Original Article - DOI:10.33594/000000826
Accepted 13 October 2025 - Published online
2 November 2025
Epistemology of the Origin of Cancer III: Fundamentals of How Metastasis Arises
bTheodor-Billroth-Academy® with its INCORE, International Consortium of Research Excellence, Munich, Germany, Sacramento, CA, USA,
cCancer Metastases Research Fund, Sacramento, California, United States of America,
dDepartment of Surgery, Medical University Lausitz – Carl-Thiem, Cottbus, Germany,
Keywords
Abstract
Metastasis, like carcinogenesis, involves the disruption of homeostasis such that cancer cells travel from the primary tumor to distant parts of the body. Almost all cancer deaths are due to metastatic spread. The prevailing theory of metastasis is an incomplete doctrine and far from sufficient as only 0.2% of free cancer cells result in the spread of cancer. To develop reasonable and effective cancer therapies and to prevent (or reverse) carcinogenesis and metastasis, a comprehensive understanding of how both carcinogenesis and metastasis arise is necessary. Fundamental questions in cancer biology have been asked and answered over decades of research: How do most cancers develop (Epistemology of the Origin of Cancer I, 2014–2022)? Which is the first cancer cell (II, 2023)? The third basic question in cancer biology remaining to be addressed is: What are the fundamentals of how metastasis develops? The pre-cancerous niche (PCN) that forms during carcinogenesis is altered by ongoing complex signaling into a premetastatic niche 1 (PMN-1): p130(cas)/crk/DOCK180 formation is necessary for lamellipodia formation, thereby enabling cell mobility. Cancer-associated fibroblasts (CAFs) begin to release fibronectin CXCL12 and Keratin 19. PMN-1 is transformed into PMN-2 during ongoing crosstalk and transformation of anti- into pro-tumorigenic platelets, macrophages, and neutrophils. Finally, persistent signaling and immune evasion result in the conversion of PMN-2 to PMN-3 with heterogeneous cancer satellites – the term “satellite” is used herein in accordance with its original meaning (a cell or particle escorting another). PMN-3 serves as a prerequisite for intravasation, traveling, and dissemination of cancer cells away from the primary tumor. Eight heterogeneous cancer satellites, including Trojan horses (immune evasion), travel alone or in combination: (1) cancer cells and (2) CAFs migrate along the CXCL12 and fibronectin gradient; (3) cancer cells surrounded by CAFs are shielded from the immune system and travel away from the primary cancer; (4) CXCL12 and Keratin 19 coat cancer cells; (5) platelets surround cancer cells and (6) CAFs, thereby facilitating cancer spread; and (7) neutrophil extracellular traps shield cancer cells and (8) CAFs. Metastasis in epithelial cancer occurs in parallel with carcinogenesis after the pre-cancerous niche is transformed into pre-metastatic niches (PMNs), which are indispensable to the origin of metastasis. Eight heterogeneous cancer satellites, including Trojan horses responsible for immune evasion, alongside reciprocally affecting sequences, wander alone or in conjunction with other cancer cells. This elucidates why the current practice of multimodal anti-cancer cell therapy should now be seen in a new light in which the benefits depend not on direct cancer cell effects, but on indirect cytopenic effects, which have previously been regarded merely as adverse effects.
Introduction
Homeostasis is a fundamental biochemical and physiological regulatory process that maintains a balance of all systems within the body, thereby sustaining a state of health. Homeostasis is an equilibrium state and a law of nature, without which no life would be possible. When the homeostatic balance is disrupted, diseases begin to develop. A simple analogy for homeostasis is the ability of a rubber band to be stretched and return to its original shape and size when released, but to break when stretched too far. This analogy of breakage applies to homeostasis in chronic diseases such as cancer. In science, homeostasis has largely been ignored in the understanding of disease processes [1].
The disruption of homeostasis, in all its complexity, is a hallmark of cancer and metastasis. Because homeostasis is critical for maintaining health, nature maintains homeostasis through multiple routes, such that biochemical and physiological redundancies protect against disease and enable health. More detailed information on homeostasis is provided in the supplemental material (Supplement part 1, Homeostasis).
To achieve homeostasis, cells must “talk” to one another to gain information on what is happening in their own neighborhood and across the living organism. This process is referred to as cell-cell communication, which has been described as “the music that the nucleus hears” [2, 3]; moreover, “biological processes as well as cell-cell communication and signaling are themselves a multidimensional musical opera in different acts, which are played differently by different symphony orchestras rather than by a soloist.” Additionally, as a colleague of ours stated, there is a lot of music, which is too fast to be heard by the nucleus.
Cancer involves the disruption of homeostasis, including dissonant and aberrant cell-cell communication. Most cancers (80.5%) are epithelial cancers, which markedly differ in their development, spread, and response to therapy [4]; epithelial cancer relapse rates in adults have not changed substantially, and cancer incidence continues to rise and is expected to double by 2070. The absolute (unadjusted) cancer mortality rates have not changed considerably over the past 80 years, although tangible benefits have been achieved in certain subpopulations where cause-based approaches have been adopted; for example, hepatitis C virus-induced liver cancer and human papillomavirus-induced cervical cancer have recently been prevented by vaccines.
Age-adjusted data are useful for comparing potential risks but should not be mistaken for precise measures, particularly when demographics change over long time periods. This important aspect is particularly evident in cancer statistics, wherein relative age-adjusted figures are used to demonstrate progress against cancer but often contradict the absolute cancer mortality rates [4]. Even gains in early cancer detection have achieved only small benefits in terms of mortality.
Both clinical and experimental data show that genetics (e.g., mutations), despite having been consistently described as key to understanding and treating cancers and having remained a major cancer research focus, does not explain the cause of most cancers. To date, only approximately 5% of cancers have been demonstrated to be caused by genetic mutations [5]. This ongoing focus on mutations explains why approximately 80% of cancers are first diagnosed in advanced stages, and approximately 50% of patients with cancer have metastasis at the time of initial diagnosis [6]. Somber findings have indicated that cancer incidence, metastasis, and mortality statistics have not changed substantially over the past century [4].
The identification of certain somatic mutations like TP53, KRAS, EGFR and others have proven helpful in the treatement of certain epithelial cancer and most notably in the selection of immunotherapeutic agents that work better in patients with such somatic mutations. That said, the application of the mutation theory has, to date, not substantially improved patient survival even though it has improved disease free progression in certain cancers.
One persisting challenge is that no morphological features can be used to distinguish tumorigenic from non-tumorigenic strains [7]. For example, no discernible difference in DNA repair exists between normal and pre-neoplastic breast tissues, thus further confirming the minor (or absent) role of somatic mutations as the cause of most cancers [8].
A known, yet largely ignored, finding is that genetic mutations do not clinically explain most epithelial cancers, because genes are not merely blueprints containing information [3] and do not function as blueprints for life [9, 10]. Thus, a “gene delusion” applies to the understanding of most cancers [11]. Genome-wide association studies, as part of the Human Genome Project, cost $3 billion from 1988 to 2003 in the U.S. and had an economic impact of approximately $796 billion [12] and an estimated economic return to the U.S. economy of $1 trillion [13]. But where are the benefits to patients with cancer in terms of quality of life or overall survival, measured in years, not in mere weeks or months, as continually indicated by numerous clinical studies? Despite investments of billions of dollars, the incidence of epithelial cancers is predicted to rise. The development of effective cancer therapies and metastasis prevention can be achieved only with proper etiological understanding.
To help patients most in need, essential cancer biology questions have been asked and answered over decades of research on disrupted homeostasis, including signaling and crosstalk in carcinogenesis: [1] How do most cancers develop? (2) Which is the first cancer cell?
These two fundamental cancer biology questions were answered by complex research in 2014–2022 (Epistemology of the Origin of Cancer I) (FIGURE 1) [3, 14] and 2023 (Epistemology of the Origin of Cancer II) (Please see figures 1 to 3 in [15]).
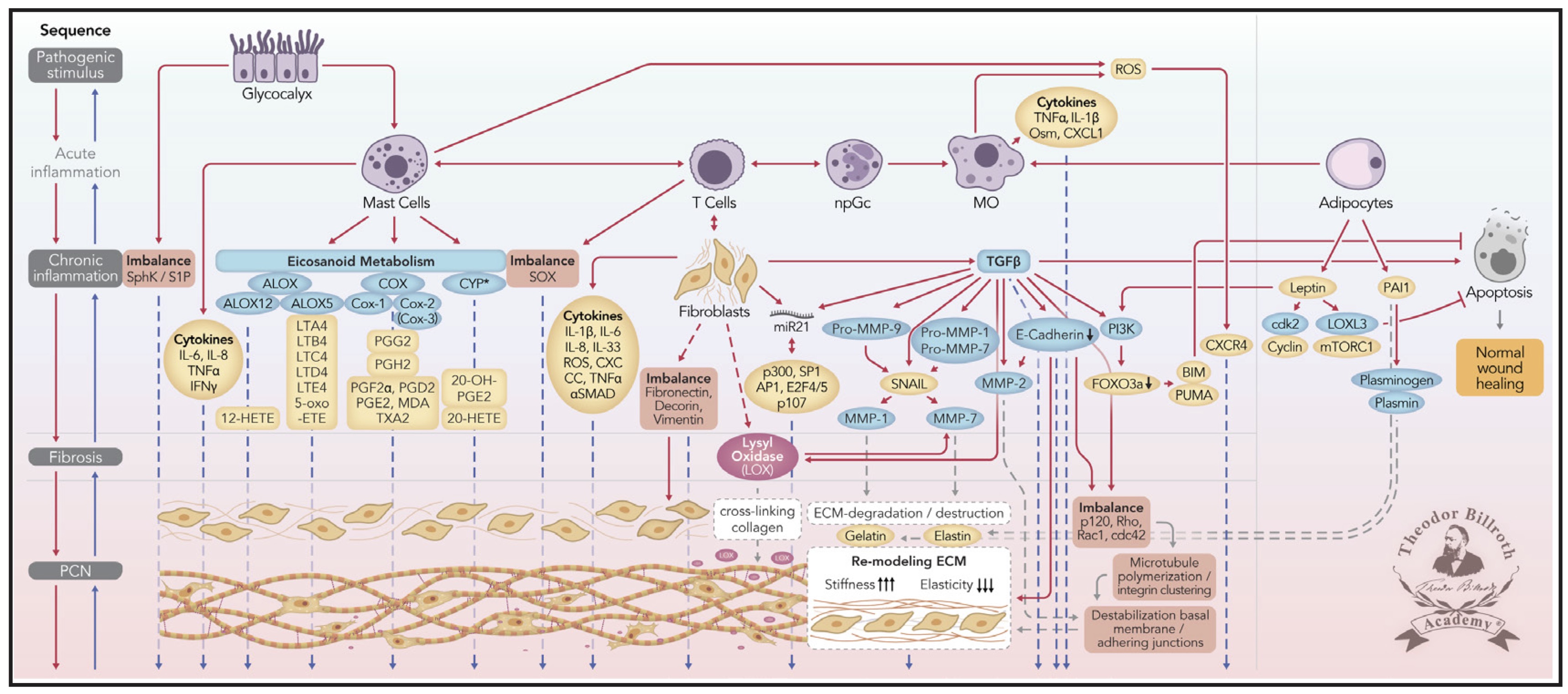
Fig. 1: This Fig. combines findings from prior research [3, 14, 16, 17] with signaling crosstalk, without applying the Chronic Stress Escape Strategy (CSES) and the Normal Cell to Cancer Cell Transition (NCCCT). The nomenclature of common abbreviations is shown in bold and is followed by common names or International Union of Pure and Applied Chemistry (IUPAC) names, as available: PCN: pre-cancerous niche; SphK: sphingosine kinase isoform; S1P: sphingosine-1-phosphate; IL-6: interleukin 6; IL-8: interleukin 8; TNFα: tumor necrosis factor alpha; IFNγ: interferon gamma; ALOX: lipoxygenase, arachidonate lipoxygenase; ALOX12: 12-lipoxygenase, 12-LOX, 12S-LOX, arachidonate 12-lipoxygenase 12S type; ALOX5: 5-lipoxygenase, 5-LOX, arachidonate 5-lipoxygenase; 12-HETE: 12-hydroxyeicosatetraenoic acid; LTA4: leukotriene A4, 4-[(2S,3S)-3-[(1E,3E,5Z,8Z)-tetradeca-1,3,5,8-tetraenyl]oxiran-2-yl]butanoic acid; LTB4: leukotriene B4, (5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,14-tetraenoic acid; LTC4: leukotriene C4, (5S,6R,7E,9E,11Z,14Z)-6-[(2R)-2-[[(4S)-4-amino-4-carboxybutanoyl]amino]-3-(carboxymethylamino)-3-oxopropyl]sulfanyl-5-hydroxyicosa-7,9,11,14-tetraenoic acid; LTD4: leukotriene D4, (5S,6R,7E,9E,11Z,14Z)-6-[(2R)-2-amino-3-(carboxymethylamino)-3-oxopropyl]sulfanyl-5-hydroxyicosa-7,9,11,14-tetraenoic acid; LTE4: leukotriene E4, (5S,6R,7E,9E,11Z,14Z)-6-[(2R)-2-amino-2-carboxyethyl]sulfanyl-5-hydroxyicosa-7,9,11,14-tetraenoic acid; 5-oxo-ETE: (6E,8Z,11Z,14Z)-5-oxoicosa-6,8,11,14-tetraenoic acid; Cox: cyclooxygenase; Cox-1: cyclooxygenase 1; Cox-2: cyclooxygenase 2; Cox-3: isoform of Cox-2 (therefore in brakes); PGG2: prostaglandin G2, (Z)-7-[(1S,4R,5R,6R)-5-[(E,3S)-3-hydroperoxyoct-1-enyl]-2,3-dioxabicyclo[2.2.1]heptan-6-yl]hept-5-enoic acid; PGH2: prostaglandin H2, (Z)-7-[(1S,4R,5R,6R)-5-[(E,3S)-3-hydroxyoct-1-enyl]-2,3-dioxabicyclo[2.2.1]heptan-6-yl]hept-5-enoic acid; PGFF2α: prostaglandin F2 alpha, (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]cyclopentyl]hept-5-enoic acid; PGD2: prostaglandin D2, (Z)-7-[(1R,2R,5S)-5-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-3-oxocyclopentyl]hept-5-enoic acid; PGE2: prostaglandin E2, (Z)-7-[(1R,2R,3R)-3-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]hept-5-enoic acid; MDA: malondialdehyde, propanedial; TXA2: thromboxane A2, (Z)-7-[(1S,2S,3R,5S)-3-[(E,3S)-3-hydroxyoct-1-enyl]-4,6-dioxabicyclo[3.1.1]heptan-2-yl]hept-5-enoic acid; CYP*: cytochrome P450 isoforms; 20-OH-PGE2: 20-hydroxy prostaglandin E2; 20-HETE: 20-hydroxyeicosatetraenoic acid, (5Z,8Z,11Z,14Z)-20-hydroxyicosa-5,8,11,14-tetraenoic acid; SOX: [sex-determining region Y (Sry) box-containing] transcription factor family; IL-β1: interleukin beta 1; IL-33: interleukin 33; ROS: reactive oxygen species; CXC CC: chemokine receptors; αSMAD: alpha-smooth muscle actin; miR21: microRNA-21; p300: protein 300 (p300-CBP coactivator family); SP1: specificity protein 1; AP1: activator protein 1; E2F4/5: cytoplasmic complex of Smad3, retinoblastoma-like protein 1 (P107, RBL1), E2F4/5 and D-prostanoid (DP1); p107: retinoblastoma-like protein 1, RBL1; TGFβ: transforming growth factor beta; Pro-MMP-9: pro-matrix metalloproteinase 9; Pro-MMP-1: pro-matrix metalloproteinase 1; Pro-MMP-7: pro matrix metalloproteinase 7; SNAIL: zinc finger protein SNAI1; MMP-1: matrix metalloproteinase 1; MMP-7: matrix metalloproteinase 7; MMP-2: matrix metalloproteinase 2; E-cadherin: CAM 120/80 or epithelial cadherin, cadherin-1, epithelial cadherin; CXCL1: chemokine (C-X-C motif) ligand 1; Osm: oncostatin-M; PI3K: phosphatidylinositide 3-kinase; FOXO3a: forkhead box protein O3a; p120: catenin delta-1, protein 120; Rho: Ras homolog gene family, member A; Rac1: Ras-related C3 botulinum toxin substrate 1; cdc42: cell division control protein 42 homolog; BIM: Bcl-2 interacting mediator of cell death; PUMA: BH3-only protein; CXCR4: C-X-C motif of chemokine receptor 4; cdk2: cyclin-dependent kinase 2; LOXL3: lysyl oxidase homolog 3; mTORc1: rapamycin complex 1; PAI1: plasminogen activator inhibitor-1.
Simplified scheme representing ongoing disruption of homeostatic crosstalk. This illustration was originally discussed in the paradigm presented in our prior publication [3, 14] and modified in 2019 [16-19]. The first four carcinogenesis sequences are included: [1] a pathogenic stimulus is followed by [2] chronic inflammation, thus resulting in [3] fibrosis with associated remodeling of the cellular microenvironment; after these changes, a [4] pre-cancerous niche (PCN) is formed as a product of fibrosis and it’s remodeling by lysyl oxidase (LOX) through persistent inflammation.
Finally, CAFs undergo mesenchymal-epithelial transition (MET) and express epithelial markers that facilitate their integration into the target tissue. The continual increase in CAFs ultimately leads to complete and unresolvable disruption of physiologic homeostasis. CAFs then undergo MET, and these cells, which continue to express epithelial markers, become the first cancer cells. The former fibroblasts are then integrated into the epithelium. This neoplastic transformation to a cancerous phenotype also explains the very high heterogeneity of cancers.
Overall cancer mortality rates and rates of metastasis in epithelial cancers have not substantially changed in the past 80 years [4].
Metastasis starts soon after the first cancer cell has developed
Readers are referred to the further details regarding many aspects of metastasis complexity and heterogeneity presented in the supplemental materials.
The injection of radiolabeled cancer cells were found to result in only 0.1–0.2% of the cells becoming metastatic [20]. The lack of change in metastatic rates in recent decades suggests that the origins and development of metastasis must differ from the prevailing understanding. Therefore, a third fundamental question in cancer research that remains to be answered is: What are the fundamentals of how metastasis occurs?
Herein, we provide potential explanations of the fundamentals of how metastasis arises in epithelial cancers. Our findings are concordant with clinical, scientific, and experimental findings, which together demonstrate the diversity of metastatic patterns. Deciphering these metastatic patterns requires an in-depth understanding of complex signaling and crosstalk.
Metastasis comes from the Greek term μετάστασις, meaning migration or displacement. In cancer, this term describes the secondary cancers that occur in areas of the body distant from the primary cancer. Therefore, cancer cell formation is temporally followed by metastasis.
Henry Earle (1789–1838), in 1823, reported that local irritation was the reason for many cancers and their spread [21]. Several years later, in 1829, Joseph-Claude-Anthelme Récamier (1774–1852) first used the term “metastase” (metastasis), in a report of a case of breast cancer with brain metastasis [22 reviewed in 23].
The supplemental material provides detailed information regarding proposed models of metastasis (various vascular hematogeneous lymphatic theories and their combination, such as the embolic theory; preliminary work and the subsequent development of seed and soil theory; and cancer implantation models) (Supplement, part 2, Metastasis theories); metastasis models (Supplement, part 3, Metastasis models); and metastasis injection models (Supplement, part 4, Metastasis injection models).
This knowledge is indispensable for understanding the biology of cancer and the origins of metastasis, but still is incomplete. The prevailing wisdom in cancer research is that circulating cancer cells are the main source of metastasis. We discuss those data underlying these theories of metastasis and show why they are insufficient to explain the spread of cancer from the primary tumor to distant sites.
Circulating tumor cells
Circulating tumor cells (CTCs) consist of red blood cells (erythrocytes), white blood cells (leucocytes), lymphocytes (antibody producing B- and T-cells), monocytes and macrophages, dendritic cells (type 2 dendritic cells and plasmacytoid dendritic cells), basophils, and platelets. CTCs have been used to explain the detection of cancer cells in circulation (a correct observation but an incorrect interpretation) [24, 25] as follows: epithelial cells break through the basal membrane; enter vascular structures (veins, arteries, and lymphatic vessels); are carried away by the blood and lymph streams; and implant elsewhere, thus giving rise to metastasis. However, detection of cancer cells in the blood is not synonymous with cancer dissemination, despite widely being accepted as such. The data disproving the CTC theory is discussed below.
In prior studies, highly aggressive cancer cells were cultivated [20, 26], and all inoculated cells were radiolabeled with 125I-iodo-2´-deoxyuridine (125IUDR). Immediately after injection, most cancer cell emboli arrested within the lungs, and a few very rarely entered the pulmonary circulation. Within 24 hours, less than 1% of cancer cells survived. After 2 weeks, only 0.2% of cancer cells injected into the bloodstream (400 of 200, 000) had survived in the lungs. No injected cancer cells survived in the liver, spleen, kidney, blood, or urine. Notably, only a small number of patients with disseminated tumor cells and CTCs develop clinically evident metastatic cancer [27]. Consequently, most (99.8%) cancer cells in the bloodstream do not survive to cause metastasis [20, 26]. Injected 125I-iodo-2´-deoxyuridine (125IUDR)-labeled cancer cells killed by heat or radiation showed no radioactivity in the lungs 8 hours later. Injecting the same cells without inactivation revealed that 24 hours post-injection, no radioactivity was evident.
These data reveal that the efficiency of the immune system and the limitations of the seed and soil-theory of CTCs. A cancer nodule of 1 cm contains 1 billion cells (1, 000, 000, 000 cells, 109 cells), only 1 million of which (1, 000, 000 cells, 106 cells; 0.1%) can be shed into the circulation per day. However, injection of 2 × 106 cells (2, 000, 000 cells) per animal rarely resulted in lung metastasis [28]. These data demonstrate that the existing explanations for how metastasis occurs are incomplete, and approximately 99.8% of metastasis occurs through a different route(s).
Moreover, a similar increase in CTCs was observed in a different experiment: induced peritoneovenous shunts aimed at decreasing malignant ascites and improving quality of life in patients with ovarian cancer, caused millions of cancer cells to enter the bloodstream but did not increase metastasis [29, 30]. Various terms are used to describe CTCs, including clusters, microemboli, and collective tumor cell migration with high metastatic potential [31, 32]. Additional information regarding CTCs is provided in the supplemental material (Supplement, part 5, Circulating tumor cells).
Clinical findings
Clinical findings for metastases are heterogeneous, given the distinct microenvironments of cancers across different organs [6, 20, 23, 30].
Various epithelial cancers, such as those from the ovaries, colon, pancreas, rectum, and stomach, heterogeneously spread to the lymph nodes, liver, and lungs, and subsequently the peritoneum. Furthermore, pancreatic and gastric cancers are upper gastrointestinal cancers without major venous flow through the inferior mesenteric vein. Esophageal cancer, despite its location and histology (adenocarcinoma versus squamous cell carcinoma), can spread to the lymph nodes, lungs, liver, bones, and peritoneum. In contrast, breast cancers preferentially metastasize to the bones, followed by the brain, liver, and lungs. Prostate and kidney cancers metastasize to the adrenal glands, bones, lymph nodes, liver, lungs, and brain. Bladder, thyroid, and uterine cancers preferentially spread to the bones, liver, and lungs.
Lymph node metastasis in primary breast cancer occurs in approximately 30–50% of cases [33, 34]. A higher likelihood of lymph node metastasis is associated with age (particularly younger age in women), race (particularly Black), primary site (particularly the upper-outer quadrant), histology (particularly lobular carcinoma), breast subtype (particularly HR−/Her2neu+), grade (particularly grade 3), and T-stage (particularly the T3 category). Tumor size (in 10 mm increments), lymph nodes, and distant spread do not demonstrate linear relationships, thus suggesting that larger tumors do not necessarily spread to distant organs or the lymphonodular regions [35]. This is in agreement with findings in other epithelial cancers, in which T4 (larger) cancers do not always demonstrate metastasis.
Lymph node metastasis in animal models aids in understanding the time course of metastasis. Lymphatic spread was found to occur as early as the 4th day after tumor transplantation in a mouse model [36]. In the stage of nidation, no metastasis occurred. Therefore, lymph node metastasis occurs early [37] and after non-visible cancer spread. Dormancy of cancer cells can be considered a rule rather than an exception.
Friedrich Stelzner (1921–2020) showed that a sharp separation of hematogenous venous metastases between the portal vein and the cava system is not possible because of a network of natural short-circuit anastomoses [38]. Furthermore, if lymphatic drainage were the major route of lymphatic cancer spread, several questions should be asked, including why patients undergoing esophagectomy for esophageal squamous cell carcinoma (ESCC), including thoracic duct resection, have a 4% higher rate of distant metastasis [39, 40].
Large colon carcinomas result in metastasis less often than moderately sized or small cancers, as shown in a study of 3, 000 colorectal cancers (CRCs) [41]. This finding has been widely reported [42], but has been disproven, given that Frederick Allen Coller (1887–1964), approximately 85 years ago, demonstrated that lymph node metastasis occurs at higher rates in smaller rather than larger bowel cancers [43]. However, in breast cancer, greater tumor size is associated with more metastatic lymph nodes.
These observations indicate that not only the size of the primary tumor but also the organ where the primary cancer occurs determines metastatic potential. This suggests that signaling and crosstalk biology, which have not been considered as necessary so far, seem to play a much more important role.
In a prostate cancer study, approximately 33% of bone metastases were observed in the sternum and ileum, but only 9% showed widespread bone metastases [44], thus indicating that the distribution of these metastases could not be explained by vascular theory.
Intravenous inoculation of radiolabeled CRC cells showed 66% lymph node metastasis, thereby suggesting that venous vascular cancer cell inoculation was responsible for more than two-thirds of the spread into the lymph nodes [45].
According to the Fuchs-Paget seed and soil theory [46, 47], cancer cells (the “seed”) are distributed through the vascular system, and metastases can develop only in locations with favorable conditions (the “soil”). Ewing, in 1928, also suggested that cancers spread to areas where the vascular system (blood and lymphatics) allows them to travel [48]. Although early cancer surgery was long believed to prevent metastasis, clinical data have revealed that even after one or two decades, patients with epithelial cancer can develop heterogeneous metastasis [15, 49], and animal studies have demonstrated that such lesions can lead to metastatic spread [50].
A study of 213 CRC cases has examined hypermutable DNA regions and/or conducted phylogenetic analysis to elucidate the origins of lymphatic or distant metastasis [51]. In approximately 65% of cases, lymphatic and distant metastases arose from independent subclones in the primary tumor, and only 35% of cases shared a common subclonal origin. Why were these percentages so low? If the seed and soil theory of metastasis were correct, the percentages should exceed 80–90%.
These heterogeneities cannot be explained by free cancer cells, free circulating DNA, or somatic mutations, or by applying an anatomical mechanistic vascular (lymphatic and hematogenous) approach, which nonetheless remains widely taught in oncology training programs. Training suggesting that anatomy facilitates cancer cell spread through “highways” does not provide a sufficient explanation. A more nuanced view of the cancer microenvironment would aid in understanding the roles of signaling and crosstalk necessary for cell migration and subsequent increases in motility.
Cancer microenvironment
The heterogeneous cancer microenvironment, including cell compartments, signaling, and cancer spread, together with transformation of the pre-cancerous niche (PCN) to the premetastatic niche (PMN) and increased cell mobility can explain the heterogeneous clinical findings.
In gastric cancer cells, CAFs maintain the chronic inflammation stage, with ongoing activation of the PCN, by increasing p300, which in turn activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, thus resulting in mesenchymal stem cell (MSC) differentiation into CAFs [52]. This self-driven, persistent inflammatory stimulation has also been observed in breast cancer cells [53]. Fibroblasts and CAFs are prominent in the cancer microenvironment. Essential information regarding heterogeneity is provided in the supplement (Supplement, part 6, Fibroblast heterogeneity).
Fibroblasts themselves show high heterogeneity and are known to convert to CAFs and to promote metastasis in cancers of the breast [54], prostate [55], pancreas [56], and colon [57]. Co-cultivation of cancer cells with fibroblasts increases the transformation of fibroblasts into CAFs [58].
Do fibroblasts derive from EMT during the chronic stress escape strategy (CSES)? Or do fibroblasts derive from the remodeled tumor microenvironment during carcinogenesis through the PCN sequence or perhaps from a normal cell to cancer cell transition (NCCCT) sequence? These questions prompt additional questions: are migrating cells the source of metastasis in distant organs, or do these secondary cancers intrinsically arise from the organ where the metastasis is found?
The fibroblast atlas cannot explain the spatial dynamics of fibroblasts, nor can it clarify the locations from which each fibroblast derives, although it advances knowledge regarding heterogeneity and orthologs in different species. Intriguingly, gene expression studies [59] have related the heat maps of the relative average expression of the most strongly enriched genes for each cluster to particular organs, such as Ccl19, Ccl21a, and Bst1 in lymph nodes; Cxcl12, Lepr, and Sp7 in bone tissue; Npnt and Ces1d in alveolar lung tissue versus Hhip and Aspn in peribronchial lung tissue; and Fbln1and Sfrp1 in intestinal tissue. However, none of these location-specific gene expression findings answer the key question of how these relate to metastasis.
Next to fibroblasts and fibrocytes, transition to CAFs can occur in mesenchymal stem cells (MSCs) [60], hematopoietic stem cells [61], and adipose-derived MSCs [62]. The transition from MSCs is dependent on myeloid zinc finger 1 (MZF1) and transforming growth factor β (TGFβ), which are mediated by osteopontin (OPN) [63]. OPN is an acidic hydrophilic glycophosphoprotein that influences αvB3-integrin and CD44 and serves as a cell attachment protein. The c-Jun homodimer of AP-1 is necessary for OPN promoter activity and binds the OPN promoter in 4T1 cells; its levels are increased by PI3K-dependent JNK- and c-Jun activation, thus promoting metastatic behavior, such as adhesion, migration, and invasion in vitro [64].
To increase cancer drug efficiency, CAF research has focused on overcoming anticancer drug resistance [65–68] and promoting understanding of the CAF signature to evaluate immunotherapy responses [69].
Furthermore, MSCs can be recruited from the bone marrow, transform into fibroblasts, and subsequently transition into CAFs [70]. A pH decrease within tissue can result in the transitioning of MSCs to CAFs [71].
CAFs show neuroendocrine differentiation in a CD105-dependent manner [72]. This neuroendocrine differentiation has been associated with circulating tissue inhibitor of metalloproteinase-1 (TIMP-1) in a manner dependent on ERK and NF-κB in metastatic castration-resistant prostate cancer [73]. These findings support the theory that the primary sources of epithelial cancers are fibroblasts and CAFs and not epithelial cells [15].
To shed light on the heterogeneity of fibroblasts and CAFs, studies have conducted single-cell RNA sequencing in pancreatic cancer tissues from six human patients and adjacent normal pancreatic tissue from two of these patients, as well as from Kras+/LSL-G12D; Trp53+/LSL-R172H; Pdx1-Cre (KPC) pancreatic cancer in mice [74 and Supplement, part 4]. Studies focusing on p53 downstream of CAFs [75–77] have suggested that CAF heterogeneity is associated with Yes protein 1 (YAP1), as indicated by the activation of MSCs [78], in cancers of the breast [79], pancreas [80], prostate [81], and colorectum [82].
Furthermore, disruption of homeostatic signaling in Dickkopf-3 (DKK3), an effector of heat-shock factor 1 (HSF1), induces the transcriptional co-factors YAP (gene Yap1) and TAZ (gene Wwtr1) via Wnt signaling, which is crucial for the tumor-promoting ability of CAFs [83]. The complexity of YAP1 homeostasis is indicated by the influence of cytoskeletal dynamics, because myocardin-associated transcription factors are required for the YAP pathway for CAF contractility and for its proinvasive properties [84].
Extracellular matrix (ECM) stiffness induces the activity of Rho-associated coiled-Coil kinase (ROCK), integrin clustering, mitogen-activated protein kinase 1 (MAPK 1, extracellular signal-regulated kinase 2, ERK2) signaling, and subsequent nuclear accumulation of SNAIL1, which in turn interacts with YAP1 [19, 85].
A more detailed signaling and crosstalk information about YAP, and fatty acid desaturase 2 (FADS2, ω-6-desaturase, D6D) signaling in the disruption of signaling homeostasis induced crosstalk in the carcinogenesis paradigm “Epistemology of the origin of cancer” can be found elsewhere (Please see figures 1 to 3 in [19]). More detailed information on the associations between CAF interactions and Neuropilin-1 (NRP1) is provided in the supplemental material (Supplement, part 7, CAF interaction with Neuropilin-1 and EGF-like domain-containing protein 7).
The copper-dependent enzyme lysyl oxidase (LOX) [14, 15, 86–88] is activated by fibroblasts under stress [89], crosslinks collagen [90], and promotes cancer in vivo [91]. Moreover, if the remodeled matrix persists in the lamina fibroreticularis, a PCN with enhanced stiffness and cell transition properties is generated [14–19].
H. pylori induces NF-κB and the YAP1 axis in gastric pre-cancerous Helicobacter-induced lesions [92] and increases SALL4, which induces the CAF phenotype [93, 94]. H. pylori infection promotes hepatoma-derived growth factor (HDGF) expression in human gastric cancer. Subsequently, HDGF recruits MSCs and regulates their differentiation into myofibroblast-like cells expressing α-smooth muscle actin (α-SMA), procollagen α1, tropomyosin I, desmin, fibroblast activation protein (FAP), and fibroblast markers prolyl-4-hydroxylase A1 (PHA1) and fibroblast specific protein-1 (FSP-1)/S100A4 [95].
More detailed information on the association of Sal-like protein 4 (SALL4) and miR-33 is provided in the supplemental material (Supplement, part 8, Sal-like protein 4 and miR-33).
CAFs can also derive from epithelial cells, pericytes, adipocytes, and endothelial cells [96, 97]. Furthermore, inhibition of the histone methyltransferase enzyme euchromatic histone-lysine N-methyltransferase 2 (EHMT2, G9a) decreases active CAFs to a less proliferative/invasive state [98].
In vitro, macrophages (THP-1 cells) induce differentiation to the CAF phenotype, which can lead to gastric epithelial lesions with malignant features via EMT [99]. Again, these findings indicate that the primary sources of epithelial cancers are fibroblasts and CAFs, but not epithelial cells [15]. The tumor microenvironment provides the prerequisites for cell migration from the primary tumor to distant sites (metastasis).
Cell migration
Cell migration is a complex and poorly understood process with central roles in embryogenesis, physiology, and the pathophysiology of diseases [100]. The following statement by Erwin Büning (1906–1990), who discovered the internal clock together with Jürgen Walther Ludwig Aschoff (1913–1998) and Colin Stephenson Pittendrigh (1918–1996), remains relevant: “Perhaps even today we do not fully realize the inherent complexity of many biological phenomena” [101].
The dynamic combination of local and peripheral conditions, cell-cell cell-microenvironment communication and adhesion results in signaling and crosstalk, wherein the information processing is akin to changes in language and grammar with modifications of polarity alignment and direct locomotion and velocity. The cellular microenvironment with its modifications of stiffness and elasticity by crosslinking changes, not just cell communication locally, but also within the cell network, its organization (tissue). Together, these factors can result in cell transition with malignant consequences for the local microenvironment, an organ, or the whole organism, thereby resulting in metastasis.
Additionally, a difference exists between single cells or compartments and multiple cells, such as cancer satellites that move and travel alone or together. According to a simulation analysis, whether cells join in small or large numbers during cell movement appears irrelevant, because cell polarity determines cell orientation and the direction of migration [102].
Stiffness gradient
A rigid matrix promotes cell proliferation [103] through a process called mechanotransduction [104, 105], which is dependent on modulation of integrin expression [106, 107]. Independently of cell size or cell division, cell migration typically aligns along with the stiffness gradient [108].
Other variables influencing cell migration, beyond the composition of the cytoskeleton and cytoplasm [109], include ion channels [110], various types of signaling [111], and chemotaxis [112].
Chemotaxis
Chemotaxis, locomotion along a gradient [101], was observed and reported in bacteria by Theodor Wilhelm Engelmann (1843–1909) in 1881 [113] and by Wilhelm Friedrich Philipp Pfeffer (1845–1920) in 1884 [114]. A statement by Pfeffer, in 1893, that “the senses of unicellulars are not poorer than those of vertebrates” [101], was considered sacrilegious at the time. Changes in chemotaxis always impair cell motility [115].
However, polarity and lamellipodia are also important for cell mobility, thereby enabling pre-cancerous and cancer cells to cross the basement membranes, enter the circulation, and become dispersed.
Cells use modifications of plasticity and actin-rich protrusions (filopodia, lamellipodia, invadopodia, podosomes, and lamellipodia) to migrate through the extracellular matrix (ECM), access the endothelium, and consequently undergo transendothelial migration and entry into the vascular system [116].
Spatial asymmetry and polarization
Spatial asymmetry causes morphological polarization with front and rear differentiation, thus defining the direction of cell migration. This polarization can occur in both individual and collective cell migration on the basis of changes in orientation and composition of filamentous F-actin distribution [100, 117]. However, over a distance of several cell lengths (~100 μm), no cell always follows a constant path in space [118]. Contractile myosin components are preferentially localized at the rear of the cell, whereas actin is localized in the front [119]. Turning cells have asymmetric shapes and actin distributions. Myosin contractions at the rear of the cell use functions similar to rear-wheel steering in a car to remain stable during sustained rotation.
Human bladder epithelial cancer cells are more deformable than normal cells [120]. This finding has also been observed in breast cancer, in which an approximately ~50% decrease in filamentous actin (F-actin) during cytoskeletal reorganization has been associated with increased metastatic potential [121, 122]. The loss of polarity in cancer tissue was reported in 1941 in carcinoma in situ (CIS) [123], which was first described in 1932 as a noninvasive carcinoma of the breast [124]. Broders described CIS in the epithelial layer as a lesion of malignant epithelial cells, with their progeny located at or near positions previously occupied by their ancestors before malignant transformation or migration and breaching of the basement membrane. However, noninvasive means non-migrating, in concordance with actin findings. CIS does not show lymph node or other metastasis, but CIS in the periphery with multifocal or multicentric localization is present in as many as 40% of breast cancers [125, 126]. More detailed information regarding CIS is provided in the supplemental material (Supplement, part 9, Carcinoma in situ).
Migration induced by signaling
The anti-inflammatory lipid mediator Resolvin D1 (RvD1) [127], within a co-culture of CAFs isolated from hepatocellular carcinoma (HCC), has been found to suppress cartilage oligomeric matrix protein (COMP) secretion, thereby inhibiting CAF-induced EMT [128]. Plasminogen activator inhibitor-1 (PAI1) from CAFs in esophageal squamous cell carcinoma (ESCC) binds the PAI-1 receptor low-density lipoprotein receptor-related protein 1 (LRP1), thereby activating Akt and Erk1/2 signaling, and inducing migration and invasion of ESCC and macrophages [129].
CAF-inducing migrating phenotype
CAFs increase the expression of alpha smooth muscle actin (α-SMA), fibroblast activation protein (FAP), fibroblast-specific protein 1 (FSP1), platelet-derived growth factor receptor (PDGFR)-α/β, and vimentin [130]. These increases are associated with cell contractility (α-SMA), FAP, cell differentiation (FSP-1, S100A4), receptor tyrosine kinase activity (PDGFR-α/β), and cell motility (vimentin). Coexpression of vimentin and keratin intermediate filaments in cancer cells enhances the migratory and invasive subtype [131–133].
FSP-1 binds Ca2+ and is expressed by inflammatory macrophages [134], thus explaining the close interaction of cancer cell migration with parts of the immune system. Changes in transcription factor expression contribute to the disruption of homeostasis and consequent transformation to heterogeneous CAFs [135]. This heterogeneity has been reported to be associated with the provenance of CAFs [136].
Furthermore, the heterogeneity suggests that [137] CAR-T cell technology is unlikely to be helpful in the short or mid-term for heterogeneous cancers. In contrast, if, for example, leukemia were caused by one heritable mutation, the CAR-T approach would be reasonable.
Highly aggressive hepatocellular carcinoma (HCC) cell lines with high metastatic potential promote liver fibroblast migration and transformation into CAFs [138]. The mechanism involves the EGF-like domain multiple 7 (Egfl7). Early HCC recurrence (within 2 years) exhibits greater CAF infiltration than observed in patients without early recurrence, thus indicating an important role of CAFs. More detailed information on the association of CAFs with Egfl7 is provided in the supplemental material (Supplement, part 7, CAF interaction with Neuropilin-1 and EGF-like domain-containing protein 7).
Changes in the stiffness gradient, chemotaxis, spatial asymmetry, and polarization induce enhanced cell migration and immune system suppression, thereby increasing cell motility and cell mobility, and enabling metastasis.
Cell mobility
Decreased cell-cell interactions result in changes in epithelial morphology, cell transition, and cell movement [3, 139, 140]. Downregulation of Ca2+-dependent homophilic adhesion receptors and cadherins, such as E-cadherin, during carcinogenesis is associated with increases in mesenchymal markers, such as fibronectin, and vimentin. As E-cadherin decreases, greater cell motility and higher rates of cell transformation are observed [141]. This response appears to be a last attempt by the biological system to restore homeostasis.
The actin protein Espin is overexpressed in cancer and promotes filopodial formation, thereby facilitating cancer cell migration and metastasis [142]. The microfilamentous cytoskeleton turnover in polymorphonuclear neutrophils is activated by chemotactic factors [143]. Exposure to the carcinogen, 7, 12-dimethylbenz [a]anthracene (DMBA), induces premalignant lesions in hamster cheek pouch mucosa, and is followed by formation of pseudopodia in stromal tissue cells and epithelia, including surrounding leukocytes [144]. Joseph Locker, in 1970, demonstrated that 48 hours after liver cancer cell inoculation in chick embryos, the tissue was invaded, and pseudopodia were observed together with loss of tight junctions, disruption of the epithelial organization, and liver metastases after 72 hours [145].
Lamellipodial formation
Lamellipodial formation is a complex actin-dependent process that results in the formation of flat sheet-like protrusions, thus leading to slow adhesive movement [146]. Lamellipodia preferentially extend in the direction of gradients [147]. Elongation of connective tissue in vivo and ex vivo results in the formation of new lamellipodia in fibroblasts within minutes, whereas in unstretched tissue, the fibroblasts show normal dendritic morphology [148, 149]. Although movement is generally slow, changes in the composition of actin filaments within lamellipodia occur rapidly with polymerization and depolymerization [150]. However, for cell migration, another precondition beyond the deformation of the cytoplasm must be met. Deformation and expansion of the laminar surface of the nucleus are required to enable cell migration through narrow spaces [151], such as the stiff ECM.
Furthermore, transformation of MSCs into CAFs has been described [152]. Ongoing TGFβ elevation (chronic inflammation) results in CAF elongation mediated via Rac, RhoA, and ROCK, and leads to cell spreading, lamellipodial formation, and spheroid invasion [153].
Cancer cells, as well as the PCN, induce LOX activity, and increased focal adhesion kinase (FAK), src, and Crc [154]. CAFs induce p130(cas), thus resulting in the formation of p130(cas)/crk/DOCK180 and, via RAC-GTP, increased lamellipodia, decreased actin fiber function, and elevated cell motility and mobility (see below FIGURE 2). No rapid movement is necessary, because the initial barrier after the first cancer cell has developed and integrated into the target tissue is loss of adhesiveness mediated by a decrease in E-cadherin [3, 14].
Crossing the basement membrane
When epithelial cancer cells grow, the basement membrane, including collagen type IV, poses a barrier to cell movement or a so-called restriction line. Alpha 3 beta 1 integrin [155] and metalloproteinase 9 (MMP-9 or gelatinase B) [156] are associated with increased collagen IV degradation, invasiveness, and migration [157], thus facilitating the transport of cancer cells across the basement membrane [158].
Extracellular vesicles
Long distance cell communication influences metastasis in a different manner. Cells can release extracellular vesicles (EVs), which are nanoparticles 50–500 nm in diameter [159]. Despite having been known for approximately 30 years, EVs were initially assumed to be cellular “junk” [160]. EVs are secreted vesicles, including exosomes and microvesicles, that contain proteins, lipids, and nucleic acids (such as mRNA and miRNA). In 1981, EVs were recognized as vesicles containing enzymes [161]. Later, EVs were found to be able to communicate over long distances [162].
Furthermore, EVs can modulate the immune system around primary tumors, thus creating pro-metastatic conditions. This phase, in accordance with seed and soil theory, is described as “preparing the soil” [163]. Autocrine, paracrine, and endocrine signaling induced by EVs does not require direct cell-to-cell contact [160]. EVs can contain the small GTPase Rab27a, which is overexpressed in breast cancer [164]. Inhibition of Rab27a in breast cancer decreases EV secretion, the number and size of lung metastases [165], and the accumulation of tumor-promoting tumor-associated neutrophils (TANs) [160]. EVs easily travel through the ECM. Aquaporin-1 (AQP1) within the EV membrane mediates EV deformability [159] and therefore facilitate easy and efficient EV travel through the ECM to target cells. Plant-derived EVs might provide a novel tumor-targeting delivery system for cancer treatment [166].
Blebbing (motility without adhesion)
Blebs are short-lived cell membrane protrusions which are bulky and have a rounded morphology [167]. Blebs undergo rapid expansion followed by a short static phase and slow retraction lasting approximately 1 minute [168–170]. Blebs were first observed as transient changes in corneal endothelium by doctoral student Steve Zantos and his supervisor Brien Anthony Holden (1942–2015) [171]. After lens insertion, blebs can be seen for approximately 10 minutes. The expansion time with intracellular pressure on the cytosol initiates bleb formation and is estimated to be approximately 5–30 s [172]. Polarized bleb accumulation can persist for at least an hour and is integrin dependent [173]. Signaling by FAK, integrin α2, and junctional adhesion molecule-1 (JAM-1) is involved in blebbing [174].
Blebs alter cell movement in vitro and in vivo, thus enabling amoeboid-like cell motility [175] without adhesion [176]. Early blebs are initially actin negative but later become actin positive, and the hydrostatic pressure necessary for movement relies on non-muscle myosin II (NMII) contractility [172]. Blebs are associated with decreases in lamellipodia [172, 177]. If the proportion of blebs becomes disproportional, the cell’s locomotion markedly slows and the movement becomes less directed.
Furthermore, blebbing promotes cancer invasion, migration, and metastasis [170, 173, 174, 179, 180]. Increased blebbing is associated with aggressiveness and metastatic behavior [173]. Changing the ratio of lamellipodia to blebs also influences the straightness of movement. The locomotion strategy of progenitor cells in tissue appears to involve a combination of blebbing and rapid straight-line forward movement. Lamellipodia are believed to be necessary for fast movement, whereas blebs are believed to be required for tumbling movements. The combination of both is believed to provide an optimized strategy for the precise movement of cancer cells.
Switching cancer cell motility
Established liver tumors recruit CAFs and stimulate their own growth [181, 182]. Cancer cells can be cohesive or mobile. TGFβ signaling, together with Smad4, EGFR, Nedd9, M-RIP, FARP, and RhoC, can collectively alter the motility of single or multiple cancer cells [183]. In contrast to primary cancers, lymph node metastases intriguingly involve a collection of initially non-motile cancer cells.
CAFs suppress the immune system
High SMAD3, even independently of TGFβ, recruits CAFs in lung adenocarcinoma, and CAFs in turn increase matrix metalloproteinases (MMPs) [184] and downregulate MHC class I-restricted T-cells (CD8+ T-cells), which can kill cancer cells [185]. CD4+ T-helper (TReg) cells are increased by CAFs [186], whereas CCL2 increases macrophage suppression [187]. Furthermore, CAFs regulate the immune system [188, 189] and can act as MHC class II-expressing CAFs presenting antigens to CD4+ T cells, thereby modulating the immune response in pancreatic tumors [74]. However, the disruption of homeostasis by CAFs can also suppress the immune system and facilitate pro-cancerous growth [190]. More information regarding the important interactions of CAFs with Transgelin-2 and cluster of differentiation 74 (CD74) is provided in the supplemental material (Supplement, part 10, CAF, Transgelin-2, and cluster of differentiation 74).
The cancer microenvironment consists of tumor-associated cells (TACs), which further influence cell migration and mobility, and create the preconditions for metastasis—the actual process of cancer cell spread, which is followed by traveling (‘circulation’) and extravasation.
Tumor-associated cells
TACs are found in the tumor center and in its vicinity. These cells include tumor-associated macrophages (TAMs), tumor-associated platelets (TAPs), tumor-associated neutrophils (TANs), tumor-associated lymphocytes, tumor-infiltrating lymphocytes, and tumor-associated dendritic cells (myeloid-derived suppressor cells). In principle, most TACs are found in cancers with a pro- or anti-cancer role, and each type can be heterogeneous.
Tumor-associated macrophages and M2 macrophages
TAMs are broadly classified into anti-tumor M1 macrophages (classically activated M1 macrophages) and cancer-inducing M2 macrophages (alternatively activated M2 macrophages). These macrophages are associated with cancers of the breast [191–193], stomach [194], and liver [195]; cholangiocellular carcinoma (CCC) [196]; HCC [197]; and prostate carcinoma [198–200]. TAMs can derive from so-called cancer stem cells [201].
M1 macrophages are classically activated in response to interferon-gamma (IFN-γ) and lipopolysaccharide (LPS), and are anti-tumorigenic. They are characterized by TNF-α, IL-1β, IL-6, nitric oxide synthase (iNOS), and reactive oxygen species. They express CD80 (B7–1), CD32, CD68, and CD11b (integrin alpha M or ITGAM), and they are crucial for immunity by eliminating pathogens and cancer cells [202–208].
M2 macrophages are activated by IL-4, IL-10, and IL-13, and express various receptors, such as TGFβ, CCR2, CD163, CD206 (mannose receptor), CXCR1, CXCR2, Dectin-1, scavenger receptors A (CD204) and B-1 (SR-B1), macrophage receptor with collagenous structure (MARCO, SR-A6), and arginase 1 (ARG1). These findings might explain why a TH2-type immune response is observed in schistosomes, which causes bladder cancer [209]. More information regarding the interactions of Alpha-1-B glycoprotein (A1BG) is provided in the supplemental material (Supplement, part 11, Alpha-1-B glycoprotein).
When stimulated by cytokines, macrophage polarization and transition can change from M1 to M2, and vice versa. Subsets of M2 macrophages include M2a, M2b, M2c, and M2d. Cancer cells expressing cluster of differentiation 47 (CD47) induce further immunosuppression by inhibiting phagocytosis and promoting cell migration [210]. More information regarding the different M0 expression profiles, as well as CD47 expressing cancer cells suppressing phagocytosis is provided in the supplemental material (Supplement, part 12, TAM profiles and cluster of differentiation 47).
Tumor-associated platelets
TAPs have a more complex role in nature and therefore are not differentiated by type 1 or 2. Pro-tumorigenic TAPs protect cancer cells against immune attack; mediate adhesion, extravasation, and angiogenesis. They also induce cell transition or the release of soluble platelet growth factors. Anti-tumorigenic TAPs increase macrophage recruitment and, after activation, can destroy cancer cells [211, 212]. More information on platelets and TAPs is provided in the supplemental material (Supplement, part 13, Thrombocytes).
Thrombocytosis (high platelet count) and/or megakaryocytosis is associated with cancers and poor survival in epithelial cancers [reviewed in 213], whereas inhibiting platelets decreases metastasis [214]. Furthermore, cancer cells activate and form aggregates with platelets and leukocytes and consequently are able to evade the immune system [215, 216].
Platelets themselves are activated by multiple pathways, such as neutrophil-released cathepsin G [217], thromboxane A2 (TXA2) [218, 219], high-mobility group box 1 (HMGB1)/Toll-like receptor 4 (TLR4) axis, and adhesion G protein-coupled receptor (GPCR) cluster of differentiation 97 (CD97). Platelets support lung microvascular integrity independently of their classic hemostatic pathways [220–222]. Platelets contain factors that promote cell survival and support the endothelial barrier [223, 224], and influence the programmed cell death pathway [225–227]. Moreover, platelets are present not only in the vascular space during lung inflammation but also actively enter the alveolar space during experimental lung injury [228, 229].
Although platelets play major roles in cancer metastasis by facilitating tumor cell survival, immune evasion, and cancer spread to distant sites, one aspect and role in metastasis might not have been interpreted correctly.
Aspirin has anti-platelet effects that correlate with favorable prognosis and diminished clinically observed metastases [127, 230]. These observations might explain experimental data from the 1980s on the anti-COX effects of aspirin, which indicated negative findings [231]. The anti-platelet aggregation effects of aspirin were ignored. Chemotherapy suppresses platelet production in the bone marrow, and this cytotoxic effect alone might explain why the role of platelets in epithelial cancer metastasis were underestimated.
Platelets bind lysophosphatidic acid in breast cancer [232–234], ovarian cancer [235, 236], prostate cancer [237], kidney cancer [238], and oral squamous cell carcinoma [239]. Subsequently, they stimulate osteoclast resorption through IL6 and IL8, thus resulting in bone metastasis [240] and osteosarcoma progression [241].
For metastasis, complement activation and thrombin [242] are also necessary. Thrombin increases metastasis [243] and activates proteinase-activated receptor 1 (PAR1), a receptor highly expressed on platelets and endothelial cells [244]. In contrast, the inhibitor hirudin decreases metastasis [245]. Thrombospondin-1 (TSP-1) and prosaposin (PSAP) have diverse functions. More detailed information regarding TSP-1 and PSAP is provided in the supplemental material (Supplement, part 14, Thrombocytosis, Thrombospondin-1).
Metastatic spread is highly heterogeneous, but spleen metastasis is notably very rare. The spleen is distinct from other organs in that it filters platelets highly effectively, thus suggesting that platelets play a critical role in metastasis. Furthermore, the liver is involved, and metastasis occurs far more often to the liver than any other organ. An important factor, sialic acid, must also be considered as further discussed.
Platelet clearance
Platelets have a lifespan of approximately 10 days. Platelet clearance occurs within the spleen by splenic macrophages. Additionally, aging platelets are eliminated in the liver by Kupffer cells, probably through desialylation, a process in which sialic acid on the cell surface is removed from glycans, glycoproteins, or glycolipids [246–248]. Asialoglycoprotein receptor 1 (ASGR1), a sialic acid receptor, participates in clearance of sialic acid from the liver and thereby functions as a tumor suppressor. This receptor is often downregulated in HCC versus normal liver tissue and is associated with a poor prognosis.
ASGR1 knockout promotes HCC development [249, 250]. In contrast, asialoglycoprotein receptor 2 (ASGR2) acts as a tumor promoter in gastric cancer [251] and is associated with cancer recurrence [252]. Moreover, high expression of ASGR2 is associated with gastric lymph node metastasis and venous invasion [253]. More detailed information regarding desialylation and platelet clearance is provided in the supplemental material (Supplement, part 15, Desialylation and thrombocyte clearance).
Blood transfusion activates TAPs
Controversial positive and negative prognostic studies have described outcomes after blood transfusion in patients with cancer [254]. However, red blood cell transfusion results in cancer cell activation and aggregation by the adenosine diphosphate (ADP)-P2Y12 receptor pathway [255]. Furthermore, red blood cell transfusion is associated with decreased survival, earlier recurrence, and increased metastasis [256–260]. Furthermore, patients with early stage I breast cancer patients who receive blood transfusions have poorer survival and earlier recurrence than patients without transfusion post-surgery [261].
The associations found in patients with cancer after blood transfusion (decreased survival and disease-free time, higher metastasis rates) are logical, particularly in the context of TACs, because transfusion-induced activation of TACs, particularly by TAPs, might explain these observations.
Tumor-associated neutrophils
TANs have various subtypes: N1 has anti-tumoral roles, whereas N2 facilitates migration of cancer cells [262, 263]. In CRC, IL-8 promotes neutrophil activation [264], and TAN promotes peritoneal cancer cell adhesion [265]. N1-TAN releases hydrolytic enzymes, or activates macrophages, dendritic cells, or T-cells. N2-TAN induces gelatinase B, an important enzyme for cancer growth and metastasis [266]. TANs release TIMP-free MMP-9, which is associated with very aggressive cancer [267].
Moreover, CRCs induce polarization between N1 and N2 by TGFβ with consequent increase in the N2 subtype [262, 268].
N2 recruitment occurs via CXCR2 in a manner regulated by IFN-γ [269]. Precursors of TAMs and TANs are located in the spleen and are readily recruited [270]. TANs promote invasion of cancer cells into lymph nodes [271].
Circulating TANs suppress peripheral leukocyte activation [272], and CTCs protect themselves via CD44 building clusters [273]. Cathepsin C released by neutrophils is associated with cancer progression and metastasis [274], and is also correlated with M2 macrophages in lung cancer [275]. The inhibition of TAPs diminishes this effect and decreases metastasis [276].
Cancer cells, as well as CAFs, induce the formation of neutrophil extracellular traps (NETs), which promote migration and metastasis. NETs are found in various epithelial cancer tissues, the vascular system, and in distant metastases. An anti-NET approach might inhibit both migration and metastasis [277–279].
Abundant polymorphonuclear myeloid-derived suppressor cells in primary and metastatic lung cancer have been measured in the blood and found to be inversely correlated with low frequencies of NK cells [280]. Furthermore, NETs promote liver metastasis and have been measured in patients undergoing curative liver resection for metastatic CRC [281]. NETs can trap CTCs [282] and serve as an adhesion substrate [283]. TANs appear to facilitate omental metastasis in ovarian cancer [284]. NETs promote liver micro-metastasis and have been measured in pancreatic cancer through the activation of CAFs [285]. NETs from TANs wrap around and consequently protect CTCs against immune attack [286]. In addition, inhibition of NETs protects against adhesion to liver sinusoids and metastasis [287].
Furthermore, TAPs, via αIIbβ3, shield CTCs in the bloodstream and consequently protect them against immune attack [288]. TAN attachment, via ICAM-1 and Mac-1, to CTCs enables transendothelial migration and extravasation [289]. Neutrophil-derived leukotrienes facilitate colonization of distant tissues [290]. Furthermore, TANs form clusters in the bloodstream [291].
Each tumor-associated cell (TAC) type has anti- and pro-tumor functions. As cancer growth increases, pro-tumor functions increase, and immuno-competent cells are suppressed (immune escape). Therefore, pro-tumor TACs promote cancer cell dissemination from primary tumors, as well as transendothelial migration, vascular stream survival, nidation, colonization, and metastatic growth at distant sites.
Chemokine CXCL12 (stromal cell-derived factor 1, SDF-1) signaling
CAFs produce the chemokine CXCL12 (stromal cell-derived factor 1, SDF-1), which is chemotactic for mesenchymal cells [292]. Furthermore, fibroblasts [293], bone marrow fibroblasts [294], CAFs [295], and particularly platelets are major sources of CXCL12 [296, 297]. Information regarding butyrophilin signaling and crosstalk in this context is provided in the supplemental material (Supplement, part 16, Butyrophilin molecules).
CAFs coat cancer cells, thereby suppressing T-cell infiltration by CXCL12 and resulting in quiescence of the cancer-immune response, with expression of Cxcl9 [298].
The maintenance of homeostasis includes diverse responses across all biological systems. Therefore, the disruption of homeostasis in cancer is both complex and heterogeneous. The CAF subtype can switch from an immune-suppressive (CXCL12+/CXCL9–) to an immune-activating (CXCL12–/CXCL9+) subtype. Furthermore, chronic inflammation increases CXCL12 [299] which in turn induces cancer progression through AKT and ERK signaling [300–302].
Cancer cells proliferate through PI3k/Akt signaling by CXCL12 [303–306]. Increased TGFβ signaling induces EMT via Wnt in breast cancer [307] and, TGFβ, together with CXCL12 expression, promotes cervical and breast cancer growth [308]. Furthermore, cancer cells follow a CXCL12 gradient [309]. More detailed information on CXCL12 is provided in the supplemental material (Supplement, part 17, Chemokine CXCL12).
Egfl7 increases FAK and Akt phosphorylation via the ανβ3 integrin receptor, and both recruit and activate liver fibroblasts to adopt the CAF phenotype [138]. For example, integrin α6β4 induces formation of lamellae and cell migration [310], with upregulation of PI3K and NF-κB [18]. Furthermore, integrin activation results in the production of fibronectin and insoluble fibrils, decreased p38 and cell proliferation [311]; consequently, cell binding promotes migration [312].
Cancer cell coating by CXCL12-Keratin 19
Cancer cells can be coated through CXCL12–Keratin-19 filamentous formation and consequently hidden from T cell–mediated immune reactions [313]. Subsequently, intravasation, traveling (‘circulation’), extravasation, and colonization of distant organs increase. Cancer cells can assemble CXCL12 together with Keratin 19 as a coating for immune evasion. Further details are discussed below.
Keratin 19 [314] is part of the cytoskeleton in epithelial cells and is found in epithelial cancer cells [315, 316]. CXCL12, a small cytokine, was identified in 1993 from a bone marrow stromal cell line and described as an intercrine-macrophage inflammatory protein [317]. Its structure was identified in 1994 [318]. While the CXCL12 mRNA is decreased [319], its expression in tissues is associated with metastasis.
The interplay of chemokines with the receptor as CXCL12/SDF-1alpha and CCL21/6Ckine exhibit peak levels of expression in organs that are the first destinations of breast cancer metastases [309].
The underlying complex framework described herein provides a basis for understanding metastasis in a new light. Several terms used herein are defined below.
Niche
The term niche comes from the Latin nidus, meaning nest. Pre-metastatic niches are a set of prerequisite conditions for tumor growth and access to the vascular system (intravasation: hematogeneous and lymphatic). Pre-metastatic niches are followed by circulation (better: traveling). Only after nidation can colonization occur at sites distant from the primary cancer, thus resulting in metastasis. To date, the term “metastatic niche” has been characterized as a region of increasing metastatic potential [320], followed by perivascular conditions [321]. In the bone marrow, the metastatic niche takes the form of an endosteal niche in regard to osteoblasts [322].
Cancer satellite (CS)
The definition of cancer satellites provided by the National Cancer Institute is “discrete tumor cell clusters near the primary tumor.” However, the original meaning of satellite comes from the Latin satelles, meaning one who escorts or follows e.g. behind an important person. We therefore use the term “satellite” to describe cancer satellite complexes in metastasis in the original (non-modified) manner, as a cell or particle escorting another from one location to another more distant location.
Circulation
The term circulation is not completely correct, because it incorrectly implies that cancer satellites can circulate within the human body for more than one round of circulation. Only one cancer cell is found per million cells [323, 324 reviewed in 325]. Only 0.2% of cancer cells in circulation form metastases, whereas 99.8% are effectively eliminated by the immune system. The term “circulation” in the context of cancer cells and metastasis is simply an incorrect assumption. However, to avoid confusion, we use this term because it has become commonplace, together with the much more appropriate, correct term of “traveling”.
Gradient
A gradient in cell biology, and within the extracellular matrix, is a fundamental process, enabling embryogenesis, as well as adapting conditions (compensate) disruption of homeostasis. A gradient is a measure of the spatial variations over a certain space in the composition (secretion by cells of types and amount of protein and/or enzymatic modification), mechanics (change of mechanical forces by properties in stiffness and elasticity) and chemistry (changes in the concentration), resulting into a physical quantity. By this, the gradient by component, mechanical, chemical and physical composition, guide movements, such as cell migration towards a direction.
Adhesion
Adhesion is fundamental to cell-cell communication and crosstalk [3, 14]. Adhesion after ECM migration and invasion precedes transendothelial migration and is mediated by the carbohydrate-binding molecules known as selectins [326–328]. The three main types of selectins are E-selectin (expressed on activated endothelial cells and promyeloblast cells), P-selectin (expressed on endothelial cells and platelets), and L-selectin (expressed on activated platelets and leukocytes). To plausibly explain how metastasis occurs, pre-conditions for cell spread in terms of adhesion, E-selectin, P-selectin, and L-selectin are important, and are detailed in the supplemental material (Supplement, part 19, E-, P-, and L-selectin). Transendothelial migration is multifaceted and has been reported in detail elsewhere [100, 102, 108, 110, 116, 119, 176, 294, 303].
Metastasis
In cancer, metastases describe secondary cancers that occur at distant sites in the body from primary cancers. We recognize that 107 cells (i.e., 10 million or 10, 000, 000 cells) must be present for a tumor nodule to be detected by imaging, and the average number of cells in a tumor when it is first palpable is 108 cells (i.e., 100 million or 100, 000, 000 cells) [19].
Therefore, how carcinogenesis and its first cancer cell occurs must be identified. Such identification has been systematically performed over 25 years [3, 4, 14–19, 127]. The same methods have also been applied to metastasis, to identify the essential sine qua non prejudices and to elucidate what comes first and later results in transendothelial migration, intravasation, and traveling before metastasis can occur.
Fundamental sequence of events in metastasis
Pre-metastatic niche 1 (PMN-1) (FIGURE 2)
PCN transforms into a local PMN-1 involving cancer cell development and an increase in CAFs and subsequent
cancer growth and angiogenesis, thereby ensuring cancer cell nutrition and lymphogenesis for the necessary
lymphatic drainage. This serves for the growth of cancer cells, but also in parallel as a proclivity for
metastasis. Ongoing mesenchymal-epithelial transition (MET) in CAFs increases cancer cell numbers and
growth,
with heterogeneous CAFs and pools of cancer cells.
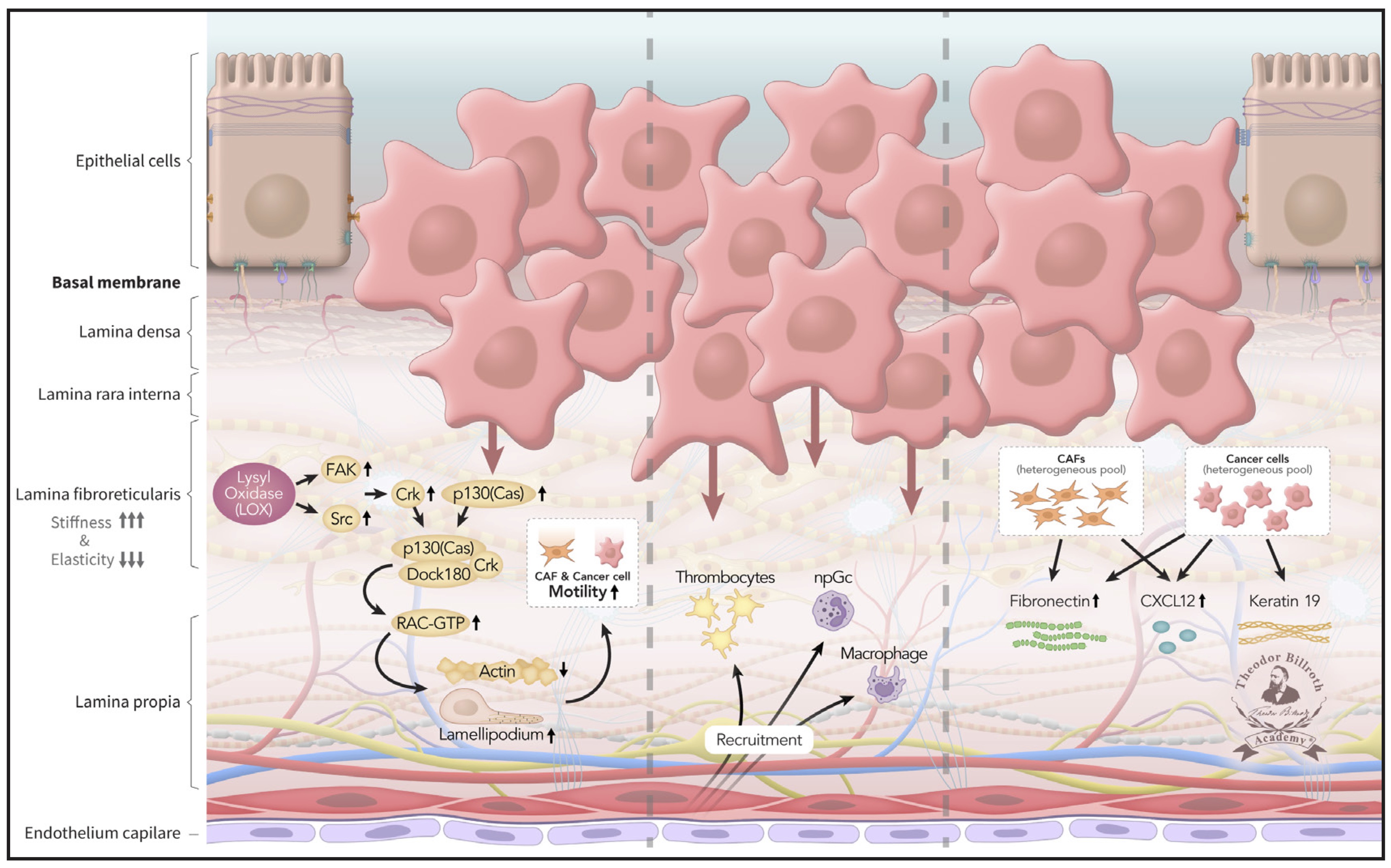
Fig. 2: Pre-metastatic niche 1 (PMN-1): With increasing disruption of homeostasis, the pre-cancerous niche transforms into a premetastatic niche 1 (PMN-1): the heterogeneous CAF cell pool together with lysyl oxidase by heterogeneous cancer cells induce p130(cas)/crk/DOCK180 complexes and, via RAC-GTP, increased lamellipodia, decreased actin fiber function, and enhanced cell motility and mobility. Decreased apoptosis and loss of cell-cell contact inhibition, reinforces cancer cell growth, and invasion of the basal lamina. CAFs begin to release fibronectin and CXCL12, and cancer cells release CXCL12 and Keratin 19. Platelets, neutrophils, and macrophages are increasingly recruited and later serve as prerequisites for pre-metastatic niche 2 (PMN-2) formation.
CAFs and LOX signaling induce crosstalk between FAK and p130(cas)-Src-Crk and formation of the p130(cas)/crk/DOCK180 complex, a necessary precursor for the formation of lamellipodia. Subsequently, lamellipodia enable cell mobility and motility and are a prerequisite for increasing migration toward the endothelium.
In parallel, an induced decrease in apoptosis (cell death), as well as loss of cell-cell contact inhibition, reinforces cancer cell growth, and encourages invasion of the basal lamina and subepithelial layers and subsequent population of the deeper stroma. This process is recognized in the TNM classification as T-stage evolution from T1 to T4.
Subsequently, additional fibroblasts are recruited, and CAFs increase and begin to release fibronectin and CXCL12 into the stroma, as well as Keratin 19. Platelets, macrophages, neutrophils, monocytes, and T-cells are recruited to the tumor microenvironment.
Pre-metastatic niche 2 (PMN-2) (FIGURE 3)
Pre-metastatic nice 1 (PMN-1) is transformed into PMN-2. Various subsets of tumor-associated cells (TACs),
such
as tumor-associated macrophage (TAMs), tumor-associated plateles (TAPs), and tumor-associated neutrophils
(TANs), develop during ongoing signaling and crosstalk. Anti-tumorigenic TACs increasingly transform
into pro-tumorigenic TACs with local immunosuppression and a lack of CD8+ T-cell immune
evasion.
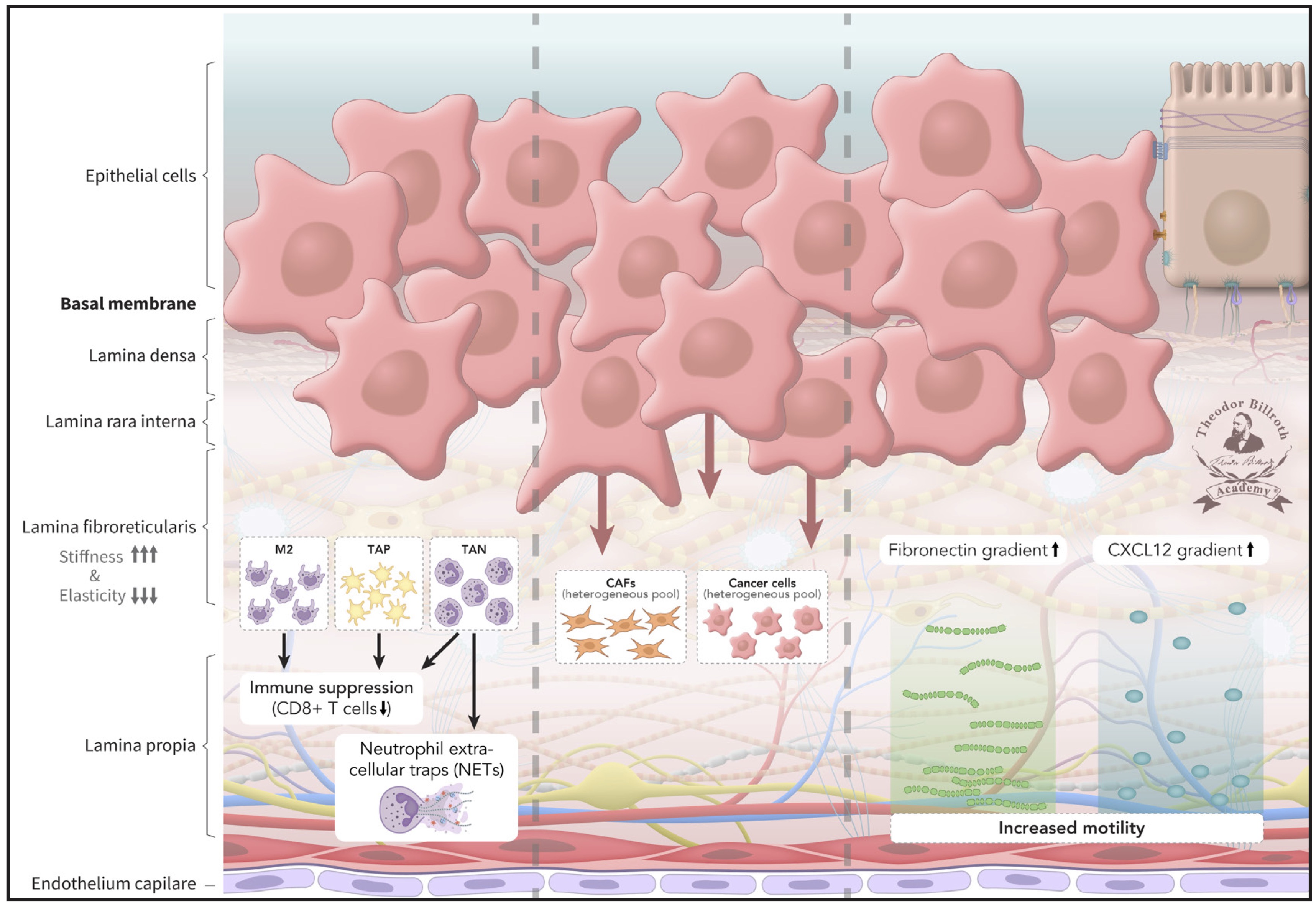
Fig. 3: Pre-metastatic niche 2 (PMN-2): Various subsets of tumor-associated cells (TACs), such as tumor-associated macrophages (TAMs), tumor-associated platelets (TAPs), and tumor-associated neutrophils (TANs), develop during ongoing signaling and crosstalk. Anti-tumorigenic TACs increasingly transform into pro-tumorigenic TACs with local immunosuppression and a lack of CD8+ T-cell immune evasion. Neutrophil extracellular traps (NETs) from TANs are increasingly released, and heterogeneous CAFs and cancer cell pools develop. The graphical icon of NETs in Figure 4 was adapted with modifications from prior research [329].
Furthermore, cancer cells, as well as CAFs, additionally induce the formation of neutrophil extracellular traps (NETs), which promote migration and metastasis, and are found in various epithelial cancer tissues, the vascular system, and distant metastases [329]. The CXCL12 and fibronectin gradients expand. Cell mobility is also facilitated by ongoing LOX-induced formation of lamellipodia, thus promoting cell motility. Blebbing and EVs further increase the motility and travel of CAFs and cancer cells. Meanwhile, increased fibronectin, CXCL12 release, and decreased F-actin result in formation of a CXCL12- and fibronectin-gradient, along which increasingly mobile cells migrate toward the endothelium as a step towards travel away from the primary tumor.
Pre-metastatic niche 3 (PMN-3) (FIGURE 4)
PMN-2 transforms to PMN-3 as further disruption of homeostasis occurs, and serves as a prerequisite for
metastasis. The evolution of the pre-metastatic niche (PMN) leads to PMN-3, in which intravasation of
cancer
satellites, involving trans-endothelial migration, provides access to the
vascular
system. This process is recognized in the TNM classification as further cancer evolution
with hematogeneous (V), lymphatic (L), and perineural (Pn) invasion.
Transendothelial migration alone is complex with its adhesion (Supplement, part 18, E-, P-, and
L-selectin).
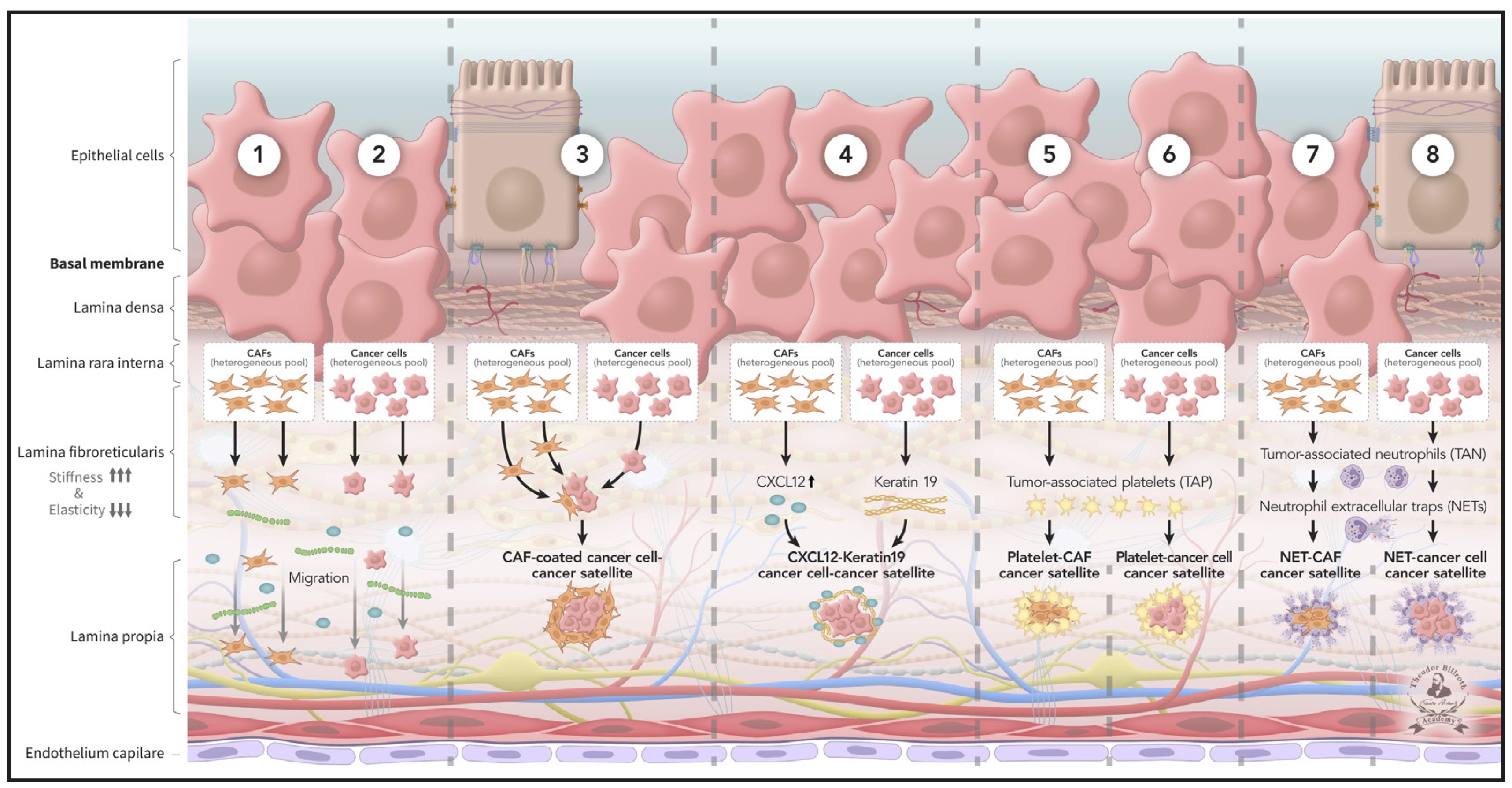
Fig. 4: Pre-metastatic niche 3 (PMN-3): Metastasis in epithelial cancer occurs in parallel with carcinogenesis after the pre-cancerous niche transformed into pre-metastatic niches (PMNs), which are indispensable to the origin of metastasis. Pre-metastatic niche 2 (PMN-2) transforms to pre-metastatic niche 3 (PMN-3) by ongoing signaling and crosstalk. PMN-3 serves as prequisite for intravasation, traveling, and how metastasis arises. According to current knowledge, a series of eight heterogeneous cancer satellites develop, including Trojan horses (immune evasion), alongside reciprocally affecting sequences, and subsequently travel (or, as usually described, “circulate”) alone or in combination, far from the primary tumor. The fundamental prerequisites for metastasis are met as follows: (1) Cancer cells and (2) CAFs both migrate along the CXCL12 and fibronectin gradient. (3) CAFs surround cancer cells and migrate. (4) CXCL12 and Keratin 19 coated cancer cells migrate. (5) CAFs and (6) cancer cells are surrounded by platelets and migrate. (7) Neutrophils form neutrophil extracellular traps (NETs) that shield CAFs and (8) cancer cells, thus facilitating their migration and traveling.
PMN-3, according to current knowledge, comprises eight cancer satellites, which access intravasation and subsequently travel (or, as usually described, “circulate”) far from the primary tumor. The fundamental prerequisites for metastasis are met by eight cancer satellites, including Trojan horses, which enable immune escape, as follows: (1) Cancer cells and (2) CAFs both migrate along the CXCL12 and fibronectin gradient. (3) CAFs surround cancer cells and migrate. (4) CXCL12 and Keratin 19 coated cancer cells migrate. (5) CAFs and (6) cancer cells are surrounded by platelets and migrate. (7) Neutrophils form NETs that shield CAFs and (8) cancer cells, thus facilitating migration and traveling.
The dissemination of cancer satellites is not synonymous with metastasis despite often being described as such. It sets the stage for later metastases. Maybe even M2 macrophages surround CAFs and cancer cells. However, cancer cells are surrounded by CAFs [330]. Platelet inhibition decreases metastasis and cancer cell adherence to platelets via P-selectin [214, 331]. Cancer cells are surrounded by platelets [332], which impede natural killer cell-mediated elimination of tumor cells [333]. Therefore, various cancer satellites to facilitate metastasis are created.
As neutrophils surround cancer cells or CAFs they expel NETs, together with nuclear histones, and granular and cytoplasmic proteins [334–336]. NETs facilitate liver metastasis [337], and have been observed in gastric, liver, and pancreatic cancers [337–339], as well as other epithelial cancers. Moreover, NETs aid in building clusters with growth at the peritoneal in gastric cancer [277, 340].
Pre- and malignant cells and various aggregates with various metastatic capabilities have been found in cancer nodules and observed to have high heterogeneity [341, 342]. These findings might explain why both single cells and clusters of cancer cells have been detected in cancer blood samples [343]. Platelet-cancer cell cancer satellites are fundamental for metastasis. The cancer satellites created from platelets with cancer cells and CAFs explain also early reports of local recurrence, such as that observed 19 years after resection of a primary tongue carcinoma or a long-term course in a breast cancer with a duration of 15 years in 1885 [344].
Metastasis is a complex phenomenon. After transendothelial migration, cancer satellites travel and if not eliminated by the immune system, extravasate after arriving at vascular endothelial capillaries. Furthermore, when cells grow, transendothelial migration occurs synchronously with local micro-metastasis, i.e., the metastatic colonization observable under a microscope.
Implications
A brief overview of implications to minimize or prevent metastasis is as follows:
As discussed, in patients with cancer, platelet aggregation and inhibition should be considered immediately after diagnosis.
This approach could also be used to mitigate the potential for metastasis.
Even patients post surgery should receive a low molecular anticoagulation regimen to inhibit platelet aggregation. The drugs should be coated to facilitate resorption in the jejunum and minimize gastric adverse effects.
Anti-inflammatory therapy: a much more effective anti-cancer therapy approach would balance the treatment with an anti-chronic inflammatory regimen.
Anti-fibrotic therapy: a much more effective anti-cancer therapy approach would include an anti-fibrotic regimen.
Although measured and observed daily worldwide, changes in the values of platelets, macrophages, neutrophils, monocytes, as well as other immunocompetent cells, are interpreted primarily as adverse effects of multimodal therapy. However, as described herein, these routine laboratory parameters could be used to create models to predict cancer progression and the potential for metastasis before it occurs.
Current cancer treatment regimens comprising multimodal therapy with chemotherapy and/or combined immune therapy and/or radiotherapy protocols. These can lead to thrombocytopenia, leukocytopenia, neutropenia, and monocytopenia, as well as decreases in macrophages and other immunocompetent cells, such as T-cells, as well as TACs. Therefore, whether therapy regimes used over several decades might be largely effective in terms of anti-cancer effects, and/or in which percentage ranges induced by these standard-of-care regimens or might have promoted metastatic development, because of their cytotoxic effects on the cells listed, remains unknown.
Real-world data on the anti-cancer effects of multimodal treatment protocols must measure each cell type together with TACs to determine whether the therapies are effective or largely reflect the adverse effects of therapy, such as decreasing TACs with observed, if minimal, anticancer effects.
This would apply to CAR-T therapy as it affects each cell type.
Applying such strategies in cancer surgery would be a challenge, because detailed understanding of inflammatory and fibrosis and its remodeling [3, 14–19, 127] is necessary: performing surgery too early might severely affect postoperative wound healing. Therefore, in pre-cancer surgery: anti-inflammatory and/or anti-fibrotic approaches should not be performed 4 weeks pre-surgery, to avoid adversely affecting post-surgery wound healing.
Post-cancer surgery: both anti-inflammatory and/or anti-fibrotic approaches would require an uneventful post-surgery course and a time delay of at least 4 weeks postoperatively (particularly for critical anastomosis).
Knowledge and understanding of molecular signaling are continually increasing. The fundamental complexity of how metastasis arises could not have been observed or recognized previously, because of the reasons discussed above.
The achievements of the giants, on whose shoulders we stand, are available but less widely taught. Although many possible explanations exist, education has increased overall, but the emphasis on basic sciences has decreased. Subjects such as biochemistry and physiology are nearly globally underemphasized in the education of scientists and physicians.
For example, in human medicine, the field in which clinicians and scientists treat patients with cancer, popular ideas have been implemented. The teaching content for, e.g., biochemistry and physiology has already decreased by nearly 50% under the rubric of the so-called new (modern) medical course model (German: Modelstudiengang). This new way of teaching has been described as an innovative approach blurring traditional disciplinary boundaries and achieving an integrated practice-oriented approach for future scientists and physicians. However, the warnings raised in many disciplines were ignored and subsequently went silent, and the propagated declarations louder, especially, we argue in spiritu to Billroth, that scientists keep both eyes on the past go blind, but in science, keeping on eye on the past, may allow us to be more critical. Giulio Bizzozero (1846–1910) has already taught us that, in science approximately 150 years ago, ”the teacher should not present science as a series of dogmas supported by the prestige of a name […] but instead expose it in its true condition, with its doubts and its questions” [English translation] [345].
Genetic and stem cell research have greatly influenced current knowledge and thinking, and have been essential to many achievements in science. Over approximately 100 years, the focus on promoting success in cancer and metastasis has been on genetics, while cancer incidence has continued to increase [4]. However, as clearly indicated, neither somatic mutation theory nor stem cell theory can explain carcinogenesis [4, 5] or metastasis [Authors’ note: we have omitted to cite the abundant literature on this topic, as it is available here: 5].
Additionally, a major paradoxical transformation occurred through misinterpretation of correct statistical analysis, such that the data were incorrectly interpreted to be the cause of cancer and metastasis.
One non-genetic example of the global cancer burden is the predicted increase in gastric cancer incidence [346]. However, there might be an incorrect focus on the gastric cancer diagnostics market, which has been predicted to increase within the next 10 years, to USD 970.95 million by 2034 [347].
Declarations that somatic mutations are “the” cause of cancer and metastasis have not helped materially to date and are extensively reviewed earlier [5].
Aspects that cannot be explained are regularly phrased by statements such as "we assume some aspects of genetics remain unknown”; more concerningly, most editors accept such sentences. A quotation by Mechtilde Lichnowsky (1879–1958) from 1924 still resonates:” Nothing is easier to learn than the art of approximation, nothing succeeds so much, nothing prevails so convincingly, nothing is more stubborn in assertion, nothing is harder to unmask because of the false resemblance to genuine” [English translation] [348].
A much more nuanced and differentiated way is psychologically contrary to a more realistic but complex explanation, possibly because binary views are easy to accept, believe, and propagate (please see “marketing and perception” in [4]).
In Summary
The thought processes described herein suggest new previously underappreciated aspects of most epithelial cancers and how they metastasize. Cancer can now be considered a parasite, developed from one’s own cells, which lives in and harms its host by actively transforming host cells into cancer cells. Various cell compartments are subverted, thereby enabling cancer cells to undergo transendothelial migration and travel to distant parts of the body (metastasis), with markedly increased mortality.
Cancer and metastasis are, in essence, states of disruption of homeostasis. The complex framework of cell-to-cell communication, including signaling and crosstalk, highlights a series of sequences positioned in relation to each other. The pre-cancerous niche (PCN) during carcinogenesis is transformed through ongoing disruption of homeostasis, thus leading to the evolution of pre-metastatic stages.
A series of eight heterogeneous cancer satellites, including Trojan horses to enable immune evasion, alongside reciprocally affecting sequences, develop, which are indispensable for intravasation, traveling, and mixed dissemination (metastasis). In parallel, the main multimodal anti-cancer cell effects can now be seen in a new, previously unrecognized light. The benefits might rely not on direct cancer cell effects but on indirect cytopenic effects, which, to date, have been regarded merely as adverse effects.
Multimodal therapy in cancer, including metastatic cancer, interferes with the anti-cancer functions of platelets, macrophages, and neutrophils, and further exacerbates disease progression and metastasis.
Consequently, these findings might provide a new explanation for why combinations of multimodal therapy can result in sudden observed efficiency, even in highly aggressive cancers, such as triple negative breast cancer [349], which are subseqzently lost.
To date, studies on cytotoxic multimodal therapy efficiency in the past 50 years have focused on observed effects of cancer cells, diminished cancer nodule growth in imaging, and decreases in cancer cells and variables (such as prolonged time to recurrence and disease-free survival), despite only minimal effects on overall survival. Now, a clearer understanding of metastasis provides opportunities to treat cancer at multiple points, and to block or even mitigate metastasis.
Consequently, multimodal treatments in patients with epithelial cancers cannot achieve healing, but can only slow disease progression until drug resistance or death occurs. Therefore, homeostasis is not merely a figurative term but clearly has a basis in multiple biochemical, physiological, and pathophysiological pathways. Given the known high redundancy in nature, and that homeostasis involves a balance among processes within the body to maintain a state of health, additional cancer satellite complexes are likely to be found and reported in the future.
Should Theodor Billroth’s (1829–1894) quotation from 1883, ”The completeness of a textbook always remains an illusion [...]“ [English translation] [350], or from 1881, “Everyone sees the world only from their own perspective, considers themselves to be the center around which everything emerges“ [English translation] [351], be invoked as excuses? Or is it the cancer research community, writ large, that has been on the wrong path?
Friedrich Schiller (1759–1805), in 1796 [352], stated: “What’s the hardest of all? What you think is the easiest, to see with your eyes what’s in front of your eyes” [English translation]. This quotation applies not only to the long time not seen origin of the first derived epithelial cancer cell. Although it was recognized by Rudolf Virchow (1821–1902) in 1856 [353], more than a century was required to address it, on the basis of current knowledge of signaling and crosstalk, and the identification of a mesenchymal cell precursor of the first cancer cell [15].
Scientific progress regarding cancer and metastasis has been slowed by a viewpoint in which all its solutions are seen from solely a genetic perspective, and a symptom-based therapeutic approach is followed.
The understanding of carcinogenesis and metastasis can no longer be viewed so myopically. The efficiency of many currently applied therapies must be re-visited by addressing the aspects described herein. Many clinically and experimentally observed adverse effects due to cytotoxic therapies are likely to be the reason for anti-metastatic efficiency. Therefore, cytotoxic therapies in epithelial cancers are often associated with transient (i.e., time-limited) responses and lower metastatic rates, but not patients’ quality of life and rarely long-term survival.
Conclusion
Many great successes have been achieved in healthcare and science in the past 200 years [4]. However, success in most epithelial cancers, despite being discussed daily in the media, have remained marginal. Survival continues to be reported in weeks or months, yet is reported as “breakthroughs.” The theories of how metastasis occurs consist of vascular and embolic genesis, the seed and soil theory, CTCs, and others [5] (Supplement, part 2–4) which are insufficient to explain metastases as documented in this paper.
We addressed the fundamental questions in cancer biology between 2014 and 2022, regarding how most cancers (80.5% epithelial) develop (Epistemology of the Origin of Cancer I) [3, 5, 14, 16–19, 127] and which is the first cancer cell (Epistemology of the Origin of Cancer II) [15]. In essence, carcinogenesis is a disruption of homeostasis. More specifically, the persistent disruption of homeostatic crosstalk by (1) ongoing pathogenic stimuli leads to (2) chronic inflammation, which is followed by (3) fibrosis and (4) remodeling to a PCN. If this step is unresolved, chronic tissue stress in the PCN results in an escape strategy, (5) CSES (FIGURE 1). To resolve the CSES and restore homeostasis, a heterogeneous fibroblast pool is recruited, which then undergoes EMT. Consequently, a heterogeneous pool of CAFs develops and undergoes (6) MET. These CAFs express epithelial markers, which facilitate their integration into the target tissue. This process explains why, later in carcinogenesis, cancer cells "appear" as an epithelial type (Please see figures 1 to 3 in [15]). This neoplastic transformation to a cancerous phenotype also explains the heterogeneity of tumors.
The third unanswered basic question in cancer biology pertains to the fundamentals of how metastases begin. Here, we demonstrated the further breakdown of homeostasis, in all its elegant complexities, to highlight the critical roles of pre-metastatic niches (PMNs) (FIGURE 2 and 3), together with cancer satellites, in transforming various cell compartments into Trojan horses enabling cancer cells and CAFs to successfully evade the immune system (FIGURE 4).
Whether the newly reported organelle structures called hemifusomes—which consist of two vesicles, one larger (~300 nm) and one smaller (~150 nm), partially fused via a thin, lens-shaped membrane (hemifusion diaphragm) [354]—have roles in cancer and/or metastasis is not currently known. However, we would not be surprised if such a role were discovered.
The findings described herein and in the supplement are summarized in FIGURE 4 to illustrate a complex but plausible explanation for how metastasis in epithelial cancer occurs in parallel with carcinogenesis after the pre-cancerous niche is transformed into pre-metastatic niches (PMNs), which are indispensable in the origin of metastasis. Eight heterogeneous cancer satellites develop, including Trojan horses responsible for immune evasion, alongside reciprocally affecting sequences, travel alone or in combination, far from the primary tumor. The fundamental prerequisites for metastasis are met as follows: (1) cancer cells and (2) CAFs migrate along the CXCL12 and fibronectin gradient; (3) cancer cells surrounded by CAFs are shielded from the immune system and consequently travel away from the primary cancer; (4) CXCL12 and Keratin 19 coat cancer cells; (5) platelets surround cancer cells and (6) CAFs, thus facilitating cancer spread; and (7) neutrophil extracellular traps shield cancer cells and (8) CAFs.
Not all questions are answered in detail because of the complexity of the processes involved. Of course, new questions will arise from new thinking on this topic. However, these findings explain not only the heterogeneity of clinical and experimental findings, but also the marginal benefits of existing anti-cancer treatments observed to date, in terms of patient survival measured in weeks or months, and why the cancer community remains far from developing anticancer treatments that measure patient survival in years with improcements in quality of life.
Metastasis, like carcinogenesis, occurs through a persistent disruption of homeostatic crosstalk, and the direction of shifts at any given point is critical
Cancer research must be less dogmatic and decrease emphasis on therapies that show significant p values, while placing greater emphasis on ameliorating the lives of patients with metastatic cancer. Much of the existing understanding of cancer biology, particularly metastasis, represents modest gains despite substantial effort. Therefore, as Johann Wolfgang von Goethe (1749–1832) stated, the content provided is just a part of a detour: “Science, as a whole, always moves away from life, and only returns to it through a detour” [English translation] [355].
Abbreviations
5-oxo-ETE (6E,8Z,11Z,14Z)-5-oxoicosa-6, 8,11, 14-tetraenoic acid; 12-HETE 12-hydroxyeicosatetraenoic acid; 20-HETE 20-hydroxyeicosatetraenoic acid, (5Z,8Z,11Z,14Z)-20-hydroxyicosa-5, 8,11, 14-tetraenoic acid; 20-OH-PGE2 20-hydroxy prostaglandin E2; 125IUDR 125I-iodo-2´-deoxyuridine; αSMAD alpha-smooth muscle actin; A1BG alpha-1-B glycoprotein; ALOX lipoxygenase, arachidonate lipoxygenase; ALOX5 5-lipoxygenase, 5-LOX, arachidonate 5-lipoxygenase; ALOX12 12-lipoxygenase, 12-LOX, 12S-LOX, arachidonate 12-lipoxygenase 12S type; AP1 activator protein 1; ARG1 arginase 1; ASGR1 asialoglycoprotein receptor 1; ASGR2 asialoglycoprotein receptor 2; B7-1 cluster of differentiation 80 (CD80); BIM Bcl-2 interacting mediator of cell death; CAF cancer-associated fibroblast; CC cancer cell; CCC cholangiocellular carcinoma; CD8+ T-cells MHC class I-restricted T-cells; CD47 cluster of differentiation 47; CD74 cluster of differentiation 74; CD80 cluster of differentiation 80 (B7-1); CD97 cluster of differentiation 97; cdc42 cell division control protein 42 homolog; cdk2 cyclin-dependent kinase 2; CIS carcinoma in situ; COMP cartilage oligomeric matrix protein; Cox cyclooxygenase; Cox-1 cyclooxygenase 1; Cox-2 cyclooxygenase 2; Cox-3: isoform of Cox-2 (therefore in brakes); CRC colorectal cancer; CS cancer satellite complex; CSES chronic stress escape strategy; CTC circulating tumor cell; CXC CC chemokine receptors; CXCL1 chemokine (C-X-C motif) ligand 1; CXCL12 chemokine CXCL12 (stromal cell-derived factor 1, SDF-1); CXCR4 C-X-C motif of chemokine receptor 4; CYP* cytochrome P450 isoforms; DOCK180 dedicator of cytokinesis; DMBA 7, 12-dimethylbenz [a]anthracene; E2F4/5 cytoplasmic complex of Smad3, retinoblastoma-like protein 1 (P107, RBL1), E2F4/5 and D-prostanoid (DP1); E-cadherin CAM 120/80 or epithelial cadherin, cadherin-1, epithelial cadherin; Egfl7 EGF-like domain multiple 7; EMT epithelial-mesenchymal transition; ERK2 extracellular signal-regulated kinase 2, mitogen-activated protein kinase 1, MAPK1; ESCC esophageal squamous cell carcinoma; EV extracellular vesicle; FADS2 fatty acid desaturase 2 (FADS2, ω-6-desaturase, D6D); FAK focal adhesion kinase; FAP fibroblast activation protein, surface gelatinase; FOXO3a forkhead box protein O3a; FSP-1 fibroblast specific protein-1; GPCR adhesion G protein-coupled receptor (high-mobility group box 1, HMGB1); HCC hepatocellular carcinoma; IFNγ interferon gamma; iNOS nitric oxide synthase; IL-6 interleukin 6; IL-8 interleukin 8; IL-β1 interleukin beta 1; IL-33: interleukin 33; JAM-1 junctional adhesion molecule-1; LPS lipopolysaccharide; LTA4 leukotriene A4, 4-[(2S,3S)-3-[(1E,3E,5Z,8Z)-tetradeca-1, 3,5, 8-tetraenyl]oxiran-2-yl]butanoic acid; LTB4 leukotriene B4, (5S,6Z,8E,10E,12R,14Z)-5, 12-dihydroxyicosa-6, 8,10, 14-tetraenoic acid; LTC4 leukotriene C4, (5S,6R,7E,9E,11Z,14Z)-6-[(2R)-2-[[(4S)-4-amino-4-carboxybutanoyl]amino]-3-(carboxymethylamino)-3-oxopropyl]sulfanyl-5-hydroxyicosa-7, 9,11, 14-tetraenoic acid; LTD4: leukotriene D4, (5S,6R,7E,9E,11Z,14Z)-6-[(2R)-2-amino-3-(carboxymethylamino)-3-oxopropyl]sulfanyl-5-hydroxyicosa-7, 9,11, 14-tetraenoic acid; LTE4 leukotriene E4, (5S,6R,7E,9E,11Z,14Z)-6-[(2R)-2-amino-2-carboxyethyl]sulfanyl-5-hydroxyicosa-7, 9,11, 14-tetraenoic acid; LOX lysyl oxidase; LOXL3 lysyl oxidase homolog 3; LRP1 low-density lipoprotein receptor-related protein 1; M1 MO classically activated M1 macrophages; anti-tumorigenic phenotype; M2 MO alternately activated M2 macrophages; pro-tumorigenic phenotype; M metastasis; MAPK1 mitogen-activated protein kinase 1, extracellular signal-regulated kinase 2, ERK2; MDA malondialdehyde, propanedial; MDSC myeloid-derived suppressor cells; MET mesenchymal epithelial transition; miR21: microRNA-21; MMP-1: matrix metalloproteinase 1; MMP-2 matrix metalloproteinase 2; MMP-7 matrix metalloproteinase 7; MMP-9 matrix metalloproteinase 9, gelatinase B; MO macrophage; MSCs mesenchymal stem cells; mTORc1 rapamycin complex 1; MZF1 myeloid zinc finger 1; N neutrophil; N1 anti-tumorigenic neutrophil phenotype; N2 pro-tumorigenic neutrophil phenotype; NCCCT normal cell to cancer cell transition; NETs neutrophil extracellular traps; NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells; NMII non-muscle myosin II; NRP1 neuropilin-1; Osm oncostatin-M; p107 retinoblastoma-like protein 1, RBL1; p120 catenin delta-1, protein 120; p130(cas) breast cancer anti-estrogen resistance protein 1, BCAR1; p300 protein 300 (p300-CBP coactivator family); P platelets (thrombocytes); PAR1 proteinase-activated receptor 1; PAI1 plasminogen activator inhibitor-1; PCN pre-cancerous niche; PDGF platelet-derived growth factor; PGD2 prostaglandin D2, (Z)-7-[(1R,2R,5S)-5-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-3-oxocyclopentyl]hept-5-enoic acid; PGE2 prostaglandin E2, (Z)-7-[(1R,2R,3R)-3-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]hept-5-enoic acid; PGFF2α prostaglandin F2 alpha, (Z)-7-[(1R,2R,3R,5S)-3, 5-dihydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]cyclopentyl]hept-5-enoic acid; PGG2 prostaglandin G2, (Z)-7-[(1S,4R,5R,6R)-5-[(E,3S)-3-hydroperoxyoct-1-enyl]-2, 3-dioxabicyclo [2.2.1]heptan-6-yl]hept-5-enoic acid; PGH2 prostaglandin H2, (Z)-7-[(1S,4R,5R,6R)-5-[(E,3S)-3-hydroxyoct-1-enyl]-2, 3-dioxabicyclo [2.2.1]heptan-6-yl]hept-5-enoic acid; PHA1 prolyl-4-hydroxylase A1; PI3K phosphatidylinositide 3-kinase; PMN-1 pre-metastatic niche 1; PMN-2 pre-metastatic niche 2; PMN-3 pre-metastatic niche 3; Pro-MMP-1 pro-matrix metalloproteinase 1; Pro-MMP-7 pro matrix metalloproteinase 7; Pro-MMP-9 pro-matrix metalloproteinase 9; PSAP prosaposin; PUMA BH3-only protein; Rac1 Ras-related C3 botulinum toxin substrate 1; Rho Ras homolog gene family, member A; ROCK Rho-associated coiled-coil kinase; ROS reactive oxygen species; S1P sphingosine-1-phosphate; SALL4 Sal-like protein 4; SNAIL zinc finger protein SNAI1; SOX [sex-determining region Y (Sry) box-containing] transcription factor family; SP1 specificity protein 1; SphK sphingosine kinase isoform; TAC tumor-associated cell; TAM tumor-associated macrophage; TAN tumor-associated neutrophil; TAP tumor-associated platelet; TGFβ transforming growth factor beta; TIMP-1 tissue inhibitor of metalloproteinases-1; TLR4 Toll-like receptor 4; TNFα tumor necrosis factor alpha; TReg CD4+ T-helper cells; TSP-1 thrombospondin-1; TXA2 thromboxane A2, (Z)-7-[(1S,2S,3R,5S)-3-[(E,3S)-3-hydroxyoct-1-enyl]-4, 6-dioxabicyclo [3.1.1]heptan-2-yl]hept-5-enoic acid; YAP yes associated protein.).
Acknowledgements
We are thankful to the countless scientists, clinicians, and individuals of various disciplines and professions from Asia, Europe, North and South America, and Australia for the personal exchanges during the last few decades. We acknowledge the intense and bias-free discussions, critical thinking, exchanges, and independent critical pro bono peer review provided to us by Prof. Dr. Marjan Slak Rupnik, Physiologist & Pathophysiologist, Vienna, Austria (ORCID: 0000-0002-3744-4882); Dr. Gudrun Schüler, Hematologist & Oncologist, Cottbus, Germany; Prof. Dr. Volkmar Weissig, Department of Pharmaceutical Sciences, Midwestern University, College of Pharmacy, Glendale, AZ, USA (ORCID: 0000-0002-6466-2367); José M Correia da Costa, National Institute of Health Ricardo Jorge, Porto, Portugal (ORCID: 0000-0001-6591-4303); Prof. Dr. Lúcio Lara Santos Surgical Oncology Department, Portuguese Institute of Oncology, Porto, Portugal (ORCID: 0000-0002-0521-5655); Prof. em. Dr. Masaharu Seno, Department of Cancer Stem Cell Engineering, Faculty of Interdisciplinary Science and Engineering in Health Systems, Okayama University, Okayama, Japan (ORCID: 0000-0001-8547-6259); Dr. Ray Perkins, New Liberty Proteomics Corporation, New Liberty, KY, USA; Dr. Sarah-Ellen Leonard, Chief Bioscience Constultant, New Liberty Proteomics Corporation, New Liberty, KY, USA; and Prof. em. Dr. Jose Florencio F Lapeña Jr., Otolaryngologist, World Association of Medical Editors, Manilla, the Philippines (ORCID: 0000-0002-5794-1878). Each of these individuals has approved this acknowledgment. The creation of the MS was supported by the Theodor-Billroth-Academy®, Munich, Germany, and Sacramento, CA, USA, its International Consortium of Research Excellence and the Cancer Metastases Research Fund.
Disclosure Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this manuscript. This manuscript contains original material that has been previously published and is appropriately cited. The opinions or assertions contained herein are those of the authors alone and are not to be construed as official or reflecting the views of the publisher or their employers. BB wrote the first MS draft. Both authors contributed to the content and approved the final version of the manuscript. The manuscript was created from our intellectual input only. No artificial intelligence software, e.g., ChatGPT or others, was used in the preparation of this manuscript.
References
| 1 | Billman GE: Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing
Principle of Physiology. Front Physiol 2020;11:200. https://doi.org/10.3389/fphys.2020.00200.
https://doi.org/10.3389/fphys.2020.00200 |
| 2 | McCrea PD, Gu D, Balda MS: Junctional music that the nucleus hears: cell-cell contact signaling
and the modulation of gene activity. Cold Spring Harb Perspect Biol 2009;1(4):a002923.
https://doi.org/10.1101/cshperspect.a002923.
https://doi.org/10.1101/cshperspect.a002923 |
| 3 | Brücher BL, Jamall IS: Cell-cell communication in the tumor microenvironment, carcinogenesis, and
anticancer treatment. Cell Physiol Biochem 2014;34(2):213-243. https://doi.org/10.1159/000362978.
https://doi.org/10.1159/000362978 |
| 4 | Brücher BLDM: The Erosion of Healthcare and Scientific Integrity: A Growing Concern. J Healthc
Leadersh 2025;17:23-43. https://doi.org/10.2147/JHL.S506767.
https://doi.org/10.2147/JHL.S506767 |
| 5 | Brücher BL, Jamall IS: Somatic Mutation Theory - Why it's Wrong for Most Cancers. Cell Physiol
Biochem 2016;38(5):1663-1680. https://doi.org/10.1159/000443106.
https://doi.org/10.1159/000443106 |
| 6 | Kumar V, Cotran RS, Robbins SL: Neoplasia. In: Basic Pathology, 6th edn, pp. 531-580. W.B.
Saunders Co., Philadelphia, St. Louis. 1997.
|
| 7 | Colburn NH, Bruegge WF, Bates JR, Gray RH, Rossen JD, Kelsey WH, Shimada T: Correlation of
anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells.
Cancer Res 1978;38(3):624-634. PMID: 626967.
|
| 8 | Bodell WJ, Banerjee MR: DNA repair in normal and preneoplastic mammary tissues. Cancer Res
1978;38(3):736-740. PMID: 626977.
|
| 9 | Noble D, Joyner M: The physiology of evolution. J Physiol 2024;602(11):2361-2365.
https://doi.org/10.1113/JP284432.
https://doi.org/10.1113/JP284432 |
| 10 | Noble D: It's time to admit that genes are not the blueprint for life. Nature
2024;626(7998):254-255. https://doi.org/10.1038/d41586-024-00327-x.
https://doi.org/10.1038/d41586-024-00327-x |
| 11 | Noble R, Noble D: Understanding living systems. Cambridge University Press. 2023. ISBN
978-1-00-927736-5.
|
| 12 | Grosjean M: $3.8B Investment in Human Genome Project Drove $796B in Economic Impact Creating 310,
000 Jobs and Launching the Genomic Revolution. Apr 29, 2015. Website:
https://ec.europa.eu/futurium/en/content/38b-investment-human-genome-project-drove-796b-economic-impact-creating-310000-jobs-and.html.
|
| 13 | Wadman M: Economic return from Human Genome Project grows. Nature June 12, 2013.
https://doi.org/10.1038/nature.2013.13187.
https://doi.org/10.1038/nature.2013.13187 |
| 14 | Brücher BL, Jamall IS: Epistemology of the origin of cancer: a new paradigm. BMC Cancer
2014;14:331. https://doi.org/10.1186/1471-2407-14-331.
https://doi.org/10.1186/1471-2407-14-331 |
| 15 | Brücher BLDM, Jamall IS: Epistemology of the Origin of Cancer II: Fibroblasts Are the First Cells
to Undergo Neoplastic Transformation. Cell Physiol Biochem 2023;57(6):512-537.
https://doi.org/10.33594/000000672.
https://doi.org/10.33594/000000672 |
| 16 | Brücher BLDM, Jamall IS: Chronic inflammation evoked by pathogenic stimulus during carcinogenesis.
4open 2019;2(8):1-22. https://doi.org/10.1051/fopen/2018006.
https://doi.org/10.1051/fopen/2018006 |
| 17 | Brücher BL, Jamall IS: Precancerous niche (PCN), a product of fibrosis with remodeling by
incessant chronic inflammation. 4open 2019;2(11):1-21. https://doi.org/10.1051/fopen/2018009.
https://doi.org/10.1051/fopen/2018009 |
| 18 | Brücher BLDM, Lang F, Jamall IS: NF-κB signaling and crosstalk during carcinogenesis. 4open
2019;2(13):1-35. https://doi.org/10.1051/fopen/2019010.
https://doi.org/10.1051/fopen/2019010 |
| 19 | Brücher BLDM, Jamall IS: Synopsis: Special Issue on "Disruption of signaling homeostasis induced
crosstalk in the carcinogenesis paradigm Epistemology of the origin of cancer". 4open
2019;2(28):1-30. https://doi.org/10.1051/fopen/2019023.
https://doi.org/10.1051/fopen/2019023 |
| 20 | Fidler JJ: Metastasis: quantitative analysis of distribution and fate of tumor embolilabeled with
125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst 1970;45(4):773-782. PMID: 5513503.
|
| 21 | Earle H: On the influence of local irritation in the production of diseases resembling cancer and
other morbid structur. Med Chir Trans 1823;12(Pt2):268-295.
https://doi.org/10.1177/09595287230120p202.
https://doi.org/10.1177/09595287230120P202 |
| 22 | Recamier JC: Recherches sur le traitment du cancer, par la compression méthodique simple ou
combinée, sur l_histoire générale de la méme maladie. Chez Gabon, Libraire-Editeur, Paris. 1829.
|
| 23 | Wilder RJ: The historical development of the concept of metastasis. J Mt Sinai Hosp N Y
1956;23(5):728-734. PMID: 13377138.
|
| 24 | Ashworth TR: A case of cancer in which cells similar to those in the tumors were seen in the blood
after death. Aust Med J 1869;14(3):146-149. CRID: 1573105976092422016. Article available at the
website of the University of Melbourne:
https://collections.mdhs.unimelb.edu.au/objects/27718/photocopied-article-a-case-of-cancer-in-which-cells-similar-to-whose-in-the-tumours-were-seen-in-the-blood-after-death.
|
| 25 | Goldmann EE: Anatomische Untersuchungen über die Verbreitungswege bösartiger Geschwülste. Beitr z
klin Chir 1897;18:595. Website: https://wellcomecollection.org/works/fn5v944u/items.
|
| 26 | Langley RR, Fidler IJ: The seed and soil hypothesis revisited-the role of tumor-stroma
interactions in metastasis to different organs. Int J Cancer 2011;128(11):2527-2535.
https://doi.org/10.1002/ijc.26031.
https://doi.org/10.1002/ijc.26031 |
| 27 | Zhe X, Cher ML, Bonfil RD: Circulating tumor cells: finding the needle in the haystack. Am J
Cancer Res 2011;1(6):740-751. PMID: 22016824.
|
| 28 | Fidler IJ: Cancer biology is the foundation for therapy. Cancer Biol Ther 2005;4(9):1036-1039.
https://doi.org/10.4161/cbt.4.9.2111.
https://doi.org/10.4161/cbt.4.9.2111 |
| 29 | Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B: Mechanisms of human tumor
metastasis studied in patients with peritoneovenous shunts. Cancer Res 1984;44(8):3584-3592. PMID:
6744281.
|
| 30 | Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B: Clinicopathological observations
on metastasis in man studied in patients treated with peritoneovenous shunts. Br Med J (Clin Res Ed)
1984;288(6419):749-751. https://doi.org/10.1136/bmj.288.6419.749.
https://doi.org/10.1136/bmj.288.6419.749 |
| 31 | Ikeda T, Arao K: Critical observation on the atypical hypernephroma with widespread intravascular
and intracardial tumor-embolism. Gan 1951;42(2-4):311-313. PMID: 14906581.
|
| 32 | Paterlini-Brechot P, Benali NL: Circulating tumor cells (CTC) detection: clinical impact and
future directions. Cancer Lett 2007;253(2):180-204. https://doi.org/10.1016/j.canlet.2006.12.014.
https://doi.org/10.1016/j.canlet.2006.12.014 |
| 33 | Davis HH, Neis DD: Distribution of axillary lymph node metastases in carcinoma of the breast. Ann
Surg 1952;136(4):604-609. PMID: 12986643.
|
| 34 | Luo M, Lin X, Hao D, Shen KW, Wu W, Wang L, Ruan S, Zhou J: Incidence and risk factors of lymph
node metastasis in breast cancer patients without preoperative chemoradiotherapy and neoadjuvant
therapy: analysis of SEER data. Gland Surg 2023;12(11):1508-1524.
https://doi.org/10.21037/gs-23-258.
https://doi.org/10.21037/gs-23-258 |
| 35 | Sopik V, Narod SA: The relationship between tumour size, nodal status and distant metastases: on
the origins of breast cancer. Breast Cancer Res Treat 2018;170(3):647-656.
https://doi.org/10.1007/s10549-018-4796-9.
https://doi.org/10.1007/s10549-018-4796-9 |
| 36 | Plenk HP, Sorenson FM, Eichwald EJ: Time interval between tumor inoculation and metastatic spread
to lymph nodes. Cancer Res 1954;14(8):580-581. PMID: 13199801.
|
| 37 | Romsdahl MD, Chu EW, Hume R, Smith RR: The time of metastasis and release of circulating tumor
cells as determined in an experimental system. Cancer 1961;14:883-888.
https://doi.org/10.1002/1097-0142(199007/08)14:4<883::aid-cncr2820140426>3.0.co;2-8.
https://doi.org/10.1002/1097-0142(199007/08)14:4<883::AID-CNCR2820140426>3.0.CO;2-8 |
| 38 | Stelzner F: The emergence of generalized carcinosis. Chirurg 1948;19(5):203-210. PMID: 18871014.
|
| 39 | Oshikiri T, Numasaki H, Oguma J, Toh Y, Watanabe M, Muto M, Kakeji Y, Doki Y: Is Thoracic Duct
Resection Necessary for Esophageal Squamous Cell Carcinoma Patients Treated with Neoadjuvant
Chemoradiotherapy? A Propensity-Matched Analysis Based on the Comprehensive Registry of Esophageal
Cancer in Japan. Ann Surg Oncol 2022;30(5):2691-2698. https://doi.org/10.1245/s10434-022-12891-5.
https://doi.org/10.1245/s10434-022-12891-5 |
| 40 | Oshikiri T, Numasaki H, Oguma J, Toh Y, Watanabe M, Muto M, Kakeji Y, Doki Y: Prognosis of
Patients with Esophageal Carcinoma following Routine Thoracic Duct Resection: A Propensity-matched
Analysis of 12, 237 Patients based on the Comprehensive Registry of Esophageal Cancer in Japan. Ann
Surg 2023;277(5):e1018-e1025. https://doi.org/10.1097/SLA.0000000000005340.
https://doi.org/10.1097/SLA.0000000000005340 |
| 41 | Decker P, Mosimann R: [The surgical treatment of cancer of the rectum and lower sigmoid]. Helv
Chir Acta 1953;20(1):1-15. PMID: 13060853.
|
| 42 | Guleke N: Die bösartigen Geschwülste des Dickdarms und Mastdarms. Ferdinand Enke Verlag,
Stuttgart. 1957.
https://doi.org/10.1097/00000658-195711000-00013 |
| 43 | Coller FA, Kay EB, Macintyre RS: Regional lymphatic metastases of carcinoma of the colon. Ann Surg
1941;114(1):56-67. https://doi.org/10.1097/00000658-194107000-00007.
https://doi.org/10.1097/00000658-194107000-00007 |
| 44 | Clifton JA, Philipp RJ, Ludovic E, Fowler WM: Bone marrow and carcinoma of the prostate. Am J Med
Sci 1952; 224(2):121-130. https://doi.org/10.1097/00000441-195208000-00001.
https://doi.org/10.1097/00000441-195208000-00001 |
| 45 | Shah SA, Gallagher BM, Sands H: Lymphoscintigraphy of human colorectal carcinoma metastases in
athymic mice by use of radioiodinated B72.3 monoclonal antibody. J Natl Cancer Inst
1987;78(6):1069-1077. PMID: 3473248.
|
| 46 | Fuchs E: Das Sarkom des Uvealtractus. Habilitationsschrift. Wilhelm Braunmüller, Wien und Graefe's
Arch Ophthalmo 1882;l,:XII. Wien. 1882. Book available here:
https://books.google.de/books?id=0JFL4mz_bHIC&printsec=frontcover&hl=de&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false.
|
| 47 | Paget S: The distribution of secondary growth in cancer of the breast. Lancet
1899;133(3421):571-573. https://doi.org/10.1016/S0140-6736(00)49915-0.
https://doi.org/10.1016/S0140-6736(00)49915-0 |
| 48 | Ewing J: Neoplastics. 3rd. Philadelphia: Saunders; 1928. Metastasis; pp. 77-89.
|
| 49 | Walker AS, Zwintscher NP, Johnson EK, Maykel JA, Stojadinovic A, Nissan A, Avital I, Brücher BL,
Steele SR: Future directions for monitoring treatment response in colorectal cancer. J Cancer
2014;5(1):44-57. https://doi.org/10.7150/jca.7809.
https://doi.org/10.7150/jca.7809 |
| 50 | Königsrainer I, Zieker D, Beckert S, von Weyhern C, Löb S, Falch C, Brücher BL, Königsrainer A,
Glatzle J: Local peritonectomy highly attracts free floating intraperitoneal colorectal tumour cells
in a rat model. Cell Physiol Biochem 2009;23(4-6):371-378. https://doi.org/10.1159/000218183.
https://doi.org/10.1159/000218183 |
| 51 | Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H,
Swanton C, Nowak MA, Elledge SJ, Jain RK: Origins of lymphatic and distant metastases in human
colorectal cancer. Science 2017;357(6346):55-60. https://doi.org/10.1126/science.aai8515.
https://doi.org/10.1126/science.aai8515 |
| 52 | Gu J, Li X, Zhao L, Yang Y, Xue C, Gao Y, Li J, Han Q, Sun Z, Bai C, Zhao RC: The role of PKM2
nuclear translocation in the constant activation of the NF-κB signaling pathway in cancer-associated
fibroblasts. Cell Death Dis 2021;12(4):291. https://doi.org/10.1038/s41419-021-03579-x.
https://doi.org/10.1038/s41419-021-03579-x |
| 53 | Rubinstein-Achiasaf L, Morein D, Ben-Yaakov H, Liubomirski Y, Meshel T, Elbaz E, Dorot O, Pichinuk
E, Gershovits M, Weil M, Ben-Baruch A: Persistent Inflammatory Stimulation Drives the Conversion of
MSCs to Inflammatory CAFs That Promote Pro-Metastatic Characteristics in Breast Cancer Cells.
Cancers (Basel) 2021;13(6):1472. https://doi.org/10.3390/cancers13061472.
https://doi.org/10.3390/cancers13061472 |
| 54 | Petersen OW, Lind Nielsen H, Gudjonsson T, Villadsen R, Rønnov-Jessen L, Bissell MJ: The
plasticity of human breast carcinoma cells is more than epithelial to mesenchymal conversion. Breast
Cancer Res 2001;3(4):213-217. https://doi.org/10.1186/bcr298.
https://doi.org/10.1186/bcr298 |
| 55 | Wen S, Niu Y, Yeh S, Chang C: BM-MSCs promote prostate cancer progression via the conversion of
normal fibroblasts to cancer-associated fibroblasts. Int J Oncol 2015;47(2):719-727.
https://doi.org/10.3892/ijo.2015.3060.
https://doi.org/10.3892/ijo.2015.3060 |
| 56 | Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn
SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT,
Crawford JM, Clevers H, Park Y, Tuveson DA: Distinct populations of inflammatory fibroblasts and
myofibroblasts in pancreatic cancer. J Exp Med 2017;214(3):579-596.
https://doi.org/10.1084/jem.20162024.
https://doi.org/10.1084/jem.20162024 |
| 57 | Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C,
Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massagué J, Sancho E, Batlle E: Dependency
of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer
Cell 2012;22(5):571-584. https://doi.org/10.1016/j.ccr.2012.08.013.
https://doi.org/10.1016/j.ccr.2012.08.013 |
| 58 | Kim YJ, Lee HS, Kim D, Byun HK, Koom WS, Koh WG: Bilayer 3D co-culture platform inducing the
differentiation of normal fibroblasts into cancer-associated fibroblast like cells: New in vitro
source to obtain cancer-associated fibroblasts. Bioeng Transl Med 2024;10(1):e10708.
https://doi.org/10.1002/btm2.10708.
https://doi.org/10.1002/btm2.10708 |
| 59 | Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, Wang AW, Yang YA, Tam L, Caothien
R, Roose-Girma M, Modrusan Z, Arron JR, Bourgon R, Müller S, Turley SJ: Cross-tissue organization of
the fibroblast lineage. Nature 2021;593(7860):575-579. https://doi.org/10.1038/s41586-021-03549-5.
https://doi.org/10.1038/s41586-021-03549-5 |
| 60 | Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D:
Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res
2008;68(11):4331-4229. https://doi.org/10.1158/0008-5472.CAN-08-0943.
https://doi.org/10.1158/0008-5472.CAN-08-0943 |
| 61 | McDonald LT, LaRue AC: Hematopoietic stem cell derived carcinoma-associated fibroblasts: a novel
origin. Int J Clin Exp Pathol 2012;5(9):863-873. PMID: 23119103.
|
| 62 | Miyazaki Y, Oda T, Mori N, Kida YS: Adipose-derived mesenchymal stem cells differentiate into
pancreatic cancer-associated fibroblasts in vitro. FEBS Open Bio 2020;10(11):2268-2281.
https://doi.org/10.1002/2211-5463.12976.
https://doi.org/10.1002/2211-5463.12976 |
| 63 | Weber CE, Kothari AN, Wai PY, Li NY, Driver J, Zapf MA, Franzen CA, Gupta GN, Osipo C, Zlobin A,
Syn WK, Zhang J, Kuo PC, Mi Z: Osteopontin mediates an MZF1-TGF-beta1-dependent transformation of
mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene
2015;34(37):4821-4833. https://doi.org/10.1038/onc.2014.410.
https://doi.org/10.1038/onc.2014.410 |
| 64 | Mi Z, Guo H, Wai PY, Gao C, Wei J, Kuo PC: Differential osteopontin expression in phenotypically
distinct subclones of murine breast cancer cells mediates metastatic behavior. J Biol Chem
2004;279(45):46659-46667. https://doi.org/10.1074/jbc.M407952200.
https://doi.org/10.1074/jbc.M407952200 |
| 65 | Kieffer Y, Hocine HR, Gentric G, Pelon F, Bernard C, Bourachot B, Lameiras S, Albergante L,
Bonneau C, Guyard A, Tarte K, Zinovyev A, Baulande S, Zalcman G, Vincent-Salomon A, Mechta-Grigoriou
F: Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer.
Cancer Discov 2020;10(9):1330-1351. https://doi.org/10.1158/2159-8290.CD-19-1384.
https://doi.org/10.1158/2159-8290.CD-19-1384 |
| 66 | Galbo PM Jr, Zang X, Zheng D: Molecular Features of Cancer-associated Fibroblast Subtypes and
their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin Cancer Res
2021;27(9):2636-2647. https://doi.org/10.1158/1078-0432.CCR-20-4226.
https://doi.org/10.1158/1078-0432.CCR-20-4226 |
| 67 | Rizzolio S, Giordano S, Corso S: The importance of being CAFs (in cancer resistance to targeted
therapies). J Exp Clin Cancer Res 2022;41(1):319. https://doi.org/10.1186/s13046-022-02524-w.
https://doi.org/10.1186/s13046-022-02524-w |
| 68 | Nishimura CD, Corrigan D, Zheng XY, Galbo PM Jr, Wang S, Liu Y, Wei Y, Suo L, Cui W, Mercado N,
Zheng D, Zhang CC, Zang X: TOP CAR with TMIGD2 as a safe and effective costimulatory domain in CAR
cells treating human solid tumors. Sci Adv 2024;10(19):eadk1857.
https://doi.org/10.1126/sciadv.adk1857.
https://doi.org/10.1126/sciadv.adk1857 |
| 69 | Xia H, Feng P, Wang W, Gong Z, Ran J, Lu P, Dai B: Development of a Cancer-associated Fibroblast
Signature for Evaluating Immunotherapy Response and Prognosis of Hepatocellular Carcinoma. Curr Med
Chem 2024;32(30):2246-6665. https://doi.org/10.2174/0109298673322216240711113419.
https://doi.org/10.2174/0109298673322216240711113419 |
| 70 | Wen S, Niu Y, Yeh S, Chang C: BM-MSCs promote prostate cancer progression via the conversion of
normal fibroblasts to cancer-associated fibroblasts. Int J Oncol 2015;47(2):719-27.
https://doi.org/10.3892/ijo.2015.3060.
https://doi.org/10.3892/ijo.2015.3060 |
| 71 | Zhu H, Guo S, Zhang Y, Yin J, Yin W, Tao S, Wang Y, Zhang C: Proton-sensing GPCR-YAP Signalling
Promotes Cancer-associated Fibroblast Activation of Mesenchymal Stem Cells. Int J Biol Sci
2016;12(4):389-396. https://doi.org/10.7150/ijbs.13688.
https://doi.org/10.7150/ijbs.13688 |
| 72 | Kato M, Placencio-Hickok VR, Madhav A, Haldar S, Tripathi M, Billet S, Mishra R, Smith B,
Rohena-Rivera K, Agarwal P, Duong F, Angara B, Hickok D, Liu Z, Bhowmick NA: Heterogeneous
cancer-associated fibroblast population potentiates neuroendocrine differentiation and castrate
resistance in a CD105-dependent manner. Oncogene 2019;38(5):716-730.
https://doi.org/10.1038/s41388-018-0461-3.
https://doi.org/10.1038/s41388-018-0461-3 |
| 73 | Gong Y, Chippada-Venkata UD, Galsky MD, Huang J, Oh WK: Elevated circulating tissue inhibitor of
metalloproteinase 1 (TIMP-1) levels are associated with neuroendocrine differentiation in castration
resistant prostate cancer. Prostate 2015;75(6):616-627. https://doi.org/10.1002/pros.22945.
https://doi.org/10.1002/pros.22945 |
| 74 | Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G,
Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee
EM, Califano A, Robson P, Tuveson DA: Cross-Species Single-Cell Analysis of Pancreatic Ductal
Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov
2019;9(8):1102-1123. https://doi.org/10.1158/2159-8290.CD-19-0094.
https://doi.org/10.1158/2159-8290.CD-19-0094 |
| 75 | Procopio MG, Laszlo C, Al Labban D, Kim DE, Bordignon P, Jo SH, Goruppi S, Menietti E, Ostano P,
Ala U, Provero P, Hoetzenecker W, Neel V, Kilarski WW, Swartz MA, Brisken C, Lefort K, Dotto GP:
Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol
2015;17(9):1193-204. https://doi.org/10.1038/ncb3228.
https://doi.org/10.1038/ncb3228 |
| 76 | Arandkar S, Furth N, Elisha Y, Nataraj NB, van der Kuip H, Yarden Y, Aulitzky W, Ulitsky I, Geiger
B, Oren M: Altered p53 functionality in cancer-associated fibroblasts contributes to their
cancer-supporting features. Proc Natl Acad Sci USA 2018;115(25):6410-6415.
https://doi.org/10.1073/pnas.1719076115.
https://doi.org/10.1073/pnas.1719076115 |
| 77 | Vennin C, Mélénec P, Rouet R, Nobis M, Cazet AS, Murphy KJ, Herrmann D, Reed DA, Lucas MC, Warren
SC, Elgundi Z, Pinese M, Kalna G, Roden D, Samuel M, Zaratzian A, Grey ST, Da Silva A, Leung W;
Australian Pancreatic Genome Initiative (APGI); Mathivanan S, Wang Y, Braithwaite AW, Christ D,
Benda A, Parkin A, Phillips PA, Whitelock JM, Gill AJ, Sansom OJ, Croucher DR, Parker BL, Pajic M,
Morton JP, Cox TR, Timpson P: CAF hierarchy driven by pancreatic cancer cell p53-status creates a
pro-metastatic and chemoresistant environment via perlecan. Nat Commun 2019;10(1):3637.
https://doi.org/10.1038/s41467-019-10968-6.
https://doi.org/10.1038/s41467-019-10968-6 |
| 78 | Zhu H, Guo S, Zhang Y, Yin J, Yin W, Tao S, Wang Y, Zhang C: Proton-sensing GPCR-YAP Signalling
Promotes Cancer-associated Fibroblast Activation of Mesenchymal Stem Cells. Int J Biol Sci
2016;12(4):389-396. https://doi.org/10.7150/ijbs.13688.
https://doi.org/10.7150/ijbs.13688 |
| 79 | Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P,
Moeendarbary E, Charras G, Sahai E: Mechanotransduction and YAP-dependent matrix remodelling is
required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol
2013;15(6):637-646. https://doi.org/10.1038/ncb2756.
https://doi.org/10.1038/ncb2756 |
| 80 | Garcia PE, Adoumie M, Kim EC, Zhang Y, Scales MK, El-Tawil YS, Shaikh AZ, Wen HJ, Bednar F, Allen
BL, Wellik DM, Crawford HC, Pasca di Magliano M: Differential Contribution of Pancreatic Fibroblast
Subsets to the Pancreatic Cancer Stroma. Cell Mol Gastroenterol Hepatol 2020;10(3):581-599.
https://doi.org/10.1016/j.jcmgh.2020.05.004.
https://doi.org/10.1016/j.jcmgh.2020.05.004 |
| 81 | Shen T, Li Y, Zhu S, Yu J, Zhang B, Chen X, Zhang Z, Ma Y, Niu Y, Shang Z: YAP1 plays a key role
of the conversion of normal fibroblasts into cancer-associated fibroblasts that contribute to
prostate cancer progression. J Exp Clin Cancer Res 2020;39(1):36.
https://doi.org/10.1186/s13046-020-1542-z.
https://doi.org/10.1186/s13046-020-1542-z |
| 82 | Naktubtim C, Payuhakrit W, Uttarawichien T, Hassametto A, Suwannalert P: YAP, a novel target
regulates F-actin rearrangement-associated CAFs transformation and promotes colorectal cancer cell
progression. Biomed Pharmacother 2022;155:113757. https://doi.org/10.1016/j.biopha.2022.113757.
https://doi.org/10.1016/j.biopha.2022.113757 |
| 83 | Ferrari N, Ranftl R, Chicherova I, Slaven ND, Moeendarbary E, Farrugia AJ, Lam M, Semiannikova M,
Westergaard MCW, Tchou J, Magnani L, Calvo F: Dickkopf-3 links HSF1 and YAP/TAZ signalling to
control aggressive behaviours in cancer-associated fibroblasts. Nat Commun 2019;10(1):130.
https://doi.org/10.1038/s41467-018-07987-0.
https://doi.org/10.1038/s41467-018-07987-0 |
| 84 | Foster CT, Gualdrini F, Treisman R: Mutual dependence of the MRTF-SRF and YAP-TEAD pathways in
cancer-associated fibroblasts is indirect and mediated by cytoskeletal dynamics. Genes Dev
2017;31(23-24):2361-2375. https://doi.org/10.1101/gad.304501.117.
https://doi.org/10.1101/gad.304501.117 |
| 85 | Zhang K, Grither WR, Van Hove S, Biswas H, Ponik SM, Eliceiri KW, Keely PJ, Longmore GD:
Mechanical signals regulate and activate SNAIL1 protein to control the fibrogenic response of
cancer-associated fibroblasts. J Cell Sci 2016;129(10):1989-2002.
https://doi.org/10.1242/jcs.180539. Correction in https://doi.org/10.1242/jcs.232348.
https://doi.org/10.1242/jcs.180539 |
| 86 | Pinnell SR, Martin GR: The cross-linking of collagen and elastin: enzymatic conversion of lysine
in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc
Natl Acad Sci USA 1968;61(2):708-716. https://doi.org/10.1073/pnas.61.2.70.
https://doi.org/10.1073/pnas.61.2.708 |
| 87 | Kagan HM, Williams MA, Calaman SD, Berkowitz EM: Histone H1 is a substrate for lysyl oxidase and
contains endogenous sodium borotritide-reducible residues. Biochem Biophys Res Commun
1983;115(1):186-192. https://doi.org/10.1016/0006-291X(83)90987-7.
https://doi.org/10.1016/0006-291X(83)90987-7 |
| 88 | Kagan HM: Characterization and regulation of lysyl oxidase. In: Biology of the Extracellular
Matrix, ed. by Mecham RP (ed), Regulation of Matrix Accumulation, Academic Press, Orlando, FL, 1986.
eBook ISBN: 9780323149006.
https://doi.org/10.1016/B978-0-12-487425-1.50013-9 |
| 89 | Panchenko MV, Stetler-Stevenson WG, Trubetskoy OV, Gacheru SN, Kagan HM: Metalloproteinase
activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of
procollagen C-proteinase. J Biol Chem 1996;271(12):7113-7119.
https://doi.org/10.1074/jbc.271.12.7113.
https://doi.org/10.1074/jbc.271.12.7113 |
| 90 | Smith-Mungo LI, Kagan HM: Lysyl oxidase: properties, regulation and multiple functions in biology.
Matrix Biol 1998;16(7):387-398. https://doi.org/10.1016/S0945-053X(98)90012-9.
https://doi.org/10.1016/S0945-053X(98)90012-9 |
| 91 | Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman
M, Neufeld G: Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo.
Cancer Res 2003;63(7):1657-1666. PMID: 12670920.
|
| 92 | Chen B, Liu X, Yu P, Xie F, Kwan JSH, Chan WN, Fang C, Zhang J, Cheung AHK, Chow C, Leung GWM,
Leung KT, Shi S, Zhang B, Wang S, Xu D, Fu K, Wong CC, Wu WKK, Chan MWY, Tang PMK, Tsang CM, Lo KW,
Tse GMK, Yu J, To KF, Kang W: H. pylori-induced NF-κB-PIEZO1-YAP1-CTGF axis drives gastric cancer
progression and cancer-associated fibroblast-mediated tumour microenvironment remodelling. Clin
Transl Med 2023;13(11):e1481. https://doi.org/10.1002/ctm2.1481.
https://doi.org/10.1002/ctm2.1481 |
| 93 | Fujii Y, Yoshihashi K, Suzuki H, Tsutsumi S, Mutoh H, Maeda S, Yamagata Y, Seto Y, Aburatani H,
Hatakeyama M: CDX1 confers intestinal phenotype on gastric epithelial cells via induction of
stemness-associated reprogramming factors SALL4 and KLF5. Proc Natl Acad Sci
USA;109(50):20584-20589. https://doi.org/10.1073/pnas.120865110.
https://doi.org/10.1073/pnas.1208651109 |
| 94 | Saberi S, Esmaeili M, Tashakoripour M, Eshagh Hosseini M, Baharvand H, Mohammadi M: Infection with
a hypervirulent strain of Helicobacter pylori primes gastric cells toward intestinal
transdifferentiation. Microb Pathog 2022;162:105353. https://doi.org/10.1016/j.micpath.2021.105353.
https://doi.org/10.1016/j.micpath.2021.105353 |
| 95 | Liu CJ, Wang YK, Kuo FC, Hsu WH, Yu FJ, Hsieh S, Tai MH, Wu DC, Kuo CH: Helicobacter pylori
Infection-Induced Hepatoma-Derived Growth Factor Regulates the Differentiation of Human Mesenchymal
Stem Cells to Myofibroblast-Like Cells. Cancers (Basel) 2018;10(12):479.
https://doi.org/10.3390/cancers10120479.
https://doi.org/10.3390/cancers10120479 |
| 96 | Liao Z, Tan ZW, Zhu P, Tan NS: Cancer-associated fibroblasts in tumor microenvironment -
Accomplices in tumor malignancy. Cell Immunol 2019;343:103729.
https://doi.org/10.1016/j.cellimm.2017.12.003.
https://doi.org/10.1016/j.cellimm.2017.12.003 |
| 97 | Simon T, Salhia B: Cancer-Associated Fibroblast Subpopulations With Diverse and Dynamic Roles in
the Tumor Microenvironment. Mol Cancer Res 2022;20(2):183-192.
https://doi.org/10.1158/1541-7786.MCR-21-0282.
https://doi.org/10.1158/1541-7786.MCR-21-0282 |
| 98 | Wu NC, Quevedo R, Nurse M, Hezaveh K, Liu H, Sun F, Muffat J, Sun Y, Simmons CA, McGaha TL, Prinos
P, Arrowsmith CH, Ailles L, D'Arcangelo E, McGuigan AP: The use of a multi-metric readout screen to
identify EHMT2/G9a-inhibition as a modulator of cancer-associated fibroblast activation state.
Biomaterials 2025;314:122879. https://doi.org/10.1016/j.biomaterials.2024.122879.
https://doi.org/10.1016/j.biomaterials.2024.122879 |
| 99 | Zhang Q, Chai S, Wang W, Wan C, Zhang F, Li Y, Wang F: Macrophages activate mesenchymal stem cells
to acquire cancer-associated fibroblast-like features resulting in gastric epithelial cell lesions
and malignant transformation in vitro. Oncol Lett 2019;17(1):747-756.
https://doi.org/10.3892/ol.2018.9703.
https://doi.org/10.3892/ol.2018.9703 |
| 100 | Lauffenburger DA, Horwitz AF: Cell migration: a physically integrated molecular process. Cell
1996;84(3):359-369. https://doi.org/10.1016/s0092-8674(00)81280-5.
https://doi.org/10.1016/S0092-8674(00)81280-5 |
| 101 | Bünning E: Fifty years of research in the wake of Wilhelm Pfeffer. Ann Rev Plant Physiol
1977;28:1-22. https://doi.org/10.1146/annurev.pp.28.060177.000245.
https://doi.org/10.1146/annurev.pp.28.060177.000245 |
| 102 | Vercruysse, E., Brückner, D.B., Gómez-González, M., Remson A, Luciano M, Kalukula Y, Rossetti L,
Trpat X, Hannezo E, Gabriele S: Geometry-driven migration efficiency of autonomous epithelial cell
clusters. Nature Phys 2024;20:1492-1500. https://doi.org/10.1038/s41567-024-02532-x
https://doi.org/10.1038/s41567-024-02532-x |
| 103 | Folkman J, Moscona A: Role of cell shape in growth control. Nature 1978;273(5661):345-349.
https://doi.org/10.1038/273345a0.
https://doi.org/10.1038/273345a0 |
| 104 | Gadasi H, Oplatka A, Lamed R, Hochberg A, Lowe W: Possible uncoupling of the mechanochemical
process in the actomyosin system by covalent crosslinking of F-actin. Biochim Biophys Acta
1974;333(1):161-168. https://doi.org/10.1016/0005-2728(74)90172-8.
https://doi.org/10.1016/0005-2728(74)90172-8 |
| 105 | Parsons DW: The morphology and ultrastructure of tension receptors in the walking legs of the
crab, Carcinus maenas. Cell Tissue Res 1980;211(1):139-149. https://doi.org/10.1007/BF00233729.
https://doi.org/10.1007/BF00233729 |
| 106 | Huang S, Ingber DE: Cell tension, matrix mechanics, and cancer development. Cancer Cell
2005;8(3):175-176. https://doi.org/10.1016/j.ccr.2005.08.009.
https://doi.org/10.1016/j.ccr.2005.08.009 |
| 107 | Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS,
Dembo M, Boettiger D, Hammer DA, Weaver VM: Tensional homeostasis and the malignant phenotype.
Cancer Cell 2005;8(3):241-254. https://doi.org/10.1016/j.ccr.2005.08.010.
https://doi.org/10.1016/j.ccr.2005.08.010 |
| 108 | Saez A, Ghibaudo M, Buguin A, Silberzan P, Ladoux B: Rigidity-driven growth and migration of
epithelial cells on microstructured anisotropic substrates. Proc Natl Acad Sci USA
2007;104(20):8281-8286. https://doi.org/10.1073/pnas.0702259104.
https://doi.org/10.1073/pnas.0702259104 |
| 109 | Lambert RA: The effect of dilution of plasma medium on the growth and fat accumulation of cells in
tissue cultures. J Exp Med 1914;19(4):398-405. https://doi.org/10.1084/jem.19.4.398.
https://doi.org/10.1084/jem.19.4.398 |
| 110 | Schwab A, Reinhardt J, Schneider SW, Gassner B, Schuricht B: K(+) channel-dependent migration of
fibroblasts and human melanoma cells. Cell Physiol Biochem 1999;9(3):126-132.
https://doi.org/10.1159/000016309.
https://doi.org/10.1159/000016309 |
| 111 | Kozal KA, Jarosiewicz M, Szustka AE, Mądrecki M, Jankowski M, Jóźwiak PJ, Krześlak A: HIF-O-Glcnac
Axis - Implications for Breast Cancer Metastasis. Cell Physiol Biochem 2025;59(3):404-418.
https://doi.org/10.33594/000000782.
https://doi.org/10.33594/000000782 |
| 112 | Bonnert JT: Evidence for the formation of cell aggregates by chemotaxis in the development of the
slime mold Dictyostelium discoideum. J Exp Zool 1947;106(1):1-26.
https://doi.org/10.1002/jez.1401060102.
https://doi.org/10.1002/jez.1401060102 |
| 113 | Engelmann TW: Neue Methode zur Untersuchung der Sauerstoffausscheidung pflanzlicher und
thierischer Organismen. Pflüger Arch 1881;25:285-292. https://doi.org/10.1007/BF01661982.
https://doi.org/10.1007/BF01661982 |
| 114 | Pfeffer W: Lokomotorische Richtungsbewegungen durch chemische Reize. Untersuchungen aus dem
Botanischen Institut zu Tübingen 1884;1(3):363-482. Available here:
https://www.biodiversitylibrary.org/item/24682#page/5/mode/1up.
|
| 115 | Harris H, Lotz M: Factors influencing chemotaxis of the polymorphonuclear leucocyte. Br J Exp
Pathol 1956;37(5):477-480. PMID: 13374205.
|
| 116 | Friedl P: Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell
Biol 2004;16(1):14-23. https://doi.org/10.1016/j.ceb.2003.11.001.
https://doi.org/10.1016/j.ceb.2003.11.001 |
| 117 | Coates TD, Watts RG, Hartman R, Howard TH: Relationship of F-actin distribution to development of
polar shape in human polymorphonuclear neutrophils. J Cell Biol 1992;117(4):765-774.
https://doi.org/10.1083/jcb.117.4.765.
https://doi.org/10.1083/jcb.117.4.765 |
| 118 | Dunn GA: Characterising a kinesis response: time averaged measures of cell speed and directional
persistence. Agents Actions Suppl. 1983;12:14-33. https://doi.org/10.1007/978-3-0348-9352-7_1.
https://doi.org/10.1007/978-3-0348-9352-7_1 |
| 119 | Allen GM, Lee KC, Barnhart EL, Tsuchida MA, Wilson CA, Gutierrez E, Groisman A, Theriot JA,
Mogilner A: Cell Mechanics at the Rear Act to Steer the Direction of Cell Migration. Cell Syst
2020;11(3):286-299.e4. https://doi.org/10.1016/j.cels.2020.08.008.
https://doi.org/10.1016/j.cels.2020.08.008 |
| 120 | Lekka M, Laidler P, Gil D, Lekki J, Stachura Z, Hrynkiewicz AZ: Elasticity of normal and cancerous
human bladder cells studied by scanning force microscopy. Eur Biophys J 1999;28(4):312-316.
https://doi.org/10.1007/s002490050213.
https://doi.org/10.1007/s002490050213 |
| 121 | Moustakas A, Stournaras C: Regulation of actin organisation by TGF-beta in H-ras-transformed
fibroblasts. J Cell Sci 1999;112(Pt8):1169-1179. https://doi.org/10.1242/jcs.112.8.1169.
https://doi.org/10.1242/jcs.112.8.1169 |
| 122 | Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM,
Ananthakrishnan R, Mitchell D, Käs J, Ulvick S, Bilby C: Optical deformability as an inherent cell
marker for testing malignant transformation and metastatic competence. Biophys J
2005;88(5):3689-3698. https://doi.org/10.1529/biophysj.104.045476.
https://doi.org/10.1529/biophysj.104.045476 |
| 123 | Foote FW, Stewart FW: Lobular carcinoma in situ: A rare form of mammary cancer. Am J Pathol
1941;17(4):491-496.3. https://doi.org/10.3322/canjclin.32.4.234.
https://doi.org/10.3322/canjclin.32.4.234 |
| 124 | Broders AC: Carcinoma in situ contrasted with benign penetrating epithelium. JAMA
1932;99(20):1670-1674. https://doi.org/10.1001/jama.1932.02740720024007.
https://doi.org/10.1001/jama.1932.02740720024007 |
| 125 | Gunhan-Bilgen I, Oktay A: Paget's disease of the breast: clinical, mammographic, sonographic and
pathologic findings in 52 cases. Eur J Radiol 2006;60(2):256-263.
https://doi.org/10.1016/j.ejrad.2006.06.010.
https://doi.org/10.1016/j.ejrad.2006.06.010 |
| 126 | Caliskan M, Gatti G, Sosnovskikh I, Rotmensz N, Botteri E, Musmeci S, Rosali dos Santos G, Viale
G, Luini A: Paget's disease of the breast: the experience of the European Institute of Oncology and
review of the literature. Breast Cancer Res Treat 2008;112(3):513-521.
https://doi.org/10.1007/s10549-007-9880-5.
https://doi.org/10.1007/s10549-007-9880-5 |
| 127 | Brücher BL, Jamall IS: Eicosanoids in carcinogenesis. 4open 2019;2(9):1-34.
https://doi.org/10.1051/fopen/2018008.
https://doi.org/10.1051/fopen/2018008 |
| 128 | Sun L, Wang Y, Wang L, Yao B, Chen T, Li Q, Liu Z, Liu R, Niu Y, Song T, Liu Q, Tu K: Resolvin D1
prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular
carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP. J Exp Clin Cancer
Res 2019;38(1):170. https://doi.org/10.1186/s13046-019-1163-6.
https://doi.org/10.1186/s13046-019-1163-6 |
| 129 | Sakamoto H, Koma YI, Higashino N, Kodama T, Tanigawa K, Shimizu M, Fujikawa M, Nishio M, Shigeoka
M, Kakeji Y, Yokozaki H: PAI-1 derived from cancer-associated fibroblasts in esophageal squamous
cell carcinoma promotes the invasion of cancer cells and the migration of macrophages. Lab Invest
2021;101(3):353-368. https://doi.org/10.1038/s41374-020-00512-2.
https://doi.org/10.1038/s41374-020-00512-2 |
| 130 | Chen X, Song E: Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug
Discov 2019;18(2):99-115. https://doi.org/10.1038/s41573-018-0004-1.
https://doi.org/10.1038/s41573-018-0004-1 |
| 131 | Chu YW, Seftor EA, Romer LH, Hendrix MJC: Experimental coexpression of vimentin and keratin
intermediate filaments in human melanoma cells augments motility. Am J Pathol 1996;148:63-69. PMID:
8546227.
|
| 132 | Hendrix MJC, Seftor EA, Chu Y-W, Trevor KT, Seftor REB: Role of intermediate filaments in
migration, invasion and metastasis. Cancer Metast Rev 1996;15:507-525.
https://doi.org/10.1007/BF00054016.
https://doi.org/10.1007/BF00054016 |
| 133 | Hendrix MJC, Seftor EA, Seftor REB, Trevor KT: Experimental coexpression of vimentin and keratin
intermediate filaments in human breast cancer cells results in phenotypic interconversion and
increased invasive behavior. Am J Pathol 1997;150:483-495. PMID: 9033265.
|
| 134 | Österreicher CH, Penz-Österreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R,
Hardiman G, Karin M, Brenner DA: Fibroblast-specific protein 1 identifies an inflammatory
subpopulation of macrophages in the liver, Proc Natl Acad Sci USA 2011;108(1):308-313.
https://doi.org/10.1073/pnas.1017547108.
https://doi.org/10.1073/pnas.1017547108 |
| 135 | Melchionna R, Trono P, Di Carlo A, Di Modugno F, Nisticò P: Transcription factors in fibroblast
plasticity and CAF heterogeneity. J Exp Clin Cancer Res 2023;42(1):347.
https://doi.org/10.1186/s13046-023-02934-4.
https://doi.org/10.1186/s13046-023-02934-4 |
| 136 | Harryvan TJ, Verdegaal EME, Hardwick JCH, Hawinkels LJAC, van der Burg SH: Targeting of the
Cancer-Associated Fibroblast-T-Cell Axis in Solid Malignancies. J Clin Med 2019;8(11):1989.
https://doi.org/10.3390/jcm8111989.
https://doi.org/10.3390/jcm8111989 |
| 137 | Taylor CA, Glover M, Maher J: CAR-T cell technologies that interact with the tumour
microenvironment in solid tumours. Expert Rev Clin Immunol 2024:1-23.
https://doi.org/10.1080/1744666X.2024.2380894.
https://doi.org/10.1080/1744666X.2024.2380894 |
| 138 | Sun B, Lei X, Cao M, Li Y, Yang LY: Hepatocellular carcinoma cells remodel the pro-metastatic
tumour microenvironment through recruitment and activation of fibroblasts via paracrine Egfl7
signaling. Cell Commun Signal 2023;21(1):180. https://doi.org/10.1186/s12964-023-01200-6.
https://doi.org/10.1186/s12964-023-01200-6 |
| 139 | Gumbiner BM: Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell
1996;84(3):345-357. https://doi.org/10.1016/S0092-8674(00)81279-9.
https://doi.org/10.1016/S0092-8674(00)81279-9 |
| 140 | Adams CL, Nelson WJ: Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol
1998;10(5):572-577. https://doi.org/10.1016/S0955-0674(98)80031-8.
https://doi.org/10.1016/S0955-0674(98)80031-8 |
| 141 | Gottardi CJ, Wong E, Gumbiner BM: E-cadherin suppresses cellular transformation by inhibiting
beta-catenin signaling in an adhesion-independent manner. J Cell Biol 2001;153(5):1049-1060.
https://doi.org/10.1083/jcb.153.5.1049.
https://doi.org/10.1083/jcb.153.5.1049 |
| 142 | Wang Y, Shi P, Liu G, Chen W, Wang YJ, Hu Y, Yang A, Wei T, Chen YC, Liang L, Liu Z, Liu YJ, Wu C:
Espin enhances confined cell migration by promoting filopodia formation and contributes to cancer
metastasis. EMBO Rep 2025;26(10):2574-2596. https://doi.org/10.1038/s44319-025-00437-1.
https://doi.org/10.1038/s44319-025-00437-1 |
| 143 | Southwick FS, Dabiri GA, Paschetto M, Zigmond SH: Polymorphonuclear leukocyte adherence induces
actin polymerization by a transduction pathway which differs from that used by chemoattractants. J
Cell Biol 1989;109(4 Pt 1):1561-1569. https://doi.org/10.1083/jcb.109.4.1561.
https://doi.org/10.1083/jcb.109.4.1561 |
| 144 | Woods DA, Smith CJ: Ultrastructure and development of epithelial cell pseudopodia in chemically
induced premalignant lesions of the hamster cheek pouch. Exp Mol Pathol 1970;12(2):160-174.
https://doi.org/10.1016/0014-4800(70)90047-X.
https://doi.org/10.1016/0014-4800(70)90047-X |
| 145 | Locker J, Goldblatt PJ, Leighton J: Ultrastructural features of invasion in chick embryo liver
metastasis of Yoshida ascites hepatoma. Cancer Res 1970;30(6):1632-1644. PMID: 4301460.
|
| 146 | Ballestrem C, Wehrle-Haller B, Hinz B, Imhof BA: Actin-dependent lamellipodia formation and
microtubule-dependent tail retraction control-directed cell migration. Mol Biol Cell
2000;11(9):2999-3012. https://doi.org/10.1091/mbc.11.9.2999.
https://doi.org/10.1091/mbc.11.9.2999 |
| 147 | Zigmond SH: Mechanisms of sensing chemical gradients by polymorphonuclear leukocytes. Nature
1974;249(456):450-452. https://doi.org/10.1038/249450a0.
https://doi.org/10.1038/249450a0 |
| 148 | Langevin HM, Bouffard NA, Badger GJ, Iatridis JC, Howe AK: Dynamic fibroblast cytoskeletal
response to subcutaneous tissue stretch ex vivo and in vivo, Am J Physiol Cell Physiol 2005;288(3),
C747-756. https://doi.org/10.1152/ajpcell.00420.2004.
https://doi.org/10.1152/ajpcell.00420.2004 |
| 149 | Abbott RD, Koptiuch C, Iatridis JC, Howe AK, Badger GJ, Langevin HM: Stress and matrix-responsive
cytoskeletal remodeling in fibroblasts. J Cell Physiol 2013;228(1):50-57.
https://doi.org/10.1002/jcp.24102.
https://doi.org/10.1002/jcp.24102 |
| 150 | Cassimeris L, McNeill H, Zigmond SH: Chemoattractant-stimulated polymorphonuclear leukocytes
contain two populations of actin filaments that differ in their spatial distributions and relative
stabilities. J Cell Biol 1990;110(4):1067-1075. https://doi.org/10.1083/jcb.110.4.1067.
https://doi.org/10.1083/jcb.110.4.1067 |
| 151 | McKee B, Abolghasemzade S, Wang TC, Harsh K, Kaur S, Blanchard R, Menon KB, Mohajeri M, Dickinson
RB, Lele TP: Excess surface area of the nuclear lamina enables unhindered cell migration through
constrictions. Sci Adv 2025;11(13):eads6573. https://doi.org/10.1126/sciadv.ads6573.
https://doi.org/10.1126/sciadv.ads6573 |
| 152 | Saxena N, Chakraborty S, Dutta S, Bhardwaj G, Karnik N, Shetty O, Jadhav S, Zafar H, Sen S:
Stiffness-dependent MSC homing and differentiation into CAFs - implications for breast cancer
invasion. J Cell Sci 2024;137(1):jcs261145. https://doi.org/10.1242/jcs.261145.
https://doi.org/10.1242/jcs.261145 |
| 153 | Stylianou A, Gkretsi V, Stylianopoulos T: Transforming growth factor-β modulates pancreatic cancer
associated fibroblasts cell shape, stiffness and invasion. Biochim Biophys Acta Gen Subj
2018;1862(7):1537-1546. https://doi.org/10.1016/j.bbagen.2018.02.009.
https://doi.org/10.1016/j.bbagen.2018.02.009 |
| 154 | Juste-Lanas Y, Díaz-Valdivia N, Llorente A, Ikemori R, Bernardo A, Arshakyan M, Borau C, Ramírez
J, Ruffinelli JC, Nadal E, Reguart N, García-Aznar JM, Alcaraz J: 3D collagen migration patterns
reveal a SMAD3-dependent and TGF-β1-independent mechanism of recruitment for tumour-associated
fibroblasts in lung adenocarcinoma, Br J Cancer 2023;128(6): 967-981.
https://doi.org/10.1038/s41416-022-02093-x.
https://doi.org/10.1038/s41416-022-02093-x |
| 155 | Melchiori A, Mortarini R, Carlone S, Marchisio PC, Anichini A, Noonan DM, Albini A: The alpha 3
beta 1 integrin is involved in melanoma cell migration and invasion. Exp Cell Res
1995;219(1):233-242. https://doi.org/10.1006/excr.1995.1223.
https://doi.org/10.1006/excr.1995.1223 |
| 156 | Gerg M, Kopitz C, Schaten S, Tschukes A, Kahlert C, Stangl M, von Weyhern CW, Brücher BL, Edwards
DR, Brand K, Krüger A: Distinct functionality of tumor cell-derived gelatinases during formation of
liver metastases, Mol Cancer Res 2008;6(3);341-351. https://doi.org/10.1158/1541-7786.MCR-07-2032.
https://doi.org/10.1158/1541-7786.MCR-07-2032 |
| 157 | Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, Noonan DM, Natali PG, Albini
A: The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and
gelatinase B (MMP-9) activity. Int J Cancer 2000;87(3):336-342.
https://doi.org/10.1002/1097-0215(20000801)87:3<336::AID-IJC5>3.0.CO;2-3.
https://doi.org/10.1002/1097-0215(20000801)87:3<336::AID-IJC5>3.0.CO;2-3 |
| 158 | Condeelis J, Segall JE: Intravital imaging of cell movement in tumours, Nat Rev Cancer
2003;3(12):921-930. https://doi.org/10.1038/nrc1231.
https://doi.org/10.1038/nrc1231 |
| 159 | Lenzini S, Bargi R, Chung G, Shin JW: Matrix mechanics and water permeation regulate extracellular
vesicle transport. Nat Nanotechnol 2020;15(3):217-223. https://doi.org/10.1038/s41565-020-0636-2.
https://doi.org/10.1038/s41565-020-0636-2 |
| 160 | Wang SE: Extracellular Vesicles and Metastasis. Cold Spring Harb Perspect Med 2020;10(7):a037275.
https://doi.org/10.1101/cshperspect.a037275.
https://doi.org/10.1101/cshperspect.a037275 |
| 161 | Trams EG, Lauter CJ, Salem N Jr, Heine U: Exfoliation of membrane ecto-enzymes in the form of
micro-vesicles. Biochim Biophys Acta 1981;645(1):63-70.
https://doi.org/10.1016/0005-2736(81)90512-5.
https://doi.org/10.1016/0005-2736(81)90512-5 |
| 162 | Théry C, Zitvogel L, Amigorena S: Exosomes: composition, biogenesis and function. Nat Rev Immunol
2002;2(8):569-579. https://doi.org/10.1038/nri855.
https://doi.org/10.1038/nri855 |
| 163 | Kaplan RN, Rafii S, Lyden D: Preparing the "soil": the premetastatic niche. Cancer Res
2006;66(23):11089-11093. https://doi.org/10.1158/0008-5472.CAN-06-2407.
https://doi.org/10.1158/0008-5472.CAN-06-2407 |
| 164 | Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN,
Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S,
Moita LF, Thery C: Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat
Cell Biol 2010;12(1):19-30; sup pp 1-13. https://doi.org/10.1038/ncb2000.
https://doi.org/10.1038/ncb2000 |
| 165 | Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C: Rab27a
supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and
can promote tumor progression. Cancer Res 2012;72(19):4920-4930.
https://doi.org/10.1158/0008-5472.CAN-12-0925.
https://doi.org/10.1158/0008-5472.CAN-12-0925 |
| 166 | Wu H, Shi M, Meng L, Qiu J, Jiang Y, Qian D, Shen F: Plant-derived extracellular vesicles as a
novel tumor-targeting delivery system for cancer treatment. Front Cell Dev Biol 2025;13:1589550.
https://doi.org/10.3389/fcell.2025.1589550.
https://doi.org/10.3389/fcell.2025.1589550 |
| 167 | Fackler OT, Grosse R: Cell motility through plasma membrane blebbing. J Cell Biol
2008;181(6):879-884. https://doi.org/10.1083/jcb.200802081.
https://doi.org/10.1083/jcb.200802081 |
| 168 | Erickson C. A., Trinkaus J. P: Microvilli and blebs as sources of reserve surface membrane during
cell spreading. Exp Cell Res 1976;99(2):375-384. https://doi.org/10.1016/0014-4827(76)90595-4.
https://doi.org/10.1016/0014-4827(76)90595-4 |
| 169 | Dixon SJ, Pitaru S, Bhargava U, Aubin JE: Membrane blebbing is associated with Ca2+-activated
hyperpolarizations induced by serum and alpha 2-macroglobulin. J Cell Physiol 1987;132(3):473-482.
https://doi.org/10.1002/jcp.1041320309.
https://doi.org/10.1002/jcp.1041320309 |
| 170 | Cunningham CC: Actin polymerization and intracellular solvent flow in cell surface blebbing. J
Cell Biol 1995;129(6):1589-1599. https://doi.org/10.1083/jcb.129.6.1589.
https://doi.org/10.1083/jcb.129.6.1589 |
| 171 | Zantos SG, Holden BA: Transient endothelial changes soon after wearing soft contact lenses. Am J
Optom Physiol Opt 1977;54(12):856-858. https://doi.org/10.1097/00006324-197712000-00010.
https://doi.org/10.1097/00006324-197712000-00010 |
| 172 | Charras G, Paluch E: Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell
Biol 2008;9(9):730-736. https://doi.org/10.1038/nrm2453.
https://doi.org/10.1038/nrm2453 |
| 173 | Guzman A, Avard RC, Devanny AJ, Kweon OS, Kaufman LJ: Delineating the role of membrane blebs in a
hybrid mode of cancer cell invasion in three-dimensional environments. J Cell Sci
2020;133:jcs236778. https://doi.org/10.1242/jcs.236778.
https://doi.org/10.1242/jcs.236778 |
| 174 | Jia W, Czabanka M, Broggini T: Cell blebbing novel therapeutic possibilities to counter
metastasis. Clin Exp Metastasis 2024;41(6):817-828. https://doi.org/10.1007/s10585-024-10308-z.
https://doi.org/10.1007/s10585-024-10308-z |
| 175 | Albrecht-Buehler G: Autonomous movements of cytoplasmic fragments. Proc Natl Acad Sci USA
1980;77(11):6639-6643. https://doi.org/10.1073/pnas.77.11.6639.
https://doi.org/10.1073/pnas.77.11.6639 |
| 176 | Schick J, Raz E: Blebs-formation, regulation, positioning, and role in amoeboid cell migration.
Front Cell Dev Biol 2022;10:926394. https://doi.org/10.3389/fcell.2022.926394.
https://doi.org/10.3389/fcell.2022.926394 |
| 177 | Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG: Analysis of the actin-myosin II system in fish
epidermal keratocytes: mechanism of cell body translocation. J Cell Biol 1997;139(2):397-415.
https://doi.org/10.1083/jcb.139.2.397.
https://doi.org/10.1083/jcb.139.2.397 |
| 178 | Keller H, Eggli P: Protrusive activity, cytoplasmic compartmentalization, and restriction rings in
locomoting blebbing Walker carcinosarcoma cells are related to detachment of cortical actin from the
plasma membrane. Cell Motil Cytoskeleton 1998;41:181-193.
https://doi.org/10.1002/(SICI)1097-0169(1998)41:2<181::AID-CM8>3.0.CO;2-H.
https://doi.org/10.1002/(SICI)1097-0169(1998)41:2<181::AID-CM8>3.0.CO;2-H |
| 179 | Tozluoğlu M, Tournier AL, Jenkins RP, Hooper S, Bates PA, Sahai E: Matrix geometry determines
optimal cancer cell migration strategy and modulates response to interventions. Nat Cell Biol
2013;15:751-762. https://doi.org/10.1038/ncb2775.
https://doi.org/10.1038/ncb2775 |
| 180 | Cantelli G, Orgaz JL, Rodriguez-Hernandez I, Karagiannis P, Maiques O, Matias-Guiu X, Nestle FO,
Marti RM, Karagiannis SN, Sanz-Moreno V: TGF-β-induced transcription sustains amoeboid melanoma
migration and dissemination. Curr Biol 2015;25(2):2899-2914.
https://doi.org/10.1016/j.cub.2015.09.054.
https://doi.org/10.1016/j.cub.2015.09.054 |
| 181 | Suetsugu A, Osawa Y, Nagaki M, Saji S, Moriwaki H, Bouvet M, Hoffman RM: Imaging the recruitment
of cancer-associated fibroblasts by liver-metastatic colon cancer. J Cell Biochem
2011;112(3):949-953. https://doi.org/10.1002/jcb.23011.
https://doi.org/10.1002/jcb.23011 |
| 182 | Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S, Moriwaki H, Bouvet M, Hoffman RM:
Imageable fluorescent metastasis resulting in transgenic GFP mice orthotopically implanted with
human-patient primary pancreatic cancer specimens Anticancer Res 2012;32(4):1175-1180. PMID:
22493347.
|
| 183 | Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E: Localized and reversible TGFbeta
signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol
2009;11(11):1287-1296. https://doi.org/10.1038/ncb1973.
https://doi.org/10.1038/ncb1973 |
| 184 | Fullár A, Dudás J, Oláh L, Hollósi P, Papp Z, Sobel G, Karászi K, Paku S, Baghy K, Kovalszky I:
Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical
cancer progression. BMC Cancer 2015;15:256. https://doi.org/10.1186/s12885-015-1272-3.
https://doi.org/10.1186/s12885-015-1272-3 |
| 185 | Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C,
Gribben JG, Ramsay AG, Kocher HM: Activated pancreatic stellate cells sequester CD8+ T cells to
reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma.
Gastroenterology 2013;145(5):1121-1132. https://doi.org/10.1053/j.gastro.2013.07.025.
https://doi.org/10.1053/j.gastro.2013.07.025 |
| 186 | Kinoshita T, Ishii G, Hiraoka N, Hirayama S, Yamauchi C, Aokage K, Hishida T, Yoshida J, Nagai K,
Ochiai A: Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are
correlated with a poor outcome in lung adenocarcinoma. Cancer Sci 2013;104(4):409-415. PMID:
23305175.
https://doi.org/10.1111/cas.12099 |
| 187 | Chun E, Lavoie S, Michaud M, Gallini CA, Kim J, Soucy G, Odze R, Glickman JN, Garrett WS: CCL2
Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell
Population and Function. Cell Rep 2015;12(2):244-257. https://doi.org/10.1016/j.celrep.2015.06.024.
https://doi.org/10.1016/j.celrep.2015.06.024 |
| 188 | Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and
future perspectives. Mol Cancer 2021;20(1):131. https://doi.org/10.1186/s12943-021-01428-1.
https://doi.org/10.1186/s12943-021-01428-1 |
| 189 | Arpinati L, Scherz-Shouval R: From gatekeepers to providers: regulation of immune functions by
cancer-associated fibroblasts. Trends Cancer 2023;9(5):421-443.
https://doi.org/10.1016/j.trecan.2023.01.007.
https://doi.org/10.1016/j.trecan.2023.01.007 |
| 190 | Cohen N, Shani O, Raz Y, Sharon Y, Hoffman D, Abramovitz L, Erez N: Fibroblasts drive an
immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase
3-like 1. Oncogene 2017;36(31):4457-4468. https://doi.org/10.1038/onc.2017.65.
https://doi.org/10.1038/onc.2017.65 |
| 191 | Müller H, Hashimoto H, Stutte HJ: [Tumor-associated macrophage subpopulation and the prolific
activity of malignant tumors--an immunohistochemical study of 109 breast carcinomas]. Verh Dtsch Ges
Pathol 1986;70:251-256. PMID: 3548129.
|
| 192 | Athanasou NA, Wells CA, Quinn J, Ferguson DP, Heryet A, McGee JO: The origin and nature of stromal
osteoclast-like multinucleated giant cells in breast carcinoma: implications for tumour osteolysis
and macrophage biology. Br J Cancer 1989;59(4):491-498. https://doi.org/10.1038/bjc.1989.102.
https://doi.org/10.1038/bjc.1989.102 |
| 193 | Zhang Y, Zhong F, Liu L: Single-cell transcriptional atlas of tumor-associated macrophages in
breast cancer. Breast Cancer Res 2024;26(1):129. https://doi.org/10.1186/s13058-024-01887-6.
https://doi.org/10.1186/s13058-024-01887-6 |
| 194 | Chen D, Tong W, Ang B, Bai Y, Dong W, Deng X, Wang C, Zhang Y: Revealing the crosstalk between
LOX+ fibroblast and M2 macrophage in gastric cancer by single-cell sequencing. BMC Cancer
2024;24(1):1117. https://doi.org/10.1186/s12885-024-12861-y.
https://doi.org/10.1186/s12885-024-12861-y |
| 195 | Fan L, Wang Q, Qian Q, Wang Q, Liu D, Gong Y, Xiong Z: Single-Cell RNA Sequencing Revealing that
MMP12+ Macrophages are Associated with Cancer Liver Metastasis. Curr Med Chem 2024 Sep 12.
https://doi.org/10.2174/0109298673337385240827061539.
https://doi.org/10.2174/0109298673337385240827061539 |
| 196 | Chen F, Sheng J, Li X, Gao Z, Hu L, Chen M, Fei J, Song Z: Tumor-associated macrophages:
orchestrators of cholangiocarcinoma progression. Front Immunol 2024;15:1451474.
https://doi.org/10.3389/fimmu.2024.1451474.
https://doi.org/10.3389/fimmu.2024.1451474 |
| 197 | Yu M, Yu H, Wang H, Xu X, Sun Z, Chen W, Yu M, Liu C, Jiang M, Zhang X: Tumor-associated
macrophages activated in the tumor environment of hepatocellular carcinoma: Characterization and
treatment (Review). Int J Oncol 2024;65(4):100. https://doi.org/10.3892/ijo.2024.5688.
https://doi.org/10.3892/ijo.2024.5688 |
| 198 | Bigotti G, Coli A, Castagnola D: Distribution of Langerhans cells and HLA class II molecules in
prostatic carcinomas of different histopathological grade. Prostate 1991;19(1):73-87.
https://doi.org/10.1002/pros.2990190108.
https://doi.org/10.1002/pros.2990190108 |
| 199 | Vukanovic J, Isaacs JT: Linomide inhibits angiogenesis, growth, metastasis, and macrophage
infiltration within rat prostatic cancers. Cancer Res 1995;55(7):1499-504. PMID: 7533663.
|
| 200 | Wei QJ, Liang HQ, Liang YW, Huang ZX: TET3 is expressed in prostate cancer tumor-associated
macrophages and is associated with anti-androgen resistance. Clin Transl Oncol 2024;27:1712-1727.
https://doi.org/10.1007/s12094-024-03708-w.
https://doi.org/10.1007/s12094-024-03708-w |
| 201 | Osman A, Oze M, Afify SM, Hassan G, El-Ghlban S, Nawara HM, Fu X, Zahra MH, Seno A, Winer I,
Salomon DS, Seno M: Tumor-associated macrophages derived from cancer stem cells. Acta Histochem
2020;122(8):151628. https://doi.org/10.1016/j.acthis.2020.151628.
https://doi.org/10.1016/j.acthis.2020.151628 |
| 202 | Stein M, Keshav S, Harris N, Gordon S: Interleukin 4 potently enhances murine macrophage mannose
receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med
1992;176(1):287-292. https://doi.org/10.1084/jem.176.1.287.
https://doi.org/10.1084/jem.176.1.287 |
| 203 | DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM: CD4(+) T cells
regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages.
Cancer Cell 2009;16(2):91-102. https://doi.org/10.1016/j.ccr.2009.06.018.
https://doi.org/10.1016/j.ccr.2009.06.018 |
| 204 | Martinez FO, Helming L, Gordon S: Alternative activation of macrophages: an immunologic functional
perspective. Annu Rev Immunol 2009;27:451-483.
https://doi.org/10.1146/annurev.immunol.021908.132532.
https://doi.org/10.1146/annurev.immunol.021908.132532 |
| 205 | Müller E, Christopoulos PF, Halder S, Lunde A, Beraki K, Speth M, Øynebråten I, Corthay A:
Toll-Like Receptor Ligands and Interferon-γ Synergize for Induction of Antitumor M1 Macrophages.
Front Immunol 2017;8:1383. https://doi.org/10.3389/fimmu.2017.01383.
https://doi.org/10.3389/fimmu.2017.01383 |
| 206 | Perry CJ, Muñoz-Rojas AR, Meeth KM, Kellman LN, Amezquita RA, Thakral D, Du VY, Wang JX, Damsky W,
Kuhlmann AL, Sher JW, Bosenberg M, Miller-Jensen K, Kaech SM: Myeloid-targeted immunotherapies act
in synergy to induce inflammation and antitumor immunity. J Exp Med 2018;215(3):877-893.
https://doi.org/10.1084/jem.20171435.
https://doi.org/10.1084/jem.20171435 |
| 207 | Lauterbach MA, Hanke JE, Serefidou M, Mangan MSJ, Kolbe CC, Hess T, Rothe M, Kaiser R, Hoss F,
Gehlen J, Engels G, Kreutzenbeck M, Schmidt SV, Christ A, Imhof A, Hiller K, Latz E: Toll-like
Receptor Signaling Rewires Macrophage Metabolism and Promotes Histone Acetylation via ATP-Citrate
Lyase. Immunity 2019;51(6):997-1011.e7. https://doi.org/10.1016/j.immuni.2019.11.009.
https://doi.org/10.1016/j.immuni.2019.11.009 |
| 208 | Pan Y, Yu Y, Wang X, Zhang T: Tumor-Associated Macrophages in Tumor Immunity. Front Immunol
2020;11:583084. https://doi.org/10.3389/fimmu.2020.583084.
https://doi.org/10.3389/fimmu.2020.583084 |
| 209 | Bernardo C, Cunha MC, Santos JH, da Costa JM, Brindley PJ, Lopes C, Amado F, Ferreira R, Vitorino
R, Santos LL: Insight into the molecular basis of Schistosoma haematobium-induced bladder cancer
through urine proteomics. Tumour Biol 2016;37(8):11279-12287.
https://doi.org/10.1007/s13277-016-4997-y.
https://doi.org/10.1007/s13277-016-4997-y |
| 210 | Zhang Y, Sime W, Juhas M, Sjölander A: Crosstalk between colon cancer cells and macrophages via
inflammatory mediators and CD47 promotes tumour cell migration. Eur J Cancer 2013;49(15):3320-3334.
https://doi.org/10.1016/j.ejca.2013.06.005.
https://doi.org/10.1016/j.ejca.2013.06.005 |
| 211 | Dymicka-Piekarska V, Koper-Lenkiewicz OM, Zińczuk J, Kratz E, Kamińska J: Inflammatory
cell-associated tumors. Not only macrophages (TAMs), fibroblasts (TAFs) and neutrophils (TANs) can
infiltrate the tumor microenvironment. The unique role of tumor associated platelets (TAPs). Cancer
Immunol Immunother 2021;70(6):1497-1510. https://doi.org/10.1007/s00262-020-02758-7.
https://doi.org/10.1007/s00262-020-02758-7 |
| 212 | Zhou L, Zhang Z, Tian Y, Li Z, Liu Z, Zhu S: The critical role of platelet in cancer progression
and metastasis. Eur J Med Res 2023;28(1):385. https://doi.org/10.1186/s40001-023-01342-w.
https://doi.org/10.1186/s40001-023-01342-w |
| 213 | Refvem O: Thrombocythemia as an independent phenomenon in cancer. Nord Med 1960;64:950-953. PMID:
14436949.
https://doi.org/10.1021/j100836a515 |
| 214 | Skolnik G, Alpsten M, Ivarsson L: Studies on mechanisms involved in metastasis formation from
circulating tumor cells. Factors influencing tumor cell lodgement during normal and post-traumatic
conditions. J Cancer Res Clin Oncol 1980;97(3):249-256. https://doi.org/10.1007/BF00405776.
https://doi.org/10.1007/BF00405776 |
| 215 | Gasic GJ, Gasic TB, Stewart CC: Antimetastatic effects associated with platelet reduction. Proc
Natl Acad Sci USA 1968;61(1):46-52. https://doi.org/10.1073/pnas.61.1.46.
https://doi.org/10.1073/pnas.61.1.46 |
| 216 | Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR: Platelets,
protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 2004;104(2):397-401.
https://doi.org/10.1182/blood-2004-02-0434.
https://doi.org/10.1182/blood-2004-02-0434 |
| 217 | Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L, Varki A, McEver RP: Carcinoma mucins
trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome.
Blood 2011;118(15):4015-4023. https://doi.org/10.1182/blood-2011-07-368514.
https://doi.org/10.1182/blood-2011-07-368514 |
| 218 | Gryglewski RJ, Dembínska-Kieć A, Korbut R: A possible role of thromboxane A2 (TXA2) and
prostacyclin (PGI2) in circulation. Acta Biol Med Ger 1978;37(5-6):715-723. PMID: 369254.
|
| 219 | Aitokallio-Tallberg A, Kärkkäinen J, Pantzar P, Wahlström T, Ylikorkala O: Prostacyclin and
thromboxane in breast cancer: relationship between steroid receptor status and medroxyprogesterone
acetate. Br J Cancer 1985;51(5):671-674. https://doi.org/10.1038/bjc.1985.101.
https://doi.org/10.1038/bjc.1985.101 |
| 220 | Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O'Donnell E, Zhao BQ, Cifuni SM, Wagner DD:
Inflammation induces hemorrhage in thrombocytopenia. Blood 2008;111(10):4958-4964.
https://doi.org/10.1182/blood-2007-11-123620.
https://doi.org/10.1182/blood-2007-11-123620 |
| 221 | Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, Mackman N, Owens AP 3rd, Ware J, Kahn ML,
Bergmeier W: Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin
Invest 2013;123(2):908-916. https://doi.org/10.1172/JCI65154.
https://doi.org/10.1172/JCI65154 |
| 222 | Gros A, Syvannarath V, Lamrani L, Ollivier V, Loyau S, Goerge T, Nieswandt B, Jandrot-Perrus M,
Ho-Tin-Noé B: Single platelets seal neutrophil-induced vascular breaches via GPVI during
immune-complex-mediated inflammation in mice. Blood 2015;126(8):1017-1026.
https://doi.org/10.1182/blood-2014-12-617159.
https://doi.org/10.1182/blood-2014-12-617159 |
| 223 | Kitchens CS, Pendergast JF: Human thrombocytopenia is associated with structural abnormalities of
the endothelium that are ameliorated by glucocorticosteroid administration. Blood
1986;67(1):203-206. PMID: 3940548
https://doi.org/10.1182/blood.V67.1.203.bloodjournal671203 |
| 224 | Mammoto T, Jiang A, Jiang E, Mammoto A: Platelet-rich plasma extract prevents pulmonary edema
through angiopoietin-Tie2 signaling. Am J Respir Cell Mol Biol 2015;52(1):56-64.
https://doi.org/10.1165/rcmb.2014-0076OC.
https://doi.org/10.1165/rcmb.2014-0076OC |
| 225 | Hisakura K, Murata S, Takahashi K, Matsuo R, Pak S, Ikeda N, Kawasaki T, Kohno K, Myronovych A,
Nakano Y, Ikeda O, Watanabe M, Ohkohchi N: Platelets prevent acute hepatitis induced by anti-fas
antibody. J Gastroenterol Hepatol 2011;26(2):348-355.
https://doi.org/10.1111/j.1440-1746.2010.06334.x.
https://doi.org/10.1111/j.1440-1746.2010.06334.x |
| 226 | Au AE, Sashindranath M, Borg RJ, Kleifeld O, Andrews RK, Gardiner EE, Medcalf RL, Samson AL:
Activated platelets rescue apoptotic cells via paracrine activation of EGFR and DNA-dependent
protein kinase. Cell Death Dis 2014;5(9):e1410. https://doi.org/10.1038/cddis.2014.373.
https://doi.org/10.1038/cddis.2014.373 |
| 227 | Chatterjee M, von Ungern-Sternberg SN, Seizer P, Schlegel F, Büttcher M, Sindhu NA, Müller S, Mack
A, Gawaz M: Platelet-derived CXCL12 regulates monocyte function, survival, differentiation into
macrophages and foam cells through differential involvement of CXCR4-CXCR7. Cell Death Dis
2015;6(11):e1989. https://doi.org/10.1038/cddis.2015.233.
https://doi.org/10.1038/cddis.2015.233 |
| 228 | Ortiz-Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR: Aspirin-triggered
15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in
mice. Blood 2014;124(17):2625-2634. https://doi.org/10.1182/blood-2014-03-562876.
https://doi.org/10.1182/blood-2014-03-562876 |
| 229 | Lax S, Rayes J, Wichaiyo S, Haining EJ, Lowe K, Grygielska B, Laloo R, Flodby P, Borok Z, Crandall
ED, Thickett DR, Watson SP: Platelet CLEC-2 protects against lung injury via effects of its ligand
podoplanin on inflammatory alveolar macrophages in the mouse. Am J Physiol Lung Cell Mol Physiol
2017;313(6):L1016-L1029. https://doi.org/10.1152/ajplung.00023.2017.
https://doi.org/10.1152/ajplung.00023.2017 |
| 230 | Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z: Effect of daily aspirin on risk of
cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet
2012;379(9826):1591-1601. https://doi.org/10.1016/S0140-6736(12)60209-8.
https://doi.org/10.1016/S0140-6736(12)60209-8 |
| 231 | Karpatkin S, Ambrogio C, Pearlstein E: Lack of effect of in vivo prostacyclin on the development
of pulmonary metastases in mice following intravenous injection of CT26 colon carcinoma, Lewis lung
carcinoma, or B16 amelanotic melanoma cells. Cancer Res 1984;44(9):3880-3883. PMID: 6378376.
|
| 232 | Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, Mills GB, Babwah AV,
Bhattacharya M: Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and
invasion of human breast tumor cells. Mol Cancer Res 2009;7(7):1064-1077.
https://doi.org/10.1158/1541-7786.MCR-08-0578.
https://doi.org/10.1158/1541-7786.MCR-08-0578 |
| 233 | David M, Wannecq E, Descotes F, Jansen S, Deux B, Ribeiro J, Serre CM, Grès S, Bendriss-Vermare N,
Bollen M, Saez S, Aoki J, Saulnier-Blache JS, Clézardin P, Peyruchaud O: Cancer cell expression of
autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent
activation of osteoclasts. PLoS One 2010;5(3):e9741. https://doi.org/10.1371/journal.pone.0009741.
https://doi.org/10.1371/journal.pone.0009741 |
| 234 | David M, Ribeiro J, Descotes F, Serre CM, Barbier M, Murone M, Clézardin P, Peyruchaud O:
Targeting lysophosphatidic acid receptor type 1 with Debio 0719 inhibits spontaneous metastasis
dissemination of breast cancer cells independently of cell proliferation and angiogenesis. Int J
Oncol 2012;40(4):1133-1141. https://doi.org/10.3892/ijo.2011.1309.
https://doi.org/10.3892/ijo.2011.1309 |
| 235 | Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clézardin P, Peyruchaud O: The type 1
lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci USA
2006;103(25):9643-9648. https://doi.org/10.1073/pnas.0600979103.
https://doi.org/10.1073/pnas.0600979103 |
| 236 | Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, Stephens C, Fang X, Mills GB: Lysophosphatidic acid
receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst
2008;100(22):1630-1642. https://doi.org/10.1093/jnci/djn378.
https://doi.org/10.1093/jnci/djn378 |
| 237 | Ward Y, Lake R, Yin JJ, Heger CD, Raffeld M, Goldsmith PK, Merino M, Kelly K: LPA receptor
heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate
cancer cells. Cancer Res 2011;71(23):7301-7311. https://doi.org/10.1158/0008-5472.CAN-11-2381.
https://doi.org/10.1158/0008-5472.CAN-11-2381 |
| 238 | Su SC, Hu X, Kenney PA, Merrill MM, Babaian KN, Zhang XY, Maity T, Yang SF, Lin X, Wood CG:
Autotaxin-lysophosphatidic acid signaling axis mediates tumorigenesis and development of acquired
resistance to sunitinib in renal cell carcinoma. Clin Cancer Res 2013;19(23):6461-6472.
https://doi.org/10.1158/1078-0432.CCR-13-1284.
https://doi.org/10.1158/1078-0432.CCR-13-1284 |
| 239 | Hwang YS, Lee SK, Park KK, Chung WY: Secretion of IL-6 and IL-8 from lysophosphatidic
acid-stimulated oral squamous cell carcinoma promotes osteoclastogenesis and bone resorption. Oral
Oncol 2012;48(1):40-48. https://doi.org/10.1016/j.oraloncology.2011.08.022.
https://doi.org/10.1016/j.oraloncology.2011.08.022 |
| 240 | Boucharaba A, Serre CM, Grès S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clézardin P,
Peyruchaud O: Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone
metastases in breast cancer. J Clin Invest 2004;114(12):1714-1725. https://doi.org/10.1172/JCI22123.
https://doi.org/10.1172/JCI22123 |
| 241 | Rastegar F, Gao JL, Shenaq D, Luo Q, Shi Q, Kim SH, Jiang W, Wagner ER, Huang E, Gao Y, Shen J,
Yang K, He BC, Chen L, Zuo GW, Luo J, Luo X, Bi Y, Liu X, Li M, Hu N, Wang L, Luther G, Luu HH,
Haydon RC, He TC: Lysophosphatidic acid acyltransferase beta (LPAATbeta) promotes the tumor growth
of human osteosarcoma. PLoS One 2010;5(12):e14182. https://doi.org/10.1371/journal.pone.0014182.
https://doi.org/10.1371/journal.pone.0014182 |
| 242 | Lerner WA, Pearlstein E, Ambrogio C, Karpatkin S: A new mechanism for tumor induced platelet
aggregation. Comparison with mechanisms shared by other tumor with possible pharmacologic strategy
toward prevention of metastases. Int J Cancer 1983;31(4):463-469.
https://doi.org/10.1002/ijc.2910310411.
https://doi.org/10.1002/ijc.2910310411 |
| 243 | Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR: Role of thrombin signalling in platelets in
haemostasis and thrombosis. Nature 2001;413(6851):74-78. https://doi.org/10.1038/35092573.
https://doi.org/10.1038/35092573 |
| 244 | Versteeg HH, Schaffner F, Kerver M, Ellies LG, Andrade-Gordon P, Mueller BM, Ruf W:
Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary
adenocarcinoma in polyoma middle T mice. Cancer Res 2008;68(17):7219-7227.
https://doi.org/10.1158/0008-5472.CAN-08-0419.
https://doi.org/10.1158/0008-5472.CAN-08-0419 |
| 245 | Palumbo JS, Kombrinck KW, Drew AF, Grimes TS, Kiser JH, Degen JL, Bugge TH: Fibrinogen is an
important determinant of the metastatic potential of circulating tumor cells. Blood
2000;96(10):3302-3309. PMID: 11071621.
https://doi.org/10.1182/blood.V96.10.3302.h8003302_3302_3309 |
| 246 | Morell AG, Irvine RA, Sternlieb I, Scheinberg IH, Ashwell G: Physical and chemical studies on
ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J Biol Chem
1968;243(1):155-159. PMID: 5635941.
https://doi.org/10.1016/S0021-9258(18)99337-3 |
| 247 | Gregoriadis G: The Role of Sialic Acid in the Catabolism of Plasma Glycoproteins. In: Allison,
A.C. (eds) Structure and Function of Plasma Proteins. Springer, Boston, MA. 1976.
https://doi.org/10.1007/978-1-4684-2679-3_5.
https://doi.org/10.1007/978-1-4684-2679-3_5 |
| 248 | Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G: The role of sialic acid in
determining the survival of glycoproteins in the circulation. J Biol Chem 1971;246(5):1461-1467.
PMID: 5545089.
https://doi.org/10.1016/S0021-9258(19)76994-4 |
| 249 | Gu D, Jin H, Jin G, Wang C, Wang N, Hu F, Luo Q, Chu W, Yao M, Qin W: The asialoglycoprotein
receptor suppresses the metastasis of hepatocellular carcinoma via LASS2-mediated inhibition of
V-ATPase activity. Cancer Lett 2016;379(1):107-116. https://doi.org/10.1016/j.canlet.2016.05.030.
https://doi.org/10.1016/j.canlet.2016.05.030 |
| 250 | Qianyu L, Wenyun G, Yifei Q, Songling L, Zijun Z, Yanfeng L: Study on the significance and
mechanism of ASGR1 in hepatocellular carcinoma. J Shanghai Jiao Tong University (Medical Science)
2023;43(9):1107-1114. https://doi.org/10.3969/j.issn.1674-8115.2023.09.005.
|
| 251 | Nie K, Shi L, Wen Y, Pan J, Li P, Zheng Z, Liu F: Identification of hub genes correlated with the
pathogenesis and prognosis of gastric cancer via bioinformatics methods. Minerva Med
2020;111(3):213-225. https://doi.org/10.23736/S0026-4806.19.06166-4.
https://doi.org/10.23736/S0026-4806.19.06166-4 |
| 252 | Tanaka H, Kanda M, Miwa T, Tanaka C, Kobayashi D, Umeda S, Shibata M, Suenaga M, Hattori N,
Hayashi M, Iwata N, Yamada S, Nakayama G, Fujiwara M, Kodera Y: Pattern-Specific Transcriptomics
Identifies ASGR2 as a Predictor of Hematogenous Recurrence of Gastric Cancer. Mol Cancer Res
2018;16(9):1420-1429. https://doi.org/10.1158/1541-7786.MCR-17-0467.
https://doi.org/10.1158/1541-7786.MCR-17-0467 |
| 253 | Oshima T, Hashimoto I, Hiroshima Y, Kimura Y, Tanabe M, Onuma S, Nagasawa S, Kanematsu K, Aoyama
T, Yamada T, Ogata T, Rino Y, Saito A, Miyagi Y: Asialoglycoprotein Receptor 2 Expression in
Patients With Locally Advanced Gastric Cancer After Curative Resection. Anticancer Res
2024;44(1):397-402. https://doi.org/10.21873/anticanres.16824.
https://doi.org/10.21873/anticanres.16824 |
| 254 | Dzik WH, Sherburne B: Intraoperative blood salvage: medical controversies. Transfus Med Rev
1990;4(3):208-235. https://doi.org/10.1016/s0887-7963(90)70266-0.
https://doi.org/10.1016/S0887-7963(90)70266-0 |
| 255 | Silvain J, Pena A, Cayla G, Brieger D, Bellemain-Appaix A, Chastre T, Vignalou JB, Beygui F,
Barthelemy O, Collet JP, Montalescot G: Impact of red blood cell transfusion on platelet activation
and aggregation in healthy volunteers: results of the TRANSFUSION study. Eur Heart J
2010;31(22):2816-2821. https://doi.org/10.1093/eurheartj/ehq209.
https://doi.org/10.1093/eurheartj/ehq209 |
| 256 | Beynon J, Davies PW, Billings PJ, Channer JL, Protheroe D, Umpleby HC, Mortensen NJ, Williamson
RC: Perioperative blood transfusion increases the risk of recurrence in colorectal cancer. Dis Colon
Rectum 1989;32(11):975-979. https://doi.org/10.1007/BF02552276.
https://doi.org/10.1007/BF02552276 |
| 257 | Cheslyn-Curtis S, Fielding LP, Hittinger R, Fry JS, Phillips RK: Large bowel cancer: the effect of
perioperative blood transfusion on outcome. Ann R Coll Surg Engl 1990;72(1):53-59. PMID: 2405765.
|
| 258 | Lim MC, Kim JY, Kim TH, Park S, Kong SY, Yoon JH, Kang S, Seo SS, Park SY: Allogeneic blood
transfusion given before radiotherapy is associated with the poor clinical outcome in patients with
cervical cancer. Yonsei Med J 2008;49(6):993-1003. https://doi.org/10.3349/ymj.2008.49.6.993.
https://doi.org/10.3349/ymj.2008.49.6.993 |
| 259 | Li XX, Meng J, Sun GP, Tang YX, Liang GF, Wang MF, Lu XB: Effects of perioperative blood
transfusion on the prognosis in hereditary and sporadic colon cancer. Biomarkers
2015;20(6-7):481-486. https://doi.org/10.3109/1354750X.2015.1096306.
https://doi.org/10.3109/1354750X.2015.1096306 |
| 260 | Qiu L, Wang DR, Zhang XY, Gao S, Li XX, Sun GP, Lu XB: Impact of perioperative blood transfusion
on immune function and prognosis in colorectal cancer patients. Transfus Apher Sci
2016;54(2):235-241. https://doi.org/10.1016/j.transci.2015.07.004.
https://doi.org/10.1016/j.transci.2015.07.004 |
| 261 | Crowe JP, Gordon NH, Fry DE, Shuck JM, Hubay CA: Breast cancer survival and perioperative blood
transfusion. Surgery 1989;106(5):836-841. PMID: 2683172.
|
| 262 | Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell
2009;16(3):183-194. https://doi.org/10.1016/j.ccr.2009.06.017.
https://doi.org/10.1016/j.ccr.2009.06.017 |
| 263 | Wang Y, Ma J, Liu Y, Cui W, Chu X, Lin Y, Wang L: Unraveling the complex role of tumor-associated
neutrophils within solid tumors. Cancer Immunol Immunother 2025;74(7):210.
https://doi.org/10.1007/s00262-025-04049-5.
https://doi.org/10.1007/s00262-025-04049-5 |
| 264 | Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, Liu P, Li Z, Xia Y, Jiang W: Neutrophil Extracellular
Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin
Cancer Res 2019;25(6):1867-1879. https://doi.org/10.1158/1078-0432.CCR-18-1226.
https://doi.org/10.1158/1078-0432.CCR-18-1226 |
| 265 | Lepsenyi M, Algethami N, Al-Haidari AA, Algaber A, Syk I, Rahman M, Thorlacius H: CXCL2-CXCR2 axis
mediates αV integrin-dependent peritoneal metastasis of colon cancer cells. Clin Exp Metastasis
2021;38(4):401-410. https://doi.org/10.1007/s10585-021-10103-0.
https://doi.org/10.1007/s10585-021-10103-0 |
| 266 | Van Coillie E, Van Aelst I, Wuyts A, Vercauteren R, Devos R, De Wolf-Peeters C, Van Damme J,
Opdenakker G: Tumor angiogenesis induced by granulocyte chemotactic protein-2 as a countercurrent
principle. Am J Pathol 2001;159(4):1405-1414. https://doi.org/10.1016/S0002-9440(10)62527-8.
https://doi.org/10.1016/S0002-9440(10)62527-8 |
| 267 | Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI:
Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor
angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 2011;179(3):1455-1470.
https://doi.org/10.1016/j.ajpath.2011.05.031.
https://doi.org/10.1016/j.ajpath.2011.05.031 |
| 268 | Shang A, Gu C, Wang W, Wang X, Sun J, Zeng B, Chen C, Chang W, Ping Y, Ji P, Wu J, Quan W, Yao Y,
Zhou Y, Sun Z, Li D: Exosomal circPACRGL promotes progression of colorectal cancer via the
miR-142-3p/miR-506-3p- TGF-β1 axis. Mol Cancer 2020;19(1):117.
https://doi.org/10.1186/s12943-020-01235-0.
https://doi.org/10.1186/s12943-020-01235-0 |
| 269 | Jablonska J, Wu CF, Andzinski L, Leschner S, Weiss S: CXCR2-mediated tumor-associated neutrophil
recruitment is regulated by IFN-β. Int J Cancer 2014;134(6):1346-1358.
https://doi.org/10.1002/ijc.28551.
https://doi.org/10.1002/ijc.28551 |
| 270 | Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y,
Marinelli B, Gorbatov R, Forghani R, Novobrantseva TI, Koteliansky V, Figueiredo JL, Chen JW,
Anderson DG, Nahrendorf M, Swirski FK, Weissleder R, Pittet MJ: Origins of tumor-associated
macrophages and neutrophils. Proc Natl Acad Sci USA 2012;109(7):2491-2496.
https://doi.org/10.1073/pnas.1113744109.
https://doi.org/10.1073/pnas.1113744109 |
| 271 | Tokumoto M, Tanaka H, Ohira M, Go Y, Okita Y, Sakurai K, Toyokawa T, Kubo N, Muguruma K, Maeda K,
Sawada T, Hirakawa K: A positive correlation between neutrophils in regional lymph nodes and
progression of gastric cancer. Anticancer Res 2014;34(12):7129-7136. PMID: 25503140.
|
| 272 | Zhang J, Qiao X, Shi H, Han X, Liu W, Tian X, Zeng X: Circulating tumor-associated neutrophils
(cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation.
Tumour Biol 2016;37(4):5397-5404. https://doi.org/10.1007/s13277-015-4349-3.
https://doi.org/10.1007/s13277-015-4349-3 |
| 273 | Liu X, Taftaf R, Kawaguchi M, Chang YF, Chen W, Entenberg D, Zhang Y, Gerratana L, Huang S, Patel
DB, Tsui E, Adorno-Cruz V, Chirieleison SM, Cao Y, Harney AS, Patel S, Patsialou A, Shen Y, Avril S,
Gilmore HL, Lathia JD, Abbott DW, Cristofanilli M, Condeelis JS, Liu H: Homophilic CD44 Interactions
Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models.
Cancer Discov 2019;9(1):96-113. https://doi.org/10.1158/2159-8290.CD-18-0065 .
https://doi.org/10.1158/2159-8290.CD-18-0065 |
| 274 | Dang YZ, Chen XJ, Yu J, Zhao SH, Cao XM, Wang Q: Cathepsin C promotes colorectal cancer metastasis
by regulating immune escape through upregulating CSF1. Neoplasma 2023;70(1):123-135.
https://doi.org/10.4149/neo_2023_220726N757.
https://doi.org/10.4149/neo_2023_220726N757 |
| 275 | Tong X, Zhu T, Ma L, Yang X, Li C, Liu Y, Qin X, Ding Y, Xia H, Liu Y: Cathepsin C correlates with
M2 macrophage infiltration and regulates the tumor growth and metastasis in non-small cell lung
cancer. BMC Cancer 2025;25(1):1001. https://doi.org/10.1186/s12885-025-14341-3.
https://doi.org/10.1186/s12885-025-14341-3 |
| 276 | Zhang Y, Wei J, Liu S, Wang J, Han X, Qin H, Lang J, Cheng K, Li Y, Qi Y, Anderson GJ, Sukumar S,
Li S, Nie G: Inhibition of platelet function using liposomal nanoparticles blocks tumor metastasis.
Theranostics 2017;7(5):1062-1071. https://doi.org/10.7150/thno.17908.
https://doi.org/10.7150/thno.17908 |
| 277 | Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee
Y, Won NH, Nakasone ES, Hearn SA, Küttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg
MS, Egeblad M: Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci
Transl Med 2016;8(361):361ra138. https://doi.org/10.1126/scitranslmed.aag1711.
https://doi.org/10.1126/scitranslmed.aag1711 |
| 278 | Yang D, Liu J: Neutrophil Extracellular Traps: A New Player in Cancer Metastasis and Therapeutic
Target. J Exp Clin Cancer Res 2021;40(1):233. https://doi.org/10.1186/s13046-021-02013-6.
https://doi.org/10.1186/s13046-021-02013-6 |
| 279 | Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri
L: Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin
Invest 2013;123(8):3446-3458. https://doi.org/10.1172/JCI67484.
https://doi.org/10.1172/JCI67484 |
| 280 | Lok LSC, Dennison TW, Mahbubani KM, Saeb-Parsy K, Chilvers ER, Clatworthy MR: Phenotypically
distinct neutrophils patrol uninfected human and mouse lymph nodes. Proc Natl Acad Sci USA
2019;116(38):19083-19089. https://doi.org/10.1073/pnas.1905054116.
https://doi.org/10.1073/pnas.1905054116 |
| 281 | Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H,
Tsung A: Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases
after Surgical Stress. Cancer Res 2016;76(6):1367-1380.
https://doi.org/10.1158/0008-5472.CAN-15-1591.
https://doi.org/10.1158/0008-5472.CAN-15-1591 |
| 282 | Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J,
Rousseau S, Ferri LE, Spicer JD: Neutrophil extracellular traps sequester circulating tumor cells
via β1-integrin mediated interactions. Int J Cancer 2017;140(10):2321-2330.
https://doi.org/10.1002/ijc.30635.
https://doi.org/10.1002/ijc.30635 |
| 283 | Monti M, De Rosa V, Iommelli F, Carriero MV, Terlizzi C, Camerlingo R, Belli S, Fonti R, Di Minno
G, Del Vecchio S: Neutrophil Extracellular Traps as an Adhesion Substrate for Different Tumor Cells
Expressing RGD-Binding Integrins. Int J Mol Sci 2018;19(8):2350.
https://doi.org/10.3390/ijms19082350.
https://doi.org/10.3390/ijms19082350 |
| 284 | Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H: Neutrophils facilitate ovarian cancer
premetastatic niche formation in the omentum. J Exp Med 2019;216(1):176-194.
https://doi.org/10.1084/jem.20181170.
https://doi.org/10.1084/jem.20181170 |
| 285 | Takesue S, Ohuchida K, Shinkawa T, Otsubo Y, Matsumoto S, Sagara A, Yonenaga A, Ando Y, Kibe S,
Nakayama H, Iwamoto C, Shindo K, Moriyama T, Nakata K, Miyasaka Y, Ohtsuka T, Toma H, Tominaga Y,
Mizumoto K, Hashizume M, Nakamura M: Neutrophil extracellular traps promote liver micrometastasis in
pancreatic ductal adenocarcinoma via the activation of cancer-associated fibroblasts. Int J Oncol
2020;56(2):596-605. https://doi.org/10.3892/ijo.2019.4951.
https://doi.org/10.3892/ijo.2019.4951 |
| 286 | Shaul ME, Eyal O, Guglietta S, Aloni P, Zlotnik A, Forkosh E, Levy L, Weber LM, Levin Y, Pomerantz
A, Nechushtan H, Eruslanov E, Singhal S, Robinson MD, Krieg C, Fridlender ZG: Circulating neutrophil
subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis.
FASEB J 2020;34(3):4204-4218. https://doi.org/10.1096/fj.201902467R.
https://doi.org/10.1096/fj.201902467R |
| 287 | Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V,
Bertos N, Cools-Lartigue J, Ferri LE, Spicer JD: Primary tumors induce neutrophil extracellular
traps with targetable metastasis promoting effects. JCI Insight 2019;5(16):e128008.
https://doi.org/10.1172/jci.insight.128008.
https://doi.org/10.1172/jci.insight.128008 |
| 288 | Zhang C, Liu Y, Gao Y, Shen J, Zheng S, Wei M, Zeng X: Modified heparins inhibit integrin
alpha(IIb)beta(3) mediated adhesion of melanoma cells to platelets in vitro and in vivo. Int J
Cancer 2009;125(9):2058-2065. https://doi.org/10.1002/ijc.24561.
https://doi.org/10.1002/ijc.24561 |
| 289 | Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE: Neutrophils
promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res
2012;72(16):3919-3927. https://doi.org/10.1158/0008-5472.CAN-11-2393.
https://doi.org/10.1158/0008-5472.CAN-11-2393 |
| 290 | Wculek SK, Malanchi I: Neutrophils support lung colonization of metastasis-initiating breast
cancer cells. Nature 2015;528(7582):413-417. https://doi.org/10.1038/nature16140. Correction:
https://doi.org/10.1038/s41586-019-1328-7.
https://doi.org/10.1038/s41586-019-1328-7 |
| 291 | Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C,
Scherrer R, Singer J, Beisel C, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP,
Beerenwinkel N, Aceto N: Neutrophils escort circulating tumour cells to enable cell cycle
progression. Nature 2019;566(7745):553-557. https://doi.org/10.1038/s41586-019-0915-y.
https://doi.org/10.1038/s41586-019-0915-y |
| 292 | Takano T, Li YJ, Kukita A, Yamaza T, Ayukawa Y, Moriyama K, Uehara N, Nomiyama H, Koyano K, Kukita
T: Mesenchymal stem cells markedly suppress inflammatory bone destruction in rats with
adjuvant-induced arthritis. Lab Invest 2014;94(3):286-296.
https://doi.org/10.1038/labinvest.2013.152.
https://doi.org/10.1038/labinvest.2013.152 |
| 293 | Ahirwar DK, Nasser MW, Ouseph MM, Elbaz M, Cuitiño MC, Kladney RD, Varikuti S, Kaul K, Satoskar
AR, Ramaswamy B, Zhang X, Ostrowski MC, Leone G, Ganju RK: Fibroblast-derived CXCL12 promotes breast
cancer metastasis by facilitating tumor cell intravasation. Oncogene 2018;37(32):4428-4442.
https://doi.org/10.1038/s41388-018-0263-7.
https://doi.org/10.1038/s41388-018-0263-7 |
| 294 | Imai K, Kobayashi M, Wang J, Shinobu N, Yoshida H, Hamada J, Shindo M, Higashino F, Tanaka J,
Asaka M, Hosokawa M: Selective secretion of chemoattractants for haemopoietic progenitor cells by
bone marrow endothelial cells: a possible role in homing of haemopoietic progenitor cells to bone
marrow. Br J Haematol 1999;106(4):905-911. https://doi.org/10.1046/j.1365-2141.1999.01644.x.
https://doi.org/10.1046/j.1365-2141.1999.01644.x |
| 295 | Jiang H, Ge H, Shi Y, Yuan F, Yue H: CAFs secrete CXCL12 to accelerate the progression and
cisplatin resistance of colorectal cancer through promoting M2 polarization of macrophages. Med
Oncol 2023;40(3):90. https://doi.org/10.1007/s12032-023-01953-7.
https://doi.org/10.1007/s12032-023-01953-7 |
| 296 | Flad HD, Brandt E: Platelet-derived chemokines: pathophysiology and therapeutic aspects. Cell Mol
Life Sci 2010;67(14):2363-2386. https://doi.org/10.1007/s00018-010-0306-x.
https://doi.org/10.1007/s00018-010-0306-x |
| 297 | Chatterjee M, Gawaz M: Platelet-derived CXCL12 (SDF-1α): basic mechanisms and clinical
implications. J Thromb Haemost 2013;11(11):1954-1967. https://doi.org/10.1111/jth.12404.
https://doi.org/10.1111/jth.12404 |
| 298 | Yan R, Moresco P, Gegenhuber B, Fearon DT: T cell-mediated development of stromal fibroblasts with
an immune-enhancing chemokine profile. Cancer Immunol Res 2023;May 22:CIR-22-0593.
https://doi.org/10.1158/2326-6066.CIR-22-0593.
https://doi.org/10.1158/2326-6066.CIR-22-0593 |
| 299 | Verbeke H, Geboes K, Van Damme J, Struyf S: The role of CXC chemokines in the transition of
chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta 2012;1825(1):117-129.
https://doi.org/10.1016/j.bbcan.2011.10.008.
https://doi.org/10.1016/j.bbcan.2011.10.008 |
| 300 | Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR: Multiple
actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res
2002;62(20):5930-5938. PMID: 12384559.
|
| 301 | Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG: Stromal cell derived factor-1:
its influence on invasiveness and migration of breast cancer cells in vitro, and its association
with prognosis and survival in human breast cancer. Breast Cancer Res 2005;7(4): R402-10.
https://doi.org/10.1186/bcr1022.
https://doi.org/10.1186/bcr1022 |
| 302 | Teicher BA, Fricker SP: CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 2010;16(11):
2927-2931. https://doi.org/10.1158/1078-0432.CCR-09-2329.
https://doi.org/10.1158/1078-0432.CCR-09-2329 |
| 303 | Lee BC, Lee TH, Avraham S, Avraham HK: Involvement of the chemokine receptor CXCR4 and its ligand
stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular
endothelial cells. Mol Cancer Res 2004;2(6):327-338. PMID: 15235108.
https://doi.org/10.1158/1541-7786.327.2.6 |
| 304 | Matteucci E, Locati M, Desiderio MA: Hepatocyte growth factor enhances CXCR4 expression favoring
breast cancer cell invasiveness. Exp Cell Res 2005;10(1):176-185.
https://doi.org/10.1016/j.yexcr.2005.07.008.
https://doi.org/10.1016/j.yexcr.2005.07.008 |
| 305 | Yin X, Liu Z, Zhu P, Wang Y, Ren Q, Chen H, Xu J: CXCL12/CXCR4 promotes proliferation, migration,
and invasion of adamantinomatous craniopharyngiomas via PI3K/AKT signal pathway. J Cell Biochem
2019;120(6):9724-9736. https://doi.org/10.1002/jcb.28253.
https://doi.org/10.1002/jcb.28253 |
| 306 | Xiaowei C, Jia M, Xiaowei W, Yina Z: Overexpression of CXCL12 chemokine up-regulates connexin and
integrin expression in mesenchymal stem cells through PI3K/Akt pathway. Cell Commun Adhes
2013;20(3-4):67-72. https://doi.org/10.3109/15419061.2013.791682.
https://doi.org/10.3109/15419061.2013.791682 |
| 307 | Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL,
Weinberg RA: Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in
the breast. Cell 2011;145(6):926-940. https://doi.org/10.1016/j.cell.2011.04.029.
https://doi.org/10.1016/j.cell.2011.04.029 |
| 308 | Mezawa Y, Daigo Y, Takano A, Miyagi Y, Yokose T, Yamashita T, Morimoto C, Hino O, Orimo A: CD26
expression is attenuated by TGF-β and SDF-1 autocrine signaling on stromal myofibroblasts in human
breast cancers. Cancer Med 2019;8(8):3936-3948. https://doi.org/10.1002/cam4.2249.
https://doi.org/10.1002/cam4.2249 |
| 309 | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN,
Barrera JL, Mohar A, Verástegui E, Zlotnik A: Involvement of chemokine receptors in breast cancer
metastasis. Nature 2001;410(6824):50-56. https://doi.org/10.1038/35065016.
https://doi.org/10.1038/35065016 |
| 310 | O'Connor KL, Nguyen BK, Mercurio AM: RhoA function in lamellae formation and migration is
regulated by the alpha6beta4 integrin and cAMP metabolism. J Cell Biol 2000;148(2):253-258.
https://doi.org/10.1083/jcb.148.2.253.
https://doi.org/10.1083/jcb.148.2.253 |
| 311 | Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L: Urokinase receptor and fibronectin
regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or
dormancy in vivo. Mol Biol Cell 2001;12(4):863-879. https://doi.org/10.1091/mbc.12.4.863.
https://doi.org/10.1091/mbc.12.4.863 |
| 312 | Kleinman HK, Klebe RJ, Martin GR: Role of collagenous matrices in the adhesion and growth of
cells. J Cell Biol 1981;88(3):473-485.
https://doi.org/10.1083/jcb.88.3.473 |
| 313 | Wang Z, Moresco P, Yan R, Li J, Gao Y, Biasci D, Yao M, Pearson J, Hechtman JF, Janowitz T, Zaidi
RM, Weiss MJ, Fearon DT: Carcinomas assemble a filamentous CXCL12-keratin-19 coating that suppresses
T cell-mediated immune attack. Proc Natl Acad Sci USA 2022;119(4):e2119463119.
https://doi.org/10.1073/pnas.2119463119.
https://doi.org/10.1073/pnas.2119463119 |
| 314 | Stasiak PC, Lane EB: Sequence of cDNA coding for human keratin 19. Nucleic Acids Res
1987;15(23):10058. https://doi.org/10.1093/nar/15.23.10058.
https://doi.org/10.1093/nar/15.23.10058 |
| 315 | Bartek J, Taylor-Papadimitriou J, Miller N, Millis R: Patterns of expression of keratin 19 as
detected with monoclonal antibodies in human breast tissues and tumours. Int J Cancer
1985;36(3):299-306. PMID: 2411673.
https://doi.org/10.1002/ijc.1985.36.3.299 |
| 316 | Taylor-Papadimitriou J, Stampfer M, Bartek J, Lewis A, Boshell M, Lane EB, Leigh IM: Keratin
expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to
in vivo phenotypes and influence of medium. J Cell Sci 1989;94(Pt3):403-413.
https://doi.org/10.1242/jcs.94.3.403.
https://doi.org/10.1242/jcs.94.3.403 |
| 317 | Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T: Signal sequence trap: a cloning
strategy for secreted proteins and type I membrane proteins. Science 1993;261(5121):600-603.
https://doi.org/10.1126/science.8342023.
https://doi.org/10.1126/science.8342023 |
| 318 | Nagasawa T, Kikutani H, Kishimoto T: Molecular cloning and structure of a pre-B-cell
growth-stimulating factor. Proc Natl Acad Sci USA 1994;91(6):2305-2309.
https://doi.org/10.1073/pnas.91.6.2305.
https://doi.org/10.1073/pnas.91.6.2305 |
| 319 | Shibuta K, Begum NA, Mori M, Shimoda K, Akiyoshi T, Barnard GF: Reduced expression of the CXC
chemokine hIRH/SDF-1alpha mRNA in hepatoma and digestive tract cancer. Int J Cancer
1997;73(5):656-662. https://doi.org/10.1002/(sici)1097-0215(19971127)73:5<656::aid-ijc8>3.0.co;2-w.
https://doi.org/10.1002/(SICI)1097-0215(19971127)73:5<656::AID-IJC8>3.0.CO;2-W |
| 320 | Psaila B, Lyden D: The metastatic niche: adapting the foreign soil. Nat Rev Cancer
2009;9(4):285-293. https://doi.org/10.1038/nrc2621.
https://doi.org/10.1038/nrc2621 |
| 321 | Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA,
Stainier DY, Chen EI, Lyden D, Bissell MJ: The perivascular niche regulates breast tumour dormancy.
Nat Cell Biol 2013;15(7):807-817. https://doi.org/10.1038/ncb2767.
https://doi.org/10.1038/ncb2767 |
| 322 | Fornetti J, Welm AL, Stewart SA: Understanding the Bone in Cancer Metastasis. J Bone Miner Res
2018;33(12):2099-2113. https://doi.org/10.1002/jbmr.3618.
https://doi.org/10.1002/jbmr.3618 |
| 323 | Maheswaran S, Haber DA: Circulating tumor cells: a window into cancer biology and metastasis. Curr
Opin Genet Dev 2010;20(1):96-99. https://doi.org/10.1016/j.gde.2009.12.002.
https://doi.org/10.1016/j.gde.2009.12.002 |
| 324 | Yu M, Stott S, Toner M, Maheswaran S, Haber DA: Circulating tumor cells: approaches to isolation
and characterization. J Cell Biol 2011;192(3):373-382. https://doi.org/10.1083/jcb.201010021.
https://doi.org/10.1083/jcb.201010021 |
| 325 | Rejniak KA: Circulating Tumor Cells: When a Solid Tumor Meets a Fluid Microenvironment. Adv Exp
Med Biol 2016;936:93-106. https://doi.org/10.1007/978-3-319-42023-3_5.
https://doi.org/10.1007/978-3-319-42023-3_5 |
| 326 | McEver RP: Selectins: novel receptors that mediate leukocyte adhesion during inflammation. Thromb
Haemost 1991;65(3):223-228.PMID: 1710835.
https://doi.org/10.1055/s-0038-1647488 |
| 327 | Bevilacqua MP, Nelson RM. Selectins: J Clin Invest 1993;91(2):379-387.
https://doi.org/10.1172/JCI116210.
https://doi.org/10.1172/JCI116210 |
| 328 | Varki A: Selectin ligands. Proc Natl Acad Sci USA 1994;91(16):7390-7397.
https://doi.org/10.1073/pnas.91.16.739.
https://doi.org/10.1073/pnas.91.16.7390 |
| 329 | Liu Z, Dou Y, Lu C, Han R, He Y: Neutrophil extracellular traps in tumor metabolism and
microenvironment. Biomark Res 2025;13(1):12. https://doi.org/10.1073/10.1186/s40364-025-00731-z.
https://doi.org/10.1186/s40364-025-00731-z |
| 330 | Barbazan J, Pérez-González C, Gómez-González M, Dedenon M, Richon S, Latorre E, Serra M, Mariani
P, Descroix S, Sens P, Trepat X, Vignjevic DM: Cancer-associated fibroblasts actively compress
cancer cells and modulate mechanotransduction. Nat Commun 2023;14(1):6966.
https://doi.org/10.1038/s41467-023-42382-4.
https://doi.org/10.1038/s41467-023-42382-4 |
| 331 | Yeini E, Satchi-Fainaro R: The role of P-selectin in cancer-associated thrombosis and beyond.
Thromb Res 2022;213 Suppl 1:S22-S28. https://doi.org/10.1016/j.thromres.2021.12.027.
https://doi.org/10.1016/j.thromres.2021.12.027 |
| 332 | Jones DS, Wallace AC, Fraser EE: Sequence of events in experimental metastases of Walker 256
tumor: light, immunofluorescent, and electron microscopic observations. J Natl Cancer Inst
1971;46(3):493-504. PMID: 4100699.
|
| 333 | Nieswandt B, Hafner M, Echtenacher B, Männel DN: Lysis of tumor cells by natural killer cells in
mice is impeded by platelets. Cancer Res 1999;59(6):1295-1300. PMID: 1009656.2.
|
| 334 | Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD:
Cancers predispose neutrophils to release extracellular DNA traps that contribute to
cancer-associated thrombosis. Proc Natl Acad Sci U S A 2012;109(32):13076-13081.
https://doi.org/10.1073/pnas.1200419109.
https://doi.org/10.1073/pnas.1200419109 |
| 335 | Kaplan MJ, Radic M: Neutrophil extracellular traps: double-edged swords of innate immunity. J
Immunol 2012;189(6):2689-2695. https://doi.org/10.4049/jimmunol.1201719.
https://doi.org/10.4049/jimmunol.1201719 |
| 336 | Erpenbeck L, Schön MP: Neutrophil extracellular traps: protagonists of cancer progression?
Oncogene 2017;36(18):2483-2490. https://doi.org/10.1038/onc.2016.406.
https://doi.org/10.1038/onc.2016.406 |
| 337 | Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H,
Tsung A: Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases
after Surgical Stress. Cancer Res 2016;76(6):1367-1380.
https://doi.org/10.1158/0008-5472.CAN-15-1591.
https://doi.org/10.1158/0008-5472.CAN-15-1591 |
| 338 | Merza M, Hartman H, Rahman M, Hwaiz R, Zhang E, Renström E, Luo L, Mörgelin M, Regner S,
Thorlacius H: Neutrophil Extracellular Traps Induce Trypsin Activation, Inflammation, and Tissue
Damage in Mice With Severe Acute Pancreatitis. Gastroenterology 2015;149(7):1920-1931.e8.
https://doi.org/10.1053/j.gastro.2015.08.026.
https://doi.org/10.1053/j.gastro.2015.08.026 |
| 339 | Yang C, Sun W, Cui W, Li X, Yao J, Jia X, Li C, Wu H, Hu Z, Zou X: Procoagulant role of neutrophil
extracellular traps in patients with gastric cancer. Int J Clin Exp Pathol 2015;8(11):14075-14086.
PMID: 26823721.
|
| 340 | Kanamaru R, Ohzawa H, Miyato H, Matsumoto S, Haruta H, Kurashina K, Saito S, Hosoya Y, Yamaguchi
H, Yamashita H, Seto Y, Lefor AK, Sata N, Kitayama J: Low density neutrophils (LDN) in postoperative
abdominal cavity assist the peritoneal recurrence through the production of neutrophil extracellular
traps (NETs). Sci Rep 2018;8(1):632. https://doi.org/10.1038/s41598-017-19091-2.
https://doi.org/10.1038/s41598-017-19091-2 |
| 341 | Fidler IJ, Kripke ML: Metastasis results from preexisting variant cells within a malignant tumor.
Science 1977;197(4306):893-895. https://doi.org/10.1126/science.887927.
https://doi.org/10.1126/science.887927 |
| 342 | Nicolson GL, Brunson KW, Fidler IJ: Specificity of arrest, survival, and growth of selected
metastatic variant cell lines. Cancer Res 1978;38(11 Pt 2):4105-4111. PMID: 359132,
|
| 343 | Talmadge JE, Fidler IJ: AACR centennial series: the biology of cancer metastasis: historical
perspective. Cancer Res 2010;70(14):5649-5669. https://doi.org/10.1158/0008-5472.CAN-10-1040.
https://doi.org/10.1158/0008-5472.CAN-10-1040 |
| 344 | Jordan H: Ueber Spätrezidive des Karzinoms. DMW - Deutsche Medizinische Wochenschrift
1904;30(25):912-914. https://doi.org/10.1055/s-0029-1187580.
https://doi.org/10.1055/s-0029-1187580 |
| 345 | Bizzozero G: Prelezione al Corso di Patologia Generale Nella Università di Torino (Tip. Lit.
Camilla e Bertolero). Torino. 1873.
|
| 346 | Park JY, Georges D, Alberts CJ, Bray F, Clifford G, Baussano I: Global lifetime estimates of
expected and preventable gastric cancers across 185 countries. Nat Med 2025 Jul 7.
https://doi.org/10.1038/s41591-025-03793-6.
https://doi.org/10.1038/s41591-025-03793-6 |
| 347 | Biospace. Market Size, Share and Growth Report 2034. July 7, 2025. Website
https://www.biospace.com/press-releases/u-s-gastric-cancer-diagnostics-market-size-share-and-growth-report-2034.
Access July 8, 2025.
|
| 348 | Lichnowsky M: Der Kampf mit dem Fachmann. Jahoda& Siegel Verlag. Wien, Leipzig. 1924.
|
| 349 | Botteri E, Hjorth S, Conforti F, Bagnardi V, Andreassen BK, Støer NC, Bhargava S, Ursin G, Gandini
S, Sloan EK, Chang A: Aprepitant use during chemotherapy and association with survival in women with
early breast cancer. J Natl Cancer Inst 2025 Jul 14:djaf178. https://doi.org/10.1093/jnci/djaf178.
https://doi.org/10.1093/jnci/djaf178 |
| 350 | Billroth T: Brief Professor Theodor Billroth an Professor Dr Johann von Mikulicz in Krakau, Wien,
12. Februar 1883. In: Briefe von Theodor Billroth. Von Dr. Georg Fischer. Erste Auflage. Georg
Fischer Verlag. 1896.
|
| 351 | Billroth T: Die Krankenpflege im Haus und im Spital. Carl Gerold's Sohn Verlag, Wien. 1881
|
| 352 | Schiller F: Xenien und Votivtafeln. Hofenberg. 1796. ISBN 978-3-8430-7081-2.
|
| 353 | Virchow R: Die Cellularpathologie in Ihrer Begruendung auf Physiologische und Pathologische
Gewebelehre. Hirschwald A Verlag, Berlin. 1858.
|
| 354 | Tavakoli A, Hu S, Ebrahim S, Kachar B: Hemifusomes and interacting proteolipid nanodroplets
mediate multi-vesicular body formation. Nat Commun 2025;16(1):4609.
https://doi.org/10.1038/s41467-025-59887-9.
https://doi.org/10.1038/s41467-025-59887-9 |
| 355 | Goethe JW: Wilhelm Meisters Wandjahre oder die Entsagenden, 3. Buch, 18. Kapitel, Cotta'sche
Buchhandlung Stuttgart und Tübingen. 1821.
|