Peripheral miRNA-155 Expression and Circulating Inflammatory Cytokines in Patients with Helicobacter Pylori–Associated Chronic Gastritis: An Exploratory Case–Control Study
Keywords
Abstract
Background/Aims:
Infection with Helicobacter pylori is the most common cause of chronic gastritis and has been linked to persistent inflammation of the stomach. miRNA-155 and inflammatory cytokines play crucial roles in immune regulation but their systemic profiles of expression are not fully understood in H. pylori–associated chronic gastritis. This pilot study was conducted to evaluate peripheral miRNA-155 expression accompanied by circulating levels of IL-6 IFN-γ and IL-10 in chronic gastritis H. pylori –related patients.Methods:
Twenty-five patients with endoscopically and histologically diagnosed H. pylori–associated chronic gastritis and 25 age-matched healthy subjects were included in this study. The expression of miRNA-155 was determined in peripheral blood by using quantitative real-time PCR • Whereas the levels of serum cytokines were measured by ELISA. Group comparison and an exploratory association were carried out.Results:
The H. pylori–associated chronic gastritis patients had significantly higher peripheral miRNA-155 levels compared with controls (p < 0.001). Serum IL-6 IFN-γ and IL-10 were all statistically higher in patients (all p < 0.001). Unadjusted logistic regression analyses showed significant correlations between biomarker levels and H.pylori infection status among the study population.Conclusion:
This exploratory case–control study provides evidence of systemic perturbations in miRNA-155 expression and inflammatory cytokines in subjects with H. pylori–associated chronic gastritis. These data indicate immune-regulatory changes are related in whole blood and justify validation studies on additional cohorts. Due to the exploratory nature and small size of our cohort • The results need to be interpreted with caution and verified by independent cohorts.Introduction
Helicobacter pylori (H. pylori) is one of the most common bacterial pathogens, infecting over 50% of the world population [1, 2]. The H. pylori infection rate differs widely in different locations and is closely related to the social economy there are more infections in developing countries caused by factors like poor living environments and overcrowding [3]. Chronic H. pylori infection is the principal cause of chronic gastritis and a risk factor for sequential damage to the gastric mucosa leading to atrophic gastritis, intestinal metaplasia and gastric cancer [4]. H. pylori causes a chronic inflammation by its virulence factors secretion, which include cytotoxin associated gene A (CagA) and vacuolating cytotoxin A (VacA), that facilitate disruption of the epithelial barrier and alter local immune signaling [5]. Such chronic inflammatory setting provides the ability of bacteria to persist, together with induction of tissue damage and disease progression [6]. Epidemiological studies in Africa and the Middle East regions have also indicated a significant correlation between H. pylori infection and gastric precancerous lesions, thereby emphasizing its clinical relevance as an obligatory risk factor of global public health importance [7, 8]. Furthermore, H. pylori interactions with the gastric microbiota have been linked to changes in gastric acid secretion and disease progression [9]. Eradication therapy has been shown to cause regression of chronic gastritis whereas the reversibility of advanced gastric lesions is a matter of debate [10], emphasizing the importance for better understanding regarding host-pathogen interaction [11].
MicroRNAs have been identified as critical post-transcription mediators of immunity and inflammation. Among them, miRNA-155 is involved in immune cells activation and cytokine expression and inflammatory regulation. Overexpression of miRNA-155 has been observed in different inflammatory diseases, including H. pylori-associated gastritis [12, 13]. At the mechanistic level, miRNA-155 has been demonstrated to potentially regulate immune-related target genes and potentiate pro-inflammation signaling pathways upon bacterial infection [12, 14]. These data indicate that miRNA-155 could be a marker of systemic immune activation in the context of chronic gastric inflammation.
Cytokines including IL-6, IFN-γ and IL-10 are important factors of immune response against H. pylori. IL-6 as well as IFN-γ are participating in the activation of both innate and adaptive immune cells and creation of chronic inflammation, while IL-10 has a suppressive and anti-inflammatory activity which may counteract exaggerated immune reaction [15–18]. Imbalance of cytokine networks has been associated with the severity and progression of H. pylori-associated gastritis [16–18].
Although miRNA-155 expression (or cytokine responses) has been previously reported individually (all at the levels of gastric tissue or isolated immune cells), little is known regarding their concomitant systematic profiles expressed in peripheral blood from patients with H. pylori-associated chronic gastritis. Accordingly, in this study we investigated the relationship of expression of peripheral miRNA-155 with circulating levels of IL-6, IFN-γ and IL-10 in patients suffering from H. pylori-related chronic gastritis to help elucidate the systemic immunity changes occurring in this disease.
Materials and Methods
Study design
This study was organized as an exploratory case–control study to evaluate the levels of systemic miRNA-155
and circulating cytokines in H. pylori-associated chronic gastritis. This cohort consisted of 25 patients
with known H. pylori infection and chronic active gastritis, who were age- and gender-matched to 25
healthy controls without a history of gastrointestinal disease. Biomarker profiles were compared between
groups to identify possible relationships among H. pylori infection, miRNA-155 expression, and
inflammatory cytokines. Peripheral blood sample instead of sampling at the lesion site was selected as a
non-invasive method to evaluate systemic immune response related to chronic gastritis.
Study population
The study population included 25 patients with H. pylori-associated chronic gastricitis and of 25 healthy
control persons. H pylori positive patients were enrolled at gastroenterology clinic between April 2023
and October 2023 based on endoscopic and histopathological diagnosis in addition to H. pylori confirmation
tests. Inclusion criteria included age between 18 and 65 years, no previous GI surgery, and the absence of
other chronic inflammatory diseases. The healthy population had no symptoms of gastrointestinal disease,
chronic systemic disease. Participants with acute infections, autoimmune diseases and immunosuppressive
treatment were excluded to reduce potential confounds. Demographic and clinical features, such as age, sex
and body mass index (BMI), were obtained in all the participants.
Sample Collection
Peripheral venous blood (about 5 mL) was gathered from every patient under aseptic circumstances. The
samples were clotted at room temperature and then centrifuged to obtain the plasma and serum layers. The
aliquoted plasma and serum samples were frozen at −80 °C until analyzed in order to avoid biomarker
degradation.
Ethical considerations
The study protocol was checked and approved by the Local Ethics Committee of Hammurabi College of
Medicine, University of Babylon -Iraq (Issue No. 38, dated 26/03/2023). All participants provided written
informed consent before they were enrolled. The investigation was performed in compliance with the
Declaration Helsinki and all information was treated anonymously.
miRNA-155 Expression Analysis
Plasma total RNA was prepared with miRNeasy Serum/Plasma Kit (Qiagen, Germany) following the
manufacturer’s instructions. The RNA concentration and purity were measured by NanoDrop Spectrophotometer.
Reverse transcription was conducted with miScript II RT Kit (Qiagen). miRNA-155 and U6 snRNA mRNA
expression was measured by quantitative real-time PCR using the miScript SYBR Green PCR Kit with miRNA-155
primers as specific primers, U6 snRNA was used as internal references. The ΔΔCt method was utilized to
determine the relative expression of miRNA-155.
IL-6 Cytokine Quantification (IL-6, IFN-γ, IL-10)
Serum levels of IL-6, IFN-γ, and IL-10 were determined by sandwich ELISAs according to the manufacturer's
instructions. All samples were done in duplicates, and standard curves were prepared of each recombinant
cytokine supplied with kits. Optical density was read at 450 nm on a microtiter plate reader.
Statistical Analysis
All statistical analyses were conducted using SPSS (version 26.0) and GraphPad Prism (version 9.0). Data
for continuous variable are mean ± SD. Student’s t-test (for normally distributed variables) and the
chi-square test (for categorical variables) were used for group comparisons. Logistic regression analyses
were conducted to examine relationships in the sample.
Results
Participant Characteristics
Demographic characteristics of the study population are summarized in Table 1. Patient and control groups
were comparable with respect to age and sex distribution. However, body mass index was significantly
higher in patients with H. pylori-associated chronic gastritis compared with healthy controls (p <
0.001; Table 1).
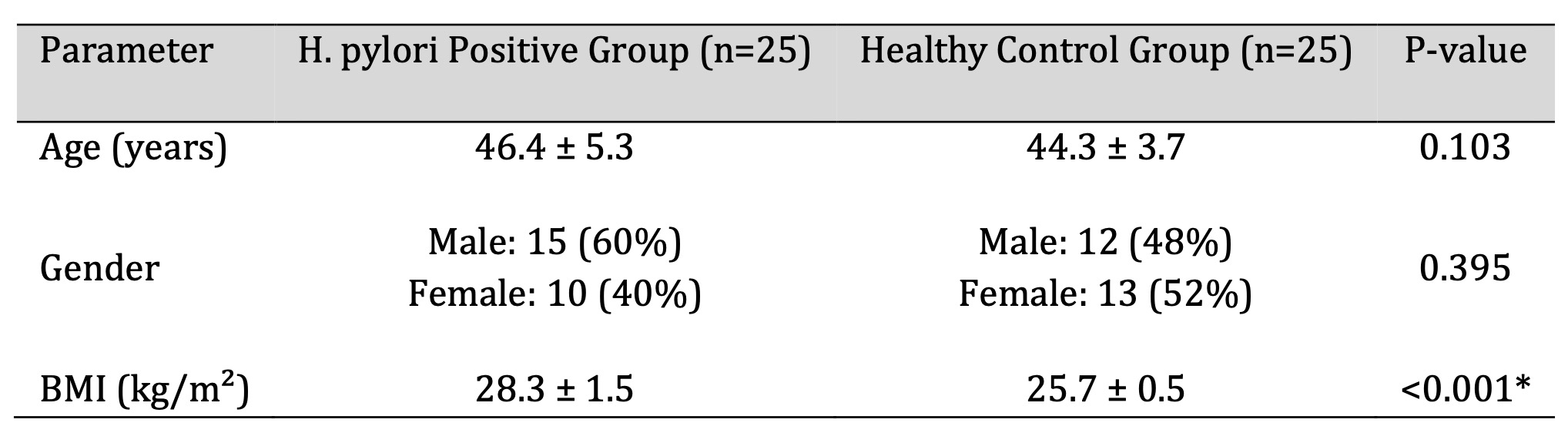
Table 1: Demographic Comparison between H. pylori Positive Patients and Healthy Controls
miRNA-155 Expression
Peripheral miRNA-155 expression was significantly higher in patients with H. pylori-associated chronic
gastritis than in healthy controls (p < 0.001; Table 2). Ct values of the housekeeping gene did not
differ significantly between groups, indicating reliable normalization. The relative increase in miRNA-155
expression in the patient group is illustrated in Fig. 1.
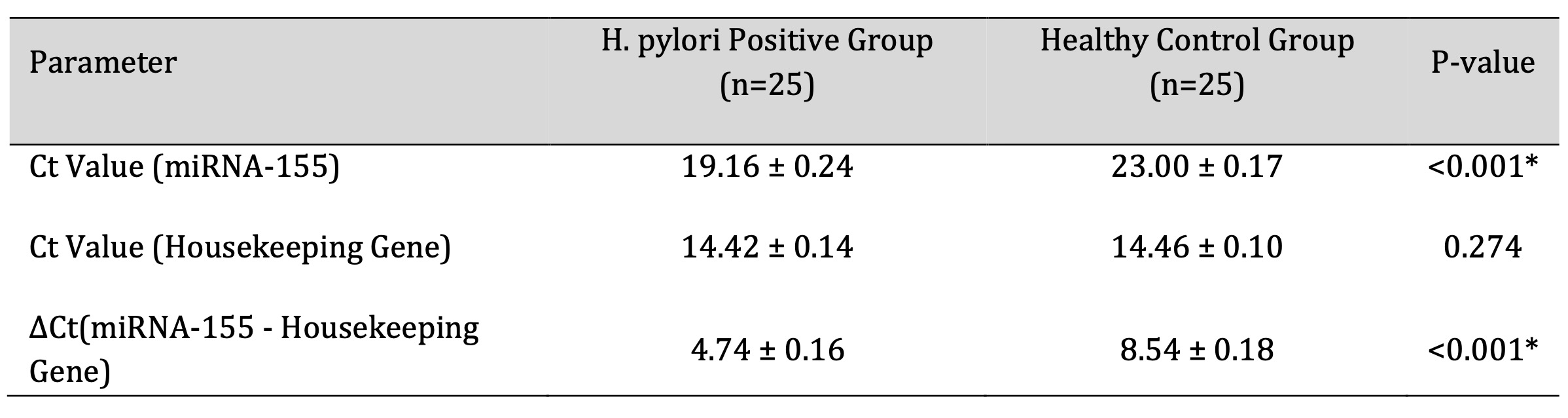
Table 2: Comparison of miRNA-155 Expression between H. pylori Positive Patients and Healthy Controls. P-value <0.05 was considered statistically significant
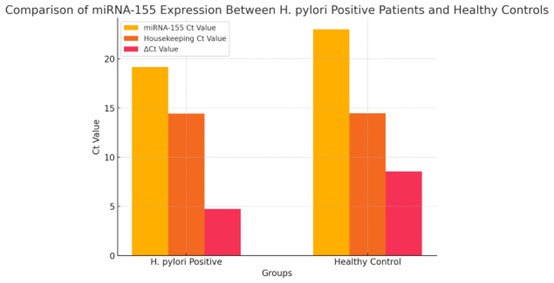
Fig. 1: Comparison of miRNA-155 Expression between H. pylori Positive Patients and Healthy Controls.
Cytokine Levels
The serum levels of IL-6, IFN-γ, and IL-10 in patients were all significantly higher than those in
controls (all p < 0.001; Table 3). These results point to systemic immune activation in the H.
pylori-positive population. Cytokine profiles in the two groups are illustrated and compared in Fig. 2.
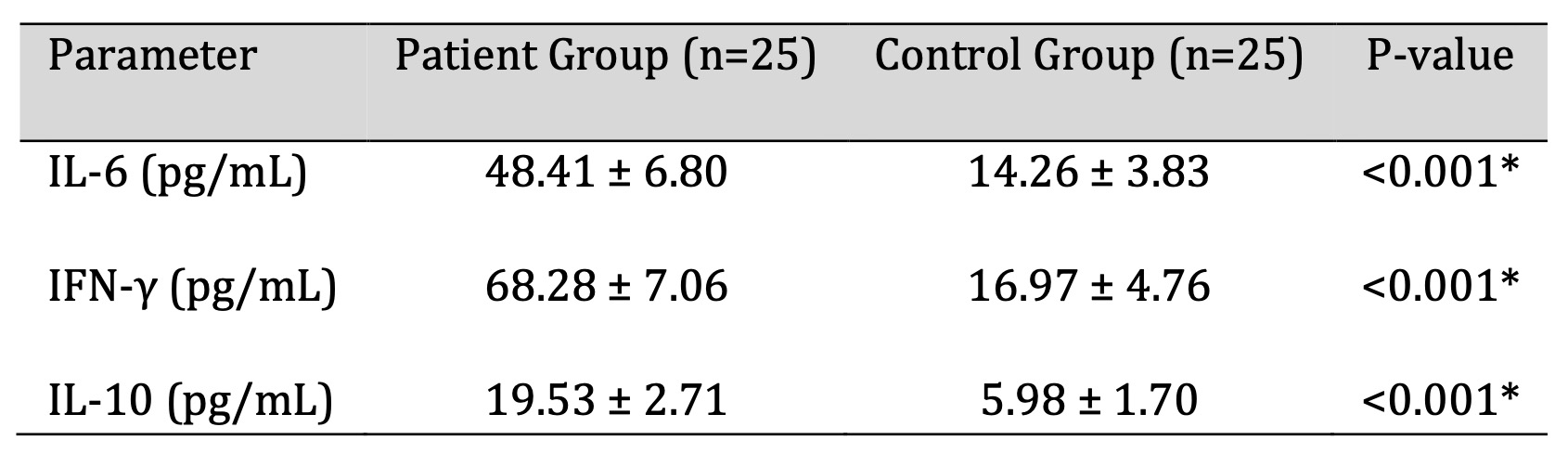
Table 3: Comparison of Cytokine Levels between H. pylori Positive Patients and Healthy Controls. P-value <0.05 was considered statistically significant
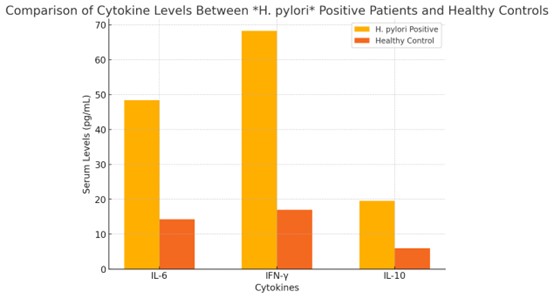
Fig. 2: Comparison of Cytokine Levels between H. pylori Positive Patients and Healthy Controls.
Exploratory Association Analyses
Exploratory logistic regression analysis was used to examine the relation between miRNA-155
expression, level of circulating cytokines and H. pylori infection among study subjects (Fig. 3 and
Table
4). Because of the relatively small number of cases, regression analysis was performed for descriptive
and
hypothesis-generating reasons only, and not for predictive modeling.
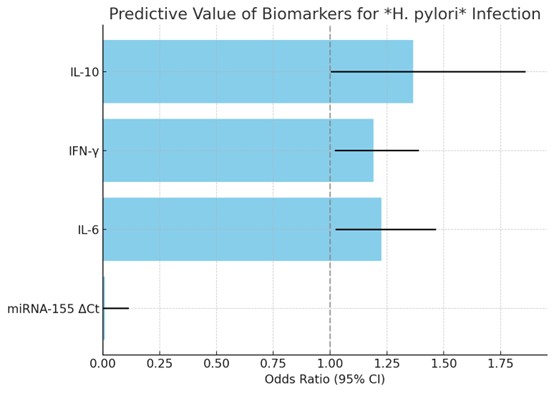
Fig. 3: Exploratory logistic regression analysis illustrating associations with Helicobacter pylori infection within the study cohort. The analysis is descriptive and not intended for predictive purposes
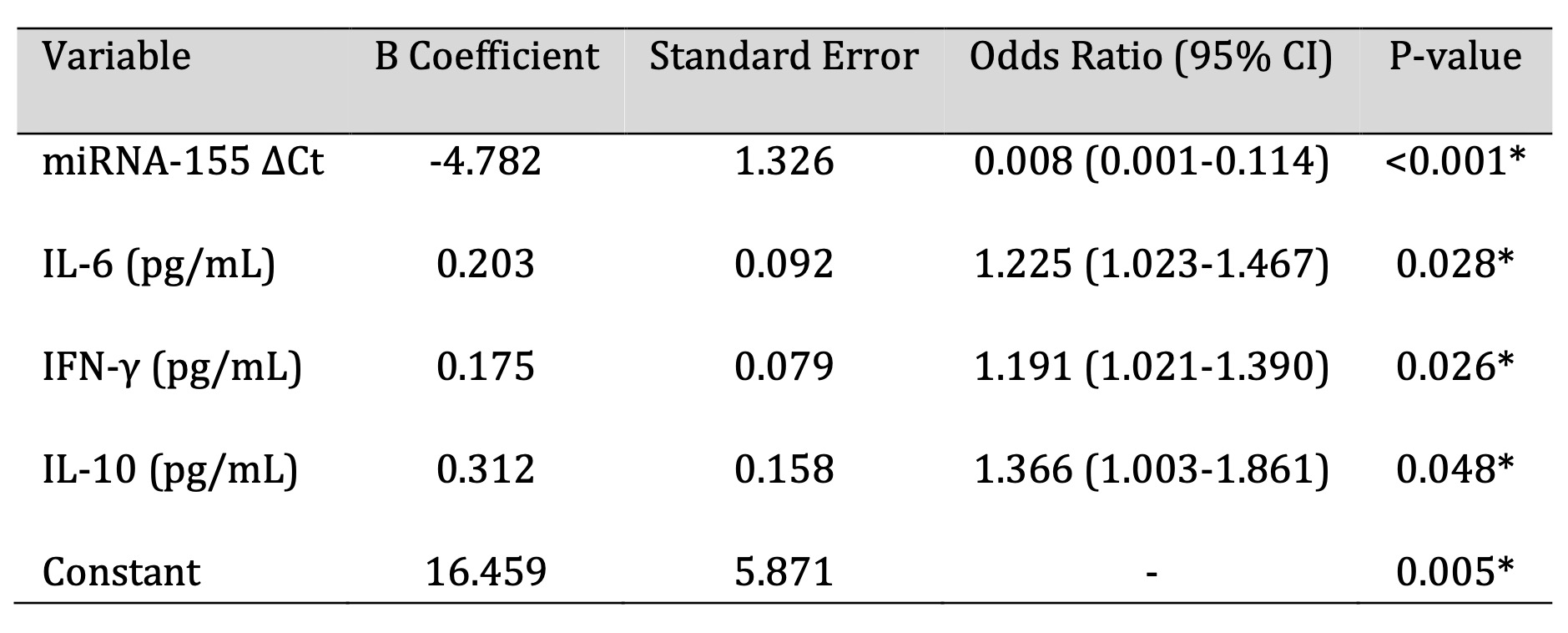
Table 4: Exploratory logistic regression analysis assessing associations between miRNA-155 expression, circulating cytokine levels, and Helicobacter pylori infection status within the study cohort. *Note: Odds ratios (OR) [95% onfidence intervals] are shown to determine the direction and strength of associations. Model fit indices are resented for descriptive purposes only. Because of he small sample size and exploratory nature of this analysis, no inference can be made about predictive or diagnostic performance from this finding
Discussion
In this case–control exploration, higher levels of peripheral expression of miRNA-155 and circulating IL-6, IFN-γ, and IL-10 were observed in H. pylori-infected subjects with chronic gastritis. These results indicate H. pylori chronic infection that leads to global immune modifications. Physiologically, it appears that a combination distinctive of systemic immune modulation may operate during chronic H. pylori infection through the modulation of miRNA-155 expression with cytokines in circulation.
miRNA-155 is a well-documented central player in immune and inflammatory signaling networks. Its higher expression level in peripheral blood might indicate persistent immune activation due to chronic bacterial stimulation. Increased levels of miRNA-155 were found in gastric tissue and immune cells of H. pylori-infected individuals in previous studies [12, 13, 23, 24] and, and the present findings extend these observations to the systemic circulation.
The elevated levels of IL-6 and IFN-γ in the present study are suggestive for a pro-inflammatory immune response whereas an increased level of IL-10 may indicate a compensatory anti-inflammatory mechanism which is beneficial in restricting uncontrolled inflammation [15–18]. Comparable cytokine profiles have been described in other studies of H. pylori-associated gastritis [25–32], lending biological credibility to the reported observations.
Several limitations should be acknowledged. The number of patients is relatively small and the lack of power statistically does lead to overfitting in multivariate analysis. Given the variation in BMI between groups, BMI may be a confounder of the inflammatory marker levels. Moreover, the analysis was limited by being restricted to peripheral blood and no validation in stomach tissues or functional experiments. Lack of independent validation cohort prevents clinical or diagnostic interpretation. The logistic regression analysis is also exploratory and should not be considered as evidence of predictive or diagnostic ability.
These results should not be taken to support their use in the clinical setting but as hypothesis-generating. Additional analyses are needed to dissect the physiological relevance of H. pylori-associated miRNA-155-mediated immune regulation in chronic gastritis, including investigation of more extensive and independent cohorts and experiments examining mechanisms.
Conclusion
In Summary, our findings show that The levels of peripheral miRNA-155 and circulating inflammatory cytokines in H. pylori–related chronic gastritis were up-regulated. The results show system host immune activation in H. pylori infection, and suggest a possibility that miRNA-155 might be associated with cytokine-mediated inflammatory response. Despite the exploratory and small-sample-size nature of the current study, additional work in larger or independent cohorts would be needed, along with mechanistic studies, to establish the biological and clinical significance of these observations.
Acknowledgements
The author acknowledges all participants, particularly those who generously agreed to be part of this study.
Author’s Contributions
S.K.S.A. designed the study, conducted the experiments and analyzed data, and drafted the manuscript.
Funding
The study was not supported by any funding.
Disclosure Statement
The author declares no conflicts of interest.
References
| 1 | Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S.
Helicobacter pylori infection. Nat Rev Dis Primers. 2023 Apr 20;9(1):19. doi:
10.1038/s41572-023-00431-8.
https://doi.org/10.1038/s41572-023-00431-8 |
| 2 | Al Mutawa OA, Izhari MA, Alharbi RA, Sindi AAA, Alqarni AM, Alotaibi FE, Gosady ARA, Dardari
DMM,
Almutairi AM, Alshehri M, Athathi AIE. Helicobacter pylori (H. pylori) Infection-Associated
Anemia
in the Asir Region, Saudi Arabia. Diagnostics (Basel). 2023 Jul 18;13(14):2404. doi:
10.3390/diagnostics13142404.
https://doi.org/10.3390/diagnostics13142404 |
| 3 | Al-Shamahy HA. Seroprevalence of Helicobacter pylori among children in Sana'a, Yemen. Ann
Saudi
Med. 2005 Jul-Aug;25(4):299-303. doi: 10.5144/0256-4947.2005.299.
https://doi.org/10.5144/0256-4947.2005.299 |
| 4 | Holleczek B, Schöttker B, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis
and
risk of stomach and esophagus cancer: Results from the prospective population-based ESTHER
cohort
study. Int J Cancer. 2020 May 15;146(10):2773-2783. doi: 10.1002/ijc.32610.
https://doi.org/10.1002/ijc.32610 |
| 5 | Bulgar K, Pavlovich I, Parcernyak A, Chumak B. The effect of Helicobacter pylori on the
morphological picture of the gastric mucosa in patients with chronic gastritis. Exp Clin
Gastroenterol. 2023;(220):19-26. doi:10.31146/1682-8658-ecg-220-12-19-26.
https://doi.org/10.31146/1682-8658-ecg-220-12-19-26 |
| 6 | Yang H, Hu B. Immunological Perspective: Helicobacter pylori Infection and Gastritis.
Mediators
Inflamm. 2022 Mar 8;2022:2944156. doi: 10.1155/2022/2944156.
https://doi.org/10.1155/2022/2944156 |
| 7 | Faujo Nintewoue GF, Kouitcheu Mabeku LB. Helicobacter pylori Infection Promotes Gastric
Premalignancies and Malignancies Lesions and Demotes Hyperplastic Polyps: A 5 Year Multicentric
Study among Cameroonian Dyspeptic Patients. Asian Pac J Cancer Prev. 2023 Jan 1;24(1):171-183.
doi:
10.31557/APJCP.2023.24.1.171.
https://doi.org/10.31557/APJCP.2023.24.1.171 |
| 8 | Nizeyimana T, Rugwizangoga B, Manirakiza F, Laga AC. Occurrence of Helicobacter Pylori in
Specimens of Chronic Gastritis and Gastric Adenocarcinoma Patients: A Retrospective Study at
University Teaching Hospital, Kigali, Rwanda. East Afr Health Res J. 2021;5(2):159-163. doi:
10.24248/eahrj.v5i2.667.
https://doi.org/10.24248/eahrj.v5i2.667 |
| 9 | Li H, Hu Y, Huang Y, Ding S, Zhu L, Li X, Lan M, Huang W, Lin X. The mutual interactions among
Helicobacter pylori, chronic gastritis, and the gut microbiota: a population-based study in
Jinjiang, Fujian. Front Microbiol. 2024 Feb 14;15:1365043. doi: 10.3389/fmicb.2024.1365043.
https://doi.org/10.3389/fmicb.2024.1365043 |
| 10 | Yang H, Zhou X, Hu B. The 'reversibility' of chronic atrophic gastritis after the eradication
of
Helicobacter pylori. Postgrad Med. 2022 Jun;134(5):474-479. doi:
10.1080/00325481.2022.2063604.Epub
2022 Apr 13. PMID: 35382697.
https://doi.org/10.1080/00325481.2022.2063604 |
| 11 | Toro DH, Bofill-Garcia A, Anzalota-Del Toro M. Helicobacter Pylori and Gastric Cancer: An
Update
in the Literature. P R Health Sci J. 2024 Mar;43(1):9-17. PMID: 38512756.
|
| 12 | Mahboobi R, Fallah F, Yadegar A, Dara N, Kazemi Aghdam M, Asgari B, Hakemi-Vala M. Expression
analysis of miRNA-155 level in Helicobacter pylori related inflammation and chronic gastritis.
Iran
J Microbiol. 2022 Aug;14(4):495-502. doi: 10.18502/ijm.v14i4.10235.
https://doi.org/10.18502/ijm.v14i4.10235 |
| 13 | Prinz C, Weber D. MicroRNA (miR) dysregulation during Helicobacter pylori-induced gastric
inflammation and cancer development: critical importance of miR-155. Oncotarget. 2020 Mar
10;11(10):894-904. doi: 10.18632/oncotarget.27520.
https://doi.org/10.18632/oncotarget.27520 |
| 14 | Vasapolli R, Venerito M, Schirrmeister W, Thon C, Weigt J, Wex T, Malfertheiner P, Link A.
Inflammatory microRNAs in gastric mucosa are modulated by Helicobacter pylori infection and
proton-pump inhibitors but not by aspirin or NSAIDs. PLoS One. 2021 Apr 15;16(4):e0249282. doi:
10.1371/journal.pone.0249282.
https://doi.org/10.1371/journal.pone.0249282 |
| 15 | Mogharehabed A, Yaghini J, Aminzadeh A, Rahaiee M. Comparative evaluation of microRNA-155
expression level and its correlation with tumor necrotizing factor α and interleukin 6 in
patients
with chronic periodontitis. Dent Res J (Isfahan). 2022 Apr 27;19:39. PMID: 35669607; PMCID:
PMC9164665.
https://doi.org/10.4103/1735-3327.344162 |
| 16 | Oana SM, Claudia B, Lelia RA, Simona M, Claudia C, Daniela DE. Differential Expression of
Tissular
miRNA-155 in Pediatric Gastritis. J Clin Med. 2022 Jun 10;11(12):3351 doi: 10.3390/jcm11123351.
https://doi.org/10.3390/jcm11123351 |
| 17 | Pachathundikandi SK, Backert S. Helicobacter pylori controls NLRP3 expression by regulating
hsa-miR-223-3p and IL-10 in cultured and primary human immune cells. Innate Immun. 2018
Jan;24(1):11-23. doi: 10.1177/1753425917738043.
https://doi.org/10.1177/1753425917738043 |
| 18 | Kim J, Cho YA, Choi IJ, Lee YS, Kim SY, Shin A, Cho SJ, Kook MC, Nam JH, Ryu KW, Lee JH, Kim
YW.
Effects of interleukin-10 polymorphisms, Helicobacter pylori infection, and smoking on the risk
of
noncardia gastric cancer. PLoS One. 2012;7(1):e29643. doi: 10.1371/journal.pone.0029643.
https://doi.org/10.1371/journal.pone.0029643 |
| 19 | Hu H, Zhu X, Lin X. GuaLou GuiZhi decoction represses LPS-induced BV2 activation via miR-155
induced inflammatory signals. Pak J Pharm Sci. 2020 Jan;33(1(Special)):403-408 PMID: 32173634.
|
| 20 | Lu S, Dong L, Jing X, Gen-Yang C, Zhan-Zheng Z. Abnormal lncRNA CCAT1/microRNA-155/SIRT1 axis
promoted inflammatory response and apoptosis of tubular epithelial cells in LPS caused acute
kidney
injury. Mitochondrion. 2020 Jul;53:76-90. doi: 10.1016/j.mito.2020.03.010.
https://doi.org/10.1016/j.mito.2020.03.010 |
| 21 | Sumiyoshi N, Toyonaga T, Shumway A, Nakagawa F, Tanaka M, Saeki C, Saruta M. P001
MicroRNA-155-5p
contributes to the development of ulcerative colitis by regulating T lymphocyte proliferation
and
functions. J Crohns Colitis. 2024;18(Suppl 1):i244. doi:10.1093/ecco-jcc/jjad212.0131.
https://doi.org/10.1093/ecco-jcc/jjad212.0131 |
| 22 | Acosta IC, Alonzo F 3rd. The Intersection between Bacterial Metabolism and Innate Immunity. J
Innate Immun. 2023;15(1):782-803. doi: 10.1159/000534872.
https://doi.org/10.1159/000534872 |
| 23 | Wu K, Zhu C, Yao Y, Wang X, Song J, Zhai J. MicroRNA-155-enhanced autophagy in human gastric
epithelial cell in response to Helicobacter pylori. Saudi J Gastroenterol. 2016
Jan-Feb;22(1):30-6.
doi: 10.4103/1319-3767.173756.
https://doi.org/10.4103/1319-3767.173756 |
| 24 | Alı PSS. Role of miRNAs in immune regulation and bacterial infections. J Microbiol Infect Dis.
2023;13(1):1-7. doi:10.5799/jmid.1264855.
https://doi.org/10.5799/jmid.1264855 |
| 25 | Shehab MJ, Mahdi BM, Al-Tabra RH, Namaa DS. Evaluation of serum levels of proinflammatory
cytokines IL-8, IL-17, and IL-22 in Helicobacter pylori infection and their association with the
degree of gastritis histopathology in a sample of Iraqi patients. Baghdad Sci J.
2023;20(3):Article
49. doi:10.21123/bsj.2023.8621.
https://doi.org/10.21123/bsj.2023.8621 |
| 26 | Alaskeri HMO, Pumbuk CIA. Interleukin-13 level accompanying with Helicobacter pylori gastric
infection in Kirkuk city. Tikrit J Pure Sci. 2021;26(6):28-31. doi:10.25130/tjps.v26i6.19.
https://doi.org/10.25130/tjps.v26i6.190 |
| 27 | Smirnova OV, Sinyakov AA. Influence of Helicobacter pylori on cytokine regulation in chronic
atrophic gastritis. Russ J Infect Immun. 2020;10(1):187-192. doi:10.15789/2220-7619-IOH-1167.
https://doi.org/10.15789/2220-7619-IOH-1167 |
| 28 | Siregar G, Tala Z. Differences of IL-12 levels between positive and negative Helicobacter
pylori
gastritis. Int J Res Rev. 2022;9(4). doi:10.52403/ijrr.20220401.
https://doi.org/10.52403/ijrr.20220401 |
| 29 | Zhao CN, Xiao LL, Zhang Y. Effects of Helicobacter pylori Infection on the Prognosis of
Chronic
Atrophic Gastritis by Inducing the Macrophage Polarization. Gastroenterology Res. 2023
Aug;16(4):226-233. doi: 10.14740/gr1636.
https://doi.org/10.14740/gr1636 |
| 30 | Soyocak A, Ergun DD, Koc G, Ergun S, Ozsobaci NP. Investigation of Aryl Hydrocarbon Receptor,
Zinc, and Vitamin B12 Levels in Chronic Gastritis with Helicobacter pylori Infection. Biol Trace
Elem Res. 2021 Jul;199(7):2431-2437 doi: 10.1007/s12011-021-02667-5.
https://doi.org/10.1007/s12011-021-02667-5 |
| 31 | Mokhonova EV, Lapin VA, Melentiev DA, Novikov DV, Neumoina NV, Perfilova KM, et al. Analysis
of
the state of immunity in patients with chronic gastritis infected with Helicobacter pylori. Russ
J
Infect Immun. 2022;12(2):306-314 doi:10.15789/2220-7619-AOT-1725.
https://doi.org/10.15789/2220-7619-AOT-1725 |
| 32 | Mahmoodzadeh AS, Moazamian E, Shamsedin SA, Kaydani GA. Prevalence of virulence genes and
antigen
pattern in Helicobacter pylori-infected patients and the level of some inflammatory cytokines
compared with non-infected individuals. Jundishapur J Microbiol. 2022;15(2):1-10.
doi:10.5812/jjm-121144.
https://doi.org/10.5812/jjm-121144 |