Acid-Sensitive Outwardly Rectifying Cl- Current in OV2944 Mouse Ovarian Cancer Cells
Keywords
Abstract
Background/Aims:
Extracellular acidic conditions impair cellular activities; however, some cancer cells drive cellular signaling to adapt to the acidic environment. It remains unclear how ovarian cancer cells sense changes in extracellular pH. This study was aimed at characterizing acid-inducible currents in an ovarian cancer cell line and evaluating the involvement of these currents in cell viability.Methods:
The biophysical and pharmacological properties of membrane currents in OV2944, a mouse ovarian cancer cell line, were studied using the whole-cell configuration of the patch-clamp technique. Viability of this cell type in acidic medium was evaluated using the MTT assay.Results:
OV2944 had significant acid-sensitive outwardly rectifying (ASOR) Cl− currents at a pH50 of 5.3. The ASOR current was blocked by pregnenolone sulfate (PS), a steroid ion channel modulator that blocks the ASOR channel as one of its targets. The viability of the cells was reduced after exposure to an acidic medium (pH 5.3) but was slightly restored upon PS administration.Conclusion:
These results offer first evidence for the presence of ASOR Cl− channel in ovarian cancer cells and indicate its involvement in cell viability under acidic environment.Introduction
Cancer cells are characterized by the overactivity of anaerobic glycolysis, which causes the accumulation of acidic metabolites, such as lactate; this phenomenon is referred to as the Warburg effect [1-3]. To maintain intracellular pH homeostasis, cancer cells export cytosolic lactate mainly through lactate-H+ symporters that work together with Na+/H+ exchangers and H+-ATPases [3, 4], leading to extracellular acidosis (i.e. , acidic pH in the extracellular environment). Although the extracellular pH (pHe) in human tumors ranges between 6.3 and 7.0, it sometimes falls below 6.0 [5-7].
In the brain, the acidic pHe caused by ischemia can reach approximately 6.0, which ultimately results in brain infarction [8, 9]. However, in cancerous tissues, the acidic extracellular microenvironment promotes cancer development and progression [6]. In fact, pHe in the 6.4–6.8 range increases the metastatic activity of cancer cells, as reported for human melanoma, colorectal, and pancreatic cell lines [10-13]. For adaptation to the acidic microenvironment, cancer cells need to sense the increase in protons in the extracellular space and transduce the information about this change to the intracellular signaling network, which drives cellular responses, including alterations in metabolic processes and gene expression profiles [4, 14]. Nonetheless, it remains unclear as to how cancer cells sense changes in pHe.
Proton-sensing ion channels play a fundamental role in sensing the extracellular proton concentration by conducting Na+ through acid-sensing ion channels (ASICs) [15] and Cl− through acid-sensitive outwardly rectifying (ASOR) channels [16- 18]. ASOR currents have been observed in cancer cell lines, including cervical cancer (HeLa), epidermoid carcinoma (KB-3-1), neuroblastoma (SK-N-MC), lung capillary carcinoma (H441), and pancreatic duct adenocarcinoma (CFPAC) as well as in HEK-293 cells [19- 21].
Ovarian cancer tissue is influenced by an acidic pHe [22, 23]. Nonetheless, it remains unclear as to how ovarian cancer cells sense changes in pHe. To unravel the mechanisms by which ovarian cancer cells sense the extracellular proton, we examined acid-sensing currents in OV2944, a mouse ovarian cancer cell line, using whole-cell patch clamp configuration. We found prominent ASOR Cl− current in OV2944 cells at pHe less than 5.3. This ASOR current was inhibited by niflumic acid (NFA), a Cl− channel blocker, and pregnenolone sulfate (PS), an ion channel modulator, as reported for TMEM206-mediated ASOR currents [16]. Furthermore, decreased viability of OV2944 cells in acidic pHe was slightly restored by the administration of PS. These results indicate the presence of ASOR current in mouse ovarian cancer cells under highly acidic pH conditions and its involvement in the viability of these cells.
Materials and Methods
Cell culture
The mouse ovarian cancer cell line (OV2944) was obtained from Riken Bioresource (Tsukuba, Japan) and cultured in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, MA, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific) and 1% penicillin-streptomycin (Thermo Fisher Scientific).
Electrophysiology
Membrane currents of OV2944 cells were recorded using the whole-cell configuration of the patch-clamp technique at room temperature (~25 °C). Patch pipettes, with tip resistance of 3–5 MΩ for whole-cell recordings, were constructed from borosilicate glass. The electrodes were connected to an Axopatch 200B amplifier (Molecular Devices, CA, USA) controlled using the pCLAMP 8.0 analysis software (Molecular Devices), and the current or voltage output was viewed directly on the screen of a computer attached to the amplifier via a DigiData 1200 interface (Molecular Devices). The digitized data were analyzed using the OriginPro 8 J software (OriginLab, MA, USA). The frequency of the low-pass Bessel filter in the amplifier was set at 1 kHz. The sampling frequency was set at 5 kHz. Current recordings were performed by holding the cells at −100 mV and applying voltage step pulses (100 ms duration) from −100 to +100 mV in 20 mV increments, with compensating membrane capacitance. The capacitance (mean ± SD) of a sample of 62 cells used for data analysis was 23 ± 6 pF. Ramp-pulse protocol for current–voltage relationship was from −100 to +100 mV at a ramp speed of 0.05 mV/ms, with 18 s time intervals. Series resistance was maintained at <15 MΩ and was uncompensated. Twenty-four to forty-eight hours after plating on a coverslip, the cells were used for electrophysiological experiments.
The composition of the extracellular standard solution was as follows: 150 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose (pH 7.3, ~320 mOsm/kg; herein referred to as “standard solution”). For eliminating extracellular Cl−, 150 mM NaCl in the standard solution was replaced with 120 mM Na2SO4. For characterizing hypotonicity-induced currents, the concentration of NaCl was reduced to 120 mM, and this solution, with a lower osmolarity (~260 mOsm/kg), was used as a “hypotonic solution”; 60 mM mannitol (rendering ionic activity equivalent to that of 30 mM NaCl) was supplemented to the hypotonic solution to obtain a “control isotonic solution” (~320 mOsm/kg). To test for the presence of potassium-involved currents, 30 mM NaCl in the standard solution was replaced either with 30 mM TEA-Cl (a broad-spectrum K+ channel blocker), 30 mM KCl, or 30 mM choline-Cl. The pH of acidic solutions was adjusted with HCl. The composition of extracellular solutions used in each experiment is provided in Supplementary Table 1. An inlet tube for perfusion was positioned 400 μm from the recorded cell. The extracellular solution was perfused under gravity at a rate of 0.3 mL/min and was changed using a valve-controlled solution changer. The pipette solution contained 145 mM CsCl, 5 mM NaCl, 10 mM HEPES, 1 mM MgCl2, 3.5 mM EGTA, and 10 mM glucose (pH was adjusted to 7.2 with CsOH; final osmolality was ~310 mOsm/kg). The liquid junction potentials of the control and low-Cl− extracellular solutions (+3 mV: measured using a 3 M KCl agar bridge) were not corrected. All chemicals, except pregnenolone sulfate and niflumic acid (Cayman Chemical, MI, USA), were purchased from Fujifilm Wako (Osaka, Japan) or Sigma-Aldrich (MO, USA). We routinely checked and confirmed that the effect of changing the external solution was reversible during stable recordings. Statistical values are given as mean ± SE; confidence limits were determined using the Student’s t -test.
MTT assay
To evaluate cell viability after treatment, a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetra-zolium bromide (MTT) assay was performed as previously described [24]. Briefly, MTT was converted from yellow, water-soluble tetrazolium to blue, water-insoluble MTT-formazan by cellular mitochondrial dehydrogenases. Because the rate of this reaction is proportional to the number of living cells, the absorbance of the formazan product reflects cell viability. In this assay, cells were resuspended and cultured in one of the following three media: (i) control medium (pH 7.4; DMEM supplemented with 100 mM mannitol), (ii) acid-treated medium (pH 5.3; DMEM supplemented with 100 mM 2-morpholinoethanesulfonic acid (MES)), and (iii) acid- and pregnenolone sulfate (PS)-treated medium (pH 5.3; DMEM supplemented with 100 mM MES and 20 μM PS). After 2 h, 100 μM MTT was added, and after further 24 h, 20% SDS in dimethylformamide was added to dissolve the insoluble formazan product, and the OD560 was measured. Statistical values are given as mean ± SE; confidence limits were determined using the Student’s t -test.
Results
Acid-sensitive outwardly rectifying current in OV2944 cells
Perfusion of an acidic bath solution (pH 4.5) elicited strong outwardly rectifying currents at positive holding potentials (Fig. 1A). The current–voltage relationships, as revealed by the ramp-pulse protocol, exhibited steep outward rectification in a pH-dependent manner (Fig. 1B). The normalized current–pH relationship, by fitting with a Hill function, yielded a pH50 of 5.3 and a Hill coefficient of 3.7 (Fig. 1C), suggesting current activation by cooperative binding of protons to the corresponding channel. This current–pH relationship was comparable to that of the ASOR current, which has been reported in wild-type (e.g. , epithelial cells: [21]; neurons: [25]; glial cells: [26]) and TMEM206-transfected [16] cells. Accordingly, the acid-sensitive outwardly rectifying current in OV2944 cells is hereafter referred to as the ASOR current.
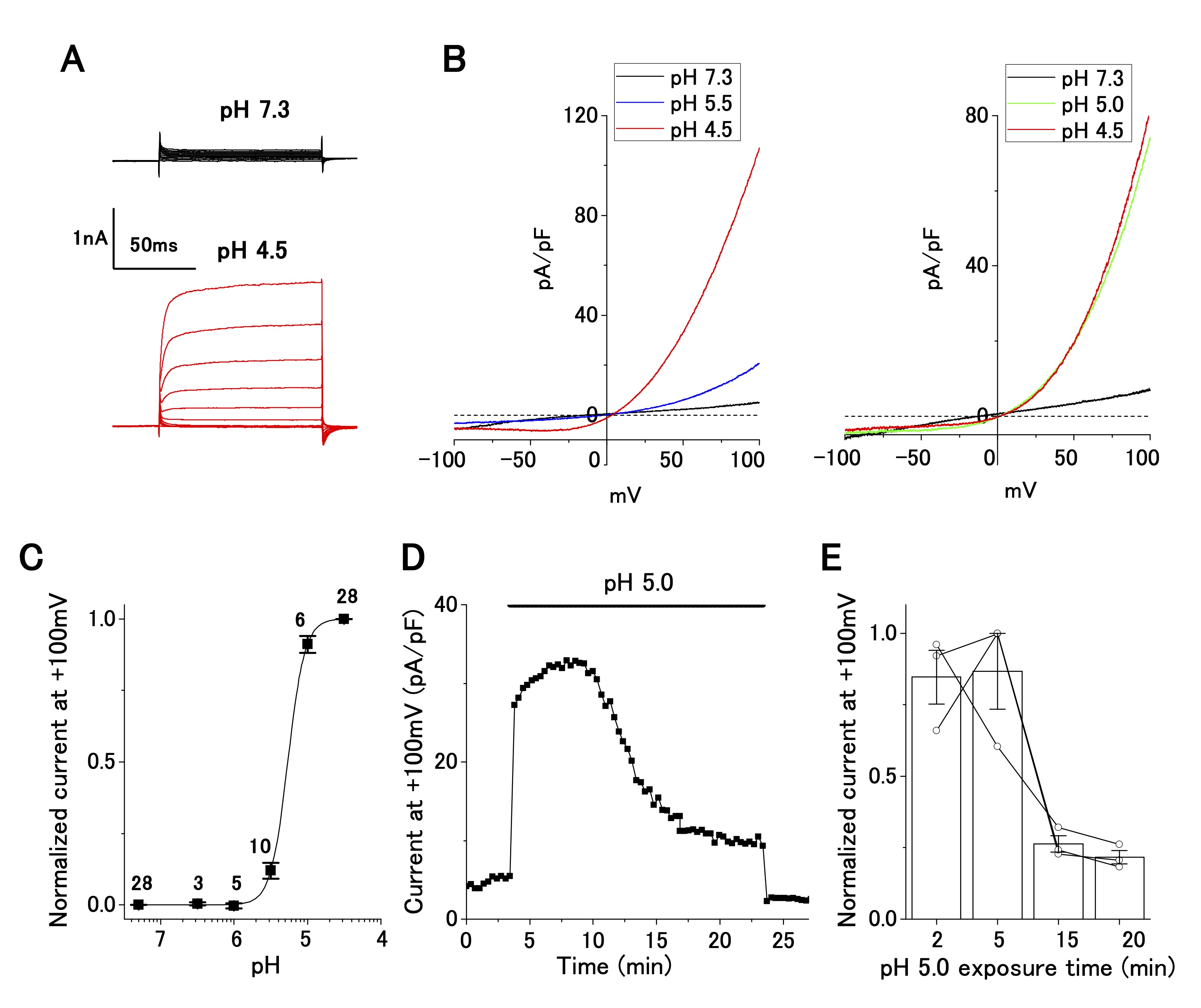
Fig. 1: Characterization of acid-sensitive outwardly rectifying (ASOR) current in cultured OV2944 cells. A. Typical time course of ASOR current activated using the voltage step protocol at pH 4.5. B. Current–voltage relationship of the ASOR current activated using the voltage ramp protocol (Left: from pH 7.3 through 5.5 to 4.5; Right: from pH 7.3 through 5.0 to 4.5). C. Normalized current (background-subtracted current at pH 4.5 and +100 mV as 1) to pH relationship fitted with the Hill equation curve (pH50: 5.3; Hill coefficient: 3.7). The number of cell analyzed for each pH value is indicated in the graph. D. ASOR current at +100 mV persisted for 20 min at pH 5.0. E. Changes in normalized current at a persistent pH of 5.0 (background-subtracted current at +100 mV as 1; n = 3 shown with circles).
ASOR current persists after a long exposure of OV2944 cells to the acidic medium
The change in pHe probably occurs chronically (i.e. , in a slow progression) in cancerous tissues. Our patch-clamp recording was completed several minutes after the replacement of the bath solution. We were uncertain about the presence of the ASOR current after long exposure to the acidic medium. To clarify this, we examined whether the ASOR current persisted after a long exposure of the cells to the acidic medium. Twenty minutes after changing the pHe from 7.3 to 5.0, the ASOR current decreased to ~20% of that recorded at the time of replacement, confirming that the ASOR current was present after persistent exposure of the cells to the acidic medium (Fig. 1D and E).
ASOR current is caused by the influx of Cl−, which is blocked by niflumic acid and pregnenolone sulfate
Next, we investigated ion selectivity and inhibitor sensitivity of ASOR current in OV2944 cells. The replacement of [Cl−]o with [SO42−]o abolished the outward current (Fig. 2A), suggesting that the influx of extracellular Cl− generated the outward ASOR current. In addition, this replacement shifted reversal potential of the current positively (Inset: −0.7 ± 0.5 mV ([Cl−]o) and 11.1 ± 3.8 mV ([SO42−]o); n = 5; p < 0.05), consistent with the notion that the current is caused by a Cl− permeable channel. This ion replacement also significantly decreased the inward current due to anion efflux, as reported for TMEM206-mediated ASOR [16] (Fig. 2A Right). Because intracellular Cl− was not replaced, this result may be ascribed to the blocking of Cl− efflux by extracellular SO42− (or HSO4−) [16]; alternatively, the activation of ASOR may require the presence of extracellular Cl− [16].
The outward ASOR current was significantly suppressed by extracellular administration of 20 and 100 μM NFA, a broad-spectrum anion channel blocker (Fig. 2C). The extent of suppression was significant (~65% suppression; 5, 35, and 10 pA/pF for pH 7.4, pH 4.5, and pH 4.5+100 μM NFA, respectively, Fig. 2B), which was similar to the effect of NFA on ASOR currents in other cell lines, such as HEK-293, HeLa, and SK-N-MC [21, 27]. Furthermore, the ASOR current in OV2944 cells was inhibited by 20 μM PS, which shuts off several ion channels [28], including TMEM206, a transmembrane protein conducting the ASOR current [16, 29] (Fig. 2D). The effect of PS on the ASOR current was drastic: the extent of inhibition was ~90% and ~100% for 5 and 20 μM PS treatments, respectively (Fig. 2D and E). Recording in KCl-based intracellular solution with the application of TEA (30 mM), a broad-spectrum blocker of K+ channels, and of high-[K+]o (+ 30 mM), in the bath showed no significant effect on the current–voltage relationship (Supplementary Fig. 1), which indicates that voltage-dependent K+ channels are unlikely to have a significant function in OV2944 cells under the bath conditions that were examined in this study.
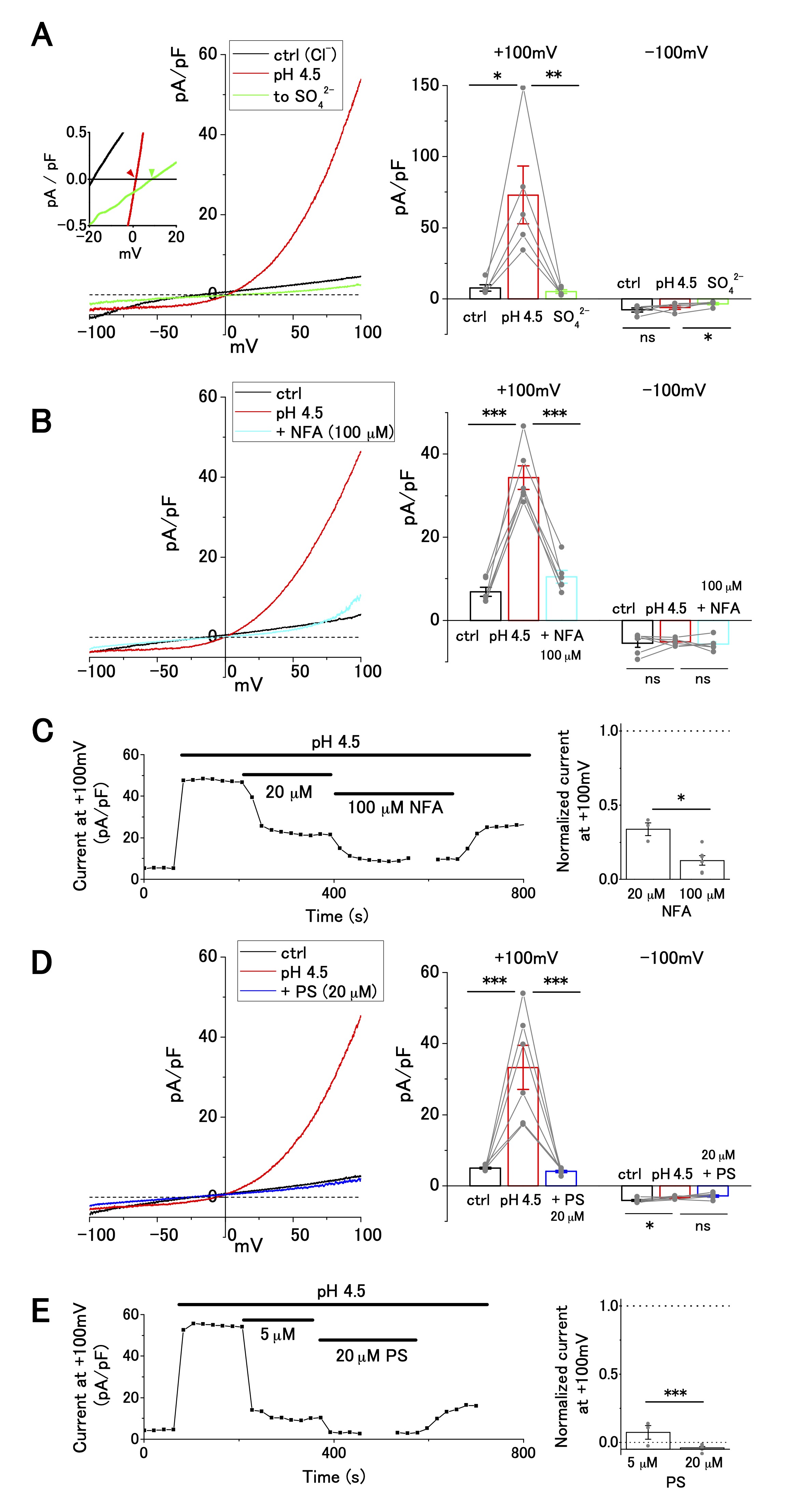
Fig. 2: Cl− selectivity and inhibitor sensitivity of acid-sensitive outwardly rectifying (ASOR) current in OV2944 cells. A. Left: Current–voltage relationship of the ASOR current recorded using the voltage ramp protocol and current inhibition by replacement of [Cl-]o with [SO42−]o at pH 4.5. Inset: Traces of the recording (smoothed with Savitzky–Golay filter) at a higher magnification. Right: Summary of current density at +100 and −100 mV in control, at pH 4.5, and in [SO42−]o at pH 4.5. B. Left: Current–voltage relationship of the ASOR current recorded using the voltage ramp protocol and current inhibition by 100 μM NFA at pH 4.5. Right: Summary of current density at +100 and −100 mV in control, at pH 4.5, and in NFA (100 μM) at pH 4.5. C. Left: Effect of NFA on the ASOR current at +100 mV. Right: Changes in normalized current in the presence of NFA (background-subtracted current at +100 mV as 1; n = 3 for 20 μM and n = 6 for 100 μM). D. Left: Current–voltage relationship of the ASOR current recorded using the voltage ramp protocol and current inhibition by 20 μM pregnenolone sulfate (PS) at pH 4.5. Right: Summary of current density at +100 and −100 mV in control, at pH 4.5, and in PS (20 μM) at pH 4.5. E. Left: Effect of PS on ASOR current at +100 mV. Right: Changes in normalized current in the presence of PS (background-subtracted current at +100 mV as 1; n = 3 for 5 μM and n = 6 for 20 μM). (Bar: mean ± SEM. ***p<0.01, **p<0.02, and *p<0.05; Circles: individual data).
Hypotonicity-induced current, partial Cl− current, is insensitive to pregnenolone sulfate
In addition to the ASOR Cl− current, hypotonicity-induced anion current is present in human cancer cell lines, including Ehrlich ascites, epidermoid, and pancreatic cancer cell lines [30-34]. OV2944 cells exhibited an outwardly rectifying current upon perfusion of a hypotonic extracellular solution (Fig. 3). The replacement of [Cl−]o (120 mM) with [SO42−]o (102 mM) partially suppressed the outward current at a positive potential by ~50% (Fig. 3A); the extent of this suppression was distinct from that of the ASOR current, which was suppressed by ~90% (Fig. 2A). Extracellular administration of PS (20 μM) had no significant effect on the hypotonicity-induced current (Fig. 3B), whereas PS administration suppressed the ASOR current by ~90% (Fig. 2D and E). These results indicate that ion selectivity and drug sensitivity of hypotonicity-induced current are distinct from those of the ASOR current. Furthermore, the outward ASOR current was evoked by the acidic change of pHe under persistent hypotonic stimulation (Supplementary Fig. 2), strengthening the contention that the ASOR and hypotonicity-induced currents originate from distinct channel types.
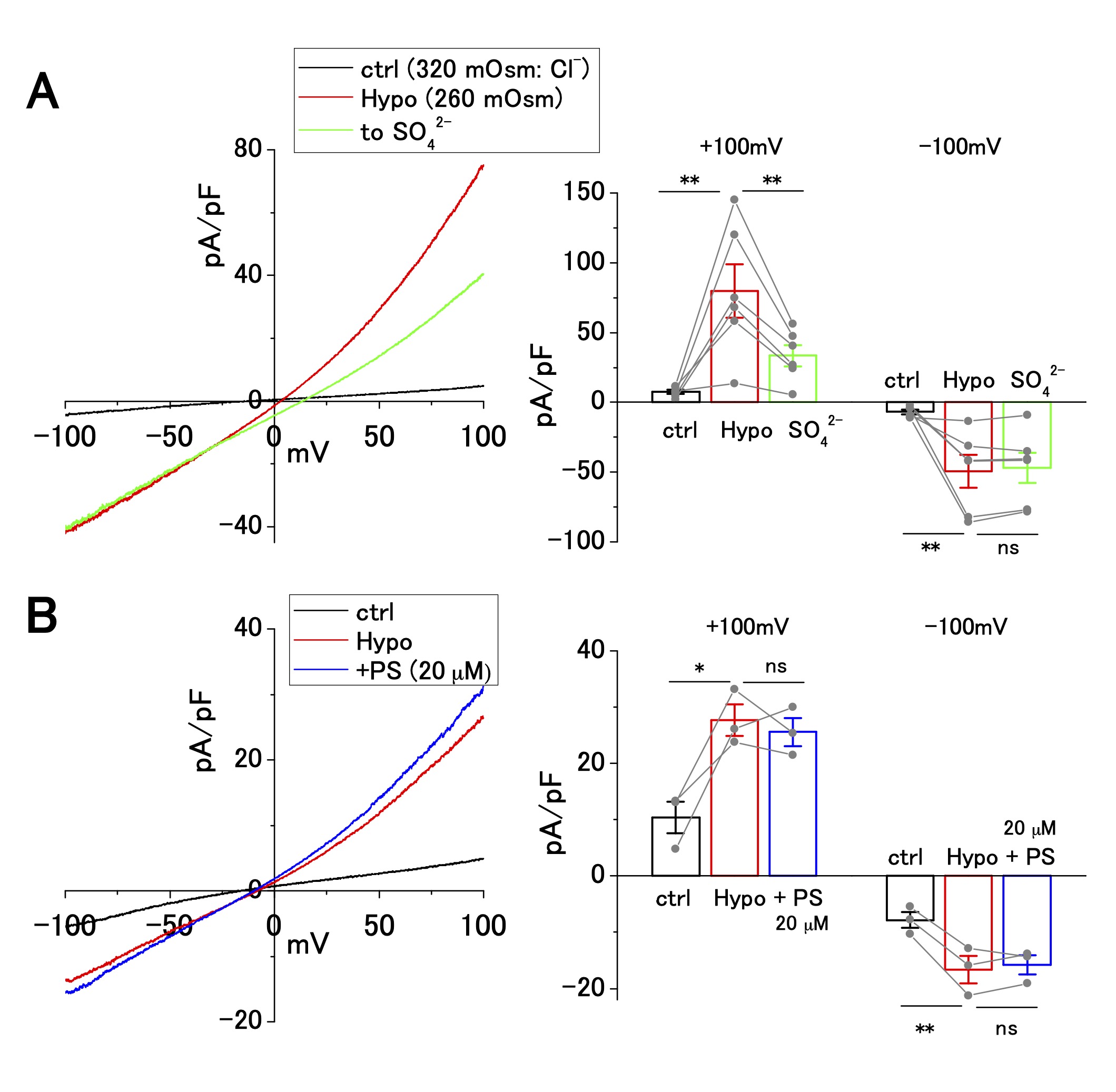
Fig. 3: Cl− selectivity and pregnenolone sulfate (PS) sensitivity of hypotonicity-induced current in OV2944 cells. A. Left: Current–voltage relationship of hypotonicity-induced current recorded using the voltage ramp protocol and current inhibition by replacement of [Cl -] o with [SO42−]o under hypotonic conditions. Right: Summary of current density at +100 and −100 mV in control, under hypotonic conditions, and in [SO42−]o under hypotonic conditions. (Bar: mean ± SEM. **p<0.02; ns: non-significant; Circles: individual data). B. Left: Current–voltage relationship of hypotonicity-induced current recorded using the voltage ramp protocol and the effect of 20 μM PS under hypotonic conditions. Right: Summary of current density at +100 and −100 mV in control, under hypotonic conditions, and in PS (20 μM) under hypotonic conditions. (Bar: mean ± SEM. **p<0.02 and *p<0.05. ns: non-significant; Circles: individual data).
Pregnenolone sulfate slightly improves cell viability under acidic environment
The ASOR channels decrease cell viability below pHe 6.0 [16, 17, 19, 25]. We performed the MTT assay to investigate whether ASOR channels affect the viability of OV2944 cells. The viability of the cells decreased to 22% and 16% of that in the control after 3 and 6 h of exposure to the acidic medium, respectively (Fig. 4). Addition of PS to the acidic medium slightly increased the cell viability after 3 h of exposure compared with that in the absence of PS (22% and 27% in the absence and presence of PS; p < 0.01), and this tendency persisted after 6 h of exposure (17% and 19% in the absence and presence of PS; p < 0.02). These results indicated that ASOR current (i.e. , channel(s) that opens in response to acidic pHe, resulting in the ASOR current) contributes slightly to facilitate acidic pHe-caused damage to OV2944 cells.
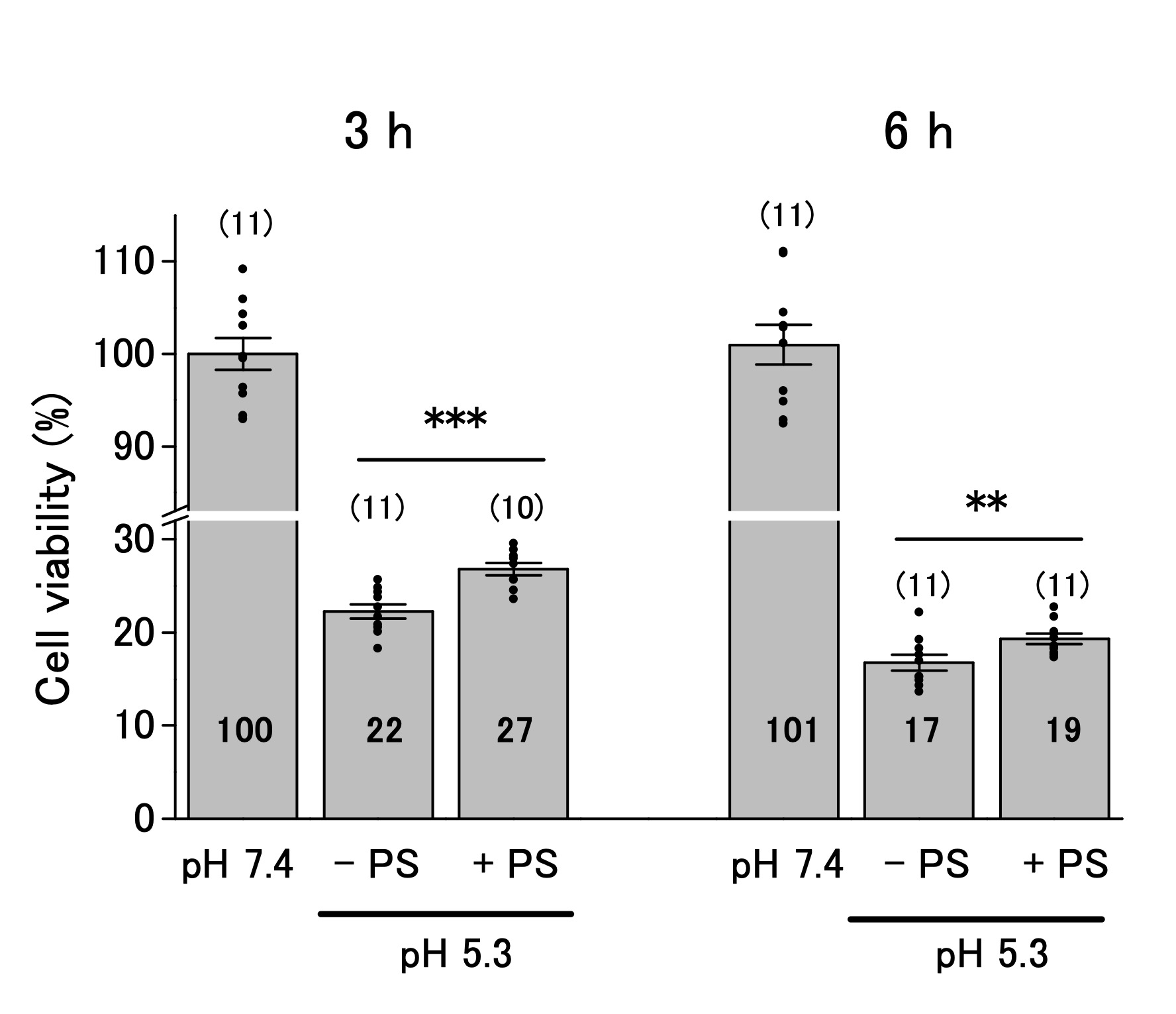
Fig. 4: Viability of OV2944 cells incubated at pH 7.4 or 5.3 without (−) or with (+) 20 μM PS for 3 (Left) or 6 h (Right). (Bar: mean ± SEM. ***p<0.01 and **p<0.02. ns: non-significant; Circles: individual data. Numbers in the bars represent averaged values for each condition. The number of samples for each condition is indicated in parenthesis).
Discussion
Besides cancer cell lines, ASOR Cl− currents have also been reported in various mammalian cell types, including murine cardiac myocytes, human monocytes, human vein, endothelial cells, and astrocytes [18, 26, 35-38]. OV2944 cells recorded with CsCl-based pipette solution exclusively showed the Cl− current as the ASOR current (Fig. 2), suggesting that the ASOR current in this study may be ascribed to acid-sensitive Cl− channel. The ASOR channel has been identified as TMEM206 (alternatively named proton-activated chloride (PAC) channel) [16, 17]. The biophysical and pharmacological properties of ASOR current in OV2944 cells, including Cl− selectivity, extracellular pH dependency (pH50: 5.3), pH-cooperative activation (Hill coefficient: 3.7), and blockade by NFA and PS, were similar to those of the TMEM206-mediated ASOR current [16], implying that the ASOR current in our study was brought about by opening of the TMEM206 Cl− channel. As of date, specific blockers for ASOR/TMEM206 are not available. NFA is known to block several types of anion channels, such as calcium-activated and volume-regulated ones [39]. PS modulates several types of ion channels; for example, it activates TRPM1 and 3, potentiates NMDA receptor-based current, and inhibits GABAA current [28]. TMEM206 is the only non-ligand-gated anion channel blocked by both NFA and PS among the channels mentioned above; therefore, TMEM206 may be responsible for the ASOR current that was observed under acidic pHe in OV2944 cells. The channel responsible for the ASOR current observed in our study remains to be identified.
In electrophysiological experiments using acidic bath solutions, we used extracellular buffer containing HEPES. The pH of the 10 mM HEPES buffer was kept constant at 4.5 over 24 h, ensuring the consistency of pH throughout our experiments. However, the buffering range of HEPES is between pH 6.8 and 8.2 at 25°C; therefore, it would be necessary to use a different buffer, such as MES (buffering range: pH 5.5–6.7) [20, 21, 27] and/or citrate buffer (buffering range: below pH 6.2) [16, 17, 26] in future studies.
In cancerous tissue, the pHe probably changes slowly; therefore, the functional channel under the acidic pHe in the cancerous tissue must be persistently activated. As shown in Fig. 1D and E, the ASOR current in OV2944 cells persisted even when the cells were settled under acidic pHe over 20 min, indicating that the activation of ASOR channel in response to an acidic environment is not transient but persistent. This suggests that the ASOR channel in OV2944 cells is functional under persistent acidic pHe. Administration of PS significantly suppressed the ASOR current whereas it increased the cell viability slightly (Figs. 2 and 4). Thus, ASOR may be activated under severe acidosis and may, in turn, be involved in the acidosis-induced cell death of OV2944 cells.
Conclusion
We provide the first evidence for the presence of ASOR Cl− channels in ovarian cancer cells and indicate its involvement in cell viability. However, molecular identification of the ASOR channel in this cell type is warranted.
Acknowledgements
Author Contributions
H.H. and N.M. designed the experiments. H.H. performed electrophysiological experiments and analyzed data. N.M. and K.A. performed the MTT assay. H.H. and N.M. discussed the data and wrote the manuscript.
Funding Sources
This work was supported in part by JSPS KAKENHI Grant Number 22K06835.
Statements of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
| 1 | Warburg O, Wind F, Negelein E: The metabolism of tumors in the body. J Gen Physiol 1927;8:519-530.
https://doi.org/10.1085/jgp.8.6.519 |
| 2 | Potter M, Newport E, Morten KJ: The Warburg effect: 80 years on. Biochem Soc Trans 2016;44:1499-1505.
https://doi.org/10.1042/BST20160094 |
| 3 | Vaupel P, Multhoff G: Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol 2021;599:1745-1757.
https://doi.org/10.1113/JP278810 |
| 4 | Corbet C, Feron O: Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer 2017;17:577-593.
https://doi.org/10.1038/nrc.2017.77 |
| 5 | Gatenby RA, Gillies RJ: Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891-899.
https://doi.org/10.1038/nrc1478 |
| 6 | Boedtkjer E, Pedersen SF: The Acidic Tumor Microenvironment as a Driver of Cancer. Annu Rev Physiol 2020;82:103-126.
https://doi.org/10.1146/annurev-physiol-021119-034627 |
| 7 | Lee SH, Griffiths JR: How and Why Are Cancers Acidic? Carbonic Anhydrase IX and the Homeostatic Control of Tumour Extracellular pH. Cancers 2020;12:1616.
https://doi.org/10.3390/cancers12061616 |
| 8 | Rehncrona S: Brain acidosis. Ann Emerg Med 1985;14:770-776.
https://doi.org/10.1016/S0196-0644(85)80055-X |
| 9 | Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA: Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol 1991;260:R581-R588.
https://doi.org/10.1152/ajpregu.1991.260.3.R581 |
| 10 | Martínez-Zaguilán R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ: Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis 1996;14:176-186.
https://doi.org/10.1007/BF00121214 |
| 11 | Rofstad EK, Mathiesen B, Kindem K, Galappathi K: Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res 2006;66:6699-6707.
https://doi.org/10.1158/0008-5472.CAN-06-0983 |
| 12 | Zhou ZH, Song JW, Li W, Liu X, Cao L, Wan LM, Tan YX, Ji SP, Liang YM, Gong F: The acid-sensing ion channel, ASIC2, promotes invasion and metastasis of colorectal cancer under acidosis by activating the calcineurin/NFAT1 axis. J Exp Clin Cancer Res 2017;36:130.
https://doi.org/10.1186/s13046-017-0599-9 |
| 13 | Zhu S, Zhou HY, Deng SC, Deng SJ, He C, Li X, Chen JY, Jin Y, Hu ZL, Wang F, Wang CY, Zhao G: ASIC1 and ASIC3 contribute to acidity-induced EMT of pancreatic cancer through activating Ca2+/RhoA pathway. Cell Death Dis 2017;8:e2806
https://doi.org/10.1038/cddis.2017.189 |
| 14 | Rauschner M, Lange L, Hüsing T, Reime S, Nolze A, Maschek M, Thews O, Riemann A: Impact of the acidic environment on gene expression and functional parameters of tumors in vitro and in vivo. J Exp Clin Cancer Res 2021;40:10.
https://doi.org/10.1186/s13046-020-01815-4 |
| 15 | Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M: A proton-gated cation channel involved in acid-sensing. Nature 1997;386:173-177.
https://doi.org/10.1038/386173a0 |
| 16 | Ullrich F, Blin S, Lazarow K, Daubitz T, von Kries JP, Jentsch TJ: Identification of TMEM206 proteins as pore of PAORAC/ASOR acid-sensitive chloride channels. Elife 2019;8:e49187.
https://doi.org/10.7554/eLife.49187 |
| 17 | Yang J, Chen J, Del Carmen Vitery M, Osei-Owusu J, Chu J, Yu H, Sun S, Qiu Z: PAC, an evolutionarily conserved membrane protein, is a proton-activated chloride channel. Science 2019;364:395-399.
https://doi.org/10.1126/science.aav9739 |
| 18 | Okada Y, Sato-Numata K, Sabirov RZ, Numata T: Cell Death Induction and Protection by Activation of Ubiquitously Expressed Anion/Cation Channels. Part 2: Functional and Molecular Properties of ASOR/PAC Channels and Their Roles in Cell Volume Dysregulation and Acidotoxic Cell Death. Front Cell Dev Biol 2021;9:702317.
https://doi.org/10.3389/fcell.2021.702317 |
| 19 | Wang HY, Shimizu T, Numata T, Okada Y: Role of acid-sensitive outwardly rectifying anion channels in acidosis-induced cell death in human epithelial cells. Pflügers Arch 2007;454:223-233.
https://doi.org/10.1007/s00424-006-0193-z |
| 20 | Sato-Numata K, Numata T, Okada T, Okada Y: Acid-sensitive outwardly rectifying (ASOR) anion channels in human epithelial cells are highly sensitive to temperature and independent of ClC-3. Pflügers Arch 2013;465:1535-1543.
https://doi.org/10.1007/s00424-013-1296-y |
| 21 | Capurro V, Gianotti A, Caci E, Ravazzolo R, Galietta LJV, Zegarra-Moran O: Functional analysis of acid-activated Cl⁻ channels: properties and mechanisms of regulation. Biochim Biophys Acta 2015;1848(1 Pt A):105-114.
https://doi.org/10.1016/j.bbamem.2014.10.008 |
| 22 | Xu L, Fidler IJ: Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res 2000;60:4610-4616
|
| 23 | Jones KM, Randtke EA , Yoshimaru ES, Howison CM , Chalasani P, Klein RR , Chambers SK , Kuo PH, Pagel MD: Clinical Translation of Tumor Acidosis Measurements with AcidoCEST MRI. Mol Imaging Biol 2017;19:617-625.
https://doi.org/10.1007/s11307-016-1029-7 |
| 24 | Hansen MB, Nielsen SE, Berg K: Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 1989;119:203-210.
https://doi.org/10.1016/0022-1759(89)90397-9 |
| 25 | Sato-Numata K, Numata T, Okada Y: Temperature sensitivity of acid-sensitive outwardly rectifying (ASOR) anion channels in cortical neurons is involved in hypothermic neuroprotection against acidotoxic necrosis. Channels (Austin) 2014;8:278-283.
https://doi.org/10.4161/chan.27748 |
| 26 | Lambert S, Oberwinkler J: Characterization of a proton-activated, outwardly rectifying anion channel. J Physiol 2005;567:191-213.
https://doi.org/10.1113/jphysiol.2005.089888 |
| 27 | Sato-Numata K, Numata T, Inoue R, Okada Y: Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel. Pflügers Arch 2016;468:795-803.
https://doi.org/10.1007/s00424-015-1786-1 |
| 28 | Harteneck C: Pregnenolone sulfate: from steroid metabolite to TRP channel ligand. Molecules 2013;18:12012-12028.
https://doi.org/10.3390/molecules181012012 |
| 29 | Marx S, Dal Maso T, Chen JW, Bury M, Wouters J, Michiels C, Le Calvé B: Transmembrane (TMEM) protein family members: Poorly characterized even if essential for the metastatic process. Semin Cancer Biol 2020;60:96-106.
https://doi.org/10.1016/j.semcancer.2019.08.018 |
| 30 | Pedersen SF, Okada Y, Nilius B: Biophysics and Physiology of the Volume-Regulated Anion Channel (VRAC)/Volume-Sensitive Outwardly Rectifying Anion Channel (VSOR). Pflügers Arch 2016;468:371-383.
https://doi.org/10.1007/s00424-015-1781-6 |
| 31 | Ise T, Shimizu T, Lee EL, Inoue H, Kohno K, Okada Y: Roles of volume-sensitive Cl- channel in cisplatin-induced apoptosis in human epidermoid cancer cells. J Membr Biol 2005;205:139-145.
https://doi.org/10.1007/s00232-005-0779-y |
| 32 | Sauter DR, Novak I, Pedersen SF, Larsen EH, Hoffmann EK: ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). Pflügers Arch 2014;467:1495-1508.
https://doi.org/10.1007/s00424-014-1598-8 |
| 33 | Pedersen SF, Prenen J, Droogmans G, Hoffmann EK, Nilius B: Separate swelling- and Ca2+ -activated anion currents in Ehrlich ascites tumor cells. J Membr Biol 1998;163:97-110.
https://doi.org/10.1007/s002329900374 |
| 34 | Okada Y, Sabirov RZ, Sato-Numata K, Numata T: Cell Death Induction and Protection by Activation of Ubiquitously Expressed Anion/Cation Channels. Part 1: Roles of VSOR/VRAC in Cell Volume Regulation, Release of Double-Edged Signals and Apoptotic/Necrotic Cell Death. Front Cell Dev Biol 2021;8:614040.
https://doi.org/10.3389/fcell.2020.614040 |
| 35 | Ma ZY, Zhang W, Chen L, Wang R, Kan XH, Sun GZ, Liu CX, Li L, Zhang Y: A proton-activated, outwardly rectifying chloride channel in human umbilical vein endothelial cells. Biochem Biophys Res Commun 2008;371:437-440.
https://doi.org/10.1016/j.bbrc.2008.04.090 |
| 36 | Nobles M, Higgins CF, Sardini A: Extracellular acidification elicits a chloride current that shares characteristics with ICl (swell). Am J Physiol Cell Physiol 2004;287:C1426-C1435.
https://doi.org/10.1152/ajpcell.00549.2002 |
| 37 | Shi CY, Wang R, Liu CX, Jiang H, Ma ZY, Li L, Zhang W: Simvastatin inhibits acidic extracellular pH-activated, outward rectifying chloride currents in RAW264.7 monocytic-macrophage and human peripheral monocytes. Int Immunopharmacol 2009;9:247-252.
https://doi.org/10.1016/j.intimp.2008.11.011 |
| 38 | Yamamoto S, Ehara T: Acidic extracellular pH-activated outwardly rectifying chloride current in mammalian cardiac myocytes. Am J Physiol Heart Circ Physiol 2006;290: H1905-H1914.
https://doi.org/10.1152/ajpheart.00965.2005 |
| 39 | Hille B: Ion Channels of Excitable Membranes. 3rd ed, Sunderland, Sinauer Associates, 2001, pp 162-163.
|