Original Article - DOI:10.33594/000000829
Accepted 1 November 2025 - Published online
13 November 2025
Dietary Vitamin E Ameliorate Production Performance Via Pyruvate Metabolism Regulation in An Aged Laying Quails
bBranch of Animal Husbandry and Veterinary of Heilongjiang Academy of Agricultural Sciences, Qiqihar, China
Keywords
Abstract
Background/Aims:
This study aimed to clarify the optimal amount of vitamin E required in the late stage of egg laying to explore the aging mechanism of the ovaries in this stage and the regulatory role of vitamin E in female reproductive aging using multiple experimental methods and multiomics joint analysis.Methods:
The development of follicles was analyzed using Hematoxylin-eosin staining and terminal deoxynucleotidyl transferase deoxyuridine phosphate nick-end labeling staining. The content of follicle-stimulating hormone (FSH), luteinizing hormone (LH) and Estradiol (E2) was determined by Enzyme-linked Immunoassay Kit. The activities of superoxide dismutase (SOD) and malondialdehyde (MDA) in the serum using enzyme-linked immunosorbent assay kits. RNA-seq and Untargeted metabolomics analyses was used to to investigate the molecular mechanisms of vitamin E during ovarian aging.Results:
The findings revealed that quail reproductive organs rapidly aged at the 11th egg-laying month. Adding vitamin E to the diet could significantly improve the egg production performance of quails. Furthermore, the vitamin E could promote the development of small yellow follicles (SYFs) by enhancing the antioxidant capacity and inhibiting cell apoptosis in quails. Moreover, we confirmed through RNA-seq and liquid chromatography with tandem mass spectrometry that a series of genes and metabolites could serve as biomarkers of vitamin E to improve ovarian aging.Conclusion:
Altogether, these data demonstrated supplementing the diet with 250 mg/kg vitamin E enhanced the body's energy metabolism, regulated SOD and MDA levels, enhanced antioxidant capacity, reduced cell apoptosis in preovulatory follicles, stimulated E2 secretion, promoted follicular development, and improved egg production performance.Introduction
For laying birds, egg production performance is an important economic indicator that directly determines the economic benefits of the farm. Mammalian reproductive performance is inversely correlated with age [1]. Similarly, the reproductive performance of birds declines rapidly with age. Quail has a strong ability to lay eggs, but in the 11th egg-laying month, the egg-laying rate decreases and the abnormal egg rate increases significantly. The reasons for these phenomena are ovarian aging and related functional impairments, especially a decrease in the number of follicles [2]. Therefore, alleviating ovarian aging is a key step in increasing the egg production of quails in the later stages of egg production.
The ovary is a highly dynamic organ that affects the quantity and quality of ovulation by selecting follicles, thus determining the reproductive ability of female animals. The development of the ovaries is mainly mediated by reproductive hormones produced by the hypothalamic-pituitary-gonadal axis [3]. Therefore, the reproductive aging of female animals not only refers to the aging of the ovaries but also includes the disruption of the feedback regulation mechanism between the gonadal axes, decreased hormone levels, follicular atresia, and decreased egg production. DNA damage, disrupted free radical balance, and mitochondrial dysfunction are molecular pathways that induce ovarian aging [4].
Vitamin E is a natural antioxidant. As birds cannot synthesize vitamin E within their bodies, they must rely on exogenous additives. The vitamin E in the diet improves the reproductive and antioxidant capacities of breeding chickens [5]. The recommended dosage of vitamin E (25 mg/kg feed) for egg-type quails by the National Research Council (NRC)[6] is extremely low and no longer suitable for modern quail breeding. Previously, the focus was mainly on regulating egg production performance by vitamin E in young quails [7-9]. Whether vitamin E could improve the egg production performance of quails in the late stage of egg production, and the optimal dosage and mechanism of vitamin E addition, were unclear. Therefore, this study focused on elderly Peking white quails in the late stage of egg production and fed them with feed containing vitamin E 10, 20, and 40 times the recommended amount of NRC. The study aimed to clarify the optimal amount of vitamin E added in the late stage of egg laying, explore the aging mechanism of ovaries in this stage, and investigate the regulatory role of vitamin E in female reproductive aging using multiple experimental methods and multiomics joint analysis. The research results provided important information for elucidating the reproductive aging mechanism of egg-laying birds, data support for clarifying the optimal amount of vitamin E required in animal feed, and solutions for the problem of decreased egg production performance in quails due to aging.
Materials and Methods
Animals, diets and experimental design
A total of 120 female Peking white quails (293-day-old) were purchased from the local market. The
experiment was
approved by the Institutional Animal Ethical Committee. The birds were randomly assigned, according to
their
initial body weights, to four treatment groups, three replicates of 10 birds each. Treatment groups
consisted of
basal diet addition 0 mg of VE (C-1) according to the NRC guidelines, basal diet addition 250 mg of VE
(V-1),
basal diet addition 500 mg of VE (V-2), basal diet addition 1000 mg of VE (V-3). dl-α-Tocopherol (Sigma,
Germany)was used as the source of VE. After the experiment (4 weeks), calculate the following metrics per
cage:
average daily feed intake, average egg weight, average daily egg production rate, feed conversion rate for
egg-type poultry, and abnormal egg ratio. Ingredients and chemical composition of the basal diet are shown
in
Table 1. All birds used in this study were kept under similar environmental conditions and given free
access to
food and water. After the experiment, quails were intramuscular injection(ketamine&diazepam, 20-40
mg/kg
& 1-1.5mg/kg) and euthanized using cervical dislocation method.
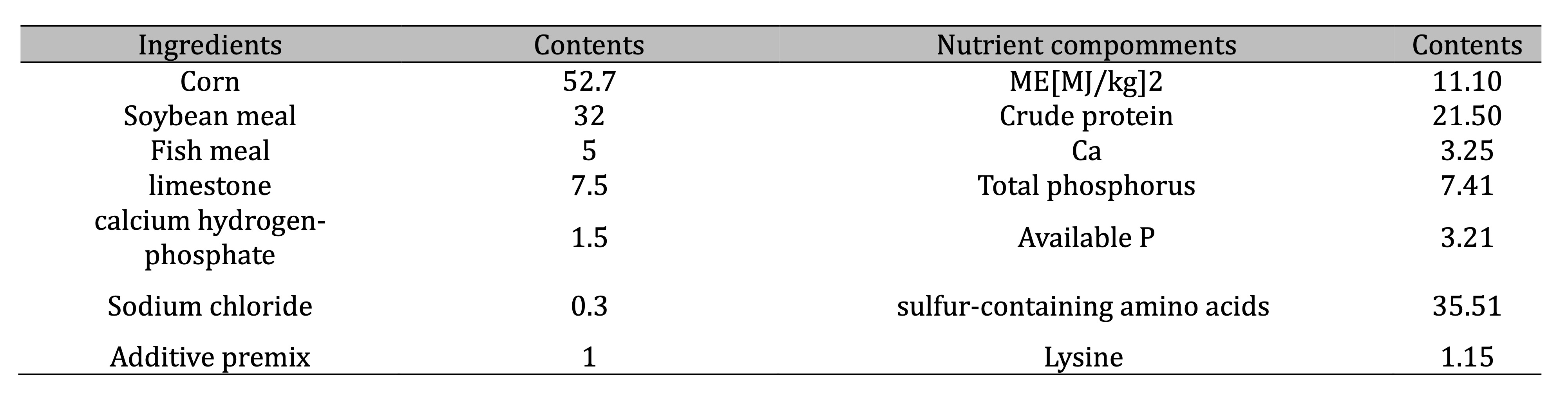
Table 1: Composition and nutrient levels of basal diet (air-dry basis) %. Additive premix supplied (per kg of diet): Vitamin A, 12 000 IU; Vitamin D, 33 000 IU; Vitamin E, 25 mg; Vitamin K, 20 mg; Vitamin B2, 70 mg; Vitamin B6, 60 mg; Folic acid, 20 mg; calcium pantothenate, 300 mg; Mn, 60 mg; Zn, 50 mg; Fe, 25 mg; Cu, 5 mg; I, 0.5 mg
Tissue collection
After the experiment, quails (n=20) were euthanized and their ovaries were taken out for weighing.
According to
Rodler's follicle classification method [10], follicles (>2 mm), small yellow follicles (SYF;1~2 mm),
and the
other follicles were isolated in descending order (<1 mm). Count the number of follicles and SYFs, and
weigh the
ovaries where the preovulatory follicles(<2 mm) are located.
Determination of reproductive hormones
The blood of quails (n=5) was centrifuged at 2, 000 g for 10 min at 4°C, then the supernatants were
collected
for reproductive hormones analyses. The content of follicle-stimulating hormone (FSH), luteinizing hormone
(LH)
and Estradiol (E2) was determined by Enzyme-linked Immunoassay Kit (Jiangsu Meimianindustrial Co, Ltd)
according
to the manufacturer's protocol.
Hematoxylin and eosin staining
Five quials in each group were humanely euthanized. The ovarian tissue was fixed with 4% paraformaldehyde
and
dehydrated step by step with increasing concentration of alcohol to immerse paraffin in the tissues. Then,
slicing, degreasing, step hydration, hematoxylin and eosin staining, and sealing were performed to
complete the
slicing. Three sections were observed for each bird, and the images were obtained. The thickness of the
granulosa cell layer and membrane layer of SYFs was measured using ImageJ software (ImageJ 1.5, NIH, USA).
Terminal deoxynucleotidyl transferase deoxyuridine phosphate nick-end labeling assay
Five quials in each group were humanely euthanized. The cell apoptosis in the ovary was examined using the
terminal deoxynucleotidyl transferase deoxyuridine phosphate nick-end labeling (TUNEL) staining kit
(Roche,
Mannheim, Germany) following the manufacturer’s protocol. The tissue sections were treated with proteinase
K,
washed with phosphate-buffered saline (PBS), incubated with the TUNEL reaction mixture, washed again with
PBS,
and stained with 4’,6-diamidino-2-phenylindole. Finally, the sections were sealed with an
anti-fluorescence
quenching solution. The results of cell staining were observed under a fluorescence microscope (Leica,
Wetzlar,
Germany). Three sections were observed for each bird, the images were obtained, and the number of
positively
stained cells was calculated using ImageJ 1.46R software (NIH, Bethesda, MD, USA). The ratio of apoptosis
positive cells (%) equals the number of red fluorescent cells/the number of blue fluorescent cells ×100 %.
Serum antioxidant properties
The blood of quails (n=5) was centrifuged at 2,000 g for 10 min at 4°C, then the supernatants were
collected
for antioxidant capacity. To evaluate the antioxidant capacity of vitamin E, we quantified the levels and
activities of superoxide dismutase (SOD) and malondialdehyde (MDA) in the serum using enzyme-linked
immunosorbent assay kits (Jiangsu Meimian industrial Co., Ltd) following the manufacturer's instructions.
RNA-seq
RNA-Seq was performed at Majorbio Company (Shanghai, China). For RNA-Seq, total RNA of the ovary (n=3) was
extracted using TRIzol (Invitrogen, CA, USA), following the manufacturer’s protocol. This was used to
prepare
the mRNA sequencing library, which was sequenced on a Hiseq X Ten system (Illumina, CA, USA). After
quality
control and data filtering, reads were mapped to the Coturnix_japonica genome (GCF_001577835.2).
Significant
differences in gene expressions were filtered with the criteria of p-value <0.05 and fold change>1.5
as
our previous study [11].
Untargeted metabolomics analyses
Liquid chromatography with tandem mass spectrometry (LC-MS/MS) analyses were performed using a UHPLC-Q
Exactive
HF-X system (Thermo Fisher Scientific, Inc.). The extract analysis, metabolite identification and
quantification
of ovaries(n=6) were performed by Majorbio Company (Shanghai, China) following their standard procedures
and
previous study [12]. The Human Metabolome Database (HMDB) and the Majorbio Database were used to retrieve
and
identify detected metabolites. The ropls (Version 1.6.2) in the R package were used for partial least
squares
discriminant analysis (PLS-DA) and orthogonal PLS-DA of the identified metabolites between the groups. The
selection of significantly differential metabolites was determined based on the variable importance in
projection (VIP) obtained using the PLS-DA model and the P value of the Student t test with a VIP score
>1
and P <0.05. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database was used to
assess
the functional differences related to differential metabolites.
RT-qPCR
The RNA-seq results were validated by qPCR. The method of RT-qPCR and the data were calculated as
previously
detailed [11]. The primers used in qPCR are listed in Table 2.
Statistical analysis
SPSS 16.0 software(IBM, USA) and GraphPad 8.0 softwares (San Diego, California, USA) were used for
statistical
analyses. All experiments were repeated three times. Data are shown as the mean ± standard deviation (SD).
Comparisons between the two groups were performed using Student’s t-tests. Comparisons among multiple
groups
were performed using two-way ANOVA followed by Duncan’s test. Differences were considered statistically
significant at P < 0.05.
Results
Impact of increasing age on reproductive organs
To investigate the effect of vitamin E on the egg production performance of quails, we initially assessed the aging of reproductive organs with advancing age. The results indicated that, compared with 300-day-old quails (Fig. 1A), 328-day-old quails (Fig. 1B) exhibited reduced ovarian volume, decreased follicle count, and an increase in the number of atretic follicles. Moreover, the fallopian tubes appeared shortened, swollen, and deformed (Fig. 1B). Concurrently, the production rate of different types of abnormal eggs also increased. These findings demonstrated that the quail reproductive organs underwent rapid aging during the 11th month of egg laying.

Fig. 1: Morphology of reproductive organs in quails. (A) Ovaries and fallopian tube of 300-day-old quails; (B) Ovaries and fallopian tubes of 328-day-old quails; (C) Quail eggs of different ages.
xVitamin E improved the egg production performance of quails
To address the issue of aging of quail reproductive organs in the late stage of egg laying, we conducted a
feeding experiment with vitamin E. No significant change was observed in the average daily feed intake
compared
with C-1 (Table 3). However, the average egg weight significantly increased in all groups
(P<0.05).
Specifically, the average egg production rate significantly increased in the group receiving the lowest
dose of
vitamin E (V-1) (P<0.05), slightly increased in the group receiving a medium dose (V-2), and did
not
exhibit significant changes in the group receiving the highest dose (V-3). Additionally, the feed
conversion
rate significantly decreased in all groups (P<0.05). The abnormal egg ratio decreased after
adding
vitamin E, with a significant difference observed in V-1 (P<0.05). These results indicated that
vitamin E supplementation could increase the quail egg production rate and improve overall performance.
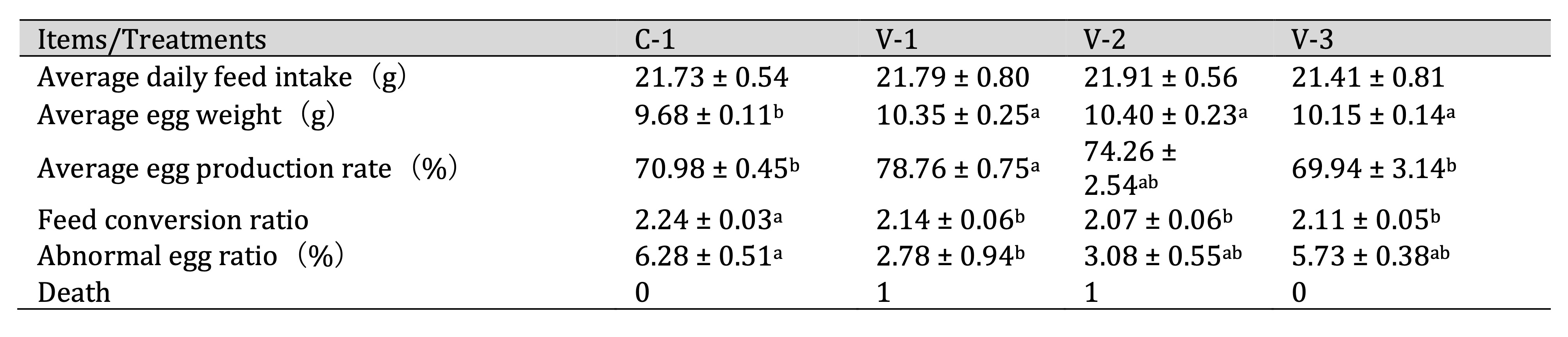
Table 3: Effect of vitamin E on the egg production performance of quails. a-bThe different lowercase letters represent a signiffcant difference (P < 0.05)
Vitamin E promoted ovarian development
Ovarian weight and the number of follicles are crucial indicators for assessing ovarian development and
physiological function. Therefore, we investigated the effect of vitamin E on quail ovarian development.
As
shown in Table 4, the addition of vitamin E increased ovarian weight, with a significant difference
observed in
groups V-2 and V-3 compared with the control group (C-1) (P<0.05). This change was not
determined by
the number of follicles but rather by the number of small yellow follicles (SYFs). With the
supplementation of
vitamin E, the number of SYFs increased and a significant difference was noted in groups V-1 and V-2
(P<0.05).
FSH, LH, and E2 are the primary hormones regulating follicular development. Hence, we examined their
serum
levels. The findings revealed no significant effect of vitamin E on FSH and LH levels. However, it
significantly
increased E2 levels in V-1 (P<0.05; Fig. 2). Therefore, vitamin E might promote ovarian development
by
upregulating E2 levels.
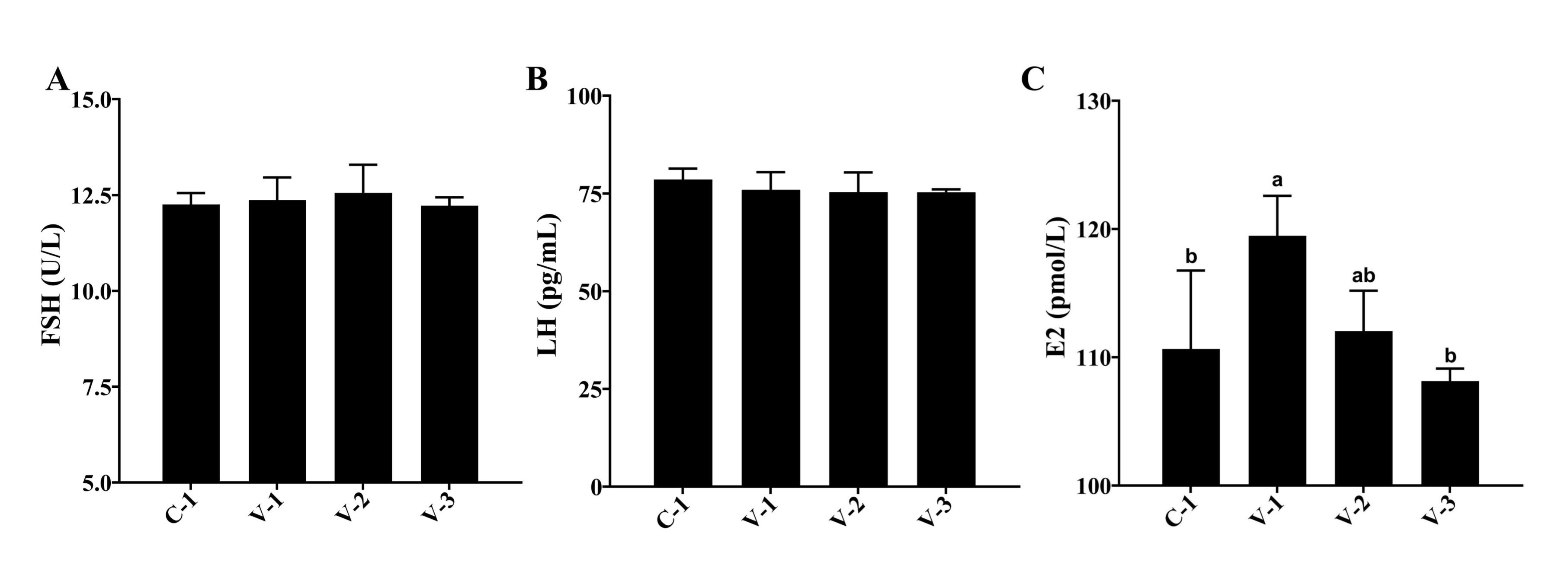
Fig. 2: Hormonal changes in quail serum. (A) Effect of vitamin E addition on FSH levels; (B) Effect of vitamin E addition on LH levels; (C) Effect of vitamin E addition on E2 levels. a-bThe different lowercase letters represent a signiffcant difference (P<0.05).
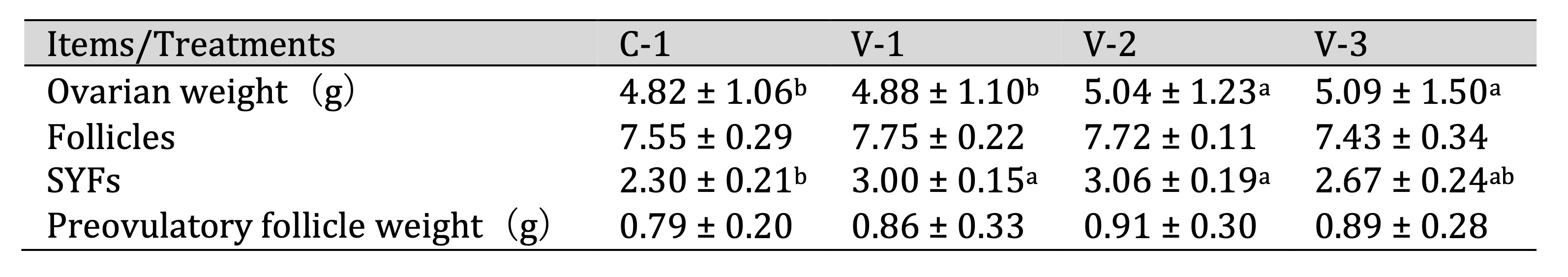
Table 4: Effect of vitamin E on the development of quail ovaries. a-bThe different lowercase letters represent a signiffcant difference (P < 0.05)
Vitamin E promoted the development of SYFs
During the development of avian follicles, SYFs play a crucial role in the maturation of
preovulatory
follicles.
The histological examinations of SYFs revealed notable changes. Both the V-1 and V-2 groups
exhibited a
significant increase in the granulosa cell layer compared with the C-1 group (P<0.05; Fig.
3A
and 3B).
Additionally, the follicular membrane layer thickened significantly upon vitamin E supplementation
(P<0.05; Fig. 3A and 3C). These observations suggested that vitamin E promoted SYF
development
by
stimulating the growth of granulosa cells and follicular membrane cells.
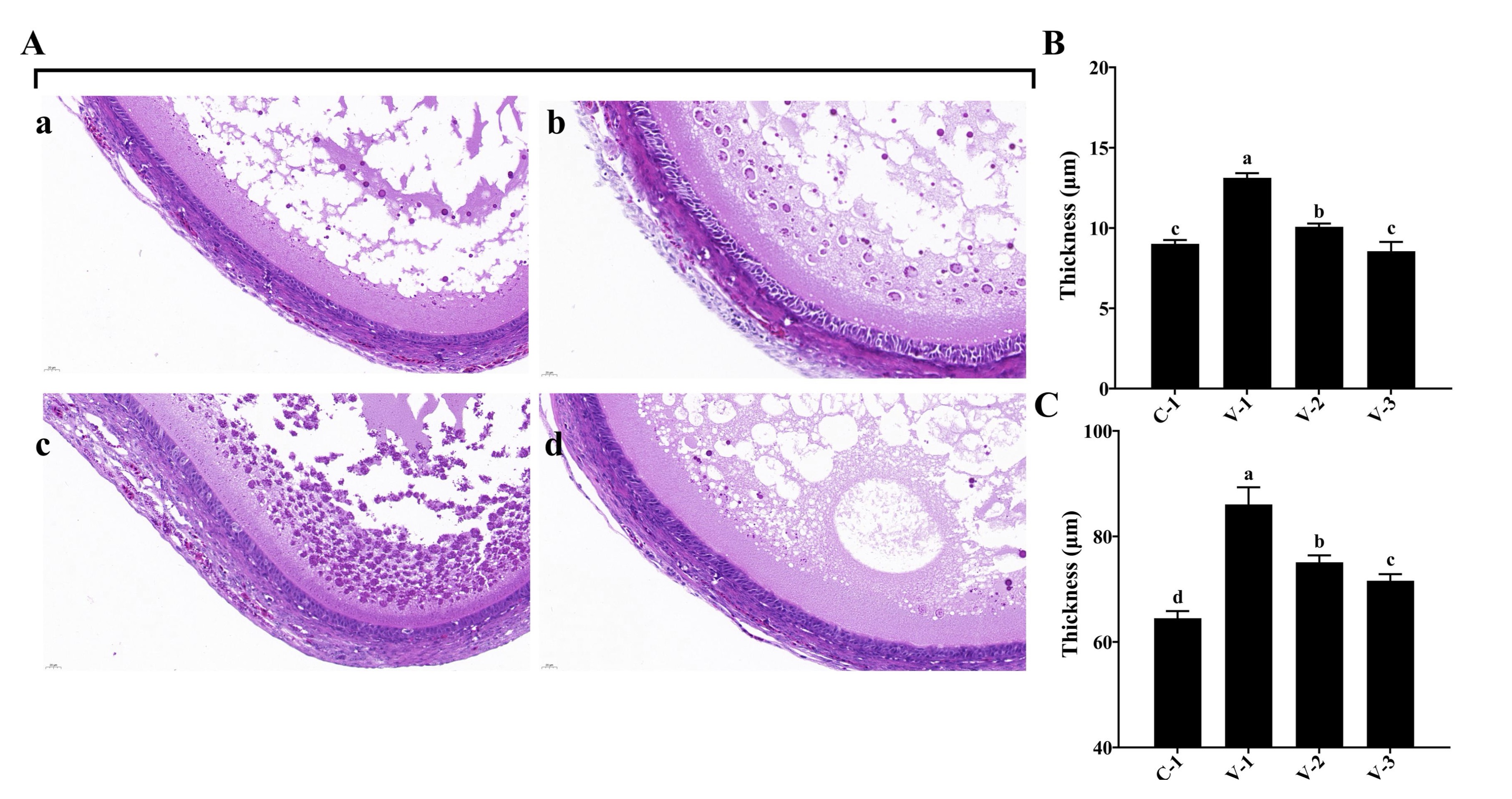
Fig. 3: Tissue observation of SYF development in quails. (A) Hematoxylin-eosin staining of SYFs in different groups (40×); (B) Thickness of granular cell layers in different groups; (C) Thickness of the follicular membrane layer in different groups. a-cThe different lowercase letters represent a signiffcant difference (P<0.05).
Vitamin E inhibited cell apoptosis in SYFs
The follicle selection is accompanied by cell proliferation and apoptosis, and the fate of cells
in
preovulatory
follicles determines follicle selection. Therefore, we conducted a statistical analysis of cell
apoptosis
in
SYFs. The TUNEL staining results showed that the addition of vitamin E significantly inhibited
cell
apoptosis in
SYFs compared with that in the C-1 group, and the cell apoptosis rate was the lowest in the V-1
group
(P<0.05; Fig. 4A and 4B). Similar results were also observed in other preovulatory
follicles.
These
results suggested that vitamin E might promote SYF development by inhibiting cell apoptosis.
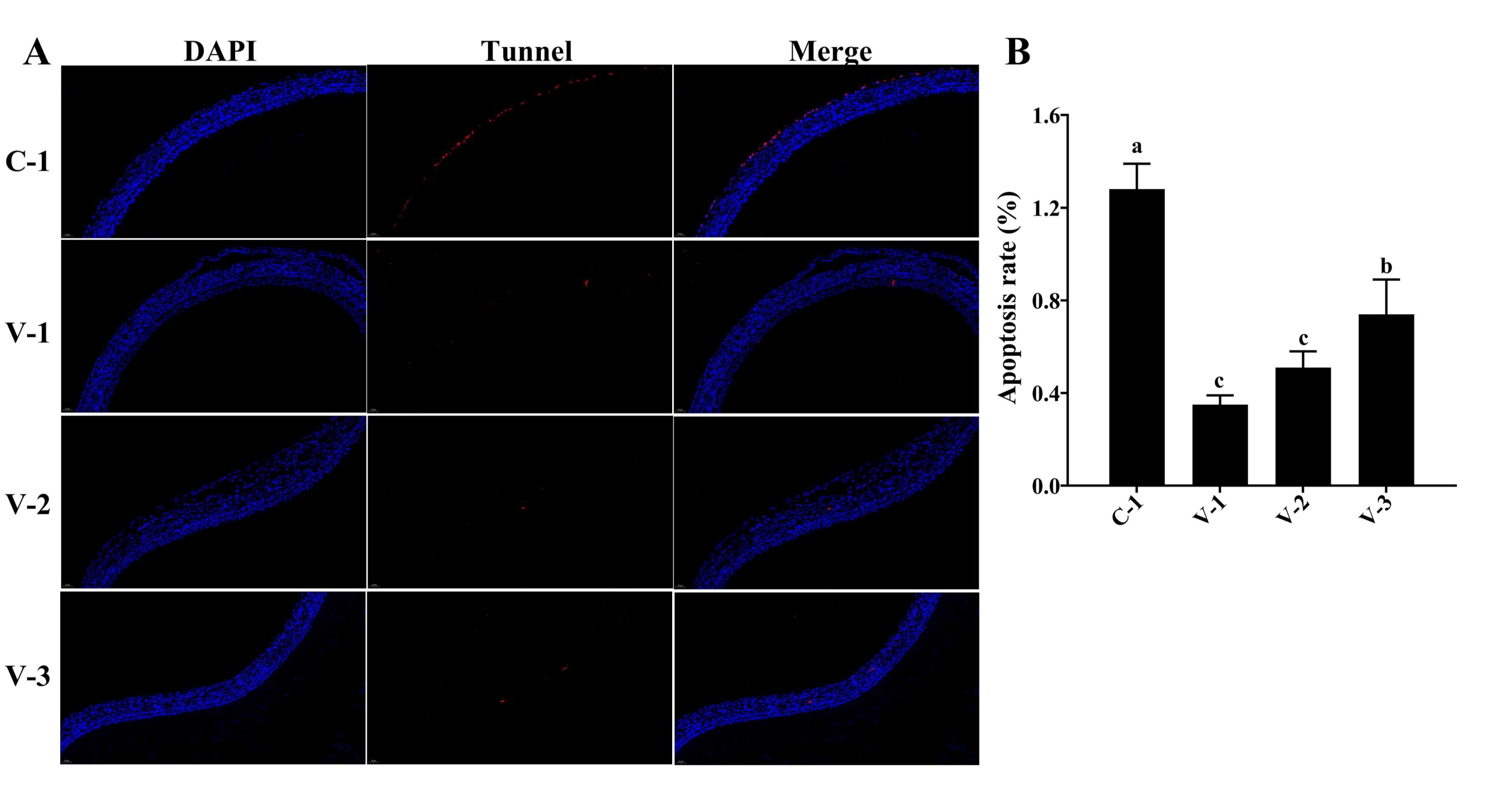
Fig. 4: SYFs apoptosis in quails. (A) TUNEL staining of SYFs in different groups (40×); (B) Cell apoptosis rate in different groups. a-cThe different lowercase letters represent a signiffcant difference (P<0.05).
Vitamin E enhanced the antioxidant capacity of quails
The improvement in ovarian oxidative stress capacity can also affect follicular development.
Therefore, we
evaluated the body's antioxidant capacity after adding vitamin E. As depicted in Fig. 5, the SOD
activity
increased in the V-1 and V-2 groups but significantly decreased in the V-3 group compared with
the C-1
group
(P<0.05). Moreover, the MDA concentration was significantly reduced in all groups
(P<0.05;
Fig. 5C). The aforementioned findings suggested that vitamin E supplementation might enhance the
antioxidant
capacity in quails.
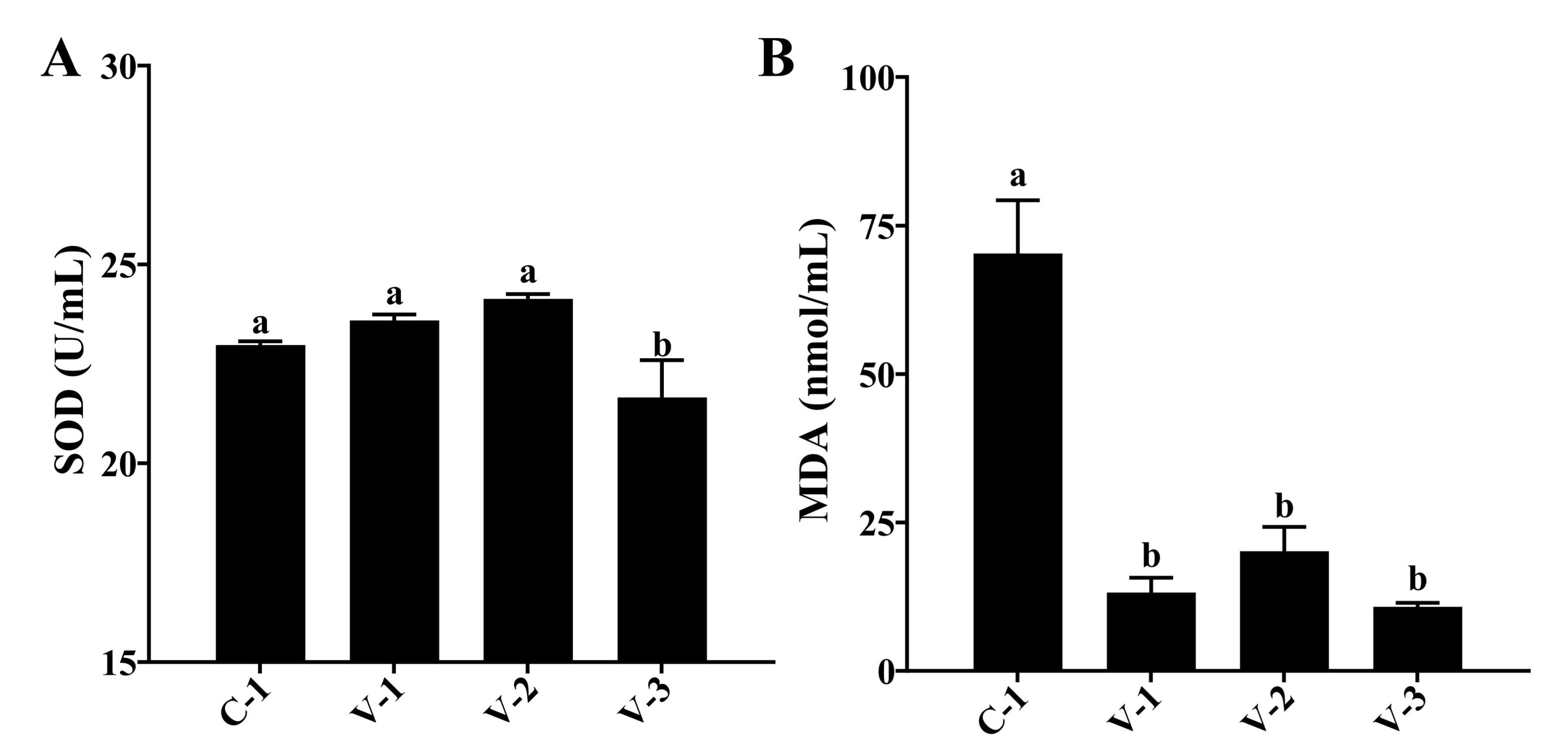
Fig. 5: Effect of vitamin E on the antioxidant capacity of quails. (A) Activity of SOD in different groups; (B) Concentration of MDA in different groups. a-bThe different lowercase letters represent a signiffcant difference (P<0.05).
Effect of vitamin E on gene expression in quail ovarian tissue
We performed RNA-seq on the ovaries of quails in the groups (n = 3, age 328 days and 300 days)
to
investigate
the gene expression regulation during ovarian aging. Compared with the O-1 group (300 days),
the C-1 group
exhibited 371 differentially expressed genes (DEGs); Compared with the C-1 group, the V-1
group exhibited
1591
DEGs; The common genes in all groups exhibited 87 DEGs (Fig. 6A). These DEGs have the same
trend in O-1
and V-1
group, but have the opposite trend in C-1 group (Fig. 6B). Additionally, KEGG functional
enrichment
analysis
identified enrichment in 38 pathways. The top 20 pathways are shown in Fig. 6C, such as
Primary
immunodeficiency,Hematopoietic cell lineage,Staphylococcus aureus infection etc. SOCS3, ADPOQ,
EPHX2,
IGF1, and
GPX4 were random selected for qPCR validation. The findings indicated that the expression of
SOCS3, ADPOQ,
and
EPHX2 significantly decreased whereas the expression of IGF1 and GPX4 significantly increased
compared
with that
in the control group. These results aligned with the sequencing outcomes (P<0.05;
Fig. 6D).
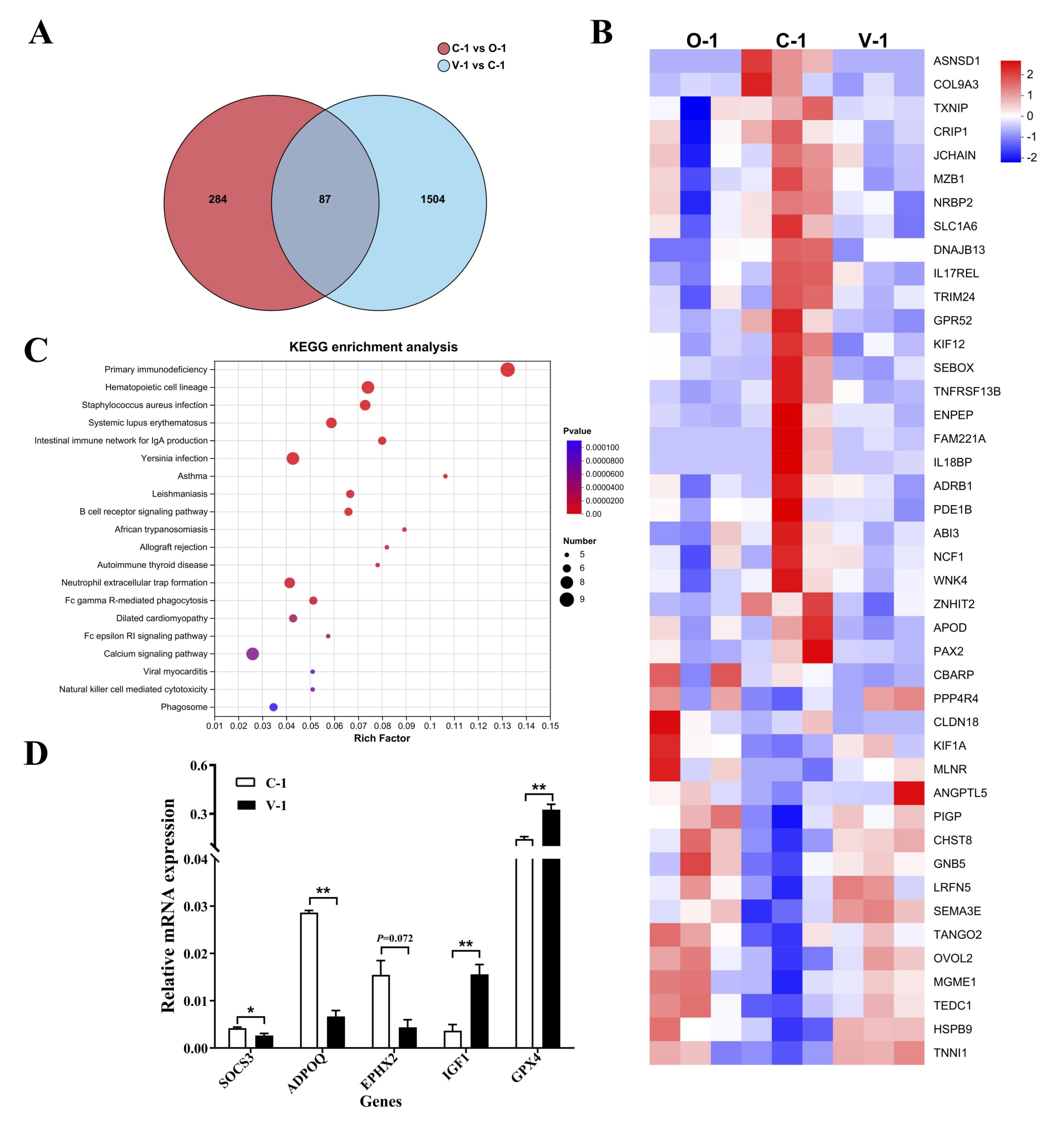
Fig. 6: RNA-seq analysis. (A) Volcano plot of DEGs; (B) Heatmap of DEGs; (C) Top 20 KEGG pathways; (E) qPCR of DEGs. *P<0.05 represented a significant difference and **P<0.01 represented a highly significant difference.
Effect of vitamin E on ovarian metabolites in quails
Partial least squares discriminant analysis (PLS-DA) was performed on each sample to examine
the impact of
vitamin E on ovarian metabolism in quails. The figure illustrates a clear separation trend
between the
three
sample groups, indicating alterations in the metabolic profile of quail ovaries following
the addition of
vitamin E (Fig. 7A). Employing the criteria of P<0.05, VIP>1, and |log2FC|≥1,
we identified
283
differentially expressed metabolites (DEMs) in the C-1 group, we identified 96 DEMs in the
V-1 group, in
all
groups exhibited the same 62 DEMs (Fig. 7B). Based on the HMDB, these DEMs were categorized
into seven
groups,
including organic acids and derivatives, organicheterocyclic compounds, lipids and
lipid-like molecules,
and so
on (Fig. 7C). Hierarchical clustering was performed on the top 20 VIP DEMs to further
evaluate the changes
in
metabolites caused by vitamin E (Fig. 7D). These DEMs have the same trend in O-1 and V-1
group, but have
the
opposite trend in C-1 group. The KEGG database was used for the pathway analysis of DEMs.
The results
showed
that 7 metabolic pathways were significantly affected (P<0.05) (Fig. 7E),
including Lysine
degradation, Tryptophan metabolism, Pyruvate metabolism, and so on.
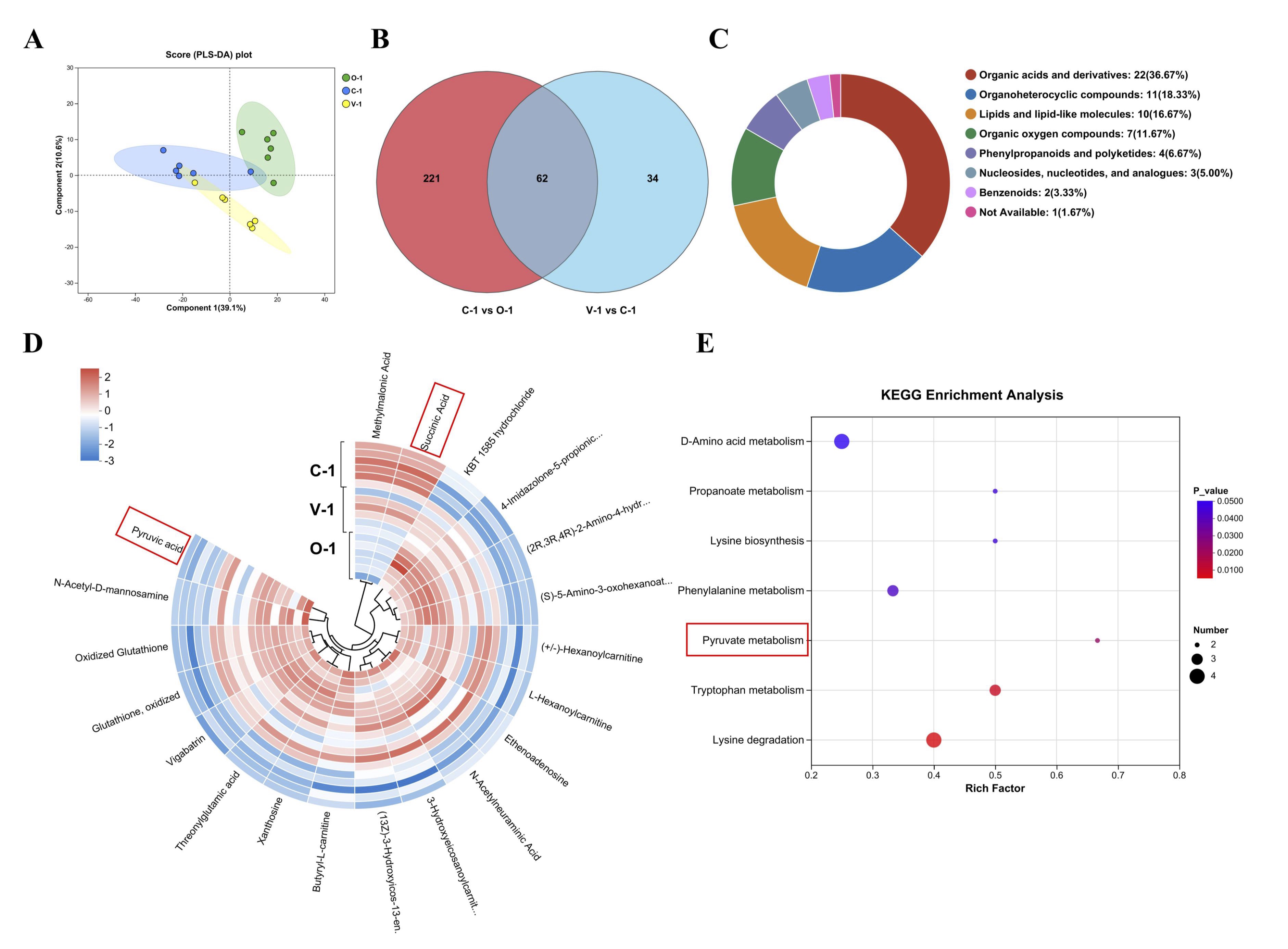
Fig. 7: Metabolome analysis. (A) PLS-DA; (B) Venn of DEMs; (C) Classification diagram of differential metabolites (n = 62); (D) Heat map of differential metabolites; (E) Pathway diagram of differential metabolites.
Combined analysis of transcriptome and metabolome
To investigate the relationship between the DEGs and DEMs, 43 DEGs and 11 DEMs were selected
for
Spearman’s
correlation analysis. As shown in Fig. 8, the relative abundance of MLNR, HSPB9, PIGP, KIF1A
positively
correlated with that of Succinic Acid, but negatively correlated with that of Pyruvic acid.
The relative
abundance of ASNSD1, COL9A3 positively correlated with that of Pyruvic Acid, but negatively
correlated
with that
of Succinic acid, suggesting that vitamin E promotes pyruvate metabolism by increasing the
abundance of
genes.
Therefore, these DEGs and DEMs were key factors for vitamin E in regulating and delaying
ovarian aging in
quail
ovaries.
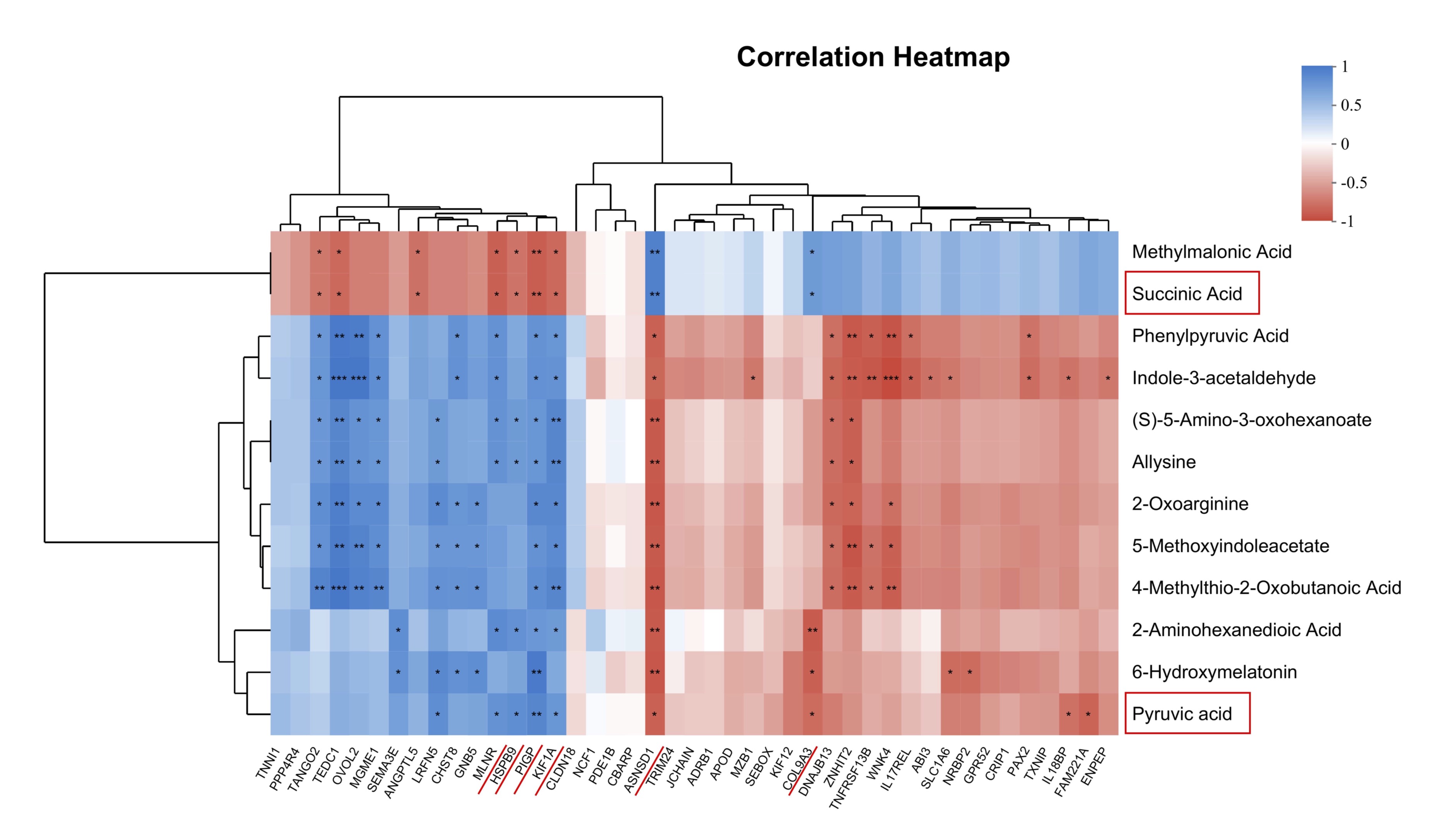
Fig. 8: Combined analysis of transcriptome and metabolome. Correlation analysis between DEGs and DEMs. *P< 0.05, **P< 0.01, and ***P< 0.001 represent significant difference between groups.
Discussion
This study used 300-day-old Peking white quails to explore the molecular mechanisms underlying the regulation of ovarian aging by vitamin E. It was observed that vitamin E supplementation improved egg production performance, increased egg production rate, reduced feed conversion ratio, and decreased the abnormal egg ratio. Further studies revealed that vitamin E promoted the development of preovulatory follicles, inhibited cell apoptosis, increased serum E2 levels, and enhanced antioxidant capacity, thereby delaying ovarian aging and improving egg production. Subsequent analyses involving RNA-seq and LC-MS/MS revealed the enrichment of DEGs and DEMs in the pyruvate metabolism. Spearman’s correlation analysis indicated that MLNR, HSPB9, PIGP, KIF1A, ASNSD1, COL9A3, Succinic Acid, Pyruvic Acid were centrally positioned. This suggested that these molecules played a crucial role in delayed ovarian aging induced by vitamin E.
On aging, laying birds often experience issues such as reproductive organ atrophy, weight loss, and a decline in both the quantity and quality of follicles [13]. These factors can negatively impact egg production. This study confirmed that quails faced these challenges in their 11th month of egg laying (Fig. 1). Ovarian aging typically involves a reduction in antioxidant capacity [14]. We investigated the regulatory effects of natural antioxidant vitamin E on ovarian aging to solve the issue of ovarian aging in egg-laying birds in the later stages. The findings revealed that adding vitamin E to the diet did not affect feed intake but significantly improved quail egg production performance, including average egg weight, average egg production rate, feed conversion ratio, and abnormal egg ratio (Table 2). A previous study reported that supplementing Japanese quails with 250 mg/kg vitamin E under heat or cold stress conditions improved the feed intake and egg production [15]. The male breeding quails in the moderate vitamin E supplementation group (75-150 IU/kg) exhibited improved reproductive performance [16]. Further, adding 120 mg/kg vitamin E significantly increased the serum estrogen levels in female quails, whereas the addition of 240 mg/kg vitamin E significantly increased the serum testosterone levels in male quails [17]. These results aligned with our findings. The NRC’s recommended vitamin E dosage for egg-type quails was extremely low, and appropriately increasing the proportion of vitamin E in the diet positively impacted quail production performance. However, excessive vitamin E dosage led to metabolite accumulation and had negative impacts.
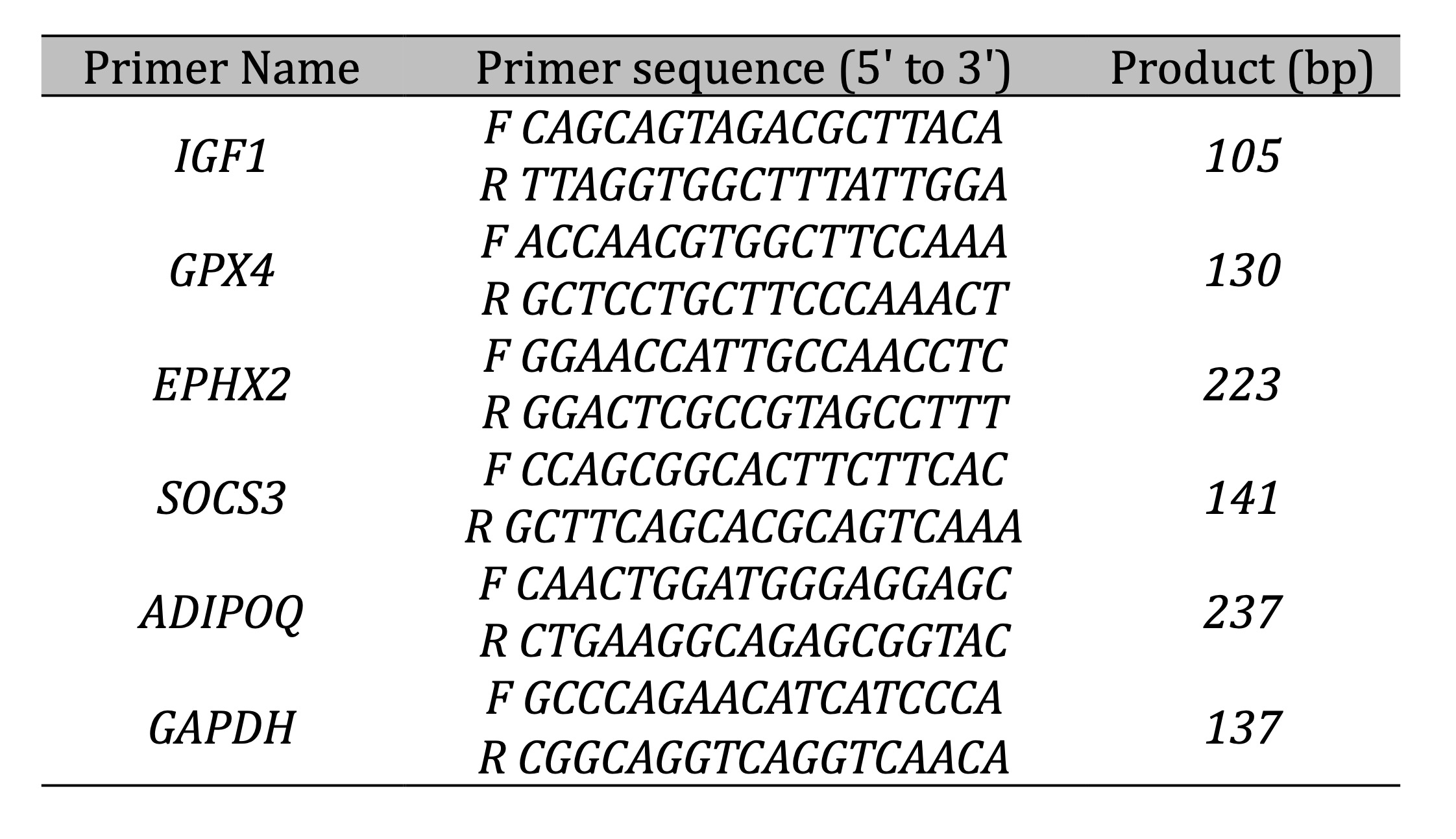
Table 2: The primers used in qPCR
The egg production rate in laying birds is closely related to the quality of their ovaries and follicles. Ovarian weight and follicle count are crucial indicators of ovarian development [18]. Therefore, we assessed ovarian development after introducing vitamin E. We observed a significant increase in the number of SYFs (Table 3). SYFs play a pivotal role in follicular development in birds, influencing the orderly progression of follicles and sustained egg laying [19]. The results of this study were consistent with the notion that SYFs are crucial for preovulatory follicle development in Peking white quails. To further verify this finding, we examined the morphology of SYFs. The results revealed that vitamin E supplementation led to the thickening of both the granulosa cell layer and the membrane layer of SYFs, accompanied by reduced apoptosis rates (Fig. 3 and 4). Granulosa cell and membrane cells are crucial in the development of follicles. Granular cells are the site for the synthesis and secretion of estradiol (E2), which is crucial for the formation and development of follicles in ovarian tissue and can directly promote follicle growth and maturation [20]. As expected, the E2 content in serum significantly increased, whereas the LH and FSH levels did not change as expected (Fig. 2). Recent studies suggest that this might be related to the negative feedback regulation of E2 on the pituitary gland. When the ovarian function is normal, the developing follicles secrete E2 and inhibin B, inhibiting the secretion of gonadotropins and keeping them at normal levels. However, with the aging of the ovaries, granulosa and follicular membrane cells lose their sensitivity to gonadotropins, and the E2 and inhibin B levels decrease, resulting in a reduced negative feedback effect on the pituitary gland. Hence, the secretion of pituitary gonadotropins increases, stimulating E2 production and follicle growth [3]. Based on our study, we speculated that the unchanged FSH and LH levels might result from improved ovarian aging due to vitamin E supplementation.
Oxidative stress plays a pivotal role in ovarian aging. Normally, the body maintains a balance between the production and elimination of free radicals. However, this equilibrium is disrupted as individuals age, leading to increased oxidative stress. This heightened oxidative stress can significantly increase follicular atresia, affect ovarian hormone secretion, and accelerate ovarian aging [21]. This study found that vitamin E improved quail ovarian aging and enhanced egg production performance. We hypothesized whether this improvement was linked to enhancing the body's antioxidant capacity. The results aligned with our expectations; with vitamin E supplementation, SOD activity increased and MDA concentration significantly decreased (Fig. 5). Previous studies showed that adding 250 mg/kg vitamin E to the diet of Japanese quails notably reduced the concentration of MDA in tissues and blood [22]. Co-Administration of cyclophosphamide, N-acetylcysteine and vitamin E at 200 mg/kg,a significantly lower concentration of MDA was observed in Female Rats [23]. Treated rats combination of vitamin C+E at a dose of 200 mg/kg, Concentrations of CAT, and SOD enzymes in the treated group were significantly highter than other groups, and MDA showed a significant reduction in the treatment groups compared to the control group [24]. Supplementing the diet with 200 IU/kg vitamin E significantly improved antioxidant indicators (Total antioxidant capacity, catalase, and superoxide dismutase) in 40-day-old quails [7]. Our findings supported these conclusions. Vitamin E enhanced the body's antioxidant capacity, potentially delaying ovarian aging, changing follicle development, and increasing egg production.
The correlation analysis between DGEs and DGMs revealed that Succinic Acid, and Pyruvic Acid at the centrally positioned (Fig. 8). The energy required for ovarian development originates from the tricarboxylic acid cycle (TCA), in which pyruvic acid is upregulated. The addition of pyruvic acid and lactic acid to culture media significantly boosts the proliferation of granulosa cells [25]. Further, in vitro studies demonstrated that pyruvic acid and lactic acid supported the maturation of bovine oocytes [26]. As an intermediate substrate for gluconeogenesis, the increase in pyruvic acid levels represents an increase in glucose metabolism, potentially supplying more energy to sustain the enhanced egg production performance of quails after vitamin E supplementation. Succinic acid is a key metabolite in the TCA cycle in cell mitochondria. It acts as an essential metabolic intermediate that promotes energy expenditure and prevents obesity [27]. However, succinic acid was downregulated in this study, indicating that vitamin E could alter the body's energy metabolism level. These findings highlighted MLNR, HSPB9, PIGP, KIF1A, ASNSD1, COL9A3, Succinic Acid, Pyruvic Acid as central molecules, indicating their pivotal role in delaying ovarian aging through vitamin E intervention (Fig. 8).
Due to the relatively small sample size in each group, While our results showed statistically significant differences, a larger cohort would enhance the statistical power and robustness, allowing for more reliable subgroup analyses and reducing the potential impact of biological variability. Then, the exploratory nature of our multi-omics approach, while a strength in generating novel hypotheses, also introduces limitations. The sample size, though sufficient for an initial discovery-phase investigation, imposes constraints on the statistical power of the transcriptomic and metabolomic analyses. This increases the risk of both Type I errors (false positives) and Type II errors (false negatives), meaning that some potentially important differentially expressed genes or metabolites might have been overlooked, while others identified may require further validation(Fig.6D). Future studies employing higher sample numbers are warranted to confirm and extend our observations.
Conclusion
In conclusion, our findings indicated that supplementing the diet with 250 mg/kg vitamin E can enhanced the body's energy metabolism-related pathways such as amino acid metabolism, glucose metabolism, and fatty acid metabolism. Further more, 250 mg/kg vitamin E can enhanced antioxidant capacity, reduced cell apoptosis in preovulatory follicles, promoted follicular development, improved ovarian aging and quails egg production performance (Fig. 9). In summary, the research results provided a important method to solve the problem of decreased egg production performance in quails due to aging. Further studies are needed to determine the optimal vitamin E dosage to ensure maximum growth performance in animals and the mechanism of vitamin E improve the reproductive aging of quails need more deep research.

Fig. 9: Mechanism diagram illustrating how vitamin E improves ovarian aging.
Acknowledgements
We thank International Science Editing for editing this manuscript ( http://www.internationalscienceediting.com ) .
Author Contributions
Conceptualization, Hongyan Chen and Zhigang Wang; Data curation, Fei Gao, Shutong Deng,
Zheng Chen and
Qingying
Li; Formal analysis, Tingting Cui and Xiaotong Qin; Methodology, Hongyan Chen and Mengqian
Zou; Writing -
original draft, Hongyan Chen; Writing - review & editing, Hongyan Chen and Zhigang
Wang.
Funding
This work was supported by Program for Young Talents of Basic Research in Universities of
Heilongjiang
Province
(YQJH2024273), the Natural Scientific Foundation of Heilongjiang Province (LH2023C115),
the Heilongjiang
Province Education Department Fundamental Scientific Research Funds (145209218), and the
Graduate
Innovation
Project of Qiqihar University (QUZLTS_CX2024018).
Data Availability
Raw RNA-Seq data have been deposited in the Sequence Read Archive (SRA) with accession
number PRJNA123518.
Raw
untargeted metabolomics data have been deposited in the MetaboLights database under
accession number
MTBLS13008.
All data underlying this article will be shared on reasonable request to the corresponding
author
(03692@qqhru.edu.cn; wangzhigang@qqhru.edu.cn).
Ethics Approval
The animal study protocol was approved by the Ethics Committee of Qiqihar University
(Permit No. 20230309)
and
was carried out in accordance with the guidelines on the care and use of experimental
animals established
by the
Ministry of Science and Technology of the People's Republic of China (approval number
2006-398).
Disclosure Statement
The authors declare no competing interests.
References
| 1 | Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, Andersen CY.
Fertility
preservation for agerelated fertility decline. Lancet. 2015 Feb 7;385(9967):506-7.
doi:
10.1016/S0140-6736(15)60198-2. PMID: 25705839.
https://doi.org/10.1016/S0140-6736(15)60198-2 |
| 2 | Hao EY, Chen H, Wang DH, Huang CX, Tong YG, Chen YF, Zhou RY, Huang RL. Melatonin
regulates the
ovarian function and enhances follicle growth in aging laying hens via activating
the mammalian
target of rapamycin pathway. Poultry Sci. 2020 Apr;99(4):2185-2195. doi:
10.1016/j.psj.2019.11.040.
PMID:32241504. PMCID:PMC7587849.
https://doi.org/10.1016/j.psj.2019.11.040 |
| 3 | Rose EM, Haakenson CM, Ball GF. Sex differences in seasonal brain plasticity and
the
neuroendocrine regulation of vocal behavior in songbirds. Horm. Behav. 2022
Jun:142:105160. doi:
10.1016/j.yhbeh.2022.105160. PMID:35366412.
https://doi.org/10.1016/j.yhbeh.2022.105160 |
| 4 | Liu Z, Li F, Xue J, Wang M, Lai S, Bao H, He S. Esculentoside A rescues granulosa
cell apoptosis
and folliculogenesis in mice with premature ovarian failure. Aging. 2020 Aug
5;12(17):16951-16962.
doi: 10.18632/aging.103609. PMID:32759462. PMCID:PMC7521512.
https://doi.org/10.18632/aging.103609 |
| 5 | Lin YF, Chang SJ, Yang JR, Lee YP, Hsu AL. Effects of supplemental vitamin E
during the mature
period on the reproduction performance of Taiwan Native Chicken cockerels. British
Poultry Science.
2005 Jun;46(3):366-73. doi: 10.1080/00071660500098186. PMID:16050192.
https://doi.org/10.1080/00071660500098186 |
| 6 | NRC-National Research Council, Nutrient requirements for poultry. 9th rev. ed.
National Academy
Press, Washington, DC, 1994.
|
| 7 | Gouda A, El-Moniary MM, Hamouda Y, Youssef AW, Hassan HMA. Vitamin E
supplementation and early age
heat conditioning to alleviate the negative effects of heat stress in quail chicks.
Pakistan Journal
Biological Sciences. 2020 Mar;23(4):526-532. doi: 10.3923/pjbs.2020.526.532.
PMID:32363838.
https://doi.org/10.3923/pjbs.2020.526.532 |
| 8 | Nemati Z, Ahmadian H, Besharati M, Lesson S, Alirezalu K, Domínguez R, Lorenzo JM.
Assessment of
dietary selenium and vitamin e on laying performance and quality parameters of fresh
and stored eggs
in japanese quails. Foods. 2020 Sep 20;9(9):1324. doi: 10.3390/foods9091324.
PMID:32962208.
PMCID:PMC7555285.
https://doi.org/10.3390/foods9091324 |
| 9 | Rahimian Y, Kheiri F, Faghani M. Evaluation the effect of dietary vitamin E,
sesamin and
thymoquinone bioactive compounds on immunological response, intestinal traits and
MUC-2 gene
expression in broiler Japanese quails (Coturnix japonica). Anim Biotechnol. 2024
Nov;35(1):2259437.
doi: 10.1080/10495398.2023.2259437. PMID:37729462.
https://doi.org/10.1080/10495398.2023.2259437 |
| 10 | Rodler D, Sasanami T, Sinowatz F. Assembly of the Inner Perivitelline Layer, a
Homolog of the
Mammalian Zona Pellucida:An Immunohistochemical and Ultrastructural Study. Cells
Tissues Organs.
2012;195(4):330-9. doi: 10.1159/000327013. PMID:21778679.
https://doi.org/10.1159/000327013 |
| 11 | Chen H, Zhou S, Wang Y, Zhang Q, Leng L, Cao Z, Luan P, Li Y, Wang S, Li H, Cheng
B. HBP1 promotes
chicken preadipocyte proliferation via directly repressing SOCS3 transcription. Int.
J. Biol.
Macromol. 2024 Jan;256(Pt 2):128414. doi: 10.1016/j.ijbiomac.2023.128414.
PMID:38029903.
https://doi.org/10.1016/j.ijbiomac.2023.128414 |
| 12 | Zhang Z, Yi P, Yang J, Huang J, Xu P, Hu M, Zhang C, Wang B, Peng W. Integrated
network
pharmacology analysis and serum metabolomics to reveal the cognitive improvement
effect of Bushen
Tiansui formula on Alzheimer's disease. J. Ethnopharmacol. 2020 Mar 1:249:112371.
doi:
10.1016/j.jep.2019.112371. PMID:31683034.
https://doi.org/10.1016/j.jep.2019.112371 |
| 13 | Tardif SD, Ziegler TE. Features of female reproductive senescence in tamarins
(Saguinus spp.), a
New World primate. J Reprod Fertil. 1992 Mar;94(2):411-21. doi:
10.1530/jrf.0.0940411. PMID:1317448.
https://doi.org/10.1530/jrf.0.0940411 |
| 14 | Liu X, Lin X, Mi Y, Li J, Zhang C. Grape seed proanthocyanidin extract prevents
ovarian aging by
inhibiting oxidative stress in the hens. Oxid. Med. Cell. Longev. 2018 Jan
9:2018:9390810. doi:
10.1155/2018/9390810.
https://doi.org/10.1155/2018/9390810 |
| 15 | Sahin N, Tuzcu M, Orhan C, Onderci M, Eroksuz Y, Sahin K. The effects of vitamin C
and E
supplementation on heat shock protein 70 response of ovary and brain in
heat-stressed quail. British
Poultry Science. 2009 Mar;50(2):259-65. doi: 10.1080/00071660902758981.
PMID:19373727.
https://doi.org/10.1080/00071660902758981 |
| 16 | Hooda S, Tyagi PK, Mohan J, Mandal AB, Elangovan AV, Pramod KT. Effects of
supplemental vitamin E
in diet of Japanese quail on male reproduction, fertility and hatchability. British
Poultry Science.
2007 Feb;48(1):104-10. doi: 10.1080/00071660601157378. PMID:17364548.
https://doi.org/10.1080/00071660601157378 |
| 17 | Abedi P, Vakili ST, Mamouei M, Aghaei A. Effect of different levels of dietary
vitamin E on
reproductive and productive performances in Japanese quails (Coturnix coturnix
japonica). Vet. Res.
Forum. 2017;8(4):353-359. PMID:29326796. PMCID:PMC5756257.
|
| 18 | Durnerin CI, Erb K, Fleming R, Hillier H, Hillier SG, Howles CM, Hugues JN, Lass
A, Lyall H,
Rasmussen P, Thong J, Traynor I, Westergaard L, Yates R, Luveris Pretreatment Group.
Effects of
recombinant LH treatment on folliculogenesis and responsiveness to FSH stimulation.
Hum. Reprod.
2008 Feb;23(2):421-6. doi: 10.1093/humrep/dem388. PMID:18084048.
https://doi.org/10.1093/humrep/dem388 |
| 19 | Johnson AL. Ovarian follicle selection and granulosa cell differentiation. Poultry
Science. 2015
Apr;94(4):781-5. doi: 10.3382/ps/peu008. PMID:25535403.
https://doi.org/10.3382/ps/peu008 |
| 20 | Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions
between
androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the
human normal and
polycystic ovary. Hum. Reprod. 2016 Nov;22(6):709-724. doi: 10.1093/humupd/dmw027.
PMID:27566840.
https://doi.org/10.1093/humupd/dmw027 |
| 21 | Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction.
Reprod. Biol.
Endocrin. 2005 Jul 14:3:28. doi: 10.1186/1477-7827-3-28. PMID:16018814.
PMCID:PMC1215514.
https://doi.org/10.1186/1477-7827-3-28 |
| 22 | Şahin K, Küçük O, Sahin N, Gürsu F. Optimal dietary concentration of vitamin E for
alleviating the
effect of heat stress on performance, thyroid status, ACTH and some serum metabolite
and mineral
concentrations in broilers. Vet. Med. 2002, 47(4):110-116. doi:
10.17221/5813-VETMED.
https://doi.org/10.17221/5813-VETMED |
| 23 | Raeeszadeh M, Hosseini SMS, Amiri AA. Impact of Co-Administration of
N-Acetylcysteine and Vitamin
E on Cyclophosphamide-Induced Ovarian Toxicity in Female Rats. J. Toxicol. 2022 Aug
23:2022:9073405.
doi: 10.1155/2022/9073405. PMID:36051383. PMCID:PMC9427260.
https://doi.org/10.1155/2022/9073405 |
| 24 | Raeeszadeh M, Shokrollahi B, Khademi N, Akbari A. Superior effect of broccoli
methanolic extract
on control of oxidative damage of sperm cryopreservation and reproductive
performance in rats:A
comparison with vitamin C and E antioxidant. Theriogenology. 2022 Mar 15:181:50-58.
doi:
10.1016/j.theriogenology.2022.01.010. PMID:35063921.
https://doi.org/10.1016/j.theriogenology.2022.01.010 |
| 25 | Peralta OA, Bucher D, Angulo C, Castro MA, Ratto MH, Concha I. Tissue localization
of GM-CSF
receptor in bovine ovarian follicles and its role on glucose uptake by mural
granulosa cells. Anim.
Reprod. Sci. 2016;170:157-169. 2016 Jul:170:157-69. doi:
10.1016/j.anireprosci.2016.04.014.
PMID:27236376.
https://doi.org/10.1016/j.anireprosci.2016.04.014 |
| 26 | Huang G, Wang L, Jian Li J, Hou R, Wang M, Wang Z, Qu Q, Zhou W, Nie Y, Hu Y, Ma
Y, Yan L, Wei H,
Wei F. Seasonal shift of the gut microbiome synchronizes host peripheral circadian
rhythm for
physiological adaptation to a low-fat diet in the giant panda. Cell Rep.
2022;38:110203. 2022 Jan
18;38(3):110203. doi: 10.1016/j.celrep.2021.110203. PMID:35045306.
https://doi.org/10.1016/j.celrep.2021.110203 |
| 27 | Liu K, Lin L, Li Q, Xue Y, Zheng F, Wang G, Zheng C, Du L, Hu M, Huang Y, Shao C,
Kong X, Melino
G, Shi Y, Wang Y. Scd1 controls de novo beige fat biogenesis through
succinate-dependent regulation
of mitochondrial complexⅡ. Proc Natl Acad Sci USA. 2020;117:2462-2472. 2020 Feb
4;117(5):2462-2472.
doi: 10.1073/pnas.1914553117. PMID:31953260. PMCID:PMC7007576.
https://doi.org/10.1073/pnas.1914553117 |