilitation protocols to optimize tissue repair, enhance joint function, and reduce injury risk. Tailoring rehabilitation strategies to modulate specific signaling pathways and cellular responses improves recovery and long-term knee joint health (Table 6).
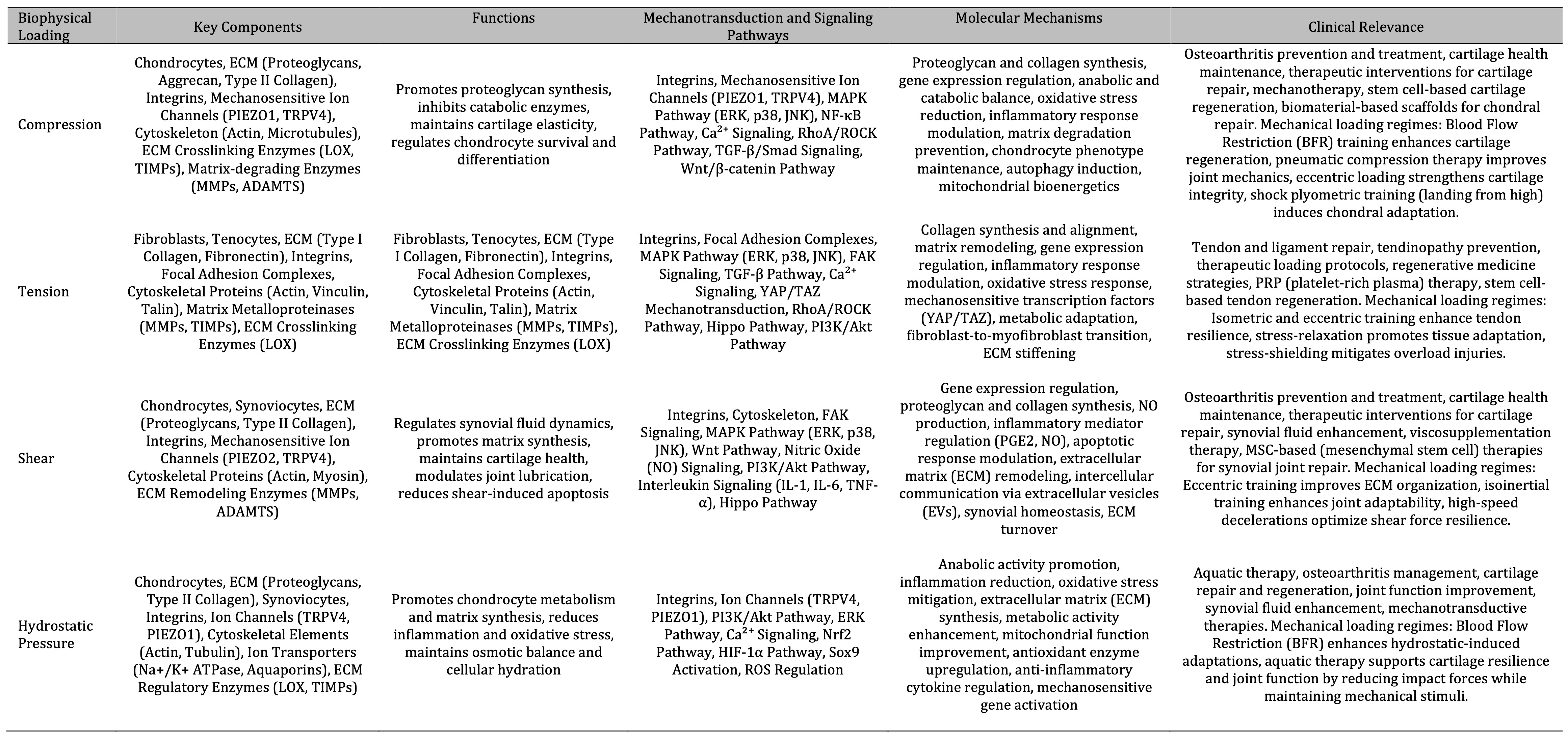
Table 6: The table summarizes the impact of mechanical forces on cartilage health and repair, detailing key components, functions, signaling pathways, molecular mechanisms, and clinical relevance for compression, tension, shear, and hydrostatic pressure. Each force type influences chondrocytes, ECM, and related pathways to promote tissue synthesis, reduce inflammation, and maintain cartilage elasticity, with clinical applications in osteoarthritis prevention, tendon and ligament repair, and regenerative medicine strategies.)
1. Compression
Compression loading is critical for cartilage, ligament, and tendon integrity, despite distinct structural
and
molecular demands [201]. In cartilage, moderate compression activates chondrocytes to synthesize
proteoglycans
like aggrecan while suppressing catabolic enzymes degrading the extracellular matrix (ECM) [202].
Chondrocytes
sense compression via integrins and mechanosensitive ion channels (PIEZO1, TRPV4), triggering
intracellular
signaling cascades such as MAPK (ERK1/2, p38, JNK) and NF-κB pathways [203]. Mechanical deformation
induces Ca²⁺
influx, activating enzymes like CaMKII and calcineurin, modulating transcription factors that regulate ECM
synthesis and stress responses [204, 205].
Integrin clustering at focal adhesions recruits adaptor proteins (talin, vinculin, paxillin) and activates
focal
adhesion kinase (FAK), which triggers downstream kinases (Src, Ras/Raf), amplifying MAPK and NF-κB
pathways.
ERK1/2 enhances anabolic gene expression, whereas overactivated p38/JNK drives MMP and aggrecanase
production,
leading to ECM degradation [206, 207]. NF-κB fine-tunes homeostasis but, under excessive compression,
promotes
cytokine and protease expression, exacerbating tissue damage [208].
Ligaments and tendons, despite primarily experiencing tensile loading, also respond to compression via
integrins
(α5β1, αvβ3) and mechanosensitive ion channels [209]. PIEZO1-mediated Ca²⁺ influx fosters collagen I
fibril
organization and proteoglycan synthesis, enhancing shock absorption in fibrocartilaginous regions [210].
Growth
factors like TGF-β and IGF-1 elevate under mild compression, activating SMAD and PI3K/Akt cascades to
regulate
matrix formation and cytoskeletal dynamics, ensuring resilience [211, 212].
Aggrecan in cartilage assembles with hyaluronic acid to form water-retentive aggregates that resist
compressive
cycles, stabilized by type II collagen [213]. Ligaments and tendons rely on type I collagen but
incorporate type
II in fibrocartilaginous zones under compressive forces. Moderate compression sustains ECM turnover, but
excessive loads induce deleterious pathways—p38/JNK overactivation and prolonged NF-κB signaling
upregulate MMPs
and aggrecanases, dismantling ECM [214, 215]. Reactive oxygen species (ROS) accumulate, impairing proteins
and
disrupting ion channel function, further amplifying NF-κB/MAPK-driven inflammation [216, 217].
Chronic overloading predisposes cartilage to osteoarthritis, marked by cartilage erosion, bone sclerosis,
and
inflammation [218]. In ligaments, excessive compression weakens collagen, increasing tear risk and joint
instability [219]. In tendons, maladaptive loading at entheses fosters tendinopathy [220]. Inflammatory
cascades
spread via synovial fluid, exacerbating joint dysfunction.
Understanding these molecular mechanisms enables targeted interventions to prevent compressive overuse
injuries
[221]. Biomechanical strategies like orthotics and exercise protocols optimize loading thresholds [222].
Pharmacological inhibitors of MMPs and ROS scavengers protect ECM integrity [223]. Regenerative
approaches,
including stem cells and growth factor therapies, balance beneficial compression-induced signaling while
mitigating destructive pathways [224]. Controlled mechanical loading can harness anabolic responses,
sustaining
tissue function and reducing inflammation across cartilage, ligaments, and tendons.
2. Tension
Tensile loading affects ligaments, tendons, and fibrocartilage, driving collagen synthesis and alignment
while
also influencing cartilage regions subjected to tensile forces, such as menisci and entheses [225].
Fibroblasts
(in ligaments) and tenocytes (in tendons) detect mechanical tension via integrin-mediated signaling [226].
Integrins link the ECM to the cytoskeleton, clustering upon stretch and recruiting focal adhesion proteins
like
vinculin and paxillin [227]. This activates focal adhesion kinase (FAK), which phosphorylates downstream
targets, initiating MAPK signaling cascades (ERK1/2, p38, JNK) that regulate ECM production, cell
survival, and
remodeling [228]. Mechanosensitive ion channels (PIEZO1, TREK-1) also permit Ca²⁺ influx, triggering
CaMKII and
calcineurin pathways to control collagen and proteoglycan synthesis [229, 230].
Proper tensile loading upregulates type I collagen synthesis, the dominant structural protein in ligaments
and
tendons, with alignment facilitated by lysyl oxidase cross-linking collagen fibrils for improved tensile
strength [231]. In fibrocartilage, tensile cues drive type II collagen production in menisci and mixed
type I/II
collagen expression in entheses, ensuring structural adaptation [232]. TGF-β and CTGF increase under
tension,
activating SMAD and PI3K/Akt pathways to enhance ECM assembly [233]. These responses support tissue
resilience
against multi-directional forces [234].
Mechanotransduction also involves nuclear translocation of transcription factors like YAP/TAZ via the
Hippo
pathway [235]. Under tension, YAP/TAZ enter the nucleus and interact with TEAD to regulate ECM remodeling
and
cytoskeletal organization [236]. While moderate tensile loading promotes adaptation, excessive tension
induces
microtears, releasing damage-associated molecular patterns (DAMPs) that upregulate cytokines IL-1β and
TNF-α
[237, 238]. These cytokines activate NF-κB, leading to increased MMP-1 and MMP-13 expression, degrading
collagen
and weakening tissue integrity, which contributes to tendinopathy and ligament laxity [239].
Excessive tension also disrupts homeostasis by increasing reactive oxygen species (ROS), exacerbating
oxidative
stress and activating JNK/p38 MAPK, which promote apoptosis and ECM degradation [240, 241]. ROS-driven
NF-κB
signaling intensifies inflammation, perpetuating tissue breakdown [242]. However, controlled tensile
loading is
integral to rehabilitation, as it modulates integrin signaling and growth factor release to reinforce ECM
integrity [243, 244]. Proper activation of mechanosensitive ion channels fine-tunes intracellular Ca²⁺
signaling, balancing matrix turnover and preventing catabolic shifts [245].
Therapeutic strategies leverage tensile loading’s anabolic effects while mitigating overstimulation risks.
Pharmacological approaches targeting MMP inhibition and ROS scavenging protect ECM integrity [246].
Regenerative
techniques, such as mesenchymal stem cell (MSC) therapy, exploit mechanosensitive differentiation to
enhance
tenocyte and fibroblast ECM production under controlled tension [247]. Gene therapy holds potential for
modifying transcription factors and growth factor expression to optimize tissue repair.
In summary, tensile loading drives molecular adaptations in ligaments, tendons, and fibrocartilage via
integrin
signaling, MAPK activation, and growth factor-mediated ECM regulation [248]. Mechanosensitive ion channels
modulate calcium-dependent gene transcription, directing matrix synthesis. While physiological tension
aligns
collagen fibers and maintains tissue strength, excessive tension triggers inflammatory and degradative
pathways.
Understanding the balance between adaptive and pathological tensile stimuli is critical for
rehabilitation,
pharmacological interventions, and regenerative medicine aimed at preserving and restoring load-bearing
tissue
function.
3. Shear
Shear Shear stress influences synovial fluid dynamics, cartilage health, and the behavior of ligaments and
tendons, adapting their responses through distinct molecular pathways [249]. In cartilage, moderate shear
stress
enhances proteoglycan and type II collagen synthesis via integrin-mediated signaling, activating Wnt and
MAPK
pathways (ERK1/2, p38, JNK) that regulate transcription factors and ECM remodeling [250]. Integrins anchor
to
the actin cytoskeleton, forming focal adhesions under shear force, recruiting focal adhesion kinase (FAK),
and
linking mechanical cues to biochemical signals for chondrocyte proliferation and matrix remodeling [251].
Shear
forces also activate mechanosensitive ion channels (TRPV4, PIEZO1), inducing Ca²⁺ influx, which modulates
metabolism, gene expression, and ECM composition. Additionally, shear regulates nitric oxide (NO) and
prostaglandin E2 (PGE2) synthesis [252]. Moderate NO and basal PGE2 support ECM integrity, while excessive
shear
upregulates inducible nitric oxide synthase (iNOS) and amplifies COX-mediated PGE2 production, leading to
inflammation, matrix degradation, and apoptosis through NF-κB and ROS accumulation [253].
Ligaments and tendons, while primarily experiencing tensile forces, endure localized shear at entheses and
bony
prominences [254]. Fibroblasts and tenocytes transduce shear via integrins, activating FAK and MAPK
cascades.
Mechanosensitive ion channels (TREK-1, TRPV4) allow Ca²⁺ influx, regulating collagen fiber organization
and ECM
turnover [255]. Moderate shear aligns fibers and sustains ECM integrity, but excessive shear induces DAMP
release, upregulating IL-1β, TNF-α, and PGE2, which promote MMP-1 and MMP-13 expression, degrading
collagen and
weakening tissue structure [256]. ROS accumulation exacerbates matrix breakdown, further activating NF-κB
signaling [257].
Across cartilage, ligaments, and tendons, shear stress modulates metabolism via AMPK and mTOR pathways,
enhancing glucose and amino acid uptake for ECM synthesis [258]. When excessive, metabolic dysfunction
reduces
nutrient availability, lowers TIMP/MMP ratios, and increases ECM degradation [259]. Elevated shear alters
synovial fluid composition, decreasing hyaluronic acid synthesis and increasing friction, accelerating
cartilage
wear [260]. In ligaments and tendons, shear-induced remodeling at entheses can weaken structural integrity
and
cause pain.
Shear stress also influences extracellular vesicle (EV) release, mediating molecular communication between
tissues [261]. Cartilage-derived EVs under moderate shear propagate anabolic signals, while excessive
shear
releases inflammatory EVs that drive catabolic responses [262]. Similar processes in ligaments and tendons
may
dictate tissue adaptation or degeneration, depending on shear intensity [263].
Ultimately, shear stress regulates ECM turnover, inflammation, and cell survival via integrin and ion
channel-mediated pathways [264]. Controlled shear fosters tissue adaptation, while excessive shear
triggers
inflammatory cascades, ROS production, and matrix degradation through MMP activation and cytokine
upregulation
[265]. Understanding these mechanisms aids in developing therapeutic strategies, including biomechanical
adjustments to reduce abnormal shear, pharmacological inhibitors targeting inflammatory pathways, and
regenerative approaches such as tissue-engineered scaffolds or stem cell therapies to optimize cellular
responses [266-268].
4. Hydrostatic Pressure
Hydrostatic pressure, as experienced in aquatic therapy, influences cartilage, ligaments, and tendons
through
distinct molecular mechanisms [269]. In cartilage, chondrocytes respond by increasing proteoglycan and
type II
collagen synthesis via mechanoreceptors such as integrins, which recruit focal adhesion kinase (FAK) and
activate PI3K/Akt and ERK pathways [270, 271]. PI3K/Akt signaling promotes cell survival and ECM synthesis
by
phosphorylating BAD and caspase-9, while ERK upregulates genes encoding collagen and aggrecan [272, 273].
Mechanosensitive ion channels (TRPV4, PIEZO1) mediate Ca²⁺ influx, activating CaMKII and other enzymes
that
regulate gene transcription and protein synthesis, further supporting ECM stability and chondrocyte
viability
[274, 275]. Hydrostatic pressure also mitigates inflammation by reducing NF-κB activity and downregulating
IL-1β
and TNF-α expression, while simultaneously enhancing antioxidant enzymes like superoxide dismutase (SOD)
and
catalase, protecting against ROS-induced damage [276, 277]. This anti-inflammatory effect extends to
synoviocytes, promoting hyaluronic acid and lubricin synthesis, improving synovial fluid viscosity, and
reducing
cartilage wear [278, 279].
In ligaments and tendons, fibroblasts and tenocytes primarily adapt to tensile forces but can benefit from
controlled hydrostatic pressure in therapeutic settings [280]. Immersion in an aquatic environment reduces
gravitational forces and applies mild hydrostatic pressure, subtly activating integrin-based signaling and
MAPK
pathways [281, 282]. FAK phosphorylation, PI3K/Akt and ERK signaling, and mechanosensitive ion channels
(TRPV4,
PIEZO1) contribute to modest increases in collagen (type I) and proteoglycan synthesis, aiding tissue
resilience
and repair [283, 284]. Hydrostatic pressure also enhances nutrient diffusion, optimizing the metabolic
environment for tendon and ligament healing.
Inflammatory responses in tendons and ligaments are modulated via hydrostatic pressure by suppressing
NF-κB and
activating the Nrf2 pathway, which governs antioxidant defenses and cytoprotective gene expression [285].
Reduced oxidative stress prevents the ROS-driven breakdown of collagen fibers, limiting tendinopathy and
ligament degeneration [286]. Additionally, growth factors such as TGF-β and IGF-1 are upregulated,
supporting
ECM synthesis and collagen cross-linking, crucial for structural integrity and repair [287].
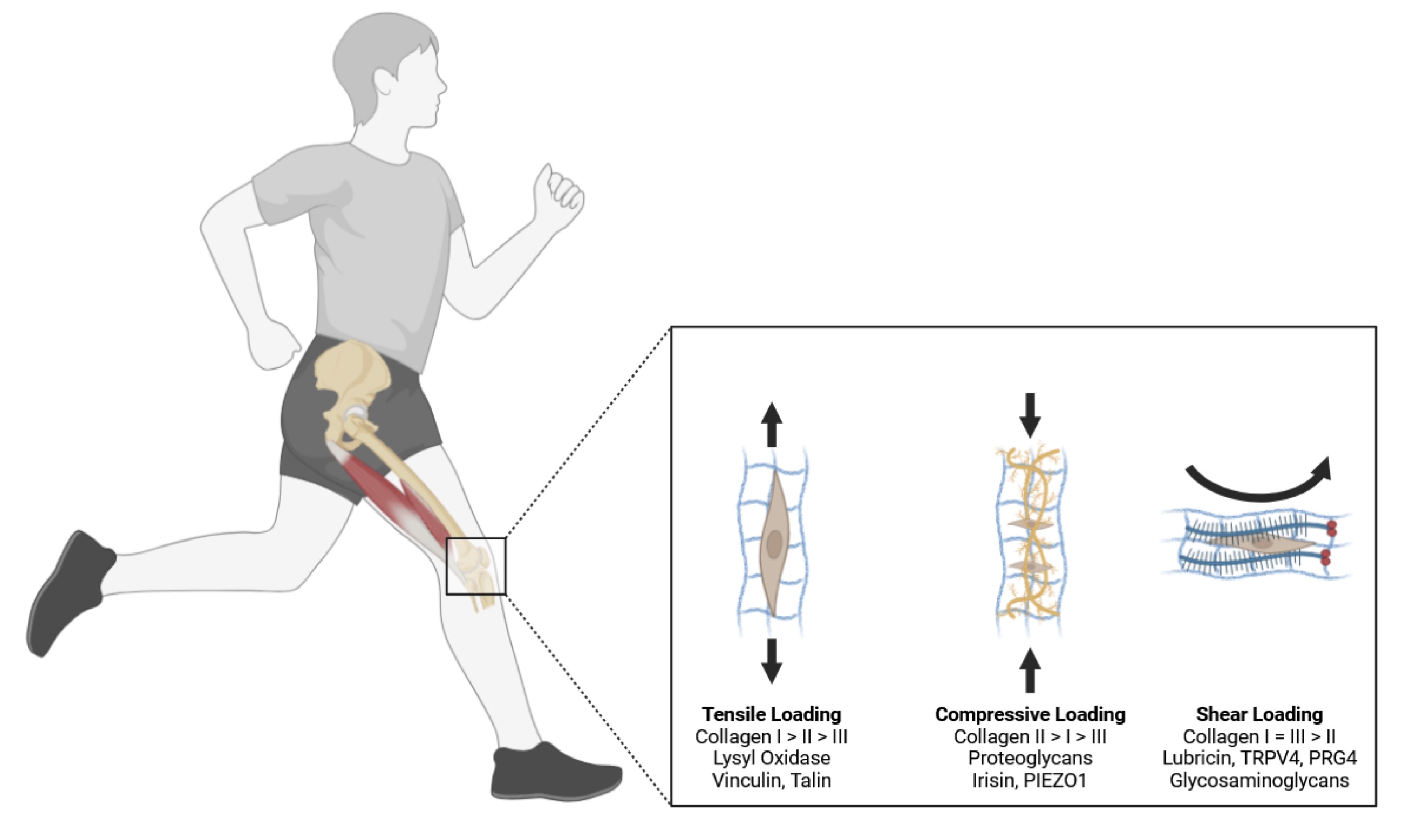
Fig. 2: Knee joints a remarkable capacity to adapt to different types of mechanical loads, with the most well-documented changes occurring in response to tensile and compressive stresses. The musculoskeletal system experiences three primary types of mechanical loads: tension (cells make more type I collagen and lysyl oxidase, resulting in a stiff aligned collagen matrix), compression (the same cells induce the expression of large proteoglycans that contain a protein-like hyaluronic acid and gylcosaminoglycans, such as chondroitin and keratin sulfate), and shear (leads to the production of proteoglycans, hyaluronic acid, superficial zone protein, and lubricin at the edge of the tissue, resulting in a collagen matrix that holds fluid at the edge of the tissue to lubricate movement). Knee soft tissues developing under tensile load show a dense, aligned matrix predominantly composed of type I collagen fibers. In contrast, musculoskeletal tissues subjected to compressive forces display a fibrocartilaginous phenotype characterized by sparsely connected, unaligned, and smaller type I collagen fibers along with larger proteoglycans. Knee joint tissues exposed to shear stress develop a partially aligned matrix and produce high levels of surface lubricating proteins such as lubricin, proteoglycan 4, and hyaluronic acid. Adapted from Kenneth Tam and Keith Baar, 2025).
Hydrostatic pressure also influences extracellular vesicle (EV) release, modulating intercellular communication among chondrocytes, tenocytes, ligament fibroblasts, and synoviocytes [288]. EVs generated under controlled pressure conditions can carry anabolic signals that enhance regeneration, while those under excessive pressure may propagate inflammatory mediators [289, 290].
Clinically, hydrostatic pressure reduces joint load while promoting beneficial cellular responses, making aquatic therapy and Blood Flow Restriction (BFR) a valuable intervention for osteoarthritis and ligament or tendon injuries [291]. By optimizing PI3K/Akt, ERK, ion channel activity, and Ca²⁺ signaling, hydrostatic pressure enhances ECM integrity, downregulates inflammation, and improves tissue recovery [292]. As research advances, therapeutic strategies will further refine aquatic therapy, pharmacological approaches, and regenerative techniques like stem cell therapy and gene modulation to maximize the protective and reparative benefits of hydrostatic pressure [293].
Rehabilitation and Mechanotransduction
Effective knee rehabilitation strategies leverage mechanotransduction principles to optimize tissue repair and functional recovery [294]. Mechanotransduction converts mechanical stimuli into biochemical signals, regulating gene expression, protein synthesis, and ECM remodeling, which are critical for musculoskeletal tissue adaptation after injury or surgery [295]. Properly controlled loading enhances healing, while excessive or improper loading disrupts repair, triggering inflammation, tissue breakdown, or re-injury [296] (Fig. 3).
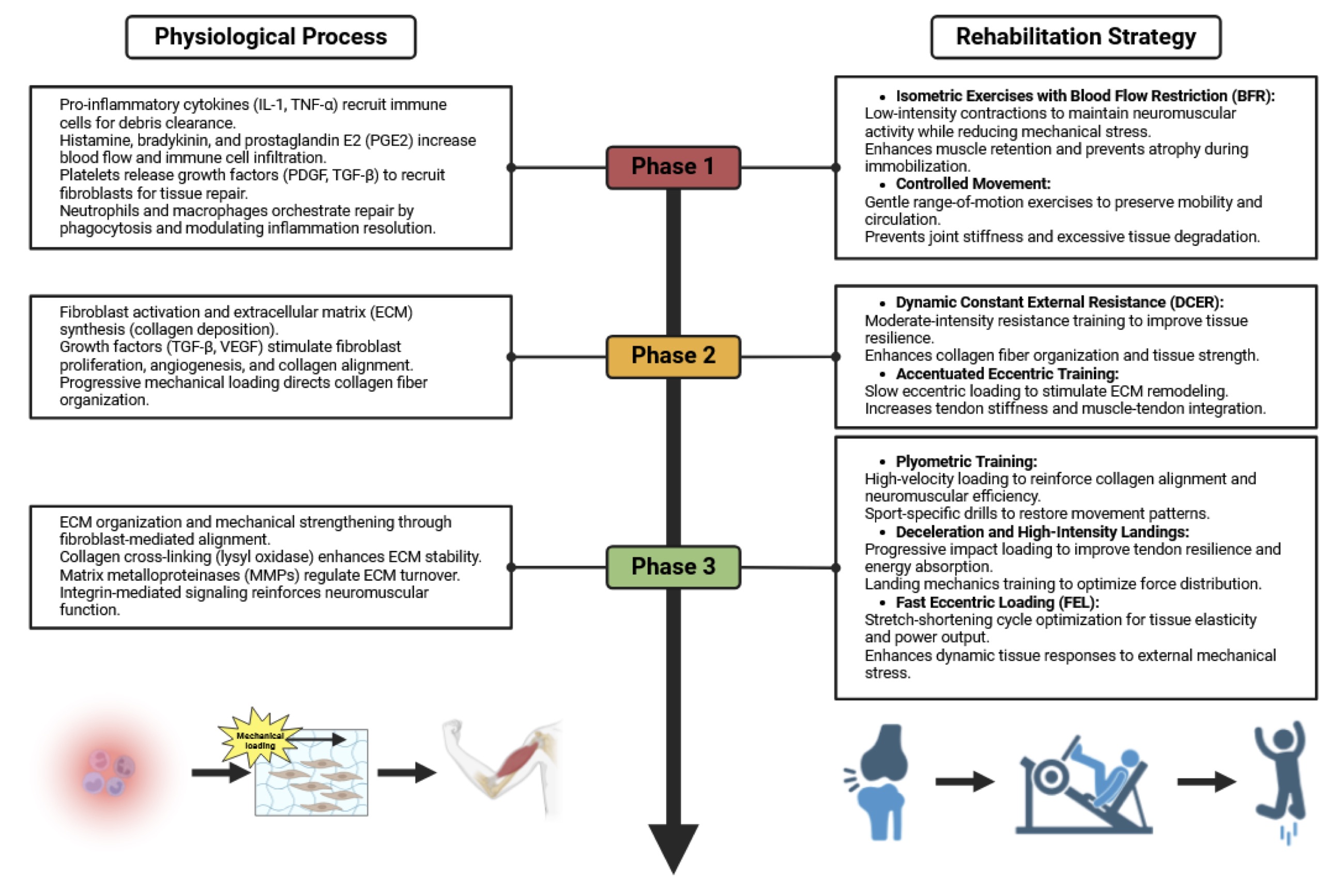
Fig. 3: This Fig. illustrates the integration of the natural healing process of musculoskeletal tissues with a progressively increasing mechanical load during rehabilitation. It is divided into three phases: inflammation, proliferation, and remodeling. The physiological processes in each phase guide the rehabilitation strategies, beginning with isometric exercises and blood flow restriction (BFR) to maintain neuromuscular activity while minimizing stress. In the proliferation phase, controlled mechanical loading through dynamic constant external resistance (DCER) and accentuated eccentric training enhances collagen organization and tissue resilience. The remodeling phase introduces plyometric training, high-speed decelerations, and fast eccentric loading (FEL) to reinforce neuromuscular efficiency, optimize tendon resilience, and restore functional capacity. The flow of recovery is visually represented by a transition from injury to functional movement, emphasizing the synergy between biological healing and progressive loading.
Mechanical forces deform cells, activating mechanoreceptors such as integrins and mechanosensitive ion channels, which initiate intracellular signaling cascades [297]. Integrins cluster at focal adhesions, recruiting focal adhesion kinase (FAK), triggering MAPK (ERK, JNK, p38) and PI3K/Akt pathways [298]. ERK signaling supports ECM synthesis and cell proliferation, while excessive JNK/p38 activation promotes inflammation and catabolic responses, accelerating tissue degradation [299]. Mechanosensitive ion channels (PIEZO, TRPV4) regulate calcium influx, activating calmodulin and calcineurin, modulating transcription factors that influence ECM dynamics and cellular survival [300].
Proper rehabilitation applies progressive mechanical loading to stimulate anabolic pathways without overstressing tissues [301]. Chondrocytes, fibroblasts, and tenocytes respond to controlled stress by producing collagen and proteoglycans, strengthening tissue integrity [302]. However, excessive loading induces NF-κB activation, increasing MMPs, cytokine release (TNF-α, IL-1), and oxidative stress, which hinder healing and may lead to osteoarthritis or tendinopathy [303].
Optimizing loading parameters—type, magnitude, frequency, and duration—is crucial [304]. Underloading leads to muscle atrophy and inadequate collagen deposition, whereas excessive loading upregulates catabolic genes, increasing ECM degradation [305]. The timing of loading is also critical; early moderate mechanical stress activates growth factors like TGF-β and IGF-1, which drive ECM remodeling and cell proliferation, enhancing tissue regeneration [306]. TGF-β strengthens collagen networks, while IGF-1 promotes muscle and tendon repair, reducing injury recurrence [307]. Conversely, excessive early loading hyperactivates JNK/p38 and NF-κB, increasing inflammation and delaying healing [308].
Clinical studies reinforce the benefits of structured loading [309]. Progressive eccentric loading improved collagen organization and pain reduction in Achilles tendinopathy, demonstrating its effectiveness in knee rehabilitation [310]. A controlled loading protocol post-ACL reconstruction accelerated functional recovery and reduced re-injury rates, linking systematic load progression to enhanced ECM remodeling [311]. Similarly, early mild loading in acute ankle sprains expedited swelling reduction and improved tissue organization, reinforcing the role of mechanotransduction in recovery [312].
Khan K.M et al., [313] explores the role of mechanotransduction in musculoskeletal rehabilitation. The authors highlight how targeted exercise can stimulate cellular repair mechanisms in the knee joint, leading to improved rehabilitation outcomes. Mechanotransduction underlies the effectiveness of rehabilitation protocols for chronic knee pain, particularly in conditions like patellar tendinopathy.
Another study from Longerstedt et al., [314] examines how mechanical loading affects knee rehabilitation by influencing structural tissue adaptation. This research emphasizes the importance of monitoring training loads to optimize knee rehabilitation. The findings suggest that different mechanical stimuli can either enhance or hinder tissue healing, depending on intensity and duration.
In conclusion, mechanotransduction-based rehabilitation optimizes tissue repair by modulating MAPK, NF-κB, and ion channel signaling, enhancing ECM integrity while preventing inflammatory degeneration [315-317]. Adjusting exercise intensity and timing activates TGF-β and IGF-1, reinforcing musculoskeletal strength while minimizing fibrosis and chronic inflammation [318]. Understanding these molecular processes allows clinicians to develop tailored rehabilitation protocols, integrating biomechanics with cellular biology to maximize recovery, minimize re-injury, and improve long-term joint function [319-325].
1. Controlled Loading
Gradual and controlled mechanical loading is essential for tissue repair and strength, as it stimulates
mechanotransductive pathways without causing further damage [326]. Mechanoreceptors like integrins sense
these
forces, triggering intracellular signaling cascades that regulate ECM synthesis and remodeling [327].
To optimize physiological adaptations in tissue following regenerative medicine, precise loading
strategies must
be applied progressively in magnitude, direction, and rate, targeting specific tissues at appropriate
healing
stages [327]. Different phases of tissue repair necessitate varied loading applications, as mechanical
stimuli
influence the composition, structure, and function of musculoskeletal tissue through mechanotransduction
[328].
In knee rehabilitation, controlled loading promotes collagen fibrillogenesis, enhancing structural
integrity
[328]. Fibroblasts synthesize procollagen, which assembles into mature fibers, cross-linking to increase
tensile
strength [329]. Growth factors such as TGF-β and VEGF facilitate ECM deposition and angiogenesis, ensuring
oxygen and nutrient supply to healing tissues [330, 331].
Primary cilia and stretch-activated ion channels contribute to mechanotransduction. Primary cilia detect
mechanical changes, influencing cell division and differentiation, while PIEZO channels mediate ion flux,
activating downstream repair pathways [332, 333].
One study from Jin et al., [334] explores a novel wearable A-mode ultrasound system designed to measure
joint
torque in real-time, providing critical insights into mechanical loading patterns during rehabilitation
exercises. The researchers examined how controlled mechanical loading affects knee joint torque during
dynamic
movements, offering real-time biofeedback to optimize rehabilitation strategies [335]. At the molecular
level,
findings suggest that mechanotransduction through integrin signaling and TGF-β activation enhances
collagen
fiber alignment and fibroblast proliferation, which are essential for ligament and cartilage healing
[336]. By
leveraging ultrasound imaging to fine-tune mechanical loading, this study introduces a non-invasive method
for
personalizing rehabilitation protocols, potentially reducing reinjury risks and improving long-term joint
function.
Another study from Sharma et al., 2024 [337] evaluates the biomechanical effects of controlled mechanical
loading via carbon fiber dynamic orthoses in patients recovering from lower limb traumatic injuries,
including
ACL and meniscal tears. Using gait analysis, the researchers demonstrated that custom dynamic orthoses
improve
joint loading symmetry, reducing excessive shear stress on cartilage and ligaments [338]. The study also
highlights how controlled loading influences proteoglycan turnover and chondrocyte mechanosensation,
preventing
cartilage degradation. Molecularly, YAP/TAZ and FAK signaling pathways were implicated in the cellular
response
to controlled loading, promoting tissue adaptation and repair [339]. These findings support the
integration of
adaptive external supports in rehabilitation programs to optimize knee joint mechanics and reduce
secondary
injury risks.
The last randomized controlled trial from Jacobs et al., 2024 [340] investigates the impact of controlled
mechanical loading combined with Vascular Occlusion Training (VOT) well known as Blood Flow Restriction
Training
(BFRT) in patients with knee osteoarthritis. The study found that low-intensity mechanical loading with
intermittent vascular occlusion enhances muscle hypertrophy, joint stabilization, and cartilage integrity
compared to traditional rehabilitation approaches. Mechanistically, VOT was shown to stimulate
hypoxia-inducible
factor (HIF-1α) and VEGF expression, promoting angiogenesis and enhancing chondrocyte survival.
Additionally,
controlled loading modulated MMP-13 and ADAMTS5 activity, reducing excessive cartilage catabolism [341].
These
findings provide compelling evidence that vascular occlusion training, when combined with controlled
mechanical
loading, may optimize knee joint function and slow OA progression.
Glasgow et al [342]. reported that variable loading may enhance mechanotransductive effects by introducing
controlled micro-stresses that facilitate adaptation while preventing repetitive strain injury and delayed
healing. Variability in tensile, compressive, and torsional forces may promote the deposition of a
structurally
resilient extracellular matrix (ECM), strengthening the biological scaffold and improving tissue load
tolerance
[343]. Mechanosensitive ion channels, such as PIEZO1 and TRPV4, respond to these dynamic forces by
modulating
intracellular calcium signaling, which activates downstream pathways like MAPK and PI3K/Akt, enhancing ECM
remodeling and cellular proliferation [339].
Molecular mechanisms involved in controlled loading include the Wnt/β-catenin and Hippo pathways.
Wnt/β-catenin
signaling regulates cell proliferation and differentiation in response to mechanical stress, while the
Hippo
pathway modulates cell growth and apoptosis, impacting tissue remodeling [342, 343].
Controlled loading also regulates MMP activity, balancing ECM degradation and synthesis for optimal tissue
remodeling [344, 345]. Proper MMP control prevents excessive breakdown while ensuring new ECM deposition.
In conclusion, controlled loading optimizes knee rehabilitation by leveraging mechanotransduction to
stimulate
tissue repair and restore function [346]. Tailoring protocols based on molecular insights enhances
recovery,
structural integrity, and long-term joint function. Integrating molecular biology into rehabilitation
strategies
enables targeted interventions, ensuring effective tissue regeneration and improved patient outcomes.
2. Exercise Therapy
It is well-recognized that resistance exercise stimulates an increase in skeletal muscle protein synthesis
and
promotes hypertrophy [347]. When skeletal muscle fibers adapt to resistance training, they do so through
incremental protein accretion, necessitating enhanced ribosomal function and protein translation. These
two
processes are strictly regulated by the mTOR signaling pathway [348]. Increasing evidence also indicates
that
the mTOR pathway intersects with MAPKs at multiple points, contributing to hypertrophic outcomes [349,
350].
Notably, resistance exercise strongly activates MAPKs; however, a sufficient intensity threshold is
required to
trigger ERK1/2 and p38, both part of the MAPK family [351, 352]. Another study highlighted that JNK, also
a
MAPK, is particularly sensitive to mechanical load, with its activation correlating to increases in
exercise
intensity [353]. Overall, MAPK activation is heavily influenced by exercise parameters. For instance,
high-intensity, low-repetition resistance protocols elicit more robust ERK1/2 and p38 activation compared
to
low-intensity, high-repetition regimens [354]. Despite the wealth of data on acute MAPK responses
following
resistance exercise, there remains a gap in understanding MAPK contributions to long-term exercise
adaptations
in humans. While MAPKs are clearly integral to mechanotransduction, additional research is needed to
clarify
their roles in sustained resistance training adaptations in human skeletal muscle [355, 356].
Several factors drive satellite cell activation, thereby influencing the hypertrophic response to
resistance
exercise [357]. Each nucleus in a multinucleated fiber governs only a fixed volume of cytoplasm—the
myonuclear
domain—so substantial muscle fiber hypertrophy beyond that domain limit requires adding new nuclei. These
additional nuclei are thought to come from satellite cells that differentiate and fuse with existing
fibers
[358]. Previous human studies have shown a marked rise in satellite cell numbers within 24 hours after
acute
lower-body resistance exercise, remaining elevated for 72–96 hours and then tapering off, with intensity
serving
as a key determinant of the acute response [359, 360]. This immediate response is minimal when exercise
intensity is under 40% of one-repetition maximum (1 RM), but increases two- to three-fold at intensities
exceeding 60% of 1 RM [361]. Likewise, long-term studies involving resistance training (comparing
high-intensity
to lower-intensity protocols) reported a notable increase in satellite cell proliferation over training
periods
of 9–16 weeks [362-372]. These findings collectively support the idea that satellite cells are activated
during
hypertrophy, supplying additional nuclei to accommodate the enlarged cytoplasmic volume in growing muscle
fibers.
Contrary to the notion of continuous myonuclear addition, some investigations have observed muscle fiber
hypertrophy without a clear increase in satellite cell–mediated myonuclear content [373–382]. More recent
evidence, however, emphasizes that the hypertrophic response to mechanical overload largely depends on
satellite
cell activity [383-389]. Taken together, mechanical loading stands out as a major stimulus for muscle
hypertrophy in resistance exercise. Hypertrophy is initially facilitated by protein accretion—regulated by
the
mTOR pathway alongside MAPKs (ERK1/2, p38, and JNK)—and is sustained by ongoing myonuclear addition via
satellite cell activation. Although early muscle fiber enlargement may rely primarily on protein
accretion,
continuous hypertrophy over time likely requires additional myonuclei contributed by satellite cells as
the
muscle remains subject to mechanical loading through resistance exercise [390-395].
Study drom Du J. et al., [396] investigates how eccentric training impacts muscle and tendon remodeling at
the
molecular level in human knee rehabilitation. Eccentric loading activates the Akt/mTOR pathway, which
enhances
protein synthesis and muscle hypertrophy, leading to improved tendon resilience in the knee joint. The
study
identifies YAP/TAZ signaling activation, which is crucial for tendon mechanotransduction and fibroblast
proliferation, promoting collagen type I and III synthesis. Eccentric training also triggers
mechanosensitive
ion channels like PIEZO1, which influence calcium influx and ECM remodeling, helping in ligament
adaptation.
Additionally, the study highlights a protective effect against oxidative stress, mediated by NRF2/KEAP1
signaling, reducing tissue degradation and inflammation.
Another study from Cheng L. et al., [397] investigates how isometric quadriceps training influences
chondrocyte
activity and cartilage regeneration in knee osteoarthritis (KOA). Isometric contractions activate the
PI3K/Akt/mTOR pathway, promoting chondrocyte survival and cartilage matrix synthesis. This research
highlights
that mechanical stress from isometric exercises enhances autophagy in chondrocytes, a process crucial for
cartilage homeostasis and degradation prevention. Increased autophagic flux protects chondrocytes from
apoptosis, reducing oxidative stress and inflammation through the NRF2/KEAP1 pathway. This findings
suggest that
controlled isometric exercise could be an effective non-pharmacological strategy to slow cartilage
degradation
and enhance knee joint rehabilitation.
3. Manual Therapy
Manual therapy, including joint mobilization and manipulation, modulates mechanical stimuli to enhance
mechanotransduction and tissue repair. By applying pressure and movement, these techniques alleviate pain,
improve joint mobility, and activate cellular signaling pathways essential for regeneration [398].
Integrins,
crucial mechanoreceptors, link the ECM to the cytoskeleton, clustering upon mechanical stimulation and
initiating MAPK and PI3K-Akt pathways, promoting protein synthesis and cellular repair [399].
At the molecular level, mechanical forces activate integrins, triggering conformational changes that
facilitate
ECM protein binding (e.g., fibronectin, collagen, laminin) [400]. This recruits focal adhesion kinase
(FAK),
leading to phosphorylation cascades activating Ras-Raf-MEK-ERK and PI3K-Akt pathways [401]. These cascades
regulate protein synthesis, cell proliferation, and survival, vital for tissue regeneration.
Gentle mobilization techniques enhance synovial fluid production, improving joint lubrication and
cartilage
health [402]. Synoviocytes increase hyaluronic acid and lubricin secretion, reducing friction and
delivering
nutrients to chondrocytes, supporting cartilage maintenance [403].
Manual therapy also influences inflammation by modulating cytokine and growth factor expression [404].
Mechanical forces upregulate IL-10, an anti-inflammatory cytokine, suppressing pro-inflammatory pathways,
while
TGF-β enhances ECM synthesis and cellular differentiation, facilitating tissue remodeling and repair [405,
406].
Combining manual therapy with exercise therapy sustains mechanotransductive effects, improving joint
strength
and flexibility while reducing re-injury risk [407]. Exercise-induced mechanical loading further activates
integrins, promoting ECM protein production and growth factor release [408]. Additionally, exercise
upregulates
genes involved in muscle hypertrophy via the mTOR pathway, enhancing muscle protein synthesis and
functional
recovery [409].
Study from Mellinger et al., [410] examines the role of manual therapy and mechanical loading
interventions in
treating knee injuries, particularly patellofemoral pain syndrome (PFPS) and ACL rehabilitation in
runners. The
research compares manual therapy techniques (joint mobilization, soft tissue therapy) combined with
controlled
loading exercises versus standard physiotherapy. The results showed that mechanical loading, when
introduced in
a structured manner, improved pain levels, enhanced running biomechanics, and reduced knee joint stress.
Last study from L NG et al., [411] study focuses on mechanobiology-based rehabilitation, emphasizing how
manual
therapy and controlled loading affect cellular healing and knee joint regeneration. The research explores
how
joint mobilization and external forces modulate chondrocyte mechanotransduction via YAP/TAZ and FAK
signaling
pathways, leading to enhanced cartilage repair. Findings indicate that gradual mechanical loading after
knee
injuries improves ligamentous remodeling (COL1A1 and COL3A1 expression) and enhances meniscus
fibrocartilage
integrity. The authors suggest that combining manual therapy (to modulate synovial fluid mechanics and
joint
congruency) with weight-bearing exercises (to promote collagen realignment) accelerates knee joint
healing. This
study provides strong evidence that physiotherapy protocols should integrate mechanobiology principles to
maximize knee rehabilitation efficiency.
The integration of manual and exercise therapy optimizes rehabilitation by leveraging molecular pathways
to
enhance healing, reduce pain, and improve functional outcomes. This synergistic approach maximizes joint
stability and tissue regeneration, providing a comprehensive strategy for long-term joint health.
4. Early Mechanical Loading
Early mechanical loading significantly impacts rehabilitation by stimulating molecular and cellular
mechanisms
that drive tissue repair [412]. For instance, a randomized controlled trial [412] found that partial
weight-bearing exercises introduced within two weeks post-knee surgery led to more robust collagen
alignment
compared to delayed loading protocols, indicating that early intervention significantly enhances tissue
quality.
Another systematic review [413] reported that beginning light functional exercises within the first three
weeks
post-ACL reconstruction correlated with improved tendon structure and reduced postoperative stiffness,
suggesting a narrow therapeutic window in which mechanotransduction can be most effectively harnessed.
Research suggests that controlled loading initiated within two weeks post-injury enhances collagen
alignment and
reduces postoperative stiffness, optimizing tendon and ligament structure [413].
Mechanotransduction plays a critical role by activating integrins, which link the ECM to the cytoskeleton
[414].
Mechanical forces trigger integrin clustering, leading to FAK phosphorylation and activation of Src family
kinases, MAPK (ERK, JNK, p38), and PI3K-Akt pathways [415, 416]. MAPK signaling regulates ECM protein
synthesis
(e.g., collagen, fibronectin), while PI3K-Akt promotes cell survival and mTOR-mediated protein synthesis,
accelerating tissue regeneration [417]. Delayed loading beyond six weeks often results in suboptimal
collagen
organization and prolonged recovery [418].
Early controlled mechanical loading following orthobiologic procedures accelerates tissue repair and
reduces
pain by activating molecular pathways involved in mechanotransduction, including integrin-mediated focal
adhesion kinase (FAK) signaling [48, 49]. Muscle contraction type also influences healing kinetics.
Eccentric
contractions generate greater tension and induce robust mechanosensitive responses compared to concentric
contractions, while isometric contractions activate muscles without altering fiber length, maintaining
joint
stability and reducing pain [418-419].
Isometric exercises in early rehabilitation facilitate neural and structural adaptations, minimizing pain
while
improving muscle activation and proprioception [414]. Pain reduction through controlled loading enables
increased joint range of motion, progressively transitioning to isotonic loading strategies that introduce
further tensile and compressive stress, stimulating collagen synthesis and ECM reorganization. Load
progression
should integrate neural adaptation mechanisms involving proprioceptive feedback loops and motor unit
recruitment
patterns, ensuring effective tissue remodeling and functional recovery [415-416].
Early loading should be carefully monitored, progressing from isometric to isotonic exercises based on
pain
levels and healing progress [419, 420]. This gradual approach provides mechanical stimuli to activate
cellular
pathways without exacerbating injury, supporting a more functional repair process.
A major benefit of early mechanical loading is the reduction of excessive scar tissue formation, which can
restrict mobility [421]. TGF-β signaling regulates fibroblast activity and ECM synthesis, promoting
organized
collagen deposition [422]. Clinical trials demonstrate that gentle weight-bearing within two weeks
post-meniscal
repair minimizes scar tissue and accelerates functional recovery [423].
This study from Mae et al., [424] examines the effects of early mechanical loading on graft tension
following
double-bundle ACL reconstruction. The research investigates how active knee extension exercises influence
the
biomechanical properties of the reconstructed ACL. The authors analyzed graft tension variations in
response to
quadriceps activation and knee extension angles, finding that certain loading conditions could improve
graft
integration while excessive stress could risk over-stretching the ligament. The molecular response to
loading
involved increased fibroblast proliferation, collagen synthesis (primarily COL1A1 and COL3A1), and
extracellular
matrix (ECM) remodeling, mediated by mechanotransduction pathways, such as TGF-β and integrin signaling.
Understanding these loading parameters helps optimize post-operative rehabilitation strategies, allowing
controlled early mechanical stimulation without compromising graft integrity.
Another study from Capin et al., [425] explores the long-term biomechanical effects of early
weight-bearing
mechanical loading following medial meniscectomy compared to meniscal repair in patients with ACL
reconstruction. Using gait analysis, the study found that partial meniscectomy significantly altered knee
joint
kinematics and load distribution even two years post-surgery. These changes were associated with increased
compressive forces on the medial tibiofemoral compartment, accelerated cartilage degradation, and a shift
in
subchondral bone remodeling. The researchers identified changes in cartilage proteoglycan content
(aggrecan
loss) and an upregulation of MMP-13 and ADAMTS5, enzymes involved in cartilage catabolism. In contrast,
patients
who underwent meniscal repair maintained more natural joint biomechanics, preserving type II collagen and
chondrogenic markers such as SOX9. The study suggests that mechanical loading post-meniscectomy should be
carefully regulated to minimize long-term degenerative changes.
Last study from Uzuner et al., [426] investigates how meniscectomy-induced mechanical changes affect ACL
loading
during weight-bearing activities. Researchers found that early mechanical loading post-meniscectomy led to
a
redistribution of forces across the knee joint, significantly increasing ACL strain and anterior tibial
translation. Molecularly, the altered mechanical environment induced an upregulation of pro-inflammatory
cytokines (IL-1β, TNF-α) and matrix-degrading enzymes (MMP-1, MMP-13), accelerating ACL microstructural
damage.
The study further showed that partial meniscectomy caused greater ACL loading asymmetry compared to total
meniscectomy, suggesting that incomplete meniscus removal may create an uneven force distribution leading
to
focal stress on the ligament. The findings highlight the importance of adaptive neuromuscular training and
early
controlled weight-bearing exercises to mitigate excessive ACL loading while optimizing recovery.
In conclusion, incorporating early mechanical loading into rehabilitation leverages mechanotransduction to
enhance recovery outcomes. By structuring controlled loading strategies—such as partial weight-bearing
within
two to three weeks post-injury—clinicians can optimize ECM remodeling, minimize scar tissue, and regulate
inflammation, ultimately improving functional outcomes and reducing recovery time.
Rehabilitation Strategies Based on Musculoskeletal Healing Stages: Early Mechanical Loading
Rehabilitation strategies for musculoskeletal injuries must align with the distinct healing stages: inflammation, proliferation, and remodeling. Tailoring interventions to these stages optimizes tissue repair, restores function, and minimizes reinjury risk [427]. Molecular insights into cellular mechanisms enhance rehabilitation effectiveness [428].
During inflammation, the body responds with pro-inflammatory cytokines like IL-1 and TNF-α, which recruit immune cells to clear debris [429]. Rehabilitation at this stage aims to reduce inflammation while maintaining muscle activation through gentle range-of-motion exercises and isometric contractions, modulating inflammatory responses and preventing excessive tissue degradation [430].
The proliferation stage involves fibroblast activation and ECM synthesis, primarily collagen deposition, driven by growth factors such as TGF-β and VEGF [431]. Controlled mechanical loading is crucial for collagen fiber organization. Low-intensity resistance training and balance exercises stimulate IGF-1 expression, further enhancing tissue regeneration [432].
In remodeling, new tissue undergoes maturation through MMP-regulated ECM remodeling and collagen cross-linking [433]. Rehabilitation shifts toward progressive overload with increased exercise intensity. Plyometric exercises and sport-specific drills promote collagen alignment along mechanical stress lines, improving tissue strength and function [434, 435].
Stage-specific rehabilitation enhances healing, reduces reinjury risk, and accelerates functional recovery. Early mechanical loading, tailored to each stage, is critical. Molecular biology insights guide timing and intervention selection, ensuring rehabilitation strategies support natural healing at the cellular level (Table 7).
1. Inflammation Stage
The inflammation stage is the initial and crucial response to musculoskeletal injury, marked by
vasodilation,
platelet activation, and the recruitment of inflammatory cells, including neutrophils, monocytes, and
macrophages [436]. These processes are regulated by complex chemical mediators such as histamine,
bradykinin,
and prostaglandin E2 (PGE2), each playing distinct roles in the inflammatory cascade. At the molecular
level,
interconnected signaling pathways orchestrate cellular responses to promote tissue repair and recovery
[437].
Vasodilation increases blood flow to the injured site, ensuring the delivery of essential nutrients and
immune
cells. This response is mediated by histamine, bradykinin, and PGE2, which trigger the relaxation of
vascular
smooth muscle cells [438]. Histamine, released from mast cells, basophils, and platelets, binds to H1
receptors
on endothelial cells, increasing vascular permeability and allowing immune cells to infiltrate the tissue.
Additionally, histamine stimulates endothelial nitric oxide synthase (eNOS), producing nitric oxide (NO),
a
potent vasodilator that further enhances blood flow and nutrient exchange [439]. This activation involves
secondary messengers such as cyclic AMP (cAMP) and intracellular calcium ions, amplifying the inflammatory
response [440].
Bradykinin, generated from kininogen via kallikrein activity, binds to B2 receptors on endothelial cells,
promoting NO and prostacyclin (PGI2) release, which dilate blood vessels and enhance permeability [441].
This
process facilitates immune cell infiltration and supports tissue repair. Bradykinin also sensitizes
nociceptors,
increasing pain perception as a protective mechanism to limit movement and prevent further damage [442].
Intracellularly, bradykinin signaling activates phospholipase C (PLC), generating inositol triphosphate
(IP3)
and diacylglycerol (DAG), leading to calcium release and protein kinase C (PKC) activation, which
propagate
inflammatory responses [443].
PGE2, synthesized from arachidonic acid via the cyclooxygenase (COX) pathway, plays a pivotal role in
inflammation. COX-2, upregulated in response to injury, drives PGE2 synthesis, which binds to EP2 and EP4
receptors on smooth muscle cells, increasing cAMP and causing vasodilation [444]. PGE2 also sensitizes
sensory
nerves, heightening pain perception. This biosynthetic cascade is tightly regulated by phospholipase A2
(PLA2),
COX enzymes, and specific synthases, ensuring controlled inflammatory signaling [445].
Platelets initiate hemostasis and tissue repair by adhering to exposed subendothelial collagen and von
Willebrand factor (vWF), which engage glycoprotein receptors GPVI and GPIb, triggering platelet activation
[446]. Activated platelets release adenosine diphosphate (ADP) and thromboxane A2 (TXA2), amplifying
aggregation
and stabilizing the injury site [447]. They also secrete growth factors such as platelet-derived growth
factor
(PDGF) and transforming growth factor-beta (TGF-β), which recruit fibroblasts and smooth muscle cells to
drive
tissue regeneration [448]. These processes are mediated by PI3K/Akt signaling and small GTPases like Rap1,
which
regulate cytoskeletal reorganization and integrin activation, stabilizing thrombus formation [449].
Inflammatory cell recruitment is critical for clearing debris and orchestrating repair. Neutrophils,
attracted
by IL-8, complement C5a, and leukotriene B4 (LTB4), engulf pathogens via phagocytosis, release proteolytic
enzymes (e.g., elastase, collagenase), and generate reactive oxygen species (ROS) to neutralize threats
[450,
451]. Monocytes migrate to the injury site under the influence of monocyte chemoattractant protein-1
(MCP-1/CCL2) and differentiate into macrophages [452]. M1 macrophages produce IL-1, IL-6, and TNF-α,
sustaining
inflammation and promoting debris clearance, while M2 macrophages secrete IL-10 and TGF-β, resolving
inflammation and supporting tissue repair. The transition from M1 to M2 macrophages is crucial for
shifting from
inflammation to healing, regulated by transcription factors such as NF-κB and STAT3 [453].
Key chemical mediators fine-tune these responses. Histamine facilitates vasodilation and immune cell
influx,
aiding debris clearance and early healing [454]. Bradykinin increases vascular permeability and nociceptor
sensitivity, amplifying pain signaling and the inflammatory response. PGE2 enhances vasodilation and
immune cell
recruitment while modulating immune responses to transition from acute inflammation to tissue repair
[455].
Leukotrienes, synthesized via the lipoxygenase pathway, act as potent neutrophil chemoattractants, while
NO,
produced by endothelial (eNOS) and inducible nitric oxide synthase (iNOS), promotes vasodilation and
antimicrobial defense [456, 457].
At the molecular level, inflammation is tightly regulated by interconnected pathways. The NF-κB pathway is
central to the transcriptional control of pro-inflammatory cytokines and adhesion molecules, governing
leukocyte
recruitment and activation. The MAPK and JAK-STAT pathways transduce cytokine and growth factor signals,
activating genes involved in inflammation, cell proliferation, and survival [458, 459]. These pathways
ensure
the inflammatory response transitions efficiently into the proliferative phase, facilitating tissue
repair.
Understanding these molecular mechanisms provides insight into therapeutic targets for modulating
inflammation
and accelerating recovery.
In the early phase of healing, the immune system releases IL-1 and TNF-α to clear debris and prevent
further
injury [460]. Rehabilitation strategies aim to minimize excessive inflammation while preserving muscle
activation and circulation. Gentle range-of-motion exercises and isometric contractions help stimulate
blood
flow, reduce stiffness, and prevent atrophy, balancing inflammatory responses and promoting efficient
healing
[461].
A randomized controlled trial [462] evaluated early-stage interventions for acute lateral ankle sprains.
Patients who performed gentle dorsiflexion and plantarflexion exercises within 72 hours of injury
demonstrated
faster resolution of swelling, decreased pain, and improved proprioception compared to a control group
receiving
only immobilization. These findings support the idea that mild mechanical stimulation modulates local
inflammation and helps prevent the deleterious effects of disuse, aligning with the molecular premise of
curbing
excessive cytokine-mediated tissue breakdown.
Mild loading in the inflammation stage can help
regulate the
expression of matrix metalloproteinases (MMPs), ensuring that collagen degradation does not outpace repair
[463]. Early motion also encourages nutrient delivery to injured tissues, aiding the clearance of
inflammatory
byproducts.
2. Fibroblastic Stage
The fibroblastic stage follows the initial inflammatory response and involves the activation and
proliferation
of fibroblasts, which synthesize and organize extracellular matrix (ECM) components necessary for tissue
repair
[464]. Key growth factors, including Transforming Growth Factor-beta 1 (TGF-β1), Bone Morphogenetic
Proteins
(BMPs), and Connective Tissue Growth Factor (CTGF), play essential roles. TGF-β1 binds to TGF-β receptors
(TGF-βRI and TGF-βRII) on fibroblasts, initiating the phosphorylation of Smad2/3 proteins [465]. These
phosphorylated Smads form complexes with Smad4, translocating to the nucleus to regulate ECM gene
transcription,
promoting ECM synthesis and fibroblast proliferation [466].
TGF-β1 enhances type I and III collagen, fibronectin, and integrin production, stabilizing the ECM [468].
It
suppresses matrix metalloproteinases (MMPs) to prevent premature ECM degradation, supporting robust tissue
repair [469]. BMPs, binding to BMPR-I and BMPR-II, activate Smad1/5/8 proteins, which complex with Smad4
to
promote fibroblast differentiation and ECM production [470]. BMPs drive fibroblast-to-myofibroblast
differentiation, a key process in wound contraction and matrix organization [471]. BMP signaling via
Smad1/5/8
regulates collagen synthesis, with myofibroblasts expressing alpha-smooth muscle actin (α-SMA) for
mechanical
tissue stabilization [473].
CTGF interacts with integrins and heparan sulfate proteoglycans, activating downstream MAPK/ERK signaling
pathways to promote fibroblast proliferation, migration, and ECM synthesis [474]. This pathway upregulates
genes
controlling fibroblast adhesion, migration, and matrix remodeling [475]. CTGF enhances collagen,
fibronectin,
and proteoglycan synthesis, strengthening fibroblast-ECM interactions and optimizing structural recovery
[476].
Fibroblast proliferation is essential for ECM production, regulated by the TGF-β/Smad, MAPK, and PI3K/Akt
pathways [478]. Type I and III collagen synthesis forms a provisional matrix, initially arranged in a
disorganized fashion, providing mechanical stability [479]. Over time, fibroblast-mediated mechanical
forces
attempt to align collagen fibers along tension lines, though this reorganization remains incomplete in
scar
tissue [480]. Lysyl oxidase (LOX) catalyzes collagen cross-linking, increasing ECM tensile strength and
ensuring
durability [482]. However, excessive fibroblast activity can lead to fibrosis, reducing tissue
functionality and
elasticity [481].
Other molecules, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor
(PDGF),
regulate fibroblast recruitment and angiogenesis, ensuring adequate oxygen and nutrient supply [485].
VEGF, via
receptor tyrosine kinases, activates the PI3K/Akt and Ras/MAPK pathways, driving endothelial cell
proliferation
and vessel formation [487]. PDGF stimulates fibroblast migration and ECM synthesis, ensuring efficient
tissue
repair [486].
At the molecular level, the fibroblastic stage is governed by signaling pathways that coordinate
fibroblast
activity and ECM remodeling [488]. Regulation of these pathways ensures controlled collagen deposition and
scar
formation, improving clinical outcomes [489]. Understanding these molecular mechanisms enables targeted
therapeutic strategies to enhance tissue repair while minimizing fibrosis.
Once inflammation subsides, fibroblasts and other repair cells proliferate and begin ECM synthesis,
primarily
collagen [490]. Growth factors such as TGF-β, VEGF, and insulin-like growth factor-1 (IGF-1) regulate
angiogenesis, cell proliferation, and collagen deposition [491]. Controlled mechanical loading directs
collagen
fiber alignment, optimizing functional tissue recovery and reducing the risk of excessive scar formation.
In this study [492], patients recovering from partial Achilles tendon tears participated in a progressive
loading protocol consisting of low-intensity resistance exercises and balance training. The intervention
significantly increased local IGF-1 expression and improved collagen fiber alignment when compared to
immobilization. Ultrasound imaging at 12 weeks showed better tissue echogenicity and organized fiber
architecture in the intervention group, correlating with greater tensile strength.
Mechanical cues
during
the proliferation stage activate integrin-mediated signaling pathways (FAK, MAPK) in fibroblasts,
stimulating
collagen gene transcription and enhancing cross-link formation via enzymes such as lysyl oxidase [493].
These
processes result in a more robust ECM scaffold capable of withstanding increasing loads.
3. Remodeling Stage
The remodeling stage enhances extracellular matrix (ECM) organization and mechanical properties through
coordinated cellular, enzymatic, and signaling interactions [494]. Fibroblasts and myofibroblasts
synthesize and
remodel collagen, driving structural integrity. This stage is regulated by complex molecular pathways that
control ECM turnover and tissue strengthening.
Collagen remodeling is central to this stage. Lysyl oxidase (LOX) catalyzes cross-linking between collagen
molecules, enhancing ECM stability [495]. LOX modifies lysine residues, forming covalent bonds that
reinforce
ECM structure. Concurrently, matrix metalloproteinases (MMPs), particularly MMP-1 and MMP-9, degrade
disorganized collagen, ensuring ECM homeostasis. Their activity is tightly regulated by tissue inhibitors
of
metalloproteinases (TIMPs) [496]. Myofibroblasts exert contractile forces that align collagen fibers along
mechanical stress lines, improving tissue resilience [497]. This alignment is facilitated by
integrin-mediated
focal adhesion kinase (FAK) and RhoA/ROCK signaling, which drive cytoskeletal reorganization and ECM
remodeling
[498].
Fibroblasts and myofibroblasts continue ECM synthesis while generating contractile forces essential for
tissue
contraction and collagen fiber alignment [499]. Myofibroblast differentiation, regulated by Transforming
Growth
Factor-beta (TGF-β), activates SMAD and non-SMAD pathways, leading to α-SMA expression and enhanced matrix
remodeling [500]. Integrin-mediated cell-ECM interactions activate intracellular signaling pathways,
including
MAPK/ERK and PI3K/Akt, promoting fibroblast survival, migration, and ECM production [501].
Key growth factors regulate ECM remodeling. TGF-β stimulates fibroblast proliferation, myofibroblast
differentiation, and collagen synthesis, activating SMAD2/3 proteins that translocate to the nucleus and
regulate ECM-related gene expression [502, 503]. TGF-β modulates MMP and TIMP expression, balancing ECM
turnover. Connective Tissue Growth Factor (CTGF) enhances collagen synthesis and fibroblast adhesion,
activating
MAPK/ERK and PI3K/Akt pathways [504]. Platelet-Derived Growth Factor (PDGF) recruits fibroblasts and
stimulates
ECM production through receptor tyrosine kinases, activating Ras/MAPK and PI3K/Akt pathways [505].
ECM components such as fibronectin and elastin contribute to tissue stability. Fibronectin binds
integrins,
facilitating cell adhesion and migration, while elastin ensures resilience to mechanical stress. Elastin
precursor tropoelastin undergoes LOX-mediated cross-linking to form stable elastic fibers [507].
Proteoglycans
and glycosaminoglycans (GAGs) regulate ECM hydration, maintaining tissue viscoelasticity [508]. Their
controlled
production supports ECM integrity and functional recovery [509].
Excessive collagen synthesis can lead to fibrosis and tendon adhesions. Persistent fibroblast activation
results
in excessive ECM deposition, reducing elasticity and function [510]. Scar tissue, though mechanically
supportive, lacks the biomechanical properties of native tissue, leading to impaired flexibility and
function
[511]. Continuous collagen deposition around tendons can cause adhesions, restricting mobility and
necessitating
therapeutic intervention [512].
Molecular pathways in adhesion formation involve inflammatory mediators. Chronic inflammation, driven by
elevated IL-1 and TNF-α, activates NF-κB and JAK/STAT pathways, upregulating fibrotic genes and sustaining
fibroblast and myofibroblast activity [514]. The fibroblast-to-myofibroblast transition, induced by TGF-β,
enhances collagen production and ECM contraction, exacerbating adhesion formation [515]. Targeting TGF-β,
MMP,
and cytokine pathways may improve therapeutic interventions by reducing fibrosis and optimizing ECM
remodeling
[516].
Over time, tissue maturation involves ECM turnover, collagen realignment, and cross-linking to enhance
mechanical properties [517]. MMPs regulate balanced ECM degradation, while progressive overload exercises
align
collagen fibers along stress lines, strengthening tissue and improving flexibility and function [518].
A prospective cohort study [519] followed athletes undergoing anterior cruciate ligament (ACL)
reconstruction
through a structured remodeling-phase program. Participants progressed from closed-chain exercises to
plyometric
drills and eventually to sport-specific agility training over four months. Biomechanical assessments and
MRI
evaluation revealed superior graft integrity, better neuromuscular control, and reduced re-injury rates in
those
who adhered to progressive loading principles compared to those in a less structured protocol.
Advanced
loading protocols reinforce collagen cross-linking and ECM reorganization, partially mediated by signaling
pathways such as SMAD (downstream of TGF-β) and NF-κB regulation of MMPs [520]. By carefully escalating
mechanical demand, the tissue remodels efficiently without triggering excessive inflammatory or catabolic
responses.
Blood Flow Restriction Training
Blood flow restriction (BFR) training offers a multidimensional molecular framework that can be harnessed in knee joint rehabilitation by driving controlled inflammation, hypoxia-mediated gene expression, anabolic hormone secretion, and fibrinolysis—all while preventing excessive mechanical stress on newly forming cartilage [521]. During low-load resistance exercises with partial venous occlusion, the localized hypoxia and metabolite accumulation trigger a cascade of intracellular signals that benefit not only skeletal muscle but also the cartilage matrix, tendons, and surrounding soft tissues [522].
Acute inflammation is induced by elevated shear stress upon reperfusion, which prompts the release of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [120]. IL-6 binds its IL-6R/gp130 receptor complex and activates Janus kinase (JAK1/JAK2/Tyk2), phosphorylating signal transducer and activator of transcription 3 (STAT3) [523]. Phosphorylated STAT3 translocates to the nucleus and induces genes that promote tissue repair, including processes relevant to chondroprogenitor cell recruitment. TNF-α, though harmful in chronic excess, can transiently assist cartilage healing by activating the IκB kinase (IKK) complex, freeing nuclear factor kappa B (NF-κB) to upregulate immune cell recruitment and debris clearance [524]. Critically, macrophage populations eventually shift toward an M2 anti-inflammatory state, supporting a more regenerative and less degradative environment conducive to cartilage matrix deposition and remodeling [525].
Simultaneously, reduced venous outflow stabilizes hypoxia-inducible factor-1 alpha (HIF-1α). Under sufficient hypoxia, prolyl hydroxylase domain (PHD) enzymes are inhibited, preventing HIF-1α degradation by the von Hippel–Lindau (VHL) pathway. Stabilized HIF-1α forms heterodimers with HIF-1β, binding hypoxia-responsive elements (HREs) to induce transcription of vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS) [526]. VEGF promotes endothelial cell proliferation and vascular sprouting, potentially benefiting the subchondral bone region that supplies nutrients to the microfracture repair site. eNOS-derived nitric oxide (NO) augments local vasodilation, supporting blood flow within the vicinity of the forming cartilage. HIF-1α signaling is also central to chondrocyte viability in the low-oxygen niche of repaired cartilage, safeguarding ECM integrity while coordinating collagen and proteoglycan synthesis [527].
Metabolite buildup—particularly lactate and hydrogen ions—heightens sympathetic drive and fosters a systemic endocrine response that amplifies growth hormone (HGH) and insulin-like growth factor 1 (IGF-1) release. IGF-1, whether liver-derived or produced locally in skeletal muscle (e.g., mechano growth factor, MGF), binds the IGF-1 receptor to activate phosphoinositide 3-kinase (PI3K) and downstream AKT/mTORC1 signaling [528]. This pathway phosphorylates p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), unleashing cap-dependent translation of anabolic mRNAs [529]. For knee joint rehabilitation, the net effect is heightened protein synthesis in periarticular musculature as well as tendon and possibly joint tissues, reinforcing both skeletal support and the local environment in which new chondral tissue forms. IGF-1 further bolsters collagen gene transcription in tenocytes, which supports improved tendon stiffness—particularly valuable in joint stabilization during rehabilitation [530].
BFR preferentially recruits type II muscle fibers at loads well below those usually necessary for fast-twitch activation, an advantage for patients with arthrogenic muscle inhibition (AMI) or limited tolerance for high-intensity exercise [531] [532]. The hypoxic and metabolite-rich milieu triggers group IV afferent fibers, boosting central motor drive and promoting the contraction of higher-threshold motor units. For knee joint rehabilitation, preserving or increasing type II fiber mass helps stabilize the joint and stress-shielding from undue forces while allowing functional gains in strength [533].
Alongside these anabolic and immunomodulatory processes, partial occlusion stimulates tissue plasminogen activator (tPA) release from Weibel–Palade bodies in the vascular endothelium [534]. tPA catalyzes the conversion of plasminogen to plasmin, sustaining fibrinolysis and mitigating thrombotic concerns, while also degrading extraneous fibrin that could hinder nutrient diffusion or ECM organization in the microfracture site [535]. This safeguard, in concert with muscle contractions, maintains adequate fibrin turnover and limits the risk of deep vein thrombosis (DVT) [536].
Overall, BFR training weaves together transient inflammation, hypoxia-driven angiogenesis, robust anabolic signaling, and fibrinolytic activity to facilitate muscle and connective tissue repair while posing minimal mechanical stress on the knee joint [537]. This molecular synergy—manifested in cellular preservation, regulated macrophage activation, enhanced growth factor profiles, and stable clot remodeling—makes BFR an innovative option in designing comprehensive and effective rehabilitation programs for knee joint rehabilitation and performance [538] [539] [540].
Neuromuscular Fatigue and Recovery
The complexities of exercise-induced neuromuscular fatigue and recovery must be carefully considered, particularly when addressing different types of training such as explosive power exercises versus traditional strength training. While it's helpful to provide general recovery guidelines based on research, as Tim Gabbett's work [541] suggests, it is crucial to emphasize that recovery times are influenced by numerous factors, including the training modality, intensity, and volume. Generalized prescriptions can lead to misinterpretations and ineffective training plans.
A clear distinction should be made between different training parameters, such as velocity loss (VL), which significantly impacts both recovery time and supercompensation effects. Research indicates that a 10% velocity loss (VL10) threshold during resistance training results in similar total repetitions as a VL20 protocol, but VL10 induces faster recovery and potentially better supercompensation [542]. Trainers and therapists must be cautious, especially when applying eccentric training protocols, as these can induce earlier neuromuscular fatigue and more pronounced delayed onset muscle soreness (DOMS) compared to concentric training [543] [544] [545].
The central challenge is that protocols designed for concentric training often cannot be directly applied to eccentric exercises without resulting in excessive neuromuscular fatigue. Eccentric training generates greater central and peripheral fatigue, primarily through impaired excitation-contraction coupling, which requires adjusted parameters for effective results [546] [547]. For example, studies prescribing 12 repetitions per set in eccentric training may not account for this increased fatigue, leading to suboptimal recovery strategies.
In order to make significant progress in understanding neuromuscular fatigue, it is imperative that future studies define the specific training parameters under investigation. Currently, much of the literature tends to generalize recovery outcomes without sufficiently accounting for variations in training intensity, contraction types, and other critical factors [548] [549] [550].
For instance, studies on recovery rates between power and strength sessions highlight the importance of eccentric phases in inducing muscle damage and slower recovery times. Eccentric force and velocity, particularly in stretch-shortening cycle exercises, appear to contribute substantially to neuromuscular impairment [551]. While power-oriented sessions involving faster eccentric velocities and moderate loads can induce substantial mechanical stress, the recovery rates vary compared to heavier strength-oriented sessions. Both types of sessions can affect recovery, but the effects of eccentric phases during explosive exercises should be further explored [552] [553].
Trainers and therapists should take these variations into account, as generalized recovery times and protocols may not apply uniformly across different exercises, muscle groups, or training statuses. For instance, upper body muscles tend to sustain more damage and require longer recovery times than lower body muscles during eccentric exercise [552] [554], although recovery rates between traditional strength training for upper and lower body exercises appear similar [555] [556] [557].
Moreover, the balance between fatigue and potentiation or supercompensation is critical for optimizing training outcomes. While heavy loads and large exercise volumes can induce long-lasting neuromuscular fatigue, low-volume, high-intensity exercises may result in potentiation and enhanced performance, sometimes even after 24–48 hours [558]. These findings highlight the need for individualized approaches to both training and recovery, as supercompensation effects are often influenced by specific training volumes and intensities.
Lastly, the interpretation of subjective and objective recovery measures should be approached cautiously. While subjective recovery scores (e.g., PRS) can provide insight into an athlete’s perceived readiness, they may not always correlate with objective neuromuscular recovery markers [559] [560] [561] [562]. Trainers should use a combination of these measures to assess recovery status, ensuring a more comprehensive understanding of the athlete's neuromuscular readiness for the next session.
In summary, recovery from resistance training varies greatly depending on the specific training parameters, especially concerning eccentric versus concentric workloads [563]. To optimize both performance and recovery, trainers and therapists should consider adjusting protocols based on the specific demands of the training, rather than relying on generalized recovery guidelines [564].
In terms of future research and practical application, we must consider the cognitive load athletes experience during training. Fatigue and recovery are not just physical processes but involve significant cognitive dimensions [565]. Fatigue and recovery are not just about the physical demands placed on the body; they are multifaceted processes that require us to also consider the cognitive aspects of training. Research suggests that the anterior cingulate cortex (ACC) plays a key role in regulating attention, helping athletes maintain efficient activation of motor units even during fatigue [566]. This has a significant effect on both motor performance and likely also on recovery [567].
As we move forward, it is crucial to take into account the cognitive load placed on athletes during training tasks. The concept of motor-cognitive interference—how cognitive load impacts movement mechanics and efficiency, even more pronounced under circumstances of fatigue—must be integrated into our understanding of fatigue and recovery [568] [569]. Tasks involving more complex decision-making place higher cognitive demands, which can reduce movement efficiency and economy, ultimately increasing the load on the body and potentially extending recovery times [570].
The Influence of Loading History on Musculoskeletal Adaptations. Application to Structured Prevention Program
The mechanical environment that muscles, tendons, ligaments, and cartilage encounter profoundly influences their cellular and molecular responses [571] [572]. When these tissues are repeatedly challenged by physical exercise or rehabilitative protocols, they develop a protective adaptation often referred to as the repeated bout effect (RBE). This phenomenon is underpinned by intricate signaling cascades involving oxidative stress, inflammatory mediators, mechanotransduction pathways, and metabolic regulators [573] [574]. Moreover, the outcomes of these molecular processes are highly individualized, reflecting differences in loading history, genetic background, and the extent of prior tissue adaptation (Fig. 4 &5).
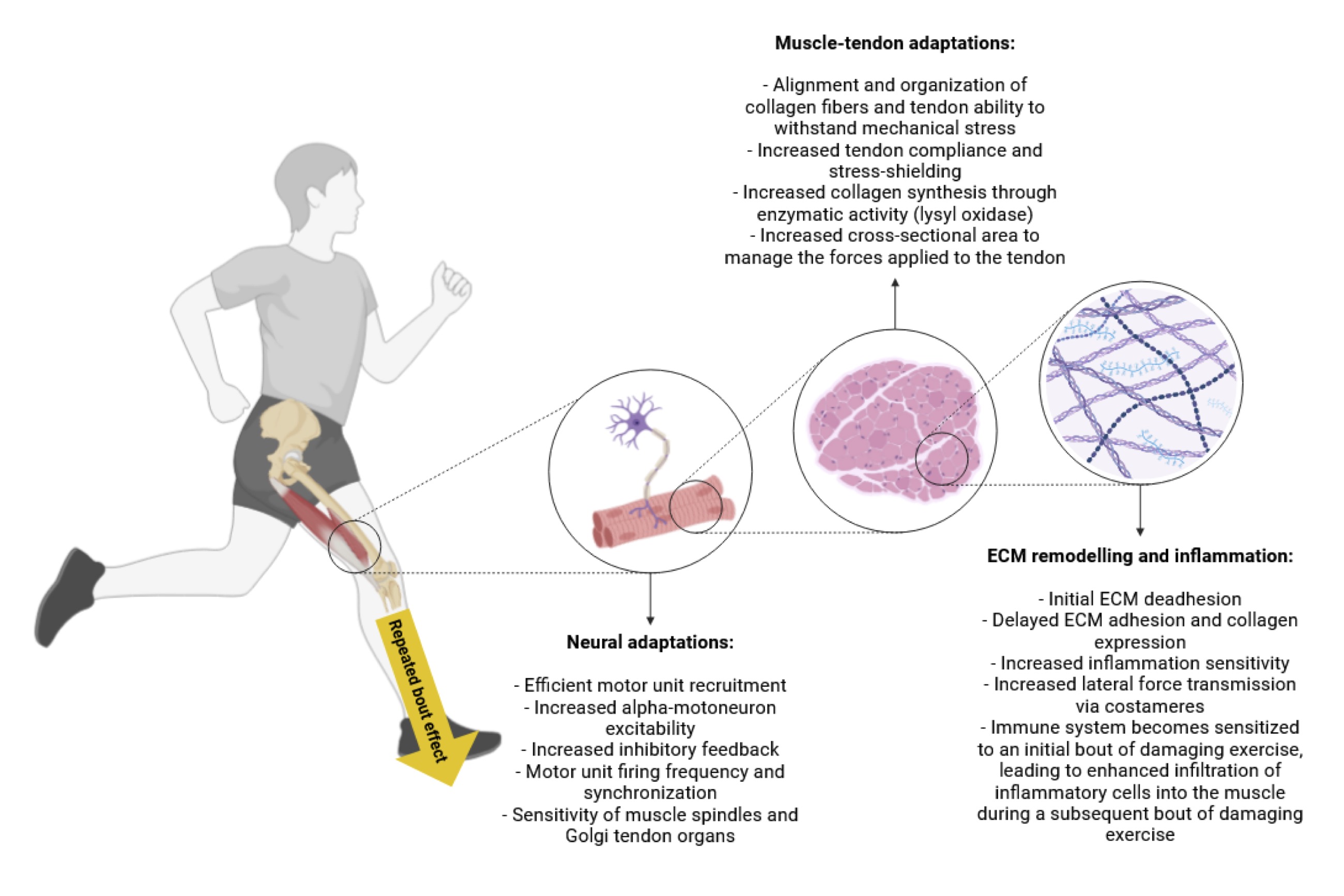
Fig. 4: The repeated bout effect describes how muscle damage is attenuated after multiple sessions of eccentric exercise. During an initial bout of unfamiliar eccentric contractions, muscle fibers experience microtears in the sarcomeres and disruption of the extracellular matrix. On the molecular level, the muscle damage triggers a pronounced inflammatory response, characterized by elevated pro-inflammatory cytokines and infiltration of neutrophils and macrophages. These immune cells help clear debris and release signals that activate satellite cells—muscle stem cells critical for fiber repair and growth. As the muscle adapts with repeated exposure, several protective and reparative processes are enhanced. First, the inflammatory response becomes more regulated, limiting excessive inflammation and tissue breakdown. Second, structural proteins such as titin, desmin, and nebulin are reinforced or reorganized, improving the muscle fiber’s cytoskeletal integrity and resilience to mechanical stress. Satellite cell activation and fusion also become more efficient, bolstering the myofiber’s capacity for repair and hypertrophy. Meanwhile, remodeling of the extracellular matrix—through changes in collagen deposition and modulation of matrix metalloproteinases—provides a sturdier scaffold for muscle tissue. Neural adaptations further contribute by optimizing motor unit recruitment and synchrony, and changes in muscle-tendon properties (e.g., improved stiffness and compliance) help distribute forces more evenly. Collectively, these molecular and structural modifications—spanning inflammation, cytoskeletal fortification, satellite cell activity, and neural coordination—culminate in reduced muscle damage, faster recovery, and less soreness following subsequent bouts of eccentric exercise. This phenomenon encapsulates the repeated bout effect, highlighting the body’s remarkable ability to adapt and protect itself against repeated mechanical stress.
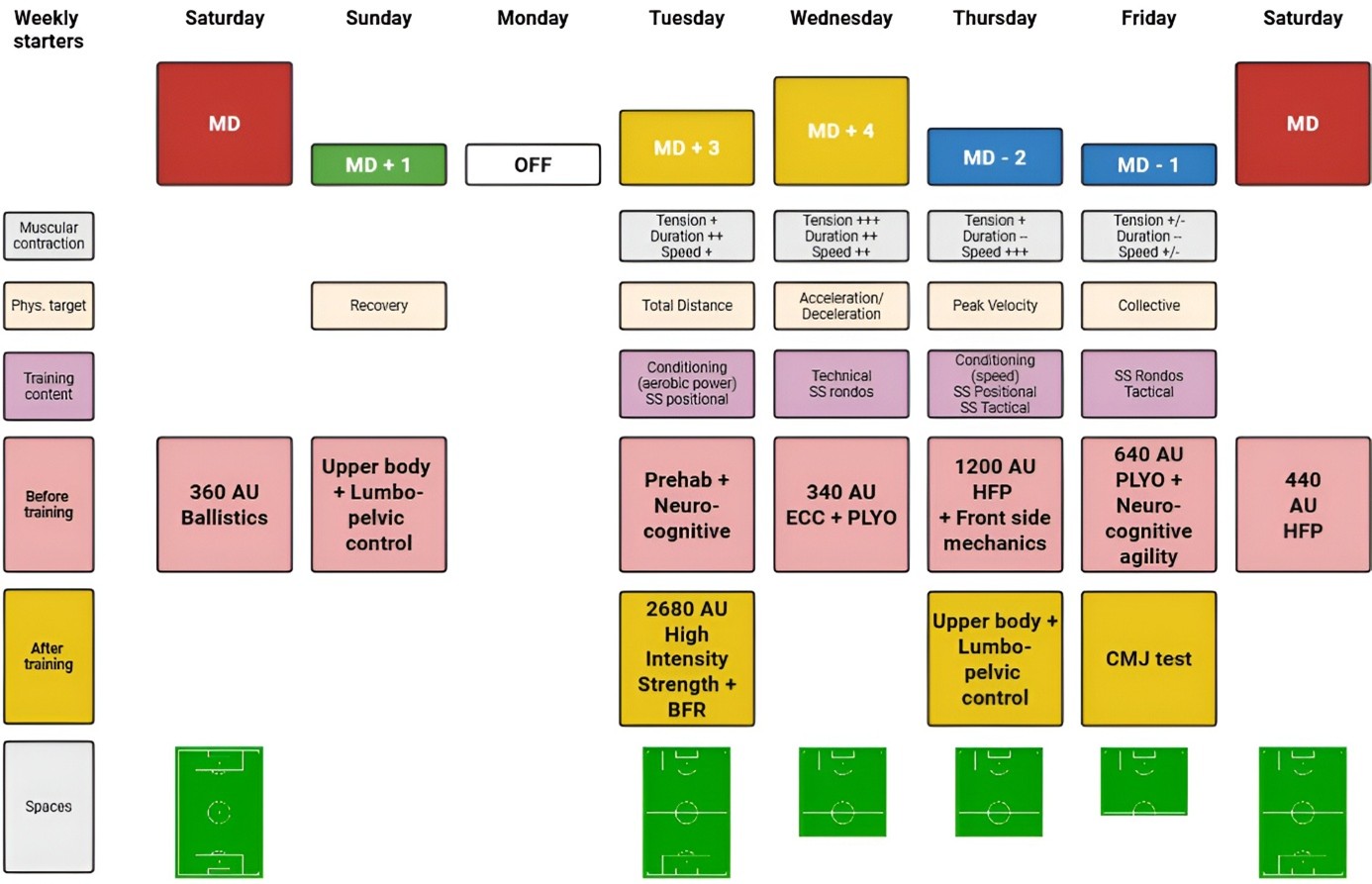
Fig. 5: The figure illustrates a weekly microdosing plan around match days, where small, high-quality stimuli (e.g., ECC + plyo, high-force plyo, neuro-cognitive work, BFR strength, lumbopelvic control) are delivered frequently with long recovery gaps to cumulate adaptations while limiting fatigue. Mechanical stretching causes matrix deformation, which transmits force through integrins to the cytoskeleton, activates FAK/Src kinases, engages the MAPK cascade, and results in phosphorylated ERK. Phosphorylated ERK increases the expression of collagen genes such as COL1A1 and COL3A1 and upregulates post-translational regulators P4HA and LOX, enhancing collagen synthesis and maturation in the extracellular matrix. The programming principle for tendons and ligaments is to use short bouts of about 10 minutes at moderate force, separated by at least six hours of complete rest. Practically, one or two sessions per day—morning and evening—satisfy the spacing requirement, maximizing connective-tissue anabolism while minimizing overload risk. Abbreviations: MD, Match Day; MD±X, Day relative to the match (e.g., MD+3 = three days after MD; MD−2 = two days before); OFF, Rest day; AU, Arbitrary Units (training-load metric, often RPE × duration); ECC, Eccentric (eccentric muscle work); PLYO, Plyometrics; HFP, Horizontal Force Production (sprint emphasis); BFR, Blood Flow Restriction training; SS, Small-Sided (small-sided games/drills); Rondos, Keep-away/possession games in a small area; Prehab, Prehabilitation (injury-prevention work); CMJ, Countermovement Jump (power/monitoring test); TD, Total Distance (running volume); ACC/DEC, Acceleration/Deceleration exposures; PV, Peak Velocity (max sprint speed); UB, Upper Body; LPC, Lumbopelvic Control (trunk/hip stability); Neuro-cog, Neurocognitive (perception–decision–action tasks); Front-side mechanics, Sprint front-side mechanics (knee lift/thigh recovery); + / ++ / +++ and − / −−, Relative emphasis or magnitude (low/medium/high; reduced/very low).
One of the key insights into muscle adaptation emerges from investigations into eccentric exercise. In a study exploring the RBE at the cellular level, it was found that initial eccentric contractions rapidly elevate reactive oxygen species (ROS) production, which serves as a signal to activate transcription factors involved in antioxidant defenses [575]. Central to this is the NRF2/KEAP1 axis: when intracellular ROS levels rise, NRF2 dissociates from KEAP1 and translocates to the nucleus, where it promotes the transcription of numerous antioxidant genes. The heightened oxidative defense established during this process diminishes muscle fiber damage in subsequent exercise bouts. Parallel proteomic analyses reveal the upregulation of FOXO3—an essential transcription factor for cellular stress responses—and heat shock proteins, which further enhance muscle resilience by stabilizing misfolded or damaged proteins. This suggests a form of molecular memory, where repeated exposure to mechanical and oxidative stress primes muscle fibers for future challenges, resulting in faster repair and improved functionality.
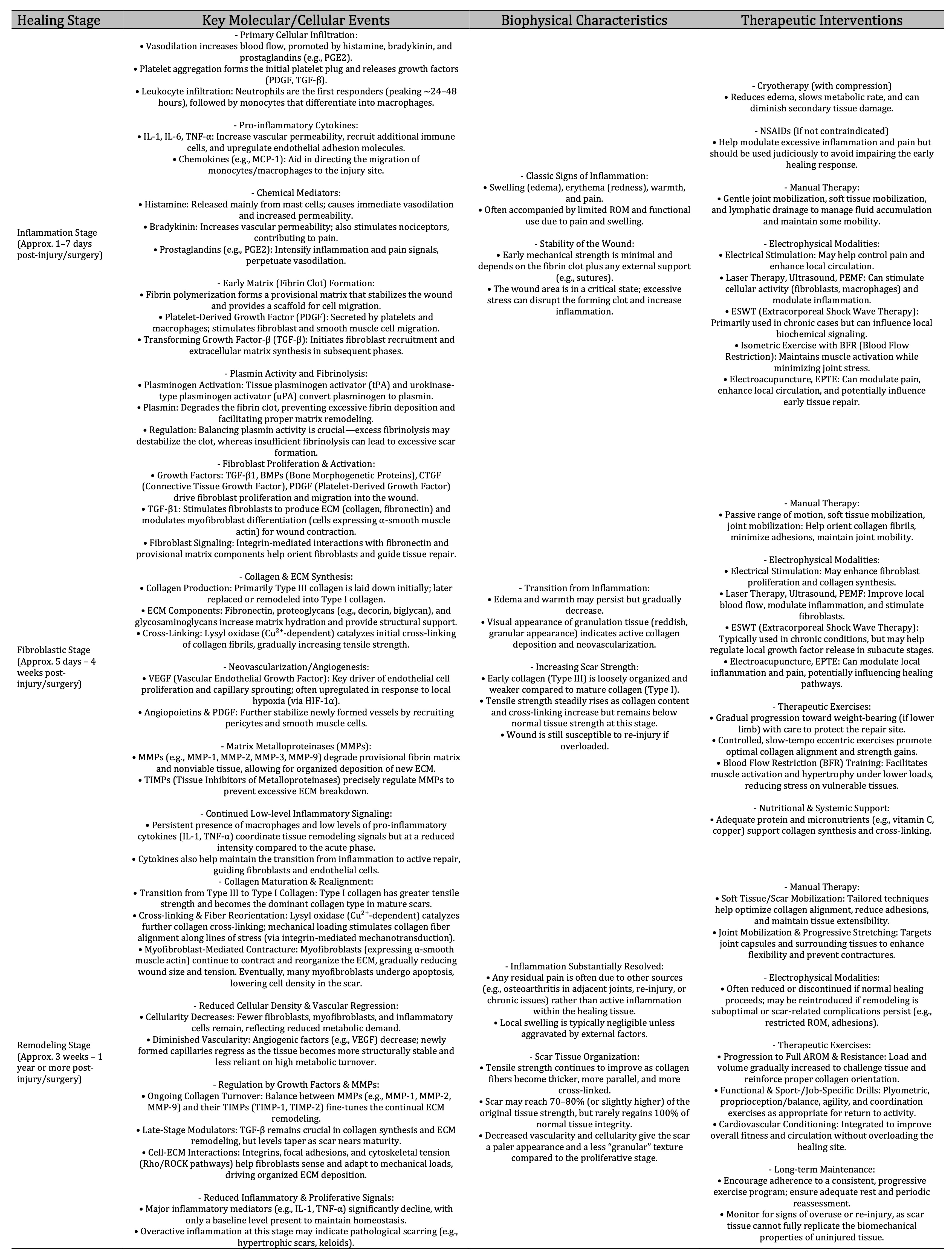
Table 7: The table outlines the healing stages of inflammation, fibroblastic, and remodeling, detailing cellular processes, biophysical characteristics, and therapeutic interventions. During inflammation, vasodilation and inflammatory cell invasion cause swelling and pain, treated with cryotherapy and NSAIDs; the fibroblastic stage involves growth factor-driven ECM synthesis, managed with manual therapy and therapeutic exercises; and the remodeling stage focuses on ECM organization and mechanical property enhancement, requiring tailored manual therapy and exercises to restore function and strength. Abbreviations: BMP, bone morphogenetic protein; CTGF, connective tissue growth factor; DOMS, delayed onset muscle soreness; ECM, extracellular matrix; ESWT, extracorporeal shock wave therapy; NSAIDs, non-steroidal anti-inflammatory drugs; PEMF, pulsed electromagnetic field therapy; BFR, blood flow restriciton; EPTE, percutaneous electrolysis therapy; PGE2, prostaglandin E2; ROM, range of motion; TGF-β1, transforming growth factor-β1
Complementing these antioxidant adaptations are changes in muscle regenerative capacity. Exercise-induced muscle damage (EIMD) activates satellite cells that reside between the basal lamina and sarcolemma of muscle fibers [576]. These progenitor cells are governed by the Pax7/Myf5/MyoD pathway, a hierarchical network of myogenic regulatory factors crucial for muscle repair. Upon mechanical injury, Pax7+ satellite cells proliferate, then differentiate under the guidance of Myf5 and MyoD, ultimately fusing to damaged fibers or forming new fibers. Over time, repeated loading reduces the surge of pro-inflammatory cytokines such as IL-6 and TNF-α, while simultaneously stabilizing mitochondrial function through the PGC-1α signaling axis. PGC-1α co-activates genes involved in oxidative phosphorylation and mitochondrial biogenesis, fostering an environment where muscle cells recover more quickly and sustain higher workloads with fewer signs of damage.
Adaptations to repeated bouts of mechanical loading are not confined to muscle alone. In the knee joint, for instance, chondrocytes and fibroblasts respond to repeated compression or tensile forces with marked changes in gene expression and extracellular matrix (ECM) remodeling [577]. Integrin-FAK signaling is pivotal here: integrins on the cell surface detect mechanical distortion of the ECM, transmitting signals via focal adhesion kinase (FAK) to initiate cascades that increase the synthesis of collagen type II, lubricin, and other ECM components. Repeated activation of this pathway, however, leads to a refined homeostatic balance in subsequent loading sessions—initially, NF-κB drives a transient inflammatory response, but repeated exposure triggers upregulation of anti-inflammatory mediators like IL-10 and the inhibition of NF-κB. This modulatory capacity underscores the tissue’s ability to “learn” from prior mechanical stress, bolstering cartilage lubrication and reducing wear over time.
These joint-level adaptations are part of a broader systemic network of mechanotransduction. Mechanosensitive ion channels such as PIEZO1 and TRPV4 open in response to fluid shear or tensile strain, permitting calcium influx that activates downstream pathways relevant to both muscle hypertrophy and tendon/ligament remodeling [578]. In tandem, metabolic regulators such as AMPK, SIRT1, and PGC-1α promote adjustments in energy substrate utilization, mitochondrial density, and overall cellular endurance. This synergy of mechanical and metabolic signaling optimizes tissue remodeling, fosters adaptation to higher training loads, and minimizes catabolic or pro-inflammatory cascades that might otherwise impair recovery.
Intriguingly, the evolution of these molecular adaptations varies significantly among individuals. Personal training history, prior injuries, and even epigenetic modifications can influence how robustly these pathways are activated. Individuals with a long-standing background of consistent loading—be it through endurance sports, resistance training, or repetitive occupational tasks—often exhibit rapid upregulation of antioxidant and anti-inflammatory defenses, swiftly recalibrating the muscle’s or joint’s response to stress. Conversely, novices or those with limited conditioning may encounter more pronounced inflammatory and oxidative responses, necessitating a more cautious progression in exercise or rehabilitation to avoid injury.
Taken together, these molecular insights underscore the importance of systematically structured loading protocols that consider not only the intensity, volume, and frequency of exercise but also each individual’s unique loading history. When appropriately calibrated, repeated mechanical stresses harness the beneficial effects of the RBE, augmenting antioxidant capacity, enhancing tissue repair mechanisms, and refining inflammatory responses. Through these interconnected pathways, muscles, tendons, ligaments, and cartilage become progressively more resilient, a transformation that holds significant promise for both athletic performance and long-term joint health. Ultimately, by acknowledging and leveraging the body’s capacity for molecular adaptation, clinicians and strength and conditioning professionals can develop periodized training and rehabilitation programs that maximize recovery and minimize injury risk, tailored to the nuanced biochemical and biomechanical profiles of each individual.
Conclusion
This review highlights the impact of mechanical loading on the knee joint at the molecular and cellular levels, emphasizing the pathways and factors involved in cartilage maintenance, synovial fluid regulation, and structural integrity. By analyzing these mechanisms, the study provides a scientific foundation for developing precise rehabilitation programs that adapt loading conditions to individual patient needs. Beyond advancing knee joint biomechanics, the findings support the translation of mechanobiological insights into clinical practice, aiming to accelerate recovery, prevent overuse injuries, and improve therapeutic outcomes.
The analysis of mechanotransduction mechanisms in cartilage, synovium, ligaments, and tendons underscores the critical role of different loading modalities—compression, tension, shear, and hydrostatic pressure—in shaping tissue responses. Understanding how key cells, such as chondrocytes, synoviocytes, and fibroblasts, process mechanical stimuli through integrins, ion channels, and signaling pathways like MAPK, NF-κB, and Wnt, is essential for optimizing rehabilitation strategies.
A mechanobiology-driven approach to rehabilitation enables the personalization of therapeutic interventions, including controlled loading, exercise regimens, manual therapy, and biophysical stimulation. By integrating biomechanics with cellular biology, these strategies enhance tissue repair, restore joint function, and prevent further degeneration. Ultimately, this review establishes a comprehensive framework for improving knee joint health and optimizing rehabilitation outcomes, contributing to more effective and patient-centered musculoskeletal therapies.
Limitations
Despite the promising insights into how mechanical loading can be harnessed to optimize knee joint health, several limitations and considerations warrant attention. First, while in vitro and animal studies have shed light on molecular pathways, translating these findings to human clinical practice can be challenging due to inter-individual variability in genetics, comorbidities, and lifestyles. Mechanotransduction pathways are highly interconnected, and their responses can be influenced by factors such as inflammation, hormonal changes, and biomechanical compensation patterns. Consequently, a one-size-fits-all loading paradigm may overlook the nuanced ways in which individuals respond to different mechanical stimuli.
Second, the complexity of knee pathologies—ranging from degenerative osteoarthritis to acute ligament injuries—demands tailored approaches that consider not only mechanical but also biochemical and inflammatory contexts. Inconsistencies in the literature regarding optimal loading protocols underscore the need for robust, controlled clinical trials that can validate specific dosing regimens of exercise or physical therapy. Furthermore, while emerging technologies (e.g., wearable sensors, motion-capture systems) hold promise for monitoring joint mechanics in real-time, their cost, accessibility, and integration into standard clinical workflows remain practical hurdles.
Third, ensuring adherence to personalized rehabilitation programs can be difficult, especially given varying patient motivations, socioeconomic barriers, and differences in healthcare access. Long-term patient follow-up and engagement are critical for maintaining therapeutic gains, yet these aspects are often underreported or inconsistently addressed in current research. Lastly, mechanobiological interventions cannot be viewed in isolation; complementary strategies—such as nutritional support, pharmacological management of pain and inflammation, and psychosocial interventions—must be integrated for truly holistic musculoskeletal rehabilitation.
By recognizing these challenges and systematically addressing them in future research, clinicians and scientists can refine mechanobiology-driven protocols to achieve more reliable and generalized benefits for knee joint health. This critical perspective ensures that the field continues to evolve toward evidence-based, individualized rehabilitation practices that maximize therapeutic impact while minimizing risks.
Disclosure Statement
The authors have nothing to disclose.
References
| 1 | Maffulli N, Cuozzo F, Migliorini F, Oliva F. The tendon unit: biochemical, biomechanical, hormonal
influences. J Orthop Surg Res. 2023 Apr 21;18(1):311 doi: 10.1186/s13018-023-03796-4.
https://doi.org/10.1186/s13018-023-03796-4 |
| 2 | Kramer, W. C., Hendricks, K. J., & Wang, J. (2011). Pathogenetic mechanisms of posttraumatic
osteoarthritis: Opportunities for early intervention. International Journal of Clinical Practice.
Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3228584/
|
| 3 | Li, H., Chen, C., & Chen, S. (2015). Posttraumatic knee osteoarthritis following anterior cruciate
ligament injury: Potential biochemical mediators of degenerative alteration and specific biomarkers.
Biomedical Reports. Retrieved from https://www.spandidos-publications.com/10.3892/br.2014.404
https://doi.org/10.3892/br.2014.404 |
| 4 | Varady, N. H., & Grodzinsky, A. J. (2016). Osteoarthritis year in review 2015: Mechanics.
Osteoarthritis and Cartilage. Retrieved from
https://www.sciencedirect.com/science/article/pii/S1063458415013175
https://doi.org/10.1016/j.joca.2015.08.018 |
| 5 | Hodgkinson, T., Amado, I. N., & O'Brien, F. J. (2022). The role of mechanobiology in bone and
cartilage model systems in characterizing initiation and progression of osteoarthritis. APL
Bioengineering. Retrieved from https://pubs.aip.org/aip/apb/article/6/1/011501/2820313
https://doi.org/10.1063/5.0068277 |
| 6 | Gangi, L. R., Petersen, C. A., & Oungoulian, S. R. (2022). A friction testing-bioreactor device
for the study of synovial joint biomechanics, mechanobiology, and physical regulation. Journal of
Biomechanics. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC10258606/
https://doi.org/10.3791/63880-v |
| 7 | Wang, N., Lu, Y., & Rothrauff, B. B. (2023). Mechanotransduction pathways in articular
chondrocytes and the emerging role of estrogen receptor-α. Bone Research. Retrieved from
https://www.nature.com/articles/s41413-023-00248-x
https://doi.org/10.1038/s41413-023-00248-x |
| 8 | Hodgkinson, T., Kelly, D. C., & Curtin, C. M. (2022). Mechanosignalling in cartilage: An emerging
target for the treatment of osteoarthritis. Nature Reviews Rheumatology. Retrieved from
https://www.nature.com/articles/s41584-021-00724-w
https://doi.org/10.1038/s41584-021-00724-w |
| 9 | Carter, D. R., Beaupré, G. S., & Wong, M. (2004). The Mechanobiology of Articular Cartilage
Development and Degeneration. Clinical Orthopaedics and Related Research. Retrieved from
https://journals.lww.com/clinorthop/fulltext/2004/10001/The_Mechanobiology_of_Articular_Cartilage.14.aspx
https://doi.org/10.1097/01.blo.0000144970.05107.7e |
| 10 | Michalaki, E., & Nepiyushchikh, Z. (2022). Effect of human synovial fluid from osteoarthritis
patients and healthy individuals on lymphatic contractile activity. Journal of Biomechanics.
Retrieved from
https://asmedigitalcollection.asme.org/biomechanical/article-abstract/144/7/071012/1135120
https://doi.org/10.1115/1.4053749 |
| 11 | Sanchez-Adams, J., Leddy, H. A., & McNulty, A. L. (2014). The mechanobiology of articular
cartilage: Bearing the burden of osteoarthritis. Current Rheumatology Reports. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4682660/
https://doi.org/10.1007/s11926-014-0451-6 |
| 12 | Gilbert, S. J., Bonnet, C. S., & Blain, E. J. (2021). Mechanical cues: Bidirectional reciprocity
in the extracellular matrix drives mechano-signalling in articular cartilage. International Journal
of Molecular Sciences. Retrieved from https://www.mdpi.com/1422-0067/22/24/13595
https://doi.org/10.3390/ijms222413595 |
| 13 | Popov, V. L., Poliakov, A. M., & Pakhaliuk, V. I. (2021). Synovial joints: Tribology,
regeneration, regenerative rehabilitation, and arthroplasty. Lubricants. Retrieved from
https://www.mdpi.com/2075-4442/9/2/15
https://doi.org/10.3390/lubricants9020015 |
| 14 | Wang, Y., Yan, Y., & Rothrauff, B. B. (2023). Biomechanical and mechanotransductive responses in
articular cartilage under dynamic mechanical loading. Bone Research. Retrieved from
https://www.nature.com/articles/s41413-023-00248-x
|
| 15 | Kokubun, T., Kanemura, N., & Murata, K. (2016). Effect of changing the joint kinematics of knees
with a ruptured anterior cruciate ligament on molecular biological responses. American Journal of
Sports Medicine. Retrieved from https://journals.sagepub.com/doi/abs/10.1177/0363546516654687
https://doi.org/10.1177/0363546516654687 |
| 16 | Nakagawa, Y., Sekiya, I., & Carballo, C. B. (2017). Basic science of articular cartilage. Clinics
in Sports Medicine. Retrieved from
https://www.sportsmed.theclinics.com/article/S0278-5919(17)30003-0/abstract
|
| 17 | Sakata, R., McNary, S. M., & Miyatake, K. (2015). Stimulation of the superficial zone protein and
lubrication in the articular cartilage by human platelet-rich plasma. American Journal of Sports
Medicine. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4930492/
https://doi.org/10.1177/0363546515575023 |
| 18 | Carter, D. R., & Wong, M. (2004). Mechanobiology of articular cartilage and osteoarthritis.
Clinical Orthopaedics and Related Research. Retrieved from
https://journals.lww.com/clinorthop/fulltext/2004/10001/The_Mechanobiology_of_Articular_Cartilage.14.aspx
|
| 19 | Michalaki, E., Nepiyushchikh, Z., & Welch, W. C. (2022). Synovial fluid dynamics in healthy and
osteoarthritic joints: Lymphatic contributions. Journal of Biomechanics. Retrieved from
https://asmedigitalcollection.asme.org/biomechanical/article-abstract/144/7/071012/1135120
https://doi.org/10.1115/1.4053749 |
| 20 | Li, Y., Zhong, H., & Yang, H. (2021). Recent advances in understanding the role of cartilage
lubrication in osteoarthritis. Molecules. Retrieved from https://www.mdpi.com/1420-3049/26/20/6122
https://doi.org/10.3390/molecules26206122 |
| 21 | O'Conor, C. J., Ramalingam, S., & Zelenski, N. A. (2016). Cartilage-specific knockout of the
mechanosensory ion channel TRPV4 decreases age-related osteoarthritis. Scientific Reports. Retrieved
from https://www.nature.com/articles/srep29053
https://doi.org/10.1038/srep29053 |
| 22 | Xu, B. Y., Jin, Y., & Ma, X. H. (2020). The potential role of mechanically sensitive ion channels
in the physiology, injury, and repair of articular cartilage. Journal of Orthopaedic Research.
Retrieved from https://journals.sagepub.com/doi/abs/10.1177/2309499020950262
https://doi.org/10.1177/2309499020950262 |
| 23 | Gilbert, S. J., Bonnet, C. S., & Blain, E. J. (2021). Mechanical cues in articular cartilage
mechanotransduction. International Journal of Molecular Sciences. Retrieved from
https://www.mdpi.com/1422-0067/22/24/13595
https://doi.org/10.3390/ijms222413595 |
| 24 | Wang, N., Lu, Y., & Rothrauff, B. B. (2023). Mechanotransduction pathways and estrogen receptor-α
in chondrocytes. Bone Research. Retrieved from https://www.nature.com/articles/s41413-023-00248-x
|
| 25 | Hodgkinson, T., Kelly, D. C., & Curtin, C. M. (2022). Mechanosignalling in cartilage: A target for
osteoarthritis treatment. Nature Reviews Rheumatology. Retrieved from
https://www.nature.com/articles/s41584-021-00724-w
https://doi.org/10.1038/s41584-021-00724-w |
| 26 | Segarra-Queralt, M., Crump, K., & Pascuet-Fontanet, A. (2024). The interplay between biochemical
mediators and mechanotransduction in chondrocytes: Unraveling the differential responses in primary
knee osteoarthritis. Physics of Life Reviews. Retrieved from
https://www.sciencedirect.com/science/article/pii/S1571064524000101
https://doi.org/10.1016/j.plrev.2024.02.003 |
| 27 | Liu, K., Zhang, B., & Zhang, X. (2024). Promoting articular cartilage regeneration through
microenvironmental regulation. Journal of Immunology Research. Retrieved from
https://onlinelibrary.wiley.com/doi/abs/10.1155/2024/4751168
https://doi.org/10.1155/2024/4751168 |
| 28 | Fox, D. B., & Warnock, J. J. (2011). Cell-based meniscal tissue engineering: A case for
synoviocytes. Clinical Orthopaedics and Related Research. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3171537/
https://doi.org/10.1007/s11999-011-1824-z |
| 29 | Maglaviceanu, A., Wu, B., & Kapoor, M. (2021). Fibroblast‐like synoviocytes: Role in synovial
fibrosis associated with osteoarthritis. Wound Repair and Regeneration. Retrieved from
https://onlinelibrary.wiley.com/doi/abs/10.1111/wrr.12939
https://doi.org/10.1111/wrr.12939 |
| 30 | McNulty, A. L., Sanchez-Adams, J., & Leddy, H. A. (2014). The mechanobiology of articular
cartilage: Bearing the burden of osteoarthritis. Current Rheumatology Reports. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4682660/
https://doi.org/10.1007/s11926-014-0451-6 |
| 31 | Jay, G. D. (2004). Lubricin and surfacing of articular joints. Current Opinion in Orthopaedics.
Retrieved from
https://journals.lww.com/co-ortho/fulltext/2004/10000/Lubricin_and_surfacing_of_articular_joints.8.aspx
https://doi.org/10.1097/01.bco.0000136127.00043.a8 |
| 32 | Steinecker-Frohnwieser, B., Lohberger, B., & Schuh, E. (2023). Activation of the mechanosensitive
ion channels Piezo1 and TRPV4 in primary human healthy and osteoarthritic chondrocytes.
International Journal of Molecular Sciences. Retrieved from https://www.mdpi.com/1422-0067/24/9/7868
https://doi.org/10.3390/ijms24097868 |
| 33 | Hu, T., Zhang, Z., Deng, C., Ma, X., & Liu, X. (2022). Effects of β2 integrins on osteoclasts,
macrophages, chondrocytes, and synovial fibroblasts in osteoarthritis. Biomolecules. Retrieved from
https://www.mdpi.com/2218-273X/12/11/1653
https://doi.org/10.3390/biom12111653 |
| 34 | Kim, J. H., Lee, G., Won, Y., & Lee, M. (2015). Matrix cross-linking-mediated mechanotransduction
promotes posttraumatic osteoarthritis. Proceedings of the National Academy of Sciences. Retrieved
from https://www.pnas.org/doi/pdf/10.1073/pnas.1505700112
https://doi.org/10.1073/pnas.1505700112 |
| 35 | Blewis, M. E., Lao, B. J., & Schumacher, B. L. (2010). Interactive cytokine regulation of
synoviocyte lubricant secretion. Tissue Engineering Part A. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2862605/
https://doi.org/10.1089/ten.tea.2009.0210 |
| 36 | Wang, B. Y., Xu, Y., & Jin, X. H. (2020). The potential role of mechanically sensitive ion
channels in the physiology, injury, and repair of articular cartilage. Journal of Orthopaedic
Research. Retrieved from https://journals.sagepub.com/doi/abs/10.1177/2309499020950262
|
| 37 | Sato, M., Nagata, K., Kuroda, S., & Horiuchi, S. (2014). Low-intensity pulsed ultrasound activates
integrin-mediated mechanotransduction pathway in synovial cells. Annals of Biomedical Engineering.
Retrieved from https://link.springer.com/article/10.1007/s10439-014-1081-x
https://doi.org/10.1007/s10439-014-1081-x |
| 38 | Pap, T., & Korb-Pap, A. (2015). Cartilage damage in osteoarthritis and rheumatoid arthritis: Two
unequal siblings. Nature Reviews Rheumatology. Retrieved from
https://www.nature.com/articles/nrrheum.2015.95
https://doi.org/10.1038/nrrheum.2015.95 |
| 39 | Salter, D. M., Millward-Sadler, S. J., & Nuki, G. (2001). Integrin-interleukin-4
mechanotransduction pathways in human chondrocytes. Clinical Orthopaedics and Related Research.
Retrieved from
https://journals.lww.com/corr/fulltext/2001/10001/Integrin_Interleukin_4_Mechanotransduction.6.aspx
https://doi.org/10.1097/00003086-200110001-00006 |
| 40 | Walsh, D. A., & Bonnet, C. S. (2005). Osteoarthritis, angiogenesis and inflammation. Rheumatology.
Retrieved from https://academic.oup.com/rheumatology/article-abstract/44/1/7/1784578
https://doi.org/10.1093/rheumatology/keh344 |
| 41 | Selig, M., Lauer, J. C., & Hart, M. L. (2020). Mechanotransduction and stiffness-sensing:
Mechanisms and opportunities to control cell phenotype. International Journal of Molecular Sciences.
Retrieved from https://www.mdpi.com/1422-0067/21/15/5399
https://doi.org/10.3390/ijms21155399 |
| 42 | Böker, K. O., Taheri, S., & Shang, X. (2021). Extracellular vesicles allow epigenetic
mechanotransduction between chondrocytes and osteoblasts. International Journal of Molecular
Sciences. Retrieved from https://www.mdpi.com/1422-0067/22/24/13282
https://doi.org/10.3390/ijms222413282 |
| 43 | Millward-Sadler, S. J., Wright, M. O., & Lee, H. S. (1999). Integrin-regulated secretion of
interleukin 4: A novel pathway of mechanotransduction in human articular chondrocytes. The Journal
of Cell Biology. Retrieved from https://rupress.org/jcb/article-abstract/145/1/183/29270
https://doi.org/10.1083/jcb.145.1.183 |
| 44 | Segarra-Queralt, M., Piella, G., & Noailly, J. (2023). Network-based modelling of
mechano-inflammatory chondrocyte regulation in early osteoarthritis. Frontiers in Bioengineering and
Biotechnology. Retrieved from https://www.frontiersin.org/articles/10.3389/fbioe.2023.1006066/full
https://doi.org/10.3389/fbioe.2023.1006066 |
| 45 | Shang, X., Böker, K. O., & Lehmann, W. (2021). Crosstalk between cartilage and bone:
Mechanotransduction through miRNA regulation. International Journal of Molecular Sciences. Retrieved
from https://www.mdpi.com/1422-0067/22/24/13282
https://doi.org/10.3390/ijms222413282 |
| 46 | Pap, T., & Korb-Pap, A. (2015). Inflammatory responses and cartilage degeneration in arthritis:
Molecular mechanisms. Nature Reviews Rheumatology. Retrieved from
https://www.nature.com/articles/nrrheum.2015.95
|
| 47 | Salter, D. M., Millward-Sadler, S. J., & Nuki, G. (2001). Mechanotransduction pathways in
chondrocytes: Role of integrin signaling. Clinical Orthopaedics and Related Research. Retrieved from
https://journals.lww.com/corr/fulltext/2001/10001/Integrin_Interleukin_4_Mechanotransduction.6.aspx
|
| 48 | Feng, Rui & Hu, Wenhui & Li, Yuheng & Yao, Xuan & Li, Jianmei & Li, Xiaoming & Zhang, Jing & Wu,
Yu & Kang, Fei & Dong, Shiwu. (2024). Mechanotransduction in subchondral bone microenvironment and
targeted interventions for osteoarthritis. Mechanobiology in Medicine. 100043
10.1016/j.mbm.2024.100043.
https://doi.org/10.1016/j.mbm.2024.100043 |
| 49 | Nims R, Palmer DR, Kassab J, Zhang B, Guilak F. The chondrocyte "mechanome": Activation of the
mechanosensitive ion channels TRPV4 and PIEZO1 drives unique transcriptional signatures. FASEB J.
2024 Jul 15;38(13):e23778 doi: 10.1096/fj.202400883R. PMID: 38959010; PMCID: PMC11327906.
https://doi.org/10.1096/fj.202400883R |
| 50 | Glyn-Jones, S., Palmer, A. J. R., Agricola, R., Price, A. J., Vincent, T. L., Weinans, H., & Carr,
A. J. (2015). Osteoarthritis. The Lancet, 386(9991), 376-387 DOI: 10.1016/S0140-6736(14)60802-3.
https://doi.org/10.1016/S0140-6736(14)60802-3 |
| 51 | Goldring, M. B., & Goldring, S. R. (2007). Osteoarthritis. Journal of Cellular Physiology, 213(3),
626-634 DOI: 10.1002/jcp.21258.
https://doi.org/10.1002/jcp.21258 |
| 52 | Millward-Sadler, S. J., & Salter, D. M. (2004). Integrin-dependent signal cascades in chondrocyte
mechanotransduction. Annals of Biomedical Engineering, 32(3), 435-446 DOI:
10.1023/B:ABME.0000017544.87366.41.
https://doi.org/10.1023/B:ABME.0000017538.72511.48 |
| 53 | Li, Y., Xiao, W., Wang, X., & Luo, H. (2018). The role of MAPK/NF-κB pathway in the development of
osteoarthritis. Frontiers in Physiology, 9, 706 DOI: 10.3389/fphys.2018.00706.
https://doi.org/10.3389/fphys.2018.00706 |
| 54 | Kyriakis, J. M., & Avruch, J. (2012). Mammalian MAPK signal transduction pathways activated by
stress and inflammation: A 10-year update. Physiological Reviews, 92(2), 689-737 DOI:
10.1152/physrev.00028.2011.
https://doi.org/10.1152/physrev.00028.2011 |
| 55 | Manning, G., Whyte, D. B., Martinez, R., Hunter, T., & Sudarsanam, S. (2002). The protein kinase
complement of the human genome. Science, 298(5600), 1912-1934 DOI: 10.1126/science.1075762.
https://doi.org/10.1126/science.1075762 |
| 56 | Fan, Z., & Guan, J. L. (2011). Compensatory role of ERK1/2 signaling in osteoarthritis
pathogenesis. Current Opinion in Rheumatology, 23(4), 448-452 DOI: 10.1097/BOR.0b013e3283475b93.
|
| 57 | Loeser, R. F. (2014). Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix
Biology, 39, 11-16 DOI: 10.1016/j.matbio.2014.08.007.
https://doi.org/10.1016/j.matbio.2014.08.007 |
| 58 | Henrotin, Y., Pesesse, L., & Sanchez, C. (2011). Subchondral bone and osteoarthritis: Biological
and cellular aspects. Osteoarthritis and Cartilage, 20(3), 288-293 DOI: 10.1016/j.joca.2012.01.013.
https://doi.org/10.1016/j.joca.2012.01.013 |
| 59 | Vincent, T. L., & Saklatvala, J. (2006). Basic science: Mechanisms of joint destruction.
Baillière's Best Practice & Research Clinical Rheumatology, 20(4), 723-740 DOI:
10.1016/j.berh.2006.05.009.
https://doi.org/10.1016/j.berh.2006.05.009 |
| 60 | Robinin protects chondrocytes injury via TLR2/TLR4/NF-κB signaling in osteoarthritis. Cell
Biochemistry and Biophysics, 2024 Dec. DOI: 10.1007/s12013-024-01497-1.
https://doi.org/10.1007/s12013-024-01497-1 |
| 61 | Cinnamaldehyde-Mediated Suppression of MMP-13, COX-2, and IL-6 Through MAPK and NF-κB Signaling
Inhibition in Chondrocytes and Synoviocytes Under Inflammatory Conditions. International Journal of
Molecular Sciences, 25(23), 2024 DOI: 10.3390/ijms252312914.
https://doi.org/10.3390/ijms252312914 |
| 62 | CRNDE alleviates IL-1β-induced chondrocyte damage by modulating miR-31/NF-κB pathway. Journal of
Orthopaedic Surgery and Research, 19(1), 860 DOI: 10.1186/s13018-024-05182-0.
https://doi.org/10.1186/s13018-024-05182-0 |
| 63 | Soyasaponin Bb/Gelatin-Methacryloyl Hydrogel for Cartilage Inflammation Inhibition. ACS Omega,
9(50), 49597-49608 DOI: 10.1021/acsomega.4c07489.
https://doi.org/10.1021/acsomega.4c07489 |
| 64 | Sliding Hydrogels Reveal the Modulation of Mechanosensing Attenuates the Inflammatory Phenotype of
Osteoarthritic Chondrocytes in 3D. Journal of Biomedical Materials Research Part A, 113(1), e37861
DOI: 10.1002/jbm.a.37861.
https://doi.org/10.1002/jbm.a.37861 |
| 65 | Mechanical loading rescues mechanoresponsiveness in a human osteoarthritis explant model despite
Wnt activation. Osteoarthritis and Cartilage, 2024 Mar. DOI: 10.1016/j.joca.2024.02.945.
https://doi.org/10.1016/j.joca.2024.02.945 |
| 66 | Cbfβ regulates Wnt/β-catenin, Hippo/Yap, and TGFβ signaling pathways in articular cartilage
homeostasis and protects from ACLT surgery-induced osteoarthritis. eLife, 13, 2024 May. DOI:
10.7554/eLife.95640.
https://doi.org/10.7554/eLife.95640 |
| 67 | Dissecting SOX9 dynamics reveals its differential regulation in osteoarthritis. Journal of
Cellular Physiology, 239(12), e31443 DOI: 10.1002/jcp.31443.
https://doi.org/10.1002/jcp.31443 |
| 68 | β-catenin Orchestrates Gli1+ Cell Fate in Condylar Development and TMJOA. Journal of Dental
Research, 103(12), 1291-1301 DOI: 10.1177/00220345241274354.
https://doi.org/10.1177/00220345241274354 |
| 69 | IGF1 drives Wnt-induced joint damage and is a potential therapeutic target for osteoarthritis.
Nature Communications, 15(1), 9170 DOI: 10.1038/s41467-024-53604-8.
https://doi.org/10.1038/s41467-024-53604-8 |
| 70 | Selenium nanoparticles ameliorate lumbar disc degeneration by restoring GPX1-mediated redox
homeostasis and mitochondrial function of nucleus pulposus cells. Journal of Nanobiotechnology,
22(1), 634 DOI: 10.1186/s12951-024-02890-x.
https://doi.org/10.1186/s12951-024-02890-x |
| 71 | The Therapeutic Potential of Adipose-Derived Mesenchymal Stem Cell Secretome in Osteoarthritis: A
Comprehensive Study. International Journal of Molecular Sciences, 25(20), 11287 DOI:
10.3390/ijms252011287.
https://doi.org/10.3390/ijms252011287 |
| 72 | Duhuo Jisheng Decoction in reduction of inflammatory response via Transforming growth
factor-β/Smad signaling pathway for repairing rabbit articular cartilage injury: A Randomized
Controlled Trial. International Immunopharmacology, 144, 113646 DOI: 10.1016/j.intimp.2024.113646.
https://doi.org/10.1016/j.intimp.2024.113646 |
| 73 | Matrigel-encapsulated articular cartilage-derived fibronectin adhesion assay-derived
chondroprogenitors for enhanced chondrogenic differentiation: An in vitro evaluation. Tissue & Cell,
92, 102638 DOI: 10.1016/j.tice.2024.102638.
https://doi.org/10.1016/j.tice.2024.102638 |
| 74 | Chondroprotective Effect of Campylaephora hypnaeoides Extract in Primary Chondrocytes and Rat OA
Model. International Journal of Molecular Sciences, 25(24), 13391 DOI: 10.3390/ijms252413391.
https://doi.org/10.3390/ijms252413391 |
| 75 | Magnetic-Responsive Carbon Nanotubes Composite Scaffolds for Chondrogenic Tissue Engineering.
Advanced Healthcare Materials, 12(30), e2301787 DOI: 10.1002/adhm.202301787.
https://doi.org/10.1002/adhm.202301787 |
| 76 | Comparison of Different Methods of Semiquantitative Assessment and Subjective Scores for
Retropatellar Articular Cartilage Evaluation in Advancing Osteoarthritis. Ortopedia, Traumatologia,
Rehabilitacja, 25(6), 297-305 DOI: 10.5604/01.3001.0054.2881.
https://doi.org/10.5604/01.3001.0054.2881 |
| 77 | Extracellular vesicles derived from mesenchymal stem cells mediate extracellular matrix remodeling
in osteoarthritis through the transport of microRNA-29a. World Journal of Stem Cells, 16(2), 191-206
DOI: 10.4252/wjsc.v16.i2.191.
https://doi.org/10.4252/wjsc.v16.i2.191 |
| 78 | Transient receptor potential vanilloid 4 regulates extracellular matrix composition and mediates
load-induced intervertebral disc degeneration in a mouse model. Osteoarthritis and Cartilage, 32(7),
881-894 DOI: 10.1016/j.joca.2024.04.001.
https://doi.org/10.1016/j.joca.2024.04.001 |
| 79 | Repair of annulus fibrosus defects using decellularized annulus fibrosus matrix/chitosan hybrid
hydrogels. Journal of Orthopaedic Surgery and Research, 19(1), 535 DOI: 10.1186/s13018-024-05017-y.
https://doi.org/10.1186/s13018-024-05017-y |
| 80 | Functional Analysis of OA-Associated PIEZO1 Human Variants on OA Susceptibility. Matheson, Derek
et al. Osteoarthritis and Cartilage, Volume 32, S374
https://doi.org/10.1016/j.joca.2024.02.543 |
| 81 | Holm PM, Juhl CB, Culvenor AG, Whittaker JL, Crossley KM, Roos EM, Patterson BE, Larsson S,
Struglics A, Bricca A. The Effects of Different Management Strategies or Rehabilitation Approaches
on Knee Joint Structural and Molecular Biomarkers Following Traumatic Knee Injury: A Systematic
Review of Randomized Controlled Trials for the OPTIKNEE Consensus. J Orthop Sports Phys Ther. 2023
Apr;53(4):172-193 doi: 10.2519/jospt.2023.11576
https://doi.org/10.2519/jospt.2023.11576 |
| 82 | Golovach, I., Rekalov, D., & Akimov, Y. (2023). Molecular mechanisms and potential applications of
chondroitin sulphate in managing post-traumatic osteoarthritis. Journal of Orthopedic Research.
Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10634410/
https://doi.org/10.5114/reum/172211 |
| 83 | Qadri, M. M. (2023). Targeting CD44 receptor pathways in degenerative joint diseases: Involvement
of Proteoglycan-4 (PRG4). Pharmaceuticals. Retrieved from https://www.mdpi.com/1424-8247/16/10/1425
https://doi.org/10.3390/ph16101425 |
| 84 | De Oliveira, P. G., Farinon, M., & Sanchez-Lopez, E. (2019). Fibroblast-like synoviocytes glucose
metabolism as a therapeutic target in rheumatoid arthritis. Frontiers in Immunology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fimmu.2019.01743/full
https://doi.org/10.3389/fimmu.2019.01743 |
| 85 | Roggio, F., Petrigna, L., & Trovato, B. (2023). The role of lubricin, irisin, and exercise in the
prevention and treatment of osteoarthritis. International Journal of Molecular Sciences. Retrieved
from https://www.mdpi.com/1422-0067/24/6/5126
https://doi.org/10.3390/ijms24065126 |
| 86 | Hu, Z., Li, Y., & Zhang, L. (2024). Metabolic changes in fibroblast-like synoviocytes in
rheumatoid arthritis: State of the art review. Frontiers in Immunology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fimmu.2024.1250884/full
https://doi.org/10.3389/fimmu.2024.1250884 |
| 87 | Saha, S., & Rebouh, N. Y. (2023). Anti-Osteoarthritis Mechanism of the Nrf2 Signaling Pathway.
Biomedicines. Retrieved from https://www.mdpi.com/2227-9059/11/12/3176
https://doi.org/10.3390/biomedicines11123176 |
| 88 | Jeon, O. H., & David, N. (2018). Senescent cells and osteoarthritis: A painful connection. Journal
of Clinical Investigation. Retrieved from https://www.jci.org/articles/view/95147
https://doi.org/10.1172/JCI95147 |
| 89 | De Sire, A., Marotta, N., & Marinaro, C. (2021). Role of physical exercise and nutraceuticals in
modulating molecular pathways of osteoarthritis. International Journal of Molecular Sciences.
Retrieved from https://www.mdpi.com/1422-0067/22/11/5722
https://doi.org/10.3390/ijms22115722 |
| 90 | Tong, Y., Deng, Q., & Li, X. (2023). Advances of small molecule drugs regulating fibroblast-like
synovial proliferation for rheumatoid arthritis. Frontiers in Pharmacology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fphar.2023.1230293/full
https://doi.org/10.3389/fphar.2023.1230293 |
| 91 | Bustamante, M. F., & Garcia-Carbonell, R. (2017). Fibroblast-like synoviocyte metabolism in the
pathogenesis of rheumatoid arthritis. Arthritis Research & Therapy. Retrieved from
https://link.springer.com/article/10.1186/S13075-017-1303-3
https://doi.org/10.1186/s13075-017-1303-3 |
| 92 | Mousavi, M. J., Karami, J., & Aslani, S. (2021). Transformation of fibroblast‐like synoviocytes in
rheumatoid arthritis: From friend to foe. Autoimmunity Highlights. Retrieved from
https://link.springer.com/article/10.1186/s13317-020-00145-x
https://doi.org/10.1186/s13317-020-00145-x |
| 93 | Li, Z., & Alexander, P. G. (2020). Pathogenesis of osteoarthritis: Risk factors, regulatory
pathways in chondrocytes, and experimental models. Biology. Retrieved from
https://www.mdpi.com/2079-7737/9/8/194
https://doi.org/10.3390/biology9080194 |
| 94 | Zhou, R., Fu, W., & Waxman, S. G. (2024). Ion channels in osteoarthritis: Emerging roles and
potential targets. Nature Reviews Rheumatology. Retrieved from
https://www.nature.com/articles/s41584-024-01146-0
https://doi.org/10.1038/s41584-024-01146-0 |
| 95 | Sanchez, C., & Florin, A. (2021). The damage-associated molecular patterns (DAMPs) as potential
targets to treat osteoarthritis. Frontiers in Medicine. Retrieved from
https://www.frontiersin.org/articles/10.3389/fmed.2020.607186/full
|
| 96 | Xu, Q., Kong, H., & Ren, S. (2023). Coix seed oil alleviates synovial angiogenesis in arthritis.
Chinese Medicine. Retrieved from https://link.springer.com/article/10.1186/s13020-023-00833-6
|
| 97 | Busa, P., & Kuthati, Y. (2022). Carnosine alleviates knee osteoarthritis via the Nrf2/HO-1
signaling pathway. Antioxidants. Retrieved from https://www.mdpi.com/2076-3921/11/6/1209
https://doi.org/10.3390/antiox11061209 |
| 98 | Elsaid, K. A., & Jay, G. D. (2023). Proteoglycan 4 (PRG4)/Lubricin and the extracellular matrix in
gout. Gout, Urate, and Crystal Diseases. Retrieved from https://www.mdpi.com/2813-4583/1/3/12
https://doi.org/10.3390/gucdd1030012 |
| 99 | Behl, T., & Chigurupati, S. (2021). Polyphenols targeting MAPK-mediated oxidative stress in
rheumatoid arthritis. Molecules. Retrieved from https://www.mdpi.com/1420-3049/26/21/6570
https://doi.org/10.3390/molecules26216570 |
| 100 | Lambert, C., & Sanchez, C. (2021). Damage-associated molecular patterns and their potential role
in osteoarthritis therapy. Frontiers in Medicine. Retrieved from
https://www.frontiersin.org/articles/10.3389/fmed.2020.607186/full
|
| 101 | Li, Q., Chen, Y., & Xie, Q. (2023). Targeting glycolytic pathways in synoviocytes for rheumatoid
arthritis therapy. Inflammation Research. Retrieved from
https://link.springer.com/article/10.1007/s00011-023-01807-y
https://doi.org/10.1007/s00011-023-01807-y |
| 102 | Schröder A, Nazet U, Muschter D, Grässel S, Proff P, Kirschneck C. Impact of Mechanical Load on
the Expression Profile of Synovial Fibroblasts from Patients with and without Osteoarthritis. Int J
Mol Sci. 2019 Jan 30;20(3):585 doi: 10.3390/ijms20030585 PMID: 30704030; PMCID: PMC6387339.
https://doi.org/10.3390/ijms20030585 |
| 103 | Wang, H., Zhang, W., Yuan, Z., Chu, G., & Sun, H. (2021). Moderate mechanical stimulation rescues
degenerative annulus fibrosus by suppressing caveolin-1 mediated pro-inflammatory signaling pathway.
The Journal of Biological Chemistry. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8040478/
|
| 104 | Schulz, H., & Wehland, M. (2024). Omics studies of specialized cells and stem cells under
microgravity conditions. International Journal of Molecular Sciences. Retrieved from
https://pmc.ncbi.nlm.nih.gov/articles/PMC11431953/
|
| 105 | Qin, H., Du, L., Luo, Z., He, Z., & Wang, Q. (2022). The therapeutic effects of low-intensity
pulsed ultrasound in musculoskeletal soft tissue injuries: Focusing on the molecular mechanism.
Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2022.1080430/full
https://doi.org/10.3389/fbioe.2022.1080430 |
| 106 | Jia, Y., Le, H., Wang, X., Zhang, J., & Liu, Y. (2023). Double-edged role of mechanical stimuli
and underlying mechanisms in cartilage tissue engineering. Frontiers in Bioengineering and
Biotechnology. Retrieved from https://www.frontiersin.org/articles/10.3389/fbioe.2023.1271762/full
https://doi.org/10.3389/fbioe.2023.1271762 |
| 107 | Rodeo, S. A., Ma, R., & Frawley, R. (2013). What's new in orthopaedic research. The Journal of
Bone and Joint Surgery. Retrieved from
https://journals.lww.com/jbjsjournal/FullText/2013/12040/What_s_New_in_Orthopaedic_Research.11.aspx
https://doi.org/10.2106/JBJS.M.01178 |
| 108 | Liu, K., Zhang, B., & Zhang, X. (2024). Promoting articular cartilage regeneration through
microenvironmental regulation. Journal of Immunology Research. Retrieved from
https://onlinelibrary.wiley.com/doi/abs/10.1155/2024/4751168
https://doi.org/10.1155/2024/4751168 |
| 109 | Jansen, M. P., & Mastbergen, S. C. (2022). Joint distraction for osteoarthritis: Clinical evidence
and molecular mechanisms. Nature Reviews Rheumatology. Retrieved from
https://www.nature.com/articles/s41584-021-00695-y
https://doi.org/10.1038/s41584-021-00695-y |
| 110 | Ma, Z., Li, D. X., & Lan, X. (2024). Short-term response of primary human meniscus cells to
simulated microgravity. Cell Communication and Signaling. Retrieved from
https://link.springer.com/article/10.1186/s12964-024-01684-w
https://doi.org/10.1186/s12964-024-01684-w |
| 111 | Goldring, M. B. (2012). Chondrogenesis, chondrocyte differentiation, and articular cartilage
metabolism in health and osteoarthritis. Therapeutic Advances in Musculoskeletal Disease. Retrieved
from https://journals.sagepub.com/doi/abs/10.1177/1759720x12448454
https://doi.org/10.1177/1759720X12448454 |
| 112 | Yokota, H., Leong, D. J., & Sun, H. B. (2011). Mechanical loading: Bone remodeling and cartilage
maintenance. Current Osteoporosis Reports. Retrieved from
https://link.springer.com/article/10.1007/s11914-011-0067-y
https://doi.org/10.1007/s11914-011-0067-y |
| 113 | Vaiciuleviciute, R., Bironaite, D., & Uzieliene, I. (2021). Cardiovascular drugs and
osteoarthritis: Effects of targeting ion channels. Cells. Retrieved from
https://www.mdpi.com/2073-4409/10/10/2572
https://doi.org/10.3390/cells10102572 |
| 114 | Liu, Y., & Shah, K. M. (2021). Strategies for articular cartilage repair and regeneration.
Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2021.770655/full
https://doi.org/10.3389/fbioe.2021.770655 |
| 115 | Gu, J., Rao, W., & Huo, S. (2022). MicroRNAs and long non-coding RNAs in cartilage homeostasis and
osteoarthritis. Frontiers in Cell and Developmental Biology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fcell.2022.1092776/full
https://doi.org/10.3389/fcell.2022.1092776 |
| 116 | Lu, M., Zhu, M., & Wu, Z. (2024). The role of YAP/TAZ on joint and arthritis. The FASEB Journal.
Retrieved from https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fj.202302273RR
https://doi.org/10.1096/fj.202302273RR |
| 117 | Du, Y., Xu, B., & Li, Q. (2024). Mechanosensitive ion channel Piezo1 in bone remodeling. Frontiers
in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2024.1342149/full
https://doi.org/10.3389/fbioe.2024.1342149 |
| 118 | Bellini, M. R. (2020). Mitogen Inducible Gene-6 in Joint Health and Osteoarthritis. ProQuest
Dissertations. Retrieved from https://search.proquest.com
|
| 119 | Semenistaja, S., Skuja, S., & Kadisa, A. (2023). Healthy and osteoarthritis-affected joints facing
the cellular crosstalk. International Journal of Molecular Sciences. Retrieved from
https://www.mdpi.com/1422-0067/24/4/4120
https://doi.org/10.3390/ijms24044120 |
| 120 | Shah, K. M., Liu, Y., & Luo, J. (2021). PRP targeting NF-κB signaling in cartilage regeneration.
Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2021.770655/full
|
| 121 | Shah, K. M., Liu, Y., & Luo, J. (2021). PRP targeting NF-κB signaling in cartilage regeneration.
Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2021.770655/full
|
| 122 | Liu, Y., Rao, W., & Huo, S. (2022). MicroRNAs in mechanotransduction and cartilage homeostasis.
Frontiers in Cell and Developmental Biology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fcell.2022.1092776/full
|
| 123 | Ma, Z., Li, D. X., & Lan, X. (2024). Mechanotransduction in meniscus cells: Pathways and potential
therapies. Cell Communication and Signaling. Retrieved from
https://link.springer.com/article/10.1186/s12964-024-01684-w
|
| 124 | Jansen, M. P., Mastbergen, S. C., & Lafeber, F. P. (2022). Clinical and molecular mechanisms of
joint distraction in osteoarthritis. Nature Reviews Rheumatology. Retrieved from
https://www.nature.com/articles/s41584-021-00695-y
https://doi.org/10.1038/s41584-021-00695-y |
| 125 | Yokota, H., Sun, H. B., & Leong, D. J. (2011). The role of mechanical loading in bone and
cartilage health. Current Osteoporosis Reports. Retrieved from
https://link.springer.com/article/10.1007/s11914-011-0067-y
https://doi.org/10.1007/s11914-011-0067-y |
| 126 | Vaiciuleviciute, R., Bironaite, D., & Mobasheri, A. (2021). Ion channel targeting in
osteoarthritis management. Cells. Retrieved from https://www.mdpi.com/2073-4409/10/10/2572
https://doi.org/10.3390/cells10102572 |
| 127 | Semenistaja, S., Skuja, S., & Kadisa, A. (2023). Cellular crosstalk in osteoarthritis and meniscal
degeneration. International Journal of Molecular Sciences. Retrieved from
https://www.mdpi.com/1422-0067/24/4/4120
https://doi.org/10.3390/ijms24044120 |
| 128 | Shah, K. M., & Liu, Y. (2021). NF-κB pathway modulation in cartilage repair. Frontiers in
Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2021.770655/full
|
| 129 | Goldring, M. B. (2012). Chondrocyte metabolism in osteoarthritis. Therapeutic Advances in
Musculoskeletal Disease. Retrieved from
https://journals.sagepub.com/doi/abs/10.1177/1759720x12448454
|
| 130 | Gu, J., & Rao, W. (2022). Long non-coding RNAs in osteoarthritis: Implications for therapy.
Frontiers in Cell and Developmental Biology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fcell.2022.1092776/full
https://doi.org/10.3389/fcell.2022.1092776 |
| 131 | Du, Y., Xu, B., & Peng, C. (2024). The role of Piezo1 in bone remodeling and mechanotransduction.
Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2024.1342149/full
https://doi.org/10.3389/fbioe.2024.1342149 |
| 132 | Ma Z, Li DX, Lan X, Bubelenyi A, Vyhlidal M, Kunze M, Sommerfeldt M, Adesida AB. Short-term
response of primary human meniscus cells to simulated microgravity. Cell Commun Signal. 2024 Jun
21;22(1):342 doi: 10.1186/s12964-024-01684-w. PMID: 38907358; PMCID: PMC11191296.
https://doi.org/10.1186/s12964-024-01684-w |
| 133 | Liu, Y., & Shah, K. M. (2021). Mechanotransduction in meniscus repair: Insights and strategies.
Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2021.770655/full
|
| 133 | Okesola, B. O., Pearce, O. M., & Mata, A. (2018). TGF-β and PDGFs in ECM protein synthesis:
Insights from a scar-in-a-jar cell model. International Journal of Experimental Pathology. Retrieved
from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6384498/
|
| 134 | Thankam, F. G., Fouda, M. B., & Dilisio, M. F. (2017). Alterations in tendon microenvironment in
response to mechanical load. American Journal of Physiology. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5666046/
|
| 135 | Zhao, B., Hu, M., Wu, H., & Ren, C. (2017). The role of TGF-β/MAPK pathways in ligament
fibroblasts. Molecular Medicine Reports. Retrieved from
https://www.spandidos-publications.com/10.3892/mmr.2017.6329
|
| 136 | Wang, H. N., Huang, Y. C., & Ni, G. X. (2020). Mechanotransduction in tendon stem cells. World
Journal of Stem Cells. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7524696/
https://doi.org/10.4252/wjsc.v12.i9.952 |
| 137 | Grier, W. K., Moy, A. S., & Harley, B. A. C. (2017). Cyclic tensile strain enhances mesenchymal
stem cell differentiation. European Cells and Materials. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5453510/
https://doi.org/10.22203/eCM.v033a17 |
| 138 | Potter, R. M., Huynh, R. T., & Volper, B. D. (2017). TGF-β receptor inhibition and MAPK pathways
in tendon ECM remodeling. American Journal of Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/ajpregu.00439.2016
|
| 139 | El Haj, A. J., & Gomes, M. E. (2020). Mechanotransduction in tendon fibroblasts. Nanoscale
Advances. Retrieved from https://pubs.rsc.org/en/content/articlehtml/2020/na/c9na00615j
|
| 140 | Nourissat, G., Berenbaum, F., & Duprez, D. (2015). Tendon injury: From biology to tendon repair.
Nature Reviews Rheumatology. Retrieved from https://www.nature.com/articles/nrrheum.2015.26
https://doi.org/10.1038/nrrheum.2015.26 |
| 141 | Kjaer, M. (2004). The role of ECM in tendon adaptation to mechanical loading. Physiological
Reviews. Retrieved from https://journals.physiology.org/doi/abs/10.1152/physrev.00031.2003
|
| 142 | Maffulli, N., Cuozzo, F., & Migliorini, F. (2023). Biochemical influences in tendon
mechanotransduction. Journal of Orthopaedic Surgery. Retrieved from
https://link.springer.com/article/10.1186/s13018-023-03796-4
|
| 143 | Russo, V., El Khatib, M., & Prencipe, G. (2022). Scaffold-mediated immunoengineering for tendon
regeneration. Cells. Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 144 | Lu, C. C., Chou, S. H., & Shen, P. C. (2020). Activation of ACL remnant cells for collagen
synthesis. Bone & Joint Research. Retrieved from
https://boneandjoint.org.uk/article/10.1302/2046-3758.98.BJR-2019-0365.R1
|
| 145 | Dyment, N. A., & Bodle, J. (2016). Signaling pathways in tenocyte responses to load. Springer
Series in Tendon Science. Retrieved from
https://link.springer.com/chapter/10.1007/978-3-319-33943-6_7
|
| 146 | Li, H., Luo, S., & Wang, H. (2023). Mechanotransduction and TGF-β pathways in tendon healing.
Injury. Retrieved from https://www.sciencedirect.com/science/article/pii/S0020138323007568
|
| 147 | Chiquet, M., & Tunç-Civelek, V. (2007). Gene regulation by mechanotransduction in fibroblasts.
Applied Physiology and Bioengineering. Retrieved from
https://cdnsciencepub.com/doi/abs/10.1139/h07-053
https://doi.org/10.1139/H07-053 |
| 148 | Li, H., Korcari, A., & Mendias, C. L. (2023). Ca²⁺ signaling and collagen fibrillogenesis in
tendon hypertrophy. BioRxiv. Retrieved from
https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 149 | Wang, W., Li, J., & Zhang, Z. (2016). Induction of tenogenic phenotype in fibroblasts through
TGF-β. American Journal of Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/ajpcell.00300.2015
|
| 150 | Gumucio, J. P., & Sugg, K. B. (2013). TGF-β pathways in skeletal muscle and tendon ECM healing.
Journal of Applied Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00137.2013
|
| 151 | Fragoulis, A., Wruck, C. J., & Kubo, Y. (2023). Nrf2/ARE system in musculoskeletal tissues.
International Journal of Molecular Sciences. Retrieved from https://www.mdpi.com/1422-0067/24/9/7722
https://doi.org/10.3390/ijms24097722 |
| 152 | Davis, M. E., Gumucio, J. P., & Sugg, K. B. (2013). ECM remodeling in tendon healing. Journal of
Applied Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00137.2013
|
| 153 | Russo, V., El Khatib, M., & Prencipe, G. (2022). Immunomodulatory scaffolds for tendon repair.
Cells. Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 154 | Stańczak M, Kacprzak B, Gawda P. Tendon Cell Biology: Effect of Mechanical Loading. Cell Physiol
Biochem. 2024 Nov 21;58(6):677-701 doi: 10.33594/000000743 PMID: 39568406.
https://doi.org/10.33594/000000743 |
| 155 | Gumucio, J. P., Sugg, K. B., & Bedi, A. (2013). MMP inhibition in tendon ECM healing. Journal of
Applied Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00137.2013
|
| 156 | Russo, V., Prencipe, G., & El Khatib, M. (2022). Scaffold innovations in tendon repair. Cells.
Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 157 | Mendias, C. L., Gumucio, J. P., & Sugg, K. B. (2023). Calcium signaling in tendon hypertrophy.
BioRxiv. Retrieved from https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 158 | Zhang, Z., Wang, W., & Li, J. (2016). ECM component production in fibroblasts through TGF-β and
elongated cell shape. American Journal of Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/ajpcell.00300.2015
|
| 159 | El Haj, A. J., & Gomes, M. E. (2020). Magnetic biomaterials as mediators in tendon
mechanotransduction. Nanoscale Advances. Retrieved from
https://pubs.rsc.org/en/content/articlehtml/2020/na/c9na00615j
|
| 160 | Nourissat, G., Berenbaum, F., & Duprez, D. (2015). Role of mechanotransduction in tendon biology
and repair. Nature Reviews Rheumatology. Retrieved from
https://www.nature.com/articles/nrrheum.2015.26
|
| 161 | Kjaer, M. (2004). ECM adaptation to mechanical stress in tendons. Physiological Reviews. Retrieved
from https://journals.physiology.org/doi/abs/10.1152/physrev.00031.2003
|
| 162 | Li, H., Korcari, A., & Mendias, C. L. (2023). Increased calcium signaling and tendon mechanics.
BioRxiv. Retrieved from https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 163 | Russo, V., El Khatib, M., & Cerveró-Varona, A. (2022). Scaffold-mediated immunomodulation for
tendon regeneration. Cells. Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 164 | Zhang, Z., Wang, W., & Li, J. (2016). Mechanotransduction and ECM signaling in tendon fibroblasts.
American Journal of Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/ajpcell.00300.2015
|
| 165 | Davis, M. E., & Gumucio, J. P. (2013). Mechanical loading in tendon biology. Journal of Applied
Physiology. Retrieved from https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00137.2013
|
| 166 | Wang, W., Li, J., & Zhang, Z. (2016). Induction of tenogenic phenotypes in human fibroblasts.
American Journal of Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/ajpcell.00300.2015
|
| 167 | Kjaer, M. (2004). The importance of ECM stiffness in tendon mechanics. Physiological Reviews.
Retrieved from https://journals.physiology.org/doi/abs/10.1152/physrev.00031.2003
|
| 168 | Li, H., Luo, S., & Korcari, A. (2023). The interplay of calcium and TGF-β in tendon
mechanotransduction. BioRxiv. Retrieved from
https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 169 | Russo, V., & Cerveró-Varona, A. (2022). YAP/TAZ signaling in tendon cell mechanobiology. Cells.
Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 170 | Nourissat, G., & Duprez, D. (2015). Mechanical and biochemical regulation of tendon repair. Nature
Reviews Rheumatology. Retrieved from https://www.nature.com/articles/nrrheum.2015.26
|
| 171 | Russo, V., & Prencipe, G. (2022). Tendon cell mechanotransduction through ion channels. Cells.
Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 172 | Mendias, C. L., & Gumucio, J. P. (2023). Calcium-mediated signaling in fibroblast
mechanotransduction. BioRxiv. Retrieved from
https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 173 | Davis, M. E., & Gumucio, J. P. (2013). ECM remodeling in tendon biology and regeneration. Journal
of Applied Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00137.2013
|
| 174 | Russo, V., & El Khatib, M. (2022). The role of growth factors in tendon ECM repair. Cells.
Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11030434 |
| 175 | Li, H., Korcari, A., & Mendias, C. L. (2023). Calcium signaling pathways in tendon hypertrophy.
BioRxiv. Retrieved from https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 176 | Russo, V., & Prencipe, G. (2022). Mechanotransduction and matrix remodeling in tendon fibroblasts.
Cells. Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 177 | Nourissat, G., & Duprez, D. (2015). The molecular biology of tendon repair. Nature Reviews
Rheumatology. Retrieved from https://www.nature.com/articles/nrrheum.2015.26
|
| 178 | Russo, V., & El Khatib, M. (2022). YAP/TAZ-mediated signaling in tendon mechanobiology. Cells.
Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 179 | Mendias, C. L., & Gumucio, J. P. (2023). Calcium influx in tendon fibroblasts. BioRxiv. Retrieved
from https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 180 | Li, H., & Korcari, A. (2023). Mechanotransduction pathways in tendon healing. BioRxiv. Retrieved
from https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 181 | Russo, V., & Prencipe, G. (2022). Immunomodulatory scaffolds for ECM regeneration in tendons.
Cells. Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 182 | Davis, M. E., & Gumucio, J. P. (2013). TGF-β and ECM remodeling in tendon biology. Journal of
Applied Physiology. Retrieved from
https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00137.2013
|
| 183 | Russo, V., & Prencipe, G. (2022). ECM stiffness and fibroblast mechanobiology. Cells. Retrieved
from https://www.mdpi.com/2073-4409/11/2/266
|
| 184 | Nourissat, G., & Duprez, D. (2015). Insights into tendon repair at the molecular level. Nature
Reviews Rheumatology. Retrieved from https://www.nature.com/articles/nrrheum.2015.26
|
| 185 | Li, H., & Mendias, C. L. (2023). Calcium signaling pathways in fibroblasts. BioRxiv. Retrieved
from https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 186 | Russo, V., & El Khatib, M. (2022). TGF-β signaling in fibroblast ECM remodeling. Cells. Retrieved
from https://www.mdpi.com/2073-4409/11/2/266
|
| 187 | Mendias, C. L., & Gumucio, J. P. (2023). ECM dynamics in tendon repair. BioRxiv. Retrieved from
https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 188 | Russo, V., & El Khatib, M. (2022). Mechanobiology and ECM remodeling in tendons. Cells. Retrieved
from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 189 | Davis, M. E., & Gumucio, J. P. (2013). TGF-β's role in tendon matrix healing. Journal of Applied
Physiology. Retrieved from https://journals.physiology.org/doi/abs/10.1152/japplphysiol.00137.2013
|
| 190 | Russo, V., & Prencipe, G. (2022). Mechanotransduction mechanisms in tendon fibroblasts. Cells.
Retrieved from https://www.mdpi.com/2073-4409/11/2/266
https://doi.org/10.3390/cells11020266 |
| 191 | Nourissat, G., & Duprez, D. (2015). Mechanotransduction in tendon repair biology. Nature Reviews
Rheumatology. Retrieved from https://www.nature.com/articles/nrrheum.2015.26
|
| 192 | Mendias, C. L., & Gumucio, J. P. (2023). Calcium influx and ECM modulation in tendon cells.
BioRxiv. Retrieved from https://www.biorxiv.org/content/10.1101/2023.01.24.525119.abstract
|
| 193 | Gallo, J., Raska, M., Kriegova, E., & Goodman, S. B. (2017). Inflammation and its resolution and
the musculoskeletal system. Journal of Orthopaedic Translation, 10, 52-67.
https://doi.org/10.1016/j.jot.2017.05.007 |
| 194 | Guilak, F., Nims, R. J., Dicks, A., Wu, C. L., & Meulenbelt, I. (2018). Osteoarthritis as a
disease of the cartilage pericellular matrix. Matrix Biology, 71-72, 40-50.
https://doi.org/10.1016/j.matbio.2018.05.008 |
| 195 | Sun, H. B. (2010). Mechanical loading, cartilage degradation, and arthritis. Annals of the New
York Academy of Sciences, 1211, 37-50.
https://doi.org/10.1111/j.1749-6632.2010.05808.x |
| 196 | Chow, Y. Y., & Chin, K. Y. (2020). The role of inflammation in the pathogenesis of osteoarthritis.
Mediators of Inflammation, 2020, 8293921.
https://doi.org/10.1155/2020/8293921 |
| 197 | Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J. P., & Fahmi, H. (2011). Role of
proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature Reviews Rheumatology,
7(1), 33-42.
https://doi.org/10.1038/nrrheum.2010.196 |
| 198 | Wang, M., & Sampson, E. R. (2013). MMP13 as a potential therapeutic target for osteoarthritis.
Current Pharmaceutical Biotechnology, 14(9), 1241-1250.
|
| 199 | Burrage, P. S., Mix, K. S., & Brinckerhoff, C. E. (2006). Matrix metalloproteinases: Role in
arthritis. Frontiers in Bioscience, 11, 529-543.
https://doi.org/10.2741/1817 |
| 200 | Wang, J. H.-C., & Thampatty, B. P. (2006). An introductory review of cell mechanobiology.
Biomechanics and Modeling in Mechanobiology, 5(1), 1-16.
https://doi.org/10.1007/s10237-005-0012-z |
| 201 | Millward-Sadler, S. J., & Salter, D. M. (2004). Integrin-dependent signal cascades in chondrocyte
mechanotransduction. Annals of Biomedical Engineering, 32(3), 435-446.
https://doi.org/10.1023/B:ABME.0000017538.72511.48 |
| 202 | Lee, W., Leddy, H. A., Chen, Y., Lee, S. H., Zelenski, N. A., McNulty, A. L., ... & Guilak, F.
(2014). Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to
articular cartilage. Proceedings of the National Academy of Sciences, 111(47), E5114-E5122.
https://doi.org/10.1073/pnas.1414298111 |
| 203 | Millward-Sadler, S. J., Wright, M. O., Lee, H., Nishida, K., Caldwell, H., Nuki, G., & Salter, D.
M. (2000). Integrin-regulated secretion of interleukin 4: A novel pathway of mechanotransduction in
human articular chondrocytes. Journal of Cell Biology, 148(1), 29-40.
https://doi.org/10.1083/jcb.145.1.183 |
| 204 | Fanning, P. J., Emkey, G., Smith, R. J., Grodzinsky, A. J., Szasz, N., & Trippel, S. B. (2003).
Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. Journal
of Biological Chemistry, 278(51), 50940-50948.
https://doi.org/10.1074/jbc.M305107200 |
| 205 | Millward-Sadler, S. J., Wright, M. O., Davies, L. W., Nuki, G., & Salter, D. M. (2000).
Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix
metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes.
Arthritis & Rheumatism, 43(9), 2091-2099.
https://doi.org/10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C |
| 206 | Millward-Sadler, S. J., & Salter, D. M. (2004). Integrin-dependent signal cascades in chondrocyte
mechanotransduction. Annals of Biomedical Engineering, 32(3), 435-446.
https://doi.org/10.1023/B:ABME.0000017538.72511.48 |
| 207 | Millward-Sadler, S. J., Wright, M. O., Lee, H., Nishida, K., Caldwell, H., Nuki, G., & Salter, D.
M. (2000). Integrin-regulated secretion of interleukin 4: A novel pathway of mechanotransduction in
human articular chondrocytes. Journal of Cell Biology, 148(1), 29-40.
https://doi.org/10.1083/jcb.145.1.183 |
| 208 | Wang, J. H.-C., & Thampatty, B. P. (2006). An introductory review of cell mechanobiology.
Biomechanics and Modeling in Mechanobiology, 5(1), 1-16.
https://doi.org/10.1007/s10237-005-0012-z |
| 209 | Millward-Sadler, S. J., & Salter, D. M. (2004). Integrin-dependent signal cascades in chondrocyte
mechanotransduction. Annals of Biomedical Engineering, 32(3), 435-446.
https://doi.org/10.1023/B:ABME.0000017538.72511.48 |
| 210 | Lee, W., Leddy, H. A., Chen, Y., Lee, S. H., Zelenski, N. A., McNulty, A. L., ... & Guilak, F.
(2014). Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to
articular cartilage. Proceedings of the National Academy of Sciences, 111(47), E5114-E5122.
https://doi.org/10.1073/pnas.1414298111 |
| 211 | Millward-Sadler, S. J., Wright, M. O., Lee, H., Nishida, K., Caldwell, H., Nuki, G., & Salter, D.
M. (2000). Integrin-regulated secretion of interleukin 4: A novel pathway of mechanotransduction in
human articular chondrocytes. Journal of Cell Biology, 148(1), 29-40.
https://doi.org/10.1083/jcb.145.1.183 |
| 212 | Roughley, P. J., & Mort, J. S. (2014). The role of aggrecan in normal and osteoarthritic
cartilage. Journal of Experimental Orthopaedics, 1(1), 8.
https://doi.org/10.1186/s40634-014-0008-7 |
| 213 | Millward-Sadler, S. J., Wright, M. O., Lee, H., Nishida, K., Caldwell, H., Nuki, G., & Salter, D.
M. (2000). Integrin-regulated secretion of interleukin 4: A novel pathway of mechanotransduction in
human articular chondrocytes. Journal of Cell Biology, 148(1), 29-40.
https://doi.org/10.1083/jcb.145.1.183 |
| 214 | Fanning, P. J., Emkey, G., Smith, R. J., Grodzinsky, A. J., Szasz, N., & Trippel, S. B. (2003).
Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. Journal
of Biological Chemistry, 278(51), 50940-50948.
https://doi.org/10.1074/jbc.M305107200 |
| 215 | Wang, J. H.-C., & Thampatty, B. P. (2006). An introductory review of cell mechanobiology.
Biomechanics and Modeling in Mechanobiology, 5(1), 1-16.
https://doi.org/10.1007/s10237-005-0012-z |
| 216 | Millward-Sadler, S. J., Wright, M. O., Lee, H., Nishida, K., Caldwell, H., Nuki, G., & Salter, D.
M. (2000). Integrin-regulated secretion of interleukin 4: A novel pathway of mechanotransduction in
human articular chondrocytes. Journal of Cell Biology, 148(1), 29-40.
https://doi.org/10.1083/jcb.145.1.183 |
| 217 | Zhao, Z., Li, Y., Wang, M., Liu, Y., & Wang, Z. (2020). Instability and excessive mechanical
loading mediate subchondral bone changes to induce osteoarthritis. Journal of Orthopaedic
Translation, 21, 111-121.
|
| 218 | Khan, K. M., & Scott, A. (2009). Mechanotherapy: how physical therapists' prescription of exercise
promotes tissue repair. British Journal of Sports Medicine, 43(4), 247-252.
https://doi.org/10.1136/bjsm.2008.054239 |
| 219 | Rees, J. D., Stride, M., & Scott, A. (2014). Tendons-time to revisit inflammation. British Journal
of Sports Medicine, 48(21), 1553-1557.
https://doi.org/10.1136/bjsports-2012-091957 |
| 220 | Beyer, R., & Kongsgaard, M. (2018). Heavy slow resistance versus eccentric training as treatment
for Achilles tendinopathy: a randomized controlled trial. The American Journal of Sports Medicine,
43(7), 1704-1711.
https://doi.org/10.1177/0363546515584760 |
| 221 | Barton, C. J., Lack, S., Hemmings, S., Tufail, S., & Morrissey, D. (2015). The 'Best Practice
Guide to Conservative Management of Patellofemoral Pain': incorporating level 1 evidence with expert
clinical reasoning. British Journal of Sports Medicine, 49(14), 923-934.
https://doi.org/10.1136/bjsports-2014-093637 |
| 222 | Paoloni, J. A., Milne, C., Orchard, J., & Hamilton, B. (2011). Non-steroidal anti-inflammatory
drugs in sports medicine: guidelines for practical but sensible use. British Journal of Sports
Medicine, 45(3), 198-202.
|
| 223 | Molloy, T., Wang, Y., & Murrell, G. A. C. (2003). The roles of growth factors in tendon and
ligament healing. Sports Medicine, 33(5), 381-394.
https://doi.org/10.2165/00007256-200333050-00004 |
| 224 | Thomopoulos, S., Marquez-Lago, T. T., & Genin, G. M. (2015). Structure and biomechanics of the
tendon enthesis. Journal of Orthopaedic Research, 33(6), 832-839.
https://doi.org/10.1002/jor.22806 |
| 225 | Wang, J. H.-C., & Thampatty, B. P. (2016). Mechanobiology of adult and stem cells. International
Review of Cell and Molecular Biology, 313, 253-278.
|
| 226 | Geiger, B., Spatz, J. P., & Bershadsky, A. D. (2009). Environmental sensing through focal
adhesions. Nature Reviews Molecular Cell Biology, 10(1), 21-33.
https://doi.org/10.1038/nrm2593 |
| 227 | Zhou, J., Aponte-Santamaría, C., Sturm, S., Bullerjahn, J. T., Bronowska, A., & Gräter, F. (2015).
Mechanism of focal adhesion kinase mechanosensing. PLoS Computational Biology, 11(11), e1004593.
https://doi.org/10.1371/journal.pcbi.1004593 |
| 228 | Coste, B., Mathur, J., Schmidt, M., Earley, T. J., Ranade, S., Petrus, M. J., Dubin, A. E., &
Patapoutian, A. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically
activated cation channels. Science, 330(6000), 55-60.
https://doi.org/10.1126/science.1193270 |
| 229 | Gautel, M., & Djinović-Carugo, K. (2016). The sarcomeric cytoskeleton: from molecules to motion.
Journal of Experimental Biology, 219(Pt 2), 135-145.
https://doi.org/10.1242/jeb.124941 |
| 230 | Kagan, H. M., & Li, W. (2003). Lysyl oxidase: properties, specificity, and biological roles inside
and outside of the cell. Journal of Cellular Biochemistry, 88(4), 660-672.
https://doi.org/10.1002/jcb.10413 |
| 231 | Benjamin, M., & McGonagle, D. (2009). Entheses: tendon and ligament attachment sites. Scandinavian
Journal of Medicine & Science in Sports, 19(4), 520-527.
https://doi.org/10.1111/j.1600-0838.2009.00906.x |
| 232 | Kondo, S., & Kubota, S. (2015). Connective tissue growth factor (CTGF/CCN2) in cartilage tissue
regeneration. Journal of Biochemistry, 158(2), 73-81.
|
| 233 | Makris, E. A., Hadidi, P., & Athanasiou, K. A. (2011). The knee meniscus: structure-function,
pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials, 32(30),
7411-7431.
https://doi.org/10.1016/j.biomaterials.2011.06.037 |
| 234 | Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., ... & Piccolo, S.
(2011). Role of YAP/TAZ in mechanotransduction. Nature, 474(7350), 179-183.
https://doi.org/10.1038/nature10137 |
| 235 | Piccolo, S., Dupont, S., & Cordenonsi, M. (2014). The biology of YAP/TAZ: Hippo signaling and
beyond. Physiological Reviews, 94(4), 1287-1312.
https://doi.org/10.1152/physrev.00005.2014 |
| 236 | Thorpe, C. T., Riley, G. P., Birch, H. L., & Clegg, P. D. (2016). Fascicles and the
interfascicular matrix show decreased fatigue life with ageing in energy storing tendons. Acta
Biomaterialia, 56, 58-64.
https://doi.org/10.1016/j.actbio.2017.03.024 |
| 237 | Millar, N. L., Murrell, G. A. C., & McInnes, I. B. (2017). Inflammatory mechanisms in
tendinopathy: Towards translation. Nature Reviews Rheumatology, 13(2), 110-122.
https://doi.org/10.1038/nrrheum.2016.213 |
| 238 | Dean, B. J. F., Gettings, P., Dakin, S. G., & Carr, A. J. (2016). Are inflammatory cells increased
in painful human tendinopathy? A systematic review. British Journal of Sports Medicine, 50(4),
216-220.
https://doi.org/10.1136/bjsports-2015-094754 |
| 239 | Bestwick, C. S., & Maffulli, N. (2004). Reactive oxygen species and tendinopathy: Do they matter?
British Journal of Sports Medicine, 38(6), 672-674.
https://doi.org/10.1136/bjsm.2004.012351 |
| 240 | Khan, M. H., Li, Z., & Wang, J. H.-C. (2005). Repeated exposure of tendon to prostaglandin-E2
leads to localized tendon degeneration. Clinical Journal of Sport Medicine, 15(1), 27-33.
https://doi.org/10.1097/00042752-200501000-00006 |
| 241 | Li, H.-Y., & Hua, Y.-H. (2016). Achilles tendinopathy: Current concepts about the basic science
and clinical treatments. BioMed Research International, 2016, 6492597.
https://doi.org/10.1155/2016/6492597 |
| 242 | Wang, J. H.-C., & Thampatty, B. P. (2016). Mechanobiology of adult and stem cells. International
Review of Cell and Molecular Biology, 313, 253-278.
|
| 243 | Gautel, M., & Djinović-Carugo, K. (2016). The sarcomeric cytoskeleton: from molecules to motion.
Journal of Experimental Biology, 219(Pt 2), 135-145.
https://doi.org/10.1242/jeb.124941 |
| 244 | Kjaer, M. (2004). Role of extracellular matrix in adaptation of tendon and skeletal muscle to
mechanical loading. Physiological Reviews, 84(2), 649-698.
https://doi.org/10.1152/physrev.00031.2003 |
| 245 | Gulotta, L. V., Kovacevic, D., Packer, J. D., Deng, X. H., & Rodeo, S. A. (2011). Bone
marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a
rat model. The American Journal of Sports Medicine, 39(6), 1282-1289.
https://doi.org/10.1177/0363546510395485 |
| 246 | Wang, J. H.-C. (2006). Mechanobiology of tendon. Journal of Biomechanics, 39(9), 1563-1582.
https://doi.org/10.1016/j.jbiomech.2005.05.011 |
| 247 | Thorpe, C. T., Riley, G. P., Birch, H. L., & Clegg, P. D. (2016). Fascicles and the
interfascicular matrix show decreased fatigue life with ageing in energy storing tendons. Acta
Biomaterialia, 56, 58-64.
https://doi.org/10.1016/j.actbio.2017.03.024 |
| 248 | Guilak, F., & McNulty, A. L. (2017). Mechanobiology of articular cartilage and chondrocytes. In P.
D. Clegg, J. E. Hardingham, & J. S. Mort (Eds.), Cartilage Biology (pp. 193-217). Springer.
|
| 249 | Zhou, S., Cui, Z., Urban, J. P. G., & Huang, C. Y. (2015). Influence of cyclic tensile strain on
expression of integrins and MAPK pathway in chondrocytes. Connective Tissue Research, 56(6),
483-490.
|
| 250 | Millward-Sadler, S. J., & Salter, D. M. (2004). Integrin-dependent signal cascades in chondrocyte
mechanotransduction. Annals of Biomedical Engineering, 32(3), 435-446.
https://doi.org/10.1023/B:ABME.0000017538.72511.48 |
| 251 | O'Conor, C. J., Leddy, H. A., Benefield, H. C., Liedtke, W. B., & Guilak, F. (2014).
TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic
loading. Proceedings of the National Academy of Sciences, 111(4), 1316-1321.
https://doi.org/10.1073/pnas.1319569111 |
| 252 | Sun, Y., & Yokota, H. (2012). Reduction of oxidative stress and enhancement of chondrocyte
survival by low-intensity pulsed ultrasound. Osteoarthritis and Cartilage, 20(10), 1263-1271.
|
| 253 | Benjamin, M., & McGonagle, D. (2009). Entheses: tendon and ligament attachment sites. Scandinavian
Journal of Medicine & Science in Sports, 19(4), 520-527.
https://doi.org/10.1111/j.1600-0838.2009.00906.x |
| 254 | Kuo, T. F., Lin, M. F., Chen, Y. S., & Su, W. R. (2010). The role of TRPV4 channels in
mechanically and chemically induced ATP release in 3T3-L1 preadipocytes. Pflugers Archiv - European
Journal of Physiology, 460(4), 799-807.
|
| 255 | Wang, J. H.-C. (2006). Mechanobiology of tendon. Journal of Biomechanics, 39(9), 1563-1582.
https://doi.org/10.1016/j.jbiomech.2005.05.011 |
| 256 | Millar, N. L., Murrell, G. A. C., & McInnes, I. B. (2017). Inflammatory mechanisms in
tendinopathy: Towards translation. Nature Reviews Rheumatology, 13(2), 110-122.
https://doi.org/10.1038/nrrheum.2016.213 |
| 257 | Dean, B. J. F., Gettings, P., Dakin, S. G., & Carr, A. J. (2016). Are inflammatory cells increased
in painful human tendinopathy? A systematic review. British Journal of Sports Medicine, 50(4),
216-220.
https://doi.org/10.1136/bjsports-2015-094754 |
| 258 | Hardie, D. G., & Lin, S. C. (2017). AMP-activated protein kinase - not just an energy sensor.
F1000Research, 6, 1724.
https://doi.org/10.12688/f1000research.11960.1 |
| 259 | Zhou, S., Cui, Z., Urban, J. P. G., & Huang, C. Y. (2015). Influence of cyclic tensile strain on
expression of integrins and MAPK pathway in chondrocytes. Connective Tissue Research, 56(6),
483-490.
|
| 260 | Guilak, F., & McNulty, A. L. (2017). Mechanobiology of articular cartilage and chondrocytes. In P.
D. Clegg, J. E. Hardingham, & J. S. Mort (Eds.), Cartilage Biology (pp. 193-217). Springer.
|
| 261 | Lopez-Verrilli, M. A., & Court, F. A. (2013). Exosomes: mediators of communication in eukaryotes.
Biological Research, 46(1), 5-11.
https://doi.org/10.4067/S0716-97602013000100001 |
| 262 | Kolhe, R., Hunter, M., Liu, S., Moore, L., Mendhe, B., Freitas, A., ... & Rojkind, M. (2017).
Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with
osteoarthritis. Scientific Reports, 7(1), 2029.
https://doi.org/10.1038/s41598-017-01905-y |
| 263 | Gatti, S., Bruno, S., Deregibus, M. C., Sordi, A., Cantaluppi, V., Tetta, C., & Camussi, G.
(2011). Microvesicles derived from human adult mesenchymal stem cells protect against
ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrology Dialysis Transplantation,
26(5), 1474-1483.
https://doi.org/10.1093/ndt/gfr015 |
| 264 | Millward-Sadler, S. J., & Salter, D. M. (2004). Integrin-dependent signal cascades in chondrocyte
mechanotransduction. Annals of Biomedical Engineering, 32(3), 435-446.
https://doi.org/10.1023/B:ABME.0000017538.72511.48 |
| 265 | O'Conor, C. J., Leddy, H. A., Benefield, H. C., Liedtke, W. B., & Guilak, F. (2014).
TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic
loading. Proceedings of the National Academy of Sciences, 111(4), 1316-1321.
https://doi.org/10.1073/pnas.1319569111 |
| 266 | Sun, Y., & Yokota, H. (2012). Reduction of oxidative stress and enhancement of chondrocyte
survival by low-intensity pulsed ultrasound. Osteoarthritis and Cartilage, 20(10), 1263-1271.
|
| 267 | Millar, N. L., Murrell, G. A. C., & McInnes, I. B. (2017). Inflammatory mechanisms in
tendinopathy: Towards translation. Nature Reviews Rheumatology, 13(2), 110-122.
https://doi.org/10.1038/nrrheum.2016.213 |
| 268 | Dong, Y., Yuan, H., Ma, G., & Cao, H. (2024). Bone-muscle crosstalk under physiological and
pathological conditions. Cellular and Molecular Life Sciences. Retrieved from
https://link.springer.com/content/pdf/10.1007/s00018-024-05331-y.pdf
https://doi.org/10.1007/s00018-024-05331-y |
| 269 | Savadipour, A. (2023). Mechanosensitive ion channels as therapeutics targets for osteoarthritis.
Retrieved from https://openscholarship.wustl.edu/cgi/viewcontent.cgi?article=2053&context=eng_etds
|
| 270 | Petrigna, L., Trovato, B., Roggio, F., & Castorina, A. (2023). Molecular assessment of healthy
pathological articular cartilages in physically active people: A scoping review. International
Journal of Molecular Sciences, 24(4), 3662 Retrieved from https://www.mdpi.com/1422-0067/24/4/3662
https://doi.org/10.3390/ijms24043662 |
| 271 | Kamalathevan, P. (2022). The role of retinoic acid in cartilage mechanoflammation. Retrieved from
https://ora.ox.ac.uk/objects/uuid:ed804ffd-f948-4614-a3f3-a5f973cd2249
|
| 272 | Teixeira, C. A., Haas, L., Frata, B., & Bortoli, A. F. (2024). Effects of a low, medium, and
high-intensity aquatic physiotherapy protocol on functional and biochemical parameters in
individuals with knee osteoarthritis: Protocol. Retrieved from
https://pmc.ncbi.nlm.nih.gov/articles/PMC11409911/
|
| 273 | Zhao, J. (2020). The degradation of articular cartilage extracellular matrix through aerobic
exercise. Retrieved from
https://go.gale.com/ps/i.do?id=GALE%7CA626504692&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=05355133&p=AONE&sw=w
|
| 274 | Lawyer, T. J. (2014). Evaluation of chondrocytes and bone marrow mesenchymal stem cells exposed to
pro-inflammatory cytokines and triamcinolone acetonide under... Retrieved from
https://search.proquest.com/openview/6bcdd4dae3a1f78f105780c8731422f6/1?pq-origsite=gscholar&cbl=18750
|
| 275 | Najafi, Z., & Rahmanian-Devin, P. (2024). Challenges and opportunities of medicines for treating
tendon inflammation and fibrosis: A comprehensive and mechanistic review. Fundamental & Clinical
Pharmacology, 38(1). Retrieved from https://onlinelibrary.wiley.com/doi/abs/10.1111/fcp.12999
https://doi.org/10.1111/fcp.12999 |
| 276 | Chan, C., & Vincent, T. (2023). The early cellular events that control mechano-inflammatory
signalling after cartilage injury. Retrieved from
https://ora.ox.ac.uk/objects/uuid:96b87b64-27e9-46e8-b51a-d08818686371
|
| 277 | Teixeira, C. A., Haas, L., & Frata, B. (2024). Effects of a low, medium, and high-intensity
aquatic physiotherapy protocol on functional and biochemical parameters in individuals with knee
osteoarthritis. Retrieved from https://f1000research.com/articles/12-1605
https://doi.org/10.12688/f1000research.140342.2 |
| 278 | Fayazi, M. (2021). Intersection of mechanobiology and musculoskeletal regenerative rehabilitation.
Retrieved from
https://search.proquest.com/openview/d5536d40f2ea66ea2b0ad089a4c36649/1?pq-origsite=gscholar&cbl=18750&diss=y
|
| 279 | Luttrell, T. (2024). Trauma and inflammation of soft tissue: Rehabilitation and wound healing and
remodeling of collagen. Retrieved from
https://api.taylorfrancis.com/content/chapters/edit/download?identifierName=doi&identifierValue=10.4324/9781003524212-3&type=chapterpdf
https://doi.org/10.4324/9781003524212-3 |
| 280 | Coglianese, D. (2024). Individuals with localized musculoskeletal and connective tissue disorders.
Retrieved from
https://api.taylorfrancis.com/content/chapters/edit/download?identifierName=doi&identifierValue=10.4324/9781003523048-23&type=chapterpdf
https://doi.org/10.4324/9781003523048-23 |
| 281 | Husby, K. A. (2016). In vitro evaluation of therapeutic laser treatment on equine tendon
fibroblasts. Retrieved from https://ir.library.oregonstate.edu/downloads/fn1072128
|
| 282 | Sharma, P., & Maffulli, N. (2012). Soft tissue physiology and healing. Retrieved from
https://api.taylorfrancis.com/content/chapters/edit/download?identifierName=doi&identifierValue=10.1201/b13543-14&type=chapterpdf
https://doi.org/10.1201/b13543-10 |
| 283 | Luttrell, T. (2024). Trauma and inflammation of soft tissue. Retrieved from
https://books.google.com/books?hl=en&lr=&id=iucLEQAAQBAJ&oi=fnd&pg=PT11&dq=hydrostatic+pressure+effects+synoviocytes+ligaments+tendons+fibroblasts+tenocytes+cytokine+modulation+oxidative+stress+aquatic+therapy&ots=XhCpqO6ed5&sig=j5qP_6UtWD3uKd5CsrCTjTFKtfo
|
| 284 | Healing, G. W. (2013). Tissue healing: Tendons, ligaments, bone, muscles, and cartilage. Retrieved
from
https://books.google.com/books?hl=en&lr=&id=ObIKAQAAQBAJ&oi=fnd&pg=PA79&dq=hydrostatic+pressure+effects+synoviocytes+ligaments+tendons+fibroblasts+tenocytes+cytokine+modulation+oxidative+stress+aquatic+therapy&ots=595riTOj6W&sig=GaMmRbQbt0e3kaXlJzkFghLMOpk
|
| 285 | Ajalik, R. E. (2023). Human tissue-on-a-chip platform for the study of tendon fibrovascular injury
and drug screening of therapeutic candidates. Retrieved from
https://search.proquest.com/openview/ab0e94447e252e7507f2a57aec9c5e48/1?pq-origsite=gscholar&cbl=18750&diss=y
|
| 286 | Sharma, P., & Maffulli, N. (2005). Tendon injury and tendinopathy: Healing and repair. Retrieved
from
https://www.researchgate.net/profile/Nicola-Maffulli/publication/7520346_Basic_biology_of_tendon_injury_and_healing/links/5a5722b60f7e9bf2a537607f/Basic-biology-of-tendon-injury-and-healing.pdf
|
| 287 | Coglianese, D. (2024). Individuals with localized musculoskeletal and connective tissue disorders.
Retrieved from
https://api.taylorfrancis.com/content/chapters/edit/download?identifierName=doi&identifierValue=10.4324/9781003523048-23&type=chapterpdf
https://doi.org/10.4324/9781003523048-23 |
| 288 | Ai, M. (2022). Pain alleviating effects of mesenchymal stem/stromal cells and derived
extracellular vesicles in osteoarthritis. Retrieved from
https://www.repository.cam.ac.uk/bitstreams/5f3f8f57-4c15-4bb3-a442-a66b67edc46b/download
|
| 289 | Qin, H., Du, L., Luo, Z., He, Z., Wang, Q., & Chen, S. (2022). The therapeutic effects of
low-intensity pulsed ultrasound in musculoskeletal soft tissue injuries: Focusing on the molecular
mechanism. Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2022.1080430/pdf
https://doi.org/10.3389/fbioe.2022.1080430 |
| 290 | Duatti, A. (2023). Lactate-induced COL1A1/DDR1 axis promotes prostate cancer aggressiveness and
enhances metastatic colonization. Retrieved from
https://usiena-air.unisi.it/bitstream/11365/1227854/6/phd_unisi_093977.pdf
|
| 291 | Dong, Y., Yuan, H., Ma, G., & Cao, H. (2024). Bone-muscle crosstalk under physiological and
pathological conditions. Cellular and Molecular Life Sciences. Retrieved from
https://link.springer.com/content/pdf/10.1007/s00018-024-05331-y.pdf
https://doi.org/10.1007/s00018-024-05331-y |
| 292 | Ai, M. (2022). Pain alleviating effects of mesenchymal stem/stromal cells and derived
extracellular vesicles in osteoarthritis. Retrieved from
https://www.repository.cam.ac.uk/bitstreams/5f3f8f57-4c15-4bb3-a442-a66b67edc46b/download
|
| 293 | Leahy, T. P. (2023). In situ and in vivo roles of focal adhesion kinase in tendon development and
mechanotransduction. Retrieved from
https://search.proquest.com/openview/395cbfc0e3a722f0cb7994d6230db622/1?pq-origsite=gscholar&cbl=18750&diss=y
|
| 294 | Dieterle, M. P., Husari, A., & Rolauffs, B. (2021). Integrins, cadherins and channels in cartilage
mechanotransduction: Perspectives for future regeneration strategies. Expert Reviews in Molecular
Medicine. Retrieved from
https://www.cambridge.org/core/services/aop-cambridge-core/content/view/807D5D5D5820811CAC938CF4B898DC54/S1462399421000168a.pdf
https://doi.org/10.1017/erm.2021.16 |
| 295 | Fayazi, M. (2021). Intersection of mechanobiology and musculoskeletal regenerative rehabilitation.
Retrieved from
https://digital.lib.washington.edu/researchworks/bitstream/handle/1773/46975/Fayazi_washington_0250E_22684.pdf?sequence=1
|
| 296 | Sup, M. K. (2024). The inflammatory response in tendon fibroblasts is multi-factorial and alters
their responses to mechanical stimulation. Retrieved from
https://search.proquest.com/openview/74b6fcc53f7d5387519597e4089f630a/1?pq-origsite=gscholar&cbl=18750&diss=y
|
| 297 | Lohberger, B., Weigl, L., Mann, A., Kullich, W., & Leithner, A. (2018). Mechanical exposure and
diacerein treatment modulates integrin-FAK-MAPKs mechanotransduction in human osteoarthritis
chondrocytes. Annals of the Rheumatic Diseases. Retrieved from
https://ard.bmj.com/content/annrheumdis/77/Suppl_2/1241.3.full.pdf
https://doi.org/10.1136/annrheumdis-2018-eular.6810 |
| 298 | Liu, Y., Okesola, B. O., Pearce, O. M., & Mata, A. (2018). Matrix biology and mechanotransduction
in cartilage and connective tissues. Retrieved from
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6384498/
|
| 299 | Lohberger, B., Weigl, L., Mann, A., Kullich, W., & Leithner, A. (2018). Mechanical exposure and
diacerein treatment modulates integrin-FAK-MAPKs mechanotransduction in human osteoarthritis
chondrocytes. Retrieved from https://ard.bmj.com/content/annrheumdis/77/Suppl_2/1241.3.full.pdf
https://doi.org/10.1136/annrheumdis-2018-eular.6810 |
| 300 | Dieterle, M. P., Husari, A., & Rolauffs, B. (2021). Integrins, cadherins and channels in cartilage
mechanotransduction: Perspectives for future regeneration strategies. Expert Reviews in Molecular
Medicine. Retrieved from
https://www.cambridge.org/core/services/aop-cambridge-core/content/view/807D5D5D5820811CAC938CF4B898DC54/S1462399421000168a.pdf
https://doi.org/10.1017/erm.2021.16 |
| 301 | Fayazi, M. (2021). Intersection of mechanobiology and musculoskeletal regenerative rehabilitation.
Retrieved from
https://search.proquest.com/openview/d5536d40f2ea66ea2b0ad089a4c36649/1?pq-origsite=gscholar&cbl=18750&diss=y
|
| 302 | Qin, H., Du, L., Luo, Z., He, Z., Wang, Q., & Chen, S. (2022). The therapeutic effects of
low-intensity pulsed ultrasound in musculoskeletal soft tissue injuries: Focusing on the molecular
mechanism. Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2022.1080430/pdf
https://doi.org/10.3389/fbioe.2022.1080430 |
| 303 | Graham, Z. A., Gallagher, P. M., & Cardozo, C. P. (2015). Focal adhesion kinase and its role in
skeletal muscle. Journal of Muscle Research and Cell Motility. Retrieved from
https://link.springer.com/content/pdf/10.1007/s10974-015-9415-3.pdf
https://doi.org/10.1007/s10974-015-9415-3 |
| 304 | Chen, J., Zhou, R., Feng, Y., & Cheng, L. (2022). Molecular mechanisms of exercise contributing to
tissue regeneration. Signal Transduction and Targeted Therapy. Retrieved from
https://www.nature.com/articles/s41392-022-01233-2.pdf
https://doi.org/10.1038/s41392-022-01233-2 |
| 305 | Huang, H., Kamm, R. D., & Lee, R. T. (2004). Cell mechanics and mechanotransduction: Pathways,
probes, and physiology. American Journal of Physiology-Cell Physiology. Retrieved from
https://journals.physiology.org/doi/pdf/10.1152/ajpcell.00559.2003
https://doi.org/10.1152/ajpcell.00559.2003 |
| 306 | Lohberger, B., Weigl, L., Mann, A., Kullich, W., & Leithner, A. (2018). Mechanical exposure and
diacerein treatment modulates integrin-FAK-MAPKs mechanotransduction in human osteoarthritis
chondrocytes. Annals of the Rheumatic Diseases. Retrieved from
https://ard.bmj.com/content/annrheumdis/77/Suppl_2/1241.3.full.pdf
https://doi.org/10.1136/annrheumdis-2018-eular.6810 |
| 307 | Kacprzak, B., & Stańczak, M. (2024). Biophysics of ACL injuries. Orthopedic Reviews. Retrieved
from https://orthopedicreviews.openmedicalpublishing.org/article/126041.pdf
https://doi.org/10.20944/preprints202408.0988.v1 |
| 308 | Qin, H., Du, L., Luo, Z., He, Z., Wang, Q., & Chen, S. (2022). The therapeutic effects of
low-intensity pulsed ultrasound in musculoskeletal soft tissue injuries: Focusing on the molecular
mechanism. Frontiers in Bioengineering and Biotechnology. Retrieved from
https://www.frontiersin.org/articles/10.3389/fbioe.2022.1080430/pdf
https://doi.org/10.3389/fbioe.2022.1080430 |
| 309 | Graham, Z. A., Gallagher, P. M., & Cardozo, C. P. (2015). Focal adhesion kinase and its role in
skeletal muscle. Journal of Muscle Research and Cell Motility. Retrieved from
https://link.springer.com/content/pdf/10.1007/s10974-015-9415-3.pdf
https://doi.org/10.1007/s10974-015-9415-3 |
| 310 | Wang, M. O., & Fisher, J. P. (2018). Signal expression in engineered tissues. Retrieved from
https://api.taylorfrancis.com/content/chapters/edit/download?identifierName=doi&identifierValue=10.1201/9781351228770-45&type=chapterpdf
|
| 311 | Dong, Y., Yuan, H., Ma, G., & Cao, H. (2024). Bone-muscle crosstalk under physiological and
pathological conditions. Cellular and Molecular Life Sciences. Retrieved from
https://link.springer.com/content/pdf/10.1007/s00018-024-05331-y.pdf
https://doi.org/10.1007/s00018-024-05331-y |
| 312 | Khan KM, Scott A. Mechanotherapy: how physical therapists' prescription of exercise promotes
tissue repair. Br J Sports Med. 2009 Apr;43(4):247-52 doi: 10.1136/bjsm.2008.054239
https://doi.org/10.1136/bjsm.2008.054239 |
| 313 | Logerstedt DS, Ebert JR, MacLeod TD, Heiderscheit BC, Gabbett TJ, Eckenrode BJ. Effects of and
Response to Mechanical Loading on the Knee. Sports Med. 2022 Feb;52(2):201-235 doi:
10.1007/s40279-021-01579-7.
https://doi.org/10.1007/s40279-021-01579-7 |
| 314 | Lavagnino, M., Wall, M. E., & Little, D. (2015). Tendon mechanobiology: Current knowledge and
future research opportunities. Journal of Orthopaedic Research. Retrieved from
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/jor.22871
https://doi.org/10.1002/jor.22871 |
| 315 | Kwon, H., Paschos, N. K., Hu, J. C., & Athanasiou, K. (2016). Articular cartilage tissue
engineering: The role of signaling molecules. Cellular and Molecular Life Sciences. Retrieved from
https://link.springer.com/article/10.1007/s00018-015-2115-8
https://doi.org/10.1007/s00018-015-2115-8 |
| 316 | Fouda, M. B., Thankam, F. G., & Dilisio, M. F. (2017). Alterations in tendon microenvironment in
response to mechanical load: Potential molecular targets for treatment strategies. Journal of
Molecular Biology. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5666046/
|
| 317 | Beyer R, Kongsgaard M, Hougs Kjær B, Øhlenschlæger T, Kjær M, Magnusson SP. Heavy Slow Resistance
Versus Eccentric Training as Treatment for Achilles Tendinopathy: A Randomized Controlled Trial. Am
J Sports Med. 2015 Jul;43(7):1704-11 doi: 10.1177/0363546515584760 Epub 2015 May 27 PMID: 26018970.
https://doi.org/10.1177/0363546515584760 |
| 318 | Barajaa, M.A., Nair, L.S. & Laurencin, C.T. Bioinspired Scaffold Designs for Regenerating
Musculoskeletal Tissue Interfaces. Regen. Eng. Transl. Med. 6, 451-483 (2020).
https://doi.org/10.1007/s40883-019-00132-3
https://doi.org/10.1007/s40883-019-00132-3 |
| 319 | Bleakley CM, O'Connor SR, Tully MA, Rocke LG, Macauley DC, Bradbury I, Keegan S, McDonough SM.
Effect of accelerated rehabilitation on function after ankle sprain: randomised controlled trial.
BMJ. 2010 May 10;340:c1964 doi: 10.1136/bmj.c1964 PMID: 20457737.
https://doi.org/10.1136/bmj.c1964 |
| 320 | McIntyre, T. D. (2013). Altering tissue mechanics and collagen synthesis in engineered ligament
using insulin-like growth factor-1 (IGF-1) and mechanical stretch. Retrieved from
https://search.proquest.com/openview/b0004c7d9ac6d68d2b740a8df7b18947/1?pq-origsite=gscholar&cbl=18750
|
| 321 | Fayazi, M. (2021). Intersection of mechanobiology and musculoskeletal regenerative rehabilitation.
Retrieved from
https://digital.lib.washington.edu/researchworks/bitstream/handle/1773/46975/Fayazi_washington_0250E_22684.pdf?sequence=1
|
| 322 | Chatterjee, M., & Muljadi, P. M. (2022). The role of the tendon ECM in mechanotransduction:
Disruption and repair following overuse. Clinical Orthopaedics and Related Research, 36(7),
1125-1134 Retrieved from
https://ecommons.cornell.edu/bitstream/handle/1813/111761/Muljadi_cornellgrad_0058F_12970.pdf?sequence=1#page=12
https://doi.org/10.1080/03008207.2021.1925663 |
| 323 | Fouda, M. B., Thankam, F. G., & Dilisio, M. F. (2017). Alterations in tendon microenvironment in
response to mechanical load: Potential molecular targets for treatment strategies. Journal of
Molecular Biology. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5666046/
|
| 324 | Chen, J., Zhou, R., Feng, Y., & Cheng, L. (2022). Molecular mechanisms of exercise contributing to
tissue regeneration. Signal Transduction and Targeted Therapy. Retrieved from
https://www.nature.com/articles/s41392-022-01233-2.pdf
https://doi.org/10.1038/s41392-022-01233-2 |
| 325 | Guzzoni, V., & Selistre-de-Araújo, H. S. (2018). Tendon remodeling in response to resistance
training, anabolic androgenic steroids, and aging. Cells, 7(12), 251 Retrieved from
https://www.mdpi.com/2073-4409/7/12/251/pdf
https://doi.org/10.3390/cells7120251 |
| 326 | Chao, Y. H., & Sun, J. S. (2020). Biomechanics of skeletal muscle and tendon. In Biomechanics of
Human Motion (pp. 46-62). Retrieved from
https://link.springer.com/chapter/10.1007/978-981-15-3159-0_2
https://doi.org/10.1007/978-981-15-3159-0_2 |
| 327 | Chen, L., Zhang, Z., & Liu, X. (2024). Role and mechanism of mechanical load in the homeostasis of
the subchondral bone in knee osteoarthritis: A comprehensive review. Journal of Immunology Research.
Retrieved from https://www.tandfonline.com/doi/pdf/10.2147/JIR.S492415
https://doi.org/10.2147/JIR.S492415 |
| 328 | Visnes, H. (2014). Risk factors for jumper's knee. Retrieved from
https://bora.uib.no/bora-xmlui/bitstream/handle/1956/8824/dr-thesis-2014-Håvard-Visnes.pdf?sequence=1&isAllowed=y
|
| 329 | Fayazi, M. (2021). Intersection of mechanobiology and musculoskeletal regenerative rehabilitation.
Retrieved from
https://digital.lib.washington.edu/researchworks/bitstream/handle/1773/46975/Fayazi_washington_0250E_22684.pdf?sequence=1
|
| 330 | Chatterjee, M., & Muljadi, P. M. (2022). The role of the tendon ECM in mechanotransduction:
Disruption and repair following overuse. Clinical Orthopaedics and Related Research, 36(7),
1125-1134 Retrieved from
https://ecommons.cornell.edu/bitstream/handle/1813/111761/Muljadi_cornellgrad_0058F_12970.pdf?sequence=1#page=12
https://doi.org/10.1080/03008207.2021.1925663 |
| 331 | Fouda, M. B., Thankam, F. G., & Dilisio, M. F. (2017). Alterations in tendon microenvironment in
response to mechanical load: Potential molecular targets for treatment strategies. Journal of
Molecular Biology. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5666046/
|
| 332 | Chen, J., Zhou, R., Feng, Y., & Cheng, L. (2022). Molecular mechanisms of exercise contributing to
tissue regeneration. Signal Transduction and Targeted Therapy. Retrieved from
https://www.nature.com/articles/s41392-022-01233-2.pdf
https://doi.org/10.1038/s41392-022-01233-2 |
| 333 | Jin, Y., Alvarez, J.T., Suitor, E.L. et al. Estimation of joint torque in dynamic activities using
wearable A-mode ultrasound. Nat Commun 15, 5756 (2024). https://doi.org/10.1038/s41467-024-50038-0
https://doi.org/10.1038/s41467-024-50038-0 |
| 334 | Dong, Y., Yuan, H., Ma, G., & Cao, H. (2024). Bone-muscle crosstalk under physiological and
pathological conditions. Cellular and Molecular Life Sciences. Retrieved from
https://link.springer.com/content/pdf/10.1007/s00018-024-05331-y.pdf
https://doi.org/10.1007/s00018-024-05331-y |
| 335 | Sup, M. K. (2024). The inflammatory response in tendon fibroblasts is multi-factorial and alters
their responses to mechanical stimulation. Retrieved from
https://search.proquest.com/openview/74b6fcc53f7d5387519597e4089f630a/1?pq-origsite=gscholar&cbl=18750&diss=y
|
| 336 | Sharma S, Anderson KM, Pacha MS, Falbo KJ, Severe C, Hansen AH, Hendershot BD, Wilken JM; CARBon
fiber Orthosis research Network (CARBON). The effect of carbon fiber custom dynamic orthosis type on
kinematics and kinetics of lower extremity joints in individuals with lower limb traumatic injuries.
Gait Posture. 2025 Mar;117:228-234 doi: 10.1016/j.gaitpost.2024.12.024 Epub 2024 Dec 27 PMID:
39787880.
https://doi.org/10.1016/j.gaitpost.2024.12.024 |
| 337 | Chen, L., Zhang, Z., & Liu, X. (2024). Role and mechanism of mechanical load in the homeostasis of
the subchondral bone in knee osteoarthritis: A comprehensive review. Journal of Immunology Research.
Retrieved from https://www.tandfonline.com/doi/pdf/10.2147/JIR.S492415
https://doi.org/10.2147/JIR.S492415 |
| 338 | Guzzoni, V., & Selistre-de-Araújo, H. S. (2018). Tendon remodeling in response to resistance
training, anabolic androgenic steroids, and aging. Cells, 7(12), 251 Retrieved from
https://www.mdpi.com/2073-4409/7/12/251/pdf
https://doi.org/10.3390/cells7120251 |
| 339 | 340 Jacobs E, Stroobant L, Victor J, Elewaut D, Tampere T, Wallaert S, Witvrouw E, Schuermans J,
Wezenbeek E. Vascular occlusion for optimising the functional improvement in patients with knee
osteoarthritis: a randomised controlled trial. Ann Rheum Dis. 2024 Oct 30:ard-2024-226579 doi:
10.1136/ard-2024-226579 Epub ahead of print. PMID: 39477487.
https://doi.org/10.1136/ard-2024-226579 |
| 340 | Glasgow P, Phillips N, Bleakley C (2015) Optimal loading: key variables and mechanisms. Br J
Sports Med 49:278-279.
https://doi.org/10.1136/bjsports-2014-094443 |
| 341 | He, Y., Li, Z., Alexander, P. G., Ocasio-Nieves, B. D., & Yocum, L. (2020). Pathogenesis of
osteoarthritis: Risk factors, regulatory pathways in chondrocytes, and experimental models. Biology,
9(8), 194 Retrieved from https://www.mdpi.com/2079-7737/9/8/194/pdf
https://doi.org/10.3390/biology9080194 |
| 342 | Huang, C., Xiao, Y., Qing, L., Tang, J., & Wu, P. (2025). Exosomal non-coding RNAs in the
regulation of bone metabolism homeostasis: Molecular mechanism and therapeutic potential. Heliyon.
Retrieved from https://www.cell.com/heliyon/pdf/S2405-8440(25)00011-8.pdf
https://doi.org/10.1016/j.heliyon.2025.e41632 |
| 343 | Stanczak, M., Kacprzak, B., & Gawda, P. (2024). Tendon cell biology: Effect of mechanical loading.
Retrieved from
https://www.researchgate.net/profile/Mikolaj-Stanczak/publication/386015709_Tendon_Cell_Biology_Effect_of_Mechanical_Loading/links/6741bd507ca4cb2842a4b04a/Tendon-Cell-Biology-Effect-of-Mechanical-Loading.pdf
https://doi.org/10.33594/000000743 |
| 344 | Gambari, L., Cellamare, A., Grassi, F., & Grigolo, B. (2023). Targeting the inflammatory hallmarks
of obesity-associated osteoarthritis: Towards nutraceutical-oriented preventive and complementary
therapeutic strategies. International Journal of Molecular Sciences, 24(11), 9340 Retrieved from
https://www.mdpi.com/1422-0067/24/11/9340/pdf
https://doi.org/10.3390/ijms24119340 |
| 345 | Schulz, H., Strauch, S. M., & Richter, P. (2022). Latest knowledge about changes in the proteome
in microgravity. Current Protein & Peptide Science. Retrieved from
https://www.tandfonline.com/doi/abs/10.1080/14789450.2022.2030711
https://doi.org/10.1080/14789450.2022.2030711 |
| 346 | Chen, L., Zhang, Z., & Liu, X. (2024). Role and mechanism of mechanical load in the homeostasis of
the subchondral bone in knee osteoarthritis: A comprehensive review. Journal of Immunology Research.
Retrieved from https://www.tandfonline.com/doi/pdf/10.2147/JIR.S492415
https://doi.org/10.2147/JIR.S492415 |
| 347 | Feng, R., Hu, W., Li, Y., Yao, X., Li, J., Li, X., & Zhang, J. (2024). Mechanotransduction in
subchondral bone microenvironment and targeted interventions for osteoarthritis. Mechanics and
Biology. Retrieved from https://www.sciencedirect.com/science/article/pii/S2949907024000068
https://doi.org/10.1016/j.mbm.2024.100043 |
| 348 | Qin, Y. X., & Zhao, J. (2023). Mechanobiology in cellular, molecular, and tissue adaptation.
Mechanobiology Journal. Retrieved from
https://www.sciencedirect.com/science/article/pii/S2949907023000220
https://doi.org/10.1016/j.mbm.2023.100022 |
| 349 | Lauretta, G. (2023). Study of novel approaches in tissue engineering for cartilage repair and
prevention of osteoarthritis. PhD Dissertation. Retrieved from
https://www.iris.unict.it/bitstream/20.500.11769/582150/1/PhD%20thesis_Giovanni%20Lauretta.pdf
|
| 350 | Knapik, D. M., Perera, P., Nam, J., & Blazek, A. D. (2014). Mechanosignaling in bone health,
trauma, and inflammation. Mechanosignaling Journal. Retrieved from
https://www.liebertpub.com/doi/abs/10.1089/ars.2013.5467
https://doi.org/10.1089/ars.2013.5467 |
| 351 | Feng, R., Hu, W., Li, Y., Yao, X., Li, J., Li, X., & Zhang, J. (2024). Mechanotransduction in
subchondral bone microenvironment and targeted interventions for osteoarthritis. Mechanics and
Biology. Retrieved from https://www.sciencedirect.com/science/article/pii/S2949907024000068
https://doi.org/10.1016/j.mbm.2024.100043 |
| 352 | Chen, L., Zhang, Z., & Liu, X. (2024). Role and mechanism of mechanical load in the homeostasis of
the subchondral bone in knee osteoarthritis: A comprehensive review. Journal of Immunology Research.
Retrieved from https://www.tandfonline.com/doi/pdf/10.2147/JIR.S492415
https://doi.org/10.2147/JIR.S492415 |
| 353 | Qin, Y. X., & Zhao, J. (2023). Mechanobiology in cellular, molecular, and tissue adaptation.
Mechanobiology Journal. Retrieved from
https://www.sciencedirect.com/science/article/pii/S2949907023000220
https://doi.org/10.1016/j.mbm.2023.100022 |
| 354 | Lauretta, G. (2023). TGF-β/Smad pathway regulation in cartilage regeneration. Retrieved from
https://www.iris.unict.it/bitstream/20.500.11769/582150/1/PhD%20thesis_Giovanni%20Lauretta.pdf
|
| 355 | Hornberger, T. A. (2011). Mechanotransduction and the regulation of mTORC1 signaling in skeletal
muscle. The international journal of biochemistry & cell biology, 43(9), 1267- 1276.
https://doi.org/10.1016/j.biocel.2011.05.007 |
| 356 | Bamman, M. M., Roberts, B. M., & Adams, G. R. (2018). Molecular regulation of exercise- induced
muscle fiber hypertrophy. Cold Spring Harbor perspectives in medicine, 8(6), a029751
https://doi.org/10.1101/cshperspect.a029751 |
| 357 | Hawley, J. A., Hargreaves, M., Joyner, M. J., & Zierath, J. R. (2014). Integrative biology of
exercise. Cell, 159(4), 738-749.
https://doi.org/10.1016/j.cell.2014.10.029 |
| 358 | West, D. W., Baehr, L. M., Marcotte, G. R., Chason, C. M., Tolento, L., Gomes, A. V., ...& Baar,
K. (2016). Acute resistance exercise activates rapamycin‐sensitive and‐insensitive mechanisms that
control translational activity and capacity in skeletal muscle. The Journal of physiology, 594(2),
453-468.
https://doi.org/10.1113/JP271365 |
| 359 | Burd, N. A., West, D. W., Staples, A. W., Atherton, P. J., Baker, J. M., Moore, D. R., & Phillips,
S. M. (2010). Low-load high-volume resistance exercise stimulates muscle protein synthesis more than
high-load low-volume resistance exercise in young men. PloS One, 5(8), e12033
https://doi.org/10.1371/journal.pone.0012033
https://doi.org/10.1371/journal.pone.0012033 |
| 360 | Holm, L., Van Hall, G., Rose, A. J., Miller, B. F., Doessing, S., Richter, E. A., & Kjaer, M.
(2010). Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates
differently in human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism,
298(2), E257-E269 https://doi.org/10.1152/ajpendo.00650.2009
https://doi.org/10.1152/ajpendo.00609.2009 |
| 361 | Gehlert, S., Suhr, F., Gutsche, K., Willkomm, L., Kern, J., Jacko, D., ... & Bloch, W. (2015).
High force development augments skeletal muscle signaling in resistance exercise modes equalized for
time under tension. Pflügers Archiv - European Journal of Physiology, 467(6), 1343-1356
https://doi.org/10.1007/s00424-014-1575-7
https://doi.org/10.1007/s00424-014-1579-y |
| 362 | Hulmi, J. J., Walker, S., Ahtiainen, J. P., Nyman, K., Kraemer, W. J., & Häkkinen, K. (2012).
Molecular signaling in muscle is affected by the specificity of resistance exercise protocol.
Scandinavian Journal of Medicine & Science in Sports, 22(2), 240-248
https://doi.org/10.1111/j.1600-0838.2010.01156.x
https://doi.org/10.1111/j.1600-0838.2010.01156.x |
| 363 | Mackey, A. L., Karlsen, A., Couppe, C., Mikkelsen, U. R., Nielsen, R. H., Magnusson, S. P., &
Kjaer, M. (2014). Differential satellite cell density of type I and II fibers with lifelong
endurance running in old men. Acta Physiologica, 210(3), 612-627 https://doi.org/10.1111/apha.12206
https://doi.org/10.1111/apha.12206 |
| 364 | Martin, N. R., & Lewis, M. P. (2012). Satellite cell activation and number following acute and
chronic exercise: A mini review. Cellular and Molecular Exercise Physiology, 1(1), e3
https://doi.org/10.7771/2157-618X.1012
https://doi.org/10.7457/cmep.v1i1.e3 |
| 365 | Kumar, V., Selby, A., Rankin, D., Patel, R., Atherton, P., Hildebrandt, W., ... & Rennie, M. J.
(2009). Age-related differences in the dose-response relationship of muscle protein synthesis to
resistance exercise in young and old men. The Journal of Physiology, 587(1), 211-217
https://doi.org/10.1113/jphysiol.2008.164483
https://doi.org/10.1113/jphysiol.2008.164483 |
| 366 | Mackey, A. L., Holm, L., Reitelseder, S., Pedersen, T. G., Doessing, S., Kadi, F., & Kjaer, M.
(2011). Myogenic response of human skeletal muscle to 12 weeks of resistance training at light
loading intensity. Scandinavian Journal of Medicine & Science in Sports, 21(6), 773-782
https://doi.org/10.1111/j.1600-0838.2010.01179.x
https://doi.org/10.1111/j.1600-0838.2010.01179.x |
| 367 | Sakamoto, K., Arnolds, D. E. W., Ekberg, I., Thorell, A., & Goodyear, L. J. (2004). Exercise
regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochemical and
Biophysical Research Communications, 319(2), 419-425 https://doi.org/10.1016/j.bbrc.2004.04.190
https://doi.org/10.1016/j.bbrc.2004.04.190 |
| 368 | Ishido, M., Uda, M., Masuhara, M., & Kami, K. (2006). Alterations of M-cadherin, neural cell
adhesion molecule and beta-catenin expression in satellite cells during overload-induced skeletal
muscle hypertrophy. Acta Physiologica, 187(3), 407-418
https://doi.org/10.1111/j.1748-1716.2006.01564.x
https://doi.org/10.1111/j.1748-1716.2006.01564.x |
| 369 | Akiho, M., Nakashima, H., Sakata, M., Yamasa, Y., Yamaguchi, A., & Sakuma, K. (2010). Expression
profile of Notch-1 in mechanically overloaded plantaris muscle of mice. Life Sciences, 86(1-2),
59-65 https://doi.org/10.1016/j.lfs.2009.11.024
https://doi.org/10.1016/j.lfs.2009.11.011 |
| 370 | MacKenzie, M. G., Hamilton, D. L., Pepin, M., Patton, A., & Baar, K. (2013). Inhibition of
Myostatin Signaling through Notch Activation following Acute Resistance Exercise. PLoS ONE, 8(7),
e68743 https://doi.org/10.1371/journal.pone.0068743
https://doi.org/10.1371/journal.pone.0068743 |
| 371 | American College of Sports Medicine. (2009). American College of Sports Medicine position stand:
Progression models in resistance training for healthy adults. Medicine & Science in Sports &
Exercise, 41(3), 687-708 https://doi.org/10.1249/MSS.0b013e3181915670
https://doi.org/10.1249/MSS.0b013e3181915670 |
| 372 | Burd, N. A., Mitchell, C. J., Churchward-Venne, T. A., & Phillips, S. M. (2012). Bigger weights
may not beget bigger muscles: Evidence from acute muscle protein synthetic responses after
resistance exercise. Applied Physiology, Nutrition, and Metabolism, 37(3), 551-554
https://doi.org/10.1139/h2012-022
https://doi.org/10.1139/h2012-022 |
| 373 | Schoenfeld, B. J., Grgic, J., Ogborn, D., & Krieger, J. W. (2017). Strength and hypertrophy
adaptations between low- vs. high-load resistance training: A systematic review and meta-analysis.
Journal of Strength and Conditioning Research, 31(12), 3508-3523
https://doi.org/10.1519/JSC.0000000000002200
https://doi.org/10.1519/JSC.0000000000002200 |
| 374 | McCarthy, J. J., Mula, J., Miyazaki, M., Erfani, R., Garrison, K., Farooqui, A. B., ... & Van
Zant, G. (2011). Effective fiber hypertrophy in satellite cell-depleted skeletal muscle.
Development, 138(17), 3657-3666 https://doi.org/10.1242/dev.068858
https://doi.org/10.1242/dev.068858 |
| 375 | Lee, S. J., Huynh, T. V., Lee, Y. S., Sebald, S. M., Wilcox-Adelman, S. A., Iwamori, N., ... &
Fan, C. M. (2012). Role of satellite cells versus myofibers in muscle hypertrophy induced by
inhibition of the myostatin/activin signaling pathway. Proceedings of the National Academy of
Sciences, 109(35), E2353-E2360 https://doi.org/10.1073/pnas.1118027109
https://doi.org/10.1073/pnas.1206410109 |
| 376 | Fry, C. S., Lee, J. D., Jackson, J. R., Kirby, T. J., Stasko, S. A., Liu, H., ... & Peterson, C.
A. (2014). Regulation of the muscle fiber microenvironment by activated satellite cells during
hypertrophy. The FASEB Journal, 28(4), 1654-1665 https://doi.org/10.1096/fj.13-239426
https://doi.org/10.1096/fj.13-239426 |
| 377 | Owens, D. J. (2015). The role of vitamin D in skeletal muscle function and regeneration. Retrieved
from http://researchonline.ljmu.ac.uk/id/eprint/4391/1/158236_2015OwensPhd.pdf
|
| 378 | Reich, K. A. (2009). Molecular changes following skeletal muscle disuse in humans. Retrieved from
https://scholarworks.umass.edu/cgi/viewcontent.cgi?article=1147&context=open_access_dissertations
|
| 379 | Baehr, L. M. (2012). The role of MuRF1 in glucocorticoid-induced muscle atrophy. Retrieved from
https://search.proquest.com/openview/74891d4bbcb6a29f399f5a931c38a9df/1?pq-origsite=gscholar&cbl=18750
|
| 380 | Harish, P. (2018). Modulating the myostatin signalling axis ameliorates tissue atrophy in
oculopharyngeal muscular dystrophy. Retrieved from https://core.ac.uk/download/pdf/328914173.pdf
|
| 381 | Arvanitidis, A. (2017). Novel biomarkers of changes in muscle mass or muscle pathology. Retrieved
from
https://orbit.dtu.dk/en/publications/novel-biomarkers-of-changes-in-muscle-mass-or-muscle-pathology
|
| 382 | Shorter, E., Engman, V., & Lanner, J. T. (2024). Cancer-associated muscle weakness-From triggers
to molecular mechanisms. Current Opinion in Clinical Nutrition & Metabolic Care. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0098299724000190
https://doi.org/10.1016/j.mam.2024.101260 |
| 383 | Harish, P. (2018). Modulating the myostatin signalling axis ameliorates tissue atrophy in
oculopharyngeal muscular dystrophy. Retrieved from https://core.ac.uk/download/pdf/328914173.pdf
|
| 384 | Stantzou, A. (2016). BMP signaling controls postnatal muscle development. Retrieved from
https://refubium.fu-berlin.de/bitstream/handle/fub188/3942/Thesis_Stantzou_FU_library_online.pdf?sequence=1&isAllowed=y
|
| 385 | Arvanitidis, A. (2017). Novel biomarkers of changes in muscle mass or muscle pathology. Retrieved
from
https://orbit.dtu.dk/en/publications/novel-biomarkers-of-changes-in-muscle-mass-or-muscle-pathology
|
| 386 | Kjaer, M., Magnusson, S. P., Krogsgaard, M., Boysen-Møller, T., Olesen, J. L., Heinemeier, K., ...
& Langberg, H. (2015). Extracellular matrix adaptation of tendon and skeletal muscle to exercise.
Journal of Anatomy, 208(4), 445-450 https://doi.org/10.1111/j.1469-7580.2006.00541.x
https://doi.org/10.1111/j.1469-7580.2006.00541.x |
| 387 | Harish, P. (2018). Modulating the myostatin signalling axis ameliorates tissue atrophy in
oculopharyngeal muscular dystrophy. Retrieved from https://core.ac.uk/download/pdf/328914173.pdf
|
| 388 | Arvanitidis, A. (2017). Novel biomarkers of changes in muscle mass or muscle pathology. Retrieved
from
https://orbit.dtu.dk/en/publications/novel-biomarkers-of-changes-in-muscle-mass-or-muscle-pathology
|
| 389 | Owens, D. J. (2015). The role of vitamin D in skeletal muscle function and regeneration. Retrieved
from http://researchonline.ljmu.ac.uk/id/eprint/4391/1/158236_2015OwensPhd.pdf
|
| 390 | Harish, P. (2018). Modulating the myostatin signalling axis ameliorates tissue atrophy in
oculopharyngeal muscular dystrophy. Retrieved from https://core.ac.uk/download/pdf/328914173.pdf
|
| 391 | Chen, L., Zhang, Z., & Liu, X. (2024). Role and mechanism of mechanical load in the homeostasis of
the subchondral bone in knee osteoarthritis: A comprehensive review. Journal of Immunology Research.
Retrieved from https://www.tandfonline.com/doi/pdf/10.2147/JIR.S492415
https://doi.org/10.2147/JIR.S492415 |
| 392 | Reich, K. A. (2009). Molecular changes following skeletal muscle disuse in humans. Retrieved from
https://scholarworks.umass.edu/cgi/viewcontent.cgi?article=1147&context=open_access_dissertations
|
| 393 | Owens, D. J. (2015). Vitamin D and muscle repair following injury and exercise. Retrieved from
http://researchonline.ljmu.ac.uk/id/eprint/15240/1
|
| 394 | Baehr, L. M. (2012). The role of MuRF1 in glucocorticoid-induced muscle atrophy. Retrieved from
https://search.proquest.com/openview/74891d4bbcb6a29f399f5a931c38a9df/1?pq-origsite=gscholar&cbl=18750
|
| 395 | Goh, Q., & Millay, D. P. (2017). Requirement of myomarker-mediated stem cell fusion for skeletal
muscle hypertrophy. eLife, 6, e20007 https://doi.org/10.7554/eLife.20007
https://doi.org/10.7554/eLife.20007 |
| 396 | Du J, Yun H, Wang H, Bai X, Su Y, Ge X, Wang Y, Gu B, Zhao L, Yu JG, Song Y. Proteomic Profiling
of Muscular Adaptations to Short-Term Concentric Versus Eccentric Exercise Training in Humans. Mol
Cell Proteomics. 2024 Apr;23(4):100748 doi: 10.1016/j.mcpro.2024.100748 Epub 2024 Mar 15 PMID:
38493954; PMCID: PMC11017286.
https://doi.org/10.1016/j.mcpro.2024.100748 |
| 397 | Cheng L, Chang S, Tan Y, He B. Platelet-rich plasma combined with isometric quadriceps contraction
regulates autophagy in chondrocytes via the PI3K/AKT/mTOR pathway to promote cartilage repair in
knee osteoarthritis. Regen Ther. 2024 Dec 4;28:81-89 doi: 10.1016/j.reth.2024.11.013 PMID: 39703816;
PMCID: PMC11655694.
https://doi.org/10.1016/j.reth.2024.11.013 |
| 398 | 399 Glatt, V., Evans, C. H., & Stoddart, M. J. (2019). Regenerative rehabilitation: The role of
mechanotransduction in orthopaedic regenerative medicine. Journal of Orthopaedic Research.
https://app.scholarai.io/paper?paper_id=DOI:10.1002/jor.24205&original_url=https%3A%2F%2Fonlinelibrary.wiley.com%2Fdoi%2Fabs%2F10.1002%2Fjor.24205
|
| 399 | Ng, J. L., Kersh, M. E., Kilbreath, S., & Knothe Tate, M. (2017). Establishing the basis for
mechanobiology-based physical therapy protocols to potentiate cellular healing and tissue
regeneration. Frontiers in Physiology.
https://www.frontiersin.org/articles/10.3389/fphys.2017.00303/full
https://doi.org/10.3389/fphys.2017.00303 |
| 400 | Loghmani, M. T., & Whitted, M. (2016). Soft tissue manipulation: a powerful form of
mechanotherapy. Semantics Scholar.
https://pdfs.semanticscholar.org/18a2/59acf36bbb3753a92210b737c1e4c3679ddd.pdf
https://doi.org/10.4172/2573-0312.1000122 |
| 401 | Thompson, W. R., Scott, A., & Loghmani, M. T. (2016). Understanding mechanobiology: physical
therapists as a force in mechanotherapy and musculoskeletal regenerative rehabilitation. Physical
Therapy, 96(4), 560-573 https://academic.oup.com/ptj/article-abstract/96/4/560/2686523
https://doi.org/10.2522/ptj.20150224 |
| 402 | Dunn, S. L., & Olmedo, M. L. (2016). Mechanotransduction: Relevance to physical therapist
practice-Understanding our ability to affect genetic expression through mechanical forces. Physical
Therapy, 96(5), 712-723 https://academic.oup.com/ptj/article-abstract/96/5/712/2686418
https://doi.org/10.2522/ptj.20150073 |
| 403 | Huang, C., Holfeld, J., Schaden, W., & Orgill, D. (2016). Mechanotherapy: revisiting physical
therapy and recruiting mechanobiology for a new era in medicine. Trends in Molecular Medicine.
https://www.cell.com/trends/molecular-medicine/fulltext/S1471-4914(13)00092-0
|
| 404 | Lederman, E. (2005). The science & practice of manual therapy. Books Google.
https://books.google.com/books?hl=en&lr=&id=L9OMvc42Kf4C&oi=fnd&pg=PR7&dq=manual+therapy+mechanotransduction+tissue+repair&ots=7kXmfd3nWS&sig=Kil4KcLHs8mpnmUvYXmqyOSew34
|
| 405 | Dunn, S. L., & Olmedo, M. L. (2016). Mechanotransduction: Relevance to physical therapist
practice-Understanding our ability to affect genetic expression through mechanical forces. Physical
Therapy. https://academic.oup.com/ptj/article-abstract/96/5/712/2686418
https://doi.org/10.2522/ptj.20150073 |
| 406 | Huang, C., Holfeld, J., Schaden, W., & Orgill, D. (2016). Mechanotherapy: revisiting physical
therapy and recruiting mechanobiology for a new era in medicine. Trends in Molecular Medicine.
|
| 407 | Cook, C. E., Keter, D., Cade, W. T., & Winkelstein, B. A. (2024). Manual therapy and exercise
effects on inflammatory cytokines: A narrative overview. Frontiers in Rehabilitation Sciences.
https://www.frontiersin.org/articles/10.3389/fresc.2024.1305925/full
https://doi.org/10.3389/fresc.2024.1305925 |
| 408 | Smith, E. A. (2024). Manual therapy in musculoskeletal injury rehabilitation: A pain-modulation
approach. Scientific Research Journal.
https://app.scholarai.io/paper?paper_id=DOI:10.5281/zenodo.14168230&original_url=https%3A%2F%2Fcspjournals.org%2Findex.php%2FScientific-Research%2Farticle%2Fview%2F424
|
| 409 | S, Neurohr GA. Evidence based treatment options for common knee injuries in runners. Ann Transl
Med. 2019 Oct;7(Suppl 7):S249 doi: 10.21037/atm.2019.04.08 PMID: 31728373; PMCID: PMC6829001.
https://doi.org/10.21037/atm.2019.04.08 |
| 410 | Ng JL, Kersh ME, Kilbreath S, Knothe Tate M. Establishing the Basis for Mechanobiology-Based
Physical Therapy Protocols to Potentiate Cellular Healing and Tissue Regeneration. Front Physiol.
2017 Jun 6;8:303 doi: 10.3389/fphys.2017.00303 PMID: 28634452; PMCID: PMC5460618.
https://doi.org/10.3389/fphys.2017.00303 |
| 411 | Kim MS, Koh IJ, Choi YJ, Pak KH, In Y. Collagen Augmentation Improves the Quality of Cartilage
Repair After Microfracture in Patients Undergoing High Tibial Osteotomy: A Randomized Controlled
Trial. Am J Sports Med. 2017 Jul;45(8):1845-1855 doi: 10.1177/0363546517691942 Epub 2017 Mar 10
PMID: 28282221.
https://doi.org/10.1177/0363546517691942 |
| 412 | Zhang K, Beshay T, Murphy B, Sheean A, de Sa D. Quadriceps Tendon Anterior Cruciate Ligament
Reconstruction: A Systematic Review of Postoperative Rehabilitation and Complication Profiles.
Arthroscopy. 2022 Jun;38(6):2062-2072.e1 doi: 10.1016/j.arthro.2021.12.020 Epub 2021 Dec 21 PMID:
34942315.
https://doi.org/10.1016/j.arthro.2021.12.020 |
| 413 | Di, X., Gao, X., Peng, L., Ai, J., Jin, X., Qi, S., & Li, H. (2023). Cellular mechanotransduction
in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduction and
Targeted Therapy. PDF
https://doi.org/10.1038/s41392-023-01501-9 |
| 414 | Xie, N., Xiao, C., Shu, Q., Cheng, B., Wang, Z., Xue, R., & Wen, Z. (2023). Cell response to
mechanical microenvironment cues via Rho signaling: From mechanobiology to mechanomedicine. Journal
of the Mechanical Behavior of Biomedical Materials. PDF
https://doi.org/10.2139/ssrn.4246343 |
| 415 | Kuehlmann, B., Bonham, C. A., Zucal, I., & Prantl, L. (2020). Mechanotransduction in wound healing
and fibrosis. Journal of Clinical Medicine, 9(5), 1423 PDF
https://doi.org/10.3390/jcm9051423 |
| 416 | Popov, C., Burggraf, M., Kreja, L., & Ignatius, A. (2015). Mechanical stimulation of human tendon
stem/progenitor cells results in upregulation of matrix proteins, integrins, and MMPs, and
activation of p38 and ERK1/2 pathways. Cell and Tissue Research. PDF
https://doi.org/10.1186/s12867-015-0036-6 |
| 417 | Rendek Z, Bon Beckman L, Schepull T, Dånmark I, Aspenberg P, Schilcher J, Eliasson P. Early
Tensile Loading in Nonsurgically Treated Achilles Tendon Ruptures Leads to a Larger Tendon Callus
and a Lower Elastic Modulus: A Randomized Controlled Trial. Am J Sports Med. 2022
Oct;50(12):3286-3298 doi: 10.1177/03635465221117780 Epub 2022 Aug 25 PMID: 36005394; PMCID:
PMC9527451.
https://doi.org/10.1177/03635465221117780 |
| 418 | Sup, M. K. (2024). The inflammatory response in tendon fibroblasts is multi-factorial and alters
their responses to mechanical stimulation. ProQuest Dissertations & Theses Global. Link
|
| 419 | Steffen, D. (2023). A better treatment for tendinopathy: Molecular insights from tendon
development, injury, and exercise studies. ProQuest Dissertations & Theses Global. PDF
|
| 420 | Steffen, D., Mienaltowski, M. J., & Baar, K. (2022). Scleraxis and collagen I expression increase
following pilot isometric loading experiments in a rodent model of patellar tendinopathy. Matrix
Biology Plus. Link
https://doi.org/10.1016/j.matbio.2022.03.006 |
| 421 | Mueller BT, Moulton SG, O'Brien L, LaPrade RF. Rehabilitation Following Meniscal Root Repair: A
Clinical Commentary. J Orthop Sports Phys Ther. 2016 Feb;46(2):104-13 doi: 10.2519/jospt.2016.6219
Epub 2016 Jan 11 PMID: 26755403.
https://doi.org/10.2519/jospt.2016.6219 |
| 422 | Heidenberger, J., Hangel, R., & Reihs, E. I. (2024). The modulating role of uniaxial straining in
the IL-1β and TGF-β mediated inflammatory response of human primary ligamentocytes. Frontiers in
Bioengineering and Biotechnology. PDF
https://doi.org/10.3389/fbioe.2024.1469238 |
| 423 | Mae T, Shino K, Matsumoto N, Maeda A, Nakata K, Yoneda M. Graft tension during active knee
extension exercise in anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy.
2010 Feb;26(2):214-22 doi: 10.1016/j.arthro.2009.07.016 Epub 2009 Dec 21 PMID: 20141984.
https://doi.org/10.1016/j.arthro.2009.07.016 |
| 424 | Capin JJ, Khandha A, Buchanan TS, Snyder-Mackler L. Partial medial meniscectomy leads to altered
walking mechanics two years after anterior cruciate ligament reconstruction: Meniscal repair does
not. Gait Posture. 2019 Oct;74:87-93 doi: 10.1016/j.gaitpost.2019.08.017 Epub 2019 Aug 27 PMID:
31491565; PMCID: PMC6790293
https://doi.org/10.1016/j.gaitpost.2019.08.017 |
| 425 | Uzuner S, Li LP. Alteration in ACL loading after total and partial medial meniscectomy. BMC
Musculoskelet Disord. 2024 Jan 25;25(1):94 doi: 10.1186/s12891-024-07201-x. PMID: 38273316; PMCID:
PMC11395656
https://doi.org/10.1186/s12891-024-07201-x |
| 426 | Luttrell, T. (2024). Trauma and inflammation of soft tissue: Rehabilitation and wound healing and
remodeling of collagen. Taylor & Francis. Link
https://doi.org/10.4324/9781003524212-3 |
| 427 | Jiang, F., Zhao, H., Zhang, P., Bi, Y., & Zhang, H. (2024). Challenges in tendon-bone healing:
Emphasizing inflammatory modulation mechanisms and treatment. Frontiers in Endocrinology. PDF
https://doi.org/10.3389/fendo.2024.1485876 |
| 428 | Janakiram, N. B., Valerio, M. S., & Goldman, S. M. (2021). The role of the inflammatory response
in mediating functional recovery following composite tissue injuries. International Journal of
Molecular Sciences, 22(24), 13552 PDF
https://doi.org/10.3390/ijms222413552 |
| 429 | Gan, Q. F., Choy, K. W., Foo, C. N., Leong, P. P., & Cheong, S. K. (2021). Incorporating insulin
growth factor-1 into regenerative and personalized medicine for musculoskeletal disorders: A
systematic review. Journal of Tissue Engineering and Regenerative Medicine. Link
https://doi.org/10.1002/term.3192 |
| 430 | Muire, P. J., Mangum, L. H., & Wenke, J. C. (2020). Time course of immune response and
immunomodulation during normal and delayed healing of musculoskeletal wounds. Frontiers in
Immunology. PDF
https://doi.org/10.3389/fimmu.2020.01056 |
| 431 | Chisari, E., Rehak, L., & Khan, W. S. (2019). Tendon healing in presence of chronic low-level
inflammation: A systematic review. British Medical Bulletin, 132(1), 97 PDF
https://doi.org/10.1093/bmb/ldz035 |
| 432 | 433Fouda, M. B., Thankam, F. G., & Dilisio, M. F. (2017). Alterations in tendon microenvironment
in response to mechanical load: Potential molecular targets for treatment strategies. National
Center for Biotechnology Information. Link
|
| 433 | Hofer, H. R., & Tuan, R. S. (2016). Secreted trophic factors of mesenchymal stem cells support
neurovascular and musculoskeletal therapies. Stem Cell Research & Therapy. PDF
https://doi.org/10.1186/s13287-016-0394-0 |
| 434 | Jiang, F., Zhao, H., Zhang, P., Bi, Y., & Zhang, H. (2024). Challenges in tendon-bone healing:
Emphasizing inflammatory modulation mechanisms and treatment. Frontiers in Endocrinology. PDF
https://doi.org/10.3389/fendo.2024.1485876 |
| 435 | Ely, M. R. (2019). Histamine and the exercise responsome. University of Oregon Dissertations. PDF
|
| 436 | Erthal, V., Maria-Ferreira, D., & de Paula Werner, M. F. (2016). Anti-inflammatory effect of laser
acupuncture in ST36 (Zusanli) acupoint in mouse paw edema. Lasers in Medical Science. Link
https://doi.org/10.1007/s10103-015-1845-z |
| 437 | Barr, A. E., & Barbe, M. F. (2004). Inflammation reduces physiological tissue tolerance in the
development of work-related musculoskeletal disorders. Journal of Electromyography and Kinesiology.
Link
https://doi.org/10.1016/j.jelekin.2003.09.008 |
| 438 | Ekman, E. F., & Koman, L. A. (2004). Acute pain following musculoskeletal injuries and orthopedic
surgery: Mechanisms and management. The Journal of Bone and Joint Surgery. PDF
https://doi.org/10.2106/00004623-200406000-00029 |
| 439 | Delforge, G. (2002). Musculoskeletal trauma: Implications for sports injury management. Human
Kinetics Publishers. Link
|
| 440 | Omoigui, S. (2007). The biochemical origin of pain: The origin of all pain is inflammation and the
inflammatory response. Part 2 of 3-inflammatory profile of pain syndromes. Medical Hypotheses. Link
https://doi.org/10.1016/j.mehy.2007.06.033 |
| 441 | Tidball, J. G. (2005). Inflammatory processes in muscle injury and repair. American Journal of
Physiology-Regulatory, Integrative and Comparative Physiology, 288(2), R345-R353 PDF
https://doi.org/10.1152/ajpregu.00454.2004 |
| 442 | Markenson, J. (1999). Nonnarcotic analgesics in short-term pain: Musculoskeletal disorders.
Journal of Back and Musculoskeletal Rehabilitation, 6(2S), 107-114 PDF
https://doi.org/10.1177/107327489900602S03 |
| 443 | Povo-Retana, A., & Sánchez-García, S. (2024). Crosstalk between P2Y receptors and cyclooxygenase
activity in inflammation and tissue repair. Purinergic Signalling. PDF
https://doi.org/10.1007/s11302-023-09938-x |
| 444 | Cheng, H., Huang, H., Guo, Z., Chang, Y., & Li, Z. (2021). Role of prostaglandin E2 in tissue
repair and regeneration. Frontiers in Immunology. Link
https://doi.org/10.7150/thno.63396 |
| 445 | Davis, F. M., Tsoi, L. C., Wasikowski, R., Joshi, A., & Wilke, C. (2020). Epigenetic regulation of
the PGE2 pathway modulates macrophage phenotype in normal and pathologic wound repair. Journal of
Immunology. Link
https://doi.org/10.1172/jci.insight.138443 |
| 446 | Loynes, C. A., Lee, J. A., Robertson, A. L., & Steel, M. J. G. (2018). PGE2 production at sites of
tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of
inflammation resolution in vivo. Science Advances. PDF
https://doi.org/10.1126/sciadv.aar8320 |
| 447 | Zhang, S., Liu, Y., Zhang, X., Zhu, D., Qi, X., Cao, X., & Fang, Y. (2018). Prostaglandin E2
hydrogel improves cutaneous wound healing via M2 macrophage polarization. Frontiers in Pharmacology.
Link
https://doi.org/10.7150/thno.27385 |
| 448 | Linke, B., Schreiber, Y., & Picard-Willems, B. (2017). Activated platelets induce an
anti-inflammatory response of monocytes/macrophages through cross-regulation of PGE2 and cytokines.
Mediators of Inflammation. PDF
https://doi.org/10.1155/2017/1463216 |
| 449 | Lu, L. Y., Loi, F., Nathan, K., & Lin, T. (2017). Pro-inflammatory M1 macrophages promote
osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. Journal of
Orthopaedic Research. PDF
https://doi.org/10.1002/jor.23553 |
| 450 | Fernando, M. R., & Giembycz, M. A. (2016). Crosstalk via IL-6, PGE2, and PGD2 between murine
myofibroblasts and alternatively activated macrophages enhances anti-inflammatory phenotype in both
cells. British Journal of Pharmacology. PDF
https://doi.org/10.1111/bph.13409 |
| 451 | Ulivi, V., Tasso, R., & Cancedda, R. (2014). Cell paracrine activity is modulated by platelet
lysate: Induction of an inflammatory response and secretion of factors maintaining macrophages in a
proinflammatory phenotype. Stem Cells and Development. PDF
https://doi.org/10.1089/scd.2013.0567 |
| 452 | 453Rozman, P., & Bolta, Z. (2007). Use of platelet growth factors in treating wounds and
soft-tissue injuries. International Journal of Wound Care. PDF
|
| 453 | Althurwi, S. A. H., Alanazi, F. F., & Kariri, M. A. (2024). Biochemical basis of inflammatory
response in nursing: Implications for patient care and treatment. Egyptian Journal of Chemistry. PDF
https://doi.org/10.21608/ejchem.2024.336228.10798 |
| 454 | Choi, H. W., Jo, M. J., Go, H. J., Park, N. G., & Ahn, D. H. (2024). Anti-inflammatory effects of
a model peptide, αAL14, via regulation of ERK/MAPK and NF-κB pathway on LPS-stimulated RAW264.7
macrophages. International Journal of Peptide Research and Therapeutics. Link
https://doi.org/10.1007/s10989-024-10638-2 |
| 455 | Soares, C. L. R., Wilairatana, P., & Silva, L. R. (2023). Biochemical aspects of the inflammatory
process: A narrative review. Clinical Biochemistry. Link
https://doi.org/10.1016/j.biopha.2023.115764 |
| 456 | Barker, K. (2022). Targeting pro-inflammatory mediators to treat visceral pain. Cambridge
University Repository. PDF
|
| 457 | Laavola, M. (2019). Immunomodulatory properties of wood biochemicals: Effects on immunomodulatory
gene expression and inflammatory responses in vivo. Tampere University Dissertations. PDF
|
| 458 | Ribeiro, D., Freitas, M., & Lima, J. L. F. C. (2015). Proinflammatory pathways: The modulation by
flavonoids. Medicinal Research Reviews. Link
https://doi.org/10.1002/med.21347 |
| 459 | Chu, A. J. (2014). Antagonism by bioactive polyphenols against inflammation: A systematic view.
Inflammation & Allergy - Drug Targets. Link
https://doi.org/10.2174/1871528112666131119211002 |
| 460 | Vendramini-Costa, D. B. (2012). Molecular link mechanisms between inflammation and cancer. Current
Pharmaceutical Design. PDF
https://doi.org/10.2174/138161212802083707 |
| 461 | Brison, R. J., Day, A. G., Pelland, L., Pickett, W., & Johnson, A. P. (2016). Effect of early
supervised physiotherapy on recovery from acute ankle sprain: A randomized controlled trial. BMJ.
https://doi.org/10.1136/bmj.i5650 |
| 462 | Xia, Y., Wang, H., Yang, R., Hou, Y., Li, Y., & Zhu, J. (2023). Biomaterials delivery strategies
to repair degenerated intervertebral discs by regulating the inflammatory microenvironment.
Frontiers in Immunology. PDF
https://doi.org/10.3389/fimmu.2023.1051606 |
| 466 | Xu, F., Liu, C., Zhou, D., & Zhang, L. (2016). TGF-β/Smad pathway and its regulation in hepatic
fibrosis. Journal of Histochemistry & Cytochemistry, 64(3), 157-167
https://journals.sagepub.com/doi/10.1369/0022155415627681
https://doi.org/10.1369/0022155415627681 |
| 467 | Yu, X. Y., Sun, Q., Zhang, Y. M., & Zou, L. (2022). TGF-β/Smad signaling pathway in
tubulointerstitial fibrosis. Frontiers in Pharmacology, 13, 860588
https://www.frontiersin.org/articles/10.3389/fphar.2022.860588/full
https://doi.org/10.3389/fphar.2022.860588 |
| 468 | Hanna, A., Humeres, C., & Frangogiannis, N. G. (2021). The role of Smad signaling cascades in
cardiac fibrosis. Current Opinion in Physiology, 1(1), 37-47
https://www.sciencedirect.com/science/article/pii/S089865682030303X
https://doi.org/10.1016/j.cellsig.2020.109826 |
| 469 | Munoz-Felix, J. M., & Gonzalez-Nunez, M. (2015). TGF-β/BMP proteins as therapeutic targets in
renal fibrosis. Pharmacological Research, 101, 1-10
https://www.sciencedirect.com/science/article/pii/S0163725815001849
|
| 470 | Lecarpentier, Y., Schussler, O., & Claes, V. (2017). The myofibroblast: TGFβ-1, a conductor which
plays a key role in fibrosis by regulating the balance between PPARγ and the canonical WNT pathway.
Retrieved from
https://www.kenzpub.com/articles/the-myofibroblast-tgf1-a-conductorwhich-plays-a-key-role-in-fibrosis-by-regulating-the-balance-between-ppar-andthe-canon.pdf
https://doi.org/10.11131/2017/101299 |
| 471 | Walton, K. L., Johnson, K. E., & Harrison, C. A. (2017). Targeting TGF-β mediated SMAD signaling
for the prevention of fibrosis. Frontiers in Pharmacology, 8, 461
https://www.frontiersin.org/articles/10.3389/fphar.2017.00461/full
https://doi.org/10.3389/fphar.2017.00461 |
| 472 | Peng, D., Fu, M., Wang, M., Wei, Y., & Wei, X. (2022). Targeting TGF-β signal transduction for
fibrosis and cancer therapy. Cancer Cell International. Retrieved from
https://link.springer.com/article/10.1186/s12943-022-01569-x
https://doi.org/10.1186/s12943-022-01569-x |
| 473 | Chang, H. H., Wu, S. B., & Tsai, C. C. (2024). A review of pathophysiology and therapeutic
strategies targeting TGF-β in Graves' ophthalmopathy. Cells, 13(17), 1493
https://www.mdpi.com/2073-4409/13/17/1493
https://doi.org/10.3390/cells13171493 |
| 474 | Hosseinzadeh, A., Javad-Moosavi, S. A., & Reiter, R. J. (2018). Idiopathic pulmonary fibrosis
(IPF) signaling pathways and protective roles of melatonin. Life Sciences. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0024320518301383
https://doi.org/10.1016/j.lfs.2018.03.032 |
| 475 | Dolivo, D. M., Larson, S. A., & Dominko, T. (2017). Fibroblast growth factor 2 as an antifibrotic:
Antagonism of myofibroblast differentiation and suppression of pro-fibrotic gene expression.
Cytotherapy, 19(9), 1081-1096 https://www.sciencedirect.com/science/article/pii/S1359610117301478
https://doi.org/10.1016/j.cytogfr.2017.09.003 |
| 476 | Jin, G., Hong, W., Guo, Y., Bai, Y., & Chen, B. (2020). Molecular mechanism of pancreatic stellate
cells activation in chronic pancreatitis and pancreatic cancer. Frontiers in Oncology. Retrieved
from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6995390/
https://doi.org/10.7150/jca.38616 |
| 477 | Fang, Z., Meng, Q., Xu, J., Wang, W., & Zhang, B. (2023). Signaling pathways in cancer-associated
fibroblasts: Recent advances and future perspectives. Cancer Communications, 43(1), 12392
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/cac2.12392
https://doi.org/10.1002/cac2.12392 |
| 478 | Nakerakanti, S., & Trojanowska, M. (2012). The role of TGF-β receptors in fibrosis. Arthritis
Research & Therapy, 14(1), 119 Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3396054/
https://doi.org/10.2174/1874312901206010156 |
| 479 | Raza, A., Chohan, T. A., Zaidi, S. H. H., & Hai, A. (2024). A systematic review on biochemical
perspectives on natural products in wound healing: Exploring phytochemicals in tissue repair and
scar prevention. Chemistry & Biodiversity.
https://onlinelibrary.wiley.com/doi/10.1002/cbdv.202400615
https://doi.org/10.1002/cbdv.202400615 |
| 480 | Li, Y. Y., Ji, S. F., Fu, X. B., Jiang, Y. F., & Sun, X. Y. (2024). Biomaterial-based mechanical
regulation facilitates scarless wound healing with functional skin appendage regeneration. Military
Medical Research. https://link.springer.com/article/10.1186/s40779-024-00519-6
https://doi.org/10.1186/s40779-024-00519-6 |
| 481 | Karppinen, S. M., Heljasvaara, R., & Gullberg, D. (2019). Toward understanding scarless skin wound
healing and pathological scarring. Matrix Biology.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6556993/
https://doi.org/10.12688/f1000research.18293.1 |
| 482 | Theocharis, A. D., Manou, D., & Karamanos, N. K. (2019). The extracellular matrix as a
multitasking player in disease. The FEBS Journal, 286(15), 2830-2869
https://febs.onlinelibrary.wiley.com/doi/pdf/10.1111/febs.14818
https://doi.org/10.1111/febs.14818 |
| 483 | Mannan, A., Dhiamn, S., Garg, N., & Singh, T. G. (2023). Pharmacological modulation of Sonic
Hedgehog signaling pathways in angiogenesis: A mechanistic perspective. Developmental Biology.
https://www.sciencedirect.com/science/article/pii/S001216062300163X
https://doi.org/10.1016/j.ydbio.2023.09.009 |
| 484 | Hunt, M., Torres, M., & Bachar-Wikstrom, E. (2024). Cellular and molecular roles of reactive
oxygen species in wound healing. Communications Biology.
https://www.nature.com/articles/s42003-024-07219-w
https://doi.org/10.1038/s42003-024-07219-w |
| 485 | de Castro Brás, L. E., & Frangogiannis, N. G. (2020). Extracellular matrix-derived peptides in
tissue remodeling and fibrosis. Matrix Biology Plus.
https://www.sciencedirect.com/science/article/pii/S0945053X20300512
https://doi.org/10.1016/j.matbio.2020.04.006 |
| 486 | Ricard-Blum, S., Baffet, G., & Théret, N. (2018). Molecular and tissue alterations of collagens in
fibrosis. Matrix Biology. https://univ-rennes.hal.science/hal-01808771/document
https://doi.org/10.1016/j.matbio.2018.02.004 |
| 487 | Häkkinnen, L., Koivisto, L., Heino, J., & Larjava, H. (2015). Cell and molecular biology of wound
healing. Comprehensive Physiology.
https://www.sciencedirect.com/science/article/pii/B9780123971579000540
https://doi.org/10.1016/B978-0-12-397157-9.00054-0 |
| 488 | Cordeiro, J. V., & Jacinto, A. (2013). The role of transcription-independent damage signals in the
initiation of epithelial wound healing. Nature Reviews Molecular Cell Biology, 14(4), 249-262
https://www.nature.com/articles/nrm3541
https://doi.org/10.1038/nrm3541 |
| 489 | Miescher, I., Rieber, J., & Calcagni, M. (2023). In vitro and in vivo effects of IGF-1 delivery
strategies on tendon healing: A review. International Journal of Molecular Sciences, 24(3), 2370
https://www.mdpi.com/1422-0067/24/3/2370
https://doi.org/10.3390/ijms24032370 |
| 490 | Asparuhova, M. B., Riedwyl, D., & Aizawa, R. (2023). Local concentrations of TGF-β1 and IGF-1
appear determinant in regulating bone regeneration in human postextraction tooth sockets.
International Journal of Molecular Sciences, 24(9), 8239 https://www.mdpi.com/1422-0067/24/9/8239
https://doi.org/10.3390/ijms24098239 |
| 491 | Wang, Y., & Li, J. (2023). Current progress in growth factors and extracellular vesicles in tendon
healing. International Wound Journal. https://onlinelibrary.wiley.com/doi/10.1111/iwj.14261
https://doi.org/10.1111/iwj.14261 |
| 492 | Freedman BR, Gordon JA, Soslowsky LJ. The Achilles tendon: fundamental properties and mechanisms
governing healing. Muscles Ligaments Tendons J. 2014 Jul 14;4(2):245-55 PMID: 25332943; PMCID:
PMC4187594.
|
| 493 | Laczko, R., & Csiszar, K. (2020). Lysyl oxidase (LOX): Functional contributions to signaling
pathways. Biomolecules, 10(8), 1093 https://doi.org/10.3390/biom10081093
https://doi.org/10.3390/biom10081093 |
| 494 | Tenti, P., & Vannucci, L. (2020). Lysyl oxidases: Linking structures and immunity in the tumor
microenvironment. Springer Immunology. Retrieved from
https://link.springer.com/article/10.1007/s00262-019-02404-x
https://doi.org/10.1007/s00262-019-02404-x |
| 495 | Walraven, M., & Hinz, B. (2018). Therapeutic approaches to control tissue repair and fibrosis:
Extracellular matrix as a game changer. Matrix Biology. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0945053X17304900
https://doi.org/10.1016/j.matbio.2018.02.020 |
| 496 | Liu, X. (2022). Extracellular matrix in muscle regeneration, adipose tissue function, and meat
quality. ProQuest Dissertations. Retrieved from
https://search.proquest.com/openview/4267e5f13339749818eba4c026f76287/1
|
| 497 | Potekaev, N. N., Borzykh, O. B., & Medvedev, G. V. (2021). The role of extracellular matrix in
skin wound healing. Journal of Clinical Medicine, 10(24), 5947
https://www.mdpi.com/2077-0383/10/24/5947
https://doi.org/10.3390/jcm10245947 |
| 498 | Li, L., Zhao, Q., & Kong, W. (2018). Extracellular matrix remodeling and cardiac fibrosis. Matrix
Biology. Retrieved from https://www.sciencedirect.com/science/article/pii/S0945053X17303980
https://doi.org/10.1016/j.matbio.2018.01.013 |
| 499 | Theocharis, A. D., Manou, D., & Karamanos, N. K. (2019). The extracellular matrix as a
multitasking player in disease. FEBS Journal, 286(15), 2830-2869
https://febs.onlinelibrary.wiley.com/doi/pdf/10.1111/febs.14818
https://doi.org/10.1111/febs.14818 |
| 500 | Lin, W., Song, Y., Li, T., Yan, J., Zhang, R., Han, L., & Ba, X. (2023). Triptolide attenuates
pulmonary fibrosis by inhibiting fibrotic extracellular matrix remodeling mediated by
MMPs/LOX/integrin. Respiratory Medicine. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0753332223011927
https://doi.org/10.1016/j.biopha.2023.115394 |
| 501 | Khalil, R. A. (2017). Matrix metalloproteinases and tissue remodeling in health and disease:
Cardiovascular remodeling. Retrieved from https://books.google.com/books?hl=en&lr=&id=qhZ2DQAAQBAJ
|
| 502 | Bonnans, C., Chou, J., & Werb, Z. (2014). Remodelling the extracellular matrix in development and
disease. Nature Reviews Molecular Cell Biology, 15(12), 786-801
https://www.nature.com/articles/nrm3904
https://doi.org/10.1038/nrm3904 |
| 503 | Afshar, K., Sanaei, M. J., & Ravari, M. S. (2023). An overview of extracellular matrix and its
remodeling in the development of cancer and metastasis with a glance at therapeutic approaches. Cell
Biochemistry and Function, 41(4), 621-636 https://onlinelibrary.wiley.com/doi/10.1002/cbf.3846
https://doi.org/10.1002/cbf.3846 |
| 504 | Goodier, H. (2016). Investigating mechanisms driving fibrosis in pathological rotator cuff
tendons. Retrieved from https://ora.ox.ac.uk/objects/uuid:21f7f117-8222-4682-9255-186a4ab697c5
|
| 505 | Vinhas, A., Almeida, A. F., & Rodrigues, M. T. (2023). Prospects of magnetically based approaches
addressing inflammation in tendon tissues. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0169409X23001308
https://doi.org/10.1016/j.addr.2023.114815 |
| 506 | Vinhas, A., Almeida, A. F., & Rodrigues, M. T. (2023). Prospects of magnetically based approaches
addressing inflammation in tendon tissues. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0169409X23001308
https://doi.org/10.1016/j.addr.2023.114815 |
| 507 | Karsdal, M. A., & Manon-Jensen, T. (2015). Novel insights into the function and dynamics of
extracellular matrix in liver fibrosis. Retrieved from
https://journals.physiology.org/doi/10.1152/ajpgi.00447.2014
https://doi.org/10.1152/ajpgi.00447.2014 |
| 508 | Kwan, K. Y. C., Ng, K. W. K., Rao, Y., Zhu, C., & Qi, S. (2023). Effect of aging on tendon
biology, biomechanics, and implications for treatment approaches. International Journal of Molecular
Sciences, 24(20), 15183 Retrieved from https://www.mdpi.com/1422-0067/24/20/15183
https://doi.org/10.3390/ijms242015183 |
| 509 | Meng, L., Chen, H., Zhang, J., Wu, Y., & Xu, Y. (2024). Matricellular proteins: From cardiac
homeostasis to immune regulation. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0753332224013490
https://doi.org/10.1016/j.biopha.2024.117463 |
| 510 | Liu, Y. (2015). Fibrosis: A key alteration of adipose tissue in obesity with metabolic
consequences. Retrieved from https://theses.hal.science/tel-01403854/
|
| 511 | Desai, N., Sahel, D., Kubal, B., & Postwala, H. (2025). Role of the extracellular matrix in
cancer: Insights into tumor progression and therapy. Retrieved from
https://onlinelibrary.wiley.com/doi/10.1002/adtp.202400370
https://doi.org/10.1002/adtp.202400370 |
| 512 | Vinhas, C. A. A. (2021). Magnetic actuation and magnetic responsive materials to modulate
inflammation and their impact in cell behavior for tendon regeneration. Retrieved from
https://search.proquest.com/openview/8a33eaa56fd3643fe7a76783c9134763/1?pq-origsite=gscholar&cbl=2026366&diss=y
|
| 513 | Theocharis, A. D., Manou, D., & Karamanos, N. K. (2019). The extracellular matrix as a
multitasking player in disease. FEBS Journal, 286(15), 2830-2869
https://febs.onlinelibrary.wiley.com/doi/pdf/10.1111/febs.14818
https://doi.org/10.1111/febs.14818 |
| 514 | Khalil, R. A. (2017). Matrix metalloproteinases and tissue remodeling in health and disease:
Cardiovascular remodeling. Retrieved from https://books.google.com/books?hl=en&lr=&id=qhZ2DQAAQBAJ
|
| 515 | Bonnans, C., Chou, J., & Werb, Z. (2014). Remodeling the extracellular matrix in development and
disease. Nature Reviews Molecular Cell Biology, 15(12), 786-801
https://www.nature.com/articles/nrm3904
https://doi.org/10.1038/nrm3904 |
| 516 | Afshar, K., Sanaei, M. J., & Ravari, M. S. (2023). An overview of extracellular matrix and its
remodeling in the development of cancer and metastasis with a glance at therapeutic approaches. Cell
Biochemistry and Function, 41(4), 621-636 https://onlinelibrary.wiley.com/doi/10.1002/cbf.3846
https://doi.org/10.1002/cbf.3846 |
| 517 | Lin, W., Song, Y., Li, T., Yan, J., Zhang, R., Han, L., & Ba, X. (2023). Triptolide attenuates
pulmonary fibrosis by inhibiting fibrotic extracellular matrix remodeling mediated by
MMPs/LOX/integrin. Respiratory Medicine. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0753332223011927
https://doi.org/10.1016/j.biopha.2023.115394 |
| 518 | Vinhas, A., Almeida, A. F., & Rodrigues, M. T. (2023). Prospects of magnetically based approaches
addressing inflammation in tendon tissues. Retrieved from
https://www.sciencedirect.com/science/article/pii/S0169409X23001308
https://doi.org/10.1016/j.addr.2023.114815 |
| 519 | Joreitz R, Lynch A, Rabuck S, Lynch B, Davin S, Irrgang J. PATIENT-SPECIFIC AND SURGERY-SPECIFIC
FACTORS THAT AFFECT RETURN TO SPORT AFTER ACL RECONSTRUCTION. Int J Sports Phys Ther. 2016
Apr;11(2):264-78 PMID: 27104060; PMCID: PMC4827369.
|
| 520 | Di Vito, A., Donato, A., Presta, I., & Mancuso, T. (2021). Extracellular matrix in calcific aortic
valve disease: Architecture, dynamic and perspectives. International Journal of Molecular Sciences,
22(2), 913 https://www.mdpi.com/1422-0067/22/2/913
https://doi.org/10.3390/ijms22020913 |
| 521 | Rossi FE, de Freitas MC, Zanchi NE, Lira FS and Cholewa JM (2018) The Role of Inflammation and
Immune Cells in Blood Flow Restriction Training Adaptation: A Review. Front. Physiol. 9:1376 doi:
10.3389/fphys.2018.01376
https://doi.org/10.3389/fphys.2018.01376 |
| 522 | Cho, C.; Lee, S. The Effects of Blood Flow Restriction Aerobic Exercise on Body Composition,
Muscle Strength, Blood Biomarkers, and Cardiovascular Function: A Narrative Review. Int. J. Mol.
Sci. 2024, 25, 9274 https://doi.org/10.3390/ijms25179274
https://doi.org/10.3390/ijms25179274 |
| 523 | Ferlito JV, Rolnick N, Ferlito MV, De Marchi T, Deminice R, et al. (2023) Acute effect of low-load
resistance exercise with blood flow restriction on oxidative stress biomarkers: A systematic review
and meta-analysis. PLOS ONE 18(4): e0283237 https://doi.org/10.1371/journal.pone.0283237
https://doi.org/10.1371/journal.pone.0283237 |
| 524 | Jørgensen, S.L., Kierkegaard-Brøchner, S., Bohn, M.B. et al. Effects of blood-flow restricted
exercise versus conventional resistance training in musculoskeletal disorders-a systematic review
and meta-analysis. BMC Sports Sci Med Rehabil 15, 141 (2023).
https://doi.org/10.1186/s13102-023-00750-z
https://doi.org/10.1186/s13102-023-00750-z |
| 525 | Chua, M.T., Sim, A. & Burns, S.F. Acute and Chronic Effects of Blood Flow Restricted
High-Intensity Interval Training: A Systematic Review. Sports Med - Open 8, 122 (2022).
https://doi.org/10.1186/s40798-022-00506-y
https://doi.org/10.1186/s40798-022-00506-y |
| 526 | de Queiros VS, Rolnick N, de Alcântara Varela PW, Cabral BGdAT, Silva Dantas PM (2022)
Physiological adaptations and myocellular stress in short-term, high-frequency blood flow
restriction training: A scoping review. PLOS ONE 17(12): e0279811.
https://doi.org/10.1371/journal.pone.0279811
https://doi.org/10.1371/journal.pone.0279811 |
| 527 | Lauber, B., König, D., Gollhofer, A. et al. Isometric blood flow restriction exercise: acute
physiological and neuromuscular responses. BMC Sports Sci Med Rehabil 13, 12 (2021).
https://doi.org/10.1186/s13102-021-00239-7
https://doi.org/10.1186/s13102-021-00239-7 |
| 528 | Franz, A., Praetorius, A., Raeder, C. et al. Blood flow restriction training in the pre- and
postoperative phases of joint surgery. Arthroskopie 36, 252-260 (2023).
https://doi.org/10.1007/s00142-023-00615-0
https://doi.org/10.1007/s00142-023-00615-0 |
| 529 | B. C. Clark, T. M. Manini, R. L. Hoffman, P. S. Williams, M. K. Guiler, M. J. Knutson, M. L.
McGLynn, M. R. Kushnick Relative safety of 4 weeks of blood flow-restricted resistance exercise in
young, healthy adults. https://doi.org/10.1111/j.1600-0838.2010.01100.x
https://doi.org/10.1111/j.1600-0838.2010.01100.x |
| 530 | Hu C, Zhu B, Wang Y, Yang F, Zhang J, Zhong W, Lu S and Luo C (2023) Effectiveness of blood flow
restriction versus traditional weight-bearing training in rehabilitation of knee osteoarthritis
patients with MASLD: a multicenter randomized controlled trial. Front. Endocrinol. 14:1220758 doi:
10.3389/fendo.2023.1220758
https://doi.org/10.3389/fendo.2023.1220758 |
| 531 | Cervini G A, Rice M, Jasperse J L (August 09, 2023) Potential Local Mechanisms for
Exercise-Induced Hypoalgesia in Response to Blood Flow Restriction Training. Cureus 15(8): e43219
doi:10.7759/cureus.43219
https://doi.org/10.7759/cureus.43219 |
| 532 | Thomas, K. (2023). Blood Flow Restriction and Other Innovations in Musculoskeletal Rehabilitation.
In: Miller, T.L. (eds) Endurance Sports Medicine. Springer, Cham.
https://doi.org/10.1007/978-3-031-26600-3_17
https://doi.org/10.1007/978-3-031-26600-3_17 |
| 533 | dos Santos L, Andreatta MV, Curty VM, Marcarini WD, Ferreira LG and Barauna VG (2020) Effects of
Blood Flow Restriction on Leukocyte Profile and Muscle Damage. Front. Physiol. 11:572040 doi:
10.3389/fphys.2020.572040
https://doi.org/10.3389/fphys.2020.572040 |
| 534 | Jensen, K.Y., Jacobsen, M., Schrøder, H.D. et al. The immune system in sporadic inclusion body
myositis patients is not compromised by blood-flow restricted exercise training. Arthritis Res Ther
21, 293 (2019). https://doi.org/10.1186/s13075-019-2036-2
https://doi.org/10.1186/s13075-019-2036-2 |
| 535 | Thomas, K. (2023). Blood Flow Restriction and Other Innovations in Musculoskeletal Rehabilitation.
In: Miller, T.L. (eds) Endurance Sports Medicine. Springer, Cham.
https://doi.org/10.1007/978-3-031-26600-3_17
https://doi.org/10.1007/978-3-031-26600-3_17 |
| 536 | Jacobs, E.; Witvrouw, E.; Calders, P.; Stroobant, L.; Victor, J.; Schuermans, J.; Wezenbeek, E.
Blood Flow Restriction Exercise as a Novel Conservative Standard in Patients with Knee
Osteoarthritis-A Narrative Review. Appl. Sci. 2024, 14, 6150 https://doi.org/10.3390/app14146150
https://doi.org/10.3390/app14146150 |
| 537 | Li N, Yang J and Liao Y (2023) The effect of blood flow restriction training combined with
electrical muscle stimulation on neuromuscular adaptation: a randomized controlled trial. Front.
Physiol. 14:1182249 doi: 10.3389/fphys.2023.1182249
https://doi.org/10.3389/fphys.2023.1182249 |
| 538 | Skouras, A.Z.; Antonakis-Karamintzas, D.; Tsantes, A.G.; Triantafyllou, A.; Papagiannis, G.;
Tsolakis, C.; Koulouvaris, P. The Acute and Chronic Effects of Resistance and Aerobic Exercise in
Hemostatic Balance: A Brief Review. Sports 2023, 11, 74 https://doi.org/10.3390/sports11040074
https://doi.org/10.3390/sports11040074 |
| 539 | Christopher S. Fry, Erin L. Glynn, Micah J. Drummond, Kyle L. Timmerman, Satoshi Fujita, Takashi
Abe, Shaheen Dhanani, Elena Volpi, and Blake B. Rasmussen Blood flow restriction exercise stimulates
mTORC1 signaling and muscle protein synthesis in older men Journal of Applied Physiology 2010 108:5,
1199-1209 https://doi.org/10.1152/japplphysiol.01266.2009
https://doi.org/10.1152/japplphysiol.01266.2009 |
| 540 | Gibson BHY, Duvernay MT, Moore-Lotridge SN, Flick MJ, Schoenecker JG. Plasminogen activation in
the musculoskeletal acute phase response: Injury, repair, and disease. Res Pract Thromb Haemost.
2020;4:469-480 https://doi.org/10.1002/rth2.12355
https://doi.org/10.1002/rth2.12355 |
| 541 | Gabbett TJ, Oetter E. From Tissue to System: What Constitutes an Appropriate Response to Loading?
Sports Med. 2024 Nov 11 doi: 10.1007/s40279-024-02126-w. Epub ahead of print. PMID: 39527327.
https://doi.org/10.1007/s40279-024-02126-w |
| 542 | Helland C, Hole EM, Ørn S, Østerås H. 2020 Velocity-based training: The effects of 10% vs. 20%
velocity loss thresholds on recovery and performance. Journal of Strength and Conditioning Research
34(6):1670-1678 DOI 10.1519/JSC.0000000000002831
|
| 543 | Lieber RL, Friden J. 2002 Mechanisms of muscle injury after eccentric contraction. Journal of
Science and Medicine in Sport 5(3):253-265 DOI 10.1016/S1440-2440(02)80010-8
https://doi.org/10.1016/S1440-2440(99)80177-7 |
| 544 | Enoka RM, Duchateau J. 2011 Translating fatigue to human performance. Medicine and Science in
Sports and Exercise 43(6):975-982 DOI 10.1249/MSS.0b013e31820c6f0d
|
| 545 | Paulsen G, Mikkelsen UR, Raastad T, Peake JM. 2012 Leucocytes, cytokines and satellite cells: What
role do they play in muscle damage and regeneration following eccentric exercise? Exercise
Immunology Review 18:42-97
|
| 546 | Faulkner JA, Opiteck JA, Brooks SV. 1992 Injury to skeletal muscle fibers during contractions:
Conditions of occurrence and prevention. Physical Therapy 72(6):893-903 DOI 10.1093/ptj/72.6.893
|
| 547 | Black CD, Elder CP, Gorgey AS, Dudley GA. 2008 High-fat diet attenuates unloading-induced
adaptations in neuromuscular function. Muscle & Nerve 37(1):28-36 DOI 10.1002/mus.20904
|
| 548 | Jones DA, Newham DJ, Torgan C. 1989 Mechanical influences on long-lasting human muscle fatigue and
delayed-onset pain. The Journal of Physiology 412(1):415-427 DOI 10.1113/jphysiol.1989.sp017626
https://doi.org/10.1113/jphysiol.1989.sp017624 |
| 549 | Lee HD, Suter E, Herzog W. 1999 Force depression induced by eccentric muscle action. Journal of
Biomechanics 32(5):523-530 DOI 10.1016/S0021-9290(99)00033-2
|
| 550 | Carson RG, Riek S, Shahbazpour N. 2002 Central and peripheral mediation of maximal voluntary force
enhancement. Journal of Applied Physiology 92(6):2298-2306 DOI 10.1152/japplphysiol.00975.2001
|
| 551 | Nicol C, Avela J, Komi PV. 2006 The stretch-shortening cycle: A model to study naturally occurring
neuromuscular fatigue. Sports Medicine 36(11):977-999 DOI 10.2165/00007256-200636110-00004
https://doi.org/10.2165/00007256-200636110-00004 |
| 552 | Jamurtas AZ, Theocharis V, Tofas T, Tsiokanos A, Yfanti C, Paschalis V, Koutedakis Y, Kouretas D.
2005 Comparison between leg and arm eccentric exercises of the same relative intensity on indices of
muscle damage. European Journal of Applied Physiology 95(2-3):179-185 DOI 10.1007/s00421-005-1386-0
https://doi.org/10.1007/s00421-005-1345-0 |
| 553 | Chen TC, Nosaka K, Tu JH. 2011 Changes in running economy following downhill running. Journal of
Strength and Conditioning Research 25(12):3181-3188 DOI 10.1519/JSC.0b013e318212de5b
|
| 554 | Chen TC, Huang HC, Lin KY, Chen HL, Chen CH, Nosaka K. 2019 Physiological and neuromuscular
responses to isokinetic eccentric exercise. Scandinavian Journal of Medicine & Science in Sports
29(3):453-462 DOI 10.1111/sms.13345
https://doi.org/10.1111/sms.13388 |
| 555 | Mclester JR, Bishop P, Guilliams ME. 2003 Comparison of 1 day and 3 days per week of equal-volume
resistance training in experienced subjects. Journal of Strength and Conditioning Research
14(3):273-281 DOI 10.1519/00124278-200308000-00002
https://doi.org/10.1519/00124278-200008000-00006 |
| 556 | Korak J, Green JM, O'Neal EK. 2015 Resistance training recovery: Considerations for single vs.
multi-joint exercises and upper vs. lower body muscles. Journal of Strength and Conditioning
Research 29(12):3382-3387 DOI 10.1519/JSC.0000000000001077
|
| 557 | Moran-Navarro R, Pérez CE, Mora-Rodríguez R, de la Cruz-Sánchez E, González-Badillo JJ, Pallares
JG. (2017). Time course of recovery following resistance training leading or not to failure.
European Journal of Applied Physiology, 117(12), 2387-2399 DOI: 10.1007/s00421-017-3725-7
https://doi.org/10.1007/s00421-017-3725-7 |
| 558 | Tsoukos A, Veligekas P, Brown LE, Terzis G. (2018). Delayed potentiation after low-volume agonist
vs. high-volume antagonist heavy-resistance exercise. Journal of Strength and Conditioning Research,
32(8), 2188-2195 DOI: 10.1519/JSC.0000000000002410
https://doi.org/10.1519/JSC.0000000000002410 |
| 559 | Zourdos MC, Dolan C, Quiles JM, Klemp A, Jo E, Loenneke JP, Blanco R, Whitehurst M. (2016).
Efficacy of daily one-repetition maximum training in well-trained powerlifters and weightlifters: a
case series. Nutrición Hospitalaria, 33(2), 437-443 DOI: 10.20960/nh.8
https://doi.org/10.20960/nh.8 |
| 560 | Ferreira DV, Pereira B, Souza H, Couto BP, Ferreira L. (2017a). Recovery following resistance
exercise: The role of perceived exertion. Journal of Strength and Conditioning Research, 31(1),
20-30 DOI: 10.1519/JSC.0000000000001928
https://doi.org/10.1519/JSC.0000000000001928 |
| 561 | Ferreira DV, Pereira B, Couto BP, Souza H, Ferreira L. (2017b). Use of perceived exertion in
monitoring and adjusting recovery. International Journal of Sports Science & Coaching, 12(2),
229-235 DOI: 10.1177/1747954117697268
|
| 562 | Marshall PW, Cross R, Haynes M. (2018). Performance and muscle activity differences between
concentric and eccentric isoinertial contractions following stretching. Journal of Strength and
Conditioning Research, 32(1), 10-18 DOI: 10.1519/JSC.0000000000001443
https://doi.org/10.1519/JSC.0000000000001443 |
| 563 | Banyard HG, Nosaka K, Haff GG. (2017). Reliability and validity of the load-velocity relationship
to predict the 1RM back squat. Journal of Strength and Conditioning Research, 31, 1897-1904 DOI:
10.1519/JSC.0000000000001657
https://doi.org/10.1519/JSC.0000000000001657 |
| 564 | Banyard HG, Nosaka K, Vernon AD, Haff GG. (2018). The reliability of individualized load-velocity
profiles. International Journal of Sports Physiology and Performance, 13, 763-769 DOI:
10.1123/ijspp.2017-0610
https://doi.org/10.1123/ijspp.2017-0610 |
| 565 | Bosco C, Viitasalo JT, Komi PV, Luhtanen P. (1982). Combined effect of elastic energy and
myoelectrical potentiation during stretch-shortening cycle exercise. Acta Physiologica Scandinavica,
114, 557-565 DOI: 10.1111/j.1748-1716.1982.tb07024.x
https://doi.org/10.1111/j.1748-1716.1982.tb07024.x |
| 566 | Buckthorpe MW, Hannah R, Pain TG, Folland JP. (2012). Reliability of neuromuscular measurements
during explosive isometric contractions, with special reference to electromyography normalization
techniques. Muscle and Nerve, 46, 566-576 DOI: 10.1002/mus.23322
https://doi.org/10.1002/mus.23322 |
| 567 | Buckthorpe M, Pain MT, Folland JP. (2014). Central fatigue contributes to the greater reductions
in explosive than maximal strength with high-intensity fatigue. Experimental Physiology, 99, 964-973
DOI: 10.1113/expphysiol.2013.075614
https://doi.org/10.1113/expphysiol.2013.075614 |
| 568 | Enoka RM, Baudry S, Rudroff T, Farina D, Klass M, Duchateau J. (2011). Unraveling the
neurophysiology of muscle fatigue. Journal of Electromyography and Kinesiology, 21, 208-219 DOI:
10.1016/j.jelekin.2010.10.006
https://doi.org/10.1016/j.jelekin.2010.10.006 |
| 569 | Farup J, Rahbek SK, Bjerre J, De PF, Vissing K. (2016). Associated decrements in rate of force
development and neural drive after maximal eccentric exercise. Scandinavian Journal of Medicine &
Science in Sports, 26, 498-506 DOI: 10.1111/sms.12481
https://doi.org/10.1111/sms.12481 |
| 570 | Gathercole RJ, Sporer BC, Stellingwerff T, Sleivert GG. (2015). Comparison of the capacity of
different jump and sprint field tests to detect neuromuscular fatigue. Journal of Strength and
Conditioning Research, 29, 2522-2531 DOI: 10.1519/JSC.0000000000000912
https://doi.org/10.1519/JSC.0000000000000912 |
| 571 | Thankam FG, Fouda MB, Dilisio MF (2024). Alterations in Tendon Microenvironment in Response to
Mechanical Load: Potential Molecular Targets for Treatment Strategies. American Journal of Sports
Medicine.
|
| 572 | Iijima H, Tajino J, Nagai M, Yamaguchi S (2024). Effects of Exercise Loading on Post-Traumatic
Osteoarthritis Progression: Bone Morphogenetic Proteins Expression in Experimental Rat Knee Models.
Osteoarthritis and Cartilage.
|
| 573 | Bleakley C, Netterström-Wedin F (2023). Does Mechanical Loading Restore Ligament Biomechanics
After Injury? A Systematic Review of Animal Models. BMC Musculoskeletal Disorders.
https://doi.org/10.1186/s12891-023-06653-x |
| 574 | Logerstedt DS, Ebert JR, MacLeod TD (2024). Effects of and Response to Mechanical Loading on the
Knee. Sports Medicine.
|
| 575 | Calvo-Rubio M, Garcia-Domiguez E, et al. (2024). The Repeated Bout Effect Evokes the
Training-Induced Skeletal Muscle Cellular Memory. Free Radical Biology & Medicine.
https://doi.org/10.1016/j.freeradbiomed.2024.09.047 |
| 576 | Stožer A, Vodopivc P, Bombek LK (2024). Pathophysiology of Exercise-Induced Muscle Damage and Its
Structural, Functional, and Metabolic Consequences. Physiological Research.
|
| 577 | León F, Mestre A, Priego L (2023). Morphological Adaptations in Response to Chronic Exercise
Across Musculoskeletal Tissues: A Systematic Review. En Movimiento: Revista de Ciencias del
Ejercicio y la Salud.
https://doi.org/10.15517/pensarmov.v21i1.51450 |
| 578 | Furrer R, Hawley JA, Handschin C (2023). The Molecular Athlete: Exercise Physiology from
Mechanisms to Medals. Physiological Reviews.
https://doi.org/10.1152/physrev.00017.2022 |